The Trajectory of Caregiver Burden and Risk Factors in Dementia Progression: A Systematic Review
Abstract
Background:
Caring for patients with dementia at home is often a long-term process, in which the independence of the patient declines, and more responsibility and supervision time is required from the informal caregiver.
Objective:
In order to minimize and reduce caregiver burden, it is important to explore its trajectory and the accompanying risk factors as dementia progresses; the objective of this systematic review.
Methods:
PRISMA guidelines were followed in this systematic review. Three databases, PubMed, PsycINFO, and EMbase, were systematically searched in November 2019 using specific keywords.
Results:
1,506 hits emerged during the systematic search but only eleven articles actually met the inclusion criteria for this review. The trajectory of caregiver burden is highly variable and depends on multiple factors. Important risk factors included: patients’ behavioral and neuropsychiatric symptoms, and their decline in functioning in (I)ADL; the caregiver’s age, gender, and physical and mental health; and, within the dyads (patient/caregiver), cohabitation and kinship.
Conclusion:
There is no one-size-fits-all for predicting how caregiver burden will change over time, but specific factors (like being a spouse and increased behavioral impairment and decline in functional status in the patient) may heighten the risk. Other factors, not yet comprehensively included in the published studies, might also prove to be important risk factors. Future research in the field of reducing caregiver burden is recommended to integrate the patient, caregiver, and context characteristics in the trajectory of caregiver burden, and to assess more clearly the phase of the dementia progression and use of external resources.
INTRODUCTION
The majority of the approximately 50 million patients with dementia worldwide [1] is cared for at home by their family caregivers, typically their spouses or children [2]. Although this caring can give joy and fulfillment [3], it is often also difficult and burdensome, especially if it is prolonged over time. The negative impact of caring for a person with dementia is often conceptualized in terms of caregiver burden [4]. This burden and its risk factors have been investigated in numerous studies over the past years. Van der Lee et al. [5] conducted a comprehensive systematic review on subjective caregiver burden and burden-related concepts (depression and mental health). This review included patient characteristics (i.e., behavioral problems, cognitive function, and self-care) and caregiver characteristics (i.e., health, social functioning, competence, coping, and personality traits). The behavioral problems and mood disorders of patients were consistently reported as important risk factors for caregiver burden, depression, and mental health. Caregiver characteristics like personality traits, coping styles, and competences were also strong determinants and considered as mediators between the impact of the patients’ behavioral problems and caregiver burden, depression, and mental health. A systematic review of Chiao et al. [6] reported patients’ functional status, behavioral problems, and levels of neuropsychiatric symptoms as most burdensome to informal caregivers. From the caregiver’s perspective, sociodemographic factors (monthly income, gender, educational level, cohabitation) and psychological factors (psychological health, perceived well-being, depressive symptoms, religious coping skills and anxiety) were found as main risk factors of caregiver burden.
The majority of the studies included in both van der Lee [5] and Chiao [6] reviews were cross-sectional in nature. However, dementia is a progressive disease in which independence declines [7] and behavioral problems and mood disorders generally become more present with increasing severity [8], although this decline is influenced by many factors over time. As a result, more responsibility and supervision time is required from the informal caregiver as time passes [9, 10], which can lead to heightened caregiver burden and burden-related complaints with increasing severity [11]. Often when caregiver burden reaches a threshold at a certain time point, admission of the patient to an institutional long-term care facility is inevitable [12].
Risk factors of caregiver burden and burden-related concepts have been previously investigated but most studies are cross-sectional in nature. However, the trajectory of caregiver burden and the risk factors associated with it has not yet been systematically analyzed over time in patients with dementia and their caregivers living in the community. This was the aim of the current review.
METHODS
This systematic review was guided by the Preferred Reporting Items for Systematic Review and Meta-Analysis: the PRISMA statement [13], which comprises a 27-item checklist and three-phase flow diagram. The checklist includes items essential for systematic review reporting and was used for the critical appraisal of the included studies.
Search strategy
A search strategy was developed to identify published studies describing the trajectory of caregiver burden and risk factors in dementia progression. The search was conducted in November 2019. Three databases (PubMed, PsycINFO, and EMBASE) were searched using combinations of the following key-words “burden”, “stress”, “depression”, “caregiver”, “dementia”, “Alzheimer’s disease”, “predictors”, “determinants”, “longitudinal study”, “trajec-tory”, “disease progression”, and “course”.
Limitations were language (English) and publication date (5 years). Eligibility criteria were cohort studies or prospective studies investigating the trajectory of caregiver burden or stress in informal caregivers who are caring for a patient with dementia. Studies must have multiple assessment of caregiver burden as the dementia progresses and included either patient, caregiver, or context risk factors.
