Anti-Dementia Drug Persistence Following Donepezil Initiation Among Alzheimer’s Disease Patients in Japan: LIFE Study
Abstract
Background:
Donepezil is frequently used to treat Alzheimer’s disease (AD) symptoms but is associated with early discontinuation. Determining the persistence rates of anti-dementia drug use after donepezil initiation may inform the development and improvement of treatment strategies, but there is little evidence from Japan.
Objective:
To determine anti-dementia drug persistence following donepezil initiation among AD patients in Japan using insurance claims data.
Methods:
Insurance claims data for AD patients with newly prescribed donepezil were obtained from 17 municipalities between April 2014 and October 2021. Anti-dementia drug persistence was defined as a gap of ≤60 days between the last donepezil prescription and a subsequent prescription of donepezil, another cholinesterase inhibitor, or memantine. Cox proportional hazards models were used to analyze the association between care needs levels and discontinuation.
Results:
We analyzed 20,474 AD patients (mean age±standard deviation: 82.2±6.3 years, women: 65.7%). The persistence rates were 89.1% at 30 days, 79.4% at 90 days, 72.6% at 180 days, 64.5% at 360 days, and 58.3% at 540 days after initiation. Among the care needs levels, the hazard ratio (95% confidence interval) for discontinuation was 1.01 (0.94–1.07) for patients with support needs, 1.12 (1.06–1.18) for patients with low long-term care needs, and 1.31 (1.21–1.40) for patients with moderate-to-high long-term care needs relative to independent patients.
Conclusion:
Japanese AD patients demonstrated low anti-dementia drug persistence rates that were similar to those of other countries. Higher long-term care needs were associated with discontinuation. Further measures are needed to improve drug persistence in AD patients.
INTRODUCTION
As the world’s population continues to grow and age, the prevalence of dementia is projected to surge from 57 million cases in 2019 to 153 million cases by 2050 [1]. Alzheimer’s disease (AD) is the most common form of dementia, and accounts for over 60% of dementia cases [2]. Treatments for AD include pharmacologic and non-pharmacologic therapies, but none of the available options are effective in stopping or reversing the underlying neurodegenerative processes [2]. Aducanumab is a monoclonal antibody aimed at reducing the formation of cerebral amyloid plaques, and received accelerated approval in the US as the first disease-modifying treatment for AD [3]. However, this medication has not been approved in the European Union or Japan. Due to the lack of pharmacologic treatments for AD that target disease progression, there is a strong interest in aducanumab and other disease-modifying therapies.
Donepezil, a cholinesterase inhibitor (ChE-I), is frequently used as a first-line therapy to treat AD symptoms. However, numerous studies from various countries have reported high discontinuation rates, with low tolerability to adverse events cited as a major reason for discontinuation [4–17]. Furthermore, cognitive function can decline quickly after donepezil discontinuation [18], and there are few alternatives that physicians can choose to replace donepezil. Accurately determining the anti-dementia drug persistence rates following donepezil initiation in AD patients and identifying the medications prescribed after discontinuation may inform the development and improvement of treatment strategies, including those for disease-modifying therapies that are currently being developed.
Despite Japan’s unprecedented population aging and rising prevalence of AD, there is little information on its use of anti-dementia drugs. Therefore, this study was conducted to determine anti-dementia drug persistence following donepezil initiation and subsequent drug switching patterns among AD patients in Japan using insurance claims data.
METHODS
Study data and patients
In order to achieve the study aim, it was necessary to obtain comprehensive prescription records of the study patients. For this reason, we chose to use insurance claims data as these would allow us to follow a patient’s prescriptions over time regardless of where each prescription was issued. This study was conducted using a database from the Longevity Improvement & Fair Evidence (LIFE) Study, which is a multi-region database project managed by Kyushu University (Fukuoka, Japan) [19]. Our database comprised health care claims data and long-term care (LTC) claims data collected from 17 municipalities. Under Japan’s health insurance system, municipal governments fulfill the role of insurer for persons enrolled in the National Health Insurance System and the Latter-Stage Older Persons Health Care System. The National Health Insurance System is a public health insurance program that covers citizens and long-term residents who are not employed in companies or the civil service; its enrollees mostly include the self-employed, part-time workers, and retirees. Over 80% of persons aged 65–74 years are enrolled in the National Health Insurance System. The Latter-Stage Older Persons Health Care System is a public health insurance program that covers all citizens and long-term residents aged ≥75 years. Municipal governments receive claims from health facilities for the reimbursement of insurance-covered services provided under these 2 systems. The LIFE Study enters into agreements with the participating municipal governments and collects and processes anonymized claims data for secondary analyses. The data collection periods varied among the municipalities, but generally included data from April 2014 to October 2021.
The initial study population comprised patients with a new prescription of donepezil following an AD diagnosis. We defined new prescriptions as the first donepezil prescription after 180 days or more from the first claims record within each patient’s claims data. This first prescription date was designated the “index prescription date”.
