Structural Brain Magnetic Resonance Imaging to Rule Out Comorbid Pathology in the Assessment of Alzheimer’s Disease Dementia: Findings from the Ontario Neurodegenerative Disease Research Initiative (ONDRI) Study and Clinical Trials Over the Past 10 Years
Abstract
Background/Objective:
Structural brain magnetic resonance imaging (MRI) is not mandatory in Alzheimer’s disease (AD) research or clinical guidelines. We aimed to explore the use of structural brain MRI in AD/mild cognitive impairment (MCI) trials over the past 10 years and determine the frequency with which inclusion of standardized structural MRI acquisitions detects comorbid vascular and non-vascular pathologies.
Methods:
We systematically searched ClinicalTrials.gov for AD clinical trials to determine their neuroimaging criteria and then used data from an AD/MCI cohort who underwent standardized MRI protocols, to determine type and incidence of clinically relevant comorbid pathologies.
Results:
Of 210 AD clinical trials, 105 (50%) included structural brain imaging in their eligibility criteria. Only 58 (27.6%) required MRI. 16,479 of 53,755 (30.7%) AD participants were in trials requiring MRI. In the observational AD/MCI cohort, 141 patients met clinical criteria; 22 (15.6%) had relevant MRI findings, of which 15 (10.6%) were exclusionary for the study.
Discussion:
In AD clinical trials over the last 10 years, over two-thirds of participants could have been enrolled without brain MRI and half without even a brain CT. In a study sample, relevant comorbid pathology was found in 15% of participants, despite careful screening. Standardized structural MRI should be incorporated into NIA-AA diagnostic guidelines (when available) and research frameworks routinely to reduce diagnostic heterogeneity.
INTRODUCTION
Neurodegeneration and neuronal injury biomarkers, including structural brain magnetic resonance imaging (MRI), have been a key component in the study of Alzheimer’s disease (AD) over the past few decades [1]. While amyloid-β (Aβ) plaques and pathologic tau remain the most important neuropathological biomarkers that characterize AD [2], other biomarkers of neurodegeneration, such as those measured by MRI, often provide insight into disease topography and severity [1]. Hippocampal and medial temporal lobe atrophy are signature markers of AD [3, 4] that are often present before clinical symptoms become manifest [5] and can signify the extent of pathology [6]. These neuroanatomical changes are especially important during the preclinical stage and may hold prognostic significance for mild cognitive impairment (MCI) to AD conversion [7]. Numerous complex image analysis methods and tools to quantify such pathology also exist and could potentially play a role in AD diagnosis [8–11]. However, since structural MRI is a nonspecific indicator of damage that could be a result of numerous etiologies [1], universally accepted quantitative biomarkers of MRI to detect AD have not been established [8, 9]. Thus, the current National Institute on Aging and Alzheimer’s Association (NIA-AA) diagnostic criteria for dementia due to AD [8] and MCI due to AD [9] do not specifically recommend either qualitative or quantitative structural brain MRI for the diagnostic workup.
The current diagnostic guidelines mention consideration of cerebrovascular disease, “extensive infarcts or severe white matter hyperintensity burden” (p. 265–266 [8]), and use of structural MRI as a marker of neuronal injury when determining diagnosis. However, in practice and likely even in clinical trials, AD or MCI patients are often diagnosed using clinical criteria and brain computed tomography (CT) scans. CT is less sensitive to small vessel disease markers such as microbleeds, perivascular spaces, small lacunes, white matter hyperintensities, and micro-infarcts [12, 13]. In addition, the presence of lobar microbleeds may suggest cerebral amyloid angiopathy [14]. These small vessel markers are predictive of future dementia risk (as well as stroke and mortality risks) [15–17] and represent potentially treatable or preventable targets that could reduce dementia expression. Although there are currently few studies comparing the accuracy of CT and MRI to detect a vascular component to dementia [18], Beynon et al. [19] found greater accuracy of MRI compared to CT for lacunar infarcts, non-lacunar infarcts, white matter hyperintensities, periventricular hyperintensities, basal ganglia hyperintensities, and global assessment (presence of two or more findings). CT may also be less sensitive to vascular changes in the hippocampus and thalamus than MRI, yet small lacunes in these areas may contribute to overall cognitive decline in the absence of a clear step-wise progression [20].
Moreover, the NIA-AA research framework [1] suggests that, while structural MRI is not specific enough to be used as a biomarker of the Alzheimer’s continuum, it may play an important role as a measure of nonspecific neurodegeneration (the “N” in the AT(N) categorization schema) [1]. For research purposes, this framework stopped short of providing specific methodologies or volumetric cut-offs (globally or regionally) that could be defined as neurodegeneration positive (N+). Similarly, the research framework steered clear of including vascular pathologies formally, although left room for addition of AT(N)(V) criteria in the future. Given that the NIA-AA diagnostic criteria did not set a cut-off for “extensive infarcts or severe white matter disease burden”, and the literature on MRI small vessel disease biomarkers is still evolving, defining a precise cut-off for vascular disease remains a challenge. Without formally requiring structural brain MRI, comorbid pathologies are often missed in routine clinical diagnosis of AD, and clinical trials in AD/MCI using NIA-AA diagnostic criteria [8, 9] can suffer from increased heterogeneity, even though recent anti-amyloid trials now report amyloid biomarker progression in cerebrospinal fluid and in brain using PET. Given the almost universally negative results of treatment trials, and the 99.6% failure rate of AD clinical drug trials between 2002 and 2012 [21], consideration of vascular factors and comorbid or competing pathologies is vital [22, 23].
The aim of this paper was to 1) explore the use of structural brain MRI in AD/MCI trials over the past 10 years by examining data from ClinicalTrials.gov and 2) to determine the frequency with which inclusion of standardized structural MRI acquisitions detects comorbid vascular and non-vascular pathologies by examining the frequency of incidental or exclusionary MRI findings in a cohort of patients with detailed, clinically-defined AD/MCI participating in the Ontario Neurodegenerative Disease Research Initiative (ONDRI) study.
METHODS
Aim 1
We examined data from ClinicalTrials.gov (accessed May 17, 2018) for AD clinical trials over the past 10 years. ClinicalTrials.gov is web-based resource maintained by the National Library of Medicine (NLM) at the National Institutes of Health (NIH) that provides information on publicly and privately supported clinical studies.
We used the search term “Alzheimer’s Disease” as categorized by ClinicalTrials.gov within the “condition or disease” search field. We limited our search to completed, suspended and terminated interventional studies in phase II and III which started on or after January 1, 2008. Extension and follow-up studies were excluded. Inclusion and exclusion criteria listed on ClinicalTrials.gov for each study were reviewed to determine whether brain imaging-based eligibility criteria were included in each trial. We extracted study title and ID, study status, trial phase, start date, number of subjects enrolled or estimated enrollment (if results were not yet reported), intervention/treatment and specifically, neuroimaging-based eligibility criteria for each study.
Aim 2
The overall design of the ONDRI study has previously been reported elsewhere [24]. A total of 521 participants with vascular cognitive impairment (n = 161), AD/MCI (n = 126), amyotrophic lateral sclerosis (n = 40), frontotemporal dementia (n = 53), and Parkinson’s disease (n = 141) have been enrolled into this longitudinal study from centers throughout Ontario. General inclusion and exclusion criteria can be found in Supplementary Table 1. Inclusion criteria for the AD/MCI cohort were: 1) 45–90 years of age, 2) meet the NIA-AA core clinical criteria for probable AD or amnestic single or multiple domain MCI, and 3) non-AD causes of dementia ruled out by standardized work up for dementia including brain imaging and blood work screen. Participant with untreated major depression within 90 days of the screening visit, substance abuse, or other significant psychiatric disorder and a non-amnestic presentation (e.g., language, visuospatial, or executive function) of AD or MCI were excluded. Each participant underwent six different platform assessments including neuroimaging, neuropsychology, neuropathology, genomics, ocular (eye movements and retinal imaging), and gait and balance assessments. Assessments details are reported in detail elsewhere [24].
