Impact of 3-Day Combined Anodal Transcranial Direct Current Stimulation-Visuospatial Training on Object-Location Memory in Healthy Older Adults and Patients with Mild Cognitive Impairment
Abstract
Background:
Associative object-location memory (OLM) is known to decline even in normal aging, and this process is accelerated in patients with mild cognitive impairment (MCI). Given the lack of curative treatment for Alzheimer’s disease, activating cognitive resources during its preclinical phase might prevent progression to dementia.
Objective:
To evaluate the effects of anodal transcranial direct current stimulation (atDCS) combined with an associative episodic memory training on OLM in MCI patients and in healthy elderly (HE).
Methods:
In a single-blind cross-over design, 16 MCI patients and 32 HE underwent a 3-day visuospatial OLM training paired with either 20 min or 30 s (sham) atDCS (1 mA, right temporoparietal cortex). Effects on immediate (training success) and long-term memory (1-month) were investigated by conducting Mixed Model analyses. In addition, the impact of combined intervention on within-session (online) and on between-session (offline) performance were explored.
Results:
OLM training+atDCS enhanced training success only in MCI patients, but not HE (difference n.s.). Relative performance gain was similar in MCI patients compared to HE under atDCS. No beneficial effect was found after 1-month. Exploratory analyses suggested a positive impact on online, but a negative effect on offline performance in MCI patients. In both groups, exploratory post-hoc analyses indicated an association between initially low-performers and greater benefit from atDCS.
Conclusion:
Cognitive training in MCI may be enhanced by atDCS, but further delineation of the impact of current brain state, as well as temporal characteristics of multi-session atDCS-training application, may be needed to induce longer-lasting effects.
INTRODUCTION
Cognitive decline leading to dementia is one of the most devastating aspects of aging as it threatens independent functioning, and severely decreases quality of life [1]. Even during normal aging, episodic memory tends to decline [2], a process that is accelerated in pathological conditions like mild cognitive impairment (MCI) and Alzheimer’s disease (AD) [3]. One aspect of episodic memory is visuospatial memory, for instance acquired during object-location learning. This object-location memory (OLM) is an integral part of everyday life, including for example the task to find one’s way around a new town, or to remember where the car was parked some hours earlier. Of note, deficits in visuospatial memory may even precede deficits in verbal memory, but have received much less attention so far in the context of studies [4]. Specifically, the binding process in terms of integration of object (visual) and location (spatial) information to form OLM might be affected in earliest stages of a disease that compromises hippocampus and adjacent structures [5–9]. Due to the lack of curative treatment for AD [10], neuro-enhancing strategies aiming to activate respective cognitive functions and brain resources at an early stage of the disease process are of utmost importance [11]. Such strategies could help to counteract memory decline and to prevent or slow progression of MCI to dementia [12].
Cognitive training (CT) might be a promising approach as it beneficially modulates plasticity of the brain [13] and does not carry the risk of severe adverse events [14]. CT usually involves stimulation and strengthening of pre-existing cognitive reserve, and reorganization of neuronal circuits implicated with task demands [15]. However, given that studies using CT have shown only small to moderate effects in both healthy older adults (healthy elderly, HE) [16, 17] and older patients with MCI [18–20], CT may be combined with other neuroplasticity-enhancing techniques in order to boost the training effect [21]. Specifically, anodal transcranial direct current stimulation (atDCS) has been suggested to foster not only immediate (online) effects via subthreshold alterations of resting membrane potential [22], but also to induce prolonged after-effects, possibly by modulating long-term-potentiation (LTP)-like processes. Such after-effects may underlie the offline gains (consolidation processes) in performance reported in several previous studies [23–26]. Thus, combined with CT, atDCS might influence neural activity in networks engaged during training, enhancing the synaptic strength of neurons. Repeated application of a combined training-atDCS approach may result in cumulative behavioral effects over consecutive sessions [24, 27].
So far, few studies involving multi-day atDCS application in combination with CT have been reported in older adults. Most of them focused on HE and evaluated atDCS effects on working memory [28–30]. Importantly, results have not been unequivocal, with some reports indicating beneficial effects of combined intervention [31–33], while others did not [34, 35]. Factors contributing to these conflicting findings may include variations between study populations in terms of age, gender, education, health status, genetic background, brain state, or electrode montage [21, 36]. In addition, given that atDCS is considered a weak form of modulation, it may be most effective at near-threshold (fragile) performance level [37]. Moreover, it may show the largest benefit in individuals with rather low baseline performance [38–41]. Thus, MCI patients, in whom low memory performance is a hallmark of the clinical symptomatology, may be particularly susceptible to the beneficial effects of atDCS. Encouraging results have been reported for repeated atDCS in MCI due to AD [42, 43] and atDCS combined with CT in MCI due to idiopathic Parkinson’s disease [44, 45], but a negative effect has been reported in a study applying atDCS before CT in MCI due to AD [46]. Conflicting results might result from heterogeneity between stimulation protocols, e.g., application of atDCS before versus during training [38, 47]. Most of these studies did not directly compare the impact of stimulation in MCI with the impact in a healthy control group, though. Interestingly, Meinzer et al. found that atDCS could improve performance of MCI patients in a semantic word retrieval task to the level of HE [48]. Although this evidence is preliminary, any possible restoring effect may be of clinical relevance and warrants further investigation.
The sham-controlled study was based on the rationale 1) that task-induced engagement of target brain area during stimulation is important for modulating behavior [49, 50], and 2) that stimulating key nodes of a given network would suffice to active this network [51]. Given the central role of hippocampus [52] and the right temporoparietal cortex [25, 53] in OLM, atDCS was applied over right temporoparietal cortex during a 3-day OLM training aiming to stimulate one node of a broader network that is highly interconnected to the hippocampus [54]. The impact of joint intervention on training success in MCI was directly compared to the impact of stimulation in HE (data of HE was reported previously [21]; in brief, no difference between stimulation conditions was found). Memory strengthening induced by training can be described as improved learning and retention [55]. Training success (primary outcome) was operationalized as increase in performance assessed immediately after the end of multi-day training, assuming that it reflects peak performance (and thus training success) after training [55]. This score comprises different processes such as learning, consolidation, reconsolidation, and forgetting. Moreover, the impact of training with atDCS or sham stimulation on memory was assessed after one month (secondary outcome) to investigate long-term effect of combined intervention. According to the seminal study from Reis et al. [24], we further explored the impact of atDCS (relative to sham) on different temporal components of learning and memory. Thus, we tried to evaluate the influence of atDCS separately on immediate (within-session ‘online’ effect) and prolonged after-effects (between-session ‘offline’ effect) on training performances.
We hypothesized that in MCI patients, performance immediately after the end of training (training success) would be significantly higher in the OLM training+atDCS group, as compared to the OLM training+sham group, and that this difference would be retained one month later (long-term memory). Given the role of sleep in memory consolidation [56] and a possible modulation of emotional state by tDCS [57] which in turn might affect memory processing [58], subjective sleep and affective state were monitored throughout the training. Moreover, in an exploratory approach, we aimed to assess if atDCS differently modulates on- and offline effects in this cognitive task, possibly with a greater positive impact on offline effects.
METHODS
Participants
Data reported here were assessed during a randomized interventional trial comprising elderly subjects with MCI (registered at https://clinicaltrials.gov: NCT02110043, https://clinicaltrials.gov/show/NCT02110043). Data were compared with previously reported data of HE (NCT02110056, https://clinicaltrials.gov/show/NCT02110056 [21]), who underwent the identical study protocol. Participants signed informed written consent prior to the first study related assessment and received a small compensation for study participation. The studies were carried out between 2014–2017 at the Department of Neurology at Charit
In total, 31 MCI patients (amnestic: single and multiple domain), aged 50–90 years, fluent German speakers, were recruited from the memory clinic of the Department of Neurology, Charit
HE (n = 56) were recruited via advertisements in Berlin, Germany. They were native German speakers between 50-90 years, without subjective cognitive complaints, or objective cognitive impairment as indicated by results of CERAD. For a complete description of HE, see Külzow et al. [21]. Exclusion criteria comprised severe untreated neurological or psychiatric disorders, e.g., epilepsy, manifest dementia, and major brain pathologies like brain tumor or previous stroke identified in the MRI scan.
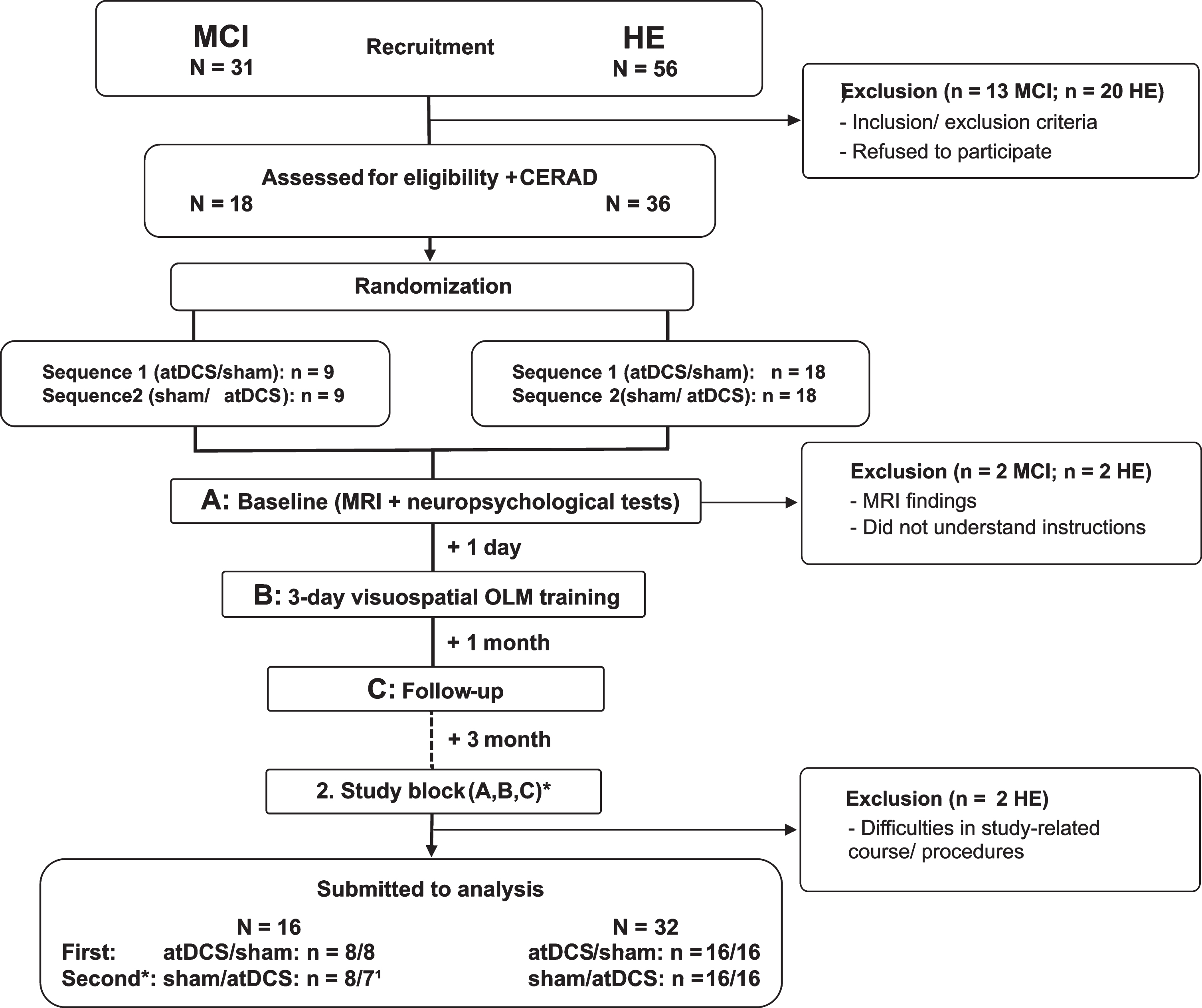
From 31 recruited MCI patients and 56 recruited HE, 11 MCI patients and 19 HE declined participation because of time constraints, as well as 2 MCI patients and 1 HE did not meet inclusion criteria. From the remaining 18 MCI patients and 36 HE, 2 MCI patients and 4 HE had to be excluded due to MRI findings (nMCI =1; nHE =2), and problems during training sessions (nMCI = 1; nHE = 2), thus leaving 16 MCI patients (5 females) and 32 HE (22 females) for analysis (see Fig. 1).
