Quality of Life and the Experience of Living with Early-Stage Alzheimer’s Disease
Abstract
Background:
There is a need to better understand the experience of patients living with Alzheimer's disease (AD) in the early stages.
Objective:
The aim of the study was to evaluate the perception of quality of life in patients with early-stage AD.
Methods:
A multicenter, non-interventional study was conducted including patients of 50–90 years of age with prodromal or mild AD, a Mini-Mental State Examination (MMSE) score ≥22, and a Clinical Dementia Rating-Global score (CDR-GS) of 0.5.–1.0. The Quality of Life in Alzheimer ’s Disease (QoL-AD) questionnaire was used to assess health-related quality of life. A battery of self-report instruments was used to evaluate different psychological and behavioral domains. Associations between the QoL-AD and other outcome measures were analyzed using Spearman’s rank correlations.
Results:
A total of 149 patients were included. Mean age (SD) was 72.3 (7.0) years and mean disease duration was 1.4 (1.8) years. Mean MMSE score was 24.6 (2.1). The mean QoL-AD score was 37.9 (4.5). Eighty-three percent (n = 124) of patients had moderate-to-severe hopelessness, 22.1% (n = 33) had depressive symptoms, and 36.9% (n = 55) felt stigmatized. The quality of life showed a significant positive correlation with self-efficacy and negative correlations with depression, emotional and practical consequences, stigma, and hopelessness.
Conclusion:
Stigma, depressive symptoms, and hopelessness are frequent scenarios in AD negatively impacting quality of life, even in a population with short disease duration and minimal cognitive impairment.
INTRODUCTION
Alzheimer’s disease (AD) is currently one of the most important health problems due to an estimated prevalence of prodromal and AD dementia of 69 and 32 million people, respectively [1]. Despite different efforts to reframe the management of AD based on a patient-centered approach, patients are still given a passive role [2– 4]. Different drug development programs targeting amyloid and tau proteins in patients with AD are underway in recent years [5]. However, the measures traditionally included in these clinical trials are not sufficient to assess the full range of disease severity from the perspective of patients and their caregivers [2, 3, 6].
Subjective perception of life satisfaction and quality of life are indicators of the ability to live well in patients with chronic diseases [3]. Most patients with AD want to be informed of the diagnosis in order to plan their long-term care, to participate in medical decisions and clinical trials, and to maintain meaningful social relationships within their community [2, 7]. Quality of life is the priority for both patients with mild cognitive impairment and their partners, followed by maintaining their memory, mental status, and autonomy [6]. Optimism, self-esteem, and self-efficacy were associated with increased coping ability in patients with dementia [9].
Identifying the most meaningful outcomes for patients and their caregivers is essential to incorporate the voice of AD patients according to their beliefs and preferences [4, 7, 10]. The aim of this study was to assess the perception of quality of life in patients with early-stage AD using a battery of different patient-reported measurements.
METHODS
We conducted a non-interventional, cross-sectional study at 21 hospital-based memory clinics in Spain. Patients between 50– 90 years old with a diagnosis of prodromal or mild AD (National Institute on Aging/Alzheimer’s Association criteria) [11], a Clinical Dementia Rating-Global score (CDR-GS) of 0.5 to 1.0 [12], and a Mini-mental State Examination (MMSE) score ≥22 [13] were included. The Research Ethics Board of Hospital de la Santa Creu i Sant Pau (Barcelona, Spain) approved the study design. Written informed consent was obtained from all participants.
Outcome measures
The Quality of Life in Alzheimer’s Disease (QoL-AD) questionnaire [14] was used to assess health-related quality of life. Total score ranges from 13 to 52 with higher points indicating better quality of life. In addition, a battery of different self-report instruments was used to evaluate functioning (Functional Activities Questionnaire, FAQ) [15], mood (Beck Depression Inventory-Fast Screen, BDI-FS) [16], hopelessness (Beck Hopelessness Scale, BHS) [17], perception of stigma (Stigma Scale for Chronic Illness, SSCI-8) [18], understanding of the illness and its consequences (Representations and Adjustment to Dementia Index, RADIX) [19], the ability to overcome obstacles and setbacks or self-efficacy (General Self-Efficacy Scale, GSES) [20], and life satisfaction (Satisfaction With Life Scale, SWLS) [21]. The characteristics and scoring of each of these instruments have been published elsewhere [22].