We excluded patients who fulfilled any of the following criteria: 1) cases who did not have any claims records after the index prescription date, 2) cases who had been prescribed any anti-dementia drug other than memantine within 30 days before or after the index prescription date, 3) cases with an initial prescription of an anti-dementia drug other than donepezil, and 4) cases whose first donepezil prescription was within 180 days from the first claims record.
Anti-dementia drug persistence
As we could not ascertain each patient’s actual drug intake, we analyzed drug utilization from prescription initiation (index prescription date) until discontinuation. The claims data contain prescription dates and durations (days). We identified prescription episodes using the prescription start dates and end dates and assumed continuous drug utilization within each episode. Next, we assessed anti-dementia drug persistence and discontinuation based on the gap between the prescription end date of one episode and the prescription start date of the subsequent episode. For this study, anti-dementia drug persistence was defined as a gap of ≤60 days between the last donepezil prescription and a subsequent prescription of donepezil, another ChE-I, or memantine (regardless of when the other prescriptions had been initially issued). Discontinuation was defined as a gap of ≥61 days between these prescriptions.
Statistical analysis
Patient characteristics were examined using sex, age, comorbidities (based on those included in the Charlson comorbidity), LTC facility institutionalization status, prescriptions of drugs other than anti-dementia drugs (hereinafter referred to as “other drug prescriptions”), and index prescription dates as recorded in their claims data. Next, we identified each patient’s certified LTC needs level (recorded in the LTC claims data) from 6 months before his/her index prescription date; we included these levels as we posited that they could be a factor in anti-dementia drug persistence. In Japan, LTC insurance enrollees are eligible to receive LTC needs certifications according to their degree of physical and/or cognitive impairments. These certified LTC needs levels are as follows: independent (no certification), LTC prevention program eligibility, support needs level 1, support needs level 2, LTC needs level 1, LTC needs level 2, LTC needs level 3, LTC needs level 4, and LTC needs level 5. For our analysis, we grouped these levels into 4 categories: independent, support needs (LTC prevention program eligibility and both support needs levels), low LTC needs (LTC levels 1–2), and moderate-to-high LTC needs (LTC levels 3–5). We analyzed comorbidities, LTC facility institutionalization status, and other drug prescriptions 6 months before each patient’s index prescription date.
Anti-dementia drug persistence rates were analyzed at 30 days, 90 days, 180 days, 360 days, and 540 days after the index donepezil prescription date. As there are no minimum durations for donepezil prescriptions in Japan, we were able to analyze persistence at an early stage (30 days). In addition to the persistence rates for all study patients, we estimated the persistence rates according to sex and LTC needs categories. As the study population comprised older adults that would have relatively high mortality rates, we did not examine the persistence rates according to age. Kaplan– Meier curves were plotted for anti-dementia drug persistence, with cases censored at the end of the study period or at death. Cox proportional hazards models were constructed to analyze the associations of the following factors with discontinuation: age, sex, comorbidities, LTC facility institutionalization status, other drug prescriptions, and LTC needs categories. These analyses were performed for the overall study sample as well as for a subgroup that excluded patients with moderate-to-high LTC needs (LTC levels 3–5). The subgroup analysis was conducted to focus on patients with lower or no LTC needs as these individuals are less likely to have progressive AD and may have a greater capacity to persist with their treatments.
To examine cases of drug switching from donepezil to another anti-dementia drug (i.e., galantamine, rivastigmine, or memantine), we identified patients who had used both donepezil and another anti-dementia drug during the study period. Among these patients, we calculated the durations (days) between the start dates of the other anti-dementia drugs relative to the donepezil discontinuation date.
All statistical analyses were performed using Stata 15.1 (StataCorp, College Station, TX, US), and statistical significance was set at p < 0.05. This study was approved by the Kyushu University Institutional Review Board for Clinical Research (Approval No. 2021-399).
Sensitivity analysis
In the main analysis, anti-dementia drug persistence was defined as a gap of ≤60 days between the last donepezil prescription and a subsequent prescription of donepezil, another ChE-I, or memantine. For the sensitivity analysis, we focused on donepezil persistence rates where persistence was defined as a gap of ≤60 days between donepezil prescription episodes and ignored switching to another ChE-I or memantine.
RESULTS
Figure 1 shows a flowchart of patient selection for this study. We first identified 57,288 patients who had been prescribed donepezil in the LIFE Study database. Among these, 52,814 patients had a new prescription of donepezil after receiving a diagnosis of AD. After applying the exclusion criteria, we obtained a sample of 20,474 patients for the final analysis.
Fig. 1
Flowchart of patient selection.
The patient characteristics are summarized in Table 1. The mean age (standard deviation) was 82.2 (6.3) years, and 65.7% were women. Cerebrovascular disease was the most prevalent (14.6%) comorbidity, and 3.6% of patients were institutionalized in an LTC facility. In addition, the majority of patients had other drug prescriptions. Among the LTC needs categories, 62.5% of the patients were independent, 10.7% had support needs, 19.3% had low LTC needs, and 7.6% had moderate-to-high LTC needs. The patients were followed-up for a mean duration of 483 days.
