End Stage Clinical Features and Cause of Death of Behavioral Variant Frontotemporal Dementia and Young-Onset Alzheimer’s Disease
Abstract
Background:
Limited literature exists regarding the clinical features of end stage behavioral variant frontotemporal dementia (bvFTD). This data is indispensable to inform and prepare family members as well as professional caregivers for the expected disease course and to anticipate with drug-based and non-pharmacological treatment strategies.
Objective:
The aim of the present study was to describe end stage bvFTD in a broad explorative manner and to subsequently evaluate similarities and dissimilarities with the end stage of the most prevalent form of young-onset dementia, Alzheimer’s disease (yoAD).
Methods:
We analyzed medical files on patients, using a mixed model of qualitative and quantitative approaches. Included were previously deceased patients with probable bvFTD and probable yoAD. End stage was defined as the last 6 months prior to death. Primary outcome measures comprised somatic, neurological, and psychiatric symptoms and the secondary outcome measure was cause of death.
Results:
Out of 89 patients, a total of 30 patients were included (bvFTD; n = 12, yoAD; n = 18). Overall, the end stages of bvFTD and yoAD were characterized by a broad spectrum of clinical symptoms including severe autonomic dysfunction and an increased muscle tone. Patients with bvFTD displayed more mutism compared with yoAD while compulsiveness was only present in bvFTD.
Conclusion:
Our study describes the full clinical spectrum of end stage bvFTD and yoAD. In this study, symptoms extend far beyond the initial behavioral and cognitive features. By taking both somatic, psychiatric, and neurological features into account, family members and professional caregivers may anticipate (non) pharmacological treatment.
INTRODUCTION
Frontotemporal dementia (FTD) is an incurable progressive neurodegenerative disease that is characterized by degeneration of the frontal and temporal lobes. It is the second most common type of young-onset dementia after Alzheimer’s disease (AD) [1, 2]. Frontotemporal dementia is a heterogeneous disease with different phenotypes, including primary progressive aphasia which can be further subdivided in the semantic variant and progressive non fluent aphasia (PNFA), and the behavioral variant (bvFTD) which is the most prevalent form [3]. bvFTD is characterized by behavioral disturbances including apathy, stereotyped behavior, disinhibition, hyperorality, and loss of empathy [4–6].
Due to the absence of curative treatment, all patients eventually will face the end stage, unless they decease earlier due to comorbid diseases [7–9]. Young-onset dementia in particular is accompanied with severe behavioral disturbances; caregivers experience high levels of burden and suffer from depressive symptoms and a broad spectrum of psychosocial problems [10, 11]. Due to the characteristic key features of bvFTD such as apathy and disinhibition, the behavioral variant is associated with a far higher caregiver burden in comparison with late-onset AD or other subtypes of FTD [12–14]. Besides, in young-onset AD (age < 65) the most prevalent neuropsychiatric symptom is apathy [15], which is likewise accompanied with severe caregiver burden [16] and is the main predictor of institutionalization in young-onset dementia [17]. Within the nursing home environment, it is acknowledged that providing care to residents with younger-onset dementia can be highly demanding [18, 19]. As a result, a high prevalence of psychotropic drug use is reported in young-onset dementia in nursing homes. In particular, this is described with regard to neuropsychiatric symptoms, where it has limited efficacy and is often accompanied with severe side effects [20–22]. Moreover, quality of life of the patient is negatively associated with advanced dementia and psychotropic drugs use [23].
Yet, despite these severe clinical issues, literature regarding clinical features of end stage bvFTD as well as the cause of death is insufficient. In fact, merely an increase of aphasia is reported as a feature, in some cases resulting in mutism, as well as development of dysphagia causing aspiration pneumonia thereby contributing to mortality [24]. Other studies focused solely on functional status, degree of suffering, or neuropsychiatric symptoms [25–30], whereas dementia has devastating effects on multiple facets leading to somatic, neurological, and psychiatric complications [31–33]. Literature regarding cause of death in bvFTD reports a premature and sudden death in the majority of patients whereas the underlying cause remained undiscovered. Additionally, a few cases of suffocation due to severe hyperorality have been described [24, 34].
At present, specific evidence-based guidelines for end stage bvFTD are lacking. Overcoming the gap in knowledge concerning the clinical appearance of advanced bvFTD is indispensable to adequately inform and prepare family members as well as professional caregivers for the expected disease course. Gaining more clarity about clinical features in the end stage is essential for planning of non-drug treatment strategies and in reducing unnecessary pharmacological treatment. Moreover, defining the end stage can contribute in developing palliative guidelines for bvFTD, thus optimizing care including adequate symptom control, which can decrease disease burden for family members as well.
We hypothesize that it is conceivable that as the disease progresses and expands beyond the frontal and temporal brain regions, symptoms of bvFTD become more similar to other types of dementia. On the other hand, FTD might display unique symptoms that relate to specific disease mechanisms, even within the end stage.
Therefore, our first aim was to investigate the full clinical picture of symptoms that define the end stage of bvFTD and compare these findings with young-onset AD (yoAD). Secondly, we aimed to study the cause of death in bvFTD and yoAD.
METHODS
Design
An explorative approach was chosen by performing a mixed model of qualitative and quantitative research techniques by using an exploratory sequential design [35, 36].
First, qualitative research was initiated with a literature review for hypothetical features outside the diagnostic criteria of bvFTD [37] and AD [38] that are associated with the end stage of dementia. The literature review was performed in PubMed, in March 2019 by ME. Articles between 1982 and 2019 were retrieved. The following search terms were used in various combinations: ‘Frontotemporal dementia’, ‘FTD’, ‘behavioural variant frontotemporal dementia’, ‘behavioral variant frontotemporal dementia’, ‘bvFTD’, ‘FTLD’, ‘Frontotemporal Lobe Degeneration’, ‘Alzheimer’s disease’, ‘AD’, ‘early onset’, ‘young onset’, ‘end stage’, ‘end of life’, ‘final stage’, ‘advanced dementia’, ‘palliative’, ‘palliative care’, ‘terminal’, ‘neuropsychiatry’, ‘survival’, ‘progression’, ‘cause of death’, ‘die’, ‘dying’, ‘mortality’, ‘mortal’, ‘nursing home’. The search led to twenty-four articles [25–30, 32, 33, 39–54] and the features deriving from this literature review were noted.
Subsequently, qualitative research was further carried out in the patient’s medical files of the Lisidunahof nursing home by examining all daily reports from the nursing staff, physicians, speech therapist, physiotherapist, occupational therapist, dentist, dental hygienist, as well as the semi-annual multidisciplinary patient evaluation, for emerging features apart from the features derived from literature review and diagnostic criteria of both bvFTD [37] and AD [38]. Additionally, somatic events were noted. The daily medical reports were generally written by the same group of staff. Emerged features from the patient files and literature review were combined with the diagnostic criteria and further categorized by subdividing them into themes and subthemes.
Thereafter, all patient medical files were reassessed and all features mentioned were categorized into the previously formed themes and subthemes. In order to avoid missing features that might have been present yet were not mentioned in the patient files, the features were verified during informal interviews with the nurse and the physician whom had a treatment relationship with the patient in the last six months before death. The informal interviews took place after reassessment of the patients files. Features that had not been assessed or could not be retrieved from the patient files nor from the informal interviews were reported as missing. Assessed features that were absent were reported as absent. From the qualitative categorized data, nominally scaled variables were expressed in relative frequencies of features mentioned across the patient files, which were sequentially used for descriptive statistics to outline the end stage of bvFTD and yoAD. In this manner, explorative qualitative data could be subsequently converted to quantitative data (relative frequencies), previously referred to as the explorative sequential design.
