Prevalence and Safety of COVID-19 Vaccination in Community-Dwelling People with Dementia: Findings from a Tertiary Memory Clinic in Italy
Abstract
This study aimed to explore the prevalence and safety of SARS-CoV-2 vaccination in individuals with dementia. Patients with mild cognitive impairment or dementia were recruited at a tertiary memory clinic, from March 15 to September 15, 2021. Information on COVID-19 vaccination and adverse events experienced after vaccine administration were collected from caregivers. Two-hundred-seventy subjects were finally recruited. Among them, 253 (93.7%) had received the vaccine and only 69 (27.3%) experienced adverse events. Cognitive and behavioral changes following immunization were only rarely reported. COVID-19 vaccination is safe and well-tolerated in patients with cognitive impairment who should be prioritized in the vaccination campaign.
INTRODUCTION
The coronavirus disease 19 (COVID-19) pandemic is having a dramatic effect on people with dementia. Indeed, high hospitalization and fatality rates have been observed in cognitively impaired people, and several studies reported dementia among the main risk factors for severe COVID-19 [1, 2]. The higher lethality of the SARS-CoV-2 infection in people with dementia may be explained by the fact that cognitively impaired individuals are generally older, more frail and less likely to be admitted to intensive care units, further reducing their chance of survival [3]. The presentation of COVID-19 with atypical and misleading clinical manifestations (e.g., delirium, worsening of behavioral disturbances) may also have contributed to an unfavorable disease course in these patients [4].
It is widely demonstrated that vaccines are among the most effective tools for reducing the spread and mortality of COVID-19 [5]. Accordingly, since the beginning of the vaccination campaign, some stakeholders supported the prioritization of individuals with dementia for vaccination against SARS-CoV-2 [6]. Nevertheless, it has been previously shown that individuals with cognitive impairment have a lower likelihood to receive vaccinations (e.g., the flu and pneumococcal vaccine) compared to their cognitively unimpaired counterparts [7, 8]. Few reports exist of a worsening, albeit transitory, of cognitive or behavioral symptoms in cognitively impaired people after vaccine administration [9]. However, the adverse effects related to vaccination may be underestimated due to communication barriers. Another factor that limits the possibility of ascertaining the safety and tolerability of vaccines in people with dementia is that they are often underrepresented in registration randomized clinical trials (RCTs) [10]. For instance, in the RCTs that led to the approval of the currently commercialized vaccines against SARS-Co-V-2, the number of enrolled participants with dementia was either not reported or extremely low (Table 1). It is therefore imperative to collect “real world” data for exploring the efficacy and safety profiles of COVID-19 vaccines in people with dementia to foster their evidence-based use and contrast vaccine hesitancy [11].
Table 1
Enrollment of participants with dementia in phase III clinical trials testing COVID-19 vaccines
| Vaccine Manufacturer(s) | N of participants | Mean/median age (age range) | Dementia as an exclusion criterion | N participants with dementia (%) |
| BNT162b2 [21] BioNTech, Pfizer | 37,706 | 52.0 (16–91) | No | 18 (< 0.1%) |
| mRNA-1273 [22] Moderna | 30,351 | 51.4 (18–95) | No | Not reported |
| JNJ-78436735 (Ad26.COV2.S) [28] Johnson &Johnson | 43,783 | 52 (18–100) | Yes* | Not reported |
| AZD1222 (ChAdOx1 nCoV-19) [29] Oxford, AstraZeneca | 32,379 | 51 (18–100) | No | 15 (< 0.1%) |
| BBV152 [30] Bharat Biotech | 25,753 | 41.0 (18–97) | No | Not reported |
| CoronaVac [31] Sinovac | 10,214 | 45 (18–59) | No | Not reported |
| BBIBP-CorV [32] Sinopharm | 38,206 | 36.1 | No | Not reported |
| Gam-COVID-Vac [33] Gamaleya Research Institute | 21,977 | 45.3 | No | Not reported |
| NVX-CoV2373 [34] Novavax | 14,039 | 56 (18–84) | Yes | None |
*only at the Stage 1a and 2a of the enrollment.
Based on these premises, the present study aimed to 1) evaluate the prevalence of COVID-19 vaccination among people with mild cognitive impairment (MCI) or dementia attending a university memory clinic; 2) estimate the incidence of adverse events in this population; and 3) identify the most common adverse events related to vaccination, with particular focus on cognitive and behavioral effects.
