Promoting Successful Cognitive Aging: A Ten-Year Update
Abstract
A decade has passed since we published a comprehensive review in this journal addressing the topic of promoting successful cognitive aging, making this a good time to take stock of the field. Because there have been limited large-scale, randomized controlled trials, especially following individuals from middle age to late life, some experts have questioned whether recommendations can be legitimately offered about reducing the risk of cognitive decline and dementia. Despite uncertainties, clinicians often need to at least make provisional recommendations to patients based on the highest quality data available. Converging lines of evidence from epidemiological/cohort studies, animal/basic science studies, human proof-of-concept studies, and human intervention studies can provide guidance, highlighting strategies for enhancing cognitive reserve and preventing loss of cognitive capacity. Many of the suggestions made in 2010 have been supported by additional research. Importantly, there is a growing consensus among major health organizations about recommendations to mitigate cognitive decline and promote healthy cognitive aging. Regular physical activity and treatment of cardiovascular risk factors have been supported by all of these organizations. Most organizations have also embraced cognitively stimulating activities, a heart-healthy diet, smoking cessation, and countering metabolic syndrome. Other behaviors like regular social engagement, limiting alcohol use, stress management, getting adequate sleep, avoiding anticholinergic medications, addressing sensory deficits, and protecting the brain against physical and toxic damage also have been endorsed, although less consistently. In this update, we review the evidence for each of these recommendations and offer practical advice about behavior-change techniques to help patients adopt brain-healthy behaviors.
INTRODUCTION
In 2010, we published an article in this journal entitled, “Promoting successful cognitive aging: a comprehensive review” [1]. A decade has passed, making this a good time to take stock of the field, which has garnered increasing interest. The number of articles on cognitive/brain health and dementia risk reduction has grown substantially. A Pubmed search of papers linked to relevant key words, including “brain health,” “healthy brain aging,” “promoting cognitive health,” “preventing cognitive decline,” “dementia risk reduction,” “dementia prevention,” and “Alzheimer prevention,” went from 917 published in 2001-2010 to 6,243 published in 2011-2020. Many of the suggestions made in 2010 have been supported by additional research and have been increasingly embraced clinically. Several major health organizations have published brain health recommendations based on research studies investigating how various behaviors and lifestyle factors affect the risk of developing cognitive decline and dementia [2–7]. In this update, we will summarize the findings of past and recent research, present them in an accessible format, and offer practical advice about helping patients to adopt brain-healthy behaviors.
An aging population
According to a 2019 United Nations report, the population over the age of 60 is growing faster than other age groups [8]. Average life expectancy has dramatically increased in the last several decades and is predicted to continue increasing on a similar trajectory. In 2019, the global life expectancy at birth reached 72.6 years, an increase of more than eight years since 1990. By 2050, the global life expectancy is predicted to reach 77.1 years [8]. As of 2019, there were an estimated one billion people over the age of 60 globally. This number is expected to grow to 2.1 billion by 2050, and to 3.1 billion by 2100 [8]. Already in Europe, more than a quarter of the population (190 million) is older than age 60 [8]. In the United States (U.S.), the older population is also growing rapidly, as 75 million Baby Boomers (the cohort born between 1946 and 1964) turn 65. Currently, 53 million people over the age of 65 live in the U.S. In the next decade, that number is projected to grow to 74 million [8]. Members of this age group have been particularly concerned about maximizing longevity and quality of life. The prospect of losing memory and independence is among the most feared aspects of aging. In fact, studies have suggested that more than half of adults over the age of 65 have concerns about their memory [9–15].
The many costs of dementia
Dementia is a general term that describes a set of symptoms, including difficulties with thinking and memory, which interfere with the ability to perform activities of daily living [13, 16]. The most common cause of dementia, Alzheimer’s disease (AD), is associated with the abnormal accumulation of the proteins amyloid-β (Aβ) and tau in the brain [13]. Relatedly, mild cognitive impairment (MCI) is a term used to describe the intermediate stage between normal cognitive aging and dementia. Activities of daily living remain intact with MCI, but the brain is often already undergoing early changes due to Alzheimer’s disease and related dementias (ADRDs) [17, 18]. ADRDs are the fifth-leading cause of disability and death among older individuals globally [19]. There are an estimated 50 million people in the world who suffer from ADRDs, and this number is expected to more than triple to 152 million by 2050 [13]. Additionally, 15–20% of adults over 65 have MCI due to ADRDs [13, 18]. It is generally acknowledged that ADRDs are some of the costliest diseases to society, due to many direct and indirect costs associated with caring for patients [20]. Compared to their peers, patients with dementia have worse overall health, increased utilization of healthcare services, higher out-of-pocket healthcare spending, shorter life expectancy, and greater likelihood of dying in hospitals [21–27]. Similarly, the health outcomes and general wellbeing of caregivers and families of patients with ADRDs are notably worse than their age-matched peers, with higher rates of anxiety and depression, worse sleep quality, and poorer overall quality of life [25, 28–30]. The financial impact of cognitive decline on health systems is largest in the transition from MCI to dementia [31, 32]. Projection models demonstrate that delaying the onset of dementia by five years can reduce its prevalence by 50%, substantially decreasing financial and social burdens on patients, families, and healthcare systems [33, 34]. Specifically, some economic models demonstrate that delaying the transition from MCI to dementia by five years could result in a cost-savings of more than $500,000 per patient [32]. Therefore, it is imperative to develop clinical care models that focus on stabilizing patients with MCI and reducing the risk of dementia for healthy older adults. In the aggregate, even small effects on preserving cognitive abilities may have a significant impact on society.
PROMOTING SUCCESSFUL COGNITIVE AGING
Many theories about normal aging have suggested that aging is associated with the risk of declining neurophysiological functions, which often leads to reductions in cognitive processing and capacity [35]. As we [1] and others [36, 37] have suggested, the promotion of healthy cognitive aging can be linked to the dual goals of 1) preventing loss of and 2) facilitating enhancement of brain and cognitive reserve. Brain reserve refers to differences in the “hardware” of the brain, including the number of neurons and synapses, which allow brains to withstand a greater burden of disease or injury before cognitive function is affected [38]. Cognitive reserve includes the ability to use brain networks more proficiently in response to every-day cognitive, emotional, and functional demands, as well as the capacity to utilize alternative cognitive strategies or neural networks in response to cerebral injury or decline [38].
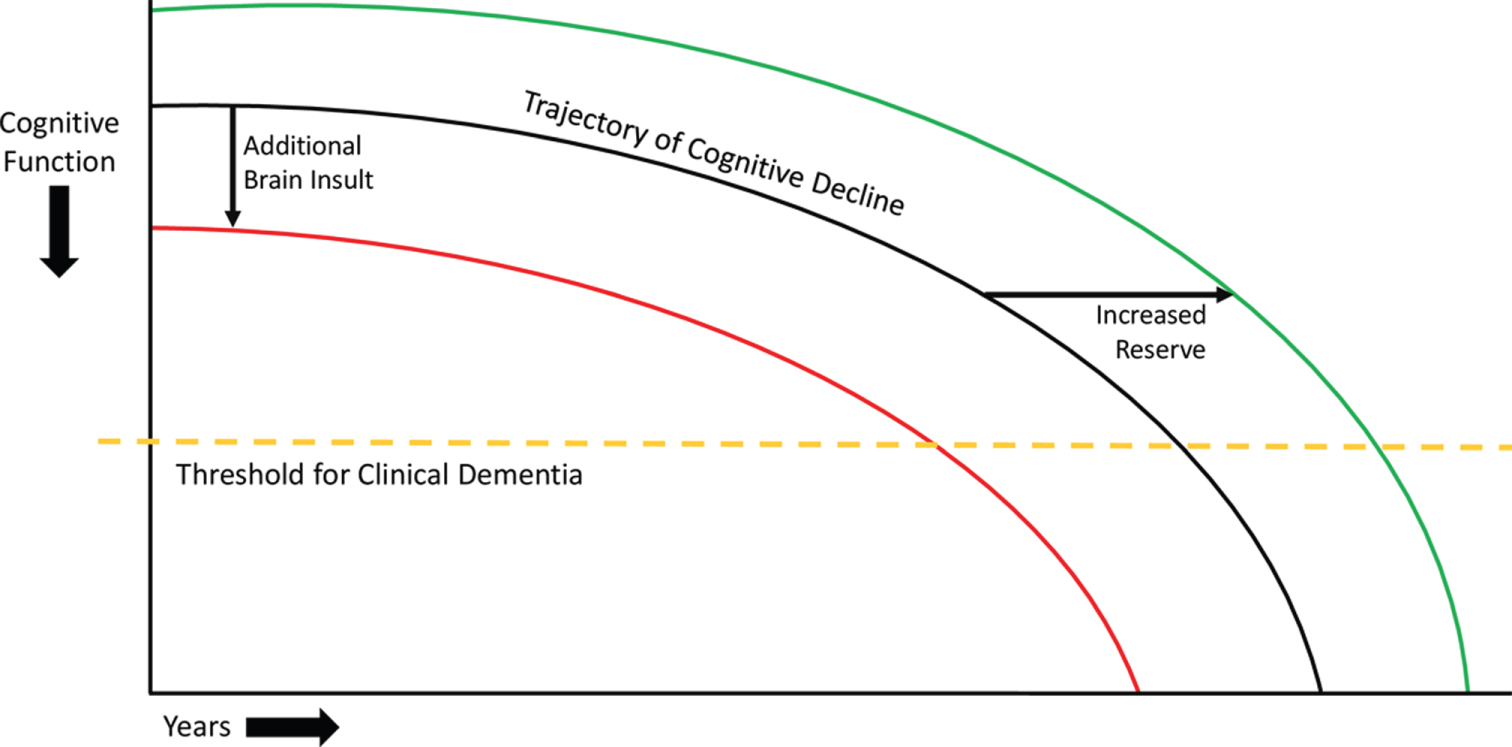
A simple model of brain reserve and deterioration suggests that, when the number of healthy, functioning neurons or their connections falls below a critical level or threshold, individuals manifest symptoms of cognitive impairment and, eventually, dementia [39]. Figure 1 illustrates the trajectory of cognitive decline in individuals with neurodegenerative disease. Additional cerebral insults (e.g., due to strokes) would shift the theoretical trajectory of cognitive decline to the left, causing patients to cross the threshold for dementia earlier in life. Conversely, boosting cognitive or brain reserve (e.g., through education or cognitively stimulating activities) would shift this theoretical curve to the right, allowing patients to cross this threshold later in life.
Fig. 1
Model of cognitive decline. The black line represents the trajectory of cognitive decline due to neurodegenerative disease. The yellow dashed line represents the threshold for clinical dementia, i.e., the inability to manage activities of daily living. The red line represents the impact of additional injuries to the brain, which can decrease brain reserve and cause a leftward shift of the trajectory of cognitive decline, leading patients to cross the threshold for clinical dementia earlier. The green line represents the effect of increased cognitive and brain reserve, which can cause a rightward shift in the trajectory of cognitive decline, leading patients to cross the threshold for clinical dementia later. These principles involving theoretical shifts in the trajectory of cognitive decline also apply to adults without neurodegenerative disease, though the initial downward trajectory is much less steep.
Blue Ribbon Panels: inadequate evidence that interventions can reduce dementia risk
Over the past decade, several systematic reviews by prestigious academic and expert governmental panels have concluded that there is inadequate high-quality evidence that the risk of cognitive decline and dementia can be modified. For example, in 2010, a 15-member panel at the National Institutes of Health Consensus Development Conference released a statement on preventing AD and cognitive decline, saying that firm conclusions could not be drawn about the association of any potential modifiable risk factor with cognitive decline or AD [40]. In 2011, an eight-member panel for the U.S. Centers for Disease Control completed a systematic review of intervention trials investigating the effect of physical activity and exercise on cognition in older adults. This review acknowledged some positive results but concluded that there was insufficient evidence that physical activity improved cognition in older adults [41]. More recently, in 2017, the Minnesota Evidence-based Practice Center conducted systematic reviews on the effects of physical activity [42], cognitive training [43], and prescription and over-the-counter medications [44] on cognitive decline and dementia; it concluded that no interventions approached the evidence level necessary to make clinical recommendations. In general, these review panels call for large-scale, randomized control trials (RCTs) to address the issue. Ideally investigators would initiate RCT interventions during midlife and follow participants over decades to determine the impact on developing cognitive deterioration and dementia in late life. Unfortunately, such studies would be expensive and impractical to carry out, and financial support for such endeavors seems unlikely. A critical question is whether clinicians or public health officials should wait for the results of large-scale RCTs like these to become available before advising patients or the wider public on behavioral and lifestyle changes to reduce dementia risk
Converging evidence
Despite uncertainties and incomplete information regarding the management of many health risks and medical conditions, clinicians often need to address challenging issues currently facing their patients. Most clinicians are not in a position to simply tell patients that they should come back in 15–20 years when we may have more definitive answers. Rather, clinicians are obligated to offer at least provisional recommendations to patients and policy makers based on the highest quality of evidence available. Healthcare providers can also play an important role in identifying and treating conditions that reduce brain reserve and cognitive capacity, including cardiovascular risk factors, metabolic syndrome, sleep disorders, mood disorders, and side effects from medication. They are well-positioned to serve as advocates for brain health and wellbeing.
Epidemiological evidence suggests that up to 35% of ADRD cases may be due to modifiable risk factors [4], and that healthy lifestyles can offset genetic risk of developing ADRDs [45]. Thus, encouraging ways to promote brain health and reduce risk of cognitive decline is particularly important, especially given the absence of disease-modifying treatments for neurodegenerative diseases.
As detailed in our 2010 review, there are several different ways to evaluate whether a proposed factor or intervention may have an impact on healthy cognitive aging. Four major lines of evidence have been used: 1) epidemiological/cohort studies, 2) animal/basic science studies, 3) human proof-of-concept studies, and 4) human intervention studies. Often, the strongest recommendations are a reflection of converging lines of evidence.
Epidemiological studies follow a cohort of subjects longitudinally to determine the impact of specified risk factors on outcomes, while attempting to control for other relevant factors. Animal or basic science studies allow for more controlled environments. Animals can be exposed to a risk factor or a hypothesized healthy behavior can be elicited. Systemic, cellular, or molecular effects can be investigated to help identify underlying mechanisms. Biological outcomes including changes in brain structure, synaptic and receptor activity, and levels of neurotransmitters, hormones, growth factors, and inflammatory markers can be measured. Human proof-of-concept studies examine markers of plasticity, brain and cognitive reserve, efficiency, and neural compensation in relevant populations or in small, controlled investigations, using methods such as structural and functional imaging, or measurements in serum or cerebral spinal fluid. In human RCTs, individuals are randomized to treatment or control groups, baseline measurements are obtained, and outcomes are measured to delineate treatment effects. Human RCTs are considered the “gold standard” in supporting treatment and public health recommendations, especially if they include large numbers of participants. Table 1, adapted from our original article, provides a brief summary of the advantages and limitations of the different sources of evidence.
Table 1
Advantages and limitations of different lines of evidence
| Lines of Evidence | Advantages | Limitations |
| Epidemiological/Cohort Studies | •Large number of subjects | •Association not causation; difficult to determine the ‘direction’ of cause/effect for exposure/outcome (reverse causality problem) |
| •Data on multiple factors | ||
| •Longitudinal follow-up over many years | ||
| •Uncertainty about the influence of unidentified factors | ||
| •Uncertainty about whether an intervention aimed at an identified factor would have an impact on biological or clinical outcome | ||
| Basic Science/Animal Studies | •Investigation under highly controlled conditions | •Reduction of complex issues into simple ones |
| •Determination of underlying mechanisms •Identification of intervening variables that help explain clinical outcomes (e.g., changes in brain structure, neurotransmitters, growth factors, inflammatory markers, etc.) | •Need to translate findings from basic science or animal models to the study of humans | |
| •Elucidating underlying mechanisms does not signify that manipulating an identified factor will alter clinical outcome | ||
| Human “Proof-of-Concept” Studies | •Opportunity to examine markers of cerebral plasticity, reserve, efficiency, and neural compensation | •Similar to limitations of basic science/animal studies noted above |
| •Utilization of tools of cognitive neuroscience to elucidate underlying mechanism | ||
| •Opportunity for further hypothesis testing and generation | ||
| Human Intervention Studies | •Often considered the “gold standard” •Randomization helps control for selection bias and other variables that may influence clinical outcomes •Outcome measures can include cognitive performance as well as biological or neuroimaging markers | •Potential feasibility concerns, including financial, logistical, or ethical barriers |
| •Uncertainty about the generalizability of observed effects to other populations or settings | ||
| •Uncertainty about whether statistically significant results are clinically relevant | ||
| •Uncertainty about whether a study outcome is due to a particular dose/duration of an intervention, or characteristics of the particular sample |
Table modified from Daffner 2010 [1].
Consensus recommendations
Based on the available lines of evidence, there is growing consensus among major health organizations that certain healthy behaviors and lifestyles can mitigate cognitive decline and promote successful cognitive aging [2–7]. Regular physical activity and treatment of cardiovascular risk factors have been supported by all of these organizations. Most organizations have also embraced cognitively stimulating activities, a heart healthy diet, countering metabolic syndrome, and smoking cessation. Other behaviors such as regular social engagement, limiting alcohol use, stress management, getting adequate sleep, avoiding medications with anticholinergic properties, addressing sensory deficits, and protecting the brain against physical and toxic damage have received less consistent endorsement. Research has provided evidence that adoption of these brain-healthy behaviors can improve cognition, reduce the risk of brain injury or dysfunction, and augment overall wellbeing and quality of life for cognitively normal older adults. The recommendations of several major organizations are outlined in Table 2.
Table 2
Recommendations from major health organizations for maintaining brain health and mitigating cognitive decline
| Institute of Medicine [2] | Alzheimer’s Association [3] | Lancet Commissions [4] | American Heart Association [5] | National Academies [6] | World Health Organization [7] | |
| Physical activity | √ | √ | √ | √ | √ | √ |
| Cognitive stimulation/education | √ | √ | √ | √ | √ | |
| Social engagement | √ | √ | √ | |||
| Heart healthy diet | √ | √ | √ | √ | ||
| Treat cardiovascular risk factors (hypertension) | √ | √ | √ | √ | √ | √ |
| Treat cardiovascular risk factors (diabetes mellitus) | √ | √ | √ | √ | √ | |
| Treat cardiovascular risk factors (hyperlipidemia) | √ | √ | ||||
| Maintain a healthy weight/counter metabolic syndrome | √ | √ | √ | √ | ||
| Stop or reduce smoking | √ | √ | √ | √ | √ | |
| Manage stress and depression | √ | √ | √ | |||
| Maintain adequate sleep | √ | √ | ||||
| Manage hearing and visual impairments | √ | |||||
| Avoid medications with anticholinergic properties | √ | |||||
| Limit alcohol use | √ | √ | √ | |||
| Protect the brain from physical and toxic injuries | √ | √ |
For each major recommendation for maintaining brain health and mitigating cognitive decline identified, we have provided a narrative summary of the best available evidence. We relied on high-caliber systematic reviews and meta-analyses, especially of RCTs and long-term observational studies, preferably that included assessments of publication bias and quality of methodology and outcome data. These reviews and meta-analyses were identified by searching for relevant key terms in the PubMed, EMBASE, and PsycInfo databases. We scrutinized individual studies that were highlighted in these reviews and meta-analyses or were identified through additional searches of the same databases for the most recent publications on the subject. We also conducted systematic searches for articles on relevant basic science/animal investigations and human proof-of-concept studies. Our major aim was to synthesize converging evidence from epidemiological, basic science/animal, proof-of-concept, and RCT studies, with the goal of producing a coherent narrative that would be readily accessible to clinicians and researchers in the field.
Physical activity
There is robust evidence that participation in physical activity is associated with a decreased risk of cognitive decline and dementia. The U.S. Department of Health and Human Services recommends that all adults do at least 150 minutes of moderate-intensity aerobic activity, or at least 75 minutes of vigorous-intensity aerobic activity a week, or an equivalent combination of moderate- and vigorous-intensity aerobic activity [46]. These guidelines also call for strength training exercises targeting all major muscle groups at least two times a week for additional health benefits [46]. Between 2006 and 2018, however, only 23% of adults 18 years and older in the U.S. were meeting recommendations for aerobic and muscle-strengthening exercise [47], based on aggregate survey data. This number dropped to 16% of adults between the ages of 65 and 74, and to 10% of adults 75 years and over [47].
Epidemiological studies have demonstrated that high levels of exercise in mid- and late life are associated with increased cognitive performance, decreased rate of cognitive decline, and decreased risk of all-cause dementia as well as dementia subtypes [48–52]. For example, the Nurses’ Health Study of over 18,000 women, aged 70 to 81 years, found that regular, long-term physical activity was associated with better cognitive performance, including tests of general cognition, verbal memory, category fluency, and attention [48]. However, as with many of the factors being discussed, the causal direction of the association is hard to determine. Perhaps, reduced physical activity is due to the early impact of an underlying neurodegenerative process. Against this hypothesis are epidemiological studies that suggest a relationship between earlier-in-life physical activity and the incidence of dementia in older age. For example, a longitudinal study of women, followed for 44 years starting in midlife, found that high fitness during middle age was associated with a decreased risk of dementia compared to medium and low fitness, and a delay of 9.5 years for onset of dementia symptoms compared to medium fitness [49]. These results are consistent with findings from large meta-analyses of prospective studies [53, 54].
Although many cohort studies demonstrate positive outcomes associated with physical activity, others have shown conflicting results. One epidemiological study of the Whitehall cohort (10,308 participants, aged 35–55 years at study inception) found no association between exercise and cognitive decline or exercise and incidence of dementia over 15 and 27 years, respectively [55]. Notably, this study relied on self-report questionnaires to determine level of physical activity, and over 50% of participants reported adhering to consensus recommendations of more than 2.5 hours of moderate to vigorous exercise per week, which seems implausible based on rates in the general population. If the amount of physical activity was inaccurately reported, it would undermine conclusions that could be drawn about the relationship between exercise and dementia risk.
Studies of animals have indicated that exercise promotes neurogenesis, synaptogenesis, and improved learning and memory, through upregulation of proteins including brain-derived neurotrophic factor (BDNF), vascular endothelial growth factor, and insulin-like growth factor [56–60]. In animal models of AD, exercise is also associated with reduced Aβ deposition and tau pathology [61–63].
In humans, physical activity plays a major role in promoting cardiovascular health, which is closely linked to brain health. Managing cardiovascular risk factors, including hypertension, diabetes mellitus (DM), hyperlipidemia, and obesity/metabolic syndrome, can reduce the risk of cognitive decline and dementia. (See “Treat cardiovascular risk factors”, below, for a more detailed discussion on cardiovascular health and brain health.) Exercise has also been shown to diminish the risk of falls, a common cause of head injury, in older adults [64]. In addition to enhancing cardiovascular health and reducing fall risk, exercise has been shown to promote neurogenesis, synaptogenesis, and brain vascularization in humans through upregulation of insulin-like growth factor-1 [65–67] and BDNF [68]. Serum levels of BDNF, which promotes neuroplasticity, synaptic growth and density, and hippocampal neurogenesis [69–72], have been shown to increase both immediately after exercise [73, 74] and following a longer intervention period [75, 76]. One study of 19 older adults found that serum BDNF immediately following a single 35-minute physical activity session was significantly elevated compared to BDNF levels immediately following other potential brain-healthy endeavors (i.e., cognitive activity and meditation) [74]. Aerobic physical activity has also been shown to attenuate loss of brain volume and decrease white matter changes [77–80], which can be seen on magnetic resonance imaging (MRI) scans and have been associated with increased incidence of cognitive impairment and dementia [81]. In one study, 120 older adults were randomized to an aerobic exercise group or a stretching and toning control group. Although the two groups had similar hippocampal volume at baseline, over one year, the control group exhibited an approximate 1.5% decline in hippocampal volume, while the exercise group showed an approximate 2% increase. Within the aerobic exercise group, increased hippocampal volume was associated with improvement on a spatial memory task [77]. Additionally, both aerobic [82] and resistance [83] training have been shown to increase measures of functional neuroplasticity.
Physical activity also may protect against the adverse consequences of cerebral Aβ and tau deposition, which include accelerated cognitive decline and the development of AD. For example, one study of 43 cognitively healthy older adults found that self-reported levels of current physical activity were associated with a decreased positron emission tomography (PET)-quantified tau burden [84]. A recent report from the Harvard Aging Brain Study, a longitudinal investigation of cognitively normal older individuals, indicated that increased baseline physical activity markedly reduced the negative impact of Aβ burden, as measured by Pittsburgh Compound B (PiB)-PET, on cognition and cerebral atrophy over a median of six years [85]. The potential benefits of physical activity also may apply to other neurodegenerative conditions. For example, in a longitudinal study of both mutation carriers and non-carriers in families with autosomal dominant frontotemporal lobar degeneration, greater physical activity was associated with > 55% slowing of clinical decline per year [52].
Aerobic exercise interventions have been associated with decreased age-related cognitive decline [86, 87], improved executive function [88, 89], and improved memory [77, 90, 91] in cognitively normal older adults, and improved memory in individuals with MCI [80]. In one intervention trial, Lautenschlager and colleagues randomized 170 older adults with subjective cognitive decline to a six-month home-based physical activity intervention or an education and usual care control group. Compared to usual care, the six-month program of physical activity provided a modest improvement in cognition, which was sustained over an 18-month follow-up period [92]. Blumenthal and colleagues randomized 160 sedentary older adults with risk factors for dementia to six months of one of four groups: aerobic exercise, Dietary Approaches to Stop Hypertension (DASH) diet nutritional counseling, aerobic exercise plus DASH nutritional counseling, or health education. Participants who engaged in aerobic exercise or aerobic exercise plus DASH nutritional counseling had improved scores on measures of executive function compared to DASH nutritional counseling or health education alone [88]. Additionally, an RCT conducted by de Oliveira Silva and colleagues found that an exercise intervention improved mobility and executive function in those with MCI, but not those with AD dementia, suggesting that exercise interventions may be more effective when implemented earlier in disease progression [93]. Resistance training interventions have also been associated with improved attention [65] and executive function [65, 94] in cognitively normal older adults, and improved attention [82], memory [82, 95], and global cognitive function [96, 97] in individuals with MCI.
Although not all exercise interventions have been associated with improved cognition, a systematic review found that exercising for at least 52 hours during the length of an intervention, regardless of how many weeks the intervention lasted or duration of exercise events, was associated with improved cognitive performance, including global cognition, processing speed/attention, and executive function. All studies associated with no improvement in cognitive outcomes had interventions lasting less than 52 total hours [98].
Cognitive stimulation and education
Converging evidence highlights the importance of remaining cognitively engaged throughout one’s life. In early life, fewer years of education is associated with reduced cognitive reserve and increased risk of dementia in late life [4]. Education may have a greater impact on dementia risk in low- and middle-income countries, where secondary education might not be available to everyone. This risk factor is most effectively addressed by implementing public policy that supports high-quality education for all children.
Although early-life education is critically important, cognitive stimulation during all stages of life has been shown to influence brain health and cognition. Epidemiological studies have shown that cognitive activity in both mid- and late life is associated with improved cognitive performance and reduced risk of cognitive decline and dementia [50, 99–103]. Najar and colleagues found that cognitive activity in midlife was associated with a 34% reduced risk of all-cause dementia and 46% reduced risk of AD over a follow-up period of 44 years, after adjusting for age, education, socioeconomic status, hypertension, body mass index, smoking, DM, angina pectoris, stress, and major depression [50]. For cognitive activity in late life, a meta-analysis of 19 studies in cognitively healthy older adults found that participation in cognitive leisure activities (e.g., crossword puzzles, card games, computer use, arts and crafts) was associated with 31% risk reduction for cognitive impairment and 42% risk reduction for dementia. Cognitive leisure activities were also significantly associated with improved memory, processing speed, and executive function [103]. It is important to note that the studies included in this meta-analysis differed in the confounding variables for which they adjusted and rarely took other lifestyle factors like physical activity or diet into account.
Studies of animals have demonstrated that environmental enrichment, which provides cognitive stimulation, is associated with improved cognitive functioning across the lifespan [104], as well as increased neurogenesis, upregulated neuronal growth factors, and increased synaptic density and plasticity [105, 106]. Environmental enrichment has also been shown to decrease cognitive deficits and Aβ burden in animal models of AD [107, 108].
The mechanisms by which cognitive activity reduces the risk of cognitive decline and dementia in humans are still unclear [109–113]. One hypothesis is that regular cognitive engagement contributes to increased neuroplasticity that partially offsets otherwise expected cognitive losses or the actual accumulation of pathologic changes due to aging or neurodegenerative conditions [114]. The evidence to support or refute this hypothesis is inconclusive. For example, an intervention trial, in which our Center was one of two sites, found that cognitive training for 35 minutes per day, five days per week for five weeks was associated with a 10% increase in serum BDNF [109], a neurotrophic factor that, as noted above, has been associated with neuroplasticity. Another intervention trial, however, found that cognitive training for one hour per day, five days per week for ten weeks was not associated with serum BDNF levels [75]. Other possible mechanisms include reduction in AD pathology or decreased loss of brain volume. A cross-sectional study of 65 healthy older adults, ten AD patients, and 11 young controls demonstrated that cognitive activity, especially in early and midlife, was associated with decreased Aβ deposition, as measured by amyloid PET [110]. Cognitive training for executive function specifically has been linked to attenuated loss of grey matter [115]. Conversely, multiple cross-sectional imaging studies found neither current nor past cognitive activity had any effect on Aβ burden, brain glucose metabolism, or hippocampal volume [111, 112]. More human research is needed to elucidate the relationship between cognitive activity and brain health.
Cognitive training interventions have shown promising results, including increased hippocampal activation during memory tasks [116, 117], improved cognition in several domains, and decreased risk of cognitive and functional decline [118–125]. Some of the most impressive outcomes have been reported for the Advanced Cognitive Training for Independent and Vital Elderly (ACTIVE) study, in which 2,832 older adults (mean age 73.6) were randomized to either a ten-session group training focused on one of three cognitive domains (memory, reasoning, or processing speed) or to a no-contact control group. After ten sessions, all groups showed significant improvement in the domain trained. After two years, cognitive improvement from baseline was maintained for all training groups [118]. Ten years after the ACTIVE intervention, all cognitive training groups reported less functional decline compared to the no-contact control group and measured improvement in the reasoning and processing speed groups was retained [122]. Additionally, individuals randomized to the processing speed training group reportedly had a 29% risk reduction for dementia [123]. The dropout rate (often due to the death of these aging participants) was similar across groups and the attrition rate was similar to that of other studies over the ten-year follow-up period.
Not all cognitive training interventions have shown positive results. For example, one study by Kallio and colleagues found no improvement in cognition after cognitive training for adults with mild to moderate dementia [126], suggesting that cognitive interventions may be more effective when they occur before the onset of dementia. Additionally, many cognitive training interventions have demonstrated enhanced performance on the task trained as well as near-transfer effects (i.e., improvement in an untrained task that involves the same cognitive domain used in the trained task), but have been less successful in showing far-transfer effects (i.e., improvement in an untrained task in a different cognitive realm than had been trained) or an impact on activities of daily living [122–125].
Although it is important to encourage patients to engage cognitively, not all activities will necessarily yield equal cognitive benefit. In the Synapse Project, McDonough and colleagues randomized 39 older adults to High-Challenge (quilting and/or digital photography) or Low-Challenge (e.g., socializing, listening to music) groups. Compared to the Low-Challenge group, the High-Challenge group showed increased ability to modulate brain activity, as measured by functional MRI, in regions associated with attention and semantic processing [127]. Additionally, despite evidence supporting cognitive stimulation for brain health, many popular commercial cognitive or memory training platforms have limited supporting evidence [128, 129]. To date there has been no compelling evidence that computerized cognitive training is superior to other less expensive, readily-available, intellectually-stimulating activities. However, it remains to be determined whether continuously-titrated task difficulty based on real-time performance or having to pay for cognitive training may be motivating influences to achieve the desired outcome. Our view is that if individuals enjoy using these platforms, there is no harm in supporting their use. Perhaps most importantly, existing research suggests that engaging in new, challenging cognitive activities may yield a greater benefit for brain health and cognition in older adults than carrying out familiar, undemanding mental activities.
Social engagement
Epidemiological evidence suggests that reduced social participation, less frequent social contact, and feelings of loneliness are associated with cognitive impairment and incident dementia [100, 130–134]. However, the causal direction of this association is difficult to prove, because more impaired individuals may have fewer social interactions. Decreased social engagement may be a prodromal symptom of dementia, but increasing evidence has demonstrated that it is also an independent risk factor. For example, individuals living with a partner in mid- to late life have a reduced risk of cognitive impairment and all-cause dementia compared to those who are single, separated, or widowed [130, 131]. Maintaining a large social network may also help promote healthy cognitive aging. In a five-year prospective study of 2,249 older women without dementia, social network size was directly associated with reduced risk of dementia and cognitive impairment. The women who had daily contact with friends and family cut their risk of dementia by nearly half [132]. Additionally, a meta-analysis of 51 longitudinal cohort studies examining social network size and social activity found that aspects of social isolation, including low levels of social activity and small social networks, were associated with decreased cognitive function in late life [133].
In animals, social isolation (via individual housing) has been shown to exacerbate memory deficits and promote cognitive decline by increasing systemic inflammation [135, 136], oxidative stress [135], neuroinflammation [137], and Aβ levels [135, 137], and by decreasing BDNF levels and neurogenesis [135, 138, 139].
