Expression and Localization of AβPP in SH-SY5Y Cells Depends on Differentiation State
Abstract
Neuroblastoma cell line SH-SY5Y, due to its capacity to differentiate into neurons, easy handling, and low cost, is a common experimental model to study molecular events leading to Alzheimer’s disease (AD). However, it is prevalently used in its undifferentiated state, which does not resemble neurons affected by the disease. Here, we show that the expression and localization of amyloid-β protein precursor (AβPP), one of the key molecules involved in AD pathogenesis, is dramatically altered in SH-SY5Y cells fully differentiated by combined treatment with retinoic acid and BDNF. We show that insufficient differentiation of SH-SY5Y cells results in AβPP mislocalization.
INTRODUCTION
Alzheimer’s disease (AD) is the most common form of dementia characterized by memory loss and cognitive decline [1]. Despite intensive research over the last few decades, the exact molecular mechanism describing the development of AD is not sufficiently clarified. Thus, there is an urgent need for suitable in vitro AD models. For characterization of fundamental molecular events and processes involved in AD development, a simple cell-based system is required. Inducible pluripotent human stem cells (iPSC), which are subsequently differentiable into neurons, represent physiologically highly relevant model, but their wider use is limited by uncertain differentiation efficiency and higher experimental costs [2]. Therefore, well-established cell lines are often selected by many researchers. Among those, chromosomally stable human neuroblastoma SH-SY5Y is probably the most common [3].
Undifferentiated SH-SY5Y cells are non-polarized, rapidly proliferative and do not express mature neuronal markers. These neuroblastoma cells can be differentiated into mature neurons through several established protocols; e.g., serum deprivation, treatment with retinoic acid (RA), 12-O-tetradecanoylphorbol-13-acetate (TPA), dibutyryl cyclic AMP (dbcAMP), or specific neurotrophins (brain-derived neurotrophic factor (BDNF) or nerve growth factor (NGF)) [4–10]. During SH-SY5Y differentiation into functional neurons, cells adopt a neuronal morphology characterized by a reduction of the cell body size and extension of branched neurites that are interconnected into functional networks [6, 11]. Full differentiation of SH-SY5Y cells alter their properties and have an impact on survival and stress signaling pathways [12]. Significant changes in gene and miRNA expression have been reported for RA treatment alone [13]. RA differentiation followed by BDNF (RA/BDNF) treatment results in even stronger changes in expression of many genes, including those encoding neuronal markers such as tau, synaptostatin, and synaptophysin [6, 11–14]. Even though the presence of amyloid plaques composed of amyloid-β (Aβ) peptide derived from amyloid-β protein precursor (AβPP) is one of the main hallmarks of AD [15–17], the impact of neuronal differentiation (RA/BDNF) on AβPP distribution and processing has not been studied.
In 2019, only 2% of published works used fully differentiated SH-SY5Y cells (RA + BDNF); in 76% of cases, the undifferentiated cells were used. This practice did not change with time [13]. The applied method of differentiation may strongly affect AβPP expression, processing and its localization, and therefore influence the experimental outcome. We have thus investigated the effect of differentiation on AβPP expression and distribution and tested functionally associated proteins. We demonstrate that undifferentiated cells show important distinctions compared to fully differentiated, neuron-like SH-SY5Y cells.
METHODS
Cell culture and treatment
SH-SY5Y cells (ECACC, 94030304) were grown in high glucose Dulbecco’s Modified Eagle’s medium (DMEM; Sigma-Aldrich, D6429) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Gibco, 10270-106) in a humidified incubator (Eppendorf) at 37°C and 5% CO2. Cells were routinely split 3 times a week at 80–90% confluence. All SH-SY5Y cells used in this study were at a low passage number (< 15) and tested for mycoplasma contamination. Cellular differentiation was induced as described by Hromadkova et al., 2020 [11] with minor modifications. Briefly, 5×104 cells/cm2 in DMEM with 5% FBS were grown on coverslips or in treated T25 flasks. After three days, the media was replaced with DMEM containing 2% FBS and 10μM all-trans-retinoic acid (RA; Sigma-Aldrich, R2625) and cultured for another 5 days. For the full differentiation, cells were washed thoroughly with PBS, the medium was replaced with Neurobasal medium (Thermo Fisher Scientific, 21103049) supplemented with N-2 supplement (Thermo Fisher Scientific, 17502001) and 50 ng/ml BDNF (Thermo Fisher Scientific, PHC7074) and the cells were cultured for another 7–9 days. We refer to the three phases of differentiation: 1) undifferentiated cells (UN); 2) cells differentiated with retinoic acid for 5 or 6 days (RA); and 3) followed by BDNF treatment (RA/BDNF).