Fig.1
Flowchart of cross-over studies including patients with mild cognitive impairment (MCI) and healthy elderly (HE). Thirty-one patients with mild cognitive impairment (MCI) and 56 healthy elderly (HE) were recruited and pre-screened. 13 MCI patients and 20 HE were excluded due to refusal or study-related constraints. One MCI patient and 2 HE matched exclusion criteria, and 1 MCI patient and 2 HE had to be excluded due to other difficulties, leaving 16 MCI patients and 32 HE for analysis. Participants were randomly assigned to either anodal transcranial direct stimulation (atDCS) or sham stimulation (sham) condition, which was applied simultaneous to training. *In the second study block, the same procedures were conducted, but according to a cross-over design training was done under the other (not yet applied) stimulation condition. 1Due to technical problems one MCI patient did not received the same training versions across training days during the second study block. Thus, performance data has to be excluded from analysis. Hence, n for MCI patients differed between study blocks.
Mean age of MCI patients was 70 years (SD: 6). They did not significantly differ from HE in terms of age (69 years old; SD: 7) and education (15 years; SD: 3). To characterize MCI patients and HE with regard to cognitive status, emotional state, and other psychological factors, subjects underwent a comprehensive standardized test battery at baseline. In addition, blood samples were drawn at baseline visit to determine genotypes of learning relevant polymorphisms (ApoE4, COMT Val158Met, BDNF Val66Met). Genotyping on coded samples was performed by the laboratory of Prof. Dr. Dan Rujescu, University Halle, Germany. Baseline characteristics of the study sample are described in details in Table 1.
Table 1
Characteristics, screening- and baseline assessments of older adults with (MCI) and without (HE) memory impairment
| MCI | HE | p | |
| n - men/women | 11/5 | 10/22 | |
| Age in years | 70 (6) | 69 (7) | 0.49 |
| Education in years | 15 (3)e | 15 (3)a | 0.46 |
| Oldfield Handedness | 88 (28)e | 97 (11)c | 0.13 |
| Polymorphisms | |||
| ApoE genotype ɛ4 allele carriers - n (%) | 6 (61%)f | 9 (28%) | 0.24 |
| BDNF met allele - N (%) | 4 (31%)f | 10 (32%)a | 0.92 |
| COMT met allele - N (%) | 12 (75%)f | 24 (75%) | 0.19 |
| CERAD subtests | |||
| Boston Naming Test | 13.8 (1.0) | 14.5 (0.84) | 0.15 |
| Words (learning) | 16.7 (3.9) | 22.1 (4.14)d | <0.001 |
| Figure (drawing) | 10.2 (1.1) | 10.7 (0.60)a | 0.70 |
| Words - recall | 5.6 (2.5) | 8.1 (1.84) | <0.001 |
| Figure - recall | 8.4 (3.2) | 9.5 (2.17)a | 0.21 |
| MMSE | 27.7 (1.8) | 29.1 (1.25) | 0.02 |
| Baseline assessment | |||
| Cognitive function | |||
| TMT-A (s) | 51.7 (19.9) | 42.3 (13.7) | 0.03 |
| TMT-B (s) | 128.1 (60.1) | 77.9 (26.8) | <0.001 |
| TMT-B/TMT-A | 2.57 (1.1) | 1.9 (0.6) | 0.04 |
| Digit Span | |||
| forward | 7.1 (1.8) | 8.7 (1.9)a | 0.02 |
| backward | 6.1 (2.1) | 6.6 (2.1)a | 0.42 |
| Verbal Fluency | |||
| S | 12.2 (4.4) | 17.6 (4.7) | 0.001 |
| M | 12.4 (5.4) | 14.8 (4.7) | 0.13 |
| G-R | 11.9 (4.3) | 15.6 (4.8) | <0.001 |
| Animals | 16.9 (3.2) | 23.6 (5.9) | <0.001 |
| Sport-fruits | 10.8 (2.4) | 15.1 (2.7) | <0.001 |
| MWT | 30.7 (3.7) | 32.9 (1.8) | 0.03 |
| LOCATO-15 learning | 49.7 (9.2) e | 57.4 (9.7) | 0.02 |
| LOCATO-15 cued recall | 42.5 (11.1)e | 51.0 (16.9) | 0.16 |
| Non-cognitive functions | |||
| PANAS positive score | 32.7 (4.3) | 34.2 (7.4) | 0.45 |
| PANAS negative score | 12.6 (4.4) | 11.1 (2.5) | 0.15 |
| BDI | 7.4 (4.8) | 3.4 (2.7)a | 0.001 |
| Quality of life: WHOQoL | |||
| Physical | 78. (12.7) | 84.9 (12.6) | 0.09 |
| Psychological | 69.3 (13.9) | 78.9 (10.0) | 0.002 |
| Social | 69.3 (15.7) | 72.3 (14.8) | 0.19 |
| Environmental | 79.8 (14.4) | 82.3 (10.2) | 0.48 |
| Overall score | 68.7 (12.9) | 75.0 (14.4)a | 0.06 |
| PSQI (sleep) | 5.3 (3.6) | 5.2 (3.1)b | 0.66 |
| Coping with stress (SVF) | |||
| Positive strategies | 13.2 (2.5) | 15.9 (15.4) | 0.48 |
| Negative strategies | 8.1 (3.1) | 9.1 (16.7) | 0.82 |
Data are given as mean and standard deviations otherwise mentioned. In some parameters N is reduced due to missing data: na = 31, nb = 29, nc = 26, nd = 23, ne = 15, nf = 13. ApoE genotype ɛ4 allele carriers (Apolipoprotein E-DNA), BDNF (Brain derived neurotropic factor) and COMT (Catechol-O-Methyl-Transferase) were extracted from whole blood using a blood mini-kit (Qiagen, Hilden, Germany); Oldfield handedness [64]; CERAD, Consortium to Establish a Registry for Alzheimer’s Disease test battery (Memory Clinic Basel, www.memoryclinic.ch); MMSE, Mini-Mental State Examination scores [63]; TMT, Trail Making Test [65]; Digit span [66]; Verbal Fluency, Regensburger Verbal Fluency Test [67]; MWT, Multiple-Choice Vocabulary Intelligence Test [68]; PANAS, Positive and Negative Affective Schedule [69|; BDI, Becks depression inventory 70]; WHOQoL,WHO Quality of life [71]; PSQI, habitual sleep score (Pittsburgh Sleep Quality) [72]; SVF120, stress coping strategies - habitual form [73].
Experimental design and procedures
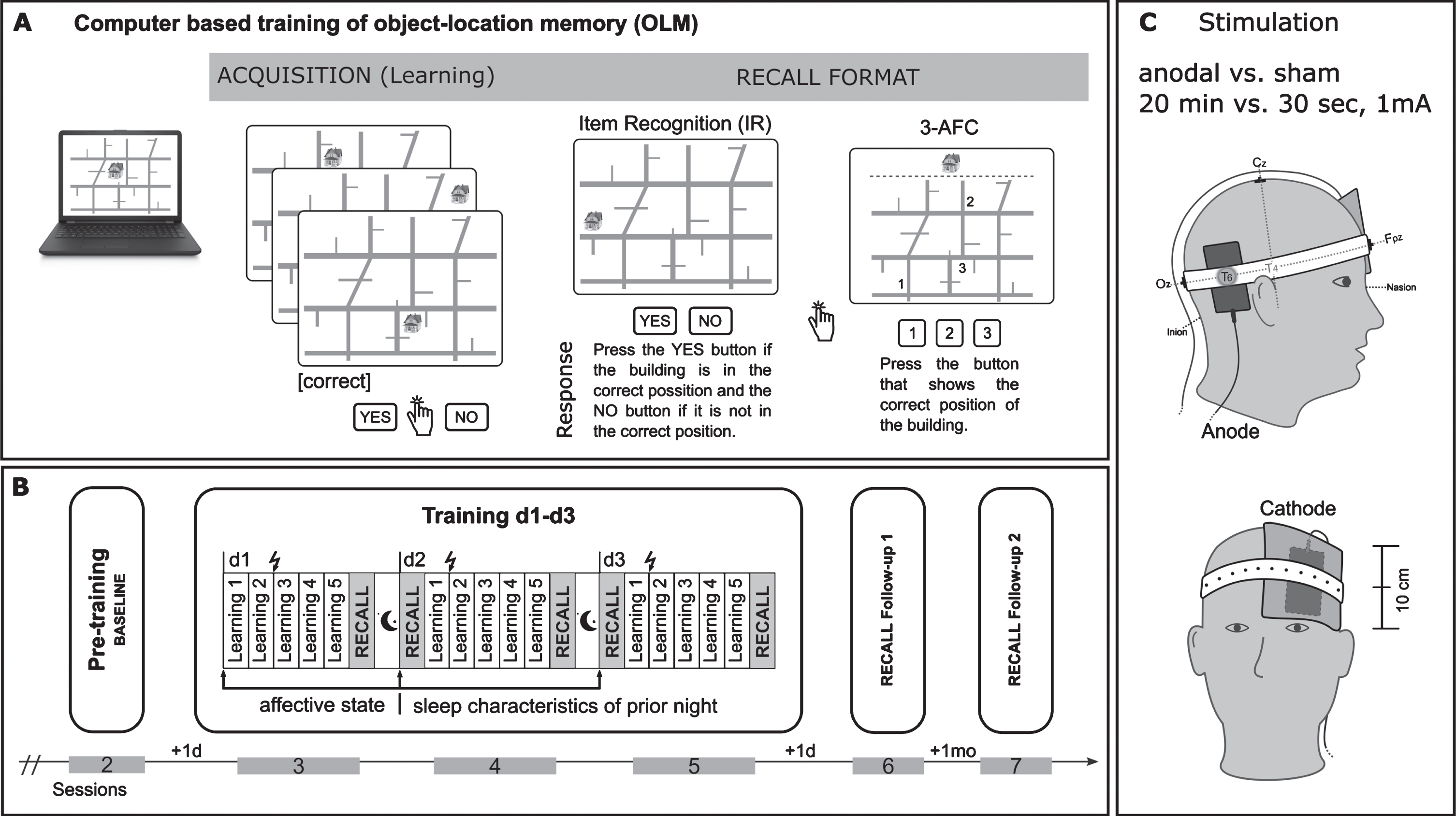
This study was conducted in a counterbalanced subject-blind placebo-controlled cross-over design involving 2 blocks of testing, separated by a period of 3 months to prevent carry-over effects (see also Fig. 1). Each block comprised a 3-day visuospatial training task called LOCATO (see Fig. 2A) [21] paired with either atDCS (20 min, 1 mA) or sham (30 s) stimulation (see Fig. 2 C). Conditions were applied in randomized order. OLM performance (recall) was also tested in follow-up session with 1-day (FU1) and 1-month (FU2) delay after training (see Fig. 2B).
Fig.2
Overview of procedures related to associative object-location memory paradigm (LOCATO). A) Learning task (acquisition) and recall format (Item Recognition: IR and 3-Alternative Forced Choice: 3-AFC). B) Distribution of learning blocks over the training days, pre-training baseline assessment and follow-up post training measurements (RECALL Follow-up 1,+1 d: after 1 day; RECALL Follow-up 2,+1 mo: after 1-month); each study block comprised 6 sessions (2–7; first session (not shown)) with 3 months in-between. Training (session 3–5) consisted of three consecutive days, each comprised five learning blocks and subsequent cued recall test (IR, 3-AFC). C) Electrodes positioning.