The neuroimaging protocol for ONDRI has been previously described [24]. Briefly, six different sequences are run on each study participant, require approximately 1 h and include the following: 1) three-dimensional T1-weighted anatomical scan used for volumetric assessment of brain structures, 2) proton density/T2-weighted scan used for the assessment of tissue ischemia and skull stripping and better distinction of perivascular spaces from lacunes, 3) fluid-attenuated inversion recovery for the assessment of white matter hyperintensities, 4) gradient echo for the assessment of tissue microbleeds, 5) resting state functional MRI for the evaluation of brain network activity, and 6) diffusion tensor imaging for the evaluation of white matter structural integrity and connectivity. A board-certified research neuroradiologist reviewed the structural images to identify incidental findings or exclusionary pathology [24]. Incidental findings were defined as possible non-AD causes of dementia (e.g., strokes, lacunes, tumors, etc.) ruled out by standardized brain imaging as outlined in the ONDRI imaging protocol [24]. ONDRI AD/MCI and neuroimaging lead investigators reviewed the findings to determine whether the findings could be contributory to dementia symptom expression and/or should be excluded by current clinical criteria for probable AD/MCI [8] (Table 1). These findings were initially either not identified, not disclosed or not reported through standardized clinical screening and assessment.
Table 1
Incidental MRI Findings in the ONDRI AD/MCI Cohort
| Pathology | Exclusionary | |
| 1 | Basilar aneurysm | No |
| 2 | Multiple cortical lesions | Yes |
| 3 | Infarct in pons | No |
| 4 | Bilateral subdural hematoma | No |
| 5 | Severe white matter hyperintensity burden | Yes |
| 6 | Temporal lobe infarct &severe white matter hyperintensity burden | Yes |
| 7 | Mass lesion in gyrus rectus | No |
| 8 | Severe white matter hyperintensity burden µ/macrobleeds | Yes |
| 9 | Sphenoid mass lesion | No |
| 10 | Severe white matter hyperintensity burden &bifrontal hygroma | Yes |
| 11 | Severe white matter hyperintensity burden &sub-arachnoid cyst | Yes |
| 12 | Basal ganglia infarct (bilateral putaminal) | Yes |
| 13 | Inferior frontal infarct | Yes |
| 14 | Cortical infarct | Yes |
| 15 | Left thalamic lacune | Yes |
| 16 | Traumatic brain injury &severe white matter hyperintensity burden | Yes |
| 17 | Subdural hygroma | No |
| 18 | Temporal lobectomy | Yes |
| 19 | Venous ischemic edema | No |
| 20 | Medial temporal lobe infarct | Yes |
| 21 | Basal ganglia, occipital, cerebellar infarcts | Yes |
| 22 | Thalamic lacunes | Yes |
RESULTS
Aim 1
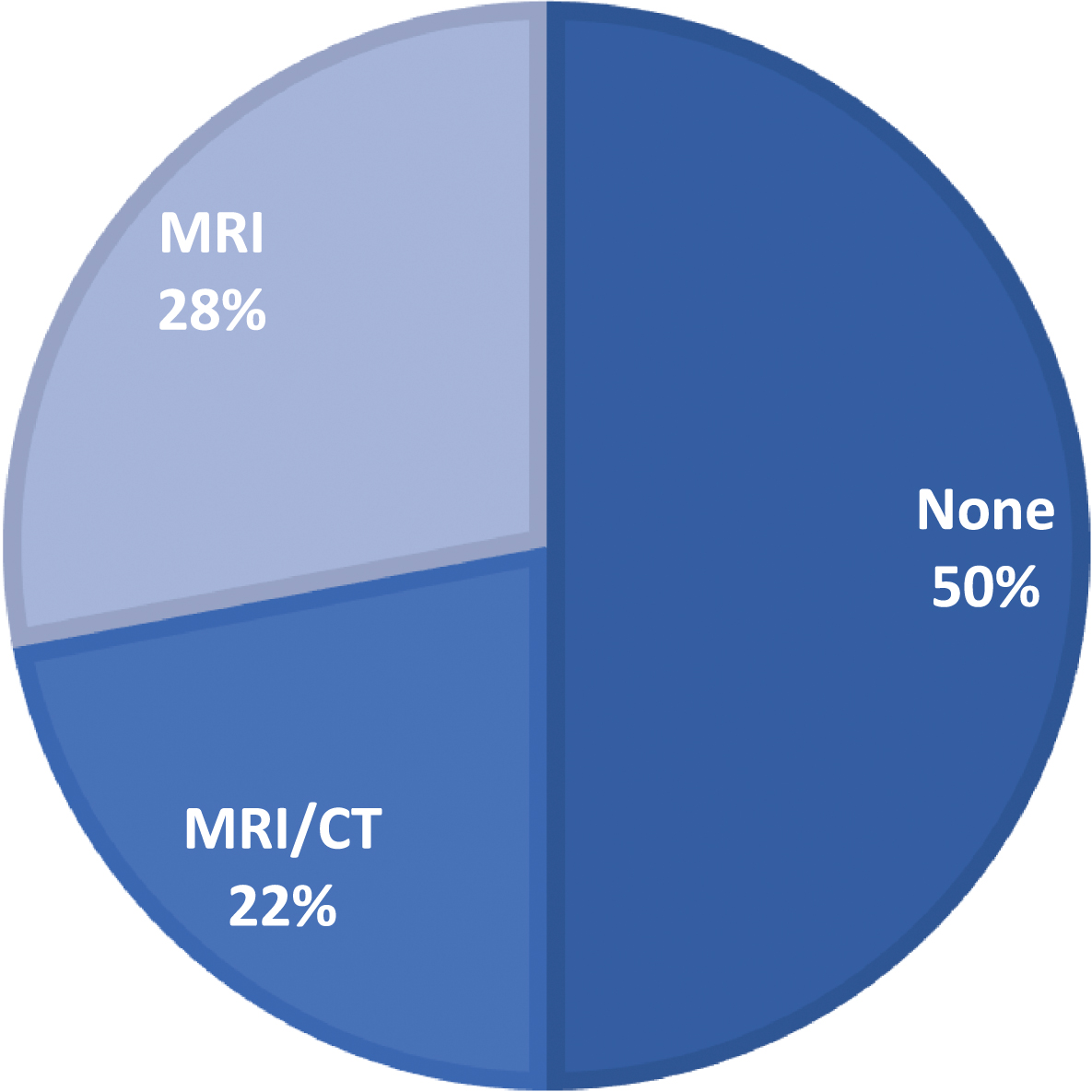
A review of 210 AD interventional clinical trials since 2008, including 53,755 patients, revealed that only 105 (50%) trials included any neuroimaging (i.e., indicated “CT”, “MRI”, “brain imaging”, or “neuroimaging”) in the exclusion or inclusion criteria (Fig. 1; neuroimaging-based eligibility criteria for each study can be found in Supplementary Table 4). Of those that did include neuroimaging in the exclusion or inclusion or both criteria, only 58 (27.6% total; 55.2% of trials with any brain imaging) specified MRI. The trials requiring MRI included 16,479 patients, representing 30.7% of all patients in AD interventional trials. We did not observe a trend of increasing MRI inclusion with time. Running the same search within the past year identified 6 new studies, and similar trends were observed, with some studies that included CT or MRI and none that specifically required MRI within the eligibility criteria.