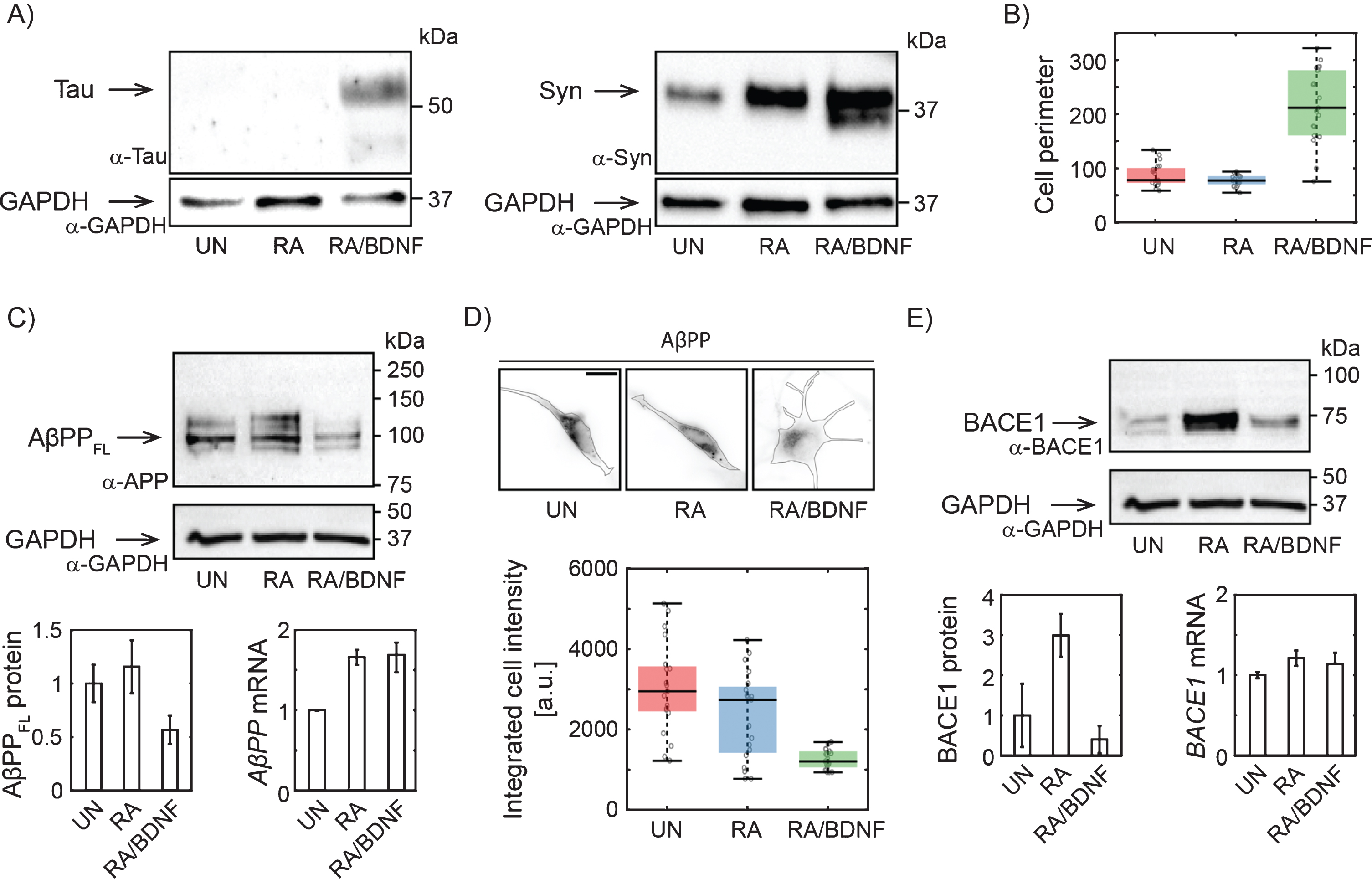
Fig. 1
Changes in AβPP expression in SH-SY5Y cells upon differentiation. A) Western blots showing increased expression of Tau upon full differentiation with RA and BDNF and Syn (synaptophysin) upon RA treatment. Antibodies: anti-tau, AXON Neuroscience, DC25; Anti-Syn, Abcam, ab32594; Anti-GAPDH, Merck, ABS16. B) Box-plot of cell perimeter changing with a differentiation state. Cell perimeters were determined using particle analysis plugin in ImageJ. C) Immunoblot showing full lengths AβPP protein levels (antibody to N terminus; aa 66–81: clone 22C11, Merck, MAB348) in SH-SY5Y cells differentiated with RA or RA/BDNF. AβPP mRNA levels were determined in total RNA extract by RT-qPCR. The expression of AβPPFL was normalized to the GAPDH data from 4 (WB), or 3 (RT-qPCR) independent experiments; error bars represent standard error of the mean. D) Representative immunofluorescence images of SH-SY5Y cells treated (or not) with RA or RA/BDNF. The scale bar 10μm. The graph shows integrated signal for AβPP quantified from the images of fixed, immunofluorescently-labeled SH-SY5Y cells (same antibody as in C). Final intensity values were normalized with respect to the total cell area. E) BACE1 protein (antibody: Cell Signaling Technology, D10E5) and mRNA levels at different differentiation states. UN, undifferentiated cells; RA, cells differentiated with retinoic acid; RA/BDNF, cells differentiated with RA and BDNF.
Immunofluorescence