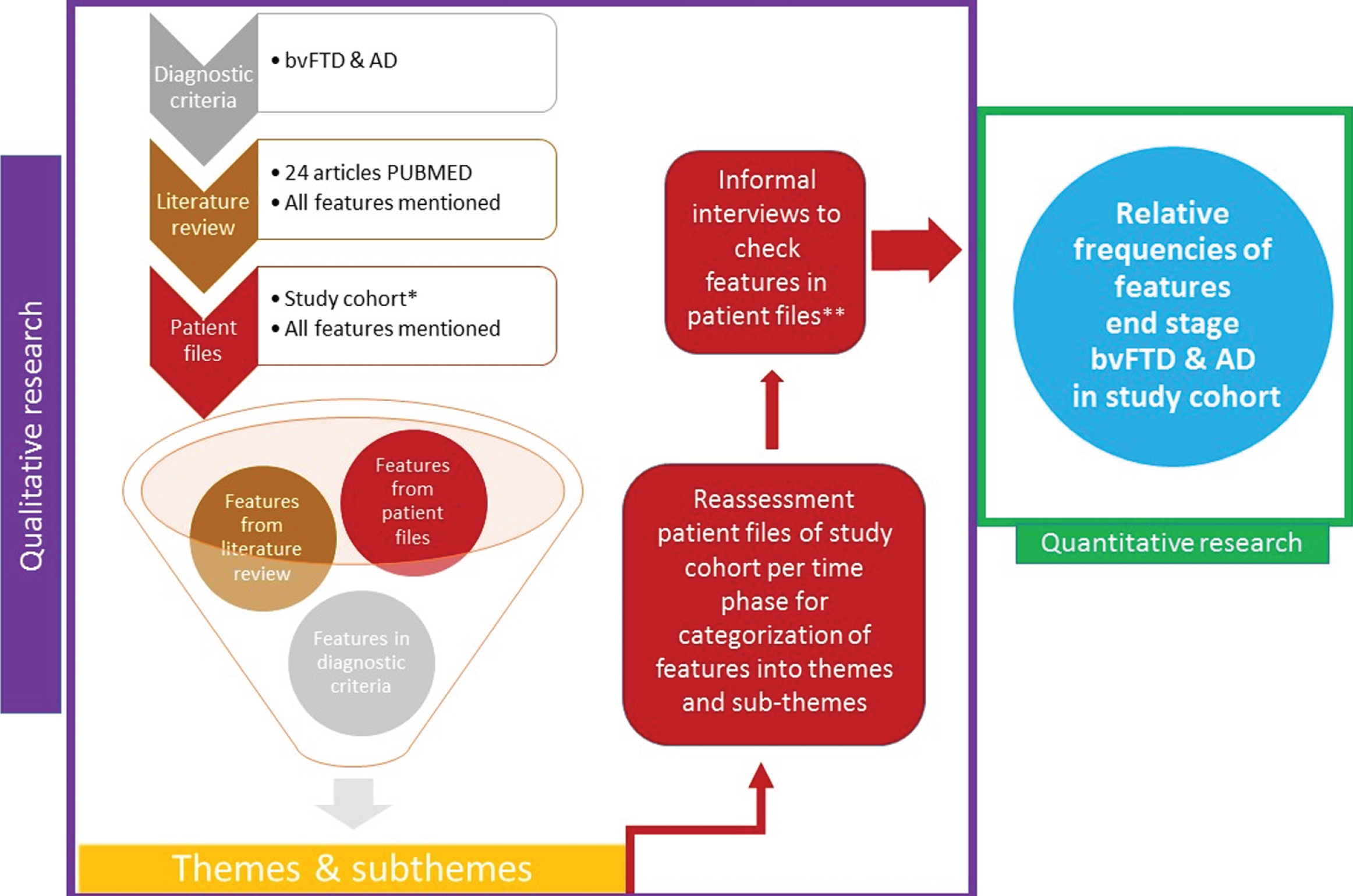
All features were examined per time phase, subdivided into 1) six to four months before death, 2) four to two months before death, and 3) two months until death, in order to map the time course of end stage symptoms. Figure 1 provides a schematic overview of the study design.
Fig. 1
Flowchart exploratory sequential design. *Patients with advanced bvFTD and AD from nursing home Lisidunahof. The last 6 months prior to death were assessed, separated per time phase: 6–4 months before death, 4–2 months before death, 2 months until death. **Informal interviews with the nurse and geriatric physician to check for missing features that might have been present yet were not mentioned in the patient files of the study cohort.
Cause of death was determined with a physical examination performed by the geriatric physician, taking into account the disease course and medical history of the patient.
Assessment of pain was performed by the geriatric physician that was interviewed by ME. Attention was paid to an excessive or reduced response to situations that can be painful or uncomfortable such moments where patients received physical care, by assessing facial expressions, groaning, changed behavior, restlessness, change in intake, micturition, defecation, and overall functioning. By working in a permanent team and involving the informal caregiver, changes could be quickly noticed.
Subjects
In our study, we focused on the clinical profile of end-stage bvFTD (case-group) and young-onset AD (control group), being the most frequent causes of young-onset dementia. All patient files were collected from the Lisidunahof, a nursing home in the Netherlands, specialized in young-onset dementia. All patients were admitted between 2002-2019 and all deceased in this same period of time.
Participants were included if they were deceased and met the criteria for ‘probable’ bvFTD [37] or ‘probable’ Alzheimer’s disease [38]. In order to verify if they met these diagnostic criteria, their charts were reviewed, taking into account the results of additional examinations, by ME and FG. Three patients had a normal MRI of the brain during initial diagnostic workup, therefore meeting the criteria of possible bvFTD [37]. Yet, there was a gradual slow progression of the disease with clinical symptoms remaining congruent with bvFTD, resulting in admission in the nursing home, eventually leading to death. Due to the evident clinical picture of bvFTD, the treating physician decided it was redundant to repeat neuroimaging. Thus, it was considered highly likely that these patients had (probable) bvFTD and therefore they were included.
All participants were in the “very severe” or “profound” stage of the disease six months prior to death, based on the Frontotemporal Dementia Rating Scale (FRS) [26] for bvFTD and within the “severe” stage of AD using the Clinical Dementia Rating (CDR) scale [55].
Patients were excluded in case of language variants of FTD, missing documentation in the last six months before death, a mixture of forms of dementia, diagnosis of epilepsy before diagnosis of dementia, a neurodegenerative disorder other than bvFTD or (young-onset) AD in the medical history, (chronic) delirium in the last six months before death and the frontal variant of AD.
Measures
The following demographic and clinical characteristics were collected: age at death, gender, year of symptom-onset, year of death, education in years, medical history, cause of death and previously performed additional diagnostics (genetics, imaging of the brain including computed tomography (CT), magnetic resonance imaging (MRI), fluorodeoxyglucose positron emission tomography [18F]-2-deoxy-2-fluoro-D-glucose (FDG) positron-emission tomography (FDG-PET), amyloid positron emission tomography (amyloid-PET), neuropsychological examination, cerebrospinal fluid (CSF) and drug treatment.
The primary outcome measures consisted of the clinical symptoms present in the end stage of bvFTD and early-onset AD, divided in somatic features, including somatic events that occurred in the last six months, neurological and psychiatric features. The secondary outcome measure consisted of cause of death.