Table 1
Patient characteristics
| Overall | LTC needs categories | ||||
| (n = 20,474) | Independent | Support needs | Low LTC needs | Moderate-to-high LTC needs | |
| (n = 12,794) | (n = 2,181) | (n = 3,949) | (n = 1,550) | ||
| Mean age [standard deviation], y | 82.2 [6.3] | 80.7 [6.1] | 84.4 [5.3] | 84.7 [5.9] | 85 [6.7] |
| Age groups, n (%) | |||||
| ≤64 y | 137 (0.7%) | 120 (0.9%) | 2 (0.1%) | 10 (0.3%) | 5 (0.3%) |
| 65–74 y | 2128 (10.4%) | 1752 (13.7%) | 84 (3.9%) | 198 (5.0%) | 94 (6.1%) |
| 75–84 y | 10676 (52.1%) | 7505 (58.7%) | 969 (44.4%) | 1607 (40.7%) | 595 (38.4%) |
| 85–94 y | 7144 (34.9%) | 3326 (26%) | 1084 (49.7%) | 1984 (50.2%) | 750 (48.4%) |
| ≥95 y | 389 (1.9%) | 91 (0.7%) | 42 (1.9%) | 150 (3.8%) | 106 (6.8%) |
| Women, % | 13459 (65.7%) | 7920 (61.9%) | 1636 (75.0%) | 2819 (71.4%) | 1084 (69.9%) |
| Comorbidities | |||||
| Myocardial infarction | 245 (1.2%) | 137 (1.1%) | 24 (1.1%) | 62 (1.6%) | 22 (1.4%) |
| Congestive heart failure | 1769 (8.6%) | 872 (6.8%) | 242 (11.1%) | 451 (11.4%) | 204 (13.2%) |
| Peripheral vascular disease | 1300 (6.3%) | 815 (6.4%) | 187 (8.6%) | 231 (5.8%) | 67 (4.3%) |
| Cerebrovascular disease | 2980 (14.6%) | 1798 (14.1%) | 348 (16%) | 591 (15.0%) | 243 (15.7%) |
| Dementia | 4833 (23.6%) | 2903 (22.7%) | 523 (24.0%) | 1005 (25.4%) | 402 (25.9%) |
| Chronic pulmonary disease | 1932 (9.4%) | 1132 (8.8%) | 249 (11.4%) | 376 (9.5%) | 175 (11.3%) |
| Rheumatic disease | 296 (1.4%) | 165 (1.3%) | 50 (2.3%) | 62 (1.6%) | 19 (1.2%) |
| Peptic ulcer disease | 1193 (5.8%) | 696 (5.4%) | 160 (7.3%) | 246 (6.2%) | 91 (5.9%) |
| Mild liver disease | 1669 (8.2%) | 1111 (8.7%) | 186 (8.5%) | 281 (7.1%) | 91 (5.9%) |
| Diabetes without chronic complication | 285 (1.4%) | 176 (1.4%) | 28 (1.3%) | 57 (1.4%) | 24 (1.5%) |
| Diabetes with chronic complication | 521 (2.5%) | 335 (2.6%) | 71 (3.3%) | 89 (2.3%) | 26 (1.7%) |
| Hemiplegia or paraplegia | 182 (0.9%) | 74 (0.6%) | 26 (1.2%) | 57 (1.4%) | 25 (1.6%) |
| Renal disease | 640 (3.1%) | 329 (2.6%) | 89 (4.1%) | 148 (3.7%) | 74 (4.8%) |
| Any malignancy | 1010 (4.9%) | 634 (5.0%) | 99 (4.5%) | 202 (5.1%) | 75 (4.8%) |
| Moderate or severe liver disease | 34 (0.2%) | 27 (0.2%) | 2 (0.1%) | 2 (0.1%) | 3 (0.2%) |
| Metastatic solid tumor | 111 (0.5%) | 77 (0.6%) | 9 (0.4%) | 16 (0.4%) | 9 (0.6%) |
| HIV/AIDS | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| LTC facility institutionalization | 734 (3.6%) | 1 (0.0%) | 34 (1.6%) | 310 (7.9%) | 389 (25.1%) |
| Number of other drug prescriptionsa | |||||
| 0 | 596 (2.9%) | 421 (3.3%) | 25 (1.1%) | 81 (2.1%) | 69 (4.5%) |
| 1–5 | 3093 (15.1%) | 2290 (17.9%) | 189 (8.7%) | 505 (12.8%) | 109 (7.0%) |
| 6–10 | 4757 (23.2%) | 3100 (24.2%) | 441 (20.2%) | 947 (24.0%) | 269 (17.4%) |
| 11–15 | 4417 (21.6%) | 2710 (21.2%) | 480 (22.0%) | 886 (22.4%) | 341 (22.0%) |
| ≥16 | 7611 (37.2%) | 4273 (33.4%) | 1046 (48.0%) | 1530 (38.7%) | 762 (49.2%) |
aNumber of drug prescriptions (other than anti-dementia drugs) based on Anatomical Therapeutic Chemical Level 4 codes. LTC, long-term care.