Cells were grown on poly-D-lysine-coated coverslips, rinsed with preheated PBS and fixed with fixation solution (4% paraformaldehyde, Electron Microscopy Sciences, 15714; 2% sucrose, Sigma-Aldrich, S7903; in PBS). After quenching with 50 mM NH4Cl in PBS, cells were washed several times with PBS and then permeabilized with 0.2% Triton X-100 in PBS for 10 min at room temperature (this last step was skipped in the case of membrane AβPP quantification experiments). After blocking with 1% bovine serum albumin in PBS for 30 min at room temperature (RT), cells were incubated with primary antibodies at 4°C overnight. Coverslips were then washed with PBS and incubated with a secondary antibody conjugated to a fluorescent probe in darkness for 1 h at RT. After several washing steps, cells were incubated with 300 nM DAPI in PBS for 5 min.
For colocalization analysis of AβPP with mitochondria, cells were incubated in full growth media containing 50 nM MitoTracker Red FM (Thermo Fisher Scientific, M22425) for 20 min at 37°C and then fixed. For visualization of the Golgi apparatus, cells were transfected with 6μg of the mApple-Golgi-7 (gift from Michael Davidson; Addgene plasmid # 54907) using FuGene HD Transfection Reagent (Promega, E2311).
Cells were imaged on a home-built widefield fluorescence microscope setup (IX71, Olympus) equipped with 100x TIRF objective (UAPON, NA = 1.49, Olympus), 405, 643 nm (both Cube, Coherent), 488, 561 nm (both Sapphire, Coherent) lasers and iXon DU-897 EMCCD camera (Andor). Membrane AβPP was imaged by total internal reflection fluorescence microscopy with 60x TIRF objective (UPLAPO60HR, NA = 1.5, Olympus). System was controlled using a home programmed software based on the LabVIEW interface (version 2011). For further processing images were z-projected by the averaging method in ImageJ [18, 19]. Wavelet-based background subtraction method [20] was used to remove intracellular signal in the case of membrane AβPP quantification.
