Integrating the Synergy of the Gut Microbiome into Regenerative Medicine: Relevance to Neurological Disorders
Abstract
A new paradigm of cell therapy-based approaches as a solution to several diseases caused by damage or loss of cells/tissues leading to organ failure heralded the birth of a new branch in medicine called regenerative medicine (RM), which was further fueled by in vitro cell expansion and tissue engineering (TE) technologies, including the ability to grow embryonic stem cells, induce pluripotent stem cells, and so on. RM addresses organ failure by repair, regeneration, or restoration, rejuvenation using cells, stem cells, or progenitor cells as tools having added cell-derived products also as a tool, and extracellular matrix component–based support, either direct or indirect (e.g., matrix induced autologous chondrocyte implantation) using scaffolds. Now, the main objective of RM is to solve the functional loss of cells that have evolved from cells as tools to cell-derived factors and scaffolds per se as tools. In this context, an important yet indispensable group of cells that constitute the major portion of the human body in terms of the number of cells having several essential roles to play, both directly and indirectly, starting from digestion and the immune system to the growing evidence of influencing neuronal function, aging, and carcinogenesis has been ignored. We would like to focus on these in this review as they should essentially be considered as a tool of RM, especially for neurological disorders for their vital role. What we are indicating is the second genome or the gut microbiome.
Regenerative medicine (RM) is the branch of medicine that deals with restoration, replacement, rejuvenation, or regeneration of the lost/dysfunctional/damaged cells, tissues and organs, and their function. History has noted that RM practices can be seen in myths such as Prometheus, whose liver regenerated every night after being eaten by an eagle during the day. Ancient Greek, Egyptian, Chinese, and Indian physicians all attempted processes like skin grafting [1]. In modern times, some examples of regenerative medicine applications include attempts by the Swiss doctor Paul Niehans to slow the process of aging in humans by infusing the cells of young animals and Dr. E. Donnall Thomas’s bone marrow transplants in 1956 for treating leukemia. The term “regenerative medicine” is believed to have been coined by William Haseltine during a 1999 conference in an attempt to describe an emerging field, which would involve tissue engineering (TE), cell transplantation, stem cell biology, biomechanics prosthetics, nanotechnology, and biochemistry to advance therapies. For the first time in the literature, Leland Kaiser found a record for the term in a 1992 paper, stating “A new branch of medicine will develop that attempts to change the course of chronic disease and in many instances will regenerate tired and failing organ systems” [1, 2].
However, the public’s recognition of the RM field is closely associated with the discovery of stem cells in the 1960s [3]. Thereafter, cell- and stem cell-based therapies became the major tools of RM.
CELLS AS TOOLS OF RM
Cell-based therapies use various kinds of cells and stem cells from different sources with varying differentiation potential, including but not limited to adult cells, progenitor cells, adult stem cells (e.g., hematopoietic stem cells), mesenchymal stem cells, and pluripotent stem cells (e.g., embryonic stem cells, induced pluripotent stem cells). Since 1997, over 300,000 patients have been treated with regulatory-approved cell therapy products [4]. Mesenchymal stem cells are the most widely used ones for both autologous and allogeneic applications. Immune cells, such as natural killer cells, tumor infiltrating lymphocytes, dendritic cells, γδ T cells, regulatory T cells (Treg), and macrophages, are also being developed as cell therapies. Ex vivo gene modifications of the cells have been utilized for T cells, hematopoietic stem cells, and mesenchymal stem cells (MSCs) for treating the genetic diseases adenosine deaminase severe combined immunodeficiency disease and advanced adenocarcinoma. In vivo gene therapy for modifying the endogenous cells by direct introduction of genetic material into the human body has been indicated for cancer gene therapy, neurological disorders, (mono)genetic disorders, infectious diseases, and cardiovascular abnormalities. Targeted genome editing using meganucleases, zinc finger nucleases (ZFNs) and transcription activator-like effector nucleases, and the latest using CRISPR-Cas9 systems have been used for cell-based therapy applications in a wide range of gene-based disorders [4]. In this, autologous HIV treatment by gene editing T cells (CCR5 gene dysfunction) using ZFN technology has reached clinical application [5].
SCAFFOLDS AS TOOLS OF RM
Three-dimensional (3D) scaffolds using biomaterials were initially employed to provide a substrate akin to the extra-cellular matrix for better cell growth and support in vitro, which gave rise to the branch of TE. In a conventional cell culture in a culture dish (2D), the proliferation stops once the cells reach confluence. 3D biomaterials provide a larger surface for cell attachment and proliferation. Also, because they are able to mimic the in vivo environment to a certain extent, the cells aggregate to form tissue-like structures with better functional characteristics than 2D cultures [6–8]. The diffusion of nutrients, oxygen, and bioactive factors helps the cells growing in them survive for extended periods of time. A wide range of natural and synthetic biomaterials have been employed as 3D scaffolds. Some examples of naturally derived biomaterials are collagen, alginate, silk, and chitosan. Although they offer excellent biocompatibility, natural scaffolds have issues in mechanical properties. Synthetic scaffolds were developed to overcome this issue. Examples of widely used synthetic biomaterials include poly(caprolactone), poly(glycolic acid), poly(lactic acid), and their copolymers. Biocompatibility is an issue with synthetic scaffolds, but techniques such as surface modification techniques that provide accessible functional groups for the immobilization of drugs, enzymes, antibodies, or other biologically active species have been used to improve the biocompatibility of such scaffolds [6–8]. Decellularized ECMs from allogenic or xenogenic tissues have been used for engineering tissues and organs in order to replace an analogous structure that has been damaged. Examples of decellularized ECMs include small intestinal submucosa for vascular graft, tendon, dura mater, skin and other tissues, and amniotic membrane. Whole organ TE utilizes decellularized scaffolds, and this technique helps to simulate the architectural growth pattern of cells that are seeded into it with better vasculature. Tracheas have been tissue engineered and transplanted. Organs such as the liver, kidney, and heart are widely being studied for the benefits of this kind of replacement technology [9, 10]. 3D-printing technology uses cells and scaffolds to precisely fabricate tissues layer by layer. This has led to applications for RM in different types of organs such as bone, cartilage, heart valve, liver, and skin [11].
