Trend in the Incidence and Prevalence of Dementia in the Faroe Islands
Abstract
Background:
Dementia has become an important public health, economic, and social issue. Knowledge about prevalence, incidence, and trends of dementia in a country is of crucial importance. However, no studies of incidence or prevalence of dementia have been undertaken in the Faroe Islands.
Objectives:
The aim was to estimate the overall and trend in incidence and prevalence of dementia among individuals ≥60 years in the Faroe Islands from 2010-2017.
Methods:
Population-based register study where all individuals ≥60 years with a dementia diagnosis from January 2010 to December 2017 were identified. The overall crude and age-and-sex-specific incidence and prevalence was assessed.
Results:
The overall crude incidence among individuals ≥60 years from 2010 to 2017 was 5.1 per 1000 individuals and the prevalence 22.5 per 1000 individuals. The age-and sex-standardized annual incidence of dementia fluctuated between 4.8 and 6.7 per 1000, with no clear secular trend while the age-and sex-standardized prevalence increased steadily from 14.5 in 2010 to 30.8 per 1000 individuals in 2017.
Conclusion:
The age-standardized incidence or prevalence estimates in the Faroes seem to be lower than in other countries. The incidence was relatively stable in the period while the prevalence of dementia simultaneously increased.
INTRODUCTION
Dementia has become an important public health, economic, and social issue [1]. Knowledge about prevalence, incidence, and trends of dementia in a country is of crucial importance to inform policy development, planning, and costing of health services [2]. Therefore, robust and up-to-date estimates of incidence and prevalence are needed. The incidence and prevalence of dementia has been estimated in several countries [3–6]. In a 2013 meta-analysis, the age-standardized prevalence (based on Western European population) was found to be between 5–7% [4], while a 2016 meta-analysis reported the incidence proportion to range from 8.7 per 1000 in a Japanese study to 142.22 per 1000 in a US study [3].
To date, only few studies have investigated time trends [6–9], and most studies were based on local or regional data using population-based cohorts, rather than nationwide registries within real-world settings [10]. A 2016 review concluded there was moderately consistent evidence to suggest a declining incidence of dementia in high-income countries while evidence on trends in the prevalence were inconsistent across studies and did not suggest any clear overall effect [8]. In contrary, a 2017 and a 2018 review indicated stable or declining incidence and prevalence the past 10–15 years, especially in the Western countries [7, 9]. The Global Burden of Disease study 2016 reported an increase in prevalence of 117% from 1990 to 2016 but only a 1.7% increase in age-standardized prevalence of dementia [6].
No studies of incidence or prevalence of dementia in the Faroe Islands have been undertaken, although this knowledge is crucial to inform policy development [2]. Of note, the prevalence of both Parkinson’s disease [11, 12] and primary focal dystonia [13] are high, while multiple sclerosis [14, 15], essential tremor [16], and amyotrophic lateral sclerosis are comparable [17] to other European countries. Thus, the aim of this study was to estimate the overall and trend in incidence and prevalence of dementia in the entire population aged ≥60 years of the Faroe Islands from 2010–2017, using nationwide data within real-world setting from the only specialized, hospital-based memory clinic in the Faroe Islands.
MATERIALS AND METHODS
Setting
Located in the North Atlantic, the Faroe Islands comprise 18 small islands with a population of approximately 50,000 people (Supplementary Figure 1). The population is of Nordic and Irish origin and comparable in many aspects to other Western populations [18, 19]. The demographics of the Faroes have been quite stable for decades and the life expectancy is among the highest in the world [20]. The Faroes have a high-quality health care system financed through statutory health insurance and taxes, guaranteeing unfettered access to general practitioners (GP) and hospitals as well as partial reimbursement for prescribed medications. There are three hospitals in the Faroes of which the National Hospital, located in the capital, is the main hospital and holds the only specialized memory clinic, the Dementia Clinic, in the islands which was established in 2007. An Electronic Health Record (EHR) system named Cosmic Gambio connects the National Health Service, i.e., the hospitals and the 30 GPs. The EHR contains all journal entries written by the GPs or hospital doctors, referrals, laboratory, and radiological findings and drug prescriptions.
Clinical evaluation of dementia in the Faroe Islands
Referrals to the Dementia Clinic for diagnostic evaluation are made through GPs who conduct the initial screening for dementia using the Mini-Mental State Examination (MMSE) [21] and the Clock Drawing Test [22], including information about observed changes and medical history. Decision regarding specialist referral is based on these pieces of information.
The first appointment at the Dementia Clinic is with a medical doctor who performs a thorough clinical and neurological examination to rule out other neurologic disease and known reasons for memory deficiency. Thorough medical history, assessment of the patient’s comorbidities, functional performance, and level of care are recorded, and lab tests and cranial computed tomography (CT) scans reviewed. The MMSE and Clock Drawing Test [22] are performed and depression assessed based on the Geriatric Depression Scale (GDS-15) [23] allowing for the identification of potentially reversible conditions (e.g., depression) or indications for secondary prophylaxis (e.g., vascular dementia). If there is a confirmed suspicion of dementia, a second appointment is scheduled a few weeks later.
At the second appointment at the Dementia Clinic, the patient is re-tested with the MMSE, Clock Drawing Test, and GDS-15 by the trained staff and the psychiatrist (SJ) evaluates the patient based on the test results, functional performance, and CT scan, also taking into account blood test results, medical history, and the patient’s comorbidities, i.e., the diagnosis is established by the psychiatrist using a two-step procedure with an initial identification of a dementia syndrome using MMSE and Clock Drawing Test and the exclusion of other possible etiologies of dementia with blood investigations to rule out other causes and with brain neuroimaging (CT) to exclude infarcts and other conditions [24]. The diagnoses are established according to ICD-10 criteria but the criteria used for Alzheimer’s disease (AD) and other dementia sub-types (mixed dementia, vascular dementia, Lewy body dementia, frontotemporal dementia, dementia associated with Parkinson’s disease, alcohol-induced dementia, and non-specified dementia) are comparable to DMS IV criteria [24].
After evaluation and establishment of a diagnosis, the common regime is to have a follow-up scheduled 3 months, 6 months, and 1 year after the diagnosis and thereafter, based on need.
