Diabetic Retinopathy Predicts Risk of Alzheimer’s Disease: A Danish Registry-Based Nationwide Cohort Study
Abstract
Background:
Retinal neurodegeneration is evident in early diabetic retinopathy (DR) which may be associated with other neurodegenerative diseases like Alzheimer's disease (AD).
Objective:
To investigate diabetes and DR as a risk marker of present and incident AD.
Methods:
A register-based cohort study was performed. We included 134,327 persons with diabetes above 60 years of age, who had attended DR screening, and 651,936 age- and gender-matched persons without diabetes.
Results:
At baseline, the prevalence of AD was 0.7% and 1.3% among patients with and without diabetes, respectively. In a multivariable regression model, patients with diabetes were less likely to have AD at baseline (adjusted OR 0.63, 95% CI 0.59–0.68). During follow-up, incident AD was registered for 1473 (0.35%) and 6,899 (0.34%) persons with and without diabetes, respectively. Compared to persons without diabetes, persons with diabetes and no DR had a lower risk to develop AD (adjusted HR 0.87, 95% CI 0.81–0.93), while persons with diabetes and DR had higher risk of AD (adjusted HR 1.24, 95% CI 1.08–1.43). When persons with diabetes and no DR were used as references, a higher risk of incident AD was observed in persons with DR (adjusted HR 1.34, 95% CI 1.18–1.53).
Conclusion:
Individuals with diabetes without DR were less likely to develop AD compared to persons without diabetes. However, individuals with DR had a 34% higher risk of incident AD, which raise the question whether screening for cognitive impairment should be done among individuals with DR.
INTRODUCTION
Diabetes is a world-wide health concern with a global prevalence of 463 million, which is projected to increase to 700 million by year 2040 [1]. People with diabetes have an elevated risk to develop various micro- and macrovascular complications, of which diabetic retinopathy (DR) is the most common [2].
DR has classically been described as a progressive microvascular angiopathy, leading to retinal ischemia, inner blood-barrier alteration, neovascularization, and macular edema [3]. However, in recent years, retinal neurodegeneration has been observed as an early event in DR, which may even precede microvascular changes [4, 5]. As the eye and brain share common anatomical and physiological properties, a connection between DR and neurodegenerative diseases has also been suggested [6].
Alzheimer’s disease (AD) is a progressive neurodegenerative disease, which is characterized by cognitive and functional impairments [7]. AD is a subtype of dementia, accounting for 60–70% of overall dementia cases [8]. Currently, 47 million people live with dementia worldwide, which is predicted to increase to 132 million by year 2050 [8]. AD pathology is primarily associated with amyloid plaques and neurofibrillary tangles, leading to cerebral neurodegeneration [7].
In a recent meta-analysis comprised of 17 cohort studies, Zhang et al. [9] demonstrated an increased risk of AD in persons with diabetes (relative risk: 1.53, 95% CI 1.42–1.63). Studies have also reported DR in association with an increased risk of cognitive impairment [10, 11], along with an increased risk of overall dementia [12] and increased risk of AD; however, the association between DR and AD has been inconsistent [12–14]. Several possible common features have been described, including chronic inflammation, oxidative stress, insulin resistance, adiponectin deficiency, plasma cholinesterase activity, and vascular damage [15, 16].
The previous studies that have examined the association between DR and AD have overall been limited by small samples sizes. Given that DR and AD may both be viewed as progressive neurovascular disorders, we hypothesized that DR may act as a risk factor for AD. With the present study, we aimed to examine diabetes and DR as a risk marker of present and incident AD in a national cohort study including all individuals screened for DR in Denmark compared with age- and gender-matched persons without diabetes.
MATERIALS AND METHODS
The Danish Health Registries
In Denmark, all citizens diagnosed with diabetes are offered tax-funded DR screening. The Danish Registry of Diabetic Retinopathy (DiaBase) has since 2013 collected data on all DR screening results of persons with diabetes above the age of 18 years, including the level of DR, corrected visual acuity, presence/absence of macular edema, prior eye surgery, and planned time interval to next eye screening. DR screening is performed by practicing ophthalmologists or at selected hospitals, and it is mandatory for the screening physician to report findings to DiaBase. The screening procedure is primarily based on retinal fundus images, according to national guidelines [17]. DR level is graded according to the International Clinical Retinopathy Disease Severity Scale [18], which consist of five steps: level 0 (no DR), level 1–3 (mild, moderate, and severe non-proliferative DR), and level 4 (proliferative diabetic retinopathy or prior panretinal photocoagulation).
The Danish National Patient Registry (DNPR) was established in 1977 and holds, on an individualized basis, information of all hospital contacts including hospitalization and outpatient visits, e.g., admission date and medical diagnosis, as given by the International Classification of Diseases (ICD) 10th revision codes [19]. Regarding AD diagnosis, DNPR has previously been validated and found suitable for use in register-based studies [20].
The Danish National Prescription Registry [21] contains all dispensed prescriptions issued by general practitioners or specialists since 1995 in accordance with the Anatomical Therapeutic Chemical Classification (ATC) system [22].
Each Danish citizen is registered in The Danish Civil Registration System by a unique personal identifier, the Central Personal Registration number, which was used to link data between the registries mentioned above. Furthermore, this registry holds information on age, gender as well as marital and vital status.