Training task
Visuospatial memory training was administered using the OLM paradigm [25, 74], which was adopted for use in a 3-day OLM training (LOCATO-30) and is described in detail elsewhere [21]. In brief, subjects had to learn 30 object-location pairings on a two-dimensional street map over the course of five learning blocks at each day, followed by a recall task (see also Fig. 2A). Buildings occurred on their “correct” and “incorrect” locations. The “correct” object-location pairings were repeated and were shown more often (occurred twice within each learning block) than “incorrect” pairings (presented once during whole training). In each learning block subjects responded to 120 object-location pairings (2 x 30 “correct”, 60 “incorrect” pairings) by button press (‘YES’, ‘NO’) on a response pad as accurate as possible to indicate whether the buildings were presented at their “correct” or “incorrect” location. Immediately after five learning blocks recall performance was tested using two cued recall tests. To avoid contaminations due to task order, and to reduce overall testing time, 50% of associations were tested via item recognition (IR) and 50% via 3-alternative forced choice (3-AFC) task. For IR 15 correct object-location pairings were intermixed with 15 (not shown before) incorrect pairings. Stimulus presentation was identical to learning blocks and subjects responded by button press whether the position was “correct” (‘YES’) or not (‘NO’). In the subsequent 3-AFC test three possible locations for a particular building were shown on the street map marked with “1,” “2,” and “3” and subjects chose the “correct” location by pressing the corresponding number on the keyboard. Neither in learning trials nor during cued recall tests a performance feedback was provided. Two parallel versions (A, B) of LOCATO-30 were used, each one with a different set of buildings, and with the street map rotated for 180° for version B. Versions were assigned in counterbalanced manner to respective intervention. LOCATO-30 was presented on a laptop (14.17 X 8 inches) using Presentation Software (Neurobehavioral Systems, Albany, CA, USA).
Brain stimulation
atDCS was delivered by a direct current stimulator (NeuroConn GmbH, Ilmenau, Germany) using two saline-soaked synthetic surface sponge electrodes and were attached on the scalp by rubber bands. The active electrode (anode, 5x7 cm2) was positioned over the right temporoparietal cortex, centered on T6 (according to international EEG 10-20 System) known to be implicated in acquisition of OLM [75]. Moreover, atDCS over this area has been shown to improve performance on a similar version of the task employed in our study [25, 76]. The return electrode (cathode, 10×10 cm2) was placed contralateral over the left supraorbital area (see also Fig. 2 C). The larger size of the cathode renders the current functionally ineffective. In addition, the current density was below the required threshold (0.017 mA/cm2) to alter cortical excitability by tDCS in humans. A current of 1 mA was applied for 20 min (atDCS) or 30 s (sham) in a ramp-like fashion of 10 s. Regarding perception of stimulation, subjects had first to indicate, if they experienced the stimulation (YES/NO) and second, to rate their level of discomfort due to stimulation on a scale from 0 (not at all) to 6 (very strongly) retrospectively.
Affective state and sleep characteristics
Immediately before application of atDCS or sham affective state [63] of previous night were assessed (see also Fig. 2B). To control for confounders subjects had to indicate on a 7-point rating scale (scale: from 0 ‘not at all’ to 6 ‘very strongly’) how they feel with regard to specific (e.g., anger, anxiety) and unspecific (e.g., activation, excitation) positive and negative affective states (10 in total) by means of an adjective checklist (“Befindlichkeitsskalierung anhand von Kategorien und Eigenschaftswörtern”; BSKE, [77]; German multidimensional checklist similar to the Positive and Negative Affective Schedule, PANAS, [69]). Moreover, sleep duration (“How many hours did you sleep last night?”) and sleep quality (“How did you sleep last night on a scale from 0 ‘lousy’ to 6 ‘excellent’?”) of prior night were self-rated by participants on each training day.
Data aggregation
Percent correct scores (PC) were calculated for every learning block (L1 - L5, acquisition) and IR-test (cued recall) on the basis of number of hits (‘YES’ response to a “correct” object-location pairing) and correct rejections (‘NO’ response to an “incorrect” object-location pairing). PC score were calculated as follows: [number of hits + number of correct rejections] * 100 / total number of presented buildings. Performance in the other memory test used in the study (3-AFC) was measured by number of correct selected responses in %. The primary outcome “training success” was pre-specified before start of study (see clinical trials registry). Training success was defined by PC at fifth learning block (L5) on last training day (day 3) and adjusted (difference score) for baseline performance in the very first learning block (L1, day 1) to account for inter-individual differences (training success: [PCL5day3 – PCL1day1]). Secondary outcome comprised long-term memory after 1-month (FU2). Here, cued recall performance (IR, 3-AFC) was used and expressed as difference to learning performance at fifth block on first training day (delayed memory: IRFU2 – PCL5day1, 3-AFCFU2 – PCL5day1). For exploratory analyses, online (within-session changes) and offline effects (between-session changes) were also determined. Online scores were related to daily improvements (difference) within each training day (ONday n =PCL5_day n – PCL1_day n). Offline scores represented overnight retention and included cued recall performance (IR and 3-AFC) before start of next OLM training in relation (difference) to learning performance of previous day (OFFday n = Cued recallday n + 1 before OLM–training – PCL5_day n).
Statistical analysis
Statistical analysis was conducted using the Statistical Package for the Social Sciences (IBM SPSS, version 24.0) and the free statistical software R [78]. Baseline differences (cognitive and other characteristics) between MCI patients and HE were tested by using chi-square tests (categorical variables), t-tests (differences in means), and Mann-Whitney tests (differences in ranks), respectively. Primary and secondary outcome measures were first analyzed by separate linear mixed models (LMM; random intercept models) with no further covariates taken into account (referred to as MODEL 0) [79]. Repeated measurements under different conditions (“atDCS” or “sham”) were entered as level one unit nested in different individuals as level two units (16 MCI patients, 31 data points; 32 HE, 64 data points in total). Effects of INTERVENTION (atDCS versus sham) and differences between GROUP (MCI patients versus HE) for each dependent variable (training success, delayed memory IR, delayed memory 3-AFC) were evaluated in separate LMM analyses including sham (INTERVENTION) and HE (GROUP) as reference value in the models. Regarding explorative analysis of online and offline effects (dependent variables), a further factor DAY (time) was added to the models (16 MCI patients, 93 data points; 32 HE, 192 data points in total). In order to statistically control for potential confounders, LMM analyses were repeated including covariates (referred as MODEL 1). Given the small sample size, the adjusted Model aimed to include not too many covariates to gain stable estimates and to avoid an overfit. Therefore, we followed a heuristic approach including a forward selection of potential contributors. Selection was guided by 1) avoidance of variables with high inter-correlations (avoid collinearity), and 2) changes in Akaike information criteria (AIC) as estimator for model fit. Impact of intervention and group was reported by model based estimated mean difference, and 95% confidence interval (mean Diff [95% CI]). Semi-partial R2 (R2β *; [80]) as implemented in the r package r2glmm were computed as measures of effect size for fixed effects. Model-based post-hoc pairwise comparisons of the fixed effects were calculated and presented as mean differences in % (atDCS – sham or MCI – HE), and a 95 % CI. A two-sided significance level of α=0.05 was used for testing the primary hypothesis of beneficial effects of atDCS in MCI patients compared to HE with regard to training success. All secondary analyses were done within an exploratory framework. No adjustment for multiple testing was applied.
RESULTS
Primary outcome: Training success
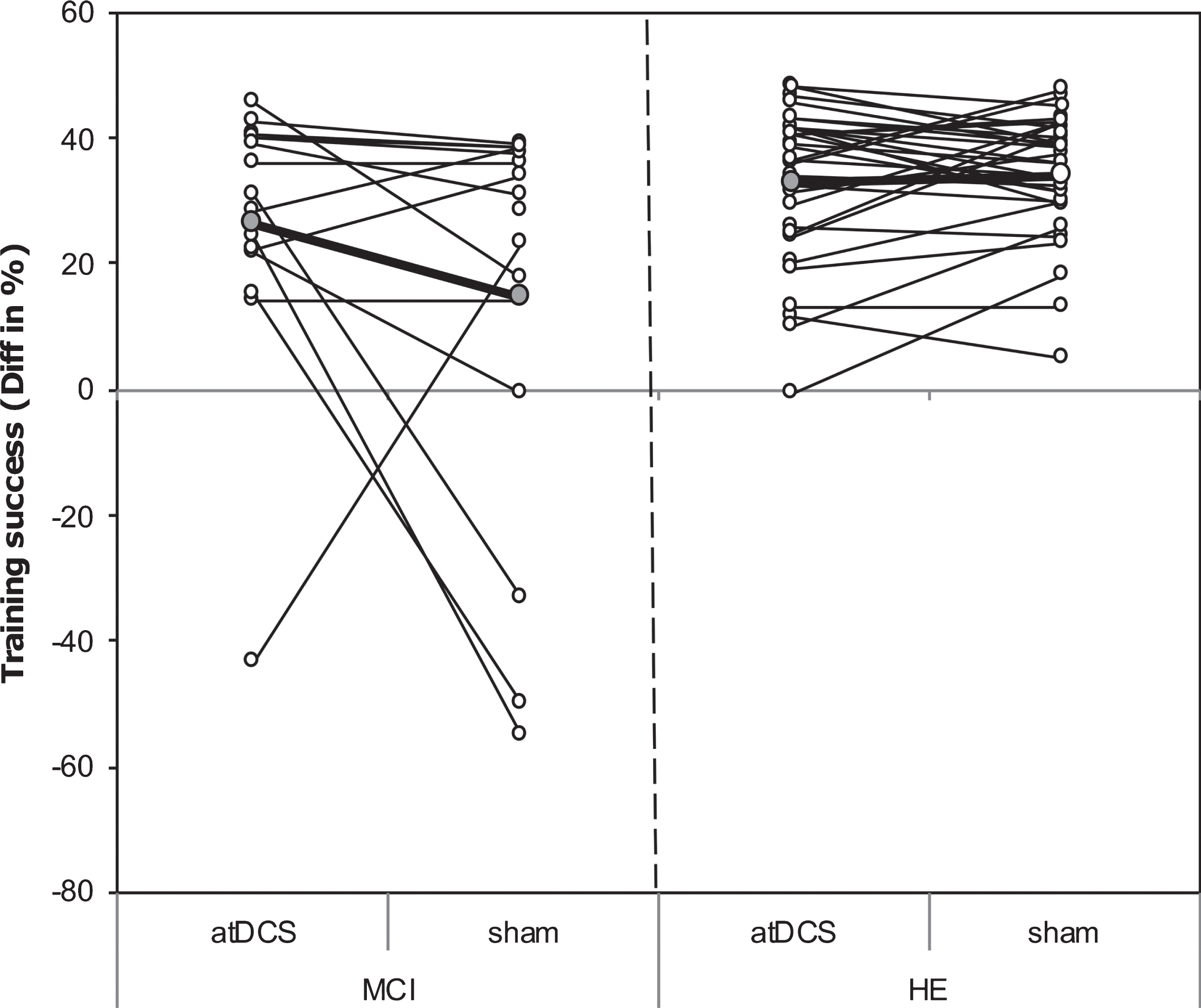
LMM analysis revealed a differential impact of atDCS condition on groups as reflected by a moderate although not statistically significant GROUP X INTERVENTION interaction effect (Model 0 without covariates; for details see Table 2, p = 0.08, R2β * =0.03). MCI patients showed a small benefit from training under atDCS compared to training under sham (10.9 [–0.2,22.0], p = 0.05). In contrast, in HE no substantial difference in training success between atDCS and sham condition was obtained (–1.2 [–8.9,6.4], p = 0.74), see Fig. 3). Moreover, under sham, relative training gain in MCI was lower than in HE (MCI versus HE: –19.0 [–30.1,–7.9], p = 0.001), while under atDCS, relative training gain was similar between groups (MCI versus HE: –6.8 [–18.1, 4.5], p = 0.23).