Figure 2A shows the Kaplan– Meier curve of anti-dementia drug persistence in all 20,474 study patients. The persistence rates were 89.1% at 30 days, 79.4% at 90 days, 72.6% at 180 days, 64.5% at 360 days, and 58.3% at 540 days after the index prescription date. Figure 2 also shows the Kaplan– Meier curves of anti-dementia drug persistence according to sex, LTC needs categories, LTC facility institutionalization status, number of Charlson comorbidities, and number of other drug prescriptions. From the Cox proportional hazards analysis (Table 2), women had a hazard ratio (95% confidence interval) for discontinuation of 0.99 (0.95–1.03) relative to men after adjusting for age. Using “independent” as the reference category, the hazard ratio (95% confidence interval) for discontinuation was 1.01 (0.94–1.09) for patients with support needs, 1.13 (1.07–1.19) for patients with low LTC needs, and 1.30 (1.20–1.41) for patients with moderate-to-high LTC needs. In addition, LTC facility institutionalization was significantly associated with higher donepezil discontinuation (hazard ratio: 1.12; p = 0.049), whereas other drug prescriptions were significantly associated with lower donepezil discontinuation (p < 0.05). For the subgroup analysis, we excluded patients with moderate-to-high LTC needs; this left a sample of 18,924 patients (mean age: 82.0 years; women: 65.4%). The persistence rates in this subgroup were 89.4% at 30 days, 79.9% at 90 days, 73.2% at 180 days, 65.2% at 360 days, and 59.0% at 540 days after the index prescription date.
Fig. 2
Kaplan– Meier curves of anti-dementia drug persistence following donepezil initiation according to patient characteristics.
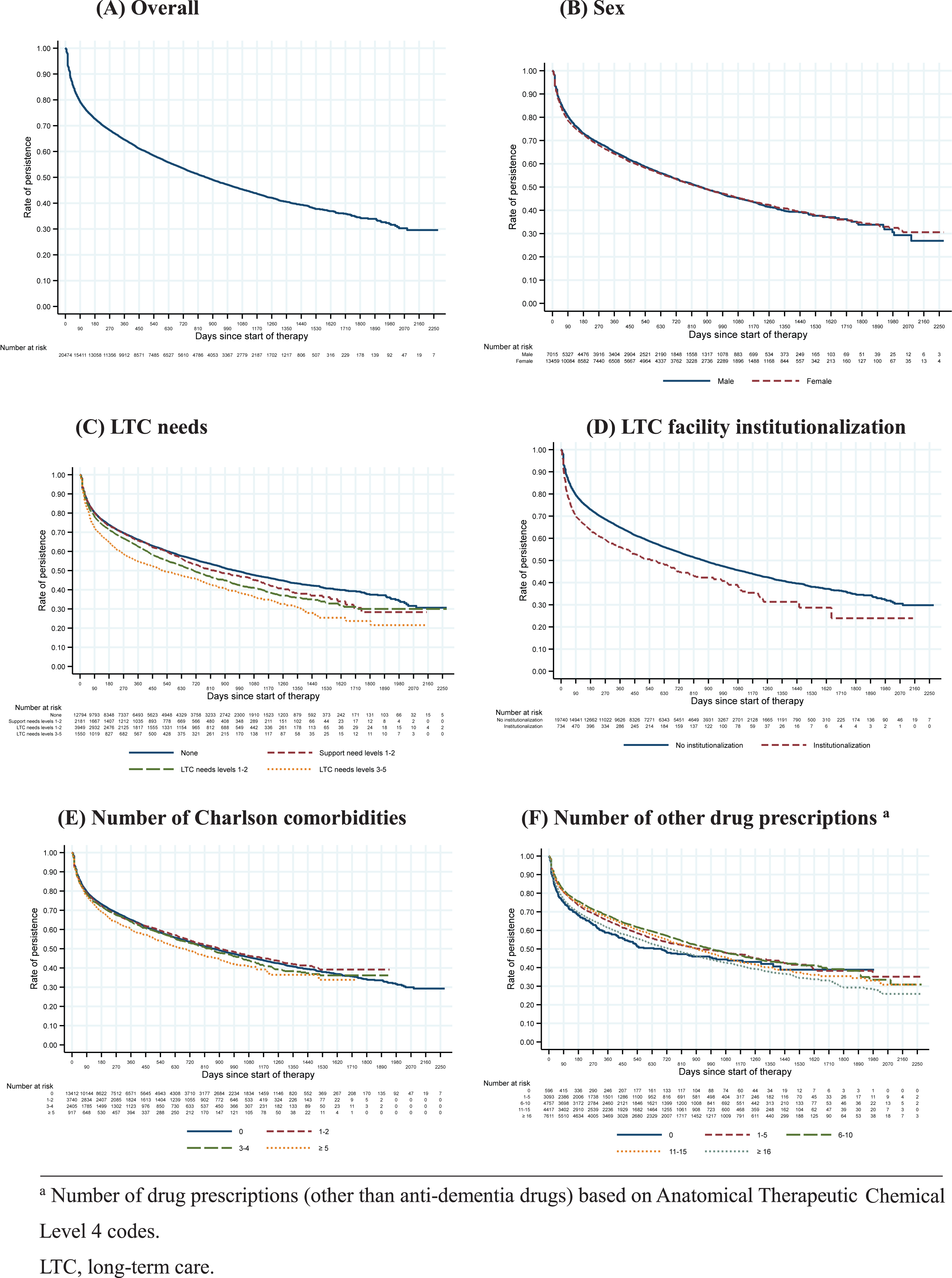
Table 2
Associations of patient characteristics with discontinuation
| Hazard ratio | 95% Confidence interval | p | |
| Age | 1.01 | 1.00–1.01 | <0.001 |
| Female | 0.99 | 0.95–1.04 | 0.816 |
| LTC needs categories | |||
| Independent | REF | ||
