Psychosocial Effects of COVID-19 Measures on (Pre-)Dementia Patients During Second Lockdown
Abstract
Background:
The COVID-19 pandemic poses enormous social challenges, especially during lockdown. People with cognitive decline and their caregivers are particularly at risk of lockdown consequences.
Objective:
To investigate psychosocial effects in (pre-)dementia patients and caregivers during second lockdown and compare effects between first and second lockdown.
Methods:
We included n = 511 (pre-)dementia patients and n = 826 caregivers from the Amsterdam Dementia Cohort and via Alzheimer Nederland. All respondents completed a self-designed survey on psychosocial effects of COVID-19. We examined relations between experienced support and psychosocial and behavioral symptoms using logistic regression. In a subset of patients and caregivers we compared responses between first and second lockdown using generalized estimating equation (GEE).
Results:
The majority of patients (≥58%) and caregivers (≥60%) reported that family and friends, hobbies, and music helped them cope. Support from family and friends was strongly related to less negative feelings in patients (loneliness: OR = 0.3[0.1–0.6]) and caregivers (loneliness: OR = 0.2[0.1–0.3]; depression: OR = 0.4[0.2–0.5]; anxiety: OR = 0.4[0.3–0.6]; uncertainty: OR = 0.3[0.2–0.5]; fatigue: OR = 0.3[0.2–0.4]; stress: OR = 0.3[0.2–0.5]). In second lockdown, less psychosocial and behavioral symptoms were reported compared to first lockdown (patients; e.g., anxiety: 22% versus 13%, p = 0.007; apathy: 27% versus 8%, p < 0.001, caregivers; e.g., anxiety: 23% versus 16%, p = 0.033; patient’s behavioral problems: 50% versus 35%, p < 0.001). Patients experienced more support (e.g., family and friends: 52% versus 93%, p < 0.001; neighbors: 28% versus 66%, p < 0.001).
Conclusion:
During second lockdown, patients and caregivers adapted to challenges posed by lockdown, as psychosocial and behavioral effects decreased, while patients experienced more social support compared to first lockdown. Support from family and friends is a major protective factor for negative outcomes in patients and caregivers.
INTRODUCTION
The COVID-19 pandemic poses enormous social challenges, especially during lockdown when social distancing is imposed [1]. Older people are particularly at risk of the consequences of COVID-19: they have direct risk of severe COVID-19 symptoms, and they are at risk of social isolation, as they are more likely to live alone, and less often use online communication tools [1–3]. Moreover, people with cognitive decline and dementia and their caregivers are affected by disrupted support services [1, 4, 5]. Decrease in structure and routine, and the closure of facilities and services, may cause distress and anxiety for both people with cognitive decline and dementia, as well as for their caregivers [4, 6, 7]. This loss of structure and support in daily life constitutes a risk of swifter cognitive deterioration [4, 5,8, 9].
In December 2020, the second wave of COVID-19 reached the Netherlands, and the government issued a second lockdown. This second lockdown was more strict than the first, as restrictions on social contact and reduced access to services were more severe: non-essential stores were closed, the number of visitors at home was reduced to one visitor a day, and from 23 January 2021, a curfew was imposed [10]. This time, society was better prepared when the second lockdown started. More health care services (e.g., day care) remained open, leading to a decrease in caregivers’ burden. By contrast, the informal support network of children, neighbors, and volunteers was still largely ineffective as a result of the measures on social contact [10].
Previous studies conducted during the first lockdown in 2020 report more cognitive decline, worsened neuropsychiatric symptoms, and increased caregiver burden in patients with dementia [8, 11–14]. Even in the general population, studies found that restrictive measures negatively affected perceived cognition [15–17]. Moreover, we found that these changes are not restricted to the dementia stage, but also occur in patients with mild cognitive impairment (MCI) and subjective cognitive decline (SCD) [8]. With the onset of the second lockdown, we aimed to investigate psychosocial and behavioral effects, experienced social support, and discontinuation of care in a large sample of (pre-)dementia patients and caregivers. We deliberately focused on potential positive aspects such as experienced support and activities that helped most to endure lockdown. In addition, we compared responses between first and second lockdown to investigate the long-term effects of COVID-19 times.
METHODS
Participants
Between 22 December 2020 and 22 March 2021, we invited memory clinic patients (SCD, MCI, and dementia) and caregivers to complete a self-designed COVID-19 survey. Patients were recruited if they were actively enrolled in one of the following four ongoing sub studies of the Amsterdam Dementia cohort (ADC) [18, 19]: 1) SCIENCe project – including individuals with a diagnosis of SCD [20]. All participants attended our memory clinic for cognitive complaints, but performed normal on cognitive testing; 2) DEvELOP project – including patients with a diagnosis of dementia with Lewy bodies (DLB) [21]; 3) ABIDE-PET project [22]; and 4) ADDITION project, ABIDE-PET and ADDITION included patients with different types of dementia, MCI, and SCD. In total, n = 1,504 patients were invited, of which n = 511 (34%) responded.
Loved ones and informal caregivers of these patients were invited to complete a similar COVID-19 survey, with additional questions on caregiver burden. In total, n = 366 caregivers (n = 204 patient-caregiver dyads, n = 162 caregiver only) participated. A subset of ADC patients (n = 196) and caregivers (n = 178) completed a similar survey on the psychosocial effects of COVID-19 measures during the first lockdown in the Netherlands, three to six months earlier [8].
To extend our findings with reports of caregivers of patients in a more severe disease stage and foster generalizability across the Netherlands, we additionally recruited caregivers via Alzheimer Nederland (Dutch association for people living with dementia). N = 460 caregivers completed the survey via Alzheimer Nederland.