Fig.1
Frequency of inclusion of structural MRI in eligibility criteria of AD clinical trials.
We then further restricted our review to: 1) Phase III trials, 2) with results available on ClinicalTrials.gov, 3) sample size greater than 100, and 4) cognitive primary outcome. Twelve studies were identified (Table 2). Review of published articles and protocols (if available) and results reported on ClinicalTrials.gov from these studies revealed that all studies required CT or MRI; however, MRI specifically was only required by half the studies (N = 6, 50%). Three of the twelve studies were conducted by the same investigators [25–27]. Of the studies that did not report requiring MRI, one study specifically mentioned that patients were not excluded for vascular abnormalities on CT or MRI [28], and one reported that only a subset of patients received MRI at baseline [25]. We conducted this same search using the EU Clinical Trials Register and WHO Clinical Trial Database, which yielded similar results, with few trials specifying MRI in the inclusion/exclusion criteria.
Table 2
Phase III Clinical Trials over Past 10 Years with Cognitive Primary Outcome
| ID | N | Interventions | Primary Outcome | Severity | MRI/CT Required | MRI Specifically Required | |
| AD Drug Trials with Cognitive Primary Outcome | |||||||
| 1 | NCT00762411 | 1111 | Drug: LY450139 | ADAS-Cog &ADCS-ADL | Mild-Moderate | x | x |
| 2 | NCT00679627 [28, 41] | 2051 | Drug: Galantamine | MMSE &Number of Deaths Reported | Mild-Moderate | x | |
| 3 | NCT01900665 [42] | 2129 | Drug: Solanezumab | ADAS-Cog | Mild | x | x |
| 4 | NCT02006641 [27] | 858 | Drug: Idalopirdine | ADAS-Cog | Mild-Moderate | x | |
| 5 | NCT01955161 [27] | 933 | Drug: Idalopirdine | ADAS-Cog | Mild-Moderate | x | |
| 6 | NCT02006654 [27] | 734 | Drug: Idalopirdine | ADAS-Cog | Mild-Moderate | x | |
| 7 | NCT01524887 | 508 | Biological: IGIV, 10% | ADAS-Cog &ADCS-ADL | Mild-Moderate | x | x |
| 8 | NCT01399125 | 501 | Drug: Rivastigmine | ADAS-Cog | Moderate | x | |
| 9 | NCT00818662 [43] | 390 | Biological: IGIV, 10% | ADAS-Cog &ADCS-ADL | Mild-Moderate | x | x |
| 10 | NCT00594568 [25] | 1537 | Drug: LY450139 | ADAS-Cog &ADCS-ADL | Mild-Moderate | x | |
| 11 | NCT00676143 [26] | 1100 | Drug: Bapineuzumab | ADAS-Cog &DAD | Mild-Moderate | x | x |
| 12 | NCT00667810 [26] | 901 | Drug: Bapineuzumab | ADAS-Cog &DAD | Mild-Moderate | x | x |
ADAS-Cog, Alzheimer’s Disease Assessment Scale-Cognitive subscale; ADCS-ADL, Alzheimer’s Disease Cooperative Study - Activities of Daily Living; DAD, Disability Assessment for Dementia; MMSE, Mini-Mental State Examination.
Aim 2
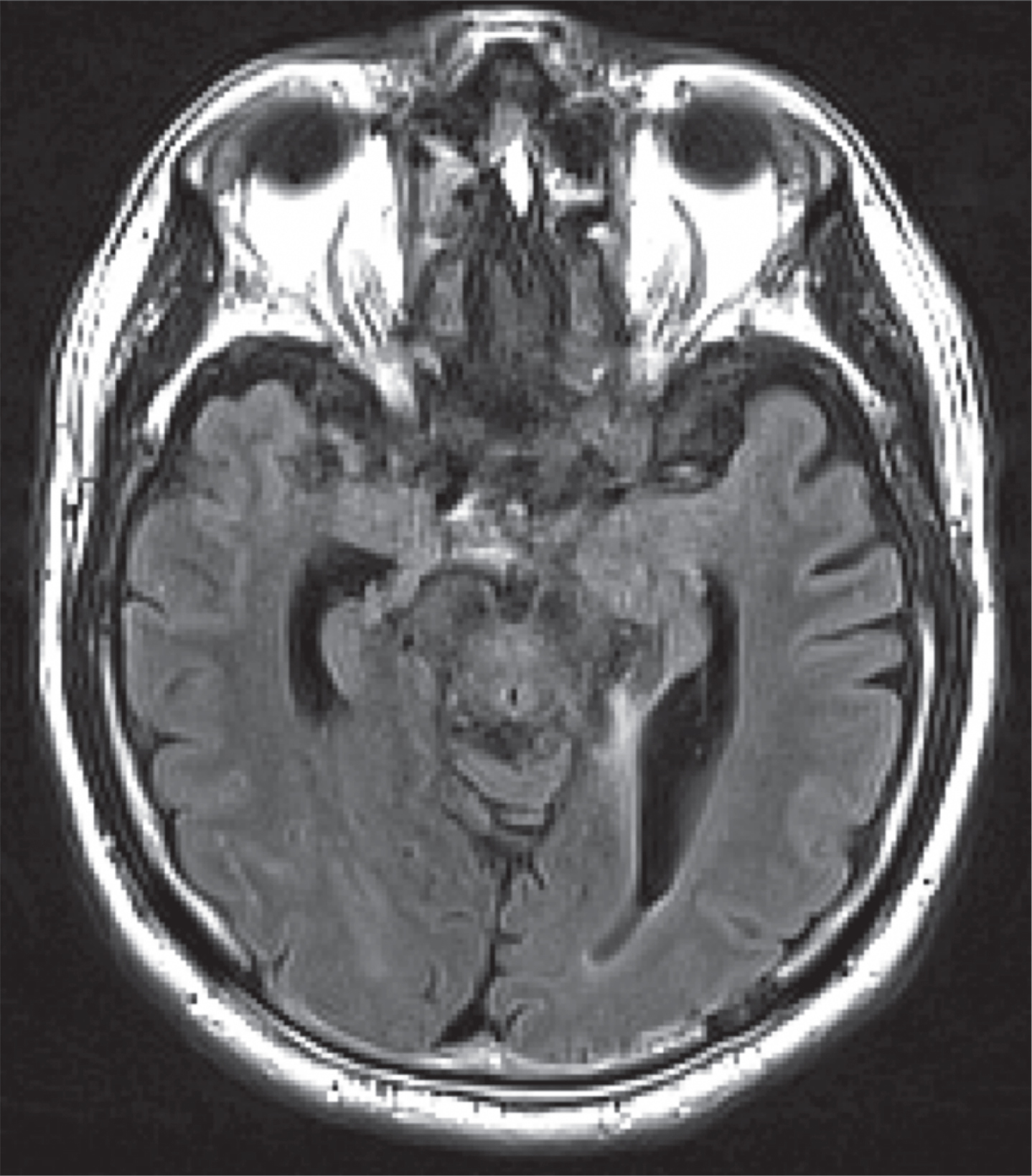
One hundred and forty-one patients met clinical criteria for the ONDRI AD/MCI cohort. Review of standardized structural MRIs from these patients revealed 22 cases (15.6%) of incidental findings, of which 15 cases (10.6%) were identified as exclusionary pathology from the AD/MCI cohort (Table 1). After the exclusion of those 15 cases, 126 patients remained in the AD/MCI cohort. Imaging exclusions included large vessel strokes, smaller strategic infarcts, tumors and prior undisclosed neurosurgeries (Figs. 2–5). Of those excluded from the AD/MCI cohort, 8 transferred to the vascular cognitive impairment cohort (which could include mixed disease) and 7 were excluded from the study due to pathologies such as traumatic brain injury, temporal lobectomy and sub-arachnoid cyst (Table 1).