| Support needs | 1.01 | 0.94–1.09 | 0.719 |
| Low LTC needs | 1.13 | 1.07–1.19 | <0.001 |
| Moderate-to-high LTC needs | 1.30 | 1.20–1.41 | <0.001 |
| LTC facility institutionalization | 1.12 | 1.00–1.25 | 0.049 |
| Number of Charlson comorbidities | |||
| 0 | REF | ||
| 1–2 | 1.00 | 0.95–1.06 | 0.918 |
| 3–4 | 1.02 | 0.95–1.08 | 0.645 |
| ≥5 | 1.08 | 0.97–1.19 | 0.149 |
| Number of other drug prescriptionsa | |||
| 0 | REF | ||
| 1–5 | 0.87 | 0.77–1.00 | 0.042 |
| 6–10 | 0.81 | 0.71–0.92 | 0.001 |
| 11–15 | 0.85 | 0.75–0.97 | 0.015 |
| ≥16 | 0.97 | 0.86–1.10 | 0.640 |
aNumber of drug prescriptions (other than anti-dementia drugs) based on Anatomical Therapeutic Chemical Level 4 codes. LTC, long-term care.
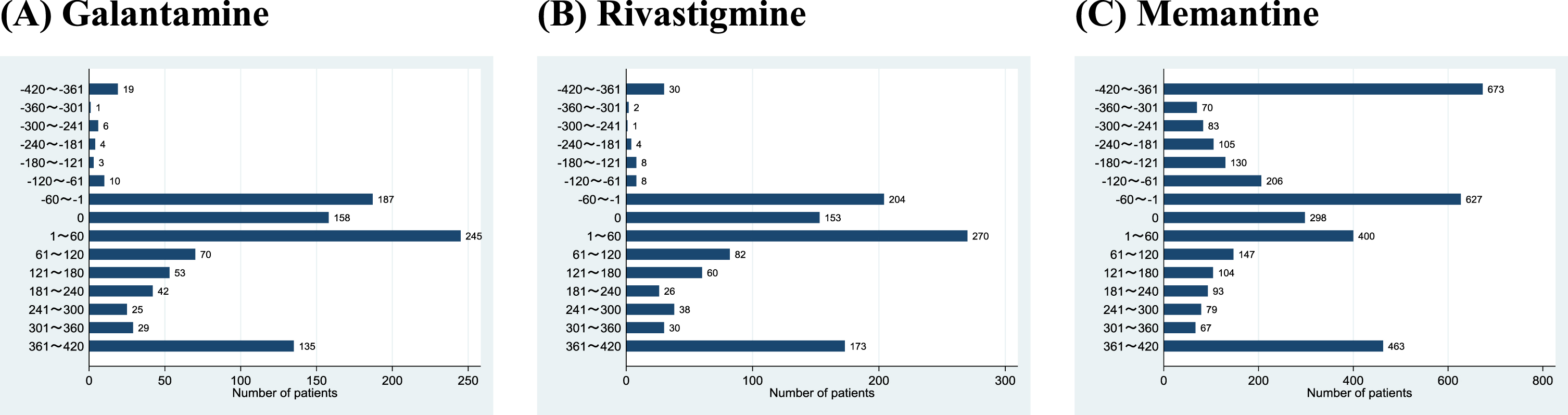
Among the 20,474 study patients, we identified 987 patients (4.8%) who had also used galantamine, 1089 patients (5.3%) who had also used rivastigmine, and 3545 patients (17.3%) who had also used memantine. Figure 3 shows the patient distributions by duration from the start date of the other anti-dementia drugs relative to the donepezil discontinuation date. Only 43 patients (0.2%) were prescribed galantamine and 53 patients (0.3%) were prescribed rivastigmine 61 days or earlier before donepezil discontinuation. Next, 590 patients (2.9%) were prescribed galantamine and 627 patients (3.1%) were prescribed rivastigmine within 60 days before or after donepezil discontinuation. Finally, 354 (1.7%) patients were prescribed galantamine and 409 patients (2.0%) were prescribed rivastigmine 61 days or later after donepezil discontinuation. In contrast, memantine was used more frequently: 1267 patients (6.2%) were prescribed memantine 61 days or earlier before donepezil discontinuation, 1325 patients (6.5%) were prescribed memantine within 60 days before or after donepezil discontinuation, and 953 patients (4.7%) were prescribed memantine 61 days or later after donepezil discontinuation.
