Cognitive, Functional, and Emotional Changes During the COVID-19 Pandemic in Greek Patients with Neurocognitive Disorders
Abstract
Background:
Prolonged periods of social deprivation, such as COVID-19-related lockdowns, are associated with deleterious effects on cognitive functions.
Objective:
The aim of this study was to gauge the effect of prolonged social isolation on the cognitive function of older adults with neurocognitive disorders.
Methods:
We recruited 125 older adults with minor or major neurocognitive disorders divided into two groups. The control group was tested at the first period of the study (October 2018–May 2019), whereas the experimental group was evaluated at the second chronological period of the study (October 2020–May 2021) during the second wave of COVID-19. Neuropsychological tests were performed at baseline and six months after baseline.
Results:
In the control group, significant changes in the scores from the Montreal Cognitive Assessment (MoCA; p = 0.049) and the Functional Rating Scale for Symptoms of Dementia (FRSSD; p = 0.005) were found between baseline and follow-up assessments, whereas no changes were identified in Mini-Mental State Examination (MMSE; p = 0.229) and Geriatric Depression Scale (GDS; p = 0.619) scores. In the experimental group, the scores from all neuropsychological tests (MoCA, MMSE, GDS, and FRSSD; p < 0.001 for all) were significantly different at follow-up when compared with those at baseline measurements. Moreover, significant deterioration of specific functions assessed in MMSE and FRSSD was detected, especially in the experimental group.
Conclusion:
This study highlights cognitive functions directly affected by social deprivation of individuals with neurocognitive disorders. The findings can be used in the rehabilitation from confinement and its negative consequences.
INTRODUCTION
Data provided by the World Health Organization (WHO) indicate that older people and people with underlying medical conditions are at higher risk for severe coronavirus disease of 2019 (COVID-19) [1]. A recent large-scale study shows that patients with dementia have a higher risk of developing COVID-19 than the elderly without dementia. There are several causal factors that may explain the higher risks, including cognitive impairments that prevent the understanding of precautionary procedures and an inability to follow self-quarantine measures [2]. In addition, patients with dementia have a significantly increased risk of more serious complications of COVID-19, a finding provided by a cohort study in United Kingdom (UK) [3]. The risk factors for dementia, such as age, obesity, cardiovascular disease, hypertension, and diabetes mellitus are also risk factors for severe acute respiratory syndrome coronavirus 2 infection (SARS-CoV-2) [4] and for severe COVID-19. Lastly, pre-existing brain pathology can increase the risk of manifesting neurological complications from COVID-19 [5]. Thus, people with neurocognitive disorders are especially vulnerable to COVID-19, as well as to its immediate and long-term complications.
Since the outbreak of the disease, lockdown measures have been a common preventive strategy to slow down the transmission of SARS-CoV-2 worldwide. Strict lockdowns, however, had a great impact on the psychosocial health of individuals. Prolonged periods of lockdown result in stress and social isolation, which is associated with manifestation of neuropsychiatric symptoms, even in cognitively healthy older adults [6]. An increase in the prevalence of negative symptoms associated with anxiety and depression has motivated governments to adopt policies aimed at protecting the mental health of its citizens. In this scenario, older adults with dementia require additional attention, since social isolation contributes to the appearance and gradual deterioration of neuropsychiatric symptoms and severe behavioral disturbance. Indeed, the pandemic further aggravates the vulnerability of individuals with cognitive impairment, especially those who need daily care [7]. To address this issue, the WHO has provided supporting information on how to deal with patients with dementia during the pandemic [8]. The preexisting neurophysiological alterations may put patients with dementia at an increased risk of various neurological complications, including a decline in cognitive functions, which may be irreversible [2]. Still, research in the field of dementia warrants further studies to verify the impact of COVID-19 lockdown on the brain and cognition.
The aim of the present study was to gauge the effect of prolonged social isolation on the cognitive function of older adults with minor neurocognitive disorders or dementia. To do so, we assessed the cognitive function of older patients with neurocognitive disorders before and after a period of strict lockdown and compared the outcome with that of older patients assessed during a period of no social deprivation before the pandemic.
METHODS
Participants
The study included 125 participants who visited the outpatient dementia clinic of the Neurology Department of University Hospital of Alexandroupolis in Greece and examined as a part of their routine clinical and neuropsychological assessment. This academic outpatient center is a medical reference in the city, and most appointments come from primary care referrals across the city, as well as from rural districts in northern-eastern Greece. All participants signed an informed consent form prior to their participation. Approval was also obtained from the caregivers and/or a legal representative. The study was approved by the Ethics Committee of the University Hospital of Alexandroupolis.
The recruitment process in this community-based cohort study covered two different periods. The first was between October 2018 and May 2019 (for the control group) and the second was from October 2020 to May 2021 (for the experimental group). The latter period corresponds to the second strict lockdown in Greece. For each group (control or experimental), patients were classified into two subgroups: 1) people with dementia and 2) people with mild cognitive impairment (MCI). Patients in the control group were assessed during the first period (October 2018–May 2019) and classified (MCI or dementia) based on the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5). Participants in the experimental group were assessed during the second period (October 2020–May 2021) and were divided into two groups (MCI or dementia) based on the diagnostic criteria of the DSM-5.This last period corresponds to the second wave of COVID-19; people in this group were tested before the confinement and had a follow-up evaluation at the end of the confinement period. All participants underwent a battery of neuropsychological tests at baseline and after six months, conducted by the same health professionals. The evaluation comprised of biographical information and medical data regarding any medical data collected, including any previous medical diagnosis, such as history of cardiovascular, metabolic, neurological, and affective disorders. All participants underwent neurological examination, neuropsychological and neuroimaging assessments, as well specific biochemical and hematological exams. All diagnoses were determined by a team of experienced physicians specialized in cognitive disorders. The diagnostic protocol of the Outpatient Dementia Clinic includes neurological and neuropsychological assessments, neuroimaging (CT scan or magnetic resonance imaging) and blood tests to exclude other types of reversible cognitive impairment or dementia. The neuropsychological assessment consists of psychometric tools that measure cognitive, functional, and emotional abilities. Before neuropsychological testing, we conducted a semi-structured interview first with patients and then with caregivers to gather information about the pre-disease history, the estimated duration of the disease, the onset of symptoms, the main symptoms of dysfunction in the first years of the disease, potential changes in behavior, relationship functionality, mobilization, motivation, interests, and pursuits.
Inclusion and exclusion criteria
The main inclusion criterion was to be diagnosed with MCI or dementia of any etiology, according to DSM-5 guidelines for mild and major neurocognitive disorders. To be diagnosed with MCI, patients had to show a noticeable decline in cognitive functioning, which is supported by Mini-Mental State Examination (MMSE) score ≥26 and Montreal Cognitive Assessment (MoCA) score ≥26. Additionally, individuals with MCI are autonomous and perform daily activities independently. Individuals with major neurocognitive disorders show a greater decline in overall cognitive functions, identified by a MMSE score < 24, as well as the ability to independently meet the demands of daily living, documented by caregivers/family [9]. All forms of dementia were included: 1) Alzheimer’s disease, 2) dementia with Lewy bodies, 3) frontotemporal dementia, and 4) vascular dementia.
The exclusion criteria were: 1) inability or unwillingness on the part of the patient or caregiver to come to the hospital; 2) previous head trauma resulting in unconsciousness; 3) a history of alcohol or substance abuse; 4) current radiotherapy or chemotherapy; 5) major depression or other major psychiatric disorder; 6) unstable medical illness; and 7) uncorrected visual or hearing impairment. None of the participants had received a laboratory-confirmed diagnosis of SARS-CoV-2 infection.
Mental function assessments
We used the Greek version of the MMSE [10, 11] and the official Greek translation of the MoCA test [12] for detecting cognitive dysfunction. Emotional status was assessed with the Geriatric Depression Scale (GDS) [13], while the Functional Rating Scale for Symptoms of Dementia (FRSSD) [14] was used to assess from the caregiver’s perspective, the patient’s ability to carry out routine tasks and identify the functional difficulties in everyday living.