Study population
A matched register-based cohort design was employed, comprising both cross-sectional and prospective analyses. Data was collected between January 2, 2013 and December 28, 2018. We included persons registered in DiaBase aged 60 years or above as exposed. Index date was defined by the first occurrence of a screening registered in the DiaBase. Each person with diabetes was assigned a single level of DR defined by the most severely affected eye at the index date. For each person in DiaBase, five random individuals (unexposed), matched by gender and year of birth, who were not registered in DiaBase, were selected from the Danish Civil Registration System. Unexposed persons were assigned the index date of their matched exposed persons with diabetes. In order to determine the type of diabetes among those registered in DiaBase, we combined ICD-10 codes for diabetes and ATC codes of redeemed prescription of insulin (A10A*), and glucose lowering drugs excl. insulin (A10B*) given before 12 months after the index date (Supplementary Table 1). Duration of diabetes was given by the difference between the index date and the first date of ICD-10 codes for diabetes or date of redeemed prescription of insulin or glucose-lowering drugs excl. insulin, whichever came first. Unexposed persons were excluded if they had been given an ICD-10 code for diabetes or ATC codes of redeemed prescription of insulin or glucose-lowering drugs excl. insulin given before 12 months after the assigned index date.
Outcome
We used the ICD-10 diagnose codes F00* (Dementia in Alzheimer’s disease) and G30* (Alzheimer’s disease) to assess the outcome. Diagnoses were only regarded as valid if they had been given by departments specialized in dementia diagnosis, which in Denmark includes departments in neurology, psychiatry, and geriatrics.
In the cross-sectional study, we assessed the presence of AD at the index date. In the prospective study, persons with and without diabetes with comorbid AD before the index date were excluded, and incident AD was registered. Follow-up duration was calculated as the time between index date and date of registration of AD, death, migration or censoring at December 28, 2018, whichever came first.
Covariates
Comorbidity were assessed by the Charlson comorbidity index score and was calculated from ICD-10 codes as described by Quan et al. [23]. Marital status was categorized in never married, married, and widowed or divorced. Depression was defined by ICD-10 codes F32 (depressive episode), F33 (recurrent depressive disorder), and F34.1 (dysthymia).
Use of medication was assessed by ATC-codes for insulin (A10A*), glucose lowering drugs excl. insulin (A10B*), antihypertensive treatment (C02*, C07*, C08*, C09*), or lipid lowering therapy (C10*), as given one year prior or after the index date.
Ethics committee approval
The present study was part of the Ocular And Systemic complications In diabetic retinopathy Study (OASIS), emerging from the Danish Excellence Centre in Ophthalmic Epidemiology (DECODE-EYE) [24]. The study was performed according to the tenets of the Helsinki Declaration, and relevant permissions were obtained from the Region of Southern Denmark’s record of data processing activities (Journal nr. 18/61231) and the Danish Clinical Registries (DIABASE-2018-12-11). In Denmark, informed consent and permissions from the Danish National Committee on Health Research Ethics are not required for register-based studies.
Statistical analyses
Continuous variables are presented with mean and standard deviation and categorical variables with frequencies and percentages. We applied Cuzick’s extension of the Wilcoxon rank-sum test for trend for several groups and Pearson Chi-squared test (χ2) to test differences between groups. DR levels were pooled to obtain greater statistical power. In the cross-sectional study (Table 2 and Supplementary Table 3), we calculated odds ratios (ORs) with 95% confidence intervals (CIs) for AD in crude, age and gender adjusted, and multivariable logistic regression models. In the prospective study (Tables 3 and 4), we calculated hazards ratios (HRs) for AD in crude, age, and gender adjusted and multivariable Cox regression models including age, gender, marital status, depression, use of antihypertensive and lipid lowering drugs, and an adapted Charlson Comorbidity index. While diabetes are part of Charlson Comorbidity index, we were not able to include the full index score but adjusted for the rest of the disease from Charlson Comorbidity index in the multivariable model. For reference, we used persons without diabetes in Tables 2 and 3, and persons with diabetes without DR in Table 4 and Supplementary Table 3. While persons with diabetes are associated with increased mortality, death before AD may be a competing risk factor. To account for this aspect, a competing risk analysis was performed with death as a competing cause to AD. p}-values below 0.05 and CI that did not include 1.0 were considered statistically significant. All statistics were performed using STATA version 16.1 (StataCorp LLC, College Station, TX, USA).
RESULTS
At baseline, the total cohort comprised 786,263 individuals, including 134,327 persons with diabetes, who had attended the screening program for DR, and 651,936 individuals without diabetes (Fig. 1). A total of 115,106 (85.7%) had no DR and the prevalence of DR was 9.2% (n = 12,300), 2.7% (n = 3,652), 0.3% (n = 463), and 2.1% (n = 2,806) for DR levels 1–4, respectively (Supplementary Table 2 and Supplementary Figure 1). Prevalence of AD at baseline was 0.7% (n = 1,007) and 1.3% (n = 8460) among persons with and without diabetes respectively (Table 1). In both groups, persons with AD were older, more likely to be female and diagnosed with depression, and had a higher Charlson comorbidity index score (Table 1). When evaluating AD among persons with diabetes, individuals with AD were more often prescribed insulin, but less often prescribed glucose-lowering drugs excl. insulin, antihypertensive and lipid lowering drug medications (Table 1).
