Benefits of Gut Microbiota Reconstitution by Beta 1,3–1,6 Glucans in Subjects with Autism Spectrum Disorder and Other Neurodegenerative Diseases
Abstract
Background:
Aureobasidium pullulans (black yeast) AFO-202 strain-produced beta glucan, Nichi Glucan, has been shown to improve the behavior and sleep pattern along with an increase in α-synuclein and melatonin in children with autism spectrum disorder (ASD).
Objective:
In this randomized pilot clinical study, we have evaluated the gut microbiota of subjects with ASD after consumption of Nichi Glucan.
Methods:
Eighteen subjects with ASD were randomly allocated: six subjects in the control group (Group 1): conventional treatment comprising remedial behavioral therapies and L-carnosine 500 mg per day, and 12 subjects (Group 2) underwent supplementation with Nichi Glucan 0.5 g twice daily along with the conventional treatment for 90 days.
Results:
Whole genome metagenome (WGM) sequencing of the stool samples at baseline and after intervention showed that among genera of relevance, the abundance of Enterobacteriaceae was decreased almost to zero in Group 2 after intervention, whereas it increased from 0.36% to 0.85% in Group 1. The abundance of Bacteroides increased in Group 1, whereas it decreased in Group 2. The abundance of Prevotella increased while the abundance of Lactobacillus decreased in both Group 1 and Group 2. Among species, a decrease was seen in Escherichia coli, Akkermansia muciniphila CAG:154, Blautia spp., Coprobacillus sp., and Clostridium bolteae CAG:59, with an increase of Faecalibacterium prausnitzii and Prevotella copri, which are both beneficial.
Conclusion:
AFO-202 beta 1,3–1,6 glucan, in addition to balancing the gut microbiome in children with ASD and its role in effective control of curli-producing Enterobacteriaceae that leads to α-synuclein misfolding and accumulation, may have a prophylactic role in Parkinson’s and Alzheimer’s diseases as well.
TRIAL REGISTRATION
The study was registered in India’s clinical trial registry CTRI, Ref no: CTRI/2020/10/028322. URL: http://ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=47623.
INTRODUCTION
The emerging evidence of constant communication and interaction between the gut and the brain through the gut–brain axis has begun to unravel its significance associated with the health of the central nervous system (CNS). Any dysbiosis of the gut microbiota has been shown to influence the development and progression of neurological pathologies of developmental (autism spectrum disorder [ASD]), inflammatory (multiple sclerosis [MS]), and degenerative (Alzheimer’s disease [AD] and Parkinson’s disease [PD]) disorders [1]. The mechanisms involve activation of the immune system; production of inflammatory cytokines and chemokines (e.g., IL-6 and TNF-α); and alteration of the gut barrier permeability, which in turn is due to the increased levels of circulating lipopolysaccharide in these neurological disorders. These mechanisms modulate the neurotrophic factors, activity of the CNS and peripheral nervous system, and the endocrine pathways, all of which contribute to the onset or the phenotypic expression of neuropsychiatric and neurodevelopmental disorders [2]. Another important player is the amyloid protein, which has self-aggregation properties. Even non-identical amyloid proteins can accelerate reciprocal amyloid aggregation in a prion-like fashion. Nearly 30 amyloidogenic proteins are encoded by humans, whereas some functional amyloids are produced by the gut microbiome [3]. Of importance are cell-surface amyloid proteins called curli, which are produced by certain Enterobacteriaceae that in turn accelerate formation of α-synuclein (αSyn), which is a presynaptic neurotransmitter that is crucial in the initiation and pathogenesis of neurological disorders such as ASD, PD, AD, and MS [4]. Though these reports [2–4] discuss the correlation of the levels of these amyloids, enteric bacteria, and neural diseases, there has been no simple and safe intervention with subjective and objective correlation to a clinical benefit that can be derived based on an associated balancing of the gut microbiota. We herein report the outcome of beneficial reconstitution of gut microbiota, especially those of the bacteria associated with αSyn and curli amyloids after consumption of beta 1,3–1,6 glucans in children with ASD in a clinical pilot study. The beta-glucan studied (Nichi Glucan) was obtained from the AFO-202 strain of a black yeast called Aureobasidium pullulans that has beneficial advantages in metabolic disorders by alleviating glucotoxicity [5], lipotoxicity [6, 7], lipidemia-induced hepatic fibrosis [8], and inflammation [9], apart from immune-enhancement [9] and modulation in COVID-19 [10], which have been reported in translational and clinical studies. The effects of this AFO-202 beta glucan from the same pilot study in terms of behavioral [11] and sleep pattern improvement [12], increase in levels of plasma αSyn [11], and serum melatonin [12] levels in children with ASD has already been reported. We report the effects of AFO-202 beta glucan on the gut microbiome in children with ASD who participated in our pilot study.
METHODS
The study was registered in India’s clinical trial registry CTRI, Ref no: CTRI/2020/10/028322. URL: http://ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=47623. The study was approved by the Institutional Ethics Committee (IEC) of Saravana Multispeciality Hospital, Madurai, India on 24 August 2019 (Ref ID: GLU/2020/01). The caregivers of each subject gave their informed consent for inclusion before participation in the study. The study was conducted in accordance with the Declaration of Helsinki.
Patient involvement
Patients were involved in the design and conduct of this research. During the feasibility stage, priority of the research question, choice of outcome measures, and methods of recruitment were informed by discussions with patients through a focus group session and structured interviews. Once the trial has been published, participants will be informed of the results through a study newsletter suitable for a non-specialist audience.
Study design
The subjects enrolled in the study had received a diagnosis of ASD from a developmental pediatrician, which was verified by a psychologist using a clinical interview for a behavioral pattern that incorporated the Childhood Autism Rating Scale score.
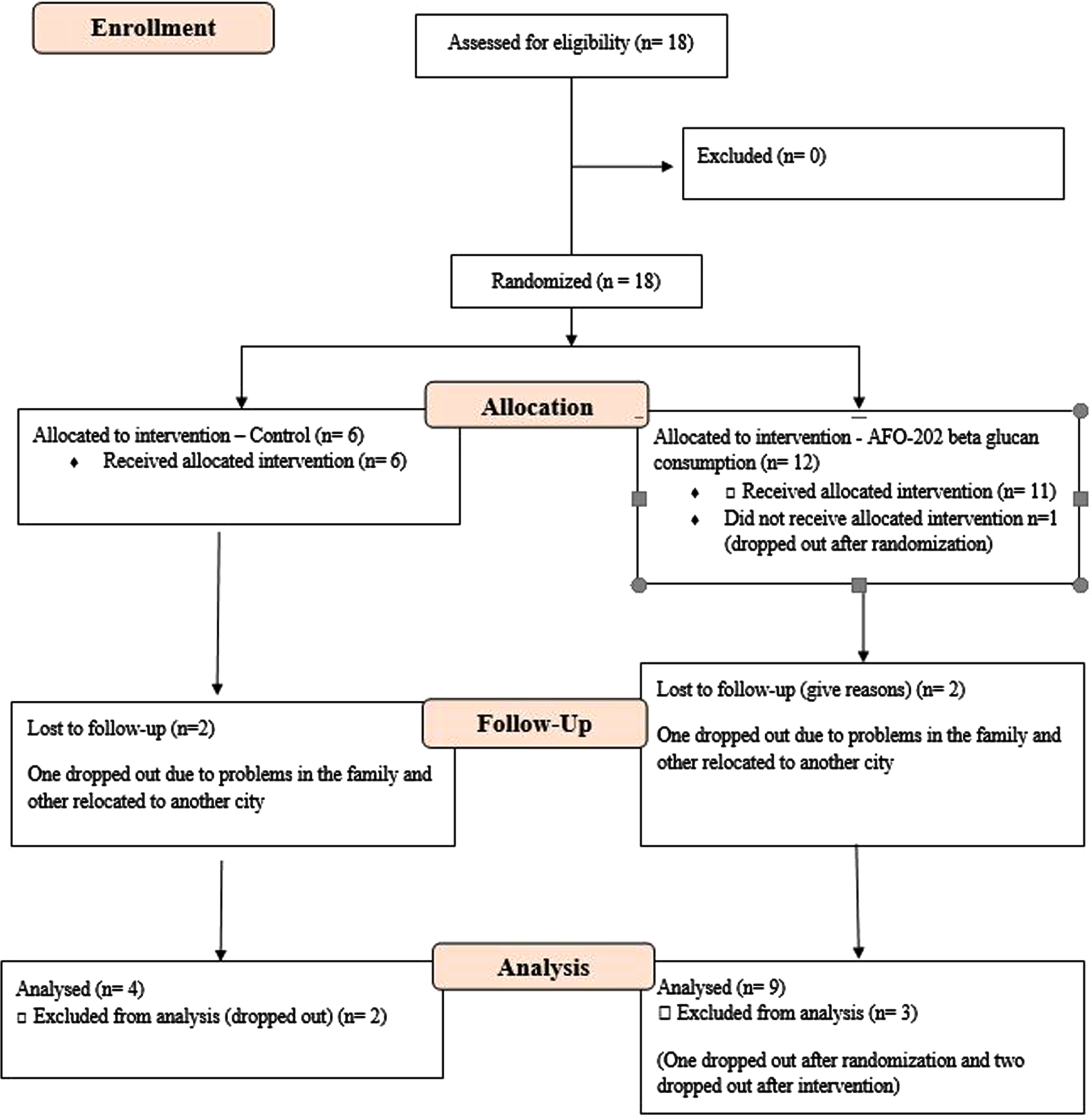
Eighteen subjects with ASD were enrolled in this prospective, open-label, pilot clinical trial comprised of two arms. The CONSORT flow diagram is presented in Fig. 1.