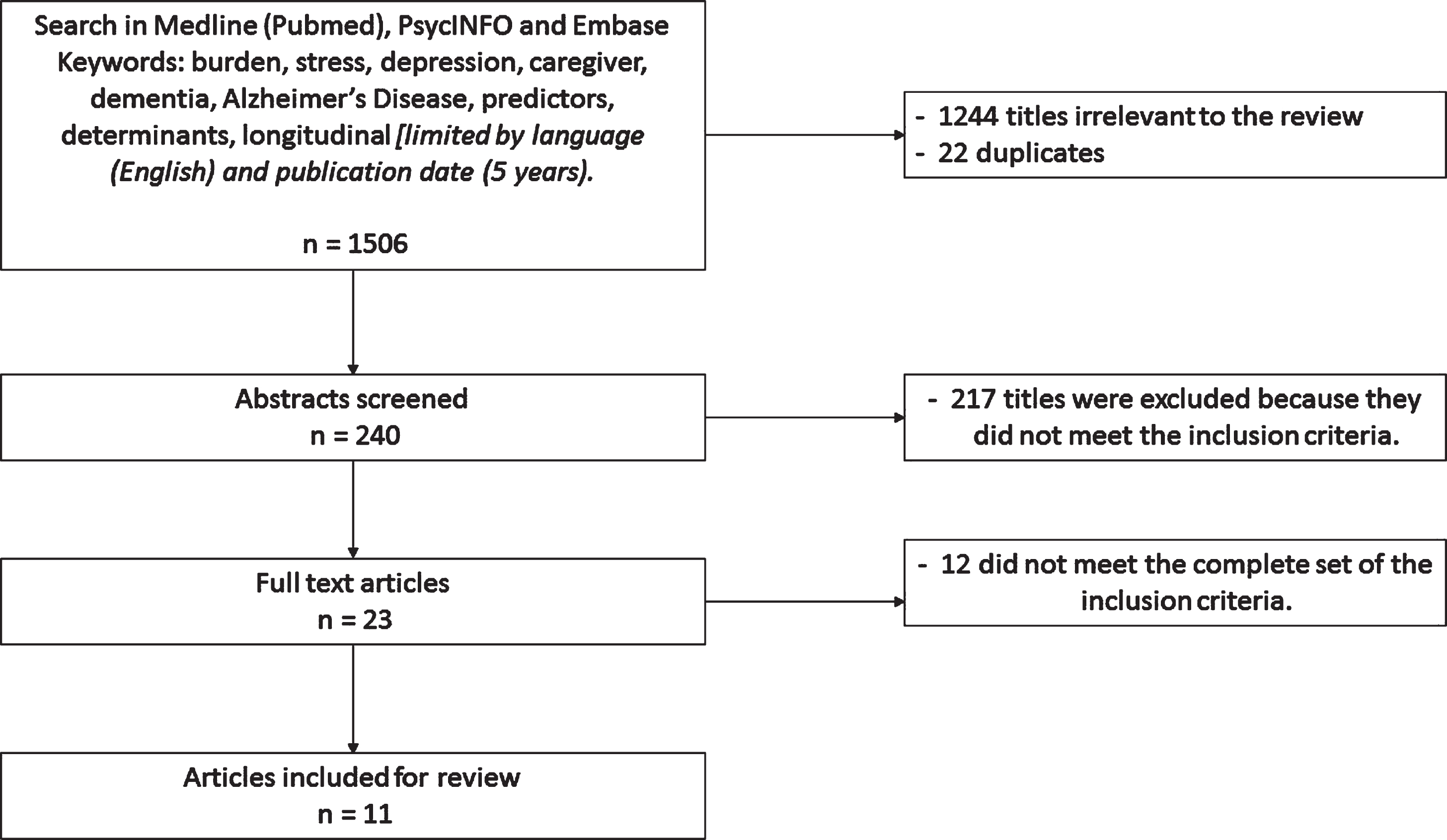
One researcher (R.K) screened the titles and abstracts of the journals on the inclusion criteria. Any uncertainties during the screening and selection process were discussed with a second researcher (L.S.). In total six articles where discussed with the second researcher in which there were no disagreements between the two researchers. The following inclusion criteria guided the search efforts to identify all the relevant studies. The search results are given in the flow chart (see Fig. 1).
Fig. 1
Search and analysis strategy.
