Comparison of Steady-State Pharmacokinetics of Donepezil Transdermal Delivery System with Oral Donepezil
Abstract
Background:
Donepezil is approved for treatment of dementia of the Alzheimer type and is currently available only in tablet forms in the United States.
Objective:
To compare steady-state pharmacokinetics of once-weekly 10-mg/d and 5-mg/d Corplex™ donepezil transdermal delivery systems (TDS) with once-daily 10-mg oral donepezil.
Methods:
Open-label, randomized, crossover study (NCT04617782) enrolled healthy participants aged 18–55 years. All participants received 5-mg/d donepezil TDS during the 5-week Period 1, followed by 10-mg/d TDS or 10-mg/d oral donepezil in the 5-week Period 2; treatments were switched in Period 3. Bioequivalence was assessed at steady state on Week 5.
Results:
All 60 enrolled participants received 5-mg/d TDS, 55 received 10-mg/d TDS, and 56 received oral donepezil. Adjusted geometric mean ratio (% [90% CI]) for maximum plasma concentration and area under the plasma concentration versus time curve (0–168 h) were 88.7 (81.7–96.2) and 108.6 (100.5–117.4) for 10-mg/d and 86.1 (79.8–92.9) and 105.3 (97.6–113.6) for dose-normalized 5-mg/d TDS and were generally within the 80% –125% range for establishing bioequivalence with oral donepezil. Skin adhesion was similar for both TDSs (>80% of patches remaining ≥75% adhered throughout the wear period). Overall incidence of adverse events (AEs) was similar across treatments. Compared with 10-mg/d TDS, oral donepezil was associated with higher incidence of gastrointestinal and nervous system AEs (14.5% versus 53.6% and 14.5% versus 30.4%, respectively).
Conclusion:
Donepezil TDSs are bioequivalent to oral donepezil at steady state and have a safety profile that supports their use in treating dementia of the Alzheimer type.
INTRODUCTION
Donepezil, a reversible acetylcholinesterase inhibitor, is approved by the United States Food and Drug Administration (FDA) for the treatment of dementia of the Alzheimer type in patients with mild, moderate, and severe disease [1, 2]. It is currently available in 5-, 10-, and 23-mg tablet forms or as an orally disintegrating tablet. All forms are administered once daily [2]. The initial dose of donepezil is 5 mg/d for mild to moderate dementia, which can be increased to 10 mg/d after 4 weeks [1]. For moderate to severe dementia, the dosage can be further increased up to 23 mg/d after the patient has been on 10-mg/d donepezil for at least 3 months [1]. However, the 23-mg/d dosage did not produce significantly greater improvement in global functioning when compared with 10-mg/d dosage and was associated with higher incidence of treatment-emergent adverse events (TEAEs) [3]. Dose escalation of oral donepezil can result in gastrointestinal distress, such as nausea, diarrhea, and vomiting, the most common adverse events (AEs) leading to treatment discontinuation in patients with Alzheimer’s disease [1].
Transdermal delivery of medications offers several benefits over the oral route of administration, including ease-of-use, maintenance of steady concentrations of the drug within the therapeutic range, and the possibility of reducing drug exposure faster by removing the patch. Transdermal delivery can also potentially reduce side effects, such as gastrointestinal AEs, by bypassing the gastrointestinal tract [4]. However, as with all transdermal products, skin adhesion and skin irritation are potential issues that require evaluation. Caregivers are instrumental in helping persons with Alzheimer’s disease take their medications, since a person with dementia may be unable to comply with instructions [5]. Thus, any treatment that allows for less frequent medication administration can potentially be of benefit to both caregivers and the ones for whom they provide care. Corplex™ donepezil transdermal delivery system (TDS), approved as Adlarity® for the treatment of mild, moderate, and severe dementia of the Alzheimer type [6], is designed to deliver donepezil through the skin over a 7-day wear period and is available in 5- and 10-mg/d doses. In this study, we compared the steady-state pharmacokinetics (PK) of once-weekly 5- and 10-mg/d donepezil TDS application with once-daily oral 10-mg donepezil (oral donepezil) administration in healthy volunteers. Donepezil TDS adhesion performance to the skin, safety, and tolerability, including skin tolerability, were also evaluated.
MATERIALS AND METHODS
Study design, participants, and treatment
This was an open-label, randomized, 3-period, 3-treatment, crossover phase 1 study (NCT04617782) enrolling healthy male and female volunteers between the ages of 18 and 55 years (inclusive), with a body mass index of 18–32 kg/m2 (inclusive), body weight of 60–100 kg (inclusive), and a Fitzpatrick skin type of I (always burns, never tans), II (usually burns, tans with difficulty), or III (may burn initially, but tans easily) or skin colorimeter scores equivalent to the allowed Fitzpatrick skin type. Participants did not have any medical conditions that prevented them from participating in the trial.
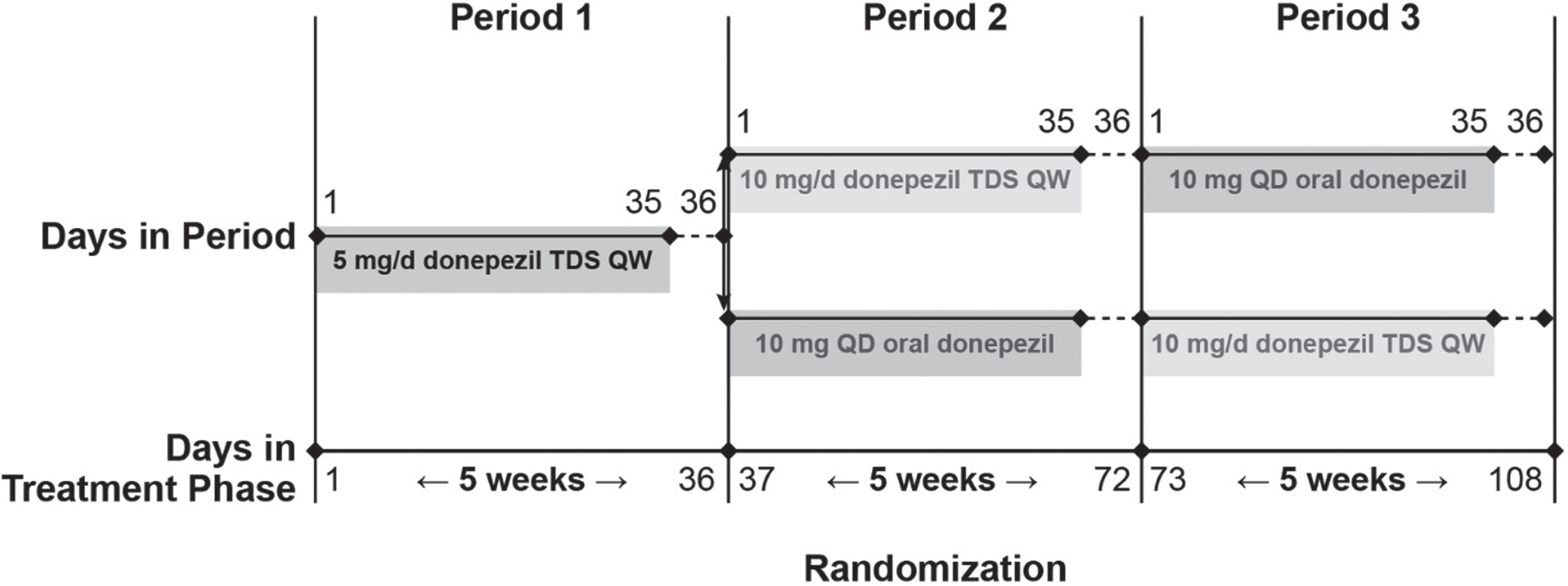
The study consisted of a 28-day screening period followed by 3 treatment periods of 36 days each (Fig. 1). There were no treatment blinding or washout periods between treatments. However, an additional day was included at the end of each treatment period (Days 36, 72, and 108) for PK measurements before starting the next treatment. During Period 1, all participants received 5-mg/d donepezil TDS (52.5 cm2 active drug area) applied weekly for 5 consecutive weeks to acclimate them to the potential cholinergic effects of donepezil. The 5 weeks of 5-mg/d treatment also allowed donepezil to reach steady-state levels for measurement of steady-state PK in Week 5. During treatment Period 2, the participants were randomized to receive either 10-mg/d donepezil TDS (105 cm2 active drug area) applied weekly or 10-mg/d oral donepezil (10-mg Aricept® tablet, Esai, Inc.); the treatment was switched for treatment Period 3. Randomization to the two treatment sequences was stratified by participant’s sex using the computer-generated randomization scheme. All participants resided at the study site for the duration of the study. A safety follow-up visit was conducted ∼5 days after the day of the last oral donepezil administration or last donepezil TDS removal during the treatment phase.
