Electrocardiographic Predictors of Cognitive Decline and Dementia: A Systematic Review
Abstract
Background:
Markers of altered cardiac function might predict cognitive decline and dementia.
Objective:
This systematic review aims to review the literature that examines the associations of various electrocardiogram (ECG) markers with cognitive decline and dementia in middle-aged and elderly populations.
Methods:
We searched PubMed, Embase, and Web of Science through 1 July 2020 for literature and conducted a systematic literature review. We included studies examining the associations of ECG markers (e.g., left ventricular hypertrophy [LVH], spatial QRS-T angle, and QT prolongation) with cognitive function and dementia in adult populations regardless of study setting and design, but excluded studies examining atrial fibrillation and heart rate variability.
Results:
Fourteen community-based cross-sectional and longitudinal studies were identified. ECG markers were investigated in association with dementia in four prospective studies, and with cognitive decline in ten prospective studies. ECG-assessed LVH was associated with dementia in one study while five heterogeneous prospective studies yielded inconsistent associations with cognitive decline. Regarding ventricular repolarization markers, spatial QRS-T angle was associated with cognitive decline in one study while another study found no association between QT prolongation and cognitive decline. High resting heart rate was associated with both dementia and cognitive decline in one study but not associated with dementia in another study. P-wave abnormality was significantly associated with incident dementia and cognitive decline in one prospective study.
Conclusion:
Some ECG markers were associated with incident dementia and cognitive decline. However, limited number of heterogeneous studies did not allow us to make firm conclusions. Further studies are needed.
INTRODUCTION
Dementia is a global epidemic. In 2018, worldwide 50 million people lived with dementia, more than double since 1990 [1]. It is projected that by 2050, the number of people living with dementia will reach 152 million, with 68%residing in low- and middle-income countries (LMICs) [2]. Dementia is the major cause of disability and institutionalization among older adults, and thus, significantly affects the quality of life of patients, their families, and the healthcare systems. Indeed, the future burden of dementia will likely overwhelm already overloaded healthcare systems. It is therefore of significant interest to identify high-risk populations for early interventions to prevent and delay the progression of cognitive decline. Current neuroimaging and biological markers are promising to identify high-risk patients for dementia, but they are usually costly, limiting their use in LMIC settings. Inexpensive and widely available methods to stratify high-risk populations for dementia are crucial for early preventive interventions.
Cardiovascular disease (CVD) is another prevalent condition in the elderly population. In the past decades, a great deal of attention has been paid to a close link between CVDs and dementia. CVDs not only share vascular risk factors with dementia, but also increase the risk of dementia [3]. The heart-brain continuum hypothesis suggests that impaired cardiac function resulting from long-term exposures to cardiovascular risk factors contributes to accelerated cognitive decline, possibly through cerebral hypoperfusion and increased arterial stiffness [4, 5]. Considering the close relationship between CVDs and brain aging, it could be argued that markers of heart damage may also be employed to identify individuals at higher risk of cognitive decline and dementia.
Electrocardiogram (ECG) is an inexpensive, non-invasive, and widely available diagnostic modality, which has been used to detect and monitor various subclinical and clinical CVDs. Substantial evidence has shown that some ECG findings have independent clinical prognostic values for cardiovascular events. By contrast, the association of ECG parameters with cognitive decline or dementia appears to be insufficiently investigated.
If some ECG markers can be used to predict the risk of cognitive decline or dementia, careful assessment and follow-up of not only cardiac function but also cognitive function in patients with such ECG markers might be useful to detect early signs of cognitive decline and intervene earlier.
In this systematic review, we sought to summarize the current evidence from epidemiological studies that investigate the association of various ECG findings with cognitive decline and dementia and identify the potential ECG predictors for cognitive decline and dementia, particularly in the general population settings of adults.
MATERIALS AND METHODS
Data sources and searches
We conducted a systematic search of literature via PubMed, Web of Science, and Embase through 1 July 2020 for full-text articles in English by using the keywords, and their synonyms, electrocardiogram and cognitive dysfunction. Search strategy is shown in the Supplementary Material. Two reviewers (YI and DLV) independently screened the titles and abstracts. In addition, we searched reference lists of studies included in our review and relevant review articles in accordance with a snowball strategy. Any disagreements were resolved through discussion between two reviewers.
Study selection
We included cross-sectional, case-control, and cohort studies as well as secondary analyses of randomized controlled trials quantifying the association between ECG findings and cognitive outcomes in adults published in English. Studies needed to consider either diagnosis of dementia or cognitive function assessed by the validated cognitive tests as outcomes. Exposure variables were various ECG parameters other than atrial fibrillation (AF) and heart rate variability (HRV). AF and heart rate variability were excluded because several systematic reviews have examined their associations with cognitive outcomes recently [6–10]. Studies using composite ECG findings (e.g., any ECG abnormalities) or the combination of clinical findings and ECG markers (e.g., ECG changes and chest pain) as exposures were excluded because we were interested in the independent association between specific ECG findings and cognitive outcomes. To have a comprehensive overview of existing literature, studies in both community setting and a clinical setting (e.g., recruiting people with cognitive impairment or people with cardiovascular diseases) were included.
To ensure study results in this review were comparable, first, eligible studies were divided into two groups: studies reporting crude associations and those reporting results adjusted for at least age and sex. We present only the adjusted results here because age and sex are strong confounders in the association we are interested in. The unadjusted results are available as online supplemental material (Supplementary Table 2).
Supplementary Table 1 shows the summary of excluded studies and the reasons for the exclusion.
Then, studies were further divided into community-based studies and hospital-based studies to make results comparable.
Data extraction and quality assessment
One author (YI) extracted study characteristics and study results using a standardized form, and the accuracy of extracted data was reviewed by a second author (DLV). Data abstracted included participant characteristics, effect estimates, and all covariates in the statistical model. We extracted all effect estimates reported (hazard ratio, odds ratio, or beta coefficient). When effect estimates were unavailable, information to compare exposure group and non-exposure group was extracted (e.g., the proportion of dementia in each group).
The quality of the included observational studies was assessed using the Newcastle-Ottawa Scale (NOS), which includes the assessment of three domains of studies (selection, comparability, and outcome). One author (YI) assessed risks of bias for each study and, any uncertain points were resolved through discussion with the second author (DLV).
RESULTS
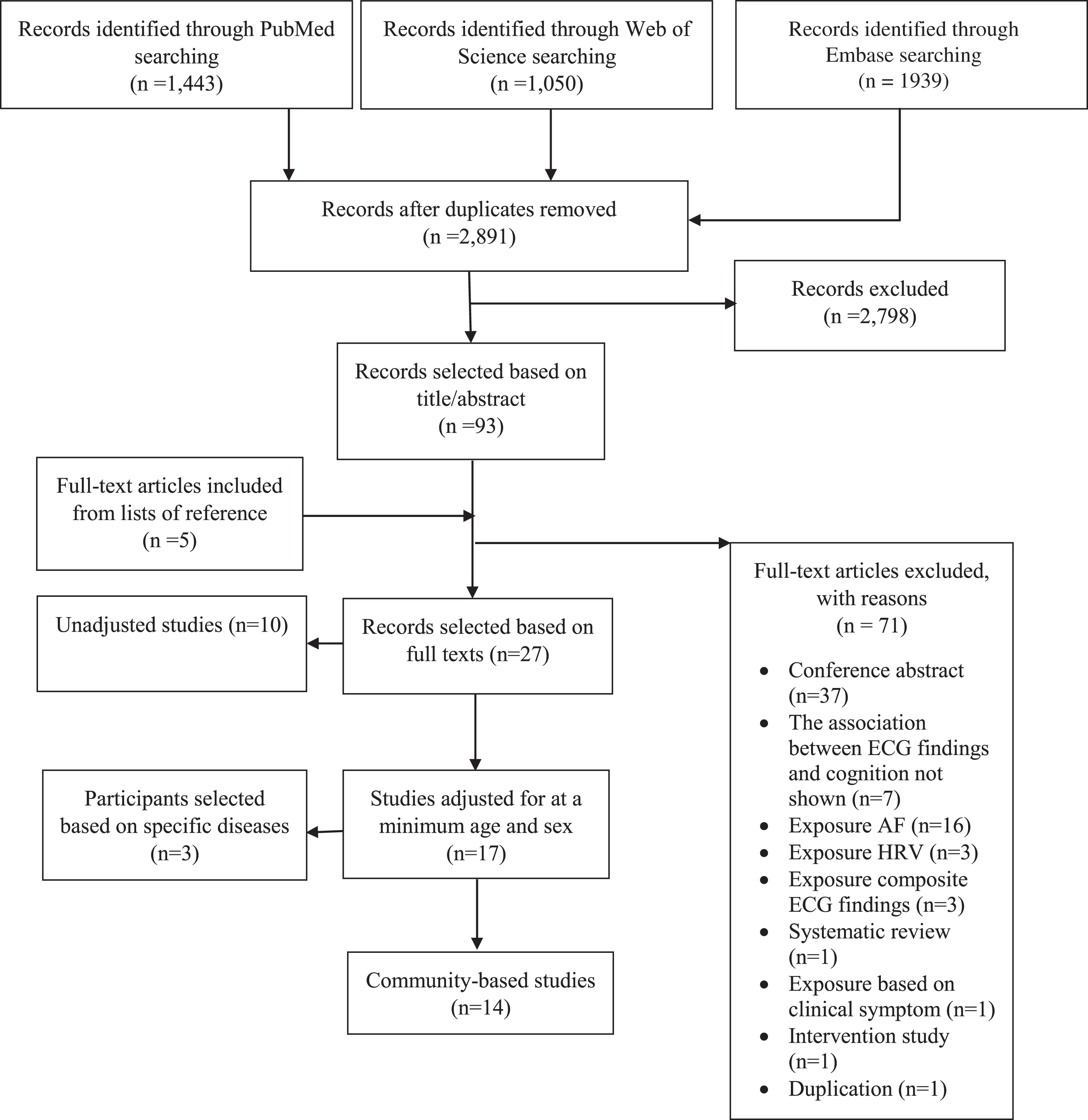
A total of 4,432 articles were retrieved. After removing duplicates, we reviewed 2,891 studies. We further identified additional five studies through searching reference lists of eligible studies. Screening by two reviewers yielded 27 full-text articles for inclusion (Fig. 1). Excluded studies and reasons for exclusion are presented in Supplementary Table 1. Among 27 eligible studies, ten studies showed crude associations (Supplementary Table 2). Among 17 studies adjusted for at least age and sex, three studies recruited participants with specific diseases (Supplementary Table 3). Results from 14 community-based studies are presented in Table 1.