Western blotting, real time PCR analysis, super-resolution imaging, and table with primer sequences are described in the Supplementary Material.
RESULTS
To determine the efficiency of our differentiation method, we have first monitored the expression of well-established neuronal markers and morphological characteristics of treated SH-SY5Y cells. Tau and Synaptophysin expression levels increased significantly upon combined treatment with RA and BDNF (Fig. 1A), suggesting effective differentiation of SH-SY5Y cells into neuron-like cells. RA/BDNF treatment, but not with RA alone, also dramatically changed cell morphology (Fig. 1B, D). We concluded that treatment with RA followed by BDNF resulted in a differentiated neuronal line.
To verify the hypothesis that a adequate study of AD-related protein distribution and dynamics in neuroblastoma cells requires their effective differentiation, we monitored changes in the expression of full length AβPP (AβPPFL) by combination of quantitative protein and RNA analysis. By immunoblotting with antibody to the N-terminal part, we confirm that RA differentiation of SH-SY5Y increases AβPPFL expression levels (Fig. 1C) [21]. However, fully differentiated RA/BDNF (12-days) cells express a significantly lower level of AβPPFL compared to untreated (UN) or RA-treated cells (Fig. 1C). The observed reduction of intracellular AβPP was confirmed by quantitative fluorescence microscopy of fixed immuno-labeled SH-SY5Y cells (Fig. 1D). The abundance of membrane AβPP was reduced accordingly (data not shown). Interestingly, the decrease in protein expression in fully differentiated cells is not due to the changes in mRNA levels, as revealed by RT-qPCR (Fig. 1C). This indicates that AβPP is rapidly processed by proteases in fully differentiated SH-SY5Y cells. We also quantified the abundance of AβPP proteolytic product Aβ peptide. Immunolabeling of fixed cells was used (Merck, AB5078P) and Aβ abundance in UN, RA, and RA/BDNF cells was determined by fluorescence microscopy. We did