Midlife Insulin Resistance as a Predictor for Late-Life Cognitive Function and Cerebrovascular Lesions
Abstract
Background:
Type 2 diabetes (T2DM) increases the risk for Alzheimer’s disease (AD) but not for AD neuropathology. The association between T2DM and AD is assumed to be mediated through vascular mechanisms. However, insulin resistance (IR), the hallmark of T2DM, has been shown to associate with AD neuropathology and cognitive decline.
Objective:
To evaluate if midlife IR predicts late-life cognitive performance and cerebrovascular lesions (white matter hyperintensities and total vascular burden), and whether cerebrovascular lesions and brain amyloid load are associated with cognitive functioning.
Methods:
This exposure-to-control follow-up study examined 60 volunteers without dementia (mean age 70.9 years) with neurocognitive testing, brain 3T-MRI and amyloid-PET imaging. The volunteers were recruited from the Finnish Health 2000 survey (n = 6062) to attend follow-up examinations in 2014–2016 according to their insulin sensitivity in 2000 and their APOE genotype. The exposure group (n = 30) had IR in 2000 and the 30 controls had normal insulin sensitivity. There were 15 APOE ɛ4 carriers per group. Statistical analyses were performed with multivariable linear models.
Results:
At follow-up the IR+group performed worse on executive functions (p = 0.02) and processing speed (p = 0.007) than the IR- group. The groups did not differ in cerebrovascular lesions. No associations were found between cerebrovascular lesions and neurocognitive test scores. Brain amyloid deposition associated with slower processing speed.
Conclusion:
Midlife IR predicted poorer executive functions and slower processing speed, but not cerebrovascular lesions. Brain amyloid deposition was associated with slower processing speed. The association between midlife IR and late-life cognition might not be mediated through cerebrovascular lesions measured here.
INTRODUCTION
Type 2 diabetes (T2DM) is an independent risk factor for cognitive decline and Alzheimer’s disease (AD) [1], but the mechanisms behind this relationship are not fully understood. Although epidemiological studies have shown an association between T2DM and AD [1], several large neuropathological studies [2, 3] have found no differences between individuals with and without diabetes in the histopathological changes typical for AD, i.e., neuritic plaques consisting of amyloid-β (Aβ) and neurofibrillary tangles consisting of hyperphosphorylated tau [2, 3]. However, lacunar brain infarcts were more common in diabetic individuals in the postmortem examinations [2]. Thus, it has been suggested that diabetes would increase the risk for dementia mainly through a cerebrovascular mechanism [4]. Interestingly however, insulin resistance (IR), the hallmark of the metabolic syndrome and T2DM [5], has been shown to associate with both brain amyloid accumulation [6–8] and with cerebrovascular lesions [9, 10], already in the prediabetic stage.
The overlap between cerebrovascular disease and AD pathology is well known and both types of lesions presumably have additive effects on cognitive function. [11–14] According to the neuropathological Nun Study, cerebrovascular disease might promote clinical symptoms of AD, and on the other hand, patients with AD pathology but without cerebrovascular disease seem to tolerate more AD lesions in their brain before developing symptoms [15]. Brain hypoxia caused by atherosclerosis of cerebral vessels has been shown to increase the cleavage of Aβ from the amyloid-β protein precursor (AβPP). It has also been suggested that cerebrovascular disease impairs Aβ clearance due to cerebral hypoperfusion resulting in increased cerebral Aβ accumulation. Aβ, in turn, might promote atherogenesis through vascular oxidative stress and endothelial dysfunction leading to further vascular damage [12]. Therefore, cerebrovascular pathology associated with diabetes and IR could also promote to the neuropathology of AD and vice versa.
IR has been demonstrated to occur at cellular level in the brains of patients with AD [16]. There are several potential mechanisms that link IR directly to the neuropathological changes of AD [17]. For example, insulin and Aβ share a common degrading enzyme in the brain (IDE, insulin degrading enzyme) and in mice with IR this enzyme is downregulated, resulting in elevated amyloidosis in the brain [17]. In addition, IR contributes to endothelial dysfunction and arterial stiffening which can lead to the cerebrovascular changes often found in age-related dementia [18]. However, to date, the associations among IR, cerebrovascular lesions on magnetic resonance imaging (MRI), cognition, and the neuropathology of AD remain unclear.
There are only few studies exploring the independent influence of IR on cerebrovascular lesions [9, 10, 19]. Metabolic syndrome increases the risk of silent infarcts [20] and white matter hyperintensities (WMHs) [21], which in turn have been associated with poorer cognitive performance [22–24] and with an increased risk of AD [25]. We have previously shown that IR predicts cognitive decline in the Finnish adult population [26].
The pathological process of AD is slow, and the accumulation of Aβ is estimated to begin 10–20 years prior to the onset of the disease [27]. Similarly, cardiovascular diseases occur over a long time period contributing to changes in the cerebrovascular system. Many epidemiological studies have shown an association between midlife but not late-life vascular risk factors and cognitive decline [28]. Therefore, there is a need to examine the longitudinal associations between midlife risk factors and late-life cerebral changes associated with dementia, to elucidate the time course of these cerebral changes and their association with cognitive performance.
We have previously shown that midlife IR predicts brain Aβ accumulation, the earliest biomarker of preclinical AD, in the present study population [6]. The objective of the present study was to evaluate if midlife IR predicts late-life cognitive performance and/or cerebrovascular lesions (WMHs or brain total vascular burden), and whether cerebrovascular lesions or brain Aβ accumulation associate with cognitive functioning.
Based on previous literature, we hypothesized that midlife IR—in the absence of diabetes—would predict poorer cognitive performance and cerebrovascular lesions on MRI 14–16 years later, and that cerebrovascular lesions and brain Aβ accumulation would associate with poorer neurocognitive performance. To test this hypothesis, we examined 30 volunteers with midlife IR and 30 controls with normal midlife insulin sensitivity with comprehensive neurocognitive testing, brain MRI imaging and amyloid-PET imaging with [11C]Pittsburgh compound B (PIB).
METHODS
Health 2000 study
The study participants were recruited from the Health 2000 survey which is a Finnish population-based, nationwide study, representative of the Finnish adult population, and conducted by the Finnish National Institute for Health and Welfare. The Health 2000 study was carried out in the years 2000–2001. In total, 8,028 individuals aged 30 or older were selected randomly from the Finnish population register. The participation rate in the health examination proper was 79% (n = 6,354). The mean age of the participants was 55.4 years. The participants underwent a thorough physical examination, and venous blood samples were drawn after the participants had been fasting for at least four hours [29, 30]. They were tested for verbal fluency and encoding and retaining verbal material by means of selected tasks from the Finnish version of the CERAD (Consortium to Establish a Registry for Alzheimer’s Disease) test battery [26, 31]. APOE genotyping was performed using the MassARRAY System (Sequenom, San Diego, CA, USA) with a modified protocol, which has been described elsewhere [32].
Study population and recruitment
We recruited 60 volunteers in the year 2014 from those who had attended the Health 2000 survey (n = 6,354) for this neuroimaging study, which was conducted at the Turku PET Centre. This study was designed to consist of an exposure group (IR + group, n = 30, IR in the Health 2000 study) and a control group (IR–group, n = 30, normal insulin sensitivity in the Health 2000 study). IR and insulin sensitivity were determined using Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) [33]. Both groups were designed to contain an equal number, i.e., 50% (n = 15) of APOE ɛ4 carriers.