The study was approved by the local Medical Ethical Committee. All patients provided written informed consent for their clinical data to be used for research purposes.
Survey on psychosocial effects of COVID-19 measures in second lockdown
The survey (see Supplementary Material) contained questions on COVID-19 infection, psychosocial and behavioral effects of lockdown measures, worries for cognitive decline, experienced (in)formal support, and (dis)continuation of care. Questions regarding COVID-19 infection consisted of questions on being infected with COVID-19 and worries for a possible COVID-19 infection. With regard to psychosocial and behavioral effects, questions were included on loneliness, anxiety, uncertainty, depression, apathy, change in sleeping behavior, fatigue, stress, and patient’s behavioral and repetitive behavior (the latter two caregiver only). With regard to experienced support, we included questions on support from the general practitioner (GP), home care, day care, case manager, volunteers, neighbors, family and friends, sports, music, and hobbies. Finally, questions regarding (dis)continuation of care consisted of continuation of day care and (digital or physical) visits to the GP or hospital. The survey was designed together with professionals in the dementia field (health care professionals and employees of Alzheimer Nederland, the Dutch patient and caregiver association). To promote an inclusive approach, the survey was tested and adapted by Pharos and Stichting ABC (i.e., a Dutch association for low literacy people) ensuring the language used was appropriate for at least B1 proficiency. All questions were measured using either categorical (for example: less lonely/I feel the same/I do not know/I am never lonely) or dichotomous (for example: yes/no) answers options (see Supplementary Material). Survey questions contained two to five answer options. In case a question contained three or more answers, these answers were dichotomized into present if participants agreed or completely agreed with a statement, and absent if disagreed or completely disagreed.
Statistical analyses
For analyses, we analyzed respondents as four groups: SCD subjects, patients with MCI or dementia, ADC caregivers, and caregivers of Alzheimer Nederland. Descriptive statistics were used to report on frequencies of COVID-19 infection, psychosocial and behavioral effects, experienced support, and (dis)continuation of care. We explored possible relationships between psychosocial or behavioral variables and support variables using logistic regression analyses (separately for patients and caregivers). We made 64 comparisons, and we applied an FDR correction. Differences in the frequencies of reported psychosocial and behavioral effects and experienced support between first and second lockdown were compared using a generalized estimating equation (GEE) analysis. Analyses were conducted both unadjusted, and adjusted for age, sex education level (patients only), and patient/caregiver subgroup (patients: SCD and MCI/dementia; caregivers: ADC and Alzheimer Nederland). Significance was set at p < 0.05 level. All analyses were carried out in SPSS Statistics version 26.
RESULTS
Descriptive statistics
Patient and caregiver characteristics are summarized in Table 1. MCI/dementia patients were slightly older than individuals with SCD. Caregivers of Alzheimer Nederland took more frequently care of a patient in a dementia stage than ADC caregivers.
Table 1
Patient and caregiver characteristics
| SCD ADC | MCI/dementia ADC | Caregivers ADC | Caregivers AN | ||
| n = 307 (60%) | n = 204 (40%) | n = 366 (100%) | n = 460 (100%) | ||
| Age | 66±8 | 69±7 | NA | 277 | 67±11 |
| Sex, F (%) | 126 (41%) | 78 (38%) | NA | 449 | 282 (61%) |
| Education level (Verhage) | 5.7±1 | 5.4±1 | NA | NA | |
| Diagnosis of patient | 205 | ||||
| SCD | 307 (100%) | NA | 83 (23%) | 0 | |
| MCI | NA | 85 (42%) | 53 (15%) | 3 (2%) | |
| Dementia | NA | 119 (58%) | 218 (60%) | 187 (91%) | |
| Other | 12 (3%) | 15 (7%) | |||
| Living status | |||||
| With partner/family | 224 (78%) | 181 (91%) | |||
| Alone | 65 (22%) | 17 (9%) | |||
ADC, Amsterdam Dementia Cohort; AN, Alzheimer Nederland; MCI, mild cognitive impairment; SCD, subjective cognitive decline.
A small proportion of six percent of participants reported they had probably been infected with COVID-19 (patients n = 32 (6%); caregivers ADC: n = 22 (6%); caregivers Alzheimer Nederland: n = 29 (6%)). In n = 27 (5%) patients and n = 42 caregivers (ADC: n = 17 (5%); Alzheimer Nederland: n = 25 (5%)) the infection was confirmed with a COVID-19 test performed by a GP or Municipal Health Service. One third of patients (MCI/dementia: n = 64 (31%); SCD: n = 101 (33%)) and a slightly higher proportion of caregivers (ADC: n = 131 (36%); Alzheimer Nederland: n = 185 (40%)) reported that they feared to get infected with COVID-19 themselves. Moreover, half of the caregivers reported anxiety for the patient to get a COVID-19 infection (ADC: n = 205 (56%); Alzheimer Nederland: n = 262 (57%)). Over one third of the caregivers reported worries for faster cognitive decline in their beloved ones (ADC: n = 103 (38%); Alzheimer Nederland: n = 202 (44%)). These worries were also reported by some patients themselves (MCI/dementia: n = 28 (14%); SCD: n = 34 (11%)).
