Targeting the Beta-2-Adrenergic Receptor and the Risk of Developing Alzheimer’s Disease: A Retrospective Inception Cohort Study
Abstract
Background:
Animal studies suggested that β2-Adrenergic receptors (β2AR) may be a potential target for the treatment of Alzheimer’s disease (AD).
Objective:
This retrospective inception cohort study aimed to assess the association between antagonists and agonists of the β2AR and the risk of starting treatment for AD in older adults.
Methods:
A retrospective inception cohort study was conducted among older adults who initiated either non-selective βAR antagonists or selective β2AR agonists using the University Groningen IADB.nl prescription database (study period 1994–2019). For each exposed cohort, two reference cohorts (A and B) were matched on age at index date. The main outcome was defined as at least two prescriptions for cholinesterase inhibitors (rivastigmine, galantamine, and donepezil) and/or memantine. Cox proportional hazard regression models were used to estimate hazard ratios (HR).
Results:
The risk of developing AD was elevated among patients exposed to non-selective βAR antagonists (A: aHR 3.303, 95% CI 1.230–8.869, B: aHR 1.569, 95% CI 0.560–4.394) and reduced among patients exposed to selective β2AR agonists (A: aHR 0.049, 95% CI 0.003–0.795, B: aHR 0.834, 95% CI 0.075–9.273) compared to reference patients.
Conclusion:
These findings suggest that exposure to non-selective βAR antagonists is associated with an increased risk for developing AD whereas there may be a decreased risk for developing AD after exposure to selective β2AR agonists.
INTRODUCTION
Over 70% of the dementia cases in the Netherlands are due to Alzheimer’s disease (AD) [1]. More than 47 million people are affected by AD worldwide and as population in many countries ages, this number is expected to triple to over 130 million people in 2050 [2]. Besides age, there are also other factors that might increase the risk of developing AD including family history (hereditary), female gender, depression, little physical movement, being strongly overweight (from middle age), high blood pressure (from middle age), and diabetes [1–3].
AD is a progressive and irreversible brain disorder with loss of behavioral abilities and cognitive functioning among which reasoning, remembering, perception, language comprehension, and thinking [4]. Pathological hallmarks of AD are the deposition of tau and amyloid-β (Aβ) in the brain which on the long run can cause neurodegeneration due to the formation of insoluble neurofibrillary tangles and amyloid fibrils (Aβ plaques) [5]. AD is also characterized by chronic inflammation [6, 7]. Tumor necrosis factor alpha (TNF-α) plays a central role in this process, and it can increase the production of other cytokines causing a persistent inflammatory response and eventually neuronal loss [7].
Currently, there is no cure for AD, nor a way to slow or stop its progression available on the Dutch market. Drugs available on the Dutch market are cholinesterase inhibitors (rivastigmine, galantamine, and donepezil) and memantine, but these drugs are not very effective (1 in 6 to 1 in 10 patients) [8–11]. The FDA, however, approved aducanumab as newest drug to treat AD (June 2021) [12]. It is an amyloid beta-directed antibody and is able to slow the progression of AD [13].
As the β2-Adrenergic receptors (β2ARs) are present in the brain (cortex and hippocampus) and are required for memory and learning, such receptors may be a potential target for the treatment of AD [14]. Different animal studies investigated the role of compounds targeting the β2ARs and its potential to be a target for the treatment of AD, but the study findings are controversial [14–25].
In the literature, ICI 118,551 is the most studied β2AR antagonist and clenbuterol the most studied β2AR agonist when investigating the involvement of the β2AR in AD. Both drugs, however, are not approved for use in humans by the FDA. Clenbuterol is, however, approved in some countries [26]. As selective-βAR antagonists have a higher affinity for the β1 receptor, it might be of great value to investigate the involvement of non-selective βAR antagonists in AD [27]. Salbutamol is a frequently used, relatively cheap, and well established selective β2AR agonist available on the Dutch market and might therefore be an interesting drug to study [28].
In order to further assess the risk-benefit of compounds targeting β2ARs, we therefore conducted a retrospective inception cohort study to assess the association between the use of non-selective βAR antagonists or selective β2AR agonists and the risk of developing AD in older adults.
METHODS
Study design and setting
A retrospective inception cohort study was performed (study period 1994–2019) using the University of Groningen IADB.nl community pharmacy database. The database from the IADB.nl is a longitudinal database. It contains pharmacy dispensing data from approximately 120 community pharmacies in the Northern Netherlands. The database is considered to be representative for the Netherlands as a whole because registration in the database is irrespective of gender, age, and health care insurance. Each person is tracked individually throughout the database period by a unique anonymous identifier. Besides the anonymous identifier, gender, address registration number, and date of birth are known. The prescription records in the database contain information on the dose regimen, date of dispensing, how much is dispensed, the number of days a prescription is valid, the physician who prescribed the prescriptions, and the Anatomical Therapeutic Chemical code (ATC code). Indications are not recorded. Due to the fact that patients from the Netherlands generally register at one community pharmacy, the records for most patients are complete. However, medication dispensed during hospitalization and drugs bought in drug stores, so called ‘over the counter drugs’ are not included in the database [29–31].
Eligible subjects
Subjects are defined as individuals starting with the exposure drug or any of the reference drugs, where starting of the drug marks the index date (see section ‘Exposure’). Data was retrieved on all starters with any of these drugs. The starters should be free of any of the exposure, reference, and outcome drugs, respectively, 12 months prior to the index date. For each of the exposure drug users, a starting referent drug user was matched (after fulfilling the inclusion and exclusion criteria) on age. A time-window was set around the index date (±3 years) and around date of birth (±5 years) of the matched exposure group. A list with drugs and their corresponding ATC-codes relevant for this study can be found in Supplementary Table 1 [31].
Inclusion criteria
Individuals 50 years and older at the index date registered in the IADB.nl database during the period of 1994 until 2019 were included in the study. The individuals had to be enrolled in the database, at least 12 months prior to the index date. Also, a minimal follow-up time of 12 months since the index date was required.
Exclusion criteria
Excluded were individuals with prescriptions for AD (based on ATC codes N06DA; N06DA02; N06DA03; N06DA04 N06DX; N06DX01) 365 days before the index date and within 365 days after the index date. We also excluded individuals with prescriptions for any anti-Parkinson drug (N04) or beta-blocking agents for the sensory organs (S01ED). A subject could only be assigned to one group so subjects that simultaneously start with drugs from two or more cohorts (e.g., exposed cohort 1 and reference cohort 1) were also excluded [31].
Exposure
We defined two different exposure groups: exposed group 1: exposure to non-selective βAR antagonists (C07AA) and exposed group 2: exposure to selective β2AR agonists (R03AC and R03CC). Exposure was defined as three or more prescriptions of the exposure drug within the first year after the index date (where the prescription on the index date count as one of the three). Individuals with less than three prescriptions were excluded and could also not be included in the reference group [31].
For classifying the individuals to one specific drug within the overall drug category (for example salbutamol within the β2AR agonists category) exposure was defined as three or more prescriptions of the specific drug within the first year after the index date.
For each of the exposure drug users a starting referent drug user is matched on age with a time-window (±5 years). Also, a time-window was set around the index date of the matched exposure group (±3 years). In this cohort study, there were four reference groups (see Supplementary Table 2): Non-exposed group 1A and 1B are both matched to exposed group 1. Non-exposed group 2A and 2B are both matched to exposed group 2.