Fig. 1
Flowchart showing population progression in the study. Diabase: Danish Registry of Diabetic Retinopathy. DR, diabetic retinopathy; AD, Alzheimer’s disease; CPR, The Danish Civil Registration System; ICD, International Classification of Disease; ATC, Anatomical Therapeutic Chemical Classification System.
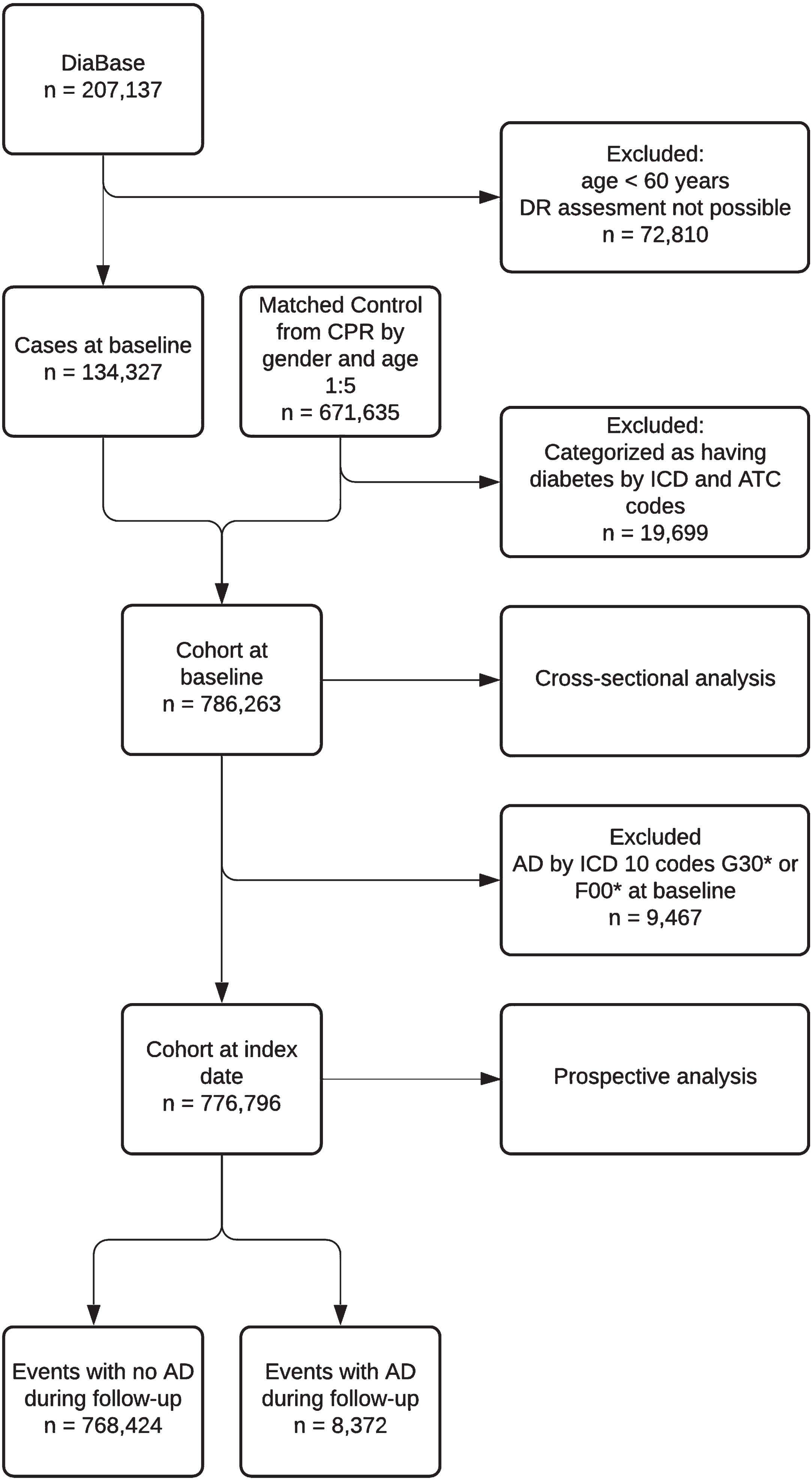
Table 1
Characteristics of persons with diabetes (exposed) and persons without diabetes (unexposed) with and without Alzheimer’s disease (AD) at the time of the first registration in the Danish Registry of Diabetic Retinopathy for persons with diabetes
| Exposed | p | unexposed | p | |||
| Alzheimer’s disease | Yes | No | Yes | No | ||
| Number of persons, n (%) | 1,007 (0.7) | 133,320 (99.3) | 8,460 (1.3) | 643,476 (98.7) | ||
| Gender, (%) male | (48.5) | (56.3) | < 0.001 | (44.3) | (56.2) | < 0.001 |
| Age, y (IQR) | 81.0 (76.1;85.3) | 70.7 (66.0;76.4) | < 0.001 | 81.1 (76.1;85.3) | 70.7 (66.0;76.3) | < 0.001 |
| Type of diabetes, n (%) | < 0.001 | N/A | ||||
| Type 1 diabetes | 20 (2.0) | 3,444 (2.6) | N/A | N/A | ||
| Type 2 diabetes | 743 (73.8) | 107,022 (80.3) | N/A | N/A | ||
| Unknown | 244 (24.2) | 22,854 (17.1) | N/A | N/A | ||
| Duration of diabetes, y (IQR)a | < 0.001 | N/A | ||||
| Type 1 diabetes | 19.4 (14.8;22.2) | 19.8 (13.3;21.5) | N/A | N/A | ||
| Type 2 diabetes | 7.5 (3.5;13.0) | 6.2 (2.7;10.7) | N/A | N/A | ||
| Unknown | 17.8 (12.8;20.4) | 15.0 (9.8;19.5) | N/A | N/A | ||
| Marital status, n (%) | < 0.001 | < 0.001 | ||||
| Never married | 40 (4.0) | 9,897 (7.4) | 361 (4.3) | 46,055 (7.2) | ||
| Married | 510 (50.6) | 79,362 (59.5) | 3,895 (46.0) | 403,758 (62.7) | ||
| Widowed or divorced | 457 (45.4) | 44,061 (33.0) | 4,204 (49.7) | 193,663 (30.1) | ||
| Charlson Comorbidity Index score, n (%) | < 0.001 | < 0.001 | ||||
| 0 (low) | 240 (23.8) | 92,089 (69.1) | 2,541 (30.0) | 529,751 (82.3) | ||
| 1 (moderate low) | 41 (4.1) | 17,923 (13.4) | 147 (1.7) | 37,555 (5.8) | ||
| 2 (Moderate high) | 430 (42.7) | 14,385 (10.8) | 4,610 (54.5) | 56,720 (8.8) | ||
| ≥3 (high) | 296 (29.4) | 8,923 (6.7) | 1,162 (13.7) | 19,450 (3.0) | ||
| Use of medication, n (%) | ||||||
| Insulin | 356 (35.4) | 36,510 (27.4) | < 0.001 | N/A | N/A | N/A |
| Glucose lowering treatment, excl. insulins | 741 (73.6) | 106,023 (79.5) | < 0.001 | N/A | N/A | N/A |
| Antihypertensive drugs | 829 (82.3) | 113,209 (84.9) | 0.022 | 4,283 (50.6) | 316,400 (49.2) | 0.008 |
| Lipid lowering drugs | 766 (76.1) | 106,921 (80.2) | 0.001 | 2,712 (32.1) | 201,595 (31.3) | 0.152 |
| Level of DR, n (%) | 0.330 | |||||
| 0 | 845 (83.9) | 114,261 (85.7) | N/A | N/A | ||
| 1 | 107 (10.6) | 12,193 (9.1) | N/A | N/A | ||
| 2 | 33 (3.3) | 3,619 (2.7) | N/A | N/A | ||
| 3 | 22b (2.2) | 461 (0.3) | N/A | N/A | ||
| 4 | 2,786 (2.1) | N/A | N/A | |||