Fig. 1
Study design. QD, once daily; QW, once weekly; TDS, transdermal delivery system.
The trial was conducted in accordance with the principles enunciated in the Declaration of Helsinki (and its amendments) and the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. The protocol was reviewed and approved by an institutional review board, and written informed consent was obtained from each participant before performing any baseline study-specific evaluations.
Study objectives
The primary objective of this study was to evaluate the bioequivalence of steady-state donepezil plasma exposure after once-weekly treatments with 10-mg/d donepezil TDS compared with once-daily 10-mg oral donepezil. Secondary objectives were to evaluate the bioequivalence of steady-state donepezil plasma exposure (after dose normalization) after once-weekly treatments with 5-mg/d donepezil TDS versus once-daily 10-mg oral donepezil and to evaluate adhesion to the skin during the wear period of 5- and 10-mg/d donepezil TDS applications. Safety and tolerability, including local skin tolerability of once-weekly donepezil TDS, were also evaluated.
Pharmacokinetic analyses
For PK assessments of all participants, blood samples were obtained predose and at specified timepoints postdose to determine the PK profiles of plasma donepezil and its active metabolite 6-O-desmethyl donepezil concentrations. Validated liquid chromatography with tandem mass spectrometry methods were used to determine the content of donepezil and 6-O-desmethyl donepezil, an active metabolite, in human plasma. The method was validated for quantitation of donepezil in human plasma over the concentration range of 0.300 to 45.0 ng/mL and for 6-O-desmethyl donepezil over the concentration range of 10 to 2000 pg/mL. PK analyses were performed using Phoenix™ WinNonlin® (Version 8.1, Certara USA, Inc.) and SAS® (Version 9.4, SAS Institute Inc.). PK parameters evaluated included the area under the plasma concentration versus time curve during a 1-week period at steady state (AUC0168,ss), the maximum observed plasma concentration at steady state (Cmax,ss; Week 5), minimum observed nonzero plasma concentration over a dosage interval at steady state (Cmin,ss; Week 5), the time to reach Cmax,ss (Tmax), and percent peak-to-trough fluctuation (FLUCPss) at steady state in Week 5.
TDS adhesion analyses
Adhesion assessments for donepezil TDS were performed in person by a trained staff member and were based on the percentage of the total surface area of the TDS that had remained adhered to the skin. The percentage was recorded based upon the measurement at each timepoint with the staff member blinded to the previous recorded percentage. A grid overlay was used to estimate the percentage of donepezil TDS adhered. Tactile pressure to the TDS was not applied during the adhesion determinations. Application of pressure to fully or partially detached TDS or reinforcing TDS adhesion to the skin by taping was not allowed throughout the study. TDS detachment was not inhibited: if a TDS completely detached from the skin, it was not reapplied, and no fresh TDS was applied for the remainder of the intended wear period. The time of full detachment of any TDS was recorded, and 0% adhesion was assigned to all remaining adhesion timepoints of that wear period. Adhesion assessments were performed at 12, 24, 36, 48, 60, 72, 84, 96, 108, 120, 132, 144, 156, and 168 h after donepezil TDS application and at the time when a TDS detached completely or was removed. The 168-h assessment was conducted before TDS removal. At each adhesion assessment timepoint, a photograph was taken as evidence of the extent of donepezil TDS adhesion to the skin.
Safety evaluation
The safety evaluation was based on AEs, laboratory evaluations, physical examinations, electrocardiogram (ECG) parameters, Columbia Suicide Severity Rating Scale (C-SSRS), and skin irritation scores. Participants informed research personnel of any AEs that occurred at any time during the study. AEs were continuously monitored from the administration of the first dose of study drug until either the safety follow-up visit or early termination from the study. For laboratory evaluations, blood samples were obtained at screening and on Days –1, 36, 72, 109, or at early termination. A complete medical history was collected at screening. Physical examination was conducted at screening, the end of treatment or at early termination, and at the safety follow-up visit. ECGs were obtained at screening, Day –1, and the end of treatment or at early termination. The C-SSRS [7] assessments were conducted at screening, on Days –1, 36, 72, 109, and at early termination (Supplementary Table 1).
Skin irritation and tolerability assessments were performed using the US FDA-recommended dermal response scale (Supplementary Table 2) and other effects scale (Supplementary Table 3) [8]. The combined skin irritation score (calculated as the sum of the dermal response and other effects scores) was recorded for the donepezil TDS treatments and for post-removal timepoints (0.5, 24, 48, and 72 h post-removal). The quantitative skin irritation assessments were not recorded as TEAEs; however, spontaneous reports of application site AEs by the participants were reported as TEAEs.
Statistical analysis
Sample size calculation
A sample size of 48 completers was estimated. We assumed a 20% dropout rate, resulting in a planned enrollment of 60 participants. The sample size was estimated to show at least 90% power to assess bioequivalence within the limits of 80% to 125% for the geometric mean ratio if the true expected geometric mean ratio was 1.05 (assuming a coefficient of variance [CV] of 29%) for AUC0168,ss.
Statistical analysis of PK and adhesion data
Statistical comparison of the PK parameters of donepezil and 6-O-desmethyl donepezil exposure at steady state was performed using an analysis of variance (ANOVA) model (SAS®; Version 9.4, SAS Institute Inc.) for a 2-way crossover design for the comparison of 10-mg donepezil TDS versus oral donepezil on the in-transformed data with sequence, period, and treatment as the fixed effects and subject within sequence as a random effect. For the comparison of 5-mg/d donepezil TDS versus oral donepezil, the analysis was performed using an ANOVA model with treatment as fixed effect and subject as random effect. The PK parameter values for 5-mg/d donepezil TDS were dose normalized (by multiplying with 2) before the analysis. A statistically significant difference was defined as p < 0.05.