not see any significant changes in Aβ levels for different treatment methods (data not shown). Next, we have found that the expression of BACE1 protease involved in amyloidogenic processing of AβPP is increased after RA, but strongly reduced by further BDNF treatment (Fig. 1E). This is in line with the earlier observation that RA-treatment of SH-SY5Y cells followed by BDNF promotes proteolytic processing of AβPP with a preference for non-amyloidogenic cleavage and that higher abundance of sAβPPα inhibits the activity of BACE1 leading to lower levels of Aβ fragments [22].
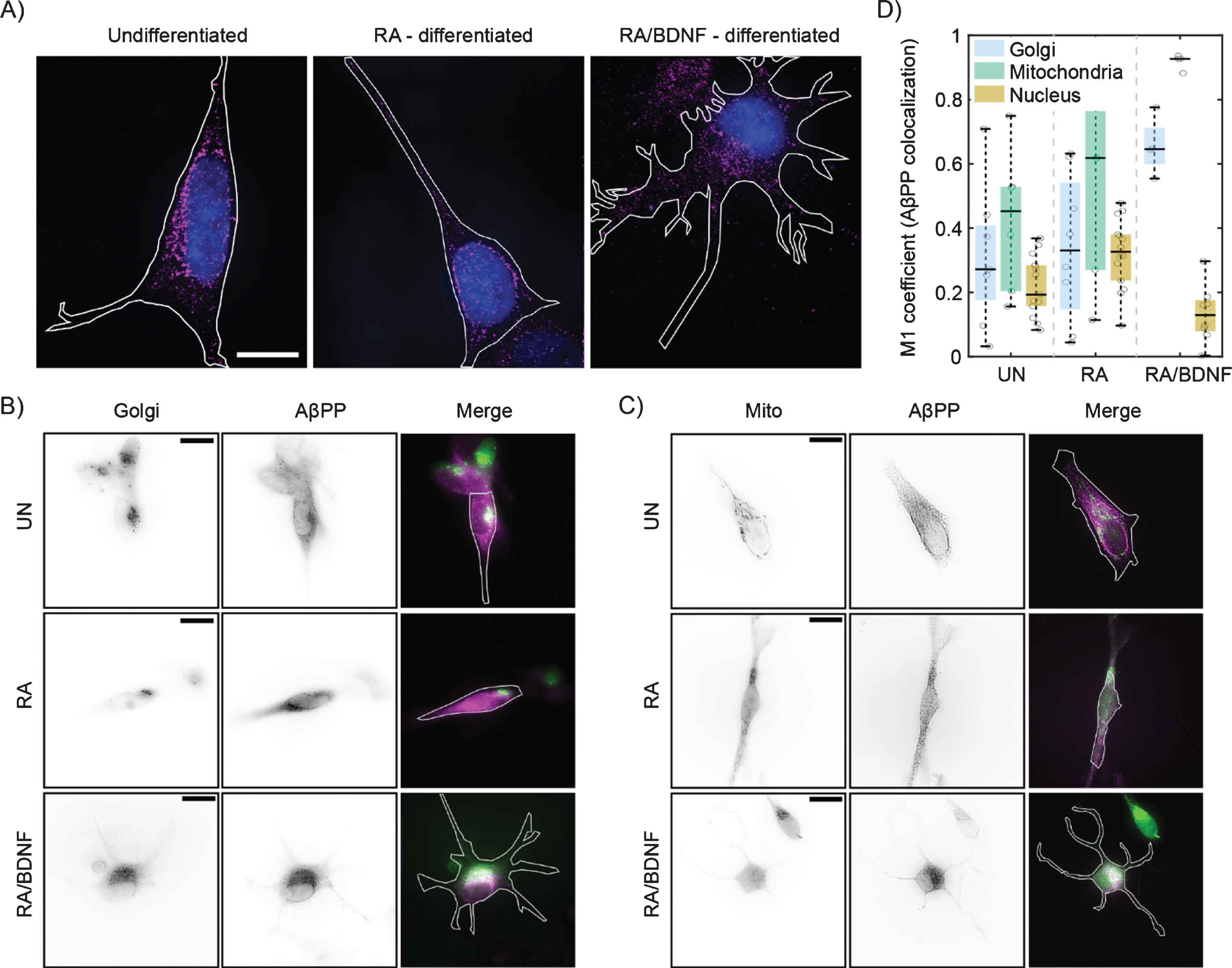
We have further analyzed distribution of AβPP in SH-SY5Y cells at different stages of differentiation using single molecule localization microscopy with spatial resolution bellow 50 nm (Fig. 2A). In UN and RA cells, the fluorescent signal is localized to the perinuclear region, while in RA/BDNF cells the signal is spread more over the cell body. This is in agreement with wide-field imaging data, which exhibit lower resolution (Fig. 1D). Broader distribution indicates altered association of AβPP with intracellular organelles in fully differentiated SH-SY5Y cells. We thus tested colocalization of AβPP with two organelles, where AβPP has been reported previously—the Golgi apparatus and mitochondria [23–25] (Fig. 2B, C). The colocalization values between AβPP and the Golgi apparatus, mitochondria, or nucleus (control) are plotted in Fig. 2D. In UN and RA-differentiated cells, AβPP does not exhibit significant colocalization with the Golgi apparatus or mitochondria markers. On the contrary, AβPP significantly overlaps with both the Golgi apparatus and mitochondria in RA/BDNF-treated cells (Fig. 2B-D). Indeed, mitochondria appear to be the preferred target for AβPP localization in such neuron-like SH-SY5Y cells. Very low overlap values with the nucleus in RA/BDNF-treated cells further support the finding that AβPP does not accumulate in the perinuclear region of fully differentiated SH-SY5Y cells.