Data analysis
Data analysis was performed using the open source statistical processing program PSPP v.1.4.0. A Barlett’s test (p < 0.05) and a sample adequacy test were concurrently conducted with data collection and a reliability analysis was performed using Cronbach’s alpha method (α> 0.05).
The scores from the tests (MoCA, MMSE, GDS, and FRSSD) were categorized according to reference cutoff scores (Table 1). Using a non-parametric design, samples were first split according to group (control versus experimental) and then compared based on diagnosis variable (MCI versus dementia) or first split according to diagnosis (MCI versus dementia) and then compared based on group variable (control versus experimental).
Table 1
The cut-off points determination
| Scales | Cut-off points | ||
| GDS | No depression: 0–5 | Mild depression: 6–10 | Severe depression: >10 |
| MoCA | MCI: 30 till 25 | <25 | |
| MMSE | MCI: Score under 26 till 30 | Dementia mild: 21–25 | Dementia Severe: 0–20 |
| FRSSD | 0–5 | Low functionality: >5 | |
GDS, Geriatric Depression Scale; MoCA, Montreal Cognitive Assessment; MMSE, Mini-Mental State Examination; FRSSD, Functional Rating Scale for Symptoms of Dementia.
Statistically significant differences between datasets were identified by using the Wilcoxon signed-rank test to compare pairs of datasets and the Wilcoxon rank-sum test to compare the datasets with different sample sizes. The significance level is considered at 5% (p < 0.05).
Considering “0” as the first moment of the patient’s assessment and “1” as the second assessment (6 months later). The score from the fist measurement was subtracted from that of the second measurement. Chi-squared tests were used to compare the scores of patients with dementia with those of patients with MCI within each group separately (control or experimental) and to compare the scores of patients in the control group (pre-pandemic) with those of patients in the experimental group (during the pandemic) within each diagnosis separately (MCI or dementia). Kruskal Wallis tests were used to make multiple comparisons between diagnosis groups and scales were applied. Regression analyses were used for diagnosis prediction, considering the first group was the control group and the second group was defined as the experimental group.
RESULTS
Among the 125 participants, 70 composed the control group, with 36 subjects diagnosed with MCI and 34 subjects diagnosed with dementia. In the experimental group, 55 participants were recruited, of which 34 were diagnosed with MCI and 21 diagnosed with dementia. Four psychometric parameters (MoCA, MMSE, GDS, and FRSSD scores) were used to compare groups with each other. The years of education ranged from 0 to 17 years (SD = 3.898) for the whole cohort (Table 2).
Table 2
Demographics in MCI and dementia patients
| Gender | Males / Females | MCI | Males: 8 | 14.5% | Females: 26 | 47.3% | |
| Dementia | 10 | 18.2% | 11 | 20.0% | |||
| Age ctg | <70 / ≥70 | MCI | 12 | 21.8% | 22 | 40.0% | |
| Dementia | 2 | 3.6% | 19 | 34.5% | |||
| Educ ctg | until 6 y | MCI | Until 6 y: | Until 6 y: | >12 y: |
| until 12 y | 13 | 23.6% | 8 | 14.5% | 13 | 23.6% | ||
| >12 y |
Differences between patients with dementia and individuals with MCI
In people older than 70 years, dementia was 70% more common than MCI. No significant differences were found between the two groups in terms of gender frequency (χ2 = 0.46, p = 0.5), and age (χ2 = 1.51, p = 0.22). However there was a statistically significant difference in years of education between the two groups (χ2 = 6.83, p = 0.03) revealing that older adults with dementia were less educated than those with MCI (Table 2). Among those with less than 6 years of education, dementia was more common than MCI (Table 2). Full demographic information is presented in Table 2.
Comparisons between control and experimental groups
Table 3 presents descriptive statistics of the psychometric parameters at the initial and the follow-up assessments for the control and experimental groups. In the experimental group, the MoCA (p = 0.049) and FRSSD (p = 0.005) scores at the initial assessment parameters, whereas GDS (p = 0.229) and MMSE (p = o.619) scores did not change significantly. Assessment differences between control and experimental group were statistically significant for all parameters (p < 0.001). This is demonstrated in the distribution of cognitive (MMSE), functional (FRSSD), and emotional (GDS) scores of patients in the two groups (Fig. 4A).
Table 3
Mean and standard deviation (SD) of psychometric parameters at the initial and follow-up assessments. The statistical differences (p-values) were obtained for the control and the experimental groups
| psychometric parameters | Control group (Oct. 2018 - May 2019) | Experimental group (Oct. 2020 – May 2021) | Assessment differences | ||||
| Initial assessments | Follow up | p | Initial assessments | Follow up | p | (p) | |
| MoCA | 22.4 (4.2) | 22.1 (4.2) | 0.049 | 22.6 (5.1) | 20.4 (6.7) | <0.001 | <0.001 |
| MMSE | 25.1 (3.4) | 25.3 (3.5) | 0.229 | 25.1 (4.9) | 22.5 (5.7) | <0.001 | <0.001 |
| GDS | 3.1 (2.8) | 3.2 (2.7) | 0.619 | 5.4 (4.2) | 6.9 (3.7) | <0.001 | <0.001 |
| FRSSD | 7.0 (5.1) | 7.8 (5.8) | 0.005 | 6.7 (6.5) | 9.8 (6.7) | <0.001 | <0.001 |
MoCA, Montreal Cognitive Assessment; MMSE, Mini-Mental State Examination; GDS, Geriatric Depression Scale; FRSSD, Functional Rating Scale for Symptoms of Dementia.
Table 4 presents the descriptive statistics of the psychometric parameters at the initial and follow-up assessments for both control and experimental groups further divided into MCI and dementia subgroup. In the control group, there were no statistically significant differences between initial and follow-up assessments in individuals with MCI (MMSE; p = 0.809, GDS; p = 0.638, FRSSD; p = 0.054), with the exception of MoCA score (p = 0.021). For those with dementia no statistically significant difference was detected (MoCA: p = 0.388, MMSE: p = 0.225, GDS: p = 0.284) with the exception of FRSSD (p = 0.034), in the control group. In the experimental group, statistically significantly differences were found for all parameters in individuals with MCI (p < 0.001). In the cases of patients with dementia, differences between control and experimental group were statistically significant for all parameters (p < 0.001), with the exception of the GDS (p = 0.516). This is demonstrated in Fig. 4B, where the distribution of cognitive (MMSE), functional (FRSSD), and emotional (GDS) scores of the two groups is presented.
Table 4
Mean and standard deviation (SD) of psychometric parameters at the initial and follow-up assessments. The statistical differences (p-values) were obtained within the control and experimental group, for the MCI and dementia diagnosis
| psychometric parameters | Control group (Oct. 2018 - May 2019) | Experimental group (Oct. 2020 – May 2021) | Assessment differences | ||||
| Initial assessments | Follow up | p | Initial assessments | Follow up | p | (p) | |
| MCI | |||||||
| MoCA | 25.6 (1.0) | 25.3 (1.0) | 0.021 | 25.1 (1.9) | 24.4 (3.3) | 0.269 | <0.001 |
| MMSE | 28.0 (1.2) | 28.0 (1.4) | 0.809 | 27.8 (1.4) | 25.9 (1.2) | <0.001 | <0.001 |
| GDS | 3.4 (3.0) | 3.3 (2.7) | 0.638 | 2.7 (1.9) | 4.8 (1.1) | <0.001 | <0.001 |
| FRSSD | 4.1 (3.4) | 4.5 (3.6) | 0.054 | 3.9 (1.6) | 6.5 (1.6) | <0.001 | <0.001 |
| Dementia | |||||||
| MoCA | 18.9 (3.4) | 18.7 (3.6) | 0.388 | 18.4 (5.9) | 14.0 (5.7) | <0.001 | <0.001 |
| MMSE | 22.1 (2.3) | 22.5 (2.7) | 0.225 | 20.6 (5.3) | 17.0 (5.9) | <0.001 | <0.001 |
| GDS | 2.8 (2.6) | 3.0 (2.7) | 0.284 | 9.8 (3.3) | 10.3 (4.0) | 0.084 | 0.516 |
| FRSSD | 10.0 (4.8) | 11.2 (5.7) | 0.034 | 11.2 (8.7) | 15.1 (8.4) | <0.001 | <0.001 |
MoCA, Montreal Cognitive Assessment; MMSE, Mini-Mental State Examination; GDS, Geriatric Depression Scale; FRSSD, Functional Rating Scale for Symptoms of Dementia.