To examine the relative bioavailability at steady state of the 5- and 10-mg/d donepezil TDS (tests) relative to oral donepezil (reference), plasma donepezil exposure, characterized by AUC0168,ss and Cmax,ss, was assessed and compared utilizing bioequivalence criteria. Similar bioavailability for donepezil was concluded if the 90% confidence intervals (CIs) of the least squares geometric means for the log-transformed AUC0168,ss and Cmax,ss ratios fell within the acceptable range of 0.80 to 1.25.
Assessment of adhesion was performed in accordance with the FDA guidance for assessment of adhesion for topical and transdermal systems [9]. Within each treatment, a one-sided 95% CI was determined for the probability (p) that a randomly selected donepezil TDS maintained ≥75% adhesion throughout the entire wear period. If the 95% lower confidence limit exceeded 80%, we concluded that ≥80% of donepezil TDSs were ≥75% adhered throughout the 7-day wear period. The one-sided 95% lower confidence limit was determined using the Jeffreys prior method [10, 11].
RESULTS
Participants
A total of 60 participants were enrolled in the study; all participants received 5-mg/d donepezil TDS, 55 received 10-mg/d donepezil TDS, and 56 received oral donepezil (Fig. 2). Overall, 8 participants discontinued early from the study: 5 because of noncompliance with the protocol or in-house rules, 2 were withdrawn by the investigator for reasons unrelated to safety, and 1 withdrew consent (Fig. 2). All 60 enrolled participants were included in the safety analysis, 57 were included in the PK analysis, and 52 were included in the relative bioavailability analysis.
Of the 60 participants enrolled in this study, most were men (63.3%) and white (93.3%). Mean age of the participants was 40 years. As a result of the crossover design, the age, sex, height, and weight of participants were similarly distributed across the treatment sequences (Table 1). Duration of treatment was similar across the treatments: mean (standard deviation [SD]) 34.1 (3.9) days for 5-mg/d donepezil TDS, 34.4 (3.7) days for 10-mg/d donepezil TDS, and 33.8 (5.1) days for oral donepezil.
Fig. 2
Participant disposition and analyses populations. *These participants were randomized to receive 10 mg/d donepezil TDS followed by oral donepezil. †Two participants discontinued from the trial in Period 3 because of noncompliance with the protocol or in-house rules. d, day; TDS, transdermal delivery system.
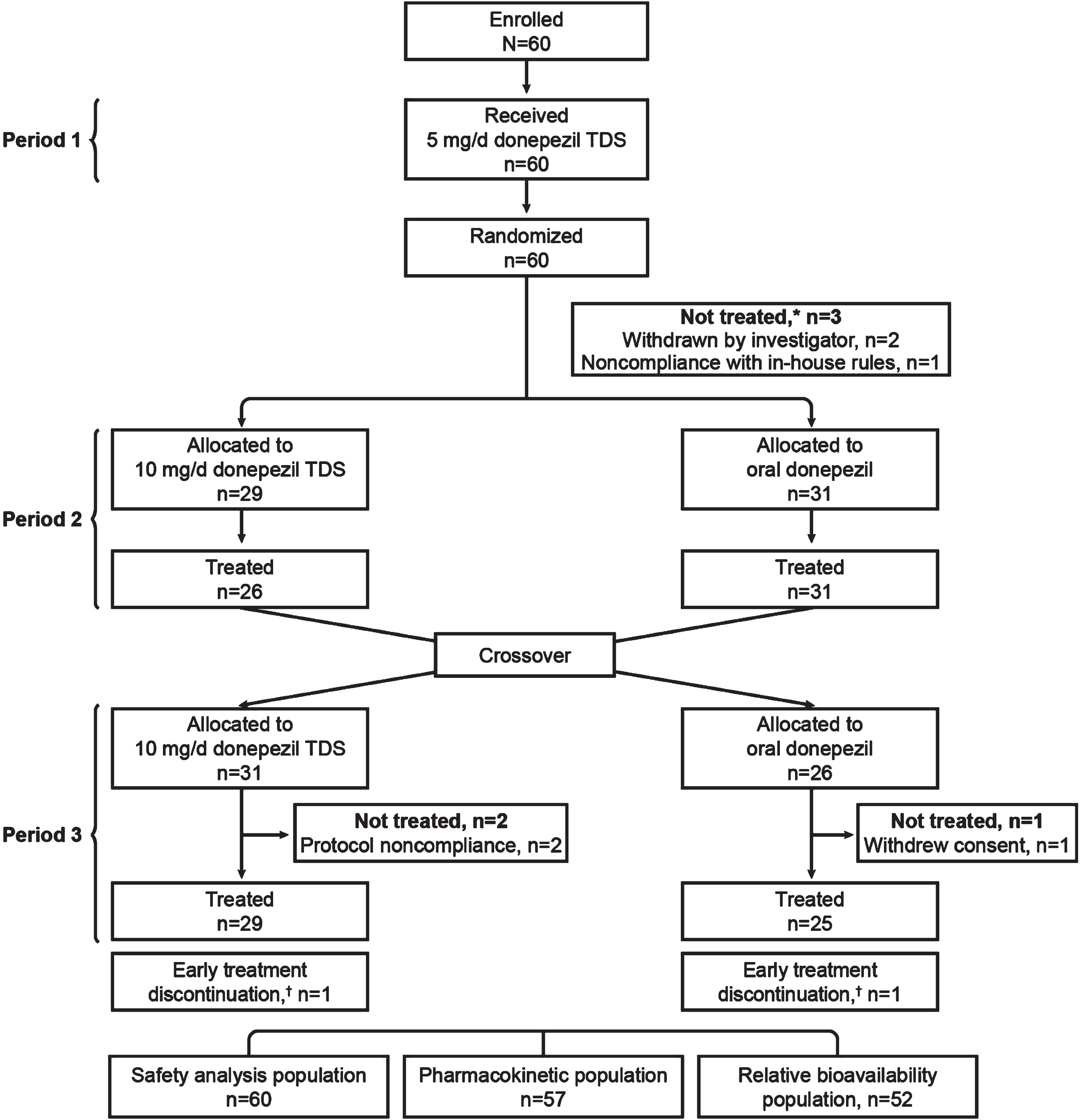
Table 1
Demographics and baseline characteristics of participants by treatment
| Donepezil TDS 5 mg/d (n = 60) | Donepezil TDS 10 mg/d (n = 55) | Oral donepezil 10 mg/d (n = 56) | Overall (N = 60) | |
| Age (y) at informed consent | ||||
| Mean (SD) [range] | 39.8 (9.91) [19–55] | 40.5 (9.97) [19–55] | 40.5 (9.84) [19–55] | 39.8 (9.91) [19–55] |
| Median (Q1, Q3) | 40.0 (32, 49) | 42.0 (34, 50) | 41.5 (34, 50) | 40.0 (32, 49) |
| Sex, n (%) | ||||
| Male | 38 (63.3) | 33 (60.0) | 35 (62.5) | 38 (63.3) |
| Female | 22 (36.7) | 22 (40.0) | 21 (37.5) | 22 (36.7) |
| Race, n (%) | ||||
| White | 56 (93.3) | 52 (94.5) | 53 (94.6) | 56 (93.3) |
| Black or African American | 3 (5.0) | 2 (3.6) | 2 (3.6) | 3 (5.0) |
| Asian | 1 (1.7) | 1 (1.8) | 1 (1.8) | 1 (1.7) |
| Height, mean (SD), cm | 170.7 (9.5) | 170.0 (9.5) | 170.3 (9.5) | 170.7 (9.5) |
| Weight, mean (SD), kg | 76.5 (10.4) | 75.8 (10.2) | 75.9 (10.2) | 76.5 (10.4) |
| BMI, mean (SD), kg/m2 | 26.2 (2.6) | 26.2 (2.6) | 26.2 (2.6) | 26.2 (2.6) |
BMI, body mass index; Q1, first quartile; Q3, third quartile; SD, standard deviation; TDS, transdermal delivery system.