METHODS
Study design and population
A single-center, cross-sectional study was conducted at the Center for Cognitive Disorders and Dementia of the Department of Human Neuroscience, Sapienza University of Rome. We consecutively enrolled individuals with MCI or dementia attending the service from March 15, 2021, to September 15, 2021, according to the following inclusion criteria: 1) diagnosis of MCI or dementia, according to the criteria of the National Institute on Aging and the Alzheimer’s Association [12, 13], and 2) presence of a formal or informal caregiver capable of providing reliable information on the patient.
Reporting of COVID-19 vaccination and related side effects
A questionnaire was designed to collect information on previous SARS-CoV-2 infection and vaccination. The questionnaire included 15 questions about previous SARS-CoV-2 infection, COVID-19 vaccination status, type of vaccine received, and short-term adverse events experienced after the administration of the first and second dose of the vaccine. Special attention was paid to possible change in patients’ cognitive and behavioral status (e.g., worsening in cognition, occurrence of new behavioral disturbances, or worsening of pre-existent neuropsychiatric symptoms) after receiving vaccination. The questionnaire was administered to caregivers after giving their informed consent to participate in the study.
In the timeframe covered by the present analysis, the vaccines commercialized in Italy were BNT162b2/Comirnaty, mRNA-1273/Spikevax, AZD1222/Vaxzevria, and JNJ-78436735/Johnson & Johnson. The proportion of people who had completed the COVID-19 vaccination in the country ranged between 3.6% (March 15, 2021) and 65.8% (September 15, 2021) [14].
Other variables of interest
Clinical information about the sociodemographic (age, sex, education), cognitive (dementia subtype diagnosis, established according to international criteria, and Mini-Mental State Examination [MMSE] [15] score) and functional (Activities of Daily Living [ADL] [16] and Instrumental ADL [IADL] [17] scores) characteristics of the enrolled patients were also retrieved from their clinical charts.
Statistical analysis
First, we estimated the prevalence of COVID-19 vaccination and the incidence of adverse events following vaccine administration in the recruited population. The most common side effects were described and categorized into the following three subgroups: “general”, “cognitive”, and “behavioral” effects.
Then, the study population was divided into two groups “Adverse Events” and “No Adverse Events” according to the reporting of any clinical disturbance following the vaccination. The characteristics of the two study groups were compared by adopting the chi-square test for categorical variables and the Mann-Whitney test for continuous variables (as they were not normally distributed). The Kolmogorov-Smirnov test was used to verify the normal distribution of continuous variables. The level of statistical significance was set at 0.05. The variables found to be statistically significant at descriptive analyses were included in a binary multivariate logistic regression model, adjusted for age, sex, and education, adopting the occurrence of adverse events as the dichotomous dependent variable of interest.
Finally, we considered individuals who were administered two vaccine shots and compared the occurrence of general adverse events after the first and second dose adopting the chi-square test. Furthermore, a binary multivariate logistic regression was performed to examine the relationship between the onset of general side effects after the first dose and after the second dose (dichotomous dependent variable of interest), adjusting for sociodemographic variables (age, sex, education), dementia diagnosis, MMSE, ADL, and IADL. The same analyses were performed for behavioral and cognitive adverse events.
The statistical analysis was performed using the Statistical Package for Social Science (SPSS) version 27.
Ethics statement
This study was performed in line with the principles of the Declaration of Helsinki. The study protocol was approved by the Ethics Committee of the Policlinico Umberto I University Hospital.
RESULTS
A total of 270 individuals (61.8% women) with MCI (17.3%) or dementia (82.7%) were recruited in the study. Enrolled patients had a mean age of 77.7 (standard deviation [SD] 7.3) years and a mean education level of 10.0 (SD 6.8) years. The most prevalent dementia subtypes were Alzheimer’s disease (59.0%), mixed dementia (8.4%), and Lewy body dementia (7.7%). Overall, recruited patients exhibited a moderate cognitive and functional impairment, with mean MMSE, ADL, and IADL scores of 18.8 (SD 6.6), 4.4 (SD 1.9), and 3.6 (SD 2.7), respectively.
At the time of the examination, 17 (6.3%) of the enrolled individuals had presented the SARS-CoV-2 infection.
Two-hundred-fifty-three patients (93.7%) had received the COVID-19 vaccine; among them, 217 (80.4% of the total sample) had completed the two-dose vaccination, while 36 (13.3%) had only received one vaccine shot. The received vaccines were BNT162b2/Comirnaty (73.5%), mRNA-1273/Spikevax (16.6%), and AZD1222/Vaxzevria (9.9%). Among the 17 individuals who did not receive the vaccination, six had previous COVID-19 infection; no information is available on the reason for the non-vaccination of the other 11 subjects.
