Longitudinally Increasing Elevated Asymmetric Flortaucipir Binding in a Cognitively Unimpaired Amyloid-Negative Older Individual
Abstract
We present the case of a cognitively unimpaired 77-year-old man with elevated, asymmetric, and longitudinally increasing Flortaucipir tau PET despite normal (visually negative) amyloid PET. His atypical tau PET signal persisted and globally increased in a follow-up scan five years later. Across eight years of observations, temporoparietal atrophy was observed consistent with tau PET patterns, but he retained the cognitively unimpaired classification. Altogether, his atypical tau PET signal is not explained by any known risk factors or alternative pathologies, and other imaging findings were not remarkable. He remains enrolled for further observation.
INTRODUCTION
During routine analysis of imaging from a cognitively unimpaired 77-year-old right-handed male participant of the Mayo Clinic Study of Aging (MCSA), investigators discovered atypically elevated, asymmetric Flortaucipir positron emission tomography (PET) positivity (T+) despite visually non-elevated amyloid PET (A–).
METHODS
The participant provided written consent with approval of the Mayo Clinic Institutional Review Board. He was only seen for regularly scheduled volunteer visits that included neuropsychiatric testing, magnetic resonance imaging (MRI), and PET. PET imaging included Fluorodeoxyglucose (FDG), amyloid PET (Pittsburgh Compound B; PiB), and tau PET (Flortaucipir) scans. Regional gray matter (GM) volume, total intracranial volume, white matter hyperintensity (WMH), and tau and amyloid PET standardized uptake value ratio (SUVR) measurements (referenced to cerebellar crus) were estimated using previously published automated methods for each scan [1–4].
RESULTS
Medical history