Fig. 1
CONSORT flow diagram of the trial.
Study groups
Arm 1 or Group 1 (control group): Six subjects with ASD underwent conventional treatment comprising remedial behavioral therapies and L-carnosine 500 mg per day.
Arm 2 or Group 2 (Nichi Glucan group): 12 subjects underwent supplementation with Nichi Glucan food supplement along with conventional treatment (remedial behavioral therapies and L-carnosine 500 mg per day). Each subject consumed two sachets (0.5 g each) of Nichi Glucan daily— one sachet with a meal twice daily— for 90 days.
Inclusion criteria: 1) Age: 3 to 18 years; 2) Gender: Both male and female; 3) ASD criteria as per Childhood autism rating scale (CARS) score; 4) Parents/caretakers willing to provide consent for their children to actively participate in the study.
Exclusion criteria: 1) Subjects aged more than 18 years old; 2) Any child with acute general illness or who has been on any antibiotic, anti-inflammatory, or antioxidant treatment in the two weeks prior to enrolment in the study; 3) Hyperallergic to any of the investigational products; 4) Subjects with long-standing infections.
Before the patients were inducted into the trial, background information was collected on gender, date of birth, age, eating habits, current medical history, medication, treatment, previous history, allergies (to drugs and food), regular use of food for specified health uses, functional foods, health foods, and intake of foods rich in β-glucan foods containing beta-glucans.
The following items were recorded during the study: intake of test foods, body temperature, intake of food for specified health uses, functional foods, and health foods, intake of restricted foods, subjective symptoms, visits to medical institutions, treatment, and use of medicines.
Examples of restricted foods: Supplements rich in beta-glucan: supplements containing beta-glucan extracted and concentrated from yeast, barley, mushrooms, and seaweed. Foods claiming to alter the gut dysbiosis: e.g., yoghurt, lactobacillus beverages, bifidobacteria powder, propolis, lactoferrin, etc.
The assessment of behavioral and sleep pattern apart from evaluation of levels of αSyn and melatonin are available in the clinical trial reported earlier. Briefly, CARS for behavior and Children’s Sleep Habits Questionnaire (CSHQ-A) for sleep pattern were employed. αSyn and melatonin levels were analyzed by ELISA [11, 12].
Fecal sample collection and preparation
Fecal samples were collected at baseline and 90 days after the intervention using a sterile fecal collection kit and the samples were kept at –20°C until they were transferred to the laboratory and processed. Samples for DNA extraction were stored at –80°C until needed for analysis.
DNA extraction
Total microbial DNA was extracted from feces of each specimen using the QIAAmp DNA Mini Kit (Qiagen) according to manufacturer’s instructions. Each batch of specimens were extracted with negative buffer control (extraction control).
Library preparation
Whole-genome metagenome sequencing libraries were prepared. In brief, the DNA was sheared using a Covaris ultrasonicator. Sheared DNA was subjected to a sequence of enzymatic steps for repairing the ends and tailing with dA by ligation of indexed adapter sequences. These adapter-ligated fragments were then cleaned up using SPRI beads. Next, the clean fragments are indexed using limited cycle PCR to enrich the adapter-ligated molecules. Finally, the amplified products were purified and checked before sequencing.
Metagenome sequencing
Prepared libraries were sequenced using Novaseq 6000 with a read length of 151 bp. The samples were taken for whole genome metagenome analysis. Initially, the reads were filtered for human DNA contamination (Supplementary Figures 1–3). The alignment to the human genome was around 18.98%. The filtered reads were then aligned to bacterial, fungal, viral, and archea genomes (Supplementary Figure 4). The overall alignment to the bacterial genome was around 40%. However, the alignment to the viral, fungi, and archaea genomes was around 0.05–0.2%. De novo assembly was carried out using the preprocessed reads to obtain the scaffolds, which were then used for gene prediction. The abundances at the phylum, genus, and species level were evaluated.
Microbiome bioinformatics
The following bioinformatics pipeline was used to perform whole-genome-sequencing metagenomic analysis. The quality of the raw data was analyzed, and the adapters were trimmed. The low-processed reads were first aligned to human genome to remove unaligned reads that were then assembled using METASPADES de novo assembler for metagenomics. After assembly, the gene prediction was performed using PRODIGAL. The predicted genes were then searched against existing genes in the NCBI database using the DIAMOND MEGAN5 program. Shannon plots were generated for Microbial alpha diversity. SEED Subsystem analysis with linear discriminant analysis (LDA) effect size (LEfSe) method was performed to find the functional changes between control and the treatment groups. The occurrence of dominant microbial population was studied at various levels (phylum, class, order, family, and genus) based on the taxonomic abundance in the given samples. The dominance was calculated based on the amount of sequence obtained from samples, community composition, and the contig size distribution. Chimeric sequences were identified and filtered from the analysis.
Statistical analysis
The statistical analysis of the study was carried out in accordance with the study protocol as follows.
Target population for analysis
After all data had been obtained, a decision was made on the handling of cases and the acceptance or rejection of data for all cases. Intention to treat (ITT) was defined as the group of all study subjects excluding those who did not consume the study food. The Full Analysis Set (FAS) was defined as the population from which the ITT excluded subjects who withdrew or dropped out of the study. The FAS excluding subjects who met the exclusion criteria for PPS analysis was defined as the Per Protocol Set (PPS).
Exclusion criteria for PPS analysis
All study subjects who consumed the test food were included in the analysis. However, if a study subject met any of the following exclusion criteria, the study investigator and sponsor discussed and decided how to handle the test results: 1) Less than 85% intake of the study food; 2) Less than 85% of the response to follow up has been made; 3) Repeated failure to take the indicated precautions; 4) If it became clear after the completion of the test that there has been a breach of the exclusion criteria; 5) When there are other obvious reasons for omission.
Statistical data were analyzed using Microsoft Excel statistics package analysis and Origin Lab Origin 2021b software. The statistical significance level was set at 5%. Non-parametric tests such as Friedman test for repeated-measures and Kruskal-Wallis test for independent measures were employed were used for metagenomic sequencing data, including taxonomy.
RESULTS
Eighteen patients who fulfilled all the selection criteria and none of the exclusion criteria were selected to start the study. These 18 patients were included in the ITT analysis.
During enrolment, in the treatment group (Group 2), one of them dropped out before the start of the study.