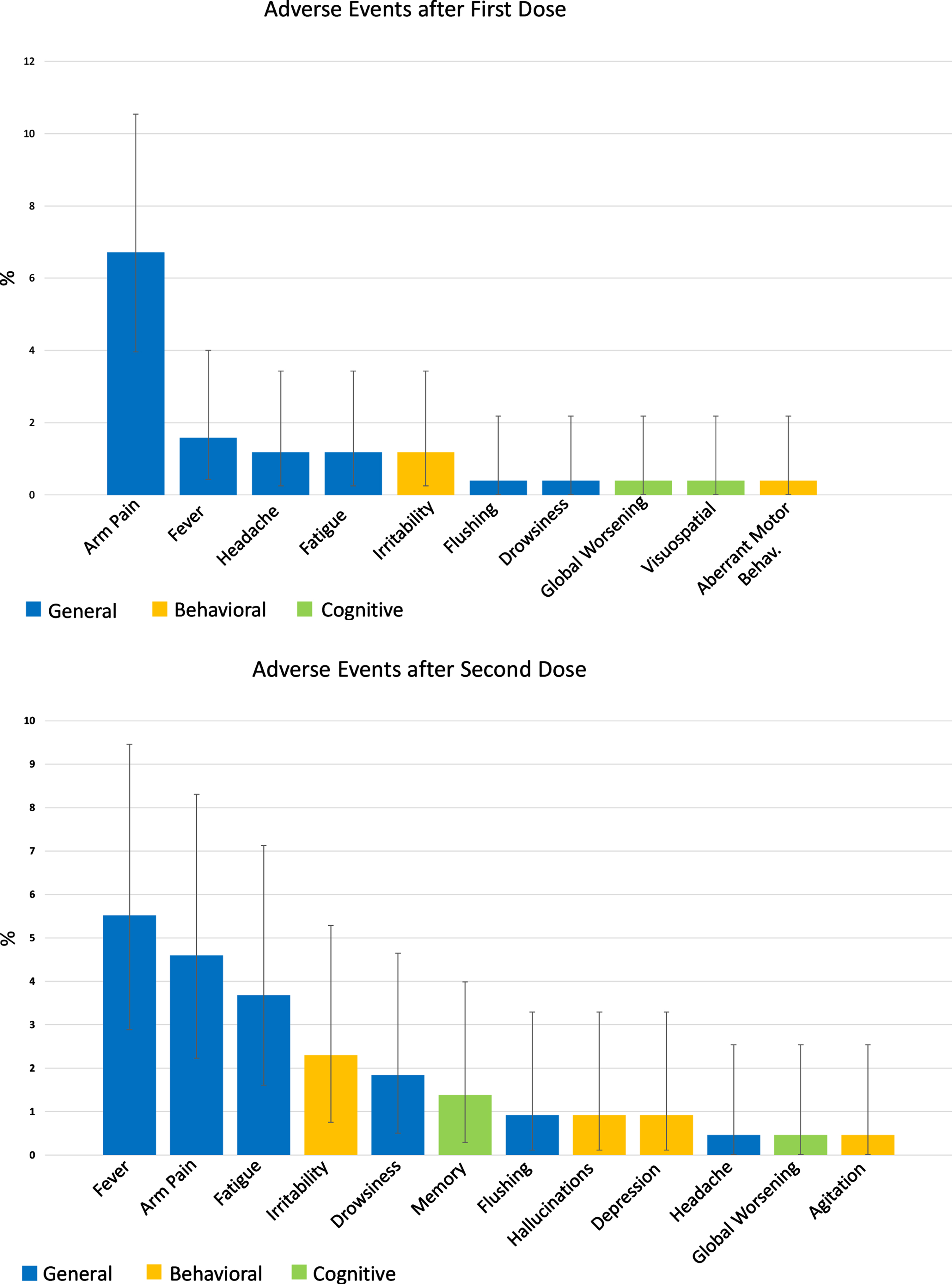
A large proportion of the vaccinated population (71.9%) did not experience any adverse events. The most common side effects were arm pain (6.71% and 4.6% of patients after first and second dose, respectively), fever (1.58% and 5.52%), and fatigue (1.18% and 3.68%). The most observed adverse behavioral change after vaccination was irritability, experienced by three (1.18%) patients after the first and five (2.3%) patients after the second dose. Moreover, after the second vaccine shot, two patients experienced depression and hallucinations, while only one experienced agitation. Cognitive adverse effects were ever rarer, with only 0.8% and 1.8% of the study population experiencing a worsening of cognitive impairment after the first and second dose. It was not possible to establish a causal relationship between such behavioral and cognitive modifications and the vaccine administration; however, it is noteworthy that all the reported events were transient, fully resolved within a few days, and did not require hospitalization or pharmacological interventions. The general, cognitive, and behavioral adverse events experienced by the study population after the administration of the first and second dose of the vaccine are detailed in Fig. 1.