Fig. 1
Flow diagram. AF, atrial fibrillation; HRV, heart rate variability.
Studies description
Out of the 14 eligible studies, four longitudinal community-based studies investigated the association between ECG markers and dementia risk (Table 1A) [11–14], and 13 examined the association with cognitive function using several cognitive tests (Table 1B) [12–24]. Of them, ten studies conducted longitudinal, or both longitudinal and cross-sectional, analyses [12–14, 18–24]. The data from the Atherosclerosis Risk in Communities (ARIC) Study and the PROspective Study of Pravastatin in the Elderly at Risk (PROSPER) were used in three studies each, which were all included because they examined different ECG markers. Therefore, this review included a large number of overlapping participants (approximately 13,000 participants in the ARIC and 4200 in the PROSPER). All studies in this review were conducted in the US or Western European countries, and no studies were from LMICs regions.
Table 1A
The association between various ECG findings and dementia in community-based studies
| Study, year, country | Study Population | Type of ECG, ECG findings | Outcome, Diagnosis criteria | Effect size from fully-adjusted model (95%confidence interval) | Adjustment | Quality |
| Frishman, 1996, USA [11] | The Bronx Aging Study, community dwelling population, N = 423, Age 75–85 y (mean 79.2 y), 36.0%males, Follow-up: mean 6.2 y | 24-h ECG, PVC, multifocal PVCs, significant ectopy, nonsustained ventricular tachycardia, sustained ventricular tachycardia, torsades des pointes, AF, paroxysmal atrial tachycardia, junctional tachycardia, sinus arrest, sinus pause, first-, second-, third-degree blocks, sinus bradycardia, | All-cause dementia, and multiinfarct dementia Detail not mentioned. | Multiinfarct dementia: AV block HR: 3.4 (0.9, 12.7), Slow heart rate HR: 2.4 (0.8, 7.1), Ventricular tachycardia HR: 6.3 (1.4, 28.7) All-cause dementia: Ventricular tachycardia HR: 1.8 (0.5, 6.2), AV block HR: 1.1 (0.4, 3.5), Slow heart rate HR: 1.5 (0.7, 3.2) | Age, gender; history of diabetes, hypertension, smoking, myocardial infarction, or stroke; LVH on baseline ECG, baseline BMI, development of diagnosable cancer after study entry; use of β adrenergic blockers, tricyclic antidepressants, centrally acting antihypertensive agents, antiarrhythmic drugs, diuretics, and digoxin | 7/9 |
| Gutierrez, 2019, USA [12] | The ARIC-NCS cohort, N = 13,714, Age 45–64 y (mean 57, SD 6), 44%, Mean 18 y | Resting 12-lead ECG, Abnormal P-wave terminal force in lead V1 (PTFV1), prolonged P-wave duration (PPWD), abnormal P-wave axis (aPWA) advanced interatrial block (aIAB) | Incident dementia, Predetermined algorithm based on NIA-AA criteria and DSM-5 criteria. | Incident dementia: PTFV1 HR: 1.60 (1.41, 2.83) PPWD HR: 1.60 (1.42, 1.80) PWA HR: 1.36 (1.17, 2.57) aIAB HR: 0.93 (0.61, 1.42) | Model 1: age, sex, race/field center | 8/9 |
| Model 2: Model 1 plus education, occupation, apolipoprotein E, smoking, BMI, SBP, DBP, antihypertensive medication, total cholesterol, diabetes, prevalent CHD, HF, and stroke | ||||||
| Model 3: Model 2 plus time-dependent incident stroke and AF | ||||||
| Norby, 2018, USA [13] | The ARIC-NCS cohort, N = 12,665, 56.9±5.7 y, 44%, Mean 18 y | Resting 12-lead ECG LVH dichotomous (yes/no): sex specific Cornell voltage criteria (SV3 + RaVL > 28 mm for men and 22 mm for women) | Incident dementia, Dementia was determined using the predetermined algorithm based on NIA-AA criteria and DSM-5. | Incident dementia in participant with LVH HR: 1.90 (1.47, 2.44) | Model 1: age, sex, and race/field center Model 2: model 1 plus education, occupation, apolipoprotein E, smoking, BMI, SBP, DBP, antihypertensive medication, total cholesterol, diabetes, prevalent CHD, prevalent HF. Model 3: model 2 plus time-dependent stroke and AF. | 8/9 |
| Wang, 2019, USA [14] | The ARIC-NCS, N = 13,172, 57.5±5.7 y, 44.1%, Over 20 y | Resting 12-lead ECG RHR in four categories (< 60, 60–69, 70–79, ≥80) | Incident dementia, Diagnosis criteria not mentioned in main text | Incident dementia in different RHR groups: RHR < 60: Reference RHR 60–69 HR: 1.08 (0.95, 1.24) RHR70–79 HR: 1.14 (0.97, 1.33) RHR≥80 HR: 1.28 (1.04, 1.57) | Model 1: age, age2, sex, race/center | 6/9 |
| Model 2: Model 1 plus education, BMI, smoking status, alcohol, physical activity | ||||||
| Model 3: Model 2 plus SBP, pulse pressure, use of hypertension medication, diabetes, HDL cholesterol, total cholesterol, cholesterol lowering medications, history of prevalent CHD, use of AV-nodal blocking medications, and APOE4 genotype |
AF, atrial fibrillation; ARIC-NCS, Atherosclerosis Risk in Communities Neurocognitive Study; BMI, body mass index; CHD, coronary heart disease; CHF, chronic heart failure; DBP, diastolic blood pressure; HF, heart failure; HR, hazard ratio; IAB, interatrial block; LVH, left ventricular hypertrophy; SBP, systolic blood pressure; NIA-AA, National Institute on Aging-Alzheimer’s Association dementia group formulations of dementia
Table 1B
The association between various ECG findings and cognitive function
| Study, year, country | Population, N, Age (mean/median), males (%) | Type of ECG, ECG findings | Outcome, Diagnosis criteria | Effect size from fully-adjusted model (95%confidence interval) | Adjustment | Quality |
| Cross-sectional association | ||||||
| Outcome: global cognitive function | ||||||
| Coppola, 2013, Italy [15] | Community living elderly registered members of social multifunctional geriatric center, N: AD 31, MCI 77, Cognitive normal 116, Age (y): AD 72.4±7.9, MCI 71.9±5.0, Cognitive normal 69.7±8.1, Male: AD 38.7%, MCI 44.2%, Cognitive normal 43.1% | Resting 12-lead ECG, QTc, QTcD | MMSE score | QTcD just p-value The association between MMSE and QTcD in MCI subjects β: not shown (p = 0.03) | Age, education, Hachinski ischemic score | 2/8 |
| Hale, 1992, USA [16] | Elderly in a health screening program, N = 1,271, Women: 77.8±6.19 y; Men: 78.0±5.97 y, 32.7% | Resting heart rate, 29 ECG abnormalities | MMSE score | The association between each ECG finding and MMSE score Left axis deviation p = 0.0038 Premature junctional contraction p = 0.021 Resting heart rate p = 0.07 | Age, sex, the Beck Depression Inventory | 3/8 |
| Lucas, 2010, USA [17] | CHAP, a stratified random sample aged over 65 y, N = 839, Median 81 (IQR 76-85) y, 37.0% | Resting 12-lead ECG, 1. T wave nondipolar voltage (TNDPV) (μV), 2. Rate-adjusted QT interval (QTrr) (ms), 3. The voltage change from the beginning to end of the ST segment in lead V5 (STV5 gradient) (μV), 4.QRS/T angle | Global cognitive performance z score derived from 17 cognitive performance tests | Difference in cognitive performance /TNDPV (μV) Core model β: –0.0290 (p = 0.01) fully-adjusted model β: –0.0240 (p = 0.03) Other ECG findings: NS | Core model: age, gender, education, race Fully-adjusted model: hypertension, diabetes, cigarette smoking, cholesterol, BMI, a quadratic term for BMI | 5/8 |
| Longitudinal association | ||||||
| Outcomes: cognitive decline/cognitive impairment defined by change in global cognitive function | ||||||