Table 2
Linear mixed models analysis with factor INTERVENTION and GROUP for training success and delayed recall
| Outcome | Training success | Delayed recall IR | Delayed recall AFC | |||||||||||||
| n | Mean diff | 95% CI | p | R-square | n | Mean diff | 95% CI | p | R-square | n | Mean diff | 95% CI | p | R-square | ||
| Model 0 | 0.13 | 0.009 | 0.03 | |||||||||||||
| nMCI, nHE | 16, 32 | 16, 32 | 16, 32 | |||||||||||||
| Total data points | 95 | 91 | 90 | |||||||||||||
| Group (HE = 0) | -12.9 | -22.0, -3.8 | 0.006 | 0.12 | 0.9 | -6.6, 8.5 | 0.81 | <0.001 | -3.9 | -12.4, 4.5 | 0.35 | 0.03 | ||||
| Intervention (stDCS = 0) | 4.8 | -1.9, 11.6 | 0.16 | 0.001 | 2.7 | -3.3, 8.7 | 0.37 | 0.003 | -0.6 | -8.4, 7.2 | 0.88 | 0.02 | ||||
| Group X Intervention | 0.03 | 0.001 | 0.02 | |||||||||||||
| GROUP (MCI - HE) | atDCS | 6.8 | -18.1, 4.5 | 0.23 | 1.6 | -7.8, 11.1 | 0.73 | 1.3 | -10.0, 12.7 | 0.81 | ||||||
| sham | -19.0 | -30.1, -7.9 | 0.001 | 0.2 | -9.4, 9.8 | 0.97 | -9.2 | -20.6, 2.2 | 0.11 | |||||||
| INTERVENTION (atDCS - sham) | MCI | 10.9 | -0.2, 22.0 | 0.05 | 3.4 | -6.5, 13.4 | 0.49 | 4.7 | -8.2, 17.7 | 0.46 | ||||||
| HE | 1.2 | -8.9, 6.4 | 0.74 | 2.0 | -4.7, 8.7 | 0.55 | -5.8 | -14.5, 3.0 | 0.18 | |||||||
| Model 1 | 0.34 | 0.17 | 0.11 | |||||||||||||
| nMCI, nHE | 13, 31 | 13, 30 | 13, 31 | |||||||||||||
| Total data points | 87 | 84 | 83 | |||||||||||||
| Group (HE = 0) | -1.6 | -15.0, 11.9 | 0.81 | 0.02 | -3.5 | -17.6, 8.5 | 0.58 | 0.006 | -4.6 | -18.6, 9.4 | 0.51 | 0.03 | ||||
| Intervention (stDCS = 0) | 6.3 | -1.3, 13.9 | 0.10 | 0.001 | 3.2 | -2.9, 9.5 | 0.29 | 0.004 | 1.2 | -7.3, 9.8 | 0.77 | 0.02 | ||||
| Group X Intervention | 0.04 | 0.001 | 0.03 | |||||||||||||
| GROUP (MCI - HE) | atDCS | 5.9 | -9.4, 21.3 | 0.44 | -2.4 | -16.4, 11.6 | 0.73 | 2.7 | -13.3, 18.7 | 0.74 | ||||||
| sham | -9.1 | -24.3, 6.2 | 0.24 | -4.7 | -18.9, 9.6 | 0.51 | -11.9 | -28.4, 4.5 | 0.15 | |||||||
| INTERVENTION (atDCS - sham) | MCI | 13.8 | 1.0, 26.7 | 0.04 | 4.4 | -6.1, 14.9 | 0.40 | 8.6 | -6.0, 23.2 | 0.24 | ||||||
| HE | -1.2 | -9.4, 7.0 | 0.77 | 2.1 | -4.4, 8.6 | 0.51 | -6.1 | -15.1, 2.9 | 0.18 | |||||||
| Covariates | β | β | β | |||||||||||||
| Gender | -9.0 | -18.1, 0.1 | 0.05 | 0.05 | 1.2 | -7.6, 10.0 | 0.78 | 0.001 | 1.6 | -8.1, 11.3 | 0.74 | 0.001 | ||||
| Age | -1.0 | -1.6, -0.4 | 0.002 | 0.12 | 0.4 | -0.2, 1.0 | 0.17 | 0.03 | -0.4 | -1.0, 0.3 | 0.25 | 0.02 | ||||
| Sequence | 0.8 | -6.1, 7.7 | 0.82 | 0.001 | 6.4 | 0.8, 11.9 | 0.02 | 0.05 | 6.9 | -0.7, 14.5 | 0.07 | 0.04 | ||||
| MWT-Score | 1.8 | 0.2, 3.5 | 0.03 | 0.06 | -1.9 | -3.5, -0.3 | 0.02 | 0.09 | -0.9 | -2.6, 0.8 | 0.31 | 0.01 | ||||
| BDI | -1.0 | -2.1, 0.2 | 0.09 | 0.04 | -0.4 | -1.5, 0.6 | 0.40 | 0.01 | 0.4 | -0.7, 1.6 | 0.47 | 0.007 | ||||
| ApoE | -0.3 | -9.3, 8.7 | 0.95 | <0.001 | -0.6 | -9.2, 8.0 | 0.88 | <0.001 | 3.0 | -6.4, 12.5 | 0.52 | 0.006 | ||||
MODEL 0: Three separate linear mixed models were performed (dependent variables: training success, item recognition (IR) and 3-alternative forced choice (3-AFC); independent variables: INTERVENTION (atDCS, sham) and GROUP (MCI, HE)). R2β* - semi-partial R2 as measure of effect size. β=regression coefficient (sham = 0, HE = 0). Positive difference scores indicated better performance for atDCS or MCI. MODEL 1: Three separate linear mixed models with adjustment for covariates: Gender, age, sequence of intervention, indicator for premorbid intelligence (MWT-scores), BDI, ApoE 4 allele carriers (a polymorphism that have been previously implicated in memory outcome, e.g., Wisdom et al. [63]; Matura et al. [64]), CI = confidence interval. Reduced data points are due to missing data. Reference values of binary covariates - gender: women = 0; sequence of intervention: study block 2 = 0; ApoE 2 and 3 = 0. atDCS = anodal transcranial direct current stimulation, MCI = mild cognitive impairment, HE = healthy elderly.
Fig.3
Scatterplot of training success. Scatterplot of training success under object-location memory (OLM) training+anodal transcranial direct current stimulation (atDCS) versus OLM training+sham condition (sham) depicted for older adults with (MCI, left) and without (HE, right) mild cognitive impairment. Grey circles, which are connected by the bolded line, refer to group mean of respective condition.
The subsequently conducted LMM analysis was adjusted (Model 1) for gender, age, sequence of intervention, premorbid intelligence (MWT-score at baseline), ApoE 4 allele genotype (a polymorphism that has previously been implicated in memory outcome, e.g., [81, 82]), and baseline depression score (BDI). The interaction effect of GROUP X INTERVENTION that demonstrated differential effects of atDCS in MCI patients and HE was similar as in the unadjusted model (p = 0.05, R2β * = 0.04). Post-hoc pairwise comparison showed that in MCI patients the beneficial effect of atDCS relative to sham condition on training success was even more pronounced (13.8 [1.0, 26.7], p = 0.04).
Given the role that initial individual performance might play for efficacy of atDCS the association between relative atDCS gain (calculated as difference in training success between atDCS and sham condition: atDCS – sham; positive scores indicate benefit in terms of better performance under atDCS compared to sham parallel to OLM training) and initial performance after first learning block (L1) of the first training day (day 1) under atDCS condition was further explored in a post-hoc correlational analysis (Pearson) separately for each group. In both groups a negative association were observed (HE: r = -0.41, p = 0.02; MCI: r = -0.33, p = 0.21), suggesting a relative greater benefit following training with atDCS for initially low-performing individuals.
Secondary outcome: Long-term memory
Analysis of long-term effects of combined intervention on memory revealed small differences between groups and conditions (Model 0: Mean diff varied between 0.2 and 3.4 for IR and between -9.2 and 1.3 for 3-AFC, for details see Table 2), but respective 95 % CI did not indicate substantial effects for MCI patients or HE in either of the cued recall tests. Pattern of results remained if LMM analysis was adjusted for the covariates as described above (Model 1, see Table 2 for details).
Explorative analysis: Behavioral measures of on- and offline effects
For within-session performance (online effects, Model 0), a substantial training effect across days was evident in both groups. Because of a training-induced increase in performance at the beginning of each further training day magnitude of online scores (improvement in performance between first (L1) and last (L5) learning block at one day) decreased across training days (DAY 1 : 17.0 [14.9, 19.1], DAY 2 : 11.3 [9.2, 13.4], DAY 3 : 3.8 [1.7, 5.9], p < 0.001, R2β* = 0.05). In addition, while in HE similar learning effects were obtained on each day irrespective of stimulation condition (Mean diff between atDCS and sham varied between 0.8 and –0.6), a slightly different pattern emerged in MCI patients. Here, although not statistically significant, online effects were enhanced by atDCS on the third training day (5.0 [–1.9, 11.9]). Results remained unchanged after adjustment for covariates (Model 1 adjusted for gender, sequence of intervention, BDI at baseline and ApoE 4; for details see Table 3).
