Associations Between Caffeine Consumption, Cognitive Decline, and Dementia: A Systematic Review
Abstract
Background:
Epidemiologic studies have provided inconclusive evidence for a protective effect of caffeine consumption on risk of dementia and cognitive decline.
Objective:
To summarize literature on the association between caffeine and 1) the risk of dementia and/or cognitive decline, and 2) cognitive performance in individuals with mild cognitive impairment (MCI) or dementia, and 3) to examine the effect of study characteristics by categorizing studies based on caffeine source, quantity and other possible confounders.
Methods:
We performed a systematic review of caffeine effects by assessing overall study outcomes; positive, negative or no effect. Our literature search identified 61 eligible studies performed between 1990 and 2020.
Results:
For studies analyzing the association between caffeine and the risk of dementia and/or cognitive decline, 16/57 (28%) studies including a total of 40,707/153,070 (27%) subjects reported positive study outcomes, and 30/57 (53%) studies including 71,219/153,070 (47%) subjects showed positive results that were dependent on study characteristics. Caffeine effects were more often positive when consumed in moderate quantities (100–400 mg/d), consumed in coffee or green tea, and in women. Furthermore, four studies evaluated the relationship between caffeine consumption and cognitive function in cognitively impaired individuals and the majority (3/4 [75% ]) of studies including 272/289 subjects (94%) reported positive outcomes.
Conclusion:
This review suggests that caffeine consumption, especially moderate quantities consumed through coffee or green tea and in women, may reduce the risk of dementia and cognitive decline, and may ameliorate cognitive decline in cognitively impaired individuals.
INTRODUCTION
Dementia is a clinical syndrome characterized by progressive deterioration of cognitive functions and loss of independence in activities of daily living. App-roximately 50 million people are living with dementia worldwide. This number is continuously rising [1], and in 2017 the World Health Organization listed dementia as a public health priority [2]. A range of neuropathological disease entities may underlie a dementia syndrome, including Alzheimer’s disease (AD), vascular pathology (VaD), Lewy bodies (DLB), Parkinson’s disease (PD), or frontotemporal lobar degeneration [1]. Many factors such as cardio- and cerebrovascular disease, metabolism, psychiatric conditions, lifestyle, and education, potentially contribute to the risk of different types of dementia [3]. Furthermore, recent studies have suggested endo- and neurocrine interactions between gut microbiota and the brain (i.e., the microbiota-gut-brain axis [4, 5]) and that dietary factors such as caffeine intake can thereby influence the risk of dementia [6].
Caffeine is a psychoactive substance that is present in many beverages and some foods. The most widely known and consumed caffeine source is coffee, but caffeine can also be found in tea, energy drinks, car-bonated soft drinks, fruits, and cocoa-containing foods [7, 8]. After caffeine ingestion the substance is absorbed into the bloodstream via the gastrointes-tinal tract. From there, caffeine is distributed throughout the entire body. Caffeine biologically acts as an adenosine A1 and A2A receptor antagonist, and these receptors are widely distributed throughout the central and peripheral nervous system [9]. By blocking adenosine receptors, caffeine is capable of exerting effects on metabolism, the cardiovascular system, the respiratory system, and neuroinflammatory, neuromodulatory, and neuroprotective processes [10, 11]. More specifically, caffeine may stimulate gastric acid secretion and vasoconstriction, elevate the heart rate and blood pressure, increase the respiratory rate, and ultimately decrease neurodegeneration. Caffeine is able to enhance alertness, wakefulness, psychomotor vigilance, and memory, possibly also through an effect on NMDA receptors [12–14]. Furthermore, caffeine may reduce neuroinflammation and afford neuroprotection, through the consecutive lowering of extracellular calcium, glutamate release from the cell, and microglial activation [15]. There are also health risks associated with excessive caffeine consumption, including anxiety, panic attacks, psychosis, mania, tension, nervousness, irritability, restlessness, nausea, palpitations, insomnia, and diuresis [16].
Research in animal models indicates that caffeine can ameliorate cognitive decline [17]. Studies assessing possible mechanisms underlying this effect have suggested that the effects of caffeine on A2A receptors can control abnormal synaptic plasticity and synaptotoxicity [18, 19]. Other studies have posited that caffeine intake may delay or reduce the risk of AD by decreasing hippocampal amyloid-β levels in transgenic mice through A2A receptor blockade [20, 21].
In human epidemiological studies, results for the protective effects of caffeine on cognitive decline and dementia have been mixed. Some studies suggest positive influences of caffeine intake on neurological disorders and dementia [22, 23], while other studies have found no associations between caffeine and de-mentia [24, 25]. The association between caffeine consumption, cognitive decline, and dementia therefore remains inconclusive.
Here, we summarize the available literature on this topic and provide a systematic review. We aimed to address whether there is an association between caffeine and 1) the risk of dementia and/or cognitive decline, and 2) cognitive function in already cognitively impaired individuals (i.e., MCI or dementia). We further aimed to examine the effects of study characteristics (e.g., caffeine source and quantity) and demographic variables of the study sample (e.g., age and sex) on study outcomes.
METHODS
Study selection procedure
We searched the PubMed and Web of Science databases for studies published before June 2, 2020, using the following (combination of) search terms: ‘caffeine’, ‘coffee’, ‘tea’, AND ‘dementia’ OR ‘Al-zheimer(’s)’, AND ‘cognitive’ or ‘cognition’. Only peer-reviewed articles on studies in humans that were published in English, were eligible for inclusion in this pre-determined systematic review. Cross references were additionally assessed for eligibility. We included cognitively unimpaired individuals as well as individuals diagnosed with any type of dementia and/or MCI. The main criteria for article selection were 1) provision of information on the relation between caffeine consumption and the risk of dementia/cognitive decline, and/or 2) assessment of the association of caffeine on cognitive function in individuals with mild cognitive impairment (MCI) or dementia. Because many studies included a mixed sample of persons with dementia and MCI, both groups were taken together and termed ‘cognitively impaired’ subjects. We included any paper that des-cribed original research, regardless of study design, and, therefore, cross-sectional, longitudinal, case-control, controlled trials, cohort, and pilot studies were all assessed in the present review.
Risk of bias assessment
This review was performed according to the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement (Supplementary Table 1) [26]. The risk of bias for each study was assessed using the Cochrane Collaboration’s tool for non-randomized studies for interventions (ROBINS-I) [27]. Several risk of bias domains were evaluated for each study, including bias due to confounding fac-tors, subject selection, classification of intervention, deviation from intended intervention, missing data, outcome measurement and reporting of results. Each domain was rated as ‘low’, ‘moderate’, ‘serious’, or ‘critical’ risk of bias. An overall risk of bias was de-rived from the quality assessment across all domains of the remaining studies. These judgements were performed independently by two authors (A.C. and C.G.) and final assessment was determined by consensus. Our analyses were confined to studies with low and moderate risk of bias, as studies with serious or critical risk of bias were excluded from the analyses.
Data analysis
Relevant data from the included studies were ex-tracted in piloted forms. Outcome measures in the primary examination were based on overall study outcomes regarding the association between caffeine and 1) the risk of dementia and/or cognitive decline and 2) cognitive function in cognitively impaired in-dividuals. Secondary analyses included examination of the effects of caffeine source (coffee, tea, pure caf-feine, or multiple caffeine containing sources), and quantity (frequency and dosage), and possible confounders (e.g., age or sex), on study outcomes. Based on a previous study [28], the quantity of caffeine con-sumption was divided into three categories: low- (<100 mg/d), moderate- (100–400 mg/d), and high caffeine consumption (>400 mg/d). In accordance with the concentrations of caffeine across sources (i.e., 71–220 mg caffeine/150 ml for coffee and 32–42 mg caffeine/150 ml for tea [29]), moderate caffeine consumption will be defined as 1–4 cups of coffee or 3–10 cups of tea per day. Low caffeine consumption will be defined as <1 cup of coffee or <3 cups of tea per day, and high caffeine consumption will be defined as >4 cups of coffee or >10 cups of tea per day. Outcomes were defined as positive (caffeine improved cognition or slowed down cognitive decline), negative (negative association with cognition), or no association (no relation between caffeine and cognition). Study outcomes could also be mixed, for instance when positive effects were only found in a subset of the sample or when study outcomes were dependent on study characteristics, like caffeine source used.
RESULTS
Study selection and characteristics
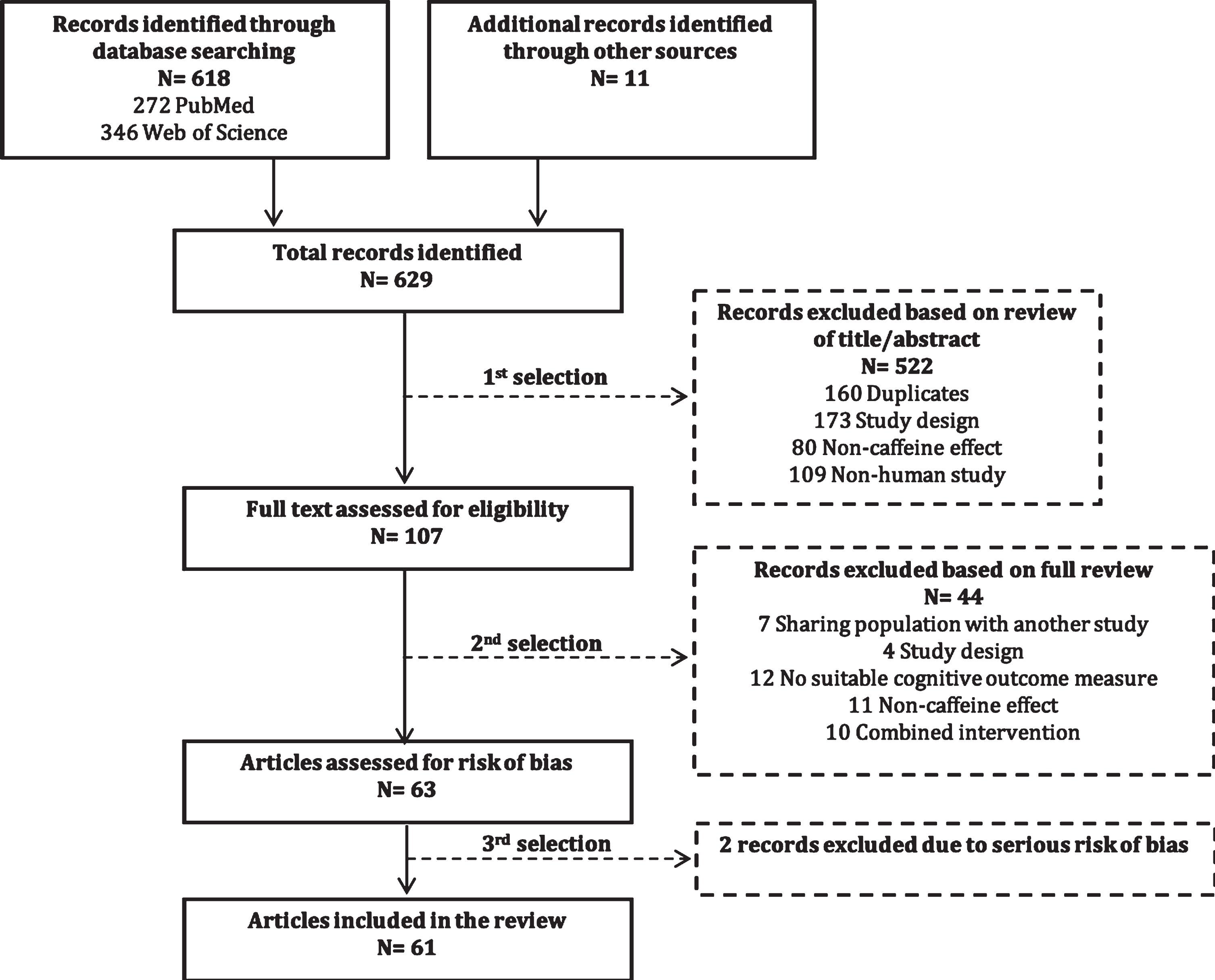
The identification of relevant studies is illust-rated in a flow diagram (Fig. 1). Through database searches on PubMed, Web of Science, and cross references, we identified a total of 629 records. First, we excluded 522 articles, including 160 duplicates, based on review of the title and abstract. After full-text assessment of the remaining 107 articles, we excluded 44 articles that had highly overlapping study populations (n = 7), incompatible study designs (n = 4), no suitable cognitive outcome measures (n = 12), only non-caffeine effects (n = 11), or combined interventions (n = 10) (Supplementary Table 2). The remaining 63 studies were assessed for risk of bias, which resulted in the exclusion of two studies [30, 31] (see “Risk of bias” section). The final selection (61 studies) comprised 48 cohort studies, nine case-control studies, three randomized controlled trials, and one pilot study.
Fig. 1
Flow diagram of identification of relevant studies.
The included studies were published between 1990 and 2020, and were executed in 24 different countries (Table 1): United States of America (n = 10) [24, 25, 32–39], Japan (n = 9) [40–48], China (n = 8) [49–56], United Kingdom (n = 4) [57–60], Finland (n = 3) [28, 61, 62], The Netherlands (n = 4) [62–65], Taipei (n = 3) [66–68], Canada (n = 2) [69, 70], France (n = 2) [71, 72], Portugal (n = 2) [73, 74], Singapore (n = 2) [75, 76], Italy (n = 2) [62, 77], Australia (n = 1) [78], Brazil (n = 1) [79], Germany (n = 1) [80], Iran (n = 1) [81], Ireland (n = 1) [82], Jordan (n = 1) [83], Norway (n = 1) [84], Scotland (n = 1) [85], South Korea (n = 1) [22], Spain (n = 1) [23], Sweden (n = 1) [86], and Switzerland (n = 1) [87]. One study [62] was performed in a multi-national collaboration between Finland, Italy, and the Netherlands. The final selection of articles comprised a total of 153,359 subjects (excluding subjects in the control group), which were either cognitively impaired (AD, DLB, PD, VaD, MCI, or undefined dementia) or cognitively unimpaired.
