Mild Behavioral Impairment and Subjective Cognitive Decline Predict Cognitive and Functional Decline
Abstract
Background:
Mild behavioral impairment (MBI) and subjective cognitive decline (SCD) are dementia risk states, and potentially represent neurobehavioral and neurocognitive manifestations, respectively, of early stage neurodegeneration. Both MBI and SCD predict incident cognitive decline and dementia, are associated with known dementia biomarkers, and are both represented in the NIA-AA research framework for AD in Stage 2 (preclinical disease).
Objective:
To assess the associations of MBI and SCD, alone and in combination, with incident cognitive and functional decline in a population of older adults. We tested the hypothesis that MBI and SCD confer additive risk for decline.
Methods:
Cognitively normal participants were followed up annually at Alzheimer’s Disease Centers. Logistic regression assessed the relationship between baseline classification (MBI-SCD-, MBI-SCD+, MBI+SCD-, or MBI+SCD+) and 3-year outcome.
Results:
Of 2,769 participants (mean age=76), 1,536 were MBI-SCD-, 254 MBI-SCD+, 743 MBI+SCD-, and 236 MBI+SCD+. At 3 years, 349 (12.6%) declined to CDR >0, including 23.1% of the MBI+group, 23.5% of the SCD+group, and 30.9% of the intersection group of both MBI+and SCD+participants. Compared to SCD-MBI-, we observed an ordinal progression in risk (ORs [95% CI]): 3.61 [2.42–5.38] for MBI-SCD+ (16.5% progression), 4.76 [3.57–6.34] for MBI+SCD- (20.7%), and 8.15 [5.71–11.64] for MBI+SCD+(30.9%).
Conclusion:
MBI and SCD together were associated with the greatest risk of decline. These complementary dementia risk syndromes can be used as simple and scalable methods to identify high-risk patients for workup or for clinical trial enrichment.
INTRODUCTION
Commonly cited reasons for high costs and poor outcomes in Alzheimer’s disease (AD) clinical trials are screen failures and poor recruitment of early phase illness [1, 2]. Identification of sensitive and specific premorbid indicators of emergent pathology is a priority [1]. A leading strategy to detect preclinical disease is to focus on subjective cognitive decline (SCD), a perceived decline in cognitive ability in the absence of objective findings [3, 4], which has been associated with amyloid burden [5] and incident cognitive decline and dementia in some [6].
An emerging strategy is to capture early behavio-ral manifestations of dementia [7] that occur in 30% of AD patients prior to cognitive manifestations [8]. Mild behavioral impairment (MBI) is a validated syndrome that serves as a sensitive transitional state marker for dementia syndromes. MBI is characterized by the emergence in later life of persistent neuro-psychiatric symptoms (NPS), and may be an index manifestation of dementia, evident before cognitive symptoms [9]. MBI is associated with cognitive imp-airment and incident cognitive decline and dementia [10–17], as well as known dementia markers including amyloid-β [18], tau [19, 20], neurofilament light [21], temporal lobe atrophy [22, 23], frontal lobe atrophy [24], white matter atrophy [25], functional dys-connectivity [26, 27], and AD genetic loci [28, 29]. This body of evidence suggests that in some older adults, MBI may be a consequence of emerging dem-entia proteinopathies which manifest independently or in concert with cognitive symptoms. What remains unclear is whether these constitute independent or synergistic prodromal manifestations with clinical utility for early detection and intervention.
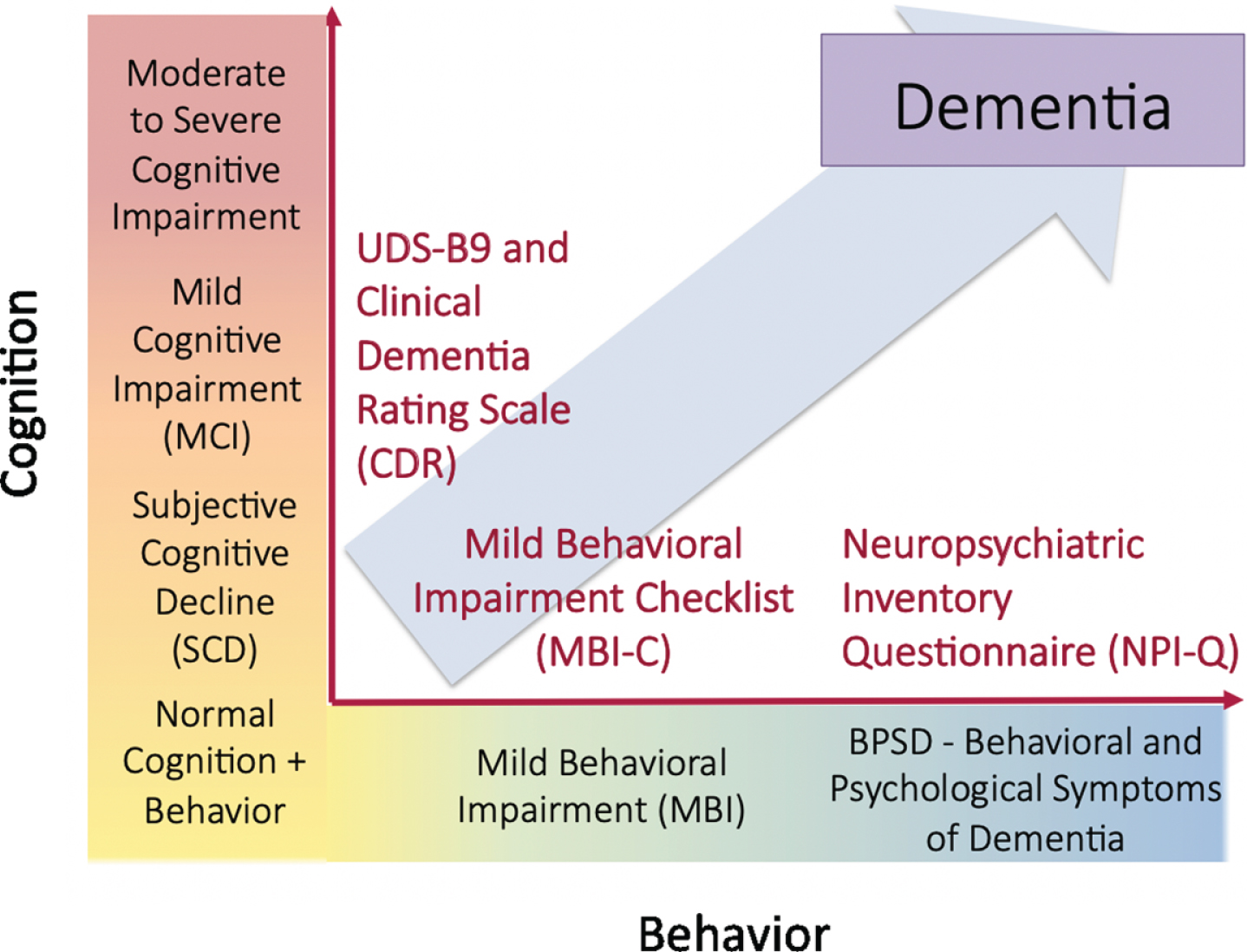
Reflecting early behavioral and cognitive signals for dementia, both MBI and SCD are included in the NIA-AA AD research framework in Stage 2 as potential preclinical manifestations of underlying neuropathology (Table 1, Fig. 1) [30]. To our knowledge, there have been no large prospective studies examining the prognostic utility of MBI and SCD in a sample of objectively normal individuals at higher risk for dementia. We hypothesized that cognitive and behavioral changes in late life may represent coherent or divergent manifestations of emerging pathology that can be leveraged to identify sensitive windows for intervention.