Pharmacokinetics and relative bioavailability
Donepezil
The mean steady-state plasma concentration-time PK profiles of donepezil TDS and oral donepezil are shown in Fig. 3. Steady state (Week 5) mean values for Cmax,ss, Cmin,ss, and AUC0168,ss were similar for 5-mg/d donepezil TDS (dose-normalized to the 10-mg/d dose before analysis by multiplying concentrations by 2), 10-mg/d donepezil TDS, and oral donepezil (Table 2). Median Tmax was considerably higher for 5-mg/d donepezil TDS (72 h) and 10-mg/d donepezil TDS (84 h) than for oral donepezil (2 h). FLUCPss was higher for oral donepezil than for 5- and 10-mg/d donepezil TDS treatments. Based on results from the steady-state assessment, steady state for donepezil was reached by Day 22 for 5- and 10-mg/d donepezil TDSs and by Day 8 for oral donepezil.
Fig. 3
Mean steady-state (Week 5) plasma concentration-time curves for 5 mg/d donepezil TDS, 10 mg/d donepezil TDS, and 10 mg/d oral donepezil. Donepezil TDS was applied weekly for 5 weeks; oral donepezil was administered daily for 5 weeks. The replicated steady-state pharmacokinetic profile for oral donepezil on Days 1–6 is shown as a dashed line to represent that they are replicated from Day 7 (144–168 h). TDS, transdermal delivery system.
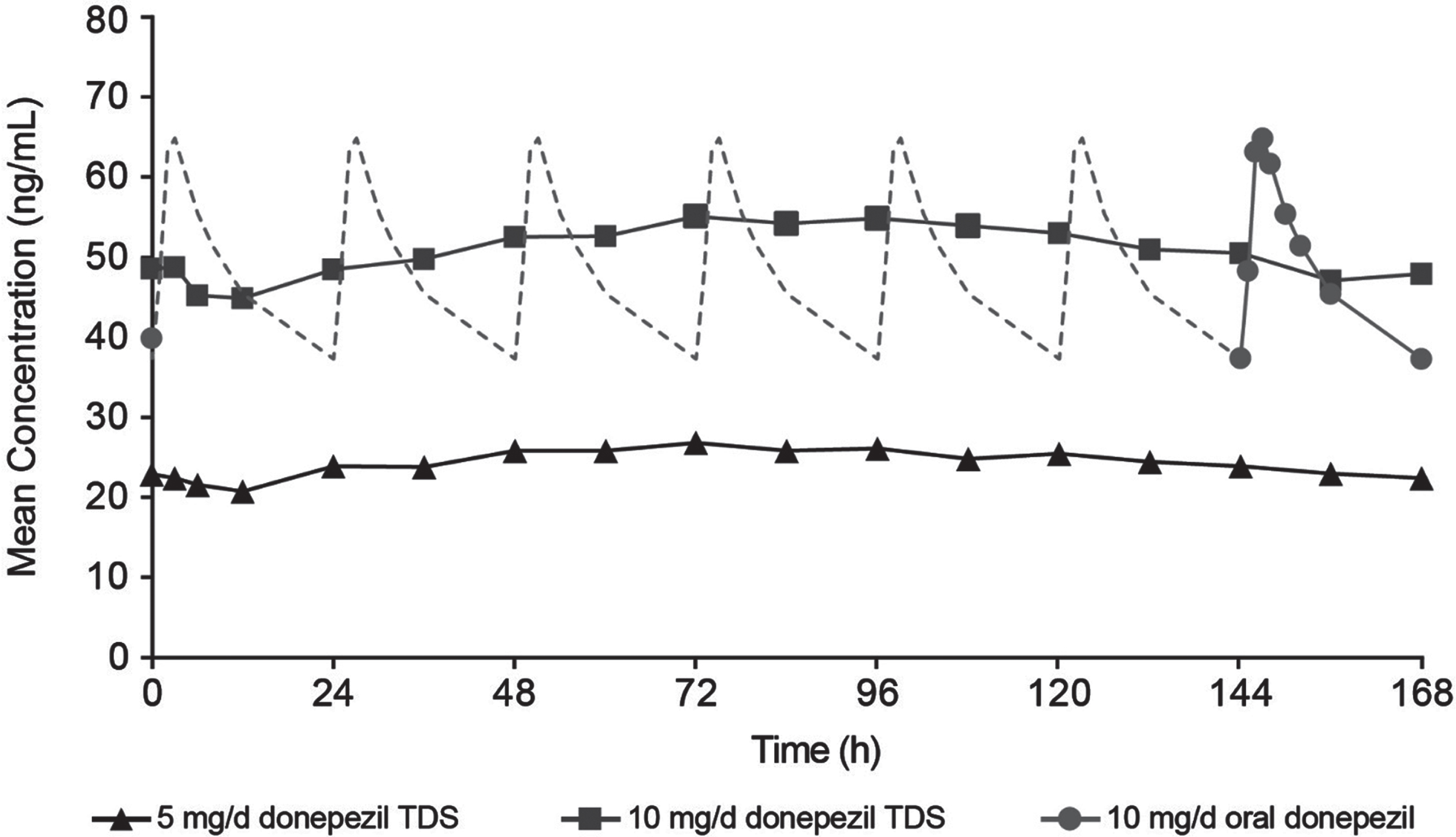
Table 2
Plasma donepezil PK parameters at Week 5
| Parameter | Donepezil TDS 5 mg/da (n = 56) | Donepezil TDS 10 mg/d (n = 53) | Oral donepezil 10 mg/d (n = 53) |
| Cmax,ss, mean (SD), ng/mL | 59.8 (18.2) | 62.5 (20.0) | 70.6 (19.4) |
| Cmin,ss, mean (SD), ng/mL | 41.0 (14.2) | 43.2 (15.6) | 39.9 (14.6) |
| AUC0168,ss (h·ng/mL) | 8732.1 (2760.3) | 9099.0 (2972.1) | 8462.6 (2558.0) |
| Tmax,ss, median (range), h | 72.0 (0–156.0) | 84.0 (0–120.0) | 2.0 (0–4.1) |
| FLUCPss, mean (SD), % | 36.7 (14.8) | 35.8 (14.5) | 64.9 (23.7) |
AUC, area under the curve; Cmax,ss, maximum concentration at steady state; Cmin,ss, minimum observed nonzero plasma concentration at steady state; FLUCPss, percent peak-to-trough fluctuation at steady-state; SD, standard deviation; TDS, transdermal delivery system; Tmax,ss, time to reach Cmax,ss. aConcentrations for 5 mg/d donepezil TDS were dose-normalized to the 10 mg/d dose before analysis by multiplying concentrations for 5 mg/d dose by 2.
