Glutathione Peroxidase Activity Is Altered in Vascular Cognitive Impairment-No Dementia and Is a Potential Marker for Verbal Memory Performance
Abstract
Background:
Coronary artery disease (CAD) increases risk for vascular cognitive impairment-no dementia (VCIND), a precursor to dementia, potentially through persistent oxidative stress.
Objective:
This study assessed peripheral glutathione peroxidase activity (GPX), which is protective against oxidative stress, in VCIND versus cognitively normal CAD controls (CN). GPX activity was also evaluated as a biomarker of cognition, particularly verbal memory.
Methods:
120 CAD patients with VCIND (1SD below norms on executive function or verbal memory (VM)) or without (CN) participated in exercise rehabilitation for 24 weeks. Neurocognitive and cardiopulmonary fitness (VO2 peak) assessments and plasma were collected at baseline and 24-weeks.
Results:
GPX was higher in VCIND compared to CN (F1,119 = 3.996, p = 0.048). Higher GPX was associated with poorer baseline VM (β= –0.182, p = 0.048), and longitudinally with VM decline controlling for sex, body mass index, VO2 peak, and education (b[SE] = –0.02[0.01], p = 0.004). Only CN participants showed improved VM performance with increased fitness (b[SE] = 1.30[0.15], p < 0.005).
Conclusion:
GPX was elevated in VCIND consistent with a compensatory response to persistent oxidative stress. Increased GPX predicted poorer cognitive outcomes (verbal memory) in VCIND patients despite improved fitness.
INTRODUCTION
Vascular cognitive impairment, no dementia
Vascular cognitive impairment (VCI) is defined as a spectrum of cognitive deficits due to cerebrovascular disease [1]. Along the spectrum of VCI, the mild stage, known as vascular cognitive impairment-no dementia (VCIND), is the most prevalent [2]. Clinically, patients are diagnosed with VCIND based on cognitive performance as well as a clear relationship between their cognitive symptoms and vascular disease history, in the absence of functional deficits. Commonly, neuroimaging is used to confirm presence of cerebrovascular disease [1, 3]. The term “possible” VCIND can be used if clinical signs and symptoms suggest vascular disease and cognitive impairment but no neuroimaging is available to corroborate the relationship [3]. Prevention and interventions that target early stages of cognitive impairment are increasingly recognized as having the potential for substantial public health, economic, and societal benefits [4]. An emerging interest in VCIND is consistent with that emphasis and the increasing recognition of the vascular contribution to dementia. VCIND may offer an ideal window to prevent or delay conversion to vascular dementia.
One population at high risk for VCIND are those with coronary artery disease (CAD). CAD patients are at increased risk for developing dementia [5, 6], consistent with the presence of hallmarks for VCI, including cerebrovascular pathology, and increased brain atrophy [7, 8] and white matter hyperintensities [9]. Cognitive impairment in CAD is associated with negative health outcomes such as poor compliance with medications, and increased rates of institutionalization and mortality [10–13]. Furthermore, even minor cognitive changes have been linked to meaningful clinical characteristics such as increased risk of non-completion of cardiac rehabilitation (CR) [14] and attenuated cognitive response to exercise intervention [15] in these patients. We previously demonstrated that up to 50% of those with CAD starting CR have cognitive impairment consistent with VCIND with the presence of white matter hyperintensities [16], and multiple cerebrovascular risk factors such as hypertension, diabetes, hypercholesterolemia, and obesity which are associated with CAD [15, 17]. As such, CAD patients represent a cognitively vulnerable population in whom early cognitive changes indicative of VCIND should be identified and studied.
Oxidative stress and exercise
Management of cardiovascular disease through exercise has been considered a strategy to modify the course of or prevent neurodegeneration attributed to vascular disease [18]. A large prospective study demonstrated that exercise may reduce the risk of cognitive impairment [19]. Moreover, a meta-analysis has shown that exercise may improve cognitive performance in patients with mild cognitive impairment (MCI) [20]. However, results in mild stages of cognitive impairment have been mixed [19–22]. While reasons for that remain unclear, our recent study found a relationship between persistent oxidative stress and decreased benefits for cognition following exercise. In 118 CAD patients, those with possible VCIND had persistent oxidative stress, indicated by higher ratios of late-stage to early-stage lipid peroxidation products [15]. Verbal memory performance was particularly related to those markers: those with higher concentrations of late-stage lipid peroxidation markers at baseline had less improvement in verbal memory performance in response to a 6-month CR program [15].
Glutathione peroxidase (GPX) activity
GPX, the main enzyme of the glutathione (GSH) antioxidant system, reduces oxidative stress species such as lipid peroxidation products [23]. Based on in vitro and animal model studies, GPX is upregulated in response to oxidative stress challenge [24]. Evidence supporting peripheral GPX activity as a diagnostic biomarker differentiating between cognitive states, as defined by the FDA-NIH Biomarkers, EndpointS, and other Tools (BEST) resource [25] is important for understanding possible mechanisms of neurodegeneration. Alterations in peripheral GPX activity have been observed in neurodegenerative processes overall [26]; however, the small existing literature on GPX activity in dementia is heterogeneous. For example, plasma GPX activity has been documented to be higher in patients with Alzheimer’s disease (AD) and in those with vascular dementia (VaD) compared to healthy controls in some studies [27–29]. Other studies in MCI and AD have found plasma GPX activity decreased compared to age-matched controls [30, 31], and another found no significant difference between dementia patients and controls [32]. The heterogeneity in GPX activity across those studies may be due to a variety of factors including the wide ranges in severity of cognitive impairments considered, as well as different sample populations, with no data to date in VCIND.
Interest in GPX and VCIND
Evidence suggests that GPX may be particularly relevant in VCIND. GPX activity is elevated in CAD [23], and a previous study found CAD patients with possible VCIND had a higher ratio of late stage to early stage lipid peroxidation markers [15], suggesting oxidative stress. There are mechanistic correlates between cardiovascular risk factors, oxidative stress, and endogenous antioxidant changes in CAD [33–36], and a link between oxidative stress and neurodegenerative processes [37, 38]. However, these correlates are not well-defined in the intersection of CAD and VCIND.
Objectives
The primary objective of this study was to compare GPX activity in those with VCIND to that in cognitively normal CAD controls (CN). As secondary objectives, GPX was assessed as a predictive and as a monitoring biomarker of verbal memory. The FDA-NIH BEST resource defines predictive biomarkers as markers that can help identify individuals likely to experience a particular outcome compared to similar individuals who do not have the same biomarker [25]. A monitoring biomarker is measured serially to assess the status of a condition for evidence of, or effect of an external influence. Verbal memory was chosen as the cognitive outcome in this study because the neuropsychological profile associated with VCIND implicates verbal memory as a primary cognitive domain affected in this condition [1, 39, 40] and because verbal memory predicts cognitive decline over time [16, 41–47].
Hypotheses
Given previous reports of increased oxidative stress in VCIND [15] but high heterogeneity in GPX activity across dementias [27, 28, 30, 31], our primary hypothesis was that GPX activity would be increased in possible VCIND participants compared to CN. Our secondary hypotheses were that baseline GPX activity would predict verbal memory, and that change in GPX activity over 6 months would be associated with change in verbal memory. Relationships with additional cognitive domains and changes in cardiopulmonary fitness were also explored.
MATERIALS AND METHODS
Study design
This study was approved by the Research Ethics Boards of Sunnybrook Health Sciences Centre and the University Health Network. Written informed consent was obtained from all participants prior to study enrolment. Patients diagnosed with CAD were recruited from the University Health Network Toronto Rehabilitation Institute CR program. The CR program consisted of weekly group education sessions, supervised exercise classes, and home exercise sessions incorporating aerobic exercises and resistance training. Cardiopulmonary fitness was assessed by exercise stress tests at entry and upon completion of CR, at which time the maximal rate of oxygen consumption measurement (VO2 peak) was obtained. Neurocognitive assessments were conducted at baseline and 24-week time points. Fasting blood was taken before noon at baseline and 24-week time points. Plasma GPX activity was subsequently measured with a specific GPX assay.