We had access to the Health 2000 data from which we identified 451 individuals based on the following recruitment criteria: 1) age (65–80 years), 2) lived in the Hospital District of Southwest Finland or the Hospital district of Helsinki and Uusimaa, 3) HOMA-IR (>2.17 for those with IR, i.e., the highest tertile of the Health 2000 study population; HOMA-IR<1.25 for those with normal insulin sensitivity, i.e., the lowest tertile of the Health 2000 study population). Individuals who had fasted for less than 4 hours at baseline (n = 229), who had insulin or unknown diabetes medication (n = 59), or who had missing HOMA-IR values (n = 4) were excluded. Because we wanted to examine the effect of midlife IR in the prediabetic stage, individuals with a diagnosis of type 2 diabetes in 2000 were also excluded (Supplementary Figure 1). Based on these criteria and further excluding those with missing APOE ɛ genotyping or ɛ2/ɛ4 genotype, and those who had died, a recruitment letter was sent via the Finnish National Institute of Health and Welfare to altogether 360 individuals (216 APOE ɛ4 negative; 97 APOE ɛ4 positive). Of those who received the recruitment letter, 98 were interested in participating the study, and they contacted us by mail. They were interviewed by telephone. In order to obtain balanced study groups, we received information on which of the study groups the persons who were interested in participating belonged to (IR–/APOE4–; IR+/APOE4–; IR–/APOE4+; or IR+/APOE4+) from the National Institution of Health and Welfare. We were blinded to all other information from the Health 2000 study until finishing data collection for the present study.
Exclusion criteria for this neuroimaging study, based on the telephone interview, were a history of a major stroke, a previous dementia diagnosis, any other major neurological disorder, and diagnosis of T2DM after the year 2000 for the IR- group. Additional exclusion criteria were any contraindication for a PET or MRI scan (such as claustrophobia or a metal object in the body, or an inability to lie still in a supine position for 90 min). The details of the recruitment process are described in Supplementary Figure 1. The primary endpoint of our neuroimaging study was brain amyloid accumulation, measured with [11C]PIB-PET imaging [6], which is why the power calculations of the present study were based on test-retest analyses of PIB-PET scans, which indicated that to detect a 15% difference in PIB accumulation in the frontal cortex, five persons per group would be sufficient [34].
Standard protocol approvals, registrations, and patient consents
The Finnish Health 2000 study was approved by the Ethics Committee for Epidemiology and Public Health in the hospital district of Helsinki and Uusimaa, Finland. This follow-up study was approved by the Ethics Committee of the Hospital District of Southwest Finland. Written informed consent was obtained from all of the participants before they attended the studies.
Laboratory examination and covariates
In the Health 2000 study, fasting blood samples were collected. The duration of the fasting time was recorded. The samples were stored in –70°C until they were analyzed. HDL cholesterol values were analyzed by a HDL-C Plus test (Roche Diagnostics, Mannheim, Germany), triglycerides by a GPO PAP test (Olympus SystemReagent), and glucose values by a hexokinase test (Olympus SystemReagent). Serum insulin was determined by a microparticle enzyme immunoassay (Abbott Laboratories Dainabot, Tokyo, Japan) [30]. In the follow-up study in 2014–2016, venous blood samples were drawn after an overnight fast (minimum 10 hours). Insulin was determined by ECLIA (electrochemiluminescence immunoassay) with a Cobas e602 immunochemistry analyzer (Roche Diagnostics GmbH, Mannheim, Germany) and glucose by enzymatic photometry with a Cobas c702 chemistry analyzer (Roche Diagnostics GmbH). HOMA-IR was calculated by the equation fasting insulin (μU/ml) times fasting glucose (mmol/l) divided by 22.5 as previously described [33].
Baseline characteristics measured in 2000 were used as covariates in the analyses. Level of education was assessed by a questionnaire and classified as 1 = primary school; 2 = middle school/comprehensive school; 3 = high school; 4 = college/university. Hypertension at baseline in 2000 was defined as systolic RR≥140 or diastolic ≥90 mmHg or use of antihypertensive treatment. Smoking was assessed by interview and defined as current smoking/no smoking.
Cognitive testing at follow-up
A thorough neurocognitive test battery was administered to the participants during 2014–2016 by trained psychology students. The test battery included parts from the Finnish version of the Wechsler Memory Scale (WMS-R) and the Wechsler Adult Intelligence Scale (WAIS-R), Boston Naming Test (BNT), Trail Making Tests A and B (TMT-A and TMT-B), S-fluency, categorical fluency, and Stroop [35–39].
MRI imaging and determining cerebrovascular lesions
The 60 study volunteers underwent MRI imaging with a 3T PET-MRI scanner (Philips Ingenuity TF PET-MR device, Philips Healthcare, the Netherlands). The sequences used were 3DT1, T2W, FLAIR (Fluid Attenuation Inversion Recovery), DTI and resting state MRI. Cerebrovascular changes were quantified from FLAIR images using an automatic image quantification tool [40] that quantifies total and regional WMHs, and cortical and lacunar infarcts. To ascertain that the computer-based analysis was accurate, the data was also inspected visually (J.K). Only the computer-based quantification was used in the analyses.
Three computer-based measures were used to evaluate cerebrovascular lesions: 1) total WMH volume normalized for age, gender, and total brain volume, 2) a computational counterpart for the Fazekas scale that was computed from the deep WMH volume using a regression-based model which has previously been defined elsewhere [41], and 3) total vascular burden measure that was calculated as the weighted combination of deep WMH volume, volume of cortical infarcts and volume of lacunar infarct volumes as described in detail in a previously published article [40].
Amyloid accumulation
PIB-PET-imaging was performed to assess Aβ accumulation in the brain. The PET-scanning protocol has been described previously elsewhere [6]. The dynamic 90 min [11C]PIB-PET scan was performed using a brain-dedicated high-resolution PET scanner, the ECAT HRRT (Siemens Medical Solutions, Knoxville, TN, USA). [11C]PIB was manufactured as previously reported [42] in high molar radioactivity (mean 680 MBq/nmol (SD±240)) at the time of injection utilizing in-target produced [11C]methane.
Voxel-by-voxel [11C]PIB standardized uptake values (SUV) were calculated using imaging data from 60 to 90 minutes after the tracer injection. Automated region of interest (ROI) generation was conducted using FreeSurfer software (version 5.3.0, http://freesurfer.net/) and individual T1 weighted MRI data as input. Six ROIs were formed (parietal cortex, prefrontal cortex, anterior cingulum, posterior cingulum, precuneus, lateral temporal cortex based on brain regions where Aβ has been shown to first accumulate in early AD [43–45]. SUV ratios (SUVRs) were obtained by using the cerebellar cortex as a reference region. A composite cortical PIB score was calculated as the average PIB SUVR over all six ROIs.
Statistical analyses
Characteristics of the study population
The study population of the present study (n = 60) and the Health 2000 study population (n = 6062) were compared with two-sample t-test for continuous variables and with Pearson’s Chi-Squared -test for categorical variables (Supplementary Table 2).
IR–and IR+ group differences were assessed with two-sample t-test or Wilcoxon rank sums test (fasting time) for continuous variables and with Pearson’s Chi-Squared -test for categorical variables except for Fazekas score. Differences between the groups in the Fazekas scores were assessed with Cochran Armitage Trend Test. Fazekas scores were obtained by rounding computed Fazekas scores to the nearest whole number (Table 1).