Exposure to the referent drug was defined as:≥3 prescriptions of the referent drug (Supplementary Table 2) within the first year after the index date. In Supplementary Table 2, is also depicted which medication the non-exposed cannot use.
Outcome
The outcome, drug-treated AD, was determined by identifying dispensed drugs to treat AD according to the pharmacological guidelines for Dutch general practitioners [8]. An individual was considered to have AD when he or she had the second prescription (starting 365 days after the index date) for cholinesterase inhibitors: rivastigmine, galantamine, and donepezil (N06DA, N06DA02, N06DA03, N06DA04) and/or memantine (N06DX01) [31]. Individuals with less than two prescriptions were treated as non-AD.
Covariates
Covariates that could potentially confound the association between the exposure of compounds targeting the β2AR and the risk of developing AD were the following: age (matching variable) and gender [1, 3]. Age was used as matching variable because the magnitude of confounding tended to 10% [32]. Potential underlying conditions that may affect the risk of developing AD, i.e. diabetes, high cholesterol levels, depression, high blood pressure, and drugs for respiratory system were taken into consideration where prescriptions of ‘drugs used in diabetes’ (A10), ‘lipid modifying agents’ (C10), ‘antidepressants’ (N06A), ‘antihypertensives’ (C02, C03, C07, C08, C09), and ‘respiratory disease’ (R03) were used as proxies for the above-mentioned conditions [1, 3, 31].
Statistical analysis
After participants’ selection the maximal follow-up time was censored at 15 years (exposed cohorts 1) and 19 years (exposed cohorts 2) for all comparisons to avoid selection bias due to differential loss to follow up after such a long follow up period. The maximal follow-up time for exposed cohort 2 differs from the maximal follow-up time for exposed cohort 1. This was done in order not to lose too many subjects in exposed cohorts 2. Differences in distribution of baseline covariates in subjects exposed and not exposed were tested with the Pearson Chi-square test (categorical variables) or the independent sample T-test (continuous variables). The Kaplan-Meier method was used to estimate cumulative incidence curves and the log-rank test was used to evaluate the differences between those cumulative incidence curves. Hazard ratios (HRs) with their 95% confidence intervals (95% CI) for developing AD after non-selective βAR antagonists/selective β2AR agonists exposure were calculated by using univariate and multivariate Cox proportional hazard regression models. The multivariate analyses were adjusted for the baseline characteristics. All statistical analyses were conducted using IBM SPSS Statistics 26 and statistically significance was considered at a p-value of < 0.05.
RESULTS
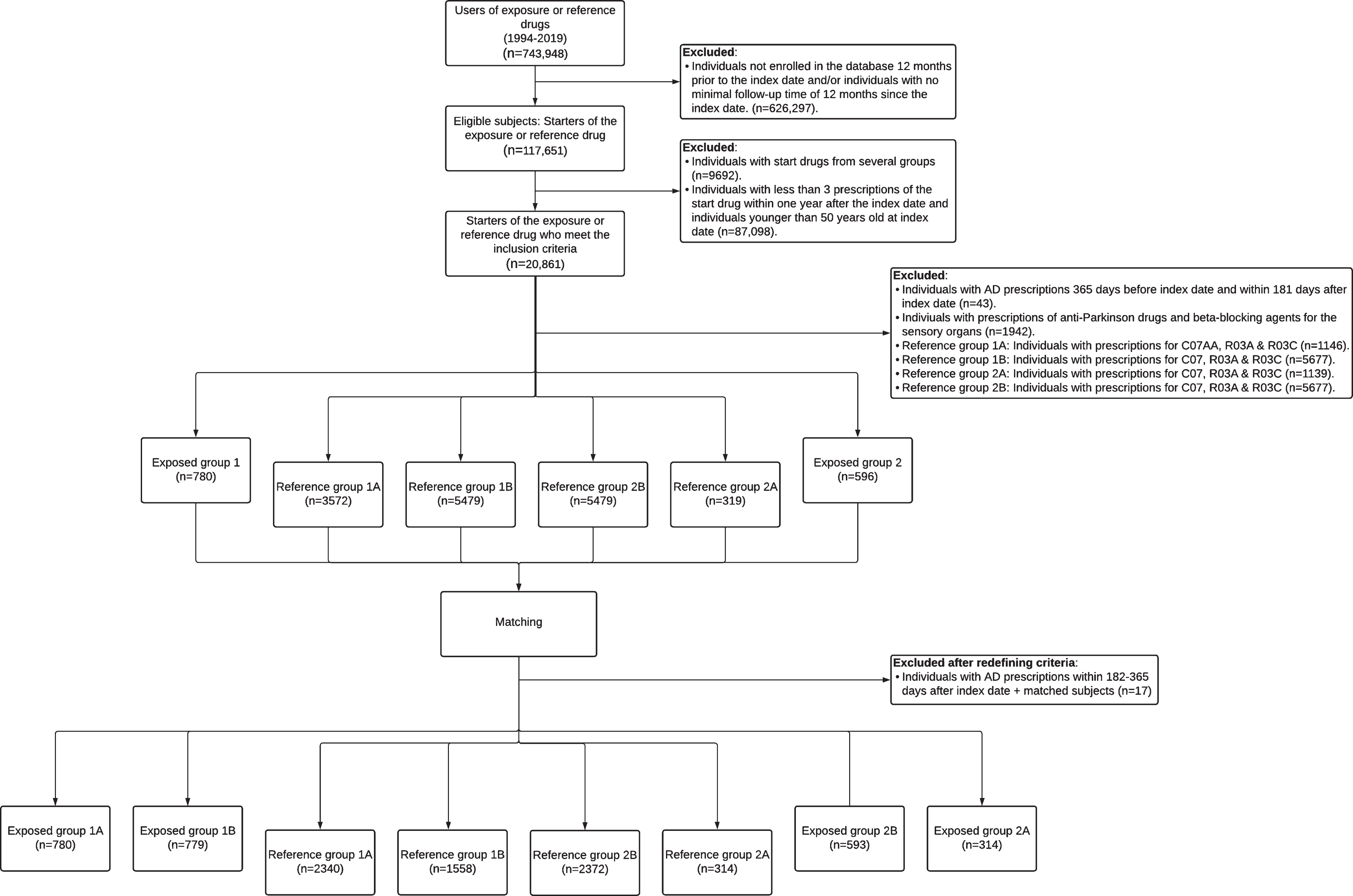
In all, 780 and 779 subjects fulfilled the inclusion and exclusion criteria in exposed cohort 1A and 1B respectively, and those were matched to 2,340 and 1,558 subjects in non-exposed cohort 1A and 1B, respectively (see Fig. 1). In exposed cohort 2A, 314 subjects were included and matched to 314 subjects (non-exposed cohort 2A). In exposed cohort 2B, 593 subjects were matched to 2,372 subjects (non-exposed cohort 2B).
Fig. 1
Flow diagram for participants’ selection.
Non-selective βAR antagonists
Most exposed subjects were exposed to propranolol (68.3%) followed by sotalol (30.6%), pindolol (0.90%), and oxprenolol (0.26%).
In Table 1 the baseline characteristics of patients exposed to non-selective β-antagonists and matched controls are depicted. Significant differences are made visible by presenting p-values in bold.