Psychosocial effects
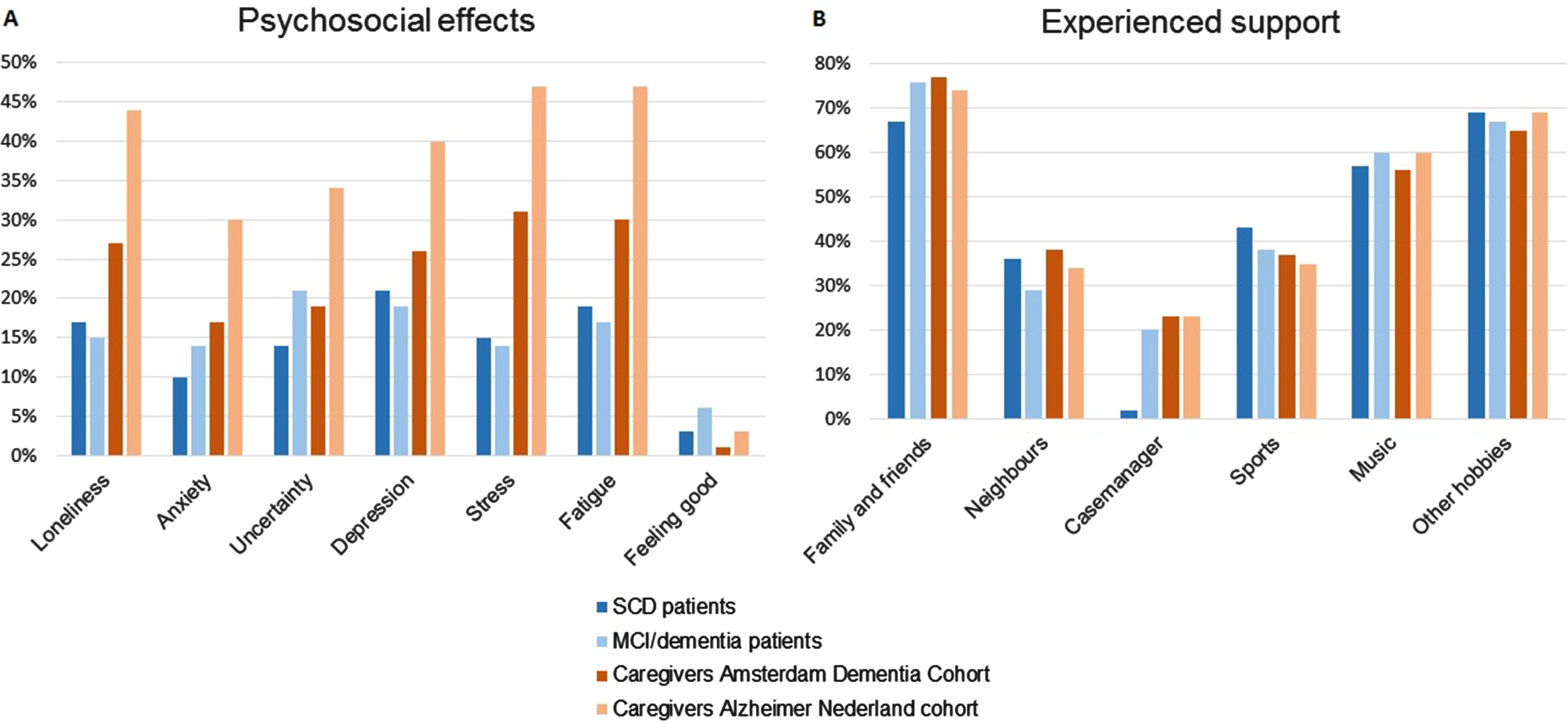
Figure 1A presents the self-reported psychosocial effects by patients and caregivers. On visual inspection, psychosocial effects were most frequently reported by caregivers, especially by caregivers of Alzheimer Nederland, almost half of which reported feelings of loneliness, stress, and fatigue. Each of the psychosocial effects under study was reported by 10–21% of patients, who relatively often mentioned feelings of uncertainty and depression.
Fig. 1
Self-reported psychosocial effects (A) and experienced support (B) in patients and caregivers.
Behavioral effects
We asked caregivers whether they saw an increase in behavioral symptoms in the patient (any behavioral problems, and specifically apathy, sleeping behavior, repetitive behavior and aggression). Highest frequencies for patient’s behavioral symptoms were reported by caregivers of Alzheimer Nederland (caregivers ADC: 13–32%; caregivers Alzheimer Nederland: 22–49%).
When we asked patients themselves about increase in apathy and change in sleeping behavior, a small group reported an increase. SCD subjects reported an increase in apathy twice as often as MCI/dementia patients (SCD: n = 32 (10%); MCI/dementia: n = 10 (5%)). Out of five reported changes in sleeping behavior (SCD: n = 58 (19%); MCI/dementia: n = 36 (18%)).
Experienced support and (dis)continuation of care
More than a quarter of caregivers reported to feel extensively burdened with giving care to the patient during lockdown (ADC: n = 98 (27%); Alzheimer Nederland: n = 157 (34%)). However, the majority of caregivers reported getting enough help in patient caregiving (ADC: n = 312 (85%); Alzheimer Nederland: n = 346 (75%)).
Over one third of patients (SCD: n = 96 (31%); MCI/dementia: n = 87 (43%)) and caregivers (ADC: n = 145 (40%); Alzheimer Nederland: n = 203 (44%)) reported to have had a physical health care appointment with regard to the patient in times of COVID-19, which they experienced as positive or neutral. In addition, a quarter of ADC patients (SCD: n = 69 (23%); MCI/dementia: n = 52 (25%)) and caregivers (ADC: n = 83 (23%); Alzheimer Nederland: n = 53 (12%)) had a digital health care appointment with regard to the patient in times of COVID-19, equally experienced as positive or neutral. For a subgroup of MCI/dementia patients (n = 36 (18%)) and caregivers (ADC: n = 67 (18%); Alzheimer Nederland: n = 131 (29%)), day care for patients continued during lockdown. Others report that an alternative for discontinued day care during lockdown had been offered (MCI/dementia patients (n = 13 (6%) and caregivers (ADC: n = 39 (11%); Alzheimer Nederland: n = 85 (19%)).