Statistical analysis
IBM SPSS Statistics 22.0 was used for all descriptive and explanatory statistics. For continuous variables, it was examined whether it followed a normal distribution. Depending on the distribution, Mann Whitney U Test was used to test for differences in age and years of education between the two groups whereas the independent Student T test was performed to test for dissimilarities in disease duration. Chi-square test was used to test for gender differences between bvFTD and yoAD.
Ethical considerations
This study was designed and conducted according to the Declaration of Helsinki. The study protocol was approved by the Ethical Committee of the VU University Medical Center deciding that The Medical Research Involving Human Subjects (MRIHS) did not apply for this study. The study was performed under the laws of General Data Protection Regulation (GDPR) and the Code of Good Conduct.
RESULTS
Study sample
Out of 89 patients, 41 patients were not deceased and therefore not eligible. Of the 48 remaining patients, 18 were excluded due to the presence of language variant of FTD (n = 11), missing documentation in the last six months before death (n = 1), the frontal variant of AD (n = 2), or a mixture of dementia (n = 4). Ultimately, the total sample size included 30 patients; n = 18 for yoAD, n = 12 for bvFTD.
Qualitative results from literature, diagnostic criteria, and patient files
Qualitative research from the literature review, diagnostic criteria, and patient files led to the following outcome measures; somatic events were categorized in ischemic stroke, hemorrhagic stroke, myocardial infarction, arrhythmia, deep vein thrombosis, renal impairment, pulmonary embolism, hematological complications, transient ischemic attack, transient loss of consciousness by unknown cause and ileus. In addition, clinical features in the end stage were divided into the following (sub)themes: somatic features including incontinence, decrease of eating, weight gain or weight loss, cachexia, autonomic disorders (including constipation, thin defecation, undigested stool, epiphoria, saliva flood, changed pain perception), reversed day-night rhythm, infections, pain, pressure ulcers, intertrigo, fever, nausea/vomiting, bedridden and dental issues; neurological features including language disorder divided in mutism/no mutism, hypersomnia, hypertonia, hyperextension of the neck, contractures, impaired trunk balance, apraxia, nystagmus, dysphagia, parkinsonism, epilepsy, myoclonus and fasciculation, gait disorders (including balance disorder due to impaired proprioception, hypokinetic rigid, paretic hypotonic, spastic, vestibular, functional, ataxia, apraxia, hanging crooked with the upper body to one side while walking). Gait disorders were assessed by a geriatric physician and physiotherapist with expertise in dementia. Psychiatric features included bradyphrenia, restlessness, suspicion, euphoria, persistent calling, wander, urge for walking, echolalia, grimaces, disinhibition, apathy, compulsive behavior, hyperorality, euphoria, anxiety, depressive symptoms, and agitation/ aggression.
Missing features
Features including weight loss, weight gain, cachexia, parkinsonism, and trunk balance could not be fully retrieved from all the patient files throughout the last six months (≥30% missing values) and were therefore excluded.
Demographics
Table 1 provides an overview of the demographic characteristics of the diagnostic groups. Overall, there were no significant differences between bvFTD and yoAD in mean age at death (65±8.0 years, p = 0.39), level of education (5.43±8.2, p = 0.72), and gender (40% male, p = 0.14).
Table 1
Demographics at death
| All | bvFTD | yoAD | P | |
| n = 30 | n = 12 | n = 18 | ||
| Sex (Male) | 12 (40) | 7 (58) | 5 (28) | 0.14 |
| Age | 64.9±8.0 | 62.3±10.2 | 66.7±5.8 | 0.39 |
| Education levela | 5.43±8.2 | 5.50±1.0 | 5.39±0.7 | 0.72 |
| Disease durationb, y | 11.7±4.5 | 12.0±4.5 | 11.6±5.4 | 0.81 |
Data represent n (%), mean±SD. P-values are based on Chi-square test for sex, Independent two-sample t-test for level of education & disease duration, Mann-Whitney U for age. aThe level of education was classified by using the Verhage system, ranging from 1 (no or little education) to 7 (highest academic degree) [92]. bRepresents the time from the onset of symptoms till death.
Clinical features
Table 2 displays the clinical features from the patient files that had a prevalence of >50% in either bvFTD or yoAD. Most of these were prevalent in both disorders. In the last six months of life, ≥70% of all participants experienced incontinence and pressure ulcers. Moreover, they were bedridden, had dental issues, and displayed severe autonomic dysfunction. Throughout the last six months, a changed pain perception was the most prevalent type of autonomic dysfunction, followed by constipation and thin defecation. The most prevalent somatic event was an infection, followed by transient loss of consciousness by unknown cause. Other events included fever due to cerebral dysfunction, cerebral ischemic attack, sclerotic bone lesions, gastric hemorrhage, hematological complication, and an ileus caused by refractory constipation, of which the last three resulted in death. Regarding the neurological features, all patients experienced language disorders (100%), comprising mutism in 75% of bvFTD cases, whereas this was less common in yoAD (23.5%). Other neurological features with a prevalence of ≥70% included hypersomnia, apraxia, dysphagia, and gait disorders. The most prevalent gait disorder was due to inability of maintaining balance caused by impaired proprioception, followed by a gait in which the patient is hanging crooked with his upper body to one side while walking. Furthermore, ≥70% of all participants were apathetic and displayed bradyphrenia. Compulsiveness was a unique feature of bvFTD (66.7% versus 0.0% in yoAD).
Table 2
Core clinical features from the patient files in the nursing homea
| bvFTD | AD | |
| n = 12 | n = 18 | |
| Somatic features | ||
| Incontinenceb | 92 | 100 |
| Autonomic function disorders | 100 | 100 |
| Changed pain perception | 90 | 100 |
| Constipation | 75 | 72 |
| Thin defecation | 50 | 67 |
| Pressure ulcers | 58 | 88 |
| Bedridden | 75 | 100 |
| Intertrigo | 33 | 65 |
| Dental issues | 67 | 100 |
| Decrease in eating | 50 | 72 |
| Pain | 42 | 50 |
| Neurological features | ||
| Hypersomnia | 83 | 100 |
| Dysphagia | 75 | 83 |
| Mutism | 75 | 24 |
| Hypertonia | 50 | 65 |
| Myoclonus | 33 | 56 |
| Gait disorder due to impaired balancec | 58 | 81 |
| Apraxia | ||
| Psychiatric features | 100 | 100 |
| Apathy | 92 | 73 |
| Restlessness | 58 | 71 |
| Disinhibition | 50 | 24 |
| Compulsiveness | 67 | 0.0 |
| Bradyphrenia | 83 | 61 |
| Grimaces | 50 | 27 |
Data represent rounded numbers of the highest percentage achieved per feature of the three measurements in time (6–4 months prior to death, 4–2 months prior to death, 2 months until death). aDisplayed features all have a total prevalence >50% in bvFTD or yoAD, or both in the last six months. bIncontinence for defecation and urine. cBalance disorder caused by impaired proprioception.
In contrast, hyperextension of the neck yielded a lower prevalence (33.3% in bvFTD, 23.5% in yoAD two months before death). Additionally, less common features included reversed day and night rhythm, epihoria, undigested stool, saliva flood, nystagmus, echolalia, and euphoria with an overall prevalence ≤10% throughout the last six months for both bvFTD and yoAD. None of the participants suffered from fasciculations or gait disorders on base of a spastic, vestibular, functional, or ataxic walking pattern.
Trajectories of clinical features
During the last six months until death, in both groups there was an increase in prevalence (≥10%, maximum of 61%) of features including pressure ulcers, decrease of eating, being bedridden, pain, fever, nausea/vomiting, dysphagia, gait disorders, myoclonus, and anxiety.
