White Matter Connectivity in Incident Mild Cognitive Impairment: A Diffusion Spectrum Imaging Study of World Trade Center Responders at Midlife
Abstract
Background:
Individuals who participated in response efforts at the World Trade Center (WTC) following 9/11/2001 are experiencing elevated incidence of mild cognitive impairment (MCI) at midlife.
Objective:
We hypothesized that white matter connectivity measured using diffusion spectrum imaging (DSI) would be restructured in WTC responders with MCI versus cognitively unimpaired responders.
Methods:
Twenty responders (mean age 56; 10 MCI/10 unimpaired) recruited from an epidemiological study were characterized using NIA-AA criteria alongside controls matched on demographics (age/sex/occupation/race/education). Axial DSI was acquired on a 3T Siemen’s Biograph mMR scanner (12-channel head coil) using a multi-band diffusion sequence. Connectometry examined whole-brain tract-level differences in white matter integrity. Fractional anisotropy (FA), mean diffusivity (MD), and quantified anisotropy were extracted for region of interest (ROI) analyses using the Desikan-Killiany atlas.
Results:
Connectometry identified both increased and decreased connectivity within regions of the brains of responders with MCI identified in the corticothalamic pathway and cortico-striatal pathway that survived adjustment for multiple comparisons. MCI was also associated with higher FA values in five ROIs including in the rostral anterior cingulate; lower MD values in four ROIs including the left rostral anterior cingulate; and higher MD values in the right inferior circular insula. Analyses by cognitive domain revealed nominal associations in domains of response speed, verbal learning, verbal retention, and visuospatial learning.
Conclusions:
WTC responders with MCI at midlife showed early signs of neurodegeneration characterized by both increased and decreased white matter diffusivity in regions commonly affected by early-onset Alzheimer’s disease.
INTRODUCTION
In the aftermath of the attacks of 9/11/2001, tens of thousands of men and women worked in search, rescue, and clean-up efforts at the World Trade Center (WTC). During this time, responders experienced a host of physical and psychological exposures with one result being severe and chronic posttraumatic stress disorder (PTSD) [1, 2]. Recent studies of WTC responders at mid-life, 15 + years post 9/11, also experienced higher than expected incidence of mild cognitive impairment (MCI) [3].
MCI is a characteristic of prodromal Alzheimer’s disease (AD) and related dementias [4, 5], while neuromotor limitations are a common non-cognitive comorbidity of neurodegenerative disease [6]. In AD, it has been demonstrated that one mechanism leading to gray matter atrophy is through decreased white matter connectivity as measured by changes in white matter tractography linking cortical networks together [7]. For example in AD, white matter connectivity has been linked to neural topography and deposition of amyloid-β [8, 9] and tau proliferation [10]. Recently, researchers have noted that interhemispheric connectivity in the posterior region is significantly worse in individuals with early-onset (aged 45–65) as compared to late-onset (aged 65 and older) AD [11].
A larger than expected group of WTC responders are experiencing early onset of cognitive impairment at midlife that may be indicative of preclinical AD or AD and related dementias. However, while measured neurocognitive functioning is impaired in this group, there have been no studies to date examining whether white matter integrity is associated with cognitive dysfunction within this population. Recognizing that many neurodegenerative conditions include widespread changes to white matter connectivity, the goal of this study was to identify changes in brain connectivity as early as possible following the onset of MCI. We hypothesized that white matter connectivity, as measured using diffusion spectrum imaging, would be restructured in WTC responders with MCI as compared to cognitively unimpaired and stable responders.
METHODS
Population
In 2002, the Centers for Disease Control and Prevention (CDC) began monitoring more than 50,000 WTC responders [12] using a comprehensive monitoring protocol as previously described [13]. Briefly, the Stony Brook University program monitors law enforcement and non-traditional (e.g., construction, utilities, and volunteer) responders who reside on Long Island, NY and prospectively monitors cognitive status [3].
Participant recruitment
Twenty WTC responders were recruited in this study for a two-hour magnetic resonance imaging (MRI) scan including a diffusion imaging component. To accomplish this task, we called 305 responders (193 with MCI, 112 without) who were believed, based on chart-review, to be eligible for a PET/MRI study investigating the etiology of WTC-related MCI and dementia, and who had previously consented to being contacted to participate in research studies and met inclusion criteria. In total, 159 responders completed phone screening (94 with MCI, 65 without) of whom 75 declined (46 with MCI, 29 without), 37 were deemed ineligible (20 with MCI, 17 without), and 54 (28 with MCI, 26 without) expressed interest in completing the study. Of the 47 responders who were scheduled for in-person screening visits, 20 (15 with MCI, 5 without) dropped out at in-person screening and four were deemed ineligible at screening interviews leaving 23 responders (11 MCI, 12 without). Of those who were scheduled to be scanned, scanning failed on one MCI responder due to patient distress on the day of the scan resulting in an incomp-lete scan, while two cognitively unimpaired responders dropped out after screening but prior to scanning. The final sample included ten responders with incident MCI and ten cognitively unimpaired responders.
Clinical measures
Responders with incident MCI were identified following the National Institute on Aging and Alzheimer’s Association (NIA-AA) criteria [14] as previously described [3]. Briefly, the Montreal Cognitive Assessment (MoCA) was utilized to objectively characterize cognitive impairment [15] and standard cut-offs (MoCA≤23) were used to identify cognitive impairment [16]. Alternate versions of the MoCA were used at each assessment to avoid test-learning effects commonly reported to increase scores in repeated use of neuropsychological tests in longitudinal studies [17]. The average length of time between diagnosis of incident MCI and scanning was 1.08 years.
Domains of cognitive functioning measured in addition to the MoCA using both paper/pen (Hopkins Verbal Learning Test-Revised [HVLT-R], Trail Making Test [TMT] A and B, Boston Naming Test [BNT], Symbol Digit Modalities [SDMT], Controlled Oral Word Association [COWA] and Wide Range Achi-evement Test –Reading Subtest [WRAT4-READ]), in addition to computer-administered (Cogstate, http://www.cogstate.com) cognitive tests [18]. Collectively, these tests measured across the following cognitive domains: reaction speed, processing speed, verbal memory, visual memory, cognitive throughput, verbal fluency. Validity checks are built into scoring. As noted in Supplementary Figure 1, cognitive impairment was detected across several domains of cognitive function but was more pronounced within domains of memory and response speed.