Table 1
Characteristics of studies included in the review (n = 61)
| Study | Study design retrospective/prospective study length of follow-up | Cohort | Subjects (N and population) | Control (N and population) | Selected cognition measure/domain | Age (y) | Sex (% men) | Caffeine | Effect and principle findings (Positive effect+, negative effect -, no effect/) (HR, OR or RR (95% CI), or p-value) |
| 1. The association between caffeine and the risk of dementia and/or cognitive decline | |||||||||
| 1. Al-khateeb et al. | Cross-sectional | Senior homes | 52 dementia | 50 cognitively | MMSE | 69.8 | 61% | Coffee | +, protective effect |
| 2014 [83] Jordan | case-control | and Jordan | healthy | (7.4) | retrospective | of coffee against | |||
| study | University | cognitive decline, | |||||||
| retrospective | Hospital | with a 6.25-fold lower | |||||||
| NA | risk with increased intake. | ||||||||
| OR = 0.16 (0.066–0.37) | |||||||||
| 2. Arab et al. | Longitudinal | The Cardiovascular | X/4,809 subjects, | X/4,809 subjects, | 3MSE | 72.6 | 43% | Coffee, | +, association coffee |
| 2011 [32] USA | cohort | Health | caffeine consumer | non-caffeine | (5.4) | Tea (NS) | and tea consumption | ||
| study | Study (CHS) | consumer | retrospective | with reduced rates | |||||
| prospective | of cognitive decline | ||||||||
| median: | in women. Tea: p = 0.007 | ||||||||
| 7.9 y | Coffee: p = 0.02 /, | ||||||||
| no association in men. | |||||||||
| Tea: p = 0.67 | |||||||||
| Coffee: p = 0.99 | |||||||||
| 3. Araújo et al. | Cross-sectional | The Longitudinal | 13,165 subjects, | 1,398 subjects, | Learning, | 52.0 | 46% | Coffee | +, moderate coffee |
| 2015 [79] Brazil | cohort study | Study of Adult | coffee consumer | non-/low coffee | recall, and | (9.0) | retrospective | consumption (2–3 cups/d) | |
| retrospective | Health (ELSA-Brasil) | consumer | word | associated with better | |||||
| 12 mo | recognition | cognitive function in | |||||||
| tests | elderly only (65–74 y). | ||||||||
| p = 0.025 /, no association | |||||||||
| for low and high coffee | |||||||||
| consumption, and | |||||||||
| for adults (35–64 y). | |||||||||
| 4. Araújo et al. 2016 | Longitudinal | The Rotterdam | 2,914 subjects | NA | LDST | 59.3 | 45% | Coffee | +, higher coffee |
| [63] The Netherlands | and cross- | Study 2005–2009 | (7.2) | retrospective | consumption (≥3 cups/d) | ||||
| sectional | associated with better | ||||||||
| cohort study | cognitive performance. | ||||||||
| prospective | p = 0.026 /, no association | ||||||||
| 5.5 y | in a longitudinal model. | ||||||||
| 5. Beydoun et al. | Longitudinal and | The Baltimore | 3,047 subjects, | 3,047 subjects, | MMSE | 58.9 | 60% | Multiple sources | +, association caffeine |
| 2014 [33] USA | cross-sectional | Longitudinal | follow-up | baseline | (18.0) | (NS) | intake with better global | ||
| cohort study | Study of | prospective | cognitive function at | ||||||
| prospective | Aging (BLSA) | baseline for age >70 y. | |||||||
| 46 y | p = 0.008 /, no association | ||||||||
| found for age <70 y. | |||||||||
| 6. Boot et al. | Cross-sectional | The Mayo Clinic | 383 cognitively | 294 cognitively | NA | 82.4 | 63% | Multiple sources | +, association caffeine |
| 2013 [34] USA | case-control | Study of Aging; | impaired | healthy | (7.5) | (coffee, tea and | intake and reduced risk | ||
| study retrospective | The Alzheimer | (236 AD, 147 DLB) | caffeinated soda) | of DLB. OR = 0.29 | |||||
| NA | Disease Patient | (0.14–0.57) retrospective | |||||||
| Registry; Alzheimer | |||||||||
| Disease Research | |||||||||
| Center Study | |||||||||
| 7. Broe et al. | Cross-sectional | The Repatriation | 170 AD | 170 cognitively | MMSE | 78.1 | 38% | Coffee, | /, no association between tea |
| 1990 [78] Australia | case-control | General Hospital | healthy | (7.3) | Tea (NS) | and coffee consumption and | |||
| study retrospective | Concord and | retrospective | reduced risk of AD. Tea: | ||||||
| NA | Lidcombe Hospital | OR = 1.42 (0.93–2.17) Coffee: | |||||||
| OR = 2.25 (0.72–7.71) | |||||||||
| 8. Chen et al. | Longitudinal | The Chinese | 1306 | 4385 | MMSE | 82.9 | 24% | Tea (NS) | +, association tea drinking |
| 2012 [49] China | case-control | Longitudinal Health | cognitive decline | cognitive healthy | (Chinese | (11.0) | retrospective | with cognitive decline. | |
| study | Longevity Study | version) | p = 0.0468 | ||||||
| prospective | (CLHLS) 2002 | ||||||||
| 3 y | |||||||||
| 9. Chin et al. | Cross-sectional | The Dublin Healthy | 466 cognitively | NA | MMSE | 75.5 | 45% | Tea (NS) | +, tea intake positively |
| 2008 [82] Ireland | cohort study | Ageing Study | healthy | (6.1) | retrospective | correlated with global | |||
| retrospective NA | cognitive performance. | ||||||||
| p = 0.042 | |||||||||
| 10. Chuang et al. | Longitudinal and | The Nutrition and | 516 subjects, | 912 subjects, | SPMSQs | 73.6 | 51% | Coffee, | +, higher intake (moderate |
| 2019 [66] Taipei | cross-sectional | Health Survey | caffeine | non-/low caffeine | and MMSE | (0.8) | Tea (NS) | consumption; ≥7 times/wk) | |
| cohort study | in Taiwan | consumer | consumer | (Chinese | retrospective | of tea and coffee associated | |||
| prospective | (NAHSIT) 2014–2016 | version) | with lower risk of dementia | ||||||
| 11 y | and 1999–2000 | Coffee: OR = 0.55 (0.30–0.98) | |||||||
| Tea: 0.46 (0.28–0.78) /, | |||||||||
| no association with low | |||||||||
| coffee and tea consumption, | |||||||||
| and in men only. | |||||||||
| 11. Corley et al. | Cross-sectional, | The Lothian Birth | 893 subjects | NA | Memory | 69.5 | 48% | Multiple sources | +, general cognitive ability |
| 2010 [85] Scotland | cohort study | Cohort 1936 Study; | (0.8) | (14 caffeine- | and memory with adjustments | ||||
| retrospective | The Scottish | containing items, | for age and sex. p = 0.02 | ||||||
| 2–3 mo | Mental Survey 1947 | e.g., coffee, tea, | /, no association with cognitive | ||||||
| chocolate, etc.) | function when additionally | ||||||||
| retrospective | adjusted for occupational | ||||||||
| social class and | |||||||||
| childhood IQ. p = 0.11 | |||||||||
| 12. Dai et al. | Longitudinal | The KAME Project | 1,275 subjects, | 315 subjects, | CASI | 71.8 | 46% | Tea (NS) | /, no association tea intake |
| 2006 [36] USA | cohort study | tea consumer | non-/low tea | (NA) | retrospective | and risk of incident probable AD. | |||
| prospective | consumer | HR = 1.29 (0.63–2.64) | |||||||
| 6.3 y | |||||||||
| 13. Dong et al. | Cross-sectional | National Health and | 1,803 subjects, | 710 subjects, | DSST | NA; | 48% | Coffee | +, association between moderate |
| 2020a [50] China | cohort study | Nutrition Examination | coffee consumer | non-coffee | >60 y | retrospective | and high coffee consumption | ||
| retrospective | Survey (NHANES) | consumer | and cognitive performance. | ||||||
| 24 h | 2011–2014 | Moderate: OR = 0.71 (0.47–0.87) | |||||||
| High: OR = 0.56 (0.39–0.79) | |||||||||
| /, no association with low | |||||||||
| and high coffee consumption. | |||||||||
| Low: OR = 1.36 (0.96–1.93) | |||||||||
| 14. Driscoll et al. | Longitudinal and | Women’s Health | 2,541 subjects, | 2,926 subjects, | 3MSE | Range: | 0% | Multiple sources | +, association between caffeine |
| 2016 [37] USA | cross-sectional | Initiative | caffeine consumer | non-low caffeine | [65–80] | (coffee, tea and | intake and probable dementia. | ||
| cohort study | Memory Study | consumer | cola) retrospective | HR = 0.74 (0.56–0.98) | |||||
| prospective | +, stronger association with | ||||||||
| 10 y | higher caffeine intake over | ||||||||
| time. p < 0.0001 | |||||||||
| 15. Eskelinen et al. | Longitudinal | The Cardiovascular | X/1,409 subjects, | X/1,409 subjects, | MMSE | 71.3 | 38% | Coffee, | +, lower risk of dementia and AD |
| 2009 [28] Finland | cohort study | Risk Factors, Aging | caffeine consumer | non-caffeine | (4.0) | Tea (NS) | for moderate (3–5 cups) coffee | ||
| prospective | and Dementia study | consumer | retrospective | consumption. Coffee (moderate): | |||||
| 21 y | (CAIDE) (North | OR = 0.34 (0.16–0.73), | |||||||
| Karelia Project | /, no association found for tea | ||||||||
| and FINMONICA study) | consumption and high | ||||||||
| (>5 cups/d) coffee consumption. | |||||||||
| Coffee (high): OR = 0.61 | |||||||||
| (0.30–1.21) Tea: | |||||||||
| OR = 1.04 (0.59–1.84) | |||||||||
| 16. Feng et al. | Longitudinal | The Chinese Longitudinal | 3,187 subjects, | 3,952 subjects, | Verbal | 91.4 | 42.9% | Tea (NS) | +, association daily tea drinking |
| 2012 [75] Singapore | cohort study | Health Longevity | tea consumer | non-tea consumer | fluency test | (7.5) | retrospective | and better cognitive function. | |
| prospective | Study (CLHLS) 1998 | p = 0.02 | |||||||
| 7 y | |||||||||
| 17. Feng et al. | Longitudinal | The Osteoporotic | 1,430 subjects, | 2,414 subjects, | 3MSE | 72.4 | 100% | Tea | /, no association black tea |
| 2018 [25] USA | cohort study | Fractures in | tea consumer | non-tea consumer | (5.2) | (black tea) | consumption and cognitive | ||
| prospective | Men (MrOS) Cohort | retrospective | decline. OR = 1.19 (0.81–1.75) | ||||||
| 6.8 y | |||||||||
| 18. Fischer et al. | Longitudinal | Aging, Cognition and | 2,622 subjects | 2,622 cognitively | CERAD | 81.2 | 35% | Coffee, Tea | /, no association coffee and green |
| 2018 [80] Germany | cohort study | Dementia in Primary | (2,204 cognitively | healthy, baseline | memory score | (3.4) | (green tea) | tea intake with memory/cognitive | |
| prospective | Care Patients | healthy, 418 incident | retrospective | decline or incident AD. Coffee: | |||||
| 10 y | (AgeCoDe) Cohort | dementia), | HR = 0.97 (0.90–1.04) Green tea: | ||||||
| HR = 0.94 (0.86–1.02) | |||||||||
| 19. Gelber et al. | Longitudinal | The Honolulu-Asia | 2,787 cognitively | 707 cognitively | CASI | 52.5 | 100% | Coffee, | /, no association between coffee |
| 2011 [24] USA | case-control | Aging Study | healthy, coffee | healthy, non-/ | (4.5) | Multiple sources | or general caffeine intake and risk | ||
| study prospective | (HAAS) | consumer | low coffee | (coffee, tea, cola) | of dementia and cognitive impairment. | ||||
| 25 y | consumer | retrospective | OR = 1.05 (0.58–1.90) | ||||||
| 20. Gu et al. | Cross-sectional | The Weitang | 1,570 subjects | 3,008 subjects (2,218 | AMT | 67.6 | 48% | Tea (green and | +, inverse association between |
| 2018 [51] China | case-control | Geratric Diseases | (1,416 cognitively | cognitively healthy, | (6.3) | other tea types) | habitual and (green) tea consumption | ||
| study | Study | healthy, 155 | 790 cognitively | retrospective | (>5 times/wk) and prevalence | ||||
| retrospective | cognitively | impaired), | of cognitive impairment. | ||||||
| NA | impaired), | non-habitual | OR = 0.74 (0.56–0.99) | ||||||
| habitual tea | tea consumers | /, no association with green tea | |||||||
| consumers | consumption of 1–5 | ||||||||
| times/wk and other tea types. | |||||||||
| 1–5 times/wk: OR = 0.56 (0.29–1.07) | |||||||||
| Other tea: OR = 0.66 (0.37–1.18) | |||||||||
| 21. Haller et al. | Longitudinal | Elderly in Geneva | 145 subjects, | 145 subjects, | MMSE | 73.8 | 44% | Coffee | +, association moderate coffee |
| 2018 [87] Switzerland | cohort study | and Lausanne | follow-up | baseline | (3.5) | retrospective | consumption and reduced risk | ||
| prospective | counties | of deteriorated cognition (dCON). | |||||||
| 3 y | OR = 0.45 (0.21–0.95) /, no association | ||||||||
| for low coffee consumption. | |||||||||
| 22. Huang et al. | Cross-sectional | The Project of | 429 cognitively | 252 cognitively | MMSE | 93.5 | 33% | Tea (NS) | +, tea consumption associated with |
| 2009 [52] China | cohort study | Longevity and Aging | impaired | healthy | (3.3) | retrospective | cognitive impairment in men. | ||
| retrospective | in Dujiangyan (PLAD) | /, no association in women. | |||||||
| 2 y | |||||||||
| 23. Iranpour et al. | Cross-sectional | National Health | 1,065 subjects, | 375 subjects, | DSST | 69.8 | 51% | Multiple sources | +, positive association between high |
| 2020a [81] Iran | cohort study | and Nutritional | ≥Q2 caffeine | Q1 caffeine | (2.3) | (e.g., tea, soda, | caffeine intake and cognitive | ||
| retrospective | Examination Surveys | consumer | consumer | chocolate, etc.) | function in an univariate model. | ||||
| 24 h | (NHANES) 2013–2014 | retrospective | p = 0.004 /, no association with | ||||||
| multiple adjustments. p = 0.99 | |||||||||