Study participants
All patients in this study had CAD, defined as having a history of myocardial infarction (MI), coronary angiographic evidence of greater than 50% stenosis in at least one major coronary artery or a prior revascularization procedure. Additionally, eligible patients were 45–80 years old, spoke and understood English, and had stable CAD defined as having no hospitalization events, such as an acute MI, unstable angina, congestive heart failure, ventricular arrhythmias, coronary revascularization, or Canadian Cardiovascular Society Class 4 angina, in the 4 weeks prior to study enrolment.
Participants were excluded if they had severely impaired liver/kidney/lung function, a current neurological condition such as epilepsy, Huntington’s disease, Parkinson’s disease or clinical stroke, a diagnosis of a major psychiatric disorder, a diagnosis of dementia, substance abuse, uncontrolled hypothyroidism, autoimmune disorders, or sepsis. Patients were also excluded if they were taking medications such as hypnotics, antipsychotics, antidepressants, and anticholinergic medications. Participants with significant cognitive impairment demonstrated by having a standardized Mini-Mental State Examination (MMSE) score of <24 were also excluded.
Patients were classified as having possible VCIND based on performing at least 1 standard deviation below the population norm based on their composite Z-scores in either executive function or verbal memory [3].
Cognitive assessments
Verbal memory and executive function were assessed based on recommendations of the National Institute of Neurological Disorders and Stroke and the Canadian Stroke Network neuropsychological battery for vascular cognitive impairment [3, 48]. To assess verbal memory, the California Verbal Learning Test 2nd Ed. (CVLT-II) [49] was used. The CVLT-II has good test-retest reliability and has been demonstrated to be sensitive in detecting cognitive impairment in patients with cardiovascular issues. Three Z-scores from the CVLT-II, verbal learning, short delay, and long delay free recall were summed, from which a composite Z-score for verbal memory was calculated. Executive function was assessed using a composite Z-score calculated from Z-scores of Trails Making Test B, Controlled Oral Word Association Test, and Category Fluency Test, as these assessments have been validated as measures of executive function [50, 51]. These tests have been used to identify cognitive impairment in CAD patients that was linked to high oxidative stress [1, 15], with cognitive impairment defined using a cut-off of at least 1 standard deviation (SD) below normative means as recommended [48, 52].
Glutathione peroxidase assay
GPX activity was measured in plasma using a GPX Assay kit (Cayman 703102), according to manufacturer’s instructions. Study participants had fasted for 12 h prior to having blood drawn from the antecubital vein for both baseline and 6-month time points. Blood samples were collected in EDTA-coated tubes and centrifuged at 1000 g for 10 min (Model 614B, The Drucker Company) from which the plasma supernatant was aliquoted and stored at –80°C until analysis. Results were expressed as nmol/min/ml.
Statistical analyses
All statistical analyses were performed on the IBM SPSS Statistics (version 20; Armonk, NY) software and were considered significant at a two-tailed p < 0.05. Demographics and associations between clinical factors and VCIND groups were assessed using Chi-Square Tests and Fisher’s Exact where appropriate, and analysis of variance (ANOVA) where appropriate. Demographic characteristics are reported in terms of means and SD for continuous variables and percentages for categorical variables. To describe the impact of CR, the change in GPX and the change inn verbal memory over time were assessed using linear mixed models. For the primary analysis, an analysis of covariance was performed to compare GPX activity between possible VCIND patients and CN controls, with a priori covariates being age, smoking status, and whether the participant was taking antioxidant/multivitamin supplementation; all of which have been previously associated with GPX activity or controlled for in previous studies [27, 53–60]. Next, the association between GPX activity and verbal memory composite Z-scores at baseline was evaluated in a linear regression, controlling for sex, BMI, VO2 peak, and years of education; covariates having previously been associated with cognitive performance [15, 45, 53, 61–71]. Baseline GPX activity was assessed as a predictor of verbal memory performance over 24 weeks of CR using a linear mixed effects analysis. Similarly, changes in GPX activity were assessed as a monitoring marker of change in verbal memory performance over 24 weeks of CR using a linear mixed model. A linear mixed model, with patient as a random effect, was also run to assess change in verbal memory over time between the CN and VCIND patient groups. A VCIND status by time interaction term were also added to this model in a post hoc analyses. All analyses included the following covariates: age, sex, total years of education, max VO2 peak, and BMI. Linear mixed model analyses were also conducted to identify associations between VO2 peak and verbal memory over 6 months to determine if changes in cognitive performance were associated with increasing physical fitness. Similarly, linear mixed model analyses were conducted to identify associations between VO2 peak and GPX activity to assess whether GPX activity was changing with increasing fitness.
RESULTS
Patient characteristics
The 120 patients recruited for this study were aged 64±6 years, mostly male (84%), Caucasian (83%), and had an average of 16±3 years of education. Demographic and clinical characteristics of those with and without possible VCIND at baseline did not differ between the groups, except for total years of education (Table 1). Baseline vascular risk factors, cardiopulmonary fitness parameters, comorbidities, and medications did not differ between groups. BMI and medications did not differ significantly between VCIND and CAD patients, and all study participants were taking statins at baseline. Cognitive performance at baseline and 6-month time points are displayed in Table 2. As expected, those with possible VCIND were significantly more cognitively impaired at both time points.
Table 1
Patient demographics and clinical characteristics between VCIND groups at baseline
| Variable | Possible VCIND (N = 36) mean±SD/% | CN controls (N = 84) mean±SD/% | Significance |
| Demographics | |||
| Age | 62.6±6.5 | 64.4±6.3 | F (1,119) = 1.95, p = 0.166 |
| Sex (male) | 89% | 82% | χ2 = 0.621, p = 0.262 |
| Total years of education | 15.1±4.3 | 16.8±2.9 | F (1,119) = 6.67, p = 0.011 |
| Ethnicity, Caucasian | 75% | 87% | χ2 = 2.57, p = 0.093 |
| Body composition | |||
| Height (cm) | 172.6±8.1 | 171.6±7.8 | F (1,119) = 0.390, p = 0.534 |
| Weight (kg) | 86.2±14.4 | 86.5±17.6 | F (1,119) = 0.008, p = 0.930 |
| BMI (kg/m2) | 28.9±4.5 | 29.3±5.4 | F (1,119) = 0.107, p = 0.744 |
| Waist circumference (cm) | 99.3±10.2 | 99.1±13.0 | F (1,119) = 0.003, p = 0.955 |
| Hip circumference (cm) | 105.6±10.8 | 105.9±11.1 | F (1,113) = 0.018, p = 0.894 |
| Body fat % | 29.3±8.0 | 32.7±11.3 | F (1,117) = 2.68, p = 0.104 |
| Vascular risk factors | |||
| Hypertension | 97% | 93% | Fisher’s Exact, p = 0.790 |
| Diabetes | 14% | 18% | χ2 = 0.286, p = 0.404 |
| Hypercholesterolemia | 100% | 100% | - |
| BMI> = 30 | 33% | 42% | χ2 = 0.734, p = 0.258 |
| History of cigarette smoking | 58% | 63% | χ2 = 0.242, p = 0.385 |
| Number of vascular risk factors | 3.0±0.8 | 3.2±1.0 | F (1,119) = 0.500, p = 0.481 |
VCIND, vascular cognitive impairment, no dementia; SD, standard deviation; BMI, body mass index.
Table 2
Cognitive performance at baseline and 6-month time-points.
| Time-point | Verbal memory composite Z-score±SD | Significance | Executive function composite Z-score±SD | Significance | ||
| VCIND | CN controls | VCIND | CN controls | |||
| Baseline | –0.94±0.96 | 0.40±0.71 | F (1,119) = 71.11, p < 0.001 | –0.99±0.73 | 0.42±0.78 | F (1,119) = 86.90, p < 0.001 |
| 6-months | –0.72±1.07 | 0.26±0.85 | F (1,99) = 22.20, p < 0.001 | –0.94±0.72 | 0.33±0.87 | F (1,119) = 44.19, p < 0.001 |
VCIND, vascular cognitive impairment, no dementia; CN, cognitively normal; SD, standard deviation.
Impact of cardiac rehabilitation
GPX activity increased over time (b[SE] =1.62[0.81], p = 0.048). However, an increase in physical fitness over CR was not associated with an increase in GPX activity over CR overall (b[SE] = 0.16[0.10], p = 0.104), or when controlling for VCIND status (b[SE] = 0.17[0.10], p = 0.09).