In humans, decreased social interaction and often attendant feelings of loneliness have been linked to factors that may increase risk of cognitive impairment and dementia, including worsened sleep [140, 141] and increased stress [140], inflammation [141–144], and systolic blood pressure [145]. Conversely, higher levels of social engagement in older adults provide increased opportunities for cognitive stimulation, problem solving, and social support, and have been linked to increased total brain and grey matter volumes [146], greater grey matter integrity in several regions relevant to social cognition [147], and increased neural network function, which boosts cognitive reserve [148]. Social network size and level of social engagement have also been shown to modify the relationship between AD pathology and cognitive function [149, 150]. In a recent report of cognitively normal older adults participating in the Harvard Aging Brain study, Biddle and colleagues observed that the negative impact of high Aβ on cognitive decline was modulated by level of social engagement [150]. Lower baseline social engagement was associated with greater Aβ-related decline in cognitive test scores, while higher baseline social engagement was associated with preserved cognitive test scores over a three-year follow-up period. These results regarding social engagement are reminiscent of the findings suggesting that higher baseline physical activity may modulate the deleterious effect of Aβ cognitive decline, as noted above [85]. Interestingly, baseline Aβ levels were not linked to a decline in social engagement after controlling for relevant co-variates, and did not modulate the negative association between lower baseline cognitive performance and subsequent reduction in social engagement.
Intervention trials aimed at promoting social engagement have also shown promise for boosting brain health and cognition. In one intervention, 149 older adults were randomized to Experience Corps [151], a team-based training group, or the waitlist control group. Experience Corps members trained in teams to work in elementary schools for 15 hours per week for six months, helping students to succeed academically and socially. After completing the program, participants showed improvement in memory and executive function compared to control. Notably, participants with impaired executive function at baseline showed the greatest improvement [151]. A functional MRI study of eight participants in Experience Corps and nine matched waitlist controls found that intervention participants also exhibited increases in prefrontal cortex and anterior cingulate cortex activation compared to controls following the six-month intervention period [152]. A subsequent two-year trial of the Experience Corps intervention found that, compared to control, participation in the program was associated with decreased volume loss in brain regions associated with dementia. In men, participating in the program was associated with a small increase in brain volume in these regions [153]. Another study compared the effects of three different social-cognitive interventions: group-based cognitive training, health promotion classes, and a social engagement-promoting book club. All three interventions resulted in a significant improvement in cognitive performance, with no significant differences between the groups [154].
Heart-healthy diet
Several heart-healthy diets have been associated with a decreased risk of cognitive decline and dementia, including the Mediterranean diet (MeDi) [155], the DASH diet [156], and the Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diet [157]. Each of these diets includes an abundance of fruits and vegetables and emphasizes consumption of antioxidant-rich foods. DASH differs from the other diets in that it encourages consumption of low-fat dairy products, aims to reduce sodium and fat intake, and does not prescribe nor prohibit wine consumption [156]. The MIND diet is unique in specifying greater intake of leafy green vegetables and berries over other plant-based foods [157]. These diets contrast with a more common diet consumed in the U.S. and other parts of the Western world, which is characterized by excess sodium, saturated fat, refined grains, red and processed meats, and calories from solid fats and added sugar [158].
Epidemiological studies have shown that all three of these heart-healthy diets are beneficial to brain health and cognition. Adherence to the MeDi has been associated with increased cognitive function [159] and decreased risk of cognitive impairment [159, 160] and AD [155, 161]. The DASH diet has been linked to decreased risk of AD [161]. The MIND diet has been associated with better cognitive function [159], decreased rate of age-related cognitive decline [162], and decreased risk of cognitive impairment [159] and AD [161]. One study of 923 older adults followed for an average of 4.5 years found that only high adherence to the MeDi or DASH diet was associated with decreased risk of AD, while moderate to high adherence to the MIND diet was associated with decreased risk of AD [161]. A study of 1,220 older adults determined that the MIND diet, but not MeDi, was associated with decreased risk of cognitive impairment over a twelve-year follow up period [163]. Taken together, these results suggest that the MIND diet may be most effective for promoting brain health and cognition, but more research, including high-quality RCTs, is needed. Meta-analyses of prospective cohort studies have affirmed that a high level of adherence to these diets is associated with reduced risk of developing MCI and AD [164–168]. One meta-analysis examined specific components of the MeDi and demonstrated that increased consumption of unsaturated fatty acids, antioxidants, and B vitamins was associated with decreased risk of dementia, and low levels of vitamin D were associated with cognitive decline [166]. Some of these studies have shown a degree-of-adherence-dependent effect of these brain-healthy diets [159, 160]. In contrast, observational evidence suggests that a more typical Western diet is linked to worsened age-related cognitive decline [169].
Like physical activity, eating a heart-healthy diet plays an important role in managing cardiovascular risk. The MeDi has been linked to beneficial effects on hypertension, DM, obesity, and hyperlipidemia [170]. Additionally, there is evidence that these diets boost cognition and reduce risk of dementia by decreasing vascular inflammation, white matter changes, and Aβ and tau pathology [171, 172]. In both animal and human studies, several components of these heart healthy diets, including omega-3 fatty acids [173, 174], antioxidant vitamins [175], and polyphenols [174, 176], have been linked to decreased neuroinflammation and neuronal death. In humans, increased adherence to the MeDi has also been associated with increased total brain volume [177, 178] and decreased hippocampal atrophy [179]. Additionally, higher MeDi scores, greater consumption of vegetables, vitamin A, and β-carotene, and mild-moderate alcohol consumption (see “Limit alcohol use” section, below) have been linked to decreased Aβ burden [180–182].
Intervention trials have also demonstrated a link between heart-healthy diets and improved cognitive function, including global cognition, memory, and executive function [183–186]. For example, the PREDIMED trial assigned 7,447 cognitively healthy older adults to either MeDi supplemented with olive oil, MeDi supplemented with nuts, or a control diet (general advice to reduce dietary fat). Both of the MeDi groups showed a reduction in myocardial infarction, stroke, and death from cardiovascular events compared to the control group [187]. Subsequent subset analyses of PREDIMED participants found that both MeDi groups also showed improved cognitive function compared to control [183, 184]. RCTs have also examined interventions using specific components of the MeDi, including polyphenol-rich berries [188–190] and omega-3 fatty acids [190–194]. Interventions using berries and berry-based supplements have shown some promising results for improving performance on cognitive tests. For example, in a small study conducted by Boespflug and colleagues, 16 older adults completed a randomized trial in which they received either daily blueberry powder or placebo powder for a 16-week period. After the intervention, participants who received blueberry supplements exhibited increased blood oxygen level-dependent signal activation in the left hemisphere network that serves verbal working memory during an n-back working memory task compared to controls. Results also suggested that participants who received blueberry supplements tended to have increased accuracy on the one-back task [188]. These results are consistent with findings from large, longitudinal cohort studies, which have demonstrated that berry consumption is associated with a decreased rate of cognitive decline [195]. The evidence on omega-3 fatty acids alone is more mixed, with some studies suggesting improved cognition [190–192] and others suggesting no effect [193, 194].
Not all studies have demonstrated positive effects of following a brain-healthy diet. For example, one population-based cohort study found that diet quality in midlife had no effect on subsequent risk of dementia [196]. A six-month RCT of 160 sedentary adults comparing the effects of aerobic activity, DASH diet, and a combination of both found that the DASH diet alone had no significant effects on executive function [88]. Participants assigned to both aerobic activity and the DASH diet, however, showed the greatest improvement in executive function, suggesting a possible synergistic effect [88]. It is important to note that this intervention only lasted for six months, compared to a median of 4.1 years in the PREDIMED trial; it may take a longer study duration to observe the effects of a heart-healthy diet on cognition.
Given the many evidence-based benefits of the MeDi, DASH, and MIND diets for both brain and overall health, patients should be encouraged to adopt manageable, sustainable changes to increase their adherence to a heart- and brain-healthy diet.
Treat cardiovascular risk factors
Cardiovascular risk factors including hypertension, DM, hyperlipemia, obesity, and metabolic syndrome are associated with an increased risk of developing cognitive impairment and dementia. Often these factors may be addressed through adopting healthy behaviors, including physical activity and a heart-healthy diet, as discussed above. In some cases, however, it may also be appropriate to prescribe medications to treat these conditions to diminish risk of both cardiovascular disease and dementia more expeditiously.
Hypertension
The connection between hypertension and cognitive function is well-documented, and controlling hypertension is recommended by all major health organizations for reducing the risk of cognitive decline. Until recently, hypertension was defined as > 140/90 mmHg, but is now separated into stage 1 hypertension (130–139 mmHg systolic, 80–89 mmHg diastolic) and stage 2 hypertension (> 140/90 mmHg) [197]. Epidemiological evidence has demonstrated that midlife hypertension is a major risk factor for all-cause dementia [198], vascular dementia [199], and cognitive dysfunction [200], including poorer executive function, memory, and processing speed [201, 202]. Several meta-analyses of both observational and intervention studies have demonstrated that use of antihypertensive medications can mitigate the increased risk of cognitive decline and dementia [203–205].
While the link between midlife hypertension and dementia risk has been established for several years [206–210], the evidence on late-life hypertension and dementia risk has been less conclusive [4]. Individuals with early dementia often have a decrease in blood pressure [211], which likely reflects the disease rather than a decrease in risk of cognitive decline. Supporting this hypothesis, steep declines in blood pressure between mid- and late life have been associated with increased risk of dementia [209, 210, 212].
Hypertension negatively impacts the brain in a number of ways. In animal models, hypertension has been associated with decreased cognitive function, increased inflammation and oxidative stress, microhemorrhages and infarcts, endothelial dysfunction, increased Aβ accumulation, and disruption of the blood-brain barrier (BBB) [213]. In humans, hypertension has also been linked to neurovascular dysfunction [214], increased risk of stroke [215, 216], increased global and regional brain atrophy [217, 218], increased white matter changes [218–220], increased Aβ [221–224] and tau [216] pathology, and increased BBB permeability [225]. The BBB, a continuous, closed membrane within all cerebral blood vessels, is critical in protecting neurons from circulating neurotoxic debris, cells, and pathogens in the blood, and increased permeability can increase neuroinflammation and trigger different pathways of neurodegeneration [226]. Treating hypertension with medication may at least partially moderate these negative effects on brain health. For example, a recent autopsy study of 96 AD cases and 53 pathological controls found that antihypertensive medication use was associated with a less extensive spread of AD pathology in the brain [227].
Recent RCTs have shown promising results for programs aimed at controlling hypertension [88, 228, 229]. In the SPRINT-MIND trial [228], 9,361 older adults were randomized to either standard or intensive blood pressure control groups (target systolic blood pressures for the groups were < 140 mmHg and < 120 mmHg, respectively). Compared to standard blood pressure control, intensive blood pressure control was associated with a 19% decreased risk of MCI alone and 15% decreased risk of MCI and dementia combined. The 17% reduction in risk of probable dementia did not reach significance, which may have been related to the early termination of the trial due to robust cardiovascular benefits. The SPRINT-MIND trial also showed promising evidence that intensive blood pressure control was well-tolerated across all ages and did not contribute to negative effects on cognition [228, 230]. Intensive blood pressure control in participants over 80 years old was associated with a greater risk of developing hypotension and possibly syncope, but was not associated with a higher risk of falls as compared to the standard blood pressure control group [230]. In most participants over 80 with at least three co-morbidities, intensive blood pressure control was associated with decreased risk of major cardiovascular events, MCI, and death [231].
In a sub-study of the SPRINT-MIND trial, cerebral white matter lesions were measured in a subset of participants who had an MRI scan at baseline and four years after randomization [232]. After four years, the participants in the intensive blood pressure control group had a smaller increase in cerebral white matter lesions compared to the participants in the standard blood pressure control group [232]. Overall, the SPRINT-MIND trial highlights the potential for early and aggressive blood pressure lowering interventions to have a positive impact on the cerebrovascular health of older adults, which may lower risk of cognitive impairment [228, 231, 232].
Evidence varies on the direct effects of blood pressure control on cognitive function or decline. A sub-analysis of the Heart Outcomes Prevention Evaluation (HOPE)-3 trial found that long-term blood pressure lowering with candesartan plus hydrochlorothiazide versus placebo did not affect cognitive decline in older adults, but did significantly reduce their blood pressure [233]. However, in HOPE-3, systolic blood pressure of the intervention group was only a mean of 6.0 mmHg lower than the control group, which was much less than the reduction achieved in SPRINT-MIND. Similarly, the Hypertension in the Very Elderly Trial cognition function assessment (HYVET-COG) also found that antihypertensive therapy in patients 80 years or older did not have a statistically significant effect in reducing incident dementia, but did significantly reduce blood pressure [234]. However, the HYVET-COG study was terminated early due to significant reductions in all-cause mortality and stroke, which could have confounded analysis of the incidence of dementia. Similar to SPRINT-MIND, the HYVET-COG study also found that older participants with higher Frailty Index scores did not have an increased risk of falls or mortality with antihypertensive therapy [235]. Several studies have confirmed that hypertension management does not contribute to negative effects on frailty and mobility, allaying a common worry for clinicians in the use of some antihypertensive regimens in this population [235–237]. Though the HOPE-3 and HYVET-COG studies did not show improvements or changes in cognition directly, the positive, “downstream” effects that reducing blood pressure have on cerebrovascular and neurodegenerative disease risk are sufficiently well-founded to support the practice of antihypertensive therapy for these at-risk, older adults.
Diabetes mellitus
The connection between DM and dementia is well established. Although both type 1 and type 2 DM are associated with increased risk of cognitive dysfunction [238], type 2 is more often studied in the context of dementia. The presence of type 2 DM has been associated with impaired cognition [239, 240], increased risk of falls [239], and an increased risk of all-cause dementia [241–244] and AD [241, 245], likely through repeated fluctuations of blood sugar levels (hyperglycemia and hypoglycemia), and consequent systemic and cerebrovascular complications [246]. A study of 10,316 patients with a new diagnosis of DM and 41,264 age- and sex-matched controls found that DM was associated with a 47% increased risk of dementia [242]. In patients with DM, hypertension and/or hyperlipidemia did not significantly increase dementia risk, suggesting that the increased risk of dementia was primarily driven by DM itself [242]. The Ginkgo Evaluation of Memory Study investigated the relationship between DM and domain-specific cognitive impairments, and found that participants with DM scored worse on cognitive baseline tests and showed greater cognitive declines over a median of 6.1 years in phonemic verbal fluency and executive function compared to their healthy peers [240]. Impaired cognition can lead to poor self-management of DM, creating a vicious cycle of worsened symptoms and worsened cognition, which may be associated with unfavorable health outcomes and decreased quality of life [247].
The mechanisms by which DM likely impacts cognitive function and dementia risk have been reviewed thoroughly [246, 248–250]. Briefly, glucose toxicity, hyperinsulinemia, and vascular damage resulting from DM may contribute to increasing risk of cognitive decline and dementia. In animal models, DM has been associated with cognitive dysfunction, neuroinflammation, vascular injury, atrophy of the hippocampus and prefrontal cortex, increased risk of stroke, and increased AD pathology [246, 248, 250]. In humans, Type 2 DM has been linked to systemic inflammation, neuroinflammation, global and regional atrophy, white matter hyperintensities, decreased functional connectivity, decreased glucose metabolism in the brain, increased AD pathology, increased risk of stroke, and comorbid depression [246, 248, 249]. Some of these changes are also observed in prediabetic populations. For example, in a cross-sectional study of 2,439 middle-aged adults from the Framingham Offspring cohort, both DM and prediabetes were associated with decreased total cerebral brain volume [251].
Some evidence demonstrates that intensive glycemic control may mitigate the increased risk of cognitive decline and dementia. A study by Yaffe and colleagues found that elderly adults with DM had lower cognitive test scores at baseline than participants without DM, and participants with DM and poor glucose control over a nine-year follow-up period had worse cognitive function and a greater rate of cognitive decline than others [252]. In an observational study of 3,433 patients with type 1 DM, individuals with more than 75% of their hemoglobin A1c (HbA1c) measurements at 8.0–8.9% or > 9% were more than twice as likely to develop dementia over a mean follow-up of 6.3 years compared to individuals with minimal exposure (less than 10% of HbA1c measurements) to these levels. Conversely, patients with better glycemic control (i.e., more than 75% of HbA1c measurements at 6.0–6.9% or 7.0–7.9%) had an approximate 60% dementia risk reduction compared to those with minimal exposure to well-controlled levels of HbA1c [253]. A secondary analysis from the Finnish Diabetes Prevention study, which randomized middle-aged adults to a four-year lifestyle intervention or control group, showed that better glycemic control was associated with better cognitive performance nine years post-intervention in individuals with impaired glucose tolerance at baseline [254]. However, most RCTs conducted to date have not found benefits of intensive glycemic control interventions on cognitive function or decline [255–258]. Despite these negative findings, there is ample evidence that untreated DM is harmful to brain health. Patients should be encouraged to adopt healthy lifestyles to prevent or delay the onset of DM. Providers should work with patients in order to detect early signs of DM, obtain an early diagnosis, manage hyperglycemia, and encourage regular monitoring of the disease.
Hyperlipidemia
Hyperlipidemia (total cholesterol > 200 mg/dL [259]) has been associated with dementia risk, although evidence is less conclusive than other cardiovascular risk factors. Some epidemiological evidence has shown that high cholesterol in midlife is associated with increased risk of all-cause dementia [260], AD [208, 260–262] and vascular dementia [261], while other studies have found no link [263]. Late-life cholesterol levels have not been linked to risk of cognitive impairment or dementia [262, 264].
The precise mechanism by which cholesterol impacts dementia risk is still unclear [265]. In a transgenic mouse model expressing human apolipoprotein B-100, hyperlipidemia has been associated with cardio- and cerebrovascular damage, vascular dysfunction, increased permeability of the BBB, and atherosclerosis [266]. In humans, hyperlipidemia increases the risk of cardiovascular disease, atherosclerosis, and stroke [267, 268]. Additionally, Bowman and colleagues investigated the relationship between the BBB and the pathology of AD and found that dyslipidemia was prevalent in 47% of study participants with mild-to-moderate AD and 75% of those who had AD with BBB impairment, suggesting that dyslipidemia may play a role in decreasing BBB integrity [269]. Total cholesterol and low-density lipoprotein cholesterol levels have also been associated with increased Aβ burden in humans [224, 270].
In order to reduce cardiovascular risk, the American Heart Association (AHA) and American College of Cardiology (ACC) recommend keeping total cholesterol levels below 170 mg/dL [259]. The AHA and ACC suggest eating a heart-healthy diet, staying physically active, quitting smoking, and maintaining a healthy weight to reduce cholesterol levels [259]. Patients with elevated cholesterol may also need to be treated with a statin. For most patients, the AHA and ACC recommend initiating statin therapy when low-density lipoprotein cholesterol levels exceed 70 mg/dL [259]. Some evidence suggests that the use of statins may mitigate the risk of all-cause dementia [271] or AD [272]. See “Other medications that may affect cognition” for a more complete review of the current evidence on statin use and risk of dementia.
Obesity and metabolic syndrome
Obesity and metabolic syndrome (or insulin resistance syndrome), which is characterized by abdominal obesity, hyperglycemia, dyslipidemia, and hypertension [273], have been well-established as risk factors for dementia [274–278]. In clinical studies, obesity is most often defined using body mass index (BMI). It is important to note that BMI does not account for differences in body composition, and therefore is not a perfect representation of adiposity. However, the association between BMI and dementia risk is still strong. For example, in a recent study of 6,582 individuals over the age of 50, Ma and colleagues found that obesity (defined as BMI≥30 kg/m2) at baseline was associated with an increased risk of dementia over a mean follow-up period of 11 years [274]. Several studies have demonstrated a positive association between midlife, but not late-life, obesity and risk of developing dementia [279, 280]. Data from the Whitehall II study demonstrated that obesity at age 50, but not at ages 60 or 70, was associated with an increased risk of dementia [280]. The lack of association between obesity in late life and dementia risk, however, may be attributable to the “preclinical” decrease in weight that can often be seen in early cognitive impairment, prior to dementia onset [280].
Obesity (particularly central or abdominal obesity) in both animals and humans has been linked to release of pro-inflammatory cytokines, including interleukin-6 and tumor necrosis factor alpha, and chronic systemic inflammation [281]. Obesity-associated inflammation and hyperinsulinemia contribute to endothelial dysfunction, which leads to increased BBB permeability and decreased insulin transport to the brain [281]. The dysregulation of adipokines, including leptin, adiponectin, and resistin, that occurs in individuals with obesity may also play a role in increasing the risk of dementia, but more research is needed to fully elucidate these relationships [282]. Together, the systemic effects of obesity have deleterious effects on the brain, but losing weight may partially mitigate these negative effects. Prehn and colleagues randomized 37 obese women to a weight-loss intervention following a caloric restriction or control condition [283]. Following the 12-week intervention period and a four-week period where the new weight was maintained, the intervention was associated with increased grey matter volume in the inferior frontal gyrus and hippocampus and augmented hippocampal functional connectivity [283].
Weight loss interventions have shown promise for improving cognition in cognitively normal older adults [284] and older adults with MCI [285–287]. For example, Napoli and colleagues randomized 107 cognitively normal older adults to weight management (diet), exercise, combination weight management and exercise, or a control group [284]. After the 52-week intervention period, participation in any of the three intervention groups was associated with increased scores on the Mini-Mental State Exam compared to control [284]. In another intervention trial, Horie and colleagues randomized 80 older adults with MCI and BMI≥30 kg/m2 to either routine medical care or routine medical care with nutritional counseling [285]. Both groups had significant decreases in BMI over the 12-month intervention period, and decreases in BMI were associated with improvements in cognition, with no difference between groups [285]. The results from these studies suggest that targeting weight loss may be a promising approach for improving cognition or slowing the rate of decline in individuals who already have cognitive impairment.
Stop or reduce smoking
Epidemiological evidence has demonstrated the detrimental effects of smoking on cognitive performance [288, 289], risk of all-cause dementia [290–293] and AD [290–295], and rate of cognitive decline [292]. For example, one cohort study of 21,123 adults followed for 20–30 years, found that smoking more than two packs per day in midlife was associated with an increased risk of dementia of over 100% [291]. Evidence suggests that amount of smoking is positively correlated with risk of dementia. In a meta-analysis of 37 observational studies, for every 20 cigarettes smoked per day, there was a 34% increase in the risk of all-cause dementia [293]. Reducing or quitting smoking, however, may partially or even fully mitigate this increased risk of dementia. Multiple studies have shown that former smokers do not have an increased risk of dementia or AD compared to never-smokers [290, 293].
In animals, exposure to cigarette smoke is associated with increased markers of oxidative stress, depleted cerebral antioxidants, and increased proinflammatory cytokines [296]. In humans, cigarette smoke has been linked to increases in oxidative stress and neuroinflammation, risk of stroke, white matter hyperintensities, subcortical atrophy, and cerebral Aβ deposition [297–299]. Additionally, in cognitively healthy individuals, chronic smoking may lead to atrophy of areas of the basal forebrain that provide cholinergic input to the brain, increasing risk of developing AD [300].
In contrast to most of the literature, a few studies have found no effect or a protective effect of cigarette smoking on dementia risk [301, 302]. However, these results may be misleading due to study participant selection bias, survivor bias, or funding by the tobacco industry [295, 303, 304]. Even after controlling for survivor bias, however, one recent study found that smoking tobacco was not associated with incident dementia, despite strong correlations with other chronic diseases and earlier mortality [305]. Despite a few conflicting results, there is overwhelming evidence that smoking is harmful to brain and overall health, and providers should encourage patients to stop or reduce smoking.
Manage stress and depression
Stress
Stress has been defined as a condition or feeling experienced when a person perceives that demands exceed the personal and social resources the individual is able to mobilize [306]. Managing life’s stressors is often recommended in order to maintain a cognitively healthy lifestyle. Demanding moments can elicit a stress response that varies in intensity, duration, and predictability across each individual experience [307]. This inherent heterogeneity complicates our understanding of the mechanisms by which stress affects cognition. Importantly, not all stress is harmful to cognition. When the stress response is moderate and controlled, it can benefit the way people learn and adapt [308]. For example, mild stress improves task accuracy by facilitating cognitive functions like selective attention [309]. Acute and chronic moderate and severe stress, however, have been shown to be deleterious to cognitive processes.
Generally, acute stress has been found to transiently degrade cognitive capacities such as working memory, processing speed, and attention [310–312]. Coping with acute stress may require cognitive resources to be diverted from tasks at hand to help contain the stressful experience, which can undermine cognitive performance and function [313]. In a meta-analysis of 51 studies, Shields and colleagues found that acute stress exerted a greater effect when working memory load was high. Additionally, acute stress is associated with significantly reduced cognitive flexibility across various conditions [312].
The transition from acute to chronic stress is marked by a prolonged, maladaptive response that can have an even greater impact on cognition. For example, a two-year study of 2,713 older adults revealed that those with higher levels of perceived stress performed worse on tests of processing speed and attention when compared to less stressed peers [314].
Critically, there is a growing body of evidence that has shown chronic life stress to be associated with an increased risk of dementia [315, 316]. In one 35-year prospective study of 1,462 women, frequent or constant stress reported in midlife was associated with an increased risk of dementia [315]. Additionally, smaller studies have found that people who suffer chronic life stressors [317], have current stress disorders [318], or who report a lack of control over their high pressure jobs [319] developed dementia at higher rates than those living less stressful lives. A review of epidemiological evidence showed that increased life stress has been linked to greater risk of MCI or dementia in a number of studies, even if stress is not the primary or direct contributing factor [316].
The precise mechanism by which acute and chronic stress affect cognitive performance and cognitive decline over time is still unclear, although several factors likely contribute. First, recent findings point to the physiologic response of chronic stress having deleterious effects on cortical structures critical to the progression of dementia [320, 321]. The hippocampus is rich in corticosteroid receptors, making it especially vulnerable to increased activity of the hypothalamic-pituitary-adrenal axis [308]. In both animals and humans, high glucocorticoid levels have been linked to increased oxidative stress and subsequent neurodegeneration in the hippocampus [321, 322]. Glucocorticoid exposure in animals has also been linked to decreased hippocampal BDNF expression [323]. In a review of animal studies, both behaviorally-induced stress and mimicry of the stress response via corticosterone treatment significantly altered hippocampal structure and function [324]. In humans, elevated cortisol levels have also been associated with decreased total brain volume [325, 326] and increased white matter changes [327]. Additionally, cortisol release may directly affect AD pathology. In a double transgenic mouse model of AD, the strain that experienced increased oxidative stress showed accelerated amyloid deposition, tau phosphorylation, and gliosis [328]. These findings are consistent with older animal models that showed in vivo cortisol administration increased levels of Aβ [320] and tau accumulation [329, 330]. A study of 99 older adults with probable AD found that plasma cortisol levels were associated with Aβ burden, as measured by PiB-PET [331]. Thus, the physiologic response to chronic stress may compound the neuropathological changes associated with AD and hasten their clinical expression.
Finding ways to manage stress may be helpful as a preventative measure in reducing the risk of dementia. One strategy is practicing meditation or mindfulness, which have been shown to have many health benefits for older adults. They are easily accessible, low-risk methods that can be practiced to reduce stress levels and improve sleep quality and mood, all of which may decrease the risk of developing MCI or dementia [332]. In a recent RCT, adults with memory concerns enrolled in a 12-week meditation program demonstrated significant improvements in measures of cognitive function, with sustained benefits after six months [333]. In another study, 14 patients with MCI reported that a mindfulness-based stress reduction program was helpful in lowering stress levels and promoting wellbeing [334]. Meditation and mindfulness practices have also been shown to diminish release of excess cortisol and increase cerebral blood flow within the frontal lobes, a brain region that is especially important for cognitive functioning [335]. Additionally, yoga has been found to have a positive impact on biomarkers of cellular aging, presumably by regulating stress and inflammatory responses [336]. Yoga has also been found to improve daily attention, memory, and executive function in individuals with MCI [337, 338]. Encouraging patients to manage stress may mitigate risk of cognitive decline.
Depression
There is conflicting evidence about whether depression in older adults is an independent risk factor for or a prodromal symptom of mild cognitive impairment and dementia. We suspect that depression may reflect either, depending on the individual. A review of observational studies reported that depression prior to age 60 was associated with a two- to four-fold increase in dementia risk, while late-life depression was associated with a two- to five-fold increase in dementia risk [339]. Not all studies, however, have found an association between both mid- and late life depression and dementia risk. One epidemiological study found that each self-reported depressive episode until approximately age 51, often of varying duration and sometimes with years separating episodes, was associated with an increased risk of developing dementia, suggesting that depression may be a dementia risk factor [340]. In individuals 51 and younger, those who had one elevated symptom of depression demonstrated an 87% increased risk of dementia [340]. In contrast, a study of 10,189 individuals followed over a 27-year period beginning at age 45 found that depressive symptoms in midlife, regardless of severity or duration, were not associated with a greater risk of dementia [341]. In the same study, however, depressive symptoms in late life, and specifically in the decade preceding the onset of dementia, were associated with an increased risk of dementia [341]. These results suggest that depression may be a feature of the “preclinical” phase of dementia rather than an independent risk factor. It is important to note that studies of late-life depression are highly variable in their timing and length of follow up, making it difficult to define depression as a risk or prodromal factor. Illustrating the complexity of the data, a meta-analysis of 34 studies on depression and dementia suggested that early or midlife depression may be a risk factor, while late-life depression is commonly a prodromal symptom [342].
Despite the conflicting evidence about its connection to dementia risk, depression has been associated with decrements in general cognitive abilities such as processing speed, attention, and executive control [343, 344]. Approximately 30–40% of depressed older adults exhibit signs of executive dysfunction during cognitive examination [345]. When compared to healthy controls, depressed adults over the age of 60 performed significantly worse on measures of episodic memory, language processing, executive function, and processing speed [346]. Notably, those who received psychotherapy for late-life depression exhibited a significant improvement in processing speed after 12 weeks of treatment [347]. Additionally, depression is known to negatively impact sleep integrity. Adults that have shortened or fragmented sleep have been found to report subsequent, suboptimal cognitive processing [348, 349]. See “Maintain adequate sleep” for further discussion on sleep, cognition, and dementia risk.
Proposed pathways for increased dementia risk include the direct physiological effects of depression on stress hormones, hippocampal volume, and neuronal growth factors [342]. In a study of 218 older adults, five years of untreated depression accurately predicted a decrease in left hippocampal volume, which was associated with cognitive impairment [350]. Additionally, Sawyer and colleagues found that depressed individuals had increased atrophy of the right hippocampus over a four-year period. This atrophy was an accurate predictor of decreased scores on the Mini-Mental State Exam [351]. The exact relationship between hippocampal volume loss and depression is unknown, but animal studies suggest that depression may contribute to the pathophysiology of neurotoxic damage [352] or decreased levels of neuronal growth factors, including BDNF [353]. Animal models of depression have demonstrated that the administration of exogenous BDNF can reverse depressive-like behaviors, including reduced exploration and behavioral despair, which are connected to decreased synaptic density in the hippocampus [353].
Despite uncertainty about the causal relationship between depression and dementia, it seems prudent to treat depression as a potential way to help prevent further stress on neurocognitive networks vulnerable to impairment.
Maintain adequate sleep
Adequate sleep is important in order to maintain brain health throughout life. The U.S. National Sleep Foundation recommends 7–9 hours of sleep per night for adults under 65 years of age, and 7-8 hours of sleep for adults 65 years and above [354]. Epidemiological evidence has shown that poor and disrupted sleep are associated with decreased cognitive performance [355], increased rate of cognitive decline [356], and elevated risk of cognitive impairment [357, 358], AD [356–359], and all-cause dementia [359, 360].
Getting enough sleep is imperative for healthy cognitive aging, as it plays a key role in consolidating memories, maintaining cognitive performance, and clearing potentially neurotoxic metabolites, including Aβ, that build up during wakefulness [361, 362]. In animal models, chronic sleep deprivation has been linked to increased inflammation, increased neuronal death, decreased neurogenesis, increased volume loss in the hippocampus and medial prefrontal cortex, and increased Aβ and tau pathology [363]. Studies in humans have shown that shorter sleep duration and lower quality of sleep are associated with increased systemic inflammation [364], decreased amyloid clearance [362, 365], and greater Aβ burden over time [366–368]. An RCT by Ooms and colleagues randomized cognitively healthy middle-aged men to a night of unrestricted sleep or total sleep deprivation. Unrestricted sleep was associated with the expected, normal morning decrease of Aβ42 levels in the cerebrospinal fluid compared to sleep deprivation after a single night, which interfered with this decrease [365]. This result suggests that poor sleep may have direct effects on the homeostatic fluctuations and clearance of aggregated neurotoxins, which may help explain the association of poor sleep with neurodegenerative processes.
Sleep-disordered breathing
Sleep-disordered breathing (SDB) is a common reason for poor and disrupted sleep in adults. Obstructive sleep apnea (OSA) is a type of SDB in which a person has frequent episodes of absent breathing (apneas) and/or slow, shallow breathing (hypopneas) linked to complete or partial blockage of air movement, respectively. These events often result in arousal from sleep and decreases in blood oxygen saturation that interfere with restorative sleep [369]. Globally, nearly one billion adults are estimated to have OSA, though most cases are undiagnosed and untreated [370]. SDB, and particularly OSA, is linked to several factors that may increase risk of cognitive decline, including sleep disruption, increased inflammation and oxidative stress, and cardiovascular comorbidities [371]. Indeed, epidemiological evidence suggests that SDB may increase risk of MCI, all-cause dementia, and AD [358, 359, 372] and decrease the age of onset of MCI and AD dementia [373]. Promisingly, observational studies [373], prospective clinical studies [374], and RCTs [375–377] have demonstrated that use of continuous positive airway pressure (CPAP) seems to at least partially mitigate the negative effects on cognition and dementia risk associated with SDB. Treatment of SDB with CPAP is also associated with improved white matter integrity and decreased Aβ burden in cognitively healthy older adults [374, 378]. Patients should be screened regularly for SDB and other sleep disorders, because diagnosis and treatment may play an important role in reducing risk of cognitive decline for many of them.