When we asked patients and caregivers what helped them cope with COVID-19 times, the majority of the respondents reported friends and family, practicing hobbies, and playing or listening to music helped them cope with COVID-19 times (Fig. 1B). Also, neighbors and practicing sports were frequently mentioned in helping to cope with COVID-19 times.
Table 2 shows the odds ratios and corresponding 95% confidence intervals between experienced support and psychosocial and behavioral symptoms in patients and caregivers during the second lockdown. We found that support from family and friends was strongly related to decreased levels of loneliness in patients, and decreased levels of loneliness, anxiety, uncertainty, depression, fatigue, and stress in caregivers. Logistic regression models remained largely unchanged after adjusting for age, sex, education level, and patient/caregiver subgroup.
Table 2
Odds ratios between experienced support and psychosocial and behavioral symptoms in patients and caregivers during second lockdown
| Loneliness | Anxiety | Uncertainty | Depression | Fatigue | Stress | Apathy in patient | Sleeping behavior in patient | ||
| OR [95% CI] | OR [95% CI] | OR [95% CI] | OR [95% CI] | OR [95% CI] | OR [95% CI] | OR [95% CI] | OR [95% CI] | ||
| Support GP | Patients | 0.9 [0.6–1.5] | 0.9 [0.5–1.5] | 0.9 [0.6–1.4] | 0.8 [0.5–1.3] | 1.1 [0.7–1.7] | 0.6 [0.3–0.9] | 1.3 [0.7–2.4] | 1.0 [0.7–1.6] |
| Caregivers | 0.9 [0.6–1.1] | 1.0 [0.7–1.4] | 0.9 [0.6–1.2] | 0.9 [0.7–1.2] | 1.0 [0.8–1.4] | 1.0 [0.8–1.3] | 1.0 [0.7–1.3] | 1.2 [0.9–1.6] | |
| Support home care | Patients | 1.1 [0.5–2.3] | 1.1 [0.5–2.5] | 1.2 [0.6–2.5] | 1.0 [0.5–2.0] | 0.9 [0.5–1.9] | 0.9 [0.4–2.0] | 1.3 [0.5–3.3] | 1.3 [0.7–2.6] |
| Caregivers | 1.2 [0.8–1.6] | 1.1 [0.7–1.5] | 1.3 [0.9–1.8] | 1.2 [0.9–1.7] | 1.5 [1.1–2.1] | 1.3 [0.9–1.8] | 0.9 [0.6–1.3] | 1.3 [0.9–1.8] | |
| Support day care | Patients | 1.5 [0.8–3.1] | 1.1 [0.5–2.6] | 1.3 [0.6–2.7] | 0.9 [0.4–1.8] | 0.8 [0.3–1.7] | 0.6 [0.2–1.4] | 1.1 [0.4–3.0] | 0.9 [0.4–1.8] |
| Caregivers | 1.2 [0.9–1.7] | 1.1 [0.7–1.5] | 1.2 [0.9–1.7] | 1.4 [1.0–1.9] | 1.5 [1.1–2.1] | 1.6 [1.2–2.2] | 1.1 [0.7–1.6] | 1.8 [1.3–2.4] | |
| Support volunteers | Patients | 1.1 [0.5–2.6] | 0.5 [0.2–1.7] | 0.8 [0.3–1.9] | 0.6 [0.2–1.4] | 0.8 [0.4–1.9] | 0.4 [0.1–1.3] | 0.5 [0.1–2.1] | 1.0 [0.4–2.1] |
| Caregivers | 1.0 [0.7–1.5] | 0.7 [0.4–1.2] | 1.2 [0.8–1.8] | 0.9 [0.6–1.3] | 1.1 [0.8–1.6] | 0.9 [0.6–1.4] | 0.8 [0.5–1.3] | 0.9 [0.6–1.4] | |
| Support case manager | Patients | 1.1 [0.6–1.8] | 0.9 [0.5–1.8] | 1.3 [0.8–2.2] | 1.0 [0.6–1.6] | 0.9 [0.5–1.5] | 0.9 [0.5–1.6] | 0.8 [0.3–1.7] | 1.0 [0.6–1.7] |
| Caregivers | 0.9 [0.7–1.2] | 0.6 [0.5–0.9] | 0.8 [0.6–1.1] | 0.7 [0.6–1.0] | 1.1 [0.8–1.5] | 1.1 [0.8–1.5] | 0.9 [0.7–1.3] | 1.1 [0.8–1.5] | |
| Support neighbors | Patients | 0.5 [0.3–0.9] | 1.0 [0.5–1.7] | 0.6 [0.4–1.1] | 0.7 [0.5–1.2] | 0.7 [0.4–1.1] | 0.7 [0.4–1.3] | 0.6 [0.3–1.2] | 0.8 [0.5–1.4] |
| Caregivers | 0.7 [0.6–1.0] | 0.9 [0.6–1.3] | 0.8 [0.6–1.2] | 0.7 [0.5–1.0] | 0.8 [0.6–1.1] | 0.7 [0.5–0.9] | 0.8 [0.6–1.1] | 0.8 [0.6–1.1] | |
| Support family and friends | Patients | 0.3 [0.1–0.6]* | 0.4 [0.2–0.8] | 0.5 [0.2–1.1] | 0.3 [0.1–0.6] | 0.7 [0.3–1.5] | 0.6 [0.3–1.4] | 0.3 [0.1–0.7] | 0.5 [0.2–1.1] |
| Caregivers | 0.2 [0.1–0.3]* | 0.4 [0.3–0.6]* | 0.3 [0.2–0.5]* | 0.4 [0.2–0.5]* | 0.3 [0.2–0.4]* | 0.3 [0.2–0.5]* | 0.6 [0.4–0.9] | 0.6 [0.4–0.8] | |
| Help from hobbies | Patients | 0.5 [0.3–0.9] | 0.8 [0.4–1.3] | 1.1 [0.7–1.9] | 0.7 [0.5–1.1] | 0.9 [0.6–1.4] | 0.8 [0.5–1.3] | 0.7 [0.3–1.3] | 0.9 [0.6–1.5] |
| Caregivers | 0.6 [0.5–0.9] | 0.8 [0.5–1.0] | 0.9 [0.6–1.2] | 0.8 [0.6–1.0] | 0.8 [0.6–1.1] | 0.9 [0.7–1.2] | 1.2 [0.8–1.7] | 1.0 [0.7–1.3] |
All analyses were performed on the same group of patients (n = 511) and caregivers (n = 826). p < 0.05 in bold. *Significant after FDR correction (p < 0.00078125). GP, general practitioner; OR, odds ratio; 95% CI, 95% confidence interval.