Study selection
Studies were selected on the basis of the inclusion criteria. The inclusion criteria were: 1) publications in English; 2) publications between 2014 and 2019 as a follow-up to van der Lee [5] focused on the longitudinal course of caregiver burden; 3) presence of patient, caregiver, or context factors related to caregiver burden or burden related concepts (depression, stress, strain); and 4) research design: longitudinal and cohort studies. The selected studies are listed in Table 1.
Table 1
Summary of included studies
| Reference | Sample size baseline at (informal caregivers) | Dementia type | Follow-up (months) | Number of assessments | Outcome measure | Caregiver burden measure |
| Kajiwara et al., 2018 [15] | 41 | Dementia | 12 | 3 | Burden | J-ZBI |
| Borsje et al., 2016 [16] | 117 | Dementia | 18 | 3 | Psychological distress | SCQ, CES-D, GHQ-12 |
| Kawaharada et al., 2019 [17] | 117 | AD | 36 | 2 | Burden | ZBI |
| Raccichini et al., 2015 [18] | 153 | AD | 6 | 2 | Burden | CBI |
| Svendsboe et al., 2018 [19] | 162 | AD and DLB | 36 | 4 | Psychological distress | RSS |
| Hallikainen et al., 2017 [20] | 226 | AD | 36 | 4 | Psychological distress | GHQ-12 |
| Conde-Sala et al., 2014 [21] | 330 | AD | 36 | 4 | Burden | ZBI |
| Viñas-Diez et al., 2017 [22] | 275 | AD | 24 | 3 | Burden | ZBI |
| Brodaty et al., 2014 [23] | 732 | Dementia | 12 | 3 | Burden | ZBI |
| Reed et al., 2019 [24] | 969 | AD | 36 | 7 | Burden | ZBI |
| Bleijlevens et al. 2014 [25] | 2014 | Dementia | 3 | 2 | Burden | ZBI, CRA |
AD, Alzheimer’s disease; DLB, dementia with Lewy bodies; J-ZBI, Japanese version of the Zarit Burden Inventory; SCQ, Sense of Competence Questionnaire; CES-D, Center for Epidemiological Studies Depression Scale; GHQ-12, General Health Questionnaire -12; ZBI, Zarit Burden Inventory; CBI, Caregiver Burden Inventory; RSS, Relative’s Stress Scale; CRA, Caregiver Reaction Assessment.
Quality appraisal
The Newcastle–Ottawa Scale (NOS) for Cohort Studies [14] was used to evaluate the included studies and to limit any potential biases from including unreliable results in our literature review. The NOS for Cohort Studies consists of 8 items related to the domains of Selection, Comparability, and Outcome. A study can be awarded a maximum of one star for each numbered item within the Selection and Outcome categories. A maximum of two stars can be given for Comparability. Thresholds for converting the Newcastle-Ottawa scales to the Agency for Health Research and Quality (AHRQ) standards (Good, Fair, and Poor) were: Good Quality (3 or 4 stars on Selection and 1 or 2 stars on Comparability and 2 or 3 stars at Outcome); Fair Quality (2 stars on Selection and 1 or 2 stars on Comparability and 2 or 3 stars at Outcome); and Poor Quality (0 or 1 star on Selection and 0 stars on Comparability and 0 or 1 star at Outcome). Table 2 represents the quality assessment per included study.
Table 2
Evaluation of individual study quality with The Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomized studies in meta-analyses
| [15] | [16] | [17] | [18] | [19] | [20] | [21] | [22] | [23] | [24] | [25] | ||
| Selection | 1) Representativeness of the exposed cohort: | a | a | a | a | a | a | a | a | a | a | a |
| a) truly representative of the average patient with dementia in the community*; b) somewhat representative of the average patient with dementia in the community*; c) selected group of users, e.g., nurses, volunteers; d) no description of the derivation of the cohort | ||||||||||||
| 2) Selection of the non-exposed cohort: | a | a | a | a | a | a | a | a | a | a | a | |
| a) drawn from the same community as the exposed cohort*; b) drawn from a different source; c) no description of the derivation of the non-exposed cohort | ||||||||||||
| 3) Ascertainment of exposure: | c | c | b | c | c | c | b | c | c | c | b | |
| a) secure record (e.g. surgical records)*; b) structured interview*; c) written self-report; d) no description | ||||||||||||
| 4) Demonstration that outcome of interest was not present at start of study: | a | a | a | a | a | a | a | a | a | a | a | |
| a) yes*; b) no | ||||||||||||
| Comparability | 1) Comparability of cohorts on the basis of the design or analysis: | |||||||||||
| a) study controls for patients or caregiver factors | X | X | X | X | X | X | X | X | X | X | X | |
| b) study controls for context factors | X | X | X | X | X | X | X | X | X | |||
| Outcome | 1) Assessment of outcome: | c | c | c | c | c | c | c | c | c | c | c |
| a) independent blind assessment*; b) record linkage; c) self-report; d) no description | ||||||||||||
| 2) Was follow-up long enough for outcomes to occur > 6 months: | a | a | a | b | a | a | a | a | a | a | b | |
| a) yes*; b) no | ||||||||||||
| 3) Adequacy of follow up of cohorts: | b | b | a | b | c | b | b | b | c | c | a | |