| Depression, n (%) | 150 (14.9) | 5,741 (4.3) | < 0.001 | 1,202 (14.2) | 22,193 (3.4) | < 0.001 |
Data are given as numbers (with percentages), medians with interquartile ranges (IQR) or percentages. aDuration of diabetes was only calculated for persons with at least one International Classification of Diseases version 10 code for diabetes or one Anatomical Therapeutic Chemical Classification codes for treatment of diabetes. DR, diabetic retinopathy; N/A, not applicable. bDR level 3 and DR level 4 were pooled because of small samples.
Compared to persons without diabetes, multivariable adjusted model for age, gender, marital status, use of hypertension and lipid lowering medication, depression, and an adjusted Charlson comorbidity index, showed a lower presence of AD in persons with diabetes for the entire group (multivariable adjusted OR 0.63, 95% CI 0.59–0.68), in persons with diabetes without DR (multivariable adjusted OR 0.62, 95% CI 0.58–0.67) as well as in persons with diabetes and DR (multivariable adjusted OR 0.69, 95% CI 0.57–0.82) (Table 2). When persons with diabetes without DR was defined as reference group, there was no difference (multivariable adjusted OR 1.16, 95% CI 0.98–1.38) of presence of AD in individuals with diabetes and DR (Supplementary Table 3).
Table 2
Odds ratio with 95% confidence interval for Alzheimer’s disease for persons screened for diabetic retinopathy (exposed) compared to age- and gender-matched persons without diabetes (unexposed) according to the level of DR at the time of the first registration in the Danish Registry of Diabetic Retinopathy for persons with diabetes
| Exposed | Unexposed | Crude model | Model adjusted for sex and age | Multivariable modela | |||
| Level of DR | Persons with AD | Persons without AD | Persons with AD | Persons without AD | OR (95% CI) | OR (95% CI) | OR (95% CI) |
| Overall | 1,007 | 133,320 | 8,460 | 643,476 | 0.57 (0.54–0.61) | 0.57 (0.53–0.60) | 0.63 (0.59–0.68) |
| Level 0 | 845 | 114,261 | 7,204 | 551,696 | 0.57 (0.53–0.61) | 0.56 (0.52–0.60) | 0.62 (0.58–0.67) |
| Level 1–4 | 162 | 19,059 | 1,256 | 91,780 | 0.62 (0.53–0.73) | 0.61 (0.52–0.72) | 0.69 (0.57–0.82) |
aMultivariable logistic regression model adjusted for age, sex, marital status, use of antihypertensive and lipid lowering drugs, depression, and Charlson comorbidity index: myocardial infarct, congestive heart failure, cerebrovascular disease, chronic pulmonary disease, connective tissue disease and rheumatologic disease, ulcer disease, renal disease, hemiplegia or hemiplegia or paraplegia, any malignancy (including leukemia and lymphoma), mild or moderate-severe liver disease, and acquired immunodeficiency syndrome. OR, odds ratio; CI, confidence interval; AD, Alzheimer’s disease; DR, diabetic retinopathy.