Compared to first lockdown
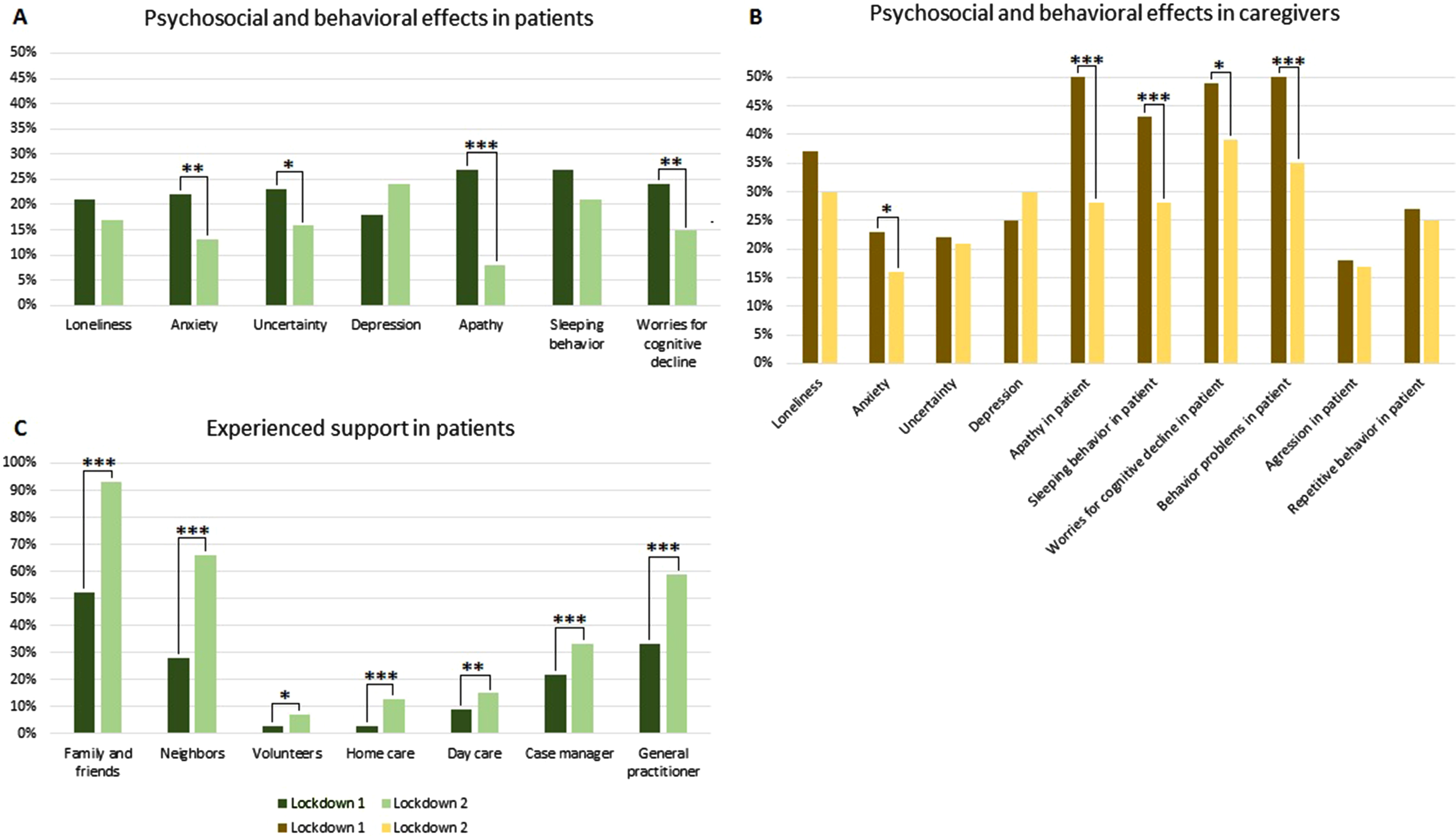
We compared self-reported psychosocial and behavioral impacts of the lockdown measures between first and second lockdown in a subgroup of patients (age = 68±6, 37% F, education level = 5±1; n = 66 (34%) SCD and n = 130 (66%) MCI/dementia) and caregivers (n = 178). We observed a consistent decline in the frequency of reported psychosocial and behavioral effects compared to the first lockdown, by both patients (anxiety: 22% versus 13%, p = 0.007; uncertainty: 23% versus 16%, p = 0.022; apathy: 27% versus 8%, p < 0.001; worries for faster cognitive decline: 24% versus 15%, p = 0.008) and caregivers (anxiety: 23% versus 16 %, p = 0.033; apathy in patient: 50% versus 28%, p < 0.001; sleeping behavior in patient: 43% versus 28%, p < 0.001; worries for faster cognitive decline in patient: 49% versus 39%, p = 0.017; behavioral problems in patient: 50% versus 35%, p < 0.001) of the ADC (Fig. 2A, B). By contrast, the proportion of patients that feared a COVID-19 infection increased (5% versus 32%, p < 0.001). This increase was also observed on the caregiver level with regard to fear for the patient getting infected (43% versus 56%, p = 0.001). Patients reported to have experienced more formal and informal support compared to the first lockdown (family and friends: 52% versus 93%, p < 0.001; neighbors: 28% versus 66%, p < 0.001; volunteers: 3% versus 7%, p = 0.025; home care: 3% versus 13%, p < 0.001; day care: 9% versus 15%, p = 0.001; case manager: 22% versus 33%, p < 0.001; GP: 33% versus 59%, p < 0.001), see Fig. 2C. GEE results remained essentially unchanged after adjusting for age, sex, and education level.