| 24. Jarvis | Cross-sectional | The Health and | X/7,414 subjects, | X/7,414 subjects, | Reaction time, | NA; ∼45 | 45% | Coffee, | +, association increased levels |
| 1993 [57] UK | cohort study | Lifestyle Survey | caffeine consumer | non-caffeine | incidental verbal | Tea (NS) | of coffee and tea consumption | ||
| retrospective | consumer | memory and visuo- | retrospective | with improved cognitive performance. | |||||
| NA | spatial reasoning | Stronger association for coffee | |||||||
| than tea intake, and | |||||||||
| older people than younger | |||||||||
| people. p < 0.05 | |||||||||
| 25. Johnson-Kozlow | Cross-sectional | The Rancho | 1,528 cognitively | NA | MMSE | 72.9 | 42% | Coffee | +, association between higher |
| et al. 2002 [38] USA | cohort study | Bernardo Study, | healthy | (9.0) | retrospective | lifetime coffee consumption | |||
| prospective | 1988–1992 | and better cognitive performance | |||||||
| NA | in women. p = 0.023 | ||||||||
| /, no association in men. | |||||||||
| 26. Kitamura et al. | Cross-sectional | The Project in | 601 subjects | 539 subjects | MMSE | 68.9 | 55% | Tea | +, green tea intake associated with |
| 2016 [42] Japan | cohort study | Sado for Total | (490 cognitively | (406 cognitively | (10.6) | (green tea) | /, no association in men. | ||
| retrospective | Health (PROST) | healthy, 111 | healthy, 133 | retrospective | OR = 0.73 (0.54–0.99) | ||||
| NA | 2008–2014 | cognitively | cognitively | ||||||
| impaired), | impaired), non- | ||||||||
| tea consumer | tea consumer | ||||||||
| 27. Konishi et al. | Cross-sectional | Healthy Japanese | 50 cognitively | 50 cognitively | SAT: executive | Range: | 50% | Pure caffeine | +, better executive function with |
| 2018 [48] Japan | RCT prospective | volunteers, 2016 | healthy, caffeine | healthy, | function | [22–59] | prospective | caffeine consumption. p = 0.03 | |
| 30 min | consumer | Placebo | |||||||
| 28. Kuriyama et al. | Cross-sectional | The Tsurugaya | 833 subjects, | 170 subjects, | MMSE | 74.7 | 43% | Coffee, | +, green tea consumption of >2 |
| 2006 [43] Japan | cohort study | Project | caffeine consumer | non-/low caffeine | (Japanese | (4.6) | Tea (green, | cups/d and prevalence of cognitive | |
| retrospective | consumer | version) | black/oolong | impairment. OR = 0.46 (0.30–0.72) | |||||
| NA | tea) | /, green tea consumption of 4–7 cups/wk. | |||||||
| retrospective | OR = 0.62 (0.33–1.19) /, no association | ||||||||
| for coffee and black/oolong tea. | |||||||||
| Coffee: OR = 1.03 (0.59–1.80) | |||||||||
| Black/oolong: OR = 0.87 (0.55–1.38) | |||||||||
| 29. Laitala et al. | Longitudinal | Finnish Twin | 2606 cognitively | NA | TELE | 74.4 | 52% | Coffee | /, no association between coffee and |
| 2009 [61] Finland | cohort study | Cohort Study | healthy | (5.3) | retrospective | cognitive performance. | |||
| prospective | OR = 1.07 (0.97–1.17) | ||||||||
| Median: | |||||||||
| 28 y | |||||||||
| 30. Larsson &Wolk | Longitudinal and | The National Research | X/28,775 subjects, | X/28,775 subjects, | NA | 83.2 | 53% | Coffee | /, coffee consumption not associated |
| 2018 [86] Sweden | cross-sectional | Infrastructure SIMPLER | caffeine consumer | non-/low caffeine | (5.1) | retrospective | with risk of AD. HR = 1.01 (0.86–1.18) | ||
| cohort study | (Swedish Infrastructure | consumer | |||||||
| prospective | for Medical Population- | ||||||||
| 12.6 y | based Life-course | ||||||||
| Environmental Research); | |||||||||
| Swedish Mammography | |||||||||
| Cohort and the | |||||||||
| Cohort of Swedish Men | |||||||||
| 31. Lee et al. | Cross-sectional | A Nationwide Survey | X/7,964 subjects, | X/7,964 subjects, | TMSE | 75.7 | 50% | Coffee, | +, inverse associations with dementia. |
| 2017 [67] Taipei | cohort study | in Japan, 2011–2013 | caffeine consumer | non-caffeine | (6.6) | Tea (green and | Coffee: OR = 0.59 (0.35–0.97) | ||
| retrospective | consumer | other tea types) | Green tea: OR = 0.51 (0.34–0.75) | ||||||
| NA | retrospective | Other tea: OR = 0.41 (0.28–0.60) | |||||||
| 32. Lesk et al. | Cross-sectional | The Oxford Project to | 57 subjects, | 32 subjects, | MMSE | Range: | 38% | Multiple sources | /, no association between caffeine- |
| 2009 [58] UK | cohort study | Investigate Memory | caffeine consumer | non-caffeine | [67–95] | (e.g., coffee, tea, | containing foodstuffs (CCFS) | ||
| retrospective | and Ageing | consumer | soft drinks, | and cognitive decline. | |||||
| 4 h | (OPTIMA) cohort | chocolate, etc.) | |||||||
| retrospective | |||||||||
| 33. Lindsay | Longitudinal | The Canadian Study | 194 AD | 3,894 cognitively | 3MSE | Range: | 42% | Coffee, | +, regular (nearly every day) coffee |
| 2002b [69] Canada | case-control | of Health and | healthy | [69–105] | Tea (NS) | consumption and a reduced risk of AD. | |||
| study prospective | Aging (CSHA) | retrospective | OR = 0.69 (0.50–0.96) /, no association | ||||||
| 5 y | with tea drinking. OR = 1.12 (0.78–1.61) | ||||||||
| 34. Maia & | Cross-sectional, | Dementia Outpatient | 54 AD | 54 cognitively | MMSE | 70.8 | 48% | Multiple sources | +, lower risk for AD, independent |
| de Mendonça 2002 | case-control | Clinics, Hospital | healthy | (7.7) | (e.g., coffee, tea, | of confounding variables. | |||
| [73] Portugal | study | Santa Maria, Lisbon | cola, etc.) | OR = 0.40 (0.25–0.67) | |||||
| retrospective | retrospective | ||||||||
| 20 y | |||||||||
| 35. Mirza et al. | Longitudinal | The Rotterdam | 3,876 subjects, | 492 subjects, | MMSE | NA; ∼70 | 41% | Coffee | +, association between coffee consumption |
| 2014 [64] | cohort study | Study 1989–1990 | coffee consumer | non-/low coffee | retrospective | (>3 cups/d) and incident dementia with | |||
| The Netherlands | prospective | consumer | short (0–4 y) follow-up. Short-term: | ||||||
| 8.7 y | HR = 0.70 (0.51–0.96) -, increased risk | ||||||||
| of incident dementia for | |||||||||
| long-term effect (>4 y), | |||||||||
| possibly due to reverse | |||||||||
| causality. Long-term: | |||||||||
| HR = 1.14 (0.83–1.56) | |||||||||
| 36. Ng et al. | Longitudinal and | The Singapore | X/2,501 subjects, | X/2,501 subjects, | MMSE | 66.0 | 36% | Coffee, Tea | +, association regular black/oolong |
| 2008 [76] Singapore | cross-sectional | Longitudinal | caffeine consumer | non-caffeine | (7.7) | (green and black/ | and green tea consumption with lower | ||
| cohort study | Ageing Studies | consumer | oolong tea) | prevalence of cognitive impairment, | |||||
| prospective | (SLAS) cohort | retrospective | and black/oolong tea with reduced risk | ||||||
| median:16 mo | of cognitive decline over time. Black/oolong: | ||||||||
| OR = 0.55 (0.40–0.76) Green tea: | |||||||||
| OR = 0.42 (0.25–0.69) /, no association | |||||||||
| for coffee. Coffee: OR = 0.99 (0.69–1.45) | |||||||||
| 37. Noguchi-Shinohara | Longitudinal | The Nakajima | X/490 subjects, | X/490 subjects, | MMSE | 71.2 | 33% | Coffee, Tea | +, reduced risk of cognitive decline |
| et al. 2014 [44]Japan | cohort study | Project | caffeine consumer | non-caffeine | (6.4) | (black and green tea) | with green tea. Green tea: | ||
| prospective | consumers | retrospective | OR = 0.53 (0.30–0.93) | ||||||
| 4.9 y | /, no effect for coffee or black tea | ||||||||
| on incidence of dementia or MCI. | |||||||||
| Coffee: OR = 1.22 (0.63–2.36) | |||||||||
| Black tea: OR = 1.19 (0.64–2.24) | |||||||||
| 38. Nurk et al. | Cross-sectional | The Hordaland | 1,083 subjects, | 948 subjects, | modified | Range: | 45% | Tea (NS) | +, habitual tea intake associated with |
| 2009 [84] Norway | cohort study | Health Study | tea consumer | non-tea consumer | MMSE | [70–74] | retrospective | better cognitive test performance. | |
| retrospective | (HUSK), | p = 0.046 | |||||||
| NA | Norway | ||||||||
| 39. Paganini-Hill | Longitudinal | The 90 + Study, | 587 subjects | 587 | MMSE/ | 93 | NA | Multiple sources | +, caffeine consumption of >200 mg/d |
| et al. 2016 [39] USA | cohort study | The Leisure World | (268 incident | cognitively healthy | CASI | (2.6) | (e.g., coffee, black | associated with reduced risk of dementia | |
| prospective | Cohort Study | dementia), | elderly, baseline | tea, green tea, soft | compared with caffeine consumption | ||||
| 36 mo | follow-up | drinks, chocolate, | of <50 mg/d at age of 90. HR = 0.66 | ||||||
| etc.) retrospective | (0.43–0.99) /, no association found | ||||||||
| 20 y earlier, at age 70, or lower | |||||||||
| caffeine consumption at age 90. | |||||||||
| 40. Ritchie et al. | Longitudinal | The Three-City | 7,017 cognitively | 7,017 cognitively | Isaacs | 73.7 | 40% | Multiple sources | +, inverse association between coffee |
| 2007 [71] France | cohort study | Study (Bordeaux, | healthy, follow-up | healthy, baseline | (5.2) | (tea and coffee) | consumption (>300 mg/day) | ||
| prospective | Dijon, Montpellier) | retrospective | and cognitive decline in women, | ||||||
| 3.5 y | especially at higher ages. | ||||||||
| OR = 0.67 (0.53–0.85) | |||||||||
| /, no effect found in men. | |||||||||
| OR = 1.18 (0.87–1.59) | |||||||||
| 41. Santos et al. | Longitudinal | Elderly in Porto | 309 cognitively | 309 cognitively | MMSE | 70 | 41% | Multiple sources | +, caffeine intake (>62 mg/day) |
| 2010 [74] Portugal | cohort study | healthy, follow-up | healthy, baseline | (1.9) | (82 caffeine-containing | associated with lower risk of | |||
| prospective | food items, e.g., coffee, | cognitive decline in women. | |||||||
| median: 48 mo | tea, soft drinks, chocolate, | RR = 0.51 (0.27–0.97) | |||||||
| etc.) retrospective | /, no effect found in men. RR = 0.51 (0.22–1.16) | ||||||||
| 42. Shen et al. | Cross-sectional | The Zhejiang Major | 2,530 subjects, | 6,845 subjects, | MMSE | 70.0 | 48.5% | Tea | +, for black tea consumption |
| 2015 [53] China | cohort study | Public Health | caffeine consumer | non-caffeine | (Chinese | (7.7) | (black, green and | and prevalence of cognitive | |
| retrospective | Surveillance | consumer | version) | multiple tea types) | impairment. Black tea: | ||||
| NA | Program (ZPHS) 2014 | retrospective | OR = 0.48 (0.29–0.80) | ||||||
| +, positive association for 2–4 cups/d | |||||||||
| and > 4 cups/d tea consumption in general. | |||||||||
| 2–4 cups/d: OR = 0.71 (0.59–0.84) | |||||||||
| ≥4 cups/d: OR = 0.76 (0.63–0.91) | |||||||||
| /, no association for green | |||||||||
| tea and low tea consumption | |||||||||
| in general. Green tea: | |||||||||
| OR = 1.00 (0.74–1.35) | |||||||||
| <2 cups/d: OR = 1.09 (0.88–1.35) | |||||||||
| 43. Shirai et al. | Longitudinal | The National Institute | X/1,305 subjects, | X/1,305 subjects, | MMSE | 66.7 | 48% | Coffee, | +, association between 2–3 times/d and |
| 2020 [45] Japan | cohort study | for Longevity Sciences, | caffeine consumer | non-caffeine | (Japanese | (6.2) | Tea (green tea) | >4 times/d green tea consumption and | |
| prospective | Longitudinal Study | consumer | version) | retrospective | reduced risk of cognitive decline. | ||||
| 5.3 y | of Aging (NILS-LSA) | 2–3 times/d: HR = 0.71 (0.52–0.97) | |||||||
| ≥4 times/d: HR = 0.72 (0.54–0.98) | |||||||||
| /, no association for <once/d green | |||||||||
| tea consumption and general | |||||||||
| coffee consumption in general. | |||||||||
| 44. Smith | Cross-sectional | The Bristol Stress and | X/3,223 subjects, | X/3,223 subjects, | CFQ | 49.6 | 43% | Multiple sources | +, caffeine consumption reduces |
| 2009 [59] UK | cohort study | Health at Work Study & | caffeine consumer | non-caffeine | (21.9) | (caffeinated drinks) | risk on cognitive failures. | ||
| respective | The Cardiff Health and | consumer | retrospective | p < 0.005 | |||||
| NA | Safety and Work Study | ||||||||
| 45. Solfrizzi et al. | Longitudinal | The Italian Longitudinal | 985 subjects, | 460 subjects, | MMSE | 71.8 | 56% | Coffee | +, lower rate of MCI incidence for |
| 2015 [77] Italy | cohort study | Study on Aging (ILSA) | coffee consumer | non-/low coffee | (5.0) | retrospective | moderate (1–2 cups/d) coffee consumption. | ||
| prospective | consumer | HR = 0.31 (0.13–0.75) /, no association | |||||||
| median: | with low coffee consumption (<1 cup/d). | ||||||||
| 3.5 y | HR = 0.47 (0.211–1.02) -, higher rate | ||||||||
| of incidence MCI for change in coffee | |||||||||
| consumption habits. | |||||||||
| HR = 1.80 (1.11–2.92) | |||||||||
| 46. Sugiyama et al. | Longitudinal | The Ohsaki Cohort 2006 | 11,089 subjects, | 2,048 subjects, | Dementia | 73.6 | 45% | Coffee | +, coffee consumption associated |