Table 1
Representation of SCD and MBI in NIA-AA Research Framework Clinical Stage 2 [30]
| NIAA -AA Stage 2 Descriptors | SCD or MBI criteria |
| Transitional cognitive decline: Cognitive performance in the | SCD: Self-experienced persistent decline in cognitive capacity |
| nonimpaired range but with a subjective complaint of cognitive | in comparison with a previously normal status and unrelated |
| decline, or a subtle decline measured on longitudinal cognitive | to an acute event, normal age-, gender-, and education-adjusted |
| testing, or neurobehavioral symptoms, or combinations of these | performance on standardized cognitive tests |
| MBI: Behavior and personality changes can precede cognitive | |
| decline and present in absence of cognitive changes, | |
| or can accompany cognitive symptoms | |
| Represents a change from individual baseline within past 1–3 | SCD: Onset of cognitive symptoms within |
| years, and persistent for at least 6 months. Although cognition | the last 5 years (SCD-plus criterion [3]) |
| is the core feature, mild neurobehavioral changes—for example, | MBI: Changes in behavior or personality, starting later in life |
| changes in mood, anxiety, or motivation—may coexist. In some | representing a clear change from usual behavior or personality, |
| individuals, the primary complaint may be neurobehavioral rather | and persisting for at least 6 months; not better accounted for by |
| than cognitive. Neurobehavioral symptoms should have a clearly | psychiatric conditions (including adjustment |
| defined recent onset, which persists and cannot be explained | difficulties secondary to life events) |
| by life events |
Fig. 1
Cognitive and behavioral pre-dementia risk axes.
MATERIALS AND METHODS
Source population: National Alzheimer’s Coordinating Center (NACC)
Data used in this study were obtained from the NACC database (https://www.alz.washington.edu/). NACC was established by the National Institute on Aging (NIA) and consists of multiple NIA-funded Alzheimer’s Disease Research Centers (ADRCs) recruiting and collecting data on subjects with cognitive function ranging from normal to dementia. The NACC Uniform Data Set (UDS) is a large longitu-dinal dataset that includes demographic and standardized clinical data collected approximately annually. All test centers administered standardized forms and informed consent was collected from all subjects and their informants. Detailed information on the cohort and neuropsychological battery of tests included in UDS is described elsewhere [31–33]. NACC-UDS with a December 2018 data freeze date was used for this study.
Patient groupings
MBI status was derived from UDS using a publi-shed algorithm [34, 35] for transformation of the Neu-ropsychiatric Inventory Questionnaire (NPI-Q) [36] items to MBI domains. Specifically, ten NPS dom-ains from the NPI-Q were used to populate the five MBI domains of decreased motivation (NPI-Q apa-thy/indifference); emotional/ affective dysregulation (NPI-Q depression/dysphoria, anxiety, elation/euph-oria); impulse dyscontrol (NPI-Q agitation/aggres-sion, irritability/lability, aberrant motor behavior); social inappropriateness (NPI-Q disinhibition); and abnormal perception or through content (NPI-Q delusions, hallucinations). To obtain the MBI total score, these five transformed domain scores were added to-gether. As the NPI-Q has a reference range of one month. Thus, to approximate MBI persistence of sy-mptoms criteria, individuals with MBI total score >0 at two consecutive annual visits were classified as MBI positive (MBI+) and their MBI scores were calculated as the average over the interval. Those with no NPS were classified as MBI negative (MBI-) for comparison. To determine subjective cognitive decline, the SCD-Initiative Workgroup criteria [3] were used as a framework and operationalized in NACC as foll-ows: 1) endorsement by participant of a decline in me-mory on the UDS B9 form; and 2) normal cognition.
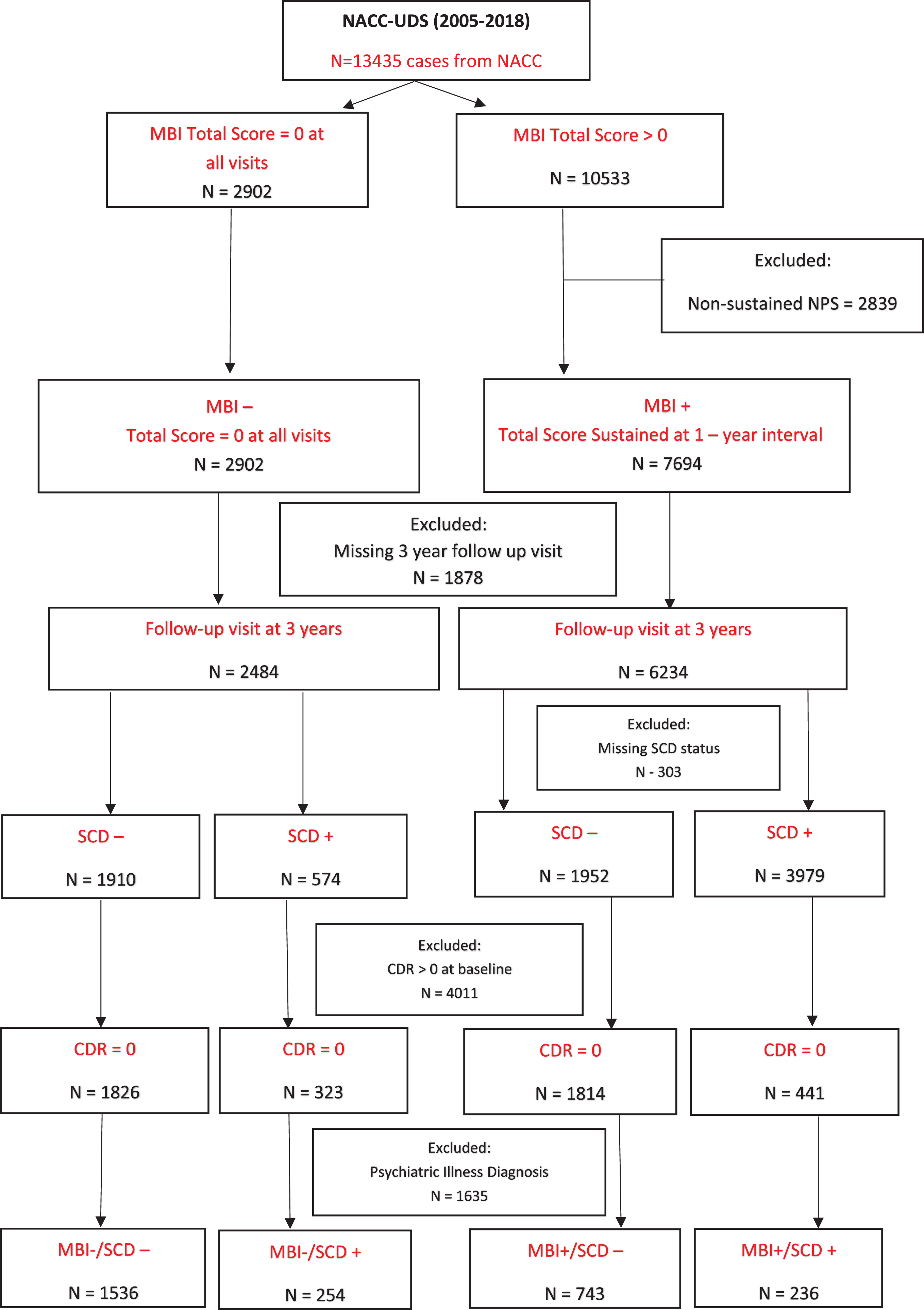
Figure 2 shows the step-by-step process for participant inclusion/exclusion. All NACC participants from 2005–2018 were initially considered for inclusion. The initial step was to classify based on MBI status (MBI+/-) and those with transient NPS not meeting MBI duration criteria were excluded. Study endpoint was chosen a priori to be 3 years to reflect clinical practice and design of observational cohort studies. This approach provided a concrete time fr-ame to assess change, in order to balance the need to wait long enough to see change, but to also minimize attrition that would accrue due to age-related mortality and other diseases that confound cognitive assessments. We included participants with a follow-up visit ∼3 years after the baseline visit to evaluate the change in Clinical Dementia Rating Scale (CDR® Dementia Staging Instrument) [37] score over time and participants were excluded if they were missing the 3-year study visit. SCD status was then determined and participants with a baseline CDR >0 were excluded. Finally, those with a baseline diagnosis of a psychiatric illness were excluded.
Fig. 2
Flowchart of participants from NACC included for analysis.