Table 1
Baseline characteristics of patients exposed to non-selective β-antagonists and matched controls
| Exposed cohort 1 (n = 780) | Non-exposed 1A (n = 2,340) | pb | Exposed cohort 1 (n = 779) | Non-exposed 1B (n = 1,558) | pb | |
| Agea at index date (mean±SD) | 61.9±9.5 | 62.0±9.3 | 0.962 | 61.9±9.5 | 62.2±9.3 | 0.516 |
| Range (y) | 50–93 | 50–92 | – | 50–93 | 50–93 | – |
| Gender (n, %) | < 0.001 | < 0.001 | ||||
| Male | 272 (34.9%) | 1,051 (44.9%) | – | 271 (34.8%) | 715 (45.9%) | – |
| Female | 508 (65.1%) | 1,289 (55.1%) | – | 508 (65.2%) | 843 (54.1%) | – |
| Co-medication (n, %) | ||||||
| Any co-medicationc | 171 (21.9%) | 545 (23.3%) | 0.432 | 171 (22.0%) | 483 (31.0%) | < 0.001 |
| Anti-diabetics | 28 (3.58%) | 87 (3.72%) | 0.869 | 28 (3.59%) | 259 (16.6%) | < 0.001 |
| Anti-depressants | 108 (13.8%) | 195 (8.33%) | < 0.001 | 108 (13.9%) | 130 (8.34%) | < 0.001 |
| Lipid Modifying agents | 56 (7.18%) | 310 (13.2%) | < 0.001 | 56 (7.19%) | 204 (13.1%) | < 0.001 |
| Drugs for obstructive airway | 0 (0.00%) | 0 (0.00%) | – | 0 (0.00%) | 0 (0.00%) | – |
| Follow-up in days (mean±SD)d | 3,876.2±1,774.6 | 3,843±4,505 | 0.649 | 3,874.2±1,774.8 | 3,197.5±1,859.0 | < 0.001 |
| Cases of Alzheimer’s disease | 8 (1.03%) | 8 (0.34%) | 0.037* | 8 (1.03%) | 8 (0.51%) | 0.156 |
aMatching variable. bp-value based on independent T-test or Chi-square test. cAnti-diabetics, anti-depressants, lipid modifying agents, and/or drugs for obstructive airway. dCensored at 5,475 days. *Fisher’s Exact Test.
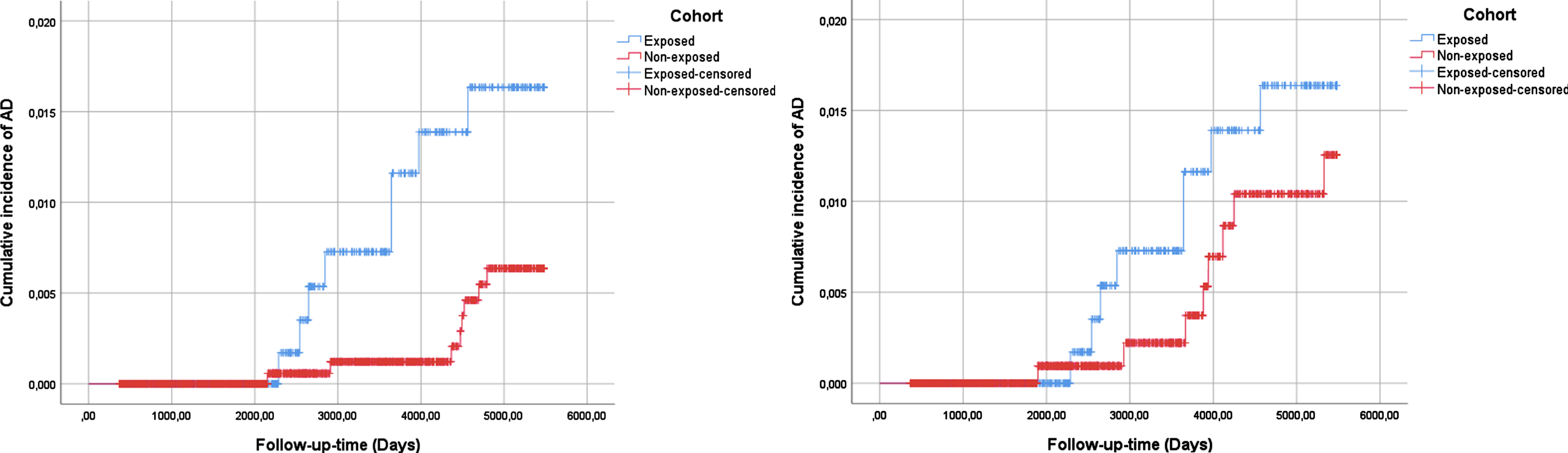
Within the exposed cohort, 8 subjects (1A) (1.03%, 9.66/10,000 person years) and 8 subjects (1B) (1.03%, 9.68/10,000 person years) suffered from medically treated AD, whereas 8 (0.34%, 3.25/10,000 person-years) and 8 (0.51%, 5.86/10,000 person-years) from the non-exposed 1A and 1B were prescribed medication for AD respectively. The Kaplan-Meier analysis revealed that the non-exposed 1A cohort had a significantly lower cumulative incidence of AD compared with the exposed cohort (log-rank test, p < 0.05). The cumulative incidence of AD was also lower in the non-exposed 1B cohort when compared to the exposed, but the difference was not found to be statistically significant (log-rank test, p = 0.443) (Fig. 2). Lifetables are presented in Supplementary Table 4.
Fig. 2
Cumulative incidence of AD according to non-selective βAR-antagonist exposure. Exposed cohort 1 compared to non-exposed cohort 1A (left diagram) and compared to non-exposed cohort 1B (right diagram).
After adjusting for the baseline characteristics presented in Table 1, the exposure to non-selective βAR-antagonists was associated with a significant increased risk of developing AD when compared to the non-exposed 1A cohort (aHR 3.303, 95% CI 1.230–8.869; see Table 2). An increased risk of developing AD was also observed when the exposed cohort was compared to the non-exposed 1B cohort (aHR 1.569, 95% CI 0.560–4.394; Table 2), but this increase was not found to be statistically significant.
Table 2
Hazard ratio of developing AD among patients exposed to either non-selective β-antagonists or selective β2-agonists and matched controls
| Exposed | Non-exposed | Univariate model | Multivariate model | |||||
| N | Cases of AD | N | Cases of AD | Crude HR (95% CI) | p | Adjusted HR (95% CI) | p | |
| Exposed 1 versus Non-exposed cohort 1A | 780 | 8 | 2,340 | 8 | 2.931 (1.100–7.808) | 0.032 | 3.303 (1.230–8.869)a | 0.018 |
| Exposed 1 versus Non-exposed cohort 1B | 779 | 8 | 1,558 | 8 | 1.465 (0.549–3.905) | 0.445 | 1.569 (0.560–4.394)a | 0.391 |
| Exposed 2 versus Non-exposed cohort 2A | 314 | 1 | 314 | 3 | 0.168 (0.017–1.614) | 0.122 | 0.049 (0.003–0.795)b | 0.034 |
| Exposed 2 versus Non-exposed cohort 2B | 593 | 2 | 2,372 | 16 | 0.391 (0.090–1.703) | 0.211 | 0.834 (0.075–9.273)b | 0.883 |
CI, Confidence interval. aadjusted for age at index date, gender, and co-medication use (anti-diabetic, anti-depressant, and lipid modifying agents). badjusted for age at index date, gender, and co-medication use (anti-diabetic, anti-depressant, anti-hypertensives, and lipid modifying agent).