Statistical analysis
Demographic and clinical characteristics were summarized using descriptive statistics. Associations between the QoL-AD and other outcome measures were analyzed using Spearman’s rank correlations. Statistical significance was set at p < 0.05. The analysis was performed using IBM SPSS Statistics software version 22.0 (IBM Corp., Armonk, NY, USA).
RESULTS
A total of 149 patients were included. Mean (SD) age was 72.3 (7.0) years and mean disease duration was 1.4 (1.8) years. Almost 90% of patients had a CDR-GS score of 0.5 with a mean MMSE score of 24.6 (2.1). Most participants were retired (79.2%) and independent for activities of daily living (72.5% with a FAQ score <9). Table 1 shows main sociodemographic and clinical characteristics of participants.
Table 1
Sociodemographic and clinical characteristics
| N = 149 | |
| Age, mean (SD), y | 72.3 (7.0) |
| Female, n (%) | 75 (50.3) |
| Marital status, married, n (%) | 125 (83.9) |
| Working status, n (%) | |
| Retired | 118 (79.2) |
| Housewife | 13 (8.7) |
| Partial or full-time employed | 7 (4.7) |
| Education, mean (SD), y | 13.1 (10.0) |
| Cardiovascular risk factors, n (%) | 107 (71.8) |
| Hyperlipidemia, % | 70.1 |
| Arterial hypertension, % | 59.8 |
| Disease duration, mean (SD), y | 1.4 (1.8) |
| MMSE score, mean (SD) | 24.6 (2.1) |
| MMSE score distribution, n (%) | |
| 22– 24 | 79 (53.0) |
| ≥25 | 70 (47.0) |
| ADAS-Cog13 score, mean (SD) | 24.4 (5.2) |
| CDR-GS score, n (%) | |
| 0.5 | 130 (87.2) |
| 1 | 19 (12.7) |
| FAQ score, mean (SD) | 6.0 (6.2) |
| FAQ score <9a, n (%) | 108 (72.5) |
| Use of anticholinesterase inhibitors, n (%) | 86 (57.7) |
ADAS-Cog13, Alzheimer's Disease Assessment Scale-Cognitive; CDR-GS, Clinical Dementia Rating-Global Score; FAQ, Functional Activities Questionnaire; MMSE, Mini-Mental State Examination; SD, standard deviation. aA score <9 indicates independence for activities of daily living.