Table 1
The study population characteristics at baseline in 2000 and at follow-up 2014 – 2016 in participants with normal (IR-) insulin tolerance and with insulin resistance (IR+) in 2000 (n = 60)
| N | IR– 30 | IR+ 30 | p |
| Baseline in 2000 | |||
| age, mean (SD) | 55.6 (3.8) | 55.2 (2.8) | 0.65 |
| women, n/% | 17/56.7 | 16/53.3 | 0.80 |
| men, n/% | 13/43.3 | 14/46.7 | 0.80 |
| Fasting time (h:min), median (range) | 6:15 (5:30–7:59) | 6:58 (5:38–12:51) | 0.24 |
| HOMA-IR, median (range) | 0.90 (0.46–1.22) | 2.82 (2.20–5.24) | <0.0001 |
| APOE ɛ4 genotype, n | 15 | 15 | 1.0 |
| Level of education, n/% | 0.04 | ||
| Primary school | 5/16.7 | 15/50.0 | |
| Middle school or comprehensive school | 12/40.0 | 9/30.0 | |
| High school | 5/16.7 | 3/10.0 | |
| College or university | 8/26.7 | 3/10.0 | |
| BMI (kg/m2), mean (SD) | 25.1 (2.9) | 29.9 (3.4) | <0.001 |
| Hypertension, n/% | 10/33.3 | 21/70.0 | 0.005 |
| HDL cholesterol (mmol/L), mean (SD) | 1.55 (0.43) | 1.23 (0.27) | 0.001 |
| Triglycerides (mmol/L), mean (SD) | 1.24 (0.46) | 1.74 (0.95) | 0.002 |
| Current smoking, n/% | 5/16.7 | 4/13.3 | 0.72 |
| CERAD | |||
| verbal fluency, mean (SD) | 28.7 (6.3) | 23.3 (5.1) | 0.0007 |
| word-list learning sum, mean (SD) | 21.8 (3.8) | 20.9 (3.1) | 0.32 |
| delayed recall, mean (SD) | 7.3 (1.7) | 6.8 (1.4) | 0.26 |
| Follow-up in 2014–2016 | |||
| age at time of MRI-scan, mean (SD) | 71.1 (3.7) | 70.8 (2.8) | 0.72 |
| HOMA-IR, median (range) | 1.73 (0.22–5.13) | 3.31 (1.2–21.4) | <0.0001 |
| Fazekas score, n/% | 0.78 | ||
| 0 | 9/30.0 | 15/50.0 | |
| 1 | 16/53.3 | 6/20.0 | |
| 2 | 3/10.0 | 7/23.3 | |
| 3 | 2/6.7 | 2/6.7 | |
| PIB-positive, n/% | 10/33.3 | 18/60.0 | 0.04 |
| PIB-negative, n/% | 20/66.7 | 12/40.0 | 0.04 |
IR–, HOMA-IR in the lowest tertile of the Health 2000 study population (HOMA-IR < 1.25); IR+, HOMA-IR in the highest tertile of the Health 2000 study population (HOMA-IR > 2.17). p-values for the differences between the IR groups assessed with Student’s t-test for continuous variables and with Pearson’s Chi-Squared test for categorical variables except for the length of the fasting time in 2000 which was assessed with Wilcoxon rank sums test and Fazekas score which was assessed with a Cochran Armitage Trend Test. Fazekas scores were obtained by rounding the computed Fazekas score to the nearest whole number. A logarithmic transformation is used for triglycerides and HOMA-IR at follow-up in order to achieve normal distribution. PIB-PET scan was considered PIB positive when PIB composite score was >1.5.
Z-scores for cognitive domains
Domain-specific neurocognitive test z-scores based on a priori hypothesis [46] by an experienced neuropsychologist (M.K.) were calculated for executive functions, processing speed, language, and episodic memory so that a higher score indicated better performance. The executive function domain included Trail Making Test A and B (TMT-B minus TMT-A), Stroop (inhibition minus naming), digit span backward and S-fluency. The processing speed domain consisted of TMT-A and digit symbol test. The episodic memory domain included WMS-R delayed logic memory and delayed verbal recall. The language domain included categorical fluency (animals), Boston naming test, and WAIS-R similarities. One person from the IR+ group refused to participate in the thorough neurocognitive evaluation. In addition, two individuals from the IR+ group had not completed all of the cognitive tests included in the z-scores (one individual did not perform TMT-B and another participant did not perform digit span test and WAIS-R similarities).
In order to combine the scores of different neurocognitive tests into domain-specific scores, z-scores for the neurocognitive tests were calculated by standardizing the raw test scores to the present study population’s mean and standard deviation of each test. In order to achieve a normal distribution a maximum of three outliers per raw test were excluded before the z-transformation (3 outliers in TMT B minus A; 2 outliers in TMT-A; 2 outliers in Boston naming test; and 2 outliers in WAIS-R similarities). All outliers performed worse on the cognitive tests than – 2 SD of the present study population, and all outliers were from the IR+ group. A skewness of –1 to 1 was accepted for the distribution of the raw test scores. The domain specific z-scores were determined by calculating the average of all the z-scores that belonged to the domain in question. In those tests (Stroop, TMT-A and TMT-B) where the scale is reverse, reciprocal numbers were used so that a higher score indicates better performance. Participants with missing test results were excluded. To ensure that excluding the outliers did not affect our results, we even calculated z-scores with the outliers included.
Group comparisons (IR–/IR+) of the neurocognitive test scores
Neurocognitive test scores were compared between the IR groups with two-sample t-test assuming equal variances. Adjusted analyses were performed using multivariable linear models. Model 1 was adjusted for level of education (Table 2). Model 2 was further adjusted for hypertension and smoking in 2000 (Table 2). Additional analyses where even body mass index (BMI), HDL-cholesterol, and triglycerides were adjusted for were also performed (Model 3). To confirm that excluding the outliers from the analyses did not affect our results, we performed nonparametric analyses with outliers included using Wilcoxon rank sum test (Supplementary Table 1). Effect sizes were calculated using Cohen’s d.
Table 2
Differences between the IR– and IR+ groups in computed Fazekas score, normalized total white matter hyperintensity volume (ml), normalized total vascular burden measure and in domain-specific neurocognitive test scores (where a higher score indicates better performance)
| IR– | IR+ | Effect size* | pa | pb | pc | |
| Cerebrovascular lesions in MRI, median (Q3–Q1) | ||||||
| Computed Fazekas score | 1.0 (0.05–1.3) | 0.5 (0–1.6) | –0.06 | 0.72 | ||
| Normalized total WMH volume (ml) | 3.7 (1.1–5.6) | 2.9 (1.5–8.4) | –0.12 | 0.88 | 0.99 | 0.97 |
| Normalized total vascular burden (ml) | 2.9 (0.6–20.9) | 4.3 (0.5–21.3) | –0.19 | 0.78 | 0.92 | 0.59 |
| Domain-specific neurocognitive test z-scores, mean (SD) | ||||||
| Executive functions (n = 54) | 0.30 (0.68) | –0.26 (0.68) | –0.78 | 0.004 | 0.02 | 0.04 |
| Processing speed (n = 57) | 0.35 (0.65) | –0.32 (0.82) | –0.83 | 0.001 | 0.007 | 0.03 |
| Episodic memory (n = 59) | 0.12 (1.0) | –0.13 (0.77) | –0.28 | 0.28 | 0.99 | 0.98 |
| Language (n = 54) | 0.24 (0.77) | –0.10 (0.65) | –0.46 | 0.09 | 0.30 | 0.18 |
*The effect sizes calculated by using Cohen’s d. aThe p-values for unadjusted differences between individuals with and without insulin resistance at baseline in 2000, assessed with a Student’s t test except for the computed Fazekas which was assessed with a Wilcoxon test. bModel 1. Analyses adjusted for level of education. cModel 2. Further adjusted for hypertension and smoking in 2000.
Group comparisons (IR–/IR+) of the cerebrovascular changes
The differences between the IR groups in cerebrovascular changes were evaluated by multivariable linear regression analysis where adjustments were made with the same models as described above (Table 2). A logarithmic transformation was performed for the normalized total WMH volume and for the normalized total vascular burden measure in order to fulfill the assumption of a normal distribution.
The associations between neurocognitive tests, cerebrovascular lesions, and amyloid accumulation
Associations between domain-specific neurocognitive z-scores, cerebrovascular lesions, and PIB SUVR composite score were evaluated with linear regression analyses (Table 3). The variables (normalized total WMH, computed Fazekas, total vascular burden measure, and PIB composite score) were analyzed in separate models. Adjusted analyses were performed with multivariable linear models. Model 1 was adjusted for age, sex, and level of education. Model 2 was further adjusted for APOE ɛ4 genotype. The residuals of the models were normally distributed. Voxel-by-voxel correlations between cognitive z-scores and PIB uptake were assessed with Statistical Parametric Mapping (SPM12; Wellcome Trust Centre for Neuroimaging, London, UK).