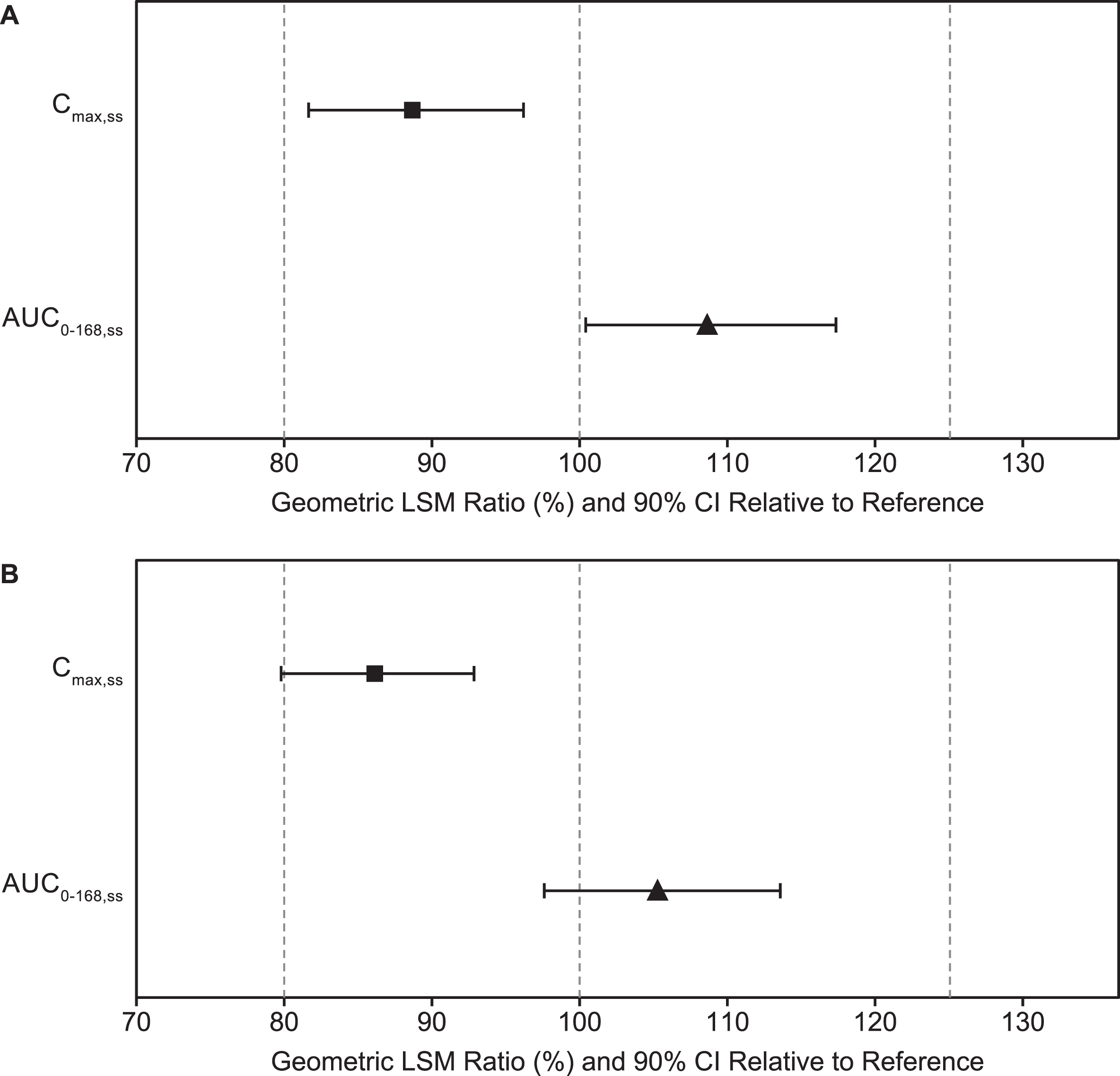
For 10-mg/d donepezil TDS versus oral donepezil, the 90% CIs for the geometric mean ratios of Cmax,ss and AUC0168,ss were within the accepted 80% to 125% range for establishing bioequivalence (Table 3 and Fig. 4A). For 5-mg/d donepezil TDS versus oral donepezil, the 90% CIs for the geometric mean ratio of AUC0168,ss were within the range for bioequivalence (Table 3 and Fig. 4B). The upper 90% CI (92.9%) of Cmax,SS geometric mean ratio for 5 mg/d donepezil TDS versus oral donepezil was within the bioequivalence range, but the lower 90% CI (79.8%) was slightly less than the lower range (80%) established for bioequivalence.
Table 3
Relative bioavailability of 10 mg/d and 5 mg/d donepezil TDS versus oral donepezil
| Dependent variable | Donepezil TDS geometric meana | Oral donepezil geometric meana | Adjusted GMR (%)b | p-valuec | 90% CI lower | 90% CI upper |
| 10 mg/d donepezil TDS versus 10 mg/d oral donepezil, n = 52 | ||||||
| Cmax,ss (ng/mL) | 59.6 | 67.2 | 88.7 | 0.02 | 81.7 | 96.2 |
| AUC0168,ss, (h·ng/mL) | 8678.7 | 7992.3 | 108.6 | 0.08 | 100.5 | 117.4 |
| 5 mg/d donepezil TDS (dose normalized)d versus 10 mg/d oral donepezil, n = 51 | ||||||
| Cmax,ss (ng/mL) | 57.8 | 67.1 | 86.1 | 0.002 | 79.8 | 92.9 |
| AUC0168,ss (h·ng/mL) | 8401.9 | 7979.1 | 105.3 | 0.26 | 97.6 | 113.6 |
AUC, area under the curve; CI, confidence interval; Cmax,ss, maximum concentration at steady state; GMR, geometric mean ratio; TDS, transdermal delivery system. aGeometric mean obtained by exponentiating the least squares mean (LS mean). bAdjusted GMR (%) = 100×[Geometric Mean (donepezil TDS)/Geometric Mean (oral donepezil)]. The GMR and its 90% CI were obtained by exponentiation of the difference between the treatment LS means on the logarithmic scale and by exponentiation of the limits of the 90% CI for the difference, respectively. cp-value from the mixed model. dConcentrations for the 5 mg/d donepezil TDS were dose-normalized to the 10 mg/d dose before analysis by multiplying concentrations for 5 mg/d dose by 2.