Study variables
Baseline variables included age, sex, education, and MBI/SCD category. Our primary outcome measure, the CDR, consists of six domains: memory, ori-entation, judgment and problem solving, community affairs, home and hobbies, and personal care, assessed by objective testing and informant report [37]. In our study, we used the global standard CDR score, which assesses the level of impairment, and ranges in sev-erity from no impairment (CDR 0), questionable im-pairment (CDR 0.5 –corresponding roughly to MCI), mild impairment (CDR 1 –corresponding to mild dementia), to moderate to severe impairment (CDR 2-3). All participants had a baseline CDR score of 0, and we measured a change in cognition and function to a CDR >0 at 3 years.
Standard protocols, registrations, and patient consent
The NACC database itself is exempt from IRB re-view and approval because it does not involve human subjects, as defined by federal and state regulations. However, all contributing ADRCs obtained informed consent from their participants and maintained their own separate IRB review and approval from their institution prior to submitting data to NACC.
Data availability statement
Data is available upon request from the corres-ponding author (ZI).
Statistical analysis
Categorical variables were analyzed with χ2 test, and the continuous variables were analyzed using on-e-way ANOVA. We defined patient groups ordinally according to the absence or presence of SCD, MBI, or both at baseline. Cognitive and functional decline was defined as progression to CDR >0 after 3 years. We tested the ordinal association of patient groups and cognitive and functional decline using linear by linear association and Somers’ D [38]. Ordinal approaches permit explicit testing of an ordinal association in the probability distribution for progression to dementia across groups. As our theoretical model posits SCD and MBI as independent axes of dementia risk, the ordinal rank of SCD+MBI- and SCD-MBI+is arbitrary between SCD-MBI- and SCD+MBI+, and therefore both permutations were tested. It is similarly worth noting that the hypotenuse in SCD*MBI space, or dementia risk space, is not expected to be additive according to our theoretical model.
We also computed odds ratios (OR) for cognitive and functional decline using logistic regression with the patient group having neither SCD nor MBI (SCD-MBI-) at baseline serving as the reference group. In this model, we included terms for all variables reaching statistical significance (p<0.05) in the univariate analyses to calculate Adjusted Odds Ratios (AOR). All analyses were conducted in SPSS v24 (IBM Corporation) with α set at 0.05.
RESULTS
The final sample consisted of 2769 participants with CDR 0 at baseline. Participants had neither MBI nor SCD (MBI-SCD-; n=1536); SCD but no MBI (MBI-SCD+; n=254); MBI but no SCD (MBI+SCD-; n=743); and both MBI and SCD (MBI+SCD+; n=236). There were significant differences in sex, age, ethnicity, and a history of hypertension but no significant differences regarding any other clinical and demographic characteristic investigated (Table 2).
Table 2
Summary statistics for demographics and MBI-C score by patient grouping in those with baseline CDR=0 (n=2769)
| Mean /Proportion | MBI-/SCD- | MBI-/SCD+ | MBI+/SCD- | MBI+/SCD+ | p | Overall |
| (SD/SE) | (n=1,536; 55.47%) | (n=254; 9.17%) | (n=743; 26.83%) | (n=236; 8.52%) | (n=2,769) | |
| Age | 72.07 (9.22) | 74.80 (8.88) | 73.46 (9.11) | 74.30 (9.53) | <0.001 | 73.00 [67.00, 80.00] |
| Sex (% female) | 1,045 (68.0) | 160 (63.0) | 402 (54.1) | 147 (62.3) | <0.001 | 1,754 (63.3) |
| Education (Years) | 15.87 (2.90) | 15.63 (3.06) | 15.72 (2.97) | 16.13 (2.66) | 0.167 | 15.83 (2.92) |
| Race (% White) | 1,167 (75.9) | 186 (73.2) | 664 (89.4) | 209 (88.6) | <0.001 | 2,226 (80.3) |
| Marital Status | 874 (56.9) | 133 (52.4) | 447 (60.2) | 131 (55.5) | 0.161 | 1,585 (57.2) |
| (% married) | ||||||
| History of | 609 (39.6) | 120 (47.2) | 360 (48.5) | 99 (41.9) | <0.001 | 1,188 (42.9) |
| hypertension | ||||||
| (% present) | ||||||
| Hypercholesterolemia | 537 (35.0) | 97 (38.2) | 331 (44.5) | 97 (41.1) | 0.191 | 1,062 (38.4) |
| (% present) | ||||||
| Diabetes (% present) | 118 (7.7) | 25 (9.8) | 70 (9.4) | 17 (7.2) | 0.500 | 230 (8.3) |
| MBI Score | 0.00 (0.00) | 0.00 (0.00) | 1.90 (1.51) | 1.82 (1.23) | <0.001 | 1.00 [0.00, 2.00] |
| CDR Score at 3 years | 0.03 (0.12) | 0.08 (0.19) | 0.13 (0.31) | 0.16 (0.25) | <0.001 | 0.00 [0.00, 1.00] |
Over the 3 years of follow-up, 349/2769 (12.6%) individuals had evidence of cognitive and functional decline. In Fig. 3, we present the incidence of decline according to the baseline presence of MBI, SCD, or their combination. Of the 1536 MBI-SCD- participa-nts, 80 (5.21%) progressed to CDR >0 at 3 years, while progression for MBI-SCD+ was 42/254 (16.54%), MBI+SCD- was 154/743 (20.73%), and MBI+SCD+was 73/236 (30.93%). This highly sig-nificant difference (linear-by-linear χ2=193.24, df=1, p<0.001) also revealed a strong ordinal by ord-inal symmetry (Somers’ D=0.22, SE=0.015, App-roximate T=12.62, p<0.001), which held whether the order of SCD+MBI- or SCD-MBI+was reversed (linear-by-linear χ2=160.41, df=1, p<0.001; Som-ers’ D=0.21, SE=0.015, Approximate T=12.25, p<0.001).
Fig. 3
Odds of CDR >0 after three years versus MBI/SCD grouping.
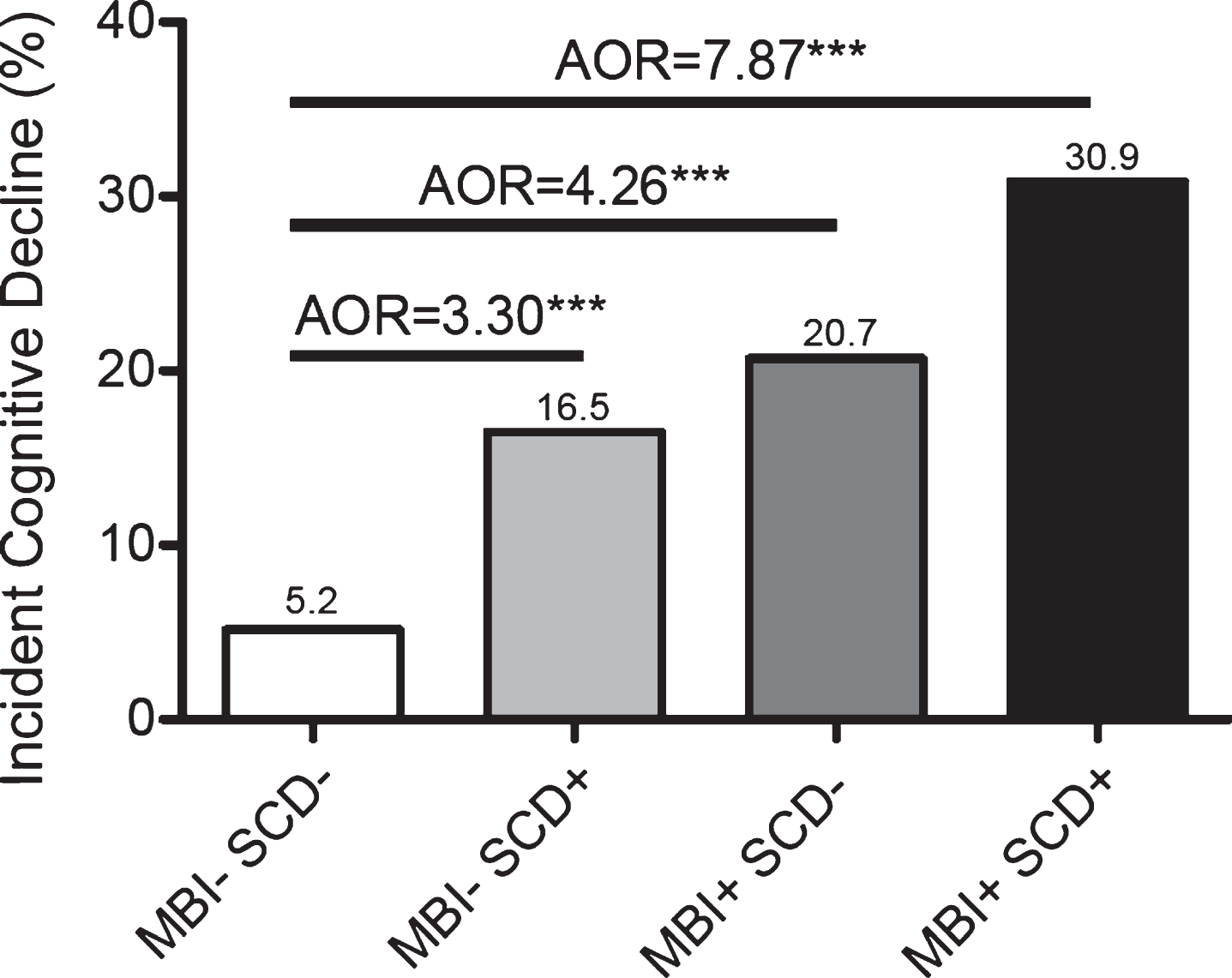
To quantify the increased risk of incident cognitive and functional decline according to these baseline risk definitions, we used logistic regression to generate adjusted odds ratios (AOR) and 95% confidence intervals (CI). The odds of change to CDR >0 was 8.15 times higher for MBI+SCD+than MBI-SCD- (95% CI 5.71–11.64, p<0.001; AOR=7.87, 95% CI: 5.46–11.35, p<0.001). Those with MBI+SCD- had 4.76 times the odds of increased CDR than MBI-SCD- individuals (95% CI 3.57–6.34, p<0.001; AOR=4.26, 95% CI: 3.17–5.73, p<0.001). Those with MBI-SCD+had 3.61 times the odds of inc-reased CDR than MBI-SCD- individuals (95% CI 2.42–5.38, p<0.001; AOR=3.30, 95% CI: 2.20–4.96, p<0.001). Covariates for all models were age, sex, race, and history of hypertension.