Table 3
The associations of MRI changes and PIB-uptake with neurocognitive tests. Variables (normalized total WMH, computed Fazekas, total vascular burden measure) are analyzed in separate models
| Executive functions | Processing speed | Episodic memory | Language | |
| Unadjusted | ||||
| Norm. total WMH, slope (95% CI) | 0.02 (–0.14 to 0.18) | 0.05 (–0.13 to 0.22) | 0.14 (–0.04 to 0.33) | 0.05 (–0.11 to 0.21) |
| Computed Fazekas, slope (95% CI) | 0.08 (–0.15 to 0.30) | 0.14 (–0.10 to 0.39) | 0.22 (–0.04 to 0.49) | 0.08 (–0.15 to 0.31) |
| Total vascular burden, slope (95% CI) | –0.01 (–0.11 to 0.09) | –0.008 (–0.12 to 0.10) | –0.007 (–0.13 to 0.12) | –0.03 (–0.13 to 0.08) |
| PIB composite score, slope (95% CI) | –0.19 (–0.75 to 0.36) | –1.10 (–1.51 to –0.70)*** | –0.52 (–1.05 to 0.01) | –0.39 (–0.91 to 0.13) |
| Model 1: age, level of education and sex adjusted | ||||
| Norm. total WMH, slope (95% CI) | 0.008 (–0.14 to 0.12) | 0.06 (–0.12 to 0.24) | 0.12 (–0.05 to 0.29) | 0.03 (–0.13 to 0.19) |
| Computed Fazekas, slope (95% CI) | 0.02 (–0.16 to 0.21) | 0.17 (–0.09 to 0.42) | 0.16 (–0.07 to 0.40) | 0.04 (–0.20 to 0.23) |
| Total vascular burden, slope (95% CI) | –0.03 (–0.11 to 0.05) | –0.02 (–0.13 to 0.09) | –0.05 (–0.15 to 0.06) | –0.03 (–0.14 to 0.06) |
| PIB composite score, slope (95% CI) | –0.17 (–0.62 to 0.23) | –1.03 (–1.43 to –0.63)*** | –0.33 (–0.80 to 0.14) | –0.33 (–0.83 to 0.18) |
| Model 2: further adjusted for APOE4 | ||||
| Norm. total WMH, slope (95% CI) | –0.007 (–0.14 to 0.12) | 0.06 (–0.11 to 0.24) | 0.13 (–0.03 to 0.29) | 0.03 (–0.13 to 0.19) |
| Computed Fazekas, slope (95% CI) | 0.02 (–0.16 to 0.21) | 0.17 (–0.08 to 0.42) | 0.17 (–0.06 to 0.41) | 0.04 (–0.19 to 0.28) |
| Total vascular burden, slope (95% CI) | –0.03 (–0.11 to 0.05) | –0.03 (–0.13 to 0.08) | –0.05 (–0.15 to 0.05) | –0.03 (–0.14 to 0.07) |
| PIB composite score, slope (95% CI) | –0.07 (–0.60 to 0.45) | –1.15 (–1.64 to –0.67)*** | –0.14 (–0.71 to 0.42) | –0.16 (–0.75 to 0.43) |
The association between MRI changes or cortical PIB uptake and neuropsychological test results assessed using a univariable regression model in unadjusted model and multivariable linear models in Model 1 and 2. A logarithmic transformation is used for normalized total white matter hyperintensity volume and normalized total vascular burden. A negative slope indicates an inverse association, i.e., that a higher vascular burden or a higher amyloid burden associates with a lower cognitive test score. *p < 0.05, **p < 0.01, ***p < 0.001.
Selection of covariates
Previously reported risk factors for cognitive decline and cerebrovascular lesions were included as covariates in the adjusted models comparing cognitive performance and presentation of cerebrovascular lesions between the IR–and IR+ groups. As education is known to be protective against cognitive decline it was included as a covariate in Model 1. Considering that hypertension and smoking are acknowledged risk factors for vascular disease, these variables were included in Model 2. Additional analyses (Model 3) were performed for cognitive test scores with including even BMI, triglycerides, and HDL cholesterol as covariates. Age, sex, or APOE ɛ4 genotype were not included in the adjusted models of group comparisons (IR–/IR+) because the groups were matched for age, sex, and APOE ɛ4 genotype at recruitment. However, age and sex, as well as APOE ɛ4 genotype were included as covariates in the models evaluating the linear associations between the neurocognitive test scores, cerebrovascular lesions, and amyloid accumulation.
Interactions
Interactions between ‘IR group×APOE ɛ4’ and neurocognitive test results, cerebrovascular lesions and amyloid accumulation were analyzed (multivariable linear models).
Two-sided statistical significance was set at p < 0.05. The statistical analyses were performed with SAS JMP 12.1 Pro (SAS Institute, Cary; NC, USA).
RESULTS
Demographics
The characteristics of the study population at baseline are shown in Table 1. The individuals in the IR–group at baseline were more educated, had a lower BMI, less hypertension, a lower triglyceride level, and a higher HDL cholesterol level than the individuals in the IR+ group. The study groups did not differ in fasting times (p = 0.24) or smoking (p = 0.78). All participants of the study were Caucasian.
The IR+ group performed worse on categorical verbal fluency at baseline (unadjusted p = 0.0007) than the IR–group, but the groups did not differ in word-list learning (p = 0.32) or word-list delayed recall (p = 0.26).
The participants of the present study (n = 60) represented the original Health 2000 study population (n = 6062) well (Supplementary Table 2). According to the recruitment criteria of the present study, the participants of this neuroimaging study were older (p < 0.0001) and more often APOE ɛ4 carriers (p = 0.02) than the participants of the nationwide Health 2000 study.