Mean QoL-AD score was 37.9 (4.5) (Table 1), and memory was the domain with the highest negative impact, followed by mood, self as a whole, and energy (Fig. 1). Depressive symptoms were found in 22.1% (n = 33) of patients and moderate-to-severe feelings of hopelessness in 83.2% (n = 124) (Table 2). “I can’t imagine what my life would be in 10 years” and “Things just don’t work out the way I want them to” were the most frequent feelings of hopelessness (64.2% and 56.7%, respectively). The prevalence of stigma was 36.9% (n = 55) with low-to-moderate severity (Table 3). Similarly, participants reported their beliefs about AD and its emotional and practical consequences (Table 4). “People treat me differently”, “I do not go out as much as I used to”, and “I get very angry about what it is happening to me” were the most common self-reported consequences.
Fig. 1
Quality of Life in Alzheimer’s Disease Scale domains.
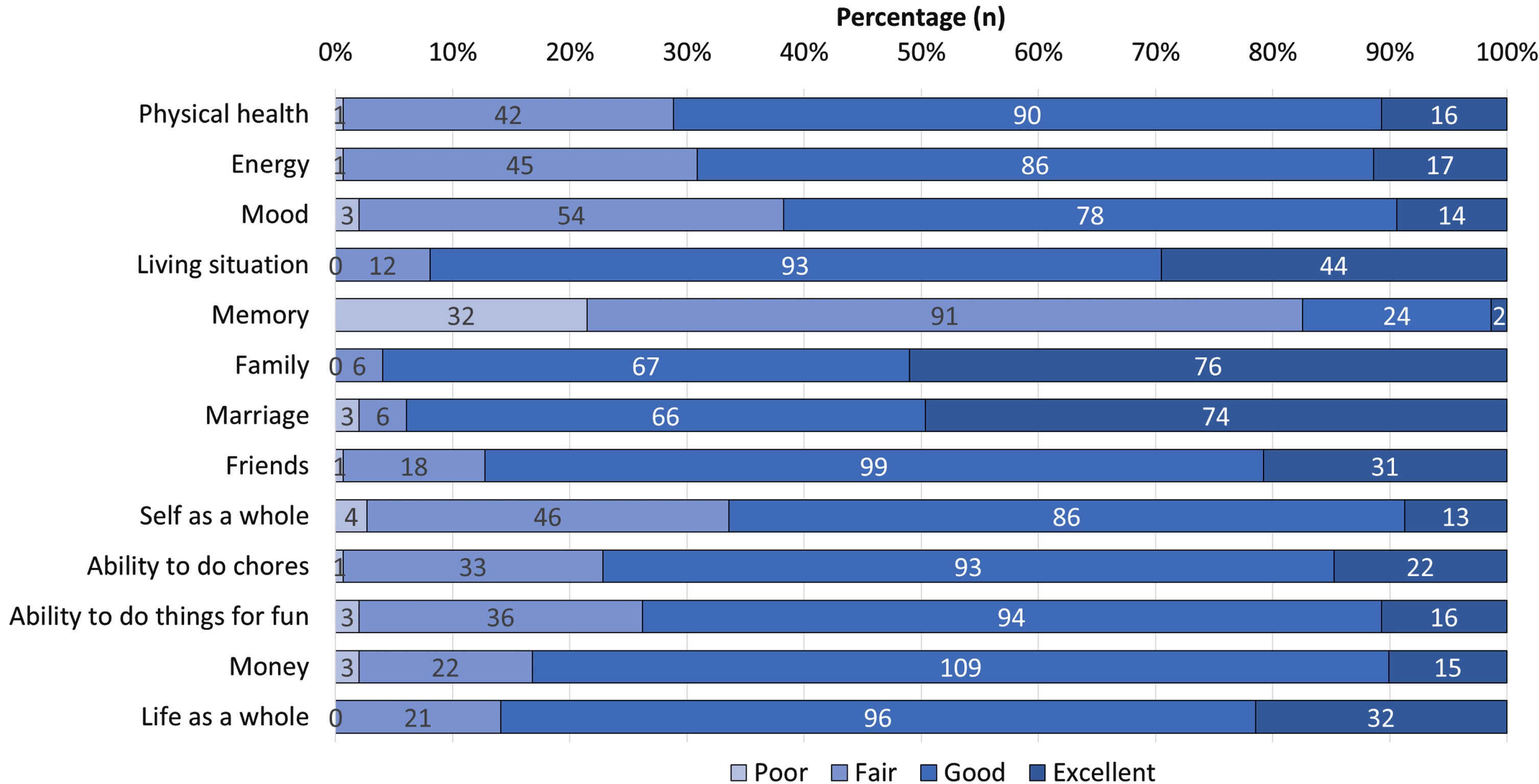
Table 2
Outcome measures
| N = 149 | |
| QoL-AD score1, mean (SD) | 37.9 (4.5) |
| SWLS score, mean (SD) | 27.5 (5.7) |
| SWLS score ≥25a, n (%) | 120 (80.5) |
| BDI-FS score, mean (SD) | 2.1 (2.2) |
| BDI-FS score ≥4b, n (%) | 33 (22.1) |
| BHS score, mean (SD) | 10.7 (2.4) |
| BHS score ≥9c, n (%) | 124 (83.2) |
| SSCI-8 score, mean (SD) | 2.1 (2.2) |
| SSCI-8 score ≥8d, n (%) | 55 (36.9) |
| Practical consequences RADIX score2, mean (SD) | 1.8 (0.6) |
| Emotional consequences RADIX score2, mean (SD) | 2.2 (0.8) |
| GSES score3, mean (SD) | 30.0 (6.3) |
BDI-FS, Beck Depression Inventory-Fast Screen; BHS, Beck Hopelessness Scale; GSES, General Self-Efficacy Scale; QoL-AD, Quality of Life in Alzheimer’s Disease; RADIX, Representations and Adjustment to Dementia Index; SD, standard deviation; SSCI8, Stigma Scale for Chronic Illness; SWLS, Satisfaction With Life Scale. aA score ≥25 indicates that respondents are satisfied or extremely satisfied with life; ba score ≥4 indicates the presence of depressive symptoms; ca score ≥9 indicates moderate-to-severe hopelessness; da score >8 indicates the presence of stigmatization. 1Total score ranges from 13 to 52 with higher points indicating better quality of life; 2total score ranges from 1 to 4 (practical) and 1 to 5 (emotional) with higher scores indicating greater negative consequences; 3total score ranges from 10 to 40 with higher scores indicating higher levels of optimistic self-belief.