During the study, four subjects were lost to follow-up: two in Group 1 (one dropped out due to social problems in the family, and the other relocated to another city) and two in Group 2 (one dropped out due to social problems in the family, and the other relocated to another city). After excluding these four subjects, 13 subjects were included in the PPS.
The results of the studies published earlier [11, 12] based on the same pilot study showed that the average serum melatonin and plasma αSyn levels increased significantly post-intervention in the Nichi Glucan group. Eight out of nine subjects in the Nichi glucan group showed improvement in their sleep pattern and quality. All the subjects in the Nichi Glucan group showed improvement in behavior evident from a significant decrease in the CARS score.
The pre-processed reads were first aligned with the human genome (hg19) using BWA-MEM aligner to remove human genome contamination from the samples. The uncontaminated sequences were then taken for further alignment with known bacteria, fungi, virus, and archaea bacteria genomes using BWA MEM aligner. Around 7–12% of the reads mapped to the human genome, and the bacterial genome with 30–60% mapped reads.
The Shannon diversity index did not show significant changes (Supplementary Figure 5). When we generated LDA for genus level (L6) for all comparisons (baseline control versus post-intervention control; baseline treatment versus post-intervention treatment; baseline control versus baseline treatment; post-intervention control versus post-intervention treatment), we did not find any significant discriminant for any of the comparisons as the data is highly correlated between the groups (Supplementary Figures 6 and 7).
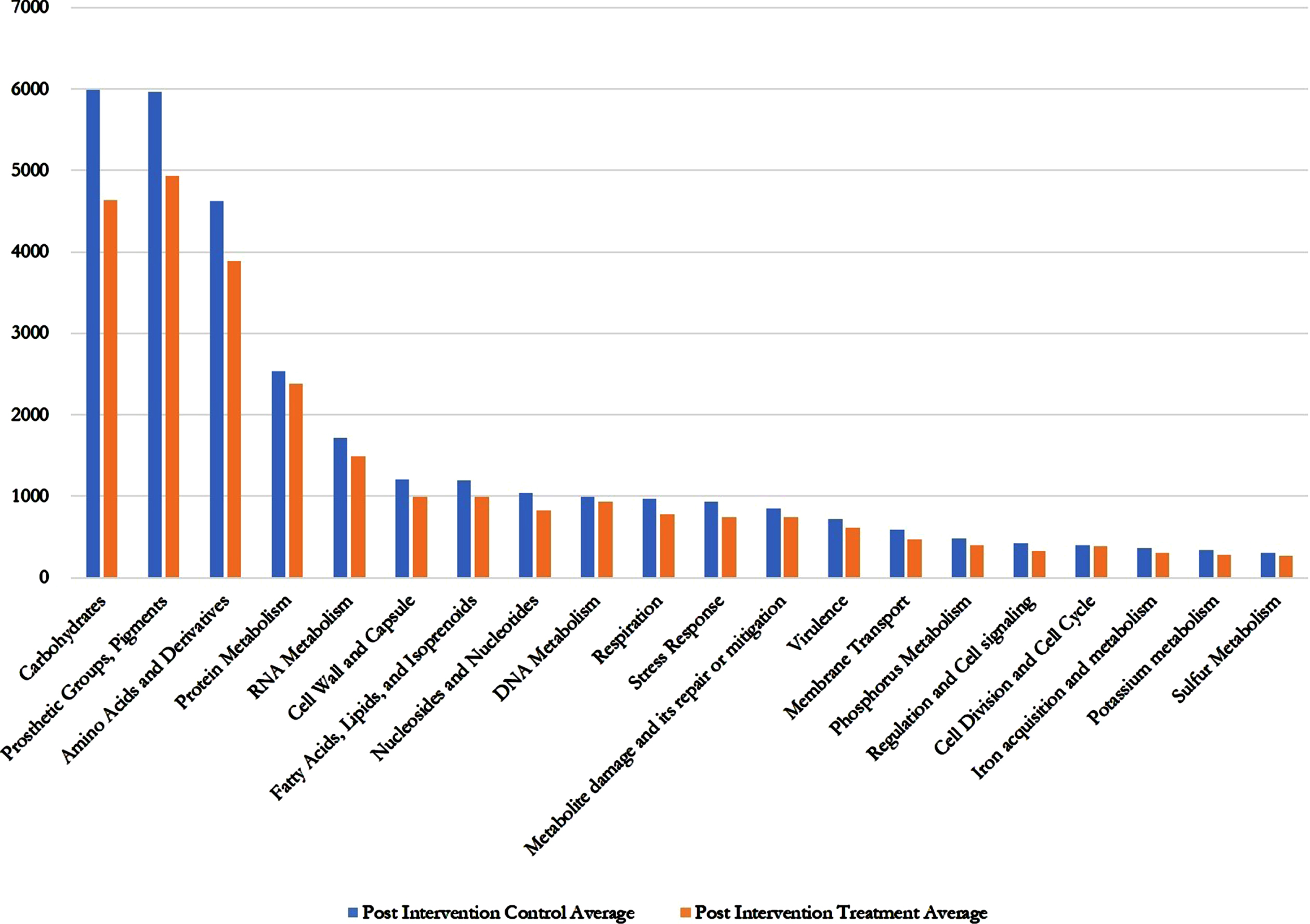
Regarding the SEED average, there was several fold decreases in all the gene annotations (metabolites and metabolic functions) in the AFO-202 Nichi Glucan treatment group including carbohydrates, fatty acids, lipids, virulence, metabolite damage, and nitrogen metabolism (Fig. 2 and Supplementary Table 1).
Fig. 2
Post intervention control versus post intervention treatment SEED Average values showing decrease in treatment (Nichi Glucan) group compared to control group for all the metabolites and metabolic functions.
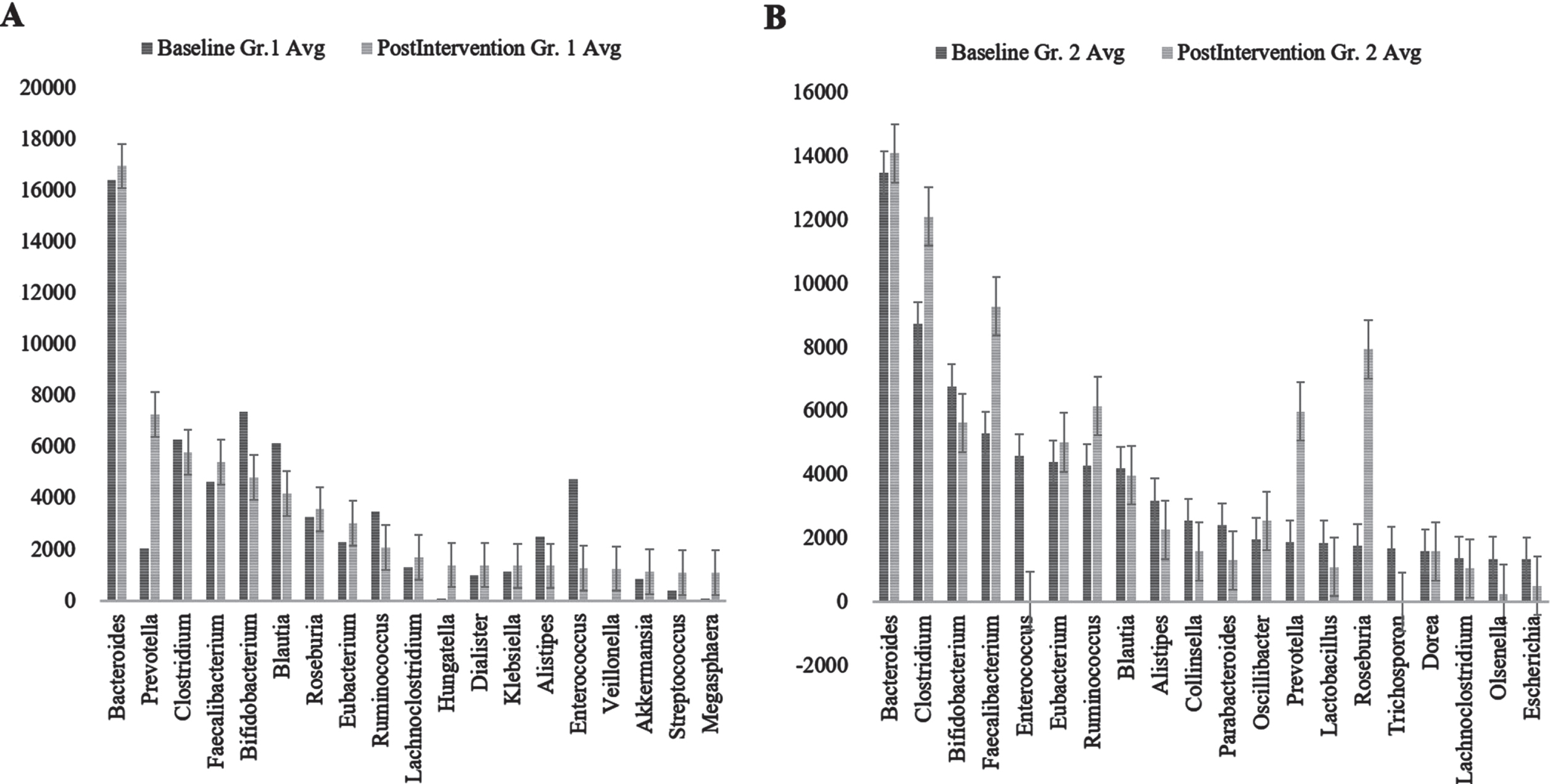
Bacterial kingdom was the most abundant organism type one. In both Group 1 (control) and Group 2 (Nichi Glucan), both before and after intervention, phylum Firmicutes was the most abundant followed by Bacteroidetes (Supplementary Figure 8). Although in Group 1 Proteobacteria was the next most abundant followed by Actinobacteria, this relationship was reversed in Group 2 (Fig. 3).