Study population
This is a population-based register study using real-time data from the Dementia Clinic. All individuals evaluated at the Dementia Clinic were identified through manual and electronic appointment records from 2007 when the Dementia Clinic was established to 31 December 2017. However, only those diagnosed from 1 January 2010 were included in the incidence estimates to allow for a wash-out period to correctly identify incident and prevalent cases over time and those alive on 1 January 2010 were included in the prevalence estimate. Medical records of all identified individuals were reviewed by MSP to identify those who had received a dementia diagnosis and data was extracted from the medical journals. Background data was obtained from records from the first clinical appointment at the Dementia Clinic while the diagnosis and anti-dementia medication use was extracted from records from the second or subsequent appointments at the clinic. Doubtful cases, e.g., where no diagnosis code was recorded or diagnosis not mentioned explicitly in the journal, were double-checked with SJ.
MMSE and GDS scores at the first examination, self-reported family history of dementia, psychiatric medication (mainly depression medication), living conditions (alone, with someone or in institution), cardiovascular or metabolic diseases (medication for type 2 diabetes, hypertension, hypercholesterolemia) prior to establishment of the dementia diagnosis and date of death if occurred were manually extracted from the medical journal and recorded into the study database. Data regarding the diagnosis and anti-dementia medication was obtained from medical journal records from second or later appointments. Age at diagnosis was calculated from the date of the first appointment at the Dementia Clinic, and disease duration was calculated from the date of the first appointment to 31 December 2017 or death whichever occurred first.
The term dementia used in this paper denotes dementia syndrome of any causes, covering all ICD codes for dementia diagnoses (AD, vascular dementia, frontotemporal dementia, Lewy body dementia, Parkinson dementia, and dementia without specification).
This registry study was approved by the Faroese Chief Medical Officer and Faroese Data Protection Agency. No personal contact has been with the individuals for this study.
Data analyses
Basic descriptive statistics are provided for population characteristics and presented as mean and standard deviation (SD) or number and percentages. Differences between years and sex were tested using one-way ANOVA for continuous variables and chi square for categorical variables.
The overall and annual crude period incidence and prevalence were calculated. The 2010 population was used as standard population and used in the calculations of incidence and prevalence for all years to permit for examining trends. Thus, the incidence was estimated using the number of incident cases as the numerator and the population above age 60+ in the Faroe Islands 31 December 2009, corresponding to the population 1 January 2010 as denominator. Incident cases were identified in each 12-month period between 1 January and 31 December, i.e., they were diagnosed between 1 January and 31 December of the respective year. The prevalence was estimated by using the number of living cases as the numerator and the population age 60+ in the Faroe Islands on the 31 December 2009 as denominator. Included as prevalent cases were all patients alive who lived in the Faroe Islands during each 12-month period from 1 January to 31 December in the years 2010 to 2017. For the overall incidence and prevalence, 95% confidence intervals (CI) were calculated.
Furthermore, the incidence and prevalence estimates were age-and sex-standardized with direct method using the 2010 Faroese populations as standard. To compare the incidence and prevalence estimated among Faroese women and men, the female incidence and prevalence estimates were adjusted using the male distribution as weight facilitating comparison between the sexes, taking the Faroese sex-ratio into account. Further, overall and sex-specific incidence and prevalence was calculated for 5-year age grouping (60–64, 65–69, 70–74, 75–79, 80–84, 85+ years).