Fig. 4
Donepezil exposure for (A) 10 mg/d donepezil TDS and (B) 5 mg/d donepezil TDS (dose normalized) versus oral donepezil. Bars are the 90% CIs. AUC0168,ss, area under the curve at steady state; CI, confidence interval; Cmax,ss, maximum concentration at steady state; LSM, least-squares mean; TDS, transdermal delivery system.
No statistically significant differences in donepezil exposure based on gender, ethnicity, and age were observed for 5-mg/d donepezil TDS, 10-mg/d donepezil TDS, and oral donepezil.
6-O-desmethyl donepezil
Mean concentration ratios of 6-O-desmethyl donepezil to donepezil were generally under 0.003 (i.e., less than 0.3%) for all treatments during Week 5; therefore, 6-O-desmethyl donepezil was determined to be a minor metabolite of donepezil for all treatments.
Adhesion
Overall, 568 donepezil TDSs were applied: 296 of 5 mg/d and 272 of 10 mg/d donepezil TDS. Of the donepezil TDS applied, 11 (1.9%) were removed prematurely: 7 (2.4%) 5-mg/d donepezil TDS (4 from lack of adhesion and 3 from premature study discontinuation) and 4 (1.5%) 10-mg/d donepezil TDS (2 from lack of adhesion and 2 from premature study discontinuation). Donepezil TDSs that were removed prematurely because of study discontinuation (3 in 5-mg/d and 2 in 10-mg/d donepezil TDS) were not included in the analysis; therefore, a total of 563 donepezil TDSs were included in the adhesion population.
The mean percentage (SD) of donepezil TDS surface area remaining adhered to skin from Weeks 1 through 5 was 92.6% (10.9%) for 5-mg/d donepezil TDS and 93.3% (8.8%) for 10-mg/d donepezil TDS. The number (%) of TDSs with adhesion ≥75% from Weeks 1 through 5 at all adhesion assessment timepoints during the 7-day wear period was 245 (83.6%) for 5-mg/d donepezil TDS and 232 (85.9%) for 10-mg/d donepezil TDS. The number (%) of donepezil TDSs with ≥50% adhesion from Weeks 1 through 5 at any timepoint was 278 (94.9%) for 5-mg/d donepezil TDS and 261 (96.7%) for 10-mg/d donepezil TDS. The number (%) of donepezil TDS with complete detachment from Weeks 1 through 5 during the 7-day wear period was 4 (1.4%) for 5-mg/d donepezil TDS and 2 (0.7%) for 10-mg/d donepezil TDS.
Using the Jeffreys prior method, the probability of a donepezil TDS maintaining ≥75% adhesion throughout the wear period was estimated as 0.8362 for 5-mg/d donepezil TDS and 0.8593 for 10-mg/d donepezil TDS. The lower limit of the one-sided 95% CIs (0.80 for 5-mg/d and 0.82 for 10-mg/d donepezil TDSs) demonstrated that ≥80% of the donepezil TDSs were ≥75% adhered throughout the 7-day wear period (Table 4).
Table 4
Probability for donepezil TDS maintaining ≥75% adhesion during the entire wear period
| Treatment | n | Point estimate | Lower limit of the one-sided 95% CI Jeffreys prior method |
| 5 mg/d donepezil TDS | 293 | 0.8362 | 0.7982 |
| 10 mg/d donepezil TDS | 270 | 0.8593 | 0.8217 |
CI, confidence interval; TDS, transdermal delivery system.
Safety
Adverse events were reported in 48 of 60 participants (80.0%)—32 of 60 participants (53.3%) for the 5-mg/d donepezil TDS, 30 of 55 participants (54.5%) for the 10-mg/d donepezil TDS, and 32 of 56 participants (57.1%) for oral donepezil (Table 5). For most participants (44 [73.3% ]), AEs were reported as mild in severity; 4 participants (6.7%; 2 on 5-mg/d donepezil TDS, 1 on 10-mg/d donepezil TDS, and 1 on oral donepezil) had AEs of moderate severity. No serious AEs or deaths were reported in the study, and no participant had AEs leading to discontinuation of study treatment or leading to early termination.
Table 5
Overall summary of treatment-emergent AEs and most frequently reported AEs in ≥5% of participants overall
| Donepezil TDS 5 mg/d (n = 60) | Donepezil TDS 10 mg/d (n = 55) | Oral donepezil 10 mg/d (n = 56) | Overall (N = 60) | |
| Participants, n (%) | ||||
| TEAE | 32 (53.3) | 30 (54.5) | 32 (57.1) | 48 (80.0) |
| Related TEAE | 25 (41.7) | 24 (43.6) | 29 (51.8) | 44 (73.3) |
| AEs by MedDRA system organ class MedDRA preferred terms | ||||
| Gastrointestinal disorders | 15 (25.0) | 8 (14.5) | 30 (53.6) | 36 (60.0) |
| Constipation | 9 (15.0) | 3 (5.5) | 10 (17.9) | 19 (31.7) |
| Nausea | 4 (6.7) | 1 (1.8) | 17 (30.4) | 19 (31.7) |
| Diarrhea | 2 (3.3) | 2 (3.6) | 7 (12.5) | 9 (15.0) |
| Abdominal pain | 0 | 3 (5.5) | 1 (1.8) | 4 (6.7) |
| Vomiting | 1 (1.7) | 0 | 3 (5.4) | 4 (6.7) |
| General disorders and administration site conditions | 18 (30.0) | 11 (20.0) | 7 (12.5) | 29 (48.3) |
| Application site pruritus | 12 (20.0) | 5 (9.1) | 0 | 14 (23.3) |
| Application site dermatitis | 5 (8.3) | 3 (5.5) | 1 (1.8) | 8 (13.3) |
| Fatigue | 2 (3.3) | 1 (1.8) | 5 (8.9) | 7 (11.7) |
| Application site irritation | 3 (5.0) | 0 | 0 | 3 (5.0) |
| Nervous system disorders | 11 (18.3) | 8 (14.5) | 17 (30.4) | 23 (38.3) |
| Headache | 8 (13.3) | 8 (14.5) | 7 (12.5) | 16 (26.7) |
| Dizziness | 3 (5.0) | 2 (3.6) | 11 (19.6) | 15 (25.0) |
| Somnolence | 0 | 0 | 6 (10.7) | 6 (10.0) |
| Mental impairment | 0 | 1 (1.8) | 2 (3.6) | 3 (5.0) |
| Psychiatric disorders | 10 (16.7) | 7 (12.7) | 6 (10.7) | 18 (30.0) |
| Insomnia | 4 (6.7) | 4 (7.3) | 0 | 7 (11.7) |
| Nightmare | 5 (8.3) | 1 (1.8) | 1 (1.8) | 6 (10.0) |
| Abnormal dreams | 1 (1.7) | 2 (3.6) | 1 (1.8) | 4 (6.7) |
| Irritability | 2 (3.3) | 0 | 2 (3.6) | 3 (5.0) |
| Musculoskeletal and connective tissue disorders | 4 (6.7) | 7 (12.7) | 6 (10.7) | 15 (25.0) |
| Muscle spasms | 4 (6.7) | 5 (9.1) | 5 (8.9) | 12 (20.0) |
| Injury, poisoning and procedural complications | 1 (1.7) | 2 (3.6) | 3 (5.4) | 6 (10.0) |
| Skin abrasion | 1 (1.7) | 0 | 2 (3.6) | 3 (5.0) |
AE, adverse event; MedDRA, Medical Dictionary for Regulatory Activities; TDS, transdermal delivery system; TEAE, treatment-emergent adverse event.