Fig. 3
Genus abundance of major genera identified (A) Group (Gr.) 1 at baseline versus postintervention and (B) Group (Gr.) 2 at baseline versus postintervention. Phylum Firmicutes was the most abundant followed by Bacteroidetes.
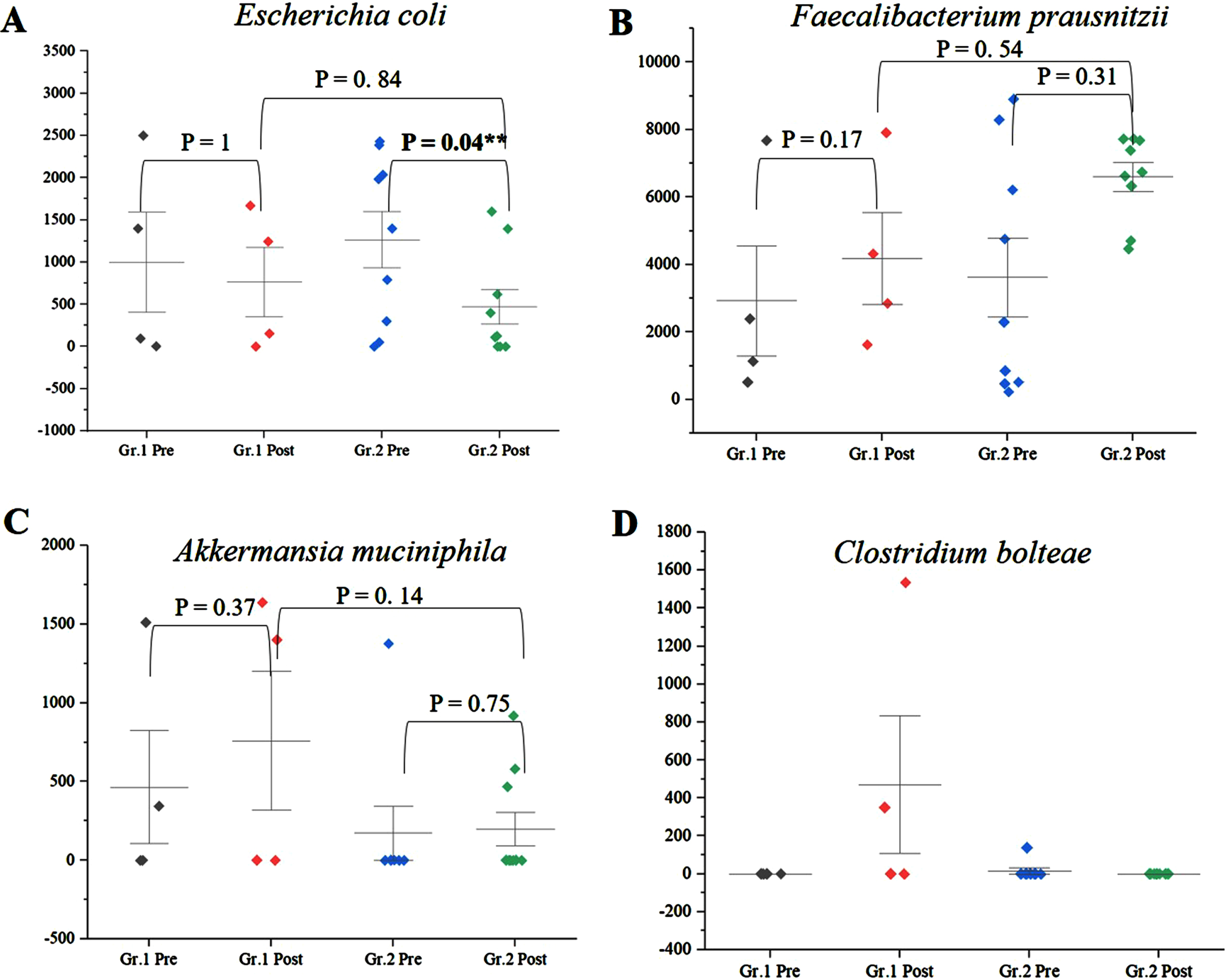
Among the genera of relevance, the abundance of Enterobacter was decreased to almost zero in Group 2 after intervention, while it increased from 0.36% to 0.85% in Group 1 (Fig. 4A) (p = 0.045). The abundance of Bacteroides increased from 16.84% to 19.09% (p = 0.65) in Group 1, while it showed little significant difference (11.60% and 11.43%) (p = 0.73) (Fig. 4B) after intervention. The abundance of Prevotella increased in both Group 1 and Group 2 (Fig. 4C). There was decrease in abundance of Lactobacillus in both the groups (Fig. 4D). Desulfovibrio decreased from 0.40% to 0.28% in Group 2. Species abundance increased in Group 2 after intervention. Faecalibacterium prausnitzii, Bifidobacterium longum, and Firmicutes bacterium CAG:124 represented the most abundant species (Fig. 5 and Supplementary Figure 9). Escherichia coli (E. coli) decreased in both Group 1 and Group 2 and but more significantly in Group 2 (p = 0.04) (Fig. 6A). Faecalibacterium prausnitzii increased in both Group 1 and Group 2 but the difference was not significant (p = 0.54) (Fig. 6B). Akkermansia muciniphila CAG:154 (Fig. 6C) and Clostridium bolteae CAG:59 increased in the Group 1, whereas it decreased in Group 2 (Fig. 6D). Prevotella copri increased in both the groups. Blautia spp., Coprobacillus sp., and several Clostridium spp. decreased in both the groups.