Manage hearing and visual impairments
Living with hearing or vision impairment can be difficult and even debilitating, especially if left untreated. Epidemiological evidence has suggested that hearing, vision, or combined sensory impairment can increase the risk of developing cognitive impairment and dementia and increase the rate of cognitive decline [379–388]. One meta-analysis found that hearing impairment in midlife nearly doubled the risk of developing dementia in late life [389]. While most epidemiological studies have found a link between diminished hearing or vison and cognitive impairment or dementia, some have found no significant effect of either sensory limitation [390] or a significant effect of hearing but not vision impairment [391].
The precise mechanism by which hearing and vision impairment affect cognition and dementia risk is still largely unknown. Several possible mechanisms have been presented. One possibility is that sensory deficits may have a direct impact on the brain. A study by Lin and colleagues found that hearing loss in humans was associated with an accelerated rate of brain atrophy in the whole brain and, regionally, in the right temporal lobe (including the superior, middle, and inferior temporal gyri) and the parahippocampal cortex [392]. Animal models of adult deafness have shown that gradual hearing loss is associated with decreased spatial memory, decreased hippocampal plasticity, and changes in neurotransmitter receptor expression [393]. The association between hearing loss and these changes in the brain may be mediated by reduced neural stimulation of the auditory cortex, decreased auditory signal strength over time, or some shared neuropathologic or intrinsic cellular aging process that affects both cochlear and brain aging [392, 394]. Another possibility is that other biological factors related to aging and microvascular pathology underlie the relationship, as these factors can contribute to both sensory deficits and impaired cognition [389, 395]. Our own research suggests that an individual’s efforts to manage degraded sensory signals, as occurs in vision impairment, may deplete limited cognitive processing resources, which likely contributes to increased cognitive difficulties [396]. Finally, hearing and vision loss can undermine an individual’s daily functioning by reducing ability to participate in physical, social, or cognitive activities [395], and can result in depression [397], social isolation, or loneliness [398], which are independent risk factors for worsened cognitive outcomes and dementia. A recent study by Maharani and colleagues found that conditions of depression and loneliness at least partially mediated the effects of hearing loss on cognition [398]. The connection between hearing and vision impairment and dementia is likely due to a combination of several of these factors. Further complicating the evidence, however, a recent study by Brenowitz and colleagues found that impaired hearing was associated with increased neurofibrillary tangle burden prior to, but not after, the onset of cognitive impairment, suggesting that impaired hearing may also be a preclinical marker of tau-related neurodegeneration [399]. More research is needed to fully elucidate the relationship between sensory deficits and dementia risk.
Observational cohort studies have suggested that correcting hearing or vision impairment may boost cognition and counter a patient’s trajectory of cognitive decline [400–403]. RCTs are needed to further investigate these findings and determine exactly what factors might explain treatment responses and impact risk. In the meantime, however, widespread efforts to treat hearing and vision loss have become increasingly important as the population ages. Two of the most easily remediable visual impairments, uncorrected refractive error (URE) and cataracts, make up 75% of the total visual impairments in older individuals (42% for URE and 33% for cataracts) [404]. Recent investigations funded by the National Eye Institute have predicted that the number of individuals in the U.S. with URE will double between 2015 and 2050, increasing from 8.2 million individuals to 16.4 million individuals [405]. Disabling hearing loss is predicted to grow at a similar rate, increasing from 466 million individuals to 900 million individuals globally between 2018 and 2050 [406]. Ensuring patients maintain updated eyeglass prescriptions, undergo cataract surgery if appropriate, and use hearing aids may help to mitigate risk of cognitive decline and dementia. It is our contention that patients should not expend limited cognitive resources on simply trying to decode sounds or decipher visual images.
Avoid medications with anticholinergic properties
Several classes of medications have been investigated to determine their effect on brain health and cognition. Thus far, medications with anticholinergic properties have the most conclusive evidence linking them to impaired cognition and increased risk of cognitive decline. Several epidemiological and cohort studies have demonstrated that use of anticholinergic medications is associated with decreased cognitive performance on tests of memory, executive function, and visual attention and increased risk of dementia [407–415]. A recent study of 688 cognitively normal older adults found that use of anticholinergic medications was associated with increased rates of memory and language decline and elevated risk of progression to MCI. These effects were enhanced in individuals who also had genetic risk of AD or cerebrospinal fluid biomarkers for AD [414]. One common tool used to quantify the effects of anticholinergic medications is the Anticholinergic Cognitive Burden (ACB) scale, a 3-point scale based on the strength of the medication’s anticholinergic properties in the brain as determined by receptor affinity assays and expert-based consensus of clinically relevant cognitive effects [416]. Observational studies that use the ACB scale have confirmed that medications with higher ACB scores are linked to increased risk of dementia [409, 410]. A recent cohort study of 8,216 older adults found that recurrent use of anticholinergic medications with the highest ACB score of 3 (but not 1 or 2) was associated with a 68% increased risk of developing dementia during a ten-year follow-up period [409]. When separated by medication class, antidepressant, antiparkinson, antipsychotic, and antimuscarinic bladder medications with anticholinergic properties, specifically, have been associated with increased risk of dementia [410, 411]. Other classes of drugs with anticholinergic properties, including antihistamines, gastrointestinal antispasmodics, and skeletal muscle relaxants, have not been definitively linked to increased risk of dementia [410, 411], although this does not preclude cognitive risks from use of these medications.
Importantly, not all studies have found a clear link between anticholinergic medication use and dementia risk. For example, a cohort study of 3,690 older adults found that, while strong anticholinergic medications more than doubled risk of MCI, there was no association between anticholinergic medications and dementia risk [417]. As the authors acknowledged, these results were likely impacted by the short duration of the study (one year).
There are several potential mechanisms by which anticholinergic medications may increase risk of cognitive decline and dementia. By definition, anticholinergic medications block cholinergic transmission, which is critical for cognitive processes such as memory and attention. A recent study by Chhatwal and colleagues using functional connectivity MRI in humans found that anticholinergic drugs disrupt neural network connectivity involved in learning and memory, which may have subsequent effects on network-based resistance to neuropathologic change [418]. Limited evidence also suggests that anticholinergic medication use may be associated with increased brain atrophy in humans [415]. Studies of animals and in vitro human cells have demonstrated that anticholinergic medications may increase Aβ formation, decrease acetylcholine-stimulated hydrolysis of amyloid-β protein precursor (AβPP), or lower levels of phosphatidylcholine, a component of the synaptic membrane [419]. In animal models of AD, anticholinergic burden has also been linked to increased tau pathology and neuroinflammation via microglial activation [420].
More research is needed to determine if discontinuing use of anticholinergic medications can improve current cognitive performance or counter the increased risk of cognitive decline and dementia that prior use may have conferred. In one population-based cohort study of 4,128 older adults, discontinuing use of anticholinergic medications was associated with a decreased risk of dementia over a four-year follow-up period [412]. To date, only a few RCTs have investigated the cognitive benefits of interventions to decrease anticholinergic medication burden and results remain inconclusive [421, 422]. In one study conducted by Kersten and colleagues, 87 older adults with no dementia (n = 27), mild dementia (n = 36), or moderate dementia (n = 24) were randomized to either a pharmacist-initiated reduction of anticholinergic burden or a control group with no medication changes. The intervention reduced anticholinergic drug burden scores as designed, but had no significant effect on cognitive function over the eight-week trial [421]. It is difficult to interpret these results. Of note, observing intervention-related differences in dementia patients who received pro-cholinergic agents (i.e., cholinesterase inhibitors) required at least six months in clinical trials that included hundreds of participants [423]. More intervention trials are needed to investigate whether reducing anticholinergic burden may improve cognition or slow cognitive decline.
In the meantime, current evidence suggests that prescribing medications with strong anticholinergic properties should be avoided when possible, and efforts to reduce or eliminate these medications in older patients already on them seem prudent.
Other medications that may affect cognition
The relationship between dementia risk and other medications like benzodiazepines, proton pump inhibitors (PPIs), and statins has also been investigated. Although the evidence on anticholinergic medications seems to point toward increased risk of dementia, the current evidence on these other medications is less conclusive.
Concerns have been raised about the negative impact of prolonged benzodiazepine use. Some observational evidence suggests that benzodiazepine use is associated with increased risk of dementia [424–427], and that risk increases with exposure duration and strength [426, 427]. A recent meta-analysis of 10 observational studies found that benzodiazepine use was associated with a 51% increased risk of dementia. Risk was higher for long-term benzodiazepine use (> 3 years) and benzodiazepines with a longer half-life (> 20 hours) [428]. Conversely, several observational studies have found no link between benzodiazepine use and dementia risk [409, 429–432]. For example, a recent Danish nationwide-cohort and nested case-control study did not find an association between use of benzodiazepines and risk of dementia, even for cumulative exposure or when divided into short- and long-acting drugs [429]. Notably, even in studies that found no association overall, there was an increased risk of dementia with small amounts of benzodiazepine use (1–30 total standard daily doses over a ten-year period) in older adulthood [431], or new benzodiazepine use in the year prior to dementia diagnosis [430]. Benzodiazepine use during those periods of time could reflect the treatment of anxiety or other prodromal neuropsychiatric symptoms of dementia. More research on benzodiazepine use and dementia risk is needed in order to determine causality and the impact of potentially confounding variables. In the absence of conclusive evidence, expert consensus currently suggests that appropriate benzodiazepine use will not lead to the development of dementia [433]. Still, to moderate risk of potential long-term effects, clinicians should consider prescribing shorter-acting benzodiazepines for limited periods of time when possible.
The evidence on PPIs, such as omeprazole, pantoprazole, and lansoprazole, and risk of dementia is similarly mixed. Some observational studies have found a link between PPI use and risk of dementia [434–436] and AD [435]. In one of these studies, a significant association was also observed between cumulative PPI use and dementia risk [436]. Conversely, many other observational studies have found no association between PPI use and risk of dementia [437–439] or AD [440, 441] regardless of cumulative exposure level or pattern of use. Meta-analyses conducted to date seem to confirm the lack of association [204, 442]. Further complicating the picture, a few studies have found that PPI use is associated with a decreased risk of cognitive decline [443] or dementia [444], suggesting a possible protective effect. For example, a prospective cohort study of 10,486 adults over 50 years old found that continuous or intermittent PPI use was associated with decreased risk of decline in cognitive function and decreased risk of conversion from MCI to dementia compared to never use. Overall, the evidence on PPI use and brain health does not point conclusively in any direction.
Statins, or HMG-CoA reductase inhibitors, are widely prescribed in the treatment of cardiovascular and cerebrovascular diseases. In addition to lowering cholesterol levels, statins have been shown to exhibit many other effects, including improving endothelial function, inhibiting platelet activation, and decreasing inflammation [445], thereby reducing the risk of cardiovascular disease and stroke [446]. Statins have also been associated with decreased cerebral Aβ deposition [447, 448]. Accordingly, several observational studies have linked statin use to decreased incidence of all-cause dementia [449–451] or decreased rate of cognitive decline [452]. Meta-analyses of pooled data seem to support these results [271, 272, 453, 454]. However, intervention trials completed to date have not supported a link between statin use and cognitive status [233, 455]. For example, in the HOPE-3 study (as discussed above in the section on “Hypertension”), 2,361 older adults were randomized to receive blood-pressuring-lowering medication (candesartan plus hydrochlorothiazide) or placebo and lipid-lowering medication (rosuvastatin) or placebo once daily until study completion, an average of 5.7 years later. Neither blood pressure lowering, lipid lowering, or their combination had a significant effect on cognitive decline over the 5.7-year follow-up period [233]. Some observational studies have also found no link between statin use and risk of dementia [456], rate of cognitive decline [457], or neuroimaging biomarkers of AD [458]. Additionally, a 2017 retrospective cohort study of 3,500 adults, ages 51 to 100, found that statins increased the risk of cognitive impairment and dementia [459]. As discussed, however, data from most studies conducted to date do not seem to support negative effects of statins on brain health. As with the other medications reviewed here, more research, including additional RCTs, is needed to further clarify the effects of statins on cognition and dementia risk. Despite the results of some epidemiological studies that suggest a correlation between statin use and reduced cognitive decline, there is currently no compelling reason to initiate treatment with a statin for the purpose of improving cognitive status or preventing decline. Likewise, there is no persuasive evidence that statin use leads to a deterioration of cognitive abilities.
Nutraceuticals and supplements
Nutraceuticals and supplements have been the subject of a number of recent reviews [460–462]. Although they fall outside the scope of this article, nutraceuticals and supplements are worth briefly mentioning due to the rapid growth of the industry in recent years. While no official legal or regulatory definition exists to date, a “nutraceutical” is generally defined as a food or food-based product that provides medical or health benefits [463]. Nutritional supplements are dietary add-ons, like vitamins, proteins, or minerals, that are also often found naturally in foods. These products are not regulated by agencies such as the U.S. Food and Drug Administration, and their safety and efficacy is often based on testimonials rather than scientific research [464]. A 2018 meta-analysis of 38 studies examined the human evidence of various supplements, including omega-3 fatty acids, soy, gingko biloba, B vitamins, vitamin D plus calcium, vitamin C or beta-carotene, multi-ingredient supplements, and other over-the-counter interventions, and found insufficient evidence that any of these supplements improved cognitive performance in a clinically meaningful way or reduced the risk of cognitive decline or dementia [461]. Despite the minimal scientific evidence supporting their efficacy, nutraceuticals and supplements targeting memory have become increasingly popular due to the lack of disease-modifying treatment options for AD and other dementias. By marketing their products as memory- or cognition-enhancing, this multibillion-dollar industry seems to be taking advantage of aging individuals eager to retain their mental capacities [465].
Limit alcohol use
Alcohol use has been identified as a risk factor for cognitive decline and dementia, but some evidence suggests that certain levels of alcohol consumption may have a protective effect. Major health organizations recommend limiting alcohol use to a moderate level, defined as up to one drink per day for women and up to two drinks per day for men [158]. Exceeding this recommended amount is considered heavy alcohol use [158]. Epidemiological evidence on alcohol consumption points toward a U-shaped dementia risk curve, with mild to moderate drinking associated with the lowest risk of dementia compared to either abstention or heavy drinking [4, 466–470]. A non-linear relationship has also been observed between alcohol consumption and cognitive function, whereby alcohol abstinence is associated with a higher risk of impaired memory and executive function compared to moderate alcohol intake [471].
Alcohol use disorder (AUD) specifically has been associated with a particularly strong risk of all-cause dementia. A nationwide, retrospective cohort study involving 31.6 million adults in France found that AUD more than tripled risk of all-cause dementia. The association was particularly clear for early-onset dementia (before age 65), although underlying dementia pathology was not determined [469]. These results are especially concerning given the prevalence of AUD. As of 2018, an estimated 5.8% of adults had AUD in the U.S. alone [472].
The risks of excessive alcohol consumption on the nervous system are well established. Longstanding evidence has demonstrated heavy alcohol use is associated with structural and functional abnormalities of the brain [473]. In animals, long-term consumption of alcohol has been linked to increased neuroinflammation via microglial activation and upregulation of pro-inflammatory cytokines [474], decreased BBB integrity [475], and increased expression of AβPP and elevated Aβ deposition [476]. In humans, heavy drinking is linked to increased risk of cardiovascular disease and stroke [477, 478], increased neuroinflammation via microglial activation and upregulation of pro-inflammatory cytokines [474], thiamine deficiency (which can contribute to cognitive and neurological dysfunction) [479], and alcohol-related dementia or alcohol-induced persisting amnestic syndrome [479]. Neuroimaging and neuropathological studies have also shown widespread loss of white matter in individuals with diagnosed AUD [479].
Conversely, mild to moderate alcohol consumption in humans has been linked to decreased risk of cardiovascular events [478, 480] and decreased Aβ deposition in older adults [180], suggesting possible reduced risk of AD. The neuroprotective effect of mild to moderate alcohol consumption may be due to antioxidant polyphenols or ethanol itself, which have each been associated with decreased neuroinflammation and oxidative stress [480]. The Mediterranean diet, as discussed in the “Heart-healthy diet” section, allows for regular, mild- moderate alcohol intake and especially emphasizes the consumption of wine. Red wine, specifically, is abundant with antioxidant polyphenols such as resveratrol and has been linked to the best cardiovascular and brain health outcomes [480, 481].
It is important to note that there is conflicting evidence about both the benefits of mild-moderate alcohol consumption and the risks of heavy alcohol consumption. For example, a recent cohort study of 3,021 older adults found that alcohol consumption within recommended limits (i.e., 7.1 to 14.0 drinks per week) was not associated with decreased risk of dementia in cognitively normal individuals or individuals with MCI compared to drinking less than 1.0 drinks per week [482]. Exceeding 14.0 drink per week, however, was associated with the most severe cognitive decline in individuals with MCI [482]. A meta-analysis found that light to moderate drinking was associated with a 25% to 28% decrease in risk of AD, vascular dementia, and all-cause dementia compared to abstinence, but heavy drinking had no impact on dementia risk. The authors acknowledge that there could be a bias due to sampling or survival effects, as heavy drinkers are less likely to participate in or finish these studies and more likely to have comorbid health conditions [483]. More research is needed to understand the effect of various levels of alcohol consumption on brain health.
Despite outstanding questions about the effect of alcohol consumption on risk of cognitive decline and dementia, the general health risks of heavy alcohol use are widely acknowledged. Patients should be encouraged to limit their alcohol consumption to a mild to moderate level, especially if they are already experiencing cognitive difficulties. Evidence suggests that the best drinking pattern for cardiovascular and brain health may be one glass of red wine per day.
Protect the brain from physical and toxic injuries
Physical injury
Traumatic brain injury (TBI) results from an impact to the head that disrupts normal brain function [484]. The most common causes of TBI are falls and motor vehicle accidents [484]. TBI has been associated with an increased risk of cognitive decline and dementia. Physical injury to the brain reduces brain reserve, thus lowering an individual’s capacity to compensate for the development of neurodegenerative processes. Whether TBI directly promotes neurodegenerative processes, however, remains an area of ongoing research.
Many epidemiological and cohort studies suggest that physical injury of the brain of any severity can increase the risk of dementia [4, 485–489], or decrease age of onset of MCI [490] or AD [491]. A nationwide cohort study in Denmark found that a history of TBI, regardless of severity, was associated with a 24% increased risk of all-cause dementia diagnosis and a 16% increased risk of a clinical AD diagnosis. The risk of dementia was also increased with multiple events (22% increased risk for one TBI versus 183% increased risk for five or more TBIs) [485]. In a retrospective cohort study of 357,558 veterans with and without TBI, even mild TBI without loss of consciousness (LOC), was associated with a doubled risk of dementia [487]. Consistent with these results, a recent meta-analysis found that history of TBI of any severity was associated with an 84% increased risk of all-cause dementia [4].
In addition to lowering brain reserve, TBI may increase dementia risk by increasing neuroinflammation, white matter changes, and AD-related pathology, although the exact etiology varies depending on the nature, frequency, and severity of injury [492, 493]. Data suggest that, in some patients, TBI initiates long-term neuroinflammation and neurodegenerative processes [494, 495]. For example, an autopsy study of 52 individuals with moderate-severe TBI and 44 healthy, age-matched controls found evidence of ongoing TBI-initiated inflammatory processes including activated microglia, axonal degeneration, and atrophy of the corpus collosum for up to 18 years following a single TBI [494]. Additionally, TBI has been linked to increased AD pathology in the brain. In animal models of TBI, brain injury is associated with increased expression of AβPP [496, 497] and accumulation of Aβ [498, 499] and tau [496]. Similarly, imaging and autopsy studies in humans have shown that TBI is linked to increased Aβ and tau accumulation [500]. The APOE ɛ4 allele has also been associated with a significantly increased risk of developing AD after TBI with LOC, suggesting a possible synergistic effect between history of TBI and genetic risk of AD [501].
Not all studies have found a clear effect of TBI on the development of dementia. One study of 706 older adults from the National Alzheimer’s Coordinating Center found that a history of TBI with LOC did not impact the course of cognitive decline in participants with or without AD [502]. Another recent study by Sugarman and colleagues found that, in 4,761 deceased participants from the National Alzheimer’s Coordinating Center, remote TBI with LOC was not associated with AD neuropathology in cases of autopsy-confirmed AD or with late-life cognitive performance in individuals with or without autopsy-confirmed AD [503]. It is worth noting that both of these studies relied on self-report data to determine history of TBI. Given these conflicting findings, more research is needed to understand the connection between TBI, cognitive decline, and AD pathology.
Even while research continues, it is wise to protect our brains from loss of cognitive capacity and brain reserve. It is important to encourage patients to wear protective equipment, especially seat belts and bicycle helmets, when participating in activities that are associated with the risk of head strike, and to reduce their risk of falls by staying physically active.
Toxic injury
Air pollutants nitrogen dioxide (NO2), carbon monoxide (CO), and fine ambient particulate matter (PM2.5) from traffic and wood burning [4, 504] as well as acute CO poisoning [505, 506] have been linked to increased risk of cognitive decline and dementia. Cohort studies have shown that living in an area with traffic-related air pollution, specifically, is associated with increased incidence of dementia [507–510]. For example, in a Canadian population-based cohort study of approximately 2.2 million older adults, individuals living within 50 meters of a highly-trafficked road had a 7% increased risk of dementia compared to individuals living greater than 300 meters away [507].
Air pollutants have also been associated with worsened cognitive performance. PM2.5 exposure has been associated with decreased performance in working memory and orientation [511], verbal learning [512], and visuospatial ability [513]. NO2 has been linked to decreased memory and executive function [512] as well as visuospatial ability [513]. A recent study of two cohorts in New York City investigated the link between long-term exposure to ambient air pollution and cognitive decline. Among 5,330 participants in the larger cohort, living in areas with higher levels of air pollution was associated with lower cognitive scores at baseline and more rapid rates of cognitive decline over time, after adjusting for sociodemographic factors, including race and ethnicity, education, and socioeconomic status. No association was found, however, in the smaller cohort with fewer repeat measures of cognition [514].
Air pollutants likely increase the risk of cognitive decline and dementia through several mechanisms [515]. Exposure to pollutants is linked to increased inflammation, oxidative stress, and Aβ accumulation in both animals [516–518] and humans [519–521]. An autopsy study of 47 clinically-healthy individuals who died suddenly, aged two to 45 years, found that exposure to high levels of air pollution was already associated with increased inflammation and Aβ accumulation in children and young adults [520]. Additionally, exposure to air pollution has been linked to increased risk of medical conditions that may worsen cognition. Long term exposure to PM2.5 has been associated with increased risk of acute coronary events [522] and cardiovascular mortality [523]. Short term exposure to NO2 and PM2.5 have been associated with increased risk of stroke and mortality due to stroke [524].
The effects of neurotoxic metals (e.g., lead, mercury, aluminum, etc.) and pesticides on dementia risk have also been investigated, but results remain inconclusive [515]. More research is needed to understand the connection between these toxic substances and dementia risk.
Although it may be difficult to eliminate pollution- and heavy metal-related exposures, it is important for clinicians and patients to understand the likely connection between these environmental hazards and cognitive decline and dementia. It is also important to understand that communities with overall lower socioeconomic status are more likely to be exposed to a higher concentration of air pollutants, and are therefore more likely to experience the health risks associated with air pollution [525]. Establishing public policies aimed at reducing air pollution in these communities is of paramount importance for mitigating this disparity. On an individual level, ways to reduce risk of toxic injury to the brain include installing a carbon monoxide detector at home and wearing protective equipment when working with toxic materials.
Multimodal interventions
Epidemiological studies have demonstrated that a combination of brain-healthy behaviors and lifestyles may yield a greater benefit than participation in any single activity [526–528]. For example, findings from two Chicago-based longitudinal studies indicated that a composite healthy lifestyle score, including moderate to vigorous physical activity, non-smoking, light to moderate alcohol consumption, MIND diet adherence, and late-life engagement in cognitive activities, was associated with a 27% decreased risk of AD for each additional healthy lifestyle factor [528].
In recent years, interventions that combine multiple behavior changes have also demonstrated a positive impact on cognition [529, 530] and a possible synergistic effect on dementia risk [531, 532]. For example, the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) study, a two-year study of 1,260 older individuals at risk for cognitive impairment, investigated a multimodal intervention that combined diet, exercise, cognitive training, and management of cardiovascular risk factors. Participation in the intervention was associated with improved BMI, dietary habits, physical activity, and cognition, including executive functioning and processing speed. Although the effect sizes measured were very small, the intervention was also associated with a 24% decreased risk of cognitive decline [531]. Not every multimodal behavioral trial, however, has shown such promising results. In the three-year Multidomain Alzheimer Prevention Trial (MAPT), a multimodal intervention for older adults, which included physical activity, nutrition, and cognitive training and consumption of polyunsaturated fatty acids, in combination or individually, had no significant effect on cognitive decline [533]. A recent report by Yu and colleagues, which analyzed many of the evidence-based suggestions for AD prevention, found insufficient evidence to draw any robust conclusions about multimodal interventions due to intervention heterogeneity and small sample sizes [534]. More clinical trials are needed to determine whether multimodal interventions are effective in preventing or delaying cognitive decline. Ongoing global research efforts, including the U.S. Study to Protect Brain Health Through Lifestyle Intervention to Reduce Risk (US-POINTER) and the Multimodal Interventions to Delay Dementia and Disability in Rural China (MIND-CHINA) study, are aiming to replicate the results of FINGER, and test the generalizability of a similar multimodal intervention to improve brain health [535, 536].
Promoting behavior change
As described in this review, there is growing consensus among health organizations about what to recommend to older adults in order to promote successful cognitive aging and reduce the risk of cognitive decline. However, a substantial gap remains between what healthcare providers and public health specialists think patients should do and what they are actually doing. For example, only 16% of adults between the ages of 65 and 74 and 10% of adults 75 years and over in the U.S. regularly meet the physical activity guidelines established by the Department of Health and Human Services [47]. Evidence that dementia risk is modifiable will not make a difference unless individuals actually change their behavior.
There are many barriers to translating knowledge about factors that may promote healthy cognitive aging into real-world practice. Changing human behavior often requires complex, multidimensional interventions, blending elements of psychology, social science, and medicine. Standard clinical practice does not systematically promote or monitor improvement of modifiable lifestyle changes, but a growing field of research is seeking to address this limitation. Recent efforts to characterize and standardize behavior change techniques (BCTs) have allowed researchers to define and replicate behavior change interventions across different populations and target behaviors [537]. Several techniques that have relied on principles derived from motivational interviewing (MI), behavioral activation (BA), and behavioral economics (BE) have shown promise in encouraging behavior change across many medical specialties and conditions.
MI focuses on reflecting and reinforcing the participant’s intrinsic motivation through a non-judgmental empathic alliance, increasing optimism, intent, and self-efficacy in patients [538–540]. Principles of MI are widely used in psychiatry and addiction medicine, where successful interventions have been developed to address several behaviors and conditions, such as smoking cessation [541] and alcohol use disorder [542]. In patient populations with or at risk for chronic health conditions, the incorporation of MI principles into behavior change counseling has effectively increased physical activity [543], weight loss [544, 545], and management of cardiovascular risk factors [546, 547]. MI has been shown to be effective even in brief or time-limited interventions [542, 548].
BA uses tangible, actionable goal setting to increase accountability and self-efficacy [549]. BA is a collaborative process between health providers and patients that accounts for individual goals and obstacles to increase positive activities and improve patients’ ability to self-monitor activity levels [550, 551]. BA also has been used to promote brain-healthy behaviors, including cognitive, physical, and social activity, relying on action plans with visual cues, written schedules, step-by-step sequencing, and procedural memory to account for cognitive deficits [549].
BE combines principles of psychology and economics to optimize behavior change. More traditional clinician education and counseling tends to appeal to patients’ slow, logical, rational cognitive system by providing facts and evidence, whereas behavior is often driven by fast, automatic, unconscious thinking [552]. BE focuses on the latter; it accounts for individuals’ often limited willpower and rationality and their use of mental shortcuts in decision-making [553]. BE-guided interventions often involve making changes to individuals’ environments or daily structures to try to develop positive, automatic, habit-based behaviors [554]. This approach has been employed in numerous public health settings, including primary care physical activity counseling [555], nutritional counseling [556], sleep interventions [557], and heart failure clinical care [558].
Table 3 offers examples of motivational strategies using each of these three principles and ways to implement them into one’s own medical practice or a program across a health system.
Table 3
Behavior change toolbox
| Strategy | BCT | Purpose | Implementation |
| Motivational Interviewing | MI | Increase willingness to change by eliciting “change talk” from patients and caregivers. | Use MI questions and interviewing techniques. |
| Activity Scheduling | BA | Lower barriers to activation by blocking out predetermined times for exercise and social interactions. Create habits by setting up a consistent schedule of activities. | Suggest use of planners and calendars, online or in print. Encourage participation in structured, pre-scheduled activities and events such as exercise classes, volunteering, community events. |
| Activity Monitoring | BA | Increase accountability by self-monitoring adherence to recommendations. | Suggest use of food and exercise logs (online or in print) to track and report activities. |
| Goal Reappraisal/Updating | BA | Ensure that goals are manageable and actionable; deal with failure to meet goals productively. | Work with patients to update/scale back goals if goals are not being met. |
| Skills Training | BA | Increase ability to change by teaching a structured skillset to compensate for memory difficulty or lack of motivation. | Teach methods of systematic goal setting; suggest referrals to specialists, where appropriate. |
| Progressive Rollout | BA | Make change more manageable by starting with a single domain and adding education and goals in new domains in a stepwise/ individualized manner. | Begin by focusing on a single domain and add additional education/support strategies as patients progress. |
| Social Networks | BE | Increase motivation by adding support from and friendly competition within peer group. | Suggest use of apps or social media that encourage communication and competition within groups. Refer patients or caregivers to resources, like support groups, that encourage social engagement. |
| Commitment Contracts | BE | Create financial or other incentives for change by requiring a specific behavior to redeem a reward. | Generate a specific short-term goal and the reward, if achieved. |
BA, behavioral activation; BCT, behavior change technique; BE, behavioral economics; MI, motivational interviewing.
Recent studies have used BCTs to change behavior in the service of promoting brain health [549, 559]. A study by Rovner and colleagues randomized Black individuals with MCI to a two-year intervention of BA for cognitive, physical, and social activities versus supportive therapy. Results demonstrated that BA participants engaged in more cognitive activities and exhibited less memory decline and greater functional stability than supportive therapy participants [549]. In the Brain Health Champion study, our team demonstrated the efficacy of a low-cost, health-coaching intervention in older adults with MCI or mild dementia. Using evidence-based BCTs, including MI and BA, the health-coaching intervention was associated with increased adherence to brain-healthy behaviors and improved self-reported quality of life [559]. The results of these studies demonstrate the promise of employing models of evidence-based BCTs to encourage the adoption of brain-healthy behaviors. More research is needed to investigate the most effective and efficient ways for clinicians to help patients to implement behavior change, including the use of mobile health platforms and other innovative technologies.
CONCLUSION
The converging evidence from epidemiological, basic science, human proof-of-concept, and available RCT studies provides a strong justification for lifestyle-modification- and brain-health-promoting interventions to help prevent and delay cognitive decline. Table 4 provides a brief summary of lifestyles and behaviors reviewed in this paper, the main evidence supporting their role in fostering brain health, and emerging consensus recommendations. The factors discussed hold promise for decreasing the risk of cognitive impairment and dementia. Of particular importance is physical activity, cognitive and social stimulation, and management of cardiovascular risk factors.