| a) complete follow up - all subjects accounted for*; b) subjects lost to follow up unlikely to introduce bias - less than 50 % lost or description of those lost suggested no difference from those followed*; c) follow up rate < 50% and no description of those lost; d) no statement | ||||||||||||
| Total number of stars | 7 | 7 | 8 | 7 | 5 | 6 | 8 | 7 | 6 | 6 | 8 | |
| Quality rating according to guideline* | Good | Good | Good | Good | Fair | Fair | Good | Good | Fair | Fair | Good |
*Thresholds for converting the NOS rating to Agency for Healthcare Research and Quality (AHRQ) standards (good, fair, and poor): Good quality: 3 or 4 stars in Selection domain AND 1 or 2 stars in Comparability domain AND 2 or 3 stars in Outcome domain. Fair quality: 2 stars in Selection domain AND 1 or 2 stars in Comparability domain AND 2 or 3 stars in Outcome domain. Poor quality: 0 or 1 star in Selection domain OR 0 stars in Comparability domain OR 0 or 1 stars in Outcome domain. Note: A study can be awarded a maximum of one star for each numbered item within the Selection and Outcome categories. A maximum of two stars can be given for Comparability.
RESULTS
Based on the search, 1,506 relevant studies were identified for further screening. After reading the titles, 1,266 publications were rejected due to irrelevance and duplication. Next, abstracts were screened and 217 were excluded because they did not meet the inclusion criteria. In total, 23 full text articles were read, of which 12 were excluded because they did not meet the complete set of the inclusion criteria. Thus, the search strategy led to 11 included studies in the review (Fig. 1). Table 1 presents the summary of the eleven included studies.
General study characteristics
Among these eleven, the number of participants at baseline ranged from n = 41 to n = 2014. One study included 41 participants [15], three included between 100 and 200 participants [16–18], two studies included between 200 and 300 participants [19, 20], and five included more than 300 participants [21–25]. The follow-up ranged from 3 to 36 months. One study [25] had a follow up of 3 months, one study [18] had a follow up of 6 months, five studies [15, 16, 22–24] had a follow up between 12 and 24 months, and four studies [17, 19–21] had a follow up of more than 24 months. Of the included studies, eight studies [15, 17, 18, 21–25] defined the negative impact of caregiving as caregiver burden. Of these eights studies with burden as outcome, seven studies [15, 17, 21–25] used the Zarit Burden Interview (ZBI), while Raccichini et al. [18] used the Caregiver Burden Inventory (CBI). Three studies [16, 19, 20] had caregiver distress as the main outcome variable. Two studies used the General Health Questionnaire–12 (GHQ-12) [15, 20] to operationalize the level of psychological distress in caregivers, while Svendsboe et al. [19] used the Relative’s Stress Scale (RSS). Of the eleven included studies, seven [15–18, 21, 22, 25] were assessed according to the AHRQ as Good quality and four [19, 20, 23, 24] of Fair quality.
Trajectory of burden
While a variety of caregiver burden trajectories were reported, the majority (eight studies) found that it increased at follow-up (after 6 months [18], 12 months [23], 24 months [22], and 36 months [17, 19–21, 24]). Only two studies reported that caregiver burden and/or distress remained stable at 12 months [15] and 18 months [16] of follow up. One study [25] found a decrease of caregiver burden, after the patient with dementia was admitted to a long-term care facility. While an overall increase of burden was reported in most (eight) studies, different trajectories were found to be influenced by many factors. For instance, while Hallikainen et al. [20] reported an overall increase in burden after 36 months for spouses, they found an initial dip in burden in the first and second year of follow-up. Viñas-Diez et al.’s study [22] found stable caregiver burden in non-spouses, but an increase in spouses after 24 months. Conde-Sala et al. [21] also reported an overall increase of burden at 36-month follow-up. On closer inspection, however, they found three different trajectories of burden over the 3-year follow-up: 1) a low burden group whose burden only slightly increased over time; 2) a high burden group whose burden significantly decreased over time; and 3) a moderate burden group whose burden significantly increased over time. Svendsboe et al. [19] reported different trajectories over a 3-year period of caregivers’ distress for patients with dementia with Lewy bodies (DLB) compared to those with Alzheimer’s disease (AD); for DLB, caregivers’ distress was higher at baseline but remained stable, while for AD it increased over time. Two studies [16, 25] found a decrease of burden or stress after the patient with dementia was transferred from home to an institutional long-term care facility.