In particularly bvFTD, there was an additional increase of ≥10% in daytime sleepiness, autonomic disorders, and epilepsy, whereas in yoAD these features included, hypertonia, restlessness,and intertrigo. On the other hand, features that decreased solely in bvFTD (≥10%) included disinhibition and hyperorality. In yoAD there was a decrease of wander (≥10%).
Cause of death
Table 3 provides an overview of causes of death. All patients died in the nursing home. The majority of the patients in both groups deceased due to an infection caused by aspiration pneumonia (mean prevalence 40%), followed by cachexia accompanied with dehydration (mean prevalence 33%). Other causes of death were gastrointestinal related including gastric hemorrhage and ileus (mean prevalence 10%), and several other divergent causes such as overall deterioration in dementia, continuous epileptic status, rapid suspected hematological process, and acute respiratory insufficiency due to heart failure.
Table 3
Cause of death
| All | bvFTD | yoAD | |
| n = 30 | n = 12 | n = 18 | |
| Infectiona | 12 (40) | 4 (33) | 8 (44) |
| Cachexia and dehydration | 10 (33) | 3 (25) | 7 (39) |
| Gastrointestinal causeb | 3 (10) | 1 (8) | 2 (11) |
| Otherc | 5 (17) | 4 (33) | 1 (6) |
Data represent n (%). All patients deceased in the nursing home. a(aspiration)pneumonia (n = 10), cause of infection unknown (n = 2), bIleus due to refractory constipation (n = 1), gastric hemorrhage (n = 2), cOverall deterioration in dementia (n = 1), cause of death unknown (n = 1), continuous epileptic status in dementia (n = 1), rapid suspected hematological process and dementia (n = 1), acute respiratory insufficiency due to heart failure (n = 1).
Drug treatment in bvFTD and yoAD
Throughout the last six months of life, the most commonly prescribed drugs included analgesics (73%), psychotropic drugs (≥70%), vitamin supplements (63%), laxatives (60%), emollients/moisturizers (40%), and proton pump inhibitors (33%). With regard to psychotropic drugs, benzodiazepines were most frequently prescribed in all patients (77%), whereas typical antipsychotics (27%), atypical antipsychotics (20%), and antiepileptic drugs (23%) were less commonly prescribed. Other psychotropic drugs included non-selective serotonin reuptake inhibitors (SNRIs) (17%) selective serotonin reuptake inhibitors (SSRIs) (7%), tricyclic antidepressants (TCAs) (3%), N-Methyl-D-aspartate receptor antagonist (3%), and cholinesterase inhibitors (3%). See Table 4 for an overview of the prevalence of all prescribed drugs in bvFTD and AD separately.
Table 4
Drug treatment
| bvFTD | AD | Total | |
| n = 12 | n = 18 | n = 30 | |
| Analgesics | 58 | 83 | 73 |
| Vitamin supplements (B, C, D) | 67 | 61 | 63 |
| Laxatives | 58 | 61 | 60 |
| Emollients/moisturizers | 25 | 50 | 40 |
| Proton pump inhibitor | 25 | 39 | 33 |
| Disinfectants | 17 | 28 | 23 |
| Antifungal cream | 8 | 28 | 20 |
| Anti-emetics | 33 | 6 | 17 |
| Corticosteroids (skin/inhalation) | 25 | 11 | 17 |
| Parasympathicolytic drug | 8 | 17 | 13 |
| Antihistamines | 0 | 22 | 13 |
| Antibiotics | 17 | 0 | 7 |
| Analogue of prostaglandin F | 0 | 6 | 3 |
| Artificial tears | 0 | 11 | 7 |
| Platelet aggregation inhibitors | 8 | 6 | 7 |
| Biguanides | 8 | 0 | 3 |
| Alpha-blockers | 8 | 6 | 7 |
| Statins | 8 | 0 | 3 |
| Vitamin K antagonists | 8 | 0 | 3 |
| Angiotensin-converting enzyme (ACE) inhibitors | 0 | 6 | 3 |
| Melatonin agonists | 8 | 0 | 3 |
| Sympathomimetic | 8 | 6 | 6 |
| Antiandrogens | 8 | 0 | 3 |
| Muscle relaxants | 8 | 0 | 3 |
| Angiotensin II receptor blockers | 0 | 6 | 3 |
| Benzodiazepines | 67 | 83 | 77 |
| Typical antipsychotics | 17 | 33 | 27 |
| Atypical antipsychotics | 25 | 17 | 20 |
| Antiepileptic drugs | 17 | 28 | 23 |
| Non-selective serotonin reuptake inhibitors | 42 | 0 | 17 |
| Selective serotonin reuptake inhibitors | 8 | 6 | 7 |
| Tricyclic antidepressants | 0 | 6 | 3 |
| N-Methyl-D-aspartate receptor antagonists | 0 | 6 | 3 |
| Cholinesterase inhibitors | 8 | 0 | 3 |
Data represents prevalence (%) of drug treatment in last six months of life till death.
DISCUSSION
Our study describes the full clinical spectrum of a nursing home sample with end stage bvFTD and yoAD patients. This novel approach reveals that the end stage of both dementias is characterized by a broad range of features extending far beyond the initial behavioral and cognitive features, including severe somatic comorbidity in form of autonomic dysfunction as well as an increased muscle tone. We found that patients with bvFTD displayed more mutism compared with yoAD, while compulsiveness was uniquely present in bvFTD. Towards death, despite nearly all features increasing over time, there was a notable decrease in prevalence (≥10%) of disinhibition and hyperorality in bvFTD. As 100% of yoAD was bedridden 2 months before death, a decrease of wander was reported (≥10%). The most common cause for death (40% of all patients) was aspiration pneumonia.
Strikingly, in both bvFTD and yoAD the majority of patients in our study displayed severe dysregulation of autonomic functions, predominately expressed as a changed pain perception and gastrointestinal dysfunction.
Our findings are in line with previous studies that report the presence of a changed pain perception in both AD and bvFTD [56–58]. Interestingly, there seems to be a syndrome dependent pattern of experience of pain as multiple studies found an increased responsiveness to pain in AD, semantic dementia, and PNFA, whereas a decreased responsiveness was related to bvFTD [56, 58–60].
Remarkably, we found severe, extensive gastrointestinal complications. Surprisingly, solely one study [61], in which an Autonomic Symptoms Questionnaire (ASQ) was obtained in informal caregivers of patients with bvFTD, semantic dementia, and AD, reported gastrointestinal dysfunction in particularly bvFTD and semantic dementia.
Other studies reveal that autonomic dysregulation is a core feature in the early disease stage of bvFTD including urinary tract dysfunction, thermodysregulation [58, 61, 62], increased heart rate and reduced heart rate variability [62–64], and a dysregulated skin conduction response [65, 66]. Research regarding the evolution of autonomic failure over the disease course could provide insight in this underlying pathological mechanism and in this way may help in the development of treatment strategies to reduce the symptom burden.
Noticeably, hypertonia was predominantly prevalent in both bvFTD and yoAD, particularly in the last two months before death. Interestingly, literature reports a corresponding characteristic change of increased muscle tone occurring during the disease course of dementia, including in bvFTD and AD, referred to as paratonia. Paratonia is defined as a variable, velocity, and force dependent kind of hypertonia, whereby the estimated prevalence is 10–48% in early stages up to 90–100% in end stage dementia [67–73].