Midlife IR predicts poorer performance in tests of executive functions and processing speed
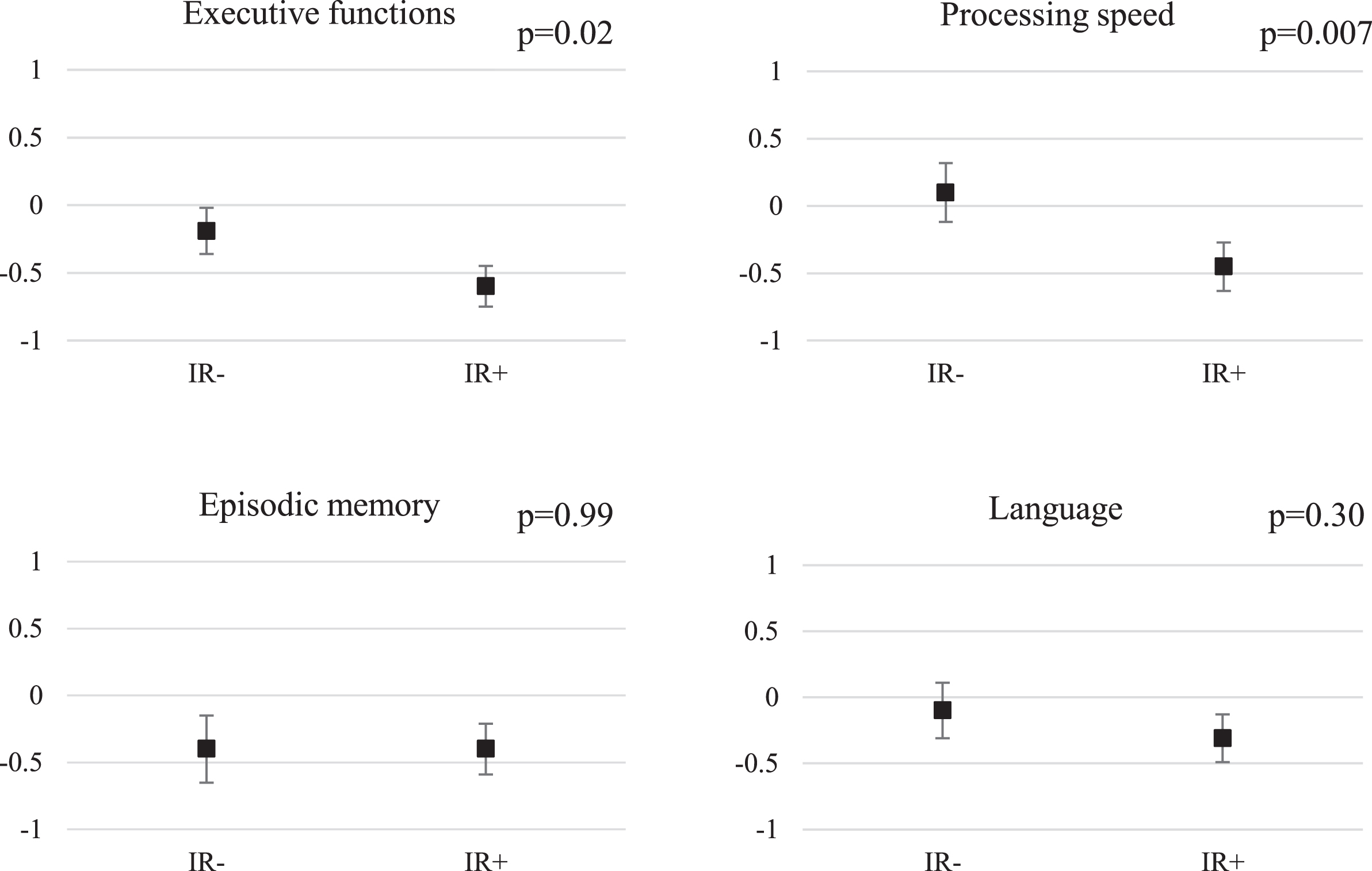
At the follow-up, the IR+ group had lower executive functions z-scores than the IR–group (unadjusted model: p = 0.004, effect size –0.78; after adjustments for level of education (Model 1) p = 0.02 and after further adjustments for hypertension and smoking in 2000 (Model 2) p = 0.04). In addition, the IR+group were slower in tests of processing speed than the IR–group (unadjusted model: p = 0.001, effect size –0.83; Model 1: p = 0.007; Model 2: p = 0.03) (Table 2). The difference between the IR groups remained significant in processing speed (p = 0.03) but not in executive functions (p = 0.32) after even further adjustments for BMI, HDL, and triglycerides (Model 3, data not shown). The differences between the IR–and IR+ groups were not significant in episodic memory (unadjusted p = 0.28; and Model 2 p = 0.98) or language function (unadjusted p = 0.09; Model 2 p = 0.18). (Table 2; Fig. 1). Using a nonparametric test and including the outliers in the analyses, the difference between the IR groups was significant for executive functions (p = 0.02, effect size –0.55), for processing speed (p = 0.0008, effect size –0.90) and also for language (p = 0.017, effect size –0.64) (Supplementary Table 1). Adjustments for education or other confounding factors could not be made in this non-parametric model. Sex-specific effects were not evaluated because of the small sample size.
Fig.1
Neurocognitive z-scores according to IR group, adjusted for education. The results are shown as education-adjusted z-score means with standard errors. A higher score indicates better performance. p-values for education-adjusted differences between IR- and IR+ groups, assessed with linear regression.
Midlife IR does not predict cerebrovascular lesions
There was no statistically significant difference between the IR groups in computer-based cerebrovascular lesions determined from MRI images: in computed Fazekas scores (p = 0.72), in normalized total WMH volume (p = 0.88 and after Model 2 adjustments p = 0.97) or in normalized total vascular burden (p = 0.78 and after Model 2 adjustments p = 0.59) (Table 2).
Aβ accumulation but not cerebrovascular lesions were associated with slower processing speed
At the follow-up, a higher PIB composite score was associated with a slower processing speed (slope –1.10, 95% CI –1.51 to –0.70, p < 0.001). After adjustments for age, level of education and sex (Model 1) the inverse association between PIB composite score and processing speed remained significant (slope –1.03, 95% CI –1.43 to –0.63, p < 0.001) as well as after further adjustments for APOE ɛ4 genotype (Model 2) (slope –1.15, 95% CI –1.64 to –0.67, p < 0.001). No associations were found between the PIB composite score and the other neurocognitive domains (executive functions p = 0.49; episodic memory p = 0.16; language function p = 0.20). In voxel-wise SPM analyses, a higher PIB SUVR correlated with a slower processing speed in the frontal regions, but also in the parietal cortex (p < 0.05, family wise error corrected for multiple comparisons, Fig. 2). No association was found between cerebrovascular changes (normalized total WMHs, computed Fazekas and total vascular burden measure) and neurocognitive z-scores (executive functions, processing speed, episodic memory and language function) (Table 3).
Fig.2
Brain regions showing a correlation between higher [11C]PIB uptake and slower processing speed z-score. The colored areas represent brain regions where the correlation between higher [11C]PIB uptake and slower processing speed z-score was significant. The color scale starts from the height threshold T = 4.8 (p < 0.05 when T = 4.8, family-wise error corrected), brighter colors represent a more significant correlation. The voxel-to-voxel analysis was performed with Statistical Parametric Mapping (SPM12).
![Brain regions showing a correlation between higher [11C]PIB uptake and slower processing speed z-score. The colored areas represent brain regions where the correlation between higher [11C]PIB uptake and slower processing speed z-score was significant. The color scale starts from the height threshold T = 4.8 (p < 0.05 when T = 4.8, family-wise error corrected), brighter colors represent a more significant correlation. The voxel-to-voxel analysis was performed with Statistical Parametric Mapping (SPM12).](https://content.iospress.com:443/media/jad/2019/72-1/jad-72-1-jad190691/jad-72-jad190691-g002.jpg)
Interactions
When comparing the neurocognitive test performances between the IR groups we did not find any interaction for ‘IR group×APOE ɛ4’ (all p-values ≥0.37). When comparing the cerebrovascular lesions between the IR groups, no interaction ‘IR group×APOE ɛ4’ was found (all p-values ≥0.48). Neither was any interaction ‘IR group×APOE ɛ4’ found when comparing the amyloid load between the IR groups (p = 0.19).
DISCUSSION
This study indicates that midlife IR associates with poorer executive functions and slower processing speed 15 years later. Contrary to our original hypothesis, midlife IR did not predict cerebrovascular lesions at follow-up. An association was found between a higher brain Aβ load and slower processing speed. No association was found between cerebrovascular changes and neurocognitive performance.
Previous studies have reported a positive association between metabolic syndrome and cerebrovascular lesions [20, 21, 47]. However, only a few studies have investigated the independent role of IR on cerebrovascular changes and the findings have been somewhat conflicting [9, 10, 19]. According to one cross-sectional study (n = 2,326) IR was independently associated with silent lacunar infarcts but not with WMH volume in otherwise healthy adults (mean age 56 years) [10]. Another cross-sectional study (n = 1,597) found an association between IR and WMHs in a young study population (mean age 40 years) but only after adjustments for BMI and hypertension [19]. The same study did not find any association between IR and cognitive performance. According to one longitudinal study (n = 934), IR did not predict lacunar infarcts nor WMH progression over a ten-year period [9]. Although these previous studies were notably larger than the present study, no previous studies have evaluated the effects of midlife IR on the aging brain after a 15-year follow-up with a combination of MRI imaging, comprehensive neurocognitive testing, and Aβ imaging. We have reported earlier that IR predicted cerebral Aβ accumulation [6]. Here, we demonstrate that midlife IR did not predict cerebrovascular lesions in a sample of elderly individuals without dementia, drawn from a large, nationally representative health examination study.