| Kähönen-Väre, 2004, Finland [18] | The Helsinki Aging Study, including three birth cohorts aged 75, 80, and 85 y. N = 650, 75 y n = 239, 80 y n = 212, 85 y n = 199, 75 y 29.6%, 80 years 29.6%, 85 y 19.8%Follow-up: 10 y | Resting 12-lead ECG LVH (detail not mentioned) | Cognitive decline (defined as an increase in the CDR class or a decrease of at least four points in the MMSE score) | LVH at baseline (%) in Cognitive decline at 1 y Yes versus No: 14.0%versus 11.0%(NS), LHV at baseline (%) in Cognitive decline at 5 y Yes versus No: 12.5%versus 10.2%(NS) LVH at baseline (%) in Cognitive decline at 10 y Yes versus No: 5.5%versus 12.0%(NS) | Age, sex | 5/9 |
| Unverzagt, 2011, US [19] | REGARDS, a national population-based cohort study of African American or white, N = 23,752, Age 45+, mean 64.3±9.2 y, 44%, Follow-up: mean 4.1 y | Resting 12-lear ECG (71%) or 7-lear ECG (39%) LVH (binary) Cornell voltage was calculated as the sum of the SV + aVL and SV3 + aVL in participants with 7-lead ECGs and 12-lead respectively. LVH was diagnosed if Cornell voltage = 2,200 ms in women or = 2,800 ms in men | Incident cognitive impairment assessed using the Six-item Screener (SIS) (a decline from an initial score of 5 or better to the most recent follow-up score of 4 or less.) | Incident cognitive impairment in patient with LVH: OR: 1.29 (1.05, 1.58) | Adjusted only for demographics: sex, race, region, education Fully adjusted models; further adjusted for FSRP, Age, SBP, antihypertensive medication, diabetes, AF, heart disease, current smoker | 8/9 |
| Outcomes: global cognitive function | ||||||
| Kaffashian, 2013, UK [20] | The Whitehall II study. N = 5,810, Age 35-55, mean 55.6±6.0 y, 71.5%, Follow-up: mean 18 y | Resting 12-leaer ECG, LVH (binary) | Change in global cognitive score that was created using all five tests (The Alice Heim 4-I, 20-word free recall test, “S” word?, “animal” word, the Mill Hill Vocabulary test) | 10-y cognitive change in change in participants with LVH: β: –0.04 (–0.08, –0.0004) | Models adjusted for demographics: age centered at the mean, sex, ethnicity, education. Health-related factors: depressive symptoms, physical activity, alcohol consumption | 7/9 |
| Suemoto, 2020, US [21] | The Honolulu Heart Study. Japanese American men born between 1990 and 1919 who lived on Oahu, N = 2,511, 54.4±4.4 y, 100% | Incident prolonged QT interval | Cognitive Abilities Screening Instrument (CASI) | The association between prolonged QT and late-life CASI-IRT β: 0.04 (–0.28, 0.35) The association between prolonged QT and change in CASI-IRT β: –0.002 (–0.013, 0.010) | Generation, alcohol use, physical activity level, education, occupation, and chest depth at Visit 1; age, height, and hypertension at Visit 2; and the presence of any APOE4 alleles at visit 4. | 6/9 |
| Outcomes: both global cognitive function &different domains of cognitive function | ||||||
| Gutierrez, 2019, US [12] | The ARIC-NCS. N = 13,714, Age 45–64, mean 57±6 y, 44%, Follow-up: mean 18 years | Resting 12-lead ECG, Abnormal P-wave terminal force in lead V1 (PTFV1), prolonged P-wave duration (PPWD), abnormal P-wave axis (aPWA) advanced interatrial block (aIAB) | The Delayed Word Recall test (DWRT), the Digit Symbol Substitution test of the Wechsler Adult Intelligence Scale-Revised (DSST), the Word Fluency test (WFT)) and global z-score based on three cognitive testing. | Associations between rate of cognitive change and ECG findings. Global z-score and PTFV1 β: –0.07 (–0.13, –0.01). Word Fluency test z-score and PTFV1 β: –0.07 (–0.13, –0.01). Other ECG findings NS | Model 1: age, sex, race/field center Model 2: Model 1 plus education, occupation, apolipoprotein E, smoking, BMI, SBP, DBP, antihypertensive medication, total cholesterol, diabetes mellitus, prevalent CHD, HF, and stroke Model 3: Model 2 plus time-dependent incident stroke and AF | 8/9 |
| Both cross-sectional and longitudinal analyses | ||||||
| Outcomes: both global cognitive function &different domains of cognitive function | ||||||
| Norby, 2018, US [13] | ARIC-NCS, N = 12,665, Age 45–64, mean 56.9±5.7 y, 44%, Follow-up: mean 18 y | LVH dichotomous (yes/no): sex specific Cornell voltage criteria (SV3 + RaVL > 28 mm for men and 22 mm for women) | DWRT, DSST, WFT, and global z-score based on three cognitive testing. | Cross-sectional analysis Difference in cognitive score in those with LVH compared to no LVH: Global z-score β: –0.16 (–0.24, –0.08) DWRT z-score β: –0.17 (–0.27, –0.06) DSST z-score β: –0.05 (–0.13, 0.02) WFT z-score β: –0.15 (–0.24, –0.05) Longitudinal analysis Additional change in cognitive testing in those with LVH compared to no LVH Global z-score β: 0.061 (–0.03, 0.155) DWRT z-score β: 0.055 (–0.091, 0.206) DSST z-score β: 0.035 (–0.041, 0.111) WFT z-score β: 0.082 (–0.008, 0.172) | Model 1: age (centered at 60), sex, race/center, time as a linear spline with a knot at 6 years, age by time spline terms, sex by time spline terms, and race/center by time spline terms. Model 2: model 1 plus education, occupation, apolipoprotein E, smoking, BMI, SBP, DBP, antihypertensive medication, total cholesterol, diabetes, prevalent CHD, prevalent HF, plus all these variables by time spline terms. Model 3: model 2 plus stroke and atrial fibrillation, plus these variables by time spline terms. | 8/9 |
| Wang, 2019, US [14] | ARIC-NCS cohort, N = 13,172, Age 44–66, mean 57.5±5.7 y, 44.1%, Follow-up: over 20 y | Resting 12-lead ECG Resting heart rate (RHR) in four categories (< 60, 60–69, 70–79, ≥80) | DWRT, DSST, WFT, and global z-score based on three cognitive testing. | Cross-sectional analysis The association between global z-score and resting heart rate groups: RHR < 60: reference, RHR≥80 β: –0.08 (–0.13, –0.03) (other NS) The association between DWRT z-score and RHR RHR≥80 β: –0.09 (–0.15, –0.03) (other NS) | Model 1: age, age2, sex, race/center, interactions between each of these variables and time Model 2: Model 1 plus education, BMI, smoking status, alcohol, physical activity, interactions between each of these variables and time | 6/8 |
| The association between DSST and RHR RHR≥80 β: –0.11 (–0.16, –0.07) (other NS) The association between WFT and RHR: NS Longitudinal analysis Difference in global cognitive decline between resting heart rate groups: Difference Difference RHR70-79 versus < 60 β: –0.07 (–0.13, –0.004) Difference RHR > = 80 versus < 60 β: –0.12 (–0.21, –0.03) (Other NS) Difference in DWRT All NS Difference in DSST Difference RHR60-69 versus < 60 β: –0.03 (–0.06, –0.003) Difference RHR70–79 versus < 60 β: –0.05 (–0.08, –0.01) Difference RHR≥80 versus < 60 β: –0.10 (–0.15, –0.05) Difference in WFT Difference RHR≥80 versus < 60 β: –0.12 (–0.18, –0.05) (Other NS) | Model 3: Model 2 plus SBP, pulse pressure, use of hypertension medication, diabetes, HDL cholesterol, total cholesterol, cholesterol lowering medications, history of prevalent CHD, use of AV-nodal blocking medications, APOE4 genotype, and interactions between each of these variables and time | |||||
| Outcomes: different domains of cognitive function | ||||||