The participant described a history of emphysema and chronic obstructive pulmonary disease, obstructive sleep apnea, hypertension, and osteoarthritis of the knee. When asked specifically, he denied any history of any cognitive disorder among first-degree relatives (parents and siblings), and he denied any history of any head injuries that caused memory loss, unconsciousness, or required a doctor visit or hospitalization. He reported less than ten years of formal education.
PET (Figs. 1 and 3, top)
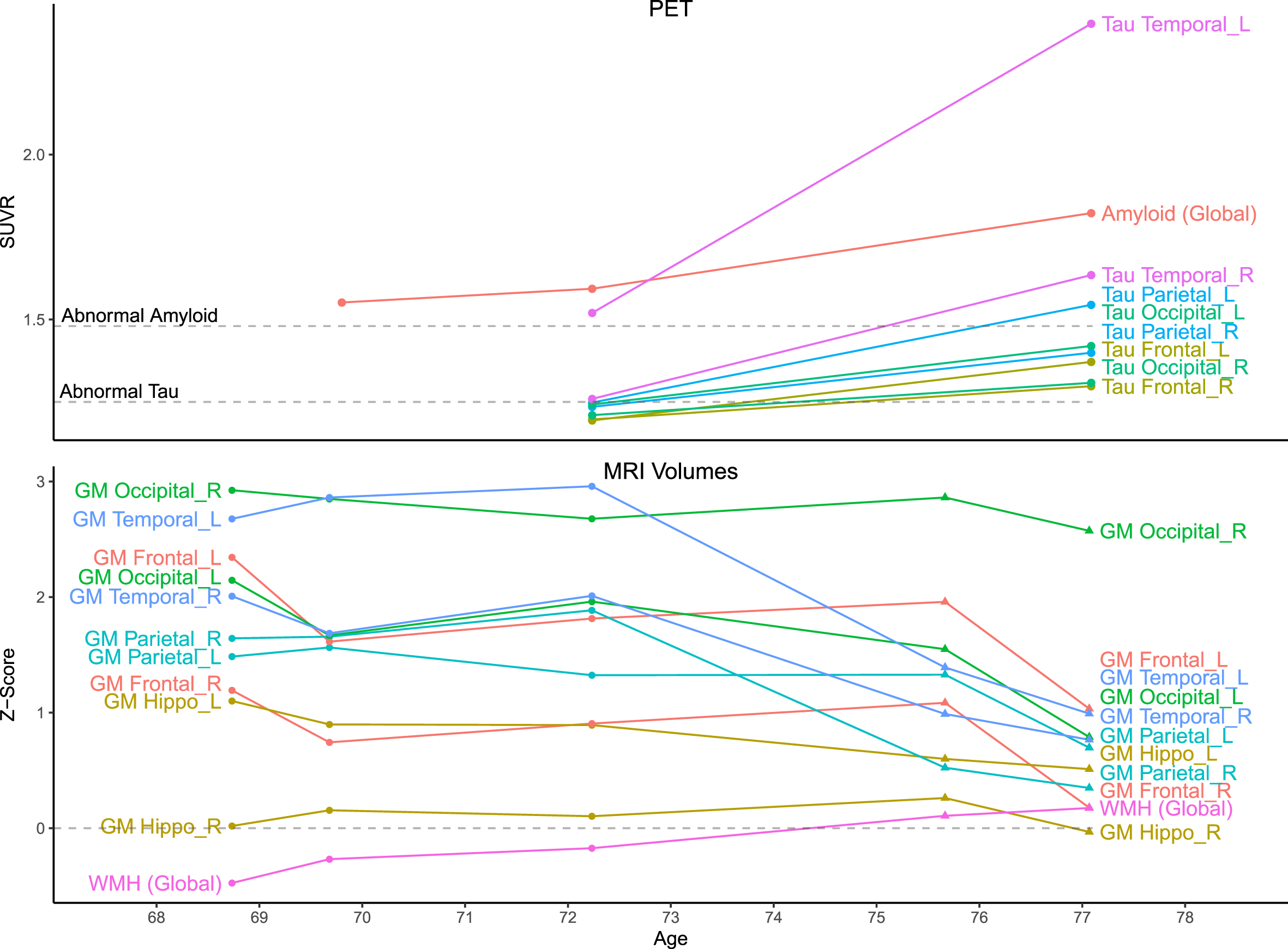
Three amyloid (PiB) PET scans at ages 69, 72, and 77 were all considered A–by an expert nuclear medicine radiologist (VJL). However, whole-brain SUVR measurements were suprathreshold (>1.48) [5, 6], which was attributed to age-typical off-target white matter (WM) binding. Flortaucipir tau PET scans at ages 72 and 77 both had substantial left-hemisphere-predominant GM signal. At age 72, only the temporal lobe was clearly suprathreshold (>1.25) [5, 6] (left: 1.52, right: 1.26). By age 77, all lobes were suprathreshold in both hemispheres with additional focal uptake in left-predominant frontal, parietal, and occipital regions. The highest SUVRs were both temporal (left 2.40, right 1.63). All scans were of good quality and all potential technical explanations for the atypically elevated signal (e.g., motion, attenuation correction error, misregistration) were excluded. A FDG PET was also performed at age 69 and was unremarkable, but this was early in his course and its later trajectory is unknown.
Fig. 1
PET findings. Amyloid (first column; PiB) and tau PET (second two columns; Flortaucipir) at age 72 (top row) and age 77 (bottom row). All amyloid scans were visually negative (A–) despite quantitative elevation due to substantial but age-expected off-target binding in adjacent WM. Tau scans showed atypically high suprathreshold (T+) signal in an asymmetric (L > R) pattern primarily in the temporal lobe with focal uptake in the frontal, parietal, and occipital lobes. All scans were of good quality and all potential technical explanations for the atypically elevated signal (e.g., motion, attenuation correction error, misregistration) were excluded.
