Pre-Diagnostic Symptoms of Young-Onset Dementia in the General Practice up to Five Years Before Diagnosis
Abstract
Background:
Young-onset dementia (YOD) has many underlying etiologies, leading to a large heterogeneity in first symptoms. This makes it difficult for general practitioners (GPs) to recognize YOD.
Objective:
Identify early symptoms that are more common in the pre-diagnostic phase of YOD.
Methods:
We performed a case-control study nested in a primary-care registry on 89 cases and 162 matched controls, where we compared symptoms of people with YOD up to 5 years before diagnosis to their matched control group without YOD. The variables included in this study were International Classification of Primary Care codes and symptoms extracted from written GP notes and categorized in groups. We used Generalized Equation Estimation to analyze symptom’s time-trajectories and logistic regression and ROC-curves to analyze differences in number of symptom categories reported.
Results:
Cognitive symptoms were more common in people with YOD 5 years before diagnosis, affective symptoms 4 years before diagnosis, social symptoms 3 years, behavioral symptoms 2 years, and daily functioning disturbances 1 year before diagnosis. The ROC-curve suggested that reporting two or more symptom categories at the GP gave the best trade-off between sensitivity (85%) and specificity (77%), for the highest percentage of correctly diagnosed persons.
Conclusion:
This study showed people with YOD present differently than people without YOD. However, it may still be difficult for GPs to use these symptom categories to distinguish people with YOD, since the symptoms also occur in people with other diseases. A combination of reported symptom categories increases the probability of an underlying cause of YOD.
INTRODUCTION
The prevalence of dementia is estimated to be approximately 55 million people worldwide [1]. Of these people, around 3.9 million people have young-onset dementia (YOD), with an onset of dementia symptoms before the age of 65 [2–4]. YOD has serious consequences as it affects people in an active life phase. Usually people are still fulfilling active roles, such as employment, family roles, and social activities. Prior to diagnosis specific symptoms or changes are likely to occur that people or their close relatives may notice. Especially in people who develop YOD, early signs and symptoms are insidious and can be very diverse due to a large heterogeneity of underlying etiologies [4, 5]. Symptoms may include cognitive impairment, changes in behavior or personality, visual, motor or language problems [6, 7]. More often than in late-onset dementia (LOD), people with YOD present with non-memory symptoms [8]. The young age, heterogeneity, and often atypical initial clinical presentation make diagnosing dementia in people with YOD a challenge in both primary and secondary care, with several studies showing a time gap of 3–5 years between the onset of first symptoms and obtaining a diagnosis of YOD [9–11]. This diagnostic delay may be due to several boundaries which people with YOD and their caregivers experience when seeking help. Previous research found delaying factors were denial from the person with YOD themselves, attribution of the symptoms to other causes, lack of confirmation from the social context, lack of help from the general practitioner (GP) or a delay in referral from the GP [12]. This delay postpones the access to appropriate healthcare, social and financial support [13].
All these barriers need to be investigated so they can be diminished, which could lead to a decrease in the diagnostic delay of YOD. In this study, we focused on the GP’s role in recognizing YOD earlier.
The GP is among the first healthcare professionals for people to contact with symptoms or health problems and therefore plays an important role in recognizing YOD. In this study, we aimed to investigate how people with YOD present at the GP up to five years before diagnosis. To do so, we investigated the time-trajectories of different symptom categories that people with YOD present up to five years prior to diagnosis and compared them with a matched group of people without YOD.
MATERIALS AND METHODS
We conducted a matched case-control study nested within the Research Network Family Medicine (RNFM) database, a regional database containing clinical data from patient encounters continuously collected by GPs in the south-eastern part of the Netherlands. The RNFM is managed by the Department of Family Medicine at Maastricht University Medical Centre (MUMC+) and, for the present study, covers about 150,000 current patients from 28 practices from 2014 onwards. Only anonymous data were used for this study. Clinical data in the RNFM are pseudonymized before being made available for research purposes. This entails that identifying data are encrypted and decryption is only possible by mediation of a trusted third party. Therefore, only encrypted, anonymous data was used for this study.
Participants
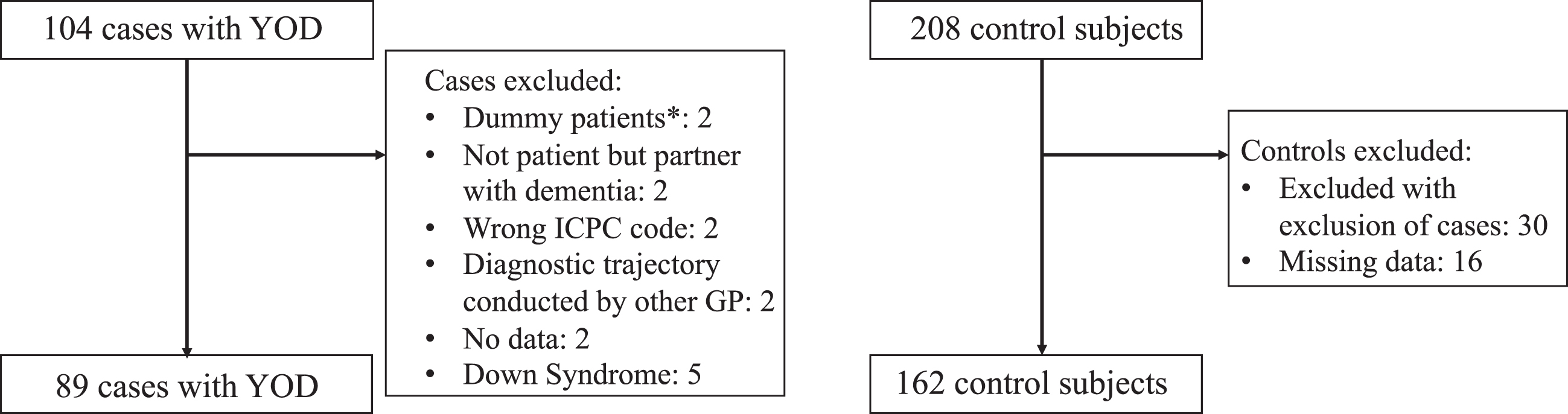
The cases in this study were diagnosed with de-mentia at an age younger than 70 years between 2016 and 2019 as registered with an ICPC-code P70. The ICPC (International Classification of Primary Care) codes are an international coding system, used to describe the evaluation of encounters, with either a diagnosis or a problem description [14]. ICPC code P70 indicates that persons are diagnosed with dementia. The cut-off at 70 years was chosen to account for the delay in diagnosis of YOD. This way, people who experienced first symptoms before the age of 65 years, but were diagnosed after the age of 65, could also be included. If there was uncertainty on the exact date of diagnosis, this was set on the day the diagnosis was first mentioned in the electronic patient records (e.g., GP written notes or first entry of ICPC code P70). For each case with YOD, we randomly selected two control participants, matched on age (e.g., a case of 51 years old was matched with two controls also 51 years old), gender, and GP practice. The only criterium for control participants was the absence of a recorded dementia diagnosis. No other exclusion criteria were used in order to keep the control group a close representation of the general population (different other diseases such as diabetes or depression could be present within the control group). A power analysis was performed to estimate the sample size needed, using different scenarios (for details, see Supplementary Method 1). A sample size of at least 84 was deemed necessary for this study. From the RNFM database, a total of 104 cases and 208 randomly matched controls were sampled. From this sample, 15 cases with their 30 matching controls were excluded due to different exclusion criteria such as discrepancies in the diagnostic coding, insufficient information, or having Down’s syndrome, as due to their condition, people with Down’s syndrome have higher risk of YOD, and they cannot be compared with age-matched controls without Down’s syndrome (see Fig. 1). We also excluded 16 controls with missing data (see Fig. 1). This led to a study population of 89 cases with YOD, and 162 controls.