Table 4
Summary of evidence and consensus recommendations for different lifestyle/behavior interventions
| Lifestyle /Behavior | Main Evidence | Consensus Recommendations |
| Physical activity | •Epidemiological: Exercise in mid- and late life is associated with decreased risk of cognitive decline and dementia. | •Participate in regular aerobic exercise and resistance training •Weekly guidelines: •150 min. moderate-intensity or •75 min. vigorous-intensity •Strength training for all muscle groups |
| •Animals: Exercise yields ↑ BDNF, ↑ VEGF, ↑ IGF-1; ↓ AD pathology | ||
| •Humans: Exercise yields ↑ BDNF, ↑ IGF-1; ↓ brain volume loss, ↓ AD pathology, ↓ cardiovascular risk factors | ||
| •RCTs: Exercise interventions yield improved memory, executive function, and functional neuroplasticity | ||
| Cognitive stimulation/education | •Epidemiological: More years of education is associated with reduced risk of dementia. Cognitive activity in mid- and late life is associated with decreased risk of cognitive decline and dementia. | •Implement public policy to ensure access to high quality education •Remain cognitively active throughout life •Engage in new or challenging activities for the greatest cognitive benefit |
| •Animals: Cognitive stimulation yields ↑ cognitive function, ↑ neurogenesis, ↑ synaptogenesis; ↓ AD pathology | ||
| •Humans: Cognitive stimulation yields ↑ neuroplasticity; ↓ brain volume loss, ↓ AD pathology | ||
| •RCTs: Cognitive interventions yield improvement in several cognitive domains and decreased risk of cognitive decline; Effects are strongest for new and/or challenging activities | ||
| Social engagement | •Epidemiological: Low levels of social activity and small social networks in mid- and late life associated with increased risk of cognitive decline and dementia. | •Participate in social activities regularly throughout life •Maintain a large, supportive social network •Stay in touch with friends and family as often as possible |
| •Animals: Social isolation yields ↑ inflammatory mediators, ↑ stress hormones, ↑ AD pathology; ↓ neurogenesis, ↓ BDNF | ||
| •Humans: | ||
| •Low social interaction: ↑ stress, ↑ inflammation; ↓ sleep | ||
| •High social interaction: ↑ brain volume | ||
| •RCTs: Social interventions yield improved cognitive performance and decreased brain volume loss | ||
| Heart healthy diet | •Epidemiological: MeDi, DASH, and MIND diets are associated with increased cognitive performance, decreased rate of cognitive decline, and decreased risk of cognitive decline and dementia. | •Emphasize consumption of vegetables, fruits, and antioxidant-rich foods •Limit refined grains, saturated fat, red and processed meats, and added sugars •Consider the Mediterranean, DASH, or MIND diets |
| •Animals: Eating a heart-healthy diet yields ↓ inflammation, ↓ neuronal death | ||
| •Humans: Eating a heart-healthy diet yields ↑ total brain volume; ↓ inflammation, ↓ hippocampal atrophy, ↓ AD pathology | ||
| •RCTs: Heart-healthy diet interventions yield improved global cognition and executive function | ||
| Treat cardiovascular risk factors (hypertension) | •Epidemiological: Hypertension in midlife is associated with increased risk of all-cause dementia and decreased memory, executive function, and processing speed. | •Control hypertension through a healthy lifestyle and/or medication •Target a systolic blood pressure < 120 mmHg |
| •Animals: Hypertension yields ↓ cognitive function; ↑ inflammation, ↑oxidative stress, ↑ microhemorrhages and infarcts, ↑ endothelial dysfunction, ↑ BBB permeability, ↑ Aβ accumulation | ||
| •Humans: Hypertension yields ↑ neurovascular dysfunction, ↑ risk of stroke, ↑ white matter changes, ↑ global and regional atrophy, ↑ AD pathology, ↑ BBB permeability | ||
| •RCTs: Intensive blood pressure control yields robust cardiovascular benefits and decreased risk of cognitive impairment | ||
| Treat cardiovascular risk factors (DM) | •Epidemiological: DM is associated with decreased cognitive function and increased risk of dementia. | •Adopt healthy lifestyles to prevent DM or delay onset if possible •Develop healthcare programs to detect the first signs of DM and make an early diagnosis •Promote regular meetings with a doctor to monitor and manage the disease |
| •Animal: DM yields ↑ neuroinflammation, ↑ vascular injury, ↑ brain atrophy, ↑ risk of stroke, ↑ AD pathology | ||
| •Humans: DM yields ↑ inflammation, ↑ global and regional atrophy, ↑ white matter hyperintensities, ↑ risk of stroke, ↑ risk of depression, ↑ AD pathology; ↓ functional connectivity, ↓ brain glucose metabolism | ||
| •RCTs: Glycemic control interventions have not demonstrated a clear effect on brain health and cognition | ||
| Treat cardiovascular risk factors (hyperlipidemia) | •Epidemiological: High cholesterol in midlife is associated with increased risk of all-cause dementia, AD, and vascular dementia. | •Follow AHA/ACC guidelines for maintaining healthy cholesterol and lipid levels •Aim to keep total cholesterol levels < 170 mg/dL •Exercise, eat a heart-healthy diet, maintain a healthy weight, and quit smoking to help lower cholesterol levels •Consider use of statins to control hyperlipidemia |
| •Animals: Hyperlipidemia yields ↑ cardio- and cerebrovascular damage, ↑ vascular dysfunction, ↑ BBB permeability, ↑ atherosclerosis | ||
| •Humans: Hyperlipidemia yields ↑ risk of cardiovascular disease, ↑ risk of stroke, ↑ atherosclerosis, ↑ BBB impairment, ↑ AD pathology | ||
| •RCTs: Interventions using statins to treat hyperlipidemia do not have a clear effect on brain health and cognition | ||
| Maintain a healthy weight/counter metabolic syndrome | •Epidemiological: A high BMI and metabolic syndrome have been associated with increased risk of dementia. | •Exercise and eat a healthy diet to maintain a healthy weight and counter metabolic syndrome •Aim to keep BMI between 18.5 and 24.9 kg/m2 •Treat components of metabolic syndrome with medication when appropriate |
| •Animal: Obesity yields ↑ inflammation, ↑ endothelial dysfunction | ||
| •Human: Obesity yields ↑ inflammation, ↑ endothelial dysfunction; ↓ brain volume | ||
| •RCTs: Weight loss interventions yield improved cognitive performance | ||
| Stop or reduce smoking | •Epidemiological: Smoking in mid- and late life is associated with decreased cognitive performance, increased rate of cognitive decline, and increased risk of dementia. Quitting or reducing smoking mitigates increased risk of dementia. | •Quit smoking or limit smoking as much as possible |
| •Animals: Exposure to cigarette smoke yields ↑ oxidative stress, ↑ proinflammatory cytokines; ↓ cerebral antioxidants | ||
| •Humans: Exposure to cigarette smoke yields ↑ oxidative stress, ↑ neuroinflammation, ↑ risk of stroke, ↑ white matter hyperintensities, ↑ subcortical atrophy, ↑ AD pathology | ||
| •RCTs: Not available | ||
| Manage stress and depression | •Epidemiological: Acute stress is associated with decreased cognitive performance, and chronic life stress is associated with increased risk of dementia. Depression has been linked to decreased cognitive performance and increased dementia risk, although it remains unclear if depression is a risk factor, prodromal symptom, or both. | •Treat depression •Consider practices like mindfulness or other forms of meditation to help reduce stress levels |
| •Animals: | ||
| •Stress: ↑ oxidative stress, ↑ hippocampal atrophy, ↑ AD pathology; ↓ BDNF | ||
| •Depression: ↑ neuronal death; ↓ BDNF | ||
| •Humans: | ||
| •Stress: ↑ oxidative stress, ↑ white matter changes, ↑ AD pathology; ↓ total brain volume | ||
| •Depression: ↓ hippocampal volume | ||
| •RCTs: Stress-reducing practice interventions yield improved cognitive function | ||
| Maintain adequate sleep | •Epidemiological: Disrupted sleep and sleep disorders are associated with decreased cognitive performance, increased rate of cognitive decline, and increased risk of dementia. | •CDC sleep recommendations: •Adults under age 65 : 7–9 hours per night •Adults ages 65 and older: 7-8 hours per night •Treat sleep-disordered breathing (e.g. OSA) and other sleep disorders |
| •Animals: Sleep deprivation yields ↑ inflammation, ↑ neuronal death, ↑ brain volume loss, ↑ AD pathology; ↓ neurogenesis | ||
| •Humans: Disrupted sleep and sleep disorders yield ↑ inflammation, ↑ cardiovascular comorbidities; ↓ memory consolidation, ↓ amyloid clearance | ||
| •RCTs: Treating sleep disorders partially mitigates negative effects on brain health and dementia risk | ||
| Manage hearing and visual impairments | •Epidemiological: Hearing and vision impairments undermine daily functioning, increase rate of cognitive decline, and increase risk of cognitive impairment and dementia. Correcting sensory impairment may counter trajectory of cognitive decline. | •Correct hearing or visual impairments with hearing aids, eyeglasses, or cataract surgery |
| •Animals: Sensory impairments yield ↓ spatial memory, ↓ hippocampal plasticity; altered neurotransmitter receptor expression | ||
| •Humans: Sensory impairments yield ↑ rate of atrophy, ↓ total brain volume; Compensatory efforts lead to depleted cognitive processing resources | ||
| •RCTs: Not available | ||
| Avoid medications with anticholinergic properties | •Epidemiological: Use of strong anticholinergic medications is associated with decreased cognitive performance (i.e., memory, executive function, and visual attention) and increased risk of dementia. | •Avoid medications with anticholinergic properties when possible •If anticholinergic medications are necessary, try to choose ones with a low anticholinergic cognitive burden |
| •Animal: Exposure to anticholinergic medications yields ↑ neuroinflammation, ↑ AD pathology | ||
| •Humans: Exposure to anticholinergic medications yields disrupted neural network connectivity important for memory and learning; ↑ brain atrophy | ||
| •RCTs: Interventions aimed at decreasing anticholinergic burden do not have a clear effect on brain health and cognition | ||
| Limit alcohol use | •Epidemiological: U-shaped dementia risk curve, where mild-moderate alcohol use has a potentially protective effect; Heavy alcohol use is associated with highly increased risk of dementia. | •If drinking alcohol, do so within recommended limits (1–7 drinks/week for women; 1–14 drinks/week for men) |
| •Animals: Alcohol exposure yields ↑ neuroinflammation, ↑ AD pathology; ↓ BBB integrity | ||
| •Humans: Heavy alcohol use yields ↑ structural and functional abnormalities, ↑ neuroinflammation, ↑ risk of cardiovascular disease and stroke; Moderate alcohol use yields ↓ AD pathology, ↓ cardiovascular risk | ||
| •RCTs: Not available | ||
| Protect the brain from physical and toxic injuries | •Epidemiological: Injury to the brain is associated with decreased cognitive performance and increased risk of all-cause dementia. | •Wear a seat belt and bicycle helmet •Wear protective equipment when working with toxic materials •Implement public policy aimed at reducing air pollution •Install a carbon monoxide detector at home |
| •Animals: Injury yields ↑ inflammation, ↑ AD pathology | ||
| •Humans: | ||
| •Physical injury yields ↑ inflammation, ↑ AD pathology, ↑ white matter changes, ↑ brain atrophy; | ||
| •Toxic injury yields ↑ inflammation, ↑ AD pathology, ↑ cardiovascular risk | ||
| •RCTs: Not available |
ACC, American College of Cardiology; AD, Alzheimer’s disease; AHA, American Heart Association; BBB, blood-brain barrier; BDNF, brain derived neurotrophic factor; BMI, body mass index; CDC, Centers for Disease Control and Prevention; DASH, Dietary Approaches to Stop Hypertension; DM, diabetes mellitus; IGF-1, insulin-like growth factor 1; MeDi, Mediterranean diet; MIND, Mediterranean-DASH Intervention for Neurodegenerative Delay; OSA, obstructive sleep apnea; RCT, randomized controlled trial; VEGF, vascular endothelial growth factor.
It is important to acknowledge that none of the recommendations made in this article have been definitively proven in the way that some experts insist is required for evidence-based treatment guidelines. In fact, as noted in the introduction, “gold-standard” large-scale RCTs that follow participants over years to decades may never be conducted for many of these recommendations.
Thus, these guidelines are best viewed as provisional ones, based on the highest-quality evidence currently available. In this context, it is worth considering the negative consequences of being wrong about specific lifestyle recommendations or medical interventions that turn out not to reduce the risk of cognitive decline and dementia. There are potential opportunity costs associated with following these guidelines. The time, effort, and resources individuals devote to following them could be invested in carrying out other activities or making different choices. Moreover, many of the recommendations involve putting restrictions on one’s life, for example, reducing access to certain enjoyable foods, alcohol, or cigarette smoking, or feeling constrained by having to wear seat belts and helmets or by having to get seven or more hours of sleep at night. Additionally, individuals may be embarrassed by wearing hearing aids or seeking treatment for depression, or may feel ashamed about being overweight or not exercising enough. Individuals may also feel uncomfortable carrying out challenging cognitive activities or doing aerobic exercise. We recognize the potential costs of these actions, many of which recapitulate the reasons why some of our patients do not embrace them.
Of note, even if these lifestyle choices and healthcare efforts do not prove to directly mitigate cognitive decline or delay dementia, they are often beneficial for other reasons. For example, management of cardiovascular risk factors, such as hypertension, DM, and elevated cholesterol, reduces the chances of suffering from a stroke or myocardial infarction.
Cardio- and cerebrovascular risk can be lowered by following a heart-healthy diet or participating in regular physical activity, the latter of which also diminishes the incidence of falls. Smoking cessation reduces the risk of heart disease, stroke, and lung disorders. Adequate sleep has been linked to improved mood, sense of wellbeing, and cognitive performance. Use of seat belts and helmets helps to diminish the severity of traumatic brain injury due to accidents. Thus, even if the recommendations reviewed in this paper do not directly result in reduced risk of cognitive deterioration and dementia, there are other very compelling reasons for providers and health systems to promulgate them. On balance, we are comfortable with the risk/benefit profile of this set of recommendations. However, clinicians will need to review the data and decide for themselves. Clinicians are in an optimal position to educate their patients about available evidence, explain why they favor certain lifestyle choices, and use behavior-change techniques to assist patients in achieving their goals.
Given the epidemiological evidence that more than a third of ADRD cases may be due to modifiable risk factors, helping individuals make brain-healthy behavior changes could improve the lives of millions of older adults around the world and reduce the social and economic burdens associated with cognitive decline and dementia. The lifestyles and behaviors examined in this review are not exhaustive, and ongoing research continues to refine our understanding of the factors that promote successful cognitive aging. We expect that work in this field will continue to expand rapidly and anticipate that our next update will need to be completed in much less than a decade.
ACKNOWLEDGMENTS
Funding for this work came from the Wimberly Family Clinical Care Research Fund, the Gaudet Family Research Fund, and the Alzheimer Innovation Fund, Brigham and Women’s Hospital. In addition, the Laboratory of Healthy Cognitive Aging at Brigham and Women’s Hospital has been strongly supported by the Muss family, the Mortimer/Grubman family, and the Herman F. Woerner Trust. The Patti Piper Memorial Fund has also supported this work and related research endeavors. The authors would like to acknowledge Hope Schwartz for her work on several prior brain health research efforts, including the ongoing Brain Health Champion Study.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/20-1462r1).
REFERENCES
[1] | Daffner KR ((2010) ) Promoting successful cognitive aging: A comprehensive review. J Alzheimers Dis 19: , 1101–1122. |
[2] | Institute of Medicine ((2015) ) Cognitive aging: Progress in understanding and opportunities for action, The National Academies Press, Washington DC. |
[3] | Alzheimer’s Association, 10 Ways to Love Your Brain, https://alz.org/help-support/brain_health/10_ways_to_love_your_brain, Accessed on 9 September 2020. |
[4] | Livingston G , Huntley J , Sommerlad A , Ames D , Ballard C , Banerjee S , Brayne C , Burns A , Cohen-Mansfield J , Cooper C , Costafreda SG , Dias A , Fox N , Gitlin LN , Howard R , Kales HC , Kivimäki M , Larson EB , Ogunniyi A , Orgeta V , Ritchie K , Rockwood K , Sampson EL , Samus Q , Schneider LS , Selbæk G , Teri L , Mukadam N ((2020) ) Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396: , 413–446. |
[5] | American Heart Association, My Life Check | Life’s Simple 7, https://www.heart.org/en/healthy-living/healthy-lifestyle/my-life-check–lifes-simple-7, Last updated 2 May 2018, Accessed on 5 September 2019. |
[6] | National Academies of Sciences, Engineering, and Medicine, Health and Medicine Division, Board on Health Sciences Policy, Committee on Preventing Dementia and Cognitive Impairment ((2017) ) Preventing Cognitive Decline and Dementia: A Way Forward, National Academies Press (US), Washington (DC). |
[7] | World Health Organization ((2019) ) Risk reduction of cognitive decline and dementia: WHO guidelines, World Health Organization, Geneva. |
[8] | United Nations, Department of Economic and Social Affairs, Population Division ((2019) ) World Population Prospects 2019: Highlights, United Nations, New York. |
[9] | Page KS , Hayslip B , Wadsworth D , Allen PA ((2019) ) Development of a multidimensional measure to examine fear of dementia. Int J Aging Hum Dev 89: , 187–205. |
[10] | Ostergren JE , Heeringa SG , Leon CFM de , Connell CM , Roberts JS ((2017) ) The influence of psychosocial and cognitive factors on perceived threat of Alzheimer’s disease. Am J Alzheimers Dis Other Demen 32: , 289–299. |
[11] | French SL , Floyd M , Wilkins S , Osato S ((2012) ) The Fear of Alzheimer’s Disease Scale: A new measure designed to assess anticipatory dementia in older adults. Int J Geriatr Psychiatry 27: , 521–528. |
[12] | Molden J , Maxfield M ((2016) ) The impact of aging stereotypes on dementia worry. Eur J Ageing 14: , 29–37. |
[13] | Alzheimer’s Association ((2020) ) Alzheimer’s disease facts and figures. Alzheimers Dement 16: , 391–460. |
[14] | Mol M , Carpay M , Ramakers I , Rozendaal N , Verhey F , Jolles J ((2007) ) The effect of perceived forgetfulness on quality of life in older adults; a qualitative review. Int J Geriatr Psychiatry 22: , 393–400. |
[15] | Corner L , Bond J ((2004) ) Being at risk of dementia: Fears and anxieties of older adults. J Aging Stud 18: , 143–155. |
[16] | Gale SA , Acar D , Daffner KR ((2018) ) Dementia. Am J Med 131: , 1161–1169. |
[17] | Petersen RC ((2016) ) Mild cognitive impairment. Continuum Minneap Minn 22: , 404–418. |
[18] | Petersen RC , Lopez O , Armstrong MJ , Getchius TSD , Ganguli M , Gloss D , Gronseth GS , Marson D , Pringsheim T , Day GS , Sager M , Stevens J , Rae-Grant A ((2018) ) Practice guideline update summary: Mild cognitive impairment: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 90: , 126–135. |
[19] | World Health Organization ((2018) ) Global Health Estimates 2016: Deaths by Cause, Age, Sex, by Country and by Region, 2000-2016, Geneva, World Health Organization. |
[20] | El-Hayek YH , Wiley RE , Khoury CP , Daya RP , Ballard C , Evans AR , Karran M , Molinuevo JL , Norton M , Atri A ((2019) ) Tip of the iceberg: Assessing the global socioeconomic costs of Alzheimer’s disease and related dementias and strategic implications for stakeholders. J Alzheimers Dis 70: , 323–341. |
[21] | Kelley AS , McGarry K , Gorges R , Skinner JS ((2015) ) The burden of health care costs for patients with dementia in the last 5 years of life. Ann Intern Med 163: , 729. |
[22] | Al-Sari UA , Tobias JH , Archer H , Clark EM ((2017) ) Do subjective memory complaints predict falls, fractures and healthcare utilization? A two-year prospective study based on a cohort of older women recruited from primary care. Int J Geriatr Psychiatry 32: , 968–976. |
[23] | Andrews JS , Desai U , Kirson NY , Enloe CJ , Ristovska L , King S , Birnbaum HG , Fleisher AS , Ye W , Kahle-Wrobleski K ((2016) ) Functional limitations and health care resource utilization for individuals with cognitive impairment without dementia: Findings from a United States population-based survey. Alzheimers Dement (Amst) 6: , 65–74. |
[24] | Zhu CW , Cosentino S , Ornstein K , Gu Y , Andrews H , Stern Y ((2015) ) Use and cost of hospitalization in dementia: Longitudinal results from a community-based study. Int J Geriatr Psychiatry 30: , 833–841. |
[25] | Zhu CW , Scarmeas N , Ornstein K , Albert M , Brandt J , Blacker D , Sano M , Stern Y ((2015) ) Healthcare use and cost in dementia caregivers: Longitudinal results from the Predictors Caregiver Study. Alzheimers Dement 11: , 444–454. |
[26] | Tom SE , Hubbard RA , Crane PK , Haneuse SJ , Bowen J , McCormick WC , McCurry S , Larson EB ((2014) ) Characterization of dementia and Alzheimer’s disease in an older population: Updated incidence and life expectancy with and without dementia. Am J Public Health 105: , 408–413. |
[27] | Pimouguet C , Rizzuto D , Fastbom J , Lagergren M , Fratiglioni L , Xu W ((2016) ) Influence of incipient dementia on hospitalization for primary care sensitive conditions: A population-based cohort study. J Alzheimers Dis 52: , 213–222. |
[28] | Goren A , Montgomery W , Kahle-Wrobleski K , Nakamura T , Ueda K ((2016) ) Impact of caring for persons with Alzheimer’s disease or dementia on caregivers’ health outcomes: Findings from a community based survey in Japan. BMC Geriatr 16: , 122. |
[29] | Stites SD , Harkins K , Rubright JD , Karlawish J ((2018) ) Relationships between cognitive complaints and quality of life in older adults with mild cognitive impairment, mild Alzheimer disease dementia, and normal cognition. Alzheimer Dis Assoc Disord 32: , 276–283. |
[30] | Brodaty H , Connors MH , Xu J , Woodward M , Ames D , PRIME study group ((2015) ) The course of neuropsychiatric symptoms in dementia: A 3-year longitudinal study. J Am Med Dir Assoc 16: , 380–387. |
[31] | Leibson CL , Long KH , Ransom JE , Roberts RO , Hass SL , Duhig AM , Smith CY , Emerson JA , Pankratz VS , Petersen RC ((2015) ) Direct medical costs and source of cost differences across the spectrum of cognitive decline: A population-based study. Alzheimers Dement 11: , 917–932. |
[32] | Ton TGN , DeLeire T , May SG , Hou N , Tebeka MG , Chen E , Chodosh J ((2017) ) The financial burden and health care utilization patterns associated with amnestic mild cognitive impairment. Alzheimers Dement 13: , 217–224. |
[33] | Brookmeyer R , Gray S , Kawas C ((1998) ) Projections of Alzheimer’s disease in the United States and the public health impact of delaying disease onset. Am J Public Health 88: , 1337–1342. |
[34] | Brookmeyer R , Johnson E , Ziegler-Graham K , Arrighi HM ((2007) ) Forecasting the global burden of Alzheimer’s disease. Alzheimers Dement 3: , 186–191. |
[35] | Glisky EL ((2007) ) Changes in cognitive function in human aging. In Brain Aging: Models, Methods, and Mechanisms, RiddleDR, ed. CRC Press/Taylor & Francis, Boca Raton, FL, pp. 3–21. |
[36] | Smith GE ((2016) ) Healthy cognitive aging and dementia prevention. Am Psychol 71: , 268–275. |
[37] | Nyberg L , Pudas S ((2019) ) Successful memory aging. Annu Rev Psychol 70: , 219–243. |
[38] | Stern Y , Arenaza-Urquijo EM , Bartrés-Faz D , Belleville S , Cantilon M , Chetelat G , Ewers M , Franzmeier N , Kempermann G , Kremen WS , Okonkwo O , Scarmeas N , Soldan A , Udeh-Momoh C , Valenzuela M , Vemuri P , Vuoksimaa E ((2020) ) Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimers Dement 16: , 1305–1311. |
[39] | Pettigrew C , Soldan A ((2019) ) Defining cognitive reserve and implications for cognitive aging. Curr Neurol Neurosci Rep 19: , 1. |
[40] | Daviglus ML ((2010) ) National Institutes of Health State-of-the-Science Conference Statement: Preventing Alzheimer disease and cognitive decline. Ann Intern Med 153: , 176. |
[41] | Snowden M , Steinman L , Mochan K , Grodstein F , Prohaska TR , Thurman DJ , Brown DR , Laditka JN , Soares J , Zweiback DJ , Little D , Anderson LA ((2011) ) Effect of exercise on cognitive performance in community-dwelling older adults: Review of intervention trials and recommendations for public health practice and research. J Am Geriatr Soc 59: , 704–716. |
[42] | Brasure M , Desai P , Davila H , Nelson VA , Calvert C , Jutkowitz E , Butler M , Fink HA , Ratner E , Hemmy LS , McCarten JR , Barclay TR , Kane RL ((2017) ) Physical activity interventions in preventing cognitive decline and Alzheimer-type dementia. Ann Intern Med 168: , 30–38. |
[43] | Butler M , McCreedy E , Nelson VA , Desai P , Ratner E , Fink HA , Hemmy LS , McCarten JR , Barclay TR , Brasure M , Davila H , Kane RL ((2017) ) Does cognitive training prevent cognitive decline? Ann Intern Med 168: , 63–68. |
[44] | Fink HA , Jutkowitz E , McCarten JR , Hemmy LS , Butler M , Davila H , Ratner E , Calvert C , Barclay TR , Brasure M , Nelson VA , Kane RL ((2017) ) Pharmacologic interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia. Ann Intern Med 168: , 39–51. |
[45] | Lourida I , Hannon E , Littlejohns TJ , Langa KM , Hyppönen E , Kuźma E , Llewellyn DJ ((2019) ) Association of lifestyle and genetic risk with incidence of dementia. JAMA 322: , 430–437. |
[46] | U.S. Department of Health and Human Services (2018) Physical Activity Guidelines for Americans, 2nd edition, U.S. Department of Health and Human Services, Washington D.C. |
[47] | U.S. Centers for Disease Control and Prevention, Early Release of Selected Estimates Based on Data From the 2018 National Health Interview Survey. https://www.cdc.gov/nchs/nhis/releases/released201905.htm, Last updated 20 May 2019, Accessed on 27 August 2019. |
[48] | Weuve J , Kang JH , Manson JE , Breteler MMB , Ware JH , Grodstein F ((2004) ) Physical activity, including walking, and cognitive function in older women. JAMA 292: , 1454–1461. |
[49] | Hörder H , Johansson L , Guo X , Grimby G , Kern S , Östling S , Skoog I ((2018) ) Midlife cardiovascular fitness and dementia. Neurology 90: , e1298–e1305. |
[50] | Najar J , Östling S , Gudmundsson P , Sundh V , Johansson L , Kern S , Guo X , Hällström T , Skoog I ((2019) ) Cognitive and physical activity and dementia: A 44-year longitudinal population study of women. Neurology 92: , e1322–e1330. |
[51] | Carlson MC , Helms MJ , Steffens DC , Burke JR , Potter GG , Plassman BL ((2008) ) Midlife activity predicts risk of dementia in older male twin pairs. Alzheimers Dement 4: , 324–331. |
[52] | Casaletto KB , Staffaroni AM , Wolf A , Appleby B , Brushaber D , Coppola G , Dickerson B , Domoto-Reilly K , Elahi FM , Fields J , Fong JC , Forsberg L , Ghoshal N , Graff-Radford N , Grossman M , Heuer HW , Hsiung G-Y , Huey ED , Irwin D , Kantarci K , Kaufer D , Kerwin D , Knopman D , Kornak J , Kramer JH , Litvan I , Mackenzie IR , Mendez M , Miller B , Rademakers R , Ramos EM , Rascovsky K , Roberson ED , Syrjanen JA , Tartaglia MC , Weintraub S , Boeve B , Boxer AL , Rosen H , Yaffe K , ARTFL/LEFFTDS Study ((2020) ) Active lifestyles moderate clinical outcomes in autosomal dominant frontotemporal degeneration. Alzheimers Dement 16: , 91–105. |
[53] | Sofi F , Valecchi D , Bacci D , Abbate R , Gensini GF , Casini A , Macchi C ((2011) ) Physical activity and risk of cognitive decline: A meta-analysis of prospective studies. J Intern Med 269: , 107–117. |
[54] | Hamer M , Chida Y ((2009) ) Physical activity and risk of neurodegenerative disease: A systematic review of prospective evidence. Psychol Med 39: , 3–11. |
[55] | Sabia S , Dugravot A , Dartigues J-F , Abell J , Elbaz A , Kivimäki M , Singh-Manoux A ((2017) ) Physical activity, cognitive decline, and risk of dementia: 28 year follow-up of Whitehall II cohort study. BMJ 357: , j2709. |
[56] | Fordyce DE , Wehner JM ((1993) ) Physical activity enhances spatial learning performance with an associated alteration in hippocampal protein kinase C activity in C57BL/6 and DBA/2 mice. Brain Res 619: , 111–119. |
[57] | Churchill JD , Galvez R , Colcombe S , Swain RA , Kramer AF , Greenough WT ((2002) ) Exercise, experience and the aging brain. Neurobiol Aging 23: , 941–955. |
[58] | Neeper SA , Gómez-Pinilla F , Choi J , Cotman CW ((1996) ) Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res 726: , 49–56. |
[59] | Vaynman S , Ying Z , Gomez-Pinilla F ((2004) ) Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci 20: , 2580–2590. |
[60] | Hillman CH , Erickson KI , Kramer AF ((2008) ) Be smart, exercise your heart: Exercise effects on brain and cognition. Nat Rev Neurosci 9: , 58–65. |
[61] | Nichol KE , Poon WW , Parachikova AI , Cribbs DH , Glabe CG , Cotman CW ((2008) ) Exercise alters the immune profile in Tg2576 Alzheimer mice toward a response coincident with improved cognitive performance and decreased amyloid. J Neuroinflammation 5: , 13. |
[62] | Kim D , Cho J , Kang H ((2019) ) Protective effect of exercise training against the progression of Alzheimer’s disease in 3xTg-AD mice. Behav Brain Res 374: , 112105. |
[63] | Ohia-Nwoko O , Montazari S , Lau Y-S , Eriksen JL ((2014) ) Long-term treadmill exercise attenuates tau pathology in P301S tau transgenic mice. Mol Neurodegener 9: , 54. |
[64] | Sherrington C , Fairhall NJ , Wallbank GK , Tiedemann A , Michaleff ZA , Howard K , Clemson L , Hopewell S , Lamb SE ((2019) ) Exercise for preventing falls in older people living in the community. Cochrane Database Syst Rev, CD012424. |
[65] | Cassilhas RC , Viana VAR , Grassmann V , Santos RT , Santos RF , Tufik S , Mello MT ((2007) ) The impact of resistance exercise on the cognitive function of the elderly. Med Sci Sports Exerc 39: , 1401–1407. |