CELL-DERIVED FACTORS AS TOOLS OF RM
Cells’ mechanism and their role in the regeneration process have been attributed to differentiation, cell fusion, and the secretion of cytokines or paracrine effects [12]. More importantly, the contribution of paracrine mechanisms to regeneration has been recently recognized, which has given rise to cell-derived/secreted factors as tools of RM. The application of these cell-derived or secreted factors is termed cell-free regeneration. This mostly involves extracellular vesicles (EVs), which are vesicular entities with lipid bilayer membranes. Among EVs, the smaller vesicles are known as exosomes, released from cells through the multivesicular endosomal pathway. Larger vesicles are termed as microvesicles and are formed by cell membrane budding, and apoptotic bodies are produced by the blebbing of aging or dying cells [13]. MSC-derived exosomes, which include a wide variety of functional proteins, mRNAs, miRNAs, and signaling lipids, are the major types of EVs used. They have been applied in preclinical models for the diseases of the liver, heart, kidney, and bone, as well as brain diseases and cancer. Stem cell–conditioned media contains the presence of cytokines and growth factors, such as vascular endothelial growth factor, FGF-2, IL-6, PIGF, and MCP-1, which are also used in cell-free regeneration approaches [14, 15].
ENDOGENOUS CELL REGENERATION
Although the abovementioned tools (e.g., cells, scaffolds, cell-derived factors) represent exogenous approaches, facilitating endogenous repair by using molecular stimuli, such as gene transfer to harness the intrinsic regenerative potential of endogenous tissues, is another recent approach. Endogenous regenerative medicine (ERM) is a cost-effective approach that uses tools, such as biological agents or chemoattractant gradients and biomaterials, to correct, augment, or engineer the local in vivo microenvironment. These factors help in mobilizing resident reparative cell populations within the host and in driving them home to the targeted area for regeneration. Injection of a vector can stimulate cell regeneration and the division or transplantation of a scaffold, which can help in the homing of stem cells and other cells to facilitate repair in the damaged area, are some of the approaches in endogenous regeneration [16]. ERM has been especially employed in cartilage healing, retinal repair musculoskeletal TE, and myocyte regeneration. ERM, also known as in situ regeneration, also uses the patient’s own biologically active proteins, growth factors, and biomaterial scaffolds (e.g., fibrin) to aid in the repair and regeneration of injured tissues by means of a controlled and local protein and growth factor delivery [17].
GUT MICROBIOME AS TOOLS OF RM
Bacteria, archaea, viruses, and eukaryotic microbes that reside in and on our bodies constitute the human microbiome. Technological advancements (e.g., 16S rRNA-encoding gene sequencing), metagenomic analysis, and analysis of the microbial transcriptome, proteome, and metabolome have been helpful in greatly furthering the knowledge on the human microbiome, especially the gut microbiome. Among the large-scale endeavors to characterize the human microbiome, European Metagenomics of the Human Intestinal Tract (MetaHIT) and the NIH-funded Human Microbiome Project (HMP) made a large contribution through its presentation of data from 1,267 gut metagenomes from 1,070 individuals showing that each sample contained about 750,000 genes or about 30 times the number of genes in the human genome and that less than 300,000 genes were shared by greater than 50% of individuals [18]. The magnitude and functional capabilities of the gut microbiome termed them as the second genome of the body. The gut microbiome differs in terms of health and disease [18]. In healthy adults, more than 1,000 species of bacteria are present, with Bacteroidetes and Firmicutes being the dominant phyla. A diverse gut microbiome represents a healthy microbiome. The gut microbiota generates nutrients from substrates that are otherwise indigestible by humans and hence are integral to digestion and nutrition. Gut microbes liberate short chain fatty acids (SCFAs) from indigestible dietary fibers [18]. SCFAs play a major role as the energy source for intestinal mucosa and are critical for immune modulation and the regulation of tumorigenesis in the gut. The gut microbiome also generates microbial products, such as uremic toxins, bile acids, trimethylamine-N-oxide, SCFAs, lipopolysaccharides (LPSs), nitric oxide, vitamin K, vitamin B complex, gut hormones, and neurotransmitters, all of which play critical roles in host metabolism and body function [19]. Gut dysbiosis or the altered gut microbiome has a two-way connection to disease. Certain disease states cause the dysbiosis due to changes in eating habits and bowel function or due to medications such as antibiotics, whereas the dysbiosis itself caused by inflammatory stimuli may lead to certain diseases [19]. Gut dysbiosis has been associated with several diseases, including but not limited to gastrointestinal disorders, obesity, diabetes, non-alcoholic fatty liver disease, cancer, neurological disorders (e.g., autism, Parkinson’s disease, Alzheimer’s disease), and cardiovascular diseases. Modulating the gut microbiome to treat the disease and aid in repair can be achieved through several strategies such as lifestyle modifications, nutritional interventions, fecal microbiota transfer, antibiotics, prebiotics and probiotics, and pharmabiotics [19].