Previous studies have suggested that T2DM [1] and IR [48, 49] are risk factors for AD. However, it is still unclear whether the increased risk associated with T2DM is explained either by AD-related or by vascular-related pathology. Vascular and metabolic diseases [18] and brain WMHs [25] have been suggested to be “second hits” that result in dementia when AD neuropathology is present. In our study Aβ deposition, but not cerebrovascular lesions, was associated with slower processing speed. Also, the IR+ group had more cerebral Aβ than the IR–group but the groups did not differ in WMHs, Fazekas score or brain total vascular burden. Thus, our study suggests that IR might increase the risk of cognitive decline rather via Aβ pathology than via vascular related pathology. However, further research with larger study populations is needed.
Previous studies have shown a negative association between cerebrovascular lesions and neurocognitive function [22, 23, 25]. A meta-analysis showed that WMHs increase the risk of future cognitive decline, especially in the domains of executive functions and processing speed [50]. In the present study the IR+ group performed worse on these neurocognitive domains than the insulin sensitive group. However, we found no association between cerebrovascular lesions and neurocognitive test performance, a finding that could be explained by our relatively small and healthy study population. Very few of our study participants had substantial white matter changes; only four individuals were classified as Fazekas 3 and they all performed well in the neurocognitive tests. It is thus possible that these somewhat atypical individuals may have influenced our results. On the other hand, in a previous study, metabolic syndrome was associated with lower performance in executive functions, but the association was not explained by WMH severity [47]. Another recently published study evaluating neurocognitive performance, brain Aβ and leukoaraiosis [51], reported similar results to ours. Among patients with hypertension, cerebrovascular lesions were not associated with neurocognitive performance. Instead, an association between Aβ and neurocognitive performance was found [51]. These findings, together with our results, might suggest that the earliest decrements in cognition in elderly individuals without dementia could be mediated through an amyloidogenic pathway, rather than through WMHs or brain total vascular burden including even lacunar and cortical infarcts. However, the associations among cognition, Aβ, and more subtle cerebrovascular lesions, white matter tract integrity and microbleeds, remain unclear, since microbleeds or diffusion tension images were not evaluated in the present study.
There are several possible mechanisms that could explain our results. According to neuropathological studies, Aβ deposits are first found in the prefrontal regions [43]. Accordingly, Aβ PET scans typically show a pattern of Aβ accumulation first in the fronto-parietal regions [52]. As processing speed is known to be subserved by the prefrontal cortex [53], the accumulation of Aβ deposits in this region could result in a slower processing speed. Our results showed no association between Aβ deposition and episodic memory, which is typically the first area of impairment in AD. However, episodic memory typically starts to decline close to the onset of symptomatic AD. [54] We assume that the volunteers of the present study who were Aβ positive represent preclinical AD, i.e., a stage where Aβ accumulation can be detected with PET imaging or cerebrospinal fluid measurements, but the clinical symptoms of the disease are not yet present. In addition to amyloid-related mechanisms, the difference in processing speed and executive functions between the IR groups could also be explained by insulin-mediated effects on the brain that were not measured here, such as the previously reported effects of insulin on neurosynaptic functioning and neuronal survival [55].
Our study had limitations. Firstly, the study population might have been too small and possibly too healthy to detect possible differences in the cerebrovascular lesions between the IR groups. Secondly, imaging modalities detecting potentially more subtle forms of cerebrovascular changes, such as diffusion tensor imaging were not evaluated here. The diffusion tensor imaging data collected in the present study is currently under analysis. Thirdly, at baseline the fasting time of the participants varied, which could have affected the HOMA-IR values. Nonetheless, at follow-up there was still a significant difference in the HOMA-IR values between the IR groups indicating that the groups did differ in IR throughout the follow-up period. Also, before deciding to use 4 hours as the minimum fasting time we compared the HOMA-IR values of all the participants of the population-based Health 2000 study (n = 6062) according to the participants’ fasting time. No significant difference was found between the HOMA-IR values among individuals who had fasted for 4–6, 6–8, or 8–10 hours before the blood samples [56].
We also had to exclude some outliers in order to achieve normal distribution before formulating the z-scores from the cognitive raw test scores. However, all the outliers excluded from the analyses were from the IR+ group and they performed worse on the tests than the average study population. Thus, excluding the outliers would have, if anything, attenuated the differences between the IR+ and IR– groups. We also performed the analyses with outliers included, and this did not change the results. We were not able to compare cognitive performance between baseline and follow-up because comprehensive neurocognitive tests were only administered at follow-up. The study groups differed in categorical verbal fluency, but not in word-list learning or word-list delayed recall at baseline. At follow-up there was no difference in categorical verbal fluency between the groups (unadjusted p = 0.06).
The strengths of this study can be summarized as: the long follow-up time of 15 years; the possibility to recruit the volunteers according to their midlife HOMA-IR values; the use of computerized programs to quantify the cerebrovascular lesions in the MRI images and PIB-PET uptake; the possibility to balance the IR groups equally according to the APOE ɛ4 status; and the possibility to adjust the results for other midlife metabolic risk factors measured at baseline.
Here, we found that midlife IR associates with poorer performance in executive functions and slower processing speed in a 15-year follow-up. IR did not predict WMHs, Fazekas score, or brain total vascular burden. Our study suggests that midlife IR predicts late-life Aβ accumulation which, in turn, seems to be associated with a slower processing speed performance. This study strengthens the contention that metabolic midlife risk factors might increase the risk for AD via an amyloidogenic pathway. Future neuroimaging studies should assess if IR associates with longitudinal changes in brain Aβ accumulation, vascular lesions, cognition and also the accumulation of tau, as this would further clarify the complex relationship between midlife vascular risk factors and AD. The recognition of midlife IR—even at prediabetic stages—as a risk factor for late-life cognitive impairment and cerebral changes related to memory disorders provides opportunities for early treatment and prevention of cognitive decline. Ongoing clinical trials on insulin-related therapies [57] will further elucidate if early treatment of IR could improve cognitive functioning and brain health.
ACKNOWLEDGMENTS
We thank all the study volunteers for their contribution. Elizabeth Nyman (University of Turku Language Centre, Turku, Finland) is acknowledged for English-language content editing.
This study was funded by Finnish Governmental Research Funding (ERVA) for Turku University Hospital and Turku City Hospital, the Pro Humanitate Foundation, the Finnish Cultural Foundation, the Sigrid Juselius Foundation, and Academy of Finland (grant #310962). In addition, Dr. Sini Toppala received personal grants from the Betania Foundation and the Uulo Arhio Foundation, and personal fees from Finnish Governmental Research Funding (ERVA). Dr. Laura Ekblad received personal grants from the Orion Research Foundation, the Paulo Foundation and the Finnish Medical Foundation and personal fees from Finnish Governmental Research Funding (ERVA).
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/19-0691r1).
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JAD-190691.