Table 3
Separate linear mixed models analysis with factor INTERVENTION and GROUP for online and offline effects
| Outcome | Online | Offline IR | Offline AFC | ||||||||||||
| n | Mean diff | 95% CI | p | R-square | n | Mean diff | 95% CI | p | R-Square | n | Mean diff | 95% CI | p | R-square | |
| Intervention effect | Model 0 | 0.27 | 0.07 | 0.08 | |||||||||||
| nMCI, nHE | 16.32 | 16, 32 | 16.32 | ||||||||||||
| Total data points | 283 | 284 | 284 | ||||||||||||
| Group (HE = 0) | -1.9 | -4.5, 0.6 | 0.13 | 0.002 | 3.3 | -1.2, 7.1 | 0.15 | <0.001 | 0.3 | -5.1, 5.7 | 0.90 | 0.006 | |||
| Intervention (atDCS = 0) | 0.1 | -2.3, 2.5 | 0.92 | 0.008 | -2.0 | -4.6, 0.6 | 0.13 | 0.03 | -3.4 | -6.9, 0.2 | 0.06 | 0.03 | |||
| Day | <0.001 | 0.05 | 0.19 | 0.003 | 0.002 | 0.002 | |||||||||
| Day 1 | 17.0 | 14.9,19.1 | -2.0 | -4.7, 0.8 | -20.5 | -24.1, -16.8 | |||||||||
| Day 2 | 11.3 | 9.1, 13.4 | -1.0 | -3.7, 1.8 | -16.7 | -20.4, -13.1 | |||||||||
| Day 3 | 3.8 | 1.7, 5.9 | 0.9 | -1.8, 3.6 | -12.7 | -16.3, -9.0 | |||||||||
| Group X Intervention X Day | 0.02 | 0.06 | 0.06 | ||||||||||||
| Day 1 | |||||||||||||||
| MCI | 1.0 | -5.9,7.9 | 0.78 | -4.7 | -12.0, 2.6 | 0.20 | -1.1 | -11.2, 8.9 | 0.82 | ||||||
| HE | 0.8 | -4.0,5.6 | 0.74 | 4.2 | -0.9, 9.2 | 0.11 | 4.1 | -2.9, 11.0 | 0.25 | ||||||
| Day 2 | |||||||||||||||
| MCI | -3.3 | -10.2,3.6 | 0.34 | 0.9 | -6.4, 8.2 | 0.81 | -6.7 | -16.9, 3.5 | 0.20 | ||||||
| HE | -2.1 | -7.0,2.7 | 0.39 | 0.5 | -4.6, 5.5 | 0.85 | -3.0 | -10.0, 3.9 | 0.39 | ||||||
| Day 3 | |||||||||||||||
| MCI | 5.0 | -1.9,11.9 | 0.16 | -11.0 | -18.3, 3.7 | 0.003 | -14.5 | -24.6, -4.5 | 0.005 | ||||||
| HE | -0.6 | -5.4,4.2 | 0.81 | -1.8 | -6.9, 3.3 | 0.48 | 1.1 | -5.9, 8.1 | 0.75 | ||||||
| Intervention effect | Model 1 | 0.28 | 0.12 | 0.10 | |||||||||||
| nMCI, nHE | 13, 31 | 13, 31 | 13, 31 | ||||||||||||
| Total data points | 259 | 260 | 260 | ||||||||||||
| Group (HE = 0) | -2.6 | -6.2, 0.9 | 0.15 | 0.006 | 2.4 | -3.6, 8.5 | 0.42 | 0.001 | 0.3 | -7.5, 8.3 | 0.93 | 0.003 | |||
| Intervention (atDCS = 0) | 0.3 | -2.3, 3.0 | 0.80 | 0.006 | -2.4 | -5.2, 0.4 | 0.10 | 0.04 | -3.5 | -7.4, 0.5 | 0.08 | 0.02 | |||
| Day | <0.001 | 0.06 | 0.07 | 0.004 | 0.002 | 0.005 | |||||||||
| Day 1 | 16.4 | 14.0, 18.7 | -2.3 | -5.8, 0.6 | -20.7 | -24.9, -16.5 | |||||||||
| Day 2 | 11.2 | 8.8, 13.6 | -0.6 | -5.5, 0.9 | -17.2 | -21.5, -13.0 | |||||||||
| Day 3 | 3.0 | 0.6, 5.4 | 1.7 | -1.7, 4.7 | -11.9 | -16.1, -7.7 | |||||||||
| Group X Intervention X Day | 0.02 | 0.10 | 0.05 | ||||||||||||
| Day 1 | |||||||||||||||
| MCI | 2.7 | -5.0, 10.5 | 0.49 | -3.6 | -11.8, 4.6 | 0.39 | -3.4 | -14.9, 8.0 | 0.56 | ||||||
| HE | 0.9 | -4.0, 5.8 | 0.72 | 4.2 | -1.0, 9.4 | 0.11 | 4.3 | -2.9, 11.6 | 0.24 | ||||||
| Day 2 | |||||||||||||||
| MCI | -3.6 | -11.4, 4.1 | 0.36 | 0.71 | -7.5, 8.9 | 0.86 | -4.7 | -16.4, 6.9 | 0.42 | ||||||
| HE | -2.2 | -7.2, 2.8 | 0.39 | 0.6 | -4.6, 5.9 | 0.80 | -3.1 | -10.3, 4.2 | 0.40 | ||||||
| Day 3 | |||||||||||||||
| MCI | 4.8 | -2.9, 12.6 | 0.22 | -14.5 | -22.7, -6.2 | 0.001 | -15.1 | -26.6, -3.7 | 0.01 | ||||||
| HE | -0.6 | -5.6, 4.3 | 0.80 | -1.8 | -7.0, 3.5 | 0.51 | 1.2 | -6.0, 8.5 | 0.73 | ||||||
| Covariates | β | β | β | ||||||||||||
| Gender | -1.0 | -3.8, 1.8 | 0.48 | 0.002 | 2.6 | -2.1, 7.3 | 0.28 | 0.01 | 0.6 | -5.6, 6.8 | 0.85 | <0.001 | |||
| Sequence | 0.6 | -1.8, 3.0 | 0.64 | 0.001 | -1.1 | -3.7, 1.4 | 0.38 | 0.003 | -2.3 | -5.8, 1.3 | 0.21 | 0.006 | |||
| BDI | -0.06 | -0.4, 0.3 | 0.73 | 0.001 | 0.2 | -0.4, 0.8 | 0.54 | 0.003 | 0.01 | -0.8, 0.8 | 0.98 | <0.001 | |||
| ApoE | 0.6 | -2.2, 3.4 | 0.66 | 0.001 | 1.0 | -3.7, 5.8 | 0.65 | 0.002 | 3.1 | -3.0, 9.3 | 0.31 | 0.009 | |||
Model-based post-hoc tests resulted from three separate linear mixed models (MODEL 0: dependent variables: online effects, offline effects for IR and 3-AFC; independent variables: INTERVENTION (atDCS, sham), DAY (day1, day2, day3) and GROUP (MCI, HE). MODEL 1: three separate linear mixed models with adjustment for covariates (for online and offline effects: Gender, sequence of intervention, BDI, and ApoE polymorphism). Reference values of binary covariates - gender: women = 0; sequence of intervention: study block 2 = 0; ApoE 2 and 3 = 0).
Between-session performance (offline effects) were analyzed separately for the two cued recall tests (Model 0; IR and 3-AFC). Post-hoc pairwise comparison suggested an adverse atDCS effect in MCI patients especially after the third night (assessed the day after training was completed). Enhanced overnight forgetting after OLM training+atDCS compared to OLM training+sham was observed in both cued recalled tests (IR: –11.0 [–18.3,–3.7], 3-AFC: –14.6 [–24.6,–4.5], see Fig. 4). A similar detrimental atDCS effect was not obtained for HE (IR: –1.8 [–6.9, 3.3], 3-AFC: 1.1 [–5.9, 8.1]). Results remained significant after adjustment for covariates (Model 1 adjusted for gender, sequence of intervention, BDI at baseline and ApoE 4; for details see also Table 3).
Fig.4
Scatterplot of behavioral on- and offline effects on DAY 3. A) Individual online scores (difference between percent correct score of last and first learning block on Day 3) for object-location memory (OLM) training+anodal transcranial direct current stimulation (atDCS) versus OLM training+sham condition (sham) depicted for older adults with (MCI, left) and without (HE, right) mild cognitive impairment. B) Individual offline scores (difference between cued recall performance in item recognition (IR) on Follow-up 1 and percent correct score of last and learning block on Day 3) for OLM training+atDCS (atDCS) versus OLM training+sham condition (sham) depicted for older adults with (MCI, left) and without (HE, right) mild cognitive impairment. C) Individual offline scores (difference between cued recall performance in 3-Alternative forced choice (3-AFC) task on Follow-up 1 and percent correct score of last and learning block on Day 3) for OLM training+atDCS (atDCS) versus OLM training+sham condition (sham) depicted for older adults with (MCI, left) and without (HE, right) mild cognitive impairment. Grey circles, which are connected by the bolded line, refer to group mean of respective condition.
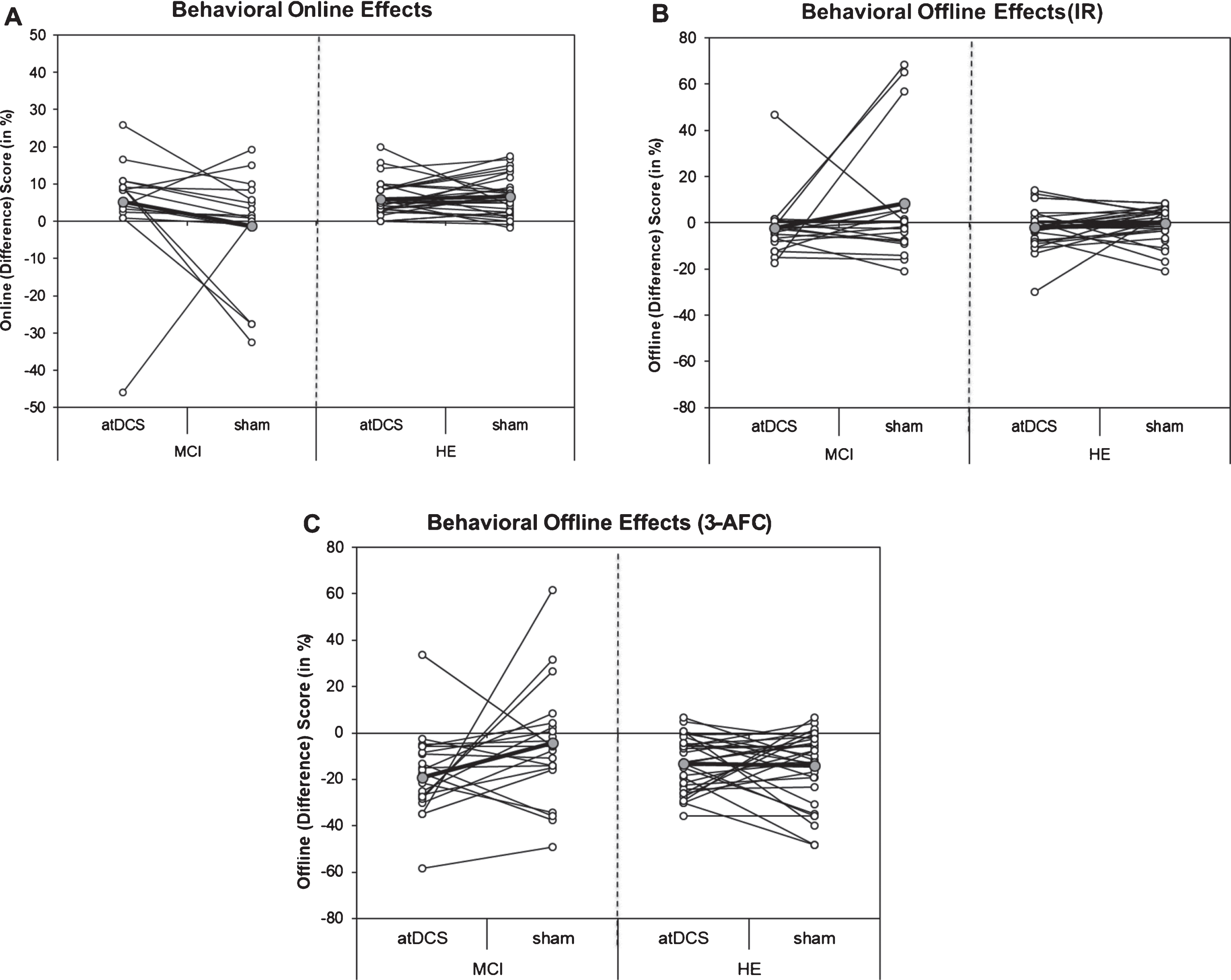
In sum, a beneficial impact of OLM training+atDCS, as compared to OLM training+sham, was observed in MCI patients, with similar rates of improvement as compared to HE. The beneficial effect in MCI patients did not persist (memory performance after 1 month). Further, atDCS seemed to differentially modulate behavioral on- and offline effects in MCI patients and HE. While online performance was enhanced on the last training day, subsequent offline performance (day after last training session was administered) declined in MCI patients. A comparable pattern of online/offline effects was not observed for HE.
Control of sleep characteristics, and affective state during training
MCI patients and HE slept on average 7 h, and reported that they had slept well the night before the respective training day (mean (SD): MCI: 4.1 (0.2); HE: 4.1 (0.1); rating scale: 0 (lousy) – 6 (excellent)). While self-rated quality of sleep did not substantially vary across training days, a difference between groups who received sham stimulation at the first study block was observed. Here, MCI patients slept on average 1 h less than HE (95% CI: 0.3, 1.7) during the night before the first training day. The difference between groups decreased over the course of the training sessions.
Positive affective state ratings, which were conducted immediately before a training session had been started, did not differ substantially between groups (–0.14 [–0.7, 0.4]), stimulation conditions (–0.04 [–0.2,0.1]), or across training days (maximal difference in positive ratings were observed between Day 1 and Day 2 : 0.5 [–0.1,0.2]). Moreover, subjects scored very low on self-assessed negative affective state. The composite negative score (mean) of the rated subscales was below 1 (on a rating scale from 0 to 6). However, irrespective of condition (atDCS or sham) MCI patients scored on average somewhat higher in negative scales compared to HE. This difference was observed especially with regard to self-rated “excitation” (1 [0.4,1.6]) and “anxiety” (0.6 [0.2,1.0]).
Stimulation perception
In the MCI group, 7 patients believed that they had received no stimulation during both sessions, 2 patients that they were stimulated in both sessions, and 3 patients that they were stimulated once during the two blocks (only one of them was able to correctly determine the atDCS condition). In the HE group, 23 subjects did not reliable discriminate between atDCS and sham condition (see [21]). Cross sectional analyses revealed that both MCI and HE were not able to reliably discern stimulation conditions in the first [both X2 (1) ≤0.4] or in the second [both X2 (1) ≤2] study block, indicating that our sham procedure was successful in blinding subjects. The low level of self-rated discomfort (scale: 0 ‘not at all’ – 6 ‘very strongly’) indicated that stimulation was well tolerated by MCI patients (atDCS: median (SD): 1 (0.9)) and HE (0.9 (1.2)).
DISCUSSION
The aim of this study was to assess the effect of 3 consecutive daily sessions of OLM training combined with right temporoparietal atDCS versus OLM training combined with sham on training success immediately after training (primary outcome) and long-term memory performance in patients with MCI in comparison to HE. In general, both groups showed improvement in performance over time (training effect), although overall performance level was greater in HE than in MCI. Importantly, only MCI patients showed an additional benefit of atDCS in terms of training success, reaching similar improvement rates for training under atDCS as seen for training in HE under sham or atDCS. Explorative analyses showed that atDCS differentially modulated on- and offline effects in MCI patients, enhancing online performance on the third training day, and impairing subsequent offline performance. None of the immediate atDCS effects persisted on follow-up assessment one month later.