| Mahinrad, 2017, Ireland, Scotland, the Netherlands [22] | The secondary analysis of a randomized controlled trial PROSPER, older subjects at high cardiovascular risk, N = 4,233, 75.2±3.3 y, 47.8% | Resting 12-lead ECG, LVH (Sokolow-Lyon voltage product level) in three categories: Low (17.11–177.16 μVs), Medium (17.20–245.55 μVs), High (245.70–902.53 μVs) | The Stroop interference test, the Letter-Digit Coding Test (LDCT), immediate and delayed Picture-Word Learning Test (PLTi and PLTd) | Cross-sectional analysis The Stroop interference test, LDCT, PLTi, PLTd per LVH category: all NS Longitudinal analysis Change in the Stroop interference test per LVH category: NS Change in LDCT β: –0.28 (–0.37, –0.20) Change in PLTi β: 0.05 (0.02, 0.09) Change in PLTd: NS | Model 1: age, sex, country, education, version of cognitive tests Model 2: age, sex, country, education, version of cognitive tests where applicable, BMI, smoking status, SBP, DBP, history of diabetes, history of vascular disease, total cholesterol level, HDL-cholesterol level, antihypertensive medications (diuretics, beta-blockers, calcium channel blockers, angiotensin converting enzyme inhibitors and angiotensin receptor blockers), anticoagulant medications. | 8/9 |
| Mahinrad, 2019, Ireland, Scotland, The Netherlands [23] | The secondary analysis of a randomized controlled trial PROSPER, older subjects at high cardiovascular risk, N = 4,172, 75.2±3.3 y, 47.9% | Spatial QRS-T angle in three categories: Low, Middle, High | The Stroop interference test, the LDCT, PLTi and PLTd | Cross-sectional analysis The Stroop interference test, LDCT, PLTi, PLTd per LVH category: all NS Longitudinal analysis Change in Stroop test β: 0.004 (0.0007, 0.007) Change in LDCT β: –0.312 (–0.37, –0.25) Change in PLTi β: 0.022 (–0.002, 0.047), Change in PLTd: β: –0.031 (–0.067, 0.005) | Model 1: age, sex, country, education, version of cognitive tests where applicable; Model 2: Model 1 plus history of vascular disease, history of diabetes, SBP, DBP, BMI, smoking status, antihypertensive medications and statin treatment groups. | 7/8 |
| Zonneveld, 2020, The Netherlands, Scotland, Ireland [24] | Secondary analysis of a randomized controlled trial PROPSER,.Older individuals at increased risk for cardiovascular disease but free of known arrhythmias. N = 4,627, 75.2±3.2 y, 46.4% | QT, QTc, JT, JTc, QRS interval | Decline in cognitive function at follow-up assessed using the Stroop Test, the LDCT, PLTi and PLTd | ross-sectional analysis Stroop test QT β: 3.02 (0.31, 5.73) QTc β:1.48 (–0.44, 3.39) JT β: 3.93 (1.23, 6.64) JTc β: 2.34 (0.39, 4.30), QTc β, QRS β: NS LDCT, digits coded QT β: –1.07 (–1.84, –0.31) QTc β: –0.62 (–1.15, –0.08) JT β: –0.81 (–1.58, –0.04) JTc β, QRS β NS PLTi, pictures remembered: All NS PLTd, pictures remembered: All NS Longitudinal analysis No associations of specific ECG findings with test were significant. | Minimally adjusted: Age, sex, country, heart rate (QT, JT, and QRS only), Fully adjusted: Age, sex, country, heart rate (QT, JT, and QRS only), alcohol intake per week, smoking, education level, BMI, serum cholesterol, antihypertensive medication, anticholinergic medication, antiarrhythmic medication, antidepressants, diabetes mellitus, SBP | 6/8 |
AD, Alzheimer’s disease; AF, atrial fibrillation; ARIC-NCS, Atherosclerosis Risk in Communities Neurocognitive Study; BMI, body mass index; CDR, Clinical Dementia Rating; CHAP, The Chicago Health and Aging Project; CHD, coronary heart disease; CHF, chronic heart failure; DBP, diastolic blood pressure; DLB, dementia with Lewy bodies; HF, heart failure; HR, hazard ratio; IAB, interatrial block; LVH, left ventricular hypertrophy; MCI, mild cognitive impairment; MI, myocardial infarction; MMSE, Mini-Mental State Examination; OR, odds ratio; PROSPER, PROspective Study of Pravastatin in the Elderly at Risk; QTc, corrected heart rate values of QT interval; QTcD, QT dispersion; REGARDS, Those without stroke from the Reasons for Geographic and Racial Differences in Stroke; SBP, systolic blood pressure; VaD, vascular dementia.
Assessment of cognitive outcomes
Dementia was diagnosed using predetermined algorithms based on the National Institute on Aging-Alzheimer’s Association working group formulations of dementia and the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition in three studies from the ARIC population [12–14], while one study did not provide detail of diagnoses criteria [11].
Global cognitive function was assessed using the Mini-Mental State Examination (MMSE), Cognitive Abilities Screening Instrument [25], or composite cognitive score derived from several cognitive subdomain tests. Multiple neuropsychological tests were used to assess specific cognitive domains, such as memory, processing speed, and executive function in studies based on the ARIC and PROSPER populations.
In the ARIC study, the Delayed Word Recall (DWR) test (verbal learning and recent memory), the Digit Symbol Substitution (DSS) test (executive function and processing speed), and the Word Fluency (WF) test (executive function and language) were performed, while the PROSPER performed the Stroop interference test (selective attention), the Letter-Digit Coding Test (LDCT) (general cognitive speed), and the Picture-Word leaning (PLTi, PLTd) (immediate and delayed memory).
Assessment of ECG markers
Most studies used a standard resting 12-lead ECG, except one that used 24-hour Holter ECG to assess various arrhythmias [11]. ECG signs of left ventricular hypertrophy (LVH) were determined in five studies, while QT interval and resting heart rate were investigated in three studies. Abnormal P waves and spatial QRS-T angle were examined in just one study. No studies with dementia outcomes except for two case-control studies included in Supplementary Table 2 mentioned the current or previous use of cholinesterase inhibitors.
Associations of ECG markers with cognitive function
The results are presented according to ECG markers and outcomes (Table 2A for dementia, Table 2B for cognitive function). We conducted a narrative synthesis of the included studies without pooling effect estimates since the availability of overlapping specific ECG markers and outcomes was limited and heterogeneous.
Table 2A
The association between various ECG findings and dementia: an overview
| Rate, rhythm, conduction | P wave | QRS wave | Repolarization, others | Quality | |||||||||||||||
| RHR | Arrhythmia | AV block | Bundle branch block | PTFV1 | PPWD | PWA | IAB | LVH | QRS low voltage | QRS interval | QTcD | QT interval | ST, T abnormality | ST depression, Q | TNDPV | STV5 gradient | QRS T angle | ||
| Longitudinal analyses | |||||||||||||||||||
| Frishman, 1996 [11] | NS | √a | NS | 7/9 | |||||||||||||||
| Gutierrez, 2019 [12] | √ | √ | √ | NS | 8/9 | ||||||||||||||
| Norby, 2018 [13] | √ | 8/9 | |||||||||||||||||
| Wang, 2019 [14] | √b | 6/9 | |||||||||||||||||
IAB: interatrial block, LVH: left ventricular hypertrophy, PPWD: prolonged P-wave duration, PTFV1: an abnormal P-wave terminal force in lead V1, PWA: abnormal P-wave axis, QTcD: QT dispersion, RHR: resting heart rate, STV5 gradient: voltage change from beginning to the end of ST-segment in lead V5, TNDPV: the T wave nondipolar voltage. aamong various ECG findings, ventricular tachycardia was significantly associated with multi-infarct dementia, bParticipants with resting heart rate (RHR) ≥80 had an increased risk for incident dementia compared to those with RHR < 60 but those with RHR 60–69 or 70–79 did not have a higher risk.