Comparison between initial and follow-up scores
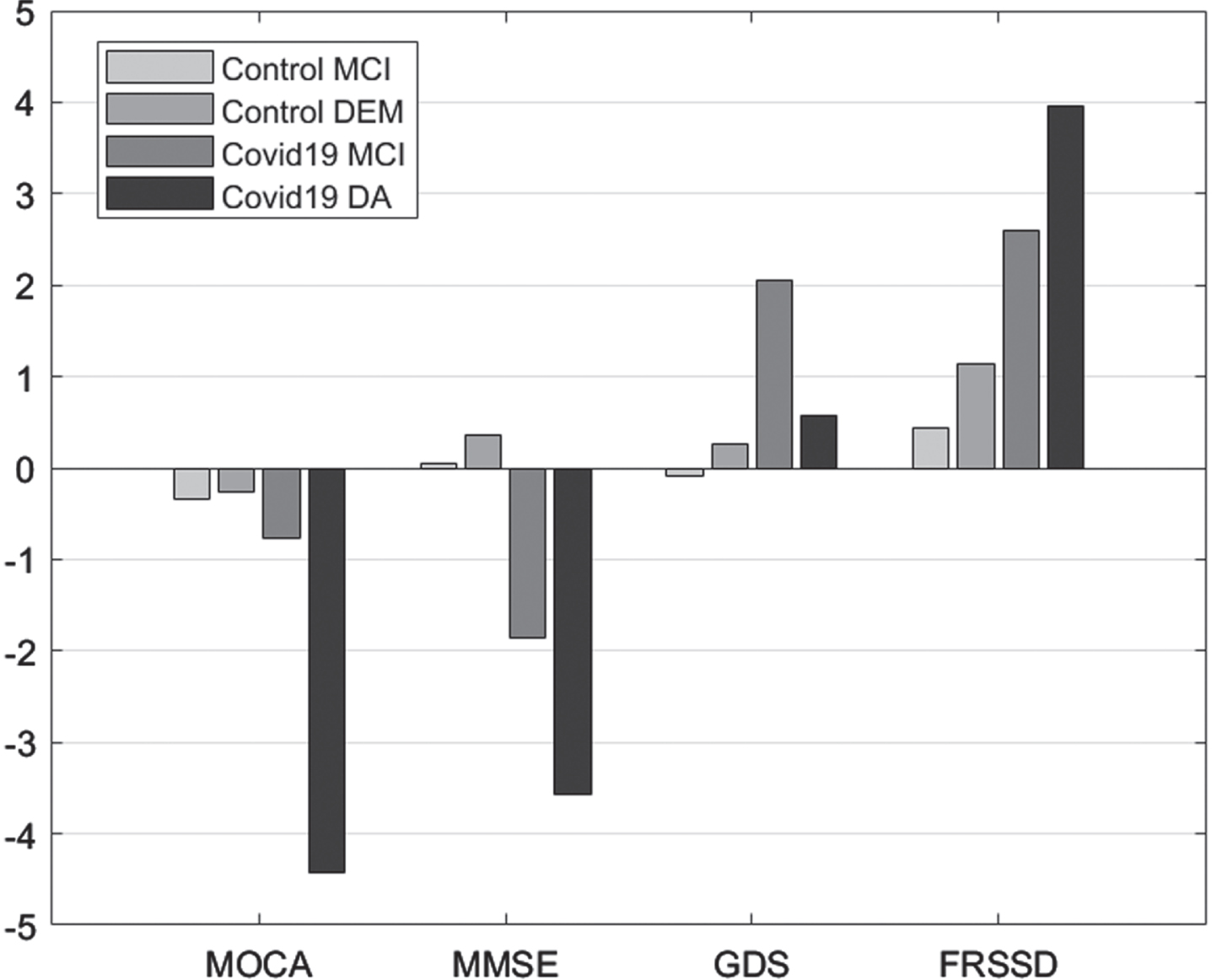
Comparisons between initial and follow-up tests revealed significant differences in MoCA scores in patients with dementia, MMSE scores in individuals with MCI or dementia, GDS score in individuals with MCI, and FRSSD scores in individuals with MCI or dementia (Fig. 1).
Fig. 1
Mean difference “initial versus follow-up”, per group, per Diagnosis for the scales Montreal Cognitive Assessment (MOCA), Mini-Mental State Examination (MMSE), Geriatric Depression Scale (GDS), and Functional Rating Scale for Symptoms of Dementia (FRSSD).
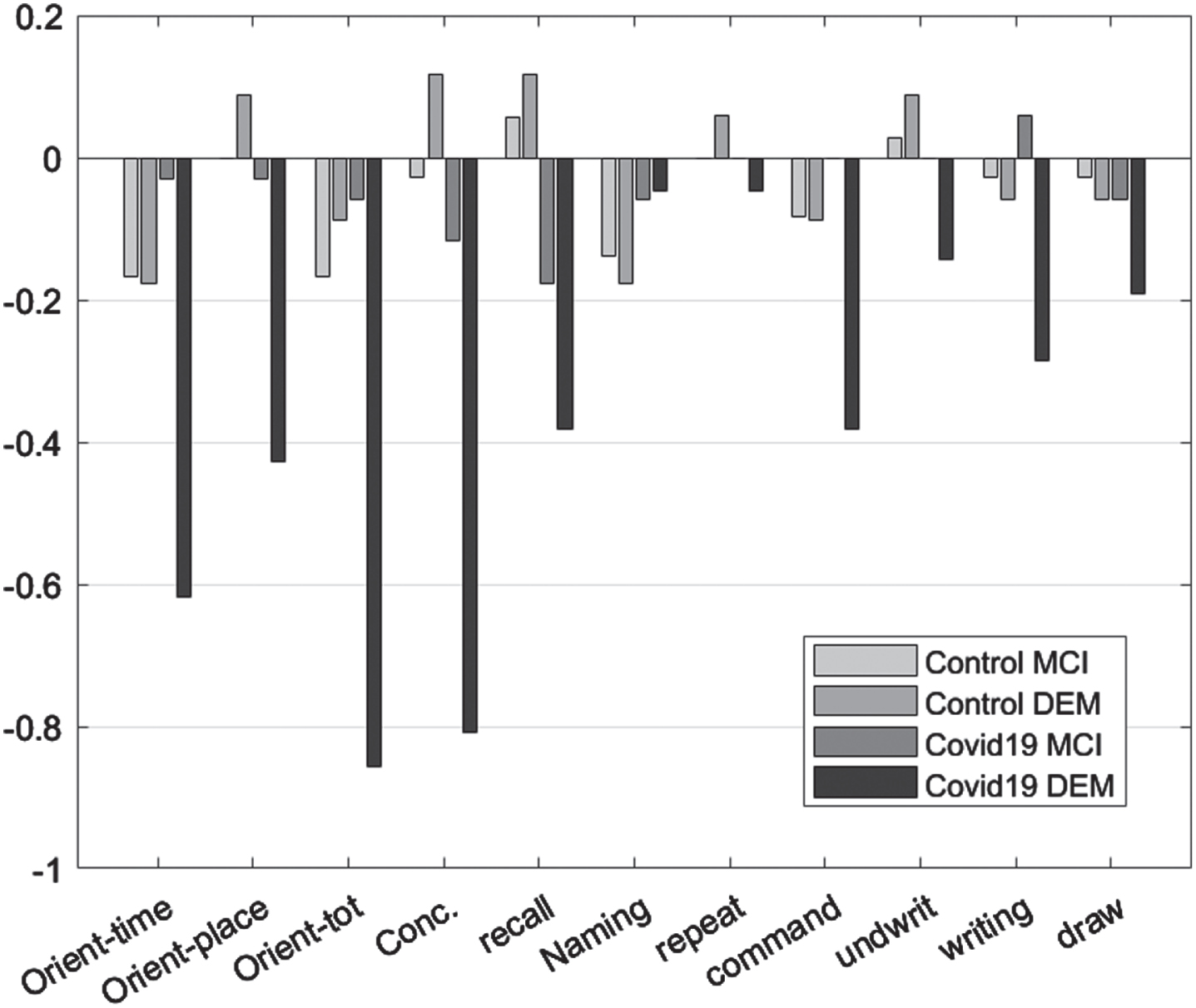
As dementia progresses, a general decline in the performance of patients in MMSE is expected. In both control experimental group, functional decline in orientation to time, recall, command execution, and ability to write were observed. Orientation to place, total orientation to space and time as well as the ability to write and draw, were affected only in the experimental group. In the control group, only orientation in space and time, recall, ability to write and follow a command changed in the period of six months between the initial and the follow-up assessment (Table 5, Fig. 2).