Gastrointestinal disorders (constipation, nausea, diarrhea, and vomiting) were more frequent when participants were taking oral donepezil than when they were using donepezil TDS (Table 5). More participants reported dizziness, fatigue, and somnolence on oral donepezil than on donepezil TDS. Application site pruritis, application site dermatitis, abdominal pain, and insomnia were more frequent with 10 mg/d donepezil TDS than oral donepezil. Application site reactions were deemed treatment related in all cases (Supplementary Table 4). Headaches occurred with similar frequency (5-mg/d donepezil TDS, 13.3%; 10-mg/d donepezil TDS, 14.5%; oral donepezil, 12.5%) with all treatments, and 14 of 16 cases were considered related to treatment.
The combined skin irritation score of ≥3 at 30 min after donepezil TDS removal was reported in 31 of 60 participants (51.7%) included in the skin irritation analysis for 5-mg/d donepezil TDS (289 patches in total) and in 30 of 55 participants (54.5%) for 10-mg/d donepezil TDS (268 patches). No participant discontinued early from the study due to skin irritation, and no donepezil TDS was removed due to unacceptable skin irritation.
There were no clinically important changes in clinical laboratory values, vital signs, ECGs, or physical examinations across all treatments. No occurrences of suicidal thoughts or ideation were reported during the study.
DISCUSSION
The study met its primary endpoint. We demonstrated that 10-mg/d donepezil TDS applied weekly was bioequivalent to 10-mg oral donepezil tablets administered once-daily for 35 days. Although the lower 90% CI (79.8%) of the Cmax,ss mean ratio for the 5-mg/d donepezil TDS was slightly below the bioequivalence range (80%), the difference was not considered clinically meaningful; therefore, the 5-mg/d donepezil TDS was also considered bioequivalent to 10-mg/d oral donepezil. Steady-state mean Cmax,ss, Cmin,ss, and AUC0168,ss values were similar for donepezil TDS and oral donepezil. Weekly donepezil TDS application produced 1 weekly Cmax value compared with 7 daily values for once-daily oral donepezil; however, this difference in plasma donepezil concentration profiles is not likely to influence the pharmacodynamic effect of donepezil on the basis of results from another phase 1, randomized, open-label, crossover, PK study of once-weekly donepezil TDS versus daily oral donepezil in healthy adults (NCT02968719). In this study, a red blood cell acetylcholinesterase (RBC AChE) inhibition assay was used to assess the pharmacodynamic effect of donepezil; similar RBC AChE inhibition versus plasma donepezil concentration profile was observed for donepezil TDS and oral donepezil (Supplementary Figure 1), supporting similar effectiveness of donepezil TDS and oral donepezil.
Adhesion was generally similar for the 5- and 10-mg/d donepezil TDS, with >80% of the donepezil TDS remaining ≥75% adhered and >94% remaining ≥50% adhered throughout the weekly wear period without having to apply pressure or taping TDS to the skin to reinforce adhesion. Adhesion for 10-mg/d donepezil TDS exceeded the FDA’s adhesion guidance, which recommends that the 95% lower confidence limit of the probability that a TDS maintains ≥75% adhesion during the entire wear period be ≥80% [9]. Adhesion of 5-mg/d donepezil TDS was marginally lower than that of 10-mg/d donepezil TDS. The larger TDSs are thought to be more sensitive to detachment than the smaller TDSs because of greater conformational and torsional strains arising from increased anatomical curvatures or a greater magnitude of flexion [9]. However, in our study, the larger donepezil TDS demonstrated better adhesion, suggesting that the marginally lower adhesion results for 5-mg donepezil TDS may reflect participants becoming accustomed to using the TDS in Period 1 before using the 10-mg donepezil TDS in Period 2 or 3. Participants in the study were allowed to shower daily, indicating that favorable donepezil TDS adhesion is likely to be maintained under real-world conditions.
All 3 treatments (5-mg/d donepezil TDS, 10-mg/d donepezil TDS, and oral donepezil each administered for 5 consecutive weeks) were generally well tolerated with no unexpected AEs or serious AEs. Fewer treatment-emergent gastrointestinal AEs were reported with donepezil TDS than with oral donepezil in our study. Gastrointestinal AEs can be a problem for patients taking oral donepezil treatment for Alzheimer’s disease [12], resulting in treatment discontinuation or dose reduction, thus lowering the effectiveness of the medication [13–15]; our results suggest that donepezil TDS may be an option for these patients. As seen with other transdermal patches [16, 17], application of donepezil TDS was associated with increased incidence of application site reactions; however, no participant discontinued early from the study due to skin irritation, and no donepezil TDSs were removed due to unacceptable skin irritation. Similar skin irritation was demonstrated between the two donepezil TDS strengths.
Transdermal patches have several advantages over oral administration, including maintenance of sustained therapeutic plasma concentrations of drugs, easy application, reduced systemic adverse effects, and better treatment compliance [4, 18]. Transdermal patch formulations of donepezil were previously investigated in clinical trials by Teikoku Pharma USA, Inc., and Eisai Co., Ltd.; however, the treatment did not achieve approval by the FDA [18]. Donepezil TDS is the first approved once-weekly patch for Alzheimer’s disease [6]. Donepezil TDS contains the most commonly prescribed Alzheimer’s disease medication—donepezil (Aricept)—formulated in a convenient patch form. A once-weekly patch of donepezil may be easier to remember than once-daily oral medication for some people with Alzheimer’s disease who have memory impairment, making it difficult to remember to take daily medication. It may also benefit caregivers who may not be able to attend to their dependents with Alzheimer’s disease every day. An added benefit of once-weekly patch administration may be reduced cost of drug administration, particularly in institutional settings or situations where treatments are provided at home by a paid caregiver.
Our results suggest that patients using donepezil TDS are more likely to receive a steady plasma exposure of donepezil than those taking oral donepezil, as FLUCPss was higher with once-daily oral administrations of donepezil than with once-weekly 5-mg/d and 10-mg/d donepezil TDS applications. Use of donepezil TDS was associated with fewer treatment-emergent gastrointestinal disorders and central nervous system-associated adverse effects, such as dizziness and somnolence, compared with oral donepezil, which may lead to better compliance among patients.
Currently, the acetylcholinesterase inhibitor rivastigmine (Exelon® Patch) is the only medication available in the transdermal patch form for treatment of mild, moderate, and severe dementia of the Alzheimer type [19, 20]. However, rivastigmine is a once-daily patch, whereas donepezil TDS only requires once-weekly application, which may be more convenient for some patients and their caregivers, potentially resulting in better treatment compliance. Similar to donepezil TDS, the rivastigmine patch was associated with fewer gastrointestinal side effects when compared with oral rivastigmine [17, 21]. Although application site reactions were observed in clinical trials of the rivastigmine patch, they were typically mild to moderate in severity and not allergic. Few patients discontinued treatment because of adverse skin reactions [22]. For donepezil TDS, skin reactions were mild and resolved quickly, suggesting that application site reactions are not a barrier to treating a patient with Alzheimer’s disease with a TDS.