Table 2B
The association between various ECG findings and cognitive function: an overview
| Test | Rate, rhythm, conduction | P wave | QRS wave | Repolarization, others | |||||||||||||||
| Study authors, year | RHR | Arrhythmia 24 h | AV block | Bundle branch block | PTFV1 | PPWD | PWA | IAB | LVH | QRS low voltage | QRS interval | QTcD | QT interval | ST, T abnormality | ST depression, Q | TNDPV | STV5 gradient | QRS T angle | |
| Cross-sectional analyses | |||||||||||||||||||
| Coppola, 2013 [15] | Global | √ | |||||||||||||||||
| Hale, 1992 [16] | Global | NS | √ | ||||||||||||||||
| Lucas, 2010 [17] | Global | NS | √ | NS | NS | ||||||||||||||
| Norby, 2018* [13] | Global | √ | |||||||||||||||||
| DWR | √ | ||||||||||||||||||
| DSS | NS | ||||||||||||||||||
| WF | √ | ||||||||||||||||||
| Wang, 2019* [14] | Global | √a | |||||||||||||||||
| DWR | √a | ||||||||||||||||||
| DSS | √a | ||||||||||||||||||
| WF | NS | ||||||||||||||||||
| Mahinrad, 2017* [22] | Stroop | NS | |||||||||||||||||
| LDCT | NS | ||||||||||||||||||
| PLTi | NS | ||||||||||||||||||
| PLTd | NS | ||||||||||||||||||
| Mahinrad, 2019* [23] | Stroop | NS | |||||||||||||||||
| LDCT | NS | ||||||||||||||||||
| PLTi | NS | ||||||||||||||||||
| PLTd | NS | ||||||||||||||||||
| Zonneveld, 2020* [24] | Stroop | NS | NS | √ | |||||||||||||||
| LDCT | NS | √ | √ | ||||||||||||||||
| PLTi | NS | NS | NS | ||||||||||||||||
| PLTd | NS | NS | NS | ||||||||||||||||
| Longitudinal analyses | |||||||||||||||||||
| Kähönen-Väre, 2004 [18] | NS | ||||||||||||||||||
| Unverzagt, 2011 [19] | √ | ||||||||||||||||||
| Kaffashian, 2013 [20] | Global | √b | |||||||||||||||||
| Suemoto, 2020 [21] | Global | ||||||||||||||||||
| NS | |||||||||||||||||||
| Gutierrez, 2019 [12] | Global | √ | NS | NS | NS | ||||||||||||||
| DWR | NS | NS | NS | NS | |||||||||||||||
| DSS | NS | NS | NS | NS | |||||||||||||||
| WF | √ | NS | NS | NS | |||||||||||||||
| Norby, 2018* [13] | Global | NS | |||||||||||||||||
| DWR | NS | ||||||||||||||||||
| DSS | NS | ||||||||||||||||||
| WF | NS | ||||||||||||||||||
| Wang, 2019* [14] | Global | √c | |||||||||||||||||
| DWR | NS | ||||||||||||||||||
| DSS | √d | ||||||||||||||||||
| WF | √e | ||||||||||||||||||
| Mahinrad, 2017* [22] | Stroop | NS | |||||||||||||||||
| LDCT | √ | ||||||||||||||||||
| PLTi | √ | ||||||||||||||||||
| PLTd | NS | ||||||||||||||||||
| Mahinrad, 2019* [23] | Stroop | √ | |||||||||||||||||
| LDCT | √ | ||||||||||||||||||
| PLTi | NS | ||||||||||||||||||
| PLTd | √ | ||||||||||||||||||
| Zonneveld, 2020* [24] | Stroop | NS | NS | NS | |||||||||||||||
| LDCT | NS | NS | NS | ||||||||||||||||
| PLTi | NS | NS | NS | ||||||||||||||||
| PLTd | NS | NS | NS | ||||||||||||||||
*Both cross-sectional and longitudinal analyses were conducted. DSS, Digit Symbol Substitution (executive function and processing speed); DWR, Delayed Word Recall (verbal learning and recent memory); IAB, interatrial block; LDCT, general cognitive speed; LVH, left ventricular hypertrophy; PLTd, delayed memory; PLTi, immediate memory; PPWD, prolonged P-wave duration; PTFV1, an abnormal P-wave terminal force in lead V1; PWA, abnormal P-wave axis; QTcD, QT dispersion; RHR, resting heart rate; Stroop, the Stroop interference test (selective attention); STV5 gradient, voltage change from beginning to the end of ST-segment in lead V5; TNDPV, the T wave nondipolar voltage; WF, Word Fluency (executive function and language). aParticipants with RHR ≥80 had significantly lower scores than those with RHR < 60, bLVH was associated with a change in global cognition in the fully adjusted model, cThose with RHR ≥80, 70–79 experienced greater decline than those with RHR < 60, dThose with RHR ≥80, 70–79, and 60–69 experienced greater decline than those with RHR < 60, eThose with RHR ≥80 experienced greater decline than those with RHR < 60.
Associations of ECG-assessed LVH with dementia and cognitive function
Dementia
Only one study investigated the association between electrocardiographic signs of LVH and dementia. The 18-year follow-up data from the ARIC cohort showed that LVH at baseline was associated with a higher risk for dementia [13].
Cognitive function
The cross-sectional associations between LVH and cognitive function were examined in two population-based studies. The ARIC Study showed association of LVH with low global cognitive z-score, DWR, and WF while not with DSS [13], whereas in the PROSPER Study, LVH was not associated with any of the four cognitive tests (Stroop, LDCT, PLTi, and PLTd) [22].
Five prospective cohort studies investigated the association between LVH at baseline and cognitive decline. For global cognitive function, LVH was associated with global cognitive decline in the Whitehall II Study and the large-scale REGARDS study [19, 20], while the ARIC Study and a relatively small Finnish study showed no significant associations [13, 18]. These four studies of global cognitive function differed substantially regarding participant characteristics, follow-up period, and statistical methods. For the associations with specific cognitive domains, LVH was associated with a decrease in LDCT and PLTi scores, but not Stroop and PLTd scores in the PROSPER Study [22]. On the contrary, LVH was not associated with any of the three cognitive tests (DWR, DSS, and WF) in the ARIC Study [13].
Associations of ventricular repolarization markers with cognitive function
Dementia
There was no study investigating the association between markers of ventricular repolarization and dementia.
Cognitive function
Three population-based studies examined cross-sectional associations between various markers of ventricular repolarization and cognitive function. The Chicago Health and Aging Project investigated the association between global cognitive z-score and four markers of cardiac repolarization abnormality (QT interval, the T wave nondipolar voltage: TNDPV, voltage change in ST-segment in V5 lead: STV5 gradient, QRS-T angle), and only TNDPV was associated with low global cognitive z-score [17]. Regarding cognitive subdomains, QT interval was associated with LDCT but not with the Stroop test, PLTi, and PLTd in the PROSPER Study [24], while QRS-T angle showed no significant associations with any cognitive tests in another PROSPER Study [23].
Two prospective cohort studies examined the association between ventricular repolarization markers and cognitive decline [21, 23]. Data from the PROSPER Study showed that spatial QRS-T angle was associated with a decline in Stroop, LDCT, and PLTd scores, but not PLTi [23]. Prolonged QT interval was not associated with cognitive decline in the Honolulu-Asia Aging Study of Japanese American men [21].
Associations of resting heart rate with dementia and cognitive function
Dementia
The study that investigated the association between resting heart rate (RHR) and dementia found that participants with RHR ≥80 beats per minute (bpm) had a higher risk for developing dementia compared to those with RHR < 60 bpm over a 20-year follow-up period in the ARIC Study [14]. One small study showed heart rate < 60 bpm assessed by 24-h Holter ECG was not associated with dementia after a 10-year follow-up [11].
Cognitive function
The ARIC study also investigated the association between RHR and cognitive function, and observed results were generally consistent with those for RHR and dementia [14]. In cross-sectional analysis, RHR ≥80 bpm was associated with poor global cognitive function and lower DWR and DSS scores at baseline. Another cross-sectional study with a small sample size showed that RHR was not associated with MMSE score [16].
In the prospective analyses, participants with RHR ≥80 bpm and RHR 70–79 bpm experienced a steeper decline in global cognitive z-score compared to those with RHR < 60 bpm in the ARIC Study [14]. Regarding cognitive subdomains, those with RHR ≥80, 70–79, and 60–69 bpm at baseline experienced a greater decline in DSS score compared to those with RHR < 60 bpm, while only those with RHR ≥80 bpm were associated with a greater decline in WF. Change in DWR was not different among RHR groups.
Associations of P-wave abnormality with dementia and cognitive function
Dementia
Only one study investigated the association between P-wave abnormality and dementia. Gutierrez et al. reported that three abnormal P-wave markers, abnormal P-wave terminal force in lead V1 (PTFV1), prolonged P-wave duration (PPWD), and abnormal P-wave axis (aPWA) at baseline, were associated with incident dementia after 18 years of follow-up in the ARIC Study [12]. No such association was observed for advanced interatrial block (aIAB).
Cognitive function
Gutierrez et al. also studied the association between four abnormal P-wave indices (PTFV1, PPWD, aPWA, and aIAB) and change in global cognitive z-score, where only PTFV1 was associated with a greater cognitive decline after the adjustment for traditional CVD risk factors [12]. Cognitive subdomains were also examined, where PTFV1 at baseline was associated with a steeper decline in WF score but not DWR and DSS scores. In contrast, the other three abnormal P-wave indices were not associated with any of the cognitive subdomain tests.
Other ECG markers
A small population-based study investigated the association between various arrhythmias assessed using 24-h Holter ECG and dementia, and only ventricular tachycardia was associated with multi-infarct dementia after a 6-year follow-up period [11].