Fig. 2
Differentiation affects the distribution of AβPP in SH-SY5Y cells. A) High-resolution AβPP localization was determined in immunolabeled (see Fig. 1), undifferentiated (left), RA differentiated (middle), and RA/BDNF differentiated (right) SH-SY5Y cells using super-resolution method –single molecule localization microscopy. N-terminal part of AβPP –magenta, nucleus –blue, cell contour –white line. The scale bar represents 10μm. B, C) Two-channel, wide-field images of AβPP in fixed SH-SY5Y cells. Green color in merged images corresponds to the Golgi (B)/Mitochondria (C) markers, magenta –N-terminal part of AβPP. The white contour shows the edge of the cell. UN, undifferentiated cells; RA, cells differentiated with retinoic acid; RA/BDNF, cells differentiated with RA and BDNF. Scale bars, 10μm. D) AβPP colocalization with Golgi apparatus, mitochondria and nucleus characterized as the M1 values using JACoP algorithms (for details, see Methods). The values were calculated from at least four independent experiments.
DISCUSSION
The use of SH-SY5Y as a model cell line for AD studies requires detailed understanding of molecular pathways involved in cell function and neurodegeneration. These aspects are expected to be affected by their differentiation state. In this work, we focused on characterization of AβPP in SH-SY5Y cells upon diverse differentiation methods. Previously, various neuronal cell lines (PC12, P19, or N27) were characterized with respect to their intrinsic properties like sensitivity to neurotoxins and oxidative stress or functional parameters such as the presence of active neurotransmitter receptors and ion channels [26–28]. However, differentiation into neuronal phenotype has not been considered in these studies. For SH-SY5Y cells, it has been reported that the method of cultivation, treatment and neuronal induction plays a critical role in the resulting phenotype [29, 30].
RA-based differentiation of SH-SY5Y cells induces TrkB receptor expression. TrkB activation is induced by neurotrophins, like BDNF, and triggers morphological changes towards neuronal phenotype [31]. Our results confirm that stimulation with BDNF after the initial RA-treatment led to a cell population with characteristics of mature neurons. Compared to cells treated with RA alone, fully differentiated cells (RA/BDNF) formed extended neurites, which were accompanied by increased cell perimeter and, importantly, expression of mature neuronal markers. The generally accepted consensus is that AβPP localizes to the Golgi apparatus and vesicles of the endo-lysosomal pathway in the perinuclear region. Here, we have presented that AβPP delocalizes from the perinuclear region and preferentially colocalizes with mitochondria in fully differentiated cells (RA/BDNF). This is associated with decreased levels of full-length AβPP, which is probably processed in a non-amyloidogenic proteolytic pathway as reported earlier [22].
Indeed, apart from the effects of the differentiation of SH-SY5Y cell on AβPP distribution and proteolytic processing mentioned, there is evidence suggesting considerable effect of RA/BDNF treatment on protein machinery of SH-SY5Y cells, implicated in pathobiology of AD. Moreover, RA/BDNF differentiated cells have been shown to display higher susceptibility to Aβ toxicity as compared to undifferentiated precursors [4]. The reasons for these are alternations in oxidative stress response [14] as well as changes in intracellular distribution and phosphorylation of another AD related protein tau [32]. These reports further consolidate our conclusion that the SH-SY5Y cell line is a superb in vitro model of addressing specific questions related to the biology of AD, assuming that methods of cultivation and testing are carefully selected.
ACKNOWLEDGMENTS
The authors thank Michael Davidson for his generous gift for Golgi apparatus staining (mApple-Golgi-7). MŠ acknowledges GAČR grant 19-08304Y. PR acknowledges AVČR grant L200401901. DB received support from project GA UK No 1834218 (CU). SVO and DB were supported by Sustainability for the National Institute of Mental Health”, nr. LO1611, and the Ministry of Education, Youth and Sports of the Czech Republic (NPU I program).
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/20-1409r1).
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-201409.