Fig. 1
Adverse events after COVID-19 vaccination in the study population. The two bar plots represent the adverse events experienced by patients after the first (253 patients) and second (217 patients) vaccine dose. Data are shown as %.
The characteristics of patients who did and who did not experience adverse events were substantially similar; indeed, no differences were found regarding sex, age, education, dementia diagnosis, and functional status between the two subgroups (Table 2). The only statistically significant difference was in the severity of cognitive impairment as measured by the MMSE, with patients presenting vaccine side effects exhibiting higher MMSE scores (20.3, SD 6.6 versus 18.3, SD 6.5; p = 0.04). However, in the logistic regression model adjusted for sociodemographic variables, the association between MMSE score and adverse events was no longer statistically significant (OR 1.05, 95% CI 0.99 – 1.10, p = 0.09) (data available upon request).
Table 2
Characteristics of the enrolled patients who received the COVID-19 vaccination according to occurrence of adverse events immunization. Data are shown as mean±standard deviation or n (%)
| Overall (n = 253) | No adverse events (n = 182) | Adverse events (n = 69) | p | |
| Sex (% F) | 156 (61.7%) | 116 (63.7%) | 40 (58.0%) | 0.46* |
| Age (y) | 77.8±7.3 | 78.1±7.3 | 77.2±7.3 | 0.46§ |
| Education (y) | 10.0±6.9 | 10.1±7.6 | 9.9±4.5 | 0.74§ |
| Diagnosis (%)# | 0.43* | |||
| MCI | 38 (16.4%) | 24 (14.1%) | 14 (22.6%) | |
| Dementia | 196 (83.6%) | 147 (85.9%) | 49 (77.4%) | |
| AD | 142 (60.3%) | 107 (62.4%) | 35 (54.8%) | |
| Vascular | 8 (3.4%) | 7 (4.1%) | 1 (1.6%) | |
| MixDem | 20 (8.6%) | 12 (7.1%) | 8 (12.9%) | |
| FTD | 5 (2.2%) | 4 (2.4%) | 1 (1.6%) | |
| PDD | 2 (0.9%) | 1 (0.6%) | 1 (1.6%) | |
| LBD | 18 (7.8%) | 15 (8.8%) | 3 (4.8%) | |
| CBD | 1 (0.4%) | 1 (0.6%) | 0 (0%) | |
| MMSE | 18.8±6.6 | 18.3±6.5 | 20.3±6.6 | 0.04§ |
| ADL | 4.5±1.9 | 4.4±1.9 | 4.6±1.9 | 0.33§ |
| IADL | 3.7±2.7 | 3.7±2.7 | 3.8±2.7 | 0.72§ |
*Chi-square test; §Mann-Whitney test. #Missing data: n = 19. No information about adverse events was available for two vaccinated patients. AD, Alzheimer’s disease; ADL, Activities of Daily Living; CBD, cortico-basal degeneration; FTD, frontotemporal dementia; IADL, Instrumental Activities of Daily Living; LBD, Lewy body dementia; MCI, Mild Cognitive impairment; MixDem, mixed dementia; MMSE, Mini-Mental State Examination; PDD, Parkinson’s disease dementia.
We also observed that the proportion of individuals experiencing general adverse events following the second vaccine dose was higher among those who experienced such effects after the first dose relative to those who did not report any effect (63.2% versus 13.7%, p < 0.001). Accordingly, in the multivariate logistic regression, the occurrence of general side effects after first dose administration was associated with an increased risk of experiencing general adverse events after the second dose (OR 14.41, 95% CI 3.14 – 66.25, p < 0.001). No association was instead found with cognitive and behavioral side effects after first and second dose administration (data available upon request).
DISCUSSION
To the best of our knowledge, the present study represents the first attempt to evaluate the prevalence of COVID-19 vaccination and to describe the short-term adverse events following vaccine administration among individuals with cognitive impairment attending a memory clinic.
Encouragingly, most patients attending our memory clinic were vaccinated at the time of the survey and did not present any vaccine-related adverse events. Specifically, behavioral changes and cognitive worsening only rarely followed vaccination.