Fig.2
MR FLAIR image of patient with basal ganglia infarcts.
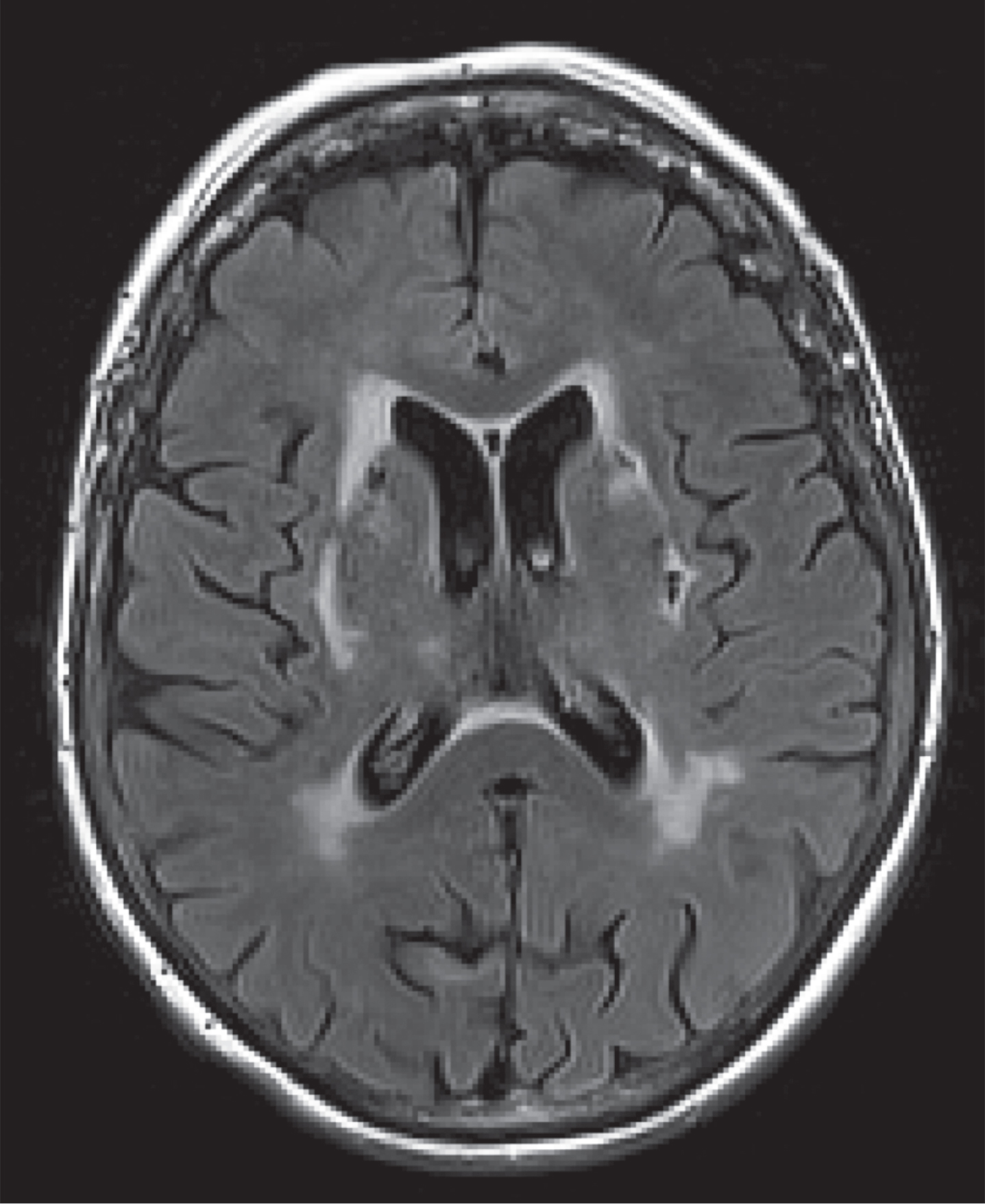
Fig.3
MR T1 of patient with remote temporal lobectomy, not reported by the patient.
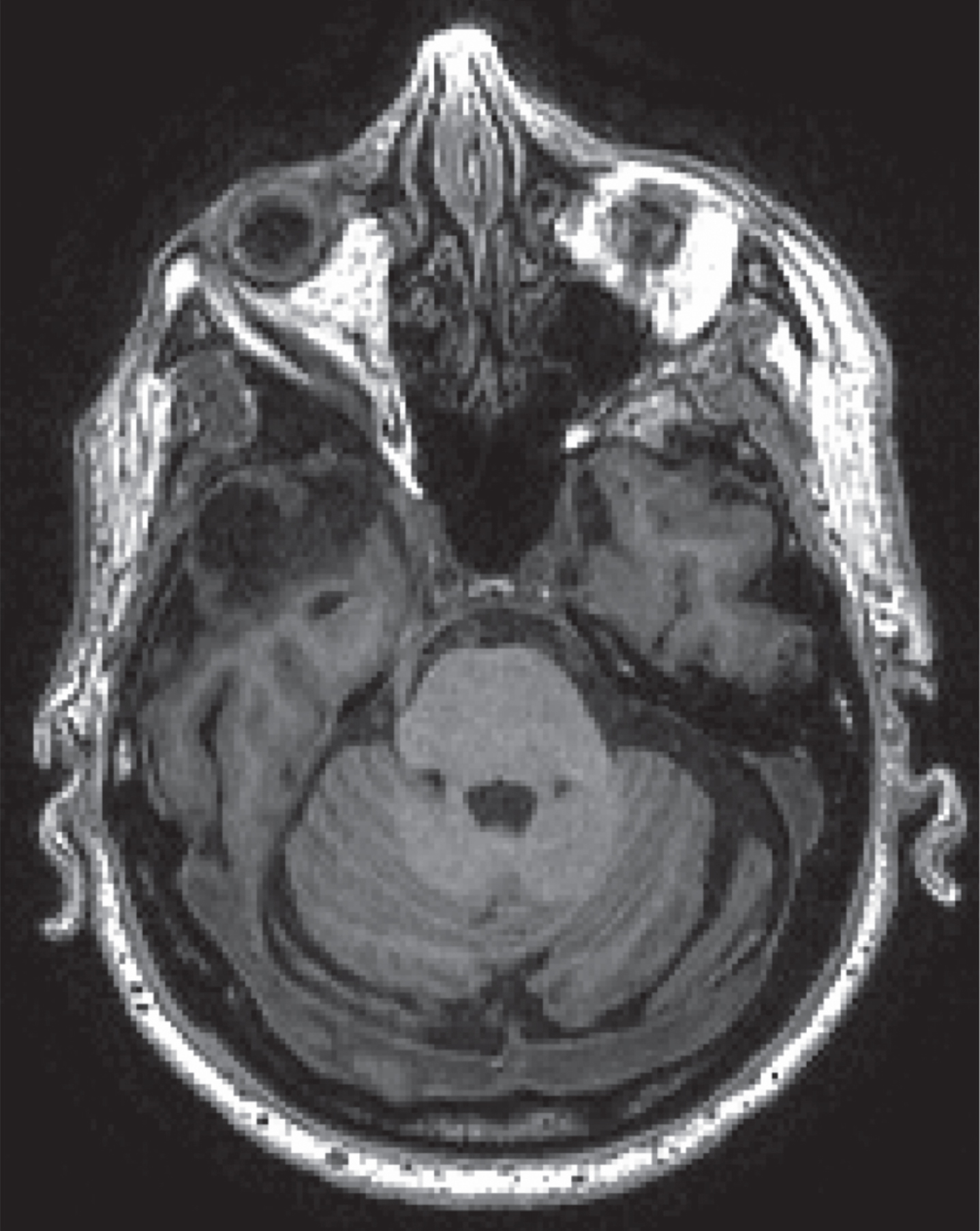
Fig.4
MR T2 of patient with left anterior thalamic lacune.