Age-standardized and age- and sex-standardized incidence or prevalence of dementia were also calculated with the direct method of standardization using the age or age- and sex-specific rates for the Western European populations in 2010 [25] to enable direct comparison with other studies [4]. Changes in incidence and prevalence rates were compared and assessed for statistically significant differences and 95% CI using Poisson regression models. Both Poisson regression models and the negative binomial distribution were considered, but based on Akaike’s Information Criterion the Poisson regression models fitted our data best.
RESULTS
Population characteristics
The study population is the entire Faroese population aged ≥60 years in the respective year (Table 1 and Supplementary Table 1). From 1 January 2010 to 31 December 2017, the total number of dementia cases identified aged 60 years and older was 539, of whom 321 (59.6%) were women (Table 1). A total of 97 individuals had a diagnosis of dementia before 1 January 2010; these individuals were included in the prevalence estimate but excluded from the incidence assessments to allow for a wash-out period. Of note and not included in the incidence or prevalence estimates, 16 (2.9%) individuals were diagnosed before the age of 60 from 2010 to 2017, of whom 13 were diagnosed with AD. The mean age at diagnosis of these 16 individuals was 55 years (SD: 3.0) and the average MMSE score was 20.38 (SD: 4.5, range: 10–28) (data not shown).
Table 1
Demographic characteristics at time of diagnosis of subjects aged ≥60 year with a dementia diagnosis between 1 January 2010 and 31 December 2017 in the Faroe Islands
| Characteristics | All n = 539 | Women n = 321 | Men n = 218 | p |
| Age at diagnosis, mean (SD), range | 78.8 (6.6), 60.0–96.9 | 79.4 (6.6), 60–96.9 | 77.9 (6.4), 60.5–96.8 | 0.01† |
| Age distribution at diagnosis, n (%) | 0.1* | |||
| 60–64 | 15 (2.8) | 10 (3.1) | 5 (2.3) | |
| 65–69 | 39 (7.2) | 20 (6.3) | 19 (8.7) | |
| 70–74 | 95 (17.7) | 52 (16.3) | 43 (19.7) | |
| 75–79 | 136 (25.3) | 71 (22.2) | 65 (29.8) | |
| 80–84 | 169 (31.4) | 108 (33.8) | 61 (28.0) | |
| 85+ | 84 (15.6) | 59 (18.4) | 25 (11.5) | |
| MMSE at first clinical appointment, mean (SD), range‡ | 20.5 (5.6), 3–30 | 20.8 (5.4), 4–30 | 20.0 (2.4), 3–30 | 0.1† |
| GDS at first clinical appointment, mean (SD), range§ | 2.8 (2.5), 0–13 | 2.7 (2.6), 0–13 | 2.9 (2.4), 0–13 | 0.4† |
| CT performed, n (%)¶ | 496 (96.9) | 294 (95.8) | 202 (98.5) | 0.08* |
| Self-informed family history of dementia, n (%)# | 181 (33.6) | 113 (35.3) | 68 (31.2) | 0.3* |
| Medication use before diagnosis, n (%) | ||||
| Depression and other psychiatric diseases | 177 (33.6) | 116 (37.5) | 61 (28.4) | 0.03* |
| Hypertension | 327 (61.5) | 187 (59.0) | 140 (65.1) | 0.2* |
| Diabetes mellitus | 66 (12.4) | 24 (7.6) | 42 (19.5) | <0.0001* |
| Hypercholesterolemia | 172 (32.3) | 89 (28.1) | 83 (38.6) | 0.01* |
| Anti-dementia medication prescribed after | 426 (79.3) | 267 (83.7) | 159 (72.9) | 0.002* |
| establishment of diagnosis, n (%) | ||||
| Living condition at first clinical appointment, n (%) | <0.0001* | |||
| Alone | 154 (29.1) | 121 (38.2) | 33 (15.5) | |
| With someone | 302 (57.0) | 157 (49.5) | 145 (68.1) | |
| Institution | 74 (14.0) | 39 (12.3) | 35 (16.4) | |
| Geographic place of living at first clinical appointment | 0.7* | |||
| Suðurstreymoy | 171 (38.7) | 108 (40.3) | 63 (36.2) | |
| Norðurstreymoy | 46 (10.4) | 29 (10.8) | 17 (9.8) | |
| Eysturoy | 103 (23.3) | 59 (22.0) | 44 (25.3) | |
| Norðoyggjar | 75 (17.0) | 46 (17.2) | 29 (16.7) | |
| Vágoy | 18 (4.1) | 11 (4.1) | 7 (4.0) | |
| Sandoy | 15 (3.4) | 6 (2.2) | 9 (5.2) | |
| Suðuroy | 14 (3.2) | 9 (3.4) | 5 (2.9) | |
| Type of dementia diagnoses, n (%) | <0.0001* | |||
| Early onset Alzheimer’s disease | 16 (3) | 12 (3.8) | 4 (1.8) | |
| Late onset Alzheimer’s disease | 378 (70.1) | 246 (76.9) | 131 (60.1) | |
| Mixed dementia | 31 (5.8) | 13 (4.1) | 18 (8.3) | |
| Vascular dementia | 37 (6.9) | 15 (4.7) | 22 (10.1) | |
| Lewy body dementia | 24 (4.5) | 13 (4.1) | 11 (5.1) | |
| Frontotemporal dementia | 11 (2.0) | 5 (1.6) | 6 (2.8) | |
| Dementia associated with Parkinson’s disease | 6 (1.1) | 2 (0.6) | 4 (1.8) | |
| Alcohol-induced dementia | 7 (1.3) | 1 (0.3) | 6 (2.8) | |
| Non-specified dementia | 29 (5.4) | 13 (4.1) | 16 (7.3) |
*chi square test; †ANOVA. ‡data missing for n = 64, §data missing for n = 88; ¶data missing for n = 26; #data missing for n = 273. SD, standard deviation; MMSE, Mini-Mental State Examination; GDS, Geriatric depression scale; CT, computed tomography.