Fig. 3
Patient distributions by duration from the start of other anti-dementia drugs relative to donepezil discontinuation.
In the sensitivity analysis, we analyzed donepezil persistence as a gap of ≤60 days between donepezil prescription episodes. Here, we calculated the persistence rates to be 86.8% at 30 days, 75.0% at 90 days, 67.4% at 180 days, 58.4% at 360 days, and 51.9% at 540 days after the index prescription date.
DISCUSSION
This large-scale medium-term retrospective cohort study examined anti-dementia drug persistence following donepezil initiation using insurance claims data from over 20,000 AD patients in Japan. This is the first study from Japan that describes persistence among AD patients who received new prescriptions of donepezil. Furthermore, this is, to our knowledge, the first study to characterize anti-dementia drug persistence according to care needs levels. Our results showed that Japanese AD patients have low anti-dementia drug persistence accompanied by very low switching rates to galantamine and rivastigmine.
Several studies from various countries have previously reported low persistence with donepezil and other anti-dementia drugs. Fisher et al. followed 33,793 new ChE-I users in British Columbia, Canada, and found that 49% had discontinued ChE-I use within a year after initiation [8]. Next, Herrmann et al. conducted a population-based cohort study on 28,961 new ChE-I users in Ontario, Canada, and reported that 55.9% had discontinued ChE-I use by the end of follow-up (mean duration of 823 days for community-dwelling patients) [9]. Brewer et al. analyzed 20,729 anti-dementia drug users in Ireland and reported that 30.1% and 43.8% of patients had discontinued their medications after 6 months and 12 months, respectively [10]. Similarly, Kostev et al. estimated that 42.2% of donepezil users in Poland had discontinued its use after 12 months [6]. In Japan, Kitamura et al. analyzed 8,025 donepezil users in a specific-cohort post-marketing observational study and found the persistence rate to be 61.2% at 12 months [5]. Our study estimated the 360-day persistence rate to be 64.5%, which was generally similar to those reported in the studies above. The small variations in the reported persistence rates may be influenced by study-based or country-based differences, such as follow-up durations, anti-dementia drug prices, and out-of-pocket paymentrates.
In this study, the patients demonstrated a rapid discontinuation of anti-dementia drugs by 6 months, with a more gradual discontinuation thereafter. This could be indicative of a subset of patients who discontinued their medications due to the occurrence of adverse events (e.g., nausea and diarrhea) soon after initiation. This is supported by the findings of 2 open-label multicenter studies, which reported that the majority of adverse events occur within 24 weeks (approximately 6 months) of donepezil initiation [20, 21]. The steeper curve observed in the initial 90 days may be due to a higher proportion of adverse events occurring during that period. The subsequent discontinuation rate was more gradual, which may have been the result of a reduction in adverse events after 6 months. This tapering off could also have been influenced by a variety of factors, including patients not feeling satisfied with the drug’s effectiveness after several months, progressive onset of behavioral and psychological symptoms of dementia, increased difficulties in taking medications due to worsening dysphagia, and the emergence of physical problems (e.g., fractures or pneumonia). The decision to discontinue donepezil may have been made by the patients, physicians, or caregivers.
Anti-dementia drug persistence rates have been associated with sex [6, 10–17], region [11, 14], and physicians’ specialties [6, 15]. Our study did not identify any sex-related differences in persistence, and the results of previous studies are conflicting. Several studies have found no sex-related differences [10, 11, 13, 17], whereas others have reported higher discontinuation rates in women [6, 12, 14, 15]. Studies have also produced varied findings on the relationship between anti-dementia drug persistence and dementia severity, with studies reporting contradictory results on the association between discontinuation and Mini-Mental State Examination scores [14, 17]. Another study found an association between donepezil discontinuation and Clinical Dementia Rating scores [16]. Gardette et al. identified Mini-Mental State Examination scores to be a predictor of anti-dementia drug switching [17]. Although our study did not have access to information on the degree of cognitive impairment, we were able to analyze LTC needs levels, which incorporate disease severity as a fundamental component. Our results showed that higher LTC needs levels were positively associated with anti-dementia drug discontinuation, which could be explained by 2 different scenarios. In the first scenario, a patient may have been initially certified with LTC needs due to physical impairment, and subsequently developed AD at a later date. These patients may have problems (e.g., polypharmacy, dysphagia, and worsening of general physical status) in persisting with anti-dementia treatment due to their high LTC needs. In the second scenario, a patient may have developed progressive dementia that increased his/her LTC needs levels after donepezil initiation. Although donepezil is thought to have beneficial effects for mild-to-moderate and severe AD for some time after initiation, these effects eventually decline [22, 23]. This could explain the increase in discontinuation as patients and their families begin to feel that the medication is no longer effective. Furthermore, patients may have a reduced understanding of their circumstances as AD progresses and refuse to take their medications.