DISCUSSION
Main findings
In this systematic review, LVH, RHR, abnormal P-waves, and spatial QRS-T angle were identified to be potential risk markers for dementia or cognitive decline in at least one prospective study of good methodological quality. However, inconsistent findings, the paucity of studies, and methodological heterogeneity make the interpretation of our findings difficult. ECG sign of LVH was the most commonly studied predictor, still only five heterogeneous studies, while other ECG predictors were investigated in only a couple of studies. This prevented us from conducting pooled quantitative analyses of these findings or drawing any strong conclusions. Further research is needed to confirm the association between ECG markers and cognitive decline.
Although ECG is a simple diagnostic tool compared to more advanced diagnostic procedures such as echocardiography and cardiac MRI scans, some ECG findings appear to have good predictive value for a wide variety of diseases. Accumulating evidence suggests that various ECG findings, such as abnormal P-waves, LVH, and markers of ventricular repolarization, predict cardiovascular morbidity and mortality [26–30]. CVDs and dementia share many risk factors, and CVDs such as stroke, AF, and heart failure are known risk factors for dementia. Therefore, it is likely that these ECG findings are also associated with dementia, possibly mediated by subclinical and clinical CVDs and subsequent hemodynamic disturbances. If ECG findings are associated with a higher risk of dementia and cognitive decline, they can be considered risk factors in a wide variety of settings.
ECG-assessed LVH
LVH is a marker of target organ damage, resulting from long-term exposure to cardiovascular risk factors, particularly high blood pressure [31]. Five longitudinal studies in our review showed inconsistent results regarding the association between LVH assessed using ECG (ECG LVH) and cognitive decline. A meta-analysis of population-based studies showed that LVH, assessed using both echocardiography and ECG, was associated with an increased risk for cognitive impairment [32], but the association was weaker in ECG LVH than LVH detected by echocardiography. This is expected because the sensitivity of LVH diagnosis using ECG is low. However, this meta-analysis of ECG LVH is based on only three studies, where one large study consists of 96%of pooled participants, affecting overall results. To provide reliable pooled results, more studies are needed regarding the association of ECG LVH with cognitive decline.
It is well known that ECG is less sensitive in the assessment of LVH compared to echocardiography or other imaging modalities [33]. However, LVH is easily assessed using ECG with no additional cost, and specificity is very high. Besides, several meta-analyses show a good predictive value of ECG LVH for stroke and cardiovascular events in both hypertensive patients and the general adult population [30, 34, 35]. Stroke and dementia share risk factors, and stroke doubles the risk of dementia [36]. Furthermore, a recent meta-analysis shows that LVH, assessed using both echocardiography and ECG, is associated with white matter hyperintensities and lacunes after the adjustment for hypertension and other vascular risk factors [37]. These cerebral small vessel diseases play an important role in the development of cognitive decline and dementia [38]. Therefore, it is possible that ECG LVH may be a marker for predicting cognitive decline as a result of the altered cerebral vasculature caused by the effect of LVH.
Ventricular repolarization markers
One study showed that a wider spatial QRS-T angle was associated with a steeper decline in cognitive performance [23]. QRS-T angle is a spatial angle between directions of ventricular depolarization and re-polarization, and a wide QRS-T angle is considered as a marker of ventricular re-polarization abnormality. Previous studies showed QRS-T angle had a good predictive ability for all-cause and cardiovascular mortality [28]. Wider QRS-T angle can be caused by scared cardiac tissues and a small ischemic area in undetected coronary disease [39]. Change in QRS-T angle also reflects pathophysiological changes in ionic channels, which makes individuals more susceptible to ventricular rhythm disturbance [40]. These mechanisms may partially explain high cardiac mortality in those with a wide QRS-T angle. And decreased brain perfusion subsequent to altered cardiac function might be associated with decreased cognitive function.
QT interval prolongation, another re-polarization abnormality, was not associated with cognitive decline in two longitudinal studies in this review [21, 24]. The association between QT interval prolongation and cardiovascular mortality is well investigated, and meta-analysis shows that prolonged QT interval is associated with all-cause, cardiovascular, and CHD deaths [29].
Resting heart rate
The ARIC study showed that higher RHR at midlife was associated with both a higher risk of incident dementia and greater cognitive decline in the middle-aged population [14]. There are two other studies that investigated the association of RHR with cognitive function, which are not included in this review because RHR was assessed by pulse palpation. A follow-up study of 20,185 patients with stroke suggested that higher RHR was associated with a greater decline in MMSE score, along with higher total and cardiovascular deaths [41]. In the population-based cross-sectional study of middle-aged and older adults from Denmark, RHR was not associated with cognitive function [42]. However, RHR was measured using pulse palpation, which may be inaccurate. Further prospective studies using ECG-assessed RHR are needed to confirm its association with cognitive decline, especially in the general population of older adults.
RHR is a very simple ECG marker that has been consistently associated with all-cause mortality, CVDs, and non-CVDs such as cancer in a dose-response manner [43–46]. Because various CVDs are well-established risk factors for dementia, RHR may also be associated with dementia partially via underlying CVDs. The potential mechanisms linking high RHR with CVDs include sympathetic overactivity, arterial stiffness caused by mechanical pulsatile stress and share stress, and ventricular arrhythmia triggered by high RHR [47].
P-wave abnormalities
There was evidence that PTFV1, PPWD, and aPWA were associated with dementia risk, but they were examined in only one study [12]. Notably, PTFV1 showed associations with both incident dementia and cognitive decline, highlighting this measure as a potential candidate marker for cognitive impairment if reproducible in future well-designed studies. P-wave abnormalities reflect atrial dilation, atrial hypertrophy, elevated atrial pressure, and delayed intra-atrial conduction [48]. PTFV1 and P-wave duration are commonly used markers of P-wave abnormalities. One meta-analysis shows that PTFV1 and P-wave duration predict incident stroke [26], while another meta-analysis shows that P-wave duration predicts heart failure and AF, but not CVD and stroke [27]. The link between the abnormal P wave and stroke may partially be explained by AF. The aforementioned atrial changes make the atrial susceptible to AF, which is an important risk factor for stroke. Furthermore, recent evidence suggests that structural and functional atrial changes increase the thrombosis risk even without AF, possibly through atrial cardiopathy [49]. Stroke is a known risk factor for dementia, and incident stroke doubles the risk for all-cause dementia [36]. Furthermore, data from the Cardiovascular Health Study showed that PTFV1 was associated with white matter hyperintensity [50]. Therefore, it is likely that P-wave abnormalities are associated with not only stroke but also with dementia.
Potential mechanisms
Overall, although the mechanism by which ECG markers affect cognitive function has been poorly understood, it is likely that altered or impaired cardiac function detected by ECG leads to cerebrovascular insult, including small vessel disease, increased risk of thrombosis, and decreased cerebral perfusion, resulting in cognitive decline. Furthermore, it is suggested that there is a synergistic interaction between cerebrovascular damages and the neurodegenerative process. For example, some studies suggest that cerebrovascular insults promote Aβ accumulation [51]. If some ECG markers are risk factors for dementia and cognitive decline, this would further support the heart-brain continuum hypothesis, emphasizing the importance of paying attention to both cardiac and cognitive function in patients with CVD risks.
Knowledge gap
By reviewing the literature, we identified four major gaps in knowledge related to the association between ECG markers and cognitive aging. Firstly, current evidence from high-quality research in support of the potential association of ECG markers with dementia was primarily derived from the ARIC cohort of middle-aged people. Thus, further high-quality research is needed to confirm these findings, especially in the elderly population. Secondly, very few ECG markers were explored in multiple studies, with LVH being the most frequently explored marker. The associations of ECG markers other than LVH (e.g., QRS-T angle and abnormal P) with cognitive decline and dementia need to be elucidated in future studies. Thirdly, ECG markers in association with cognitive subdomains are rarely investigated apart from some data from ARIC and PROSPER cohorts. It could be informative to assess which cognitive subdomain is most affected by subclinical cardiac change detected by ECG. Furthermore, global cognitive function or cognitive impairment was assessed using the MMSE test in several reviewed studies, which has potential limitations such as ceiling effect, floor effects, and insensitivity to mild cognitive impairment [52]. The selection of appropriate cognitive testing would be vital in future studies. Fourth, among all studies with dementia outcomes, only two case-control studies mentioned the use of cholinesterase inhibitors, although its use can affect ECGs. Regarding four prospective studies in our main results, prevalent dementia cases were excluded, and it is very unlikely that participants used cholinesterase inhibitors at baseline ECG examination due to the specificity of cholinesterase inhibitor use for the treatment of dementia. However, in cross-sectional or case-control studies, it would be desirable to include information on the use of cholinesterase inhibitors and exclude participants on them. Finally, all eligible studies were from the US and Western European countries. It is known that the associations identified in HICs are sometimes not replicated in LMICs due to different sociodemographic and socioeconomic structures [53]. Considering that ECG markers are particularly useful in a low-cost setting, further studies in LMICs would be necessary. Some ECG markers such as special QRS-T angle and PTFV1 require the analysis software for advanced interpretation, which may not be relevant in a low-cost setting or a daily clinical setting. On the other hand, some ECG markers such as LVH and RHR are easily obtained manually, using widely available 12-lead ECG.