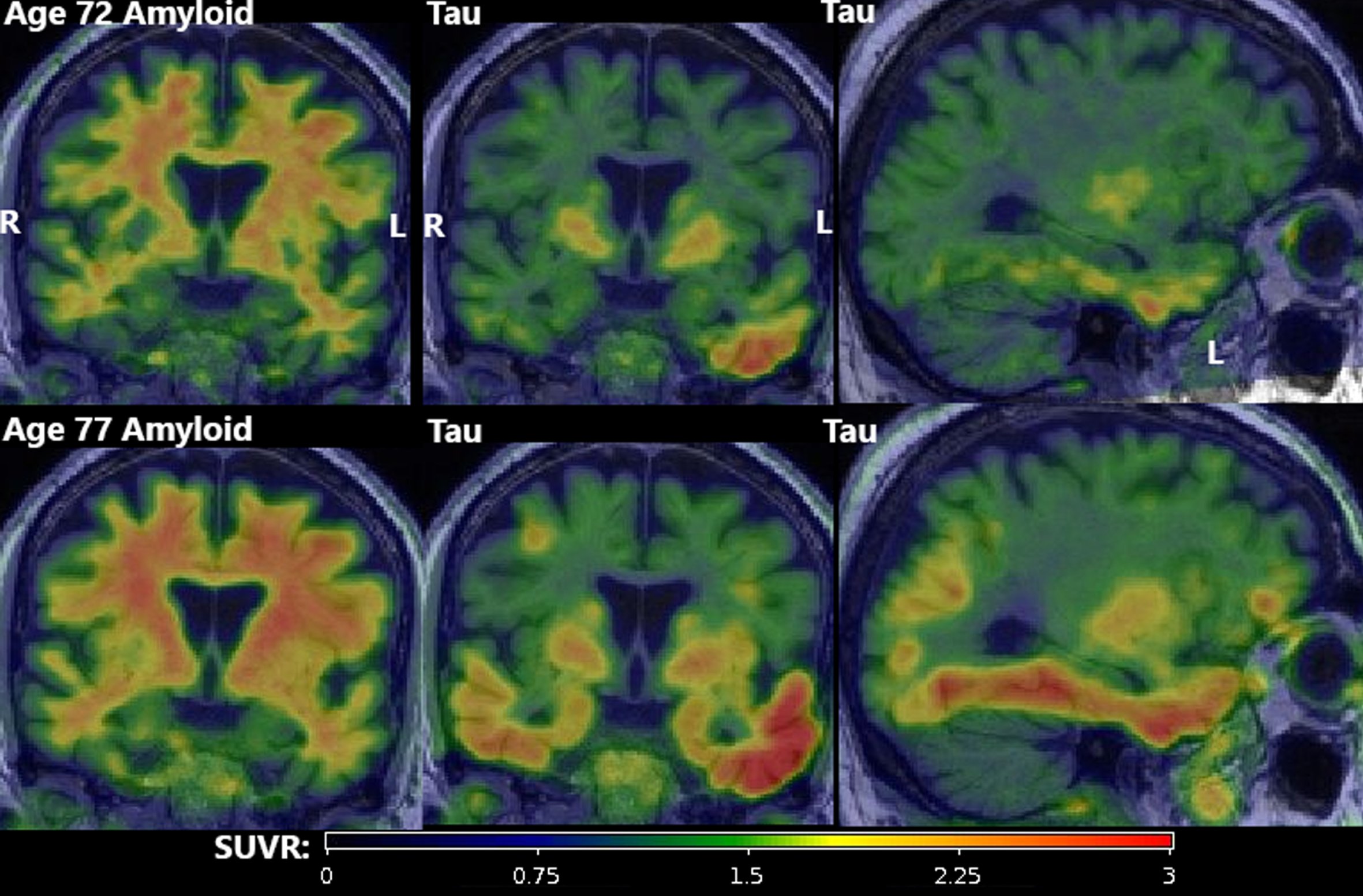
Fig. 2
MRI findings. The most recent (age 77) T1-weighted MRI (top left, top center), and T2-weighted MRI (top right) showed mild global volume loss and extensive dilated perivascular spaces throughout the cerebrum. FLAIR MRI (bottom row) showed white matter hyperintensities (biomarkers of small vessel ischemic change) with an age-typical total volume but an atypical distribution with focal lesions scattered throughout deeper white matter rather than a more typical periventricular pattern.