Fig. 1
Flow diagram of in- and excluded cases and controls. *Dummy patients were patient files created by GPs that did not belong to a single patient.
Data collection
Data used for this study included signs, symptoms and diagnoses recorded during any patient contact with the GP up to five years prior to dementia diagnosis. The RNFM database used retrospective information from 2014 until 2019. Depending on the date of diagnosis, not all persons have a recorded time pre-diagnosis of 5 years. If a person was diagnosed with YOD in 2016, this means for this person recorded pre-diagnosis time was only 2 years.
All ICPC codes up to 5 years prior to diagnosis were retrospectively collected from electronic patient records. The codes are classified in groups according to body systems (e.g., digestive, circulatory, psychological, see Supplementary Method 2).
Since not all observations are captured by the ICPC codes, symptoms were also collected from written GP notes. When patients contact their GP, the GP generally enters the reasons for the encounter, symptoms, and observations during the contact in a patient file. One author (SH) read the patient files to identify and extract all signs and symptoms relevant for this study. Another author (KP) cross-validated a subset of ten YOD patient files, after which consensus was reached. Discrepancies were discussed with a third author (MdV) if needed, using the framework from a similar study using the RNFM database for studying the pre-diagnostic phase in people with LOD (Supplementary Method 3) [15]. Symptoms were then classified into categories, following the previous study [15]. Symptom categories rather than single symptoms were used to increase power. Symptom categories were 1) cognitive symptoms; 2) affective symptoms; 3) behavioral symptoms; 4) vascular symptoms; 5) gait disturbances; 6) changes in weight or appetite. Two categories were added based on relevant symptoms which could not be classified in one of the existing categories. These categories were 7) social symptoms; 8) disturbances in daily functioning (see Supplementary Method 3 for clustering of the symptoms in the categories).
Data analysis
Statistical analyses were performed using Stata version 17 for Mac OS X (StataCorp, USA). Baseline characteristics were extracted from the RNFM dataset for age and sex, or from the written GP notes for subtypes of dementia. We used non-linear generalized estimating equations (GEE) models, which account for the correlation within individuals due to the repeated measures. We used the longitudinally assessed binary symptom categories or ICPC codes as outcome and assumed a logit-link function with an exchangeable correlation structure to account for within-subjects correlation (that is population average logistic regression). This yielded odds ratios (OR) and their 95% confidence intervals (CI).
The presence or absence of an ICPC code or symptom category was coded longitudinally over five years and dichotomized for the analysis. This means for every visit where a symptom category was recorded, this category was coded as 1, whereas visits without the symptom category were coded as 0. This led to a time-series in which the outcome was the presence or absence of a symptom category. Pre-diagnosis time was analyzed as a continuous variable, although we present results on specific time points, with t(0) being fixed at the date of diagnosis for cases, or fixed date of last available patient file information for controls, t(–1) being one year before diagnosis, t(–2) two years before diagnosis etc. To study the difference over time on the predictive value of symptom categories between cases and controls a group x time interaction term was added. Model-based odds ratios and estimated probabilities (marginal effects) were computed for each 1-year time-point to find the moment in time of the first significant deviation in odds of symptoms between the groups.
Additional analyses were performed to increase clinical relevance of our outcome for GPs. A sum score was created indicating how many symptom categories a person reported in the five years before diagnosis (or less years if a person had less than five years recorded time pre-diagnosis). For this, categories were only included if they differentiated significantly between cases and controls in the GEE analyses. Binary logistic regression was then used to analyze the odds ratio for the number of symptom categories and a diagnosis of YOD. Additionally, a ROC-curve was computed to analyze the best trade-off point between sensitivity and specificity, for the highest percentage of correctly diagnosed persons.
Ethical considerations
The study was approved by the Medical Ethics Committee of the Faculty of Health, Medicine and Lifesciences of Maastricht University, Maastricht, the Netherlands (FHML-REC/2020/115). According to their guidelines, the study did not fall under the national Act on Medical Research with People.
RESULTS
Baseline characteristics
Characteristics of the study participants on t(0), i.e., the date of diagnosis for cases with YOD and the last date of follow-up for control participants are presented in Table 1. The mean age at YOD diagnosis t(0) was 63.0 years, and the mean age of the control participants at t(0) was 61.8 years. There were slightly more females than males included in the study. For approximately half of the people with YOD, a subtype of dementia was known.
Table 1
Baseline characteristics of the study sample
| People with YOD (n = 89) | Control participants (n = 162) | |
| Age, (mean, range) | 63.0 (32–69) | 61.8 (32–69) |
| 30–39 (n) | 2 | 4 |
| 40–49 (n) | 3 | 6 |
| 50–55 (n) | 6 | 11 |
| 56–60 (n) | 12 | 23 |
| 61–65 (n) | 30 | 51 |
| 66–70 (n) | 36 | 67 |
| Subtype of dementia (if known)* | ||
| Alzheimer’s disease (n) | 22 | |
| Vascular dementia (n) | 11 | |
| Mixed dementia (n) | 3 | |
| Primary progressive aphasia (n) | 2 | |
| Parkinson’s dementia (n) | 3 | |
| Lewy body dementia (n) | 2 | |
| Frontotemporal dementia (n) | 3 | |
| Secondary dementia** (n) | 4 | |
| Unknown (n) | 49 | |
| Female, (%) | 48 (53.9%) | 84 (54.8%) |
| Retrospective information | ||
| Available before diagnosis or last follow up | ||
| 1 year (n) | 89 | 162 |
| 2 years (n) | 86 | 155 |
| 3 years (n) | 80 | 154 |
| 4 years (n) | 63 | 149 |
| 5 years (n) | 42 | 143 |
*not all subtypes of diagnoses were recorded; **secondary dementias were due to amyotrophic lateral sclerosis, Huntington’s dis-ease, brain tumor, alcohol related dementia.