Fig. 2
Self-reported psychosocial and behavioral effects (A and B) and experienced support (C) during first and second lockdown in patients and caregivers of the Amsterdam Dementia Cohort (*p < 0.05, **p < 0.01, ***p < 0.001).
DISCUSSION
The main findings of this study are that in the second lockdown, both patients and caregivers reported less psychosocial and behavioral symptoms compared to first lockdown, and patients experienced more social support. Family and friends, hobbies, and music were important factors that helped coping with COVID-19 times. Support of family and friends was also strongly related to less experience of negative feelings in patients and caregivers.
Our results extend on earlier findings, that report similarly increased levels of psychological symptoms in caregivers and increased behavioral problems in dementia patients during the first COVID-19 lockdown [13, 23]. In addition, we extend on former studies, as we deliberately asked patients and caregivers to report what helped them during lockdown, to identify ways to boost resilience in patients and caregivers. Next to social support, patients and caregivers frequently reported that hobbies and music helped them cope with COVID-19 times. Previous studies show that social support and practicing hobbies are positively related to well-being and quality of life in dementia patients and their caregivers [24–27]. Additionally, next to giving joy, practicing hobbies gives dementia patients meaning to a day and a day schedule. These findings have important implications, as we found that experienced social support and practicing hobbies increased during second lockdown compared to first lockdown. In addition, psychosocial and behavioral symptoms in patients and experienced burden in caregivers decreased during second lockdown. As we see that support is a major protective factor for negative psychosocial and behavioral outcomes, especially support from family and friends, it is conceivable that increased experienced support contribute to lower levels psychosocial and behavioral symptoms and burden in caregivers. We are sharing this knowledge nationally via an online platform for dementia patients and their caregivers.
We were pleasantly surprised by our finding that reported detrimental effects in psychosocial and behavioral symptoms declined, while experienced support increased between first and second lockdown. This might be explained by several factors. Firstly, society in general may have adapted to the “new normal”, where people have found ways to deal with the lockdown measures. Patients and caregivers may have been better prepared for a second lockdown, resulting in a decrease in psychosocial and behavioral symptoms. Secondly, there was more health care continuation during second lockdown compared to first lockdown in the Netherlands. In the first lockdown, all non-acute healthcare appointments were cancelled and day care discontinued, leading to havoc among patients and families with high need of care. We found that during second lockdown, a substantial number of patients and caregivers could continue their healthcare appointments (physically and/or digitally) and day care. This fits with the notion that patient and caregiver support was better organized compared to first lockdown and illustrates that even for vulnerable populations like patients with dementia and their families, digital technology can provide helpful alternatives for in person meetings. Previous studies show that well organized health care and support systems, like day care and case management, are essential to keep behavioral symptoms at minimum and decrease caregiver burden [28–30]. Finally, we suspect that informal care networks also adapted to the lockdown situation and found new ways of continuing their support, as (pre-)dementia patients reported both more formal and informal support compared to the first lockdown.
Among the limitations of the current study is a potential selection bias, due to the online survey approach. The accessibility of the online survey might be reduced for people with suboptimal technical knowledge, cognitive complaints, or migration background. However, with help of Pharos and Stichting ABC, we ensured the survey’s language was appropriate for low literacy people. Furthermore, the online survey system allows us to include a large group of patients and caregivers despite social distancing and reduced social contact. To counteract the fact that minorities might be less represented by our online approach, we are currently conducting interviews with patients and caregivers with a migrationbackground, on their experiences in COVID-19 times. Another limitation of this study is that all patients were included in a tertiary memory clinic, which might not give a general representation of (pre-)dementia patients in the Netherlands. Of note, the caregiver sample of Alzheimer Nederland is representative of the average dementia patient and results from this sample suggest that our findings might in fact be an underestimation. Finally, important to keep in mind is the variability in COVID-19 restrictions across various lockdowns within and between countries during lockdown. Governments across different countries worldwide issued different restrictions to counter COVID-19. Within the Netherlands, restrictions varied greatly between the first and second lockdown. Furthermore, COVID-19 is not behind us yet, as in the Netherlands and many countries we are now at the end of 2021 facing a third lockdown. Therefore, comparison with studies focusing on lockdown in other time periods or other countries must be done with caution. On the other hand, we feel that this also stresses the timeliness and relevance of our results, as they illustrate that people do adjust to the challenges posed by the COVID-19 pandemic and the COVID-19-related measures. An important factor may be that in the second lockdown, there was less discontinuation of care. Moreover, support from family and friends are of great relevance in helping caregivers through COVID-19 times.