[66] | Baker LD , Frank LL , Foster-Schubert K , Green PS , Wilkinson CW , McTiernan A , Cholerton BA , Plymate SR , Fishel MA , Watson GS , Duncan GE , Mehta PD , Craft S ((2010) ) Aerobic exercise improves cognition for older adults with glucose intolerance, a risk factor for Alzheimer’s disease. J Alzheimers Dis 22: , 569–579. |
[67] | Tsai C-L , Wang C-H , Pan C-Y , Chen F-C ((2015) ) The effects of long-term resistance exercise on the relationship between neurocognitive performance and GH, IGF-1, and homocysteine levels in the elderly. Front Behav Neurosci 9: , 23. |
[68] | Vecchio LM , Meng Y , Xhima K , Lipsman N , Hamani C , Aubert I ((2018) ) The neuroprotective effects of exercise: Maintaining a healthy brain throughout aging. Brain Plast 4: , 17–52. |
[69] | Leal G , Bramham CR , Duarte CB ((2017) ) BDNF and hippocampal synaptic plasticity. Vitam Horm 104: , 153–195. |
[70] | de Pins B , Cifuentes-Díaz C , Farah AT , López-Molina L , Montalban E , Sancho-Balsells A , López A , Ginés S , Delgado-García JM , Alberch J , Gruart A , Girault J-A , Giralt A ((2019) ) Conditional BDNF delivery from astrocytes rescues memory deficits, spine density, and synaptic properties in the 5xFAD mouse model of Alzheimer disease. J Neurosci 39: , 2441–2458. |
[71] | von Bohlen Und Halbach O , von Bohlen Und Halbach V ((2018) ) BDNF effects on dendritic spine morphology and hippocampal function. Cell Tissue Res 373: , 729–741. |
[72] | Liu PZ , Nusslock R ((2018) ) Exercise-mediated neurogenesis in the hippocampus via BDNF. Front Neurosci 12: , 52. |
[73] | Babaei P , Azali Alamdari K , Soltani Tehrani B , Damirchi A ((2013) ) Effect of six weeks of endurance exercise and following detraining on serum brain derived neurotrophic factor and memory performance in middle aged males with metabolic syndrome. J Sports Med Phys Fitness 53: , 437–443. |
[74] | Håkansson K , Ledreux A , Daffner K , Terjestam Y , Bergman P , Carlsson R , Kivipelto M , Winblad B , Granholm A-C , Mohammed AKH ((2017) ) BDNF responses in healthy older persons to 35 minutes of physical exercise, cognitive training, and mindfulness: Associations with working memory function. J Alzheimers Dis 55: , 645–657. |
[75] | Küster OC , Laptinskaya D , Fissler P , Schnack C , Zügel M , Nold V , Thurm F , Pleiner S , Karabatsiakis A , von Einem B , Weydt P , Liesener A , Borta A , Woll A , Hengerer B , Kolassa I-T , von Arnim CAF ((2017) ) Novel blood-based biomarkers of cognition, stress, and physical or cognitive training in older adults at risk of dementia: Preliminary evidence for a role of BDNF, irisin, and the kynurenine pathway. J Alzheimers Dis 59: , 1097–1111. |
[76] | Dinoff A , Herrmann N , Swardfager W , Liu CS , Sherman C , Chan S , Lanctôt KL ((2016) ) The effect of exercise training on resting concentrations of peripheral brain-derived neurotrophic factor (BDNF): A meta-analysis. PloS One 11: , e0163037. |
[77] | Erickson KI , Voss MW , Prakash RS , Basak C , Szabo A , Chaddock L , Kim JS , Heo S , Alves H , White SM , Wojcicki TR , Mailey E , Vieira VJ , Martin SA , Pence BD , Woods JA , McAuley E , Kramer AF ((2011) ) Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A 108: , 3017–3022. |
[78] | Marks BL , Madden DJ , Bucur B , Provenzale JM , White LE , Cabeza R , Huettel SA ((2007) ) Role of aerobic fitness and aging on cerebral white matter integrity. Ann N Y Acad Sci 1097: , 171–174. |
[79] | Spartano NL , Davis-Plourde KL , Himali JJ , Andersson C , Pase MP , Maillard P , DeCarli C , Murabito JM , Beiser AS , Vasan RS , Seshadri S ((2019) ) Association of accelerometer-measured light-intensity physical activity with brain volume: The Framingham Heart Study. JAMA Netw Open 2: , e192745-e192745. |
[80] | ten Brinke LF , Bolandzadeh N , Nagamatsu LS , Hsu CL , Davis JC , Miran-Khan K , Liu-Ambrose T ((2015) ) Aerobic exercise increases hippocampal volume in older women with probable mild cognitive impairment: A 6-month randomised controlled trial. Br J Sports Med 49: , 248–254. |
[81] | Alber J , Alladi S , Bae H-J , Barton DA , Beckett LA , Bell JM , Berman SE , Biessels GJ , Black SE , Bos I , Bowman GL , Brai E , Brickman AM , Callahan BL , Corriveau RA , Fossati S , Gottesman RF , Gustafson DR , Hachinski V , Hayden KM , Helman AM , Hughes TM , Isaacs JD , Jefferson AL , Johnson SC , Kapasi A , Kern S , Kwon JC , Kukolja J , Lee A , Lockhart SN , Murray A , Osborn KE , Power MC , Price BR , Rhodius-Meester HFM , Rondeau JA , Rosen AC , Rosene DL , Schneider JA , Scholtzova H , Shaaban CE , Silva NCBS , Snyder HM , Swardfager W , Troen AM , van Veluw SJ , Vemuri P , Wallin A , Wellington C , Wilcock DM , Xie SX , Hainsworth AH ((2019) ) White matter hyperintensities in vascular contributions to cognitive impairment and dementia (VCID): Knowledge gaps and opportunities. Alzheimers Dement 5: , 107–117. |
[82] | Nagamatsu LS , Handy TC , Hsu CL , Voss M , Liu-Ambrose T ((2012) ) Resistance training promotes cognitive and functional brain plasticity in seniors with probable mild cognitive impairment. Arch Intern Med 172: , 666–668. |
[83] | Liu-Ambrose T , Nagamatsu LS , Voss MW , Khan KM , Handy TC ((2012) ) Resistance training and functional plasticity of the aging brain: A 12-month randomized controlled trial. Neurobiol Aging 33: , 1690–1698. |
[84] | Brown BM , Rainey-Smith SR , Dore V , Peiffer JJ , Burnham SC , Laws SM , Taddei K , Ames D , Masters CL , Rowe CC , Martins RN , Villemagne VL ((2018) ) Self-reported physical activity is associated with tau burden measured by positron emission tomography. J Alzheimers Dis 63: , 1299–1305. |
[85] | Rabin JS , Klein H , Kirn DR , Schultz AP , Yang H-S , Hampton O , Jiang S , Buckley RF , Viswanathan A , Hedden T , Pruzin J , Yau W-YW , Guzmán-Vélez E , Quiroz YT , Properzi M , Marshall GA , Rentz DM , Johnson KA , Sperling RA , Chhatwal JP ((2019) ) Associations of physical activity and β-amyloid with longitudinal cognition and neurodegeneration in clinically normal older adults. JAMA Neurol 76: , 1203–1210. |
[86] | Muscari A , Giannoni C , Pierpaoli L , Berzigotti A , Maietta P , Foschi E , Ravaioli C , Poggiopollini G , Bianchi G , Magalotti D , Tentoni C , Zoli M ((2010) ) Chronic endurance exercise training prevents aging-related cognitive decline in healthy older adults: A randomized controlled trial. Int J Geriatr Psychiatry 25: , 1055–1064. |
[87] | Antunes HKM , Santos-Galduroz RF , De Aquino Lemos V , Bueno OFA , Rzezak P , de Santana MG , De Mello MT ((2015) ) The influence of physical exercise and leisure activity on neuropsychological functioning in older adults. Age (Dordr) 37: , 9815. |
[88] | Blumenthal JA , Smith PJ , Mabe S , Hinderliter A , Lin P-H , Liao L , Welsh-Bohmer KA , Browndyke JN , Kraus WE , Doraiswamy PM , Burke JR , Sherwood A ((2019) ) Lifestyle and neurocognition in older adults with cognitive impairments. Neurology 92: , e212–e223. |
[89] | Albinet CT , Abou-Dest A , André N , Audiffren M ((2016) ) Executive functions improvement following a 5-month aquaerobics program in older adults: Role of cardiac vagal control in inhibition performance. Biol Psychol 115: , 69–77. |
[90] | Fabre C , Chamari K , Mucci P , Massé-Biron J , Préfaut C ((2002) ) Improvement of cognitive function by mental and/or individualized aerobic training in healthy elderly subjects. Int J Sports Med 23: , 415–421. |
[91] | Antunes HK , De Mello MT , Santos-Galduróz RF , Galduróz JCF , Lemos VA , Tufik S , Bueno OFA ((2015) ) Effects of a physical fitness program on memory and blood viscosity in sedentary elderly men. Braz J Med Biol Res Rev Bras Pesqui Medicas E Biol 48: , 805–812. |
[92] | Lautenschlager NT , Cox KL , Flicker L , Foster JK , Bockxmeer FM van , Xiao J , Greenop KR , Almeida OP ((2008) ) Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: A randomized trial. JAMA 300: , 1027–1037. |
[93] | de Oliveira Silva F , Ferreira JV , Plácido J , Sant’Anna P , Araújo J , Marinho V , Laks J , Camaz Deslandes A ((2019) ) Three months of multimodal training contributes to mobility and executive function in elderly individuals with mild cognitive impairment, but not in those with Alzheimer’s disease: A randomized controlled trial. Maturitas 126: , 28–33. |
[94] | Liu-Ambrose T , Nagamatsu LS , Graf P , Beattie BL , Ashe MC , Handy TC ((2010) ) Resistance training and executive functions: A 12-month randomized controlled trial. Arch Intern Med 170: , 170–178. |
[95] | Busse A , Filho W , Magaldi R , Coelho V , Melo A , Betoni R , Santarém J ((2008) ) Effects of resistance training exercise on cognitive performance in elderly individuals with memory impairment: Results of a controlled trial. Einstein 6: , 402–407. |
[96] | Fiatarone Singh MA , Gates N , Saigal N , Wilson GC , Meiklejohn J , Brodaty H , Wen W , Singh N , Baune BT , Suo C , Baker MK , Foroughi N , Wang Y , Sachdev PS , Valenzuela M ((2014) ) The Study of Mental and Resistance Training (SMART) study—resistance training and/or cognitive training in mild cognitive impairment: A randomized, double-blind, double-sham controlled trial. J Am Med Dir Assoc 15: , 873–880. |
[97] | Yoon DH , Kang D , Kim H-J , Kim J-S , Song HS , Song W ((2017) ) Effect of elastic band-based high-speed power training on cognitive function, physical performance and muscle strength in older women with mild cognitive impairment. Geriatr Gerontol Int 17: , 765–772. |
[98] | Gomes-Osman J , Cabral DF , Morris TP , McInerney K , Cahalin LP , Rundek T , Oliveira A , Pascual-Leone A ((2018) ) Exercise for cognitive brain health in aging: A systematic review for an evaluation of dose. Neurol Clin Pract 8: , 257–265. |
[99] | Wilson RS , Leon CFM de , Barnes LL , Schneider JA , Bienias JL , Evans DA , Bennett DA ((2002) ) Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA 287: , 742–748. |
[100] | Wang H-X , Karp A , Winblad B , Fratiglioni L ((2002) ) Late-life engagement in social and leisure activities is associated with a decreased risk of dementia: A longitudinal study from the Kungsholmen Project. Am J Epidemiol 155: , 1081–1087. |
[101] | Verghese J , LeValley A , Derby C , Kuslansky G , Katz M , Hall C , Buschke H , Lipton RB ((2006) ) Leisure activities and the risk of amnestic mild cognitive impairment in the elderly. Neurology 66: , 821–827. |
[102] | Lee ATC , Richards M , Chan WC , Chiu HFK , Lee RSY , Lam LCW ((2018) ) Association of daily intellectual activities with lower risk of incident dementia among older Chinese adults. JAMA Psychiatry 75: , 697–703. |
[103] | Yates LA , Ziser S , Spector A , Orrell M ((2016) ) Cognitive leisure activities and future risk of cognitive impairment and dementia: Systematic review and meta-analysis. Int Psychogeriatr 28: , 1791–1806. |
[104] | Sampedro-Piquero P , Begega A ((2017) ) Environmental enrichment as a positive behavioral intervention across the lifespan. Curr Neuropharmacol 15: , 459–470. |
[105] | van Praag H , Kempermann G , Gage FH ((2000) ) Neural consequences of environmental enrichment. Nat Rev Neurosci 1: , 191–198. |
[106] | Li S , Jin M , Zhang D , Yang T , Koeglsperger T , Fu H , Selkoe DJ ((2013) ) Environmental novelty activates β2-adrenergic signaling to prevent the impairment of hippocampal LTP by Aβ oligomers. Neuron 77: , 929–941. |
[107] | Lazarov O , Robinson J , Tang Y-P , Hairston IS , Korade-Mirnics Z , Lee VM-Y , Hersh LB , Sapolsky RM , Mirnics K , Sisodia SS ((2005) ) Environmental enrichment reduces Abeta levels and amyloid deposition in transgenic mice. Cell 120: , 701–713. |
[108] | Jankowsky JL , Melnikova T , Fadale DJ , Xu GM , Slunt HH , Gonzales V , Younkin LH , Younkin SG , Borchelt DR , Savonenko AV ((2005) ) Environmental enrichment mitigates cognitive deficits in a mouse model of Alzheimer’s disease. J Neurosci 25: , 5217–5224. |
[109] | Ledreux A , Håkansson K , Carlsson R , Kidane M , Columbo L , Terjestam Y , Ryan E , Tusch E , Winblad B , Daffner K , Granholm A-C , Mohammed AKH ((2019) ) Differential effects of physical exercise, cognitive training, and mindfulness practice on serum BDNF levels in healthy older adults: A randomized controlled intervention study. J Alzheimers Dis 71: , 1245–1261. |
[110] | Landau SM , Marks SM , Mormino EC , Rabinovici GD , Oh H , O’Neil JP , Wilson RS , Jagust WJ ((2012) ) Association of lifetime cognitive engagement and low β-amyloid deposition. Arch Neurol 69: , 623–629. |
[111] | Gidicsin CM , Maye JE , Locascio JJ , Pepin LC , Philiossaint M , Becker JA , Younger AP , Dekhtyar M , Schultz AP , Amariglio RE , Marshall GA , Rentz DM , Hedden T , Sperling RA , Johnson KA ((2015) ) Cognitive activity relates to cognitive performance but not to Alzheimer disease biomarkers. Neurology 85: , 48–55. |
[112] | Vemuri P , Lesnick TG , Przybelski SA , Knopman DS , Roberts RO , Lowe VJ , Kantarci K , Senjem ML , Gunter JL , Boeve BF , Petersen RC , Jack CR ((2012) ) Effect of lifestyle activities on Alzheimer disease biomarkers and cognition. Ann Neurol 72: , 730–738. |
[113] | Wilson RS , Scherr PA , Schneider JA , Tang Y , Bennett DA ((2007) ) Relation of cognitive activity to risk of developing Alzheimer disease. Neurology 69: , 1911–1920. |
[114] | Park DC , Bischof GN ((2013) ) The aging mind: Neuroplasticity in response to cognitive training. Dialogues Clin Neurosci 15: , 109–119. |
[115] | Nguyen L , Murphy K , Andrews G ((2019) ) Cognitive and neural plasticity in old age: A systematic review of evidence from executive functions cognitive training. Ageing Res Rev 53: , 100912. |
[116] | Rosen AC , Sugiura L , Kramer JH , Whitfield-Gabrieli S , Gabrieli JD ((2011) ) Cognitive training changes hippocampal function in mild cognitive impairment: A pilot study. J Alzheimers Dis 26 Suppl 3: , 349–357. |
[117] | Kirchhoff BA , Anderson BA , Smith SE , Barch DM , Jacoby LL ((2012) ) Cognitive training-related changes in hippocampal activity associated with recollection in older adults. Neuroimage 62: , 1956–1964. |
[118] | Ball K , Berch DB , Helmers KF , Jobe JB , Leveck MD , Marsiske M , Morris JN , Rebok GW , Smith DM , Tennstedt SL , Unverzagt FW , Willis SL , Advanced Cognitive Training for Independent and Vital Elderly Study Group ((2002) ) Effects of cognitive training interventions with older adults: A randomized controlled trial. JAMA 288: , 2271–2281. |
[119] | Corbett A , Owen A , Hampshire A , Grahn J , Stenton R , Dajani S , Burns A , Howard R , Williams N , Williams G , Ballard C ((2015) ) The effect of an online cognitive training package in healthy older adults: An online randomized controlled trial. J Am Med Dir Assoc 16: , 990–997. |
[120] | Belleville S , Hudon C , Bier N , Brodeur C , Gilbert B , Grenier S , Ouellet M-C , Viscogliosi C , Gauthier S ((2018) ) MEMO+: Efficacy, durability and effect of cognitive training and psychosocial intervention in individuals with mild cognitive impairment. J Am Geriatr Soc 66: , 655–663. |
[121] | Han JW , Lee H , Hong JW , Kim K , Kim T , Byun HJ , Ko JW , Youn JC , Ryu S-H , Lee N-J , Pae C-U , Kim KW ((2017) ) Multimodal cognitive enhancement therapy for patients with mild cognitive impairment and mild dementia: A multi- center, randomized, controlled, double-blind, crossover trial. J Alzheimers Dis 55: , 787–796. |
[122] | Rebok GW , Ball K , Guey LT , Jones RN , Kim H-Y , King JW , Marsiske M , Morris JN , Tennstedt SL , Unverzagt FW , Willis SL , ACTIVE Study Group ((2014) ) Ten-year effects of the advanced cognitive training for independent and vital elderly cognitive training trial on cognition and everyday functioning in older adults. J Am Geriatr Soc 62: , 16–24. |
[123] | Edwards JD , Xu H , Clark DO , Guey LT , Ross LA , Unverzagt FW ((2017) ) Speed of processing training results in lower risk of dementia. Alzheimers Dement N Y 3: , 603–611. |
[124] | Simon SS , Hampstead BM , Nucci MP , Duran FLS , Fonseca LM , Martin M da GM , Ávila R , Porto FHG , Brucki SMD , Martins CB , Tascone LS , Amaro E , Busatto GF , Bottino CMC ((2018) ) Cognitive and brain activity changes after mnemonic strategy training in amnestic mild cognitive impairment: Evidence from a randomized controlled trial. Front Aging Neurosci 10: , 342. |
[125] | Wolinsky FD , Vander Weg MW , Howren MB , Jones MP , Dotson MM ((2013) ) A randomized controlled trial of cognitive training using a visual speed of processing intervention in middle aged and older adults. PloS One 8: , e61624. |
[126] | Kallio E-L , Öhman H , Hietanen M , Soini H , Strandberg TE , Kautiainen H , Pitkälä KH ((2018) ) Effects of cognitive training on cognition and quality of life of older persons with dementia. J Am Geriatr Soc 66: , 664–670. |
[127] | McDonough IM , Haber S , Bischof GN , Park DC ((2015) ) The Synapse Project: Engagement in mentally challenging activities enhances neural efficiency. Restor Neurol Neurosci 33: , 865–882. |
[128] | Simons DJ , Boot WR , Charness N , Gathercole SE , Chabris CF , Hambrick DZ , Stine-Morrow EAL ((2016) ) Do “brain-training” programs work? Psychol Sci Public Interest 17: , 103–186. |
[129] | Kable JW , Caulfield MK , Falcone M , McConnell M , Bernardo L , Parthasarathi T , Cooper N , Ashare R , Audrain-McGovern J , Hornik R , Diefenbach P , Lee FJ , Lerman C ((2017) ) No effect of commercial cognitive training on brain activity, choice behavior, or cognitive performance. J Neurosci 37: , 7390–7402. |
[130] | Håkansson K , Rovio S , Helkala E-L , Vilska A-R , Winblad B , Soininen H , Nissinen A , Mohammed AH , Kivipelto M ((2009) ) Association between mid-life marital status and cognitive function in later life: Population based cohort study. BMJ 339: , b2462. |
[131] | Sundström A , Westerlund O , Kotyrlo E ((2016) ) Marital status and risk of dementia: A nationwide population-based prospective study from Sweden. BMJ Open 6: , e008565. |
[132] | Crooks VC , Lubben J , Petitti DB , Little D , Chiu V ((2008) ) Social network, cognitive function, and dementia incidence among elderly women. Am J Public Health 98: , 1221–1227. |
[133] | Evans IEM , Martyr A , Collins R , Brayne C , Clare L ((2019) ) Social isolation and cognitive function in later life: A systematic review and meta-analysis. J Alzheimers Dis 70: , S119–S144. |
[134] | Kuiper JS , Zuidersma M , Oude Voshaar RC , Zuidema SU , van den Heuvel ER , Stolk RP , Smidt N ((2015) ) Social relationships and risk of dementia: A systematic review and meta-analysis of longitudinal cohort studies. Ageing Res Rev 22: , 39–57. |
[135] | Ali AA , Khalil MG , Elariny HA , Elfotuh karema A ((2017) ) Study on social isolation as a risk factor in development of Alzheimer’s disease in rats. Brain Disord Ther 06: , 230. |
[136] | Powell ND , Sloan EK , Bailey MT , Arevalo JMG , Miller GE , Chen E , Kobor MS , Reader BF , Sheridan JF , Cole SW ((2013) ) Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via β-adrenergic induction of myelopoiesis. Proc Natl Acad Sci U S A 110: , 16574–16579. |
[137] | Huang H , Wang L , Cao M , Marshall C , Gao J , Xiao N , Hu G , Xiao M ((2015) ) Isolation housing exacerbates Alzheimer’s disease-like pathophysiology in aged APP/PS1 mice. Int J Neuropsychopharmacol 18: , pyu116. |
[138] | Stranahan AM , Khalil D , Gould E ((2006) ) Social isolation delays the positive effects of running on adult neurogenesis. Nat Neurosci 9: , 526–533. |
[139] | Murínová J , Hlaváčová N , Chmelová M , Riečanský I ((2017) ) The evidence for altered BDNF expression in the brain of rats reared or housed in social isolation: A systematic review. Front Behav Neurosci 11: , 101. |
[140] | McHugh JE , Lawlor BA ((2013) ) Perceived stress mediates the relationship between emotional loneliness and sleep quality over time in older adults. Br J Health Psychol 18: , 546–555. |
[141] | Hawkley LC , Cacioppo JT ((2010) ) Loneliness matters: A theoretical and empirical review of consequences and mechanisms. Ann Behav Med Publ Soc Behav Med 40: , 218–227. |
[142] | Hackett RA , Hamer M , Endrighi R , Brydon L , Steptoe A ((2012) ) Loneliness and stress-related inflammatory and neuroendocrine responses in older men and women. Psychoneuroendocrinology 37: , 1801–1809. |
[143] | Jaremka LM , Fagundes CP , Peng J , Bennett JM , Glaser R , Malarkey WB , Kiecolt-Glaser JK ((2013) ) Loneliness promotes inflammation during acute stress. Psychol Sci 24: , 1089–1097. |
[144] | Uchino BN , Trettevik R , Kent de Grey RG , Cronan S , Hogan J , Baucom BRW ((2018) ) Social support, social integration, and inflammatory cytokines: A meta-analysis. Health Psychol 37: , 462–471. |
[145] | Hawkley LC , Masi CM , Berry JD , Cacioppo JT ((2006) ) Loneliness is a unique predictor of age-related differences in systolic blood pressure. Psychol Aging 21: , 152–164. |
[146] | James BD , Glass TA , Caffo B , Bobb JF , Davatzikos C , Yousem D , Schwartz BS ((2012) ) Association of social engagement with brain volumes assessed by structural MRI. J Aging Res 2012: , 512714. |
[147] | Felix C , Rosano C , Zhu X , Flatt JD , Rosso AL ((2020) ) Greater social engagement and greater gray matter microstructural integrity in brain regions relevant to dementia. J Gerontol B Psychol Sci Soc Sci, doi: 10.1093/geronb/gbaa173 |
[148] | Yu L , Boyle PA , Segawa E , Leurgans S , Schneider JA , Wilson RS , Bennett DA ((2015) ) Residual decline in cognition after adjustment for common neuropathologic conditions. Neuropsychology 29: , 335–343. |
[149] | Bennett DA , Schneider JA , Tang Y , Arnold SE , Wilson RS ((2006) ) The effect of social networks on the relation between Alzheimer’s disease pathology and level of cognitive function in old people: A longitudinal cohort study. Lancet Neurol 5: , 406–412. |
[150] | Biddle KD , d’Oleire Uquillas F , Jacobs HIL , Zide B , Kirn DR , Rentz DM , Johnson KA , Sperling RA , Donovan NJ ((2019) ) Social engagement and amyloid-β-related cognitive decline in cognitively normal older adults. Am J Geriatr Psychiatry 27: , 1247–1256. |
[151] | Carlson MC , Saczynski JS , Rebok GW , Seeman T , Glass TA , McGill S , Tielsch J , Frick KD , Hill J , Fried LP ((2008) ) Exploring the effects of an “everyday” activity program on executive function and memory in older adults: Experience Corps. Gerontologist 48: , 793–801. |
[152] | Carlson MC , Erickson KI , Kramer AF , Voss MW , Bolea N , Mielke M , McGill S , Rebok GW , Seeman T , Fried LP ((2009) ) Evidence for neurocognitive plasticity in at-risk older adults: The experience corps program. J Gerontol A Biol Sci Med Sci 64: , 1275–1282. |
[153] | Carlson MC , Kuo JH , Chuang Y-F , Varma VR , Harris G , Albert MS , Erickson KI , Kramer AF , Parisi JM , Xue Q-L , Tan EJ , Tanner EK , Gross AL , Seeman TE , Gruenewald TL , McGill S , Rebok GW , Fried LP ((2015) ) Impact of the Baltimore Experience Corps Trial on cortical and hippocampal volumes. Alzheimers Dement 11: , 1340–1348. |
[154] | Cohen-Mansfield J , Cohen R , Buettner L , Eyal N , Jakobovits H , Rebok G , Rotenberg-Shpigelman S , Sternberg S ((2015) ) Interventions for older persons reporting memory difficulties: A randomized controlled pilot study. Int J Geriatr Psychiatry 30: , 478–486. |
[155] | Scarmeas N , Stern Y , Tang M-X , Mayeux R , Luchsinger JA ((2006) ) Mediterranean diet and risk for Alzheimer’s disease. Ann Neurol 59: , 912–921. |
[156] | Appel LJ , Moore TJ , Obarzanek E , Vollmer WM , Svetkey LP , Sacks FM , Bray GA , Vogt TM , Cutler JA , Windhauser MM , Lin PH , Karanja N ((1997) ) A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med 336: , 1117–1124. |
[157] | Morris MC , Tangney CC , Wang Y , Barnes LL , Bennett D , Aggarwal N ((2014) ) MIND diet score more predictive than DASH or Mediterranean diet scores. Alzheimers Dement 10: , P166. |
[158] | U.S. Department of Health and Human Services and U.S. Department of Agriculture (2015) Dietary Guidelines for Americans 2015-2020, U.S. Department of Health and Human Services and U.S. Department of Agriculture, Washington D.C. |
[159] | McEvoy CT , Guyer H , Langa KM , Yaffe K ((2017) ) Neuroprotective diets are associated with better cognitive function: The Health and Retirement Study. J Am Geriatr Soc 65: , 1857–1862. |
[160] | Scarmeas N , Stern Y , Mayeux R , Manly JJ , Schupf N , Luchsinger JA ((2009) ) Mediterranean diet and mild cognitive impairment. Arch Neurol 66: , 216–225. |
[161] | Morris MC , Tangney CC , Wang Y , Sacks FM , Bennett DA , Aggarwal NT ((2015) ) MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement 11: , 1007–1014. |
[162] | Morris MC , Tangney CC , Wang Y , Sacks FM , Barnes LL , Bennett DA , Aggarwal NT ((2015) ) MIND diet slows cognitive decline with aging. Alzheimers Dement 11: , 1015–1022. |
[163] | Hosking DE , Eramudugolla R , Cherbuin N , Anstey KJ ((2019) ) MIND not Mediterranean diet related to 12-year incidence of cognitive impairment in an Australian longitudinal cohort study. Alzheimers Dement 15: , 581–589. |
[164] | Wu L , Sun D ((2017) ) Adherence to Mediterranean diet and risk of developing cognitive disorders: An updated systematic review and meta-analysis of prospective cohort studies. Sci Rep 7: , 41317. |
[165] | Singh B , Parsaik AK , Mielke MM , Erwin PJ , Knopman DS , Petersen RC , Roberts RO ((2014) ) Association of Mediterranean diet with mild cognitive impairment and Alzheimer’s disease: A systematic review and meta-analysis. J Alzheimers Dis 39: , 271–282. |
[166] | Cao L , Tan L , Wang H-F , Jiang T , Zhu X-C , Lu H , Tan M-S , Yu J-T ((2016) ) Dietary patterns and risk of dementia: A systematic review and meta-analysis of cohort studies. Mol Neurobiol 53: , 6144–6154. |
[167] | Tangney CC , Li H , Wang Y , Barnes L , Schneider JA , Bennett DA , Morris MC ((2014) ) Relation of DASH- and Mediterranean-like dietary patterns to cognitive decline in older persons. Neurology 83: , 1410–1416. |
[168] | van den Brink AC , Brouwer-Brolsma EM , Berendsen AAM , van de Rest O ((2019) ) The Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diets are associated with less cognitive decline and a lower risk of Alzheimer’s disease—a review. Adv Nutr 10: , 1040–1065. |
[169] | Shakersain B , Santoni G , Larsson SC , Faxén-Irving G , Fastbom J , Fratiglioni L , Xu W ((2016) ) Prudent diet may attenuate the adverse effects of Western diet on cognitive decline. Alzheimers Dement 12: , 100–109. |
[170] | Rees K , Takeda A , Martin N , Ellis L , Wijesekara D , Vepa A , Das A , Hartley L , Stranges S ((2020) ) Mediterranean-style diet for the primary and secondary prevention of cardiovascular disease: A Cochrane review. Glob Heart 15: , 56. |
[171] | McGrattan AM , McGuinness B , McKinley MC , Kee F , Passmore P , Woodside JV , McEvoy CT ((2019) ) Diet and inflammation in cognitive ageing and Alzheimer’s disease. Curr Nutr Rep 8: , 53–65. |
[172] | Kinney JW , Bemiller SM , Murtishaw AS , Leisgang AM , Salazar AM , Lamb BT ((2018) ) Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement (N Y) 4: , 575–590. |
[173] | Devassy JG , Leng S , Gabbs M , Monirujjaman M , Aukema HM ((2016) ) Omega-3 polyunsaturated fatty acids and oxylipins in neuroinflammation and management of Alzheimer disease. Adv Nutr 7: , 905–916. |
[174] | Vauzour D , Martinsen A , Layé S ((2015) ) Neuroinflammatory processes in cognitive disorders: Is there a role for flavonoids and n-3 polyunsaturated fatty acids in counteracting their detrimental effects? Neurochem Int 89: , 63–74. |
[175] | Monacelli F , Acquarone E , Giannotti C , Borghi R , Nencioni A ((2017) ) Vitamin C, aging and Alzheimer’s disease. Nutrients 9: , 670. |
[176] | Flanagan E , Müller M , Hornberger M , Vauzour D ((2018) ) Impact of flavonoids on cellular and molecular mechanisms underlying age-related cognitive decline and neurodegeneration. Curr Nutr Rep 7: , 49–57. |
[177] | Gu Y , Brickman AM , Stern Y , Habeck CG , Razlighi QR , Luchsinger JA , Manly JJ , Schupf N , Mayeux R , Scarmeas N ((2015) ) Mediterranean diet and brain structure in a multiethnic elderly cohort. Neurology 85: , 1744–1751. |
[178] | Luciano M , Corley J , Cox SR , Valdés Hernández MC , Craig LCA , Dickie DA , Karama S , McNeill GM , Bastin ME , Wardlaw JM , Deary IJ ((2017) ) Mediterranean-type diet and brain structural change from 73 to 76 years in a Scottish cohort. Neurology 88: , 449–455. |
[179] | Gu Y , Scarmeas N , Stern Y , Manly JJ , Schupf N , Mayeux R , Brickman AM ((2016) ) Mediterranean DIET is associated with slower rate of hippocampal atrophy: A longitudinal study in cognitively normal older adults. Alzheimers Dement 12: , P193–P194. |
[180] | Vassilaki M , Aakre JA , Syrjanen JA , Mielke MM , Geda YE , Kremers WK , Machulda MM , Alhurani RE , Staubo SC , Knopman DS , Petersen RC , Lowe VJ , Jack CR , Roberts RO ((2018) ) Mediterranean diet, its components, and amyloid imaging biomarkers. J Alzheimers Dis 64: , 281–290. |
[181] | Berti V , Walters M , Sterling J , Quinn CG , Logue M , Andrews R , Matthews DC , Osorio RS , Pupi A , Vallabhajosula S , Isaacson RS , de Leon MJ , Mosconi L ((2018) ) Mediterranean diet and 3-year Alzheimer brain biomarker changes in middle-aged adults. Neurology 90: , e1789–e1798. |
[182] | Rainey-Smith SR , Gu Y , Gardener SL , Doecke JD , Villemagne VL , Brown BM , Taddei K , Laws SM , Sohrabi HR , Weinborn M , Ames D , Fowler C , Macaulay SL , Maruff P , Masters CL , Salvado O , Rowe CC , Scarmeas N , Martins RN ((2018) ) Mediterranean diet adherence and rate of cerebral Aβ-amyloid accumulation: Data from the Australian Imaging, Biomarkers and Lifestyle Study of Ageing. Transl Psychiatry 8: , 238. |
[183] | Martínez-Lapiscina EH , Clavero P , Toledo E , Estruch R , Salas-Salvadó J , San Julián B , Sanchez-Tainta A , Ros E , Valls-Pedret C , Martinez-Gonzalez MÁ ((2013) ) Mediterranean diet improves cognition: The PREDIMED-NAVARRA randomised trial. J Neurol Neurosurg Psychiatry 84: , 1318–1325. |
[184] | Valls-Pedret C , Sala-Vila A , Serra-Mir M , Corella D , de la Torre R , Martínez-González MÁ , Martínez-Lapiscina EH , Fitó M , Pérez-Heras A , Salas-Salvadó J , Estruch R , Ros E ((2015) ) Mediterranean diet and age-related cognitive decline: A randomized clinical trial. JAMA Intern Med 175: , 1094–1103. |
[185] | Lee J , Pase M , Pipingas A , Raubenheimer J , Thurgood M , Villalon L , Macpherson H , Gibbs A , Scholey A ((2015) ) Switching to a 10-day Mediterranean-style diet improves mood and cardiovascular function in a controlled crossover study. Nutrition 31: , 647–652. |