Risk factors
Patient characteristics
Patient characteristics were grouped (Table 3) into three categories, namely behavioral and neuropsychiatric symptoms, functional status, and cognitive status. Six studies [15, 16, 20–22, 24] reported that higher prevalence of behavioral disturbances and higher levels of neuropsychiatric symptoms over time were associated with caregivers experiencing higher levels of burden. Hallikainen et al. [20] reported that specific factors, namely an increase in delusions, agitation, and sleep disturbance over time were associated with an increase of psychological distress in the caregiver over time.
Table 3
The different patient, caregiver, and context characteristics assessed in the included studies
| Risk factor | Study references |
| Patient characteristics | |
| Behavioral and neuropsychiatric symptoms | Kajiwara et al. [15]; Borsje et al. [16]; Hallikainen et al. [20]; Conde-Sala et al. [21]; Brodaty et al. [23]; Reed et al. [24] |
| Functional status | Kawaharada et al. [17]; Conde-Sala et al. [21]; Brodaty et al. [23]; Reed et al. [24] |
| Cognitive status | Kajiwara et al. [15]; Borsje et al. [16]; Conde-Sala et al. [21]; Brodaty et al. [23]; Reed et al. [24] |
| Caregiver characteristics | |
| Age | Borsje et al. [16]; Hallikainen et al. [20]; Conde-Sala et al. [21], Viñas-Diez et al. [22]; Brodaty et al. [23] |
| Gender | Borsje et al. [16]; Hallikainen et al. [20]; Conde-Sala et al. [21]; Viñas-Diez et al. [22], Brodaty et al. [23] |
| Physical and mental health | Conde-Sala et al. [21] |
| Context characteristics | |
| Cohabitation | Raccichini et al. [18]; Conde-Sala et al. [21]; Viñas-Diez et al. [22], Bleijlevens et al. [25] |
| Kinship | Conde-Sala et al. [21]; Viñas-Diez et al. [22] |
| Sole caregiver | Conde-Sala et al. [21] |
Four studies [17, 21, 23, 24] found a decline in independence in basic activities of daily living (ADL) as a robust risk factor for caregiver burden over time. Kawaharada et al. [17] found that specific ADL decline in feeding and bathing were significantly associated with increased caregiver burden.
Of the five studies [15, 16, 21, 23, 24] with cognitive status as a factor, four studies [15, 16, 23, 24] reported a decline in cognitive status, but found no association with an increase of caregiver burden over time. Only Conde-Sala et al. [21] reported a decline in cognitive status which was associated with an increase of burden in the total sample.
Caregiver characteristics
Caregiver characteristics were grouped into two categories, namely sociodemographic and psychological factors. Of the sociodemographic factors, the caregiver’s age and gender were found to be important determinants for caregiver burden over time [16, 20–23]. Borsje et al. [16] and Brodaty et al. [23] reported that female caregivers showed higher levels of psychological distress compared to male caregivers. Concerning the age of the informal caregivers, Borsje et al. [16] found that informal caregivers aged between 50 and 70 years reported higher levels of psychological distress over time compared to those aged >70 years. Viñas-Diez et al. [22], however, found that older age was associated with a higher burden over time, but only in the non-live-in adult-child group.
Caregivers who had themselves poor psychological [21] and mental health [21, 22] and were sole caregivers [21] experienced a greater burden over time.
Context characteristics
Context characteristics were grouped into two categories, namely kinship and cohabitation. Two studies [21, 22] reported higher levels of burden and distress over time in spouses and adult-child caregivers who cohabitated with the patient compared to adult-child caregivers who did not live with the patient. Conde-Sala et al. also [21] reported that adult-child caregivers who cohabitated with the patient reported the highest level of burden throughout the follow-up, whereas adult-caregivers who did not live with the patient consistently reported the lowest levels of burden across the 36 months of follow-up. Spouses had intermediate scores, but were the only group to show a significant increase in burden over the three years.
The study of Viñas-Diez et al. [22] reported significantly higher levels of caregiver burden in the live-in adult-child group compared to the non-live-in adult-child group, but found that the burden in the spouse group was significantly higher at follow up compared to the adult-child group.