Among the strengths of our study is the large sample of both caregivers and patients along the cognitive continuum. Caregivers were also included via Alzheimer Nederland, increasing generalizability across the Netherlands and to patients with a more severe disease stage. In addition, we were able to compare results between the first and second lockdown, which provided important insights into how patients and caregivers adapt to the challenges posed by COVID-19. Finally, we explored factors that have helped patients and caregivers cope with COVID-19 times, which we will use as input for the development of tools and recommendations to render patients and caregivers more resilient to challenges posed by the pandemic, that we will disseminate via online articles, webinars, and toolkits with tips for patients, caregivers, and professional support in times of COVID-19. In addition, we will spread our knowledge to government agencies by info sheets and reports on people living with dementia in COVID-19 times to emphasize the importance of good health care support. Of note, it could be that our results are applicable to other individuals with other diseases as well. Finally, to make sure that the results of our study are available to all communities within the Netherlands, we develop video clips in multiple languages on lessons learned about dementia and the pandemic. All tools and recommendations focus on increasing resilience to COVID-19 challenges in cognitively impaired individuals and their caregivers, and therefore have important clinical impact.
ACKNOWLEDGMENTS
Research of Alzheimer center Amsterdam is part of the neurodegeneration research program of Amsterdam Neuroscience. Alzheimer Center Amsterdam is supported by Stichting Alzheimer Nederland and Stichting VUmc fonds. The chair of Wiesje van der Flier is supported by the Pasman stichting. EDB is appointed at a ZonMW funded project on social implications of COVID19 (POLAR #10430 03201 0004). WMF and ISM are recipients of the EU Joint Programme Neurodegenerative Disease Research project ADDITION (ZonMW no. 733051083). The SCIENCe project is funded by stichting Dioraphte. WMF is recipient of ABOARD, which is a public-private partnership receiving funding from ZonMW (#73305095007) and Health Holland, Topsector Life Sciences & Health (PPP-allowance; #LSHM20106). More than 30 partners participate in ABOARD. ABOARD also receives funding from Edwin Bouw Fonds and Gieskes-Strijbisfonds.
This project has received funding from ZonMW via the COVID-19 program (POLAR #10430 03201 0004).
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/21-5342r2).
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-215342.
REFERENCES
[1] | Douglas M , Katikireddi SV , Taulbut M , McKee M , McCartney G ((2020) ) Mitigating the wider health effects of covid-19 pandemic response. BMJ 369: , m1557. |
[2] | Verity R , Okell LC , Dorigatti I , Winskill P , Whittaker C , Imai N , Cuomo-Dannenburg G , Thompson H , Walker PGT , Fu H , Dighe A , Griffin JT , Baguelin M , Bhatia S , Boonyasiri A , Cori A , Cucunubá Z , FitzJohn R , Gaythorpe K , Green W , Hamlet A , Hinsley W , Laydon D , Nedjati-Gilani G , Riley S , van Elsland S , Volz E , Wang H , Wang Y , Xi X , Donnelly CA , Ghani AC , Ferguson NM ((2020) ) Estimates of the severity of coronavirus disease 2019: A model-based analysis. Lancet Infect Dis 20: , 669–677. |
[3] | Zhou F , Yu T , Du R , Fan G , Liu Y , Liu Z , Xiang J , Wang Y , Song B , Gu X , Guan L , Wei Y , Li H , Wu X , Xu J , Tu S , Zhang Y , Chen H , Cao B ((2020) ) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 395: , 1054–1062. |
[4] | Greenberg NE , Wallick A , Brown LM ((2020) ) Impact of COVID-19 pandemic restrictions on community-dwelling caregivers and persons with dementia. Psychol Trauma 12: , S220–s221. |
[5] | Dourado MCN , Belfort T , Monteiro A , de Lucena AT , Lacerda IB , Gaigher J , Baptista MAT , Brandt M , Kimura NR , de Souza N , Gasparini P , Rangel R , Marinho V ((2020) ) COVID-19: Challenges for dementia care and research. Dement Neuropsychol 14: , 340–344. |
[6] | Ryoo N , Pyun JM , Baek MJ , Suh J , Kang MJ , Wang MJ , Youn YC , Yang DW , Kim SY , Park YH , Kim S ((2020) ) Coping with dementia in the middle of the COVID-19 pandemic. J Korean Med Sci 35: , e383. |
[7] | Borges-Machado F , Barros D , Ribeiro Ó , Carvalho J ((2020) ) The effects of COVID-19 home confinement in dementia care: Physical and cognitive decline, severe neuropsychiatric symptoms and increased caregiving burden. Am J Alzheimers Dis Other Demen 35: , 1533317520976720. |
[8] | van Maurik IS , Bakker ED , van den Buuse S , Gillissen F , van de Beek M , Lemstra E , Mank A , van den Bosch KA , van Leeuwenstijn M , Bouwman FH , Scheltens P , van der Flier WM ((2020) ) Psychosocial effects of corona measures on patients with dementia, mild cognitive impairment and subjective cognitive decline. Front Psychiatry 11: , 585686. |
[9] | Brown EE , Kumar S , Rajji TK , Pollock BG , Mulsant BH ((2020) ) Anticipating and mitigating the impact of the COVID-19 pandemic on Alzheimer’s disease and related dementias. Am J Geriatr Psychiatry 28: , 712–721. |