The majority of cases (73%) were diagnosed with AD while the other sub-types were more rare (Table 1). The median age at diagnosis was 78.8 years (SD: 6.6) and disease duration for incidence cases (from time of diagnosis to death or to end of the study period) 3.1 years (SD: 2.1), ranging from below one year to 8 years. The mean MMSE at first appointment was 20.5 (SD: 5.7), ranging from 3 to 30. A total of 44.8% had an MMSE of 20 or below at their first appointment.
Both age at diagnosis and disease duration were significantly higher in women (p = 0.01 and p = 0.03, respectively). Men were more likely to receive medication for type 2 diabetes and hypercholesterolemia, while women were more likely to receive medication for depression and related psychiatric disorders (Table 1). Annual differences were only found in regard to medication prior to diagnoses related to hypercholesterolemia, although without any clear trend. Otherwise, the case characteristics were quite similar throughout the years studied (Supplementary Table 1).
Incidence and prevalence estimates
The overall crude incidence from 2010 to 2017, with 440 new cases and the total population at risk of 86940 individuals, was 5.1 per 1000 (95% CI: 4.6–5.6).
The sex-and age-standardized annual incidence of dementia among individuals ≥60 years for the years 2010 to 2017 fluctuated between 4.8 and 6.7 per 1000 population at risk, with no clear secular trend (Fig. 1, Table 2, p = 0.7)).
Fig.1
Time trend in age-and sex standardized incidence of dementia in the Faroe Islands from 2010 to 2017. The Faroese estimates are standardized to the 2010 Faroese and Western European populations, respectively. Error bars represent 95% confidence intervals.
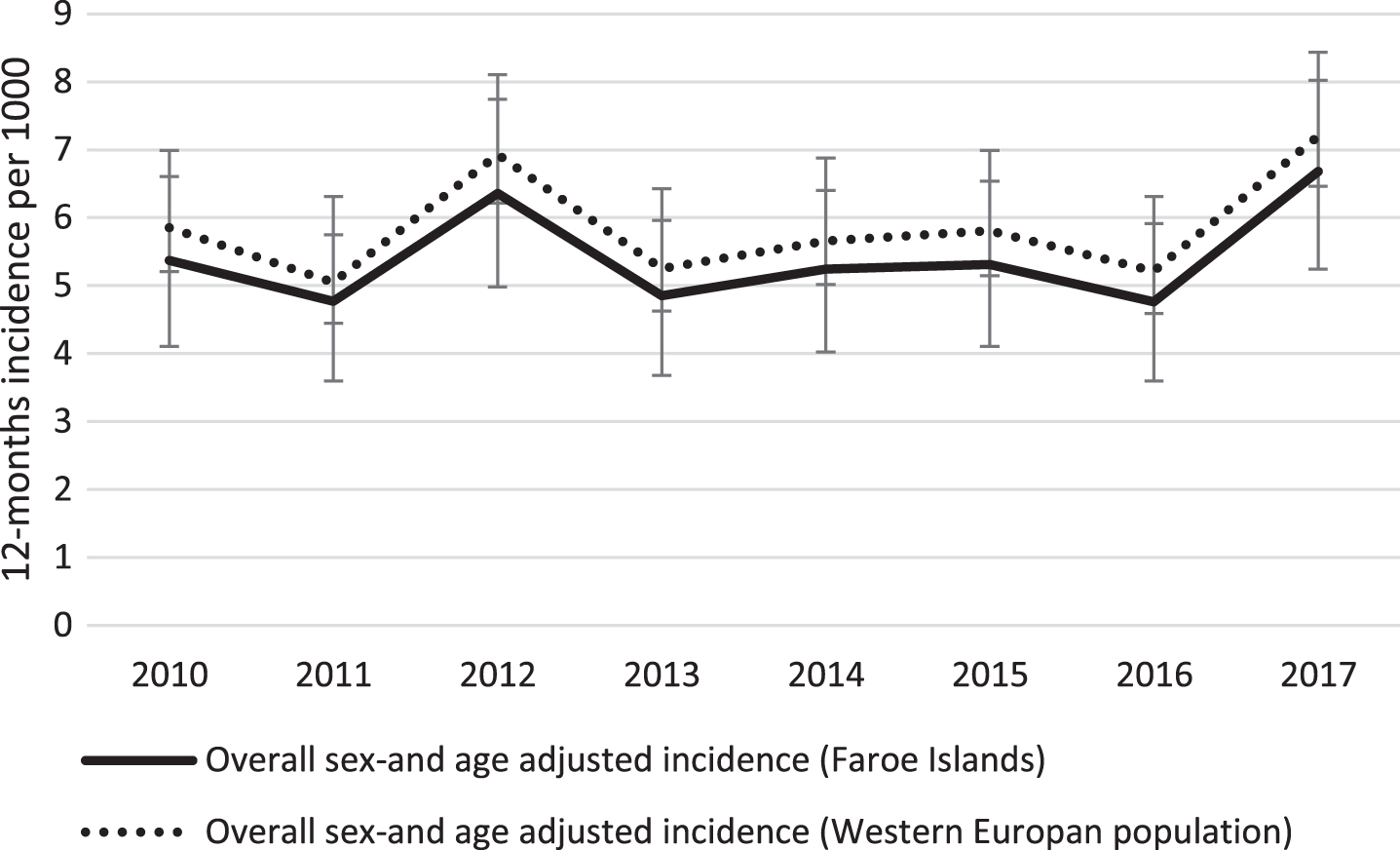
Table 2
Twelve-month incidence and prevalence of dementia (per 1000) among subjects aged ≥60 year in Faroe Islands from 2010 to 2017
| Year | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 |
| Incidence cases | 55 | 50 | 62 | 51 | 54 | 54 | 46 | 68 |
| Prevalent cases | 147 | 179 | 219 | 248 | 272 | 285 | 292 | 317 |
| Prevalence per 1000 | ||||||||
| Crude | ||||||||
| Overall | 14.59 | 17.77 | 21.74 | 24.62 | 27.00 | 28.29 | 28.98 | 31.46 |
| Female | 16.42 | 21.64 | 26.85 | 31.11 | 34.58 | 35.74 | 36.71 | 39.61 |
| Female (standardized to male population weight) | 13.62 | 16.43 | 20.17 | 22.68 | 24.74 | 25.95 | 26.77 | 28.89 |
| Male | 12.66 | 13.68 | 16.33 | 17.76 | 18.98 | 20.41 | 20.82 | 22.86 |
| Age-standardized according to the Western European population structure* | ||||||||
| Overall | 15.98 | 19.31 | 23.65 | 26.70 | 29.22 | 30.71 | 31.42 | 34.11 |
| Female | 17.66 | 23.06 | 28.78 | 33.27 | 37.03 | 38.29 | 39.29 | 42.26 |
| Male | 14.00 | 14.99 | 17.85 | 19.30 | 20.43 | 22.18 | 22.68 | 24.96 |
| Age-and sex standardized according to the Faroese populations or the Western European population structure* | ||||||||
| Overall (Faroese population) | 14.53 | 17.44 | 21.60 | 24.22 | 26.42 | 27.75 | 28.51 | 30.75 |
| Overall (Western European population) | 15.91 | 18.94 | 23.48 | 26.27 | 28.61 | 30.14 | 30.92 | 33.35 |
| Incidence per 1000 | ||||||||
| Crude | ||||||||
| Overall | 5.46 | 4.96 | 6.15 | 5.06 | 5.36 | 5.36 | 4.57 | 6.75 |
| Female | 6.18 | 6.96 | 7.15 | 5.80 | 6.18 | 6.57 | 5.22 | 7.73 |
| Female (standardized to male population weight) | 4.91 | 4.34 | 5.72 | 4.57 | 4.71 | 4.74 | 4.17 | 5.85 |
| Male | 4.69 | 2.86 | 5.10 | 4.29 | 4.49 | 4.08 | 4.29 | 5.72 |
| Age-standardized according to Western European population structure* | ||||||||
| Overall | 5.95 | 5.26 | 6.72 | 5.46 | 5.78 | 5.85 | 5.03 | 7.28 |
| Female | 6.65 | 7.25 | 7.80 | 6.06 | 6.61 | 7.02 | 5.58 | 8.04 |
| Male | 5.07 | 2.98 | 5.52 | 4.73 | 4.76 | 5.53 | 4.76 | 6.34 |
| Age-and sex standardized according to the Faroese populations or the Western European population structure* | ||||||||
| Overall (Faroese population) | 5.37 | 4.77 | 6.36 | 4.85 | 5.24 | 5.31 | 4.76 | 6.68 |
| Overall (Western European population) | 5.85 | 5.05 | 6.93 | 5.25 | 5.66 | 5.81 | 5.21 | 7.20 |
*The population and sex-distribution from 2010 is used as standard population in all estimates: total population at risk (≥60 years) in the Faroe Islands (n = 10075) and Western European population (n = 45699.068).
The overall crude prevalence from 2010 to 2017, with 1959 prevalent cases and the total population at risk of 86940, as 22.5 per 1000 (CI: 21.6–23.5).