GUT MICROBIOME AND CELL-BASED THERAPIES
Gut bacteria regulate the damage repair, regeneration, and differentiation of stem cells, either by direct contact or release of products and/or metabolites. Because the gut microbiota intensely participates in the modulation of several host metabolic pathways, immune-inflammatory axes, and signaling pathways, the synergistic effects of modulating the gut microbiome and MSC-based regeneration need mentioning, especially in diseases such as inflammatory bowel disease [20]. Cytokine gene transcription and surface protein expressions in MSCs have been found to be regulated by immune-regulatory mediator secretions of the gastrointestinal bacteria [21]. Microbiota has been found to alter the differentiation potentials and improve the immunomodulation ability of bone marrow MSCs. Nagashima et al. [22] reported that a sub-epithelial population of MSCs are capable of inducing gut microbiota diversity and regulating the production of IgA that in turn a symbiotic equilibrium in the gut. An initial alteration in the Bacteroidetes/Firmicutes ratio, which sustained intestinal mucosal function and homeostasis leading to hepatocyte repair, has been reported after MSC infusion. The gut microbiota is associated with gut epithelial cell regeneration through NOD2 sensors [22]. The gut microbiome has been found to play a critical role in various cancer treatment modalities. Several gut dysbiosis conditions have been reported in cancer patients receiving hematopoietic stem cell transplantation (HSCT) and in conditioning regimens including chemotherapy, radiotherapy, and immunosuppressive therapy [23]. Modulation of the microbiota by probiotics, prebiotics, and fecal microbiota transplantation (FMT) in hematologic cancer patients has been reported with increased positive outcome [24]. Lactobacillus administered before and after HSCT resulted in reduced graft versus host disease and improved survival in animal studies. Patients with refractory C. difficile–associated disease after HSCT after FMT had minimal adverse effects [24].
CARDIAC REPAIR
Gut microbiota by actions on cholesterol metabolism and by metabolite production, such as bile acids, coprostanol, SCFAs, and trimethylamine-N-oxide production, influences the development of coronary artery disease. Microorganisms such as Helicobacter pylori, cytomegalovirus, hepatitis C virus, Chlamydia pneumoniae, and Porphyromonas gingivalis have been linked to atherosclerosis. The composition of gut microbiota has been found to be different between symptomatic and asymptomatic atherosclerotic plaques, with asymptomatic plaques having an increased abundance of Porphyromonadaceae, Bacteroidaceae, Micrococcacaea, and Streptococcacaea, and symptomatic atherosclerotic plaques showed increased abundance of Helicobacteraceae, Neisseriaceae, and Thiotrichacaea. Heart failure has also been associated with increased Escherichia coli, Klebsiella penumoniae, and Streptococcus viridans. With Firmicutes (e.g., Lactobacillus reuteri) being associated with higher HDL and Eggerthella being associated with decreased HDL cholesterol, the gut microbiome by regulating lipids can influence coronary artery disease development [25]. The gut microbiota has been shown to be associated with the pathophysiology of and repair after myocardial infarction (MI). One mice study demonstrated that a reduction in Lactobacillus was associated with MI [26]. Antibiotic-treated mice showed dose-dependent mortality after MI. Depletion of the gut microbiota led to reductions in the proportion of myeloid cells and SCFAs, which in turn also led to decreased infiltration of CX3CR1 + monocytes to the peri-infarct zone, and impairment of repair after MI and dietary SCFA supplementation improved the physiological status and survival of mice. Supplementing with a Lactobacillus probiotic before MI restored myeloid cell proportions and yielded cardioprotective effects [26]. Larger infarct size and poorer post-stroke outcome in mice were observed after transplantation of a dysbiotic microbiome from brain-injured donor mice. Post-stroke recovery was accelerated by the presence of a healthy gut microbiome with a higher abundance of SCFA-producing bacteria (e.g., Bifidobacterium longum, Clostridium symbiosum, and Lactobacillus fermentum) [26].
LIVER REGENERATION
The liver is constantly exposed to bacterial components and gut microbial metabolites through the portal vein. A mild release of LPS from the gut has been shown to stimulate liver regeneration and tissue repair. The gut-derived LPS by binding to Toll-like receptor 4 (TLR-4) activates Kupffer cells for the activation of NF-κB and stimulates the production of tumor necrosis factor-α (TNF-α), which causes the Kupffer cells to secrete interleukin-6 (IL-6). IL-6 trans-signaling induces hepatic stellate cells to produce a hepatocyte growth factor that, in coordination with other extrahepatic factors, such as T3, insulin, and epidermal growth factor, allows the remnant hepatocytes to overcome the cell-cycle checkpoint control and to proliferate towards liver regeneration after partial hepatectomy [27]. Administration of the Lactiplantibacillus plantarum AR113 probiotic has been shown to accelerate liver regeneration by increased hepatocyte proliferation and TNF-α, hepatocyte growth factor, and transforming growth factor-β expression [28]. In a mice study, the administration of five broad-spectrum antibiotics for 14 days at the maximum dose to eliminate the gut microbes showed there was induction of inflammation through the expression of proinflammatory factors (interleukin-1beta (IL-1β), IL-6, TNF-α, and TWEAK), which impaired liver function and activated hepatic progenitor cells [29]. In a STAMtrademark murine model of non-alcoholic steatohepatitis, the supplementation of a beta glucan prebiotic has been shown to decrease fibrosis and inflammation [30].