[186] | Lehtisalo J , Levälahti E , Lindström J , Hänninen T , Paajanen T , Peltonen M , Antikainen R , Laatikainen T , Strandberg T , Soininen H , Tuomilehto J , Kivipelto M , Ngandu T ((2019) ) Dietary changes and cognition over 2 years within a multidomain intervention trial-The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER). Alzheimers Dement 15: , 410–417. |
[187] | Estruch R , Ros E , Salas-Salvadó J , Covas M-I , Corella D , Arós F , Gómez-Gracia E , Ruiz-Gutiérrez V , Fiol M , Lapetra J , Lamuela-Raventos RM , Serra-Majem L , Pintó X , Basora J , Muñoz MA , Sorlí JV , Martínez JA , Fitó M , Gea A , Hernán MA , Martínez-González MA , PREDIMED Study Investigators ((2018) ) Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med 378: , e34. |
[188] | Boespflug EL , Eliassen JC , Dudley JA , Shidler MD , Kalt W , Summer SS , Stein AL , Stover AN , Krikorian R ((2018) ) Enhanced neuronal activation with blueberry supplementation in mild cognitive impairment. Nutr Neurosci 21: , 297–305. |
[189] | Whyte AR , Cheng N , Fromentin E , Williams CM ((2018) ) A randomized, double-blinded, placebo-controlled study to compare the safety and efficacy of low dose enhanced wild blueberry powder and wild blueberry extract (ThinkBlueTM) in maintenance of episodic and working memory in older adults. Nutrients 10: , 660. |
[190] | McNamara RK , Kalt W , Shidler MD , McDonald J , Summer SS , Stein AL , Stover AN , Krikorian R ((2018) ) Cognitive response to fish oil, blueberry, and combined supplementation in older adults with subjective cognitive impairment. Neurobiol Aging 64: , 147–156. |
[191] | Külzow N , Witte AV , Kerti L , Grittner U , Schuchardt JP , Hahn A , Flöel A ((2016) ) Impact of omega-3 fatty acid supplementation on memory functions in healthy older adults. J Alzheimers Dis 51: , 713–725. |
[192] | Bo Y , Zhang X , Wang Y , You J , Cui H , Zhu Y , Pang W , Liu W , Jiang Y , Lu Q ((2017) ) The n-3 polyunsaturated fatty acids supplementation improved the cognitive function in the Chinese elderly with mild cognitive impairment: A double-blind randomized controlled trial. Nutrients 9: , 54. |
[193] | Danthiir V , Hosking DE , Nettelbeck T , Vincent AD , Wilson C , O’Callaghan N , Calvaresi E , Clifton P , Wittert GA ((2018) ) An 18-mo randomized, double-blind, placebo-controlled trial of DHA-rich fish oil to prevent age-related cognitive decline in cognitively normal older adults. Am J Clin Nutr 107: , 754–762. |
[194] | Leckie RL , Lehman DE , Gianaros PJ , Erickson KI , Sereika SM , Kuan DCH , Manuck SB , Ryan CM , Yao JK , Muldoon MF ((2020) ) The effects of omega-3 fatty acids on neuropsychological functioning and brain morphology in mid-life adults: A randomized clinical trial. Psychol Med 50: , 2425–2434. |
[195] | Devore EE , Kang JH , Breteler MMB , Grodstein F ((2012) ) Dietary intakes of berries and flavonoids in relation to cognitive decline. Ann Neurol 72: , 135–143. |
[196] | Akbaraly TN , Singh-Manoux A , Dugravot A , Brunner EJ , Kivimäki M , Sabia S ((2019) ) Association of midlife diet with subsequent risk for dementia. JAMA 321: , 957–968. |
[197] | Whelton PK , Carey RM , Aronow WS , Casey DE , Collins KJ , Dennison-Himmelfarb C , DePalma SM , Gidding S , Jamerson KA , Jones DW , MacLaughlin EJ , Muntner P , Ovbiagele B , Smith SC , Spencer CC , Stafford RS , Taler SJ , Thomas RJ , Williams KA , Williamson JD , Wright JT ((2018) ) 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 71: , e13–e115. |
[198] | Ou Y-N , Tan C-C , Shen X-N , Xu W , Hou X-H , Dong Q , Tan L , Yu J-T ((2020) ) Blood pressure and risks of cognitive impairment and dementia: A systematic review and meta-analysis of 209 prospective studies. Hypertension 76: , 217–225. |
[199] | Sharp SI , Aarsland D , Day S , Sønnesyn H , Ballard C ((2011) ) Hypertension is a potential risk factor for vascular dementia: Systematic review. Int J Geriatr Psychiatry 26: , 661–669. |
[200] | Tadic M , Cuspidi C , Hering D ((2016) ) Hypertension and cognitive dysfunction in elderly: Blood pressure management for this global burden. BMC Cardiovasc Disord 16: , 208. |
[201] | Birns J , Morris R , Donaldson N , Kalra L ((2006) ) The effects of blood pressure reduction on cognitive function: A review of effects based on pooled data from clinical trials. J Hypertens 24: , 1907–1914. |
[202] | Sun D , Thomas EA , Launer LJ , Sidney S , Yaffe K , Fornage M ((2020) ) Association of blood pressure with cognitive function at midlife: A Mendelian randomization study. BMC Med Genomics 13: , 121. |
[203] | Tully PJ , Hanon O , Cosh S , Tzourio C ((2016) ) Diuretic antihypertensive drugs and incident dementia risk: A systematic review, meta-analysis and meta-regression of prospective studies. J Hypertens 34: , 1027–1035. |
[204] | Hussain S , Singh A , Zameer S , Jamali MC , Baxi H , Rahman SO , Alam M , Altamish M , Singh AK , Anil D , Hussain MS , Ahmad A , Najmi AK ((2020) ) No association between proton pump inhibitor use and risk of dementia: Evidence from a meta-analysis. J Gastroenterol Hepatol 35: , 19–28. |
[205] | Ding J , Davis-Plourde KL , Sedaghat S , Tully PJ , Wang W , Phillips C , Pase MP , Himali JJ , Gwen Windham B , Griswold M , Gottesman R , Mosley TH , White L , Guðnason V , Debette S , Beiser AS , Seshadri S , Ikram MA , Meirelles O , Tzourio C , Launer LJ ((2020) ) Antihypertensive medications and risk for incident dementia and Alzheimer’s disease: A meta-analysis of individual participant data from prospective cohort studies. Lancet Neurol 19: , 61–70. |
[206] | Barnes DE , Yaffe K ((2011) ) The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol 10: , 819–828. |
[207] | Kennelly SP , Lawlor BA , Kenny RA ((2009) ) Blood pressure and dementia - a comprehensive review. Ther Adv Neurol Disord 2: , 241–260. |
[208] | Kivipelto M , Helkala EL , Laakso MP , Hänninen T , Hallikainen M , Alhainen K , Soininen H , Tuomilehto J , Nissinen A ((2001) ) Midlife vascular risk factors and Alzheimer’s disease in later life: Longitudinal, population based study. BMJ 322: , 1447–1451. |
[209] | McGrath ER , Beiser AS , DeCarli C , Plourde KL , Vasan RS , Greenberg SM , Seshadri S ((2017) ) Blood pressure from mid- to late life and risk of incident dementia. Neurology 89: , 2447–2454. |
[210] | Abell JG , Kivimäki M , Dugravot A , Tabak AG , Fayosse A , Shipley M , Sabia S , Singh-Manoux A ((2018) ) Association between systolic blood pressure and dementia in the Whitehall II cohort study: Role of age, duration, and threshold used to define hypertension. Eur Heart J 39: , 3119–3125. |
[211] | Delgado J , Bowman K , Ble A , Masoli J , Han Y , Henley W , Welsh S , Kuchel GA , Ferrucci L , Melzer D ((2018) ) Blood pressure trajectories in the 20 years before death. JAMA Intern Med 178: , 93–99. |
[212] | Walker KA , Sharrett AR , Wu A , Schneider ALC , Albert M , Lutsey PL , Bandeen-Roche K , Coresh J , Gross AL , Windham BG , Knopman DS , Power MC , Rawlings AM , Mosley TH , Gottesman RF ((2019) ) Association of midlife to late-life blood pressure patterns with incident dementia. JAMA 322: , 535–545. |
[213] | Lerman LO , Kurtz TW , Touyz RM , Ellison DH , Chade AR , Crowley SD , Mattson DL , Mullins JJ , Osborn J , Eirin A , Reckelhoff JF , Iadecola C , Coffman TM ((2019) ) Animal models of hypertension: A scientific statement from the American Heart Association. Hypertension 73: , e87–e120. |
[214] | Jennings JR , Muldoon MF , Ryan C , Price JC , Greer P , Sutton-Tyrrell K , van der Veen FM , Meltzer CC ((2005) ) Reduced cerebral blood flow response and compensation among patients with untreated hypertension. Neurology 64: , 1358–1365. |
[215] | Portegies MLP , Mirza SS , Verlinden VJA , Hofman A , Koudstaal PJ , Swanson SA , Ikram MA ((2016) ) Mid- to late-life trajectories of blood pressure and the risk of stroke: The Rotterdam Study. Hypertension 67: , 1126–1132. |
[216] | Arvanitakis Z , Capuano AW , Lamar M , Shah RC , Barnes LL , Bennett DA , Schneider JA ((2018) ) Late-life blood pressure association with cerebrovascular and Alzheimer disease pathology. Neurology 91: , e517–e525. |
[217] | Beauchet O , Celle S , Roche F , Bartha R , Montero-Odasso M , Allali G , Annweiler C ((2013) ) Blood pressure levels and brain volume reduction: A systematic review and meta-analysis. J Hypertens 31: , 1502–1516. |
[218] | Lane CA , Barnes J , Nicholas JM , Sudre CH , Cash DM , Parker TD , Malone IB , Lu K , James S-N , Keshavan A , Murray-Smith H , Wong A , Buchanan SM , Keuss SE , Gordon E , Coath W , Barnes A , Dickson J , Modat M , Thomas D , Crutch SJ , Hardy R , Richards M , Fox NC , Schott JM ((2019) ) Associations between blood pressure across adulthood and late-life brain structure and pathology in the neuroscience substudy of the 1946 British birth cohort (Insight 46): An epidemiological study. Lancet Neurol 18: , 942–952. |
[219] | Poels MMF , Zaccai K , Verwoert GC , Vernooij MW , Hofman A , van der Lugt A , Witteman JCM , Breteler MMB , Mattace-Raso FUS , Ikram MA ((2012) ) Arterial stiffness and cerebral small vessel disease: The Rotterdam Scan Study. Stroke 43: , 2637–2642. |
[220] | Verhaaren BFJ , Vernooij MW , de Boer R , Hofman A , Niessen WJ , van der Lugt A , Ikram MA ((2013) ) High blood pressure and cerebral white matter lesion progression in the general population. Hypertension 61: , 1354–1359. |
[221] | Hoffman LB , Schmeidler J , Lesser GT , Beeri MS , Purohit DP , Grossman HT , Haroutunian V ((2009) ) Less Alzheimer disease neuropathology in medicated hypertensive than nonhypertensive persons. Neurology 72: , 1720–1726. |
[222] | Shah NS , Vidal J-S , Masaki K , Petrovitch H , Ross GW , Tilley C , DeMattos RB , Tracy RP , White LR , Launer LJ ((2012) ) Midlife blood pressure, plasma β-amyloid, and the risk for Alzheimer disease: The Honolulu Asia Aging Study. Hypertension 59: , 780–786. |
[223] | Ashby EL , Miners JS , Kehoe PG , Love S ((2016) ) Effects of hypertension and anti-hypertensive treatment on amyloid-β (Aβ) plaque load and Aβ-synthesizing and Aβ-degrading enzymes in frontal cortex. J Alzheimers Dis 50: , 1191–1203. |
[224] | Köbe T , Gonneaud J , Pichet Binette A , Meyer P-F , McSweeney M , Rosa-Neto P , Breitner JCS , Poirier J , Villeneuve S , Presymptomatic Evaluation of Experimental or Novel Treatments for Alzheimer Disease (PREVENT-AD) Research Group ((2020) ) Association of vascular risk factors with β-amyloid peptide and tau burdens in cognitively unimpaired individuals and its interaction with vascular medication use. JAMA Netw Open 3: , e1920780. |
[225] | Muñoz Maniega S , Chappell FM , Valdés Hernández MC , Armitage PA , Makin SD , Heye AK , Thrippleton MJ , Sakka E , Shuler K , Dennis MS , Wardlaw JM ((2017) ) Integrity of normal-appearing white matter: Influence of age, visible lesion burden and hypertension in patients with small-vessel disease. J Cereb Blood Flow Metab 37: , 644–656. |
[226] | Sweeney MD , Sagare AP , Zlokovic BV ((2018) ) Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol 14: , 133–150. |
[227] | Affleck AJ , Sachdev PS , Stevens J , Halliday GM ((2020) ) Antihypertensive medications ameliorate Alzheimer’s disease pathology by slowing its propagation. Alzheimers Dement 6: , e12060. |
[228] | Williamson JD , Pajewski NM , Auchus AP , Bryan RN , Chelune G , Cheung AK , Cleveland ML , Coker LH , Crowe MG , Cushman WC , Cutler JA , Davatzikos C , Desiderio L , Erus G , Fine LJ , Gaussoin SA , Harris D , Hsieh M-K , Johnson KC , Kimmel PL , Tamura MK , Launer LJ , Lerner AJ , Lewis CE , Martindale-Adams J , Moy CS , Nasrallah IM , Nichols LO , Oparil S , Ogrocki PK , Rahman M , Rapp SR , Reboussin DM , Rocco MV , Sachs BC , Sink KM , Still CH , Supiano MA , Snyder JK , Wadley VG , Walker J , Weiner DE , Whelton PK , Wilson VM , Woolard N , Wright JT , Wright CB ((2019) ) Effect of intensive vs standard blood pressure control on probable dementia: A randomized clinical trial. JAMA 321: , 553–561. |
[229] | Zhang H , Cui Y , Zhao Y , Dong Y , Duan D , Wang J , Sheng L , Ji T , Zhou T , Hu W , Chen Y , Sun S , Gong G , Chai Q , Liu Z ((2019) ) Effects of sartans and low-dose statins on cerebral white matter hyperintensities and cognitive function in older patients with hypertension: A randomized, double-blind and placebo-controlled clinical trial. Hypertens Res 42: , 717–729. |
[230] | Sink KM , Evans GW , Shorr RI , Bates JT , Berlowitz D , Conroy MB , Felton DM , Gure T , Johnson KC , Kitzman D , Lyles MF , Servilla K , Supiano MA , Whittle J , Wiggers A , Fine LJ ((2018) ) Syncope, hypotension, and falls in the treatment of hypertension: Results from the randomized clinical systolic blood pressure intervention trial. J Am Geriatr Soc 66: , 679–686. |
[231] | Pajewski NM , Berlowitz DR , Bress AP , Callahan KE , Cheung AK , Fine LJ , Gaussoin SA , Johnson KC , King J , Kitzman DW , Kostis JB , Lerner AJ , Lewis CE , Oparil S , Rahman M , Reboussin DM , Rocco MV , Snyder JK , Still C , Supiano MA , Wadley VG , Whelton PK , Wright JT , Williamson JD ((2020) ) Intensive vs standard blood pressure control in adults 80 years or older: A secondary analysis of the systolic blood pressure intervention trial. J Am Geriatr Soc 68: , 496–504. |
[232] | SPRINT MIND Investigators for the SPRINT Research Group, Nasrallah IM , Pajewski NM , Auchus AP , Chelune G , Cheung AK , Cleveland ML , Coker LH , Crowe MG , Cushman WC , Cutler JA , Davatzikos C , Desiderio L , Doshi J , Erus G , Fine LJ , Gaussoin SA , Harris D , Johnson KC , Kimmel PL , Kurella Tamura M , Launer LJ , Lerner AJ , Lewis CE , Martindale-Adams J , Moy CS , Nichols LO , Oparil S , Ogrocki PK , Rahman M , Rapp SR , Reboussin DM , Rocco MV , Sachs BC , Sink KM , Still CH , Supiano MA , Snyder JK , Wadley VG , Walker J , Weiner DE , Whelton PK , Wilson VM , Woolard N , Wright JT , Wright CB , Williamson JD , Bryan RN ((2019) ) Association of intensive vs standard blood pressure control with cerebral white matter lesions. JAMA 322: , 524–534. |
[233] | Bosch J , O’Donnell M , Swaminathan B , Lonn EM , Sharma M , Dagenais G , Diaz R , Khunti K , Lewis BS , Avezum A , Held C , Keltai M , Reid C , Toff WD , Dans A , Leiter LA , Sliwa K , Lee SF , Pogue JM , Hart R , Yusuf S , on behalf of the HOPE-3 Investigators ((2019) ) Effects of blood pressure and lipid lowering on cognition: Results from the HOPE-3 study. Neurology 92: , e1435–e1446. |
[234] | Peters R , Beckett N , Forette F , Tuomilehto J , Clarke R , Ritchie C , Waldman A , Walton I , Poulter R , Ma S , Comsa M , Burch L , Fletcher A , Bulpitt C , HYVET investigators ((2008) ) Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG): A double-blind, placebo controlled trial. Lancet Neurol 7: , 683–689. |
[235] | Warwick J , Falaschetti E , Rockwood K , Mitnitski A , Thijs L , Beckett N , Bulpitt C , Peters R ((2015) ) No evidence that frailty modifies the positive impact of antihypertensive treatment in very elderly people: An investigation of the impact of frailty upon treatment effect in the HYpertension in the Very Elderly Trial (HYVET) study, a double-blind, placebo-controlled study of antihypertensives in people with hypertension aged 80 and over. BMC Med 13: , 78. |
[236] | Benetos A , Petrovic M , Strandberg T ((2019) ) Hypertension management in older and frail older patients. Circ Res 124: , 1045–1060. |
[237] | Odden MC , Peralta CA , Berlowitz DR , Johnson KC , Whittle J , Kitzman DW , Beddhu S , Nord JW , Papademetriou V , Williamson JD , Pajewski NM , Systolic Blood Pressure Intervention Trial (SPRINT) Research Group ((2017) ) Effect of intensive blood pressure control on gait speed and mobility limitation in adults 75 years or older: A randomized clinical trial. JAMA Intern Med 177: , 500–507. |
[238] | McCrimmon RJ , Ryan CM , Frier BM ((2012) ) Diabetes and cognitive dysfunction. Lancet 379: , 2291–2299. |
[239] | Smith MA , Else JE , Paul L , Foster JK , Walker M , Wesnes KA , Riby LM ((2014) ) Functional living in older adults with type 2 diabetes: Executive functioning, dual task performance, and the impact on postural stability and motor control. J Aging Health 26: , 841–859. |
[240] | Palta P , Carlson MC , Crum RM , Colantuoni E , Sharrett AR , Yasar S , Nahin RL , DeKosky ST , Snitz B , Lopez O , Williamson JD , Furberg CD , Rapp SR , Golden SH ((2017) ) Diabetes and cognitive decline in older adults: The Ginkgo Evaluation of Memory Study. J Gerontol A Biol Sci Med Sci 73: , 123–130. |
[241] | Ott A , Stolk RP , van Harskamp F , Pols HA , Hofman A , Breteler MM ((1999) ) Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology 53: , 1937–1942. |
[242] | Fan Y-C , Hsu J-L , Tung H-Y , Chou C-C , Bai C-H ((2017) ) Increased dementia risk predominantly in diabetes mellitus rather than in hypertension or hyperlipidemia: A population-based cohort study. Alzheimers Res Ther 9: , 7. |
[243] | Frison E , Dufouil C , Helmer C , Berr C , Auriacombe S , Chêne G ((2019) ) Diabetes-associated dementia risk and competing risk of death in the Three-City Study. J Alzheimers Dis 71: , 1339–1350. |
[244] | Xue M , Xu W , Ou Y-N , Cao X-P , Tan M-S , Tan L , Yu J-T ((2019) ) Diabetes mellitus and risks of cognitive impairment and dementia: A systematic review and meta-analysis of 144 prospective studies. Ageing Res Rev 55: , 100944. |
[245] | Zhang J , Chen C , Hua S , Liao H , Wang M , Xiong Y , Cao F ((2017) ) An updated meta-analysis of cohort studies: Diabetes and risk of Alzheimer’s disease. Diabetes Res Clin Pract 124: , 41–47. |
[246] | Lee JH , Choi Y , Jun C , Hong YS , Cho HB , Kim JE , Lyoo IK ((2014) ) Neurocognitive changes and their neural correlates in patients with type 2 diabetes mellitus. Endocrinol Metab 29: , 112–121. |
[247] | Byrn MA , Adams W , Penckofer S , Emanuele MA ((2019) ) Vitamin D supplementation and cognition in people with type 2 diabetes: A randomized control trial. J Diabetes Res 2019: , 5696391. |
[248] | Sims-Robinson C , Kim B , Rosko A , Feldman EL ((2010) ) How does diabetes accelerate Alzheimer disease pathology? Nat Rev Neurol 6: , 551–559. |
[249] | Zhao X , Han Q , Lv Y , Sun L , Gang X , Wang G ((2017) ) Biomarkers for cognitive decline in patients with diabetes mellitus: Evidence from clinical studies. Oncotarget 9: , 7710–7726. |
[250] | Biessels GJ , Despa F ((2018) ) Cognitive decline and dementia in diabetes mellitus: Mechanisms and clinical implications. Nat Rev Endocrinol 14: , 591–604. |
[251] | Tan ZS , Beiser AS , Fox CS , Au R , Himali JJ , Debette S , Decarli C , Vasan RS , Wolf PA , Seshadri S ((2011) ) Association of metabolic dysregulation with volumetric brain magnetic resonance imaging and cognitive markers of subclinical brain aging in middle-aged adults: The Framingham Offspring Study. Diabetes Care 34: , 1766–1770. |
[252] | Yaffe K , Falvey C , Hamilton N , Schwartz AV , Simonsick EM , Satterfield S , Cauley JA , Rosano C , Launer LJ , Strotmeyer ES , Harris TB ((2012) ) Diabetes, glucose control, and 9-year cognitive decline among older adults without dementia. Arch Neurol 69: , 1170–1175. |
[253] | Lacy ME , Gilsanz P , Karter AJ , Quesenberry CP , Pletcher MJ , Whitmer RA ((2018) ) Long-term glycemic control and dementia risk in type 1 diabetes. Diabetes Care 41: , 2339–2345. |
[254] | Lehtisalo J , Lindström J , Ngandu T , Kivipelto M , Ahtiluoto S , Ilanne-Parikka P , Keinänen-Kiukaanniemi S , Eriksson JG , Uusitupa M , Tuomilehto J , Luchsinger JA , Finnish Diabetes Prevention Study (DPS) ((2016) ) Diabetes, glycaemia, and cognition-a secondary analysis of the Finnish Diabetes Prevention Study. Diabetes Metab Res Rev 32: , 102–110. |
[255] | Launer LJ , Miller ME , Williamson JD , Lazar RM , Gerstein HC , Murray AM , Sullivan M , Horowitz KR , Ding J , Marcovina S , Lovato LC , Lovato J , Margolis KL , O’Connor P , Lipkin EW , Hirsch J , Coker L , Maldjian J , Sunshine JL , Truwit C , Davatzikos C , Bryan RN , ACCORD MIND investigators ((2011) ) Effects of intensive glucose lowering on brain structure and function in people with type 2 diabetes (ACCORD MIND): A randomised open-label substudy. Lancet Neurol 10: , 969–977. |
[256] | Cukierman-Yaffe T , Bosch J , Diaz R , Dyal L , Hancu N , Hildebrandt P , Lanas F , Lewis BS , Marre M , Yale J-F , Yusuf S , Gerstein HC , ORIGIN Investigators ((2014) ) Effects of basal insulin glargine and omega-3 fatty acid on cognitive decline and probable cognitive impairment in people with dysglycaemia: A substudy of the ORIGIN trial. Lancet Diabetes Endocrinol 2: , 562–572. |
[257] | Luchsinger JA , Perez T , Chang H , Mehta P , Steffener J , Pradabhan G , Ichise M , Manly J , Devanand DP , Bagiella E ((2016) ) Metformin in amnestic mild cognitive impairment: Results of a pilot randomized placebo controlled clinical trial. J Alzheimers Dis 51: , 501–514. |
[258] | Biessels GJ , Verhagen C , Janssen J , van den Berg E , Zinman B , Rosenstock J , George JT , Passera A , Schnaidt S , Johansen OE , CARMELINA Investigators ((2019) ) Effect of linagliptin on cognitive performance in patients with type 2 diabetes and cardiorenal comorbidities: The CARMELINA Randomized Trial. Diabetes Care 42: , 1930–1938. |
[259] | Grundy SM , Stone NJ , Bailey AL , Beam C , Birtcher KK , Blumenthal RS , Braun LT , de Ferranti S , Faiella-Tommasino J , Forman DE , Goldberg R , Heidenreich PA , Hlatky MA , Jones DW , Lloyd-Jones D , Lopez-Pajares N , Ndumele CE , Orringer CE , Peralta CA , Saseen JJ , Smith SC , Sperling L , Virani SS , Yeboah J ((2019) ) 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 139: , e1082–e1143. |
[260] | Anstey KJ , Lipnicki DM , Low L-F ((2008) ) Cholesterol as a risk factor for dementia and cognitive decline: A systematic review of prospective studies with meta-analysis. Am J Geriatr Psychiatry 16: , 343–354. |
[261] | Solomon A , Kivipelto M , Wolozin B , Zhou J , Whitmer RA ((2009) ) Midlife serum cholesterol and increased risk of Alzheimer’s and vascular dementia three decades later. Dement Geriatr Cogn Disord 28: , 75–80. |
[262] | Anstey KJ , Ashby-Mitchell K , Peters R ((2017) ) Updating the evidence on the association between serum cholesterol and risk of late-life dementia: Review and meta-analysis. J Alzheimers Dis 56: , 215–228. |
[263] | Mielke MM , Zandi PP , Shao H , Waern M , Östling S , Guo X , Björkelund C , Lissner L , Skoog I , Gustafson DR ((2010) ) The 32-year relationship between cholesterol and dementia from midlife to late life. Neurology 75: , 1888–1895. |
[264] | Han K-T , Kim SJ ((2021) ) Are serum cholesterol levels associated with cognitive impairment and depression in elderly individuals without dementia?: A retrospective cohort study in South Korea. Int J Geriatr Psychiatry 36: , 163–173. |
[265] | McFarlane O , Kędziora-Kornatowska K ((2020) ) Cholesterol and dementia: A long and complicated relationship. Curr Aging Sci 13: , 42–51. |
[266] | Tóth ME , Dukay B , Hoyk Z , Sántha M ((2020) ) Cerebrovascular changes and neurodegeneration related to hyperlipidemia: Characteristics of the human ApoB-100 transgenic mice. Curr Pharm Des 26: , 1486–1494. |
[267] | Nelson RH ((2013) ) Hyperlipidemia as a risk factor for cardiovascular disease. Prim Care 40: , 195–211. |
[268] | Menet R , Bernard M , ElAli A ((2018) ) Hyperlipidemia in stroke pathobiology and therapy: Insights and perspectives. Front Physiol 9: , 488. |
[269] | Bowman GL , Kaye JA , Quinn JF ((2012) ) Dyslipidemia and blood-brain barrier integrity in Alzheimer’s disease. Curr Gerontol Geriatr Res 2012: , 184042. |
[270] | Buxbaum JD , Cullen EI , Friedhoff LT ((2002) ) Pharmacological concentrations of the HMG-CoA reductase inhibitor lovastatin decrease the formation of the Alzheimer beta-amyloid peptide in vitro and in patients. Front Biosci J Virtual Libr 7: , a50–59. |
[271] | Chu C-S , Tseng P-T , Stubbs B , Chen T-Y , Tang C-H , Li D-J , Yang W-C , Chen Y-W , Wu C-K , Veronese N , Carvalho AF , Fernandes BS , Herrmann N , Lin P-Y ((2018) ) Use of statins and the risk of dementia and mild cognitive impairment: A systematic review and meta-analysis. Sci Rep 8: , 5804. |
[272] | Zissimopoulos JM , Barthold D , Brinton RD , Joyce G ((2017) ) Sex and race differences in the association between statin use and the incidence of Alzheimer disease. JAMA Neurol 74: , 225–232. |
[273] | Eckel RH , Grundy SM , Zimmet PZ ((2005) ) The metabolic syndrome. Lancet 365: , 1415–1428. |
[274] | Ma Y , Ajnakina O , Steptoe A , Cadar D ((2020) ) Higher risk of dementia in English older individuals who are overweight or obese. Int J Epidemiol 49: , 1353–1365. |
[275] | Kivipelto M , Ngandu T , Fratiglioni L , Viitanen M , Kåreholt I , Winblad B , Helkala E-L , Tuomilehto J , Soininen H , Nissinen A ((2005) ) Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol 62: , 1556–1560. |
[276] | Whitmer RA , Gustafson DR , Barrett-Connor E , Haan MN , Gunderson EP , Yaffe K ((2008) ) Central obesity and increased risk of dementia more than three decades later. Neurology 71: , 1057–1064. |
[277] | Kerwin DR , Gaussoin SA , Chlebowski RT , Kuller LH , Vitolins M , Coker LH , Kotchen JM , Nicklas BJ , Wassertheil-Smoller S , Hoffmann RG , Espeland MA , Women’s Health Initiative Memory Study ((2011) ) Interaction between body mass index and central adiposity and risk of incident cognitive impairment and dementia: Results from the Women’s Health Initiative Memory Study. J Am Geriatr Soc 59: , 107–112. |
[278] | Xu WL , Atti AR , Gatz M , Pedersen NL , Johansson B , Fratiglioni L ((2011) ) Midlife overweight and obesity increase late-life dementia risk: A population-based twin study. Neurology 76: , 1568–1574. |
[279] | Pedditzi E , Peters R , Beckett N ((2016) ) The risk of overweight/obesity in mid-life and late life for the development of dementia: A systematic review and meta-analysis of longitudinal studies. Age Ageing 45: , 14–21. |
[280] | Singh-Manoux A , Dugravot A , Shipley M , Brunner EJ , Elbaz A , Sabia S , Kivimaki M ((2018) ) Obesity trajectories and risk of dementia: 28 years of follow-up in the Whitehall II Study. Alzheimers Dement 14: , 178–186. |
[281] | Buie JJ , Watson LS , Smith CJ , Sims-Robinson C ((2019) ) Obesity-related cognitive impairment: The role of endothelial dysfunction. Neurobiol Dis 132: , 104580. |
[282] | Bednarska-Makaruk M , Graban A , Wiśniewska A , Łojkowska W , Bochyńska A , Gugała-Iwaniuk M , Sławińska K , Ługowska A , Ryglewicz D , Wehr H ((2017) ) Association of adiponectin, leptin and resistin with inflammatory markers and obesity in dementia. Biogerontology 18: , 561–580. |
[283] | Prehn K , Jumpertz von Schwartzenberg R , Mai K , Zeitz U , Witte AV , Hampel D , Szela A-M , Fabian S , Grittner U , Spranger J , Flöel A ((2017) ) Caloric restriction in older adults-differential effects of weight loss and reduced weight on brain structure and function. Cereb Cortex 27: , 1765–1778. |
[284] | Napoli N , Shah K , Waters DL , Sinacore DR , Qualls C , Villareal DT ((2014) ) Effect of weight loss, exercise, or both on cognition and quality of life in obese older adults. Am J Clin Nutr 100: , 189–198. |
[285] | Horie NC , Serrao VT , Simon SS , Gascon MRP , Dos Santos AX , Zambone MA , Del Bigio de Freitas MM , Cunha-Neto E , Marques EL , Halpern A , de Melo ME , Mancini MC , Cercato C ((2016) ) Cognitive effects of intentional weight loss in elderly obese individuals with mild cognitive impairment. J Clin Endocrinol Metab 101: , 1104–1112. |
[286] | Witte AV , Fobker M , Gellner R , Knecht S , Flöel A ((2009) ) Caloric restriction improves memory in elderly humans. Proc Natl Acad Sci U S A 106: , 1255–1260. |
[287] | Siervo M , Nasti G , Stephan BCM , Papa A , Muscariello E , Wells JCK , Prado CM , Colantuoni A ((2012) ) Effects of intentional weight loss on physical and cognitive function in middle-aged and older obese participants: A pilot study. J Am Coll Nutr 31: , 79–86. |
[288] | Durazzo TC , Meyerhoff DJ , Nixon SJ ((2012) ) A comprehensive assessment of neurocognition in middle-aged chronic cigarette smokers. Drug Alcohol Depend 122: , 105–111. |
[289] | Wagner M , Schulze-Rauschenbach S , Petrovsky N , Brinkmeyer J , von der Goltz C , Gründer G , Spreckelmeyer KN , Wienker T , Diaz-Lacava A , Mobascher A , Dahmen N , Clepce M , Thuerauf N , Kiefer F , de Millas JW , Gallinat J , Winterer G ((2013) ) Neurocognitive impairments in non-deprived smokers–results from a population-based multi-center study on smoking-related behavior. Addict Biol 18: , 752–761. |
[290] | Reitz C , den Heijer T , van Duijn C , Hofman A , Breteler MMB ((2007) ) Relation between smoking and risk of dementia and Alzheimer disease: The Rotterdam Study. Neurology 69: , 998–1005. |
[291] | Rusanen M , Kivipelto M , Quesenberry CP , Zhou J , Whitmer RA ((2011) ) Heavy smoking in midlife and long-term risk of Alzheimer disease and vascular dementia. Arch Intern Med 171: , 333–339. |
[292] | Anstey KJ , von Sanden C , Salim A , O’Kearney R ((2007) ) Smoking as a risk factor for dementia and cognitive decline: A meta-analysis of prospective studies. Am J Epidemiol 166: , 367–378. |
[293] | Zhong G , Wang Y , Zhang Y , Guo JJ , Zhao Y ((2015) ) Smoking is associated with an increased risk of dementia: A meta-analysis of prospective cohort studies with investigation of potential effect modifiers. PLoS One 10: , e0118333. |
[294] | Merchant C , Tang MX , Albert S , Manly J , Stern Y , Mayeux R ((1999) ) The influence of smoking on the risk of Alzheimer’s disease. Neurology 52: , 1408–1412. |
[295] | Cataldo JK , Prochaska JJ , Glantz SA ((2010) ) Cigarette smoking is a risk factor for Alzheimer’s disease: An analysis controlling for tobacco industry affiliation. J Alzheimers Dis 19: , 465–480. |
[296] | Khanna A , Guo M , Mehra M , Royal W ((2013) ) Inflammation and oxidative stress induced by cigarette smoke in Lewis rat brains. J Neuroimmunol 254: , 69–75. |
[297] | Swan GE , Lessov-Schlaggar CN ((2007) ) The effects of tobacco smoke and nicotine on cognition and the brain. Neuropsychol Rev 17: , 259–273. |
[298] | Durazzo TC , Mattsson N , Weiner MW ((2014) ) Smoking and increased Alzheimer’s disease risk: A review of potential mechanisms. Alzheimers Dement 10: , S122–S145. |
[299] | Giunta B , Deng J , Jin J , Sadic E , Rum S , Zhou H , Sanberg P , Tan J ((2012) ) Evaluation of how cigarette smoke is a direct risk factor for Alzheimer’s disease. Technol Innov 14: , 39–48. |