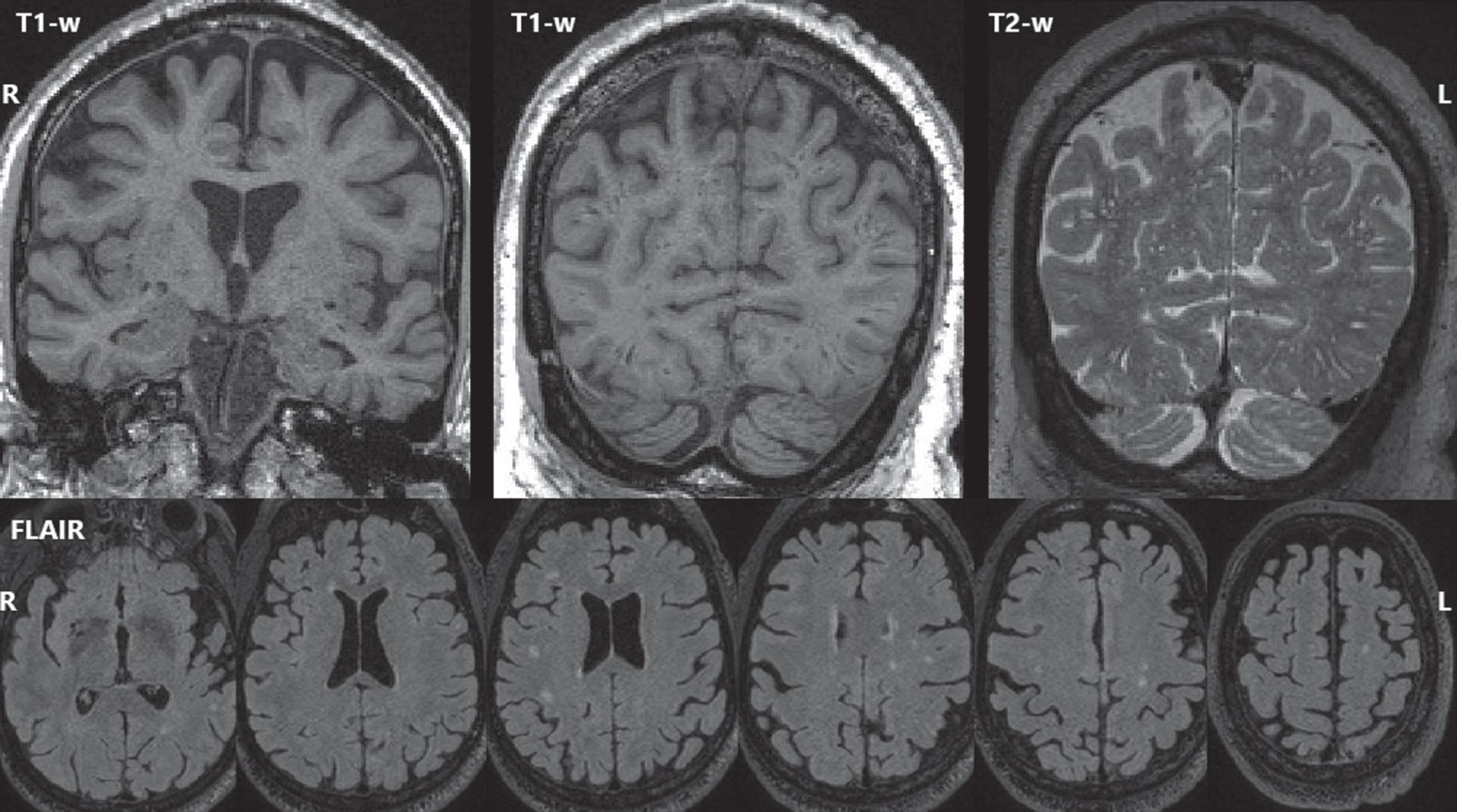
Fig. 3
Quantitative data. Top) Amyloid (PiB) and tau (Flortaucipir) PET standardized uptake value ratio (SUVR) values were measured using a fully automated pipeline with a cerebellar crus reference region. Thresholds for amyloid positivity (A+: 1.48) and tau positivity (T+: 1.25) are shown. Amyloid scans were visually negative but SUVRs were suprathreshold due to off-target white matter binding. At age 72, tau SUVRs were suprathreshold only in the temporal lobe (L > R), but all were suprathreshold by age 77. Bottom) MRI volumes for each scan were normalized by total intracranial volume and z-scored relative to a sample of 169 unimpaired men ages 65–80 with Siemens MRI. Circles indicate timepoints with GE MRI and triangles with Siemens. Before z-scoring, GE-measured volumes were adjusted to better match Siemens using linear transformations derived for each region from a scan-rescan crossover dataset (n = 110; ages 34–97), but changes between these timepoints (age 72–75) should still be interpreted with caution. GM volumes were mostly stable until temporoparietal atrophy at ages 72–75, before global atrophy at 75–77. Relative to the reference group, GM volumes mostly began far above average and remained above average after declines, except for the right hippocampus (which was near average). WMH volumes slowly grew but remained near average.
MRI (Figs. 2 and 3, bottom)
Five 3T MRIs were acquired between ages 68–77. At age 77, there was mild global volume loss, but all GM volume measurements except right hippocampus exceeded reference group averages. Longitudinally, GM volumes were largely stable until temporoparietal atrophy at ages 72–75, then greater widespread atrophy at 75–77. The greatest atrophy occurred in the left temporal lobe, consistent with tau PET distribution. WMH (biomarkers of small vessel ischemic change) were also observed with total volume similar to the reference group but an atypical distribution of scattered deeper focal lesions rather than a typical periventricular predominance. Extensive dilated perivascular spaces were observed throughout the cerebrum including the temporal lobes and the basal ganglia.