BONE REGENERATION
The gut microbiota regulates bone homeostasis in health and disease. In a study that treated mice with broad-spectrum antibiotics for 1 month to deplete over 99% of resident bacteria in the gut, the results showed there was increased trabecular bone mass compared to the control mice. The treated mice demonstrated decreased pro-osteoclastogenic cytokine production and increased bone formation reflected by the serum marker P1NP [31]. Lactobacillus reuteri probiotic treatment significantly protected mice from bone loss after surgical ovariectomy in association with reduced levels of a bone-resorption marker and decreased osteoclastogenesis. In another study, Bifidobacterium longum supplementation increased bone mineral density and an increase in bone formation [31]. Lactobacillus plantarum administration has been reported to reverse the blunted skeletal development of germ-free mice [32]. Spontaneous development of osteomyelitis in Pstpip2cmo mice predisposed to autoinflammatory osteomyelitis was able to be prevented by dietary modification of the gut microbiota, which regulated the production of IL-1β [9].
SKIN REGENERATION
The immuno-modulating potential of the microbiome on the skin via the gut–skin axis has been increasingly recognized. The Western diet and the resulting intestinal dysbiosis have been associated with the development of numerous immune-mediated inflammatory diseases, such as rheumatoid arthritis, psoriasis, and atopic dermatitis. Acne patients have been shown to have decreased diversity of the gut microbiota with lower abundance of Firmicutes and increased levels of Bacteroides [33, 34]. Clinical studies are currently researching the oral supplementation of probiotics in acne vulgari. Evidence from a meta-analysis supports the use of probiotics for the treatment of atopic dermatitis in infants mainly through prophylaxis, such as the balance of Th1/Th2 immunity and enhanced Treg activity. In psoriasis, fewer relapses in the group treated with a probiotic mixture have been reported. The promotion of wound healing by the probiotic strain Lactobacillus reuteri through stimulating oxytocin, which in turn induced the CD4 + Foxp3 + CD25 + Treg lymphocytes, has also been reported [35].
LUNG REGENERATION
The existence of a gut–lung axis has been established by studying the influence of the gut microbiome on the lung. The mesenteric lymphatic system is the connecting pathway between the lungs and the intestine, through which intact bacteria, their fragments, or metabolites (e.g., SCFAs) translocate across the intestinal barrier, reach the systemic circulation, and modulate the lung immune response. Upon stimulation of IL-25, innate lymphoid cells involved in tissue repair have been shown to be recruited from the gut to the lungs. Intestinal TLR activation has been shown to be associated with an increased influenza-related lung response in mice involving the NF-κB–dependent pathways of innate immunity and inflammation [36]. Probiotics have been proposed as potential agents to prevent and treat several allergic diseases. Administration of Lactobacillus reuteri, LGG, and Bifidobacterium breve in a murine model of asthma led to decreased airway hyperresponsiveness, the number of inflammatory cells in bronchoalveolar lavage fluid, and inflammation of lung tissue. Intranasal administration of probiotics has been reported to protect mice from H1N1 influenza virus infection by regulating respiratory immune responses. In addition, probiotic use has been associated with a lower incidence of ventilator-associated pneumonia, reduced respiratory infections in healthy and hospitalized children, and reduced duration of infection with the common cold [37].
KIDNEY REGENERATION
Gut dysbiosis affecting the integrity of the intestinal barrier may lead to bacterial translocation, production, and accumulation of dysbiotic gut-derived metabolites, such as urea, indoxyl sulfate, and p-cresyl sulfate, which cause abnormal activation of immune cells, overproduction of antibodies, immune complexes, and inflammatory factors that may directly or indirectly cause damage to the renal parenchyma [38]. Administration of the probiotic L. casei has been shown to reduce kidney inflammation by restoring the SCFA-producing gut microbiome and nicotinamide metabolism [39]. Regulating the gut microbiome has been shown to lower blood pressure, ameliorate kidney disease, and prevent complications in patients with chronic kidney disease. Studies have shown the correlation between intestinal flora and diabetes. Restoring the gut microbiota is considered to be an effective strategy in preventing and treating diabetes and diabetic nephropathy [39]. Administration of antibiotics has been shown to protect against renal ischemia-reperfusion injury by reducing the maturation status of the bone marrow monocytes and F4/80 + renal resident macrophages [39].