Fig. 4
A) Significant decrease in abundance of Enterobacteriaceae in Group (Gr.) 2 compared to Group (Gr.) 1, postintervention (p = 0.045). B) Decrease in abundance of Bacteroides in Gr. 2 and increase in Gr. 1 postintervention. C) Increase in Prevotella in Gr. 1 and Gr. 2 postintervention and D) decrease in Lactobacillus in Gr. 1 and Gr. 2 postintervention. **significance p < 0.05
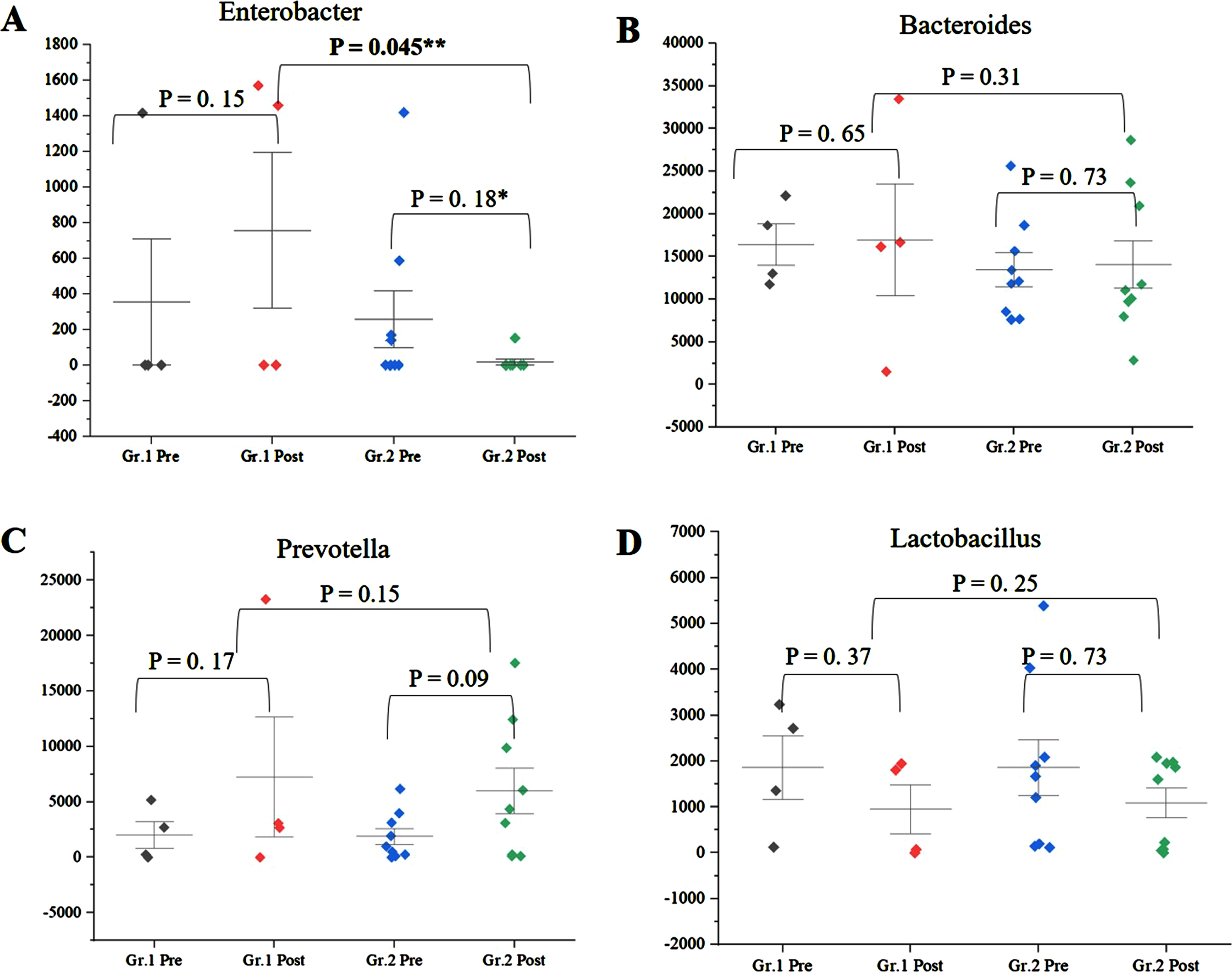
Fig. 5
Species abundance of major species analyzed; A) Group (Gr.) 1 at baseline versus postintervention and B) Group (Gr.) 2 at baseline versus postintervention. Fecalibacterium prausnitzii, Bifidobacterium longum, and Firmicutes bacterium CAG:124 represented the most abundant species.
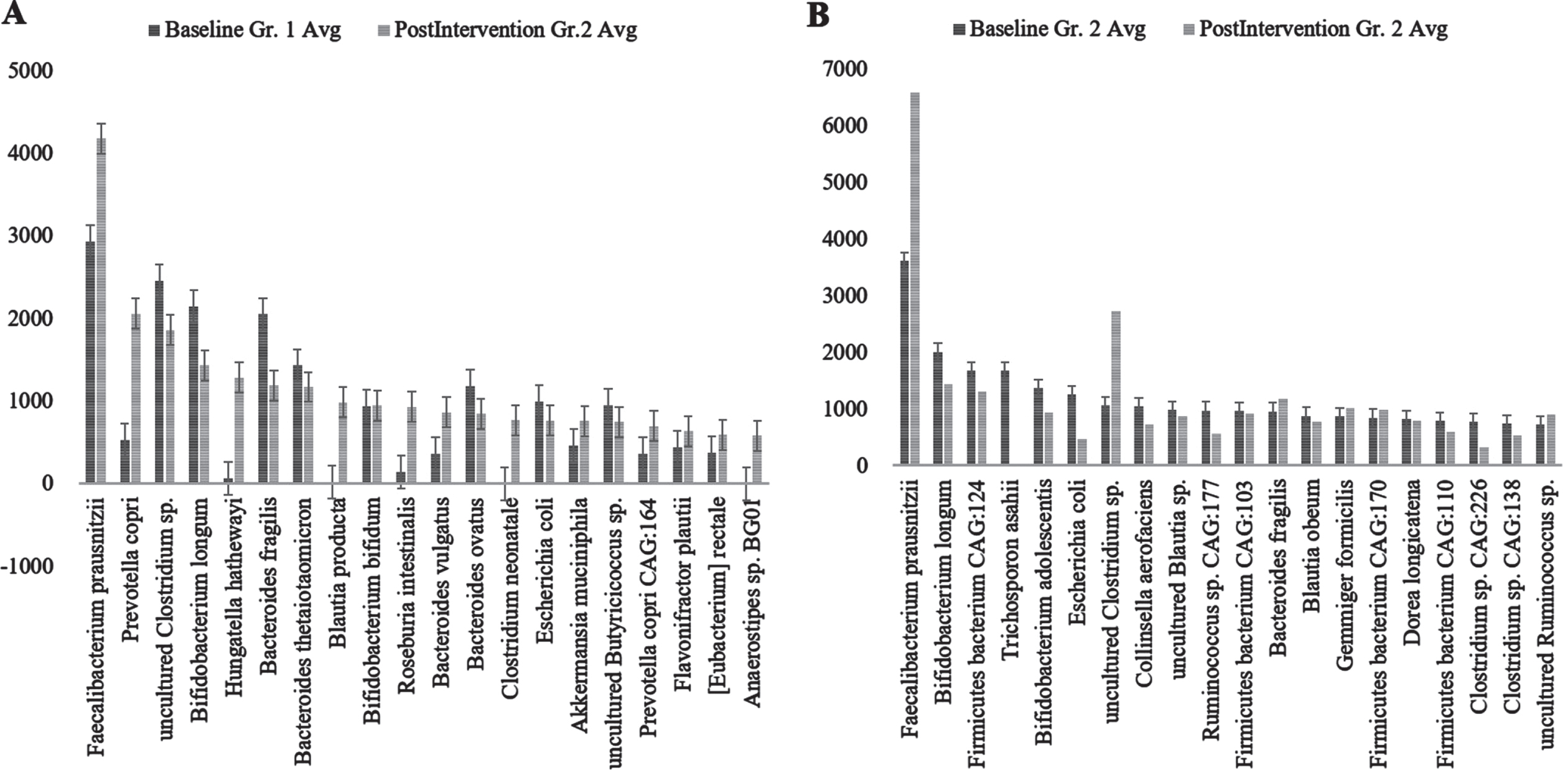
Fig. 6
A) Significant decrease in abundance of E. coli in Group (Gr.) 2 compared to Group (Gr.) 1 (p = 0.04). B) Increase in abundance of Faecalibacterium prausnitzii in Gr. 2 compared to Gr. 1 (p = 0.54). C) Increase in Akkermansia muciniphila in Gr. 1 but decrease in Gr. 2 postintervention. D) Increase in Clostridium bolteae CAG:59 in Gr. 1 but decrease in Gr. 2 postintervention. **significance p < 0.05
The data of the genus and species level abundance in Group 1 and Group 2 are presented in Supplementary Tables 2–5.