| 2016a [46] Japan | cohort study | caffeine consumer | non-caffeine | Scale | (5.8) | retrospective | with lower risk of incident dementia. | ||
| prospective | consumer | HR = 0.72 (0.61–0.84) | |||||||
| 5.7 y | |||||||||
| 47. Tomata et al. | Longitudinal | The Ohsaki Cohort 2006 | 11,411 subjects, | 2,234 subjects, | CDR | 73.8 | 44% | Tea (green, black | +, Green tea consumption of >3 cups/d |
| 2016a [47] Japan | cohort study | caffeine consumer | non-/low caffeine | (5.8) | and oolong tea) | associated with a lower risk of incident | |||
| prospective | consumer | retrospective | dementia. Green tea: OR = 0.74 (0.63–0.88) | ||||||
| 5.7 y | /, no association found for low green tea | ||||||||
| consumption (<2 cups/d) and black | |||||||||
| and oolong tea. Green tea: OR = 0.94 | |||||||||
| (0.79–1.11) Black tea: | |||||||||
| HR = 0.98 (0.61–1.59) | |||||||||
| Oolong tea: HR = 0.89 (0.54–1.45) | |||||||||
| 48. Tyas et al. | Longitudinal | The Manitoba Study | 36 AD | 658 cognitively | 3MSE | 74.0 | 37.6% | Coffee, | /, no association between coffee and |
| 2001b [70] Canada | cohort study | of Health and Aging | healthy | (5.8) | Tea (NS) | tea consumption and risk of AD. | |||
| prospective | (MSHA); The Canadian | retrospective | Coffee: RR = 1.03 (0.47–2.30) | ||||||
| 5 y | Study of Health | Tea: RR = 0.46 (0.20–1.06) | |||||||
| and Aging (CSHA) | |||||||||
| 49. Valls-Pedret et al. | Cross-sectional, | The Prevención | 447 cognitively | NA | RAVLT – | Range: | 48% | Coffee | +, better memory function and global |
| 2012 [23] Spain | cohort study | con Dieta | healthy | delayed recall | [55–80] | retrospective | cognition with coffee consumption. | ||
| retrospective | Mediterránea | Coffee: p = 0.016 | |||||||
| NA | (PREDIMED) Study | ||||||||
| 50. Van Boxtel et al. | Longitudinal | The Maastricht | 1,366 cognitively | 1,366 cognitively | VVLT | 50.2 | 52% | Multiple sources | /, no association found between |
| 2003 [65] | cohort study | Aging Study (MAAS) | healthy, follow-up | healthy, baseline | (15.4) | (coffee, tea, cola, | caffeine consumption | ||
| The Netherlands | prospective | energy-drink) | and age over time. | ||||||
| 6 y | retrospective | ||||||||
| 51. Van Gelder et al. | Longitudinal | The Finland, Italy | 531 cognitively | 145 cognitively | MMSE | 76.1 | 100% | Coffee | +, inverse association between low |
| 2007 [62] Finland, | cohort study | and the Netherlands | healthy | healthy, non-coffee | (4.2) | retrospective | and moderate coffee consumption | ||
| Italy, | prospective | (FINE) Study | consumer | (<4 cups/day) and cognitive decline. | |||||
| The Netherlands | 10 y | cohorts | 3 cups/d: | ||||||
| p < 0.001 1, 2, 4 cups/d: p < 0.05 | |||||||||
| /, no effect for high coffee consumption | |||||||||
| (>4 cups/d). p > 0.05 | |||||||||
| 52. Vercambre et al. | Longitudinal | The Women’s | X/2,475 cognitively | X/2,475 cognitively | Global | NA; | 0% | Multiple sources | +, slower rates of global cognitive decline |
| 2013 [72] France | cohort study | Antioxidant | healthy,≥Q2 | healthy, Q1 caffeine | cognitive | >65 y | (116 caffeine-containing | with increasing caffeine intake | |
| prospective | Cardiovascular | caffeine | consumer | score | items, e.g., caffeinated | (4 cups/d versus non-/low caffeine | |||
| 5 y | Study (WACS) | consumer | coffee, decaffeinated | consumption). p = 0.02 +, stronger | |||||
| Cohort | coffee, tea, chocolate) | association with multiple additional | |||||||
| retrospective | adjustments and additional vitamin B | ||||||||
| supplementation. p = 0.02 | |||||||||
| /, adjustments only for age, | |||||||||
| education and energy from diet. p = 0.066 | |||||||||
| 53. Walters &Lesk | Cross-sectional | Division of Psychology, | 20 cognitively | 20 cognitively | MMSE | 73.4 | NA | Pure caffeine | /, no significant interaction |
| 2016 [60] UK | RCT | University of | healthy, caffeine | healthy, placebo | (6.6) | prospective | of caffeine found on cognitive tests. | ||
| prospective | Bradford database | consumer | |||||||
| NA | |||||||||
| 54. Wang et al. | Longitudinal and | Elderly in Shanghai | 224 MCI | 781 cognitively | MMSE | 72.7 | 42% | Tea (NS) | +, tea can protect people >60 |
| 2017 [54] China | cross-sectional | (from Huangpu, | healthy | (8.5) | retrospective | y against MCI. | |||
| cohort study | Changning, Putuo, | OR = 0.59 (0.43–0.82) | |||||||
| prospective | Pudong districts) | /, tea consumption in the age >70 y. | |||||||
| 1 y | OR = 0.72 (0.49–1.07) | ||||||||
| 55. Wu et al. | Cross-sectional | The National Health | X/2,219 subjects, | X/2,219 subjects, | MMSE | 73.3 | 52% | Coffee, | +, decreased risk of cognitive |
| 2011 [68] Taipei | cohort study | Interview Survey 2005 | caffeine consumer | non-caffeine | (5.9) | Tea (NS) | impairment with coffee. | ||
| retrospective | consumer | retrospective | Coffee: OR = 0.51 (0.31–0.83) | ||||||
| NA | /, no effect for tea. Tea: | ||||||||
| OR = 0.99 (0.75–1.30) | |||||||||
| 56. Xu et al. | Cross-sectional | China Longitudinal | 439 MCI | 1,692 cognitively | MMSE | 70.9 | 45% | Tea | +, protective effect against MCI |
| 2018 [55] China | cohort study | Aging Study (CLAS) | healthy | (7.9) | (green, black, | for green tea consumption in men, | |||
| retrospective | oolong tea) | particularly at <70 y. Green tea | |||||||
| NA | retrospective | (age <70): OR = 0.376 (0.20–0.70) | |||||||
| /, green tea in women; black and | |||||||||
| oolong tea in general. | |||||||||
| Green tea (women): OR = 0.82 | |||||||||
| (0.58–1.16) Black tea: | |||||||||
| OR = 0.74 (0.37–1.49) | |||||||||
| 57. Yang et al. | Cross-sectional | Elderly in | 847 subjects | 11,68 subjects | MMSE | 79.5 | 42% | Tea (NS) | +, association between tea consumption |
| 2016 [56] China | cohort study | Zhejiang province | (749 cognitively | (822 cognitively | (7.6) | retrospective | and AD or severe cognitive impairment. | ||
| retrospective | healthy +98 | healthy+346 | OR = 0.5 (0.4–0.6) | ||||||
| NA | dementia), tea | dementia), | |||||||
| consumer | non-tea consumer | ||||||||
| 2. The association between caffeine and cognitive function in cognitively impaired individuals | |||||||||
| 58. Cao et al. | Longitudinal | Florida Alzheimer’s | 124 subjects | 124 subjects | MMSE | 74.9 | 40% | Multiple sources | +, caffeine/coffee intake associated |
| 2012 [35] USA | case-control | Disease Research | (69 cognitively | (69 cognitively | (1.9) | (Plasma caffeine | with reduced risk of dementia | ||
| study | Center (FADRC), | healthy, 32 MCI, | healthy, 32 MCI, | concentration) | or delayed onset, | ||||
| prospective | Miami and | 23 dementia), | 23 dementia), | retrospective | particularly for those who | ||||
| 2–4 y | Tampa cohort | follow-up | baseline | already have MCI. p < 0.02 | |||||
| 59. Cho et al. | Cross-sectional, | The Movement | 136 PD, coffee | 60 PD, | K-MMSE | 66.3 | 52% | Coffee | +, better global cognitive scores |
| 2018 [22] | cohort study | Disorders Clinic, | consumer | non-coffee | (Korean | (9.5) | retrospective | for coffee consumption | |
| South Korea | retrospective | Chonnam National | consumer | version) | in patients with PD. | ||||
| NA | University Hospital | p = 0.004 | |||||||
| 60. Ide et al. | Longitudinal | The White Cross | 12 cognitively | 12 cognitively | MMSE-J | 88 | 17% | Green tea | +, association between three-month |
| 2014 [40] Japan | pilot study | Nursing Home in | impaired (3 AD, | impaired (3 AD, | (Japanese | (7.6) | powder | green tea consumption and improved | |
| prospective | Higashi-Murayama, | 8 VaD, | 8 VaD, | version) | prospective | cognitive function or reduced | |||
| 3 months | Tokyo, Japan 2012 | 1 DLB), | 1 DLB), | progression of cognitive | |||||
| follow-up | baseline | dysfunction.s p = 0.03 | |||||||
| 61. Ide et al. | Longitudinal | The White Cross | 17 cognitively | 16 cognitively | MMSE-J | 84.8 | 12% | Green tea | /, no association between |
| 2016 [41] Japan | RCT | Nursing Home | impaired (9 AD, | impaired (8 AD, | (Japanese | (9.3) | powder | 1-y green tea consumption | |
| prospective | in Higashi-Murayama, | 7 VaD, | 8 VaD), | version) | prospective | and cognitive performance. | |||
| 12 months | Tokyo, Japan | 1 DLB), | placebo | p = 0.59 | |||||
| caffeine | |||||||||
| consumer | |||||||||
MCI, mild cognitive impairment; AD, Alzheimer’s disease; PD, Parkinson’s disease; DLB, dementia with Lewy bodies, VaD, vascular dementia; RCT, randomized controlled trial; AMT, Abbreviated Mental Test; MMSE, Mini-Mental State Examination; 3MSE, Modified Mini-Mental State Examination; CERAD. Consortium to Establish a Registry for Alzheimer’s Disease; CASI, Cognitive Abilities Screening Instrument; DSST, Digit Symbol Substitution Test; CFQ, Cognitive Failures Questionnaire; MSQ, Mental Status Questionnaire; TELE, Telephone-Assessment of Cognitive State; TMSE, Tested Thai Mental State Examination; CDR, Clinical Dementia Rating; LDST, Letter-Digit Substitution Task; RAVLT, Rey Auditory Verbal Learning Test; VVLT, Visual Verbal Learning Test; SAT, Shifting Attention Test; SPMSQs, Short Portable Mental Status Questionnaires; NA, not available; NS, non-specified; HR, hazard ratio; OR, odds ratio; RR, relative risk; CI, confidence interval; y, year; mo, month; wk, week, d, day; h, hour, min, minute. Age values represent mean (±SD), unless otherwise indicated. aOverlapping or sharing population but different study design. bSmall number of overlapping population with other included study.
Risk of bias
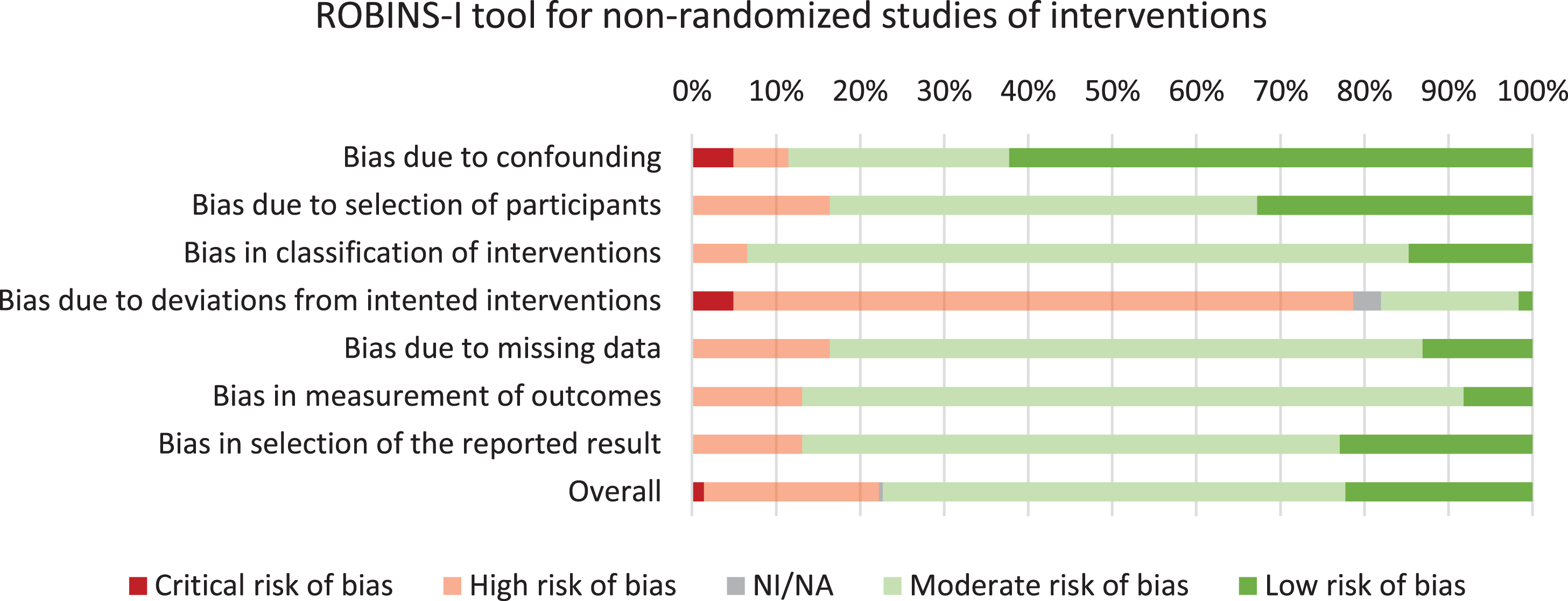
Using the Cochrane Collaboration tool, an assessment of bias was performed for all included studies, which lead to the exclusion of two studies [30, 31] (Supplementary Table 3). Furthermore, 39/61 studies had low risk of bias and 22/61 studies had moderate risk of bias. Assessment of bias across risk of bias domains revealed predominantly moderate- to low risk of bias for six out of seven domains (Fig. 2). High risk of bias was observed on the ‘deviations from intended interventions’ domain, which could be explained by most studies employing self-reported data.
Fig. 2
Risk of bias assessment of the included studies.
Associations between caffeine consumption and cognition
Caffeine and the risk of dementia/cognitive decline
Of the 61 articles included in this review, 57 studies with a total of 153,070 subjects, assessed the association between caffeine and the risk of dementia and/or cognitive decline (Fig. 3A, B). Within these studies, 16/57 (28%) studies including 40,707/153,070 (27%) subjects found a positive association for caffeine on the risk of dementia and/or cognitive decline that was independent of study related factors. Approximately half of the studies (30/57 (53%) studies including 71,219/153,070 (47%) subjects) reported positive results that were dependent on caffeine consumption quantity (n = 14), type of caffeine source (n = 11), sex (n = 7), age (n = 4), caffeine consumption duration (short- or long-term effects) (n = 2), and/or adjustments for covariates (n = 3). No association between caffeine and risk of dementia or cognitive decline was found in 11/57 (19%) studies including 41,144/153,070 (27%) subjects.
Fig. 3
A Study outcomes for the association between caffeine and dementia and/or cognitive function. Pie charts show study outcomes based on population, caffeine consumption dosage and type of caffeine source: positive effect (darker green), positive effect dependent on study characteristics (lighter green), no effect (gray), and negative effect (red [none observed]). Outlined charts indicate a predominant positive outcome.
![A Study outcomes for the association between caffeine and dementia and/or cognitive function. Pie charts show study outcomes based on population, caffeine consumption dosage and type of caffeine source: positive effect (darker green), positive effect dependent on study characteristics (lighter green), no effect (gray), and negative effect (red [none observed]). Outlined charts indicate a predominant positive outcome.](https://content.iospress.com:443/media/jad/2020/78-4/jad-78-4-jad201069/jad-78-jad201069-g003a.jpg)
Fig. 3
B Study outcomes for the association between caffeine and dementia and/or cognitive function of the included subjects. Pie charts show study outcomes based on population, caffeine consumption dosage and type of caffeine source: positive effect (darker green), positive effect dependent on study characteristics (lighter green), no effect (gray), and negative effect (red [none observed]). Outlined charts indicate a predominant positive outcome.