Presence of ICPC categories prior to dementia diagnosis
GEE-analyses showed that the ORs and marginal effects (estimated probabilities) for the psychological (P) ICPC codes were statistically significant when comparing ICPC codes over a five-year period between cases and controls (Table 2 and Supplementary Result 1). People with YOD had a time-constant higher odds of having psychological symptoms with ORs between 2.25 and 2.35 (Table 2 and Supplementary Result 1). See Supplementary Result 2 for a list of symptoms that were categorized as ICPC P in the people with YOD and their controls. GEE analysis for the ICPC L, the musculoskeletal code, was also significant. However, whereas the OR for ICPC L was significantly higher for people with YOD five years before diagnosis, it was significantly lower the year before diagnosis (Supplementary Result 1).
Table 2
Odds ratios with 95% confidence interval (CI) of the ICPC codes at the time of diagnosis and OR of the interaction term of diagnosis by time (in years prior to diagnosis)
| ICPC code | Odds ratio (95% CI) at time of diagnosis1 | p | Odds ratio (95% CI) interaction with time1 | p |
| A: General and unspecified | 1.32 (0.81–2.15) | 0.269 | 1.17 (0.99–1.38) | 0.068 |
| B: Blood, blood forming organs and immune mechanism | 0.39 (0.07–2.30) | 0.297 | 0.82 (0.39–1.72) | 0.598 |
| D: Digestive | 0.66 (0.35–1.25) | 0.199 | 0.93 (0.75–1.13) | 0.454 |
| F: Eye | 0.50 (0.23–1.08) | 0.079 | 0.89 (0.69–1.15) | 0.369 |
| H: Ear | 1.02 (0.45–2.32) | 0.960 | 1.19 (0.91–1.57) | 0.208 |
| K: Cardiovascular | 1.42 (0.77–2.62) | 0.261 | 1.05 (0.86–1.27) | 0.635 |
| L: Musculoskeletal | 0.63 (0.42–0.96) | 0.029 | 0.84 (0.74–0.96) | 0.008 |
| N: Neurological | 1.49 (0.64–3.49) | 0.356 | 1.14 (0.86–1.50) | 0.381 |
| P: Psychological | 2.25 (1.16–4.39) | 0.017 | 0.99 (0.80–1.23) | 0.939 |
| R: Respiratory | 0.87 (0.50–1.52) | 0.622 | 1.00 (0.84–1.19) | 0.996 |
| S: Skin | 0.91 (0.56–1.46) | 0.681 | 0.97 (0.84–1.11) | 0.637 |
| T: Metabolic, endocrine, nutrition | 1.73 (0.73–4.07) | 0.210 | 1.15 (0.88–1.51) | 0.307 |
| U: Urinary | 1.54 (0.77–3.07) | 0.224 | 1.02 (0.81–1.28) | 0.862 |
| W: Pregnancy, family planning | N.A.2 | |||
| X: Female genital | 0.99 (0.38–2.58) | 0.982 | 1.03 (0.74–1.41) | 0.877 |
| Y: Male genital | 1.26 (0.21–7.36) | 0.797 | 1.30 (0.76–2.23) | 0.333 |
| Z: Social | 1.19 (0.42–3.40) | 0.740 | 1.00 (0.73–1.39) | 0.976 |
1An OR at time of diagnosis > 1 shows a higher odds of symptom presence in people with YOD at time of diagnosis compared to controls; and OR interaction with time > 1 shows a stronger increase in the odds of symptoms per year before diagnosis in people with YOD compared to controls. Vice versa for OR < 1; 2No participants with this ICPC code.
Presence of other symptom categories prior to dementia diagnosis
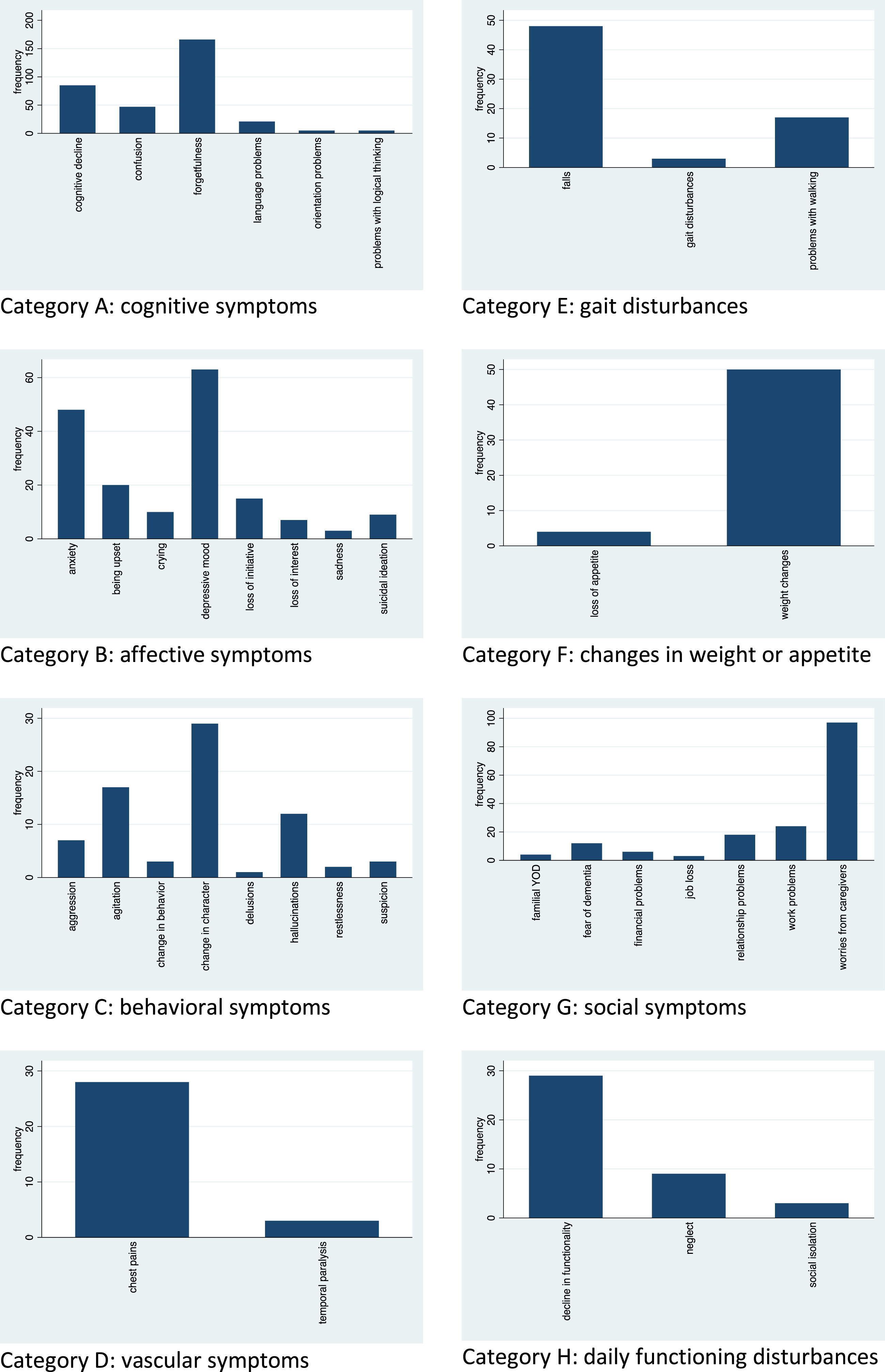
Next, symptoms mentioned in written notes from GPs were analyzed. Figure 2 shows the frequency of different reported symptoms for the people with YOD. Within the cognitive symptom category, they most often reported forgetfulness, cognitive decline, and confusion. For affective symptoms, people with YOD mostly reported depressive mood and anxiety. Behavioral symptoms were predominantly a change of character and agitation. Social symptoms were primarily worries from caregivers and work problems. Daily functioning disturbances were for the most part the experience of a general decline in daily functioning.
Fig. 2