At the time of the study conclusion, the vaccine coverage (i.e., the proportion of people who had received two vaccine doses) in the Italian population aged > 12 years was 76.9% [18]. In patients aged 60–69, 70–79, and 80+, the percentage of individuals who had received two vaccine shots was 85.3%, 89.5%, and 92.5%, respectively [18]. The share of patients enrolled in our study in the above-mentioned age groups who had completed the vaccination was 50.0%, 75.7% and 93.1%, thus lower than that observed in the general population. This could reflect the fact that, in Italy, dementia was not included among the conditions prioritized for vaccination [19]. Consequently, most patients received the vaccination at the same time, or even later, than their same-aged cognitively intact counterparts. This finding is also in line with the previous evidence of lower flu and pneumococcal vaccination rates in people with dementia [7, 8].
The incidence of adverse events following vaccination was substantially low. As in the cognitively intact population, the most frequently reported negative effects were mild local and systemic disturbances (e.g., pain at the injection site, fever, fatigue) and their occurrence was more frequent after the second dose. The overall incidence of adverse reactions was higher than that registered by the Italian National Pharmacovigilance Network. As of September 26, 2021, an adverse event reporting rate of 475 reports per 100,000 administered doses was documented in the 60 + population living in the country [20], while the estimate rate of adverse events in our study would theoretically be of 17,872 events per 100,000 administered doses. On the contrary, the frequency of adverse events documented in our population was extremely lower than that reported in phase III RCTs testing COVID-19 vaccines. For instance, local pain, headache, and fatigue occurred after the second vaccine dose in 66%, 39%, and 51% of older participants (i.e., 55+) recruited in the BNT162b2/Comirnaty trial [21] and 83.2%, 46.2%, and 58.3% of older subjects (i.e., 65+) participating to the mRNA-1273/Spikevax trial [22]. These side effects were reported only for 4.6%, 0.5%, and 3.7% of patients in our analysis. Along the same lines, the observed incidence of fever after the second dose (i.e., 5.5%) was approximately half of that documented in these RCTs (10–11%) [21, 22]. These findings are strongly suggestive for under-detection and underreporting of adverse events in people with dementia. Indeed, it is well established that pain and other subjective complaints (e.g., fatigue), potentially constituting side effects of many medications, are less frequently reported (and, thus, often poorly managed) by patients with impaired cognition [23, 24]. However, the accurate and timely detection of pain resulting from the COVID-19 vaccine in these individuals is important as it may be easily resolved with the administration of paracetamol or other painkillers. Conversely, its persistence can exacerbate and/or trigger behavioral disturbances and further increase the discomfort of the patient as well as the caregiver’s burden. The rate of under-detection of pain and other side effects is likely influenced by the severity of cognitive deficits. In this regard, in our study, subjects reporting the occurrence of any adverse events seem to have a tendency towards a better overall cognitive performance relative to those not exhibiting adverse reactions. Another factor that may have contributed to an underestimation of adverse events in our study is the full reliance on the perspective of caregivers, who may not be completely aware of all the symptoms experienced by the assisted patients. Finally, we have no information about the exact time interval between vaccination and data collection. However, we can hypothesize that, in some cases, the interview took place weeks after the vaccination, when the memory of side effects might have faded.
Some limitations of the present study should be acknowledged and discussed. First, the absence of a control group hampered the possibility of comparing the findings observed in cognitively impaired patients with that potentially documented among healthy controls. Moreover, we only considered short-term side effects of COVID-19 vaccination (thus not focusing on possible long-term reactions) without clearly defining a time limit between the vaccination and the onset of side effects. Finally, the study was conducted in a single Italian university memory clinic and considered a limited number of outpatients. Accordingly, the study results may not apply worldwide to all people living with dementia and to all care settings. For instance, different figures of vaccination prevalence and tolerability could be observed among institutionalized patients. However, despite an isolated report of higher mortality rates after BNT162b2/Comirnaty vaccine administration in older people living in Norwegian nursing homes [25], other studies showed good efficacy and tolerability of the same vaccine in institutionalized patients [26, 27].
Conclusions
Overall, the present study indicates that COVID-19 vaccination was safe and well-tolerated in patients with cognitive impairment attending an Italian tertiary memory clinic. Given the limitations of our analysis, further studies are needed to confirm our results in other clinical settings and countries.
However, considering the high risk of experiencing adverse outcomes in the event of SARS-CoV-2 infection and the apparently favorable safety/tolerability profile of available vaccines, people with dementia should be prioritized in the vaccination campaign.
DISCLOSURE STATEMENT
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/22-0077r1).