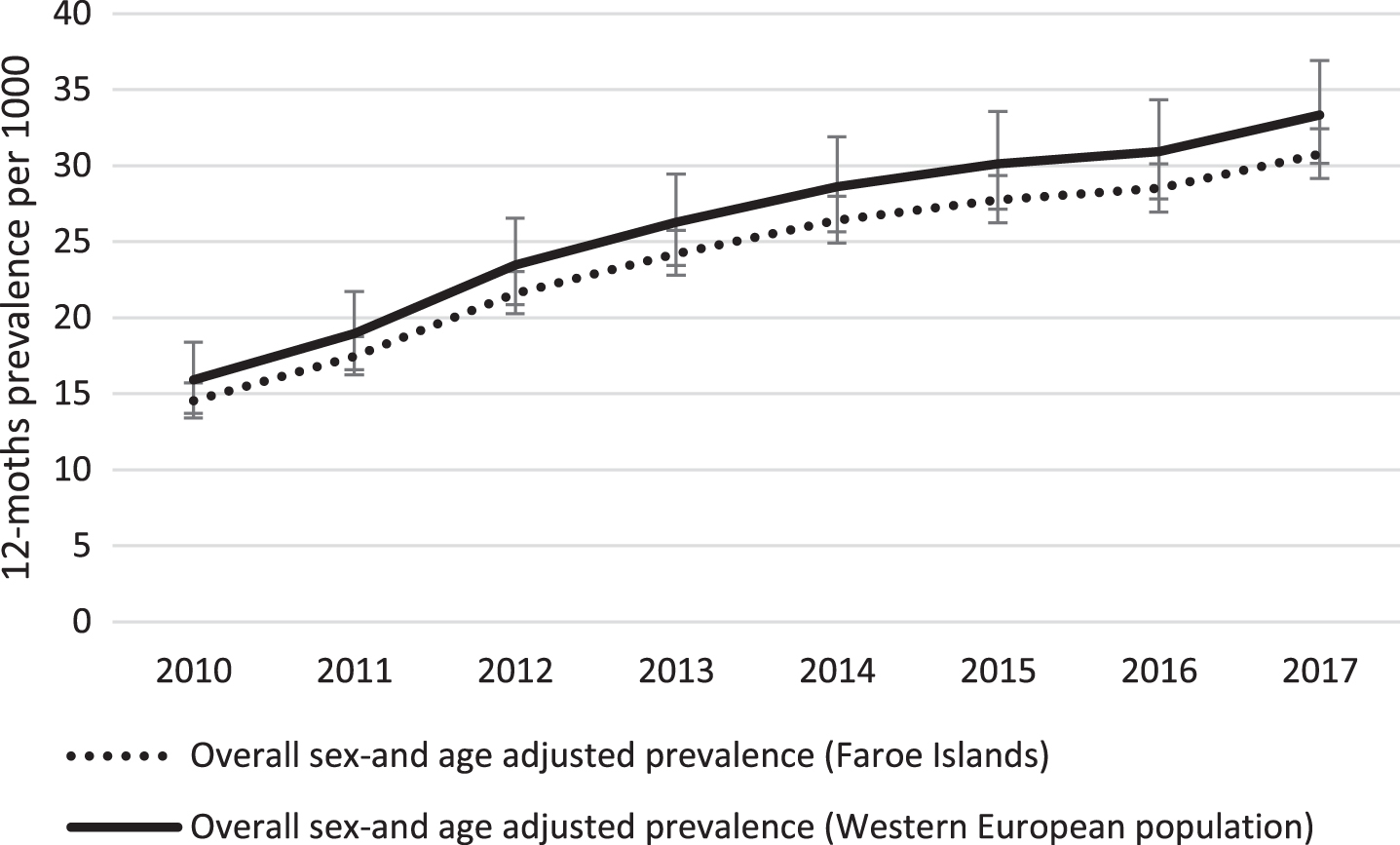
The age and sex-standardized prevalence increased steadily from 2010 to 2017 among those aged ≥60 years, from 14.5 in 2010 to 30.8 per 1000 in 2017, i.e., 112% increase (Fig. 2, Table 2, p = 0.003). Both the age- and sex standardized incidence or prevalence using the West European population as standard was slightly higher, although non-significantly (p = 0.7 and p = 0.5, respectively) (Fig. 2).
Fig.2
Time trend in age-and sex standardized prevalence of dementia in the Faroe Islands from 2010 to 2017. The Faroese estimates are standardized to the 2010 Faroese and Western European populations, respectively. Error bars represent 95% confidence intervals.
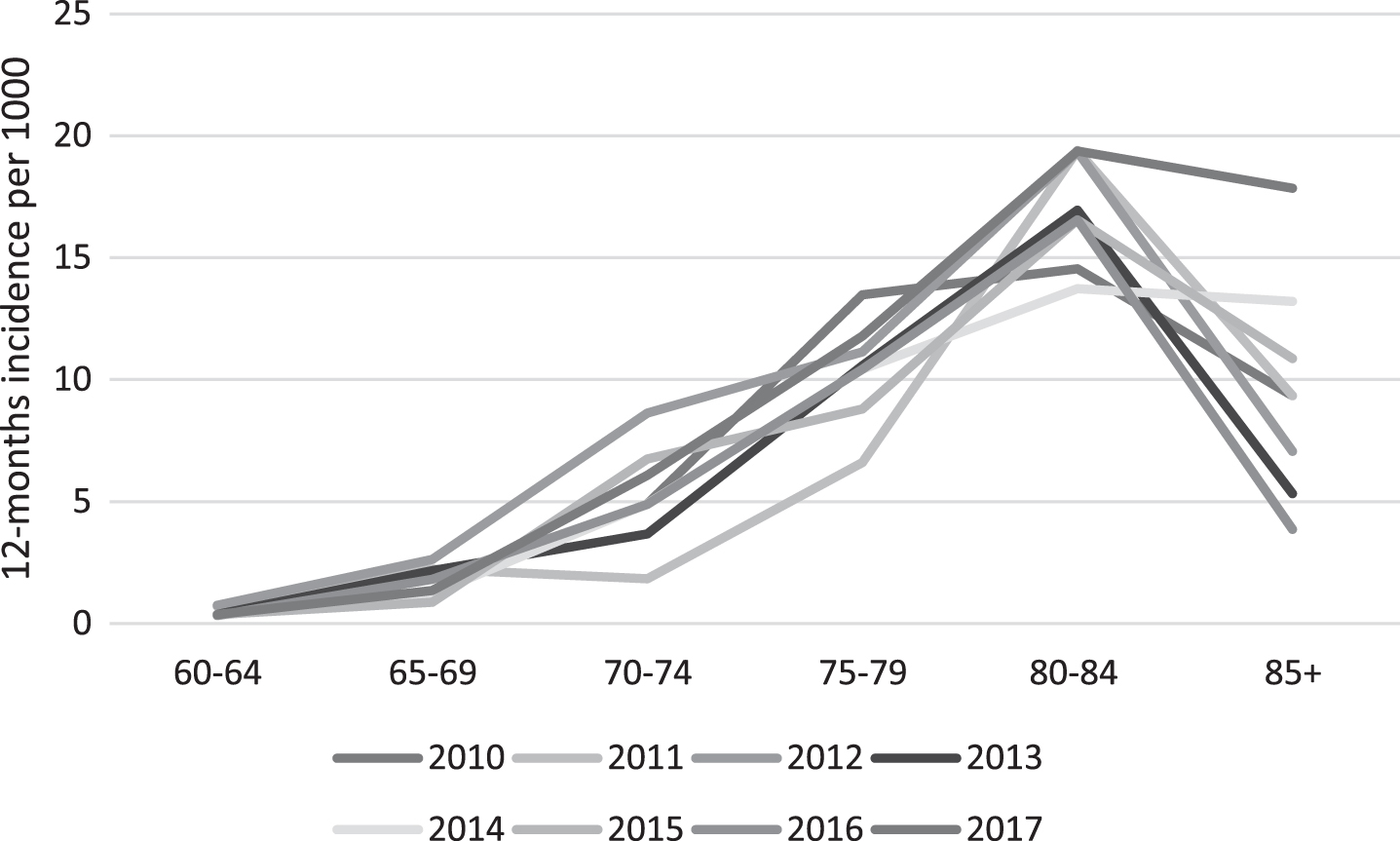
Annual incidence estimated for the six 5-year age groups are shown in Fig. 3. As expected, the incidence increased steadily with age, with the largest increase occurring between the ages of 70–74 and 75–79 year. However, at ages 85 and older the incidence estimate declined. The relationship between age and incidence was fairly consistent over the time period (Fig. 3, Supplementary Tables 2 and 3).