After individuals with AD at baseline were excluded among persons with and without diabetes, persons in these groups were followed for overall 416,009 and 2,011,062 person-years, respectively. Incidence of AD was 0.34%, 0.45%, and 0.47% for DR levels 0–2 and 0.38% for DR levels 3–4 combined. While the overall incidence of AD did not vary between persons with (n = 1,473, 0.35%) and without diabetes (n = 6,899, 0.34%), persons with diabetes without DR had a lower risk of developing AD (multivariable adjusted HR 0.87, 95% CI 0.81–0.93) in contrast to persons with diabetes and DR (multivariable adjusted HR 1.24, 95% CI 1.08–1.43) (Table 3). The competing risk analysis resulted in estimates consistent with the main analysis (multivariable adjusted HR 0.91, 95% CI 0.85–0.97 data not shown). When persons with diabetes without DR were used as references, an increased risk of incident AD was identified (multivariable adjusted HR 1.34, 95% CI 1.18–1.53) (Table 4).
Table 3
Hazard Ratio with 95% confidence interval for incident Alzheimer’s disease after the index datea for persons screened for diabetic retinopathy (Exposed) and age- and gender-matched persons without diabetes (unexposed) according to the level of DR for persons with diabetes
| Exposed | Unexposed | Crude model | Model adjusted for sex and age | Multivariable modelc | |||
| Level of DR | Events of ADb | Years of risk | Events of ADb | Years of risk | HR (95% CI) | HR (95% CI) | HR (95% CI) |
| Overall | 1,473 | 416,009 | 6,899 | 2,011,062 | 1.03 (0.98; 1.09) | 1.01 (0.95; 1.07) | 0.92 (0.87;0.98) |
| Level 0 | 1,178 | 348,937 | 5,805 | 1,675,556 | 0.97 (0.92; 1.04) | 0.95 (0.89; 1.01) | 0.87 (0.81; 0.93) |
| Level 1–4 | 295 | 67,072 | 1,094 | 335,506 | 1.35 (1.19; 1.54) | 1.34 (1.18;1.52) | 1.24 (1.08; 1.43) |
aIndex date defined as the date of the first registration in the Danish Registry of Diabetic Retinopathy for persons with diabetes (exposed). bEvents of AD given as the number of persons with new registration of Alzheimer’s disease after the index date. cCox regression model adjusted for sex, age, depression, marital status, use of antihypertensive drugs, lipid lowering drugs, and Charlson comorbidity index: myocardial infarction, congestive heart failure, cerebrovascular disease, chronic pulmonary disease, connective tissue disease and rheumatologic disease, ulcer disease, mild or moderate-severe liver disease, renal disease, hemiplegia or paraplegia, any malignancies (including leukemia and lymphoma), and acquired immunodeficiency syndrome. HR, hazard ratio; CI, confidence interval; AD, Alzheimer’s disease; DR, diabetic retinopathy.
Table 4
Hazard ratio with 95% confidence interval for incident Alzheimer’s disease after index datea for persons screened for diabetic retinopathy according to the level of DR (level 0 used as reference)
| Crude model | Model adjusted for age and sex | Multivariable modelc | |||
| Level of DR | Events of ADb | Years of risk | HR (95% CI) | HR (95% CI) | HR (95% CI) |
| Level 1–4 | 295 | 67,072 | 1.27 (1.12–1.45) | 1.34 (1.18–1.52) | 1.34 (1.18–1.53) |
| Level 0 | 1,178 | 348,937 | 1 (reference) | 1 (reference) | 1 (reference) |
aIndex date defined as the date of the first registration in the Danish Registry of Diabetic Retinopathy for persons with diabetes. bEvents of AD given as the number of persons with new registration of AD after the index date. cCox regression model adjusted for sex, age, depression, marital status, use of antihypertensive drugs, lipid lowering drugs, and Charlson comorbidity index: myocardial infarction, congestive heart failure, cerebrovascular disease, chronic pulmonary disease, connective tissue disease and rheumatologic disease, ulcer disease, mild or moderate-severe liver disease, renal disease, hemiplegia or paraplegia, any malignancies (including leukemia and lymphoma), and acquired immunodeficiency syndrome. HR, hazard ratio; CI, confidence interval; AD, Alzheimer’s disease; DR, diabetic retinopathy.
DISCUSSION
In a nationwide registry-based cohort study of almost 800,000 persons, the risk of incident AD was 13% lower in persons without DR. However, individuals with DR had a 24% higher risk of developing AD within five years compared to an age- and gender-matched population without diabetes.
In our study, the presence of AD was significantly lower at baseline for persons with diabetes compared with individuals without diabetes. Selection bias may have occurred as persons with higher levels of comorbidity or those with mental illness may be more prone to refrain from attending screening programs [25].