Current diagnoses of posttraumatic stress disorder, major depressive disorder, and substance abuse disorder were characterized in these data using the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders-IV [SCID] (DSM-IV) [19]. PTSD symptoms were also reported for the three DSM-IV specific symptom clusters of re-experiencing, avoidance and hyperarousal.
Matching variables
Matching criteria included age in years, sex, occupation dichotomized to identify individuals wo-rking in non-traditional versus law enforcement occupations, race (White, Black) and education trichotomized into high school education, those with some college-level schooling, and those with at least a university degree.
Inclusion criteria
Inclusion criteria included: aged between 45–65 years; MoCA scores between 19–23 and evidence of score decline between baseline and follow-up for in-cident MCI group, or responders who are cognitively unimpaired (MoCA > 26) in the first two waves; body mass index (BMI)≤40 (due to MRI scanner bore size); capacity to provide informed consent and willingness to undergo neuroimaging.
Exclusion criteria
Exclusion criteria: History of psychosis or substance-related or addictive disorders (determined from relevant modules within the SCID); a drinking problem or current substance abuse disorder; history of stroke; history of serious head trauma or other neurological disorders such as epilepsy; currently receiving cognitively active medications (e.g., meth-ylphenidate); brain cancer; renal failure or receiving dialysis; being treated for severe liver disease or hepatitis; indication of unmanaged diabetes; dia-gnosed major depressive disorder; chronic autoimmune disease (e.g., multiple sclerosis); heart failure or myocardial infarction in the past year; embedded ferromagnetic metal implants, history of claustrophobia or any other conditions that would impair their ability to undergo an MRI scan (e.g., a pacemaker, shrapnel, wires, including surgically implanted which are not MRI safe); current pregnancy, breastfeeding, or sexually active and of child-bearing potential without using adequate contraceptive methods.
Imaging acquisition
All WTC axial diffusion spectrum imaging (DSI) images used in this study were acquired on a 3T Siemen’s Biograph mMR scanner with software version VB20P using a 12-channel head coil. Following localizer and magnetization-prepared rapid gradient echo (MPRAGE) for structural imaging, DSI was performed axially using a 2D multi-band diffusion sequence [20] with echo time = 121.4 ms, repetition time = 6300 ms, matrix size = 112×88, multi-band factor = 2, in-plane resolution = 2×2 mm2, 64 slices, slice thickness/gaP = 2/0 mm, 4 shells with b = 1000, 2000, 3000, and 4000 with 64, 32, 32, 32 diffusion directions in the shells respectively. The diffusion direction was generated using an electrostatic repulsion algorithm for optimal coverage.
Data processing
Diffusion images were visually inspected to check for major image artifacts or significant motion during the acquisition. All images passed inspection. No incidental neurological changes were identified in the images. The diffusion data were reconstructed in the Montreal Neurological Institute (MNI) space using q-space diffeomorphic reconstruction [21] to obtain the spin distribution function (SDF; diffusion sampling length ratio = 1.25) [22]. The restricted diffusion was quantified using restricted diffusion imaging [23]. The spin distribution function (SDF) values were used in connectometry analysis.
Connectometry
Diffusion MRI connectometry [24] was used to examine associations between incident MCI and white matter connectivity using DSI Studio (May 4 2019 build). A t-score threshold of 2 was assigned to select local connectomes, and the local connectomes were tracked using a deterministic fiber tracking algorithm [25]. All tracts generated from bootstrap resampling were included. A length threshold of 20 voxel distance was used to select tracts. The seeding number for each permutation was 10,000. To estimate the false discovery rate (FDR), a total of 2,000 randomized permutations were applied to group labels to obtain the null distribution for tract length.
Based on the connectometry analysis, k-means clustering (k = 5) was conducted to find clusters of increased and decreased connectivity (10 total clusters). Quantitative metrics were then extracted from these tracts to compare differences between MCI and CN. GLM analysis was used to compare the two groups while controlling for age.
Regional analysis
Quantitative metrics, such as fractional anisotropy (FA), mean diffusivity (MD), and quantified anisot-ropy (QA) were extracted for a region of interest (ROI) based analysis using Desikan-Killiany atlas [26] and DSI studios’ FreeSurferSeg atlas. All 34 left and right ROI pairs from the Desikan-Killiany atlas [26] were studied along with left and right ROI pairs of the hippocampus and amygdala, and five subsections of the corpus callosum from the FreeSurferSeg atlas provided by DSI Studio. The DSI measure (QA) was obtained using data acquired with all b values, and the diffusion tensor image (DTI) measures (FA and MD) were obtained using images acquired with b = 1,000 only. DSI and DTI quantitative metrics were extracted across all subjects for an ROI-based approach to conduct generalized linear model (GLM) analysis in SPSS (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp.). ROI analyses relied on multiple regression models to consider associations between MCI and measures of white matter integrity (FA/MD/QA).
Whole brain volume
Three-dimensional T1-weighted MPRAGE ima-ges were acquired (long repetition time (TR) = 1900 s, echo time (TE) = 2.49 ms, medium inversion time (TI) = 900 ms, Flip Angle = 9°, acquisition mat-rix: 256×256 and voxel resolution: 0.89×0.89×0.89 mm). The Computational Anatomy Toolbox (CAT12) [27] was used to estimate volumetric differences separately for right and left hemispheres before being merged and smoothed (15 mm). For descriptive purposes, total intracranial, gray, and white matter volume were also extracted using automated volumetric processing in CAT12.
Correlation analysis
Multiple non-parametric Spearman’s rank correlation tests were performed to explore associational rho coefficients (rs) between diffusion metrics and cognitive scores in all 20 WTC responders in this study, with significance set at α= 0.05.