Cognition
Testing was performed roughly annually between ages 68-77. Details are withheld to protect participant privacy, but he retained a status of cognitively unimpaired throughout the duration.
Genotyping
We assessed his genotypes for several common genetic variants associated with risk of neurodegenerative diseases (including in APOE, MAPT, TOMM40, GRN, and C9ORF72, among others) and found no overtly explanatory patterns. However, these assessments used genome-wide association study (GWAS) data, which does not encompass all base pairs in the genome and cannot assess rarer mutations. Further details are withheld to protect participant privacy.
DISCUSSION
The participant’s tau PET had widespread, asymmetric elevation that quickly rose longitudinally despite peri-threshold, visually A–amyloid PET. Elevated tau PET signal outside the medial temporal lobe (T+) only typically occurs in A+ individuals [7–9]. Within the medial temporal lobe, elevated tau PET signal in A– individuals is common [8, 10] and associated with Primary Age-Related Tauopathy (PART) [11, 12], but this participant’s signal greatly exceeds that typical for PART in both magnitude and distribution. Weak tau PET signal is often observed in juxtacortical WM of A– individuals with primary tauopathies [13–15], but this participant’s scan had much higher SUVR than is typical for those cases, and the signal was primarily located in GM. Similar GM A–T+ signal has been associated with rare MAPT mutations [16–21] whose status in this individual are not known (not all were included in our GWAS dataset), but those cases as described have included more bilateral/symmetric involvement. Furthermore, this individual’s pattern of common genetic variation at targeted risk alleles was observed frequently within the MCSA cohort, with no other known individuals having comparable tau PET patterns. His elevated signal may also be due to a previously unknown category of off-target binding. Therefore, the imaging findings are not explained by any known genetic factors. We have never observed a similar case among the approximately 1100 unimpaired MCSA participants with amyloid and tau PET.