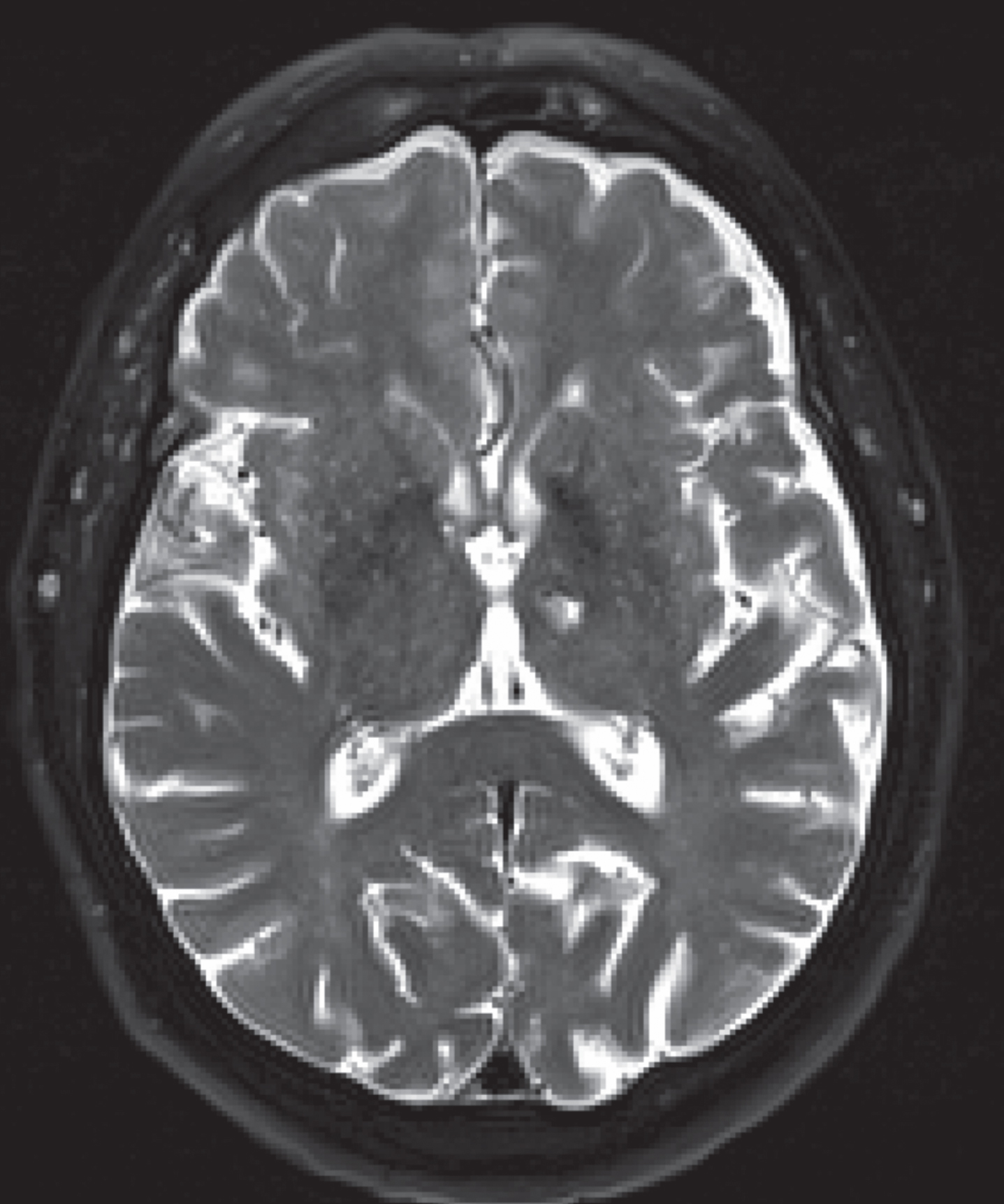
Fig.5
Left medial temporal lobe infarct taking out entire posterior hippocampus.
DISCUSSION
Quantitative measures of brain MRI may not be feasible yet as a biomarker of the Alzheimer’s continuum, but standardized, qualitative, structural brain MRI may serve an important role in the diagnosis and classification of AD by identifying comorbid pathologies that may alter treatment and prognosis, and increase heterogeneity within clinical trials. Our results demonstrate that inclusion of structural brain MRI sequences could identify comorbid or alternate pathologies in over 15% of patients, as identified in the ONDRI study as part of inclusion/exclusion criteria evaluation. Yet, less than a third of AD interventional clinical trials patients had a brain MRI as part of their inclusion/exclusion criteria. It is thus possible that over 5,500 clinical trials patients (15% of non-MRI required trials participants) may have had concomitant non-AD or non-concomitant pathologies. These results have implications for current NIA-AA AD and MCI diagnostic criteria as well as for current and future clinical trial designs.
The NIA-AA workgroups identified the “lack of acknowledgement of distinguishing features of other dementing conditions that occur in aged populations” (p. 264 [8]) as a limitation of the 1984 NINCDS-ADRDA diagnostic criteria that required revision. New guidelines recommend consideration of features of other dementias and neurological diseases and encourage efforts to incorporate biomarkers into the diagnosis of AD/MCI. However, qualitative review of brain MRI findings is currently not specifically incorporated within these guidelines. Likewise, the use of structural brain MRI to identify vascular disease, other comorbidities, and alternate diagnosis is not required in the Diagnostic and Statistical Manual of Mental Disorders (DSM-V) or in the International Classification of Diseases (ICD-10) Classification of Mental and Behavioral Disorders diagnostic guidelines for AD (Supplementary Tables 2 and 3). While all criteria mention that the absence of other neurodegenerative diseases or cerebrovascular disease must be established, none specifically acknowledges the value of clinical review of structural brain MRI findings. Moreover, structural imaging is not required by Canadian Consensus Guidelines for Dementia [29]. Yet vascular disease and other comorbidities (e.g., tumors, unrecalled traumas, or surgeries) can affect expression of dementia and must be considered.
Subtle vascular disease (lacunar small vessel disease, small cortical infarcts, and diffuse white matter changes) may impact clinical presentation, and will have significant treatment implications (e.g., detection and treatment of vascular risk factors) that could alter long-term outcomes. In a study of 61 dementia patients from the Columbia University Alzheimer’s Disease Research Center, Massoud et al. [30] found that structural MRI detected 13 cases of stroke that had not been clinically suspected. Similarly, Hentschel et al, [31] found that inclusion of neuropsychological test results and MRI findings changed the initial clinical diagnosis for 26% of dementia patients referred to a university memory clinic (e.g., neurodegenerative dementia to vascular dementia). Our findings are in line with these previous studies and reinforce the value of structural brain MRI in the differential diagnosis of AD.
ONDRI as well as other projects have contributed to the development of and adopted the harmonized Canadian Dementia Imaging Protocol [32, 33] (http://www.cdip-pcid.ca), which aims to harmonize the acquisition of magnetic resonance images. At minimum, we recommend a 3D T1-weighted scan (1 mm), FLAIR at 1.5T, and gradient echo. At 3T, 3D T1 and FLAIR allow cortical microinfarcts to be recognized [34, 35]. Such findings may suggest cardiovascular disease, and could have treatment implications, requiring a cardiac workup, including echo and arrhythmia monitoring. This could lead to use of anticoagulants; however, multiple lobar microbleeds would require caution [36], further emphasizing the need to evaluate vascular pathologies.