Statistical analysis
We began by describing sample characteristics for responders included in this study. Student’s t-tests and tests of proportions were used to examine whether characteristics varied between responders who were cognitively unimpaired and responders with incident MCI. Whole-brain analyses relied on examining differences between incident MCI and non-MCI groups. Regional analyses compared FA, MD, and QA within 34 ROIs. A two-tailed α= 0.05 was used to determine statistical significance; results from repeated testing analyses (Supplementary Figure 1) were adjusted for the false discovery rate (FDR = 0.05) [28].
Ethics
The Institutional Review Board approved this study (IRB#1113241). Responders provided infor-med written consent.
Data availability
Data can be made available by reasonable request to the corresponding author.
RESULTS
On average, the 20 responders who completed this imaging protocol (Table 1) did not differ acr-oss groups on matching criteria such as age (p = 0.785), sex (p = 1.000), race (p = 1.000), education (p = 0.362), or occupation (p = 0.558). Additionally, responders with incident MCI did not differ from cognitively unimpaired responders on total intracranial volume (p = 0.531), white (p = 0.507) or gray (p = 0.126) matter volume. Responders with incident MCI significantly underperformed cognitively unimpaired responders in measures of processing speed, working memory, verbal recognition, reaction speed, memory, and throughput (Supplementary Figure 1).
Table 1
Characteristics of World Trade Center responders with incident mild cognitive impairment [MCI] (n = 10) versus cognitively unimpaired responders (n = 10). Values expressed as mean (SD) or percentages
| Characteristic | Incident MCI | Cognitively unimpaired | p |
| Age | 56.30 (5.27) | 55.70 (4.37) | 0.785 |
| Body mass, kg/m2 | 30.65 (3.08) | 30.67 (4.13) | 0.990 |
| PTSD symptoms: | |||
| Re-experiencing | 14.50 (4.88) | 15.20 (4.10) | 0.733 |
| Avoidance | 16.50 (4.90) | 19.20 (6.14) | 0.292 |
| Hyperarousal | 14.10 (5.99) | 15.40 (5.15) | 0.609 |
| Intracranial volume, cm3 | 1506.28 (182.20) | 1553.98 (149.75) | |
| Total brain volume, cm3 | 1239.54 (147.09) | 1140.73 (89.52) | |
| Gray matter volume, cm3 | 46.65 (21.93) | 36.65 (17.54) | |
| Law enforcement, % | 40.0 | 70.0 | 0.178 |
| Female, % | 10.0 | 10.0 | 1.000 |
| Black, % | 10.0 | 10.0 | 1.000 |