Frequency of symptoms reported by people with YOD within the different symptom categories.
Overall, there was a significant diagnosis by time interaction for most symptom categories, suggesting differential change in symptoms between people with YOD and controls in the years before diagnosis. ORs, estimated from the slopes for cases and controls at discrete time points t(1–5) and t(0) as the intercept were significantly higher in people with YOD for five symptom categories (Table 3). Higher odds of symptom presence were found for: cognitive symptoms (from five years before diagnosis), affective symptoms (from four years before diagnosis), social symptoms (from three years before diagnosis), behavioral symptoms (from two years before diagnosis) and disturbances in daily functioning (from one year before diagnosis) (Table 3). For cognitive symptoms, behavioral symptoms, social symptoms, and daily functioning disturbances, the ORs increased and people with YOD had an increasingly higher odds of reporting these symptoms over the five years before diagnosis compared to controls (Table 3). Affective symptoms showed constant ORs with little change over the five years (Table 3). Vascular symptoms, in contrast, showed declining ORs, with people with YOD reporting significantly lower vascular symptoms from four years before diagnosis (Table 3).
Table 3
Odds ratios with 95% confidence interval (CI) of the symptom categories at the time of diagnosis and all 5 years before diagnosis and percentage of people with YOD and controls reporting symptoms all 5 years before diagnosis
| Symptom category | Odds ratio1 (95% CI) | p | People with YOD | Controls |
| Cognitive symptoms | ||||
| At time of diagnosis | 61.33 (24.39–154.2) | < 0.001 | ||
| 1 year before diagnosis | 34.06 (15.40–75.31) | < 0.001 | 75/89 (84.3%) | 13/162 (8.0%) |
| 2 years before diagnosis | 18.91 (9.08–39.40) | < 0.001 | 41/86 (47.7%) | 8/155 (5.2%) |
| 3 years before diagnosis | 10.50 (4.91–22.46) | < 0.001 | 22/80 (27.5%) | 7/154 (4.5%) |
| 4 years before diagnosis | 5.83 (2.46–13.84) | < 0.001 | 12/63 (19.1%) | 7/149 (4.7%) |
| 5 years before diagnosis | 3.24 (1.16–9.00) | 0.024 | 2/42 (4.8%) | 5/143 (3.5%) |
| Overall interaction with time prior to diagnosis (continuous) | 1.80 (1.40–2.32) | < 0.001 | ||
| Affective symptoms | ||||
| At time of diagnosis | 1.73 (1.02–2.93) | 0.039 | ||
| 1 year before diagnosis | 1.75 (1.11–2.74) | 0.015 | 34/89 (38.2%) | 46/162 (28.4%) |
| 2 years before diagnosis | 1.76 (1.16–2.68) | 0.008 | 37/86 (43.0%) | 42/155 (27.1%) |
| 3 years before diagnosis | 1.78 (1.13–2.77) | 0.011 | 21/80 (26.3%) | 36/154 (23.4%) |
| 4 years before diagnosis | 1.79 (1.07–3.00) | 0.028 | 14/63 (22.2%) | 30/149 (20.1%) |
| 5 years before diagnosis | 1.80 (0.97–3.36) | 0.063 | 5/42 (11.9%) | 20/143 (14.0%) |
| Overall interaction with time prior to diagnosis (continuous) | 0.99 (0.85–1.16) | 0.922 | ||
| Behavioral symptoms | ||||
| At time of diagnosis | 11.88 (4.15–33.99) | < 0.001 | ||
| 1 year before diagnosis | 6.67 (2.78–16.02) | < 0.001 | 28/89 (31.5%) | 14/162 (8.6%) |
| 2 years before diagnosis | 3.75 (1.68–8.35) | 0.001 | 21/86 (24.4%) | 13/155 (8.4%) |
| 3 years before diagnosis | 2.11 (0.90–4.95) | 0.087 | 10/80 (12.5%) | 13/154 (8.4%) |
| 4 years before diagnosis | 1.19 (0.43–3.27) | 0.743 | 2/63 (3.2%) | 10/149 (6.7%) |
| 5 years before diagnosis | 0.67 (0.19–2.30) | 0.521 | 0/42 (0%) | 6/143 (4.2%) |
| Overall interaction with time prior to diagnosis (continuous) | 1.78 (1.28–2.47) | 0.001 | ||