Our results also have significant implications for clinical trials. The NIA-AA research framework recommend AD categorizations by pathology (Aβ, tau, neurodegeneration). Neurodegenerative/neuronal injury biomarkers, including structural MRI, are not specific to AD. However, without requiring brain MRI, clinical trials may be increasing heterogeneity within study samples. This may be acceptable for trials of non-specific interventions targeting diverse or “real-world” cohorts. But for AD-specific therapies, review of previous clinical trials elucidates that solely relying on clinical diagnosis of AD may be insufficient to assess eligibility for clinical trials [37]. Even if amyloid PET or cerebrospinal fluid biomarkers are detected—as is now the case for many anti-amyloid trials [37]—confirming diagnosis and awareness of vascular pathologies through multiple biomarkers may be necessary to ensure a homogeneous sample, or should be at least be acknowledged to stratify results and ensure vascular prevention measures are in place prior to randomization.
Despite its value in the assessment of AD, there are limitations to the use of brain MRI in the assessment of AD. As a measure of neurodegeneration, atrophy patterns seen on MRI are non-specific and MRI currently does not allow quantification burden of cerebral Aβ, tau, or other proteinopathies. Nevertheless, with deep learning and quantitative volumetry, hippocampal volumes and signature cortical thinning patterns could support diagnostic impressions. This is expected to become available in the near future. In the meantime, appropriate MRI acquisitions can currently assess possible alternative contributory causes and reinforce diagnostic specificity. Sequential MRI and longitudinal measurements of hippocampal volume could further provide additional value in assessment of AD and MCI [38]. It is important to note, however, that tolerability, safety, and feasibility of MRI use, and inclusion of MRI in clinical diagnostic criteria for advanced dementia could be challenging. The findings of this study and inclusion of MRI in clinical diagnosis criteria may therefore be more applicable and feasible for MCI and mild to moderate AD. Moreover, compared to CT, the cost of MRI is higher, MRI has more contraindications and is usually less available in most jurisdictions. Access to MRI is therefore more limited for clinical use. However, these considerations are no longer limitations for research purposes at many institutions. MRI may be able to identify small vessel disease, small cortical infarcts, and vascular disease in the deep grey matter structures (especially the thalamus and hippocampus [20])—that CT is insensitive to—that could alter diagnoses, treatment, and management of AD. It can also reveal specific patterns of degeneration that can be seen in the prodromal stage of AD, which can be modified by concurrent vascular pathologies.
This study highlights the need for considering structural brain MRI in the diagnosis of AD and MCI and in the eligibility criteria for clinical trials. Many publications are examining the role of MRI-based biomarkers in improving the accuracy of MCI or AD diagnoses and in predicting conversion or decline [39]; however, the literature on frequency of management-altering findings from structural MRI scans is much more sparse. Participants with these findings are, if identified on imaging, sometimes excluded from research studies of AD/MCI. Given the results of the current study, future studies should explore the cost-effectiveness of more widespread MRI imaging in the diagnosis of AD and MCI. Moreover, to improve diagnostic accuracy and decrease heterogeneity within clinical trials, future research should explore additional strategies to aid differential diagnosis. Imaging analytics (including measures of vascular changes), neuropsychological assessments, genomics, behavioral measures (e.g., gait and balance), clinical and laboratory measures, retinal imaging, and eye tracking [24] could potentially further enhance AD and MCI assessment. Furthermore, automated and quantitative MRI measures and analyses, and the use of other MRI modalities—such as diffusion tensor imaging, magnetic resonance spectroscopy, functional fMRI, pseudo-continuous arterial spin-labelled MRI—in AD/MCI assessment warrant further exploration.
One limitation of this study is that we could not account for patients who received a brain MRI in the community prior to screening for the ONDRI study. It is possible that clinical structural brain MRIs identified comorbid or alternate diagnoses, and these patients were not subsequently screened or included in the ONDRI study. This could have resulted in an underestimation of the burden of comorbid pathologies that might be seen in a first presentation cohort; however it is likely representative of the types of patients referred for clinical trials as these often come through specialty centers where routine clinical imaging has been performed. Moreover, in the ONDRI study, we still identified pathologies during the research screening that were not reported, not identified, or perhaps not disclosed during the clinical screening. In addition, our review of previous clinical trials was based on data reported on ClinicalTrials.gov; studies that were not registered on the website, or those that did not report complete eligibility criteria or study results on the website may influence our findings. Moreover, some studies may have required imaging measures by requiring a clinical diagnosis of AD, but not as part of the research screening for inclusion. It is also noteworthy that while many studies did not specify the use of MRI, a subset of participants in those studies may still have received an MRI. Despite these limitations, this study suggests that the use and utility of structural brain MRIs in AD/MCI assessment warrants further attention.
Despite decades of AD drug trials, there have been no successful disease modifying trials. Given the lack of reliable biomarkers, most trials use clinical progression, which is highly variable, as trial endpoints. The results of this study elucidate that lack of recognition of disease modifying co-pathologies or misdiagnosis may be contributing to increased heterogeneity within study samples and negative trials. Our results indicate that, to increase their potential for success, dementia clinical trials should include structural brain imaging protocols—ideally with MRI (e.g., ONDRI imaging protocol [24] or HARNESS initiative [40])—to account for real-world disease heterogeneity, and diagnostic guidelines should consider endorsing standardized structural brain MRI protocols in disease diagnosis and management.
ACKNOWLEDGMENTS
This research was conducted with the support of the Ontario Brain Institute, an independent non-profit corporation, funded partially by the Ontario government. The opinions, results, and conclusions are those of the authors and no endorsement by the Ontario Brain Institute is intended or should be inferred. Matching funds were provided by participant hospital and research foundations, including the Baycrest Foundation, Bruyere Research Institute, Centre for Addiction and Mental Health Foundation, London Health Sciences Foundation, McMaster University Faculty of Health Sciences, Ottawa Brain and Mind Research Institute, Queen’s University Faculty of Health Sciences, the Thunder Bay Regional Health Sciences Centre, the University of Ottawa Faculty of Medicine, and the Windsor/Essex County ALS Association. The Temerty Family Foundation provided the major infrastructure matching funds.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/19-1097r1).
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-191097.