Previous prospective studies have investigated the association between DR and cognitive impairment [10, 26, 27] and overall dementia [12, 28, 29]. Still to our knowledge, the association between DR and AD has only been examined by Lee et al. [13], Hwang et al. [14], and Schrijvers et al. [29]. In a prospective study, Lee et al. [13] included 3,877 participants without dementia at baseline with 31,142 person-years follow-up, and reported an increased risk of AD in persons with DR (HR 1.50, p-value = 0.03) after adjusting for age, gender, education, Apolipoprotein E genotype, and smoking. Lee et al. also investigated AD risk in persons with diabetes with DR and without DR and reported that only persons with DR had an elevated risk of AD (HR 1.46, 95% CI 1.09–1.97 versus HR 1.01, 95% CI 0.78–1.31). In a prospective study Schrijvers et al. [29] included 5,640 participants of whom 438 individuals had retinopathy with a mean follow-up of 11.4 years. That study reported a tendency to increased risk of AD in individuals with DR (HR 1.15, 95% CI 0.86–1.55) after adjusting for age, gender, blood pressure, education, Apolipoprotein E genotype, current cigarette smoking, diabetes, total cholesterol, C-reactive protein, and coronary heart disease. However Schrijvers et al. [29] only used the central field for retinopathy grading, which may have led to underestimation of retinopathy. Furthermore, the study included both persons with and without diabetes which may have clouded the nature of the retinopathy. In a recent study, Hwang et al. [14] reported no association between DR and AD (HR 0.46, 95% CI 0.06–3.33); however, the study was limited by a small sample size. In a cross sectional study, the AGES-Reykjavik Study [30] did not find a statistically significant risk of retinopathy and AD; however, the study investigated retinopathy and not solely retinopathy caused by diabetes. The results of our much larger, national population-based study, including more than two million years of observation time are in line with the results of Lee et al. [13] and Schrijvers et al. [29] However, the 13% decreased 5-year risk of AD in persons attending eye screening without DR is quite interestingly, while a recent meta-analysis of 17 studies involving 1,746,777 persons report diabetes as a risk factor for AD (relative risk: 1.53, 95% CI 1.42–1.63) [9]. The discrepancies between these results may be due to differences in study population while the meta-analysis included individuals with diabetes without evaluating the presence of DR. The decreased risk of AD in persons with diabetes without DR compared to persons without diabetes may also be due to improved diabetic treatment. Individuals with diabetes attend a physician at least two times a year where modifiable risks factors of AD are evaluated and treated, which includes mid-life hypertension, overweight, and smoking.
In the present study, individuals with DR were at significant higher risk of developing AD, even after we adjusted for possible vascular factors including myocardial infarct, cerebrovascular disease, use of antihypertensive and lipid-lowering medication. This may indicate that non-vascular factors play an important role in the relationship between DR and AD. The higher risk of AD in persons with DR found in this study may be caused by shared risk factors between retinal and cerebral neurodegeneration. At the same time, previous findings demonstrate an association between decreased retinal nerve fiber layer in AD [31] and early DR [4]. Neural apoptosis and reactive gliosis (response to injury in glial cells) are thought to play a significant role in retinal neurodegeneration [32]. Although glial cells are responsible for neural regeneration, the release of pro-inflammatory cytokines may induce neural death. In addition, the retinal circulation has no autonomic innervation and relies on local neurovascular interaction [33]. Müller cells is one of several types of retinal glial cells, which is part of the neurovascular interaction and produces factors to modulate blood flow, vascular permeability, and cell survival. When Müller cells are injured, the neurovascular unit is impaired, and vascular changes occur, leading to the progression of DR through various downstream pathways [34]. It is well described that retinal and cerebral microvasculature share many common properties, including vascular regulatory properties [33]. Changes in the retinal vasculature may mirror similar changes in the cerebral vasculature while autopsy studies have reported that diabetes is associated with cerebral vascular pathology but not AD pathology [35]. Therefore, AD may be caused by mixed pathophysiological mechanisms, which includes both vascular and non-vascular components, where changes that occur in DR may mirror the same pathophysiological processes seen in the development of AD and therefore be detectable in an early stage by non-invasive eye examinations. In summary, both DR and AD may be viewed as progressive neurovascular disorders.
Currently, no treatment is available to stop progression in an individual suffering from AD. However, detection of groups with higher risk or early detection of AD enable medical care takers to treat adjustable risk factors of AD [36] or provide those who already have AD with the necessary aids.
Strengths of this study include our sizeable national cohort of persons attending eye screening and our design, including a cross-sectional and prospective analysis of more than two million years of risk. Each person who attended eye screening were matched with a person without diabetes of the same age and gender, and we were able to adjust for multiple systemic comorbidities as well as the use of various medications.
On the other hand, limitations should be acknowledged. The registers do not hold information regarding race and lifestyle factors such as smoking, blood glucose, or blood pressure values, and therefore made it impossible for us to adjust for these possible confounders. Diagnosis of AD in the DNPR has previously been evaluated to have high validity, although overall, 30% of AD diagnosis were misclassified as unspecific dementia [20] To compensate for this, we only included persons with AD if the diagnosis was made at a specialized department.
This study reports DR as a major risk factor for incidence of AD. Already known techniques to measure retinal neural health may provide the necessary information in order to construct a non-invasive risk assessment tool to detect individuals at a higher risk of progressive neurodegenerative diseases which may be implemented in DR screening programs.