Fig. 7
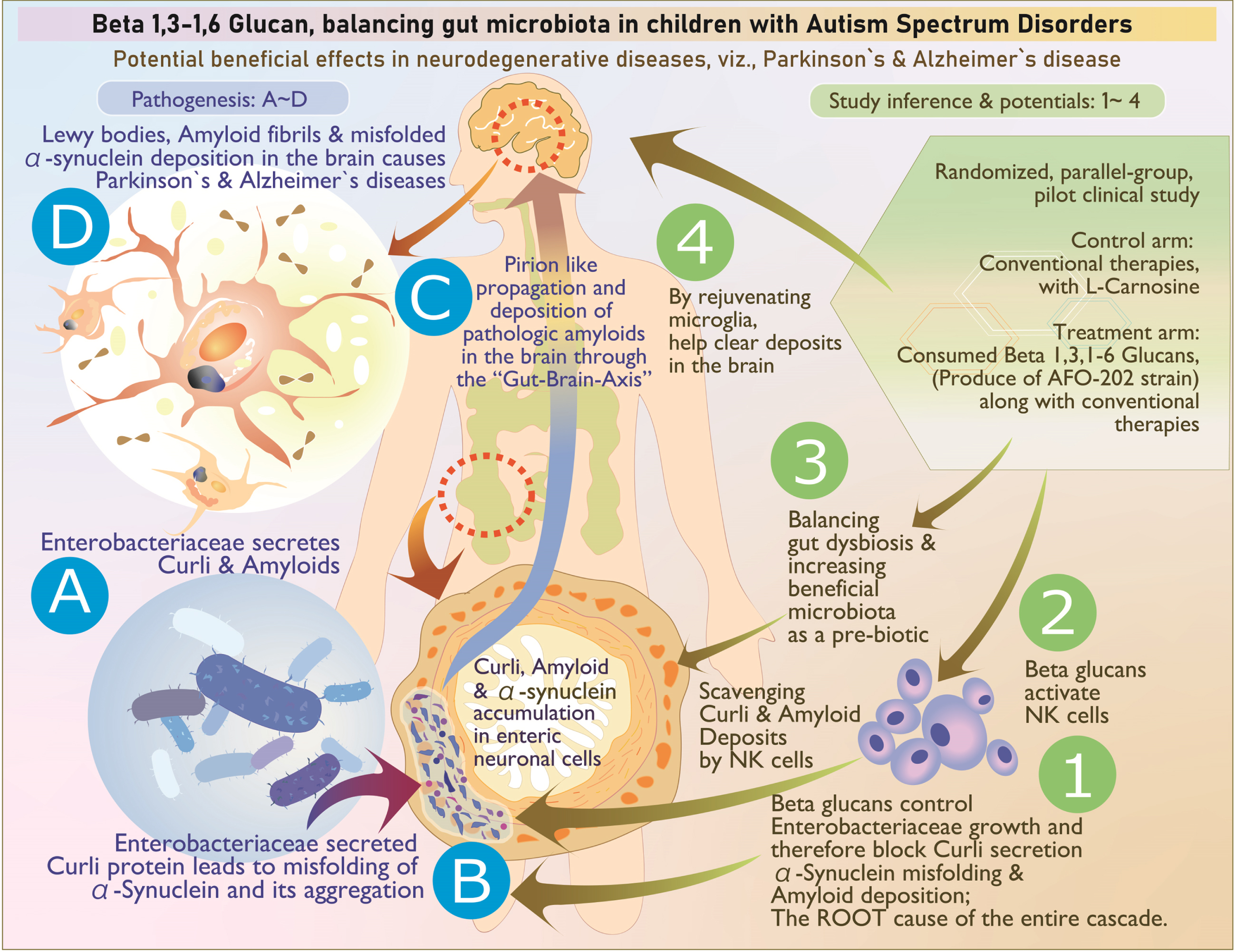
The above illustration explains, stepwise, the pathogenesis as well as the way beta glucan tackles each stage of the disease process: A, B) Enterobacteriaceae secretion of curli that causes misfolding of α-synuclein; its aggregation in enteric neuronal cells is tackled by (1) control of Enterobacteriaceae, (2) scavenging of the accumulated amyloids by activated natural killer cells, and (3) reconstitution of beneficial microbiome. C) The prion like propagation may not occur because the accumulation of curli proteins and amyloids is controlled at the level of production and aggregation (1) as well as clearing of already accumulated deposits (3). D) Deposition of Lewy bodies, amyloid fibrils, and misfolded αSyn are tackled by (4) microglial-based scavenging.
DISCUSSION
In this study, following earlier reports of clinical improvement in terms of sleep, behavioral pattern, plasma αSyn, and serum melatonin increase [11, 12], gut dysbiosis has been shown to have a strong correlation with the severity of symptoms in ASD [13]. We evaluated and compared the gut microbiota of the subjects who were supplemented with AFO-202-derived 1,3–1,6 beta glucan with those who did not take the supplement. Several studies have reported the differences in the gut microbiota between children with ASD and neurotypical children. Reduced number of bifidobacterial and increased Clostridium spp., Desulfovibrio spp., Sutterella spp., and/or Veillonellacea was reported by Souza et al. [14]. Tomova et al. [15] reported a change in Bacteroidetes/Firmicutes ratio and an increase in bifidobacterial numbers after probiotic administration. An exclusion diet and a 6-week prebiotic intervention demonstrated lower abundance of Bifidobacterium spp. and Veillonellaceae family and higher abundance of Faecalibacterium prausnitzii and Bacteroides spp. [13]. Faecalibacterium, Ruminococcus, and Bifidobacterium were relatively less abundant, whereas Caloramator, Sarcina, Sutterella ceae, and Enterobacteriaceae were more abundant in children with ASD [16]. In addition, lower abundances of the genera Prevotella, Coprococcus, and unclassified Veillonellaceae have been reported [17]. Among these bacteria, increased Bacteroidaceae, Prevotellaceae, and Ruminococcaceae and decreased Prevotella copri, Faecalibacterium prausnitzii, and Haemophilus parainfluenzae have been noted [18, 19]. In the present study, in line with these reports, the shift of the gut microbiome was towards a beneficial spectrum in Group 2 (Nichi Glucan) because there was a decrease in Enterobacter, Lactobacillus, E. coli, Akkermansia muciniphila CAG:154, Blautia spp., Coprobacillus sp., several Clostridium spp., and Clostridium bolteae CAG:59, with an increase in the abundance of Bacteroides, Prevotella, Faecalibacterium prausnitzii, and Prevotella copri. Desulfovibrio bacteria, which have been reported to be associated with PD [20], decreased in the Group 2.