Selective β2AR agonists
Cohort 2A
In exposed cohort 2A, 314 subjects were matched to 314 controls. Most subjects were exposed to salbutamol (75.2%). A detailed overview of the β2-adrenoreceptor agonists included in the analysis and the number of older adults exposed can be seen in Supplementary Table 3.
In Table 3, the baseline characteristics of patients exposed to selective β-agonists and matched controls are depicted. Exposed cohort 2A was compared to non-exposed cohort 2A. Significant differences are made visible by presenting p-values in bold.
Table 3
Baseline characteristics of patients exposed to selective β-agonists and matched controls
| Exposed cohort 2A (n = 314) | Non-exposed 2A (n = 314) | pb | Exposed cohort 2B (n = 593) | Non-exposed 2B (n = 2,372) | pb | |
| Agea at index date (mean±SD) | 63.4±10.3 | 64.4±10.3 | 0.219 | 61.5±9.5 | 63.1±9.5 | < 0.001 |
| Range (y) | 50–93 | 50–92 | – | 50–93 | 50–96 | – |
| Gender (n, %) | 0.150 | 0.005 | ||||
| Male | 139 (44.3%) | 157 (50.0%) | – | 255 (43.0%) | 1174 (49.5%) | – |
| Female | 175 (55.7%) | 157 (50.0%) | – | 338 (57.0%) | 1198 (50.5%) | – |
| Co-medication (n, %) | ||||||
| Any co-medicationc | 52 (16.6%) | 66 (21.0%) | 0.153 | 116 (19.6%) | 2201 (92.8%) | < 0.001 |
| Anti-diabetics | 12 (3.82%) | 16 (5.10%) | 0.439 | 19 (3.20%) | 399 (16.8%) | < 0.001 |
| Anti-depressants | 33 (10.5%) | 36 (11.5%) | 0.702 | 77 (13.0%) | 181 (7.62%) | < 0.001 |
| Lipid modifying agents | 16 (5.10%) | 25 (7.96%) | 0.146 | 38 (6.40%) | 407 (17.2%) | < 0.001 |
| Anti-hypertensives | 0 (0.00%) | 0 (0.00%) | – | 0 (0.00%) | 2161 (91.1%) | < 0.001 |
| Follow-up in days (mean±SD)d | 3,351.9±2,092.0 | 2,515.6±1,830.9 | < 0.001 | 3,481.7±2,209.6 | 2,990.8±2,096.9 | < 0.001 |
| Cases of Alzheimer’s disease | 1 (0.32%) | 3 (0.96%) | 0.624* | 2 (0.34%) | 16 (0.67%) | 0.554* |
aMatching variable. bp-value based on independent T-test or Chi-square test. cAnti-diabetics, anti-depressants, lipid modifying agents, and/or anti-hypertensives. dCensored at 6935 days. *Fisher’s Exact Test.
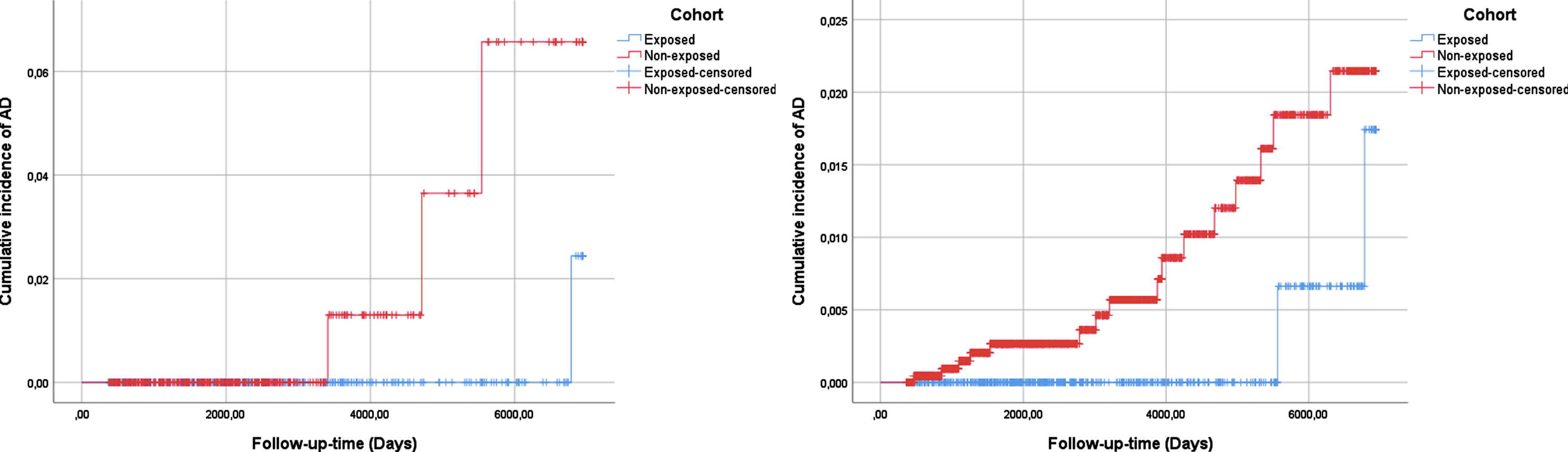
Within the exposed cohort, 1 subject (0.32%, 3.47/10,000 person-years) suffered from medically treated AD, whereas 3 (0.96%, 13.9/10,000 person-years) from the non-exposed 2A were prescribed medication for AD respectively. The Kaplan-Meier analysis revealed that the cumulative incidence of AD was higher in the non-exposed group when compared to the exposed, but this was not found to be statistically significant (log-rank test, p = 0.079) (Fig. 3). Lifetables are presented in Supplementary Table 5.
Fig. 3
Cumulative incidence of AD according to selective β2AR-agonist exposure. Exposed cohort 2A compared with non-exposed cohort 2A (left diagram) and exposed cohort 2B compared with non-exposed cohort 2B (right diagram).
After adjusting for the baseline characteristics presented in Table 3, the exposure to selective β2AR agonists was associated with a decreased risk (HR) of developing AD (aHR 0.049, 95% CI 0.003–0.795; see Table 2).
Cohort 2B
In exposed cohort 2B, 593 subjects were matched to 2,372 controls. Most subjects were exposed to salbutamol (76.7%). A detailed overview of the β2-adrenoreceptor agonists included in the analysis and the number of older adults exposed can be seen in Supplementary Table 3.
In Table 3, the baseline characteristics of patients exposed to selective β-agonists and matched controls are depicted. Exposed cohort 2B was compared to non-exposed cohort 2B. Significant differences are made visible by presenting p-values in bold.
Within the exposed group, 2 subjects (0.34%, 3.54/10,000 person-years) suffered from medically treated AD, whereas 16 (0.67%, 8.23/10,000 person-years) of the non-exposed group were prescribed anti-AD medication respectively. The Kaplan-Meier analysis revealed that the cumulative incidence of AD was higher in the non-exposed group when compared to the exposed, but this was not found to be statistically significant (log-rank test, p = 0.194) (Fig. 3). Lifetables are presented in Supplementary Table 5.
After adjusting for the baseline characteristics presented in Table 3, the exposure to selective β2AR agonists was associated with a decreased risk (HR) of developing AD (aHR 0.834, 95% CI 0.075–9.273; see Table 2) but this decrease was not found to be statistically significant.