Table 5
Related sample Wilcoxon comparisons per group
| Control | Experimental | |||||
| Z | p | sig. | Z | p | sig. | |
| Orientation_time0 - Orientation_time1 | –1.97 | 0.049 | ** | –3.30 | 0.001 | ** |
| Orientation_place0 - Orientation_place1 | –0.65 | 0.513 | –2.89 | 0.004 | ** | |
| Orient_tot0 - Orient_tot1 | –1.13 | 0.258 | –3.11 | 0.002 | ** | |
| nam0 - nam1 | –0.38 | 0.705 | –1.41 | 0.157 | ||
| Concentr0 - concentr1 | –0.30 | 0.765 | –3.04 | 0.002 | ** | |
| recall0 - recall1 | –1.90 | 0.058 | * | –2.21 | 0.027 | ** |
| nam0 - nam1 | –2.84 | 0.005 | ** | –1.34 | 0.180 | |
| repet0 - repet1 | –0.50 | 0.617 | –0.30 | 0.763 | ||
| command0 - command1 | –1.73 | 0.083 | * | –2.14 | 0.033 | ** |
| undwrit0 - undwrit1 | –1.63 | 0.102 | * | –1.73 | 0.083 | * |
| writing0 - writing1 | –1.34 | 0.180 | –1.41 | 0.157 | ||
| draw0 - draw1 | –1.13 | 0.257 | –2.45 | 0.014 | ** | |
| ffood0 - ffood1 | –1.41 | 0.157 | –1.73 | 0.083 | * | |
| fdress0 - fdress1 | 0.00 | 0.99 | –1.73 | 0.083 | * | |
| fakrat0 - fakrat1 | –0.58 | 0.564 | –1.73 | 0.083 | * | |
| ftalking0 - ftalking1 | –0.53 | 0.593 | –5.49 | 0.000 | ** | |
| fsleep0 - fsleep1 | –0.19 | 0.850 | –1.41 | 0.157 | ||
| fident0 - fident1 | –2.71 | 0.007 | ** | –4.94 | 0.000 | ** |
| fwash0 - fwash1 | –1.67 | 0.096 | * | –1.63 | 0.102 | * |
| fidentnam0 - fidentnam1 | –0.77 | 0.439 | –5.58 | 0.000 | ** | |
| fmem0 - fmem1 | –1.85 | 0.065 | * | –5.75 | 0.000 | ** |
| fattent0 - fattent1 | –2.45 | 0.014 | ** | –5.39 | 0.000 | ** |
| fconfus0 - fconfus1 | –2.92 | 0.004 | ** | –2.24 | 0.025 | ** |
| forientplace0 - forientplace1 | –0.38 | 0.705 | –1.89 | 0.059 | * | |
| ffeeling0 - ffeeling1 | –1.15 | 0.248 | –5.26 | 0.000 | ** | |
| fsocial0 - fsocial1 | –1.44 | 0.151 | –5.90 | 0.000 | ** | |
**p≤0.05, *p≤0.10.
Fig. 2
Mean difference “initial versus follow-up”, per group, per Diagnosis for the Mini-Mental State Examination subscales.
In the case of FRSSD scores, food intake, ability to get dressed, continence, verbal communication, social responsiveness, emotionality, spatial orientation, and memory for names were significantly affected only in the experimental group (p < 0.05). In the control group, no statistically significant change was detected in these parameters. On the other hand, memory for events, mental alertness hygiene, grooming and sleep, changed significantly between initial and follow-up assessments for both control and experimental groups (p < 0.05) (Table 5; Fig. 3).
Fig. 3
Mean difference “initial versus follow-up”, per group, per Diagnosis for the Functional Rating Scale for Symptoms of Dementia subscales.
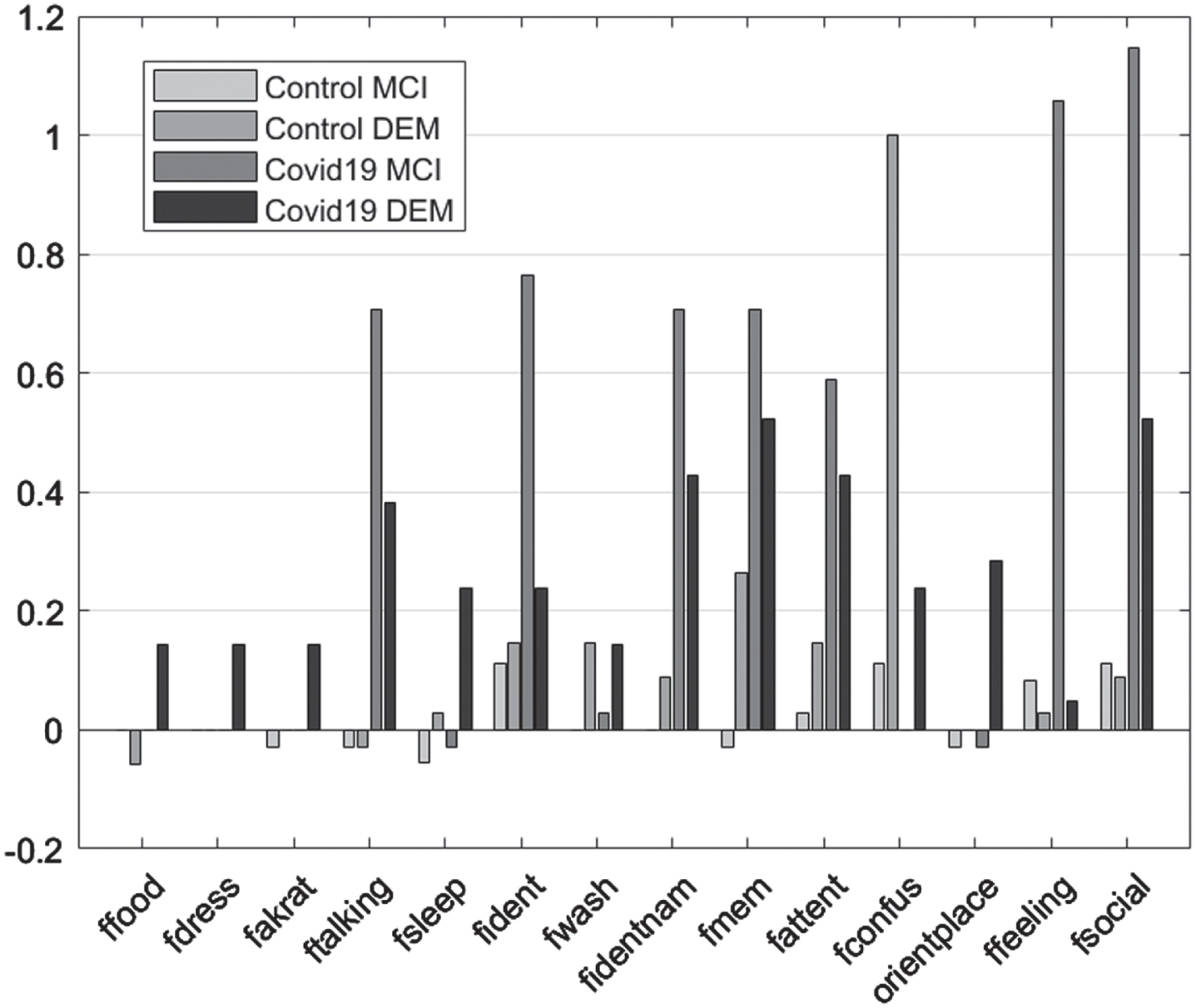
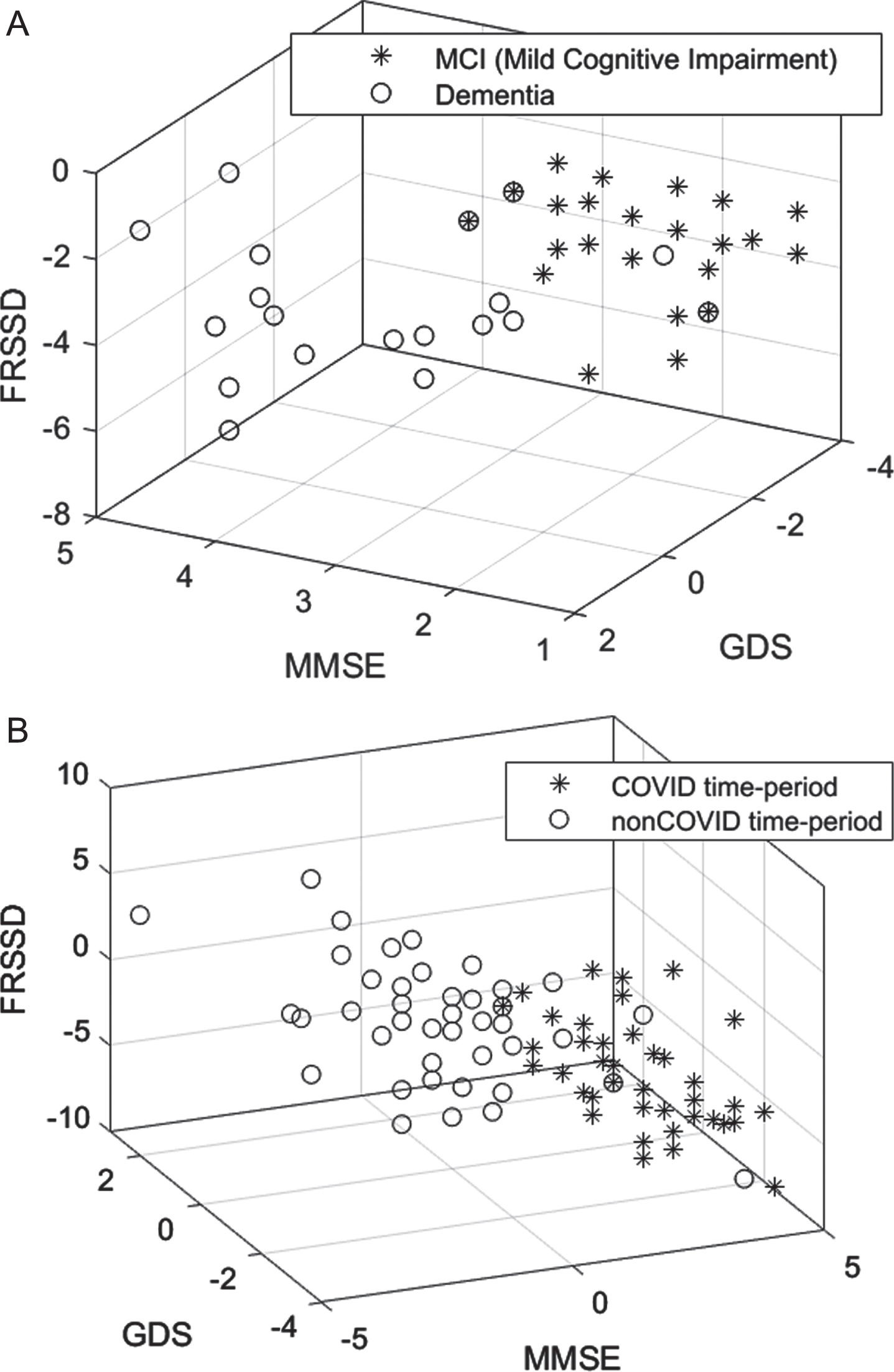
Fig. 4
Scatter diagram showing the distributions of cognitive (MMSE), functional (FRSSD) and emotional (GDS) neurophysiological assessments (A) for the COVID and non-COVID time-period and (B) for the MCI and dementia diagnosed participants within the COVID time-period.
DISCUSSION