As shown in Fig. 1, connectometry analysis indicates fiber tracts in the corpus callosum, corticothalamic pathway and cortico-striatal pathway to have decreased connectivity relating to incident MCI. Conversely, fiber tracts were found to have increased connectivity relating to MCI in the corticothalamic pathway and cortico-striatal pathway. The tracts with increased connectivity had an FDR = 0.041 and the tracts with decreased connectivity had FDR = 0.19.
Fig. 1
Connectometry analysis examining associations between incident MCI and white matter connectivity. Decreased connectivity (left) was found in the corpus callosum, corticothalamic pathways and cortico-striatal pathway. Increased connectivity (right) was also found in the corticothalamic pathway and cortico-striatal pathway.
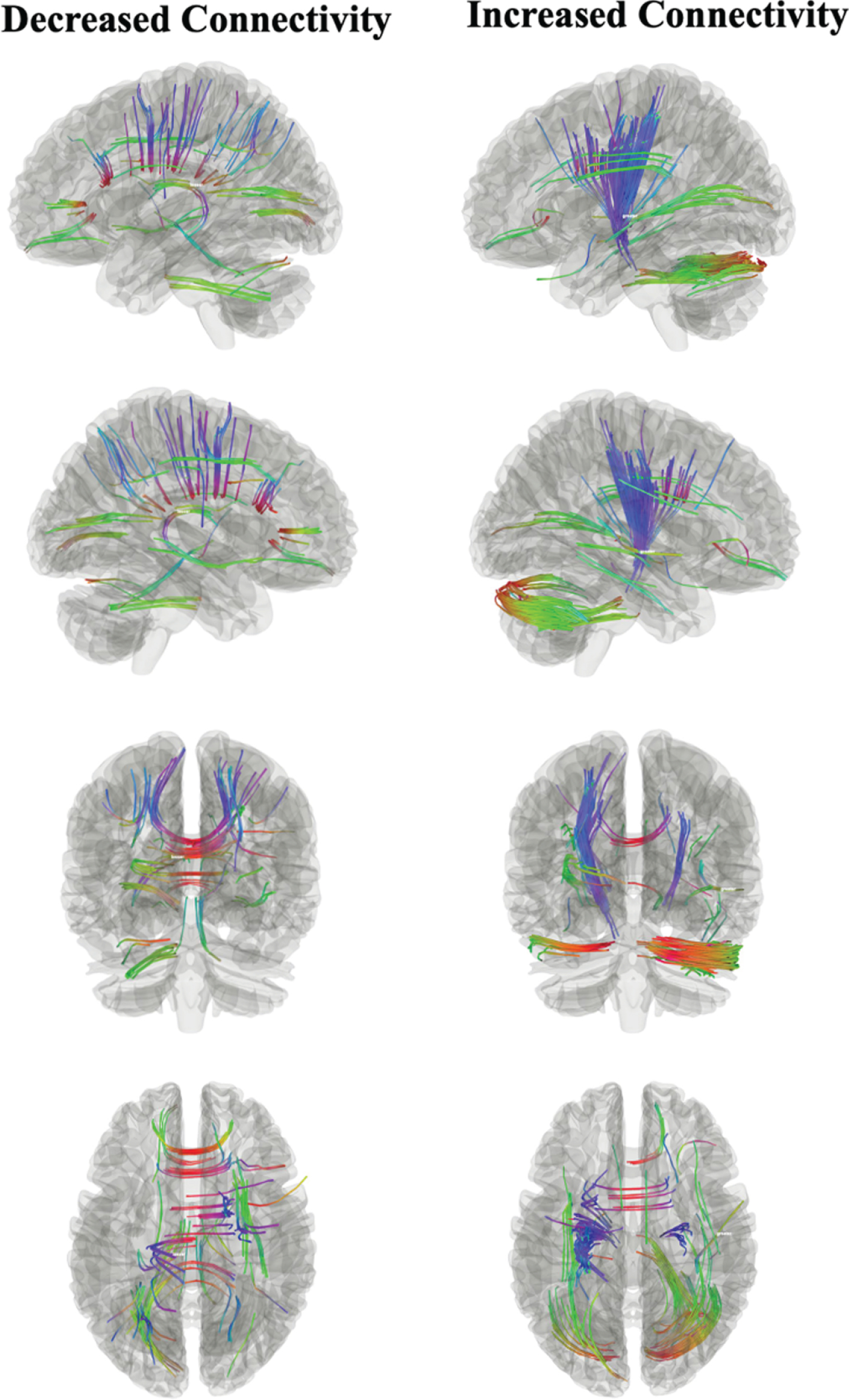
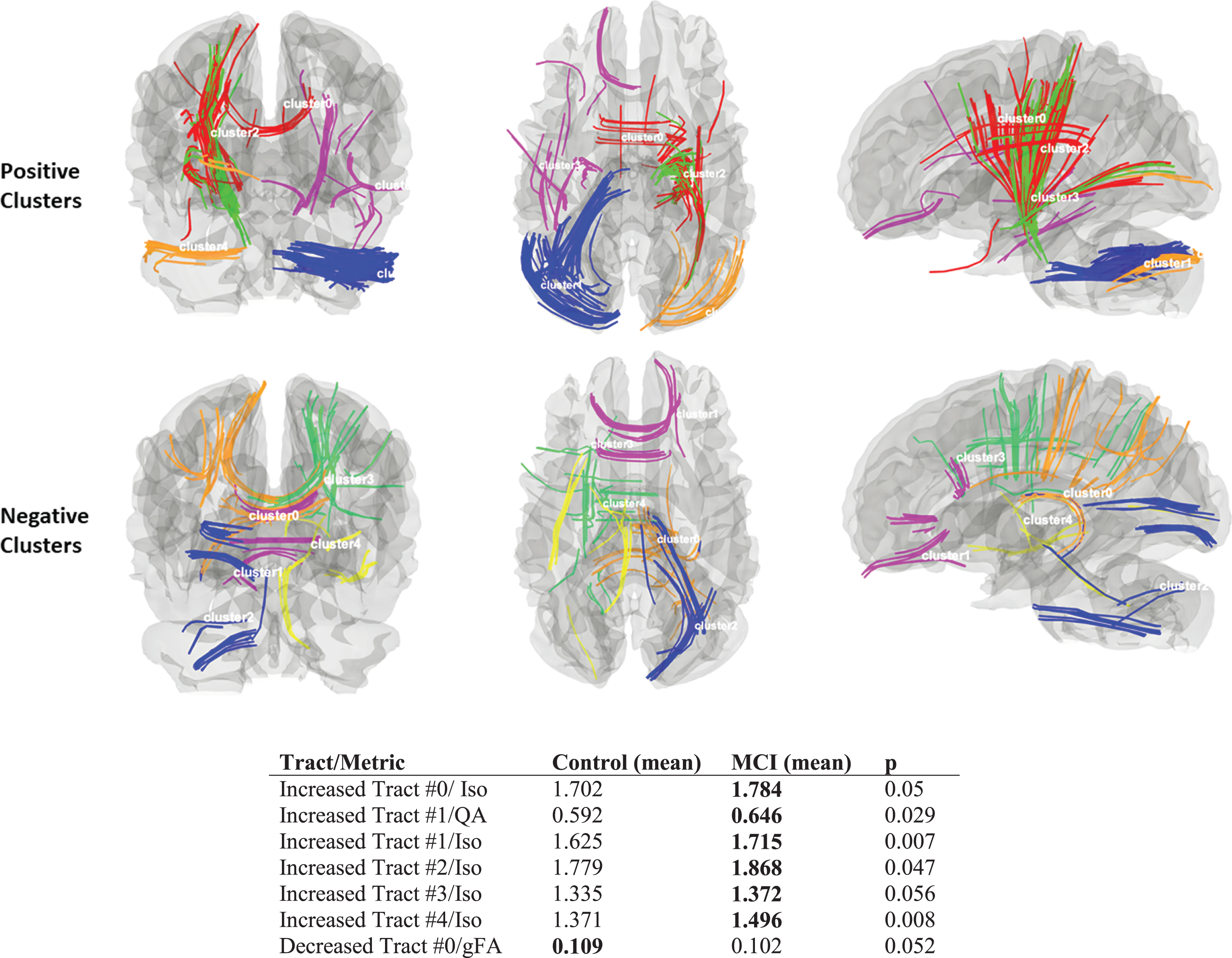
Figure 2 shows five tract-clusters identified as having increased connectivity (top) or decreased connectivity (bottom). DTI and DSI metrics were extracted from the individual clustered fibers for each subject. Multivariable-adjusted analyses also compared MCI versus controls while controlling for age. In these analyses, MCI had greater isotropic diffusion (iso) as compared to controls (p < 0.05) in all clustered tracts from the increased connectivity analysis. Consistent with the whole-brain analysis, only one clustered tract from the decreased connectivity analysis displayed a trend of lower gFA in MCI versus controls (p = 0.052).
Fig. 2
Image of ten k-means clusters created from tracts with increased connectivity (five clusters on top) and decreased connectivity (five clusters on bottom). Group means are shown in the accompanying table with p-values derived from GLM analyses with age correction.
Region-based analysis
T-tests comparing ROIs between the two groups demonstrated that responders with incident MCI had higher mean FA values compared to cognitively unimpaired responders in the right rostral anterior cingulate (0.277 versus 0.234, p = 0.002), right anterior cingulate (0.256 versus 0.227, p = 0.029), left (0.424 versus 0.292, p = 0.021) and right accumbens (0.464 versus 0.355, p = 0.004), and left anterior circular insula (0.254 versus 0.218, p = 0.024). However, responders with incident MCI demonstrated lower mean MD values versus cognitively unimpaired responders in the left rostral anterior cingulate (0.651 versus 0.695, p = 0.026), and left mid-anterior cingulate (0.688 versus 0.723, p = 0.041), left accumbens area (0.483 versus 0.616, p = 0.037), left pallidum (0.533 versus 0.611, p = 0.011) and higher MD values in right inferior circular insula (0.824 versus 0.786, p = 0.013). No significant differences were found in DSI based metrics.