Strengths and limitations
This is the first systematic overview of the studies investigating the association between various ECG markers and cognitive outcomes. However, our review has limitations. Firstly, we could not conduct quantitative meta-analyses due to the paucity of studies for specific ECG markers and heterogeneity of study design. Secondly, because this review consists of observational studies and no data from randomized controlled trials are included, we cannot assess causality. Finally, the results of our review may be subject to publication bias. We restricted our search to English-written articles, and the studies with significant results are more likely to be published in English.
Clinical implications
Although some ECG markers appear to be associated with future cognitive decline and development of dementia in our review, it would be premature to conclude that they can be used as biomarkers of dementia. However, some of the ECG markers might be considered potential risk factors for accelerated cognitive decline, implying the presence of cerebrovascular pathology caused by altered cardiac function. ECG examination is widely available and inexpensive. If physicians carefully assess and follow up both cognitive function and cardiac function in those with specific ECG markers, the early signs of cognitive decline might be captured, resulting in early intervention. Besides, physicians can modify other behavioral and cardiovascular risk factors for dementia and encourage patients to adhere to a healthy lifestyle. These approaches might delay the onset of dementia and help to reduce the burden of both dementia and CVDs in the older population.
CONCLUSION
Emerging evidence has suggested that several ECG markers might be associated with cognitive decline and dementia. However, their associations have been investigated in only a limited number of studies from US and western European countries. Given that ECG markers are non-invasive, inexpensive, and easily available in different settings, future studies that confirm the predictive role of specific ECG markers in cognitive aging are of high relevance for identifying target populations for early interventions.
ACKNOWLEDGMENTS
CQ received grants from the Swedish Research Council (grants no. 2017-00740, 2017-05819, and 2020-01574), the Swedish Foundation for International Cooperation in Research and Higher Education (CH2019-8320), and the Karolinska Institutet, Stockholm, Sweden.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/21-0606r1).
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-210606.
REFERENCES
[1] | Patterson C ((2018) ) World Alzheimer Report 2018. The state of the art of dementia research: New frontiers. Alzheimer’s Disease International, London. |
[2] | Livingston G , Huntley J , Sommerlad A , Ames D , Ballard C , Banerjee S , Brayne C , Burns A , Cohen-Mansfield J , Cooper C , Costafreda SG , Dias A , Fox N , Gitlin LN , Howard R , Kales HC , Kivimäki M , Larson EB , Ogunniyi A , Orgeta V , Ritchie K , Rockwood K , Sampson EL , Samus Q , Schneider LS , Selbæk G , Teri L , Mukadam N ((2020) ) Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396: , 413–446. |
[3] | Qiu C , Fratiglioni L ((2015) ) A major role for cardiovascular burden in age-related cognitive decline. Nat Rev Cardiol 12: , 267–277. |
[4] | Abete P , Della-Morte D , Gargiulo G , Basile C , Langellotto A , Galizia G , Testa G , Canonico V , Bonaduce D , Cacciatore F ((2014) ) Cognitive impairment and cardiovascular diseases in the elderly. A heart-brain continuum hypothesis. Ageing Res Rev 18: , 41–52. |
[5] | van Buchem MA , Biessels GJ , Brunner la Rocca HP , de Craen AJ , van der Flier WM , Ikram MA , Kappelle LJ , Koudstaal PJ , Mooijaart SP , Niessen W ((2014) ) The heart-brain connection: A multidisciplinary approach targeting a missing link in the pathophysiology of vascular cognitive impairment. J Alzheimers Dis 42: , S443–S451. |
[6] | Forte G , Favieri F , Casagrande M ((2019) ) Heart rate variability and cognitive function: A systematic review. Front Neurosci 13: , 710. |
[7] | da Silva VP , Ramalho Oliveira BR , Tavares Mello RG , Moraes H , Deslandes AC , Laks J ((2018) ) Heart rate variability indexes in dementia: A systematic review with a quantitative analysis. Curr Alzheimer Res 15: , 80–88. |
[8] | Islam MM , Poly TN , Walther BA , Yang HC , Wu CC , Lin MC , Chien SC , Li YC ((2019) ) Association between atrial fibrillation and dementia: A meta-analysis. Front Aging Neurosci 11: , 305. |
[9] | Liu DS , Chen J , Jian WM , Zhang GR , Liu ZR ((2019) ) The association of atrial fibrillation and dementia incidence: A meta-analysis of prospective cohort studies. J Geriatr Cardiol 16: , 298–306. |
[10] | Kwok CS , Loke YK , Hale R , Potter JF , Myint PK ((2011) ) Atrial fibrillation and incidence of dementia: A systematic review and meta-analysis. Neurology 76: , 914–922. |
[11] | Frishman WH , Heiman M , Karpenos A , Ooi WL , Mitzner A , Goldkorn R , Greenberg S ((1996) ) Twenty-four-hour ambulatory electrocardiography in elderly subjects: Prevalence of various arrhythmias and prognostic implications (report from the Bronx Longitudinal Aging Study). Am Heart J 132: , 297–302. |
[12] | Gutierrez A , Norby FL , Maheshwari A , Rooney MR , Gottesman RF , Mosley TH , Lutsey PL , Oldenburg N , Soliman EZ , Alonso A , Chen LY ((2019) ) Association of abnormal P-wave indices with dementia and cognitive decline over 25 years: ARIC-NCS (The Atherosclerosis Risk in Communities Neurocognitive Study). J Am Heart Assoc 8: , e014553. |
[13] | Norby FL , Chen LY , Soliman EZ , Gottesman RF , Mosley TH , Alonso A ((2018) ) Association of left ventricular hypertrophy with cognitive decline and dementia risk over 20 years: The Atherosclerosis Risk In Communities-Neurocognitive Study (ARIC-NCS). Am Heart J 204: , 58–67. |
[14] | Wang S , Fashanu OE , Zhao D , Guallar E , Gottesman RF , Schneider ALC , McEvoy JW , Norby FL , Aladin AI , Alonso A , Michos ED ((2019) ) Relation of elevated resting heart rate in mid-life to cognitive decline over 20 years (from the Atherosclerosis Risk in Communities [ARIC] Study). Am J Cardiol 123: , 334–340. |
[15] | Coppola L , Mastrolorenzo L , Coppola A , De Biase M , Adamo G , Forte R , Fiorente F , Orlando R , Caturano M , Cioffi A , Riccardi A ((2013) ) QT dispersion in mild cognitive impairment: A possible tool for predicting the risk of progression to dementia? Int J Geriatr Psychiatry 28: , 632–639. |
[16] | Hale WE , Stewart RB , Moore MT , Marks RG ((1992) ) Electrocardiographic changes and cognitive impairment in the elderly. J Clin Exp Gerontol 14: , 91–102. |
[17] | Lucas BP , Mendes de Leon CF , Prineas RJ , Bienias JL , Evans DA ((2010) ) Relation of cardiac ventricular repolarization and global cognitive performance in a community population. Am J Cardiol 106: , 1169–1173. |
[18] | Kähönen-Väre M , Brunni-Hakala S , Lindroos M , Pitkala K , Strandberg T , Tilvis R ((2004) ) Left ventricular hypertrophy and blood pressure as predictors of cognitive decline in old age. Aging Clin Exp Res 16: , 147–152. |
[19] | Unverzagt FW , McClure LA , Wadley VG , Jenny NS , Go RC , Cushman M , Kissela BM , Kelley BJ , Kennedy R , Moy CS , Howard V , Howard G ((2011) ) Vascular risk factors and cognitive impairment in a stroke-free cohort. Neurology 77: , 1729–1736. |
[20] | Kaffashian S , Dugravot A , Brunner EJ , Sabia S , Ankri J , Kivimäki M , Singh-Manoux A ((2013) ) Midlife stroke risk and cognitive decline: A 10-year follow-up of the Whitehall II cohort study. Alzheimers Dement 9: , 572–579. |