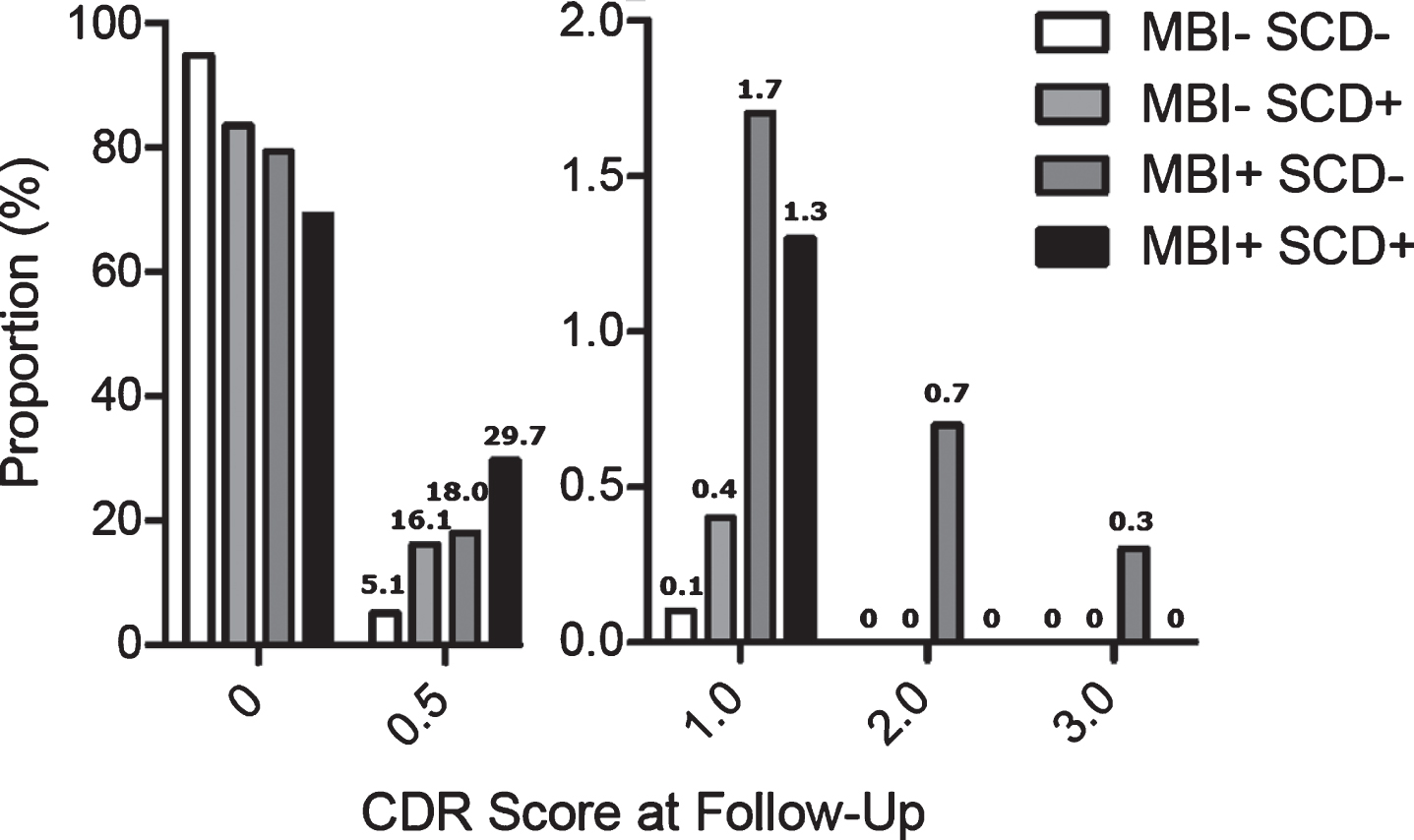
We then examined the distribution of CDR scores at follow-up according to the presence of SCD, MBI, or both at baseline (Fig. 4), which revealed that the magnitude of progression from CDR 0 in-creased incrementally according to baseline characteristics. This distribution in CDR scores was significantly different across groups (linear-by-linear χ2=165.96, df=1, p<0.001), with strong ordinal by ordinal symmetry (Somers’ D=0.22, Approximate T=12.69, p<0.001). This also held when the order of SCD+MBI- and SCD-MBI+was reversed (linear-by-linear χ2=116.32, df=1, p<0.001; Somers’ D=0.21, Approximate T=12.32, p<0.001).
Fig. 4
CDR score at follow up (baseline CDR=0).