![B Study outcomes for the association between caffeine and dementia and/or cognitive function of the included subjects. Pie charts show study outcomes based on population, caffeine consumption dosage and type of caffeine source: positive effect (darker green), positive effect dependent on study characteristics (lighter green), no effect (gray), and negative effect (red [none observed]). Outlined charts indicate a predominant positive outcome.](https://content.iospress.com:443/media/jad/2020/78-4/jad-78-4-jad201069/jad-78-jad201069-g003b.jpg)
Caffeine and cognitive function in cognitively impaired individuals
Four studies [22, 35, 40, 41] with a total of 289 subjects assessed the influence of caffeine consumption on cognitive function in cognitively impaired individuals. Cao et al. (2012) [35] assessed concurrent plasma caffeine levels in MCI subjects over a time period of 2–4 years, and observed a reduction in progression to dementia at plasma caffeine levels >1200 ng/ml in this population. Cho et al. (2018) [22] found better global cognitive scores for individuals with PD that consumed coffee, compared to their non-coffee consuming counterparts. Ide et al. (2014) [40] and Ide et al. (2016) [41] both assessed cognitively impaired individuals with AD, VaD, or DLB that consumed green tea powder over a time period of 3 months and 12 months, respectively. Only ‘short-term’ (3 months) green tea consumption was associated with improved cognitive function or reduced progression of cognitive dysfunction.
Taken together, caffeine has a positive effect on cognition in the majority of studies (3/4 (75%) studies including 272/289 (94%) subjects) including cognitively impaired subjects.
Caffeine and study characteristics
Caffeine source
Through categorization of caffeine source that were investigated in each study, we found 29 (48%; 103,321 (67%) subjects) coffee-based studies, 30 (49%; 59,309 (39%) subjects) studies based on tea, 15 (25%; 25,928 (17%) subjects) studies based on multiple caffeinated sources, and 2 (3%; 70 (0.05%) subjects) studies based on pure caffeine (Table 2A–D). Further categorization of tea-based studies revealed 13 (21%; 32,295 (21%) subjects) studies assessing green tea, 7 (11%; 19,635 (13%) subjects) studies assessing black tea and/or oolong tea, and 19 (31%; 37,648 (25%) subjects) studies with other or non-specified tea types (Fig. 3). For the coffee-based studies, we found that 8/29 (28%) studies including 29,515/103,321 (29%) subjects reported a positive association of caffeine consumption on the risk of dementia and/or cognitive decline. Furthermore, 11/29 (38%) studies including 31,681/103,321 (31%) subjects indicated that the outcome was dependent on the quantity of coffee consumed (more positive associations with moderate quantities), sex (more positive for women), age (more positive for older age, 65–74 years), and/or the assessment of short- or long-term association (more protective in the short-term than long-term). The remaining studies on coffee (10/29 (34%); 42,125/103,321 (41%) subjects) reported no association between caffeine and risk of dementia and/or cognitive function. Two studies reported negative associations when long-term effects were assessed [64] or when examining change in habitual consumption [77], but these outcomes shifted toward a positive association when assessing short-term effects and a fixed caffeine consumption frequency and/or concentration over time, respectively.
Table 2A
Association between coffee-based studies (n = 29) and cognitive decline/dementia
| Coffee-based studies | ||
| Positive association | No association | Negative association |
| Al-khateeb et al. 2014 [83] | Arab et al. 2011 | Mirza et al. 2014 [64] |
| (Sex; men) | (Caffeine consumption duration; long-term) | |
| Arab et al. 2011 [32] | Araújo et al. 2015 [79] | Solfrizzi et al. 2015 [77] |
| (Sex; women) | (Caffeine consumption quantity and age; ≤1 cup/d or ≥3 cups/d, 35–64 years) | (Change in habitual intake; increased consumption) |
| Araújo et al. 2015 [79] | Araújo et al. 2016 [63] | |
| (Caffeine consumption quantity and age; 2 –3 cups/d, 65–74 years) | (Caffeine consumption duration; long-term) | |
| Araújo et al. 2016 [63] | Broe et al. 1990 [78] | |
| (Caffeine consumption duration; short-term) | ||
| Cho et al. 2018 [22] | Chuang et al. 2019 [66] | |
| (Caffeine consumption quantity and sex; 2–6 times/wk, men) | ||
| Chuang et al. 2019 [66] | Dong et al. 2020 [50] | |
| (Caffeine consumption quantity and sex;≥ 7 times/wk, women) | (Caffeine consumption quantity; < 266.4 g/d) | |
| Dong et al. 2020 [50] | Eskelinen et al. 2009 [28] | |
| (Caffeine consumption quantity; 266.4–495 g/d or ≥495 g/d) | (Caffeine consumption quantity; <3 cups/d and > 5 cups/d) | |
| Eskelinen et al. 2009 [28] | Fischer et al. 2018 [80] | |
| (Caffeine consumption quantity; 3–5 cups/d) Haller et al. 2018 [87] | ||
| Gelber et al. 2011 [24] | ||
| (Caffeine consumption quantity; 29–60 cups/months) | ||
| Jarvis 1993 [57] | Haller et al. 2018 [87] | |
| (Caffeine consumption quantity; < 28 cups/months) | ||
| Johnson-Kozlow et al. 2002 [38] | Johnson-Kozlow et al. 2002 [38] | |
| (Sex; women) | (Sex; men) | |
| Lee et al. 2017 [67] | Kuriyama et al. 2006 [43] | |
| Lindsay, 2002 [69] | Laitala et al. 2009 [61] | |
| Mirza et al. 2014 [64] | Larsson &Wolk 2018 [86] | |
| (Caffeine consumption quantity and caffeine consumption duration; > 3 cups/d, short-term) | ||
| Solfrizzi et al. 2015 [77] | Mirza et al. 2014 [64] | |
| (Caffeine consumption quantity; 1–2 cups/d) | (Caffeine consumption quantity; 1–3 cups/d) | |
| Sugiyama et al. 2016 [46] | Ng et al. 2008 [76] | |
| Valls-Pedret et al. 2012 [23] | Noguchi-Shinohara et al. 2014 [44] | |
| Van Gelder et al. 2007 [62] | Shirai et al. 2020 [45] | |
| (Caffeine consumption quantity; < 4 cups/d) | ||
| Wu et al. 2011 [68] | Solfrizzi et al. 2015 [77] | |
| (Caffeine consumption quantity;< 1 cup/d) | ||
| Tyas et al. 2001 [70] | ||
| Van Gelder et al. 2007 [62] | ||
| (Caffeine consumption quantity;> 4 cups/d) | ||
Bold studies indicate multiple outcomes.
Table 2B
Association between tea-based studies (n = 30), subdivided into green tea (n = 13), black/oolong tea (n = 7), and other or non-specified tea types (n = 19) and cognitive decline/dementia
| Tea-based studies | ||
| Green tea | ||
| Positive association | No association | Negative association |
| Ide et al. 2014 [40] | Ide et al. 2016 [41] | |
| Gu et al. 2018 | Fischer et al. 2018 [80] | |
| (Caffeine consumption quantity and type of tea source;> 5 times/wk) | ||
| Kitamura et al. 2016 [42] | Gu et al. 2018 [51] | |
| (Caffeine consumption quantity; 1–5 times/wk) | ||
| Kuriyama et al. 2006 [43] | Kuriyama et al. 2006 [43] | |
| (Caffeine consumption quantity and type of tea source; ≥ 2 cups/d) | (Caffeine consumption quantity; 4–7 cups/wk) | |
| Lee et al. 2017 [67] | Shen et al. 2015 [53] | |
| (Type of tea source) | ||
| Ng et al. 2008 [76] | Shirai et al. 2020 [45] | |
| (Caffeine consumption quantity;< once/d) | ||
| Noguchi-Shinohara et al. 2014 [44] | Tomata et al. 2016 [47] | |
| (Type of tea source) | (Caffeine consumption quantity;< 2 cups/d) | |
| Shirai et al. 2020 [45] | Xu et al. 2018 [55] | |
| (Caffeine consumption quantity; 2–3 times/d and ≥ 4 times/d) | (Sex and age; women, ≥70 years) | |
| Tomata et al. 2016 [47] | ||
| (Caffeine consumption quantity and type of tea source;> 2 cups/d) | ||
| Xu et al. 2018 [55] | ||
| (Type of tea source, sex and age; men,< 70 years) | ||
| Black/Oolong tea | ||
| Ng et al. 2008 [76] | Feng et al. 2018 [25] | |
| Shen et al. 2015 [53] | Kuriyama et al. 2006 [43] | |
| (Type of tea source) | (Type of tea source) | |
| Noguchi-Shinohara et al. 2014 [44] | ||
| (Type of tea source) | ||
| Tomata et al. 2016 [47] | ||
| (Type of tea source) | ||
| Xu et al. 2018 [55] | ||
| (Type of tea source) | ||
| Other/non-specified tea type | ||
| Arab et al. 2011 [32] | Arab et al. 2011 [32] | |
| (Sex; women) | (Sex; men) | |
| Chen et al. 2012 [49] | Broe et al. 1990 [78] | |
| Chin et al. 2008 [82] | Chuang et al. 2019 [66] | |
| (Caffeine consumption quantity and sex; 2–6 times/wk, men) | ||
| Chuang et al. 2019 [66] | Dai et al. 2006 [36] | |
| (Caffeine consumption quantity and sex; ≥ 7 times/wk, women) | ||
| Feng et al. 2012 [75] | Eskelinen et al. 2009 [28] | |
| Huang et al. 2009 [52] | Gu et al. 2018 [51] | |
| (Sex; men) | (Type of tea source) | |
| Jarvis 1993 [57] | Huang et al. 2009 [52] | |
| (Sex; women) | ||
| Lee et al. 2017 [67] | Lindsay 2002 [69] | |
| Nurk et al. 2009 [84] | Shen et al. 2015 [53] | |
| (Caffeine consumption quantity; < 2 cups/d) | ||
| Shen et al. 2015 | Tyas et al. 2001 [70] | |
| (Caffeine consumption quantity; | ||
| 2–4 cups/d and ≥4 cups/d) | ||
| Wang et al. 2017 [54] | Wang et al. 2017 [54] | |
| (Age;> 60 years) | (Age;> 70 years) | |
| Yang et al. 2016 [56] | Wu et al. 2011 [68] | |
Bold studies indicate multiple outcomes.
Table 2C
Association between multiple caffeinated sources (n = 15) and cognitive decline/dementia
| Multiple caffeinated sources | ||
| Positive association | No association | Negative association |
| Beydoun et al. 2014 | Beydoun et al. 2014 [33] | |
| (Age; ≥70 years) | (Age;< 70 years) | |
| Boot et al. 2013 [34] | Corley et al. 2010 [85] | |
| (Model; additional adjustments for socioeconomic status and (childhood) IQ) | ||
| Cao et al. 2012 [35] | Gelber et al. 2011 [24] | |
| Corley et al. 2010 [85] | Iranpour et al. 2020 [81] | |
| (Model; adjustment for age and sex only) | (Model; multiple additional adjustments) | |
| Driscoll et al. 2016 [37] | Lesk et al. 2009 [58] | |
| Iranpour et al. 2020 [81] | Paganini-Hill et al. 2016 [39] | |
| (Model; no adjustments) | (Caffeine consumption quantity and age; 60–199 mg/d, 70 years) | |
| Maia &de Mendonça 2002 [73] | Ritchie et al. 2007 [71] | |
| (Caffeine consumption quantity and sex; | < 300 mg/d, men) | |
| Paganini-Hill et al. 2016 [39] | Santos et al. 2010 [74] | |
| (Caffeine consumption quantity and age;> 200 mg/d, 90 years) | (Caffeine consumption quantity and sex; < 62 mg/d, men) | |
| Ritchie et al. 2007 [71] | Van Boxtel et al. 2003 [65] | |
| (Caffeine consumption quantity and sex; > 300 mg/d, women) | ||
| Santos et al. 2010 [74] | Vercambre et al. 2013 [72] | |
| (Caffeine consumption quantity and sex; > 62 mg/d, women) | (Model; adjustment for age, education and energy from diet only) | |
| Smith 2009 [59] | ||
| Vercambre et al. 2013 [72] | ||
| (Model; multiple additional adjustments) | ||
Bold studies indicate multiple outcomes.
Table 2D
Association between pure caffeine (n = 2) and cognitive decline/dementia
| Pure caffeine | ||
| Positive association | No association | Negative association |
| Konishi et al. | Walters &Lesk | |
| 2018 [48] | 2016 [60] | |
For tea-based studies, we observed 10/30 (33%) studies including 25,381/59,309 (43%) subjects with positive outcomes, 11/30 (37%) studies including 24,556/59,309 (41%) subjects with mixed outcomes dependent on consumed tea source (more positive for green tea), consumed quantity (more positive with moderate quantities), sex (mixed effects), and/or age (mixed effects), Furthermore, 9/30 (30%) studies including 9,372/59,309 (16%) subjects reported no association between tea intake and cognition. No negative associations were found for tea consumption. By classifying the different tea types, we observed proportionally more beneficial associations for green tea (39%) and other/non-specified tea (37%) compared to black/oolong tea (29%). On the other hand, we found that, across most studies (5/7 (71%) studies including 14,603/19,634 (74%) subjects), black/oolong tea was not associated with dementia/ cognitive decline.
Next, we assessed studies that included more than one caffeine source, including coffee, tea, carbonated soft drinks, energy drinks, and foods. Five out of 15 (33%) studies including 6,325/25,928 (24%) subjects reported a protective association and 3/15 (20%) studies including 4,210/25,928 (16%) reported no association between caffeine consumption and cognitive decline. Mixed results were found for 7/15 (47%) studies including 15,393/25,928 (59%) subjects: these studies revealed a dependency of study outcomes according to consumed quantity of caffe-ine, sex, age, and/or covariates in the models. More positive outcomes were found for women compared to men [71, 74], and more positive associations were found for a moderate or higher caffeine quantity (>62mg/d [74], >200 mg/d [39], >300 mg/d [71]). We also found that in studies with mixed caffeine sources, more positive effects were found at ages >70 years, and particularly over 90 years. We found inconclusive findings for the impact of univariate-/basic adjustments or multiple adjustments on cognitive function [72, 81, 85].
Finally, two studies assessed the association of pure caffeine consumption: Konishi et al. (2018) reported better executive function scores, while Walters & Lesk (2016) reported no significant association on cognitive tests.
Our examination of effects in coffee, tea, mixed sources, and pure caffeine-based studies demonstrates that the study outcomes are highly dependent on the caffeine source. Among these caffeine sources, only black/oolong tea seems not to have a protective effect for dementia/cognitive decline. In addition, our data reveal that evidence of a deleterious effect of caffeine consumption on cognitive function is limited.
Caffeine consumption quantity
We assessed the associations between caffeine qu-antity based on the frequency and/or dosage. Of the 61 studies, 48 provided sufficient information to al-low assessment of these associations (Table 3, Fig. 3). Based on pre-specified criteria, the studies were divided into three quantity categories: low caffeine consumption (<100 mg/d) (n = 29, N = 68,470), moderate caffeine consumption (100–400 mg/d) (n = 35, N = 111,776), and high caffeine consumption (>400 mg/d) (n = 14, N = 69,039). For studies with low- and high quantities of caffeine consumption, we mainly found no impact on risk of dementia or cognitive function: positive associations were only observed for 11/29 (38%) and 5/14 (36%) studies respectively. Interestingly, for moderate caffeine consumption, we mainly found beneficial associations with cognitive function (27/35 (77%) studies, that were either dependent (16/35 (46%) studies) or independent of type of caffeine source and/or other study characteristics (11/35 (31%) studies). By further stratifying studies using moderate consumption according to caffeine sources (Table 3), we found that especially consumption of green tea may reduce the risk of dementia and cognitive decline.