Effects of atDCS on training success
MCI patients showed a small benefit from OLM training+atDCS with regard to training success assessed immediately at the end of 3-day training. This finding corroborates previous results reported by Lawrence et al. [45], Murugaraja et al. [90], or meta-analyses [83], who revealed a medium effect size for memory for immediate effects. Mean improvement did not persist on follow-up, also in line with our findings. At odds with our results, Das et al., using a pre-training stimulation approach, showed a negative impact on immediate cognitive performance [46]. Note, though that optimal timing of tDCS application (pre-, during, or post-training) is still an open question and certainly depends on a number of factors, like task, current brain state, or population under study. Overall, the literature rather suggests that “during-task stimulation” is more effective in MCI, while HE might rather benefit from “pre-task stimulation” due to different mechanisms of action (see also [38, 47, 90]). Timing aspects such as during-task stimulation might be another reason why we failed to demonstrate enhancing atDCS effects in HE with an identical set-up and comparable CT (for a further detailed discussion of this result, see [21]). Interestingly, and consistent with previous results from our group [48], atDCS boosted relative training gain in MCI to a similar rate as seen in HE. Although the effects are small and seems to be short-lived, we think it would be premature to conclude that atDCS had no effect in this study. Given that we used a within-subject design and balanced the sequence across conditions, it seems unlikely that increased training success under atDCS is merely a result of a practice effect. A putative hypothesis is that stabilization of memory processes in MCI might take longer, and 3 session were thus too short to induce a robust benefit. Hence, combined training-stimulation protocols for extended periods of time, e.g., 10 sessions over the course of 2 weeks, should be assessed in future trials.
Large-scale network modulation beyond stimulation sites has been shown for tDCS [84]. In the next section, we will provide some suggestions about putative mechanisms. Note, though, that our report is based on behavioral data only, therefore, we cannot demonstrate the exact network effects of the stimulation on a functional neuroimaging level. However, a large body of evidence indicates hippocampus and adjacent structures as a central hub for binding processes within the widely distributed fronto-tempo-parietal network [5, 85, 86], of which we stimulated the temporoparietal part. Although some of the neuropathological changes associated with memory decline in MCI are also common in normal aging [87], additional pathology may be present in MCI due to AD with regard to amyloid and tau pathology [88]. Given that no biomarkers were available in the MCI group, no definite conclusion can be drawn with regard to response to atDCS in individuals with amyloid/tau pathology. Other changes, including neurochemical alterations (disequilibrium between excitatory and inhibitory neurotransmitters systems [89]), and structural alterations (accelerated global atrophy with predominance in the temporoparietal regions, cortical thinning), might be more severe, and will thus exert a more pronounced impact on distributed hippocampal-cortical networks [90–92]. Consequently, dependent on individual network excitability, network-level response to stimulation might vary in MCI patients and HE. Suboptimal network activation in MCI patients [93, 94] might benefit from activation with atDCS if applied in parallel to training. In line with this hypothesis, initially low-performing HE, that likely also show suboptimal network functioning [95, 96], showed greater benefit from atDCS application compared to initially high-performing HE. We concede, though, that stimulation of other nodes of the network such as prefrontal [97], or more parietal sites [98], or combination of nodes in a dual-site approach, may be more effective in enhancing memory performance. In order to provide more compelling evidence future studies combining neuroimaging and electrophysiological methods with behavioral outcome measures and systematic variations of different network nodes are necessary to further delineate these putative mechanisms, and to determine the most effective strategy to activate a given network.
Effects of atDCS on long-term memory
No sustained beneficial effects were found in the present study for delayed memory in either HE or MCI patients, which would be important for therapeutic application. Even though our results correspond to the meta-analysis as discussed above [83], it is at odds with studies that noted more sustained effects [42, 45]. Differences may be related to variations in study specific parameters including number of sessions, and stimulation intensities. These parameters might be important modulators, but are probably insufficient to fully account for the lack of delayed effects (see also [16] for a detailed discussion). Interestingly, some previous studies did not stimulate daily, but applied 3 stimulation sessions per week for a total of 3 [42] or 4 [45] weeks. Accordingly, timing might be a critical factor to establish long-term effects when combining atDCS with interventions like training [21, 95, 96]. In addition, according to the system consolidation theory [99, 100], cortical representations are strengthened and hippocampal involvement is diminished over time, which might require dynamic adaptions in the stimulation protocol (e.g., stimulation site, or frequency of stimulation) across training days. These issues should be addressed in more detail in future studies.
Effects of atDCS on behavioral measures of online versus offline effects
Behavioral on- and offline effects may differ in underlying mechanisms and might thus be differently susceptible to atDCS [24]. Online performance (improvement during stimulation within a training day) seems to be mainly related to subthreshold alterations in membrane potential [101–104]. Similar to recent meta-analytic results, which summarized studies of working memory in HE and in patients with depression, idiopathic Parkinson’s disease, or schizophrenia [105], we found a beneficial online effect in MCI, as opposed to HE, but only on the third training day. Beneficial effects in neuropsychiatric patients were interpreted in terms of an atDCS-induced shift of the imbalance of excitation and inhibition towards a more physiological state, in turn leading to increased working memory performance. While in the meta-analysis of Hill et al. [105], positive effects were already observed with single session atDCS applications, we observed a benefit only after the third training day. Of note, the extent of suboptimal activation in networks underlying performance may differ between tasks, and may be modulated by neurochemical changes due to disease-specific changes or pharmacological treatment. Thus, our result might indicate that in MCI, co-activation in task-relevant networks induced by training and atDCS would not exert immediate effects, but would rather have to accumulate over several days to reach a level which modulates behavioral performance. In the present study, we did not record electrophysiological or neuroimaging data on a day-to-day basis, and therefore cannot validate this hypothesis further.
Offline effects involve synaptic plasticity processes which critically depend on N-Methyl-D-Aspartate (NMDA) receptors and are comprised of GABAergic and the glutamergic neurotransmitter system [23]. Surprisingly, the beneficial online effect at day 3 in the MCI group was followed by a deterioration of behavior after the third night. Given that no such impact on offline effect was found for the preceding days, on- and offline effects may have interacted. The mechanisms underlying these effects may relate to the multi-day stimulation protocol including retrieval (reactivation) at the beginning of each training day. During reactivation, consolidated memories become susceptible to modification [106], and the direction of such modification might critically depend on the timing of stimulation, that is before or after memory reactivation [107]. In sum, different effects in behavioral on- and offline measures further support the notion of distinct mechanisms involved in behavioral on- and offline effects.
Variability of atDCS effects
Including several covariates to for inter-individual differences in tDCS response [108–110] strengthened the observed beneficial effect of training success in MCI (Model 1). Specifically, lower age, female gender, a lower BDI baseline score, and higher MWT score were associated with increased training success. Initially, the pattern may seem less plausible with regard to reported antidepressant effects of atDCS [111]). However, although MCI patients scored higher in BDI compared to HE, the scores did not reach the threshold for clinical depression and may accordingly differ with respect to tonic cortical activation/excitation from depressed patients resulting in a different atDCS response. Moreover, since knowledge about confounders mostly stem from single session tDCS studies, it is largely unclear if and how individual differences of several (pathological) age-related changes such as cranial anatomy, functional connectivity, excitation/inhibition balance, psychological, and cognitive status affect both, cortical excitability and cognitive training independently and/or its combination [112]. Hence, a non-trivial, complex influence of inter-individual factors in response to multi-day tDCS seems likely. In sum, even if atDCS is applied according to the same protocol during the same tasks in a given clinical population, uniform atDCS effects seem unlikely [113], and future studies should therefore carefully control psychological traits/states, brain state, as well as specific aspects of context and tasks (see also [105]).
Limitations
Several limitations should be acknowledged when interpreting results of this study. First, low statistical power may have contributed to small effects obtained in the present study. However, given inconsistent previous findings, proof-of-concept studies are useful to refine protocols by identifying further significant contributing factors and conditions before applying protocols to larger randomized controlled trials. Due to relatively small sample size the results should be interpreted with caution. Second, the studied cohorts were unbalanced in terms of gender distribution. Gender-related differences have been reported for some aspects of visuospatial memory favoring men [114] and for response to tDCS where women are suggested to receive less current compared to men [115]. We therefore adjusted our statistical model for gender (and other known modulators). Future studies should employ a more balanced design with regard to gender to further minimize this source of variability. Third, no biomarkers (e.g., tau, p-Tau, or Aβ) [116] were available to provide additional information with regard to underlying pathology of MCI. Biomarkers are especially relevant when individual progression rate is to be estimated. This was not the goal of the present study, and thus we included patients with clinically core criteria for amnestic MCI [59]. Future studies should integrate systematic evaluation of biomarkers to determine the response to atDCS in MCI due to AD, and MCI due to vascular disease or mixed etiology.
Conclusion
The present study sheds further light on the efficacy of atDCS in MCI patients. First, our data lends some support to the concept of boosting cognitive training efficacy with atDCS in a multi-session approach. Total improvement was small though, and beneficial effects were not retained over the follow-up period rendering it difficult to draw clear recommendations about treatment options in clinical settings. Second, our study provides first insight into baseline determinants of atDCS response, as well as timing aspects in multi-session approaches and thus further highlights the need for the development of individualized treatment approaches. Dealing with the variance caused by atDCS itself in combination with neuroplastic methods such as CT, and heterogeneity present in MCI, is a major challenge for future work. Prior identification of injected electric fields using simulation software (SIMNIBS) [117], and taking into consideration the role of temporal dynamics in multi-session CT combined with atDCS, may help to refine stimulation protocols and eventually induce longer-lasting effects. Moreover, a careful delineation of baseline variables that may influence response to atDCS might help to better define patient subgroups likely to benefit most from the intervention.
ACKNOWLEDGMENTS
This work was supported by the Deutsche Forschungsgemeinschaft (DFG, FL 379-10) and the Bundesministerium für Bildung und Forschung (BMBF, 01GQ 1424A). Angelica Vieira Cavalcanti de Sousa received support from National Council for Scientific and Technological Development (CNPq) – Brazil. The authors thank Anke Nießen, Maria Meier, Magda Cesarz, Julie Hanke, Almut Dünnebeil, Thorge Profitlich, Sonja Fabian and Dr. Sven Passmann for help with data acquisition, and Barbara Lamp for proofreading and language editing.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/19-1234r1).