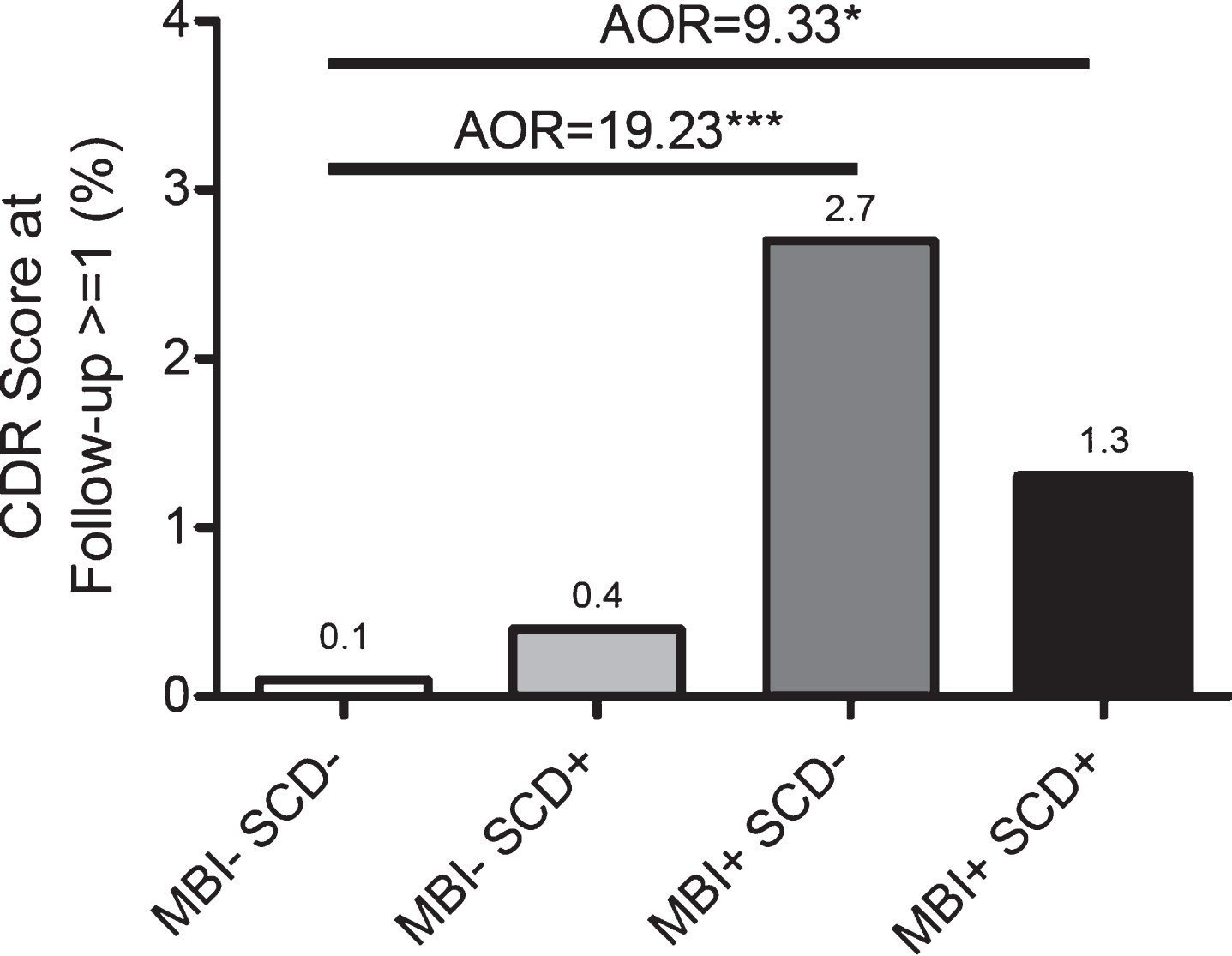
Over the 3 years of follow-up, 2/1536 of the MBI-SCD- participants (0.1%) progressed to CDR ≥1 (dementia), compared to 1/254 of the MBI-SCD+ participants (0.4%), 20/743 of the MBI+SCD- participants (2.7%), and 3/236 of the MBI+SCD+ par-ticipants (1.3%) (Fig. 5). To quantify this increased risk of incident dementia over the follow-up period, we used logistic regression. Compared to MBI-SCD-, the odds of progressing to dementia over the follow-up period were 21.21 (95% CI: 4.94–91.01, p<0.001; AOR=19.23, 95% CI: 4.40–84.03, p<0.001) for MBI+SCD- and 9.87 (95% CI: 1.64–59.41, p<0.05; AOR=9.33, 95% CI: 1.53–56.78, p<0.05) for MBI+SCD+individuals. Covariates for all models were age, sex, ethnicity, and history of hypertension.
Fig. 5
Odds of dementia (CDR ≥1) after 3 years versus MBI/SCD grouping. Adjusted Odds Ratios (AORs) are adjusted for age, sex, ethnicity, and hypertension.
DISCUSSION
In an analysis of a longitudinal cohort of 2,769 participants, we demonstrated that those who are cognitively unimpaired, have MBI, SCD, or both MBI and SCD lie on a continuum of risk for incident cognitive decline and dementia. Importantly, MBI was associated with progression to CDR >0 at the three year visit even when cognitive symptoms were absent (i.e., in the absence of SCD). The OR for progression to CDR >0 was numerically higher in persons with MBI alone (20.7% progression rate; OR 4.76) compared with SCD alone (16.5% progression rate; OR 3.76) but this difference was not statistically significant. Of all the MBI+participants, 23.19% progressed from CDR 0 to CDR >0 at 3 years, compared to 23.5% of the total SCD+group, and 30.9% of the intersection group of both MBI+and SCD+. MBI was also associated with progression from normal cognition to dementia (CDR ≥1), with very high ORs (OR of 19.23 for MBI alone and 9.87 for MBI+SCD+) but these analyses were based on only a handful of events and consequently specific estimates are likely unstable.
Nosology of psychiatric symptoms
Chronic and recurrent psychiatric syndromes are associated with an increased risk of dementia [39, 40]; however, de novo persistent psychiatric symptoms in older age constitute a unique risk marker. In order to restrict our approach to this population, we excluded participants with a psychiatric diagno-sis at baseline as MBI diagnosis is precluded by the presence of a psychiatric disorder. This is important because several large longitudinal cohorts have provided compelling evidence that the age of onset of psychiatric symptoms is a crucial factor in determi-ning the nature of these symptoms. These studies sug-gest that the later in life the onset of psychiatric symptomatology, the more likely these symptoms re-present the early stages of a neurodegenerative process, precede dementia by 5–11 years [41–44], and have a higher progression rate than early onset psy-chiatric syndromes, which are themselves at heightened risk [13]. From a community cohort of 9,931 participants, the emergence of MBI was associated with decline in attention and working memory at 1 year [12]. In psychiatry and neurology specialty clinic samples, incidence of dementia was higher for MBI than other psychiatric disorders [13, 14]. This evidence suggests that chronic and recurrent psychiatric symptoms reflect a psychiatric disorder framed in the context of psychiatric conditions, are sometimes neurodevelopmental in origin, i.e., these conditions are not a consequence of later life neurodegenerative disease, supporting exclusion from our analysis. In contrast to this, late onset psychiatric symptoms may be prodromal or precursor to cognitive decline and dementia in some and are better framed in the context of neurodegeneration. The ISTAART-AA MBI criteria were developed with an appreciation of the difference between later life de novo behavioral changes, and psychiatric disease recurring in later life [9]. Indeed, MBI has been associated with amyloid, tau, and neurodegeneration, and is now harmonized with the biological understanding of AD [30].
SCD
SCD is also represented in Stage 2 AD of the NIA-AA research framework in which there is subjective or objective evidence of subtle decline, not meeting criteria for objective impairment. On the AD continuum, subjective complaints of cognitive impairment, with or without evidence of impairment on cogni-tive testing [45], would be considered evidence of subtle cognitive decline and attributable to the pat-hologic process [30]. Meta-analysis of large longitudinal cohorts has shown that SCD is associated with ORs of 6 of progression to MCI and 2 for progression to dementia over a mean of 4.8 years [6]. In a study of older adults with SCD, ascertained using a composite score of 3 rating scales, 26% were determined to be Aβ+[5]. However, significant inter-site variability in the association between SCD and abnormal cerebrospinal fluid amyloid levels has been attributed to different recruitment approaches and a lack of standardized case definitions and ascertainment [46]. There can be other contributors to subjective complaints of cognitive decline not limited to medical issues, stressors or even medications. Nonetheless, as with MBI, SCD reflects the index clinical manifestation of a neurodegenerative process for some.
Intersection of MBI and SCD
MBI and SCD intersect in some instances. For example, a study of SCD determined that worries about self-perceived functioning were associated with Aβ positivity, rather than subjective cognitive functioning itself [47]. Worries or concerns are in-cluded in the SCD plus criteria, proposed to increase specificity for detecting preclinical AD [3]. Worry is also a component of the MBI affective dysregulation domain, which includes emergent mood and anxiety symptoms. Mood symptoms and SCD have been shown to interact to predict dementia independent of their main effects [48], and similar results have been found examining persistent neuropsychiatric symptoms and MCI [15]. The approach to psychiatric symptomatology in SCD has generally utilized traditional constructs of personality (e.g., neuroticism) and psychiatric conditions [49–51]. However, a change in personality to greater neuroticism (which is a neurodevelopmental construct) can also be framed as the emergence of MBI affective dysregulation, if considered in a neurodegenerative frame of reference [49]. This intersection of MBI and SCD is consistent with both constructs being represented in NIA-AA stage 2 AD.