Raccichini et al. [18] found that cohabitation was the main, significant predictor for caregiver burden, after 6 months. Bleijlevens et al. [25] reported higher levels of burden and lower HRQoL in informal caregivers who lived with the patient at home versus those whose patient had moved into an institutional long-term care facility. After the transition a decrease in burden and psychological distress was observed [25]. Svendsboe et al. [19] found a decrease of psychological distress after transition at 12 months the caregivers caring for patients with DLB.
DISCUSSION
Risk factors of caregiver burden and burden-related concepts have been previously investigated but most studies are cross-sectional in nature. This is the first systematic review exploring the trajectory of caregiver burden in informal caregivers for patients with dementia. This review included eleven longitudinal studies on caregiver burden and reveals that the main trajectory was an overall increase of burden over time [17–24], whereas two studies [15, 16] reported relatively stable patterns of caregiver burden during follow-up. Probably due to the fact that after certain levels of caregiver burden are reached, transition to a long-term care facility is inevitable. This review found evidence that after this transition, levels of caregiver burden or stress subsequently decrease [16, 25] as the high care demands on the informal caregiver drops.
Although the majority (eight studies) of the included studies reported an overall increase, different trajectories were found. Four studies found a relatively gradual increase during the follow-up period ranging from 6 to 36 months [17, 18, 23, 24], where others found more fluctuations over time [19, 20, 21, 22]. For example, Hallikainen et al. [20] reported an early dip in caregiver stress at 12 and 24 months, with a significant increase at 36-month follow-up. A possible explanation is that the high baseline caregiver burden could be explained by the caregivers’ inexperience and that the initial dip could reflect caregivers becoming more accustomed to their roles, and burden eventually increases over time because of the increasing higher care demands. Another explanation for the fluctuations in the trajectories of caregiver burden come from Viñas-Diez et al. [22] and Conde-Sala et al. [21], as they reported three different trajectories of burden, depending on the presence of various risk factors, highlighting the dynamic nature of informal caregiving.
Although an overall stable pattern in caregiver burden was reported by Borsje et al. [16], they found in concordance with Hallikainen [20] an initial drop in caregiver burden at 9 months follow-up after it returned to baseline levels after 18 months resulting in an overall stable pattern. In accordance with the study of Borsje et al. [16], a stable pattern of overall caregiver burden at 12-month follow-up was reported in the study of Kajiwara [15]. They argued that this stable pattern in caregiver burden is related with increases in personal strain and decreases in role strain. It could be that caregivers’ personal lives are changing due to the increase of care activities and that the role they have become much clearer during the dementia process of their love ones.
This review found consistent evidence [15, 16, 20–22, 24] that more behavioral and neuropsychiatric symptoms over time were associated with an increase of caregiver burden. Besides the presence of behavioral and neuropsychiatric symptoms, decline in functional status and independence in basic activities of daily living (ADL) were found to be robust risk factors [17, 21, 23, 24] for caregiver burden at follow-up, as more responsibility and hours of supervision from the informal caregiver are required. Besides these patients’ risk factors, this review found consistent evidence [18, 20–22] that cohabitation and kinship are associated with a stronger increase of caregiver burden as the dementia progresses. Adult-children caregivers living with the patient with dementia are most at risk to experience higher levels of caregiver burden, compared to adult-children caregivers who live elsewhere, whereas spouses remained at intermediate levels but had the most increase over time [22]. However, these factors seem related due to the fact that mostly spouses cohabitate with the patient [20], whereas adult-child caregivers usually live elsewhere. Besides, spouses are themselves older of age, which also is associated with more caregiver burden [22], although this association is not consistently found. Borsje et al. [16] found evidence that a particular age category [50–70] is more at risk for increasing caregiver burden. This inconsistency was found in previous studies of Andrén and Elmstahl [26], as they reported that elderly caregivers reported less burden than younger ones, whereas Rinaldi et al. [27] reported greater burden in older caregivers. The association of age with caregiver burden may be mediated by the physical and mental health of the informal caregiver themselves. Older caregivers tend to have a poorer physical and mental health, compared to younger caregivers, resulting in more elevated levels of caregiver burden [21]. This review found some evidence that female gender [16, 23] was a risk factor for more burden over time compared to male gender of the informal caregiver. This is in line with previous studies [28, 29], as they found caregiving wives expressed significantly higher levels of anxiety, sadness, and anger than caregiving husbands, possible due to women dedicating more time to caregiving duties.
This systematic review has certain limitations. Overall the comparison of studies is difficult due to different inclusion criteria, definition of burden, methodology, and follow-up periods. A limitation is that none of the studies assessed caregiver resources, like personality traits, competence, or coping styles of the informal caregiver. However, based on the review of van der Lee [5], these caregiver resources may be considered strong mediators between the impact of the patient’s functional decline and behavioral and neuropsychiatric symptoms and caregiver burden. A second limitation is that only the studies of Svendsboe et al. [19] and Brodaty et al. [23] included the average duration of the symptoms, although worsening of severity of dementia and long duration of the illness were associated with a greater burden from caregiving [6].