In all patients, verbal memory did not change significantly over time (b[SE] = –0.06[0.08], p = 0.43), and change in verbal memory was not associated with increased physical fitness indicated by VO2 peak over CR (b[SE] = 0.002[0.009], p = 0.861). In a post-hoc analysis including VCIND status as a covariate, only CN CAD patients had significantly improved verbal memory (b[SE] = 1.30[0.15], p < 0.005) with improved fitness.
Glutathione peroxidase activity in possible VCIND and CN controls
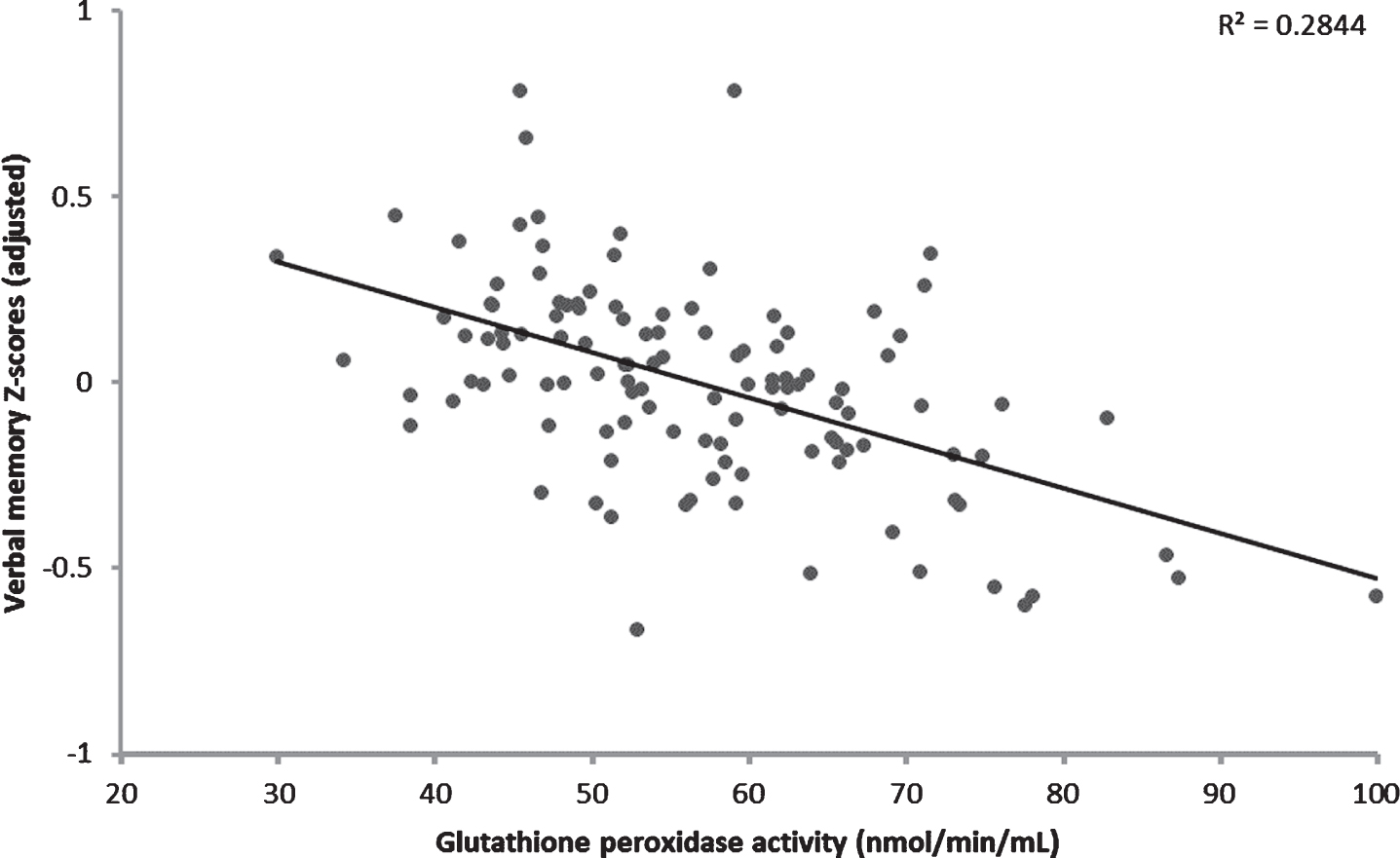
GPX activity was significantly higher in those with possible VCIND compared to CN controls (F1,119 = 3.996, p = 0.048). The adjusted mean (±standard error) GPX activities were 61.0±2.1 nmol/ml/min in possible VCIND patients and 56.2±1.4 nmol/ml/min in CN controls, when controlling for age, antioxidant supplementation use, and cigarette smoking history (Fig. 1). Of these covariates, significantly more CN controls were taking antioxidant supplements at baseline (30% versus 9%, χ2 = 5.23, P = 0.02). Upon further exploration, higher GPX activity was significantly associated with poorer verbal memory performance at baseline (β= –0.182, p = 0.048) when controlling for the covariates max VO2 peak, sex, total years of education, and BMI (Fig. 2).
Fig. 1
Glutathione peroxidase activity in vascular cognitive impairment, no dementia (VCIND) patients compared to cognitively normal (CN) controls with coronary artery disease. Activity was higher in possible VCIND patients, controlling for age, antioxidant/vitamin use and cigarette smoking history. Error bars represent ± 1 standard error.
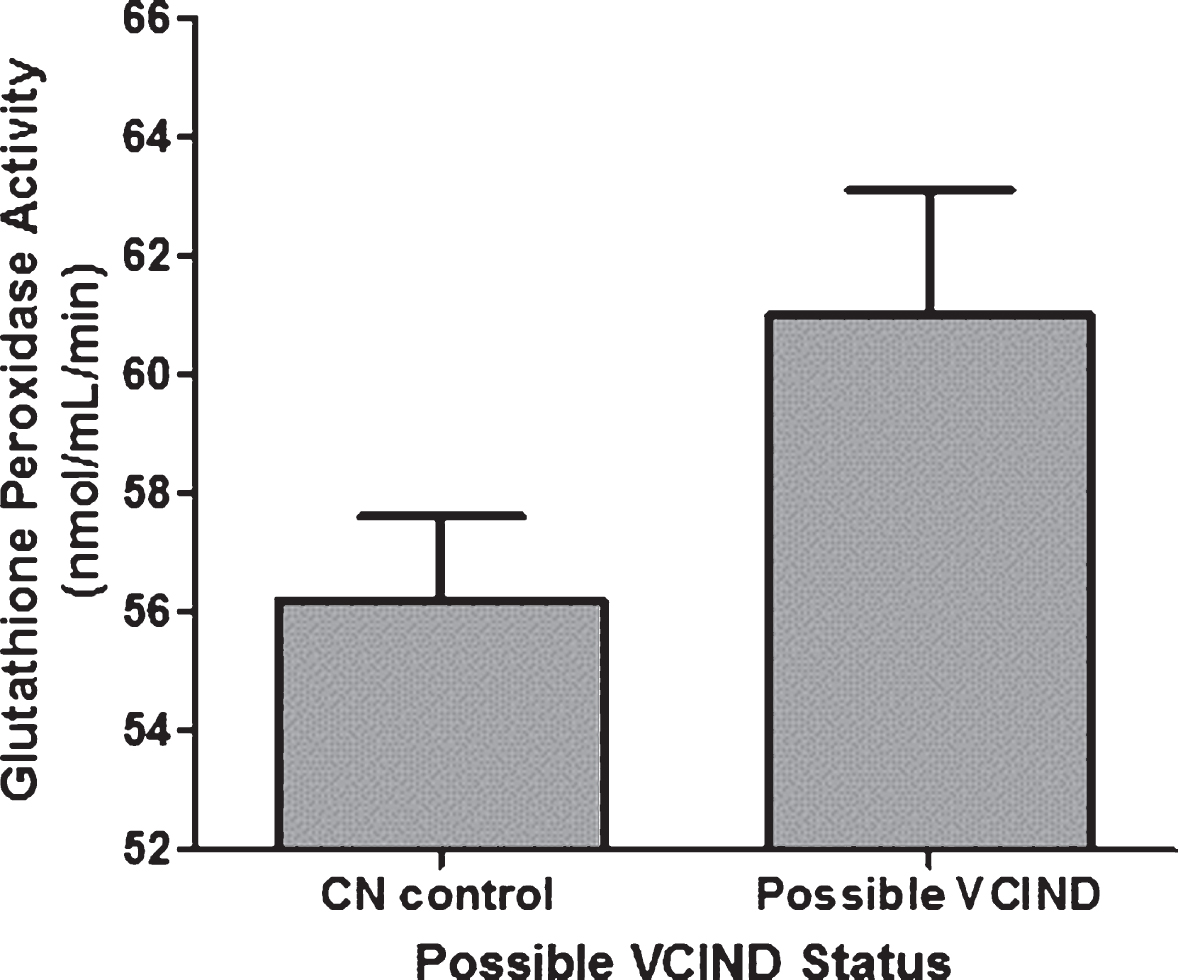
Fig. 2
Higher glutathione peroxidase activity was significantly associated with poorer verbal memory performance at baseline in coronary artery disease patients. Covariates included in this model include: sex, body mass index, cardiopulmonary fitness (VO2 peak), and years of education.