In particular, Enterobacter and E. coli significantly decreased in Group 2 compared to Group 1 after the intervention. Gram-negative enteric bacteria such as the Enterobacteriaceae and E. coli secrete the amyloid curli that constitutes 85% of the extracellular matrix of enteric biofilms. The curli has similarities and associations with pathological and immunomodulatory human amyloids such as amyloid-β implicated in AD, αSyn involved in ASD and PD, and serum amyloid A associated with neuroinflammation [21]. Curli causes misfolding [22] and accumulation of the neuronal protein αSyn in the form of insoluble amyloid aggregations, leading to inflammation and neuronal dysfunction that is central to pathogenesis of Lewy body-associated synucleinopathies, including PD and AD. Curli-producing bacteria also increase the production and aggregation of the amyloid protein αSyn, which has been shown to propagate in a prion-like fashion from the gut to the brain via the vagus nerve and/or spinal cord, thus culminating in the neurological disorders such as ASD [22]. In this study, the significant decrease in Enterobacter and E. coli will thus be of benefit in these synculeopathies. Before the start of this study, the objective to study αSyn was to understand the effects of the beta glucan supplementation on synaptic imbalance in presynaptic terminals, observed in ASD. The study results showed that plasma levels of αSyn increased in Group 2 compared with Group 1 along with improvement in CARS score and sleep pattern, which, to our knowledge, is the first of its kind intervention producing an observable change in the plasma synuclein levels [22]. In a study by Ding et al. [23], it was reported that 14 functional properties displayed differences between the ASD and healthy control groups. Four functions, including galactose metabolism, glycosyltransferase activity, glutathione metabolism, and antifolate resistance, were enriched in the ASD group. In another study by Lindefeldt et al. [24], the relative abundance of 26 metabolic pathways was diminished after 3 months on a ketogenic diet in children with epilepsy and 3 became more relative abundant. The group with most pathways changed was carbohydrate metabolism, showing reduction of fructooligosaccharides and raffinose utilization, sucrose utilization, glycogen metabolism, lacto-N-biose I, and galacto-N-biose metabolic pathway. The SEED average findings of the present study also reflect this beneficial outcome (Fig. 2 and Supplementary Table 4). The study of the gut microbiome has offered further new insights wherein Enterobacter increasing curli protein and αSyn deposition in the enteric nervous system having been controlled by the beta glucan food supplement, the increase in plasma αSyn levels point to the disintegration of the amyloid deposits leading to these αSyn entering the blood stream. Indeed, natural killer (NK) cells have been shown to act as efficient scavengers of abnormal αSyn aggregates [25], and the AFO-202 beta glucan has a proven capability to increase and activate NK cells [26], which could be another probable mechanism contributing to the increased αSyn levels in the plasma, apart from positive clinical outcomes in these children with ASD. This result highlights the potential of NK cells as a promising therapeutic strategy for prophylaxis and prevention of brain disorders, and they are likely to be used for such αSyn accumulation and propagation, after relevant research on their specific pathways and variations in their capability (Fig. 7). NK cells have been proven to clear the amyloid deposits peripherally, though not macrophages [27]. In the CNS, such a role is played by the microglia [28]. Beta glucans also rejuvenate microglia [29] that have been shown to scavenge amyloid deposits in the brain and CNS [30], thus proving to be a wholesome therapeutic strategy for neurodevelopmental and neurodegenerative diseases. Altered αSyn protein misfolding spreading to anatomically connected regions in a prion-like manner and mediating neurodegenerative diseases such as PD and the increased risk of children with ASD in developing PD at a later stage [31] suggests further research into these converging pathogenic pathways of neurodevelopmental and neurodegenerative diseases is needed as well as suggests that this safety-proven food supplement is a preventive strategy in subjects with ASD against PD. Other than research into normal and abnormal αSyn being warranted, studying the implications of soluble and insoluble αSyn [32] is important because the proportion of insoluble αSyn that was phosphorylated at Ser129 was reported to be significantly higher in brain tissue from PD patients. In addition, cell lines such as the SH-SYHY neuroblastoma [32] will reveal the correlation of the various αSyn with the severity of symptoms and pathogenesis in these neurodegenerative diseases, apart from helping to develop novel disease-modifying strategies employing such simple nutritional supplementation.
The major limitations of the study are the small sample size, proportion between the two arms of the clinical trial not being the same, the high dropout rate, and the high variability within each group being reflected in limited statistically significant differences in the results. The high dropout rate was mainly because subjects were children who had other factors influencing their compliance with the intervention. Nevertheless, the microbiota reconstituted in a beneficial manner in the present study with AFO-202 beta glucan is significant and must be further progressed into research to study the effects of other variants of Aureobasidium pullulans beta glucan that have been shown to be anti-inflammatory. Such research could lead to mechanistic insight into the molecular pathways from the local immune responses in the gut leading to systemic inflammation and, eventually, to organ-specific autoimmunity of the CNS in neuro-inflammatory conditions such as MS.
Conclusion
Favorable reconstitution of the gut microbiota after consumption of AFO-202 beta glucan in children with ASD has been demonstrated in this study, apart from the clinical improvement already reported. The decrease in Enterobacteriaceae demonstrates the potential of this beta glucan supplementation for neurodevelopmental conditions such as ASD as well as neurodegenerative disorders such as PD and AD, with converging pathways of amyloid accumulations and propagation, warranting larger clinical studies and research to recommend this as a routine food supplement or an adjunct to existing therapies for prevention and management of both neurodevelopmental and neurodegenerative diseases.
ACKNOWLEDGMENTS
The authors would like to dedicate this paper to the memory of Mr. Takashi Onaka, who passed away on 1 June 2022 at the age of 90 years, who played an instrumental role in successfully culturing an industrial scale up of AFO-202 strain of Aureobasidium pullulans after their isolation by Prof. Noboru Fujii, producing the novel beta glucan described in this study. They thank 1) The Government of Japan and the Prefectural Government of Yamanashi for a special loan and M/s Yamanashi Chuo Bank for processing the transactions; 2) Mr. Mohan Ponnusamy, Dr. Madhankumar & staff of Kenmax, Mr. Vijayakumar and Mr. Manoha of MediNippon Healthcare Pvt. Ltd., Chennai, India for their assistance during the clinical study and data collection of the manuscript; 3) Mr. Yasunori Ikeue, Dr. Mitsuru Nagataki, Mr. Yasushi Onaka, and Mr. Masato Onaka (Sophy Inc, Kochi, Japan), for necessary technical clarifications; 4) Mr. Yoshio Morozumi and Ms. Yoshiko Amikura of GN Corporation, Japan for their liaison assistance with the conduct of the study; 5) Ms. Eiko Amemiya of II Dept. of Surgery, University of Yamanashi for her secretarial assistance; and 6) Loyola-ICAM College of Engineering and Technology (LICET) for their support to our research work.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/22-0388r2).
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-220388.