Different efforts were made to minimalize the methodological limitations of this current review. The search strategy included multiple keywords to ensure all relevant articles were found. By using the NOS for Cohort Studies [14], the quality of the included studies was assessed. All included studies were of fair to good quality. This review was guided by the PRISMA guidelines to enhance transparency and reproducibility.
Further longitudinal research should be conducted to examine the complex and multidimensional concept and the trajectory of caregiver burden including all of the different patient, caregiver, and context factors combined. Duration of symptoms and the time since diagnosis also need to be considered in order to monitor dementia progression. Use of external resources may also be a beneficial factor to reduce levels of caregiver burden and this should be assessed in future studies.
Conclusion
There is no one-size-fits-all for predicting how caregiver burden will change over time but specific factors (like being a spouse and increased behavioral impairment and decline in functional status in the patient) may heighten the risk. Other factors, not yet comprehensively included in the published studies, might also prove to be important risk factors like duration of symptoms or hours of supervision needed. Future research is recommended to integrate the patient, caregiver, and context characteristics in the trajectory of caregiver burden, and to assess more clearly the phase of the dementia progression and use of external resources. Determining when and if caregiver burden becomes too much and which risk factors are important should improve not only the caregiver’s quality of life but also the patient’s care. The knowledge gained could guide treatments and policy. It may even delay institutionalization, something most caregivers and their patients, not to mention economies across the world, would welcome.
DISCLOSURE STATEMENT
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/20-0647r1).
REFERENCES
[1] | Patterson C ((2018) ) World Alzheimer Report 2018. The state of the art of dementia research: New frontiers. Alzheimer’s Disease International, London. |
[2] | Etters L , Goodall D , Harrinson BE ((2008) ) Caregiver burden among dementia patient caregivers: A review of the literature, J Am Acad Nurse Pract 20: , 423–428. |
[3] | Lim J , Griva K , Goh J , Chionh H , Yap P ((2011) ) Coping strategies influence caregiver outcome among Asian family caregivers of persons with dementia in Singapore, Alzheimer Dis Assoc Disord 21: , 34–41. |
[4] | Zarit S , Todd P , Zarit J ((1986) ) Subjective burden of husband and wives as caregivers: A longitudinal study, Gerontol Soc Am 26: , 260–266. |
[5] | Van der Lee J , Bakker T , Duivenvoorden H , Dröes R ((2014) ) Multivariate models of subjective caregiver burden in dementia: A systematic review, Ageing Res Rev 15: , 76–93. |
[6] | Chia CY , Wu HS , Hsiao CY ((2015) ) Caregiver burden for informal caregiver of patients with dementia; a systematic review, Int Nurs Rev 62: , 340–350. |
[7] | Volicer L , Hurley A ((1998) ) Hospice care for patients with advanced progressive dementia, Springer, New York. |
[8] | Reisberg B , Borenstein J , Salob SP , Ferris SH ((1987) ) Behavioral symptoms in Alzheimer’s disease: Phenomenology and treatment, J Clin Psychiatr 48: , 9–15. |
[9] | Agüera-Ortiz L , Frank-García A , Gil P , Moreno A ((2010) ) Clinical progression of moderate-to-severe Alzheimer’s disease and caregiver burden: A 12-month multicenter prospective observational study, Int Psychogeriatr 22: , 1265–1279. |
[10] | Mohamed S , Rosenheck R , Lyketsos CG , Schneider LS ((2010) ) Caregiver burden in Alzheimer disease: Cross-sectional and longitudinal patient correlates, Am J Geriatr Psychiatry 18: , 917–927. |
[11] | Haro JM , Kahle-Wrobleski K , Bruno G , Belger M , Dell’Agnello G , Dodel R , Argimon JM ((2014) ) Analysis of burden in caregivers of people with Alzheimer’s Disease using self-report and supervision hours, J Nutr Health Aging 18: , 677–684. |
[12] | Sury L , Burns K , Brodaty H ((2013) ) Moving: Adjustment of people living with dementia going into a nursing home and their families, Int Psychogeriatr 25: , 867–876. |