In the current study we investigated the potential effects of confinement during the second wave of COVID-19 on cognitive, executive, and emotional functions in Greek patients with neurocognitive disorders. The main objective was to verify whether patients had their cognitive function impairment aggravated beyond the expected progression of the neurocognitive disease by the social isolation imposed by strict lockdowns. The average scores in MMSE, MoCA, FRSSD, and GDS tests indicated a cognitive decline beyond the expected in patients assessed during the pandemic.
Cognitive skills were assessed using MoCA and MMSE tests, but differences in their results were observed. Scores from MoCA test presented a decline in both control and experimental groups but revealed more prominent changes in the experimental group (i.e., COVID-19 group), confirming the higher sensitivity of MoCA in detecting cognitive impairment compared with other tests [15]. A decrease in MMSE total scores was detected only in the experimental group. Moreover, specific subscales of the MMSE, such as orientation, concentration, recall, understanding, and drawing ability were particularly affected in COVID-19 group, especially in patients with major neurocognitive disorders. These findings indicate that confinement had a significant impact on cognitive functioning of people with neurocognitive disorders. Such impact appeared to be unrelated to the type of cognitive disorder (MCI or dementia), age, sex, and education. Previous studies, that also used objective neuropsychological measures, have highlighted the impact of pandemic restrictions on cognitive functioning [16–19]. These are further supported by findings from studies that used self-reports of patients and/or caregivers [20–26] and systematic reviews [27, 28].
The interpretation of cognitive impairment during quarantine may not be straightforward but multifactorial. Moreover, one cannot assert that it is transient, and it will be improved at the end of the confinement. However, an interpretive approach to the issue is to focus on everyday lifestyle [17] and how much the individual is involved in intellectual activities, such as participation in cultural events, discussions, and hobbies [29]. Stimulating everyday activities has been found to protect patients with neurocognitive disorders from deterioration of cognitive functioning [29–31]. Another causal explanation has been proposed by Kolb [33], who describes the experience-dependent plasticity as a process whereby neuron connections can be modified by experience, highlighting the effect of ‘enriched experiences’. The idea that both exercise and cognitive stimulation are factors that increase neuronal plasticity and resistance to cell death precedes the evidence that environmental enrichment may act directly to prevent or slow the onset of Alzheimer’s disease [34]. Moreover, several studies have confirmed the negative impact of social deprivation and the fear of infection on cognitive functions [16, 18, 22, 24, 26, 35]. Tech illiteracy in older people is an additional obstacle to social interactions, as they do not fully explore new technologies and virtual platforms to communicate with others [36, 37]. Similar studies reported a deterioration of specific cognitive domains during confinement, including memory and orientation [18, 22] and attention and working memory [38].