Because diffusion metrics are known to be age dependent, sensitivity analyses using GLM compared responders with incident MCI against cognitively un-impaired responders while controlling for age. These analyses indicated higher mean FA values for the right rostral anterior cingulate (0.0268 versus 0.227, p = 0.001) in responders with incident MCI compared to cognitively unimpaired responders. Additionally, responders with incident MCI associated with lower mean MD values in the left rostral anterior cingulate (0.652 versus 0.696, p = 0.035) and right caudal anterior cingulate (0.738 versus 0.771, p = 0.028), as well as higher mean MD values in the left amygdala (0.393 versus 0.353, p = 0.024) and lower left parahippocampus (0.0227 versus 0.216, p = 0.043), when compared to cognitively unimpaired responders.
Correlations
Spearman correlation analysis between white matter FA and cognitive tests revealed many significant pairs (see Supplementary Table 2). Table 3 shows the ROIs found to be significantly different between the two group. Among these, significant correlations were observed between FA in right rostral anterior cingulate and MoCA (rs = –0.64), MD in the left rostral anterior cingulate and visual learning (rs=–0.59), FA in the right caudal anterior cingulate and HVLT (rs = 0.49), FA in the left nucleus accumbens and WRAT (rs =–0.50), FA in the right nucleus accumbens and MoCA (rs =–0.52), FA in the left circular insula and TMTA (rs = 0.47), MD in the left circular insula and WRAT (rs = 0.46) and finally MD in the left pallidum and MoCA (rs = 0.49) and TMTA (rs=–0.50).
Table 2
Comparison of white matter connectivity in responders with in-cident mild cognitive impairment (MCI) versus cognitively unimpaired responders using Student’s t-test. Diffusion tensor image (DTI) and diffusion spectrum image (DSI) metrics are displayed with corresponding ROI showing results for mean values in incident MCI and Cognitively unimpaired. Significant, uncorrected values are bolded and defined by p < 0.05
| Metric ROI | Incident MCI | Cognitively unimpaired | p |
| Fractional Anisotropy (FA) | |||
| Right rostral anterior cingulate | 0.277 | 0.234 | 0.002* |
| Right anterior cingulate | 0.256 | 0.227 | 0.029 |
| Left accumbens | 0.424 | 0.292 | 0.021 |
| Right accumbens | 0.464 | 0.355 | 0.004* |
| Mean Diffusivity (MD) | |||
| Left rostral anterior cingulate | 0.651 | 0.695 | 0.026 |
| Left accumbens | 0.483 | 0.616 | 0.037 |
| Left pallidum | 0.533 | 0.611 | 0.011 |
| Quantified Anisotropy (QA) | |||
| Left amygdala | 0.363 | 0.326 | 0.090 |
*Statistically significant upon adjusting for the false discovery rate. (FDR = 0.2); none of the associations pass correction for the FDR.
Table 3
Spearman’s rank correlation tests between cognitive measures and diffusion metrics in ROIs that were significantly different at the group level (see Table 2), in all 20 WTC responders in this study, with significance set at α= 0.05
| Right Rostral Anterior Cingulate (FA) | Right Caudal Anterior Cingulate (FA) | Left Accumbens (FA) | Right Accumbens (FA) | Left Cortical Anterior Circular Insula (FA) | Right Rostral Anterior Cingulate (MD) | Left Cortex of the Anterior Middle Cingulate (MD) | Left Accumbens (MD) | Left Pallidum (MD) | Left Cortical Inferior Circular Insula (MD) | |
| Aphasia | 0.23 | 0.15 | 0.38 | 0.36 | 0.20 | –0.22 | –0.41 | –0.32 | –0.39 | –0.15 |
| Response speed | 0.40 | –0.22 | 0.02 | 0.24 | 0.47 | 0.11 | –0.19 | 0.03 | –0.50 | 0.01 |
| Processing speed | –0.23 | 0.08 | –0.10 | –0.21 | –0.32 | –0.02 | 0.14 | 0.01 | 0.30 | –0.01 |
| Verbal fluency | –0.14 | –0.30 | –0.26 | –0.06 | 0.23 | 0.22 | 0.14 | 0.16 | –0.03 | –0.16 |
| Overall verbal learning | –0.10 | 0.49 | –0.19 | 0.02 | –0.06 | 0.04 | –0.17 | 0.05 | –0.16 | –0.07 |
| Retention rate | 0.06 | 0.46 | –0.33 | –0.03 | –0.32 | 0.20 | 0.01 | 0.19 | –0.16 | 0.32 |
| Recognition | –0.20 | 0.16 | –0.20 | –0.09 | –0.12 | 0.18 | –0.10 | 0.04 | –0.12 | –0.05 |
| e-Response speed | –0.09 | 0.17 | –0.27 | –0.38 | –0.05 | 0.26 | 0.13 | 0.30 | 0.13 | 0.16 |
| e-Processing speed | –0.24 | 0.13 | –0.16 | –0.11 | –0.24 | 0.00 | 0.06 | 0.07 | 0.11 | 0.13 |
| e-Memory | 0.06 | –0.24 | –0.13 | 0.05 | –0.22 | –0.08 | 0.09 | –0.01 | 0.13 | 0.19 |
| e-Throughput | –0.05 | 0.43 | –0.24 | –0.19 | –0.24 | –0.21 | –0.04 | 0.08 | 0.18 | –0.01 |
| e-Paired-associate learning | 0.15 | 0.18 | –0.22 | –0.17 | 0.15 | 0.07 | 0.02 | 0.25 | –0.04 | –0.08 |
| e-Visuospatial learning | 0.25 | 0.44 | 0.03 | 0.21 | –0.07 | –0.59 | –0.26 | –0.24 | 0.12 | –0.33 |
| e-Visuospatial memory | –0.02 | –0.05 | 0.28 | –0.12 | 0.24 | –0.03 | –0.12 | –0.12 | –0.17 | –0.28 |
Bolded numbers are statistically significant (p < 0.05). Italicized notes are statistically significant upon adjusting for the False discovery rate (FDR = 0.05).