[10] | Rijksoverheid, Nederlandse aanpak en maatregelen tegen het coronavirus, https://www.rijksoverheid.nl/, Accessed 3-1-2021. |
[11] | Tsapanou A , Papatriantafyllou JD , Yiannopoulou K , Sali D , Kalligerou F , Ntanasi E , Zoi P , Margioti E , Kamtsadeli V , Hatzopoulou M , Koustimpi M , Zagka A , Papageorgiou SG , Sakka P ((2021) ) The impact of COVID-19 pandemic on people with mild cognitive impairment/dementia and on their caregivers. Int J Geriatr Psychiatry 36: , 583–587. |
[12] | Canevelli M , Valletta M , Toccaceli Blasi M , Remoli G , Sarti G , Nuti F , Sciancalepore F , Ruberti E , Cesari M , Bruno G ((2020) ) Facing dementia during the COVID-19 outbreak. J Am Geriatr Soc 68: , 1673–1676. |
[13] | Simonetti A , Pais C , Jones M , Cipriani MC , Janiri D , Monti L , Landi F , Bernabei R , Liperoti R , Sani G ((2020) ) Neuropsychiatric symptoms in elderly with dementia during COVID-19 pandemic: Definition, treatment, and future directions. Front Psychiatry 11: , 579842. |
[14] | Altieri M , Santangelo G ((2021) ) The psychological impact of COVID-19 pandemic and lockdown on caregivers of people with dementia. Am J Geriatr Psychiatry 29: , 27–34. |
[15] | Santangelo G , Baldassarre I , Barbaro A , Cavallo ND , Cropano M , Maggi G , Nappo R , Trojano L , Raimo S ((2021) ) Subjective cognitive failures and their psychological correlates in a large Italian sample during quarantine/self-isolation for COVID-19. Neurol Sci 42: , 2625–2635. |
[16] | Podlesek A , Komidar L , Kavcic V ((2021) ) The relationship between perceived stress and subjective cognitive decline during the COVID-19 epidemic. Front Psychol 12: , 647971. |
[17] | Maggi G , Baldassarre I , Barbaro A , Cavallo ND , Cropano M , Nappo R , Santangelo G ((2021) ) Mental health status of Italian elderly subjects during and after quarantine for the COVID-19 pandemic: A cross-sectional and longitudinal study. Psychogeriatrics 21: , 540–551. |
[18] | van der Flier WM , Pijnenburg YA , Prins N , Lemstra AW , Bouwman FH , Teunissen CE , van Berckel BN , Stam CJ , Barkhof F , Visser PJ , van Egmond E , Scheltens P ((2014) ) Optimizing patient care and research: The Amsterdam Dementia Cohort. J Alzheimers Dis 41: , 313–327. |
[19] | van der Flier WM , Scheltens P ((2018) ) Amsterdam Dementia Cohort: Performing research to optimize care. J Alzheimers Dis 62: , 1091–1111. |
[20] | Slot RER , Verfaillie SCJ , Overbeek JM , Timmers T , Wesselman LMP , Teunissen CE , Dols A , Bouwman FH , Prins ND , Barkhof F , Lammertsma AA , Van Berckel BNM , Scheltens P , Sikkes SAM , Van der Flier WM ((2018) ) Subjective Cognitive Impairment Cohort (SCIENCe): Study design and first results. Alzheimers Res Ther 10: , 76. |
[21] | van de Beek M , van Steenoven I , van der Zande JJ , Porcelijn I , Barkhof F , Stam CJ , Raijmakers P , Scheltens P , Teunissen CE , van der Flier WM , Lemstra AW ((2021) ) Characterization of symptoms and determinants of disease burden in dementia with Lewy bodies: DEvELOP design and baseline results. Alzheimers Res Ther 13: , 53. |
[22] | de Wilde A , van Maurik IS , Kunneman M , Bouwman F , Zwan M , Willemse EA , Biessels GJ , Minkman M , Pel R , Schoonenboom NS , Smets EM , Wattjes MP , Barkhof F , Stephens A , van Lier EJ , Batrla-Utermann R , Scheltens P , Teunissen CE , van Berckel BN , van der Flier WM ((2017) ) Alzheimer’s biomarkers in daily practice (ABIDE) project: Rationale and design. Alzheimers Dement (Amst) 6: , 143–151. |
[23] | Cohen G , Russo MJ , Campos JA , Allegri RF ((2020) ) Living with dementia: Increased level of caregiver stress in times of COVID-19. Int Psychogeriatr 32: , 1377–1381. |
[24] | Dam AE , de Vugt ME , Klinkenberg IP , Verhey FR , van Boxtel MP ((2016) ) A systematic review of social support interventions for caregivers of people with dementia: Are they doing what they promise? Maturitas 85: , 117–130. |
[25] | Willis E , Semple AC , de Waal H ((2018) ) Quantifying the benefits of peer support for people with dementia: A Social Return on Investment (SROI) study. Dementia (London) 17: , 266–278. |
[26] | Giebel CM , Challis DJ , Montaldi D ((2016) ) A revised interview for deterioration in daily living activities in dementia reveals the relationship between social activities and well-being. Dementia (London) 15: , 1068–1081. |
[27] | Giebel CM , Sutcliffe C ((2018) ) Initiating activities of daily living contributes to well-being in people with dementia and their carers. Int J Geriatr Psychiatry 33: , e94–e102. |
[28] | Yeung RR ((1996) ) The acute effects of exercise on mood state. J Psychosom Res 40: , 123–141. |
[29] | Woodhead EL , Zarit SH , Braungart ER , Rovine MR , Femia EE ((2005) ) Behavioral and psychological symptoms of dementia: The effects of physical activity at adult day service centers. Am J Alzheimers Dis Other Demen 20: , 171–179. |
[30] | Pinquart M , Sörensen S ((2006) ) Helping caregivers of persons with dementia: Which interventions work and how large are their effects? Int Psychogeriatr 18: , 577–595. |