In this cohort, none of the patients showed fasciculations. Fasciculations reflect concomitant motor neuron disease in FTD, which is particularly associated with the C9ORF72 mutation present in approximately 7–12% of the FTD cases [74]. In this sample, carrier status for the C9ORF72 mutation was unknown. Presumably, the absence of fasciculations in this study is due to the small sample size.
Strengths of this study include the use of well documented patient data, enabling elucidation of the full clinical spectrum of end stage bvFTD and yoAD. Nonetheless, as a limitation of this study we must acknowledge the relatively small number of participants, reducing the generalizability of the results. In addition, the fact that the clinicians were verbally interviewed about clinical features could have led to recall bias. However, the majority of information derived from the patient files, thereby minimalizing the possible effect on the results. Also, as missing data regarding clinical features including weight loss, weight gain, cachexia, parkinsonism, and trunk balance could not be retrieved, it remains unraveled whether these were present.
A plausible mechanism for our findings regarding the dysregulated autonomic nervous system, might be the effect of neuropathological changes occurring in dementia. Areas that mediate autonomic processes include the brainstem, amygdala, hypothalamus, insula, and the anterior cingulate cortex [62, 75–77]. In bvFTD, particularly the insula and the anterior cingulate cortex are primarily affected [78, 79]. However, as the disease progresses toward severe stages, other areas might be impaired as well, which can likewise be expected in yoAD. Interestingly, pain perception seems not to be determined by disease severity nor demographic or neuropsychological factors and the severity of symptoms can vary widely among different subtypes of dementias [58]. In a previous study, that examined the neuroanatomical association between altered behavioral responsiveness to pain and temperature processing, the right-lateralized grey matter in the mid and posterior insula (in sporadic bvFTD, semantic dementia, PNFA), the bilateral posterior thalamus (in C9orf72 mutation carriers), and the inferior parietal cortex (in AD) [58] were found to be the corresponding areas. In addition, the anterior cingulate cortex is involved in regulation of pain perception [58, 80]. Thus, as both the thalamus, insula, and anterior cingulate cortex are affected in bvFTD and the inferior parietal cortex in AD, it is conceivable that our findings of a changed pain experience are attributable to degeneration in these specific brain areas.
The underlying pathological origin of hypertonia remains yet unrevealed. Typical antipsychotics are notorious for their extrapyramidal side effects including hypertonia, due to antagonizing dopamine type 2 receptors in both the mesocortical and limbic brain area. Contrary, in atypical antipsychotics extrapyramidal side effects are less common. However, in this study hypertonia is unlikely to be a side effect of the psychotropic drugs, as the majority of patients with hypertonia did not receive antipsychotics. Moreover, of the patients with hypertonia who did receive antipsychotics, these were mainly atypical antipsychotics instead of typical antipsychotics. Frontal release symptoms, which are reflected by the reoccurrence of neonatal reflexes, are associated with paratonia, suggesting involvement of the frontal lobe [72, 81]. Other studies hypothesized the possible engagement of the substantia nigra in the advanced disease stages of AD; however, analysis of the dopaminergic nigrostriatal function by use of PET [82] and brain autopsy [67] found no association. In addition to the probable causative central cerebral pathology for hypertonia, emerging evidence suggest that there might also be a contributing role for peripheral biomechanical alterations in form of accumulation of advanced glycation end-products (AGEs) possibly decreasing muscle elasticity and increasing rigidity [83].
In this study, analgesics were frequently prescribed for divergent causes, including pressure ulcers, contractures, and in the context of palliative care. Also, because most patients had a changed pain perception and were unable to express pain adequately due to cognitive impairment, the threshold for prescription of analgesics was low. In this manner, it was attempted to make the patient as comfortable as possible. Among analgesics, opiates were prescribed (predominately for providing palliative care). A common side effect of opiates is constipation. Therefore, all patients treated with opiates were simultaneously treated with laxatives (with the exception of 2 out 22 patients with constipation that were treated with opiates but not with laxatives) [84]. For this reason, it is implausible that the high prevalence of constipation in end stage bvFTD and yoAD was caused by opiates. On the other hand, it can be debated if thin defecation was a side effect of the laxatives. Further, benzodiazepines can have several side effects including sedation, sleepiness, and constipation. It is possible that the clinical features hypersomnia and constipation are partly a side effect of the benzodiazepines. However, both constipation [85] and sleep disorders including hypersomnia are reported features of (advanced) dementia [86].
Our findings irrefutably reflect their relevance in clinical practice. It is alarming that emerging studies [87, 88] report a low prescription of analgesic medication in dementia, even speculating that this might reflect an under treatment of pain. In addition to these facts, patients are frequently unable of expressing pain adequately due to cognitive or language impairment, where there is a possibility that there might even be an increased pain experience. Together this could result in a high underestimated disease burden for patients with dementia. As an extension of this, it is well acknowledged that gastrointestinal dysfunction negatively influences quality of life whereas it is considered as an often treatable condition [89]. Thus, awareness for autonomic function disorders, particularly a changed pain perception and gastrointestinal dysfunction, is highly important to take into account when developing evidence based (palliative) guidelines.
In the current study patients displayed contractures, which originated from immobility due to hypertonia, as the patient files revealed the inability of movement of the extremities due to the increased muscle tone. In the same manner hypertonia was a contributing factor for the development of pressure ulcers, functional decline, and providing difficulties in Activities of Daily Living care due to pain which are likewise highlighted in literature [68, 90, 91]. Hence, the development of effective treatment options is important, to avert secondary complications and decrease symptom burden of pain.
This study provides insight for both caregivers and family members in the broad clinical spectrum of the end stages of bvFTD and yoAD and accentuates the need for further investigation to validate these results. The dissimilarities of features in bvFTD and yoAD emphasize further research to evaluate if a different approach with regard to palliative guidelines is necessary. Our findings underline the importance to take both somatic, psychiatric, and neurological features into account in the end stage and to initiate further development of treatment strategies for professional caregivers. Moreover, monitoring the evolvement of these features in earlier stages might be beneficial. Hence, in this manner there can be anticipated with (non) pharmacological (preventive) treatment, thus enhancing quality of life for the patient as well as decreasing caregiver burden by optimizing treatment.
ACKNOWLEDGMENTS
Research of Alzheimer center Amsterdam is part of the neurodegeneration research program of Amsterdam Neuroscience. Alzheimer Center Amsterdam is supported by Stichting Alzheimer Nederland and Stichting VUmc fonds. There was no financial nor material support for this research nor there were any potential conflicts of interest. We thank the FTD expert group for their valuable input on the design of the study and the interpretation of the results. See https://www.ftdexpertgroep.nl. Also, we thank Craig Ball for his editorial contribution.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/20-0337r2).