DISCUSSION
This is the first study to examine white matter connectivity in a sample of WTC responders, and the first study to examine DSI measures in individuals with incident MCI at midlife. The goal of this study was to understand the extent to which white matter tractography may be restructured or impaired in WTC responders with incident MCI. Results from this study were suggestive of an ongoing neurodegenerative process characterized by both increased and decreased white matter diffusivity in regions characteristic of early AD.
This study found areas with both increased and decreased connectivity. Prior studies have suggested that in the presence of clinical symptoms of AD, dysfunction in white matter integrity and connectivity is also often present, such as reduced FA in patients in all-cause MCI [29], and in both amnestic and multi-domain MCI [30]. In these studies, changes in FA did not differ between subtypes of MCI or between MCI and AD, potentially suggesting that changes in FA and MD occurred prior to the clinical characterization of MCI. Supporting that view, work using tractography identified significant disruptions in white matter connectivity of the left anterior and inferior cingulum bundles in AD prior to the onset of gray matter dysfunction [31], attesting to white matter restructuring and disruption as a biomarker with greater sensitivity than grey matter analysis. In contrast, a study of MCI subtypes found differences in MD between non-amnestic MCI and amnestic MCI, characterized by increases in the left precuneus and temporal regions for the former and increases across AD ROIs (including the cingulate, temporal, parietal, and frontal regions) for the latter, suggesting that DTI may be also sensitive to differences in disease etiology [32]. However, recent work has reported white matter connectivity increases in cognitively unimpaired individuals that tracked alongside increased tau deposition [33], suggesting that there may be a U-shape curve in early-onset connectivity changes as a process of neural compensation to pathology, which is then followed neural burn-out [34].
Recent reports are demonstrating that WTC res-ponders are experiencing incidence of MCI at a rate that is more than twice as high as individuals who are more than twenty years older [3]. While there are reasons to believe that incident MCI may sometimes revert to normal on their own, this may be more likely to occur among individuals without neuropathological features or for whom there remains sufficient cognitive reserve to overcome evidence of neuropathology [35]. The interpretation of these results depends on whether white matter connectivity changes are sufficient to fully compensate for the incident MCI-inducing insult. For example, if results reflect a non-progressive disorder, then changes in white matter connectivity may simply represent a stable condition, whereby responder brains are actively coping with low-grade neuropathology resulting in potentially variable cognitive performance. If, however, the onset of MCI reflects a progressive loss of white matter connectivity and then subsequently grey matter neuronal density, then this study may reflect the early incremental loss of cognitive reserve that is implicated when trying to compensate for neuropathology. Longitudinal, follow-up research is required to clarify to what extent changes seen in DSI may reflect a progressive neurodegenerative disease.
The affected areas observed in this study are known to be associated with cognition. Indeed, correlation analysis between diffusion metrics and cognitive scores in this study revealed many significant associations, especially in the affected areas. The anterior cingulate is involved in motor control, error detection and high-level functioning, placing the region in a unique position to translate intentions into actions [36, 37]. The accumbens is a key part in a larger striatal system that sub-serves acquisition and consolidation of different types of learning and memory [38]. It has also been reported that altered insula structure and function are related with cognitive impairments and autonomic dysfunction [39, 40], while the hippocampus and amygdala are known to play a role in cognition, learning and memory, emotion and mental state [41–44]. These brain regions are in line with the MCI literature. Interestingly, the insula, anterior cingulate, amygdala, parahippocampus were found to be among the most discriminant regions in a neuroimaging study identifying MCI patients using DTI and functional connectivity [45].
Limitations
This is the first DSI study of WTC responders and one of the first studies of incident MCI nested in a prospective cohort study of individuals at midlife. While being novel in several ways, this study also has several limitations including, crucially, a small sample size. Due to the small sample size and the population characteristics, there were only two females and a small number of minorities who participated in this study, thereby reducing capacity to complete analysis of sex and/or racial/ethnic differences. However, because diffusion metrics are age dependent, we performed post hoc connectometry analysis with sex and age correction. Furthermore, there is evidence demonstrating neurogenesis when practicing meditation [46, 47] or receiving counselling [48, 49], behavioral remedies that may apply to WTC responders considering their traumatic experiences and of which may have played a role in our findings and interpretations. However, this information is not collected at their WTC clinic visits and could therefore not be accounted for in the present study. In addition, due to the small sample size network-based analysis showing network disruption associated with altered white matter connections between the two groups and correlation analysis between diffusion metrics and cognitive scores were under-powered in this study. Further follow-up with a larger study would be helpful to improve our understanding of the range of factors that are involved in the development of WTC-related dementias. Despite these limitations, the groups were well matched, and the identified affected tracts were virtually the same as reported in Fig. 1 without these corrections, with differences appearing to arise from reduced power implicit in overmatching and similar results were found in our ROI-based analysis comparing both non-corrected and age corrected results.
CONCLUSIONS
This study examined, for the first time, white matter connectivity in WTC responders with incident MCI at midlife. Results are supportive of a mixed connectivity picture for incident MCI in this population, indicating that these responders are at a critical point in time. Certain regions of the brain appear to be compensating with increased white matter connectivity, possibly accounting for neuropathology arising in certain regions. However, other regions are demonstrating decreases in white matter connectivity, signifying irreversible damage to the neocortical connectome, which could explain certain facets of the observed incident MCI. To date, it remains unclear how WTC exposures might result in changes to white matter connectivity; however, our results also suggest that responders’ brains may be able to compensate for such exposures in the short-term. Lastly, these results support ongoing work suggesting that WTC responders with incident MCI are experiencing neurological changes consistent with an as of yet, undefined but emerging early-onset neurodegenerative disease.
ACKNOWLEDGMENTS
This works was supported by the National Ins-titutes of Health (NIH/NIA R01 AG049953), the Centers for Disease Control and Prevention (CDC/NIOSH 200-2011-39361). We would like to acknowledge and kindly thank all the participant WTC responders in this study and Ms. Melissa Carr for her supervision and coordination of participant recruitment.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/20-1237r1).
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-201237.