A comprehensive assessment of drug persistence requires access to all of a patient’s prescription-related data from health facilities, which can be acquired from insurance claims data. Therefore, we conducted our study using a LIFE Study database comprising claims data. However, the use of claims data gives rise to several limitations. First, the analysis was based on prescription data, and we could not ascertain the actual intake of anti-dementia drugs by the patients. However, this limitation is shared by the vast majority of pharmacoepidemiological studies on community-dwelling patients. Second, we could not directly assess AD severity. Access to the scores from dementia severity measures (e.g., Clinical Dementia Rating and Mini-Mental State Examination) may shed light on the clinical indications for pharmaceutical therapy and contribute to improvements in AD treatment. For example, Umegaki et al. reported that Clinical Dementia Rating scores of ≥3 were associated with higher donepezil discontinuation rates [16]. Although our analysis showed that LTC needs levels of ≥3 were associated with higher discontinuation rates, the lack of dementia severity measures in our data precluded a more in-depth exploration of this relationship. Nevertheless, our study population comprised patients with new prescriptions of donepezil, and the majority are likely to have similar levels of dementia severity at baseline. Third, we were unable to account for variations in drug doses. In Japan, donepezil prescriptions generally begin at a dose of 3 mg/day for 1–2 weeks, which is then increased to 5 mg/day if no adverse effects are observed. For patients with advanced dementia, the dose may be further increased to 10 mg/day after a month. High doses of donepezil may be associated with higher discontinuation rates [22, 24]. As these prescription patterns in Japan can change at the weekly level based on physicians’ assessments of their patients, it would be difficult to analyze and interpret the variations in doses. Finally, we could not account for variations in the patients’ education levels, which may be associated with drug adherence.
Despite these limitations, our study provides important insight into anti-dementia drug persistence among AD patients in Japan, which is struggling with rapid population aging and increasing dementia prevalence. Our study also showed that the vast majority of AD patients who had discontinued donepezil were not switched to another anti-dementia drug, highlighting the current lack of pharmaceutical options for AD in Japan.
Conclusion
Japanese patients with AD demonstrated low anti-dementia drug persistence rates that were comparable with those of other countries. Future research is needed to develop measures that support drug persistence in AD patients, including strategies for upcoming disease-modifying therapies.
ACKNOWLEDGMENTS
The construction of the study database was funded by JSPS KAKENHI Grants (Grant Numbers JP19K21590 and JP20H00563) and JST FOREST Program (Grant Number JPMJFR205J). Data analysis and publication were funded by grants from Eisai Co., Ltd. and Biogen Inc.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/22-0200r1).
REFERENCES
[1] | GBD 2019 Dementia Forecasting Collaborators ((2022) ) Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019, Lancet Public Health 7: , e105–e125. |
[2] | Alzheimer’s Association ((2016) ) 2016 Alzheimer’s disease facts and figures. Alzheimers Dement 12: , 459–509. |
[3] | Alexander GC , Knopman DS , Emerson SS , Ovbiagele B , Kryscio RJ , Perlmutter JS , Kesselheim AS ((2021) ) Revisiting FDA approval of Aducanumab. N Engl J Med 385: , 769–771. |
[4] | Park KH , Yang Y , Chen C , Shim YS , Domingueze JC , Lee CN , Kang K , Kim HJ , Jeong SK , Jeong JH , Hong Z , Yoon SJ , Zhang ZX , Kim EJ , Jang JW , Li Y , Xu Y , Lin YT , Qu Q , Hu CJ , Chou CH , Fan D , Kandiah N , Yang YH , Lau CI , Chu LW , Wang H , Jung S , Choi SH , Kim S ((2021) ) Discontinuation rate of newly prescribed donepezil in Alzheimer’s disease patients in Asia. J Clin Neurol 17: , 376–384. |
[5] | Kitamura S , Endo A , Otake M , Yamakawa S , Ishii M , Takase T ((2017) ) Post-marketing surveillance of donepezil hydrochloride: Effectiveness, safety, and factors affecting persistence rates [in J apanese]. Geriat Med 55: , 1131–1145. |