Table 3
Association between caffeine consumption quantity and cognitive decline/dementia
| Low caffeine consumption | |||
| (<100 mg/d) <1 cup coffee/d or <3 cups tea/d) | |||
| Studies | Caffeine source | Quantity | Association (+, /, –) |
| Arab et al. 2011 [32] | Tea (NS), Coffee | 0.57 cups/d | +(women), |
| 0.95 cups/d | / (men) | ||
| Araújo et al. 2015 [79] | Coffee | ≤1 cup/d | / |
| Araújo et al. 2016 [63] | Coffee | 0–1 cup/d | / |
| Chuang et al. 2019 [66] | Tea (NS), Coffee | 2–6 cups/wk | / |
| Dai et al. 2006 [36] | Tea (NS) | ≥3 cups/wk | / |
| Dong et al. 2020a [50] | Coffee | 1–266.4 mg/d | / |
| Feng et al. 2018 [25] | Tea (Black) | 1 cup/wk | / |
| Gu et al. 2018 [51] | Tea (Green), Tea (NS) | 1–5 times/wk | / |
| Haller et al. 2018 [87] | Coffee | <28 cups/month | / |
| Ide et al. 2014 [40] | Tea (Green tea powder) | 2 g/d (<100 mg/d caffeine) | + |
| Ide et al. 2016 [41] | Tea (Green tea powder) | 2 g/d (<100 mg/d caffeine) | / |
| Iranpour et al. 2020 [81] | Multiple sources | 11–102 mg/d | / |
| Kitamura et al. 2016 [42] | Tea (Green) | 1–6 cups/wk | +(univariate model), |
| / (multiple additional adjustments) | |||
| Kuriyama et al. 2006 [43] | Tea (Green), Tea | <1 cup/d | / |
| (Black/oolong), Coffee | |||
| Lee et al. 2017a [67] | Tea (Green), Tea | >3 cups/wk | + |
| (Black/oolong), Coffee | |||
| Lesk et al. 2009 [58] | Multiple sources | Mean: 70.3 (±36.2) mg/d | / |
| Maia &de Mendonça, 2002 [73] | Multiple sources | Mean: 73.9 (±97.9) mg/d | + |
| Ng et al. 2008 [76] | Tea (Green), Tea | <1 cup/d | + |
| (Black/oolong), Coffee | / | ||
| Noguchi-Shinohara et al. 2014 [44] | Tea (Green) | <1 cup/d | + |
| Tea (Black), Coffee | / | ||
| Paganini-Hill et al. 2016a [39] | Multiple sources | 50–199 mg/d | / |
| Ritchie et al. 2007a [71] | Multiple sources | 100–200 mg/d | / |
| Santos et al. 2010 [74] | Multiple sources | 22–62 mg/day | / |
| Shen et al. 2015 [53] | Tea (Black), Tea (Green) | <2 cups/d | / |
| Shirai et al. 2020 [45] | Tea (Green) | 2–3 times/d | + |
| Solfrizzi et al. 2015a [77] | Coffee | 1 cup/d | / |
| Tomata et al. 2016 [47] | Tea (Green), | 1–2 cup/d | / |
| Tea (Black/oolong) | |||
| Valls-Pedret et al. 2012 [23] | Coffee | Median: 21 ml/d | + |
| Wu et al. 2011 [68] | Coffee | >1 cup/wk | + |
| Tea (NS) | / | ||
| Xu et al. 2018 [55] | Tea (Green) | >3 cup/wk | +(men, particularly < 70 |
| Tea (Black/oolong) | years),/ (women) | ||
| Moderate caffeine consumption | |||
| (100–400 mg/d) | |||
| 1–4 cups coffee/d or 3–10 cups tea/d | |||
| Araújo et al. 2015 [79] | Coffee | 2–3 cups/d | +(65–74 years), |
| / (35–64 years) | |||
| Araújo et al. 2016 [63] | Coffee | 1–3 cups/d | / |
| Beydoun et al. 2014 [33] | Multiple sources | Mean: 132 mg/d | +(≥70 years), |
| / (< 70 years) | |||
| Broe et al. 1990 [78] | Tea (NS) | >4 cups/d | / |
| Chin et al. 2008 [82] | Tea (NS) | Mean: 4.46 cups/d | + |
| Chuang et al. 2019a [66] | Tea (NS), Coffee | ≥7 cups/wk | +(all subjects and women), |
| / (men) | |||
| Corley et al. 2010 [85] | Multiple sources | Mean: 182.5 mg/d | +(adjustment for age and), |
| sex/ (multiple additional) | |||
| adjustments | |||
| Dong et al. 2020a [50] | Coffee | 266.4–295 mg/d | + |
| Driscoll et al. 2016 [37] | Multiple sources | Mean: 261 mg/d | + |
| Eskelinen et al. 2009a [28] | Coffee | 3–5 cups/d | + |
| Feng et al. 2018a [25] | Tea (Black) | >1 cup/d | / |
| Gelber et al. 2011 [24] | Coffee, Multiple sources | 115.5–188.0 mg/d | / |
| Gu et al. 2018 [51] | Tea (Green) | >5 times/wk | + |
| Tea (NS) | / | ||
| Haller et al. 2018 [87] | Coffee | 29–60 cups/mo | + |
| Iranpour et al. 2020 [81] | Multiple sources | >209 mg/d | +(univariate model), |
| / (multiple additional adjustments) | |||
| Johnson-Kozlow et al. 2002 [38] | Coffee | Mean: 3 cups/d | +(women), |
| / (men) | |||
| Kitamura et al. 2016a [42] | Tea (Green) | >1 cup/d | + |
| Konishi et al. 2018 [48] | Pure caffeine | 200 mg/d | + |
| Kuriyama et al. 2006 [43] | Tea (Green) | ≥2 cups/d | + |
| Tea (Black/oolong), | / | ||
| Coffee | |||
| Larsson &Wolk, 2018a [86] | Coffee | 1.0–4.9 cups/d | / |
| Lindsay, 2002 [69] | Coffee | >1 cup/d | + |
| Tea (NS) | / | ||
| Mirza et al. 2014 [64] | Coffee | 1–3 cup/d | / |
| Ng et al. 2008 [76] | Tea (Green), | >1 cup/d | + |
| Tea (Black/oolong) | / | ||
| Coffee | |||
| Noguchi-Shinohara et al. 2014 [44] | Tea (Green) | >1 cup/d | + |
| Coffee | / | ||
| Paganini-Hill et al. 2016 [39] | Multiple sources | >200 mg/d | +(> 90 years),/(> 70 years) |
| Ritchie et al. 2007 [71] | Multiple sources | 200–300 mg/d | / |
| Santos et al. 2010a [74] | Multiple sources | >62 mg/day | +(women), |
| / (men) | |||
| Shen et al. 2015 [53] | Tea (Black) | ≥4 cups/d | + |
| Tea (Green) | / | ||
| Shirai et al. 2020 [45] | Tea (Green) | ≥4 times/d | + |
| Coffee | ≥2 times/d | / | |
| Smith, 2009 [59] | Multiple sources | Mean: 140 mg/d | + |
| Solfrizzi et al. 2015 [77] | Coffee | 1–2 cups/d | + |
| Sugiyama et al. 2016 [46] | Coffee | 1–2 cups/d | + |
| Tomata et al. 2016 [47] | Tea (Green) | ≥5 cups/d | + |
| Tea (Black/oolong) | / | ||
| Van Gelder et al. 2007 [62] | Coffee | 1–4 cups/d | + |
| Walters &Lesk, 2016 [60] | Pure caffeine | 200 mg/d | / |
| High caffeine consumption | |||
| (>400 mg/d) | |||
| >4 cups coffee/d,>10 cups tea/d | |||
| Araújo et al. 2015a [79] | Coffee | ≥3 cups/d | / |
| Araújo et al. 2016a [63] | Coffee | ≥3 cups/d | +(short-term),/ (long-term) |
| Broe et al. 1990 [78] | Coffee | ≥4 cups/d | / |
| Dong et al. 2020 [50] | Coffee | ≥495 mg/d | + |
| Eskelinen et al. 2009 [28] | Coffee | >5 cups/d | / |
| Gelber et al. 2011 [24] | Coffee | 415–2673 mg/d | / |
| Multiple sources | |||
| Haller et al. 2018a [87] | Coffee | 61–168 cups/mo | / |
| Laitala et al. 2009 [61] | Coffee | Mean: 5.3 cups/d | / |
| Larsson &Wolk, 2018a [86] | Coffee | ≥5.0 cups/d | / |
| Mirza et al. 2014a [64] | Coffee | >3 cups/d | +(short-term), |
| / (long-term) | |||
| Ritchie et al. 2007a [71] | Multiple sources | >300 mg/d | +(women), |
| / (men) | |||
| Van Boxtel et al. 2003 [65] | Multiple sources | Median: 5–6 cups/d | / |
| Van Gelder et al. 2007 [62] | Coffee | >4 cups/d | / |
| Vercambre et al. 2013 [72] | Multiple sources | >371 mg/d | + |
aCategorization in this group due to different categories used in the study.
Confounding factors
Most studies adjusted for age and sex, and in a subset of studies additional model adjustments were made for factors like hypertension, diabetes mellitus, hyperlipidemia, education, APOE genotype, smoking, alcohol, physical activities, body mass index (BMI), socioeconomic status, and global cognition (MMSE). Some studies reported an impact of confounding factors on outcomes.
For seven studies [32, 38, 52, 55, 66, 71, 74], outcomes were dependent on sex. These studies reported that beneficial associations are predominantly found in women (5/7 studies). In line with these findings, two studies with only female participants [37, 72] reported positive associations and two out of three studies with only male participants [24, 25, 62] reported no associations.
Four studies indicated that positive associations are dependent on age. These studies reported positive associations between caffeine consumption and dementia and/or cognitive function at older ages (65–74 years versus 35–64 years [79], >70 years versus <70 years [33], 90 years versus 70 years [39]). However, two other studies indicated the reverse, an effect at younger age (>60 years versus >70 years [54]) or that effects were particularly found at ages <70 years old [55].
Furthermore, Mirza et al. (2014) [64] and Araújo et al. (2016) [63] found different outcomes depending on the time of follow-up. Short-term follow-up (within 4 years) revealed positive associations, while the association was negative at long-term follow-up (>4 years) [64] and absent in another study implementing a long-term follow-up (5.5 years) [63].
Corley et al. (2010) [85] observed protective associations between caffeine and cognitive function when adjusting for age and sex, but when additional adjustments were made for socioeconomic status or social class and (childhood) IQ, the association did not reach the threshold for statistical significance. Similar results were observed by Iranpour et al. (2020) [81], who reported a positive association in a univariate model but no association in models where adjustments for factors like sex, age, race/ethnicity, education, and marital status, or self-rated health, disease history, and depression were made. Vercambre et al. (2013) [72], on the other hand, only found a positive association when adjusting for alcohol consumption, physical activity, BMI, and smoking, but not when only adjusting for age, education, and diet. Moreover, this study found a more pronounced positive association with caffeine when it was supplemented with vitamin B.
DISCUSSION
In this systematic review, we assessed the association between caffeine and 1) the risk of dementia and/or cognitive decline and 2) cognitive function in individuals with impaired cognition (i.e., MCI or dementia). The number of studies showing positive associations (dependent or independent of study cha-racteristics) was 46/57 (81%) including 111,926/153,070 (73%) subjects, indicating that caffeine has a beneficial effect on the risk of dementia/cognitive decline. We also found more positive results (3/4 (75%) studies including 272/289 (94%) subjects) for studies that included subjects with MCI, or any type of dementia, indicating that caffeine also has a beneficial effect in cognitively impaired subjects. Furthermore, we observed that various study characteristics affect the reported associations of caffeine such that moderate consumption seems to be more beneficial than low- or high quantities, and coffee, green- and other/non-specified tea, and multiple caffeinated sources are more beneficial than other caffeine sources like black/oolong tea. Effects were also found to be more pronounced in women compared to men, and many studies reported mixed outcomes based on other factors like age and follow-up time. Across all studies, we observed only two studies with a negative effect, suggesting that caffeine is unlikely to negatively affect cognition or dementia risk. This review highlights that dietary factors may influence risk of cognitive decline and dementia, and may also aid the future development of caffeine-based intervention studies, which might serve as a cost-effective alternative or add-on to other non-pharmacological or pharmacological treatments against cognitive decline and dementia (e.g., physical activity [88]).
Potential mechanisms
Results from this review suggest that caffeine effects are dependent on the caffeine source and quantity. Several explanations exist for this outcome. First, different types of caffeine sources contain different levels of caffeine [29], and low dosages might be inadequate to convey positive effects while with excessive dosages the negative effects (e.g., anxiety) might outweigh the positive effects. There might also be individual variability in the physiological response to caffeine (e.g., due to genetic factors that influence responsiveness of A2A receptors), which would result in differential effects of the same dose of caffeine across individuals [14, 89, 90]. Furthermore, physiological effects of other substances than caffeine that are contained within the caffeine source (e.g., coffee) may influence or strengthen the caff-eine response, by affecting the kinetics of caffeine in the body and the response of adenosine receptors, or have a caffeine independent effect that influen-ces cognitive performance [91]. For example, various sources of caffeine contain antioxidants, which have been found to play a role in protecting against oxi-dative stress, and may thereby help in preventing cognitive deterioration [92]. Coffee displays antioxidative effects through chlorogenic acid and poly-phenols [93]. Tea displays antioxidative effects through tea catechins and theaflavins, and green tea exhibits higher antioxidative effects than black or oolong tea [94]. Varying antioxidative mechanisms or degrees of antioxidative effects might contribute to the differences in study effects according to caf-feine sources observed in this report (i.e., more effects in green compared to black/oolong tea). However, further research is needed on the effect of antioxidants as studies have also reported no effect of antioxidants on cognitive function, but rather on mood [95]. Caffeine may also lead to better cognitive function and memory indirectly through an increase in alertness and wakefulness [12], and by influencing sleep and impulsivity [14, 96].
Caffeine has also been found to influence neural and vascular activity such as vasoconstriction and reduction in cerebral blood flow (CBF). Reduction in CBF leads to an increased oxygen extraction from the blood to cerebral structures in the brain [97], which, in turn, enhances cognitive performance. It seems possible that a sufficient quantity must be ingested in order to produce this effect. On the other hand, excessive caffeine consumption could lead to (acute) caffeine overdose, which could convey negative effects such as reactive oxygen species formation [98], that outweigh the positive, or indirect negative symptoms that could influence cognitive function such as restlessness, anxiety, agitation, insomnia, and headache [16].