REFERENCES
[1] | Wortmann M ((2012) ) Dementia: A global health priority - Highlights from an ADI and World Health Organization report. Alzheimer’s Res Ther 4: , 4–6. |
[2] | Hu BY , Weick JP , Yu J , Ma LX , Zhang XQ , Thomson JA , Zhang SC ((2010) ) Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc Natl Acad Sci U S A 107: , 4335–4340. |
[3] | Yusuf M , Leung K , Morris KJ , Volpi EV ((2013) ) Comprehensive cytogenomic profile of the} neuronal model SH-SY5Y. Neurogenetics 14: , 63–70. |
[4] | Krishtal J , Metsla K , Bragina O , Tõugu V , Palumaa P ((2019) ) Toxicity of amyloid-β peptides varies depending on differentiation route of SH-SY5Y cells. J Alzheimers Dis 71: , 879–887. |
[5] | Chen J , Chattopadhyay B , Venkatakrishnan G , Ross AH ((1990) ) Nerve growth factor-induced differentiation of human neuroblastoma and neuroepithelioma cell lines. Cell Growth Differ 1: , 79–85. |
[6] | Encinas M , Iglesias M , Liu Y , Wang H , Muhaisen A , Ceña V , Gallego C , Comella JX ((2000) ) Sequential treatment of SH-SY5Y cells with retinoic acid and brain-derived neurotrophic factor gives rise to fully differentiated, neurotrophic factor-dependent, human neuron-like cells. J Neurochem 75: , 991–1003. |
[7] | Kume T , Kawato Y , Osakada F , Izumi Y , Katsuki H , Nakagawa T , Kaneko S , Niidome T , Takada-Takatori Y , Akaike A ((2008) ) Dibutyryl cyclic AMP induces differentiation of human neuroblastoma SH-SY5Y cells into a noradrenergic phenotype. Neurosci Lett 443: , 199–203. |
[8] | Påhlman S , Odelstad L , Larsson E , Grotte G , Nilsson K ((1981) ) Phenotypic changes of human neuroblastoma cells in culture induced by 12-O-tetradecanoyl-phorbol-13-acetate. Int J Cancer 28: , 583–589. |
[9] | Paik S , Somvanshi RK , Kumar U ((2019) ) Somatostatin-mediated changes in microtubule-associated proteins and retinoic acid-induced neurite outgrowth in SH-SY5Y cells. J Mol Neurosci 68: , 120–134. |
[10] | Presgraves SP , Ahmed T , Borwege S , Joyce JN ((2003) ) Terminally differentiated SH-SY5Y cells provide a model system for studying neuroprotective effects of dopamine agonists. Neurotox Res 5: , 579–598. |
[11] | Hromadkova L , Bezdekova D , Pala J , Schedin-Weiss S , Tjernberg LO , Hoschl C , Ovsepian SV ((2020) ) Brain-derived neurotrophic factor (BDNF) promotes molecular polarization and differentiation of immature neuroblastoma cells into definitive neurons. Biochim Biophys Acta Mol Cell Res 1867: , 118737. |
[12] | Cheung Y , Lau WK , Yu M , Lai CS , Yeung S , So K , Chang RC ((2009) ) Effects of all-trans-retinoic acid on human SH-SY5Y neuroblastoma as} model in neurotoxicity research. Neurotoxicology 30: , 127–135. |
[13] | Goldie BJ , Barnett MM , Cairns MJ ((2014) ) BDNF and the maturation of posttranscriptional regulatory networks in human SH-SY5Y neuroblast differentiation. Front Cell Neurosci 8: , 325. |
[14] | Forster JI , Köglsberger S , Trefois C , Boyd O , Baumuratov AS , Buck L , Balling R , Antony PMA ((2016) ) Characterization of differentiated SH-SY5Y as neuronal screening model reveals increased oxidative vulnerability. J Biomol Screen 21: , 496–509. |
[15] | Brion JP ((2006) ) Immunological demonstration of tau protein in neurofibrillary tangles of Alzheimer’s disease. J Alzheimers Dis 9: , 177–185. |