NPI-Q informant report
The source of information for MBI status in our study was the NPI-Q [36] completed by an informant. The NPI-Q was developed to measure NPS in dementia, and the symptoms as described are relevant to an aging population with neurodegenerative disease. Informant reports have shown to be more reliable assessments of NPS in neurodegenerative disease to minimize the impact of anosognosia [52]. Coincidentally, in another study of SCD, confirmation of decline by an informant was the best predictor of worse cognitive performance and lower gray matter volumes [53]. Anosognosia is also important to consider in the assessment of SCD. The INSIGHT-PreAD study showed that patients with low cognitive awareness (reporting fewer difficulties than their relatives did) showed greater amyloid burden and lower cortical metabolism, compared to the high awareness group [54]. These findings suggest that self-report of symptoms alone, whether cognitive or behavioral, may not be adequate to capture early disease.
Clinical and research implications
Our data indicate that in cognitively normal older adults, the neurobehavioral axis of dementia risk represented by MBI, and neurocognitive axis of demen-tia risk represented by SCD, have complementary associations with the risk of progression to MCI and dementia. As operationalized in our study, MBI appears to be at least as strong a risk factor for progression to MCI or dementia as SCD and the two constructs have overlapping features. The combination of both MBI and SCD was associated with the highest risk (30.9% at 3 years), and this may have clinical utility by identifying a subset of individual at high risk of progression and reducing overmedi-calization of risk markers that in isolation have low specificity.
In addition to screening for subjective and obj-ective cognitive symptoms in older adults, incorporating MBI into clinical assessments may provide complementary information and better risk stratification [55, 56]. Not infrequently, dementia patients are first given a psychiatric diagnosis when presenting with a neuropsychiatric symptom, resulting in delays to treatment [57, 58]. Identifying MBI would prompt clinicians to consider neurocognitive disorders on the differential diagnosis, and flag patients who might benefit from imaging or further workup.
These findings also have clinical trial implications. Research and development costs are higher for AD than other therapy areas due to lower success rates and longer development times [2]. Despite the fact that changes in brain structure and function occur up to 20 years before gross memory impairment [59], screening for preclinical disease is expensive and inefficient. Leveraging the ease of measurement of MBI, in conjunction with SCD, could be an inexpen-sive and scalable method to select patients at highest risk for biomarker positivity and cognitive decline. For dementia prevention trials, combining MBI and SCD could increase yield and improve signal-to-noise ratios for clinical trial screening in order to identify an enriched group for assessment and workup. An associated reduction in screen failures could increase trial efficiency and decrease trial cost.
Limitations
The NACC participants are mostly white, highly educated volunteers seeking care and consultation at urban, university-based centers, and the finding may not generalize to other settings. Our choice of the CDR as the outcome measure was chosen for its clinical relevance, representing cognition-related daily function as a real-world outcome meaningful to patients, family members, and clinicians. However, this outcome does not provide as much detail on cognition as neuropsychological testing which could more accurately describe the performance of the four groups. For MBI case ascertainment, we used the NPI-Q [36] rather than the validated MBI-C [56, 60, 61] which was developed specifically to measure MBI. We used a validated algorithm to convert NPI-Q scores to MBI-C domains and required NPS at 2 consecutive visits to match the MBI criterion of symptom persistence. However, the cutoff of >0 to define MBI+may not provide optimum specificity and risks overestimating the MBI phenomenon. Although 44.5% of the participants had a dementia risk syndrome of either MBI or SCD (or both), 21.8% of these had cognitive and functional decline at 3 years (compared to 5.2% of participants with neither risk syndrome)—a substantial proportion, nonetheless. Additional research is needed to refine the group, balancing the risk of overmedicalization with the need to determine risk with sufficient sensitivity in order to not miss cases of preclinical disease; the MBI-C may help with this need. MBI criteria stipulate that symptoms are not better accounted for by life events. However, life context could not be determined using the data available and there may be a proportion of participants with reactive symptoms, even over the two-visit duration used to determine MBI status. Similarly, MBI criteria require symptoms to have an impact on interpersonal relationships, social functioning and workplace performance. These data were not included in the analysis, and it is possible for some with mild symptoms to have minimal impact in those domains.
CONCLUSION
In summary, we have demonstrated that MBI, a neurobehavioral syndrome, is an important predictor of incident cognitive and functional decline at 3 years in cognitively normal subjects, supporting the use of MBI as a powerful risk assessment tool. Our findings suggest that MBI is at least as useful as SCD in assessing risk for incident cognitive decline and dementia, and that the two constructs are likely complementary. Assessment of the neurobehavioral and neurocognitive axes at the same time are required in cognitively normal individuals to better define their risk.
ACKNOWLEDGMENTS
Data used in this study was from the NACC database which is funded by NIA/NIH Grant U01 AG016976. NACC data are contributed by the NIA-funded ADCs: P30 AG019610 (PI Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P30 AG062428-01 (PI James Leverenz, MD) P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P50 AG047266 (PI Todd Golde, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P30 AG062421-01 (PI Bradley Hyman, MD,PhD), P30 AG062422-01 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Thomas Wisniewski, MD), P30 AG013854 (PI Robert Vassar, PhD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P30 AG062429-01(PI James Brewer, MD, PhD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG053760 (PI Henry Paulson, MD, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P30 AG049638 (PI Suzanne Craft, PhD), P50 AG005136 (PI Thomas Grabowski, MD), P30 AG062715-01(PI Sanjay Asthana, MD, FRCP), P50 AG005681 (PI John Morris, MD), P50 AG047270 (PI Stephen Strittmatter, MD, PhD).
Additional support was from The Alzheimer Society of Calgary via the Hotchkiss Brain Institute. We also acknowledge the Mathison Centre for Mental Health Research & Education, and the Ron and Rene Ward Centre for Healthy Brain Aging for support.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/20-1184r2).