Our study has some limitations. The study was conducted in healthy volunteers; therefore, PK outcomes may not completely represent older patients with Alzheimer’s disease and other comorbid conditions. Participants and investigators knew which treatment was being administered, as the study was open label. The study was done in a controlled setting, and the participants were healthy and capable of complying with treatment directions; this may not reflect real-world situations. Despite these limitations, our results show that once-weekly 10- and 5-mg/d donepezil TDSs are bioequivalent to once-daily 10-mg oral donepezil (Aricept®) and have an acceptable safety profile. Donepezil TDS was associated with fewer gastrointestinal disorders than oral donepezil, supporting the feasibility of using donepezil TDS as a convenient and safe once-weekly dosing regimen for treatment of dementia of the Alzheimer type.
ACKNOWLEDGMENTS
The authors would like to thank the trial participants and the staff at sites that participated in this study. The study was sponsored by Corium, Inc. Medical writing support for the development of this manuscript under the direction of the authors was provided by Ritu Pathak, PhD, and editing support by Polina Novichenok, both of Ashfield MedComms, an Inizio company, and funded by Corium Inc.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/22-0530r1).
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-220530.
REFERENCES
[1] | Kumar A , Gupta V , Sharma S ((2021) ) Donepezil. In Stat Pearls. StatPearls Publishing, Treasure Island, FL. |
[2] | ARICEPT® (donepezil hydrochloride) tablets, fororal use [prescribing information]. Woodcliff Lake, NJ; Eisai Inc.;12/2018. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/020690s042,021720s014,022568s011lbl.pdf. Accessed November 15, 2021. |
[3] | Farlow MR , Salloway S , Tariot PN , Yardley J , Moline ML , Wang Q , Brand-Schieber E , Zou H , Hsu T , Satlin A ((2010) ) Effectiveness and tolerability of high-dose (23 mg/d) versus standard-dose (10 mg/d) donepezil in moderate to severe Alzheimer’s disease: A 24-week, randomized, double-blind study. Clin Ther 32: , 1234–1251. |
[4] | Paudel KS , Milewski M , Swadley CL , Brogden NK , Ghosh P , Stinchcomb AL ((2010) ) Challenges and opportunities in dermal/transdermal delivery. Ther Deliv 1: , 109–131. |
[5] | Lindauer A , Sexson K , Harvath TA ((2017) ) Medication management for people with dementia. Am J Nurs 117: , 60–64. |
[6] | ADLARITY® (donepezil transdermal system)[prescribing information]. Grand Rapids, MI; Corium, Inc.; 03/2022.Available at: https://corium.com/products/ADLARITY/ADLARITY_PI_ENGLISH_US.pdf .Accessed March 15, 2022. |
[7] | Posner K , Brown GK , Stanley B , Brent DA , Yershova KV , Oquendo MA , Currier GW , Melvin GA , Greenhill L , Shen S , Mann JJ ((2011) ) The Columbia-Suicide Severity Rating Scale: Initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry 168: , 1266–1277. |
[8] | U.S. Food AND Drug Administration (2018) Assessing the irritation and sensitization potential of transdermal and topical delivery systems for ANDAs: Draft guidance for industry. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/assessing-irritation-and-sensitization-potential-transdermal-and-topical-delivery-systems-andas, Last updated March 3, 2020, Accessed December 1 2021. |
[9] | U.S. Food and Drug Administration (2021) Assessment of adhesion for topical and transdermal systems submitted in new drug applications: Draft guidance for Industry. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/assessment-adhesion-topical-and-transdermal-systems-submitted-new-drug-applications, Accessed December 3, 2021. |
[10] | Cai T ((2005) ) One-sided confidence intervals in discrete distributions. J Stat Plan Inference 131: , 63–88. |
[11] | Newcombe RG ((2011) ) Measures of location for confidence intervals for proportions. Commun Stat Theory Methods 40: , 1743–1767. |
[12] | Hansen RA , Gartlehner G , Webb AP , Morgan LC , Moore CG , Jonas DE ((2008) ) Efficacy and safety of donepezil, galantamine, and rivastigmine for the treatment of Alzheimer’s disease: A systematic review and meta-analysis. Clin Interv Aging 3: , 211–225. |
[13] | Rogers SL , Doody RS , Mohs RC , Friedhoff LT ((1998) ) Donepezil improves cognition and global function in Alzheimer disease: A 15-week, double-blind, placebo-controlled study. Donepezil Study Group. Arch Intern Med 158: , 1021–1031. |
[14] | Doody RS , Geldmacher DS , Gordon B , Perdomo CA , Pratt RD , Donepezil Study G ((2001) ) Open-label, multicenter, phase 3 extension study of the safety and efficacy of donepezil in patients with Alzheimer disease. Arch Neurol 58: , 427–433. |
[15] | Campbell NL , Perkins AJ , Gao S , Skaar TC , Li L , Hendrie HC , Fowler N , Callahan CM , Boustani MA ((2017) ) Adherence and tolerability of Alzheimer’s disease medications: A pragmatic randomized trial. J Am Geriatr Soc 65: , 1497–1504. |
[16] | Watts RL , Jankovic J , Waters C , Rajput A , Boroojerdi B , Rao J ((2007) ) Randomized, blind, controlled trial of transdermal rotigotine in early Parkinson disease. Neurology 68: , 272–276. |
[17] | Winblad B , Cummings J , Andreasen N , Grossberg G , Onofrj M , Sadowsky C , Zechner S , Nagel J , Lane R ((2007) ) A six-month double-blind, randomized, placebo-controlled study of a transdermal patch in Alzheimer’s disease––rivastigmine patch versus capsule. Int J Geriatr Psychiatry 22: , 456–467. |
[18] | Sozio P , Cerasa LS , Marinelli L , Di Stefano A ((2012) ) Transdermal donepezil on the treatment of Alzheimer’s disease. Neuropsychiatr Dis Treat 8: , 361–368. |
[19] | Chan AL , Chien YW , Jin Lin S ((2008) ) Transdermal delivery of treatment for Alzheimer’s disease: Development, clinical performance and future prospects. Drugs Aging 25: , 761–775. |
[20] | EXELON® PATCH (rivastigmine transdermal system)[prescribing information]. East Hanover, NJ; Novartis PharmaceuticalCorporation; 6/2020. Available at: https://www.novartis.us/sites/www.novartis.us/files/exelonpatch.pdf. Accessed Ferbruary 15, 2022. |
[21] | Wentrup A , Oertel WH , Dodel R ((2009) ) Once-daily transdermal rivastigmine in the treatment of Alzheimer’s disease. Drug Des Devel Ther 2: , 245–254. |
[22] | Alva G , Cummings JL , Galvin JE , Meng X , Velting DM ((2015) ) Skin reactions at the application site of rivastigmine patch (4.6 mg/24 h, 9.5 mg/24 h or 13.3 mg/24 h): A qualitative analysis of clinical studies in patients with Alzheimer’s disease. Int J Clin Pract 69: , 518–530. |