Future studies should evaluate retinal and cerebral neurodegeneration in a prospective clinical manner in order to determine which and how these changes occur. Currently, a European multicentre collaboration, RECOGNISED (ClinicalTrials.gov registration no. NCT04281186) is launched which aims to assess whether retinal sensitivity to light can detect cognitive decline.
ACKNOWLEDGMENTS
This work was supported by VELUX FONDEN (grant number 00028744) and Odense University Hospital PhD Foundation (grant number 4339).
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/21-5313r1).
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-215313.
REFERENCES
[1] | Saeedi P , Petersohn I , Salpea P , Malanda B , Karuranga S , Unwin N , Colagiuri S , Guariguata L , Motala AA , Ogurtsova K , Shaw JE , Bright D , Williams R , IDF Diabetes Atlas Committee ((2019) ) Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract 157: , 107843. |
[2] | Grauslund J , Green A , Sjolie AK ((2009) ) Prevalence and 25 year incidence of proliferative retinopathy among Danish type 1 diabetic patients. Diabetologia 52: , 1829–1835. |
[3] | Antonetti DA , Klein R , Gardner TW ((2012) ) Diabetic retinopathy. N Engl J Med 366: , 1227–1239. |
[4] | Frydkjaer-Olsen U , Hansen RS , Peto T , Grauslund J ((2018) ) Structural neurodegeneration correlates with early diabetic retinopathy. Int Ophthalmol 38: , 1621–1626. |
[5] | Simo R , Stitt AW , Gardner TW ((2018) ) Neurodegeneration in diabetic retinopathy: Does it really matter? Diabetologia 61: , 1902–1912. |
[6] | Gupta VB , Chitranshi N , den Haan J , Mirzaei M , You Y , Lim JK , Basavarajappa D , Godinez A , Di Angelantonio S , Sachdev P , Salekdeh GH , Bouwman F , Graham S , Gupta V ((2020) ) Retinal changes in Alzheimer’s disease- integrated prospects of imaging, functional and molecular advances. Prog Retin Eye Res 82: , 100899. |
[7] | Lane CA , Hardy J , Schott JM ((2018) ) Alzheimer’s disease. Eur J Neurol 25: , 59–70. |
[8] | World Health Organization ((2017) ) Global action plan on the public health response to dementia 2017–2025. https://www.who.int/publications/i/item/global-action-plan-on-the-public-health-response-to-dementia-2017—2025 |
[9] | Zhang J , Chen C , Hua S , Liao H , Wang M , Xiong Y , Cao F ((2017) ) An updated meta-analysis of cohort studies: Diabetes and risk of Alzheimer’s disease. Diabetes Res Clin Pract 124: , 41–47. |
[10] | Gupta P , Gan ATL , Man REK , Fenwick EK , Sabanayagam C , Mitchell P , Cheung CY , Cheung N , Wong TY , Cheng CY , Lamoureux EL ((2019) ) Association between diabetic retinopathy and incident cognitive impairment. Br J Ophthalmol 103: , 1605–1609. |
[11] | Hugenschmidt CE , Lovato JF , Ambrosius WT , Bryan RN , Gerstein HC , Horowitz KR , Launer LJ , Lazar RM , Murray AM , Chew EY , Danis RP , Williamson JD , Miller ME , Ding J ((2014) ) The cross-sectional and longitudinal associations of diabetic retinopathy with cognitive function and brain MRI findings: The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care 37: , 3244–3252. |
[12] | Exalto LG , Biessels GJ , Karter AJ , Huang ES , Quesenberry CP, Jr. , Whitmer RA ((2014) ) Severe diabetic retinal disease and dementia risk in type 2 diabetes. J Alzheimers Dis 42: (Suppl 3), S109–117. |
[13] | Lee CS , Larson EB , Gibbons LE , Lee AY , McCurry SM , Bowen JD , McCormick WC , Crane PK ((2019) ) Associations between recent and established ophthalmic conditions and risk of Alzheimer’s disease. Alzheimers Dement 15: , 34–41. |
[14] | Hwang PH , Longstreth WT. Jr. , Thielke SM , Francis CE , Carone M , Kuller LH , Fitzpatrick AL ((2021) ) Ophthalmic conditions associated with dementia risk: The Cardiovascular Health Study. Alzheimers Dement 17: , 1442–1451. |
[15] | Fiore V , De Rosa A , Falasca P , Marci M , Guastamacchia E , Licchelli B , Giagulli VA , De Pergola G , Poggi A , Triggiani V ((2019) ) Focus on the correlations between Alzheimer’s disease and type 2 diabetes. Endocr Metab Immune Disord Drug Targets 19: , 571–579. |
[16] | Simo R , Ciudin A , Simo-Servat O , Hernandez C ((2017) ) Cognitive impairment and dementia: A new emerging complication of type 2 diabetes-The diabetologist’s perspective. Acta Diabetol 54: , 417–424. |
[17] | Grauslund J , Andersen N , Andresen J , Flesner P , Haamann P , Heegaard S , Larsen M , Laugesen CS , Schielke K , Skov J , Bek T ((2018) ) Evidence-based Danish guidelines for screening of diabetic retinopathy. Acta Ophthalmol 96: , 763–769. |