REFERENCES
[1] | Jack CR , Bennett DA , Blennow K , Carrillo MC , Dunn B , Haeberlein SB , Holtzman DM , Jagust W , Jessen F , Karlawish J , Liu E , Molinuevo JL , Montine T , Phelps C , Rankin KP , Rowe CC , Scheltens P , Siemers E , Snyder HM , Sperling R , Contributors ((2018) ) NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14: , 535–562. |
[2] | Hardy J , Selkoe DJ ((2002) ) The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 297: , 353–356. |
[3] | Jack CR , Petersen RC , O’Brien PC , Tangalos EG ((1992) ) MR-based hippocampal volumetry in the diagnosis of Alzheimer’s disease. Neurology 42: , 183–188. |
[4] | Krasuski JS , Alexander GE , Horwitz B , Daly EM , Murphy DG , Rapoport SI , Schapiro MB ((1998) ) Volumes of medial temporal lobe structures in patients with Alzheimer’s disease and mild cognitive impairment (and in healthy controls). Biol Psychiatry 43: , 60–68. |
[5] | Fox NC , Warrington EK , Freeborough PA , Hartikainen P , Kennedy AM , Stevens JM , Rossor MN ((1996) ) Presymptomatic hippocampal atrophy in Alzheimer’s disease. A longitudinal MRI study. Brain 119 (Pt 6): , 2001–2007. |
[6] | Henneman WJP , Sluimer JD , Barnes J , van der Flier WM , Sluimer IC , Fox NC , Scheltens P , Vrenken H , Barkhof F ((2009) ) Hippocampal atrophy rates in Alzheimer disease: Added value over whole brain volume measures. Neurology 72: , 999–1007. |
[7] | Jack CR , Shiung MM , Weigand SD , O’Brien PC , Gunter JL , Boeve BF , Knopman DS , Smith GE , Ivnik RJ , Tangalos EG , Petersen RC ((2005) ) Brain atrophy rates predict subsequent clinical conversion in normal elderly and amnestic MCI. Neurology 65: , 1227–1231. |
[8] | McKhann GM , Knopman DS , Chertkow H , Hyman BT , Jack CR , Kawas CH , Klunk WE , Koroshetz WJ , Manly JJ , Mayeux R , Mohs RC , Morris JC , Rossor MN , Scheltens P , Carrillo MC , Thies B , Weintraub S , Phelps CH ((2011) ) The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: , 263–269. |
[9] | Albert MS , DeKosky ST , Dickson D , Dubois B , Feldman HH , Fox NC , Gamst A , Holtzman DM , Jagust WJ , Petersen RC , Snyder PJ , Carrillo MC , Thies B , Phelps CH ((2011) ) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: , 270–279. |
[10] | Basaia S , Agosta F , Wagner L , Canu E , Magnani G , Santangelo R , Filippi M , Alzheimer’s Disease Neuroimaging Initiative ((2018) ) Automated classification of Alzheimer’s disease and mild cognitive impairment using a single MRI and deep neural networks. Neuroimage Clin 21: , 101645. |
[11] | Brickman AM , Tosto G , Gutierrez J , Andrews H , Gu Y , Narkhede A , Rizvi B , Guzman V , Manly JJ , Vonsattel JP , Schupf N , Mayeux R ((2018) ) An MRI measure of degenerative and cerebrovascular pathology in Alzheimer disease. Neurology 91: , e1402–e1412. |
[12] | Kidwell CS , Saver JL , Villablanca JP , Duckwiler G , Fredieu A , Gough K , Leary MC , Starkman S , Gobin YP , Jahan R , Vespa P , Liebeskind DS , Alger JR , Vinuela F ((2002) ) Magnetic resonance imaging detection of microbleeds before thrombolysis: An emerging application. Stroke 33: , 95–98. |
[13] | Wardlaw JM , Smith EE , Biessels GJ , Cordonnier C , Fazekas F , Frayne R , Lindley RI , O’Brien JT , Barkhof F , Benavente OR , Black SE , Brayne C , Breteler M , Chabriat H , Decarli C , de Leeuw F-E , Doubal F , Duering M , Fox NC , Greenberg S , Hachinski V , Kilimann I , Mok V , Oostenbrugge R van , Pantoni L , Speck O , Stephan BCM , Teipel S , Viswanathan A , Werring D , Chen C , Smith C , van Buchem M , Norrving B , Gorelick PB , Dichgans M , STandards for ReportIng Vascular changes on nEuroimaging (STRIVE v1) ((2013) ) Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 12: , 822–838. |
[14] | Mesker DJ , Poels MMF , Ikram MA , Vernooij MW , Hofman A , Vrooman HA , van der Lugt A , Breteler MMB ((2011) ) Lobar distribution of cerebral microbleeds. Arch Neurol 68: , 656–659. |
[15] | Romero JR , Beiser A , Himali JJ , Shoamanesh A , DeCarli C , Seshadri S ((2017) ) Cerebral microbleeds and risk of incident dementia: The Framingham Heart Study. Neurobiol Aging 54: , 94–99. |
[16] | Ding J , Sigurðsson S , Jónsson PV , Eiriksdottir G , Charidimou A , Lopez OL , van Buchem MA , Guðnason V , Launer LJ ((2017) ) Large perivascular spaces visible on magnetic resonance imaging, cerebral small vessel disease progression, and risk of dementia: The Age, Gene/Environment Susceptibility-Reykjavik Study. JAMA Neurol 74: , 1105–1112. |
[17] | Zeestraten EA , Lawrence AJ , Lambert C , Benjamin P , Brookes RL , Mackinnon AD , Morris RG , Barrick TR , Markus HS ((2017) ) Change in multimodal MRI markers predicts dementia risk in cerebral small vessel disease. Neurology 89: , 1869–1876. |
[18] | Health Quality Ontario ((2014) ) The appropriate use of neuroimaging in the diagnostic work-up of dementia: An evidence-based analysis. Ont Health Technol Assess Ser 14: , 1–64. |
[19] | Beynon R , Sterne JAC , Wilcock G , Likeman M , Harbord RM , Astin M , Burke M , Bessell A , Ben-Shlomo Y , Hawkins J , Hollingworth W , Whiting P ((2012) ) Is MRI better than CT for detecting a vascular component to dementia? A systematic review and meta-analysis. BMC Neurol 12: , 33. |
[20] | Swartz RH , Black SE ((2006) ) Anterior-medial thalamic lesions in dementia: Frequent, and volume dependently associated with sudden cognitive decline. J Neurol Neurosurg Psychiatry 77: , 1307–1312. |
[21] | Cummings JL , Morstorf T , Zhong K ((2014) ) Alzheimer’s disease drug-development pipeline: Few candidates, frequent failures. Alzheimers Res Ther 6: , 37. |
[22] | Sweeney MD , Montagne A , Sagare AP , Nation DA , Schneider LS , Chui HC , Harrington MG , Pa J , Law M , Wang DJJ , Jacobs RE , Doubal FN , Ramirez J , Black SE , Nedergaard M , Benveniste H , Dichgans M , Iadecola C , Love S , Bath PM , Markus HS , Salman RA , Allan SM , Quinn TJ , Kalaria RN , Werring DJ , Carare RO , Touyz RM , Williams SCR , Moskowitz MA , Katusic ZS , Lutz SE , Lazarov O , Minshall RD , Rehman J , Davis TP , Wellington CL , González HM , Yuan C , Lockhart SN , Hughes TM , Chen CLH , Sachdev P , O’Brien JT , Skoog I , Pantoni L , Gustafson DR , Biessels GJ , Wallin A , Smith EE , Mok V , Wong A , Passmore P , Barkof F , Muller M , Breteler MMB , Román GC , Hamel E , Seshadri S , Gottesman RF , van Buchem MA , Arvanitakis Z , Schneider JA , Drewes LR , Hachinski V , Finch CE , Toga AW , Wardlaw JM , Zlokovic BV ((2019) ) Vascular dysfunction-The disregarded partner of Alzheimer’s disease. Alzheimers Dement 15: , 158–167. |