Fig.3
Trend in age-specific, sex-standardized incidence of dementia in the Faroe Islands, by year. Standardization is done using the 2010 Faroese population as standard.
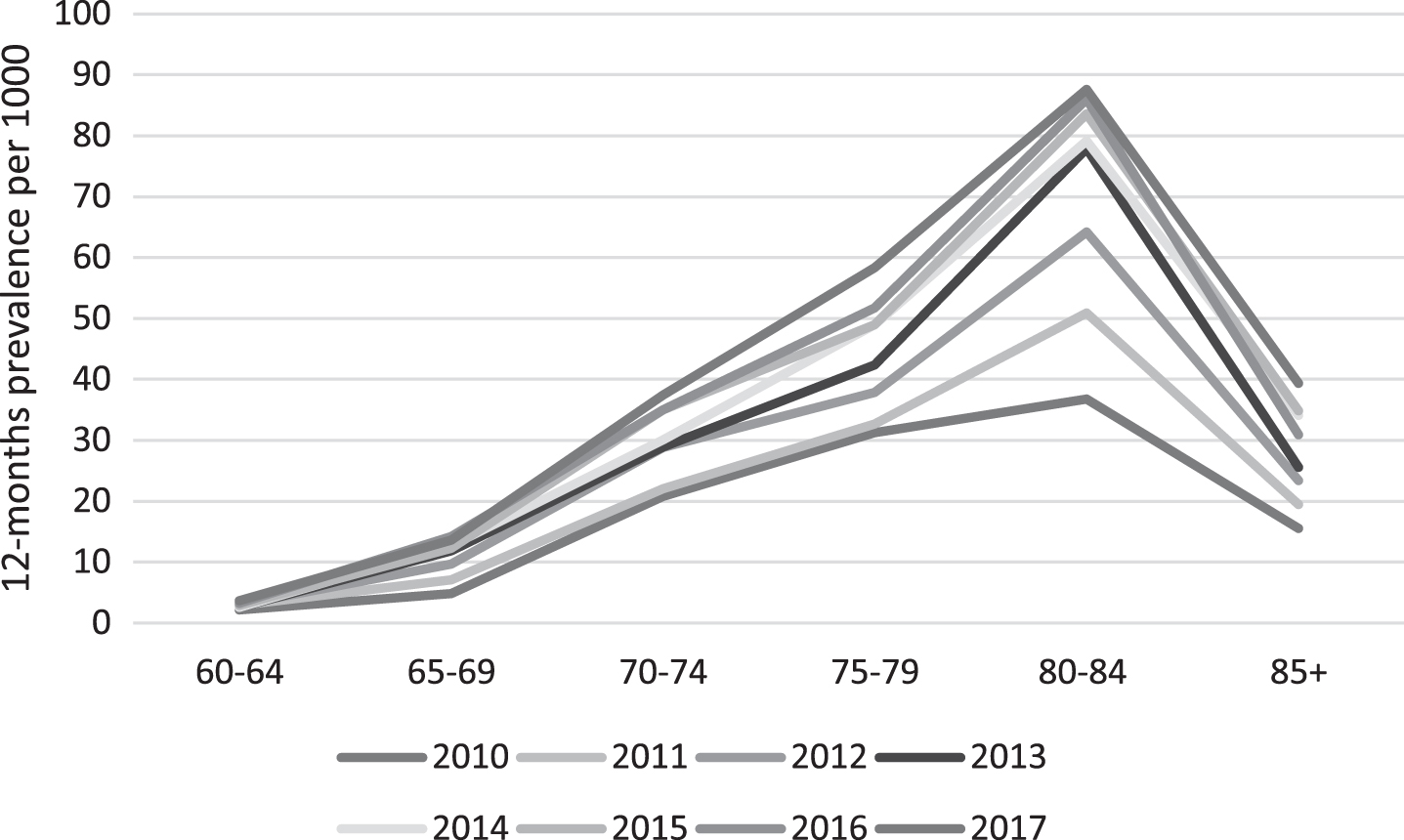
Annual prevalence estimated for the six 5-year age groups is shown in Fig. 4. As with the incidence, the sex-standardized prevalence increased likewise steadily with increasing age, with the highest increase occurring between age-group 70–74 and 75–79 year (Fig. 4, Supplementary Table 2). The highest increase in prevalence through the time period was in the age-range 80–84 where the prevalence increased with 138% from 36.7 per 1000 in 2010 to 87.61 per 1000 in 2017 (p < 0.0001) (Fig. 4).
Fig.4
Trend in age-specific, sex-standardized prevalence of dementia in the Faroe Islands, by year. Standardization is done using the 2010 Faroese population as standard.
Supplementary Figures 2 and 3 show annual sex-specific, age-standardized incidence and prevalence. There is an overall stable time trend in the annual incidence of dementia, apart from some random fluctuation while the prevalence increased steadily from 2010 to 2017 in both sexes. Both incidence and prevalence estimates were higher in females compared with males (Supplementary Figures 1 and 2), but reached only significance for the prevalence (p = 0.6 and p = 0.05).