REFERENCES
[1] | Gangolli VK ((2016) ) Recent advances in the understanding of cognitive decline among the elderly. J Geriatr Ment Health 3: , 36–43. |
[2] | Hedden T , Gabrieli JD ((2004) ) Insights into the ageing mind: A view from cognitive neuroscience. Nat Rev Neurosci 5: , 87–96. |
[3] | Bishop Na , Lu T , Yankner BA ((2010) ) Neural mechanisms of ageing and cognitive decline. Nature 464: , 529–535. |
[4] | Iachini I , Iavarone A , Senese VP , Ruotolo F , Ruggiero G ((2009) ) Visuospatial memory in healthy elderly, AD and MCI: A review. Curr Aging Sci 2: , 43–59. |
[5] | Konkel A , Cohen NJ ((2009) ) Relational memory and the hippocampus: Representations and methods. Front Neurosci 3: , 166–174. |
[6] | Gillis MM , Garcia S , Hampstead BM ((2016) ) Working memory contributes to the encoding of object location associations: Support for a 3-part model of object location memory. Behav Brain Res 311: , 192–200. |
[7] | Hampstead BM , Towler S , Stringer AY , Sathian K ((2018) ) Continuous measurement of object location memory is sensitive to effects of age and mild cognitive impairment and related to medial temporal lobe volume. Alzheimers Dement (Amst) 10: , 76–85. |
[8] | Troyer AK , Murphy KJ , Anderson ND , Hayman-Abello BA , Craik FI , Moskovitch M ((2008) ) Item and associative memory in amnestic mild cognitive impairment: Performance on standardized memory tests. Neuropsychology 22: , 10–16. |
[9] | Hampstead BM , Stringer AY , Stilla RF , Amaraneni A , Sathian K ((2011) ) Where did I put that? Patients with amnestic mild cognitive impairment demonstrate widespread reductions in activity during the encoding of ecologically relevant object-location associations. Neuropsychologia 49: , 2349–2361. |
[10] | Weinstein JD ((2018) ) A new direction for Alzheimer’s research. Neural Regen Res 13: , 190–193. |
[11] | Shatenstein B , Barberger Gateau P , Mecocci P ((2015) ) Prevention of age-related cognitive decline: Which strategies, when, and for whom? J Alzheimers Dis 48: , 35–53. |
[12] | Prehn K , Flöel A ((2015) ) Potentials and limits to enhance cognitive functions in healthy and pathological aging by tDCS. Front Cell Neurosci 9: , 355. |
[13] | Leung NTY , Tam HMK , Chu LW , Kwok TCY , Chan F , Lam LCW , Woo J , Lee TMC ((2015) ) Neural plastic effects of cognitive training on aging brain. Neural Plast 2015: , 535618. |
[14] | Gates NJ , Vernooij RWM , Di Nisio M , Karim S , March E , Martínez G , Rutjes AWS ((2019) ) Computerized cognitive training for preventing dementia in people with mild cognitive impairment. Cochrane Database Syst Rev 3: , CD012279. |
[15] | Sherman DS , Mauser J , Nuno M , Sherzai D ((2017) ) The efficacy of cognitive intervention in mild cognitive impairment (MCI): A meta-analysis of outcomes on neuropsychological measures. Neuropsychol Rev 2: , 440–484. |
[16] | Lampit A , Hallock H , Valenzuela M ((2014) ) Computerized cognitive training in cognitively healthy older adults: A systematic review and meta-analysis of effect modifiers. PloS Med 11: , e1001756. |
[17] | Passow S , Thurm F , Li SC ((2017) ) Activating developmental reserve capacity via cognitive training or non-invasive brain stimulation: Potentials for promoting fronto-parietal and hippocampal-striatal network functions in old age. Front Aging Neurosci 9: , 33. |
[18] | Hill NT , Mowszovski L , Naismith SL , Chadwick VL , Valenzuela M , Lampit A ((2017) ) Computerized cognitive training in older adults with mild cognitive impairment or dementia: A systematic review and meta-analysis. Am J Psychiatry 174: , 329–340. |
[19] | Mudar RA , Chapman SB , Rackley A , Eroh J , Chiang HS , Perez A , Venza E , Spence JS ((2017) ) Enhancing latent cognitive capacity in mild cognitive impairment with gist reasoning training: A pilot study. Int J Geriatr Psychiatry 32: , 548–555. |
[20] | Bahar-Fuchs A , Webb S , Bartsch L , Clare L , Rebok G , Cherbuin N , Anstey KJ ((2017) ) Tailored and adaptive computerized cognitive training in older adults at risk for dementia: A randomized controlled trial. J Alzheimers Dis 60: , 889–911. |
[21] | Külzow N , Sousa AVC , Cesarz M , Hanke JM , Günsberg A , Harder S , Koblitz S , Grittner U , Flöel A ((2018) ) No effects of non-invasive brain stimulation on multiple sessions of object-location memory training in healthy older adults. Front Neurosci 11: , 746. |
[22] | Elsmary J , Loo C , Martin D ((2015) ) A systematic review of transcranial electrical stimulation combined with cognitive training. Restor Neurol Neurosci 33: , 263–278. |
[23] | Stagg CJ , Nitsche MA ((2011) ) Physiological basis of transcranial direct current stimulation. Neuroscientist 17: , 37–53. |
[24] | Reis J , Schambra HM , Cohen LG , Buch ER , Fritsch B , Zarahn E , Celnik PA , Krakauer JW ((2009) ) Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci U S A 106: , 1590–1595. |
[25] | Flöel A , Suttorp W , Kohl O , Kurten J , Lohmann H , Breitenstein C ((2012) ) Non-invasive brain stimulation improves object location learning in the elderly. Neurobiol Aging 33: , 1682–1689. |
[26] | Perceval G , Flöel A , Meinzer M ((2016) ) Can transcranial direct current stimulation counteract age-associated functional impairment? Neurosci Biobehav Rev 65: , 157–172. |
[27] | Ditye T , Jacobson L , Walsh V , Lavidor M ((2012) ) Modulating behavioral inhibition by tDCS combined with cognitive training. Exp Brain Res 219: , 363–368. |
[28] | Trémolière B , Maheux-Caron V , Lepage JF , Blanchette I ((2018) ) tDCS stimulation of the dlPFC selectively moderates the detrimental impact of emotion on analytical reasoning. Front Psychol 9: , 568. |
[29] | Wager TD , Smith EE ((2003) ) Neuroimaging studies of working memory: A meta-analysis. Cogn Affect Behav Neurosci 4: , 255–274. |
[30] | Owen AM , McMillan KM , Laird AR , Bullmore E ((2005) ) N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Brain Mapp 25: , 46–59. |
[31] | Au J , Katz B , Buschkuehl M , Bunarjo K , Senger T , Zabel C , Jaeggi SM , Jonides J ((2016) ) Enhancing working memory training with transcranial direct current stimulation. J Cogn Neurosci 28: , 1419–1432. |
[32] | Park SH , Seo JH , Kim YH , Ko MH ((2014) ) Long-term effects of transcranial direct current stimulation combined with computer assisted cognitive training in healthy older adults. Neuroreport 25: , 122–126. |
[33] | Jones KT , Stephens JA , Alam M , Bikson M , Berryhill ME ((2015) ) Longitudinal neurostimulation in older adults improves working memory. PloS One 10: , e0121904. |
[34] | Stephens JA , Berryhill ME ((2016) ) Older adults improve on everyday tasks after working memory training and neurostimulation. Brain Stimul 9: , 553–559. |
[35] | Nilsson J , Lebedev AV , Rydstrom A , Lovden M ((2017) ) Direct-current stimulation does little to improve the outcome of working memory training in older adults. Psychol Sci 28: , 907–920. |
[36] | Krause B , Kadosh RC ((2014) ) Not all brains are created equal: The relevance of individual differences in responsiveness to transcranial electrical stimulation. Front Syst Neurosci 8: , 25. |
[37] | Berryhill ME , Peterson DJ , Jones KT , Stephens JA ((2014) ) Hits and misses: Leveraging tDCS to advance cognitive research. Front Psychol 5: , 800. |
[38] | Hsu WY , Ku Y , Zanto TP , Gazzaley A ((2015) ) Effects of noninvasive brain stimulation on cognitive function in healthy aging and Alzheimer’s disease: A systematic review and meta-analysis. Neurobiol Aging 36: , 2348–2359. |
[39] | Bjekića J , Vulića K , Živanovićb M , Vujičićb J , Ljubisavljevića M , Filipovića SR ((2019) ) The immediate and delayed effects of single tDCS session over posterior parietal cortex on face-word associative memory. Behav Brain Res 366: , 88–95. |
[40] | Habich A , Klöppel S , Peter J ((2017) ) Higher tDCS gain in low performers in a verbal episodic memory task. Brain Stimul 10: , 382. |
[41] | Hsu TY , Juan CH , Tseng P ((2016) ) Individual differences and state-dependent responses in transcranial direct current stimulation. Front Hum Neurosci 10: , 1–12. |
[42] | Yun K , Song IU , Chung YA ((2016) ) Changes in cerebral glucose metabolism after 3 weeks of noninvasive electrical stimulation of mild cognitive impairment patients. Alzheimers Res Ther 8: , 49. |
[43] | Manenti R , Sandrini M , Gobbi E , Binetti G , Cotelli M ((2018) ) Effects of transcranial direct current stimulation on episodic memory in amnestic mild cognitive impairment: A pilot study. J Gerontol B Psychol Sci Soc Sci, Series B 128: , 1774–11. |
[44] | Barone D , Aarsland D , Burn D , Emre M , Kulisevsky J , Weintraub D ((2011) ) Cognitive impairment in non-demented Parkinson’s disease. Mov Disord 26: , 2483–2495. |
[45] | Lawrence BJ , Gasson N , Johnson AR , Booth L , Loftus AM ((2018) ) Cognitive training and transcranial direct current stimulation for mild cognitive impairment in Parkinson’s disease: A randomized controlled trial. Parkinsons Dis 2018,: , 1–12. |
[46] | Das N , Spence JS , Aslan S , Vanneste S , Mudar R , Rackley A , Quiceno M , Chapman SB ((2019) ) Cognitive training and transcranial direct current stimulation in mild cognitive impairment: A randomized pilot trial. Front Neurosci 13: , 307. |
[47] | Dedoncker J , Brunoni AR , Baeken C , Vanderhasselt MA ((2016) ) A systematic review and meta-analysis of the effects of transcranial direct current stimulation (tDCS) over the dorsolateral prefrontal cortex in healthy and neuropsychiatric samples: Influence of stimulation parameters. Brain Stim 9: , 501–517. |
[48] | Meinzer M , Lindenberg R , Phan MT , Ulm L , Volk C , Flöel A ((2015) ) Transcranial direct current stimulation in mild cognitive impairment: Behavioral effects and neural mechanisms. Alzheimers Dement 11,: , 1032–1040. |
[49] | Miniussi C , Harris JA , Ruzzoli M ((2013) ) Modelling non-invasive brain stimulation in cognitive neuroscience. Neurosci Biobehav Rev 37: , 1702–1712. |
[50] | Nozari N , Woodard K , Thompson-Schill SL ((2014) ) Consequences of cathodal stimulation for behavior: When does it help and when does it hurt performance? PLoS One 9: , e84338. |
[51] | Kim K , Ekstrom AD , Tandon N ((2016) ) A network approach for modulating memory processes via direct and indirect brain stimulation: Toward a causal approach for the neural basis of memory. Neurobiol Learn Mem 134 (Part A): , 162–177. |
[52] | Gilbert PE , Kesner RP ((2004) ) Memory for objects and their locations: The role of the hippocampus in retention of object-place associations. Neurobiol Learn Mem 81: , 39–45. |
[53] | Kesner RP ((2009) ) The posterior parietal cortex and long-term memory representation of spatial information. Neurobiol Learn Mem 91: , 197–206. |
[54] | Wang W , Pan YW , Zou J , Li T , Abel GM , Palmiter RD , Storm DR , Xia Z ((2014) ) Genetic activation of ERK5 MAP kinase enhances adult neurogenesis and extends hippocampus-dependent long-term memory. J Neurosci 34: , 2130–2147. |
[55] | Smith CD , Scarf D ((2017) ) Spacing repetitions over long timescales: A review and a reconsolidation explanation. Front Psychol 8: , 962. |
[56] | Stickgold R ((2006) ) Neuroscience: A memory boost while you sleep. Nature 444: , 559–560. |
[57] | Plewnia C , Schroeder PA , Kunze R , Faehling F , Wolkenstein L ((2015) ) Keep calm and carry on: Improved frustration tolerance and processing speed by transcranial direct current stimulation (tDCS). PLoS One 10: , e0122578. |