Table 3
Perception of stigma (N = 149)
| Frequency, n (%) | |||
| SSCI-8 items | Never/Rarely | Sometimes | Often/Always |
| Because of my illness, some people seemed | 145 (97.3) | 4 (2.7) | 0 (0) |
| uncomfortable with me | |||
| Because of my illness, some people avoided me | 145 (97.3) | 4 (2.7) | 0 (0) |
| Because of my illness, I felt left out of things | 134 (89.9) | 15 (10.1) | 0 (0) |
| Because of my illness, people were unkind to me | 146 (98.0) | 3 (2.0) | 0 (0) |
| Because of my illness, people avoided looking at me | 149 (100) | 0 (0) | 0 (0) |
| I felt embarrassed about my illness | 138 (92.6) | 9 (6.0) | 2 (1.3) |
| I felt embarrassed because of my physical | 144 (96.6) | 3 (2.0) | 2 (2.9) |
| limitations | |||
| Some people acted as though it was my fault | 145 (97.3) | 2 (1.3) | 1 (0.7) |
| I have this illness | |||
SSCI-8, Stigma Scale for Chronic Illness.
Table 4
Practical and emotional consequences (N = 146)
| RADIX items | Frequency, n (%) | ||
| Strongly disagree/Disagree | Agree | Strongly agree | |
| Practical Consequences | |||
| People treat me differently | 17 (11.6) | 52 (35.6) | 77 (52.8) |
| I do not go out as much as I used to | 22 (15.0) | 48 (32.8) | 76 (52.0) |
| I cannot do some of the things I used to do | 42 (28.7) | 62 (42.6) | 42 (28.7) |
| I feel I have lost control over my life | 28 (19.1) | 62 (42.5) | 56 (38.4) |
| Emotional Consequences | |||
| I get annoyed or frustrated with myself | 54 (36.9) | 45 (30.8) | 47 (32.3) |
| I get very angry about what it is happening to me | 48 (32.9) | 53 (36.3) | 45 (30.8) |
| I feel I have lost confidence in myself | 53 (36.3) | 53 (36.3) | 40 (27.4) |
| I feel low or upset when I think about my condition | 56 (38.4) | 51 (34.9) | 39 (26.7) |
| I find myself worrying about my condition | 62 (42.4) | 44 (30.2) | 40 (27.4) |
RADIX, Representations and Adjustment to Dementia Index.
3.1Correlations between quality of life and other outcome measures
The QoL-AD score showed a positive correlation with GSES score and negative correlations with BDI-FS, RADIX emotional and practical consequences, SSCI-8, and BHS scores (Table 5).
Table 5
Spearman correlations between quality of life and other outcome measures
| QoL-AD rho (p) | |
| MMSE | 0.06 (0.46) |
| ADAS-Cog13 | 0.003 (0.96) |
| CDR-GS | 0.13 (0.09) |
| Years of education | 0.04 (0.60) |
| Disease duration | – 0.14 (0.07) |
| FAQ | – 0.04 (0.60) |
| BDI-FS | – 0.45 (<0.0001) |
| BHS | – 0.22 (0.006) |
| SSCI-8 | – 0.31 (<0.0001) |
| GSES | 0.34 (<0.0001) |
| SWLS | 0.44 (<0.0001) |
| Practical consequences RADIX | – 0.38 (<0.0001) |
| Emotional consequences RADIX | – 0.41 (<0.0001) |
ADAS-Cog13, Alzheimer’s Disease Assessment Scale-Cognitive; BDI-FS, Beck Depression Inventory-Fast Screen; BHS, Beck Hopelessness Scale; CDR-GS, Clinical Dementia Rating-Global Score; FAQ, Functional Activities Questionnaire; GSES, General Self-Efficacy Scale; MMSE, Mini-Mental State Examination; QoL-AD, Quality of Life in Alzheimer’s Disease; RADIX, Representations and Adjustment to Dementia Index; SD, standard deviation; SSCI-8, Stigma Scale for Chronic Illness; SWLS, Satisfaction With Life Scale.
DISCUSSION
Quality of life is a concept involving physical and mental health, cognitive performance, social interactions, and subjective well-being [23, 24]. Wehrman et al. [7] found that quality of life was the outcome most prioritized by patients with dementia or mild cognitive impairment and that this preference remained stable over time, regardless of the progression of cognitive impairment. Patients with AD can describe their beliefs and concerns throughout the disease trajectory [7, 8, 10, 25]. However, there is a lack of information regarding the subjective experiences of patients with early-stage AD [24].