Using the mean population aged ≥60 years in the Faroes from 2010 to 2017, 36% of the population aged ≥60 years lived in Suðurstreymoy, where the capital is located, 22% in Eysturoy, 12% in Norðoyggjar, 8% in Norðurstreymoy, 7% in Vágoy, 4% in Sandoy, and 12% in the most southern island Suðuroy, only connected with a 2-h boat trip (Supplementary Figure 1). Comparing the population distribution with the geographic distribution of the patients, some areas have fewer cases than expected, e.g., Suðuroy, Sandoy, and Vágar, while others have more than expected based on the population structure (Table 1).
DISCUSSION
Using a population- and register-based cohort design, for the first time, a reliable estimate of dementia incidence and prevalence in the Faroe Islands is provided. The overall crude incidence for the entire period was 5.1 per 1000 individuals and the prevalence 22.5 per 1000 individuals. With an age-and sex-standardized prevalence of 30.8 and 33.4 per 1000 for those 60 years and above, respectively (2017 figures), the Faroese estimate fits within the lower end of the global range of dementia prevalence, ranging from 5% to 7% [4] among people aged ≥60 years. However, a Danish study [26] using data from secondary health care from 1976 to 2004 and a Canadian study using administrative health databases data from 2012-2013 [28] found an overall age-standardized prevalence of 37.6 per 1000 person-years and 28.16 per 1000 population at risk respectively. These estimates are comparable with the 2017 Faroese age-standardized prevalence of 30.8 per 1000 which was the highest estimate in the study period, 112% higher than in 2010.
The standardized incidence estimates in this study (between 4.8–6.7 per 1000 individuals) seem to be lower than reported in comparable studies using registry data [10, 26–28]. The Canadian study reported an age-standardized rate of 7.5 per 1000 individuals [28], a Dutch study using nationwide primary care data found the incidence to be 5.8 per 1000 person-years [10], while the Danish study reported an age-standardized incidence of 10.5 per 1000 person-years [26]. One should always be cautious in comparing the incidence and prevalence rates for dementia across countries as discrepancy between estimates may be a true difference and reflect, e.g., difference in risk or protective factors [29] but may also reflect methodical and/or diagnostic issues.
The time trend observed with relatively stable incidence is in line with the growing body of literature that suggests a stable or potential decline in incidence, especially in high-income countries [2, 6–9]. The Dutch study found a stable incidence [10] while a German study using public health insurance records and the Canadian study reported a decline in incidence [27, 28]. The increasing prevalence has been seen in some studies but most studies report a stable prevalence of dementia and some even decline [7, 8]. One possible explanation for increasing prevalence in the Faroes but stable incidence can be that dementia ascertainment in younger age groups has remained constant, while case survival has been improving. In general, time trend estimates may be affected by increasing attention to and awareness of dementia, shifting diagnostic boundaries and medical knowledge. This increase in awareness and diagnosis probably countered the effect of reduction in occurrence through increased detection of mild cases that previously were not recognized as meeting dementia criteria [2]. Our data shows an increased number of dementia referrals in 2017 (Supplementary Table 1), which may be due to greater public awareness of dementia, both among politicians and medical staff in the primary and secondary health sector but also the general population seeking help for memory related problems at an earlier stage than before. Of note, in 2012 a working group was established by the Faroese Minister of Health to analyze the dementia situation in the Faroe Islands and to make a proposal for future actions. In 2015, the dementia plan was published entitled ‘Dementia friendly society – forgetting but not forgotten’. The report specified seven focus areas to improve or develop further and came with 14 recommendations, including establishing a national dementia policy, earlier detection, research, prevention, etc. However, evidence-based assessment of the dementia burden in the Faroe Islands has not been undertaken before this study, although this is crucial information needed in planning healthcare services. Thus, this is highly needed information.
The decline in incidence or prevalence trajectories in our oldest age group (85+) were lower than would be expected from curve trajectories in preceding age groups. This is different from what is observed in most other studies estimating the incidence and prevalence [4, 5, 26, 28, 30]. One explanation for this decline in the Faroese estimates may be that less effort or reluctance of the GP or family to refer elderly Faroese residents for dementia evaluation or maybe a general thinking that older people tend to forget, i.e., not a disease but a natural consequence of getting older. An additional explanation can be that in a small-scale society like the Faroese, life may be relatively simple and the social network is often substantial. Thus, problems with everyday tasks and living at home may not become obvious before the disease is more severe. Furthermore, the physical distance to the Dementia Clinic may also play a role due to, e.g., disabilities or limited access to transportation and also the situation with temporary and changing GPs in some places may have some influence. We observed, e.g., a reduced representation from the most southern island which is only connected to the main land by boat; only 3% of the identified cases lived in Suðuroy although 12% of the population aged 60+ lives on this island.
There was a marked sex effect as dementia prevalence among Faroese women was significantly higher than of men. This is in agreement with many other studies although some studies do not find a sex difference [4, 5, 7]. As the Faroese sex-ratio has been taken into account in our estimates, the longer survival of women cannot explain the higher incidence and prevalence. One explanation may be that women are more likely to seek help or being referred than men by themselves and/or the health care system. There may also be a selective survival of older men who have lower risk of developing dementia.
Using the regular MMSE cut-off score of 27 as an indication of dementia [21], our data indicated delayed diagnosis in the Faroe Islands as almost half of the included subjects had a MMSE of 20 or below at time of diagnosis pared with the declining incidence estimates in the age 85+ age group. Thus, it seems like Faroese people in general are seeking help or are being referred rather late in the course of the disease. This may be due to the reasons mentioned above with reluctance to refer people to the Dementia Clinic, reluctance of people to get an assessment and/or the small-scale society situation. Consequently, a number of mild cases are probably un-diagnosed. Consequently, this study may underestimate the incidence and prevalence of dementia in the Faroe Islands.