| Vascular symptoms | ||||
| At time of diagnosis | 0.26 (0.13–0.55) | < 0.001 | ||
| 1 year before diagnosis | 0.31 (0.17–0.56) | < 0.001 | 23/89 (25.8%) | 44/162 (27.2%) |
| 2 years before diagnosis | 0.37 (0.22–0.62) | < 0.001 | 18/86 (20.9%) | 35/155 (22.6%) |
| 3 years before diagnosis | 0.43 (0.24–0.78) | 0.004 | 12/80 (15%) | 31/154 (20.1%) |
| 4 years before diagnosis | 0.51 (0.25–1.04) | 0.066 | 6/63 (9.5%) | 24/149 (16.1%) |
| 5 years before diagnosis | 0.61 (0.25–1.50) | 0.278 | 2/42 (4.8%) | 17/143 (11.9%) |
| Overall interaction with time prior to diagnosis (continuous) | 0.84 (0.66–1.09) | 0.194 | ||
| Gait disturbances | ||||
| At time of diagnosis | 1.55 (0.76–3.17) | 0.232 | ||
| 1 year before diagnosis | 1.30 (0.71–2.39) | 0.398 | 35/89 (39.3%) | 35/162 (21.6%) |
| 2 years before diagnosis | 1.09 (0.61–1.95) | 0.769 | 21/86 (24.4%) | 30/155 (19.4%) |
| 3 years before diagnosis | 0.92 (0.48–1.75) | 0.790 | 14/80 (17.5%) | 26/154 (16.9%) |
| 4 years before diagnosis | 0.77 (0.35–1.68) | 0.510 | 6/63 (9.5%) | 21/149 (14.1%) |
| 5 years before diagnosis | 0.65 (0.25–1.68) | 0.371 | 2/42 (4.8%) | 15/143 (10.4%) |
| Overall interaction with time prior to diagnosis (continuous) | 1.19 (0.94–1.51) | 0.149 | ||
| Changes in weight or appetite | ||||
| At time of diagnosis | 2.03 (0.91–4.55) | 0.085 | ||
| 1 year before diagnosis | 1.88 (0.99–3.57) | 0.055 | 32/89 (36.0%) | 24/162 (14.8%) |
| 2 years before diagnosis | 1.74 (0.99–3.04) | 0.054 | 22/86 (25.6%) | 21/155 (13.5%) |
| 3 years before diagnosis | 1.60 (0.89–2.89) | 0.118 | 13/80 (16.3%) | 15/154 (9.7%) |
| 4 years before diagnosis | 1.48 (0.72–3.06) | 0.287 | 6/63 (9.5%) | 13/149 (8.7%) |
| 5 years before diagnosis | 1.37 (0.55–3.41) | 0.500 | 2/42 (4.8%) | 9/143 (6.3%) |
| Overall interaction with time prior to diagnosis (continuous) | 1.08 (0.83–1.41) | 0.555 | ||
| Social symptoms | ||||
| At time of diagnosis | 5.26 (3.01–9.22) | < 0.001 | ||
| 1 year before diagnosis | 3.95 (2.49–6.28) | < 0.001 | 60/89 (67.4%) | 36/162 (22.2%) |
| 2 years before diagnosis | 2.97 (1.94–4.55) | < 0.001 | 34/86 (39.5%) | 31/155 (20%) |
| 3 years before diagnosis | 2.23 (1.40–3.56) | 0.001 | 22/80 (27.5%) | 27/154 (17.5%) |
| 4 years before diagnosis | 1.67 (0.95–2.95) | 0.076 | 12/63 (19.1%) | 22/149 (14.8%) |
| 5 years before diagnosis | 1.26 (0.62–2.54) | 0.525 | 5/42 (11.9%) | 15/143 (10.4%) |
| Overall interaction with time prior to diagnosis (continuous) | 1.33 (1.11–1.60) | 0.002 | ||
| Daily functioning disturbances | ||||
| At time of diagnosis | 27.01 (4.51–161.81) | < 0.001 | ||
| 1 year before diagnosis | 10.34 (2.31–46.17) | 0.002 | 23/89 (25.8%) | 4/162 (2.5%) |
| 2 years before diagnosis | 3.96 (0.94–16.57) | 0.060 | 7/86 (8.1%) | 4/155 (2.6%) |
| 3 years before diagnosis | 1.51 (0.30–7.68) | 0.617 | 1/80 (1.3%) | 3/154 (1.9%) |
| 4 years before diagnosis | 0.58 (0.07–4.28) | 0.593 | 0/63 (0%) | 3/149 (2.0%) |
| 5 years before diagnosis | 0.22 (0.02–2.64) | 0.234 | 0/42 (0%) | 2/143 (1.4%) |
| Overall interaction with time prior to diagnosis (continuous) | 2.61 (1.40–4.87) | 0.003 |
1An OR at time of diagnosis or at 1–5 years before diagnosis > 1 shows a higher odds of symptom presence in people with YOD at time of diagnosis or at 1–5 years before diagnosis compared to controls; and OR interaction with time > 1 shows a stronger increase in the odds of symptoms per year before diagnosis in people with YOD compared to controls. Vice versa for OR < 1.