NEURONAL REGENERATION
The gut microbiota influences the nervous system in more profound ways than the other systems— that is, by means of the gut–brain axis through bacterial components, dietary metabolites that are systematically available, intermediates (e.g., circulating immune cells), and direct neuronal connections (i.e., the vagus nerve) [40]. The increase in multiple sclerosis (MS) incidence can be seen in subjects who move from low-risk countries to those with higher MS prevalence, usually those far north of the equator. This was initially believed to be due to climatic conditions but has later been proved to be associated with diet and gut microbiome composition. Administration of long chain fatty acids in an experimental model of MS worsened the disease course via polarization towards T-helper (Th) 1 and Th17 cells, whereas the SCFAs (i.e., propionic acids) diminished clinical symptoms due to an increase of intestine-derived Treg. Administration of high-dose propionic acid has been shown to stimulate autism-like behavior in animal models. In a clinical trial, a 2-week antibiotic treatment followed by bowel cleanse and a subsequent FMT resulted in the reduction of gastrointestinal symptoms, accompanied by a significant improvement in behavioral autism spectrum disorder (ASD) symptoms [41]. In another clinical study, the administration of Aureobasidium pullulans (i.e., black yeast AFO-202-produced beta glucan supplement) has been shown to improve sleep, behavior melatonin, and α-synuclein (αSyn) levels in ASD subjects; apart from this, the effective control of curli-producing enterobacteria in turn controls the formation of amyloids such as αSyn, a presynaptic neurotransmitter, whose propagation to the brain and aggregation has been associated with the initiation and pathogenesis of neurological disorders such as ASD, inflammatory (MS), and degenerative (Alzheimer’s disease and Parkinson’s disease) disorders [42]. The antidepressant and neurogenic effects of fluoxetine, a standard selective serotonin reuptake inhibitor, was impaired by fecal transfer from chronic mild stress mice to healthy mice. It is important to note that fluoxetine not only promotes the proliferation, differentiation, and survival of progenitor cells in the hippocampus but also influences the plasticity of new neurons generated [43]. Tryptophan-metabolizing gut microbes mediated by the metabolic- and immune-linked aryl hydrocarbon receptor have been reported to elevate transcription factors and signaling proteins that promote adult neurogenesis, as well as key markers of synaptic maturation [44].
CONCLUDING REMARKS
RM aims to repair, regenerate, or restore lost/damaged/dysfunctional tissues by utilizing tools such as cells, scaffolds, and cell-derived factors. Modern regenerative medicine started with cells as tools, was propelled by the discovery of embryonic stem cells [45], and then moved towards induced pluripotent stem cells [45] apart from progenitor subsequently adult stem cells. Thereafter, cell-secreted factors joined this bandwagon of tools because several of the positive outcomes of cell therapy were mainly attributed to their paracrine effects [12]. Engraftment of the transplanted cells by retaining the cell-produced factors in vivo was better supported by biomimicking the extracellular matrix by the scaffolds, thereby adding scaffold-assisted cell therapy as another tool of RM [6–8].
All of these tools address a damaged or dysfunctional cell or tissue. However, they do not address any hurdle, which may prevent the repair that needs to occur with tools such as cells, scaffolds, or cell-derived factors. One such phenomena pertains to degenerative diseases, especially in neurology, because the age-related decline of both the brain and the entire body has to be addressed along with the repair, which is an uphill task. An important hurdle in neurological regeneration is the abnormal protein deposits. The aberrant self-assembly of such abnormal proteins into amyloid fibers occurs as a unifying molecular event in several neurological disorders such as ASD, depression, anxiety, Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, amyotrophic lateral sclerosis, and so on [46]. These amyloids may be diverse, including αSyn, amyloid-β, cellular prion protein, and tau, which can further accelerate the amyloidogenesis of heterologous mammalian amyloid proteins [47]. Therefore, a wholesome or 360-degree tool of RM would be one which, along with the repair or regeneration process, is capable of scavenging these deposits that occur by cells of the immune system (e.g., microglia, macrophages), preventing the formation of amyloids, or working at the root level of the pathogenesis. Constipation and other GI dysfunctions years prior to the onset of movement dysfunction have been reported in individuals diagnosed with various synucleinopathies [46]. These amyloids are capable of a prion-like spread from the gut to the brain via the vagus nerve and/or spinal cord. In humans, truncal vagotomy and appendectomy have been reported with a decreased risk of Parkinson’s disease [46]. Therefore, it is evident that the gut microbiome plays a major role in the production and propagation of these amyloids. Hence, when the gut microbiome is used as a tool for RM and modulated by strategies, such as dietary interventions, they will become the foremost tool of RM and can be an adjunct to improve the outcome of all the other tools of RM (Fig. 1).
Fig. 1
A schematic illustration describing the contribution of gut microbiome in the prevention and management of neurological conditions, through gut brain axis and microglia respectively, beside supporting all three modes of action of regenerative medicine.