[23] | Jagust W , Jack CR , Bennett DA , Blennow K , Haeberlein SB , Holtzman DM , Jessen F , Karlawish J , Liu E , Molinuevo JL , Montine T , Phelps C , Rankin KP , Rowe CC , Scheltens P , Siemers E , Sperling R ((2019) ) Alzheimer’s disease is neither Alzheimer’s clinical syndrome nor dementia. Alzheimers Dement 15: , 153–157. |
[24] | Farhan SMK , Bartha R , Black SE , Corbett D , Finger E , Freedman M , Greenberg B , Grimes DA , Hegele RA , Hudson C , Kleinstiver PW , Lang AE , Masellis M , McIlroy WE , McLaughlin PM , Montero-Odasso M , Munoz DG , Munoz DP , Strother S , Swartz RH , Symons S , Tartaglia MC , Zinman L , ONDRI Investigators, Strong MJ ((2017) ) The Ontario Neurodegenerative Disease Research Initiative (ONDRI). Can J Neurol Sci 44: , 196–202. |
[25] | Doody RS , Raman R , Farlow M , Iwatsubo T , Vellas B , Joffe S , Kieburtz K , He F , Sun X , Thomas RG , Aisen PS , Alzheimer’s Disease Cooperative Study Steering Committee, Siemers E , Sethuraman G , Mohs R , Semagacestat Study Group ((2013) ) A phase 3 trial of semagacestat for treatment of Alzheimer’s disease. N Engl J Med 369: , 341–50. |
[26] | Vandenberghe R , Rinne JO , Boada M , Katayama S , Scheltens P , Vellas B , Tuchman M , Gass A , Fiebach JB , Hill D , Lobello K , Li D , McRae T , Lucas P , Evans I , Booth K , Luscan G , Wyman BT , Hua L , Yang L , Brashear HR , Black RS , Bapineuzumab 3000 and 3001 Clinical Study Investigators ((2016) ) Bapineuzumab for mild to moderate Alzheimer’s disease in two global, randomized, phase 3 trials. Alzheimers Res Ther 8: , 18. |
[27] | Atri A , Frölich L , Ballard C , Tariot PN , Molinuevo JL , Boneva N , Windfeld K , Raket LL , Cummings JL ((2018) ) Effect of idalopirdine as adjunct to cholinesterase inhibitors on change in cognition in patients with Alzheimer disease: Three randomized clinical trials. JAMA 319: , 130–142. |
[28] | Hager K , Baseman AS , Nye JS , Brashear HR , Han J , Sano M , Davis B , Richards HM ((2016) ) Effect of concomitant use of memantine on mortality and efficacy outcomes of galantamine-treated patients with Alzheimer’s disease: Post-hoc analysis of a randomized placebo-controlled study. Alzheimers Res Ther 8: , 47. |
[29] | Gauthier S , Patterson C , Chertkow H , Gordon M , Herrmann N , Rockwood K , Rosa-Neto P , Soucy J-P ((2012) ) Recommendations of the 4th Canadian Consensus Conference on the Diagnosis and Treatment of Dementia (CCCDTD4). Can Geriatr J 15: , 120–126. |
[30] | Massoud F , Devi G , Moroney JT , Stern Y , Lawton A , Bell K , Marder K , Mayeux R ((2000) ) The role of routine laboratory studies and neuroimaging in the diagnosis of dementia: A clinicopathological study. J Am Geriatr Soc 48: , 1204–1210. |
[31] | Hentschel F , Kreis M , Damian M , Krumm B , Frölich L ((2005) ) The clinical utility of structural neuroimaging with MRI for diagnosis and differential diagnosis of dementia: A memory clinic study. Int J Geriatr Psychiatry 20: , 645–650. |
[32] | Potvin O , Chouinard I , Dieumegarde L , Bartha R , Bellec P , Collins DL , Descoteaux M , Hoge R , Ramirez J , Scott CJM , Smith EE , Strother SC , Black SE , Duchesne S ; CIMA-Q group; CCNA group ((2019) ) The Canadian Dementia Imaging Protocol: Harmonization validity for morphometry measurements. Neuroimage Clin 24: , 101943. |
[33] | Duchesne S , Chouinard I , Potvin O , Fonov VS , Khademi A , Bartha R , Bellec P , Collins DL , Descoteaux M , Hoge R , McCreary CR , Ramirez J , Scott CJM , Smith EE , Strother SC , Black SE ((2019) ) The Canadian Dementia Imaging Protocol: Harmonizing National Cohorts. J Magn Reson Imaging 49: , 456–465. |
[34] | van Dalen JW , Scuric EEM , van Veluw SJ , Caan MWA , Nederveen AJ , Biessels GJ , van Gool WA , Richard E ((2015) ) Cortical microinfarcts detected in vivo on 3 Tesla MRI: Clinical and radiological correlates. Stroke 46: , 255–257. |
[35] | Ii Y , Maeda M , Kida H , Matsuo K , Shindo A , Taniguchi A , Tomimoto H ((2013) ) In vivo detection of cortical microinfarcts on ultrahigh-field MRI. J Neuroimaging 23: , 28–32. |
[36] | Charidimou A , Shoamanesh A , Al-Shahi Salman R , Cordonnier C , Perry LA , Sheth KN , Biffi A , Rosand J , Viswanathan A ((2018) ) Cerebral amyloid angiopathy, cerebral microbleeds and implications for anticoagulation decisions: The need for a balanced approach. Int J Stroke 13: , 117–120. |
[37] | Cummings J ((2018) ) Lessons learned from Alzheimer disease: Clinical trials with negative outcomes. Clin Transl Sci 11: , 147–152. |
[38] | den Heijer T , van der Lijn F , Koudstaal PJ , Hofman A , van der Lugt A , Krestin GP , Niessen WJ , Breteler MMB ((2010) ) A 10-year follow-up of hippocampal volume on magnetic resonance imaging in early dementia and cognitive decline. Brain 133: , 1163–1172. |
[39] | Frisoni GB , Fox NC , Jack CR , Scheltens P , Thompson PM ((2010) ) The clinical use of structural MRI in Alzheimer disease. Nat Rev Neurol 6: , 67–77. |
[40] | Smith EE , Biessels GJ , De Guio F , de Leeuw FE , Duchesne S , Düring M , Frayne R , Ikram MA , Jouvent E , MacIntosh BJ , Thrippleton MJ , Vernooij MW , Adams H , Backes WH , Ballerini L , Black SE , Chen C , Corriveau R , DeCarli C , Greenberg SM , Gurol ME , Ingrisch M , Job D , Lam BYK , Launer LJ , Linn J , McCreary CR , Mok VCT , Pantoni L , Pike GB , Ramirez J , Reijmer YD , Romero JR , Ropele S , Rost NS , Sachdev PS , Scott CJM , Seshadri S , Sharma M , Sourbron S , Steketee RME , Swartz RH , van Oostenbrugge R , van Osch M , van Rooden S , Viswanathan A , Werring D , Dichgans M , Wardlaw JM ((2019) ) Harmonizing brain magnetic resonance imaging methods for vascular contributions to neurodegeneration. Alzheimers Dement (Amst) 11: , 191–204. |
[41] | Hager K , Baseman AS , Nye JS , Brashear HR , Han J , Sano M , Davis B , Richards HM ((2014) ) Effects of galantamine in a 2-year, randomized, placebo-controlled study in Alzheimer’s disease. Neuropsychiatr Dis Treat 10: , 391–401. |
[42] | Honig LS , Vellas B , Woodward M , Boada M , Bullock R , Borrie M , Hager K , Andreasen N , Scarpini E , Liu-Seifert H , Case M , Dean RA , Hake A , Sundell K , Poole Hoffmann V , Carlson C , Khanna R , Mintun M , DeMattos R , Selzler KJ , Siemers E ((2018) ) Trial of solanezumab for mild dementia due to Alzheimer’s disease. N Engl J Med 378: , 321–330. |
[43] | Relkin NR , Thomas RG , Rissman RA , Brewer JB , Rafii MS , van Dyck CH , Jack CR , Sano M , Knopman DS , Raman R , Szabo P , Gelmont DM , Fritsch S , Aisen PS , Alzheimer’s Disease Cooperative Study ((2017) ) A phase 3 trial of IV immunoglobulin for Alzheimer disease. Neurology 88: , 1768–1775. |