This review revealed incongruent outcomes for other confounding factors such as sex, age, and follow-up time. It seems that caffeine consumption is particularly beneficial for cognitive function in women in comparison with men. In general, inconsi-stent results for women and men might be explained through sex-based biological variations such as tes-tosterone and estrogen hormone levels [99]. Furthermore, four studies reported an outcome that was dependent on age, but it remains to be determined at what age caffeine has the most beneficial effect as some studies reported greater effects in older subjects, while others reported greater effects in younger subjects. Follow-up time was also found to influence outcomes in two studies. These studies both reported beneficial associations at a short follow-up time, while no effects were observed at a long-term follow up. This suggests that the beneficial effects of caffeine might be temporary.
Strengths and limitations
The main strength is that we performed a systematic review and assessed all available studies, regardless of study design. Thereby, we were able to include more studies than have previously been in-cluded in other reviews and meta-analyses [100–103]. However, there are also limitations that need to be considered when interpreting this review. First of all, it is important to highlight that, in the second-ary analysis on cognitively impaired individuals, we were able to assess only four studies, and that these studies included individuals with different types of cognitive impairments, various caffeine sources and different study designs. Also, one out of four studies included patients with PD, for which the degrading underlying mechanisms are different compared to patients with dementia or MCI. Secondly, our app-roach of providing this systematic review did not allow us to perform formal statistical analyses to assess the effects of caffeine quantitatively, or stati-stically assess modifying effects. This lack of quantitative assessments means our findings were based exclusively on overall study outcomes. Thirdly, our interpretation of the included studies relied on data provided in the paper, and we did not contact the authors to provide additional information because of the wide inclusion timeframe of this review (1990–2020). As a result, not all studies could be included when assessing study characteristics. For example, accurate information on caffeine quantity was not al-ways provided. Furthermore, many studies employed self-reported caffeine consumption data resulting in a high risk of bias due to deviations from the intended intervention. Finally, information on reporting of funding sources and conflicts of interests were not considered as possible confounders in the analyses.
Conclusion
Our findings indicate that caffeine beneficially affects cognitive function and risk of dementia and that this effect is dependent on the type of caffeine source (e.g., more effects for coffee and green tea), quantity (more effects with moderate quantities), and sex (more effects in female subjects). Furthermore, we found that other factors such as age and follow-up time might influence effects and it is important for future studies to examine, and account for, these confounders. Ideally, future investigation should implement a randomized-controlled trial design, which would allow for quantitative assessme-nts of effects across studies. Future studies including various dosage levels could additionally help to extend our findings regarding the most beneficial caffeine dosage by accurately determining the optimal caffeine quantity to effect cognitive decline and risk of dementia. Furthermore, it would be interesting to map genetic factors that influence response to caffeine (e.g., A2A receptor haplotype) in future studies, as differences in responsiveness to caffeine could influence effects of caffeine on cognition. These in-sights may help in tailoring cost-effective lifestyle interventions, and possibly even aid in the development of pharmacological interventions that combat cognitive decline and dementia.
DISCLOSURE STATEMENT
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/20-1069).
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-201069.
REFERENCES
[1] | ((2019) ) 2019 Alzheimer’s disease facts and figures. Alzheimers Dement 15: , 321–387. |
[2] | World Health Organization (2017) Global action plan on the public health response to dementia 2017 - 2025. |
[3] | Livingston G , Sommerlad A , Orgeta V , Costafreda SG , Huntley J , Ames D , Ballard C , Banerjee S , Burns A , Cohen-Mansfield J , Cooper C , Fox N , Gitlin LN , Howard R , Kales HC , Larson EB , Ritchie K , Rockwood K , Sampson EL , Samus Q , Schneider LS , Selbæk G , Teri L , Mukadam N ((2017) ) Dementia prevention, intervention, and care. Lancet 390: , 2673–2734. |
[4] | Mayer EA , Tillisch K , Gupta A ((2015) ) Gut/brain axis and the microbiota. J Clin Invest 125: , 926–938. |
[5] | Carabotti M , Scirocco A , Maselli MA , Severi C ((2015) ) The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol 28: , 203–209. |
[6] | Kalaria RN , Maestre GE , Arizaga R , Friedland RP , Galasko D , Hall K , Luchsinger JA , Ogunniyi A , Perry EK , Potocnik F , Prince M , Stewart R , Wimo A , Zhang Z-X , Antuono P ((2008) ) Alzheimer’s disease and vascular dementia in developing countries: Prevalence, management, and risk factors. Lancet Neurol 7: , 812–826. |
[7] | Mitchell DC , Knight CA , Hockenberry J , Teplansky R , Hartman TJ ((2014) ) Beverage caffeine intakes in the U.S. Food Chem Toxicol 63: , 136–142. |
[8] | Heckman MA , Weil J , Gonzalez de Mejia E ((2010) ) Caffeine (1, 3, 7-trimethylxanthine) in foods: A comprehensive review on consumption, functionality, safety, and regulatory matters.R. J Food Sci 75: , 77–87. |
[9] | López-Cruz L , Salamone JD , Correa M ((2018) ) Caffeine and selective adenosine receptor antagonists as new therapeutic tools for the motivational symptoms of depression. Front Pharmacol 9: , 526. |
[10] | Gomes C V , Kaster MP , Tomé AR , Agostinho PM , Cunha RA ((2011) ) Adenosine receptors and brain diseases: Neuroprotection and neurodegeneration. Biochim Biophys Acta 1808: , 1380–1399. |
[11] | Jacobson KA , Gao Z-G ((2006) ) Adenosine receptors as therapeutic targets. Nat Rev Drug Discov 5: , 247–264. |
[12] | Temple JL , Bernard C , Lipshultz SE , Czachor JD , Westphal JA , Mestre MA ((2017) ) The safety of ingested caffeine: A comprehensive review. Front Psychiatry 8: , 80. |
[13] | Liszt KI , Ley JP , Lieder B , Behrens M , Stöger V , Reiner A , Hochkogler CM , Köck E , Marchiori A , Hans J , Widder S , Krammer G , Sanger GJ , Somoza MM , Meyerhof W , Somoza V ((2017) ) Caffeine induces gastric acid secretion via bitter taste signaling in gastric parietal cells. Proc Natl Acad Sci U S A 114: , E6260–E6269. |
[14] | Cunha RA , Agostinho PM ((2010) ) Chronic caffeine consumption prevents memory disturbance in different animal models of memory decline.S. J Alzheimers Dis 20: (Suppl 1), 95–116. |
[15] | Kolahdouzan M , Hamadeh MJ ((2017) ) The neuroprotective effects of caffeine in neurodegenerative diseases. CNS Neurosci Ther 23: , 272–290. |
[16] | Szpak A , Allen D ((2012) ) A case of acute suicidality following excessive caffeine intake. J Psychopharmacol 26: , 1502–1510. |
[17] | Assis MS , Soares AC , Sousa DN , Eudes-Filho J , Faro LRF , Carneiro FP , Silva M V , Motoyama AB , Souza GMR , Marchiori S , Lima NT , Boëchat-Barros R , Ferreira VM ((2018) ) Effects of caffeine on behavioural and cognitive deficits in rats. Basic Clin Pharmacol Toxicol 123: , 435–442. |
[18] | Kaster MP , Machado NJ , Silva HB , Nunes A , Ardais AP , Santana M , Baqi Y , Müller CE , Rodrigues ALS , Porciúncula LO , Chen JF , Tomé ÂR , Agostinho P , Canas PM , Cunha RA ((2015) ) Caffeine acts through neuronal adenosine A2A receptors to prevent mood and memory dysfunction triggered by chronic stress. Proc Natl Acad Sci U S A 112: , 7833–7838. |
[19] | Cunha RA ((2016) ) How does adenosine control neuronal dysfunction and neurodegeneration?. J Neurochem 139: , 1019–1055. |
[20] | Arendash GW , Mori T , Cao C , Mamcarz M , Runfeldt M , Dickson A , Rezai-Zadeh K , Tane J , Citron BA , Lin X , Echeverria V , Potter H ((2009) ) Caffeine reverses cognitive impairment and decreases brain amyloid-beta levels in aged Alzheimer’s disease mice. J Alzheimers Dis 17: , 661–680. |
[21] | Dall’Igna OP , Fett P , Gomes MW , Souza DO , Cunha RA , Lara DR ((2007) ) Caffeine and adenosine A(2a) receptor antagonists prevent beta-amyloid (25-35)-induced cognitive deficits in mice. Exp Neurol 203: , 241–245. |
[22] | Cho B-H , Choi S-M , Kim J-T , Kim BC ((2018) ) Association of coffee consumption and non-motor symptoms in drug-naïve, early-stage Parkinson’s disease. Parkinsonism Relat Disord 50: , 42–47. |
[23] | Valls-Pedret C , Lamuela-Raventós RM , Medina-Remón A , Quintana M , Corella D , Pintó X , Martínez-González MÁ , Estruch R , Ros E ((2012) ) Polyphenol-rich foods in the Mediterranean diet are associated with better cognitive function in elderly subjects at high cardiovascular risk. J Alzheimers Dis 29: , 773–782. |
[24] | Gelber RP , Petrovitch H , Masaki KH , Ross GW , White LR ((2011) ) Coffee intake in midlife and risk of dementia and its neuropathologic correlates. J Alzheimers Dis 23: , 607–615. |
[25] | Feng L , Langsetmo L , Yaffe K , Sun Y , Fink HA , Shikany JM , Leung PC , Lane NE , Cauley JA ((2018) ) No effects of black tea on cognitive decline among older US men: A prospective cohort study. J Alzheimers Dis 65: , 99–105. |
[26] | Moher D , Liberati A , Tetzlaff J , Altman DG , PRISMA Group ((2009) ) Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. PLOS Med 6: , 1–6. |
[27] | Sterne JAC , Hernán MA , McAleenan A , Reeves BC , Higgins JPT ((2020) ) Chapter 25: Assessing risk of bias in a non-randomized study. In: Cochrane Handbook for Systematic Reviews of Interventions, version 6.1 (updated September 2020), Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, eds. Cochrane, pp. 621-641. |
[28] | Eskelinen MH , Ngandu T , Tuomilehto J , Soininen H , Kivipelto M ((2009) ) Midlife coffee and tea drinking and the risk of late-life dementia: A population-based CAIDE study. J Alzheimers Dis 16: , 85–91. |
[29] | Eskelinen MH , Kivipelto M ((2010) ) Caffeine as a protective factor in dementia and Alzheimer’s disease.S. J Alzheimers Dis 20 Suppl 1: , 167–74. |
[30] | Arab H , Mahjoub S , Hajian-Tilaki K , Moghadasi M ((2016) ) The effect of green tea consumption on oxidative stress markers and cognitive function in patients with Alzheimer’s disease: A prospective intervention study. Casp J Intern Med 7: , 188–194. |
[31] | Lammi UK , Kivelä SL , Nissinen A , Punsar S , Puska P , Karvonen M ((1989) ) Mental disability among elderly men in Finland: Prevalence, predictors and correlates. Acta Psychiatr Scand 80: , 459–468. |
[32] | Arab L , Biggs ML , O’Meara ES , Longstreth WT , Crane PK , Fitzpatrick AL ((2011) ) Gender differences in tea, coffee, and cognitive decline in the elderly: The Cardiovascular Health Study. J Alzheimers Dis 27: , 553–566. |
[33] | Beydoun MA , Gamaldo AA , Beydoun HA , Tanaka T , Tucker KL , Talegawkar SA , Ferrucci L , Zonderman AB ((2014) ) Caffeine and alcohol intakes and overall nutrient adequacy are associated with longitudinal cognitive performance among US adults. J Nutr 144: , 890–901. |
[34] | Boot BP , Orr CF , Ahlskog JE , Ferman TJ , Roberts R , Pankratz VS , Dickson DW , Parisi J , Aakre JA , Geda YE , Knopman DS , Petersen RC , Boeve BF ((2013) ) Risk factors for dementia with Lewy bodies: A case-control study. Neurology 81: , 833–840. |
[35] | Cao C , Loewenstein DA , Lin X , Zhang C , Wang L , Duara R , Wu Y , Giannini A , Bai G , Cai J , Greig M , Schofield E , Ashok R , Small B , Potter H , Arendash GW ((2012) ) High blood caffeine levels in MCI linked to lack of progression to dementia. J Alzheimers Dis 30: , 559–572. |
[36] | Dai Q , Borenstein AR , Wu Y , Jackson JC , Larson EB ((2006) ) Fruit and vegetable juices and Alzheimer’s disease: The Kame Project. Am J Med 119: , 751–759. |
[37] | Driscoll I , Shumaker SA , Snively BM , Margolis KL , Manson JE , Vitolins MZ , Rossom RC , Espeland MA ((2016) ) Relationships between caffeine intake and risk for probable dementia or global cognitive impairment: The Women’s Health Initiative Memory Study. J Gerontol A Biol Sci Med Sci 71: , 1596–1602. |
[38] | Johnson-Kozlow M , Kritz-Silverstein D , Barrett-Connor E , Morton D ((2002) ) Coffee consumption and cognitive function among older adults. Am J Epidemiol 156: , 842–850. |
[39] | Paganini-Hill A , Kawas CH , Corrada MM ((2016) ) Lifestyle factors and dementia in the oldest-old: The 90+Study. Alzheimer Dis Assoc Disord 30: , 21–26. |
[40] | Ide K , Yamada H , Takuma N , Park M , Wakamiya N , Nakase J , Ukawa Y , Sagesaka YM ((2014) ) Green tea consumption affects cognitive dysfunction in the elderly: A pilot study. Nutrients 6: , 4032–4042. |
[41] | Ide K , Yamada H , Takuma N , Kawasaki Y , Harada S , Nakase J , Ukawa Y , Sagesaka YM ((2016) ) Effects of green tea consumption on cognitive dysfunction in an elderly population: a randomized placebo-controlled study. Nutr J 15: , 49. |
[42] | Kitamura K , Watanabe Y , Nakamura K , Sanpei K , Wakasugi M , Yokoseki A , Onodera O , Ikeuchi T , Kuwano R , Momotsu T , Narita I , Endo N ((2016) ) Modifiable factors associated with cognitive impairment in 1,143 Japanese outpatients: The Project in Sado for Total Health (PROST). Dement Geriatr Cogn Dis Extra 6: , 341–349. |