Association between baseline GPX activity and verbal memory performance in CAD patients over 6 months
Baseline glutathione peroxidase did not predict change in verbal memory performance over CR, when controlling for sex, BMI, VO2 peak, and total years of education (b[SE] = –0.01[0.01], p = 0.117). When VCIND status was added as an additional predictor, baseline GPX activity did not predict changes in verbal memory over time (b[SE] = –0.002[0.006], p = 0.677) and there was no significant group by time interaction term (b[SE] = –0.18[0.18], p = 0.33). However, VCIND status was significantly associated with verbal memory performance over 6 months (b[SE] = 1.22[0.16], p < 0.001). Specifically, after controlling for sex, BMI, VO2 peak, and total years of education, VCIND participants performed worse than CN participants by 1.22 unit Z-scores over 6 months.
Association between change in GPX activity and verbal memory performance in CAD patients over 6 months
An increase in GPX activity was significantly associated with a decline in verbal memory performance over 6 months when controlling for sex, BMI, VO2 peak, and total years of education (b[SE] = –0.02[0.01], p = 0.004). Specifically, a 1 nmol/min/mL increase in glutathione peroxidase activity was associated with a 0.02 unit Z-score decrease in verbal memory Z-score over 6 months.
DISCUSSION
This study assessed the relationship between GPX activity and VCIND, evaluating GPX activity in the FDA-NIH BEST roles of diagnostic biomarker, predictive biomarker and monitoring biomarker for cognition. In this population with coronary artery disease, baseline GPX activity was increased in those with VCIND, and while baseline GPX activity did not predict cognitive response to exercise in those with VCIND, change in GPX activity was correlated with change in verbal memory.
GPX activity was found to be higher in VCIND compared with CN control patients by approximately 4.80 nmol/mL/min, after controlling for age, antioxidant supplementation use, and cigarette smoking history. Supplements included: vitamins, selenium, zinc, cysteine, methionine and other amino acids, and omega-3 fatty acids. Of those, vitamin E, selenium, and omega-3 have been reported to affect GPX activity [72–75]. More CN controls were taking omega-3 supplements, which may increase GPX activity as demonstrated in vitro and in animal studies [74, 75]. While omega-3 supplementation was not a significant predictor in our study, the difference in GPX activity may have been greater had these groups been homogenous in the proportion of subjects taking these supplements. Age, BMI, and sex have previously been associated with GPX [54, 76, 77], but those factors did not differ among VCIND and CN controls in this study and were controlled for in our analysis. Previous findings suggest that GPX activity tends to be higher in CAD [23]; our findings expand upon this, showing GPX activity was elevated to a greater extent in those with VCIND. GPX activity is known to be increased by oxidative stress [24]. While not measured in this study, oxidative stress has previously been demonstrated to be increased in CAD and VCIND [15, 78]. As such, our finding of higher GPX in VCIND versus CN controls is consistent with increased oxidative stress in these patients.
In this study, baseline GPX activity was not predictive of changes in verbal memory over a 6-month CR program. There is variable response to exercise in those with early cognitive impairment [22, 79]. Predictors of increased cognition include improvements in cardiac health, or reduction of hypertension and/or hyperhomocysteinemia [80, 81], while oxidative stress which is associated with vascular risk factors [82–86], has predicted lack of cognitive response [15]. To fully evaluate if a biomarker is predictive of an intervention’s effect, there needs to be comparison to a control treatment arm [25]. Future studies that measure GPX activity in CAD patients who do not participate in CR may provide additional evidence to evaluate if GPX activity could be predictive of improved verbal memory performance following CR. It is also possible that the relationship between GPX activity and cognition may vary over time, making prediction difficult. Indeed, even the relationship between amyloid-beta and cognitive impairment in those with AD is complicated with a distinct period of correlated increase followed by a ceiling effect [87, 88]. Additional research will be needed to clarify this relationship in VCI.
This study also explored GPX activity as a monitoring biomarker for cognitive outcomes in CAD patients, finding that an increase in GPX activity was associated with a decline in verbal memory performance over the course of a 6-month CR program. While counter-intuitive to some degree, this finding is consistent with previous research. A single study has previously attempted to associate changes in GPX activity with changes in cognition, finding that a 100 International Unit increase in GPX activity over 6 months was associated with an average loss of 1.19 points on the MMSE (indicating cognitive decline) in 97 patients with various types and stages of neurodegenerative disease such as AD, VaD, and MCI [89]. GPX uses reduced GSH to neutralize oxidative stress species [24, 90, 91]. A longitudinal study demonstrated that a decrease in reduced GSH over time is a strong predictor of progression to AD from MCI [92]. McCaddon et al. [93] also demonstrated that a decrease in plasma GSH levels over time was associated with a significant decrease in MMSE scores. An increase in GPX activity is directly related to oxidative stress, which in turn may reflect mechanisms contributing to neuronal loss [90, 94–96].
Increased physical fitness over CR was not significantly associated with improvements in verbal memory in the total study group. Importantly, when controlling for the presence of VCIND, only CN controls had significantly improved cognition over 6 months of CR. This suggests that those with even a small degree of cognitive impairment at the start of their CR program are not deriving the same cognitive benefit from exercise as their CN control counterparts, as has been demonstrated previously [65]. Studies showing improvement in cognitive performance from exercise have been mixed, and a recent finding supported a role for underlying differences in levels of oxidative stress being a factor [15]. Some meta-analyses examining the effect of exercise on cognition in participants with AD and MCI reported significant cognitive improvements with exercise [79, 97, 98], while others reported small to no effects on cognition [22, 99]. The heterogeneous effects of exercise on cognition suggests that there are additional neurobiological barriers that may attenuate responses to exercise. Elucidating these barriers, particularly those that may be pharmacologic targets, would be beneficial. Our current study’s findings relating GPX changes to change in verbal memory, along with observations of elevated oxidative stress in VCIND patients [15], suggests that oxidative stress may be a potential target with potential interventions such as antioxidant supplementation.
In our population of CAD patients, GPX activity increased over time. While this may be expected to have positive clinical effects, the relationship between GPX activity and outcomes is complex. A previous meta-analysis of antioxidant enzymes found that increasing GPX activity was related to negative clinical outcomes in CAD [23]. GPX becomes upregulated in response to oxidative stress and uses the antioxidant GSH as a co-factor to neutralize reactive oxygen species [24]. As such, our findings may reflect the interplay between oxidative stress, GPX, and GSH. Oxidative stress is elevated in CAD patients, particularly in those with VCIND [15]. While GSH levels have not been studied in VCIND, GSH is decreased in MCI and AD [91, 100, 101], and GSH depletion is known to result in oxidative stress [102]. Taken together, one possible explanation for our findings is that the increase in GPX was insufficient to detoxify oxidative stress species in the presence of depleted GSH levels. One way to overcome this may be exercise. While exercise overall has been shown to increase cognition, VCIND participants’ verbal memory performance after 6 months remained significantly lower than that of CN controls despite exercise. Although exercise overall has been shown to increase antioxidant capabilities in individuals over time [103], in this population, improvements in fitness were not associated with increases in GPX. This again suggests an opportunity to evaluate supplemental antioxidant interventions.