[13] | Moher D , Liberati A , Tetzlaff J , Altman DG ; PRISMA Group ((2009) ) Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement, PLoS Med 6: , e1000097. |
[14] | Wells GA , Shea B , O’Connell D , Peterson J , Welch V , Losos M , Tugwell P ((2014) ) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in metaanalyses. http://www.ohri.ca/ programs/clinical_epidemiology/oxford.asp, Retrieved March 10, 2020. |
[15] | Kajiwara K , Noto H , Yamanaka M ((2018) ) Changes in caregiving appraisal among family caregivers of persons with dementia: A longitudinal study over 12 months, Psychogeriatrics 18: , 460–467. |
[16] | Borsje P , Hems MA , Lucassen PL , Bor H , Koopmans RT , Pot AM ((2016) ) Psychological distress in informal caregivers of patients with dementia in primary care: Course and determinants, Fam Pract 33: , 374–381. |
[17] | Kawaharada R , Sugimoto T , Matsuda N , Tsuboi Y , Sakurai T , Ono R ((2019) ) Impact of loss of independence in basic activities of daily living on caregiver burden in patients with Alzheimer’s disease: A retrospective cohort study, Geriatr Gerontol Int 19: , 1243–1247. |
[18] | Raccichini A , Spazzafumo L , Castellani S , Civerchia P , Pelliccioni G , Scarpino O ((2015) ) Living with mild to moderate Alzheimer patients increases the caregiver’s burden at 6 months, Am J Alzheimers Dis Other Demen 30: , 462–467. |
[19] | Svendsboe EJ , Testad I , Terum T , Jörg A , Corbett A , Rongve A ((2018) ) Patterns of carer distress over time in mild dementia, Int J Geriatr Psychiatry 33: , 987–993. |
[20] | Hallikainen I , Koivisto AM , Välimäki T ((2017) ) The influence of the individual neuropsychiatric symptoms of people with Alzheimer disease on family caregiver distress: A longitudinal ALSOVA study, Int J Geriatr Psychiatry 33: , 1207–1212. |
[21] | Conde-Sala JL , Turró-Garriga O , Calvó-Perxas L , Vilatla-Franch J , Lopez-Pousa S , Garre-Olmo J ((2014) ) Three-year trajectories of caregiver burden in Alzheimer’s disease, J Alzheimers Dis 42: , 623–633. |
[22] | Viñas-Diez V , Turró-Garriga O , Portellano-Ortiz C , Gascón-Bayarri J , Reñé-Ramirez R , Garre-Olmo J , Conde-Sala JL ((2017) ) Kinship and cohabitation in relation to caregiver burden in the context of Alzheimer’s disease: A 24-months longitudinal study, Int J Geriatr Psychiatry 32: , 72–82. |
[23] | Brodaty H , Woordward M , Boundy K , Ames D , Balshaw R ((2014) ) Prevalence and predictors of burden in caregivers of people with dementia, Am J Geriatr Psychiatry 22: , 756–765. |
[24] | Reed C , Belger M , Scott Andrews J , Tockhorn-Heidenrech A , Jones RW , Wimo A , Dodel R , Haro JM ((2019) ) Factors associated with long-term impact on informal caregivers during Alzheimer’s disease dementia progression: 36 month results from GERAS, Int Psychogeriatr 32: , 267–277. |
[25] | Bleijlevens MHC , Stolt M , Stephan A , Zabalegui A , Sutcliffe C , Lethin C , Zwakhalen SMG ((2014) ) Changes in caregiver burden and health-related quality of life of informal caregivers of older people with dementia: Evidence from the European RightTimePlaceCare prospective cohort study, J Adv Nurs 71: , 1378–1391. |
[26] | Andrén S , Elmståhl S ((2007) ) Relationships between income, subjective health and caregiver burden in caregivers of people with dementia in group living care: A cross-sectional community based study, Int J Nurs Stud 44: , 435–446. |
[27] | Rinaldi P , Spazzafumo L , Mastriforti R , Mattioli P , Marvardi M , Polidori MC , Cherubini A , Abate G , Bartorelli L , Bonaiuto S , Capurso A , Cucinotta D , Gallucci M , Giordano M , Martorelli M , Masaraki G , Nieddu A , Pettenati C , Putzu P , Tammaro VA , Tomassini PF , Vergani C , Senin U , Mecocci P ((2005) ) Predictors of high level of burden and distress in caregivers of demented patients: Results of an Italian multicenter study, Int J Geriatr Psychiatry 20: , 168–174. |
[28] | Chappell NL , Dujela C , Smith A ((2014) ) Spouse and adult child differences in caregiving burden, Can J Aging 33: , 462–472. |
[29] | Bastawrous M , Cignac MA , Kapral MK , Cameron JI ((2015) ) Factors that contribute to adult children caregivers’ well-being: A scoping review, Health Soc Care Community 23: , 449–466. |