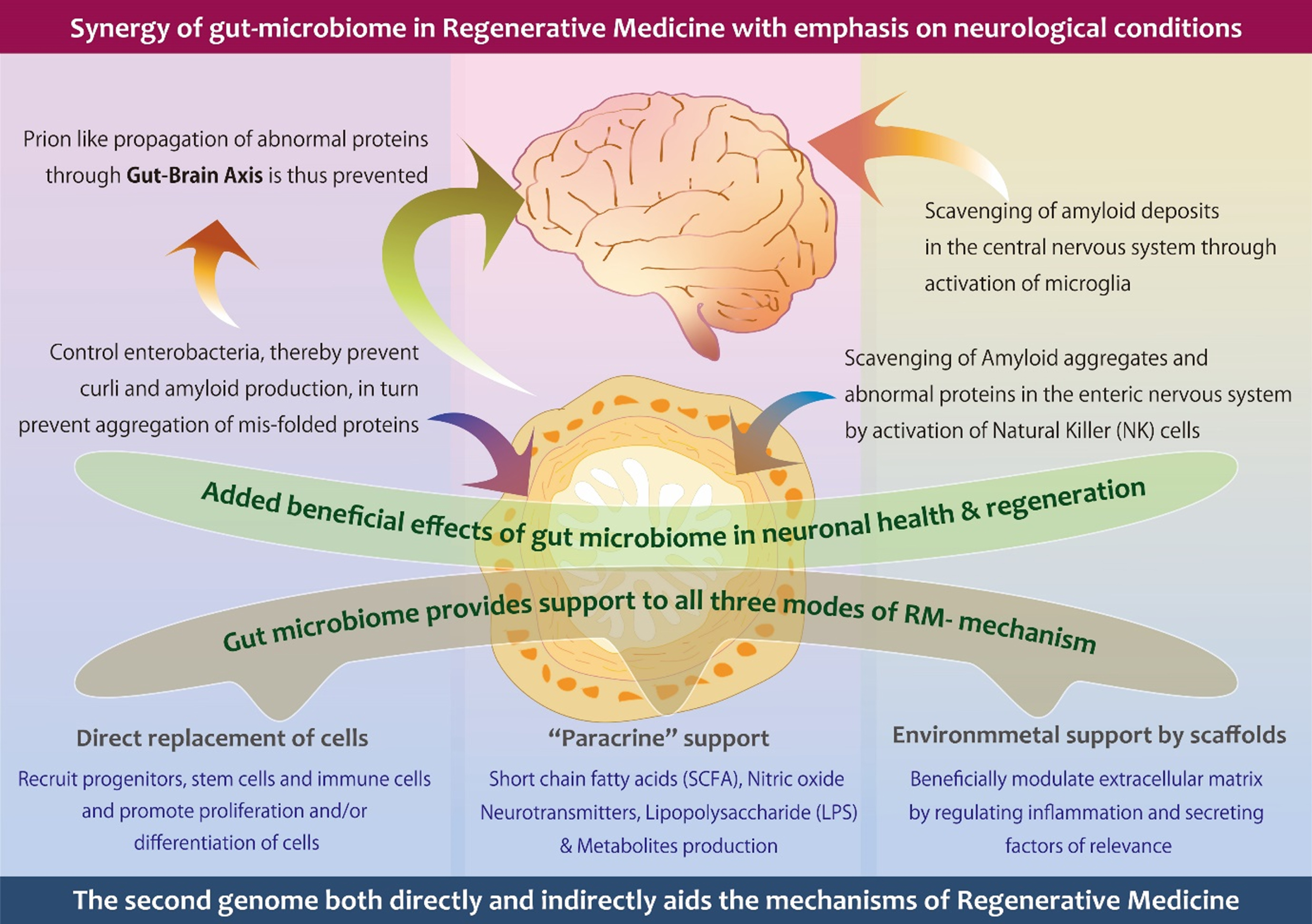
The other tools of RM are mostly exogenous and need sophisticated technologies to harness their potential and for administration. Endogenous stimulation of repair and regeneration is achieved by using growth factors, the delivery of genes, and the transplantation of scaffolds that promote cell homing, which have limitations besides laborious tasks. The other tools of RM, especially the cells, promote regeneration through differentiation, cell fusion, and so on, but more so through paracrine effects. The gut microbiome in itself is a group of cells and a source of several factors. It not only plays a role in cell differentiation, proliferation, and paracrine effects but also influences tissue repair and regeneration, even at a distant site, via its inter-organ axes such as the gut–brain, gut–lung, and gut–skin axis, to mention a few. Harnessing the regenerative potential of the gut microbiome can be achieved by simple methods such as dietary interventions, lifestyle modifications, and safe food supplements such as probiotics and prebiotics. Further, the influence of the gut microbiome is continuous, from the initial start of the repair, inflammation, and tissue regeneration, and it continues to be present even after tissue maturation for homeostasis, which is not so well orchestrated or possible through the other tools of RM, such as cell, scaffolds, and so on, thus making their role indispensable as an integral part of RM or a new terminology or subdomain of RM.
ACKNOWLEDGMENTS
The authors thank Mr. Takashi Onaka, Mr. Yasunori Ikeue, and Dr. Mitsuru Nagataki (Sophy Inc, Kochi, Japan), for necessary technical clarifications; Mr. Yoshio Morozumi and Ms. Yoshiko Amikura of GN Corporation, Japan for their liaison assistance for our research work; and Loyola-ICAM College of Engineering and Technology (LICET) for their support to our research work.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/22-0313r1).