REFERENCES
[1] | Ratnavalli E , Brayne C , Dawson K , Hodges J ((2002) ) The prevalence of frontotemporal dementia. Neurology 58: , 1615–1621. |
[2] | Rosso SM , Donker Kaat L , Baks T , Joosse M , de Koning I , Pijnenburg Y , de Jong D , Dooijes D , Kamphorst W , Ravid R , Niermeijer MF , Verheij F , Kremer HP , Scheltens P , van Duijn CM , Heutink P , van Swieten JC ((2003) ) Frontotemporal dementia in the Netherlands: Patient characteristics and prevalence estimates from a population-based study. Brain 126: , 2016–2022. |
[3] | Onyike CU , Diehl-Schmid J ((2013) ) The epidemiology of frontotemporal dementia. Int Rev Psychiatry 25: , 130–137. |
[4] | Piguet O , Hornberger M , Mioshi E , Hodges JR ((2011) ) Behavioural-variant frontotemporal dementia: Diagnosis, clinical staging, and management. Lancet Neurol 10: , 162–172. |
[5] | Devenney E , Bartley L , Hoon C , O’Callaghan C , Kumfor F , Hornberger M , Kwok JB , Halliday GM , Kiernan MC , Piguet O , Hodges JR ((2015) ) Progression in behavioral variant frontotemporal dementia: A longitudinal study. JAMA Neurol 72: , 1501–1509. |
[6] | Smits LL , van Harten AC , Pijnenburg YA , Koedam EL , Bouwman FH , Sistermans N , Reuling IE , Prins ND , Lemstra AW , Scheltens P , van der Flier WM ((2015) ) Trajectories of cognitive decline in different types of dementia. Psychol Med 45: , 1051–1059. |
[7] | Hodges J , Davies R , Xuereb J , Kril J , Halliday G ((2003) ) Survival in frontotemporal dementia. Neurology 61: , 349–354. |
[8] | Garcin B , Lillo P , Hornberger M , Piguet O , Dawson K , Nestor P , Hodges J ((2009) ) Determinants of survival in behavioral variant frontotemporal dementia. Neurology 73: , 1656–1661. |
[9] | Coyle-Gilchrist IT , Dick KM , Patterson K , Rodríquez PV , Wehmann E , Wilcox A , Lansdall CJ , Dawson KE , Wiggins J , Mead S ((2016) ) Prevalence, characteristics, and survival of frontotemporal lobar degeneration syndromes. Neurology 86: , 1736–1743. |
[10] | van Vliet D , de Vugt ME , Bakker C , Koopmans RT , Verhey FR ((2010) ) Impact of early onset dementia on caregivers: A review. Int J Geriatr Psychiatry 25: , 1091–1100. |
[11] | Lim L , Zhang A , Lim L , Choong TM , Silva E , Ng A , Kandiah N ((2018) ) High caregiver burden in young onset dementia: What factors need attention?. J Alzheimers Dis 61: , 537–543. |
[12] | Mioshi E , Foxe D , Leslie F , Savage S , Hsieh S , Miller L , Hodges JR , Piguet O ((2013) ) The impact of dementia severity on caregiver burden in frontotemporal dementia and Alzheimer disease. Alzheimer Dis Assoc Disord 27: , 68–73. |
[13] | Davis JD , Tremont G ((2007) ) Impact of frontal systems behavioral functioning in dementia on caregiver burden. J Neuropsychiatry Clin Neurosci 19: , 43–49. |
[14] | Armstrong N , Schupf N , Grafman J , Huey ED ((2013) ) Caregiver burden in frontotemporal degeneration and corticobasal syndrome. Dement Geriatr Cogn Disord 36: , 310–318. |
[15] | van Vliet D , de Vugt ME , Aalten P , Bakker C , Pijnenburg YA , Vernooij-Dassen MJ , Koopmans RT , Verhey FR ((2012) ) Prevalence of neuropsychiatric symptoms in young-onset compared to late-onset Alzheimer’s disease - part 1: Findings of the two-year longitudinal NeedYD-study. Dement Geriatr Cogn Disord 34: , 319–327. |
[16] | de Vugt ME , Riedijk SR , Aalten P , Tibben A , van Swieten JC , Verhey FR ((2006) ) Impact of behavioural problems on spousal caregivers: A comparison between Alzheimer’s disease and frontotemporal dementia. Dement Geriatr Cogn Disord 22: , 35–41. |
[17] | Bakker C , de Vugt ME , van Vliet D , Verhey FR , Pijnenburg YA , Vernooij-Dassen MJ , Koopmans RT ((2013) ) Predictors of the time to institutionalization in young- versus late-onset dementia: Results from the Needs in Young Onset Dementia (NeedYD) study. J Am Med Dir Assoc 14: , 248–253. |
[18] | van Duinen-van den IJCL , Mulders A , Smalbrugge M , Zwijsen SA , Appelhof B , Zuidema SU , de Vugt ME , Verhey FRJ , Bakker C , Koopmans R ((2018) ) Nursing staff distress associated with neuropsychiatric symptoms in young-onset dementia and late-onset dementia. J Am Med Dir Assoc 19: , 627–632. |
[19] | Mulders AJ , Zuidema SU , Verhey FR , Koopmans RT ((2014) ) Characteristics of institutionalized young onset dementia patients–the BEYOnD study. Int Psychogeriatr 26: , 1973–1981. |
[20] | Nijk RM , Zuidema SU , Koopmans RT ((2009) ) Prevalence and correlates of psychotropic drug use in Dutch nursing-home patients with dementia. Int Psychogeriatr 21: , 485–493. |
[21] | Koopmans RT , Reinders R , van Vliet D , Verhey FR , de Vugt ME , Bor H , Bakker C ((2014) ) Prevalence and correlates of psychotropic drug use in community-dwelling people with young-onset dementia: The NeedYD-study. Int Psychogeriatr 26: , 1983–1989. |
[22] | Mulders A , Zuidema SU , Leeuwis R , Bor H , Verhey FRJ , Koopmans R ((2019) ) Prevalence and correlates of psychotropic drug use in Dutch nursing home patients with young-onset dementia. Int J Geriatr Psychiatry 34: , 1185–1193. |
[23] | Appelhof B , Bakker C , Van Duinen-van den Ijssel JCL , Zwijsen SA , Smalbrugge M , Verhey FRJ , de Vugt ME , Zuidema SU , Koopmans R ((2017) ) The determinants of quality of life of nursing home residents with young-onset dementia and the differences between dementia subtypes. Dement Geriatr Cogn Disord 43: , 320–329. |
[24] | Diehl-Schmid J , Pohl C , Perneczky R , Hartmann J , Förstl H , Kurz A ((2007) ) Initial symptoms, survival and causes of death in 115 patients with frontotemporal lobar degeneration. Fortschr Neurol Psychiatr 75: , 708–713. |
[25] | Diehl-Schmid J , Pohl C , Perneczky R , Forstl H , Kurz A ((2006) ) Behavioral disturbances in the course of frontotemporal dementia. Dement Geriatr Cogn Disord 22: , 352–357. |
[26] | Mioshi E , Hsieh S , Savage S , Hornberger M , Hodges JR ((2010) ) Clinical staging and disease progression in frontotemporal dementia. Neurology 74: , 1591–1597. |
[27] | Chow TW , Fridhandler JD , Binns MA , Lee A , Merrilees J , Rosen HJ , Ketelle R , Miller BL ((2012) ) Trajectories of behavioral disturbance in dementia. J Alzheimers Dis 31: , 143–149. |
[28] | O’Connor CM , Clemson L , Hornberger M , Leyton CE , Hodges JR , Piguet O , Mioshi E ((2016) ) Longitudinal change in everyday function and behavioral symptoms in frontotemporal dementia. Neurol Clin Pract 6: , 419–428. |