Changes over time in reported symptoms in the years before diagnosis
The absolute reported percentages of people reporting symptom categories (Table 3) and marginal (model-based) probabilities (Supplementary Result 3) showed that especially cognitive symptoms are more present in people with YOD, where 84% reported cognitive symptoms in the year before diagnosis, compared to 8% of the control group. Affective symptoms were reported by 38% of people with YOD the year before diagnosis, compared to 28% of controls. Behavioral symptoms, social symptoms, and daily functioning disturbances were increasingly more reported by people with YOD in the years before diagnosis, with 31% reporting behavioral symptoms, 67% reporting social symptoms, and 25% reporting daily functioning disturbances the year before diagnosis, whereas controls reported 9%, 22%, and 3% respectively. Social symptoms increased drastically for people with YOD from 39.5% two years before diagnosis to 67.4% the year before diagnosis. This increase was mainly due to an increase in worries from caregivers, which also triggered GPs to refer people for further diagnosis. The marginal effects (Supplementary Result 3) show the changes in probability over time of the reporting of the symptom categories and the difference in probability between the people with YOD and the controls.∥
Accumulation of symptom categories
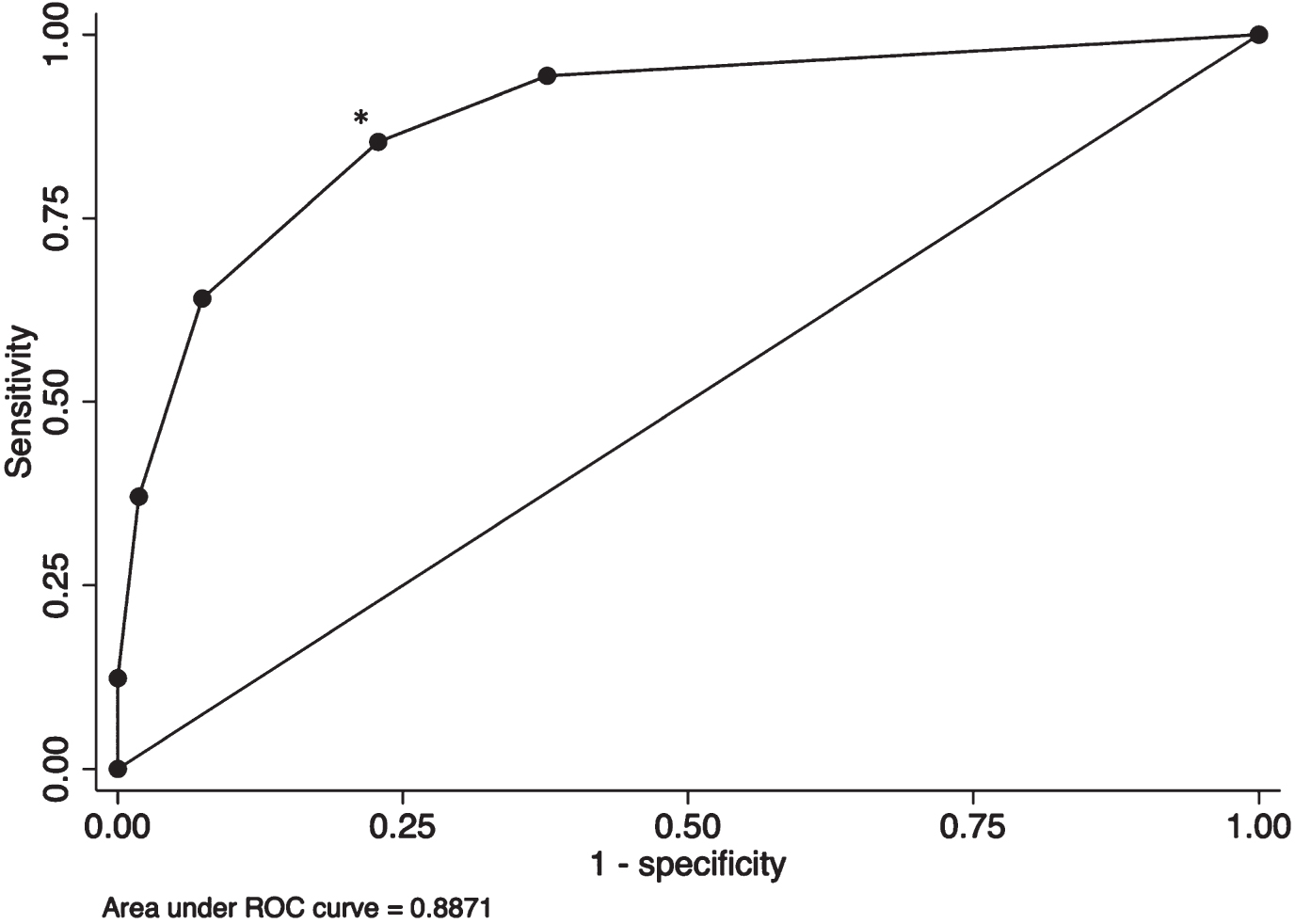
Of the eight symptom categories, five were more common in the YOD pre-diagnostic phase. A new variable was created indicating the number of significant symptom categories that a patient reported at any time during the previous five years (range 0–5). This variable was used as the independent variable in a binary logistic regression to predict group (YOD, control). This showed a steep increase in OR of YOD status when more symptom categories were present. The ROC curve had an Area Under the Curve of 0.89 (Table 4 and Fig. 3), suggesting good discriminating validity of reporting multiple symptom categories. Reporting symptoms from two or more symptom categories showed the best trade-off point with sensitivity of 85.39% and specificity of 77.16% (Table 4).
Table 4
Odds ratio of the number of categories reported in the previous five years, number of cases and controls reporting the symptoms, and percentage correctly classified persons
| Number of reported categories that predicted YOD | ||||||
| 0 symptom categories | 1 symptom category | 2 symptom categories | 3 symptom categories | 4 symptom categories | 5 symptom categories | |
| OR (95% CI) | ref | 6.73 | 15.35 | 53.87 | 148.13 | 1.0** |
| (2.02–22.42)* | (5.22–45.12)* | (16.55–175.36)* | (32.93–666.42)* | |||
| Number of cases reporting symptoms*** | 5 | 8 | 19 | 24 | 22 | 11 |
| Number of controls reporting symptoms**** | 101 | 24 | 25 | 9 | 3 | 0 |
| Sensitivity | 100% | 94.38% | 85.39% | 64.04% | 37.08% | 12.36% |
| Specificity | 0% | 62.35% | 77.16% | 92.59% | 98.15% | 100% |
*p < 0.001; **could not be calculated due to 100% prediction (0 controls reporting 5 symptom categories); ***total number of cases: 89; ****total number of controls: 162.
Fig. 3
ROC-curve. *trade-off point.
DISCUSSION
In this paper we investigated the presentation of people with YOD at the General Practice up to five years before diagnosis. Our results showed that the ICPC psychological codes, as well as the symptom categories cognitive symptoms, affective symptoms, behavioral symptoms, social symptoms, and daily functioning disturbances were more common in people with YOD. Cognitive symptoms already were more common in YOD five years before diagnosis, followed by affective symptoms, social symptoms, behavioral symptoms, and daily functioning disturbances. This indicates that people with YOD indeed present differently already several years before diagnosis. Moreover, we found that the reporting of two or more of these symptom categories in the years before diagnosis had a sensitivity of 85% and a specificity of 77%, indicating that this could be a potential trade-off mark for GPs to consider monitoring the patient or refer them for diagnostic follow-up.
All symptom categories that were more common in people with YOD showed increasing rates over the five years before diagnosis, except for affective symptoms, which remained stable. The high presence of symptoms in the final year probably started the diagnostic process. This might especially be the case for social symptoms, which increased from 40% two years before diagnosis to 67% mainly due to an increase in worries from caregivers. Based on the GPs written notes, these worries were a main trigger for GPs to refer patients for further diagnostic assessment. This is in contrast with studies of caregivers of people with YOD, who experienced a lack of understanding when they consulted their GP with their worries [13, 16–18]. This was also acknowledged in the only study we know of investigating the experiences of healthcare professionals on the diagnosis of YOD, where they emphasize the importance of better recognition by a GP [19]. It is possible that in our study caregivers did express their concerns at an earlier time point but this was not noted down by GPs.