Strengths of this study included having groups with similar vascular and clinical factors, and the generalizability of the results to the CAD patients referred a CR program. A limitation of this study was not being able to confirm whether these patients had probable VCIND as participants did not undergo neuroimaging. Nevertheless, classifying patients as having possible VCIND represents a clinically useful and convenient option, given that obtaining an MRI is expensive and may not be a readily accessible diagnostic procedure. This study defined VCIND by selecting patients who were only at least one standard deviation below age and/or education-matched population norms. Importantly, when controlling for sex, BMI, VO2 peak, and total years of education and GPX activity, VCIND patients demonstrated lower verbal memory compared to CN controls by 1.22 unit Z-scores over 6 months despite exercise. It would be interesting to see if future studies also report GXP changes in more impaired VCI populations. In addition, the majority of our participants were male and Caucasian. While this demographic is representative of the population enrolled in CR, results must be interpreted with caution when applied to the general populations of CAD and VCIND patients. Sex differences are typically observed in cognition, with female participants performing better at verbal memory tasks in cognitively healthy individuals and early stages of cognitive impairment such as MCI [71, 104]. Unfortunately, the rate of CR enrollment among women is significantly lower than that in men, with women being 36% less likely to enroll in a rehabilitation program [105]. While our current study included more male than female patients in both the VCIND and the CN control groups, both groups had a similar proportion of males. When sex was added as a covariate to control for potential sex-differences in our models, sex did not contribute, likely due to the small number of females. Additional studies with higher proportions of females will be needed to ascertain the potential influence of sex on the relationship between verbal memory and GPX.
We were not able to compare our absolute values of GPX activity to those in the literature to define a group with elevations. To date, the variability in sample types, disease states, and severity of cognitive decline across studies [27, 106, 107] have not allowed definition of a specific range of plasma GPX activity that can be characterized as physiologically abnormal. More research needs to be conducted to determine cut off points for characterizing high GPX activity that could be indicative of cognitive impairment in CAD patients. GPX activity should be measured in a larger population of possible VCIND patients and compared to healthy controls to further validate these findings.
CONCLUSION
These results suggest that the GSH antioxidant system is altered in VCIND, contributing to the body of research surrounding the role of the GSH antioxidant system and neurocognitive impairment. In this study, GPX activity was elevated in patients with possible VCIND compared to CN controls and secondary analyses found that longitudinal increases in GPX activity were significantly associated with a decline in verbal memory performance over 6 months, suggesting the possible relationship between this system and cognitive decline in the prodromal VCIND state. CAD patients are at higher risk of developing cognitive impairment. However, it is difficult to predict who is most at risk. Combining early changes in verbal memory with biomarker evidence of altered GPX may help in this. As CAD patients receive routine clinical blood testing to assess for blood lipids, assessing blood-based biomarkers, such GPX activity, may be feasible to incorporate into a clinical care routine screen. However, the greatest potential of biomarkers is likely in identifying patients who would benefit from targeted, preventative interventions.
Elevating antioxidant levels has been suggested as a potential therapeutic strategy to prevent vascular cognitive decline [108, 109]. The therapeutic benefits related to this strategy in cognitively vulnerable populations have yet to be elucidated, and there remains a need for biomarkers that can describe whether antioxidant supplementation could be beneficial, such as in VCIND patients who may not be receiving the full benefits of exercise interventions on improving cognitive outcomes. Therefore, studying GPX activity in association with cognitive performance in CAD patients at risk for cognitive decline is warranted. The results of this study highlight the potential of GPX activity as a biomarker of response to antioxidant supplementation in possible VCIND patients. Research into the role of the GSH antioxidant system to probe the antioxidant status of individuals with various forms of vascular cognitive impairment, especially in those with possible VCIND, may aid in the administration of personalized or precision medicine in the future.
ACKNOWLEDGMENTS
This work was supported by the Canadian Institutes of Health Research (Lanctot MOP-114913).
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/20-0754r3).
REFERENCES
[1] | Gorelick PB , Scuteri A , Black SE , Decarli C , Greenberg SM , Iadecola C , Launer LJ , Laurent S , Lopez OL , Nyenhuis D , Petersen RC , Schneider JA , Tzourio C , Arnett DK , Bennett DA , Chui HC , Higashida RT , Lindquist R , Nilsson PM , Roman GC , Sellke FW , Seshadri S , American Heart Association Stroke Council, Council on Epidemiology and Prevention, ouncil on Cardiovascular Nursing, Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia ((2011) ) Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 42: , 2672–2713. |
[2] | Rockwood K , Wentzel C , Hachinski V , Hogan DB , MacKnight C , McDowell I ((2000) ) Prevalence and outcomes of vascular cognitive impairment. Vascular Cognitive Impairment Investigators of the Canadian Study of Health and Aging. Neurology 54: , 447–451. |
[3] | Hachinski V , Iadecola C , Petersen R , Breteler M , Nyenhuis D , Black S , Powers W , DeCarli C , Merino J , Kalaria R , Vinters H , Holtzman D , Rosenberg G , Wallin A , Dichgans M , Marler J , Leblanc G ((2006) ) National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke 37: , 2220–2241. |
[4] | Vickland V , McDonnell G , Werner J , Draper B , Low LF , Brodaty H ((2010) ) A computer model of dementia prevalence in Australia: Foreseeing outcomes of delaying dementia onset, slowing disease progression, and eradicating dementia types. Dement Geriatr Cogn Disord 29: , 123–130. |
[5] | Roberts RO , Knopman DS , Geda YE , Cha RH , Roger VL , Petersen RC ((2010) ) Coronary heart disease is associated with non-amnestic mild cognitive impairment. Neurobiol Aging 31: , 1894–1902. |
[6] | van Oijen M , de Jong FJ , Witteman JC , Hofman A , Koudstaal PJ , Breteler MM ((2007) ) Atherosclerosis and risk for dementia. Ann Neurol 61: , 403–410. |
[7] | Barekatain M , Zahedian F , Askarpour H , Maracy MR , Hashemi-Jazi M , Aghaye-Ghazvini MR ((2014) ) Coronary artery disease and plasma apolipoprotein E4 in mild cognitive impairment. ARYA Atheroscler 10: , 244–251. |
[8] | Debette S , Bombois S , Bruandet A , Delbeuck X , Lepoittevin S , Delmaire C , Leys D , Pasquier F ((2007) ) Subcortical hyperintensities are associated with cognitive decline in patients with mild cognitive impairment. Stroke 38: , 2924–2930. |