REFERENCES
[1] | Sampogna G , Guraya SY , Forgione A ((2015) ) Regenerative medicine: Historical roots and potential strategies in modern medicine. J Microsc Ultrastruct 3: , 101–107. |
[2] | Kaiser LR ((1992) ) The future of multihospital systems. Top Health Care Financ 18: , 32–45. |
[3] | Steensma DP , Kyle RA ((2021) ) James Till and Ernest McCulloch: Hematopoietic stem cell discoverers. Mayo Clin Proc 96: , 830–831. |
[4] | Mount NM , Ward SJ , Kefalas P , Hyllner J ((2015) ) Cell-based therapy technology classifications and translational challenges. Philos Trans R Soc Lond B Biol Sci 370: , 20150017. |
[5] | Kalos M , Collman RG , Binder-Scholl G , Plesa G , Hwang WT , Levine BL , June CH ((2014) ) Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N Engl J Med 370: , 901–910. |
[6] | Chan BP , Leong KW ((2008) ) Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur Spine J 17: (Suppl 4), 467–479. |
[7] | Scaffold for Tissue Engineering. https://www.sciencedirect.com/topics/medicine-and-dentistry/scaffold-for-tissue-engineering, Accessed on March 24, 2022. |
[8] | Hosseinkhani M , Mehrabani D , Karimfar MH , Bakhtiyari S , Manafi A , Shirazi R ((2014) ) Tissue engineered scaffolds in regenerative medicine. World J Plast Surg 3: , 3–7. |
[9] | Pellegata AF , Tedeschi AM , De Coppi P ((2018) ) Whole organ tissue vascularization: Engineering the tree to develop the fruits. Front Bioeng Biotechnol 6: , 56. |
[10] | O’Brien FJ ((2011) ) Biomaterials & scaffolds for tissue engineering. Mater Today 14: , 88–95. |
[11] | Chung JJ , Im H , Kim SH , Park JW , Jung Y ((2020) ) Toward biomimetic scaffolds for tissue engineering: 3D printing techniques in regenerative medicine. Front Bioeng Biotechnol 8: , 586406. |
[12] | Yin L , Liu X , Shi Y , Ocansey DKW , Hu Y , Li X , Zhang C , Xu W , Qian H ((2020) ) Therapeutic advances of stem cell-derived extracellular vesicles in regenerative medicine. Cells 9: , 707. |
[13] | Zhao T , Sun F , Liu J , Ding T , She J , Mao F , Xu W , Qian H , Yan Y ((2019) ) Emerging role of mesenchymal stem cell-derived exosomes in regenerative medicine. Curr Stem Cell Res Therapy 14: , 482–494. |
[14] | Gupta A , Cady C , Fauser AM , Rodriguez HC , Mistovich RJ , Potty AGR , Maffulli N ((2020) ) Cell-free stem cell-derived extract formulation for regenerative medicine applications. Int J Mol Sci 21: , 9364. |
[15] | Burdon TJ , Paul A , Noiseux N , Prakash S , Shum-Tim D ((2011) ) Bone marrow stem cell derived paracrine factors for regenerative medicine: Current perspectives and therapeutic potential. Bone Marrow Res 2011: , 207326. |
[16] | Lichtbroun A ((2017) ) The therapeutic potential of stimulating endogenous stem cell mobilization. Acupunct Electrother Res 42: , 27–37. |
[17] | Anitua E , Sánchez M , Orive G ((2010) ) Potential of endogenous regenerative technology for regenerative medicine. Adv Drug Deliv Rev 62: , 741–752. |
[18] | Shreiner AB , Kao JY , Young VB ((2015) ) The gut microbiome in health and in disease. Curr Opin Gastroenterol 31: , 69–75. |
[19] | Quigley EMM , Gajula P ((2020) ) Recent advances in modulating the microbiome.. F1000Res 9: , F1000Faculty Rev-46. |
[20] | Kazemian N , Mahmoudi M , Halperin F , Wu JC , Pakpour S ((2020) ) Gut microbiota and cardiovascular disease: Opportunities and challenges. Microbiome 8: , 36. |
[21] | Ocansey DKW , Wang L , Wang J , Yan Y , Qian H , Zhang X , Xu W , Mao F ((2019) ) Mesenchymal stem cell-gut microbiota interaction in the repair of inflammatory bowel disease: An enhanced therapeutic effect. Clin Transl Med 8: , 31. |
[22] | Nagashima K , Sawa S , Nitta T , Tsutsumi M , Okamura T , Penninger JM , Nakashima T , Takayanagi H ((2017) ) Identification of subepithelial mesenchymal cells that induce IgA and diversify gut microbiota. Nat Immunol 18: , 675–682. |
[23] | Ciernikova S , Kasperova B , Drgona L , Smolkova B , Stevurkova V , Mego M ((2021) ) Targeting the gut microbiome: An emerging trend in hematopoietic stem cell transplantation. Blood Rev 48: , 100790. |
[24] | Andermann TM , Rezvani A , Bhatt AS ((2016) ) Microbiota manipulation with prebiotics and probiotics in patients undergoing stem cell transplantation. Curr Hematol Malig Rep 11: , 19–28. |
[25] | Tang TWH , Chen HC , Chen CY , Yen CYT , Lin CJ , Prajnamitra RP , Chen LL , Ruan SC , Lin JH , Lin PJ , Lu HH , Kuo CW , Chang CM , Hall AD , Vivas EI , Shui JW , Chen P , Hacker TA , Rey FE , Kamp TJ , Hsieh PCH ((2019) ) Loss of gut microbiota alters immune system composition and cripples postinfarction cardiac repair.. Circulation 139: , 647–659. |
[26] | Chak Kwong C , Yu H ((2021) ) The gut-cardiovascular connection: New era for cardiovascular therapy. Med Rev 1: , 23–46. |
[27] | Zheng Z , Wang B ((2021) ) The gut-liver axis in health and disease: The role of gut microbiota-derived signals in liver injury and regeneration. Front Immunol 12: , 775526. |