DISCUSSION
The results seem to suggest that non-selective βAR antagonist exposure was associated with a higher, and selective β2AR agonist exposure with a lower risk of developing AD compared to those who were not exposed.
Non-selective βAR antagonists
First is discussed whether the cohorts in this study are comparable to the overall population by comparing the numbers regarding co-medication use to existing literature. In the Netherlands, 1 in 14 (7.14%) people are diagnosed with diabetes [33]. As diabetics are more likely to be overweight, have disturbed fat metabolism, and chronic inflammation, most patients will sooner or later deal with additional physical complaints among which include cardiovascular diseases [34]. As β-blockers can affect the adrenergic symptoms of a hypoglycemia, the use of β-blockers is not recommended in patients with diabetes [35]. This might explain the similarity in anti-diabetic use when comparing the exposed to the non-exposed 1A cohort and also the deviation between the exposed and the non-exposed 1B cohort. 8.53% of the Dutch people are diagnosed with fat metabolism disorder [36]. The fact that diabetics are more likely to be overweight or to have disturbed fat metabolism might explain why the use of lipid modifying agents is higher in the non-exposed 1B cohort compared to the exposed [34]. That lipid modifying agent use is also higher in non-exposed cohort 1A compared to the exposed might be explained by the fact that selective β-blockers are preferred above non-selective β-blockers for several indications such as angina pectoris or after going through a myocardial infarction and those events are mostly accompanied by the use of lipid modifying agents [27]. Regarding depression, among adults each year, more than one in twenty (5.2%) suffer from depression [37]. Focusing on the elderly, 6.3% reported having a depression in the last 12 months (2018) and the prevalence of depression in nursing homes was reported around 4% [37]. The fact that the percentages regarding antidepressant use are higher than the numbers presented in literature can be explained by the fact that we are dealing with a prescription database and no indications are known. Antidepressants can namely also be prescribed for other indications; pain or behavioral and anxiety disorders, for example [38].
In this retrospective inception cohort study, we observed that the incidence density of AD was higher in the antagonist exposed cohort when compared to both non-exposed cohorts. The KM analysis showed that the cumulative incidence of AD equaled up to about 5 years and then increased with higher cumulative incidence of AD in the exposed when compared to both non-exposed cohorts (statistically significant for 1A). Thus, it seems that over the longer term there is an apparent increase in AD cases, possible due to non-selective βAR antagonist exposure. After adjusting for confounders by applying Cox proportional hazard regression, such increased risk was confirmed. The risk of developing AD after exposure more than tripled (statistically significant) and almost doubled (not statistically significant) compared to non-exposed cohort 1A and 1B, respectively. These findings can be supported by previous animal studies who suggest that blocking of the β2AR has detrimental effects on AD [15–17]. Also, some human studies also reported that exposure to β-blockers could impair cognition [39–41]. Contradicting, prior animal studies also reported that blocking of the receptor by antagonists can have beneficial effects on AD [18–20], which again is supported by other human studies investigating the effect of antihypertensives, among which include β-blockers, on cognition [42–46]. However, the apparent beneficial results on cognition reported by those previous studies may be mediated by the blood pressure lowering abilities of the antihypertensive drugs [44, 45, 47]. This can stand in contrast to the potential detrimental effects on inflammation, Aβ-, tau-, synaptic-, and thus eventually cognitive pathologies involved in AD [15–18].
Overall, this study suggests that the exposure to non-selective βAR antagonists is associated with an increased risk of AD. Since previous studies focused mainly on cognition and not on AD and showed conflicting results further observational studies and studies in humans are needed to confirm our findings. Those studies should further focus on the association between compounds targeting the β2AR receptor and the risk of developing AD.
Selective β2AR agonists
First is discussed whether the cohorts in this study are comparable to the overall population by comparing the numbers regarding co-medication use to existing literature. No significant differences were found regarding co-medication use between the exposed and matched controls 2A in contrast to matched controls 2B. As stated earlier, 1 in 14 (7.14%) people are diagnosed with diabetes in the Netherlands [33]. A reason why the percentages (anti-diabetic use) are lower in the exposed and non-exposed 2A cohort could be due to chance but might also be partially assigned to the fact that β-agonist use can lead to hyperglycemia and might therefore be avoided [28]. For the non-exposed 2B cohort, it might again be explained by indication bias as subjects with diabetics have higher risk of cardiovascular diseases [34]. 8.53% people are diagnosed with fat metabolism disorder among which includes hypercholesteremia, in the Netherlands [36]. This number is comparable to the number of lipid modifying agent users within the exposed group 2A/B. That lipid modifying agent use is higher in the non-exposed cohort 2B can be explained by the fact that in this cohort there are more anti-diabetic users [34]. As stated earlier regarding depression, each year more than one in twenty (5.2%) adults suffer from depression [37]. Among elderly in 2018, 6.3% reported having a depression in the last 12 months and in nursing homes the prevalence of depression was reported around 4% [37]. The fact that the percentages regarding anti-depressants use are higher in all cohorts (2A/B) can also here be explained by the fact that we are dealing with a prescription database and no indications are known.
In contrast to non-selective βAR antagonist exposure, we observed that the incidence density of AD was lower in the cohorts exposed to selective β2AR agonists when compared to the non-exposed. Focusing on the KM analysis, it can be seen that overall the cumulative incidence is lower in the exposed cohorts when compared to the non-exposed cohorts, although no statistically significant differences were found. After statistical analysis, we observed that the hazard ratio of developing AD was more than halved among patients exposed to selective β2AR agonists when compared to the unexposed subjects (Exposed cohort 2A versus reference cohort 2A). So, it seems that there is a trend towards a protective influence of selective β2AR agonists on the risk of developing AD. These results seem to support previous animal studies reporting that β2AR activation can positively influence neuroinflammation, Aβ-, tau-, synaptic-, and cognitive pathologies [14, 18, 21–23]. However, there are also animal studies reporting that β2AR activation could lead to an increase in Aβ accumulation (hallmark of AD) [24, 25]. But this might be attributed to abnormal β2AR activation induced by stress [14, 21, 24, 25]. To our knowledge, β2AR agonist exposure and the risk of developing AD has not been studied in humans before. Further observational studies and studies in humans are necessary to reveal whether the association found in this retrospective inception cohort is justified.
Salbutamol is a frequently used, relatively cheap, and well established selective β2AR agonist available on the Dutch market [25]. The in vitro study Townsend et al [5]. reported that salbutamol is able to decrease the rate and yield of filament formation and also that it could inhibit tau’s structural change into aggregates [5]. That salbutamol could also decrease Aβ levels and neuronal death, and that it could improve memory was found by yet another study [18]. Besides, studies already identified salbutamol as an indirect anti-TNF agent [48, 49].
Overall, this study seems to suggest that there is a trend towards a protective influence of selective β2AR agonists, including salbutamol, on the risk of developing AD. Since previous studies did not focus on β2AR activation and AD in humans, further observational studies are needed. Those studies should further focus on the association between compounds targeting β2AR and the risk of developing AD.