The earliest symptom category significantly differentiating people with YOD from the controls was cognitive symptoms, although only 5% of the people with YOD reported these symptoms 5 years before diagnosis. Although monitoring every person with cognitive symptoms is not feasible for GPs, they could advise persons and their caregivers to monitor a potential increase in severity of the symptoms themselves, and monitor if other symptoms, such as affective, behavioral, or social symptoms or daily life disturbances arise. If so, the GP can then be contacted again to discuss the trajectory of symptoms and indicate whether further assessment is necessary.
Our finding of affective symptoms being more common in people with YOD is comparable to previous studies, which found half of the people with YOD had affective symptoms as an early feature, with diagnoses of depression and anxiety being most prevalent [6, 20, 21]. In our sample, depressive and anxiety symptoms were also the most commonly reported affective symptoms. Behavioral symptoms were also more common in people with YOD, which is also indicated in previous research where they found behavioral symptoms were early features in half of the people with YOD (6), and symptoms such as verbal or physical abuse or social inappropriateness were significantly more prevalent compared to people with LOD [22].
We found vascular symptoms were negatively associated with YOD, indicating that people with YOD report fewer vascular symptoms in the years before diagnosis. However, people with YOD had more ICPC codes in the vascular category in the five years before diagnosis compared to the controls, indicating more diagnoses in this category, although the difference was not statistically significant (Supplementary Result 1). Other studies on characteristics of people with YOD also found diabetes, stroke, and coronary heart disease were the most common comorbidities in people with YOD [22, 23]. Although the discrepancy between the higher ICPC codes but lower vascular symptoms remains unclear, we could speculate that young people do not mention or notice vascular symptoms or are not worried about these symptoms, possibly due to the pre-diagnostic phase of dementia. The symptoms are therefore not reported to the GP. It could also be that the symptoms were not reported in the GP notes, or that we did not properly extract these symptoms because they were overlooked.
Finally, people with YOD and controls showed different trajectories for the musculoskeletal code (ICPC L): people with YOD had more ICPC L codes compared to controls at five years before diagnosis, which changed to fewer ICPC L codes in people with YOD the year before diagnosis. Therefore, the clinical value of this code for instantaneous decision-making appears minimal since this information can only be used retrospectively.
Studies that investigated the first symptoms of YOD as reported by caregivers, report a similar progression of symptoms [13, 17, 18]. In one study with 96 caregivers, they mentioned cognitive problems as first symptoms in most cases, sometimes combined with affective or behavioral symptoms. As time progressed, caregivers reported these symptoms to become more profound and to be accompanied by social symptoms and disturbances in daily life [13]. Another study with 12 caregivers found that caregivers were, however, not alerted to seek help by symptoms of memory loss or other cognitive symptoms, but rather by behavioral symptoms and changes in personality [17]. However, our study showed cognitive symptoms were the earliest more common symptoms in people with YOD. It is therefore important to further investigate at what moment in time further assessment is most warranted, given that not everyone with cognitive symptoms will develop YOD.
Previously, Ramakers et al. [15] conducted a similar study within the RNFM database on people with LOD. They found cognitive symptoms and gait disturbances were more common in people with LOD in the five years before diagnosis compared to a control group. In our study, gait disturbances were not associated with YOD, possibly because young people usually still have a strong and healthy physique with little frailty [22]. We found that affective and behavioral symptoms were more common in people with YOD, whereas Ramakers et al. did not. This suggests that young and older people with dementia present differently in the years before diagnosis to their GP.
Strengths
This study used data from the GP at the time of consultation, which minimizes recall bias. It gives a unique insight into the symptoms and problems with which people with YOD presented to medical services years before the diagnosis was made.
Patients with YOD and their controls were unselected and represent the population that present to their GP. In the Netherlands, the GP acts as a gatekeeper to medical care and every person is registered with a GP. Hence the study can be considered population-based and selection bias is unlikely.
We chose to perform GEE-analyses because this approach takes all available data into account. With the number of measurements being profoundly different between the participants, GEE-analyses produced the most robust results for this study [24].
Limitations
Although we were able to collect and analyze a lot of relevant information, the sample size of this study was too small to investigate individual symptoms, different subtypes of dementia and different age ranges separately. This makes our conclusions general for the total YOD population but constrains us from giving conclusions for separate subtypes of dementia or different age ranges of YOD.
The data in this article are based on ICPC codes and contact notes written by the GP. The primary goal of these written notes is not research related, but to serve as a memory aid for the GP during consultations. There may therefore be a large variety between GPs regarding the level of detail provided in their notes. It is thus possible that we missed information on early symptoms, leading to an underestimation in the frequency of the symptoms in this analysis. It could be that what the GP did not ask or record that may be important, which we were unable to investigate with this research design. Also, the written notes were interpreted by us, which can lead to interpretation bias.
For some patients it was unclear whether they had been referred to specialists for a diagnosis of YOD. Sometimes, people with cognitive problems were monitored by the GP before being referred to a specialist. It is probable that diagnoses were made in different stages of the disease for different persons, which might influence the timing of the symptoms reported in the years before diagnosis.
Implications
For a GP, there is a fine balance between referring too many false positive cases causing unnecessary distress and diagnostic workup and missing cases causing a delay in diagnosis and initiation of treatment and care. Therefore, with this study we aimed to get a better understanding of how people with YOD present themselves at the GP.
YOD is relatively rare, with a prevalence of 119/100.000 [2] and hence, GPs encounter relatively few people with YOD. Our results are a first indication that people with YOD present differently to the GP compared to people without YOD in the years before diagnosis.
This does not yet have clear clinical implications but is mainly a prelude to further research. Since cognitive symptoms were the earliest more common symptom category in people with YOD, future research should address how to differentiate the cognitive symptoms that are prodromal for YOD from cognitive symptoms that are not. Also, we found reporting multiple symptom categories increased sensitivity and specificity for YOD. However, clinical relevance of this finding is restricted, since GPs probably focus more on specific symptoms. Still, this analysis showed a combination of symptom categories could be an interesting subject for further research.
Furthermore, this study compared the people with YOD to a general control group. Future research should aim to compare people with YOD to a group of people with depression or burn-out, since symptoms between these groups overlap and distinction is difficult for a GP.