In our study, depressive symptoms, feelings of hopelessness and stigma were frequent findings impacting on quality of life in a population with a short duration of illness (mean of 1.4 years since AD diagnosis) and minimal cognitive impairment (87.2% with a CDR-GS score of 0.5). Lima et al. [26] found that poor physical activity and functionality, depression, and anxiety were associated with low quality of life in a sample of 158 patients with mild AD. Patients with mild cognitive impairment also have a reduction in their quality of life in crucial domains including cognition, mood, social relationships, and helping with household chores [27]. Quality of life is already impacted in people with subjective cognitive decline, especially in the physical and mental health domains [28, 29].
Depressive symptoms are very frequent in patients with mild cognitive impairment with a prevalence between 16.9% and 55% and are associated with an increased risk of dementia [30– 32]. Depressive symptoms and memory impairment have traditionally been considered the main factors influencing the quality of life of patients with dementia [30, 31]. Negative attitudes about the future or hopelessness is conceived as the experience of anticipating unfavorable situations or consequences that are beyond a person’s control [33]. Hopelessness is recognized as a risk factor for self-harm and suicide [34]. In line with these results, 22% of patients in our study had depressive symptoms and 83% had moderate-to-severe hopelessness, implying a potential suicide risk.
We found almost 37% of patients had some stigma perception in our study. Stigmatizing attitudes have been associated with different neurological disorders contributing to low self-esteem, depression, and reduced health-seeking activity [35– 38]. People have beliefs about AD that are based upon their personal experience of the disease, as well as its depiction in the media and artistic works. Unfortunately, the latter often depict the most advanced cases with a high loss of functional independence. Different studies found that stigma is more pronounced in those with limited AD knowledge, those with little contact with AD patients, young people, and men [39]. On the other hand, we must also consider the internal stigma due to subjective beliefs and representations about the disease generated by patients themselves, especially in the early stages after diagnosis. Using the RADIX questionnaire [19] in our study, we could detect different emotional and practical consequences based on patients’ illness representations. Patients felt that people treated them differently and also reported a spectrum of negative feelings including anger, frustration, upset and worry.
Interestingly, the mean SWLS score in our study was 27.5, with 80.5% of patients considering themselves satisfied or extremely satisfied with life. Similar findings were previously reported in the literature [24, 27]. The decline in self-reported quality of life was not accompanied by the expected parallel decline in life satisfaction, for which a possible explanation could be that the QoL-AD and SWLS measure different constructs [24, 27].
Our study has some limitations. First, the perceptions of patients’ relatives or partners were not collected in this study. The discrepancy in the assessment of quality of life between self-report and proxy report has been described from the onset of cognitive impairment regardless of the instrument administered [40]. Therefore, we cannot rule out that the negative impact on quality of life has been underestimated in our study. Second, the cross-sectional design of the study does not allow us to identify any changes in the patient’s perception of quality of life over time [25]. Third, we acknowledge a potential selection bias given that those people most motivated to collaborate or with a better relationship with their physicians may have enrolled in the study. Finally, patients were recruited from memory clinics. It would be important to replicate these results in a population-based study.
The ability to live well with AD should be the main goal of multidisciplinary teams managing this disease, especially in the early stages where patients retain their awareness of the disease and can make key decisions for their future [41]. Different psychological resources have been described that could help patients deal with negative beliefs associated with the diagnosis of AD and uncertainty about the disease progression and loss of autonomy. Langer et al. [42] found that self-efficacy was associated with a better perception of quality of life in patients with mild cognitive impairment. In addition, resilient coping was associated with greater autonomy and better quality of life than non-resilient patients in a study in mild cognitive impairment [43]. Patients’ coping strategies and family cohesion and flexibility as well as good communication among their members moderated the relationship between psychological problems and quality of life [26].
Despite successive awareness campaigns and educational programs, lack of information and misconceptions about AD and mild cognitive impairment remain a critical problem [36– 39]. Therefore, as a strategy to reduce the impact of stigma on patients, more information campaigns are needed, especially targeting those population groups identified as having the largest knowledge gaps about AD [36].
Conclusion
Stigma, depressive symptoms, and hopelessness are frequent scenarios in AD negatively impacting quality of life, even in a population with short disease duration and minimal cognitive impairment. Understanding patients' perceptions in the early stages of the disease may facilitate adopting specific strategies to develop psychological resources to foster living well with AD.
ACKNOWLEDGMENTS
The authors would like to acknowledge all patients and their families for making this study possible. The authors would also like to thank Alicia Subtil-Rodríguez (Evidenze Group) for her medical writing support. This study was funded by the Medical Department of Roche Farma Spain (ML42346).
A preliminary report of this data was presented as a poster at the 74th Annual Meeting of the American Academy of Neurology, April 2022 (P829).