Potential mechanisms
The molecular mechanism has been investigated in several studies. Studies suggesting that AβPP phosphorylation and α-secretase activity are involved are the studies of Chai et al. [14, 21], Branca et al. [15], and the study of Wu et al. [16]. Activation of the β2AR by clenbuterol decreased Aβ levels by reducing AβPP phosphorylation (specifically, phosphorylation of Thr688) and by increasing α-secretase activity [14, 21]. Blocking of the β2AR increased Aβ levels by increasing AβPP phosphorylation and by decreasing α-secretase activity [15, 16]. Besides the studies of Chai et al. [14, 21] reported that β2AR activation increased levels of PSD95, which is an important regulator of dendritic spines regarding structure and function.
Ni et al. [25] and Yu et al. [24] both attributed the deterioration of the Aβ pathology to an increase in γ-secretase activity. They proposed that the activation of the β2AR lead to endocytosis of the receptor and the bound presenilin-1 ((PS1) part of γ-secretase). As a result, PS1 travels to late endosomes and lysosomes, and γ-secretase activity is enhanced causing Aβ fragments to be formed [24, 25].
AβPP can be cleaved by α-secretase and by β-secretase forming different C-terminal fragments. Those fragments can thenceforth be cleaved by γ-secretase to produce Aβ [19]. Different studies also showed that β-secretase knockout mice produce no or little Aβ proteins suggesting that β-secretase expression can play an important role in the synthesis of Aβ proteins [50, 51]. Insulin degrading enzyme (IDE) can also play an important role in the Aβ pathology as IDE is involved in Aβ degradation and IDE knockout mice also show lower degradation of Aβ [52]. The results of Dobarro et al. [19, 20] suggest that propranolol can increase IDE expression and decrease β-secretase expression and in this way prevent the increase in Aβ levels. Focusing on the tau pathology, JNK1 is believed to be an important kinase because activation of JNK1 can phosphorylate tau. That treatment with propranolol decreased JNK1 activity in SAMP8 mice has been found in the study by Tamagno et al. [53] The decrease in JNK1 was accompanied by decreased pTau expression. Besides, they showed that JNK1 could mediate β-secretase upregulation [38]. The studies of Dobarro et al. [19, 20] also relate JNK1 activation to Aβ, tau, synaptic, and cognitive pathology. Propranolol was able to reverse these pathological features but how propranolol affect JNK1 activity should be clarified in future studies [19, 20].
The study of Ciprés-Flores et al. [18] investigated the β-arrestin (β-arr)/ERK-pathway as a potential mechanism because studies showed that propranolol and salbutamol have weak activity for this pathway [54]. They proposed that the β-arr/ERK-pathway can be activated by β2AR biased ligands resulting in poor internalization of the β2AR and γ-secretase complexes causing Aβ to be secreted poorly. Other studies already reported that ERK signaling has been related to improved memory function [55].
A protective role of formoterol against AD was reported by the study of Abdel Rasheed et al. [21]. Formoterol targeted neuroinflammation because it was able to decrease GSK-α and β (enzymes linked to neuronal loss seen in AD) and thereby neurofibrillary tangles and Aβ plaques [23].
Ryan et al. [22] also investigated inflammation and proposed a mechanism in which clenbuterol (by activating the β2AR) could block IκBα phosphorylation and thus NFκB activity in basal conditions. Thereby, TNF-α and ICAM-1 could be suppressed due to this (NFκB inducible genes). They also proposed that under LPS-stimulated conditions, clenbuterol (by the activation of the β2AR) could suppress NFκB activity by blocking IκBα phosphorylation [22].
Overall, the studies included in this study proposed different mechanisms for the pathologies that appear in AD and involvement of the β2AR. Futures studies are warranted to fully understand how β2AR are involved in the pathology of AD.
Strengths and limitations
To our knowledge this is the first study investigating the association between compounds targeting β2AR and the risk of developing AD in humans. The first strength of this study is that not only β2AR antagonists but also β2AR agonists are studied. Another strength of this study is that data was obtained from the IADB.nl prescription database and therefore likely eliminating recall bias. Besides the database made it possible to follow the patients for a longer period of time because it contained data from 1994–2019. Thereby, the database provided not only information on drugs of interest but also on co-medication use, due to this it was possible to take different confounders into account [30]. Yet another strength of this study is that we matched the patients from the exposed groups to controls by using a time window around the index date (±3 years) and date of birth (±5 years). Due to this, an equal distribution among exposed and controls was ensured and therefore limiting confounding by age. The time window around the index date was set so that eventual changes in the prescription guidelines will be corrected for. One more strength of this study is that the study population is representative for the Netherlands as a whole (external validity) because the IADB.nl database contains data from approximately 120 community pharmacies in the northern Netherlands and was therefore considered to be representative for the Netherlands as a whole [29, 30].
Aside from strengths, this study also has limitations. First, the database does not contain information on actual chronic use of the exposure drugs. This could lead to misclassification and could possibly underestimate the impact of exposure on the risk of developing AD. This, however, is unlikely because we defined exposure on multiple prescriptions within the first year after the index date. Second, not all patients diagnosed with AD actually use anti-AD medication because it is merely effective in 1 in 6 to 10 patients, so this could underestimate the effect of the exposure to non-selective βAR antagonists and overestimate the effect of the exposure to selective βAR agonists [11]. According to the Stichting Farmaceutische Kengetallen (SFK), the use of medication against AD has declined over the years. According to Alzheimer Nederland there are approximately 290,000 individuals with dementia and SFK expects fewer than 30,000 individuals to be on anti-AD medication so the use of drugs in the treatment of dementia is relatively limited [56, 57]. Third, the index date might not be the first date a subject was exposed to drugs but is the defined starting date of follow-up in this inception cohort. We have set this requirement so that we were able to identify any covariates (such as underlying conditions (anti-diabetic use as proxy for diabetes mellitus for example)) by focusing on the dispensed drugs in this 12-month period prior to the index date as the database does not contain information on indication. In general, random, misclassification of exposure may have led to bias toward the null value. However, anti-AD medication is only prescribed for AD or Parkinson’s disease and subjects with prescriptions for Parkinson’s disease were excluded. Regarding co-medication use, the numbers found in this study are explainable or similar to the numbers found in literature. Fourth, the non-exposed B cohorts differs from the exposed cohorts regarding co-medication use and this could be due to indication bias which could possibly overestimate the impact of exposure on the risk of developing AD. Fifth, the database does not contain information on personal characteristics such as body mass index and socioeconomic status for example which might also confound the association. At last, there are little events in this study and therefore the statistical power is low, possibly leading to reduced accuracy of the estimates. In the future bigger studies are needed with larger datasets to increase precision.
Conclusion
This retrospective inception cohort study shows that exposure to non-selective βAR antagonists is associated with an increased risk for developing AD and it seems to suggest that exposure to selective β2AR agonists (among which salbutamol) may be associated with a decreased risk for developing AD. Since previous studies did not split by type of β2AR antagonist/agonist used, focused mainly on cognition and not on AD and showed conflicting results, further studies are needed in to confirm our findings. Those studies should further focus on the association between compounds targeting the β2AR receptor and the risk of developing AD.
DISCLOSURE STATEMENT
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/21-5057r3).
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-215057.