[9] | Vinkers DJ , Stek ML , van der Mast RC , de Craen AJ , Le Cessie S , Jolles J , Westendorp RG , Gussekloo J ((2005) ) Generalized atherosclerosis, cognitive decline, and depressive symptoms in old age. Neurology 65: , 107–112. |
[10] | Conn V , Taylor S , Miller R ((1994) ) Cognitive impairment and medication adherence. J Gerontol Nurs 20: , 41–47. |
[11] | Ekman I , Fagerberg B , Skoog I ((2001) ) The clinical implications of cognitive impairment in elderly patients with chronic heart failure. J Cardiovasc Nurs 16: , 47–55. |
[12] | Tilvis RS , Kahonen-Vare MH , Jolkkonen J , Valvanne J , Pitkala KH , Strandberg TE ((2004) ) Predictors of cognitive decline and mortality of aged people over a 10-year period. J Gerontol A Biol Sci Med Sci 59: , 268–274. |
[13] | Takata Y , Ansai T , Soh I , Awano S , Nakamichi I , Akifusa S , Goto K , Yoshida A , Fujii H , Fujisawa R , Sonoki K ((2014) ) Cognitive function and 10 year mortality in an 85 year-old community-dwelling population. Clin Interv Aging 9: , 1691–1699. |
[14] | Swardfager W , Herrmann N , Marzolini S , Oh PI , Saleem M , Shammi P , Kiss A , Cappell J , Lanctot KL ((2011) ) Verbal memory performance and completion of cardiac rehabilitation in patients with coronary artery disease. Psychosom Med 73: , 580–587. |
[15] | Suridjan I , Herrmann N , Adibfar A , Saleem M , Andreazza A , Oh PI , Lanctot KL ((2017) ) Lipid peroxidation markers in coronary artery disease patients with possible vascular mild cognitive impairment. J Alzheimers Dis 58: , 885–896. |
[16] | Santiago C , Herrmann N , Swardfager W , Saleem M , Oh PI , Black SE , Lanctot KL ((2015) ) White matter microstructural integrity is associated with executive function and processing speed in older adults with coronary artery disease. Am J Geriatr Psychiatry 23: , 754–763. |
[17] | Grech ED ((2003) ) Pathophysiology and investigation of coronary artery disease. BMJ 326: , 1027–1030. |
[18] | Mielke MM , Bandaru VV , Haughey NJ , Rabins PV , Lyketsos CG , Carlson MC ((2010) ) Serum sphingomyelins and ceramides are early predictors of memory impairment. Neurobiol Aging 31: , 17–24. |
[19] | Verdelho A , Madureira S , Ferro JM , Baezner H , Blahak C , Poggesi A , Hennerici M , Pantoni L , Fazekas F , Scheltens P , Waldemar G , Wallin A , Erkinjuntti T , Inzitari D , Study L ((2012) ) Physical activity prevents progression for cognitive impairment and vascular dementia: Results from the LADIS (Leukoaraiosis and Disability) study. Stroke 43: , 3331–3335. |
[20] | Zheng G , Xia R , Zhou W , Tao J , Chen L ((2016) ) Aerobic exercise ameliorates cognitive function in older adults with mild cognitive impairment: A systematic review and meta-analysis of randomised controlled trials. Br J Sports Med 50: , 1443–1450. |
[21] | Gates N , Fiatarone Singh MA , Sachdev PS , Valenzuela M ((2013) ) The effect of exercise training on cognitive function in older adults with mild cognitive impairment: A meta-analysis of randomized controlled trials. Am J Geriatr Psychiatry 21: , 1086–1097. |
[22] | Young J , Angevaren M , Rusted J , Tabet N ((2015) ) Aerobic exercise to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev, CD005381. |
[23] | Flores-Mateo G , Carrillo-Santisteve P , Elosua R , Guallar E , Marrugat J , Bleys J , Covas MI ((2009) ) Antioxidant enzyme activity and coronary heart disease: Meta-analyses of observational studies. Am J Epidemiol 170: , 135–147. |
[24] | Espinosa-Diez C , Miguel V , Mennerich D , Kietzmann T , Sánchez-Pérez P , Cadenas S , Lamas S ((2015) ) Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol 6: , 183–197. |
[25] | FDA-NIH Biomarker Working Group (2016) BEST (Biomarkers, EndpointS, and other Tools) Resource, Silver Spring (MD). |
[26] | Schrag M , Mueller C , Zabel M , Crofton A , Kirsch WM , Ghribi O , Squitti R , Perry G ((2013) ) Oxidative stress in blood in Alzheimer’s disease and mild cognitive impairment: A meta-analysis. Neurobiol Aging 59: , 100–110. |
[27] | Ceballos-Picot I , Merad-Boudia M , Nicole A , Thevenin M , Hellier G , Legrain S , Berr C ((1996) ) Peripheral antioxidant enzyme activities and selenium in elderly subjects and in dementia of Alzheimer’s type–place of the extracellular glutathione peroxidase. Free Radic Biol Med 20: , 579–587. |
[28] | Martin-Aragon S , Bermejo-Bescos P , Benedi J , Felici E , Gil P , Ribera JM , Villar AM ((2009) ) Metalloproteinase’s activity and oxidative stress in mild cognitive impairment and Alzheimer’s disease. Neurochem Res 34: , 373–378. |
[29] | Krishnan S , Rani P ((2014) ) Evaluation of selenium, redox status and their association with plasma amyloid/tau in Alzheimer’s disease. Biol Trace Elem Res 158: , 158–165. |
[30] | Puertas MC , Martinez-Martos JM , Cobo MP , Carrera MP , Mayas MD , Ramirez-Exposito MJ ((2012) ) Plasma oxidative stress parameters in men and women with early stage Alzheimer type dementia. Exp Gerontol 47: , 625–630. |
[31] | Rinaldi P , Polidori MC , Metastasio A , Mariani E , Mattioli P , Cherubini A , Catani M , Cecchetti R , Senin U , Mecocci P ((2003) ) Plasma antioxidants are similarly depleted in mild cognitive impairment and in Alzheimer’s disease. Neurobiol Aging 24: , 915–919. |
[32] | Perrin R , Briancon S , Jeandel C , Artur Y , Minn A , Penin F , Siest G ((1990) ) Blood activity of Cu/Zn superoxide dismutase, glutathione peroxidase and catalase in Alzheimer’s disease: A case-control study. Gerontology 36: , 306–313. |
[33] | Steven S , Frenis K , Oelze M , Kalinovic S , Kuntic M , Bayo Jimenez MT , Vujacic-Mirski K , Helmstädter J , Kröller-Schön S , Münzel T , Daiber A ((2019) ) Vascular inflammation and oxidative stress: Major triggers for cardiovascular disease. Oxid Med Cell Longev 2019: , 7092151. |
[34] | Yang X , Li Y , Li Y , Ren X , Zhang X , Hu D , Gao Y , Xing Y , Shang H ((2017) ) Oxidative stress-mediated atherosclerosis: Mechanisms and therapies. Front Physiol 8: , 600–600. |
[35] | Petrie JR , Guzik TJ , Touyz RM ((2018) ) Diabetes, hypertension, and cardiovascular disease: Clinical insights and vascular mechanisms. Can J Cardiol 34: , 575–584. |
[36] | Gupta S , Sodhi S , Mahajan V ((2009) ) Correlation of antioxidants with lipid peroxidation and lipid profile in patients suffering from coronary artery disease. Expert Opin Ther Targets 13: , 889–894. |
[37] | Guglielmotto M , Giliberto L , Tamagno E , Tabaton M ((2010) ) Oxidative stress mediates the pathogenic effect of different Alzheimer’s disease risk factors. Front Aging Neurosci 2: , 3. |
[38] | Barnham KJ , Masters CL , Bush AI ((2004) ) Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov 3: , 205–214. |
[39] | Garrett M , Coote S ((2009) ) Multiple sclerosis and exercise in people with minimal gait impairment –a review. Phys Ther Rev 14: , 169–180. |
[40] | Vasquez BP , Zakzanis KK ((2015) ) The neuropsychological profile of vascular cognitive impairment not demented: A meta-analysis. J Neuropsychol 9: , 109–136. |
[41] | Eggermont LH , de Boer K , Muller M , Jaschke AC , Kamp O , Scherder EJ ((2012) ) Cardiac disease and cognitive impairment: A systematic review. Heart 98: , 1334–1340. |
[42] | Garrett K , Browndyke JN , Whelihan W , Paul RH , DiCarlo M , Moser DJ , Cohen RA , Ott BR ((2004) ) The neuropsychological profile of vascular cognitive impairment–no dementia: Comparisons to patients at risk for cerebrovascular disease and vascular dementia. Arch Clin Neuropsychol 19: , 745–757. |