[43] | Kuriyama S , Hozawa A , Ohmori K , Shimazu T , Matsui T , Ebihara S , Awata S , Nagatomi R , Arai H , Tsuji I ((2006) ) Green tea consumption and cognitive function: A cross-sectional study from the Tsurugaya Project. Am J Clin Nutr 83: , 355–361. |
[44] | Noguchi-Shinohara M , Yuki S , Dohmoto C , Ikeda Y , Samuraki M , Iwasa K , Yokogawa M , Asai K , Komai K , Nakamura H , Yamada M ((2014) ) Consumption of green tea, but not black tea or coffee, is associated with reduced risk of cognitive decline. PLoS One 9: , e96013. |
[45] | Shirai Y , Kuriki K , Otsuka R , Kato Y , Nishita Y , Tange C , Tomida M , Imai T , Ando F , Shimokata H ((2020) ) Green tea and coffee intake and risk of cognitive decline in older adults: The National Institute for Longevity Sciences, Longitudinal Study of Aging. Public Health Nutr 23: , 1049–1057. |
[46] | Sugiyama K , Tomata Y , Kaiho Y , Honkura K , Sugawara Y , Tsuji I ((2016) ) Association between coffee consumption and incident risk of disabling dementia in elderly Japanese: The Ohsaki Cohort 2006 Study. J Alzheimers Dis 50: , 491–500. |
[47] | Tomata Y , Sugiyama K , Kaiho Y , Honkura K , Watanabe T , Zhang S , Sugawara Y , Tsuji I ((2016) ) Green tea consumption and the risk of incident dementia in elderly Japanese: The Ohsaki Cohort 2006 Study. Am J Geriatr Psychiatry 24: , 881–889. |
[48] | Konishi Y , Hori H , Ide K , Katsuki A , Atake K , Igata R , Kubo T , Tominaga H , Beppu H , Asahara T , Yoshimura R ((2018) ) Effect of single caffeine intake on neuropsychological functions in healthy volunteers: A double-blind placebo-controlled study. PLoS One 13: , e0202247. |
[49] | Chen X , Huang Y , Cheng HG ((2012) ) Lower intake of vegetables and legumes associated with cognitive decline among illiterate elderly Chinese: A 3-year cohort study. J Nutr Heal Aging 16: , 549–552. |
[50] | Dong X , Li S , Sun J , Li Y , Zhang D ((2020) ) Association of coffee, decaffeinated coffee and caffeine intake from coffee with cognitive performance in older adults: National Health and Nutrition Examination Survey (NHANES) 2011-2014. Nutrients 12: , 840. |
[51] | Gu Y-J , He C-H , Li S , Zhang S-Y , Duan S-Y , Sun H-P , Shen Y-P , Xu Y , Yin J-Y , Pan C-W ((2018) ) Tea consumption is associated with cognitive impairment in older Chinese adults. Aging Ment Health 22: , 1232–1238. |
[52] | Huang C-Q , Dong B-R , Zhang Y-L , Wu H-M , Liu Q-X ((2009) ) Association of cognitive impairment with smoking, alcohol consumption, tea consumption, and exercise among Chinese nonagenarians/centenarians. Cogn Behav Neurol 22: , 190–196. |
[53] | Shen W , Xiao Y , Ying X , Li S , Zhai Y , Shang X , Li F , Wang X , He F , Lin J ((2015) ) Tea consumption and cognitive impairment: A cross-sectional study among Chinese elderly. PLoS One 10: , e0137781. |
[54] | Wang T , Xiao S , Chen K , Yang C , Dong S , Cheng Y , Li X , Wang J , Zhu M , Yang F , Li G , Su N , Liu Y , Dai J , Zhang M ((2017) ) Prevalence, incidence, risk and protective factors of amnestic mild cognitive impairment in the elderly in Shanghai. Curr Alzheimer Res 14: , 460–466. |
[55] | Xu H , Wang Y , Yuan Y , Zhang X , Zuo X , Cui L , Liu Y , Chen W , Su N , Wang H , Yan F , Li X , Wang T , Xiao S ((2018) ) Gender differences in the protective effects of green tea against amnestic mild cognitive impairment in the elderly Han population. Neuropsychiatr Dis Treat 14: , 1795–1801. |
[56] | Yang L , Jin X , Yan J , Jin Y , Yu W , Wu H , Xu S ((2016) ) Prevalence of dementia, cognitive status and associated risk factors among elderly of Zhejiang province, China in 2014. Age Ageing 45: , 708–712. |
[57] | Jarvis MJ ((1993) ) Does caffeine intake enhance absolute levels of cognitive performance?. Psychopharmacology (Berl) 110: , 45–52. |
[58] | Lesk VE , Honey TEM , de Jager CA ((2009) ) The effect of recent consumption of caffeine-containing foodstuffs on neuropsychological tests in the elderly. Dement Geriatr Cogn Disord 27: , 322–328. |
[59] | Smith AP ((2009) ) Caffeine, cognitive failures and health in a non-working community sample. Hum Psychopharmacol 24: , 29–34. |
[60] | Walters ER , Lesk VE ((2016) ) The effect of prior caffeine consumption on neuropsychological test performance: A placebo-controlled study. Dement Geriatr Cogn Disord 41: , 146–151. |
[61] | Laitala VS , Kaprio J , Koskenvuo M , Räihä I , Rinne JO , Silventoinen K ((2009) ) Coffee drinking in middle age is not associated with cognitive performance in old age. Am J Clin Nutr 90: , 640–646. |
[62] | van Gelder BM , Buijsse B , Tijhuis M , Kalmijn S , Giampaoli S , Nissinen A , Kromhout D ((2007) ) Coffee consumption is inversely associated with cognitive decline in elderly European men: The FINE Study. Eur J Clin Nutr 61: , 226–232. |
[63] | Araújo LF , Mirza SS , Bos D , Niessen WJ , Barreto SM , van der Lugt A , Vernooij MW , Hofman A , Tiemeier H , Ikram MA , Araujo LF , Mirza SS , Bos D , NiesLsen WJ , Barreto SM , van der Lugt A , Vernooij MW , Hofman A , Tiemeier H , Ikram MA ((2016) ) Association of coffee consumption with MRI markers and cognitive function: A population-based study. J Alzheimers Dis 53: , 451–461. |
[64] | Mirza SS , Tiemeier H , de Bruijn RFAG , Hofman A , Franco OH , Kiefte-de Jong J , Koudstaal PJ , Ikram MA ((2014) ) Coffee consumption and incident dementia. Eur J Epidemiol 29: , 735–741. |
[65] | van Boxtel MPJ , Schmitt JAJ , Bosma H , Jolles J ((2003) ) The effects of habitual caffeine use on cognitive change: A longitudinal perspective. Pharmacol Biochem Behav 75: , 921–927. |
[66] | Chuang S-Y , Lo Y-L , Wu S-Y , Wang P-N , Pan W-H ((2019) ) Dietary patterns and foods associated with cognitive function in Taiwanese older adults: The cross-sectional and longitudinal studies. J Am Med Dir Assoc 20: , 544–550.e4. |
[67] | Lee C-Y , Sun Y , Lee H-J , Chen T-F , Wang P-N , Lin K-N , Tang L-Y , Lin C-C , Chiu M-J ((2017) ) Modest overweight and healthy dietary habits reduce risk of dementia: A nationwide survey in Taiwan. J Prev Alzheimers Dis 4: , 37–43. |
[68] | Wu M-S , Lan T-H , Chen C-M , Chiu H-C , Lan T-Y ((2011) ) Socio-demographic and health-related factors associated with cognitive impairment in the elderly in Taiwan. BMC Public Health 11: , 22. |
[69] | Lindsay J , Laurin D , Verreault R , Hébert R , Helliwell B , Hill GB , McDowell I ((2002) ) Risk factors for Alzheimer’s disease: A prospective analysis from the Canadian Study of Health and Aging. Am J Epidemiol 156: , 445–453. |
[70] | Tyas SL , Manfreda J , Strain LA , Montgomery PR ((2001) ) Risk factors for Alzheimer’s disease: A population-based, longitudinal study in Manitoba, Canada. Int J Epidemiol 30: , 590–597. |
[71] | Ritchie K , Carriére I , de Mendonca A , Portet F , Dartigues JF , Rouaud O , Barberger-Gateau P , Ancelin ML ((2007) ) The neuroprotective effects of caffeine: A prospective population study (the Three City Study). Neurology 69: , 536–545. |
[72] | Vercambre M-N , Berr C , Ritchie K , Kang JH ((2013) ) Caffeine and cognitive decline in elderly women at high vascular risk. J Alzheimers Dis 35: , 413–421. |
[73] | Maia L , de Mendonça A ((2002) ) Does caffeine intake protect from Alzheimer’s disease?. Eur J Neurol 9: , 377–382. |
[74] | Santos C , Lunet N , Azevedo A , de Mendonça A , Ritchie K , Barros H ((2010) ) Caffeine intake is associated with a lower risk of cognitive decline: A cohort study from Portugal.S. J Alzheimers Dis 20: (Suppl 1), 175–85. |
[75] | Feng L , Li J , Ng T-P , Lee T-S , Kua E-H , Zeng Y ((2012) ) tea drinking and cognitive function in oldest-old Chinese. J Nutr Heal Aging 16: , 754–758. |
[76] | Ng T-P , Feng L , Niti M , Kua E-H , Yap K-B ((2008) ) Tea consumption and cognitive impairment and decline in older Chinese adults. Am J Clin Nutr 88: , 224–231. |
[77] | Solfrizzi V , Panza F , Imbimbo BP , D’Introno A , Galluzzo L , Gandin C , Misciagna G , Guerra V , Osella A , Baldereschi M , Di Carlo A , Inzitari D , Seripa D , Pilotto A , Sabbá C , Logroscino G , Scafato E ((2015) ) Coffee consumption habits and the risk of mild cognitive impairment: The Italian Longitudinal Study on Aging. J Alzheimers Dis 47: , 889–899. |
[78] | Broe GA , Henderson AS , Creasey H , McCusker E , Korten AE , Jorm AF , Longley W , Anthony JC ((1990) ) A case-control study of Alzheimer’s disease in Australia. Neurology 40: , 1698–1707. |
[79] | Araujo LF , Giatti L , dos Reis RC , Goulart AC , Schmidt MI , Duncan BB , Ikram MA , Barreto SM ((2015) ) Inconsistency of association between coffee consumption and cognitive function in adults and elderly in a cross-sectional study (ELSA-Brasil). Nutrients 7: , 9590–9601. |
[80] | Fischer K , Melo van Lent D , Wolfsgruber S , Weinhold L , Kleineidam L , Bickel H , Scherer M , Eisele M , van den Bussche H , Wiese B , König H-H , Weyerer S , Pentzek M , Röhr S , Maier W , Jessen F , Schmid M , Riedel-Heller SG , Wagner M ((2018) ) Prospective associations between single foods, Alzheimer’s dementia and memory decline in the elderly. Nutrients 10: , 852. |
[81] | Iranpour S , Saadati HM , Koohi F , Sabour S ((2020) ) Association between caffeine intake and cognitive function in adults; effect modification by sex: Data from National Health and Nutrition Examination Survey (NHANES) 2013-2014. Clin Nutr 39: , 2158–2168. |
[82] | Chin A-V , Robinson DJ , O’Connell H , Hamilton F , Bruce I , Coen R , Walsh B , Coakley D , Molloy A , Scott J , Lawlor BA , Cunningham CJ ((2008) ) Vascular biomarkers of cognitive performance in a community-based elderly population: The Dublin Healthy Ageing study. Age Ageing 37: , 559–564. |
[83] | Al-khateeb E , Al-zayadneh E , Al-dalahmah O , Alawadi Z , khatib F , Naffa R , Shafagoj Y ((2014) ) Relation between copper, lipid profile, and cognition in elderly Jordanians. J Alzheimers Dis 41: , 203–211. |
[84] | Nurk E , Refsum H , Drevon CA , Tell GS , Nygaard HA , Engedal K , Smith AD ((2009) ) Intake of flavonoid-rich wine, tea, and chocolate by elderly men and women is associated with better cognitive test performance. J Nutr 139: , 120–127. |
[85] | Corley J , Jia X , Kyle JAM , Gow AJ , Brett CE , Starr JM , McNeill G , Deary IJ ((2010) ) Caffeine consumption and cognitive function at age 70: The Lothian Birth Cohort 1936 study. Psychosom Med 72: , 206–214. |
[86] | Larsson SC , Wolk A ((2018) ) The role of lifestyle factors and sleep duration for late-onset dementia: A cohort study. J Alzheimers Dis 66: , 579–586. |
[87] | Haller S , Montandon M-L , Rodriguez C , Herrmann FR , Giannakopoulos P ((2018) ) Impact of coffee, wine, and chocolate consumption on cognitive outcome and MRI parameters in old age. Nutrients 10: , 1391. |
[88] | Groot C , Hooghiemstra AM , Raijmakers PGHM , van Berckel BNM , Scheltens P , Scherder EJA , van der Flier WM , Ossenkoppele R ((2016) ) The effect of physical activity on cognitive function in patients with dementia: A meta-analysis of randomized control trials. Ageing Res Rev 25: , 13–23. |
[89] | Erblang M , Drogou C , Merino DG , Metlaine A , Boland A , Deleuze JF , Thomas C , Sauvet F , Chennaoui M ((2019) ) The impact of genetic variations in ADORA2A in the association between caffeine consumption and sleep. Genes (Basel) 10: , 1–17. |
[90] | Nehlig A ((2018) ) Interindividual differences in caffeine metabolism and factors driving caffeine consumption. Pharmacol Rev 70: , 384 LP -411. |
[91] | Alsabri S , Mari W , Younes S , Alsadawi M , Oroszi T ((2018) ) Kinetic and dynamic description of caffeine. J Caffeine Adenosine Res 8: , 3–9. |
[92] | Peluso I , Serafini M ((2017) ) Antioxidants from black and green tea: From dietary modulation of oxidative stress to pharmacological mechanisms. Br J Pharmacol 174: , 1195–1208. |
[93] | Yashin A , Yashin Y , Wang JY , Nemzer B ((2013) ) Antioxidant and antiradical activity of coffee. Antioxidants (Basel) 2: , 230–245. |
[94] | Lee KW , Lee HJ , Lee CY ((2002) ) Antioxidant activity of black tea vs. green tea. J Nutr 132: , 785; author reply 786. |
[95] | Cropley V , Croft R , Silber B , Neale C , Scholey A , Stough C , Schmitt J ((2012) ) Does coffee enriched with chlorogenic acids improve mood and cognition after acute administration in healthy elderly? A pilot study. Psychopharmacology (Berl) 219: , 737–749. |
[96] | Grant JE , Chamberlain SR ((2018) ) Caffeine’s influence on gambling behavior and other types of impulsivity. Addict Behav 76: , 156–160. |
[97] | Kunz A , Iadecola C ((2009) ) Cerebral vascular dysregulation in the ischemic brain. Handb Clin Neurol 92: , 283–305. |
[98] | Massaad CA , Klann E ((2011) ) Reactive oxygen species in the regulation of synaptic plasticity and memory. Antioxid Redox Signal 14: , 2013–2054. |
[99] | Temple JL , Ziegler AM ((2011) ) Gender differences in subjective and physiological responses to caffeine and the role of steroid hormones. J Caffeine Res 1: , 41–48. |
[100] | Ruxton CHS ((2008) ) The impact of caffeine on mood, cognitive function, performance and hydration: A review of benefits and risks. Nutr Bull 33: , 15–25. |
[101] | McLellan TM , Caldwell JA , Lieberman HR ((2016) ) A review of caffeine’s effects on cognitive, physical and occupational performance. Neurosci Biobehav Rev 71: , 294–312. |
[102] | Liu Q-P , Wu Y-F , Cheng H-Y , Xia T , Ding H , Wang H , Wang Z-M , Xu Y ((2016) ) Habitual coffee consumption and risk of cognitive decline/dementia: A systematic review and meta-analysis of prospective cohort studies. Nutrition 32: , 628–636. |
[103] | Santos C , Costa J , Santos J , Vaz-Carneiro A , Lunet N ((2010) ) Caffeine intake and dementia: Systematic review and meta-analysis. J Alzheimers Dis 20: (Suppl 1), S187–204. |