One possible explanation for his unimpaired cognition despite high tau levels may be higher MRI GM volumes in the earliest visits, which were far above average (except right hippocampus). Even at the latest visit, GM volumes remained above average, which could explain his cognitive resilience. However, his education level (surrogate of cognitive reserve/resilience) of <10 years was not a likely explanation. The left-temporal tau predominance would be anticipated to yield language-domain decline, but he remained cognitively unimpaired. The right hippocampus had the lowest GM volume z-score relative to the population, but it was never significantly below average and remained relatively stable over time. The left hippocampus had a higher z-score than the right at baseline, but it showed stronger decline over time, consistent with the left-predominant tau pattern. The additional MRI-observed vascular abnormalities were consistent with the reported pulmonary conditions and sleep disorders but were not of a burden that would be considered unusual in this age and risk factor range.
Altogether, the participant’s unusual tau PET signal is not explained by any known pathologies or risk factors, and other imaging findings were not remarkable. He remains enrolled for further observation.
ACKNOWLEDGMENTS
The authors give thanks to all the volunteers, participants, and coordinators who contributed to this research. We gratefully thank our funding sources that made this work possible: NIH grants R37 AG011378, R01 AG041851, R01 AG056366, U01 AG006786, P50 AG016574, P30 AG062677, R01 AG034676, R01 NS097495, Gerald and Henrietta Rauenhorst Foundation, Elsie and Marvin Dekelboum Family Foundation, Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Clinic, Liston Award, Schuler Foundation, and Mayo Foundation for Medical Education and Research. We also thank AVID Radiopharmaceuticals, Inc., for their support in supplying AV-1451 precursor, chemistry production advice, and FDA regulatory cross-filing permission and documentation needed for this work.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/21-5052r1).
DATA AND CODE AVAILABILITY STATEMENT
This study describes a specific individual participant. To protect that participant’s privacy, these data will not be shared. In general, data from the Mayo Clinic Study of Aging are made available to qualified academic and industry researchers by request to the Mayo Clinic Study of Aging/Alzheimer’s Disease Research Center Executive Committee.
REFERENCES
[1] | Schwarz CG , Gunter JL , Wiste HJ , Przybelski SA , Weigand SD , Ward CP , Senjem ML , Vemuri P , Murray ME , Dickson DW , Parisi JE , Kantarci K , Weiner MW , Petersen RC , Jack CR Jr ((2016) ) A large-scale comparison of cortical thickness and volume methods for measuring Alzheimer’s disease severity. Neuroimage Clin 11: , 802–812. |
[2] | Schwarz CG , Gunter JL , Ward CP , Vemuri P , Senjem ML , Wiste HJ , Petersen RC , Knopman DS , Jack CR ((2017) ) The Mayo Clinic Adult Lifespan Template: Better quantification across the lifespan. Alzheimers Dement 13: , P792. |
[3] | Schwarz CG , Therneau TM , Weigand SD , Gunter JL , Lowe VJ , Przybelski SA , Senjem ML , Botha H , Vemuri P , Kantarci K , Boeve BF , Whitwell JL , Josephs KA , Petersen RC , Knopman DS , Jack CR ((2021) ) Selecting software pipelines for change in flortaucipir SUVR: Balancing repeatability and group separation. Neuroimage 238: , 118259. |