The use of the FRSSD allowed us to identify significant declines in functional abilities in both control and experimental groups, which were related to the severity of the neurocognitive disease. In the experimental group there was deterioration in more areas of functionality than in the control group, confirming the negative effect of confinement individuals with neurocognitive disorders. This finding is in line with previous studies [17, 18, 20, 22, 28] and supports the importance of identifying patients’ deficits and correctly addressing caregivers’ needs to alleviate the adverse effects of the pandemic on psychosocial health. Cognitive areas that were objectively confirmed to be impaired, such as memory and attention, appeared to be those reported by caregivers as especially demanding. Therefore, these results may be affected by the emotional state of caregivers, whose psychosocial health has been greatly affected by quarantine periods [19, 22, 24, 26].
Another observation of the present study was the impact of confinement on the emotional status of participants. The GDS test confirmed the presence of depressive symptoms in the COVID-19 group, but not in the control group, regardless of the severity of the neurocognitive disease. The restrictions imposed during the quarantine period, mainly the social isolation and avoidance of social contacts, even with close relatives and friends and the cessation of all systematic activities in social and cultural contexts, are possible causes of neuropsychiatric symptoms, with the predominance of the depressive symptoms [19, 21, 22, 26–29, 39, 40]. These neuropsychiatric manifestations are both part of the development of neurocognitive disorders and risk factors for dementia. In particular, the presence of depressive symptoms is one important risk factor [41]. This interplay has been explained by several, non-mutually exclusive hypotheses, [42]: 1) depression is a risk factor for cognitive impairment [43–45], 2) cognitive impairment is a risk factor for depression [46], 3) and/or the presence of a third variable (neurological condition), may simultaneously cause cognitive deterioration and depression [44, 47, 48]. Depression, as a symptom, may not always be causal, but it can exacerbate pre-existing cognitive impairment by depleting cognitive reserve [47]. If depression is treated successfully, then MCI improves, but the person may be at greater risk for developing Alzheimer’s disease [49]. Cognitive impairment is considered a symptom of depression in older patients [48]. This is supported by meta-analyses, where poor performance in neurocognitive tests was associated with depression [50, 51]. Understanding the etiology of neuropsychiatric symptoms in MCI is important in understanding the development of dementia [52]. To date, only three studies have measured the effects of prolonged lockdown on cognitive, functional and emotional abilities of Greek patients with neurocognitive disorders. Two of them conducted [25, 37] in the regional area of Athens, used self-reported questionnaires, and focused on caregivers’ assessments and perceptions. The third one conducted in the urban area of Thessaloniki [53], reported interesting clinical findings during the first COVID-19 wave, where no impact of the confinement on the functionality of the participants was found could the lack of effect can be explained by the fact that the participants continued to receive psychosocial support and remote web-based interventions during the quarantine. Our current study focused on the confinement during the second COVID-19 wave, which lasted longer than the first. Moreover, the present study included participants living in the regional area of Eastern-Northern Greece. The geographical factor plays a role in the accessibility of people to free specialized services for people with dementia. The availability of these services was greatly limited and worked precariously during the quarantine period in Eastern-Northern Greece. To our knowledge, there are no available data on the effects of the second COVID-19 wave on the cognitive function, as measured by neuropsychological tests used in clinical practice, of Greek individuals with mild and major neurocognitive disorders, living in remote areas, A major strength of the present study is the assessment of neuropsychological performance using face-to-face interviews of patients with neurocognitive disorders, which is considered a reliable strategy to detect cognitive and neuropsychiatric changes [17]. Our experimental design included the recruitment of a strict control group and the use of a variety of neuropsychological scales to evaluate the global cognitive, functional, and emotional functions at two time points to assess changes in different types of neurocognitive disorders. On the other hand, the small sample size and the inclusion of patients living at home are limitations that might weaken the generalizability of the present results. Another limitation of the study is that additional neuropsychological tests, which could provide more information about the overall functioning of the participants, were not used. Especially for depression screening, complementary assessments could provide deeper insights into the emotional status of the participants before and during the pandemic. Moreover, we did not re-test patients to investigate, if the changes were permanent or transient (i.e., they could return to baseline levels). Finally, the results obtained with older individuals with mild and major neurocognitive disorders cannot be equally applied or generalized to cognitively healthy older adults.
In conclusion, this study highlights elements of the clinical condition of individuals with neurocognitive disorders that were directly affected by the confinement during the second COVID-19 wave. The findings can be used in the course of rehabilitation of people who have experienced the negative effects of social deprivation and have been cut off from any activity. A deeper understanding of neuropsychological changes can provide useful insights into both health services and crisis management. These, in turn, can be used in the development of prevention policies for aging societies and the protection of vulnerable populations. The design of post-emergency dementia rehabilitation plan should include the involvement of a three-pronged support system that includes the patient-family-health system. Long term consequences of the social circumstances associated with isolation and stress are risk factors for subsequent cognitive decline [54]. Since many neurocognitive disorders can be prevented through modifiable risk factors, such as lifestyle routines [55], informing patients about desirable changes in lifestyle might mitigate their cognitive decline. Thus, it is crucial to educate potential patients and their caregivers about healthy habits. Moreover, awareness campaigns for memory disorders should include useful information for early detection of symptoms. A key element for the rehabilitation of individuals with neurocognitive disorders is the improvement of quality of life. This can be achieved through the development of adequate strategies to stimulate healthy activities. National efforts are essential to set up frameworks for dementia management and providing support to patients and caregivers. Further research should investigate whether the neuropsychological alterations observed herein are permanent or can be improved with appropriate approaches. The transformation of remote diagnostics and support services is an opportunity to establish a theoretical and practical model that maximizes therapeutic outcomes for patients and their families.
DISCLOSURE STATEMENT
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/22-0118r1).