[16] | Glenner G and Wong WC ((1984) ) Alzheimer’s disease and Down’s syndrome: Sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun 122: , 1131–1135. |
[17] | Gupta S , Banerjee P , Laferla FM , Selkoe DJ ((2010) ) Alzheimer’s disease: Genes, proteins, and therapy. Physiol Rev 81: , 741–766. |
[18] | Rueden CT , Schindelin J , Hiner MC , DeZonia BE , Walter AE , Arena ET , Eliceiri KW ((2017) ) ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics 18: , 529. |
[19] | Schindelin J , Arganda-Carreras I , Frise E , Kaynig V , Longair M , Pietzsch T , Preibisch S , Rueden C , Saalfeld S , Schmid B ((2012) ) Fiji: An open-source platform for biological-image analysis. Nat Methods 9: , 676–682. |
[20] | Hüpfel M , Yu. Kobitski A , Zhang W , Nienhaus GU ((2021) ) Wavelet-based background and noise subtraction for fluorescence microscopy images. Biomed Opt Express 12: , 969. |
[21] | Dreses-Werringloer U , Lambert JC , Vingtdeux V , Zhao H , Vais H , Siebert A , Jain A , Koppel J , Rovelet-Lecrux A , Hannequin D , Pasquier F , Galimberti D , Scarpini E , Mann D , Lendon C , Campion D , Amouyel P , Davies P , Foskett JK , Campagne F , Marambaud P ((2008) ) A polymorphism in CALHM1 influences Ca2+homeostasis, Aβ levels, and Alzheimer’s disease risk. Cell 133: , 1149–1161. |
[22] | Nigam SM , Xu S , Kritikou JS , Marosi K , Brodin L , Mattson MP ((2017) ) Exercise and BDNF reduce Aβ production by enhancing α-secretase processing of APP. J Neurochem 142: , 286–296. |
[23] | Dunning CJR , Black HL , Andrews KL , Davenport EC , Conboy M , Chawla S , Dowle AA , Ashford D , Thomas JR , Evans GJO ((2016) ) Multisite tyrosine phosphorylation of the N-terminus of Mint1/X11α by Src kinase regulates the trafficking of amyloid precursor protein. J Neurochem 137: , 518–527. |
[24] | LaFerla FM , Green KN , Oddo S ((2007) ) Intracellular amyloid-β in Alzheimer’s disease. Nat Rev Neurosci 8: , 499–509. |
[25] | Pavlov PF , Wiehager B , Sakai J , Frykman S , Behbahani H , Winblad B , Ankarcrona M ((2011) ) Mitochondrial γ-secretase participates in the metabolism of mitochondria-associated amyloid precursor protein. FASEB J 25: , 78–88. |
[26] | Edwards MA , Loxley RA , Williams AJ , Connor M , Phillips JK ((2007) ) Lack of functional expression of NMDA receptors in PC12 cells. Neurotoxicology 28: , 876–885. |
[27] | Heusinkveld HJ , Westerink RHS ((2017) ) Comparison of different in vitro cell models for the assessment of pesticide-induced dopaminergic neurotoxicity. Toxicol Vitr 45: , 81–88. |
[28] | Popova D , Karlsson J , Jacobsson SOP ((2017) ) Comparison of neurons derived from mouse P19, rat PC12 and human SH-SY5Y cells in the assessment of chemical- and toxin-induced neurotoxicity. BMC Pharmacol Toxicol 18: , 42. |
[29] | Datta PK ((2013) ) Neuronal cell culture. Neuronal Cell Cult Methods Protoc 1078: , 35–44. |
[30] | Xicoy H , Wieringa B , Martens GJM ((2017) ) The SH-SY5Y cell line in Parkinson’s disease research: A systematic review. Mol Neurodegener 12: , 10. |
[31] | Kaplan DR , Matsumoto K , Lucarelli E , Thielet CJ ((1993) ) Induction of TrkB by retinoic acid mediates biologic responsiveness to BDNF and differentiation of human neuroblastoma cells. Neuron 11: , 321–331. |
[32] | Chen Q , Zhou Z , Zhang L , Xu S , Chen C , Yu Z ((2014) ) The cellular distribution and Ser262 phosphorylation of tau protein are regulated by BDNF . PLoS One 9: , e91793. |