REFERENCES
[1] | Ge E , Li Y , Wu S , Candido E , Wei X ((2021) ) Association of pre-existing comorbidities with mortality and disease severity among 167,500 individuals with COVID-19 in Canada: A population-based cohort study. PLoS One 16: , e0258154. |
[2] | Liu N , Sun J , Wang X , Zhao M , Huang Q , Li H ((2020) ) The impact of dementia on the clinical outcome of COVID-19: A systematic review and meta-analysis. J Alzheimers Dis 78: , 1775–1782. |
[3] | Canevelli M , Palmieri L , Raparelli V , Lo Noce C , Colaizzo E , Tiple D , Vaianella L , Vanacore N , Brusaferro S , Onder G , Italian National Institute of Health COVID-19 Mortality Group* ((2020) ) Prevalence and clinical correlates of dementia among COVID-19-related deaths in Italy. Alzheimers Dement (Amst) 12: , e12114. |
[4] | Poloni TE , Carlos AF , Cairati M , Cutaia C , Medici V , Marelli E , Ferrari D , Galli A , Bognetti P , Davin A , Cirrincione A , Ceretti A , Cereda C , Ceroni M , Tronconi L , Vitali S , Guaita A ((2020) ) Prevalence and prognostic value of delirium as the initial presentation of COVID-19 in the elderly with dementia: An Italian retrospective study. EClinicalMedicine 26: , 100490. |
[5] | Omer SB , Benjamin RM , Brewer NT , Buttenheim AM , Callaghan T , Caplan A , Carpiano RM , Clinton C , DiResta R , Elharake JA , Flowers LC , Galvani AP , Lakshmanan R , Maldonado YA , McFadden SM , Mello MM , Opel DJ , Reiss DR , Salmon DA , Schwartz JL , Sharfstein JM , Hotez PJ ((2021) ) Promoting COVID-19 vaccine acceptance: Recommendations from the Lancet Commission on Vaccine Refusal, Acceptance, and Demand in the USA. Lancet 398: , 2186–2192. |
[6] | Castilla J , Guevara M , Miqueleiz A , Baigorria F , Ibero-Esparza C , Navascués A , Trobajo-Sanmartín C , Martínez-Baz I , Casado I , Burgui C , Ezpeleta C ((2021) ) Risk factors of infection, hospitalization and death from SARS-CoV-2: A population-based cohort study. J Clin Med 10: , 2608. |
[7] | Ashtarieh B , Grabkowski M , Bartfay E , Sun W ((2022) ) Examining the barriers of influenza vaccine hesitancy in persons with dementia: A literature review. Aging Clin Exp Res 34: , 309–324. |
[8] | Gallini A , Coley N , Andrieu S , Lapeyre-Mestre M , Gardette V ((2017) ) Effect of dementia on receipt of influenza vaccine: A cohort study in French older adults using administrative data: 2007-2012. Fundam Clin Pharmacol 31: , 471–480. |
[9] | Naharci MI , Tasci I ((2021) ) Delirium in a patient with Alzheimer’s dementia following COVID-19 vaccination. Psychogeriatrics 21: , 846–847. |
[10] | Soiza RL , Scicluna C , Thomson EC ((2021) ) Efficacy and safety of COVID-19 vaccines in older people. Age Ageing 50: , 279–283. |
[11] | Wouters OJ , Shadlen KC , Salcher-Konrad M , Pollard AJ , Larson HJ , Teerawattananon Y , Jit M ((2021) ) Challenges in ensuring global access to COVID-19 vaccines: Production, affordability, allocation, and deployment. Lancet 397: , 1023–1034. |
[12] | Albert MS , DeKosky ST , Dickson D , Dubois B , Feldman HH , Fox NC , Gamst A , Holtzman DM , Jagust WJ , Petersen RC , Snyder PJ , Carrillo MC , Thies B , Phelps CH ((2011) ) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: , 270–279. |
[13] | McKhann GM , Knopman DS , Chertkow H , Hyman BT , Jack CR , Kawas CH , Klunk WE , Koroshetz WJ , Manly JJ , Mayeux R , Mohs RC , Morris JC , Rossor MN , Scheltens P , Carrillo MC , Thies B , Weintraub S , Phelps CH ((2011) ) The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: , 263–269. |
[14] | Mathieu E , Ritchie H , Ortiz-Ospina E , Roser M , Hasell J , Appel C , Giattino C , Rodés-Guirao L ((2021) ) A global database of COVID-19 vaccinations. Nat Hum Behav 5: , 947–953. |