[18] | Wilkinson CP , Ferris FL , 3rd , Klein RE , Lee PP , Agardh CD , Davis M , Dills D , Kampik A , Pararajasegaram R , Verdaguer JT , Global Diabetic Retinopathy Project Group ((2003) ) Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 110: , 1677–1682. |
[19] | WHO. International Classification of Diseases, https://www.who.int/classifications/icd/en/. |
[20] | Phung TK , Andersen BB , Hogh P , Kessing LV , Mortensen PB , Waldemar G ((2007) ) Validity of dementia diagnoses in the Danish hospital registers. Dement Geriatr Cogn Disord 24: , 220–228. |
[21] | Kildemoes HW , Sorensen HT , Hallas J ((2011) ) The Danish National Prescription Registry. Scand J Public Health 39: , 38–41. |
[22] | ((1987) ) Techniques for scatter and local photocoagulation treatment of diabetic retinopathy: Early Treatment Diabetic Retinopathy Study Report no. 3. The Early Treatment Diabetic Retinopathy Study Research Group. Int Ophthalmol Clin 27: , 254–264. |
[23] | Quan H , Li B , Couris CM , Fushimi K , Graham P , Hider P , Januel JM , Sundararajan V ((2011) ) Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 173: , 676–682. |
[24] | Grauslund J , Stokholm L , Ohm Kyvik K , Dornonville de la Cour M , Kessel L , Hass Rubin K ((2020) ) Interactions between ocular and systemic disease using national register-based data in the Danish Excellence Centre in Ophthalmic Epidemiology (DECODE-EYE): Study perspective. Acta Ophthalmol 98: , 573–578. |
[25] | Bradley ER , Delaffon V ((2020) ) Diabetic retinopathy screening in persons with mental illness: A literature review. BMJ Open Ophthalmol 5: , e000437. |
[26] | Ryan CM , Geckle MO , Orchard TJ ((2003) ) Cognitive efficiency declines over time in adults with Type 1 diabetes: Effects of micro- and macrovascular complications. Diabetologia 46: , 940–948. |
[27] | Bruce DG , Davis WA , Starkstein SE , Davis TM ((2014) ) Mid-life predictors of cognitive impairment and dementia in type 2 diabetes mellitus: The Fremantle Diabetes Study. J Alzheimers Dis 42: (Suppl 3), S63–70. |
[28] | Rodill LG , Exalto LG , Gilsanz P , Biessels GJ , Quesenberry CP,Jr. , Whitmer RA ((2018) ) Diabetic retinopathy and dementia in type 1 diabetes. Alzheimer Dis Assoc Disord 32: , 125–130. |
[29] | Schrijvers EM , Buitendijk GH , Ikram MK , Koudstaal PJ , Hofman A , Vingerling JR , Breteler MM ((2012) ) Retinopathy and risk of dementia: The Rotterdam Study. Neurology 79: , 365–370. |
[30] | Qiu C , Cotch MF , Sigurdsson S , Jonsson PV , Jonsdottir MK , Sveinbjrnsdottir S , Eiriksdottir G , Klein R , Harris TB , van Buchem MA , Gudnason V , Launer LJ ((2010) ) Cerebral microbleeds, retinopathy, and dementia: The AGES-Reykjavik Study. Neurology 75: , 2221–2228. |
[31] | Kirbas S , Turkyilmaz K , Anlar O , Tufekci A , Durmus M ((2013) ) Retinal nerve fiber layer thickness in patients with Alzheimer disease. J Neuroophthalmol 33: , 58–61. |
[32] | Simo R , Hernandez C ((2015) ) Novel approaches for treating diabetic retinopathy based on recent pathogenic evidence. Prog Retin Eye Res 48: , 160–180. |
[33] | Patton N , Aslam T , Macgillivray T , Pattie A , Deary IJ , Dhillon B ((2005) ) Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: A rationale based on homology between cerebral and retinal microvasculatures. J Anat 206: , 319–348. |
[34] | Stitt AW , Curtis TM , Chen M , Medina RJ , McKay GJ , Jenkins A , Gardiner TA , Lyons TJ , Hammes HP , Simo R , Lois N ((2016) ) The progress in understanding and treatment of diabetic retinopathy. Prog Retin Eye Res 51: , 156–186. |
[35] | Abner EL , Nelson PT , Kryscio RJ , Schmitt FA , Fardo DW , Woltjer RL , Cairns NJ , Yu L , Dodge HH , Xiong C , Masaki K , Tyas SL , Bennett DA , Schneider JA , Arvanitakis Z ((2016) ) Diabetes is associated with cerebrovascular but not Alzheimer’s disease neuropathology. Alzheimers Dement 12: , 882–889. |
[36] | Yu JT , Xu W , Tan CC , Andrieu S , Suckling J , Evangelou E , Pan A , Zhang C , Jia J , Feng L , Kua EH , Wang YJ , Wang HF , Tan MS , Li JQ , Hou XH , Wan Y , Tan L , Mok V , Tan L , Dong Q , Touchon J , Gauthier S , Aisen PS , Vellas B ((2020) ) Evidence-based prevention of Alzheimer’s disease: Systematic review and meta-analysis of 243 observational prospective studies and 153 randomised controlled trials. J Neurol Neurosurg Psychiatry 91: , 1201–1209. |