REFERENCES
[1] | Galea S , Ahern J , Resnick H , Kilpatrick D , Bucuvalas M , Gold J , Vlahov D ((2002) ) Psychological sequelae of the September 11 terrorist attacks in New York City. N Engl J Med 346: , 982–987. |
[2] | Bromet EJ , Hobbs MJ , Clouston SA , Gonzalez A , Kotov R , Luft BJ ((2016) ) DSM-IV post-traumatic stress disorder among World Trade Center responders 11-13 years after the disaster of 11 September 2001 (9/11). Psychol Med 46: , 771–783. |
[3] | Clouston SAP , Diminich ED , Kotov R , Pietrzak RH , Richards M , Spiro A 3rd , Deri Y , Carr M , Yang X , Gandy S , Sano M , Bromet EJ , Luft BJ ((2019) ) Incidence of mild cognitive impairment in World Trade Center responders: Long-term consequences of re-experiencing the events on 9/11/2001. Alzheimers Dement (Amst) 11: , 628–636. |
[4] | Backman L , Small BJ , Fratiglioni L ((2001) ) Stability of the preclinical episodic memory deficit in Alzheimer’s disease. Brain 124: , 96–102. |
[5] | Rodrigue KM , Kennedy KM , Devous MD , Rieck JR , Hebrank AC , Diaz-Arrastia R , Mathews D , Park DC ((2012) ) β-Amyloid burden in healthy aging: Regional distribution and cognitive consequences. Neurology 78: , 387–395. |
[6] | Duggan EC , Piccinin AM , Clouston S , Koval AV , Robitaille A , Zammit AR , Wu C , Brown CL , Lee LO , Finkel D , Beasley WH , Kaye J , Muniz-Terrera G , Katz M , Lipton RB , Deeg D , Bennett DA , Bjork MP , Johansson B , Spiro A , Weuve J , Hofer SM ((2019) ) A multi-study coordinated meta-analysis of pulmonary function and cognition in aging. J Gerontol A Biol Sci Med Sci 74: , 1793–1804. |
[7] | Menon V ((2011) ) Large-scale brain networks and psychopathology: A unifying triple network model. Trends Cogn Sci 15: , 483–506. |
[8] | Pandya S , Kuceyeski A , Raj A ((2017) ) The brain’s structural connectome mediates the relationship between regional neuroimaging biomarkers in Alzheimer’s disease. J Alzheimers Dis 55: , 1639–1657. |
[9] | Prescott JW , Guidon A , Doraiswamy PM , Roy Choudhury K , Liu C , Petrella JR ((2014) ) The Alzheimer structural connectome: Changes in cortical network topology with increased amyloid plaque burden. Radiology 273: , 175–184. |
[10] | Yang F , Chowdhury SR , Jacobs HI , Johnson KA , Dutta J ((2019) ) A longitudinal model for tau aggregation in Alzheimer–s disease based on structural connectivity. In International Conference on Information Processing in Medical Imaging, Springer, pp. 384–393. |
[11] | Li K-C , Luo X , Zeng Q-Z , Xu X-J , Huang P-Y , Shen Z-J , Xu J-J , Zhou J , Zhang M-M ((2018) ) Distinct patterns of interhemispheric connectivity in patients with early- and late-onset Alzheimer’s disease. Fronti Aging Neurosci 10: , 261. |
[12] | Centers for Disease Control and Prevention, World Trade Center Health Program At A Glance, https://www.cdc.gov/wtc/ataglance.html, Accessed March 22, 2017. |
[13] | Dasaro CR , Holden WL , Berman KD , Crane MA , Kaplan JR , Lucchini RG , Luft BJ , Moline JM , Teitelbaum SL , Tirunagari US , Udasin IG , Weiner JH , Zigrossi PA , Todd AC ((2017) ) Cohort Profile: World Trade Center Health Program General Responder Cohort. Int J Epidemiol 46: , e9. |
[14] | Albert MS , DeKosky ST , Dickson D , Dubois B , Feldman HH , Fox NC , Gamst A , Holtzman DM , Jagust WJ , Petersen RC ((2011) ) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: , 270–279. |
[15] | Nasreddine ZS , Phillips NA , Bédirian V , Charbonneau S , Whitehead V , Collin I , Cummings JL , Chertkow H ((2005) ) The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriat Soc 53: , 695–699. |
[16] | Carson N , Leach L , Murphy KJ ((2018) ) A re-examination of Montreal Cognitive Assessment (MoCA) cutoff scores. Int J Geriatr Psychiatry 33: , 379–388. |
[17] | Goldberg TE , Harvey PD , Wesnes KA , Snyder PJ , Schneider LS ((2015) ) Practice effects due to serial cognitive assessment: Implications for preclinical Alzheimer’s disease randomized controlled trials. Alzheimers Dement (Amst) 1: , 103–111. |
[18] | Maruff P , Thomas E , Cysique L , Brew B , Collie A , Snyder P , Pietrzak RH ((2009) ) Validity of the CogState brief battery: Relationship to standardized tests and sensitivity to cognitive impairment in mild traumatic brain injury, schizophrenia, and AIDS dementia complex. Arch Clin Neuropsychol 24: , 165–178. |
[19] | First MB ((2014) ) Structured clinical interview for the DSM (SCID). In The Encyclopedia of Clinical Psychology, John Wiley & Sons, Inc., pp. 1–6. |
[20] | Setsompop K , Cohen-Adad J , Gagoski B , Raij T , Yendiki A , Keil B , Wedeen VJ , Wald LL ((2012) ) Improving diffusion MRI using simultaneous multi-slice echo planar imaging. Neuroimage 63: , 569–580. |
[21] | Yeh F-C , Tseng W-YI ((2011) ) NTU-90: A high angular resolution brain atlas constructed by q-space diffeomorphic reconstruction. Neuroimage 58: , 91–99. |
[22] | Yeh F-C , Wedeen VJ , Tseng W-YI ((2010) ) Generalized q-sampling imaging. IEEE Trans Med Imaging 29: , 1626–1635. |
[23] | Yeh FC , Liu L , Hitchens TK , Wu YL ((2017) ) Mapping immune cell infiltration using restricted diffusion MRI. Magn Reson Med 77: , 603–612. |