[15] | Folstein MF , Folstein SE , McHugh PR ((1975) ) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: , 189–198. |
[16] | Katz S , Ford AB , Moskowitz RW , Jackson BA , Jaffe MW ((1963) ) Studies of illness in the aged. The index of ADL: A standardized measure of biological and psychosocial function. JAMA 185: , 914–919. |
[17] | Katz S ((1983) ) Assessing self-maintenance: Activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc 31: , 721–727. |
[18] | Task force COVID-19 del Dipartimento Malattie Infettive e Servizio di Informatica, Istituto Superiore di Sanità. Epidemia COVID-19. Aggiornamento nazionale: 22 settembre (2021) . |
[19] | ((2021) ) Vaccinazione anti-SARS-CoV-2/COVID-19. Raccomandazioni ad interim sui gruppi target della vaccinazione anti-SARS-CoV-2/COVID-19. Gazzetta Ufficiale della Repubblica Italiana. |
[20] | AIFA, Agenzia Italiana del Farmaco Rapporto sulla Sorveglianza dei vaccini COVID-19. |
[21] | Polack FP , Thomas SJ , Kitchin N , Absalon J , Gurtman A , Lockhart S , Perez JL , Pérez Marc G , Moreira ED , Zerbini C , Bailey R , Swanson KA , Roychoudhury S , Koury K , Li P , Kalina WV , Cooper D , Frenck RW , Hammitt LL , Türeci Ö , Nell H , Schaefer A , Ünal S , Tresnan DB , Mather S , Dormitzer PR , Şahin U , Jansen KU , Gruber WC , C4591001 Clinical Trial Group ((2020) ) Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med 383: , 2603–2615. |
[22] | Baden LR , El Sahly HM , Essink B , Kotloff K , Frey S , Novak R , Diemert D , Spector SA , Rouphael N , Creech CB , McGettigan J , Khetan S , Segall N , Solis J , Brosz A , Fierro C , Schwartz H , Neuzil K , Corey L , Gilbert P , Janes H , Follmann D , Marovich M , Mascola J , Polakowski L , Ledgerwood J , Graham BS , Bennett H , Pajon R , Knightly C , Leav B , Deng W , Zhou H , Han S , Ivarsson M , Miller J , Zaks T , COVE Study Group ((2021) ) Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 384: , 403–416. |
[23] | Lichtner V , Dowding D , Esterhuizen P , Closs SJ , Long AF , Corbett A , Briggs M ((2014) ) Pain assessment for people with dementia: A systematic review of systematic reviews of pain assessment tools. BMC Geriatr 14: , 138. |
[24] | Jensen-Dahm C , Werner MU , Jensen TS , Ballegaard M , Andersen BB , Høgh P , Waldemar G ((2015) ) Discrepancy between stimulus response and tolerance of pain in Alzheimer disease. Neurology 84: , 1575–1581. |
[25] | Wyller TB , Kittang BR , Ranhoff AH , Harg P , Myrstad M ((2021) ) Nursing home deaths after COVID-19 vaccination. Tidsskr Nor Laegeforen, 141. doi: 10.4045/tidsskr.21.0383. |
[26] | Bardenheier BH , Gravenstein S , Blackman C , Gutman R , Sarkar IN , Feifer RA , White EM , McConeghy K , Nanda A , Mor V ((2021) ) Adverse events following mRNA SARS-CoV-2 vaccination among U.S. nursing home residents. Vaccine 39: , 3844–3851. |
[27] | Salmerón Ríos S , Mas Romero M , Cortés Zamora EB , Tabernero Sahuquillo MT , Romero Rizos L , Sánchez-Jurado PM , Sánchez-Nievas G , Señalada JJB , García Nogueras I , Estrella Cazalla J de D , Andrés-Pretel F , Murillo Romero A , Lauschke VM , Stebbing J , Abizanda P ((2021) ) Immunogenicity of the BNT162b2 vaccine in frail or disabled nursing home residents: COVID-A study. J Am Geriatr Soc 69: , 1441–1447. |
[28] | Sadoff J , Gray G , Vandebosch A , Cárdenas V , Shukarev G , Grinsztejn B , Goepfert PA , Truyers C , Fennema H , Spiessens B , Offergeld K , Scheper G , Taylor KL , Robb ML , Treanor J , Barouch DH , Stoddard J , Ryser MF , Marovich MA , Neuzil KM , Corey L , Cauwenberghs N , Tanner T , Hardt K , Ruiz-Guiñazú J , Le Gars M , Schuitemaker H , Van Hoof J , Struyf F , Douoguih M , ENSEMBLE Study Group ((2021) ) Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med 384: , 2187–2201. |