In conclusion, to investigate more in-depth which symptoms and combination of symptoms should raise a red flag for a GP, future research should aim to investigate single symptoms rather than symptom categories in individual patients. The symptom categories in this study are very broad, making decision making for GPs difficult, therefore individual symptoms could better identify pre-diagnostic symptom profiles of young people who get a diagnosis of dementia that could ultimately help GPs in the recognition of YOD. To do so, larger studies with more incident cases of YOD are needed.
ACKNOWLEDGMENTS
This research was funded by Gieskes-Strijbis fonds, Alzheimer Netherlands, and the Dutch Knowledge Centre on Young-Onset Dementia.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/22-0215r1).
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-220215.
REFERENCES
[1] | World Health Organization (2021) Dementia. https://www.who.int/news-room/fact-sheets/detail/dementia, Last updated September 2, 2021, Accessed on November 19, 2021. |
[2] | Hendriks S , Peetoom K , Bakker C , Van Der Flier WM , Papma JM , Koopmans R , Verhey F , De Vugt M , Köhler S , Young-Onset Dementia Epidemiology Study Group ((2021) ) Global prevalence of young-onset dementia: A systematic review and meta-analysis. JAMA Neurol 78: , 1080–1090. |
[3] | Koopmans R , Rosness T ((2014) ) Young onset dementia–what does the name imply? Int Psychogeriatr 26: , 1931–1933. |
[4] | Van de Veen D , Bakker C , Peetoom K , Pijnenburg Y , Papma JM , The PRECODE Study Group, De Vugt M, Koopmans R ((2021) ) An integrative literature review on the nomenclature and definition of dementia at a young age. J Alzheimers Dis 83: , 1891–1916. |
[5] | Mendez MF ((2006) ) The accurate diagnosis of early-onset dementia. Int J Psychiatry Med 36: , 401–412. |
[6] | Kelley BJ , Boeve BF , Josephs KA ((2009) ) Cognitive and noncognitive neurological features of young-onset dementia. Dement Geriatr Cogn Disord 27: , 564–571. |
[7] | Graff-Radford J , Yong KXX , Apostolova LG , Bouwman FH , Carrillo M , Dickerson BC , Rabinovici GD , Schott JM , Jones DT , Murray ME ((2021) ) New insights into atypical Alzheimer’s disease in the era of biomarkers. Lancet Neurol 20: , 222–234. |
[8] | Rossor MN , Fox NC , Mummery CJ , Schott JM , Warren JD ((2010) ) The diagnosis of young-onset dementia. Lancet Neurol 9: , 793–806. |
[9] | Van Vliet D , de Vugt ME , Bakker C , Pijnenburg YAL , Vernooij-Dassen MJFJ , Koopmans RTCM , Verhey FRJ ((2013) ) Time to diagnosis in young-onset dementia as compared with late-onset dementia. Psychol Med 43: , 423–432. |
[10] | Draper B , Cations M , White F , Trollor J , Loy C , Brodaty H , Sachdev P , Gonski P , Demirkol A , Cumming RG , Withall A ((2016) ) Time to diagnosis in young-onset dementia and its determinants: The INSPIRED study. Int J Geriatr Psychiatry 31: , 1217–1224. |
[11] | Kvello-Alme M , Bråthen G , White LR , Sando SB ((2021) ) Time to diagnosis in young onset Alzheimer’s disease: A population-based study from central Norway. J Alzheimers Dis 82: , 965–974. |
[12] | Van Vliet D , de Vugt ME , Bakker C , Koopmans RTCM , Pijnenburg YAL , Vernooij-Dassen MJFJ , Verhey FRJ ((2011) ) Caregivers’ perspectives on the pre-diagnostic period in early onset dementia: A long and winding road. Int Psychogeriatr 23: , 1393–1404. |
[13] | Van Vliet D , de Vugt ME , Bakker C , Koopmans RTCM , Verhey FRJ ((2010) ) Impact of early onset dementia on caregivers: A review. Int J Geriatr Psychiatry 25: , 1091–1100. |
[14] | Bentsen BG ((1986) ) International classification of primary care. Scand J Prim Health Care 4: , 43–50. |
[15] | Ramakers IHGB , Visser PJ , Aalten P , Boesten JHM , Metsemakers JFM , Jolles J , Verhey FRJ ((2007) ) Symptoms of preclinical dementia in general practice up to five years before dementia diagnosis. Dement Geriatr Cogn Disord 24: , 300–306. |
[16] | O’Malley M , Carter J , Stamou V , LaFountaine J , Oyebode J , Parkes J ((2021) ) Receiving a diagnosis of young onset dementia: A scoping review of lived experiences. Aging Ment Health 25: , 1–12. |
[17] | Ducharme F , Kergoat MJ , Antoine P , Pasquier F , Coulombe R ((2013) ) The unique experience of spouses in early-onset dementia. Am J Alzheimers Dis Other Demen 28: , 634–641. |
[18] | Bruinsma J , Peetoom K , Bakker C , Boots L , Verhey F , De Vugt M ((2020) ) ‘They simply do not understand’: A focus group study exploring the lived experiences of family caregivers of people with frontotemporal dementia. Aging Ment Health 26: , 277–285. |
[19] | Spreadbury JH , Kipps CM ((2018) ) Understanding important issues in young-onset dementia care: The perspective of healthcare professionals. Neurodegener Dis Manag 8: , 37–47. |
[20] | Gumus M , Multani N , Mack ML , Tartaglia MC ((2021) ) Progression of neuropsychiatric symptoms in young-onset versus late-onset Alzheimer’s disease.. Geroscience 43: , 213–223. |
[21] | Rosness TA , Barca ML , Engedal K ((2010) ) Occurrence of depression and its correlates in early onset dementia patients. Int J Geriatr Psychiatry 25: , 704–711. |
[22] | Ryan B , Martinez Ruiz A , Rivera-Rodriguez C , Curtis M , Cheung G ((2021) ) Sociodemographic and clinical characteristics of 1350 patients with young onset dementia: A comparison with older patients. Alzheimer Dis Assoc Disord 35: , 200–207. |
[23] | Heath CA , Mercer SW , Guthrie B ((2015) ) Vascular comorbidities in younger people with dementia: A cross-sectional population-based study of 616 245 middle-aged people in Scotland. J Neurol Neurosurg Psychiatry 86: , 959–964. |
[24] | Ma Y , Mazumdar M , Memtsoudis SG ((2012) ) Beyond repeated-measures analysis of variance: Advanced statistical methods for the analysis of longitudinal data in anesthesia research. Reg Anesth Pain Med 37: , 99–105. |