[4] | Raghavan S , Reid RI , Przybelski SA , Lesnick TG , Graff-Radford J , Schwarz CG , Knopman DS , Mielke MM , Machulda MM , Petersen RC , Jack CR , Vemuri P ((2021) ) Diffusion models reveal white matter microstructural changes with ageing, pathology and cognition. Brain Commun 3: , fcab106. |
[5] | Jack CR Jr , Wiste HJ , Weigand SD , Therneau TM , Lowe VJ , Knopman DS , Gunter JL , Senjem ML , Jones DT , Kantarci K , Machulda MM , Mielke MM , Roberts RO , Vemuri P , Reyes D , Petersen RC ((2017) ) Defining imaging biomarker cut points for brain aging and Alzheimer’s disease. Alzheimers Dement 13: , 205–216. |
[6] | Jack CR , Therneau TM , Weigand SD , Wiste HJ , Knopman DS , Vemuri P , Lowe VJ , Mielke MM , Roberts RO , Machulda MM , Graff-Radford J , Jones DT , Schwarz CG , Gunter JL , Senjem ML , Rocca WA , Petersen RC ((2019) ) Prevalence of biologically vs clinically defined Alzheimer spectrum entities using the National Institute on Aging-Alzheimer’s Association Research Framework. JAMA Neurol 76: , 1174–1183. |
[7] | Schöll M , Lockhart SN , Schonhaut DR , O’Neil JP , Janabi M , Ossenkoppele R , Baker SL , Vogel JW , Faria J , Schwimmer HD , Rabinovici GD , Jagust WJ ((2016) ) PET imaging of tau deposition in the aging human brain. Neuron 89: , 971–982. |
[8] | Lowe VJ , Wiste HJ , Senjem ML , Weigand SD , Therneau TM , Boeve BF , Josephs KA , Fang P , Pandey MK , Murray ME , Kantarci K , Jones DT , Vemuri P , Graff-Radford J , Schwarz CG , Machulda MM , Mielke MM , Roberts RO , Knopman DS , Petersen RC , Jack CR ((2018) ) Widespread brain tau and its association with ageing, Braak stage and Alzheimer’s dementia. Brain 141: , 271–287. |
[9] | Villemagne VL , Lopresti BJ , Dorä V , Tudorascu D , Ikonomovic MD , Burnham S , Minhas D , Pascoal TA , Mason NS , Snitz B , Aizenstein H , Mathis CA , Lopez O , Rowe CC , Klunk WE , Cohen AD ((2021) ) What is T+? A Gordian Knot of tracers, thresholds, and topographies. J Nucl Med 62: , 614–619. |
[10] | Weigand AJ , Bangen KJ , Thomas KR , Delano-Wood L , Gilbert PE , Brickman AM , Bondi MW ((2020) ) Is tau in the absence of amyloid on the Alzheimer’s continuum?: A study of discordant PET positivity. Brain Commun 2: , 1–18. |
[11] | Crary JF , Trojanowski JQ , Schneider JA , Abisambra JF , Abner EL , Alafuzoff I , Arnold SE , Attems J , Beach TG , Bigio EH , Cairns NJ , Dickson DW , Gearing M , Grinberg LT , Hof PR , Hyman BT , Jellinger K , Jicha GA , Kovacs GG , Knopman DS , Kofler J , Kukull WA , Mackenzie IR , Masliah E , McKee A , Montine TJ , Murray ME , Neltner JH , Santa-Maria I , Seeley WW , Serrano-Pozo A , Shelanski ML , Stein T , Takao M , Thal DR , Toledo JB , Troncoso JC , Vonsattel JP , White CL , Wisniewski T , Woltjer RL , Yamada M , Nelson PT ((2014) ) Primary age-related tauopathy (PART): A common pathology associated with human aging. Acta Neuropathol 128: , 755–766. |
[12] | Lowe VJ , Curran G , Fang P , Liesinger AM , Josephs KA , Parisi JE , Kantarci K , Boeve BF , Pandey MK , Bruinsma T , Knopman DS , Jones DT , Petrucelli L , Cook CN , Graff-Radford NR , Dickson DW , Petersen RC , Jack CR , Murray ME ((2016) ) An autoradiographic evaluation of AV-1451 Tau PET in dementia. Acta Neuropathol Commun 4: , 1–19. |