[29] | Falsey AR , Sobieszczyk ME , Hirsch I , Sproule S , Robb ML , Corey L , Neuzil KM , Hahn W , Hunt J , Mulligan MJ , McEvoy C , DeJesus E , Hassman M , Little SJ , Pahud BA , Durbin A , Pickrell P , Daar ES , Bush L , Solis J , Carr QO , Oyedele T , Buchbinder S , Cowden J , Vargas SL , Guerreros Benavides A , Call R , Keefer MC , Kirkpatrick BD , Pullman J , Tong T , Brewinski Isaacs M , Benkeser D , Janes HE , Nason MC , Green JA , Kelly EJ , Maaske J , Mueller N , Shoemaker K , Takas T , Marshall RP , Pangalos MN , Villafana T , Gonzalez-Lopez A , AstraZeneca AZD1222 Clinical Study Group ((2021) ) Phase 3 safety and efficacy of AZD1222 (ChAdOx1 nCoV-19) Covid-19 vaccine. N Engl J Med 385: , 2348–2360. |
[30] | Ella R , Reddy S , Blackwelder W , Potdar V , Yadav P , Sarangi V , Aileni VK , Kanungo S , Rai S , Reddy P , Verma S , Singh C , Redkar S , Mohapatra S , Pandey A , Ranganadin P , Gumashta R , Multani M , Mohammad S , Bhatt P , Kumari L , Sapkal G , Gupta N , Abraham P , Panda S , Prasad S , Bhargava B , Ella K , Vadrevu KM , COVAXIN Study Group ((2021) ) Efficacy, safety, and lot-to-lot immunogenicity of an inactivated SARS-CoV-2 vaccine (BBV152): Interim results of a randomised, double-blind, controlled, phase 3 trial. Lancet 398: , 2173–2184. |
[31] | Tanriover MD , Doğanay HL , Akova M , Güner HR , Azap A , Akhan S , Köse Ş , Erdinç FŞ , Akalın EH , Tabak ÖF , Pullukçu H , Batum Ö , Şimşek Yavuz S , Turhan Ö , Yıldırmak MT , Köksal İ , Taşova Y , Korten V , Yılmaz G , Çelen MK , Altın S , Çelik İ , Bayındır Y , Karaoğlan İ , Yılmaz A , Özkul A , Gür H , Unal S , CoronaVac Study Group ((2021) ) Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): Interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet 398: , 213–222. |
[32] | Al Kaabi N , Zhang Y , Xia S , Yang Y , Al Qahtani MM , Abdulrazzaq N , Al Nusair M , Hassany M , Jawad JS , Abdalla J , Hussein SE , Al Mazrouei SK , Al Karam M , Li X , Yang X , Wang W , Lai B , Chen W , Huang S , Wang Q , Yang T , Liu Y , Ma R , Hussain ZM , Khan T , Saifuddin Fasihuddin M , You W , Xie Z , Zhao Y , Jiang Z , Zhao G , Zhang Y , Mahmoud S , ElTantawy I , Xiao P , Koshy A , Zaher WA , Wang H , Duan K , Pan A , Yang X ((2021) ) Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: A randomized clinical trial. JAMA 326: , 35–45. |
[33] | Logunov DY , Dolzhikova IV , Shcheblyakov DV , Tukhvatulin AI , Zubkova OV , Dzharullaeva AS , Kovyrshina AV , Lubenets NL , Grousova DM , Erokhova AS , Botikov AG , Izhaeva FM , Popova O , Ozharovskaya TA , Esmagambetov IB , Favorskaya IA , Zrelkin DI , Voronina DV , Shcherbinin DN , Semikhin AS , Simakova YV , Tokarskaya EA , Egorova DA , Shmarov MM , Nikitenko NA , Gushchin VA , Smolyarchuk EA , Zyryanov SK , Borisevich SV , Naroditsky BS , Gintsburg AL , Gam-COVID-Vac Vaccine Trial Group ((2021) ) Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 397: , 671–681. |
[34] | Heath PT , Galiza EP , Baxter DN , Boffito M , Browne D , Burns F , Chadwick DR , Clark R , Cosgrove C , Galloway J , Goodman AL , Heer A , Higham A , Iyengar S , Jamal A , Jeanes C , Kalra PA , Kyriakidou C , McAuley DF , Meyrick A , Minassian AM , Minton J , Moore P , Munsoor I , Nicholls H , Osanlou O , Packham J , Pretswell CH , San Francisco Ramos A , Saralaya D , Sheridan RP , Smith R , Soiza RL , Swift PA , Thomson EC , Turner J , Viljoen ME , Albert G , Cho I , Dubovsky F , Glenn G , Rivers J , Robertson A , Smith K , Toback S , 2019nCoV-302 Study Group ((2021) ) Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N Engl J Med 385: , 1172–1183. |