[6] | Kostev K , Kurylo P , Kosik J , Jacob L ((2019) ) One-year persistence with donepezil, memantine, and rivastigmine in more than 66,000 elderly patients followed in Poland. J Alzheimers Dis 70: , 899–905. |
[7] | Fisher A , Carney G , Bassett K , Dormuth CR ((2017) ) Tolerability of cholinesterase inhibitors: A population-based study of persistence, adherence, and switching. Drugs Aging 34: , 221–231. |
[8] | Fisher A , Carney G , Bassett K , Chappell NL ((2016) ) Cholinesterase inhibitor utilization: The impact of provincial drug policy on discontinuation. Value Health 19: , 688–696. |
[9] | Herrmann N , Gill SS , Bell CM , Anderson GM , Bronskill SE , Shulman KI , Fischer HD , Sykora K , Shi HS , Rochon PA ((2007) ) A population-based study of cholinesterase inhibitor use for dementia. J Am Geriatr Soc 55: , 1517–1523. |
[10] | Brewer L , Bennett K , McGreevy C , Williams D ((2013) ) A population-based study of dosing and persistence with anti-dementia medications. Eur J Clin Pharmacol 69: , 1467–1475. |
[11] | Mucha L , Shaohung S , Cuffel B , McRae T , Mark TL , Del Valle M ((2008) ) Comparison of cholinesterase inhibitor utilization patterns and associated health care costs in Alzheimer’s disease. J Manag Care Pharm 14: , 451–461. |
[12] | Taipale H , Tanskanen A , Koponen M , Tolppanen AM , Tiihonen J , Hartikainen S ((2014) ) Antidementia drug use among community-dwelling individuals with Alzheimer’s disease in Finland: A nationwide register-based study. Int Clin Psychopharmacol 29: , 216–223. |
[13] | Kogut SJ , El-Maouche D , Abughosh SM ((2005) ) Decreased persistence to cholinesterase inhibitor therapy with concomitant use of drugs that can impair cognition. Pharmacotherapy 25: , 1729–1735. |
[14] | Amuah JE , Hogan DB , Eliasziw M , Supina A , Beck P , Downey W , Maxwell CJ ((2010) ) Persistence with cholinesterase inhibitor therapy in a population-based cohort of patients with Alzheimer’s disease. Pharmacoepidemiol Drug Saf 19: , 670–679. |
[15] | Bohlken J , Weber S , Rapp MA , Kostev K ((2015) ) Continuous treatment with antidementia drugs in Germany 2003-2013: A retrospective database analysis. Int Psychogeriatr 27: , 1335–1342. |
[16] | Umegaki H , Itoh A , Suzuki Y , Nabeshima T ((2008) ) Discontinuation of donepezil for the treatment of Alzheimer’s disease in geriatric practice. Int Psychogeriatr 20: , 800–806. |
[17] | Gardette V , Lapeyre-Mestre M , Piau A , Gallini A , Cantet C , Montastruc JL , Vellas B , Andrieu S ; ICTUS Group ((2014) ) A 2-year prospective cohort study of antidementia drug non-persistency in mild-to-moderate Alzheimer’s disease in Europe: Predictors of discontinuation and switch in the ICTUS study. CNS Drugs 28: , 157–170. |
[18] | Rogers SL , Farlow MR , Doody RS , Mohs R , Friedhoff LT ((1998) ) A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer’s disease. Donepezil Study Group. Neurology 50: , 136–145. |
[19] | Fukuda H , Ishiguro C , Ono R , Kiyohara K ((2022) ) The Longevity Improvement & Fair Evidence (LIFE) Study: Overview of the study design and baseline participant profile. J Epidemiol doi:10.2188/jea.JE20210513. |
[20] | Homma A , Imai Y , Tago H , Asada T , Shigeta M , Iwamoto T , Takita M , Arimoto I , Koma H , Takase T , Ohbayashi T ((2009) ) Long-term safety and efficacy of donepezil in patients with severe Alzheimer’s disease: Results from a 52-week, open-label, multicenter, extension study in Japan. Dement Geriatr CognDisord 27: , 232–239. |
[21] | Burns A , Gauthier S , Perdomo C ((2007) ) Efficacy and safety of donepezil over 3 years: An open-label, multicentre study in patients with Alzheimer’s disease. Int J Geriatr Psychiatry 22: , 806–812. |
[22] | Whitehead A , Perdomo C , Pratt RD , Birks J , Wilcock GK , Evans JG ((2004) ) Donepezil for the symptomatic treatment of patients with mild to moderate Alzheimer’s disease: A meta-analysis of individual patient data from randomised controlled trials. Int J Geriatr Psychiatry 19: , 624–633. |
[23] | Homma A , Imai Y , Tago H , Asada T , Shigeta M , Iwamoto T , Takita M , Arimoto I , Koma H , Ohbayashi T ((2008) ) Donepezil treatment of patients with severe Alzheimer’s disease in a Japanese population: Results from a 24-week, double-blind, placebo-controlled, randomized trial. Dement Geriatr Cogn Disord 25: , 399–407. |
[24] | Farlow M , Veloso F , Moline M , Yardley J , Brand-Schieber E , Bibbiani F , Zou H , Hsu T , Satlin A ((2011) ) Safety and tolerability of donepezil 23 mg in moderate to severe Alzheimer’s disease. BMC Neurol 11: , 57. |