Qualified researchers may request access to individual patient-level data through the corresponding author. The datasets generated during the analysis of the study are available from the corresponding author on reasonable request.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/22-0696r1).
REFERENCES
[1] | Gustavsson A , Norton N , Fast T , Frölich L , Georges J , Holzapfel D , Kirabali T , Krolak-Salmon P , Rossini PM , Ferretti MT , Lanman L , Chadha AS , van der Flier WM (2022) Global estimates on the number of 295 persons across the Alzheimer’s disease continuum. Alzheimers Dement. doi: 10.1002/alz.12694. |
[2] | Gaugler JE , Bain LJ , Mitchell L , Finlay J , Fazio S , Jutkowitz E , Alzheimer’s Association Psychosocial Measurement Workgroup ((2019) ) Reconsidering frameworks of Alzheimer’s dementia when assessing psychosocial outcomes. Alzheimers Dement (N Y) 5: , 388–397. |
[3] | Mast BT , Molony SL , Nicholson N , Kate Keefe C , DiGasbarro D ((2021) ) Person-centered assessment of people living with dementia: Review of existing measures. Alzheimers Dement (N Y) 7: , e12138. |
[4] | DiBenedetti DB , Slota C , Wronski SL , Vradenburg G , Comer M , Callahan LF , Winfield J , Rubino I , Krasa HB , Hartry A , Wieberg D , Kremer IN , Lappin D , Martin AD , Frangiosa T , Biggar V , Hauber B ((2020) ) Assessing what matters most to patients with or at risk forAlzheimer’s and care partners: A qualitative study evaluatingsymptoms, impacts, and outcomes. Alzheimers Res Ther 12: , 90. |
[5] | Cohen S , Cummings J , Knox S , Potashman M , Harrison J ((2022) ) Clinical trial endpoints and their clinical meaningfulness in early stages of Alzheimer’s disease. J Prev Alzheimers Dis 9: , 507–522. |
[6] | Tochel C , Smith M , Baldwin H , Gustavsson A , Ly A , Bexelius C , Nelson M , Bintener C , Fantoni E , Garre-Olmo J , Janssen O , Jindra C , Jørgensen IF , McKeown A , Öztürk B , Ponjoan A , Potashman MH , Reed C , Roncancio-Diaz E , Vos S , Sudlow C ; ROADMAP consortium ((2019) ) What outcomes are important to patients with mild cognitive impairment or Alzheimer’s disease, their caregivers, and health-care professionals? A systematic review. Alzheimers Dement (Amst) 11: , 231–247. |
[7] | Wehrmann H , Michalowsky B , Lepper S , Mohr W , Raedke A , Hoffmann W ((2021) ) Priorities and preferences of people living with dementia or cognitive impairment - a systematic review. Patient Prefer Adherence 15: , 2793–2807. |
[8] | Smith GE , Chandler M , Fields JA , Aakre J , Locke DEC ((2018) ) A survey of patient and partner outcome and treatment preferences in mild cognitive impairment. J Alzheimers Dis 63: , 1459–1468. |
[9] | Lamont RA , Nelis SM , Quinn C , Martyr A , Rippon I , Kopelman MD , Hindle JV , Jones RW , Litherland R , Clare L ((2020) ) Psychological predictors of ‘living well’ with dementia: Findings from the IDEAL study. Aging Ment Health 24: , 956–964. |
[10] | Clare L , Gamble LD , Martyr A , Quinn C , Litherland R , Morris RG , Jones IR , Matthews FE ((2022) ) Psychological processes in adapting to dementia: Illness representations among the IDEAL cohort. Psychol Aging 37: , 524–541. |
[11] | Jack CR Jr , Bennett DA , Blennow K , Carrillo MC , Dunn B , Haeberlein SB , Holtzman DM , Jagust W , Jessen F , Karlawish J , Liu E , Molinuevo JL , Montine T , Phelps C , Rankin KP , Rowe CC , Scheltens P , Siemers E , Snyder HM , Sperling R ; Contributors ((2018) ) NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14: , 535–562. |
[12] | Berg L ((1988) ) Clinical Dementia Rating (CDR). Psychopharmacol Bull 24: , 637–639. |
[13] | Folstein MF , Folstein SE , McHugh PR ((1975) ) “Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: , 189–198. |
[14] | Logsdon RG , Gibbons LE , McCurry SM , Teri L ((2002) ) Assessing quality of life in older adults with cognitive impairment. Psychosom Med 64: , 510–519. |