[29] | Kazui H , Yoshiyama K , Kanemoto H , Suzuki Y , Sato S , Hashimoto M , Ikeda M , Tanaka H , Hatada Y , Matsushita M , Nishio Y , Mori E , Tanimukai S , Komori K , Yoshida T , Shimizu H , Matsumoto T , Mori T , Kashibayashi T , Yokoyama K , Shimomura T , Kabeshita Y , Adachi H , Tanaka T ((2016) ) Differences of behavioral and psychological symptoms of dementia in disease severity in four major dementias. PLoS One 11: , e0161092. |
[30] | Diehl-Schmid J , Richard-Devantoy S , Grimmer T , Forstl H , Jox R ((2017) ) Behavioral variant frontotemporal dementia: Advanced disease stages and death. A step to palliative care. Int J Geriatr Psychiatry 32: , 876–881. |
[31] | Shega JW , Hougham GW , Stocking CB , Cox-Hayley D , Sachs GA ((2008) ) Patients dying with dementia: Experience at the end of life and impact of hospice care. J Pain Symptom Manage 35: , 499–507. |
[32] | Mitchell SL , Teno JM , Kiely DK , Shaffer ML , Jones RN , Prigerson HG , Volicer L , Givens JL , Hamel MB ((2009) ) The clinical course of advanced dementia. N Engl J Med 361: , 1529–1538. |
[33] | van der Steen JT ((2010) ) Dying with dementia: What we know after more than a decade of research. J Alzheimers Dis 22: , 37–55. |
[34] | Pasquier F , Richard F , Lebert F ((2004) ) Natural history of frontotemporal dementia: Comon with Alzheimer’s disease. Dement Geriatr Cogn Disord 17: , 253–257. |
[35] | Creswell JW ((2014) ) A concise introduction to mixed methods research, SAGE publications. |
[36] | Creswell JW , Klassen AC , Plano Clark VL , Smith KC ((2013) ) Best practices for mixed methods research in the health sciences. National Institutes of Health, Bethesda, pp. 541-545. |
[37] | Rascovsky K , Hodges JR , Knopman D , Mendez MF , Kramer JH , Neuhaus J , van Swieten JC , Seelaar H , Dopper EG , Onyike CU , Hillis AE , Josephs KA , Boeve BF , Kertesz A , Seeley WW , Rankin KP , Johnson JK , Gorno-Tempini ML , Rosen H , Prioleau-Latham CE , Lee A , Kipps CM , Lillo P , Piguet O , Rohrer JD , Rossor MN , Warren JD , Fox NC , Galasko D , Salmon DP , Black SE , Mesulam M , Weintraub S , Dickerson BC , Diehl-Schmid J , Pasquier F , Deramecourt V , Lebert F , Pijnenburg Y , Chow TW , Manes F , Grafman J , Cappa SF , Freedman M , Grossman M , Miller BL ((2011) ) Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134: , 2456–2477. |
[38] | McKhann GM , Knopman DS , Chertkow H , Hyman BT , Jack CR Jr, Kawas CH , Klunk WE , Koroshetz WJ , Manly JJ , Mayeux R , Mohs RC , Morris JC , Rossor MN , Scheltens P , Carrillo MC , Thies B , Weintraub S , Phelps CH ((2011) ) The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: , 263–269. |
[39] | Reisberg B , Ferris SH , de Leon MJ , Crook T ((1982) ) The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry 139: , 1136–1139. |
[40] | Ahronheim JC , Morrison RS , Baskin SA , Morris J , Meier DE ((1996) ) Treatment of the dying in the acute care hospital: Advanced dementia and metastatic cancer. Arch Intern Med 156: , 2094–2100. |
[41] | McCarthy M , Addington-Hall J , Altmann D ((1997) ) The experience of dying with dementia: A retrospective study. Int J Geriatr Psychiatry 12: , 404–409. |
[42] | Morrison RS , Siu AL ((2000) ) Survival in end-stage dementia following acute illness. JAMA 284: , 47–52. |
[43] | Meier DE , Ahronheim JC , Morris J , Baskin-Lyons S , Morrison RS ((2001) ) High short-term mortality in hospitalized patients with advanced dementia: Lack of benefit of tube feeding. Arch Intern Med 161: , 594–599. |
[44] | Allen RS , Kwak J , Lokken KL , Haley WE ((2003) ) End-of life issues in the context of Alzheimer’s disease. Alzheimers Care Quarterly 4: , 312. |
[45] | Volicer L , Hurley A ((2004) ) Hospice care for patients with advanced progressive dementia, Springer Publishing Company. |
[46] | Mitchell SL , Kiely DK , Hamel MB ((2004) ) Dying with advanced dementia in the nursing home. Arch Intern Med 164: , 321–326. |
[47] | Aminoff BZ , Adunsky A ((2006) ) Their last 6 months: Suffering and survival of end-stage dementia patients. Age Ageing 35: , 597–601. |
[48] | Aminoff BZ , Adunsky A ((2005) ) Dying dementia patients: Too much suffering, too little palliation. Am J Hosp Palliat Care 22: , 344–348. |
[49] | Black BS , Finucane T , Baker A , Loreck D , Blass D , Fogarty L , Phillips H , Hovanec L , Steele C , Rabins PV ((2006) ) Health problems and correlates of pain in nursing home residents with advanced dementia. Alzheimer Dis Assoc Disord 20: , 283–290. |
[50] | Chen JH , Lamberg JL , Chen YC , Kiely DK , Page JH , Person CJ , Mitchell SL ((2006) ) Occurrence and treatment of suspected pneumonia in long-term care residents dying with advanced dementia. J Am Geriatr Soc 54: , 290–295. |
[51] | Mitchell SL , Kiely DK , Jones RN , Prigerson H , Volicer L , Teno JM ((2006) ) Advanced dementia research in the nursing home: The CASCADE study. Alzheimer Dis Assoc Disord 20: , 166. |
[52] | Di Giulio P , Toscani F , Villani D , Brunelli C , Gentile S , Spadin P ((2008) ) Dying with advanced dementia in long-term care geriatric institutions: A retrospective study. J Palliat Med 11: , 1023–1028. |
[53] | Givens JL , Kiely DK , Carey K , Mitchell SL ((2009) ) Healthcare proxies of nursing home residents with advanced dementia: Decisions they confront and their satisfaction with decision-making. J Am Geriatr Soc 57: , 1149–1155. |
[54] | Hendriks SA , Smalbrugge M , Hertogh CM , van der Steen JT ((2014) ) Dying with dementia: Symptoms, treatment, and quality of life in the last week of life. J Pain Symptom Manage 47: , 710–720. |
[55] | Morris JC ((1997) ) Clinical dementia rating: A reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr 9: , 173–176. |
[56] | Carlino E , Benedetti F , Rainero I , Asteggiano G , Cappa G , Tarenzi L , Vighetti S , Pollo A ((2010) ) Pain perception and tolerance in patients with frontotemporal dementia. Pain 151: , 783–789. |
[57] | Rainero I , Vighetti S , Bergamasco B , Pinessi L , Benedetti F ((2000) ) Autonomic responses and pain perception in Alzheimer’s disease. Eur J Pain 4: , 267–274. |
[58] | Fletcher PD , Downey LE , Golden HL , Clark CN , Slattery CF , Paterson RW , Rohrer JD , Schott JM , Rossor MN , Warren JD ((2015) ) Pain and temperature processing in dementia: A clinical and neuroanatomical analysis. Brain 138: , 3360–3372. |
[59] | Bathgate D , Snowden J , Varma A , Blackshaw A , Neary D ((2001) ) Behaviour in frontotemporal dementia, Alzheimer’s disease and vascular dementia. Acta Neurol Scand 103: , 367–378. |