REFERENCES
[1] | Cheng G , Huang C , Deng H , Wang H G ((2012) ) Diabetes as a risk factor for dementia and mild cognitive impairment: A meta-analysis of longitudinal studies. Intern Med J 42: , 484–491. |
[2] | Abner EL , Nelson PT , Kryscio RJ , Schmitt FA , Fardo DW , Woltjer RL , Cairns NJ , Yu L , Dodge HH , Xiong C , Masaki K , Tyas SL , Bennett DA , Schneider JA , Arvanitakis Z ((2016) ) Diabetes is associated with cerebrovascular but not Alzheimer’s disease neuropathology. Alzheimers Dement 12: , 882–889. |
[3] | Dos Santos Matioli MNP , Suemoto CK , Rodriguez RD , Farias DS , da Silva MM , Leite REP , Ferretti-Rebustini REL , Farfel JM , Pasqualucci CA , Jacob Filho W , Arvanitakis Z , Naslavsky MS , Zatz M , Grinberg LT , Nitrini R ((2017) ) Diabetes is not associated with Alzheimer’s disease neuropathology. J Alzheimers Dis 60: , 1035–1043. |
[4] | MacKnight C , Rockwood K , Awalt E , McDowell I ((2002) ) Diabetes mellitus and the risk of dementia, Alzheimer’s disease and vascular cognitive impairment in the Canadian Study of Health and Aging. Dement Geriatr Cogn Disord 14: , 77–83. |
[5] | Reaven GM ((1997) ) Banting Lecture 1988. Role of insulin resistance in human disease. 1988. Nutrition 13: , 65. |
[6] | Ekblad LL , Johansson J , Helin S , Viitanen M , Laine H , Puukka P , Jula A , Rinne JO ((2018) ) Midlife insulin resistance, APOE genotype, and late-life brain amyloid accumulation. Neurology 90: , e1150–e1157. |
[7] | Willette AA , Johnson SC , Birdsill AC , Sager MA , Christian B , Baker LD , Craft S , Oh J , Statz E , Hermann BP , Jonaitis EM , Koscik RL , La Rue A , Asthana S , Bendlin BB ((2005) ) Insulin resistance predicts brain amyloid deposition in late middle-aged adults. Alzheimers Dement 11: , 504–510. |
[8] | Matsuzaki T , Sasaki K , Tanizaki Y , Hata J , Fujimi K , Matsui Y , Sekita A , Suzuki SO , Kanba S , Kiyohara Y , Iwaki T ((2010) ) Insulin resistance is associated with the pathology of Alzheimer disease: The Hisayama study. Neurology 75: , 764–770. |
[9] | Dearborn JL , Schneider AL , Sharrett AR , Mosley TH , Bezerra DC , Knopman DS , Selvin E , Jack CR , Coker LH , Alonso A , Wagenknecht LE , Windham BG , Gottesman RF ((2015) ) Obesity, insulin resistance, and incident small vessel disease on magnetic resonance imaging: Atherosclerosis Risk in Communities study. Stroke 46: , 3131–3136. |
[10] | Lee JE , Shin DW , Yun JM , Kim SH , Nam YS , Cho B , Lim JS , Jeong HY , Kwon HM , Park JH ((2016) ) Insulin resistance is a risk factor for silent lacunar infarction. Stroke 47: , 2938–2944. |
[11] | Attems J , Jellinger KA ((2014) ) The overlap between vascular disease and Alzheimer’s disease - lessons from pathology. BMC Med 12: , 206. |
[12] | Gupta A , Iadecola C ((2015) ) Impaired Aβ clearance: A potential link between atherosclerosis and Alzheimer’s disease. Front Aging Neurosci 7: , 115. |
[13] | Kaskikallio A , Karrasch M , Rinne JO , Tuokkola T , Parkkola R , Grönholm-Nyman P ((2019) ) Cognitive effects of white matter pathology in normal and abnormal aging. J Alzheimers Dis 67: , 489–493. |
[14] | Kaskikallio A , Karrasch M , Rinne JO , Tuokkola T , Parkkola R , Grönholm-Nyman P ((2019) ) Domain-specific cognitive effects of white matter pathology in old age, mild cognitive impairment and Alzheimer’s disease. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 14: , 1–18. |
[15] | Snowdon DA , Greiner LH , Mortimer JA , Riley KP , Greiner PA , Markesbery WR ((1997) ) Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA 277: , 813–817. |
[16] | Talbot K , Wang HY , Kazi H , Han LY , Bakshi KP , Stucky A , Fuino RL , Kawaguchi KR , Samoyedny AJ , Wilson RS , Arvanitakis Z , Schneider JA , Wolf BA , Bennett DA , Trojanowski JQ , Arnold SE ((2012) ) Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest 122: , 1316–1338. |
[17] | Mullins RJ , Diehl TC , Chia CW , Kapogiannis D ((2017) ) Insulin resistance as a link between amyloid-beta and tau pathologies in Alzheimer’s disease. Front Aging Neurosci 9: , 118. |
[18] | Hughes TM , Craft S ((2015) ) The role of insulin in the vascular contributions to age-related dementia. Biochim Biophys Acta 1862: , 983–991. |
[19] | Weinstein G , Maillard P , Himali JJ , Beiser AS , Au R , Wolf PA , Seshadri S , DeCarli C ((2015) ) Glucose indices are associated with cognitive and structural brain measures in young adults. Neurology 84: , 2329–2337. |
[20] | Kwon HM , Kim BJ , Lee SH , Choi SH , Oh BH , Yoon BW ((2006) ) Metabolic syndrome as an independent risk factor of silent brain infarction in healthy people. Stroke 37: , 466–470. |
[21] | Portet F , Brickman AM , Stern Y , Scarmeas N , Muraskin J , Provenzano FA , Berr C , Bonafé A , Artero S , Ritchie K , Akbaraly TN ((2012) ) Metabolic syndrome and localization of white matter hyperintensities in the elderly population. Alzheimers Dement 8: (5 Suppl), S88–95. |
[22] | Baune BT , Roesler A , Knecht S , Berger K ((2009) ) Single and combined effects of cerebral white matter lesions and lacunar infarctions on cognitive function in an elderly population. J Gerontol A Biol Sci Med Sci 64: , 118–124. |
[23] | de Groot JC , de Leeuw FE , Oudkerk M , van Gijn J , Hofman A , Jolles J , Breteler MM ((2000) ) Cerebral white matter lesions and cognitive function: The Rotterdam Scan Study. Ann Neurol 47: , 145–151. |
[24] | Ylikoski R , Ylikoski A , Erkinjuntti T , Sulkava R , Raininko R , Tilvis R ((1993) ) White matter changes in healthy elderly persons correlate with attention and speed of mental processing. Arch Neurol 50: , 818–824. |
[25] | Provenzano FA , Muraskin J , Tosto G , Narkhede A , Wasserman BT , Griffith EY , Guzman VA , Meier IB , Zimmerman ME , Brickman AM ; Alzheimer’s Disease Neuroimaging Initiative ((2013) ) White matter hyperintensities and cerebral amyloidosis: Necessary and sufficient for clinical expression of Alzheimer disease? AMA Neurol 70: , 455–461. |
[26] | Ekblad LL , Rinne JO , Puukka P , Laine H , Ahtiluoto S , Sulkava R , Viitanen M , Jula A ((2017) ) Insulin resistance predicts cognitive decline: An 11-year follow-up of a nationally representative adult population sample. Diabetes Care 40: , 751–758. |
[27] | Villemagne VL , Burnham S , Bourgeat P , Brown B , Ellis KA , Salvado O , Szoeke C , Macaulay SL , Martins R , Maruff P , Ames D , Rowe CC , Masters CL ; Australian Imaging Biomarkers and Lifestyle (AIBL) Research Group ((2017) ) Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: A prospective cohort study. Lancet Neurol 12: , 357–367. |
[28] | Winblad B , Amouyel P , Andrieu S , Ballard C , Brayne C , Brodaty H , Cedazo-Minguez A , Dubois B , Edvardsson D , Feldman H , Fratiglioni L , Frisoni GB , Gauthier S , Georges J , Graff C , Iqbal K , Jessen F , Johansson G , Jönsson L , Kivipelto M , Knapp M , Mangialasche F , Melis R , Nordberg A , Rikkert MO , Qiu C , Sakmar TP , Scheltens P , Schneider LS , Sperling R , Tjernberg LO , Waldemar G , Wimo A , Zetterberg H ((2016) ) Defeating Alzheimer’s disease and other dementias: A priority for European science and society. Lancet Neurol 15: , 455–532. |