[58] | Zlomuzica A , Preusser F , Totzeck C , Dere E , Margraf J ((2016) ) The impact of different emotional states on the memory for what, where and when features of specific events. Behav Brain Res 298 (Part B): , 181–187. |
[59] | Petersen RC , Doody R , Kurz A , Mohs RC , Morris JC , Rabins PV , Ritchie K , Rossor M , Thal L , Winblad B ((2001) ) Current concepts in mild cognitive impairment. . Arch Neurol 58: , 1985–1992. |
[60] | Petersen RC ((2004) ) Mild cognitive impairment as a diagnostic entity. J Intern Med 256: , 183–194. |
[61] | Winblad B , Palmer K , Kivipelto M , Jelic V , Fratiglioni L , Wahlund LO , Nordberg A , Bäckman L , Albert M , Almkvist O , Harai H , Basun H , Blennow K , De Leon M , DeCarli C , Erkinjuntti T , Giacobini E , Graff C , Hardy J , Jack C , Jorm A , Ritchie K , Van Duijn C , Visser P , Petersen RC ((2004) ) Mild cognitive impairment beyond controversies, towards a consensus: Report of the International Working Group on Mild Cognitive Impairment. J Intern Med 256: , 240–246. |
[62] | Jessen F , Wolfsgruber S , Wiese B , Bickel H , Mösch E , Kaduszkiewicz H , Pentzek M , Riedel-Heller SG , Luck T , Fuchs A , Weyerer S , Werle J , van den Bussche H , Scherer M , Maier W , Wagner M ((2014) ) AD dementia risk in late MCI, in early MCI, and in subjective memory impairment. Alzheimers Dement 10: , 76–83. |
[63] | Folstein MF , Folstein SE , McHugh PR ((1975) ) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: , 189–198. |
[64] | Oldfield RC ((1971) ) The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9: , 97–113. |
[65] | Tombaugh TN ((2004) ) Trail making test A and B: Normative data stratified by age and education. Arch Clin Neuropsychol 19: , 203–214. |
[66] | Härtling C , Markowitsch HJ , Neufeld H , Calabrese P , Deisinger K , Kessler J ((2000) ) Wechsler Gedächtnistest – Revidierte Fassung: Manual. Huber, Bern. |
[67] | Aschenbrenner A , Tucha O , Lange K ((2000) ) RWT Regensburger Wortflüssigkeits-Test. Hogrefe, Göttingen. |
[68] | Lehrl S ((2005) ) Mehrfachwahl-Wortschatz-Intelligenztest MWT-B. Spitta Verlag, Balingen. |
[69] | Watson D , Clark LA , Tellegen A ((1988) ) Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol 54: , 1063–1070. |
[70] | Hautzinger M , Bailer M , Worall H , Keller F ((2001) ), Beck-Depressions-Inventar (BDI). Testhandbuch. Hans Huber, Bern. |
[71] | Angermeyer R , Kilian R , Matschinger H ((2000) ) Handbuch für die deutschsprachigen Versionen der WHO Instrumente zur Erfassung von Lebensqualität. Hogrefe, Göttingen. |
[72] | Buysse DJ , Reynolds CFIII , Monk TH , Berman SR , Kupfer DJ ((1989) ) The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res 28: , 193–213. |
[73] | Erdmann G , Janke W ((2000) ) Stressverarbeitungsfragebogen: Stress, Stressverarbeitung und Ihre Erfassung durch ein Mehrdimensionales Testsystem. Hogrefe, Göttingen. |
[74] | Külzow N , Kerti L , Witte VA , Kopp U , Breitenstein C , Flöel A ((2014) ) An object location memory paradigm for older adults with and without mild cognitive impairment. J Neurosci Methods 237: , 16–25. |
[75] | Postma A , Kessels RP , Van Asselen M ((2008) ) How the brain remembers and forgets where things are: The neurocognition of object-location memory. Neurosci Biobehav Rev 32: , 1339–1345. |
[76] | Prehn K , Stengl H , Grittner U , Kosiolek R , Ölschläger A , Weidemann A , Flöel A ((2017) ) Effects of anodal transcranial direct current stimulation and serotonergic enhancement on memory performance in young and older adults. Neuropsychopharmacology 42: , 551–561. |
[77] | Janke W , Hüppe M , Erdmann G ((2000) ) Befindlichkeitsskalierung Anhand von Kategorien und Eigenschaftswörtern (BSKE): Handanweisung. Lehrstuhl für Biologische und Klinische Psychologie, Würzburg: Lübeck:Berlin. |
[78] | R Development Core Team ((2008) ) R: A language and environment forstatistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org. |
[79] | Verbeke G , Molenberghs G ((2000) ) Linear Mixed Models for Longitudinal Data. Springer, New York. |
[80] | Jaeger B , Edwards L , Das K , Sen PK ((2016) ) An r2 statistic for fixed effects in the generalized linear mixed model. J Appl Stat 44: , 1086–1105. |
[81] | Wisdom NM , Callahan JL , Hawkins KA ((2011) ) The effects of apolipoprotein E on non-impaired cognitive functioning: A meta-analysis. Neurobiol Aging 32: , 63–74. |
[82] | Matura S , Prvulovic D , Butz M , Hartmann D , Sepanski B , Linnemann K , Oertel-Knöchel V , Karakaya T , Fußer F , Pantel J , van de Ven V ((2014) ) Recognition memory is associated with altered resting-state functional connectivity in people at genetic risk for Alzheimer’s disease. Eur J Neurosci 40,: , 3128–3135. |
[83] | Gonzalez PC , Fong KNK , Chung RCK , Ting KH , Law LLF , Brown T ((2018) ) Can transcranial direct-current stimulation alone or combined with cognitive training be used as a clinical intervention to improve cognitive functioning in persons with mild cognitive impairment and dementia? A systematic review and meta-analysis. . Front Hum Neurosci 12: , 416. |
[84] | Meinzer M , Lindenberg R , Darkow R , Ulm L , Copland D , Flöel A ((2014) ) Transcranial direct current stimulation and simultaneous functional magnetic resonance imaging. J Vis Exp 86: , e51730. |
[85] | Davachi L ((2009) ) Item, context and relational episodic encoding in humans. Curr Opin Neurobiol 16: , 693–700. |
[86] | Horecka KM , Dulas MR , Schwarb H , Lucas HD , Duff M , Cohen NJ ((2018) ) Reconstructing relational information. Hippocampus 28: , 164–177. |
[87] | Nyberg L ((2017) ) Functional brain imaging of episodic memory decline in ageing. J Inter Med 281: , 65–74. |
[88] | Jack CR , Knopman DS , Jaugust WJ , Petersen RC , Weiner MW , Aisen PS , Shaw LM , Vemuri P , Wiste HJ , Weigand SD , Lesnick TG , Pankratz VS , Donohue MC , Trojanowski JQ ((2013) ) Tracking pathophysiological processes in Alzheimer’s disease: An updated hypothetical model of dynamic biomarkers. Lancet Neurol 12: , 207–216. |
[89] | Roche N , Geiger M , Bussel B ((2015) ) Mechanisms underlying transcranial direct current stimulation in rehabilitation. Ann Phys Rehabil Med 58: , 214–219. |
[90] | Murugaraja V , Shivakumar V , Sinha P , Venkatasubramanian G , Sivakumar PT ((2017) ) Transcranial direct current stimulation for mild cognitive impairment. J Geriatr Ment Health 4: , 106–114. |
[91] | Das SR , Pluta J , Mancuso L , Kliot D , Yushkevich PA , Wolk DA ((2015) ) Anterior and posterior MTL networks in aging and MCI. Neurobiol Aging 36: , 141–150. |
[92] | D Vogelaere F , Santens P , Achten E , Boon P , Vingerhoets G ((2012) ) Altered default-mode network activation in mild cognitive impairment compared with healthy aging. Neuroradiology 54: , 1195–1206. |
[93] | Melrose RJ , Gimenez AM , Riskin-Jones H , Weissberger G , Veliz J , Hasratian AS , Wilkins S , Sultzer DL ((2018) ) Alterations to task positive and task negative networks during executive functioning in mild cognitive impairment. Neuroimage Clin 19: , 970–981. |
[94] | Park DC , Reuter-Lorenz P ((2009) ) The adaptive brain: Aging and neurocognitive scaffolding. Annu Rev Psychol 60: , 173–196. |
[95] | Martin AK , Meinzer M , Lindenberg R , Sieg MM , Nachtigall L , Flöel A ((2017) ) Effects of transcranial direct current stimulation on neural networks in young and older adults. J Cogn Neurosci 29: , 1817–1828. |
[96] | Möller A , Nemmi F , Karlsson K , Klinberg T ((2017) ) Transcranial electric stimulation can impair gains during working memory training and affects the resting state connectivity. Front Hum Neurosci 11: , 364. |
[97] | Smirni D , Turriziani P , Mangano GR , Cipolotti L , Oliveri M ((2015) ) Modulating memory performance in healthy subjects with transcranial direct current stimulation over the right dorsolateral prefrontal cortex. PLoS One 10: , e0144838. |
[98] | Bjekić J , Čolić MV , Živanović M , Milanović SD , Filipović SR ((2019) ) Transcranial direct current stimulation (tDCS) over parietal cortex improves associative memory. Neurobiol Learn Mem 157: , 114–120. |
[99] | Squire LR ((2004) ) Memory systems of the brain: A brief history and currenterspective. Neurobiol Learn Mem 82: , 171–177. |
[100] | Dudai Y , Karni A , Born J ((2015) ) The consolidation and transformation of memory. Neuron 88: , 20–32. |
[101] | Nitsche MA , Paulus W ((2000) ) Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 527: , 633–639. |
[102] | Nitsche MA , Paulus W ((2001) ) Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 57: , 1899–1901. |
[103] | Nitsche MA , Schauenburg A , Lang N , Liebetanz D , Exner C , Paulus W , Tergau F ((2003) ) Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. J Cogn Neurosci 15: , 619–626. |
[104] | Giordano J , Bikson M , Kappenman ES , Clark VP , Coslett HB , Hamblin MR , Hamilton R , Jankord R , Kozumbo WJ , McKinley RA , Nitsche MA , Reilly JP , Richardson J , Wurzman R , Calabrese E ((2017) ) Mechanisms and effects of transcranial direct current stimulation. Dose Response 15: , 1559325816685467. |
[105] | Hill AT , Fitzgerald PB , Hoy KE ((2016) ) Effects of anodal transcranial current stimulation on working memory: A systematic review and meta-analysis of findings from healthy and neuropsychiatric populations. Brain Stim 9: , 197–208. |
[106] | Nader K , Hardt O ((2009) ) A single standard for memory: The case for reconsolidation. Nat Rev Neurosci 10: , 224–234. |
[107] | Marián M , Szöllösi Á , Racsmány M ((2018) ) Anodal transcranial direct current stimulation of the right dorsolateral prefrontal cortex impairs long-term retention of reencountered memories. Cortex 108: , 80–91. |
[108] | Liu CS , Rau A , Gallagher D , Rajji TK , Lanctôt KL , Herrmann N ((2017) ) Using transcranial direct current stimulation to treat symptoms in mild cognitive impairment and Alzheimer‘s disease. Neurodegener Dis Manag 7: , 317–329. |
[109] | Davis NJ ((2017) ) Brain stimulation for cognitive enhancement in the older person: State of the art and future directions. J Cogn Enhanc 1: , 337–344. |
[110] | Shin YI , Foerster Á , Nitsche MA ((2015) ) Transcranial direct current stimulation (tDCS) – application in neuropsychology. Neuropsychologia 69: , 154–175. |
[111] | Bennabi D , Haffen E ((2018) ) Transcranial direct current stimulation (tDCS): A promising treatment for major depressive disorder? Brain Sci 8: , 81. |
[112] | Katz B , Au J , Buschkuehl M , Abagis T , Zabel C , Jaeggi SM , Jonides ((2017) ) Individual differences and long-term consequences of tDCS-augmented cognitive training. J Cogn Neurosci 29: , 1498–1508. |
[113] | Sarkar A , Dowker A , Cohen Kadosh R ((2014) ) Cognitive enhancement or cognitive cost: Trait-specific outcomes of brain stimulation in the case of mathematics anxiety. J Neurosci 34: , 16605–16610. |
[114] | Millet X , Raoux N , Le Carret N , Bouisson J , Dartigues JF , Amieva H ((2009) ) Gender-related differences in visuo-spatial memory persist in Alzheimer’s disease. Neuropsychol 24: , 783–789. |
[115] | Russell M , Goodman T , Wang Q , Groshong B , Lyeth BG ((2014) ) Gender differences in current received during transcranial electrical stimulation. Front Psychiatry 5: , 104. |
[116] | Jack CR , Holtzman DM ((2013) ) Biomarker model of Alzheimer’s disease. Neuron 80: , 1347–1358. |
[117] | Antonenko D , Thielscher A , Saturnino GB , Aydin S , Ittermann B , Grittner U , Flöel A ((2019) ) Towards precise brain stimulation: Is electric field simulation related to neuromodulation? Brain Stim 12: , 1159–1168. |