REFERENCES
[1] | Kang DW , Adams JB , Coleman DM , Pollard EL , Maldonado J , McDonough-Means S , Caporaso JG , Krajmalnik-Brown R ((2019) ) Long-term benefit of Microbiota Transfer Therapy on autism symptoms and gut microbiota, Sci Rep 9: , 5821. |
[2] | Santocchi E , Guiducci L , Prosperi M , Calderoni S , Gaggini M , Apicella F , Tancredi R , Billeci L , Mastromarino P , Grossi E , Gastaldelli A , Morales MA , Muratori F ((2020) ) Effects of probiotic supplementation on gastrointestinal, sensory and core symptoms in autism spectrum disorders: A randomized controlled trial, Front Psychiatry 11: , 550593. |
[3] | Werner T , Horvath I , Wittung-Stafshede P ((2020) ) Crosstalk between alpha-synuclein and other human and non-human amyloidogenic proteins: Consequences for amyloid formation in Parkinson’s disease, J Parkinsons Dis 10: , 819–830. |
[4] | Sampson TR , Challis C , Jain N , Moiseyenko A , Ladinsky MS , Shastri GG , Thron T , Needham BD , Horvath I , Debelius JW , Janssen S , Knight R , Wittung-Stafshede P , Gradinaru V , Chapman M , Mazmanian SK ((2020) ) A gut bacterial amyloid promotes α-synuclein aggregation and motor impairment in mice, eLife 9: , e53111. |
[5] | Dedeepiya VD , Sivaraman G , Venkatesh AP , Preethy S , Abraham SJ ((2012) ) Potential effects of nichi glucan as a food supplement for diabetes mellitus and hyperlipidemia: Preliminary findings from the study on three patients from India, Case Rep Med 2012: , 895370. |
[6] | Ganesh JS , Rao YY , Ravikumar R , Jayakrishnan GA , Iwasaki M , Preethy S , Abraham SJ ((2014) ) Beneficial effects of black yeast derived 1-3, 1-6 Beta Glucan-Nichi Glucan in a dyslipidemic individual of Indian origin–a case report, J Diet Suppl 11: , 1–6. |
[7] | Ikewaki N , Onaka T , Ikeue Y , Nagataki M , Kurosawa G , Dedeepiya VD , Rajmohan M , Vaddi S , Senthilkumar R , Preethy S , Abraham SJK (2021) Beneficial effects of the AFO-202 and N-163 strains of Aureobasidium pullulans produced 1,3-1,6 beta glucans on non-esterified fatty acid levels in obese diabetic KKAy mice: A comparative study. bioRxiv 2021.07.22.453362; doi: 10.1101/2021.07.22.453362. |
[8] | Ikewaki N , Kurosawa G , Iwasaki M , Preethy S , Dedeepiya VD , Vaddi S , Senthilkumar R , Levy GA , Abraham SJK (2022) Hepatoprotective effects of Aureobasidium pullulans derived Beta 1,3-1,6 biological response modifier glucans in a STAM- animal model of nonalcoholic steatohepatitis. J Clin Exp Hepatol. doi: 10.1016/j.jceh.2022.06.008. |
[9] | Ikewaki N , Sonoda T , Kurosawa G , Iwasaki M , Dedeepiya VD , Senthilkumar R , Preethy S , Abraham SJK (2021) Immune and metabolic beneficial effects of Beta 1,3-1,6 glucans produced by two novel strains of Aureobasid- ium pullulans in healthy middle-aged Japanese men: Anexploratory study. medRxiv 2021.08.05.21261640. doi: 10.1101/2021.08.05.21261640. |
[10] | Raghavan K , Dedeepiya VD , Suryaprakash V , Rao KS , Ikewaki N , Sonoda T , Levy GA , Iwasaki M , Senthilkumar R , Preethy S , Abraham SJ ((2022) ) Beneficial effects of novel aureobasidium pullulans strains produced beta-1,3-1,6 glucans on interleukin-6 and D-dimer levels in COVID-19 patients; results of a randomized multiple-arm pilot clinical study, Biomed Pharmacother 145: , 112243. |
[11] | Raghavan K , Dedeepiya VD , Ikewaki N , Sonoda T , Iwasaki M , Preethy S , Abraham SJ ((2022) ) Improvement of behavioural pattern and alpha-synuclein levels in autism spectrum disorder after consumption of a beta-glucan food supplement in a randomised, parallel-group pilot clinical study, BMJ Neurol Open 4: , e000203. |
[12] | RaghavanK, DedeepiyaVD, KandaswamyR, BalamuruganM, IkewakiN, SonodaT, KurosawaG, IwasakiM, PreethyS, AbrahamSJK ((2022) ) Improvement of sleep patterns and serum melatonin levels in children with autism spectrum disorders after consumption of beta-1,3/1,6-glucan in a pilot clinical study. Brain and Behaviour (In Print). |
[13] | Grimaldi R , Gibson GR , Vulevic J , Giallourou N , Castro-Mejía JL , Hansen LH , Leigh Gibson E , Nielsen DS , Costabile A ((2018) ) A prebiotic intervention study in children with autism spectrum disorders (ASDs), Microbiome 6: , 133. |
[14] | Souza NC , Mendonca JN , Portari GV , Jordao Junior AA , Marchini JS , Chiarello PG ((2012) ) Intestinal permeability and nutritional status in developmental disorders, Altern Ther Health Med 18: , 19–24. |
[15] | Tomova A , Husarova V , Lakatosova S , Bakos J , Vlkova B , Babinska K , Ostatnikova D ((2015) ) Gastrointestinal microbiota in children with autism in Slovakia, Physiol Behav 138: , 179–187. |
[16] | De Angelis M , Piccolo M , Vannini L , Siragusa S , De Giacomo A , Serrazzanetti DI , Cristofori F , Guerzoni ME , Gobbetti M , Francavilla R ((2013) ) Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified, PloS One 8: , e76993. |
[17] | Kang DW , Park JG , Ilhan ZE , Wallstrom G , Labaer J , Adams JB , Krajmalnik-Brown R ((2013) ) Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children., PloS One 8: , e68322. |
[18] | Kang DW , Ilhan ZE , Isern NG , Hoyt DW , Howsmon DP , Shaffer M , Lozupone CA , Hahn J , Adams JB , Krajmalnik-Brown R ((2018) ) Differences in fecal microbial metabolites and microbiota of children with autism spectrum disorders, Anaerobe 49: , 121–131. |
[19] | Oh D , Cheon KA ((2020) ) Alteration of gut microbiota in autism spectrum disorder: An overview, Soa Chongsonyon Chongsin Uihak 31: , 131–145. |
[20] | Murros KE , Huynh VA , Takala TM , Saris PEJ ((2021) ) bacteria are associated with Parkinson’s disease, Front Cell Infect Microbiol 11: , 652617. |
[21] | Miller AL , Bessho S , Grando K , Tükel Ç ((2021) ) Microbiome orinfections: Amyloid-containing biofilms as a trigger for complexhuman diseases, Front Immunol 12: , 638867. |
[22] | Al-Mazidi S , Al-Ayadhi LY ((2021) ) Plasma levels of alpha and gamma synucleins in autism spectrum disorder: An indicator of severity, Med Princ Pract 30: , 160–167. |
[23] | Ding X , Xu Y , Zhang X , Zhang L , Duan G , Song C , Li Z , Yang Y , Wang Y , Wang X , Zhu C ((2020) ) Gut microbiota changes in patients with autism spectrum disorders, J Psychiatr Res 129: , 149–159. |
[24] | Lindefeldt M , Eng A , Darban H , Bjerkner A , Zetterström CK , Allander T , Andersson B , Borenstein E , Dahlin M , Prast-Nielsen S ((2019) ) The ketogenic diet influences taxonomic and functional composition of the gut microbiota in children with severe epilepsy, NPJ Biofilms Microbiomes 5: , 5. |
[25] | Earls RH , Menees KB , Chung J , Gutekunst CA , Lee HJ , Hazim MG , Rada B , Wood LB , Lee JK ((2020) ) NK cells clear α-synuclein and the depletion of NK cells exacerbates synuclein pathology in a mouse model of α-synucleinopathy, Proc Natl Acad Sci U S A 117: , 1762–1771. |
[26] | Ikewaki N , Fujii N , Onaka T , Ikewaki S , Inoko H ((2007) ) Immunological actions of Sophy beta-glucan (beta-1,3-1,6 glucan), currently available commercially as a health food supplement, Microbiol Immunol 51: , 861–73. |
[27] | Raghavan K , Kandaswamy RS , Ikewaki N , Iwasaki M , Abraham SJK ((2021) ) Potentials to alleviate coagulopathy and enhance microglial function of beta (β)- glucans, making them worth a clinical study for COVID-19’s neurological sequalae, J Neurol Sci 427: , 117554. |
[28] | Bartels T , De Schepper S , Hong S ((2020) ) Microglia modulate neurodegeneration in Alzheimer’s and Parkinson’s diseases, Science 370: , 66–69. |
[29] | Luna E , Luk KC ((2015) ) Bent out of shape: α-Synuclein misfolding and the convergence of pathogenic pathways in Parkinson’s disease, FEBS Lett 589: , 3749–3759. |
[30] | Morato Torres CA , Wassouf Z , Zafar F , Sastre D , Outeiro TF , Schüle B ((2020) ) The role of alpha-synuclein and other Parkinson’s genes in neurodevelopmental and neurodegenerative disorders, Int J Mol Sci 21: , 5724. |
[31] | Swirski M , Miners JS , de Silva R , Lashley T , Ling H , Holton J , Revesz T , Love S ((2014) ) Evaluating the relationship between amyloid-β and α-synuclein phosphorylated at Ser129 in dementia with Lewy bodies and Parkinson’s disease, Alzheimers Res Ther 6: , 77. |
[32] | Boziki MK , Kesidou E , Theotokis P , Mentis AA , Karafoulidou E , Melnikov M , Sviridova A , Rogovski V , Boyko A , Grigoriadis N ((2020) ) Microbiome in multiple sclerosis; where are we, what we know and do not know, Brain Sci 10: , 234. |