REFERENCES
[1] | Mortby ME , Black SE , Gauthier S , Miller DS , Porsteinsson A , Smith EE , Ismail Z ((2018) ) Dementia clinical trial implications of mild behavioral impairment. Int Psychogeriatr 30: , 171–175. |
[2] | Gauthier S , Albert M , Fox N , Goedert M , Kivipelto M , Mestre-Ferrandiz J , Middleton LT ((2016) ) Why has therapy development for dementia failed in the last two decades? Alzheimers Dement 12: , 60–64. |
[3] | Jessen F , Amariglio RE , Van Boxtel M , Breteler M , Ceccaldi M , Chételat G , Dubois B , Dufouil C , Ellis KA , Van Der Flier WM ((2014) ) A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement 10: , 844–852. |
[4] | Slot RE , Verfaillie SC , Overbeek JM , Timmers T , Wesselman LM , Teunissen CE , Dols A , Bouwman FH , Prins ND , Barkhof F ((2018) ) Subjective Cognitive Impairment Cohort (SCIENCe): Study design and first results. Alzheimers Res Ther 10: , 76. |
[5] | Amariglio RE , Becker JA , Carmasin J , Wadsworth LP , Lorius N , Sullivan C , Maye JE , Gidicsin C , Pepin LC , Sperling RA ((2012) ) Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia 50: , 2880–2886. |
[6] | Mitchell A , Beaumont H , Ferguson D , Yadegarfar M , Stubbs B ((2014) ) Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: Meta-analysis. Acta Psychiatr Scand 130: , 439–451. |
[7] | Mortby ME , Lyketsos CG , Geda YE , Ismail Z ((2018) ) Special Issue on mild behavioral impairment and non-cognitive prodromes to dementia. Int Psychogeriatr 30: , 167–169. |
[8] | Wise EA , Rosenberg PB , Lyketsos CG , Leoutsakos J-M ((2019) ) Time course of neuropsychiatric symptoms and cog-nitive diagnosis in National Alzheimer’s Coordinating Centers volunteers. Alzheimer’s Dement (Amst) 11: , 333–339. |
[9] | Ismail Z , Smith EE , Geda Y , Sultzer D , Brodaty H , Smith G , Agüera-Ortiz L , Sweet R , Miller D , Lyketsos CG ((2016) ) Neuropsychiatric symptoms as early manifestations of emergent dementia: Provisional diagnostic criteria for mild behavioral impairment. Alzheimers Dement 12: , 195–202. |
[10] | Rouse HJ , Small BJ , Schinka JA , Loewenstein DA , Duara R , Potter H ((2020) ) Mild behavioral impairment as a predictor of cognitive functioning in older adults. Int Psychogeriatr, doi: 10.1017/S1041610220000678 |
[11] | Kassam F , Chen H-Y , Nosheny RL , Williams T , Mackin RS , Weiner MW , Ismail Z ((2020) ) Cognitive profile of mild behavioral impairment (MBI) in brain health registry participants. Alzheimers Dement 16: (Suppl 6), e047673. |
[12] | Creese B , Brooker H , Ismail Z , Wesnes KA , Hampshire A , Khan Z , Megalogeni M , Corbett A , Aarsland D , Ballard C ((2019) ) Mild behavioral impairment as a marker of cognitive decline in cognitively normal older adults. Am J Geriatr Psychiatry 27: , 823–834. |
[13] | Taragano FE , Allegri RF , Heisecke SL , Martelli MI , Feldman ML , Sánchez V , García VA , Tufro G , Castro DM , Leguizamón PP ((2018) ) Risk of conversion to dementia in a mild behavioral impairment group compared to a psychiatric group and to a mild cognitive impairment group. J Alzheimers Dis 62: , 227–238. |
[14] | Matsuoka T , Ismail Z , Narumoto J ((2019) ) Prevalence of mild behavioral impairment and risk of dementia in a psychiatric outpatient clinic. J Alzheimers Dis 70: , 505–513. |
[15] | Nathan S , Gill S , Ismail Z ((2020) ) ApoE ɛ4 status in pre-dementia risk states, mild behavioural impairment and subjective cognitive decline, and the risk of incident cognitive decline. Alzheimers Dement 16: (Suppl 6), e046615. |
[16] | Rao AR , Chatterjee P , Thakral M , Dwivedi S , Dey AB ((2020) ) Behavioural issues in late life may be the precursor of dementia-A cross sectional evidence from memory clinic of AIIMS, India. PLoS One 15: , e0234514. |
[17] | Gill S , Mouches P , Hu S , Rajashekar D , MacMaster FP , Smith EE , Forkert ND , Ismail Z , Initiative AsDN ((2020) ) Using machine learning to predict dementia from neuropsychiatric symptom and neuroimaging data. J Alzheimers Dis 75: , 277–288. |
[18] | Lussier FZ , Pascoal TA , Chamoun M , Therriault J , Tissot C , Savard M , Kang MS , Mathotaarachchi S , Benedet AL , Parsons M , Qureshi MNI , Thomas ÉM , Shin M , Dion L-A , Massarweh G , Soucy J-P , Tsai I-H , Vitali P , Ismail Z , Rosa-Neto P , Gauthier S ((2020) ) Mild behavioral impairment is associated with β-amyloid but not tau or neurodegeneration in cognitively intact elderly individuals. Alzheimers Dement 16: , 192–199. |
[19] | Johansson M , Stomrud E , Insel P , Leuzy A , Johansson P , Smith R , Ismail Z , Janelidze S , Palmqvist S , van Westen D , Mattsson N , Hansson O ((2021) ) Mild behavioral impairment and its relation to tau pathology in preclinical Alzheimer’s disease. Transl Psychiatry 11: , 76. |
[20] | Lussier F , Pascoal T , Therriault J , Chamoun M , Tissot C , Savard M , Mathotaarachchi S , Ismail Z , Rosa-Neto P , Gauthier S ((2019) ) Mild behavioral impairment is associated with β-amyloid and tau across the Alzheimer’s disease spectrum. In J Cereb Blood Flow Metab 39: (1 Suppl), 158–159. |
[21] | Naude J , Gill S , Hu S , McGirr A , Forkert N , Monchi O , Stys P , Smith EE , Ismail Z ((2020) ) Plasma neurofilament light: A marker of cognitive decline in mild behavioural impairment. J Alzheimers Dis 76: , 1017–1027. |
[22] | Matuskova V , Ismail Z , Nikolai T , Markova H , Cechova K , Laczó J , Nedelska Z , Lerch O , Hort J , Vyhnalek M ((2020) ) Mild behavioral impairment is associated with atrophy in Alzheimer’s disease-related regions in non-demented older adults. Alzheimers Dement 16: (Suppl 6), e044819. |
[23] | Yoon E , Ismail Z , Hanganu A , Kibreab M , Hammer T , Cheetham J , Kathol I , Sarna JR , Martino D , Furtado S , Monchi O ((2019) ) Mild Behavioral Impairment is linked to worse cognition and brain atrophy in Parkinson’s disease. Neurology 93: , e766–e777. |
[24] | Orso B , Mattei C , Arnaldi D , Massa F , Serafini G , Plantone D , Doglione E , Grafman J , Nobili F , Pardini M ((2020) ) Clinical and MRI predictors of conversion from mild behavioural impairment to dementia. Am J Geriatr Psychiatry 28: , 755–763. |
[25] | Gill S , Wang M , Forkert ND , MacMaster FP , Smith EE , Ismail Z ((2019) ) Diffusion Tensor Imaging in pre-dementia risk states: White matter atrophy findings in mild behavioral impairment (P5. 1-025). Neurology 92: (15 Suppl), P5.1–025. |
[26] | Lang S , Ismail Z , Kibreab M , Kathol I , Sarna J , Monchi O ((2020) ) Common and unique connectivity at the interface of motor, neuropsychiatric, and cognitive symptoms in Parkinson’s disease: A commonality analysis. Hum Brain Mapp 41: , 3749–3764. |
[27] | Lang S , Yoon EJ , Kibreab M , Kathol I , Cheetham J , Hammer T , Sarna J , Ismail Z , Monchi O ((2020) ) Mild behavioral impairment in Parkinson’s disease is associated with altered corticostriatal connectivity. Neuroimage Clin 26: , 102252. |
[28] | Andrews SJ , Ismail Z , Anstey KJ , Mortby M ((2018) ) Association of Alzheimer’s genetic loci with mild behavioral impairment. Am J Med Genet B Neuropsychiatr Genet 177: , 727–735. |
[29] | Creese B , Brooker H , Aarsland D , Corbett A , Ballard C , Ismail Z ((2020) ) Genetic risk for Alzheimer disease, cognition and mild behavioral impairment in healthy older adults. Alzheimer’s & Dementia: Diagnosis and Disease Monitoring, in press. |
[30] | Jack CR Jr , Bennett DA , Blennow K , Carrillo MC , Dunn B , Haeberlein SB , Holtzman DM , Jagust W , Jessen F , Karlawish J ((2018) ) NIA-AA research framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14: , 535–562. |
[31] | Weintraub S , Salmon D , Mercaldo N , Ferris S , Graff-Radford NR , Chui H , Cummings J , DeCarli C , Foster NL , Galasko D ((2009) ) The Alzheimer’s disease centers’ uniform data set (UDS): The neuropsychological test battery. Alzheimer Dis Assoc Disord 23: , 91. |