[21] | Suemoto CK , Gibbons LE , Thacker EL , Jackson JD , Satizabal CL , Bettcher BM , Launer L , Phillips C , White LR , Power MC ((2020) ) Incident prolonged QT interval in midlife and late-life cognitive performance. PLoS One 15: , e0229519. |
[22] | Mahinrad S , Vriend AE , Jukema JW , van Heemst D , Sattar N , Blauw GJ , Macfarlane PW , Clark EN , de Craen AJM , Sabayan B ((2017) ) Left ventricular hypertrophy and cognitive decline in old age. J Alzheimers Dis 58: , 275–283. |
[23] | Mahinrad S , Ferguson I , Macfarlane PW , Clark EN , Stott DJ , Ford I , Mooijaart SP , Trompet S , van Heemst D , Jukema JW , Sabayan B ((2019) ) Spatial QRS-T angle and cognitive decline in older subjects. J Alzheimers Dis 67: , 279–289. |
[24] | Zonneveld MH , Noordam R , Grond JV , Sabayan B , Mooijaart SP , McFarlane PW , Jukema JW , Trompet S ((2020) ) Ventricular repolarization is associated with cognitive function, but not with cognitive decline and brain magnetic resonance imaging (MRI) measurements in older adults. J Clin Med 9: , 911. |
[25] | Teng EL , Hasegawa K , Homma A , Imai Y , Larson E , Graves A , Sugimoto K , Yamaguchi T , Sasaki H , Chiu D , et al. ((1994) ) The Cognitive Abilities Screening Instrument (CASI): A practical test for cross-cultural epidemiological studies of dementia.45-58; discussion . Int Psychogeriatr 6: , 62. |
[26] | He J , Tse G , Korantzopoulos P , Letsas KP , Ali-Hasan-Al-Saegh S , Kamel H , Li G , Lip GYH , Liu T ((2017) ) P-wave indices and risk of ischemic stroke: A systematic review and meta-analysis. Stroke 48: , 2066–2072. |
[27] | Kwok CS , Rashid M , Beynon R , Barker D , Patwala A , Morley-Davies A , Satchithananda D , Nolan J , Myint PK , Buchan I ((2016) ) Prolonged PR interval, first-degree heart block and adverse cardiovascular outcomes: A systematic review and meta-analysis. Heart 102: , 672–680. |
[28] | Oehler A , Feldman T , Henrikson CA , Tereshchenko LG ((2014) ) QRS-T angle: A review. Ann Noninvasive Electrocardiol 19: , 534–542. |
[29] | Zhang Y , Post WS , Blasco-Colmenares E , Dalal D , Tomaselli GF , Guallar E ((2011) ) Electrocardiographic QT interval and mortality: A meta-analysis. Epidemiology 22: , 660–670. |
[30] | Zhang H , Hu L , Wei X ((2020) ) Prognostic value of left ventricular hypertrophy in hypertensive patients: A meta-analysis of electrocardiographic studies. J Clin Hypertens 22: , 254–260. |
[31] | Diamond JA , Phillips RA ((2005) ) Hypertensive heart disease. Hypertens Res 28: , 191–202. |
[32] | Georgakis MK , Synetos A , Mihas C , Karalexi MA , Tousoulis D , Seshadri S , Petridou ET ((2017) ) Left ventricular hypertrophy in association with cognitive impairment: A systematic review and meta-analysis. Hypertens Res 40: , 696–709. |
[33] | Bacharova L , Schocken D , Estes EH , Strauss D ((2014) ) The role of ECG in the diagnosis of left ventricular hypertrophy. Curr Cardiol Rev 10: , 257–261. |
[34] | Van Kleef ME , Visseren FL , Vernooij JW , Nathoe HM , Cramer M-JM , Bemelmans RH , Van Der Graaf Y , Spiering W , Group S-S ((2018) ) Four ECG left ventricular hypertrophy criteria and the risk of cardiovascular events and mortality in patients with vascular disease. J Hypertens 36: , 1865–1873. |
[35] | You Z , He T , Ding Y , Yang L , Jiang X , Huang L ((2020) ) Predictive value of electrocardiographic left ventricular hypertrophy in the general population: A meta-analysis. J Electrocardiol 62: , 14–19. |
[36] | Kuźma E , Lourida I , Moore SF , Levine DA , Ukoumunne OC , Llewellyn DJ ((2018) ) Stroke and dementia risk: A systematic review and meta-analysis. Alzheimers Dement 14: , 1416–1426. |
[37] | Papadopoulos A , Palaiopanos K , Protogerou AP , Paraskevas GP , Tsivgoulis G , Georgakis MK ((2020) ) Left ventricular hypertrophy and cerebral small vessel disease: A systematic review and meta-analysis. J Stroke 22: , 206–224. |
[38] | Iadecola C , Duering M , Hachinski V , Joutel A , Pendlebury ST , Schneider JA , Dichgans M ((2019) ) Vascular cognitive impairment and dementia: JACC Scientific Expert Panel. J Am Coll Cardiol 73: , 3326–3344. |
[39] | Lipton JA , Nelwan SP , van Domburg RT , Kors JA , Elhendy A , Schinkel AF , Poldermans D ((2010) ) Abnormal spatial QRS-T angle predicts mortality in patients undergoing dobutamine stress echocardiography for suspected coronary artery disease. Coron Artery Dis 21: , 26–32. |
[40] | Aro AL , Huikuri HV , Tikkanen JT , Junttila MJ , Rissanen HA , Reunanen A , Anttonen O ((2012) ) QRS-T angle as a predictor of sudden cardiac death in a middle-aged general population. Europace 14: , 872–876. |
[41] | Böhm M , Cotton D , Foster L , Custodis F , Laufs U , Sacco R , Bath PM , Yusuf S , Diener HC ((2012) ) Impact of resting heart rate on mortality, disability and cognitive decline in patients after ischaemic stroke. Eur Heart J 33: , 2804–2812. |
[42] | Wod M , Jensen MT , Galatius S , Hjelmborg JB , Jensen GB , Christensen K ((2018) ) Resting heart rate is not associated with cognitive function. Neuroepidemiology 50: , 160–167. |
[43] | Aune D , Sen A , ó’Hartaigh B , Janszky I , Romundstad PR , Tonstad S , Vatten LJ ((2017) ) Resting heart rate and the risk of cardiovascular disease, total cancer, and all-cause mortality - A systematic review and dose-response meta-analysis of prospective studies. Nutr Metab Cardiovasc Dis 27: , 504–517. |
[44] | Tadic M , Cuspidi C , Grassi G ((2018) ) Heart rate as a predictor of cardiovascular risk. Eur J Clin Invest 48: , doi: 10.1111/eci.12892. |
[45] | Zhang D , Shen X , Qi X ((2016) ) Resting heart rate and all-cause and cardiovascular mortality in the general population: A meta-analysis. CMAJ 188: , E53–e63. |
[46] | Khan H , Kunutsor S , Kalogeropoulos AP , Georgiopoulou VV , Newman AB , Harris TB , Bibbins-Domingo K , Kauhanen J , Gheorghiade M , Fonarow GC , Kritchevsky SB , Laukkanen JA , Butler J ((2015) ) Resting heart rate and risk of incident heart failure: Three prospective cohort studies and a systematic meta-analysis. J Am Heart Assoc 4: , e001364. |
[47] | Fox K , Borer JS , Camm AJ , Danchin N , Ferrari R , Lopez Sendon JL , Steg PG , Tardif JC , Tavazzi L , Tendera M ((2007) ) Resting heart rate in cardiovascular disease. J Am Coll Cardiol 50: , 823–830. |
[48] | Hancock EW , Deal BJ , Mirvis DM , Okin P , Kligfield P , Gettes LS ((2009) ) AHA/ACCF/HRS Recommendations for the Standardization and Interpretation of the Electrocardiogram: Part V: Electrocardiogram changes associated with cardiac chamber hypertrophy a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol 53: , 992–1002. |
[49] | Kamel H , Okin PM , Longstreth WT Jr. , Elkind MS , Soliman EZ ((2015) ) Atrial cardiopathy: A broadened concept of left atrial thromboembolism beyond atrial fibrillation. Future Cardiol 11: , 323–331. |
[50] | Kamel H , Bartz TM , Longstreth WT Jr. , Okin PM , Thacker EL , Patton KK , Stein PK , Gottesman RF , Heckbert SR , Kronmal RA , Elkind MS , Soliman EZ ((2015) ) Association between left atrial abnormality on ECG and vascular brain injury on MRI in the Cardiovascular Health Study. Stroke 46: , 711–716. |
[51] | Santos CY , Snyder PJ , Wu WC , Zhang M , Echeverria A , Alber J ((2017) ) Pathophysiologic relationship between Alzheimer’s disease, cerebrovascular disease, and cardiovascular risk: A review and synthesis. Alzheimers Dement (Amst) 7: , 69–87. |
[52] | Nieuwenhuis-Mark RE ((2010) ) The death knoll for the MMSE: Has it outlived its purpose? J Geriatr Psychiatry Neurol 23: , 151–157. |
[53] | Brion MJ , Lawlor DA , Matijasevich A , Horta B , Anselmi L , Araújo CL , Menezes AM , Victora CG , Smith GD ((2011) ) What are the causal effects of breastfeeding on IQ, obesity and blood pressure? Evidence from comparing high-income with middle-income cohorts. Int J Epidemiol 40: , 670–680. |