[43] | Laukka E , Macdonald S , Fratiglioni L , Backman L ((2012) ) Preclinical cognitive trajectories differ for Alzheimer’s disease and Vascular Dementia. Int Neuropsychol Soc 18: , 191–199. |
[44] | Nyenhuis DL , Gorelick PB , Geenen EJ , Smith CA , Gencheva E , Freels S , deToledo-Morrell L ((2004) ) The pattern of neuropsychological deficits in Vascular Cognitive Impairment-No Dementia (Vascular CIND). Clin Neuropsychol 18: , 41–49. |
[45] | Silbert BS , Scott DA , Evered LA , Lewis MS , Maruff PT ((2007) ) Preexisting cognitive impairment in patients scheduled for elective coronary artery bypass graft surgery. Anesth Analg 104: , 1023–1028. |
[46] | Vicario A , Martinez CD , Baretto D , Diaz Casale A , Nicolosi L ((2005) ) Hypertension and cognitive decline: Impact on executive function. J Clin Hypertens (Greenwich) 7: , 598–604. |
[47] | Zheng L , Mack WJ , Chui HC , Heflin L , Mungas D , Reed B , DeCarli C , Weiner MW , Kramer JH ((2012) ) Coronary artery disease is associated with cognitive decline independent of changes on magnetic resonance imaging in cognitively normal elderly adults. J Am Geriatr Soc 60: , 499–504. |
[48] | Sachdev P , Kalaria R , O’Brien J , Skoog I , Alladi S , Black SE , Blacker D , Blazer DG , Chen C , Chui H , Ganguli M , Jellinger K , Jeste DV , Pasquier F , Paulsen J , Prins N , Rockwood K , Roman G , Scheltens P ((2014) ) Diagnostic criteria for vascular cognitive disorders: A VASCOG statement. Alzheimer Dis Assoc Disord 28: , 206–218. |
[49] | Woods SP , Delis DC , Scott JC , Kramer JH , Holdnack JA ((2006) ) The California Verbal Learning Test–second edition: Test-retest reliability, practice effects, and reliable change indices for the standard and alternate forms. Arch Clin Neuropsychol 21: , 413–420. |
[50] | Barry D , Bates ME , Labouvie E ((2008) ) FAS and CFL forms of verbal fluency differ in difficulty: A meta-analytic study. Appl Neuropsychol 15: , 97–106. |
[51] | Gaudino EA , Geisler MW , Squires NK ((1995) ) Construct validity in the Trail Making Test: What makes Part B harder? J Clin Exp Neuropsychol 17: , 529–535. |
[52] | Bondi MW , Edmonds EC , Jak AJ , Clark LR , Delano-Wood L , McDonald CR , Nation DA , Libon DJ , Au R , Galasko D , Salmon DP ((2014) ) Neuropsychological criteria for mild cognitive impairment improves diagnostic precision, biomarker associations, and progression rates. J Alzheimers Dis 42: , 275–289. |
[53] | Hatanaka H , Hanyu H , Hirose D , Fukusawa R , Namioka N , Iwamoto T ((2015) ) Peripheral oxidative stress markers in individuals with Alzheimer’s disease with or without cerebrovascular disease. J Am Geriatr Soc 63: , 1472–1474. |
[54] | Espinoza SE , Guo H , Fedarko N , DeZern A , Fried LP , Xue QL , Leng S , Beamer B , Walston JD ((2008) ) Glutathione peroxidase enzyme activity in aging. J Gerontol A Biol Sci Med Sci 63: , 505–509. |
[55] | Zeni-Graiff M , Rios AC , Maurya PK , Rizzo LB , Sethi S , Yamagata AS , Mansur RB , Pan PM , Asevedo E , Cunha GR , Zugman A , Bressan RA , Gadelha A , Brietzke E ((2019) ) Peripheral levels of superoxide dismutase and glutathione peroxidase in youths in ultra-high risk for psychosis: A pilot study. CNS Spectr 24: , 333–337. |
[56] | Yildiz L , Kayaoglu N , Aksoy H ((2002) ) The changes of superoxide dismutase, catalase and glutathione peroxidase activities in erythrocytes of active and passive smokers. Clin Chem Lab Med 40: , 612–615. |
[57] | Siedlak SL , Casadesus G , Webber KM , Pappolla MA , Atwood CS , Smith MA , Perry G ((2009) ) Chronic antioxidant therapy reduces oxidative stress in a mouse model of Alzheimer’s disease. Free Radic Res 43: , 156–164. |
[58] | Mates JM , Perez-Gomez C , Nunez de Castro I ((1999) ) Antioxidant enzymes and human diseases. Clin Biochem 32: , 595–603. |
[59] | Lloret A , Badia MC , Mora NJ , Pallardo FV , Alonso MD , Vina J ((2009) ) Vitamin E paradox in Alzheimer’s disease: It does not prevent loss of cognition and may even be detrimental. J Alzheimers Dis 17: , 143–149. |
[60] | Kolosova NG , Shcheglova TV , Sergeeva SV , Loskutova LV ((2006) ) Long-term antioxidant supplementation attenuates oxidative stress markers and cognitive deficits in senescent-accelerated OXYS rats. Neurobiol Aging 27: , 1289–1297. |
[61] | Saleem M , Bandaru VV , Herrmann N , Swardfager W , Mielke MM , Oh PI , Shammi P , Kiss A , Haughey NJ , Rovinski R , Lanctot KL ((2013) ) Ceramides predict verbal memory performance in coronary artery disease patients undertaking exercise: A prospective cohort pilot study. BMC Geriatr 13: , 135. |
[62] | Yaffe K , Blackwell T , Kanaya AM , Davidowitz N , Barrett-Connor E , Krueger K ((2004) ) Diabetes, impaired fasting glucose, and development of cognitive impairment in older women. Neurology 63: , 658–663. |
[63] | Ottens TH , Hendrikse J , Nathoe HM , Biessels GJ , van Dijk D ((2017) ) Brain volume and cognitive function in patients with revascularized coronary artery disease. Int J Cardiol 230: , 80–84. |
[64] | Momtaz YA , Haron SA , Hamid TA , Ibrahim R , Tanjani PT ((2018) ) Body mass index (BMI) and cognitive functions in later life. Curr Alzheimer Res 15: , 195–200. |
[65] | Swardfager W , Herrmann N , Marzolini S , Saleem M , Kiss A , Shammi P , Oh PI , Lanctot KL ((2010) ) Cardiopulmonary fitness is associated with cognitive performance in patients with coronary artery disease. J Am Geriatr Soc 58: , 1519–1525. |
[66] | Kim S , Kim Y , Park SM ((2016) ) Body mass index and decline of cognitive function. PLoS One 11: , e0148908. |
[67] | Steenbergen L , Colzato LS ((2017) ) Overweight and cognitive performance: High body mass index is associated with impairment in reactive control during task switching. Front Nutr 4: , 51. |
[68] | Kirton JW , Dotson VM ((2016) ) The interactive effects of age, education, and BMI on cognitive functioning. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 23: , 253–262. |
[69] | Hawkins S , Wiswell R ((2003) ) Rate and mechanism of maximal oxygen consumption decline with aging: Implications for exercise training. Sports Med 33: , 877–888. |
[70] | Hwang J , Castelli DM , Gonzalez-Lima F ((2017) ) The positive cognitive impact of aerobic fitness is associated with peripheral inflammatory and brain-derived neurotrophic biomarkers in young adults. Physiol Behav 179: , 75–89. |
[71] | Sundermann EE , Maki PM , Rubin LH , Lipton RB , Landau S , Biegon A ((2016) ) Female advantage in verbal memory: Evidence of sex-specific cognitive reserve. Neurology 87: , 1916–1924. |
[72] | Scott DL , Kelleher J , Losowsky MS ((1977) ) The influence of dietary selenium and vitamin E on glutathione peroxidase and glutathione in the rat. Biochim Biophys Acta 497: , 218–224. |
[73] | Oliveira-Silva JA , Yamamoto JUP , Oliveira RB , Monteiro VCL , Frangipani BJ , Kyosen SO , Martins AM , D’Almeida V ((2019) ) Oxidative stress assessment by glutathione peroxidase activity and glutathione levels in response to selenium supplementation in patients with Mucopolysaccharidosis I, II and VI. Genet Mol Biol 42: , 1–8. |
[74] | Joulain C , Prigent AF , Nemoz G , Lagarde M ((1994) ) Increased glutathione peroxidase activity in human blood mononuclear cells upon in vitro incubation with n-3 fatty acids. Biochem Pharmacol 47: , 1315–1323. |