REFERENCES
[1] | World Health Organization, Coronavirus Disease 2019, https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200311-sitrep-51-covid-19.pdf, Last updated March 11, 2020, Accessed on June 2, 2021. |
[2] | Mok VCT , Pendlebury S , Wong A , Alladi S , Au L , Bath PM , Biessels GJ , Chen C , Cordonnier C , Dichgans M , Dominguez J , Gorelick PB , Kim S , Kwok T , Greenberg SM , Jia J , Kalaria R , Kivipelto M , Naegandran K , Lam LCW , Lam BYK , Lee ATC , Markus HS , O’Brien J , Pai MC , Pantoni L , Sachdev P , Senanarong V , Skoog I , Smith EE , Srikanth V , Suh GH , Wardlaw J , Ko H , Black SE , Scheltens P ((2021) ) Tackling challenges in care of Alzheimer’s disease and other dementias amid the COVID-19 pandemic, now and in the future. Alzheimers Dement 17: , 906–907. |
[3] | Atkins JL , Masoli JAH , Delgado J , Pilling LC , Kuo CL , Kuchel GA , Melzer D ((2020) ) Preexisting comorbidities predicting COVID-19 and mortality in the UK Biobank Community Cohort. J Gerontol A Biol Sci Med Sci 75: , 2224–2230. |
[4] | Williamson EJ , Walker AJ , Bhaskaran K , Bacon S , Bates C , Morton CE , Curtis HJ , Mehrkar A , Evans D , Inglesby P , Cockburn J , McDonald HI , MacKenna B , Tomlinson L , Douglas IJ , Rentsch CT , Mathur R , Wong AYS , Grieve R , Harrison D , Forbes H , Schultze A , Croker R , Parry J , Hester F , Harper S , Perera R , Evans SJW , Smeeth L , Goldacre B ((2020) ) Factors associated with COVID-19-related death using OpenSAFELY. Nature 584: , 430–436. |
[5] | Kuo C-L , Pilling LC , Atkins JL , Masoli JAH , Delgado J , Kuchel GA , Melzer D ((2020) ) APOE e4 genotype predicts severe COVID-19 in the UK Biobank Community Cohort. J Gerontol A Biol Sci Med Sci 75: , 2231–2232. |
[6] | Brown EE , Kumar S , Rajji TK , Pollock BG , Mulsant BH ((2020) ) Anticipating and mitigating the impact of COVID-19 pandemic on Alzheimer’s disease and related dementias. Am J Geriatr Psychiatry 28: , 712–721. |
[7] | Alonso-Lana S , Marquié M , Ruiz A , Boada M ((2020) ) Cognitive and neuropsychiatric manifestations of COVID-19 and effects on elderly individuals with dementia. Front Aging Neurosci 12: , 369. |
[8] | World Health Organization ((2020) ) Coronavirus Disease (COVID-19) pandemic.https://www.who.int/emergencies/diseases/novel-coronavirus-2019, Last updated October 1, 2021, Accessed on January 1, 2022. |
[9] | American Psychiatric Association ((2013) ) Diagnostic and Statistical Manual of Mental Disorders (DSM-5®), American Psychiatric Publishing, Inc., Arlington, VA, US. |
[10] | PAR MMSE®, https://www.parinc.com/Products/Pkey/237, Accessed on June 7, 2021. |
[11] | Folstein MF , Folstein SE , McHugh PR ((1975) ) “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: , 189–98. |
[12] | MoCA Cognitive Assessment, MoCA, Version 7; http://www.mocatest.org, Accessed on June 7, 2021. |
[13] | Sheikh JI , Yesavage JA ((1986) ) Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. Clin Gerontol 5: , 165–173.3. |
[14] | Hutton JT ((1990) ) Alzheimer’s disease. In Conn’s Current Therapy, RakelRE, eds. Elsevier Health Sciences, Amsterdam, pp 778–781. |
[15] | Lees R , Selvarajah J , Fenton C , Pendlebury S , Langhorne P , Quinn T ((2014) ) Test accuracy of cognitive screening tests for diagnosis of dementia and multidomain cognitive impairment in stroke. Stroke 45: , 3008–3018. |
[16] | Chen Z-C , Liu S , Gan J , Ma L , Du X , Zhu H , Han J , Xu J , Wu H , Fei M , Dou Y , Yang Y , Deng P , Wang X-D , Ji Y ((2021) ) The impact of the COVID-19 pandemic and lockdown on mild cognitive impairment, Alzheimer’s disease and dementia with Lewy bodies in China: A 1-year follow-up study. Front Psychiatry 12: , 711658. |
[17] | Gan J , Liu S , Wu H , Chen Z , Fei M , Xu J , Dou Y , Wang X , Ji Y ((2021) ) The Impact of the COVID-19 pandemic on Alzheimer’s disease and other dementias. Front Psychiatry 12: , 703481. |
[18] | Ismail II , Kamel WA , Al-Hashel JY ((2021) ) Association of COVID-19 pandemic and rate of cognitive decline in patients with dementia and mild cognitive impairment: A cross-sectional study. Gerontol Geriatr Med 7: , 23337214211005224. |
[19] | Tondo G , Sarasso B , Serra P , Tesser F , Comi C ((2021) ) The impact of the COVID-19 pandemic on the cognition of people with dementia. Int J Environ Res Public Health 18: , 4285. |
[20] | Borelli WV , Augustin MC , de Oliveira PBF , Reggiani LC , Bandeira-de-Mello RG , Schumacher-Schuh AF , Chaves MLF , Castilhos RM ((2021) ) Neuropsychiatric symptoms in patients with dementia associated with increased psychological distress in caregivers during the COVID-19 pandemic. J Alzheimers Dis 80: , 1705–1712. |
[21] | Cagnin A , Di Lorenzo R , Marra C , Bonanni L , Cupidi C , Laganà V , Rubino E , Vacca A , Provero P , Isella V , Vanacore N , Agosta F , Appollonio I , Caffarra P , Pettenuzzo I , Sambati R , Quaranta D , Guglielmi V , Logroscino G , Filippi M , Tedeschi G , Ferrarese C , Rainero I , Bruni AC , SINdem COVID-19 Study Group ((2020) ) Behavioral and psychological effects of coronavirus disease-19 quarantine in patients with dementia. Front Psychiatry 11: , 578015. |
[22] | Canevelli M , Valletta M , Toccaceli Blasi M , Remoli G , Sarti G , Nuti F , Sciancalepore F , Ruberti E , Cesari M , Bruno G ((2020) ) Facing dementia during the COVID-19 outbreak. J Am Geriatr Soc 68: , 1673–1676. |
[23] | Rainero I , Bruni AC , Marra C , Cagnin A , Bonanni L , Cupidi C , Laganà V , Rubino E , Vacca A , Di Lorenzo R , Provero P , Isella V , Vanacore N , Agosta F , Appollonio I , Caffarra P , Bussè C , Sambati R , Quaranta D , Guglielmi V , Logroscino G , Filippi M , Tedeschi G , Ferrarese C , the SINdem COVID-19 Study Group, Gallo E , Grassini A , Marcinnò A , Roveta F , Martino PD , Frangipane F , Puccio G , Colao R , Mirabelli M , Terracciano C , Lino F , Mozzetta S , Gazzola G , Camporese G , Sacco S , Lechiara MC , Carrarini C , Russo M , lena AC , Sucapane P , Tiraboschi P , Caroppo P , Redaelli V , Fede GD , Coppa D , Peluso L , Insarda P , Bartolo MD , Esposito S , Iavarone A , Fuschillo C , Salvatore E , Criscuolo C , Sambati L , Santoro R , Gragnaniello D , Pedriali I , Ludovico L , Chiari A , Fabbo A , Bevilacqua P , Galli C , Magarelli S , Spalletta G , Banaj N , Caruso G , Porcari DE , Giubilei F , Casini AR , Ursini F , Bruno G , Boffelli S , Brambilla M , Magnani G , Caso F , Spinelli EG , Sinforiani E , Costa A , Luzzi S , Cacchiò G , Parma AIMA -sez , Perini M , Angeloni R , Giuli C , Fabi K , Guidi M , Paci C , Castellano A , Carapelle E , Petrucci R , Accogli M , Trevisi GN , Renna S , Giuliano AV , Re FD , Milia A , Pilia G , Mascia MG , Putzu V , Piccoli T , Cuffaro L , Monastero R , Battaglia A , Blandino V , Lupo F , Cumbo E , Luca A , Caravaglios G , Vezzosi A , Bessi V , Tognoni G , Calsolaro V , Lucarelli G , Amici S , Trequattrini A , Pezzuto S , Mecocci P , Caironi G , Boselli B , Formilan M , Coin A , Togni LD , Sala F , Sandri G , Gallucci M , Mazzarolo AP , Bergamelli C , Passoni S ((2021) ) The impact of COVID-19 quarantine on patients with dementia and family caregivers: A nation-wide survey. Front Aging Neurosci 12: , 507. |
[24] | Rising KL , Salcedo VJ , Amadio G , Casten R , Chang A , Gentsch A , O’Hayer CV , Sarpoulaki N , Worster B , Gerolamo AM ((2022) ) Living through the pandemic: The voices of persons with dementia and their caregivers. J Appl Gerontol 41: , 30–35. |