[15] | Marshall GA , Zoller AS , Lorius N , Amariglio RE , Locascio JJ , Johnson KA , Sperling RA , Rentz DM ((2015) ) Functional Activities Questionnaire items that best discriminate and predict progression from clinically normal to mild cognitive impairment. Curr Alzheimer Res 12: , 493–502. |
[16] | Elben S , Dimenshteyn K , Trenado C , Folkerts AK , Ophey A , Sulzer P , Becker S , Schmidt N , Tödt I , Witt K , Liepelt-Scarfone I , Yilmaz R , Kalbe E , Wojtecki L ((2021) ) Screen fast, screen faster: A pilot study to screen for depressive symptoms using the Beck Depression Inventory Fast Screen in Parkinson’s disease with mild cognitive impairment. Front Neurol 12: , 640137. |
[17] | Dyce JA ((1996) ) Factor structure of the beck hopelessness scale. J Clin Psychol 52: , 555–558. |
[18] | Molina Y , Choi SW , Cella D , Rao D ((2013) ) The stigma scale for chronic illnesses 8-item version (SSCI-8): Development, validation and use across neurological conditions. Int J Behav Med 20: , 450–60. |
[19] | Quinn C , Morris RG , Clare L ((2018) ) Beliefs about dementia: Development and validation of the Representations and Adjustment to Dementia Index (RADIX). Am J Geriatr Psychiatry 26: , 680–689. |
[20] | Schwarzer R , Jerusalem M (1995) Generalized Self-Efficacy scale. In Measures in Health Psychology: A User’s Portfolio. Causal and control beliefs, Weinman J, Wright S, Johnston M, eds. NFER-NELSON, Windsor, UK, pp. 35–37. |
[21] | Pavot W , Diener E , Colvin CR , Sandvik E ((1991) ) Further validation of the Satisfaction with Life Scale: Evidence for the cross-method convergence of well-being measures. J Pers Assess 57: , 149–161. |
[22] | Villarejo-Galende A , García-Arcelay E , Piñol-Ripoll G , Del Olmo-Rodríguez A , Viñuela F , Boada M , Franco-Macías E , de la Peña AI , Riverol M , Puig-Pijoan A , Abizanda-Soler P , Arroyo R , Baquero-Toledo M , Feria-Vilar I , Balasa M , Berbel Á , Rodríguez-Rodríguez E , Vieira-Campos A , García-Ribas G , Rodrigo-Herrero S , Lleó A , Maurino J ((2022) ) Awareness of diagnosis in persons with early-stage Alzheimer’s disease: An observational study in Spain. Neurol Ther 11: , 1183–1192. |
[23] | Landeiro F , Mughal S , Walsh K , Nye E , Morton J , Williams H , Ghinai I , Castro Y , Leal J , Roberts N , Wace H , Handels R , Lecomte P , Gustavsson A , Roncancio-Diaz E , Belger M , Jhuti GS , Bouvy JC , Potashman MH , Tockhorn-Heidenreich A , Gray AM; ROADMAP consortium ((2020) ) Health-related quality of life in people with predementia Alzheimer’s disease, mild cognitive impairment or dementia measured with preference-based instruments: A systematic literature review. Alzheimers Res Ther 12: , 154. |
[24] | Clarke C , Woods B , Moniz-Cook E , Mountain G , Øksnebjerg L , Chattat R , Diaz A , Gove D , Vernooij-Dassen M , Wolverson E ((2020) ) Measuring the well-being of people with dementia: A conceptual scoping review. Health Qual Life Outcomes 18: , 249. |
[25] | Alexander CM , Martyr A , Clare L ; IDEAL Programme Research Team ((2022) ) Changes in awareness of condition in people with mild-to-moderate dementia: Longitudinal findings from the IDEAL cohort. Int J Geriatr Psychiatry 37: . doi: 10.1002/gps.5702. |
[26] | Lima S , Sevilha S , Pereira MG ((2020) ) Quality of life in early-stage Alzheimer’s disease: The moderator role of family variables and coping strategies from the patients’ perspective. Psychogeriatrics 20: , 557–567. |
[27] | Bárrios H , Narciso S , Guerreiro M , Maroco J , Logsdon R , de Mendonça A ((2013) ) Quality of life in patients with mild cognitive impairment. Aging Ment Health 17: , 287–292. |
[28] | Pusswald G , Tropper E , Kryspin-Exner I , Moser D , Klug S , Auff E , Dal-Bianco P , Lehrner J ((2015) ) Health-related quality of life in patients with subjective cognitive decline and mild cognitive impairment and its relation to activities of daily living. J Alzheimers Dis 47: , 479–486. |