[75] | Patten AR , Brocardo PS , Christie BR ((2013) ) Omega-3 supplementation can restore glutathione levels and prevent oxidative damage caused by prenatal ethanol exposure. J Nutr Biochem 24: , 760–769. |
[76] | Mendoza-Núñez VM , Beristain-Pérez A , Pérez-Vera SP , Altamirano-Lozano MA ((2010) ) Age-related sex differences in glutathione peroxidase and oxidative DNA damage in a healthy Mexican population. J Womens Health (Larchmt) 19: , 919–926. |
[77] | Goyal R , Singhai M , Faizy AF ((2011) ) Glutathione peroxidase activity in obese and nonobese diabetic patients and role of hyperglycemia in oxidative stress. J Midlife Health 2: , 72–76. |
[78] | Madamanchi Nageswara R , Vendrov A , Runge Marschall S ((2005) ) Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol 25: , 29–38. |
[79] | Colcombe S , Kramer AF ((2003) ) Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychol Sci 14: , 125–130. |
[80] | Liu-Ambrose T , Best JR , Davis JC , Eng JJ , Lee PE , Jacova C , Boyd LA , Brasher PM , Munkacsy M , Cheung W , Hsiung GR ((2016) ) Aerobic exercise and vascular cognitive impairment: A randomized controlled trial. Neurology 87: , 2082–2090. |
[81] | Lee JH , Hong SM , Shin YA ((2018) ) Effects of exercise training on stroke risk factors, homocysteine concentration, and cognitive function according the APOE genotype in stroke patients. J Exerc Rehabil 14: , 267–274. |
[82] | Rodrigo R , González J , Paoletto F ((2011) ) The role of oxidative stress in the pathophysiology of hypertension. Hypertens Res 34: , 431–440. |
[83] | Tyagi N , Sedoris KC , Steed M , Ovechkin AV , Moshal KS , Tyagi SC ((2005) ) Mechanisms of homocysteine-induced oxidative stress. Am J Physiol Heart Circ Physiol 289: , H2649–2656. |
[84] | Dhalla NS , Temsah RM , Netticadan T ((2000) ) Role of oxidative stress in cardiovascular diseases. J Hypertens 18: , 655–673. |
[85] | Chrissobolis S , Miller AA , Drummond GR , Kemp-Harper BK , Sobey CG ((2011) ) Oxidative stress and endothelial dysfunction in cerebrovascular disease. Front Biosci (Landmark Ed) 16: , 1733–1745. |
[86] | Carvalho C , Moreira PI ((2018) ) Oxidative stress: A major player in cerebrovascular alterations associated to neurodegenerative events. Front Physiol 9: , 806. |
[87] | Thal DR , Ronisz A , Tousseyn T , Rijal Upadhaya A , Balakrishnan K , Vandenberghe R , Vandenbulcke M , von Arnim CAF , Otto M , Beach TG , Lilja J , Heurling K , Chakrabarty A , Ismail A , Buckley C , Smith APL , Kumar S , Farrar G , Walter J ((2019) ) Different aspects of Alzheimer’s disease-related amyloid beta-peptide pathology and their relationship to amyloid positron emission tomography imaging and dementia. Acta Neuropathol Commun 7: , 178. |
[88] | Harrington KD , Lim YY , Ames D , Hassenstab J , Laws SM , Martins RN , Rainey-Smith S , Robertson J , Rowe CC , Salvado O , Dore V , Villemagne VL , Snyder PJ , Masters CL , Maruff P ((2017) ) Amyloid beta-associated cognitive decline in the absence of clinical disease progression and systemic illness. Alzheimers Dement (Amst) 8: , 156–164. |
[89] | Revel F , Gilbert T , Roche S , Drai J , Blond E , Ecochard R , Bonnefoy M ((2015) ) Influence of oxidative stress biomarkers on cognitive decline. J Alzheimers Dis 45: , 553–560. |
[90] | Bains JS , Shaw CA ((1997) ) Neurodegenerative disorders in humans: The role of glutathione in oxidative stress-mediated neuronal death. Brain Res Brain Res Rev 25: , 335–358. |
[91] | Saharan S , Mandal PK ((2014) ) The emerging role of glutathione in Alzheimer’s disease. J Alzheimers Dis 40: , 519–529. |
[92] | Baldeiras I , Santana I , Proenca MT , Garrucho M ((2010) ) Oxidative damage and progression to Alzheimer’s disease in patients with mild cognitive impairment. J Alzheimers Dis 21: , 1165–1177. |
[93] | McCaddon A , Hudson P , Hill D , Barber J , Lloyd A , Davies G , Regland B ((2003) ) Alzheimer’s disease and total plasma aminothiols. Biol Psychiatry 53: , 254–260. |
[94] | Bennett S , Grant MM , Aldred S ((2009) ) Oxidative stress in vascular dementia and Alzheimer’s disease: A common pathology. J Alzheimers Dis 17: , 245–257. |
[95] | Pratico D , Sung S ((2004) ) Lipid peroxidation and oxidative imbalance: Early functional events in Alzheimer’s disease. J Alzheimers Dis 6: , 171–175. |
[96] | Aoyama K , Nakaki T ((2013) ) Impaired glutathione synthesis in neurodegeneration. Int J Mol Sci 14: , 21021–21044. |
[97] | Jia RX , Liang JH , Xu Y , Wang YQ ((2019) ) Effects of physical activity and exercise on the cognitive function of patients with Alzheimer disease: A meta-analysis. BMC Geriatr 19: , 181. |
[98] | Sanders LMJ , Hortobágyi T , la Bastide-van Gemert S , van der Zee EA , van Heuvelen MJG ((2019) ) Dose-response relationship between exercise and cognitive function in older adults with and without cognitive impairment: A systematic review and meta-analysis. PloS One 14: , e0210036–e0210036. |
[99] | Ludyga S , Gerber M , Pühse U , Looser VN , Kamijo K ((2020) ) Systematic review and meta-analysis investigating moderators of long-term effects of exercise on cognition in healthy individuals. Nat Hum Behav 4: , 603–612. |
[100] | Bermejo P , Martin-Aragon S , Benedi J , Susin C , Felici E , Gil P , Ribera JM , Villar AM ((2008) ) Peripheral levels of glutathione and protein oxidation as markers in the development of Alzheimer’s disease from Mild Cognitive Impairment. Free Radic Res 42: , 162–170. |
[101] | Baldeiras I , Santana I , Proenca MT , Garrucho MH , Pascoal R , Rodrigues A , Duro D , Oliveira CR ((2008) ) Peripheral oxidative damage in mild cognitive impairment and mild Alzheimer’s disease. J Alzheimers Dis 15: , 117–128. |
[102] | Vaziri ND , Wang XQ , Oveisi F , Rad B ((2000) ) Induction of oxidative stress by glutathione depletion causes severe hypertension in normal rats. Hypertension 36: , 142–146. |
[103] | de Sousa CV , Sales MM , Rosa TS , Lewis JE , de Andrade RV , Simoes HG ((2017) ) The antioxidant effect of exercise: A systematic review and meta-analysis. Sports Med 47: , 277–293. |
[104] | Asperholm M , Nagar S , Dekhtyar S , Herlitz A ((2019) ) The magnitude of sex differences in verbal episodic memory increases with social progress: Data from 54 countries across 40 years. PLoS One 14: , e0214945. |
[105] | Samayoa L , Grace SL , Gravely S , Scott LB , Marzolini S , Colella TJF ((2014) ) Sex differences in cardiac rehabilitation enrollment: A meta-analysis. Can J Cardiol 30: , 793–800. |
[106] | Youssef P , Chami B , Lim J , Middleton T , Sutherland GT , Witting PK ((2018) ) Evidence supporting oxidative stress in a moderately affected area of the brain in Alzheimer’s disease. Sci Rep 8: , 11553. |
[107] | Anneren G , Gardner A , Lundin T ((1986) ) Increased glutathione peroxidase activity in erythrocytes in patients with Alzheimer’s disease/senile dementia of Alzheimer’s type. Acta Neurol Scand 73: , 586–589. |
[108] | Pocernich CB , Butterfield DA ((2012) ) Elevation of glutathione as a therapeutic strategy in Alzheimer disease. Biochim Biophys Acta 1822: , 625–630. |
[109] | Mazereeuw G , Herrmann N , Oh PI , Ma DW , Wang CT , Kiss A , Lanctot KL ((2016) ) Omega-3 Fatty acids, depressive symptoms, and cognitive performance in patients with coronary artery disease: Analyses from a randomized, double-blind, placebo-controlled trial. J Clin Psychopharmacol 36: , 436–444. |