[25] | Tsapanou A , Papatriantafyllou JD , Yiannopoulou K , Sali D , Kalligerou F , Ntanasi E , Zoi P , Margioti E , Kamtsadeli V , Hatzopoulou M , Koustimpi M , Zagka A , Papageorgiou SG , Sakka P ((2021) ) The impact of COVID-19 pandemic on people with mild cognitive impairment/dementia and on their caregivers. Int J Geriatr Psychiatry 36: , 583–587. |
[26] | van Maurik IS , Bakker ED , van den Buuse S , Gillissen F , van de Beek M , Lemstra E , Mank A , van den Bosch KA , van Leeuwenstijn M , Bouwman FH , Scheltens P , van der Flier WM ((2020) ) Psychosocial effects of corona measures on patients with dementia, mild cognitive impairment and subjective cognitive decline. Front Psychiatry 11: , 585686. |
[27] | Lebrasseur A , Fortin-Bédard N , Lettre J , Raymond E , Bussières E-L , Lapierre N , Faieta J , Vincent C , Duchesne L , Ouellet M-C , Gagnon E , Tourigny A , Lamontagne M-È , Routhier F ((2021) ) Impact of the COVID-19 pandemic on older adults: Rapid review. JMIR Aging 4: , e26474. |
[28] | Suárez-González A , Rajagopalan J , Livingston G , Alladi S ((2021) ) The effect of COVID-19 isolation measures on the cognition and mental health of people living with dementia: A rapid systematic review of one year of quantitative evidence. EClinicalMedicine 39: , 101047. |
[29] | Kouzuki M , Furukawa S , Mitani K , Urakami K ((2021) ) Examination of the cognitive function of Japanese community-dwelling older adults in a class for preventing cognitive decline during the COVID-19 pandemic. PLoS One 16: , e0248446. |
[30] | Akbaraly TN , Portet F , Fustinoni S , Dartigues J-F , Artero S , Rouaud O , Touchon J , Ritchie K , Berr C ((2009) ) Leisure activities and the risk of dementia in the elderly: Results from the Three-City Study. Neurology 73: , 854–861. |
[31] | ShimadaH, DoiT, LeeS, MakizakoH ((2019) ) Reversible predictors of reversion from mild cognitive impairment to normal cognition: A 4-year longitudinal study. Alzheimers Res Ther 11: , 24. |
[32] | ShimadaH, MakizakoH, DoiT, ParkH, TsutsumimotoK, VergheseJ, SuzukiT ((2018) ) Effects of combined physical and cognitive exercises on cognition and mobility in patients with mild cognitive impairment: A randomized clinical trial. J Am Med Dir Assoc 19: , 584–591. |
[33] | Kolb B , Harker A , Gibb R ((2017) ) Principles of plasticity in the developing brain. Dev Med Child Neurol 59: , 1218–1223. |
[34] | Lazarov O , Robinson J , Tang Y-P , Hairston IS , Korade-Mirnics Z , Lee VM-Y , Hersh LB , Sapolsky RM , Mirnics K , Sisodia SS ((2005) ) Environmental enrichment reduces Abeta levels and amyloid deposition in transgenic mice. Cell 120: , 701–713. |
[35] | Padala KP , Parkes CM , Padala PR ((2020) ) Neuropsychological and functional impact of COVID-19 on mild cognitive impairment. Am J Alzheimers Dis Other Demen 35: , 153331752096087. |
[36] | Korczyn AD ((2020) ) Dementia in the COVID-19 period. J Alzheimers Dis 75: , 1071–1072. |
[37] | Tsapanou A , Zoi P , Kalligerou F , Blekou P , Sakka P ((2021) ) The effect of prolonged lockdown due to COVID-19 on Greek demented patients of different stages and on their caregivers. J Alzheimers Dis 83: , 907–913. |
[38] | Stewart CC , Yu L , Glover CM , Mottola G , Bennett DA , Wilson RS , Boyle PA ((2020) ) Loneliness interacts with cognition in relation to healthcare and financial decision making among community-dwelling older adults. Gerontologist 60: , 1476–1484. |
[39] | Manca R , De Marco M , Venneri A ((2020) ) The impact of COVID-19 infection and enforced prolonged social isolation on neuropsychiatric symptoms in older adults with and without dementia: A review. Front Psychiatry 11: , 585540. |
[40] | Numbers K , Brodaty H ((2021) ) The effects of the COVID-19 pandemic on people with dementia. Nat Rev Neurol 17: , 69–70. |
[41] | Gao Y , Huang C , Zhao K , Ma L , Qiu X , Zhang L , Xiu Y , Chen L , Lu W , Huang C , Tang Y , Xiao Q ((2013) ) Retracted: Depression as a risk factor for dementia and mild cognitive impairment: A meta-analysis of longitudinal studies. Int J Geriatr Psychiatry 28: , 441–449. |
[42] | Sachs-Ericsson N , Blazer DG ((2015) ) The new DSM-5 diagnosis of mild neurocognitive disorder and its relation to research in mild cognitive impairment. Aging Ment Health 19: , 2–12. |
[43] | Diniz BS , Butters MA , Albert SM , Dew MA , Reynolds CF ((2013) ) Late-life depression and risk of vascular dementia and Alzheimer’s disease: Systematic review and meta-analysis of community-based cohort studies. Br J Psychiatry 202: , 329–335. |
[44] | Ownby RL , Crocco E , Acevedo A , John V , Loewenstein D ((2006) ) Depression and risk for Alzheimer disease: Systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry 63: , 530–538. |
[45] | Zeki Al Hazzouri A , Vittinghoff E , Byers A , Covinsky K , Blazer D , Diem S , Ensrud KE , Yaffe K ((2014) ) Long-term cumulative depressive symptom burden and risk of cognitive decline and dementia among very old women. J Gerontol A Biol Sci Med Sci 69: , 595–601. |
[46] | Richard E , Reitz C , Honig LH , Schupf N , Tang MX , Manly JJ , Mayeux R , Devanand D , Luchsinger JA ((2013) ) Late-life depression, mild cognitive impairment, and dementia. JAMA Neurol 70: , 374–382. |
[47] | Jorm AF ((2001) ) History of depression as a risk factor for dementia: An updated review. Aust N Z J Psychiatry 35: , 776–781. |
[48] | Sachs-Ericsson N , Joiner T , Plant EA , Blazer DG ((2005) ) The influence of depression on cognitive decline in community-dwelling elderly persons. Am J Geriatr Psychiatry 13: , 402–408. |
[49] | Alexopoulos GS ((2005) ) Depression in the elderly. Lancet 365: , 1961–1970. |
[50] | McDermott LM , Ebmeier KP ((2009) ) A meta-analysis of depression severity and cognitive function. J Affect Disord 119: , 1–8. |
[51] | Lee RSC , Hermens DF , Porter MA , Redoblado-Hodge A ((2012) ) A meta-analysis of cognitive deficits in first episode major depressive disorder. J Affect Disord 140: , 113–114. |
[52] | Crocco EA , Loewenstein DA ((2005) ) Psychiatric aspects of mild cognitive impairment. Curr Psychiatry Rep 7: , 32–36. |
[53] | Tsatali M , Moraitou D , Poptsi E , Sia E , Agogiatou C , Gialaouzidis M , Tabakis I-M , Avdikou K , Bakoglidou E , Batsila G , Bekiaridis-Moschou D , Chatziroumpi O , Diamantidou A , Gavra A , Kouroundi E , Liapi D , Markou N , Ouzouni F , Papasozomenou C , Soumpourou A , Tsolaki M ((2021) ) Are there any cognitive and behavioral changes potentially related to quarantine due to the COVID-19 pandemic in people with mild cognitive impairment and AD dementia? A longitudinal study. Brain Sci 11: , 1165. |
[54] | Gordon MN , Heneka MT , Le Page LM , Limberger C , Morgan D , Tenner AJ , Terrando N , Willette AA , Willette SA ((2022) ) Impact of COVID-19 on the onset and progression of Alzheimer’s disease and related dementias: A roadmap for future research. Alzheimers Dement 18: , 1038–1046. |
[55] | Livingston G , Huntley J , Sommerlad A , Ames D , Ballard C , Banerjee S , Brayne C , Burns A , Cohen-Mansfield J , Cooper C , Costafreda SG , Dias A , Fox N , Gitlin LN , Howard R , Kales HC , Kivimäki M , Larson EB , Ogunniyi A , Orgeta V , Ritchie K , Rockwood K , Sampson EL , Samus Q , Schneider LS , Selbæk G , Teri L , Mukadam N ((2020) ) Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396: , 413–446. |