[29] | Stites SD , Harkins K , Rubright JD , Karlawish J ((2018) ) Relationships between cognitive complaints and quality of life in older adults with mild cognitive impairment, mild Alzheimer disease dementia, and normal cognition. Alzheimer Dis Assoc Disord 32: , 276–283. |
[30] | Pusswald G , Moser D , Pflüger M , Gleiss A , Auff E , Stögmann E , Dal-Bianco P , Lehrner J ((2016) ) The impact of depressive symptoms on health-related quality of life in patients with subjective cognitive decline, mild cognitive impairment, and Alzheimer’s disease. Int Psychogeriatr 28: , 2045–2054. |
[31] | Ma L ((2020) ) Depression, anxiety, and apathy in mild cognitive impairment: Current perspectives. Front Aging Neurosci 12: , 9. |
[32] | Tan EYL , Köhler S , Hamel REG , Muñoz-Sánchez JL , Verhey FRJ , Ramakers IHGB ((2019) ) Depressive symptoms in mild cognitiveimpairment and the risk of dementia: A systematic review andcomparative meta-analysis of clinical and community-based studies. J Alzheimers Dis 67: , 1319–1329. |
[33] | Huen JM , Ip BY , Ho SM , Yip PS ((2015) ) Hope and hopelessness: The role of hope in buffering the impact of hopelessness on suicidal ideation. PLoS One 10: , e0130073. |
[34] | Balsamo M , Carlucci L , Innamorati M , Lester D , Pompili M ((2020) ) Further Insights Into the Beck Hopelessness Scale (BHS): Unidimensionality among psychiatric inpatients. Front Psychiatry 31: , 727. |
[35] | European Federation of Neurological Associations (EFNA) (2020) Survey on stigma and neurological disorder. Available from: https://www.efna.net/wp-content/uploads/2020/07/SurveyReport2020.pdf. Accessed July, 2022. |
[36] | Herrmann LK , Welter E , Leverenz J , Lerner AJ , Udelson N , Kanetsky C ((2018) ) We move the stigma dial? Am J Geriatr Psychiatry 26: , 316–331. |
[37] | Rosin ER , Blasco D , Pilozzi AR , Yang LH , Huang X ((2020) ) A narrative review of Alzheimer’s disease stigma. J Alzheimers Dis 78: , 515–528. |
[38] | Lion K , Szcześniak D , Bulińska K , Evans SB , Evans SC , Saibene FL , d’Arma A , Farina E , Brooker DJ , Chattat R , Meiland FJM , Dröes RM , Rymaszewska J ((2020) ) Do people with dementia and mildcognitive impairments experience stigma? A cross-culturalinvestigation between Italy, Poland and the UK. Aging MentHealth 24: , 947–955. |
[39] | Garcia-Ribas G , García-Arcelay E , Montoya A , Maurino J ((2020) ) Assessing knowledge and perceptions of Alzheimer’s disease among employees of a pharmaceutical company in spain: A comparison between caregivers and non-caregivers. Patient Prefer Adherence 14: , 2357–2364. |
[40] | Hutchinson C , Worley A , Khadka J , Milte R , Cleland J , Ratcliffe J ((2022) ) Do we agree or disagree? A systematic review of the application of preference-based instruments in self and proxy reporting of quality of life in older people. Soc Sci Med 305: , 115046. |
[41] | Galvin JE , Aisen P , Langbaum JB , Rodriguez E , Sabbagh M , Stefanacci R , Stern RA , Vassey EA , de Wilde A , West N , Rubino I ((2021) ) Early stages of Alzheimer’s disease: Evolving the care team for optimal patient management. Front Neurol 11: , 592302. |
[42] | Langer K , O’Shea DM , De Wit L , DeFeis B , Mejia A , Amofa P , Chandler M , Locke DEC , Fields J , Phatak V , Dean PM , Smith G ((2019) ) Self-efficacy mediates the association between physical function and perceived quality of life in individuals with mild cognitive impairment. J Alzheimers Dis 68: , 1511–1519. |
[43] | Clement-Carbonell V , Ferrer-Cascales R , Ruiz-Robledillo N , Rubio-Aparicio M , Portilla-Tamarit I , Cabañero-Martínez MJ ((2019) ) Differences in autonomy and health-related quality of life between resilient and non-resilient individuals with mild cognitive impairment. Int J Environ Res Public Health 16: , 2317. |