[29] | Aromaa A , Koskinen S ((2004) ) Health and functional capacity in Finland. Baseline results of the Health 2000 Health Examination Survey. Publications of the National Public Health Institute, Helsinki; B12, http://www.julkari.fi/handle/10024/78534. Accessed 19 February 2018. |
[30] | Heistaro S. ((2008) ) Methodology report: Health 2000 survey. Publications of the National Public Health Institute, Helsinki; B26, http://www.julkari.fi/handle/10024/78185. Accessed 19 February 2018. |
[31] | Morris JC , Heyman A , Mohs RC , Hughes JP , van Belle G , Fillenbaum G , Mellits ED , Clark C ((1989) ) The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology 39: , 1159–1165. |
[32] | Jänis MT , Siggins S , Tahvanainen E , Vikstedt R , Silander K , Metso J , Aromaa A , Taskinen MR , Olkkonen VM , Jauhiainen M , Ehnholm C ((2004) ) Active and low-active forms of serum phospholipid transfer protein in a normal Finnish population sample. J Lipid Res 45: , 2303–2309. |
[33] | Matthews DR , Hosker JP , Rudenski AS , Naylor BA , Treacher DF , Turner RC ((1985) ) Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: , 412–419. |
[34] | Aalto S , Scheinin NM , Kemppainen NM , Någren K , Kailajärvi M , Leinonen M , Scheinin M , Rinne JO ((2009) ) Reproducibility of automated simplified voxel-based analysis of PET amyloid ligand [11C]PIB uptake using 30 min scanning data. Eur J Nucl Med Mol Imaging 36: , 1651–1660. |
[35] | Wechsler D ((1987) ) Wechsler Memory Scale - Revised: Manual, The Psychological Corporation, San Antonio. |
[36] | Wechsler D ((1981) ) Wechsler Adult Intelligence Scale - Revised, Manual, The Psychological Corporation, New York. |
[37] | Kaplan EF , Goodglass H , Weintraub S ((1983) ) The Boston Naming Test, Lea & Febiger, Philadelphia. |
[38] | Army Individual Test Battery ((1944) ) Manual of Directions and Scoring, War Department, Adjutant General’s Office, Washington DC. |
[39] | Stroop JR ((1935) ) Studies of interference in serial verbal reactions. J Exp Psychol 18: , 643–662. |
[40] | Koikkalainen J , Rhodius-Meester H , Tolonen A , Barkhof F , Tijms B , Lemstra AW , Tong T , Guerrero R , Schuh A , Ledig C , Rueckert D , Soininen H , Remes AM , Waldemar G , Hasselbalch S , Mecocci P , van der Flier W , Lötjönen J ((2016) ) Differential diagnosis of neurodegenerative diseases using structural MRI data. Neuroimage Clin 11: , 435–449. |
[41] | Koikkalainen JR , Rhodius-Meester HFM , Frederiksen KS , Bruun M , Hasselbalch SG , Baroni M , Mecocci P , Vanninen R , Remes A , Soininen H , van Gils M , van der Flier WM , Scheltens P , Barkhof F , Erkinjuntti T , Lötjönen JMP , Alzheimer’s Disease Neuroimaging Initiative ((2019) ) Automatically computed rating scales from MRI for patients with cognitive disorders. Eur Radiol 29: , 4937–4947. |
[42] | Snellman A , Rokka J , López-Picón FR , Helin S , Re F , Löyttyniemi E , Pihlaja R , Forloni G , Salmona M , Masserini M , Solin O , Rinne JO , Haaparanta-Solin M ((2017) ) Applicability of [11C]PIB micro-PET imaging for in vivo follow-up of anti-amyloid treatment effects in APP23 mouse model. Neurobiol Aging 57: , 84–94. |
[43] | Braak H , Braak E ((1991) ) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82: , 239–259. |
[44] | Jack CR Jr , Lowe VJ , Senjem ML , Weigand SD , Kemp BJ , Shiung MM , Knopman DS , Boeve BF , Klunk WE , Mathis CA , Petersen RC ((2008) ) 11C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic mild cognitive impairment. Brain 131: , 665–680. |
[45] | Rowe CC , Ellis KA , Rimajova M , Bourgeat P , Pike KE , Jones G , Fripp J , Tochon-Danguy H , Morandeau L , O’Keefe G , Price R , Raniga P , Robins P , Acosta O , Lenzo N , Szoeke C , Salvado O , Head R , Martins R , Masters CL , Ames D , Villemagne VL ((2010) ) Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging 31: , 1275–1283. |
[46] | Lezak MD , Howieson DB , Bigler ED , Tranel D ((2012) ) Neuropsychological Assessment (5th Ed). Oxford University Press, New York. |
[47] | Viscogliosi G , Chiriac IM , Andreozzi P , Ettorre E ((2015) ) Executive dysfunction assessed by Clock-Drawing Test in older non-demented subjects with metabolic syndrome is not mediated by white matter lesions. Psychiatry Clin Neurosci 69: , 620–629. |
[48] | Kuusisto J , Koivisto K , Mykkänen L , Helkala EL , Vanhanen M , Hänninen T , Kervinen K , Kesäniemi YA , Riekkinen PJ , Laakso M ((1997) ) Association between features of the insulin resistance syndrome and Alzheimer’s disease independently of apolipoprotein E4 phenotype: Cross sectional population based study. BMJ 315: , 1045–1049. |
[49] | Schrijvers EM , Witteman JC , Sijbrands EJ , Hofman A , Koudstaal PJ , Breteler MM ((2010) ) Insulin metabolism and the risk of Alzheimer disease: The Rotterdam Study. Neurology 75: , 1982–1987. |
[50] | Debette S , Markus HS ((2010) ) The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: Systematic review and meta-analysis. BMJ 341: , c3666. |
[51] | Smith EE , Muzikansky A , McCreary CR , Batool S , Viswanathan A , Dickerson BC , Johnson K , Greenberg SM , Blacker D ((2018) ) Impaired memory is more closely associated with brain beta-amyloid than leukoaraiosis in hypertensive patients with cognitive symptoms. PLoS One 13: , e0191345. |
[52] | Villemagne VL , Doré V , Bourgeat P , Burnham SC , Laws S , Salvado O , Masters CL , Rowe CC ((2017) ) Aβ-amyloid and tau imaging in dementia. Semin Nucl Med 47: , 75–88. |
[53] | Motes MA , Biswal BB , Rypma B ((2011) ) Age-dependent relationships between prefrontal cortex activation and processing efficiency. Cogn Neurosci 2: , 1–10. |
[54] | Twamley EW , Ropacki SA , Bondi MW ((2006) ) Neuropsychological and neuroimaging changes in preclinical Alzheimer’s disease. J Int Neuropsychol Soc 12: , 707–735. |
[55] | Arnold SE , Arvanitakis Z , Macauley-Rambach SL , Koenig AM , Wang HY , Ahima RS , Craft S , Gandy S , Buettner C , Stoeckel LE , Holtzman DM , Nathan DM ((2018) ) Brain insulin resistance in type 2 diabetes and Alzheimer disease: Concepts and conundrums. Nat Rev Neurol 14: , 168–181. |
[56] | Ekblad LL , Rinne JO , Puukka PJ , Laine HK , Ahtiluoto SE , Sulkava RO , Viitanen MH , Jula AM ((2015) ) Insulin resistance is associated with poorer verbal fluency performance in women. Diabetologia 58: , 2545–2553. |
[57] | Craft S , Claxton A , Baker LD1 , Hanson AJ , Cholerton B , Trittschuh EH , Dahl D , Caulder E , Neth B , Montine TJ , Jung Y , Maldjian J , Whitlow C , Friedman S ((2017) ) Effects of regular and long-acting insulin on cognition and Alzheimer’s disease biomarkers: A pilot clinical trial. J Alzheimers Dis 57: , 1325–1334. |