REFERENCES
[1] | Alzheimer Nederland (2021) Factsheet cijfers en feiten over dementia. https://www.alzheimer-nederland.nl/factsheet-cijfers-en-feiten-over-dementie#: :text=%2D%20Ruim%208%25%20van%20de%20mensen,in%20zijn%20leven%20dementie% Last updated February 2021, Accessed on December 3, 2021. |
[2] | Prince M , Comas-Herrera A , Knapp M , Guerchet M , Karagiannidou M ((2016) ) World Alzheimer Report 2016. Improving healthcare for people living with dementia: Cov-erage, quality and costs now and in the future. Alzheimer’s Disease International, London. |
[3] | Web MD (2020) Causes of Alzheimer’s Disease. https://www.webmd.com/alzheimers/guide/alzheimers-causes-risk-factors, Last updated November 2020, Accessed on December 3, 2021. |
[4] | National Institutes of Health (2021) Alzheimer’s disease fact sheet. https://www.nia.nih.gov/health/alzheimers-disease-fact-sheet#causes, Last updated July 2021, Accessed on October 8, 2021 |
[5] | Townsend DJ , Mala B , Hughes E , Hussain R , Siligardi G , Fullwood NJ , Middleton DA ((2020) ) Circular dichroism spectroscopy identifies the β-adrenoceptor agonist salbutamol as a direct inhibitor of tau filament formation in vitro. ACS Chem Neurosci 11: , 2104–2116. |
[6] | Akiyama H ((1994) ) Inflammatory response in Alzheimer’s disease. Tohoku J Exp Med 174: , 295–303. |
[7] | Decourt B , Lahiri DK , Sabbagh MN ((2017) ) Targeting tumor necrosis factor alpha for Alzheimer’s disease. Curr Alzheimer Res 14: , 412–425. |
[8] | NHG (2020) NHG-standaard dementia. https://richtlijnen.nhg.org/standaarden/dementie#volledige-tekst-patint, Last updated April 2020, Accessed on October 8, 2021 |
[9] | Alzheimer’s Association. Medications for Memory, Cog-nition and Dementia-Related Behaviors. https://www.alz.org/alzheimers-dementia/treatments/medications-for-memory, Last updated 2022, Accessed on October 8, 2021. |
[10] | Tan C-C , Yu J-T , Wang H-F , Tan M-S , Meng X-F , Wang C , Jiang T , Zhu X-C , Tan L ((2014) ) Efficacy and safety of donepezil, galantamine, rivastigmine, and memantine for the treatment of Alzheimer’s disease: A systematic review and meta-analysis. J Alzheimers Dis 41: , 615–631. |
[11] | KNMP. Dementie. https://www.apotheek.nl/klachten-ziektes/dementie#welke-medicijnen-worden-gebruikt-bij-dementie, Last updated 2022, Accessed on June 18, 2021. |
[12] | FDA (2021) Aducanumab (marketed as Aduhelm) informa-tion. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/aducanumab-marketed-aduhelm-information. Last updated 2021, Accessed on September 16, 2021. |
[13] | Amsterdam UMC (2021) Groen licht in Amerika voor Alzheimermedicijn Aducanumab. https://amsterdamumc.org/nl/vandaag/groen-licht-in-amerika-voor-het-alzheimermedicijn-aducanumab.htm. Last updated 2021, Accessed on September 16, 2021. |
[14] | Chai G-S , Wang Y-Y , Yasheng A , Zhao P ((2016) ) Beta 2-adrenergic receptor activation enhances neurogenesis in Alzheimer’s disease mice. Neural Regen Res 11: , 1617–1624. |
[15] | Branca C , Wisely Ev , Hartman LK , Caccamo A , Oddo S ((2014) ) Administration of a selective β2 adrenergic receptor antagonist exacerbates neuropathology and cognitive deficits in a mouse model of Alzheimer’s disease. Neurobiol Aging 35: , 2726–2735. |
[16] | Wu Q , Sun J-X , Song X-H , Wang J , Xiong C-Q , Teng F-X , Gao C-X ((2017) ) Blocking beta 2-adrenergic receptor inhibits dendrite ramification in a mouse model of Alzheimer’s disease. Neural Regen Res 12: , 1499–1506. |
[17] | Evans AK , Ardestani PM , Yi B , Park HH , Lam RK , Shamloo M ((2020) ) Beta-adrenergic receptor antagonism is proinflammatory and exacerbates neuroinflammation in a mouse model of Alzheimer’s disease. Neurobiol Dis 146: , 105089. |
[18] | Ciprés-Flores FJ , Segura-Uribe JJ , Orozco-Suárez S , Guerra-Araiza C , Guevara-Salazar JA , Castillo-García EL , Soriano-Ursúa MA , Farfán-García ED ((2019) ) Beta-blockers and salbutamol limited emotional memory disturbance and damage induced by orchiectomy in the rat hippocampus. Life Sci 224: , 128–137. |
[19] | Dobarro M , Orejana L , Aguirre N , Ramírez MJ ((2013) ) Propranolol restores cognitive deficits and improves amyloid and Tau pathologies in a senescence-accelerated mouse model. Neuropharmacology 64: , 137–144. |
[20] | Dobarro M , Gerenu G , Ramírez MJ ((2013) ) Propranolol reduces cognitive deficits, amyloid and tau pathology in Alzheimer’s transgenic mice. Int J Neuropsychopharmacol 16: , 2245–2257. |
[21] | Chai G-S , Wang Y-Y , Zhu D , Yasheng A , Zhao P ((2017) ) Activation of β2-adrenergic receptor promotes dendrite ramification and spine generation in APP/PS1 mice. Neurosci Lett 636: , 158–164. |
[22] | Ryan KJ , Griffin É , Yssel JD , Ryan KM , McNamee EN , Harkin A , Connor TJ ((2013) ) Stimulation of central β2-adrenoceptors suppresses NFκB activity in rat brain, A role for IkaaB. Neurochem Int 63: , 368–378. |
[23] | Abdel Rasheed NO , el Sayed NS , El-Khatib AS ((2018) ) Targeting central β2 receptors ameliorates streptozotocin-induced neuroinflammation via inhibition of glycogen synthase kinase3 pathway in mice. Prog Neuropsychopharmacol Biol Psychiatry 86: , 65–75. |
[24] | Yu N-N , Wang X-X , Yu J-T , Wang N-D , Lu R-C , Miao D , Tian Y , Tan L ((2010) ) Blocking beta2-adrenergic receptor attenuates acute stress-induced amyloid beta peptides production. Brain Res 1317: , 305–310. |
[25] | Ni Y , Zhao X , Bao G , Zou L , Teng L , Wang Z , Song M , Xiong J , Bai Y , Pei G ((2006) ) Activation of beta2-adrenergic receptor stimulates gamma-secretase activity and accelerates amyloid plaque formation. Nat Med 12: , 1390–1396. |
[26] | Drug Enforcement Administration (2019) Clenbuterol. https://www.deadiversion.usdoj.gov/drug_chem_info/clenbuterol.pdf Last updated unknown, Accessed on October 8, 2021. |
[27] | Farmacotherapeutisch Kompas. Bètablokkers, systemisch. https://www.farmacotherapeutischkompas.nl/bladeren/groepsteksten/betablokkers_systemisch. Last updated_unknown, Accessed December 4, 2020. |
[28] | Farmacotherapeutisch Kompas. Salbutamol (inhalatie) https://www.farmacotherapeutischkompas.nl/bladeren/preparaatteksten/s/salbutamolinhalatie Last updated unknown, Accessed December 4, 2020. |