[13] | Josephs KA , Whitwell JL , Tacik P , Duffy JR , Senjem ML , Tosakulwong N , Jack CR , Lowe V , Dickson DW , Murray ME ((2016) ) [18F]AV-1451 tau-PET uptake does correlate with quantitatively measured 4R-tau burden in autopsy-confirmed corticobasal degeneration. Acta Neuropathol 132: , 931–933. |
[14] | Smith R , Schöll M , Widner H , van Westen D , Svenningsson P , Hagerstrom D , Ohlsson T , Jogi J , Nilsson C , Hansson O ((2017) ) In vivo retention of 18 F-AV-1451 in corticobasal syndrome. Neurology 89: , 845–853. |
[15] | Marquiä M , Normandin MD , Meltzer AC , Siao Tick Chong M , Andrea N V. , Antón-Fernández A , Klunk WE , Mathis CA , Ikonomovic MD , Debnath M , Bien EA , Vanderburg CR , Costantino I , Makaretz S , DeVos SL , Oakley DH , Gomperts SN , Growdon JH , Domoto-Reilly K , Lucente D , Dickerson BC , Frosch MP , Hyman BT , Johnson KA , Gómez-Isla T ((2017) ) Pathological correlations of [F-18]-AV-1451 imaging in non-alzheimer tauopathies. Ann Neurol 81: , 117–128. |
[16] | Jones DT , Knopman DS , Graff-Radford J , Syrjanen JA , Senjem ML , Schwarz CG , Dheel C , Wszolek Z , Rademakers R , Kantarci K , Petersen RC , Jack CR , Lowe VJ , Boeve BF ((2018) ) In vivo 18 F-AV-1451 tau PET signal in MAPT mutation carriers varies by expected tau isoforms. Neurology 90: , e947–e954. |
[17] | Soleimani-Meigooni DN , Iaccarino L , La Joie R , Baker S , Bourakova V , Boxer AL , Edwards L , Eser R , Gorno-Tempini ML , Jagust WJ , Janabi M , Kramer JH , Lesman-Segev OH , Mellinger T , Miller BL , Pham J , Rosen HJ , Spina S , Seeley WW , Strom A , Grinberg LT , Rabinovici GD ((2020) ) 18F-flortaucipir PET to autopsy comparisons in Alzheimer’s disease and other neurodegenerative diseases. Brain 143: , 3477–3494. |
[18] | Tsai RM , Bejanin A , Lesman-Segev O , LaJoie R , Visani A , Bourakova V , O’Neil JP , Janabi M , Baker S , Lee SE , Perry DC , Bajorek L , Karydas A , Spina S , Grinberg LT , Seeley WW , Ramos EM , Coppola G , Gorno-Tempini ML , Miller BL , Rosen HJ , Jagust W , Boxer AL , Rabinovici GD ((2019) ) 18F-flortaucipir (AV-1451) tau PET in frontotemporal dementia syndromes. Alzheimers Res Ther 11: , 13. |
[19] | Spina S , Schonhaut DR , Boeve BF , Seeley WW , Ossenkoppele R , O’Neil JP , Lazaris A , Rosen HJ , Boxer AL , Perry DC , Miller BL , Dickson DW , Parisi JE , Jagust WJ , Murray ME , Rabinovici GD ((2017) ) Frontotemporal dementia with the V337M MAPT mutation. Neurology 88: , 758–766. |
[20] | Smith R , Puschmann A , Schöll M , Ohlsson T , Van Swieten J , Honer M , Englund E , Hansson O ((2016) ) 18F-AV-1451 tau PET imaging correlates strongly with tau neuropathology in MAPT mutation carriers. Brain 139: , 2372–2379. |
[21] | Wolters EE , Papma JM , Verfaillie SCJ , Visser D , Weltings E , Groot C , van der Ende EL , Giannini LAA , Tuncel H , Timmers T , Boellaard R , Yaqub M , van Assema DME , Kuijper DA , Segbers M , Rozemuller AJM , Barkhof F , Windhorst AD , van der Flier WM , Pijnenburg YAL , Scheltens P , van Berckel BNM , van Swieten JC , Ossenkoppele R , Seelaar H ((2021) ) [18F]Flortaucipir PET across various MAPT mutations in presymptomatic and symptomatic carriers. Neurology 97: , e1017–e1030. |