[300] | Teipel S , Grothe MJ , Alzheimer’s Disease Neuroimaging Initiative ((2016) ) Association between smoking and cholinergic basal forebrain volume in healthy aging and prodromal and dementia stages of Alzheimer’s disease. J Alzheimers Dis 52: , 1443–1451. |
[301] | Fratiglioni L , Wang HX ((2000) ) Smoking and Parkinson’s and Alzheimer’s disease: Review of the epidemiological studies. Behav Brain Res 113: , 117–120. |
[302] | Letenneur L , Larrieu S , Barberger-Gateau P ((2004) ) Alcohol and tobacco consumption as risk factors of dementia: A review of epidemiological studies. Biomed Pharmacother 58: , 95–99. |
[303] | Hernán MA , Alonso A , Logroscino G ((2008) ) Cigarette smoking and dementia: Potential selection bias in the elderly. Epidemiology 19: , 448–450. |
[304] | Weuve J , Tchetgen Tchetgen EJ , Glymour MM , Beck TL , Aggarwal NT , Wilson RS , Evans DA , Mendes de Leon CF ((2012) ) Accounting for bias due to selective attrition: The example of smoking and cognitive decline. Epidemiology 23: , 119–128. |
[305] | Abner EL , Nelson PT , Jicha GA , Cooper GE , Fardo DW , Schmitt FA , Kryscio RJ ((2019) ) Tobacco smoking and dementia in a Kentucky cohort: A competing risk analysis. J Alzheimers Dis 68: , 625–633. |
[306] | The American Institute of Stress, What is Stress? https://www.stress.org/dev/daily-life, Accessed on 10 October 2020. |
[307] | Sandi C ((2013) ) Stress and cognition. Wiley Interdiscip Rev Cogn Sci 4: , 245–261. |
[308] | Sousa N ((2016) ) The dynamics of the stress neuromatrix. Mol Psychiatry 21: , 302–312. |
[309] | Shields GS , Rivers AM , Ramey MM , Trainor BC , Yonelinas AP ((2019) ) Mild acute stress improves response speed without impairing accuracy or interference control in two selective attention tasks: Implications for theories of stress and cognition. Psychoneuroendocrinology 108: , 78–86. |
[310] | Munoz E , Sliwinski MJ , Scott SB , Hofer S ((2015) ) Global perceived stress predicts cognitive change among older adults. Psychol Aging 30: , 487–499. |
[311] | Stawski RS , Mogle JA , Sliwinski MJ ((2013) ) Daily stressors and self-reported changes in memory in old age: The mediating effects of daily negative affect and cognitive interference. Aging Ment Health 17: , 168–172. |
[312] | Shields GS , Sazma MA , Yonelinas AP ((2016) ) The effects of acute stress on core executive functions: A meta-analysis and comparison with cortisol. Neurosci Biobehav Rev 68: , 651–668. |
[313] | Hermans EJ , Henckens MJAG , Joëls M , Fernández G ((2014) ) Dynamic adaptation of large-scale brain networks in response to acute stressors. Trends Neurosci 37: , 304–314. |
[314] | Chen Y , Liang Y , Zhang W , Crawford JC , Sakel KL , Dong X ((2019) ) Perceived stress and cognitive decline in Chinese-American older adults. J Am Geriatr Soc 67: , S519–S524. |
[315] | Johansson L , Guo X , Waern M , Ostling S , Gustafson D , Bengtsson C , Skoog I ((2010) ) Midlife psychological stress and risk of dementia: A 35-year longitudinal population study. Brain 133: , 2217–2224. |
[316] | Greenberg MS , Tanev K , Marin M-F , Pitman RK ((2014) ) Stress, PTSD, and dementia. Alzheimers Dement 10: , S155–165. |
[317] | Peavy GM , Jacobson MW , Salmon DP , Gamst AC , Patterson TL , Goldman S , Mills PJ , Khandrika S , Galasko D ((2012) ) The influence of chronic stress on dementia-related diagnostic change in older adults. Alzheimer Dis Assoc Disord 26: , 260–266. |
[318] | Gradus JL , Horváth-Puhó E , Lash TL , Ehrenstein V , Tamang S , Adler NE , Milstein A , Glymour MM , Henderson VW , Sørensen HT ((2019) ) Stress disorders and dementia in the Danish population. Am J Epidemiol 188: , 493–499. |
[319] | Wang H-X , Wahlberg M , Karp A , Winblad B , Fratiglioni L ((2012) ) Psychosocial stress at work is associated with increased dementia risk in late life. Alzheimers Dement 8: , 114–120. |
[320] | Dong H , Csernansky JG ((2009) ) Effects of stress and stress hormones on amyloid-beta protein and plaque deposition. J Alzheimers Dis 18: , 459–469. |
[321] | Ouanes S , Popp J ((2019) ) High cortisol and the risk of dementia and Alzheimer’s disease: A review of the literature. Front Aging Neurosci 11: , 43. |
[322] | Fotuhi M , Do D , Jack C ((2012) ) Modifiable factors that alter the size of the hippocampus with ageing. Nat Rev Neurol 8: , 189–202. |
[323] | Suri D , Vaidya VA ((2013) ) Glucocorticoid regulation of brain-derived neurotrophic factor: Relevance to hippocampal structural and functional plasticity. Neuroscience 239: , 196–213. |
[324] | Tata DA , Anderson BJ ((2010) ) The effects of chronic glucocorticoid exposure on dendritic length, synapse numbers and glial volume in animal models: Implications for hippocampal volume reductions in depression. Physiol Behav 99: , 186–193. |
[325] | Geerlings MI , Sigurdsson S , Eiriksdottir G , Garcia ME , Harris TB , Gudnason V , Launer LJ ((2015) ) Salivary cortisol, brain volumes, and cognition in community-dwelling elderly without dementia. Neurology 85: , 976–983. |
[326] | Echouffo-Tcheugui JB , Conner SC , Himali JJ , Maillard P , DeCarli CS , Beiser AS , Vasan RS , Seshadri S ((2018) ) Circulating cortisol and cognitive and structural brain measures: The Framingham Heart Study. Neurology 91: , e1961–e1970. |
[327] | Cox SR , MacPherson SE , Ferguson KJ , Royle NA , Maniega SM , Hernández MDCV , Bastin ME , MacLullich AMJ , Wardlaw JM , Deary IJ ((2015) ) Does white matter structure or hippocampal volume mediate associations between cortisol and cognitive ageing? Psychoneuroendocrinology 62: , 129–137. |
[328] | Kanamaru T , Kamimura N , Yokota T , Iuchi K , Nishimaki K , Takami S , Akashiba H , Shitaka Y , Katsura K-I , Kimura K , Ohta S ((2015) ) Oxidative stress accelerates amyloid deposition and memory impairment in a double-transgenic mouse model of Alzheimer’s disease. Neurosci Lett 587: , 126–131. |
[329] | Green KN , Billings LM , Roozendaal B , McGaugh JL , LaFerla FM ((2006) ) Glucocorticoids increase amyloid-beta and tau pathology in a mouse model of Alzheimer’s disease. J Neurosci 26: , 9047–9056. |
[330] | Sotiropoulos I , Catania C , Pinto LG , Silva R , Pollerberg GE , Takashima A , Sousa N , Almeida OFX ((2011) ) Stress acts cumulatively to precipitate Alzheimer’s disease-like tau pathology and cognitive deficits. J Neurosci 31: , 7840–7847. |
[331] | Toledo JB , Toledo E , Weiner MW , Jack CR , Jagust W , Lee VMY , Shaw LM , Trojanowski JQ , Alzheimer’s Disease Neuroimaging Initiative ((2012) ) Cardiovascular risk factors, cortisol, and amyloid-β deposition in Alzheimer’s Disease Neuroimaging Initiative. Alzheimers Dement 8: , 483–489. |
[332] | Innes KE , Selfe TK ((2014) ) Meditation as a therapeutic intervention for adults at risk for Alzheimer’s disease - potential benefits and underlying mechanisms. Front Psychiatry 5: , 40. |
[333] | Innes KE , Selfe TK , Khalsa DS , Kandati S ((2016) ) Effects of meditation versus music listening on perceived stress, mood, sleep, and quality of life in adults with early memory loss: A pilot randomized controlled trial. J Alzheimers Dis 52: , 1277–1298. |
[334] | Wells RE , Kerr CE , Wolkin J , Dossett M , Davis RB , Walsh J , Wall RB , Kong J , Kaptchuk T , Press D , Phillips RS , Yeh G ((2013) ) Meditation for adults with mild cognitive impairment: A pilot randomized trial. J Am Geriatr Soc 61: , 642–645. |
[335] | Sharma A , Kumar Y ((2019) ) Nature’s derivative(s) as alternative anti-Alzheimer’s disease treatments. J Alzheimers Dis Rep 3: , 279–297. |
[336] | Tolahunase M , Sagar R , Dada R ((2017) ) Impact of yoga and meditation on cellular aging in apparently healthy individuals: A prospective, open-label single-arm exploratory study. Oxid Med Cell Longev 2017: , 7928981. |
[337] | Berk L , Warmenhoven F , van Os J , van Boxtel M ((2018) ) Mindfulness training for people with dementia and their caregivers: Rationale, current research, and future directions. Front Psychol 9: , 982. |
[338] | Marciniak R , Sheardova K , Cermáková P , Hudeček D , Sumec R , Hort J ((2014) ) Effect of meditation on cognitive functions in context of aging and neurodegenerative diseases. Front Behav Neurosci 8: , 17. |
[339] | Byers AL , Yaffe K ((2011) ) Depression and risk of developing dementia. Nat Rev Neurol 7: , 323–331. |
[340] | Dotson VM , Beydoun MA , Zonderman AB ((2010) ) Recurrent depressive symptoms and the incidence of dementia and mild cognitive impairment. Neurology 75: , 27–34. |
[341] | Singh-Manoux A , Dugravot A , Fournier A , Abell J , Ebmeier K , Kivimäki M , Sabia S ((2017) ) Trajectories of depressive symptoms before diagnosis of dementia: A 28-year follow-up study. JAMA Psychiatry 74: , 712–718. |
[342] | Bennett S , Thomas AJ ((2014) ) Depression and dementia: Cause, consequence or coincidence? Maturitas 79: , 184–190. |
[343] | Salvat-Pujol N , Labad J , Urretavizcaya M , de Arriba-Arnau A , Segalàs C , Real E , Ferrer A , Crespo JM , Jiménez-Murcia S , Soriano-Mas C , Menchón JM , Soria V ((2017) ) Hypothalamic-pituitary-adrenal axis activity and cognition in major depression: The role of remission status. Psychoneuroendocrinology 76: , 38–48. |
[344] | Morimoto SS , Alexopoulos GS ((2013) ) Cognitive deficits in geriatric depression: Clinical correlates and implications for current and future treatment. Psychiatr Clin North Am 36: , 517–531. |
[345] | Morimoto SS , Kanellopoulos D , Manning KJ , Alexopoulos GS ((2015) ) Diagnosis and treatment of depression and cognitive impairment in late life. Ann N Y Acad Sci 1345: , 36–46. |
[346] | Gandelman JA , Albert K , Boyd BD , Park JW , Riddle M , Woodward ND , Kang H , Landman BA , Taylor WD ((2019) ) Intrinsic functional network connectivity is associated with clinical symptoms and cognition in late-life depression. Biol Psychiatry Cogn Neurosci Neuroimaging 4: , 160–170. |
[347] | Mackin RS , Nelson JC , Delucchi K , Raue P , Byers A , Barnes D , Satre DD , Yaffe K , Alexopoulos GS , Arean PA ((2014) ) Cognitive outcomes after psychotherapeutic interventions for major depression in older adults with executive dysfunction. Am J Geriatr Psychiatry 22: , 1496–1503. |
[348] | Scullin MK , Bliwise DL ((2015) ) Sleep, cognition, and normal aging: Integrating a half century of multidisciplinary research. Perspect Psychol Sci 10: , 97–137. |
[349] | Alfini AJ , Tzuang M , Owusu JT , Spira AP ((2020) ) Later-life sleep, cognition, and neuroimaging research: An update for 2020. Curr Opin Behav Sci 33: , 72–77. |
[350] | Elcombe EL , Lagopoulos J , Duffy SL , Lewis SJG , Norrie L , Hickie IB , Naismith SL ((2015) ) Hippocampal volume in older adults at risk of cognitive decline: The role of sleep, vascular risk, and depression. J Alzheimers Dis 44: , 1279–1290. |
[351] | Sawyer K , Corsentino E , Sachs-Ericsson N , Steffens DC ((2012) ) Depression, hippocampal volume changes, and cognitive decline in a clinical sample of older depressed outpatients and non-depressed controls. Aging Ment Health 16: , 753–762. |
[352] | Jiang S , Zhang Q-A , Guo Q , Di Z ((2019) ) The glutamatergic system and astrocytic impairment in rat hippocampus: A comparative study of underlying etiology and pathophysiology of depression. J Integr Neurosci 18: , 387–392. |
[353] | Qiao H , An S-C , Xu C , Ma X-M ((2017) ) Role of proBDNF and BDNF in dendritic spine plasticity and depressive-like behaviors induced by an animal model of depression. Brain Res 1663: , 29–37. |
[354] | Hirshkowitz M , Whiton K , Albert SM , Alessi C , Bruni O , DonCarlos L , Hazen N , Herman J , Adams Hillard PJ , Katz ES , Kheirandish-Gozal L , Neubauer DN , O’Donnell AE , Ohayon M , Peever J , Rawding R , Sachdeva RC , Setters B , Vitiello MV , Ware JC ((2015) ) National Sleep Foundation’s updated sleep duration recommendations: Final report. Sleep Health 1: , 233–243. |
[355] | Blackwell T , Yaffe K , Ancoli-Israel S , Redline S , Ensrud KE , Stefanick ML , Laffan A , Stone KL ((2011) ) Association of sleep characteristics and cognition in older community-dwelling men: The MrOS Sleep Study. Sleep 34: , 1347–1356. |
[356] | Lim ASP , Kowgier M , Yu L , Buchman AS , Bennett DA ((2013) ) Sleep fragmentation and the risk of incident Alzheimer’s disease and cognitive decline in older persons. Sleep 36: , 1027–1032. |
[357] | Osorio RS , Pirraglia E , Agüera-Ortiz LF , During EH , Sacks H , Ayappa I , Walsleben J , Mooney A , Hussain A , Glodzik L , Frangione B , Martínez-Martín P , de Leon MJ ((2011) ) Greater risk of Alzheimer’s disease in older adults with insomnia. J Am Geriatr Soc 59: , 559–562. |
[358] | Bubu OM , Brannick M , Mortimer J , Umasabor-Bubu O , Sebastião YV , Wen Y , Schwartz S , Borenstein AR , Wu Y , Morgan D , Anderson WM ((2017) ) Sleep, cognitive impairment, and Alzheimer’s disease: A systematic review and meta-analysis. Sleep 40: , zsw032. |
[359] | Shi L , Chen S-J , Ma M-Y , Bao Y-P , Han Y , Wang Y-M , Shi J , Vitiello MV , Lu L ((2018) ) Sleep disturbances increase the risk of dementia: A systematic review and meta-analysis. Sleep Med Rev 40: , 4–16. |
[360] | Tsapanou A , Gu Y , Manly J , Schupf N , Tang M-X , Zimmerman M , Scarmeas N , Stern Y ((2015) ) Daytime sleepiness and sleep inadequacy as risk factors for dementia. Dement Geriatr Cogn Disord Extra 5: , 286–295. |
[361] | Pace-Schott EF , Spencer RMC ((2015) ) Sleep-dependent memory consolidation in healthy aging and mild cognitive impairment. Curr Top Behav Neurosci 25: , 307–330. |
[362] | Xie L , Kang H , Xu Q , Chen MJ , Liao Y , Thiyagarajan M , O’Donnell J , Christensen DJ , Nicholson C , Iliff JJ , Takano T , Deane R , Nedergaard M ((2013) ) Sleep drives metabolite clearance from the adult brain. Science 342: , 373–377. |
[363] | Owen JE , Veasey SC ((2020) ) Impact of sleep disturbances on neurodegeneration: Insight from studies in animal models. Neurobiol Dis 139: , 104820. |
[364] | Faraut B , Boudjeltia KZ , Vanhamme L , Kerkhofs M ((2012) ) Immune, inflammatory and cardiovascular consequences of sleep restriction and recovery. Sleep Med Rev 16: , 137–149. |
[365] | Ooms S , Overeem S , Besse K , Rikkert MO , Verbeek M , Claassen JAHR ((2014) ) Effect of 1 night of total sleep deprivation on cerebrospinal fluid β-amyloid 42 in healthy middle-aged men: A randomized clinical trial. JAMA Neurol 71: , 971–977. |
[366] | Spira AP , Gamaldo AA , An Y , Wu MN , Simonsick EM , Bilgel M , Zhou Y , Wong DF , Ferrucci L , Resnick SM ((2013) ) Self-reported sleep and β-amyloid deposition in community-dwelling older adults. JAMA Neurol 70: , 1537–1543. |
[367] | Sprecher KE , Bendlin BB , Racine AM , Okonkwo OC , Christian BT , Koscik RL , Sager MA , Asthana S , Johnson SC , Benca RM ((2015) ) Amyloid burden is associated with self-reported sleep in nondemented late middle-aged adults. Neurobiol Aging 36: , 2568–2576. |
[368] | Sprecher KE , Koscik RL , Carlsson CM , Zetterberg H , Blennow K , Okonkwo OC , Sager MA , Asthana S , Johnson SC , Benca RM , Bendlin BB ((2017) ) Poor sleep is associated with CSF biomarkers of amyloid pathology in cognitively normal adults. Neurology 89: , 445–453. |
[369] | Epstein LJ , Kristo D , Strollo PJ Jr , Friedman N , Malhotra A , Patil SP , Ramar K , Rogers R , Schwab RJ , Weaver EM , Weinstein MD , Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine ((2009) ) Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med 05: , 263–276. |
[370] | Benjafield AV , Ayas NT , Eastwood PR , Heinzer R , Ip MSM , Morrell MJ , Nunez CM , Patel SR , Penzel T , Pépin J-L , Peppard PE , Sinha S , Tufik S , Valentine K , Malhotra A ((2019) ) Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir Med 7: , 687–698. |
[371] | Andrade A , Bubu OM , Varga AW , Osorio RS ((2018) ) The relationship between obstructive sleep apnea and Alzheimer’s disease. J Alzheimers Dis 64: , S255–S270. |
[372] | Yaffe K , Laffan AM , Harrison SL , Redline S , Spira AP , Ensrud KE , Ancoli-Israel S , Stone KL ((2011) ) Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA 306: , 613–619. |
[373] | Osorio RS , Gumb T , Pirraglia E , Varga AW , Lu S-E , Lim J , Wohlleber ME , Ducca EL , Koushyk V , Glodzik L , Mosconi L , Ayappa I , Rapoport DM , de Leon MJ , Alzheimer’s Disease Neuroimaging Initiative ((2015) ) Sleep-disordered breathing advances cognitive decline in the elderly. Neurology 84: , 1964–1971. |
[374] | Castronovo V , Scifo P , Castellano A , Aloia MS , Iadanza A , Marelli S , Cappa SF , Strambi LF , Falini A ((2014) ) White matter integrity in obstructive sleep apnea before and after treatment. Sleep 37: , 1465–1475. |
[375] | Kushida CA , Nichols DA , Holmes TH , Quan SF , Walsh JK , Gottlieb DJ , Simon RD , Guilleminault C , White DP , Goodwin JL , Schweitzer PK , Leary EB , Hyde PR , Hirshkowitz M , Green S , McEvoy LK , Chan C , Gevins A , Kay GG , Bloch DA , Crabtree T , Dement WC ((2012) ) Effects of continuous positive airway pressure on neurocognitive function in obstructive sleep apnea patients: The Apnea Positive Pressure Long-term Efficacy Study (APPLES). Sleep 35: , 1593–1602. |
[376] | Cooke JR , Ayalon L , Palmer BW , Loredo JS , Corey-Bloom J , Natarajan L , Liu L , Ancoli-Israel S ((2009) ) Sustained use of CPAP slows deterioration of cognition, sleep, and mood in patients with Alzheimer’s disease and obstructive sleep apnea: A preliminary study. J Clin Sleep Med 5: , 305–309. |
[377] | Ancoli-Israel S , Palmer BW , Cooke JR , Corey-Bloom J , Fiorentino L , Natarajan L , Liu L , Ayalon L , He F , Loredo JS ((2008) ) Cognitive effects of treating obstructive sleep apnea in Alzheimer’s disease: A randomized controlled study. J Am Geriatr Soc 56: , 2076–2081. |
[378] | Sharma RA , Varga AW , Bubu OM , Pirraglia E , Kam K , Parekh A , Wohlleber M , Miller MD , Andrade A , Lewis C , Tweardy S , Buj M , Yau PL , Sadda R , Mosconi L , Li Y , Butler T , Glodzik L , Fieremans E , Babb JS , Blennow K , Zetterberg H , Lu SE , Badia SG , Romero S , Rosenzweig I , Gosselin N , Jean-Louis G , Rapoport DM , de Leon MJ , Ayappa I , Osorio RS ((2018) ) Obstructive sleep apnea severity affects amyloid burden in cognitively normal elderly. A longitudinal study. Am J Respir Crit Care Med 197: , 933–943. |
[379] | Maharani A , Dawes P , Nazroo J , Tampubolon G , Pendleton N ((2018) ) Visual and hearing impairments are associated with cognitive decline in older people. Age Ageing 47: , 575–581. |
[380] | Deal JA , Betz J , Yaffe K , Harris T , Purchase-Helzner E , Satterfield S , Pratt S , Govil N , Simonsick EM , Lin FR ((2017) ) Hearing impairment and incident dementia and cognitive decline in older adults: The Health ABC Study. J Gerontol Ser A 72: , 703–709. |
[381] | Gallacher J , Ilubaera V , Ben-Shlomo Y , Bayer A , Fish M , Babisch W , Elwood P ((2012) ) Auditory threshold, phonologic demand, and incident dementia. Neurology 79: , 1583–1590. |
[382] | Gurgel RK , Ward PD , Schwartz S , Norton MC , Foster NL , Tschanz JT ((2014) ) Relationship of hearing loss and dementia: A prospective, population-based study. Otol Neurotol 35: , 775–781. |
[383] | Lin MY , Gutierrez PR , Stone KL , Yaffe K , Ensrud KE , Fink HA , Sarkisian CA , Coleman AL , Mangione CM ((2004) ) Vision impairment and combined vision and hearing impairment predict cognitive and functional decline in older women. J Am Geriatr Soc 52: , 1996–2002. |
[384] | Fritze T , Teipel S , Óvári A , Kilimann I , Witt G , Doblhammer G ((2016) ) Hearing impairment affects dementia incidence. An analysis based on longitudinal health claims data in Germany. PLoS One 11: , e0156876. |
[385] | Naël V , Pérès K , Dartigues J-F , Letenneur L , Amieva H , Arleo A , Scherlen A-C , Tzourio C , Berr C , Carrière I , Delcourt C , Helmer C , Sense-Cog consortium ((2019) ) Vision loss and 12-year risk of dementia in older adults: The 3C cohort study. Eur J Epidemiol 34: , 141–152. |
[386] | Chen SP , Bhattacharya J , Pershing S ((2017) ) Association of vision loss with cognition in older adults. JAMA Ophthalmol 135: , 963–970. |
[387] | Davies-Kershaw HR , Hackett RA , Cadar D , Herbert A , Orrell M , Steptoe A ((2018) ) Vision impairment and risk of dementia: Findings from the English Longitudinal Study of Ageing. J Am Geriatr Soc 66: , 1823–1829. |
[388] | Hwang PH , Longstreth WT , Brenowitz WD , Thielke SM , Lopez OL , Francis CE , DeKosky ST , Fitzpatrick AL ((2020) ) Dual sensory impairment in older adults and risk of dementia from the GEM Study. Alzheimers Dement (Amst) 12: , e12054. |
[389] | Livingston G , Sommerlad A , Orgeta V , Costafreda SG , Huntley J , Ames D , Ballard C , Banerjee S , Burns A , Cohen-Mansfield J , Cooper C , Fox N , Gitlin LN , Howard R , Kales HC , Larson EB , Ritchie K , Rockwood K , Sampson EL , Samus Q , Schneider LS , Selbæk G , Teri L , Mukadam N ((2017) ) Dementia prevention, intervention, and care. Lancet 390: , 2673–2734. |
[390] | Hong T , Mitchell P , Burlutsky G , Liew G , Wang JJ ((2016) ) Visual impairment, hearing loss and cognitive function in an older population: Longitudinal findings from the Blue Mountains Eye Study. PLoS One 11: , e0147646. |
[391] | Michalowsky B , Hoffmann W , Kostev K ((2019) ) Association between hearing and vision impairment and risk of dementia: Results of a case-control study based on secondary data. Front Aging Neurosci 11: , 363. |
[392] | Lin FR , Ferrucci L , An Y , Goh JO , Doshi J , Metter EJ , Davatzikos C , Kraut MA , Resnick SM ((2014) ) Association of hearing impairment with brain volume changes in older adults. Neuroimage 90: , 84–92. |
[393] | Beckmann D , Feldmann M , Shchyglo O , Manahan-Vaughan D ((2020) ) Hippocampal synaptic plasticity, spatial memory, and neurotransmitter receptor expression are profoundly altered by gradual loss of hearing ability. Cereb Cortex 30: , 4581–4596. |
[394] | Peelle JE , Troiani V , Grossman M , Wingfield A ((2011) ) Hearing loss in older adults affects neural systems supporting speech comprehension. J Neurosci 31: , 12638–12643. |
[395] | Lin FR , Albert M ((2014) ) Hearing loss and dementia - who’s listening? Aging Ment Health 18: , 671–673. |
[396] | Billig AR , Feng N , Behforuzi H , McFeeley BM , Nicastri CM , Daffner KR ((2020) ) Capacity-limited resources are used for managing sensory degradation and cognitive demands: Implications for age-related cognitive decline and dementia. Cortex 133: , 277–294. |
[397] | Gopinath B , Wang JJ , Schneider J , Burlutsky G , Snowdon J , McMahon CM , Leeder SR , Mitchell P ((2009) ) Depressive symptoms in older adults with hearing impairments: The Blue Mountains Study. J Am Geriatr Soc 57: , 1306–1308. |
[398] | Maharani A , Pendleton N , Leroi I ((2019) ) Hearing impairment, loneliness, social isolation, and cognitive function: Longitudinal analysis using English Longitudinal Study on Ageing. Am J Geriatr Psychiatry 27: , 1348–1356. |
[399] | Brenowitz WD , Besser LM , Kukull WA , Keene CD , Glymour MM , Yaffe K ((2020) ) Clinician-judged hearing impairment and associations with neuropathologic burden. Neurology 95: , e1640–e1649. |
[400] | Dawes P , Emsley R , Cruickshanks KJ , Moore DR , Fortnum H , Edmondson-Jones M , McCormack A , Munro KJ ((2015) ) Hearing loss and cognition: The role of hearing aids, social isolation and depression. PLoS One 10: , e0119616. |
[401] | Maharani A , Dawes P , Nazroo J , Tampubolon G , Pendleton N ((2018) ) Longitudinal relationship between hearing aid use and cognitive function in older Americans. J Am Geriatr Soc 66: , 1130–1136. |
[402] | Maharani A , Dawes P , Nazroo J , Tampubolon G , Pendleton N , SENSE-Cog WP1 group ((2018) ) Cataract surgery and age-related cognitive decline: A 13-year follow-up of the English Longitudinal Study of Ageing. PloS One 13: , e0204833. |
[403] | Yu W-K , Chen Y-T , Wang S-J , Kuo S-C , Shia B-C , Liu CJ-L ((2015) ) Cataract surgery is associated with a reduced risk of dementia: A nationwide population-based cohort study. Eur J Neurol 22: , 1370–1377, e79-80. |
[404] | Lee SY , Mesfin FB ((2020) ) Blindness. In StatPearls, StatPearls Publishing, Treasure Island, FL. |
[405] | Varma R , Vajaranant TS , Burkemper B , Wu S , Torres M , Hsu C , Choudhury F , McKean-Cowdin R ((2016) ) Visual impairment and blindness in adults in the United States: Demographic and geographic variations from 2015 to 2050. JAMA Ophthalmol 134: , 802–809. |
[406] | Davis AC , Hoffman HJ ((2019) ) Hearing loss: Rising prevalence and impact. Bull World Health Organ 97: , 646–646A. |
[407] | Gray SL , Anderson ML , Dublin S , Hanlon JT , Hubbard R , Walker R , Yu O , Crane PK , Larson EB ((2015) ) Cumulative use of strong anticholinergics and incident dementia: A prospective cohort study. JAMA Intern Med 175: , 401–407. |
[408] | Papenberg G , Bäckman L , Fratiglioni L , Laukka EJ , Fastbom J , Johnell K ((2017) ) Anticholinergic drug use is associated with episodic memory decline in older adults without dementia. Neurobiol Aging 55: , 27–32. |
[409] | Grossi CM , Richardson K , Fox C , Maidment I , Steel N , Loke YK , Arthur A , Myint PK , Campbell N , Boustani M , Robinson L , Brayne C , Matthews FE , Savva GM ((2019) ) Anticholinergic and benzodiazepine medication use and risk of incident dementia: A UK cohort study. BMC Geriatr 19: , 276. |
[410] | Richardson K , Fox C , Maidment I , Steel N , Loke YK , Arthur A , Myint PK , Grossi CM , Mattishent K , Bennett K , Campbell NL , Boustani M , Robinson L , Brayne C , Matthews FE , Savva GM ((2018) ) Anticholinergic drugs and risk of dementia: Case-control study. BMJ 361: , k1315. |
[411] | Coupland CAC , Hill T , Dening T , Morriss R , Moore M , Hippisley-Cox J ((2019) ) Anticholinergic drug exposure and the risk of dementia: A nested case-control study. JAMA Intern Med 179: , 1084–1093. |
[412] | Carrière I , Fourrier-Reglat A , Dartigues J-F , Rouaud O , Pasquier F , Ritchie K , Ancelin M-L ((2009) ) Drugs with anticholinergic properties, cognitive decline, and dementia in an elderly general population: The 3-city study. Arch Intern Med 169: , 1317–1324. |
[413] | Campbell NL , Lane KA , Gao S , Boustani MA , Unverzagt F ((2018) ) Anticholinergics influence transition from normal cognition to mild cognitive impairment in older adults in primary care. Pharmacotherapy 38: , 511–519. |
[414] | Weigand AJ , Bondi MW , Thomas KR , Campbell NL , Galasko DR , Salmon DP , Sewell D , Brewer JB , Feldman HH , Delano-Wood L , Alzheimer’s Disease Neuroimaging Initiative ((2020) ) Association of anticholinergic medications and AD biomarkers with incidence of MCI among cognitively normal older adults. Neurology 95: , e2295–e2304. |
[415] | Risacher SL , McDonald BC , Tallman EF , West JD , Farlow MR , Unverzagt FW , Gao S , Boustani M , Crane PK , Petersen RC , Jack CR , Jagust WJ , Aisen PS , Weiner MW , Saykin AJ , Alzheimer’s Disease Neuroimaging Initiative ((2016) ) Association between anticholinergic medication use and cognition, brain metabolism, and brain atrophy in cognitively normal older adults. JAMA Neurol 73: , 721–732. |
[416] | Campbell NL , Maidment I , Fox C , Khan B , Boustani M ((2013) ) The 2012 update to the anticholinergic cognitive burden scale. J Am Geriatr Soc 61: , S142–S143. |
[417] | Cai X , Campbell N , Khan B , Callahan C , Boustani M ((2013) ) Chronic anticholinergic use and the aging brain. Alzheimers Dement 9: , 377–385. |
[418] | Chhatwal JP , Schultz AP , Hedden T , Boot BP , Wigman S , Rentz D , Johnson KA , Sperling RA ((2019) ) Anticholinergic amnesia is mediated by alterations in human network connectivity architecture. Cereb Cortex 29: , 3445–3456. |
[419] | Wurtman RJ ((2015) ) How anticholinergic drugs might promote alzheimer’s disease: More amyloid-β and less phosphatidylcholine. J Alzheimers Dis 46: , 983–987. |
[420] | Yoshiyama Y , Kojima A , Itoh K , Isose S , Koide M , Hori K , Arai K ((2015) ) Does anticholinergic activity affect neuropathology? Implication of neuroinflammation in Alzheimer’s disease. Neurodegener Dis 15: , 140–148. |
[421] | Kersten H , Molden E , Tolo IK , Skovlund E , Engedal K , Wyller TB ((2013) ) Cognitive effects of reducing anticholinergic drug burden in a frail elderly population: A randomized controlled trial. J Gerontol A Biol Sci Med Sci 68: , 271–278. |
[422] | van der Meer HG , Wouters H , Pont LG , Taxis K ((2018) ) Reducing the anticholinergic and sedative load in older patients on polypharmacy by pharmacist-led medication review: A randomised controlled trial. BMJ Open 8: , e019042. |
[423] | Birks JS ((2006) ) Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst Rev, CD005593. |
[424] | Gallacher J , Elwood P , Pickering J , Bayer A , Fish M , Ben-Shlomo Y ((2012) ) Benzodiazepine use and risk of dementia: Evidence from the Caerphilly Prospective Study (CaPS). J Epidemiol Community Health 66: , 869–873. |
[425] | Billioti de Gage S , Bégaud B , Bazin F , Verdoux H , Dartigues J-F , Pérès K , Kurth T , Pariente A ((2012) ) Benzodiazepine use and risk of dementia: Prospective population based study. BMJ 345: , e6231. |
[426] | Billioti de Gage S , Moride Y , Ducruet T , Kurth T , Verdoux H , Tournier M , Pariente A , Bégaud B ((2014) ) Benzodiazepine use and risk of Alzheimer’s disease: Case-control study. BMJ 349: , g5205. |
[427] | Gomm W , von Holt K , Thomé F , Broich K , Maier W , Weckbecker K , Fink A , Doblhammer G , Haenisch B ((2016) ) Regular benzodiazepine and Z-substance use and risk of dementia: An analysis of German claims data. J Alzheimers Dis 54: , 801–808. |
[428] | He Q , Chen X , Wu T , Li L , Fei X ((2019) ) Risk of dementia in long-term benzodiazepine users: Evidence from a meta-analysis of observational studies. J Clin Neurol 15: , 9–19. |
[429] | Osler M , Jørgensen MB ((2020) ) Associations of benzodiazepines, Z-drugs, and other anxiolytics with subsequent dementia in patients with affective disorders: A nationwide cohort and nested case-control study. Am J Psychiatry 177: , 497–505. |
[430] | Imfeld P , Bodmer M , Jick SS , Meier CR ((2015) ) Benzodiazepine use and risk of developing Alzheimer’s disease or vascular dementia: A case-control analysis. Drug Saf 38: , 909–919. |
[431] | Gray SL , Dublin S , Yu O , Walker R , Anderson M , Hubbard RA , Crane PK , Larson EB ((2016) ) Benzodiazepine use and risk of incident dementia or cognitive decline: Prospective population based study. BMJ 352: , i90. |
[432] | Nafti M , Sirois C , Kröger E , Carmichael P-H , Laurin D ((2020) ) Is benzodiazepine use associated with the risk of dementia and cognitive impairment-not dementia in older persons? The Canadian Study of Health and Aging. Ann Pharmacother 54: , 219–225. |