[32] | Beekly DL , Ramos EM , Lee WW , Deitrich WD , Jacka ME , Wu J , Hubbard JL , Koepsell TD , Morris JC , Kukull WA ((2007) ) The National Alzheimer’s Coordinating Center (NACC) database: The uniform data set. Alzheimer Dis Assoc Disord 21: , 249–258. |
[33] | Morris JC , Weintraub S , Chui HC , Cummings J , DeCarli C , Ferris S , Foster NL , Galasko D , Graff-Radford N , Peskind ER ((2006) ) The Uniform Data Set (UDS): Clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord 20: , 210–216. |
[34] | Sheikh F , Ismail Z , Mortby ME , Barber P , Cieslak A , Fischer K , Granger R , Hogan DB , Mackie A , Maxwell CJ , Menon B , Mueller P , Patry D , Pearson D , Quickfall J , Sajobi T , Tse E , Wang M , Smith EE , investigators Pr ((2018) ) Prevalence of mild behavioral impairment in mild cognitive impairment and subjective cognitive decline, and its association with caregiver burden. Int Psychogeriatr 30: , 233–244. |
[35] | Mortby ME , Ismail Z , Anstey KJ ((2018) ) Prevalence estimates of mild behavioral impairment in a population-based sample of pre-dementia states and cognitively healthy older adults. Int Psychogeriatr 30: , 221–232. |
[36] | Kaufer DI , Cummings JL , Ketchel P , Smith V , MacMillan A , Shelley T , Lopez OL , DeKosky ST ((2000) ) Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci 12: , 233–239. |
[37] | Morris JC ((1993) ) The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology 43: , 2412–2414. |
[38] | Somers RH ((1962) ) A new asymmetric measure of association for ordinal variables. Am Sociol Rev 27: , 799–811. |
[39] | Ismail Z , Malick A , Smith EE , Schweizer T , Fischer C ((2014) ) Depression versus dementia: Is this construct still relevant? Neurodegener Dis Manag 4: , 119–126. |
[40] | Panza F , Frisardi V , Capurso C , D’Introno A , Colacicco AM , Imbimbo BP , Santamato A , Vendemiale G , Seripa D , Pilotto A ((2010) ) Late-life depression, mild cognitive impairment, and dementia: Possible continuum? Am J Geriatr Psychiatry 18: , 98–116. |
[41] | Ismail Z , Gatchel J , Bateman DR , Barcelos-Ferreira R , Chantillon M , Jaeger J , Donovan NJ , Mortby ME ((2018) ) Affective and emotional dysregulation as pre-dementia risk markers: Exploring the mild behavioral impairment symptoms of depression, anxiety, irritability, and euphoria. Int Psychogeriatr 30: , 185–196. |
[42] | Almeida O , Hankey G , Yeap B , Golledge J , Flicker L ((2017) ) Depression as a modifiable factor to decrease the risk of dementia. Transl Psychiatry 7: , e1117. |
[43] | Singh-Manoux A , Dugravot A , Fournier A , Abell J , Ebmeier K , Kivimäki M , Sabia S ((2017) ) Trajectories of depressive symptoms before diagnosis of dementia: A 28-year follow-up study. JAMA Psychiatry 74: , 712–718. |
[44] | Tapiainen V , Hartikainen S , Taipale H , Tiihonen J , Tolppanen A-M ((2017) ) Hospital-treated mental and behavioral disorders and risk of Alzheimer’s disease: A nationwide nested case-control study. Eur Psychiatry 43: , 92–98. |
[45] | van Harten AC , Mielke MM , Swenson-Dravis DM , Hagen CE , Edwards KK , Roberts RO , Geda YE , Knopman DS , Petersen RC ((2018) ) Subjective cognitive decline and risk of MCI: The Mayo Clinic Study of Aging. Neurology 91: , e300–e312. |
[46] | Wolfsgruber S , Molinuevo JL , Wagner M , Teunissen CE , Rami L , Coll-Padrós N , Bouwman FH , Slot RE , Wesselman LM , Peters O ((2019) ) Prevalence of abnormal Alzheimer’s disease biomarkers in patients with subjective cognitive decline: Cross-sectional comparison of three European memory clinic samples. Alzheimers Res Ther 11: , 8. |
[47] | Verfaillie SC , Timmers T , Slot RE , Van Der Weijden CW , Wesselman LM , Prins ND , Sikkes SA , Yaqub M , Dols A , Lammertsma AA ((2019) ) Amyloid-β load is related to worries, but not to severity of cognitive complaints in individuals with subjective cognitive decline: The SCIENCe project. Front Aging Neurosci 11: , 7. |
[48] | Lee YC , Kang JM , Lee H , Kim K , Kim S , Yu TY , Lee E-M , Kim CT , Kim DK , Lewis M ((2020) ) Subjective cognitive decline and subsequent dementia: A nationwide cohort study of 579,710 people aged 66 years in South Korea. Alzheimers Res Ther 12: , 1–13. |
[49] | Agv̈era-Ortiz L , Lyketsos C , Ismail Z ((2019) ) Comment on “Personality Changes During the Transition from Cognitive Health to Mild Cognitive Impairment”. J Am Geriatr Soc 67: , 190–191. |
[50] | Caselli RJ , Langlais BT , Dueck AC , Henslin BR , Johnson TA , Woodruff BK , Hoffman-Snyder C , Locke DE ((2018) ) Personality changes during the transition from cognitive health to mild cognitive impairment. J Am Geriatr Soc 66: , 671–678. |
[51] | Sutin AR , Stephan Y , Luchetti M , Terracciano A ((2018) ) Self-reported personality traits are prospectively associated with proxy-reported behavioral and psychological symptoms of dementia at the end of life. Int J Geriatr Psychiatry 33: , 489–494. |
[52] | Verhülsdonk S , Quack R , Höft B , Lange-Asschenfeldt C , Supprian T ((2013) ) Anosognosia and depression in patients with Alzheimer’s dementia. Arch Gerontol Geriatr 57: , 282–287. |
[53] | Sánchez-Benavides G , Grau-Rivera O , Suárez-Calvet M , Minguillon C , Cacciaglia R , Gramunt N , Falcon C , Gispert JD , Molinuevo JL ((2018) ) Brain and cognitive correlates of subjective cognitive decline-plus features in a population-based cohort. Alzheimers Res Ther 10: , 123. |
[54] | Cacciamani F , Tandetnik C , Gagliardi G , Bertin H , Habert M-O , Hampel H , Boukadida L , Révillon M , Epelbaum S , Dubois B ((2017) ) Low cognitive awareness, but not complaint, is a good marker of preclinical Alzheimer’s disease. J Alzheimers Dis 59: , 753–762. |
[55] | Ismail Z , Black S , Camicioli R , Chertkow H , Herrmann N , Jr. RL , Montero-Odasso M , Rockwood K , Rosa-Neto P , Seitz D , Sivananthan S , Smith EE , Soucy J-P , Vedel I , Gauthier S ((2020) ) Recommendations of the 5th Canadian Consensus Conference on the Diagnosis and Treatment of Dementia. Alzheimers Dement 16: , 1182–1195. |
[56] | Ismail Z , Aguera-Ortiz L , Brodaty H , Cieslak A , Cummings J , Fischer CE , Gauthier S , Geda YE , Herrmann N , Kanji J , Lanctot KL , Miller DS , Mortby ME , Onyike CU , Rosenberg PB , Smith EE , Smith GS , Sultzer DL , Lyketsos C , Istaart N-P ((2017) ) The Mild Behavioral Impairment Checklist (MBI-C): A Rating Scale for Neuropsychiatric Symptoms in Pre-Dementia Populations. J Alzheimers Dis 56: , 929–938. |
[57] | Cieslak A , Smith EE , Lysack J , Ismail Z ((2018) ) Case series of mild behavioral impairment: Toward an understanding of the early stages of neurodegenerative diseases affecting behavior and cognition. Int Psychogeriatr 30: , 273–280. |
[58] | Woolley JD , Khan BK , Murthy NK , Miller BL , Rankin KP ((2011) ) The diagnostic challenge of psychiatric symptoms in neurodegenerative disease: Rates of and risk factors for prior psychiatric diagnosis in patients with early neurodegenerative disease. J Clin Psychiatry 72: , 126–133. |
[59] | Reiman EM , Quiroz YT , Fleisher AS , Chen K , Velez-Pardo C , Jimenez-Del-Rio M , Fagan AM , Shah AR , Alvarez S , Arbelaez A ((2012) ) Brain imaging and fluid biomarker analysis in young adults at genetic risk for autosomal dominant Alzheimer’s disease in the presenilin 1 E280A kindred: A case-control study. Lancet Neurol 11: , 1048–1056. |
[60] | Creese B , Griffiths A , Brooker H , Corbett A , Aarsland D , Ballard C , Ismail Z ((2020) ) Profile of mild behavioral impairment and factor structure of the mild behavioral impairment checklist in cognitively normal older adults. Int Psychogeriatr 32: , 705–717. |
[61] | Mallo SC , Ismail Z , Pereiro AX , Facal D , Lojo-Seoane C , Campos-Magdaleno M , Juncos-Rabadán O ((2019) ) Assessing mild behavioral impairment with the mild behavioral impairment checklist in people with subjective cognitive decline. Int Psychogeriatr 31: , 231–239. |