[29] | IADB. http://iadb.nl/ Accessed November 16, 2020 |
[30] | Visser ST , Schuiling-Veninga CCM , Bos JHJ , de Jong-van den Berg LTW , Postma MJ ((2013) ) The population-based prescription database IADB.nl: Its development, usefulness in outcomes research and challenges. Expert Rev Pharmacoecon Outcomes Res 13: , 285–292. |
[31] | WHO Collaborating Centre for Drug Statistics Methodology (2020) ATC/DD index. https://www.whocc.no/atcdddindex/Last updated December 14, Accessed November 17, 2020. |
[32] | (2021) Confounding and effect measure modification. Adjusting for confounding in analysis.https://sphweb.bumc.bu.edu/otlt/MPH-Modules/PH717-QuantCore/PH717-Module11-Confounding-EMM/PH717-Module11-Confounding-EMM5.html . Last updated November 11, Accessed May 18, 2021. |
[33] | Diabetes fonds. Diabetes in cijfers. https://www.diabetesfonds.nl/over-diabetes/diabetes-in-het-algemeen/diabetes-in-cijfers Last updated unknown, Accessed February 23, 2021. |
[34] | Hartstichting. Diabetes. https://www.hartstichting.nl/risicofactoren/diabetes Last updated unknown, Accessed February 23, 2021. |
[35] | KNMP (2019) Diabetes mellitus. https://www.knmp.nl/richtlijnen/diabetes-mellitus Last updated 2019, Accessed February 23, 2021. |
[36] | Volksgezondheid en zorg. Cholesterol. Cijfers & context. https://www.volksgezondheidenzorg.info/onderwerp/cholesterol/cijfers-context/huidige-situatie.. Last updated un- known, Accessed 23-02- 2021]. |
[37] | Zorgvoorbeter. Cijfersdepressiebijouderen. https://www.zorgvoorbeter.nl/depressie/cijfers-ouderen Accessed Feb-ruary 23, 2021. |
[38] | Volksgezondheid en zorg. Depressie en andere stem-mingsstoornissen. https://www.vzinfo.nl/depressie-en-andere-stemmingsstoornissen/zorg. Accessed February 23, 2021. |
[39] | O’Carroll RE , Drysdale E , Cahill L , Shajahan P , Ebmeier KP ((1999) ) Stimulation of the noradrenergic system enhances and blockade reduces memory for emotional material in man. Psychol Med 29: , 1083–1088. |
[40] | Paran E , Anson O , Lowenthal DT Cognitive function and antihypertensive treatment in the elderly: A 6-year follow-up study. Am J Ther 17: , 358–364. |
[41] | Gliebus G , Lippa CF ((2007) ) The influence of beta-blockers on delayed memory function in people with cognitive impairment. Am J Alzheimers Dis Other Demen 22: , 57–61. |
[42] | Gelber RP , Ross GW , Petrovitch H , Masaki KH , Launer LJ , White LR ((2013) ) Antihypertensive medication use and risk of cognitive impairment: The Honolulu-Asia Aging Study. Neurology 81: , 888–895. |
[43] | Rosenberg PB , Mielke MM , Tschanz J , Cook L , Corcoran C , Hayden KM , Norton M , Rabins P v , Green RC , Welsh-Bohmer KA , Breitner JCS , Munger R , Lyketsos CG ((2008) ) Effects of cardiovascular medications on rate of functional decline in Alzheimer disease. Am J Geriatr Psychiatry 16: , 883–892. |
[44] | Hajjar I , Catoe H , Sixta S , Boland R , Johnson D , Hirth V , Wieland D , Eleazer P ((2005) ) Cross-sectional and longitudinal association between antihypertensive medications and cognitive impairment in an elderly population. J Gerontol A Biol Sci Med Sci 60: , 67–73. |
[45] | Bohlken J , Jacob L , Kostev K ((2019) ) The relationship between the use of antihypertensive drugs and the incidence of dementia in general practices in Germany. J Alzheimers Dis 70: , 91–97. |
[46] | Khachaturian AS , Zandi PP , Lyketsos CG , Hayden KM , Skoog I , Norton MC , Tschanz JT , Mayer LS , Welsh-Bohmer KA , Breitner JCS ((2006) ) Antihypertensive medication use and incident Alzheimer disease: The Cache County Study. Arch Neurol 63: , 686–692. |
[47] | Duron E , Hanon O ((2010) ) Antihypertensive treatments, cognitive decline, and dementia. J Alzheimers Dis 20: , 903–914. |
[48] | Clark IA , Vissel B ((2018) ) Therapeutic implications of how TNF links apolipoprotein E, phosphorylated tau, α-synuclein, amyloid-β and insulin resistance in neurodegenerative diseases. Br J Pharmacol 175: , 3859–3875. |
[49] | Uzkeser H , Cadirci E , Halici Z , Odabasoglu F , Polat B , Yuksel TN , Ozaltin S , Atalay F ((2012) ) Anti-inflammatory and antinociceptive effects of salbutamol on acute and chronic models of inflammation in rats: Involvement of an antioxidant mechanism. Mediators Inflamm 2012: , 438912. |
[50] | Bodendorf U , Danner S , Fischer F , Stefani M , Sturchler-Pierrat C , Wiederhold K-H , Staufenbiel M , Paganetti P ((2002) ) Expression of human beta-secretase in the mouse brain increases the steady-state level of beta-amyloid. J Neurochem 80: , 799–806. |
[51] | Luo Y , Bolon B , Kahn S , Bennett BD , Babu-Khan S , Denis P , Fan W , Kha H , Zhang J , Gong Y , Martin L , Louis JC , Yan Q , Richards WG , Citron M , Vassar R ((2001) ) Mice deficient in BACE1, the Alzheimer’s beta-secretase, have normal phenotype and abolished beta-amyloid generation. Nat Neurosci 4: , 231–2. |
[52] | Farris W , Mansourian S , Chang Y , Lindsley L , Eckman EA , Frosch MP , Eckman CB , Tanzi RE , Selkoe DJ , Guenette S ((2003) ) Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc Natl Acad Sci U S A 100: , 4162–4167. |
[53] | Tamagno E , Parola M , Bardini P , Piccini A , Borghi R , Guglielmotto M , Santoro G , Davit A , Danni O , Smith MA , Perry G , Tabaton M ((2005) ) Beta-site APP cleaving enzyme up-regulation induced by 4-hydroxynonenal is mediated by stress-activated protein kinases pathways. J Neurochem 92: , 628–636. |
[54] | van der Westhuizen ET , Breton B , Christopoulos A , Bouvier M ((2014) ) Quantification of ligand bias for clinically relevant β2-adrenergic receptor ligands: Implications for drug taxonomy. Mol Pharmacol 85: , 492–509. |
[55] | Liu X , Ma L , Li HH , Huang B , Li YX , Tao YZ , Ma L ((2015) ) β-Arrestin-biased signaling mediates memory reconsolidation. Proc Natl Acad Sci U S A 112: , 4483–4488. |
[56] | Alzheimer Nederland (2021) Neemt het aantal mensen met dementie toe of af? https://www.alzheimer-nederland.nl/nieuws/toename-of-afname-mensen-met-dementie. Last updated 2019, Accessed June 25, 2021. |
[57] | Stichting Farmaceutische Kengetallen (2016) Inzet medi-cijnen bij dementia loopt terug. https://www.sfk.nl/publicaties/PW/2016/inzet-medicijnen-bij-dementie-loopt-terug. Last updated 2016, Accessed June 25, 2021. |