[433] | Salzman C ((2020) ) Do benzodiazepines cause Alzheimer’s disease? Am J Psychiatry 177: , 476–478. |
[434] | Gomm W , von Holt K , Thomé F , Broich K , Maier W , Fink A , Doblhammer G , Haenisch B ((2016) ) Association of proton pump inhibitors with risk of dementia: A pharmacoepidemiological claims data analysis. JAMA Neurol 73: , 410. |
[435] | Haenisch B , von Holt K , Wiese B , Prokein J , Lange C , Ernst A , Brettschneider C , König H-H , Werle J , Weyerer S , Luppa M , Riedel-Heller SG , Fuchs A , Pentzek M , Weeg D , Bickel H , Broich K , Jessen F , Maier W , Scherer M ((2015) ) Risk of dementia in elderly patients with the use of proton pump inhibitors. Eur Arch Psychiatry Clin Neurosci 265: , 419–428. |
[436] | Tai S-Y , Chien C-Y , Wu D-C , Lin K-D , Ho B-L , Chang Y-H , Chang Y-P ((2017) ) Risk of dementia from proton pump inhibitor use in Asian population: A nationwide cohort study in Taiwan. PLoS One 12: , e0171006. |
[437] | Gray SL , Walker RL , Dublin S , Yu O , Aiello Bowles EJ , Anderson ML , Crane PK , Larson EB ((2018) ) Proton pump inhibitor use and dementia risk: Prospective population-based study. J Am Geriatr Soc 66: , 247–253. |
[438] | Hwang IC , Chang J , Park SM ((2018) ) A nationwide population-based cohort study of dementia risk among acid suppressant users. Am J Geriatr Psychiatry 26: , 1175–1183. |
[439] | Huang S-T , Tseng L-Y , Chen L-K , Peng L-N , Hsiao F-Y ((2019) ) Does long-term proton pump inhibitor use increase risk of dementia? Not really! Results of the group-based trajectory analysis. Clin Pharmacol Ther 106: , 616–622. |
[440] | Imfeld P , Bodmer M , Jick SS , Meier CR ((2018) ) Proton pump inhibitor use and risk of developing Alzheimer’s disease or vascular dementia: A case-control analysis. Drug Saf 41: , 1387–1396. |
[441] | Taipale H , Tolppanen A-M , Tiihonen M , Tanskanen A , Tiihonen J , Hartikainen S ((2017) ) No association between proton pump inhibitor use and risk of Alzheimer’s disease. Am J Gastroenterol 112: , 1802–1808. |
[442] | Li M , Luo Z , Yu S , Tang Z ((2019) ) Proton pump inhibitor use and risk of dementia: Systematic review and meta-analysis. Medicine (Baltimore) 98: , e14422. |
[443] | Goldstein FC , Steenland K , Zhao L , Wharton W , Levey AI , Hajjar I ((2017) ) Proton pump inhibitors and risk of mild cognitive impairment and dementia. J Am Geriatr Soc 65: , 1969–1974. |
[444] | Booker A , Jacob LE , Rapp M , Bohlken J , Kostev K ((2016) ) Risk factors for dementia diagnosis in German primary care practices. Int Psychogeriatr 28: , 1059–1065. |
[445] | Oesterle A , Laufs U , Liao JK ((2017) ) Pleiotropic effects of statins on the cardiovascular system. Circ Res 120: , 229–243. |
[446] | Taylor F , Huffman MD , Macedo AF , Moore THM , Burke M , Davey Smith G , Ward K , Ebrahim S ((2013) ) Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev, CD004816. |
[447] | Refolo LM , Pappolla MA , LaFrancois J , Malester B , Schmidt SD , Thomas-Bryant T , Tint GS , Wang R , Mercken M , Petanceska SS , Duff KE ((2001) ) A cholesterol-lowering drug reduces beta-amyloid pathology in a transgenic mouse model of Alzheimer’s disease. Neurobiol Dis 8: , 890–899. |
[448] | Di Paolo G , Kim T-W ((2011) ) Linking lipids to Alzheimer’s disease: Cholesterol and beyond. Nat Rev Neurosci 12: , 284–296. |
[449] | Jick H , Zornberg GL , Jick SS , Seshadri S , Drachman DA ((2000) ) Statins and the risk of dementia. Lancet 356: , 1627–1631. |
[450] | Corrao G , Ibrahim B , Nicotra F , Zambon A , Merlino L , Pasini TS , Catapano AL , Mancia G ((2013) ) Long-term use of statins reduces the risk of hospitalization for dementia. Atherosclerosis 230: , 171–176. |
[451] | Chang C-F , Liou Y-S , Lin T-K , Ma S , Hu Y-R , Chen H-Y , Jong G-P ((2019) ) High exposure to statins decrease the risk of new-onset dementia: A nationwide population-based longitudinal cohort study. Medicine (Baltimore) 98: , e16931. |
[452] | Bettermann K , Arnold AM , Williamson J , Rapp S , Sink K , Toole JF , Carlson MC , Yasar S , Dekosky S , Burke GL ((2012) ) Statins, risk of dementia, and cognitive function: Secondary analysis of the ginkgo evaluation of memory study. J Stroke Cerebrovasc Dis 21: , 436–444. |
[453] | Zhang X , Wen J , Zhang Z ((2018) ) Statins use and risk of dementia: A dose-response meta analysis. Medicine (Baltimore) 97: , e11304. |
[454] | Poly TN , Islam MM , Walther BA , Yang H-C , Wu C-C , Lin M-C , Li Y-C ((2020) ) Association between use of statin and risk of dementia: A meta-analysis of observational studies. Neuroepidemiology 54: , 214–226. |
[455] | McGuinness B , Craig D , Bullock R , Passmore P ((2016) ) Statins for the prevention of dementia. Cochrane Database Syst Rev, CD003160. |
[456] | Rea TD , Breitner JC , Psaty BM , Fitzpatrick AL , Lopez OL , Newman AB , Hazzard WR , Zandi PP , Burke GL , Lyketsos CG , Bernick C , Kuller LH ((2005) ) Statin use and the risk of incident dementia: The Cardiovascular Health Study. Arch Neurol 62: , 1047–1051. |
[457] | Samaras K , Makkar SR , Crawford JD , Kochan NA , Slavin MJ , Wen W , Trollor JN , Brodaty H , Sachdev PS ((2019) ) Effects of statins on memory, cognition, and brain volume in the elderly. J Am Coll Cardiol 74: , 2554–2568. |
[458] | Ramanan VK , Przybelski SA , Graff-Radford J , Castillo AM , Lowe VJ , Mielke MM , Roberts RO , Reid RI , Knopman DS , Jack CR , Petersen RC , Vemuri P ((2018) ) Statins and brain health: Alzheimer’s disease and cerebrovascular disease biomarkers in older adults. J Alzheimers Dis 65: , 1345–1352. |
[459] | Roy S , Weinstock JL , Ishino AS , Benites JF , Pop SR , Perez CD , Gumbs EA , Rosenbaum JA , Roccato MK , Shah H , Contino G , Hunter K ((2017) ) Association of cognitive impairment in patients on 3-hydroxy-3-methyl-glutaryl-CoA reductase inhibitors. J Clin Med Res 9: , 638–649. |
[460] | D’Cunha NM , Georgousopoulou EN , Dadigamuwage L , Kellett J , Panagiotakos DB , Thomas J , McKune AJ , Mellor DD , Naumovski N ((2018) ) Effect of long-term nutraceutical and dietary supplement use on cognition in the elderly: A 10-year systematic review of randomised controlled trials. Br J Nutr 119: , 280–298. |
[461] | Butler M , Nelson VA , Davila H , Ratner E , Fink HA , Hemmy LS , McCarten JR , Barclay TR , Brasure M , Kane RL ((2018) ) Over-the-counter supplement interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: A systematic review. Ann Intern Med 168: , 52–62. |
[462] | Crawford C , Boyd C , Avula B , Wang Y-H , Khan IA , Deuster PA ((2020) ) A public health issue: Dietary supplements promoted for brain health and cognitive performance. J Altern Complement Med 26: , 265–272. |
[463] | Santini A , Cammarata SM , Capone G , Ianaro A , Tenore GC , Pani L , Novellino E ((2018) ) Nutraceuticals: Opening the debate for a regulatory framework. Br J Clin Pharmacol 84: , 659–672. |
[464] | Alzheimer’s Association, Alternative Treatments, https://www.alz.org/alzheimers-dementia/treatments/alternative-treatments, Accessed on 18 October 2020. |
[465] | Hellmuth J , Rabinovici GD , Miller BL ((2019) ) The rise of pseudomedicine for dementia and brain health. JAMA 321: , 543. |
[466] | Rehm J , Hasan OSM , Black SE , Shield KD , Schwarzinger M ((2019) ) Alcohol use and dementia: A systematic scoping review. Alzheimers Res Ther 11: , 1. |
[467] | Ilomaki J , Jokanovic N , Tan ECK , Lonnroos E ((2015) ) Alcohol consumption, dementia and cognitive decline: An overview of systematic reviews. Curr Clin Pharmacol 10: , 204–212. |
[468] | Sabia S , Fayosse A , Dumurgier J , Dugravot A , Akbaraly T , Britton A , Kivimäki M , Singh-Manoux A ((2018) ) Alcohol consumption and risk of dementia: 23 year follow-up of Whitehall II cohort study. BMJ 362: , k2927. |
[469] | Schwarzinger M , Pollock BG , Hasan OSM , Dufouil C , Rehm J , QalyDays Study Group ((2018) ) Contribution of alcohol use disorders to the burden of dementia in France 2008-13: A nationwide retrospective cohort study. Lancet Public Health 3: , e124–e132. |
[470] | Hersi M , Irvine B , Gupta P , Gomes J , Birkett N , Krewski D ((2017) ) Risk factors associated with the onset and progression of Alzheimer’s disease: A systematic review of the evidence. Neurotoxicology 61: , 143–187. |
[471] | Sabia S , Nabi H , Kivimaki M , Shipley MJ , Marmot MG , Singh-Manoux A ((2009) ) Health behaviors from early to late midlife as predictors of cognitive function: The Whitehall II study. Am J Epidemiol 170: , 428–437. |
[472] | National Institute on Alcohol Abuse and Alcoholism (NIAAA), Alcohol Facts and Statistics, https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/alcohol-facts-and-statistics, Last updated 25 April 2019, Accessed on 25 September 2020. |
[473] | Harper C ((2009) ) The neuropathology of alcohol-related brain damage. Alcohol Alcohol 44: , 136–140. |
[474] | Venkataraman A , Kalk N , Sewell G , Ritchie CW , Lingford-Hughes A ((2017) ) Alcohol and Alzheimer’s disease—does alcohol dependence contribute to beta-amyloid deposition, neuroinflammation and neurodegeneration in Alzheimer’s disease? Alcohol Alcohol 52: , 151–158. |
[475] | Wei J , Qin L , Fu Y , Dai Y , Wen Y , Xu S ((2019) ) Long-term consumption of alcohol exacerbates neural lesions by destroying the functional integrity of the blood-brain barrier. Drug Chem Toxicol, doi: 10.1080/01480545.2019.1681444 |
[476] | Huang D , Yu M , Yang S , Lou D , Zhou W , Zheng L , Wang Z , Cai F , Zhou W , Li T , Song W ((2018) ) Ethanol alters APP processing and aggravates Alzheimer-associated phenotypes. Mol Neurobiol 55: , 5006–5018. |
[477] | Bell S , Daskalopoulou M , Rapsomaniki E , George J , Britton A , Bobak M , Casas JP , Dale CE , Denaxas S , Shah AD , Hemingway H ((2017) ) Association between clinically recorded alcohol consumption and initial presentation of 12 cardiovascular diseases: Population based cohort study using linked health records. BMJ 356: , j909. |
[478] | Mukamal KJ , Chen CM , Rao SR , Breslow RA ((2010) ) Alcohol consumption and cardiovascular mortality among U.S. adults, 1987 to 2002. J Am Coll Cardiol 55: , 1328–1335. |
[479] | Ridley NJ , Draper B , Withall A ((2013) ) Alcohol-related dementia: An update of the evidence. Alzheimers Res Ther 5: , 3. |
[480] | O’Keefe JH , Bhatti SK , Bajwa A , DiNicolantonio JJ , Lavie CJ ((2014) ) Alcohol and cardiovascular health: The dose makes the poison... or the remedy. Mayo Clin Proc 89: , 382–393. |
[481] | Brust JCM ((2010) ) Ethanol and cognition: Indirect effects, neurotoxicity and neuroprotection: A review. Int J Environ Res Public Health 7: , 1540–1557. |
[482] | Koch M , Fitzpatrick AL , Rapp SR , Nahin RL , Williamson JD , Lopez OL , DeKosky ST , Kuller LH , Mackey RH , Mukamal KJ , Jensen MK , Sink KM ((2019) ) Alcohol consumption and risk of dementia and cognitive decline among older adults with or without mild cognitive impairment. JAMA Netw Open 2: , e1910319. |
[483] | Anstey KJ , Mack HA , Cherbuin N ((2009) ) Alcohol consumption as a risk factor for dementia and cognitive decline: Meta-analysis of prospective studies. Am J Geriatr Psychiatry 17: , 542–555. |
[484] | U.S. Centers for Disease Control and Prevention, Traumatic Brain Injury & Concussion, https://www.cdc.gov/traumaticbraininjury/index.html, Last updated 28 August 2020, Accessed on 14 November 2020. |
[485] | Fann JR , Ribe AR , Pedersen HS , Fenger-Grøn M , Christensen J , Benros ME , Vestergaard M ((2018) ) Long-term risk of dementia among people with traumatic brain injury in Denmark: A population-based observational cohort study. Lancet Psychiatry 5: , 424–431. |
[486] | Nordström A , Nordström P ((2018) ) Traumatic brain injury and the risk of dementia diagnosis: A nationwide cohort study. PLOS Med 15: , e1002496. |
[487] | Barnes DE , Byers AL , Gardner RC , Seal KH , Boscardin WJ , Yaffe K ((2018) ) Association of mild traumatic brain injury with and without loss of consciousness with dementia in US military veterans. JAMA Neurol 75: , 1055–1061. |
[488] | Abner EL , Nelson PT , Schmitt FA , Browning SR , Fardo DW , Wan L , Jicha GA , Cooper GE , Smith CD , Caban-Holt AM , Van Eldik LJ , Kryscio RJ ((2014) ) Self-reported head injury and risk of late-life impairment and AD pathology in an AD center cohort. Dement Geriatr Cogn Disord 37: , 294–306. |
[489] | Li Y , Li Y , Li X , Zhang S , Zhao J , Zhu X , Tian G ((2017) ) Head injury as a risk factor for dementia and Alzheimer’s disease: A systematic review and meta-analysis of 32 observational studies. PLoS One 12: , e0169650. |
[490] | LoBue C , Woon FL , Rossetti HC , Hynan LS , Hart J , Cullum CM ((2018) ) Traumatic brain injury history and progression from mild cognitive impairment to Alzheimer disease. Neuropsychology 32: , 401–409. |
[491] | Schaffert J , LoBue C , White CL , Chiang H-S , Didehbani N , Lacritz L , Rossetti H , Dieppa M , Hart J , Cullum CM ((2018) ) Traumatic brain injury history is associated with an earlier age of dementia onset in autopsy-confirmed Alzheimer’s disease. Neuropsychology 32: , 410–416. |
[492] | Filley CM , Kelly JP ((2018) ) White matter and cognition in traumatic brain injury. J Alzheimers Dis 65: , 345–362. |
[493] | Kokiko-Cochran ON , Godbout JP ((2018) ) The inflammatory continuum of traumatic brain injury and Alzheimer’s disease. Front Immunol 9: , 672. |
[494] | Johnson VE , Stewart JE , Begbie FD , Trojanowski JQ , Smith DH , Stewart W ((2013) ) Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain 136: , 28–42. |
[495] | Ramlackhansingh AF , Brooks DJ , Greenwood RJ , Bose SK , Turkheimer FE , Kinnunen KM , Gentleman S , Heckemann RA , Gunanayagam K , Gelosa G , Sharp DJ ((2011) ) Inflammation after trauma: Microglial activation and traumatic brain injury. Ann Neurol 70: , 374–383. |
[496] | Acosta SA , Tajiri N , Sanberg PR , Kaneko Y , Borlongan CV ((2017) ) Increased amyloid precursor protein and tau expression manifests as key secondary cell death in chronic traumatic brain injury. J Cell Physiol 232: , 665–677. |
[497] | Gao H , Han Z , Bai R , Huang S , Ge X , Chen F , Lei P ((2017) ) The accumulation of brain injury leads to severe neuropathological and neurobehavioral changes after repetitive mild traumatic brain injury. Brain Res 1657: , 1–8. |
[498] | Washington PM , Morffy N , Parsadanian M , Zapple DN , Burns MP ((2014) ) Experimental traumatic brain injury induces rapid aggregation and oligomerization of amyloid-beta in an Alzheimer’s disease mouse model. J Neurotrauma 31: , 125–134. |
[499] | Shishido H , Ueno M , Sato K , Matsumura M , Toyota Y , Kirino Y , Tamiya T , Kawai N , Kishimoto Y ((2019) ) Traumatic brain injury by weight-drop method causes transient amyloid-β deposition and acute cognitive deficits in mice. Behav Neurol 2019: , 3248519. |
[500] | Johnson VE , Stewart W , Smith DH ((2012) ) Widespread τ and amyloid-β pathology many years after a single traumatic brain injury in humans. Brain Pathol 22: , 142–149. |
[501] | Mayeux R , Ottman R , Maestre G , Ngai C , Tang MX , Ginsberg H , Chun M , Tycko B , Shelanski M ((1995) ) Synergistic effects of traumatic head injury and apolipoprotein-epsilon 4 in patients with Alzheimer’s disease. Neurology 45: , 555–557. |
[502] | Tripodis Y , Alosco ML , Zirogiannis N , Gavett BE , Chaisson C , Martin B , McClean MD , Mez J , Kowall N , Stern RA ((2017) ) The effect of traumatic brain injury history with loss of consciousness on rate of cognitive decline among older adults with normal cognition and Alzheimer’s disease dementia. J Alzheimers Dis 59: , 251–263. |
[503] | Sugarman MA , McKee AC , Stein TD , Tripodis Y , Besser LM , Martin B , Palmisano JN , Steinberg EG , O’Connor MK , Au R , McClean M , Killiany R , Mez J , Weiner MW , Kowall NW , Stern RA , Alosco ML ((2019) ) Failure to detect an association between self-reported traumatic brain injury and Alzheimer’s disease neuropathology and dementia. Alzheimers Dement 15: , 686–698. |
[504] | Peters R , Ee N , Peters J , Booth A , Mudway I , Anstey KJ ((2019) ) Air pollution and dementia: A systematic review. J Alzheimers Dis 70: , S145–S163. |
[505] | Lai C-Y , Huang Y-W , Tseng C-H , Lin C-L , Sung F-C , Kao C-H ((2016) ) Patients with carbon monoxide poisoning and subsequent dementia: A population-based cohort study. Medicine (Baltimore) 95: , e2418. |
[506] | Wong C-S , Lin Y-C , Hong L-Y , Chen T-T , Ma H-P , Hsu Y-H , Tsai S-H , Lin Y-F , Wu M-Y ((2016) ) Increased long-term risk of dementia in patients with carbon monoxide poisoning: A population-based study. Medicine (Baltimore) 95: , e2549. |
[507] | Chen H , Kwong JC , Copes R , Tu K , Villeneuve PJ , van Donkelaar A , Hystad P , Martin RV , Murray BJ , Jessiman B , Wilton AS , Kopp A , Burnett RT ((2017) ) Living near major roads and the incidence of dementia, Parkinson’s disease, and multiple sclerosis: A population-based cohort study. Lancet 389: , 718–726. |
[508] | Oudin A , Forsberg B , Adolfsson AN , Lind N , Modig L , Nordin M , Nordin S , Adolfsson R , Nilsson L-G ((2016) ) Traffic-related air pollution and dementia incidence in northern Sweden: A longitudinal study. Environ Health Perspect 124: , 306–312. |
[509] | Carey IM , Anderson HR , Atkinson RW , Beevers SD , Cook DG , Strachan DP , Dajnak D , Gulliver J , Kelly FJ ((2018) ) Are noise and air pollution related to the incidence of dementia? A cohort study in London, England. BMJ Open 8: , e022404. |
[510] | Chang K-H , Chang M-Y , Muo C-H , Wu T-N , Chen C-Y , Kao C-H ((2014) ) Increased risk of dementia in patients exposed to nitrogen dioxide and carbon monoxide: A population-based retrospective cohort study. PloS One 9: , e103078. |
[511] | Ailshire JA , Clarke P ((2015) ) Fine particulate matter air pollution and cognitive function among U.S. older adults. J Gerontol B Psychol Sci Soc Sci 70: , 322–328. |
[512] | Gatto NM , Henderson VW , Hodis HN , St John JA , Lurmann F , Chen J-C , Mack WJ ((2014) ) Components of air pollution and cognitive function in middle-aged and older adults in Los Angeles. Neurotoxicology 40: , 1–7. |
[513] | Schikowski T , Vossoughi M , Vierkötter A , Schulte T , Teichert T , Sugiri D , Fehsel K , Tzivian L , Bae I , Ranft U , Hoffmann B , Probst-Hensch N , Herder C , Krämer U , Luckhaus C ((2015) ) Association of air pollution with cognitive functions and its modification by APOE gene variants in elderly women. Environ Res 142: , 10–16. |
[514] | Kulick ER , Wellenius GA , Boehme AK , Joyce NR , Schupf N , Kaufman JD , Mayeux R , Sacco RL , Manly JJ , Elkind MSV ((2020) ) Long-term exposure to air pollution and trajectories of cognitive decline among older adults. Neurology 94: , e1782–e1792. |
[515] | Chin-Chan M , Navarro-Yepes J , Quintanilla-Vega B ((2015) ) Environmental pollutants as risk factors for neurodegenerative disorders: Alzheimer and Parkinson diseases. Front Cell Neurosci 9: , 124. |
[516] | Levesque S , Surace MJ , McDonald J , Block ML ((2011) ) Air pollution & the brain: Subchronic diesel exhaust exosure causes neuroinflammation and elevates early markers of neurodegenerative disease. J Neuroinflammation 8: , 105. |
[517] | Kim SH , Knight EM , Saunders EL , Cuevas AK , Popovech M , Chen L-C , Gandy S ((2012) ) Rapid doubling of Alzheimer’s amyloid-β40 and 42 levels in brains of mice exposed to a nickel nanoparticle model of air pollution. F1000Res 1: , 70. |
[518] | Rivas-Arancibia S , Hernández-Zimbrón LF , Rodríguez-Martínez E , Borgonio-Pérez G , Velumani V , Durán-Bedolla J ((2013) ) Chronic exposure to low doses of ozone produces a state of oxidative stress and blood-brain barrier damage in the hippocampus of rat. Adv Biosci Biotechnol 4: , 24–29. |
[519] | Calderón-Garcidueñas L , Reed W , Maronpot RR , Henríquez-Roldán C , Delgado-Chavez R , Calderón-Garcidueñas A , Dragustinovis I , Franco-Lira M , Aragón-Flores M , Solt AC , Altenburg M , Torres-Jardón R , Swenberg JA ((2004) ) Brain inflammation and Alzheimer’s-like pathology in individuals exposed to severe air pollution. Toxicol Pathol 32: , 650–658. |
[520] | Calderón-Garcidueñas L , Solt AC , Henríquez-Roldán C , Torres-Jardón R , Nuse B , Herritt L , Villarreal-Calderón R , Osnaya N , Stone I , García R , Brooks DM , González-Maciel A , Reynoso-Robles R , Delgado-Chávez R , Reed W ((2008) ) Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid beta-42 and alpha-synuclein in children and young adults. Toxicol Pathol 36: , 289–310. |
[521] | Moulton PV , Yang W ((2012) ) Air pollution, oxidative stress, and Alzheimer’s disease. J Environ Public Health 2012: , 472751. |
[522] | Cesaroni G , Forastiere F , Stafoggia M , Andersen ZJ , Badaloni C , Beelen R , Caracciolo B , de Faire U , Erbel R , Eriksen KT , Fratiglioni L , Galassi C , Hampel R , Heier M , Hennig F , Hilding A , Hoffmann B , Houthuijs D , Jöckel K-H , Korek M , Lanki T , Leander K , Magnusson PKE , Migliore E , Ostenson C-G , Overvad K , Pedersen NL , J JP , Penell J , Pershagen G , Pyko A , Raaschou-Nielsen O , Ranzi A , Ricceri F , Sacerdote C , Salomaa V , Swart W , Turunen AW , Vineis P , Weinmayr G , Wolf K , de Hoogh K , Hoek G , Brunekreef B , Peters A ((2014) ) Long term exposure to ambient air pollution and incidence of acute coronary events: Prospective cohort study and meta-analysis in 11 European cohorts from the ESCAPE Project. BMJ 348: , f7412. |
[523] | Hoek G , Krishnan RM , Beelen R , Peters A , Ostro B , Brunekreef B , Kaufman JD ((2013) ) Long-term air pollution exposure and cardio- respiratory mortality: A review. Environ Health 12: , 43. |
[524] | Shah ASV , Lee KK , McAllister DA , Hunter A , Nair H , Whiteley W , Langrish JP , Newby DE , Mills NL ((2015) ) Short term exposure to air pollution and stroke: Systematic review and meta-analysis. BMJ 350: , h1295. |
[525] | Hajat A , Hsia C , O’Neill MS ((2015) ) Socioeconomic disparities and air pollution exposure: A global review. Curr Environ Health Rep 2: , 440–450. |
[526] | Karp A , Paillard-Borg S , Wang H-X , Silverstein M , Winblad B , Fratiglioni L ((2006) ) Mental, physical and social components in leisure activities equally contribute to decrease dementia risk. Dement Geriatr Cogn Disord 21: , 65–73. |
[527] | Anastasiou CA , Yannakoulia M , Kontogianni MD , Kosmidis MH , Mamalaki E , Dardiotis E , Hadjigeorgiou G , Sakka P , Tsapanou A , Lykou A , Scarmeas N ((2018) ) Mediterranean lifestyle in relation to cognitive health: Results from the HELIAD Study. Nutrients 10: , 1557. |
[528] | Dhana K , Evans DA , Rajan KB , Bennett DA , Morris MC ((2020) ) Healthy lifestyle and the risk of Alzheimer dementia: Findings from 2 longitudinal studies. Neurology 95: , e374–e383. |
[529] | Anderson-Hanley C , Barcelos NM , Zimmerman EA , Gillen RW , Dunnam M , Cohen BD , Yerokhin V , Miller KE , Hayes DJ , Arciero PJ , Maloney M , Kramer AF ((2018) ) The Aerobic and Cognitive Exercise Study (ACES) for community-dwelling older adults with or at-risk for mild cognitive impairment (MCI): Neuropsychological, neurobiological and neuroimaging outcomes of a randomized clinical trial. Front Aging Neurosci 10: , 76. |
[530] | Karssemeijer EGA , Aaronson JA , Bossers WJR , Donders R , Olde Rikkert MGM , Kessels RPC ((2019) ) The quest for synergy between physical exercise and cognitive stimulation via exergaming in people with dementia: A randomized controlled trial. Alzheimers Res Ther 11: , 3. |
[531] | Ngandu T , Lehtisalo J , Solomon A , Levälahti E , Ahtiluoto S , Antikainen R , Bäckman L , Hänninen T , Jula A , Laatikainen T , Lindström J , Mangialasche F , Paajanen T , Pajala S , Peltonen M , Rauramaa R , Stigsdotter-Neely A , Strandberg T , Tuomilehto J , Soininen H , Kivipelto M ((2015) ) A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. Lancet 385: , 2255–2263. |
[532] | Isaacson RS , Hristov H , Saif N , Hackett K , Hendrix S , Melendez J , Safdieh J , Fink M , Thambisetty M , Sadek G , Bellara S , Lee P , Berkowitz C , Rahman A , Meléndez-Cabrero J , Caesar E , Cohen R , Lu P-L , Dickson SP , Hwang MJ , Scheyer O , Mureb M , Schelke MW , Niotis K , Greer CE , Attia P , Mosconi L , Krikorian R ((2019) ) Individualized clinical management of patients at risk for Alzheimer’s dementia. Alzheimers Dement 15: , 1588–1602. |
[533] | Andrieu S , Guyonnet S , Coley N , Cantet C , Bonnefoy M , Bordes S , Bories L , Cufi M-N , Dantoine T , Dartigues J-F , Desclaux F , Gabelle A , Gasnier Y , Pesce A , Sudres K , Touchon J , Robert P , Rouaud O , Legrand P , Payoux P , Caubere J-P , Weiner M , Carrié I , Ousset P-J , Vellas B , MAPT Study Group ((2017) ) Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): A randomised, placebo-controlled trial. Lancet Neurol 16: , 377–389. |
[534] | Yu J-T , Xu W , Tan C-C , Andrieu S , Suckling J , Evangelou E , Pan A , Zhang C , Jia J , Feng L , Kua E-H , Wang Y-J , Wang H-F , Tan M-S , Li J-Q , Hou X-H , Wan Y , Tan L , Mok V , Tan L , Dong Q , Touchon J , Gauthier S , Aisen PS , Vellas B ((2020) ) Evidence-based prevention of Alzheimer’s disease: Systematic review and meta-analysis of 243 observational prospective studies and 153 randomised controlled trials. J Neurol Neurosurg Psychiatry 91: , 1201–1209. |
[535] | WWFINGERS, US-POINTER, http://wwfingers.com/us-finger/, Accessed on 23 July 2020. |
[536] | Rosenberg A , Mangialasche F , Ngandu T , Solomon A , Kivipelto M ((2020) ) Multidomain interventions to prevent cognitive impairment, Alzheimer’s disease, and dementia: From FINGER to World-Wide FINGERS. J Prev Alzheimers Dis 7: , 29–36. |
[537] | Michie S , Richardson M , Johnston M , Abraham C , Francis J , Hardeman W , Eccles MP , Cane J , Wood CE ((2013) ) The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: Building an international consensus for the reporting of behavior change interventions. Ann Behav Med 46: , 81–95. |
[538] | Moyers TB ((2014) ) The relationship in motivational interviewing. Psychotherapy 51: , 358–363. |
[539] | Rollnick S , Mason P , Butler C ((1999) ), Health behavior change: A guide for practitioners, Churchill Livingstone, Edinburgh. |
[540] | Miller W , Rollnick S ((2013) ) Motivational interviewing: Helping people change, Guilford Press, New York, NY. |
[541] | Lindson N , Thompson TP , Ferrey A , Lambert JD , Aveyard P ((2019) ) Motivational interviewing for smoking cessation. Cochrane Database Syst Rev 7: , CD006936. |
[542] | D’Onofrio G , Pantalon MV , Degutis LC , Fiellin DA , O’Connor PG ((2005) ) Development and implementation of an emergency practitioner-performed brief intervention for hazardous and harmful drinkers in the emergency department. Acad Emerg Med 12: , 249–256. |
[543] | O’Halloran PD , Blackstock F , Shields N , Holland A , Iles R , Kingsley M , Bernhardt J , Lannin N , Morris ME , Taylor NF ((2014) ) Motivational interviewing to increase physical activity in people with chronic health conditions: A systematic review and meta-analysis. Clin Rehabil 28: , 1159–1171. |
[544] | Olson R , Wipfli B , Thompson SV , Elliot DL , Anger WK , Bodner T , Hammer LB , Perrin NA ((2016) ) Weight control intervention for truck drivers: The SHIFT Randomized Controlled Trial, United States. Am J Public Health 106: , 1698–1706. |
[545] | Barnes RD , Barber J ((2017) ) Preliminary examination of metabolic syndrome response to motivational interviewing for weight loss as compared to an attentional control and usual care in primary care for individuals with and without binge-eating disorder. Eat Behav 26: , 108–113. |
[546] | Bóveda-Fontán J , Barragán-Brun N , Campiñez-Navarro M , Pérula-de Torres LÁ , Bosch-Fontcuberta JM , Martín-Álvarez R , Arbonies-Ortiz JC , Novo-Rodríguez JM , Criado-Larumbe M , Fernández-García JA , Martín-Rioboó E , Collaborative Group Estudio Dislip-EM ((2015) ) Effectiveness of motivational interviewing in patients with dyslipidemia: A randomized cluster trial. BMC Fam Pract 16: , 151. |
[547] | Hardcastle SJ , Taylor AH , Bailey MP , Harley RA , Hagger MS ((2013) ) Effectiveness of a motivational interviewing intervention on weight loss, physical activity and cardiovascular disease risk factors: A randomised controlled trial with a 12-month post-intervention follow-up. Int J Behav Nutr Phys Act 10: , 40. |
[548] | Lundahl B , Moleni T , Burke BL , Butters R , Tollefson D , Butler C , Rollnick S ((2013) ) Motivational interviewing in medical care settings: A systematic review and meta-analysis of randomized controlled trials. Patient Educ Couns 93: , 157–168. |
[549] | Rovner BW , Casten RJ , Hegel MT , Leiby B ((2018) ) Preventing cognitive decline in black individuals with mild cognitive impairment: A randomized clinical trial. JAMA Neurol 75: , 1487–1493. |
[550] | Kanter JW , Manos RC , Bowe WM , Baruch DE , Busch AM , Rusch LC ((2010) ) What is behavioral activation? A review of the empirical literature. Clin Psychol Rev 30: , 608–620. |
[551] | Cuijpers P , van Straten A , Warmerdam L ((2007) ) Behavioral activation treatments of depression: A meta-analysis. Clin Psychol Rev 27: , 318–326. |
[552] | Kahneman D ((2011) ) Thinking Fast and Slow, Farrar, Straus and Giroux, New York, NY. |
[553] | Thorgeirsson T , Kawachi I ((2013) ) Behavioral economics: Merging psychology and economics for lifestyle interventions. Am J Prev Med 44: , 185–189. |
[554] | Kawachi I ((2015) ) Why do behavior interventions fail? Insights from behavioral economics. J Nutr Sci Vitaminol (Tokyo) 61: (Suppl), S210. |
[555] | Shuval K , Leonard T , Drope J , Katz DL , Patel AV , Maitin-Shepard M , Amir O , Grinstein A ((2017) ) Physical activity counseling in primary care: Insights from public health and behavioral economics. CA Cancer J Clin 67: , 233–244. |
[556] | Roberto CA , Kawachi I ((2014) ) Use of psychology and behavioral economics to promote healthy eating. Am J Prev Med 47: , 832–837. |
[557] | Stevens J ((2015) ) Behavioral economics strategies for promoting adherence to sleep interventions. Sleep Med Rev 23: , 20–27. |
[558] | Chang LL , DeVore AD , Granger BB , Eapen ZJ , Ariely D , Hernandez AF ((2017) ) Leveraging behavioral economics to improve heart failure care and outcomes. Circulation 136: , 765–772. |
[559] | Schwartz HEM , Bay CP , McFeeley BM , Krivanek TJ , Daffner KR , Gale SA ((2019) ) The Brain Health Champion study: Health coaching changes behaviors in patients with cognitive impairment. Alzheimers Dement (N Y) 5: , 771–779. |