[24] | Yeh F-C , Badre D , Verstynen T ((2016) ) Connectometry: A statistical approach harnessing the analytical potential of the local connectome. Neuroimage 125: , 162–171. |
[25] | Yeh F-C , Verstynen TD , Wang Y , Fernández-Miranda JC , Tseng W-YI ((2013) ) Deterministic diffusion fiber tracking improved by quantitative anisotropy. PloS One 8: , e80713. |
[26] | Desikan RS , Ségonne F , Fischl B , Quinn BT , Dickerson BC , Blacker D , Buckner RL , Dale AM , Maguire RP , Hyman BT ((2006) ) An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31: , 968–980. |
[27] | Gaser C , Dahnke R ((2016) ) CAT-a computational anatomy toolbox for the analysis of structural MRI data. HBM 2016: , 336–348. |
[28] | Benjamini Y , Hochberg Y ((1995) ) Controlling the false discovery rate - a practical and powerful approach to multiple testing. J R Stat Soc Series B Methodol 57: , 289–300. |
[29] | Liu Y , Spulber G , Lehtimäki KK , Könönen M , Hallikainen I , Gröhn H , Kivipelto M , Hallikainen M , Vanninen R , Soininen H ((2011) ) Diffusion tensor imaging and tract-based spatial statistics in Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging 32: , 1558–1571. |
[30] | Dimitra S , Verganelakis DA , Gotsis E , Toulas P , Papatriantafillou J , Karageorgiou C , Thomaides T , Kapsalaki EZ , Hadjigeorgiou G , Papadimitriou A ((2013) ) Diffusion tensor imaging (DTI) in the detection of white matter lesions in patients with mild cognitive impairment (MCI). Acta Neurol Belg 113: , 441–451. |
[31] | Chang Y-L , Chen T-F , Shih Y-C , Chiu M-J , Yan S-H , Tseng W-YI ((2015) ) Regional cingulum disruption, not gray matter atrophy, detects cognitive changes in amnestic mild cognitive impairment subtypes. J Alzheimers Dis 44: , 125–138. |
[32] | Gyebnár G , Szabó Á , Sirály E , Fodor Z , Sákovics A , Salacz P , Hidasi Z , Csibri É , Rudas G , Kozák LR , Csukly G ((2018) ) What can DTI tell about early cognitive impairment? –Differentiation between MCI subtypes and healthy controls by diffusion tensor imaging. Psychiatr Res Neuroimaging 272: , 46–57. |
[33] | Shigemoto Y , Sone D , Maikusa N , Okamura N , Furumoto S , Kudo Y , Ogawa M , Takano H , Yokoi Y , Sakata M , Tsukamoto T , Kato K , Sato N , Matsuda H ((2018) ) Association of deposition of tau and amyloid-β proteins with structural connectivity changes in cognitively normal older adults and Alzheimer’s disease spectrum patients. Brain Behav 8: , e01145. |
[34] | Zahodne LB , Reuter-Lorenz PA ((2019) ) Compensation and brain aging:Areviewand analysis of evidence. In The Aging Brain: Functional Adaptation Across Adulthood. American Psychological Association, pp. 185-216. |
[35] | Stern Y , Arenaza-Urquijo EM , Bartrés-Faz D , Belleville S , Cantilon M , Chetelat G , Ewers M , Franzmeier N , Kempermann G , Kremen WS ((2020) ) Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimers Dement 16: , 1305–1311. |
[36] | Paus T ((2001) ) Primate anterior cingulate cortex: Where motor control, drive and cognition interface. Nat Rev Neurosci 2: , 417–424. |
[37] | Pardo JV , Pardo PJ , Janer KW , Raichle ME ((1990) ) The anterior cingulate cortex mediates processing selection in the Stroop attentional conflict paradigm. Proce Natl Acad Sci U S A 87: , 256–259. |
[38] | Setlow B ((1997) ) The nucleus accumbens and learning and memory. J Neurosci Res 49: , 515–521. |
[39] | Pan P , Song W , Shang H ((2012) ) Voxel-wise meta-analysis of gray matter abnormalities in idiopathic Parkinson’s disease. Eur J Neurol 19: , 199–206. |
[40] | Criaud M , Christopher L , Boulinguez P , Ballanger B , Lang AE , Cho SS , Houle S , Strafella AP ((2016) ) Contribution of insula in Parkinson’s disease: A quantitative meta-analysis study. Hum Brain Mapp 37: , 1375–1392. |
[41] | Rubin RD , Watson PD , Duff MC , Cohen NJ ((2014) ) The role of the hippocampus in flexible cognition and social behavior. Front Hum Neurosci 8: , 742. |
[42] | Salzman CD , Fusi S ((2010) ) Emotion, cognition, and mental state representation in amygdala and prefrontal cortex. Ann Rev Neurosci 33: , 173–202. |
[43] | Bliss T ((2007) ) The hippocampus book, Oxford University Press, New York, NY, US. |
[44] | Kandel ER ((2009) ) The biology of memory: A forty-year perspective. J Neurosci 29: , 12748–12756. |
[45] | Wee C-Y , Yap P-T , Zhang D , Denny K , Browndyke JN , Potter GG , Welsh-Bohmer KA , Wang L , Shen D ((2012) ) Identification of MCI individuals using structural and functional connectivity networks. Neuroimage 59: , 2045–2056. |
[46] | Holzel BK , Carmody J , Vangel M , Congleton C , Yerramsetti SM , Gard T , Lazar SW ((2011) ) Mindfulness practice leads to increases in regional brain gray matter density. Psychiatry Res 191: , 36–43. |
[47] | Krishnakumar D , Hamblin MR , Lakshmanan S ((2015) ) Meditation and yoga can modulate brain mechanisms that affect behavior and anxiety-a modern scientific perspective. Anc Sci 2: , 13–19. |
[48] | Beeson ET , Field TA ((2017) ) Neurocounseling: A new section of the Journal of Mental Health Counseling. J Ment Health Couns 39: , 71–83. |
[49] | Ivey AE , Zalaquett CP ((2011) ) Neuroscience and counseling: Central issue for social justice leaders. J Soc Action Couns Psychol 3: , 103–116. |