[28] | Xie C , Zhang Z , Yang M , Cao C , Zhou Y , Zhu Z , Gong W , Xu C , Yan L , Hu Z , Ai L , Peng Y ((2022) ) Lactiplantibacillus plantarum AR113 exhibit accelerated liver regeneration by regulating gut microbiota and plasma glycerophospholipid. Front Microbiol 12: , 800470. |
[29] | Wang F , Sun NN , Li LL , Zhu WW , Xiu J , Shen Y , Xu Q ((2019) ) Hepatic progenitor cell activation is induced by the depletion of the gut microbiome in mice. Microbiologyopen 8: , e873. |
[30] | Ikewaki N , Kurosawa G , Iwasaki M , Preethy S , Dedeepiya VD , Vaddi S , Senthilkumar R. , Levy GA , Abraham SJK (2021) Hepatoprotective effects of Aureobasidium pul-lulans derived Beta 1,3-1,6 biological response modifier glucans in a STAM- animal model of non-alcoholic steato-hepatitis. bioRxiv 2021.07.08.451700. doi: https://doi.org/10.1101/2021.07.08.451700. |
[31] | Yan J , Charles JF ((2017) ) Gut microbiome and bone: To build, destroy, or both? Curr Osteoporos Rep 15: , 376–384. |
[32] | Schwarzer M , Makki K , Storelli G , Machuca-Gayet I , Srutkova D , Hermanova P , Martino ME , Balmand S , Hudcovic T , Heddi A , Rieusset J , Kozakova H , Vidal H , Leulier F ((2016) ) Lactobacillus plantarum strain maintains growth of infant mice during chronic undernutrition. Science 351: , 854–857. |
[33] | Lukens JR , Gurung P , Vogel P , Johnson GR , Carter RA , McGoldrick DJ , Bandi SR , Calabrese CR , Vande Walle L , Lamkanfi M , Kanneganti TD ((2014) ) Dietary modulation of the microbiome affects autoinflammatory disease. Nature 516: , 246–249. |
[34] | De Pessemier B , + Grine L , Debaere M , Maes A , Paetzold B , Callewaert C ((2021) ) Gut-skin axis: Current knowledge of the interrelationship between microbial dysbiosis and skin conditions. Microorganisms 9: , 353. |
[35] | Poutahidis T , Kearney SM , Levkovich T , Qi P , Varian BJ , Lakritz JR , Ibrahim YM , Chatzigiagkos A , Alm EJ , Erdman SE ((2013) ) Microbial symbionts accelerate wound healing via the neuropeptide hormone oxytocin. PloS One 8: , e78898. |
[36] | Enaud R , Prevel R , Ciarlo E , Beaufils F , Wieërs G , Guery B , Delhaes L ((2020) ) The gut-lung axis in health and respiratory diseases: A place for inter-organ and inter-kingdom crosstalks. Front Cell Infect Microbiol 10: , 9. |
[37] | Mortaz E , Adcock IM , Folkerts G , Barnes PJ , Paul Vos A , Garssen J ((2013) ) Probiotics in the management of lung diseases. Mediators Inflamm 2013: , 751068. |
[38] | Yu SM , He JC ((2021) ) Happy gut, happy kidneys? Restoration of gut microbiome ameliorates acute and chronic kidney disease. Cell Metab 33: , 1901–1903. |
[39] | Chi M , Ma K , Wang J , Ding Z , Li Y , Zhu S , Liang X , Zhang Q , Song L , Liu C ((2021) ) The immunomodulatory effect of the gut microbiota in kidney disease. J Immunol Res 2021: , 5516035. |
[40] | Bourassa MW , Alim I , Bultman SJ , Ratan RR ((2019) ) Impact of diet and the gut microbiome on neurodegeneration and regeneration in neurological disorders.. Neuroforum 25: , 39–47. |
[41] | Haghikia A , Jörg S , Duscha A , Berg J , Manzel A , Waschbisch A , Hammer A , Lee DH , May C , Wilck N , Balogh A , Ostermann AI , Schebb NH , Akkad DA , Grohme DA , Kleinewietfeld M , Kempa S , Thöne J , Demir S , Müller DN , Gold R , Linker RA ((2015) ) Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity 43: , 817–829. |
[42] | Raghavan K , Dedeepiya VD , Yamamoto N , Ikewaki N , Sonoda T , Kurosawa G , Iwasaki M , Kandaswamy R , Senthilkumar R , Preethy S , Abraham SJK (2021) Beneficial reconstitution of gut microbiota and control of alpha-synuclein and curli-amyloids-producing enterobacteria, by beta 1,3-1,6 glucans in a clinical pilot study of autism and potentials in neurodegenerative diseases. medRxiv 2021. 10.26.21265505. doi: https://doi.org/10.1101/2021.10.26.21265505. |
[43] | Liu C , Yang SY , Wang L , Zhou F ((2022) ) The gut microbiome: Implications for neurogenesis and neurological diseases. Neural Regener Res 17: , 53–58. |
[44] | Wei GZ , Martin KA , Xing PY , Agrawal R , Whiley L , Wood TK , Hejndorf S , Ng YZ , Low JZY , Rossant J , Nechanitzky R , Holmes E , Nicholson JK , Tan EK , Matthews PM , Pettersson S ((2021) ) Tryptophan-metabolizing gut microbes regulate adult neurogenesis via the aryl hydrocarbon receptor. Proc Natl Acad Sci U S A 118: , e2021091118. |
[45] | Zakrzewski W , Dobrzyński M , Szymonowicz M , Rybak Z ((2019) ) Stem cells: Past, present, and future. Stem Cell Res Therapy 10: , 68. |
[46] | Sampson TR , Challis C , Jain N , Moiseyenko A , Ladinsky MS , Shastri GG , Thron T , Needham BD , Horvath I , Debelius JW , Janssen S , Knight R , Wittung-Stafshede P , Gradinaru V , Chapman M , Mazmanian SK ((2020) ) A gut bacterial amyloid promotes α-synuclein aggregation and motor impairment in mice. eLife 9: , e53111. |
[47] | Werner T , Horvath I , Wittung-Stafshede P ((2020) ) Crosstalk between alpha-synuclein and other human and non-human amyloidogenic proteins: Consequences for amyloid formation in Parkinson’s disease. J Parkinsons Dis 10: , 819–830. |