[60] | Snowden JS , Bathgate D , Varma A , Blackshaw A , Gibbons ZC , Neary D ((2001) ) Distinct behavioural profiles in frontotemporal dementia and semantic dementia. J Neurol Neurosurg Psychiatry 70: , 323–332. |
[61] | Ahmed RM , Iodice V , Daveson N , Kiernan MC , Piguet O , Hodges JR ((2015) ) Autonomic dysregulation in frontotemporal dementia. J Neurol Neurosurg Psychiatry 86: , 1048–1049. |
[62] | Ahmed RM , Ke YD , Vucic S , Ittner LM , Seeley W , Hodges JR , Piguet O , Halliday G , Kiernan MC ((2018) ) Physiological changes in neurodegeneration - mechanistic insights and clinical utility. Nat Rev Neurol 14: , 259–271. |
[63] | Ahmed RM , Landin-Romero R , Collet TH , van der Klaauw AA , Devenney E , Henning E , Kiernan MC , Piguet O , Farooqi IS , Hodges JR ((2017) ) Energy expenditure in frontotemporal dementia: A behavioural and imaging study. Brain 140: , 171–183. |
[64] | Guo CC , Sturm VE , Zhou J , Gennatas ED , Trujillo AJ , Hua AY , Crawford R , Stables L , Kramer JH , Rankin K , Levenson RW , Rosen HJ , Miller BL , Seeley WW ((2016) ) Dominant hemisphere lateralization of cortical parasympathetic control as revealed by frontotemporal dementia. Proc Natl Acad Sci U S A 113: , E2430–2439. |
[65] | Hoefer M , Allison SC , Schauer GF , Neuhaus JM , Hall J , Dang JN , Weiner MW , Miller BL , Rosen HJ ((2008) ) Fear conditioning in frontotemporal lobar degeneration and Alzheimer’s disease. Brain 131: , 1646–1657. |
[66] | Werner KH , Roberts NA , Rosen HJ , Dean DL , Kramer JH , Weiner MW , Miller BL , Levenson RW ((2007) ) Emotional reactivity and emotion recognition in frontotemporal lobar degeneration. Neurology 69: , 148–155. |
[67] | Risse SC , Lampe TH , Bird TD , Nochlin D , Sumi SM , Keenan T , Cubberley L , Peskind E , Raskind MA ((1990) ) Myoclonus, seizures, and paratonia in Alzheimer disease. Alzheimer Dis Assoc Disord 4: , 217–225. |
[68] | Souren LE , Franssen EH , Reisberg B ((1997) ) Neuromotor changes in Alzheimer’s disease: Implications for patient care. J Geriatr Psychiatry Neurol 10: , 93–98. |
[69] | Pasquier F , Lebert F , Lavenu I , Guillaume B ((1999) ) The clinical picture of frontotemporal dementia: Diagnosis and follow-up. Dement Geriatr Cogn Disord 10: , 10–14. |
[70] | Hobbelen JHS , Koopmans RT , Verhey FR , Van Peppen RP , de Bie RA ((2006) ) Paratonia: A Delphi procedure for consensus definition. J Geriatr Phys Ther 29: , 50–56. |
[71] | Hobbelen JS , Tan FE , Verhey FR , Koopmans RT , de Bie RA ((2011) ) Prevalence, incidence and risk factors of paratonia in patients with dementia: A one-year follow-up study. Int Psychogeriatr 23: , 1051–1060. |
[72] | Vahia I , Cohen CI , Prehogan A , Memon Z ((2007) ) Prevalence and impact of paratonia in Alzheimer disease in a multiracial sample. Am J Geriatr Psychiatry 15: , 351–353. |
[73] | Van Deun B , Van Den Noortgate N , Van Bladel A , Palmans T , Cambier D ((2019) ) The impact of paratonia on fine and gross motor function in older adults with mild and moderate dementia. Alzheimer Dis Assoc Disord 33: , 54–61. |
[74] | Hodges J ((2012) ) Familial frontotemporal dementia and amyotrophic lateral sclerosis associated with the C9ORF72 hexanucleotide repeat. Brain 135: , 652–655. |
[75] | Jones SE ((2011) ) Imaging for autonomic dysfunction. Cleve Clin J Med 78: (Suppl 1), S69–74. |
[76] | Idiaquez J , Roman GC ((2011) ) Autonomic dysfunction in neurodegenerative dementias. J Neurol Sci 305: , 22–27. |
[77] | Issac TG , Chandra SR , Gupta N , Rukmani MR , Deepika S , Sathyaprabha T ((2017) ) Autonomic dysfunction: A comparative study of patients with Alzheimer’s and frontotemporal dementia–A pilot study. J Neurosci Rural Pract 8: , 84. |
[78] | Seeley WW , Carlin DA , Allman JM , Macedo MN , Bush C , Miller BL , DeArmond SJ ((2006) ) Early frontotemporal dementia targets neurons unique to apes and humans. Ann Neurol 60: , 660–667. |
[79] | Yang Y , Halliday GM , Hodges JR , Tan RH ((2017) ) von Economo neuron density and thalamus volumes in behavioral deficits in frontotemporal dementia cases with and without a C9ORF72 repeat expansion. J Alzheimers Dis 58: , 701–709. |
[80] | Fuchs PN , Peng YB , Boyette-Davis JA , Uhelski ML ((2014) ) The anterior cingulate cortex and pain processing. Front Integr Neurosci 8: , 35. |
[81] | Beversdorf DQ , Heilman KM ((1998) ) Facilitory paratonia and frontal lobe functioning. Neurology 51: , 968–971. |
[82] | Tyrrell PJ , Sawle GV , Ibanez V , Bloomfield PM , Leenders KL , Frackowiak RS , Rossor MN ((1990) ) Clinical and positron emission tomographic studies in the’extrapyramidal syndrome’of dementia of the Alzheimer type. Arch Neurol 47: , 1318–1323. |
[83] | Drenth H , Zuidema SU , Krijnen WP , Bautmans I , van der Schans C , Hobbelen H ((2017) ) Advanced glycation end-products are associated with the presence and severity of paratonia in early stage Alzheimer disease. J Am Med Dir Assoc 18: , 636 e637–636 e612. |
[84] | Rao VL , Micic D , Davis AM ((2019) ) Medical management of opioid-induced constipation. JAMA 322: , 2241–2242. |
[85] | Allan L , McKeith I , Ballard C , Kenny RA ((2006) ) The prevalence of autonomic symptoms in dementia and their association with physical activity, activities of daily living and quality of life. Dement Geriatr Cogn Disord 22: , 230–237. |
[86] | Peter-Derex L , Yammine P , Bastuji H , Croisile B ((2015) ) Sleep and Alzheimer’s disease. Sleep Med Rev 19: , 29–38. |
[87] | Monroe TB , Misra SK , Habermann RC , Dietrich MS , Cowan RL , Simmons SF ((2014) ) Pain reports and pain medication treatment in nursing home residents with and without dementia. Geriatr Gerontol Int 14: , 541–548. |
[88] | Tan EC , Jokanovic N , Koponen MP , Thomas D , Hilmer SN , Bell JS ((2015) ) Prevalence of analgesic use and pain in people with and without dementia or cognitive impairment in aged care facilities: A systematic review and meta-analysis. Curr Clin Pharmacol 10: , 194–203. |
[89] | Belsey J , Greenfield S , Candy D , Geraint M ((2010) ) Systematic review: Impact of constipation on quality of life in adults and children. Aliment Pharmacol Ther 31: , 938–949. |
[90] | Van Deun B , Van den Noortgate N , Cinthia S , Van Bladel A , Dirk C ((2018) ) Paratonia in Flemish nursing homes: Current state of practice. Am J Alzheimers Dis Other Demen 33: , 205–214. |
[91] | Souren LE , Franssen EH , Reisberg B ((1995) ) Contractures and loss of function in patients with Alzheimer’s disease. J Am Geriatr Soc 43: , 650–655. |
[92] | Verhage F ((1965) ) Intelligence and age in a Dutch sample. Hum Dev 8: , 238–245. |