The diagnostic procedures and treatment have been relatively stable in the period studied (Table 2). Comparing with a Danish study using the Danish Dementia Registry [31] and SweDem, the Swedish Dementia Registry [32], the characteristics of the Faroese patients are similar to Danish and Swedish patients and with similar sex ratio (1.5 in the Faroes compared with 1.6 in Denmark and 1.4 in Sweden) [31, 32]. The age of diagnosis (78.8 years in the Faroes versus 78.6 years in Denmark and 78.1 years in Sweden), baseline MMSE (20.5 versus 20.0 and 21.2) were similar while less anti-dementia medication was given in the Faroes (79.3% compared with 87.4% in Denmark and 85.2% in Sweden), more CT scans performed in the Faroes (96.9% versus 88.4% in Denmark and 88.7% in Sweden) and fewer Faroese patients lived alone (29.1% in the Faroes compared with 58.8% in Denmark and 53.3% in Sweden). The difference in living situation may reflect the traditional Faroese society with close family relations and a general expectation in the society to the families to take care of the elderly. Still, we need to take into account that the Danish and Swedish registers included all cases where we have restricted our analyses to people aged 60 and above [31].
This register-based study has some unique advantages. It used nationwide data within real-world setting, i.e., the whole Faroese population aged 60+ was used as background population, including people living in institutions. A selection bias due to nonparticipation or due to recruitment from a specific area was nonexistent. Another major strength of this study is that diagnostic evaluation of dementia was uniform through the period since all dementia evaluations were done at the Dementia Clinic using the same diagnostic standardized criteria for dementia and by the same clinician (SJ). However, there are some limitations. Using only the secondary health sector to identify dementia cases may underestimate the incidence and prevalence in the population as a relatively high number of people are expected to have un-diagnosed dementia [33], especially among the elderly and those living in institutions. However, in our study, about 13% of the patients were living at institutions and are included in the estimate limiting this bias. The decline in the oldest age group and the reduced representation from some geographic areas also indicated an under-estimation of the prevalence in the Faroes. Still, although our estimate based on EHR may be lower than in population-based studies, it is representative of the proportion of people being diagnosed, including people living in institutions. Another limitation was that we used the first contact with the Dementia Clinic as time of onset making the period-specific estimates somewhat imprecise. However, as the numbers and characteristics of the individuals are comparable through the study period, this imprecision is likely spread randomly over the period. Other limitations are also in using existing data in health records. In particular, referrals to specialized clinics may vary substantially between GPs and localities, influenced, for example, by clinician knowledge and attitudes to the usefulness of a diagnosis, its requirement or not for treatment initiation, family attitude and physical distance to the Dementia Clinic.
In conclusion, the age-standardized incidence or prevalence estimates in the Faroes seem to be lower than in other countries. The present study demonstrated a relatively stable age-and sex-standardized incidence of dementia among individuals aged ≥60 over an 8-year period, while the age- and sex-standardized prevalence of dementia simultaneously increased. These trends indicate that the average survival time with dementia is increasing, suggesting earlier detection of dementia. It is prudent that policymakers plan future policy recommendations, services, and health resource planning based upon valid incidence and prevalence assessments as, e.g., provided in this paper. Priorities should include investing in brain health promotion and prevention which are essential components to reduce the burden of dementia. In fact, consistent evidence from observational studies estimates that one-third of AD cases worldwide are attributable to seven common modifiable risk factors: diabetes mellitus, midlife hypertension, midlife obesity, physical inactivity, depression, smoking, and low education [29, 34]. Thus, prevention of dementia via risk factor modification has the potential to curb the increasing number of people living with dementia [29], also in the Faroe Islands.
ACKNOWLEDGMENTS
The study was funded by the Faroese Health Assurance Foundation; and the Independent Research Fund Denmark (grant number: DFF – 4183-00534).
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/19-0341r2).
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JAD-190341.
REFERENCES
[1] | Gustavsson A , Svensson M , Jacobi F , Allgulander C , Alonso J , Beghi E , Dodel R , Ekman M , Faravelli C , Fratiglioni L , Gannon B , Jones DH , Jennum P , Jordanova A , Jonsson L , Karampampa K , Knapp M , Kobelt G , Kurth T , Lieb R , Linde M , Ljungcrantz C , Maercker A , Melin B , Moscarelli M , Musayev A , Norwood F , Preisig M , Pugliatti M , Rehm J , Salvador-Carulla L , Schlehofer B , Simon R , Steinhausen HC , Stovner LJ , Vallat JM , Van den Bergh P , van Os J , Vos P , Xu W , Wittchen HU , Jonsson B , Olesen J ((2011) ) Cost of disorders of the brain in Europe 2010. Eur Neuropsychopharmacol 21: , 718–779. |
[2] | Wu YT , Fratiglioni L , Matthews FE , Lobo A , Breteler MM , Skoog I , Brayne C ((2016) ) Dementia in western Europe: Epidemiological evidence and implications for policy making. Lancet Neurol 15: , 116–124. |
[3] | Fiest KM , Jette N , Roberts JI , Maxwell CJ , Smith EE , Black SE , Blaikie L , Cohen A , Day L , Holroyd-Leduc J , Kirk A , Pearson D , Pringsheim T , Venegas-Torres A , Hogan DB ((2016) ) The prevalence and incidence of dementia: A systematic review and meta-analysis. Can J Neurol Sci 43: (Suppl 1), S3–S50. |
[4] | Prince M , Bryce R , Albanese E , Wimo A , Ribeiro W , Ferri CP ((2013) ) The global prevalence of dementia: A systematic review and metaanalysis. Alzheimers Dement 9: , 63–75. e62. |
[5] | Perera G , Pedersen L , Ansel D , Alexander M , Arrighi HM , Avillach P , Foskett N , Gini R , Gordon MF , Gungabissoon U , Mayer MA , Novak G , Rijnbeek P , Trifiro G , van der Lei J , Visser PJ , Stewart R ((2018) ) Dementia prevalence and incidence in a federation of European Electronic Health Record databases: The European Medical Informatics Framework resource. Alzheimers Dement 14: , 130–139. |
[6] | ((2019) ) Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 18: , 88–106. |
[7] | Wu YT , Beiser AS , Breteler MMB , Fratiglioni L , Helmer C , Hendrie HC , Honda H , Ikram MA , Langa KM , Lobo A , Matthews FE , Ohara T , Peres K , Qiu C , Seshadri S , Sjolund BM , Skoog I , Brayne C ((2017) ) The changing prevalence and incidence of dementia over time - current evidence. Nat Rev Neurol 13: , 327–339. |
[8] | Prince M , Ali GC , Guerchet M , Prina AM , Albanese E , Wu YT ((2016) ) Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimers Res Ther 8: , 23. |
[9] | Roehr S , Pabst A , Luck T , Riedel-Heller SG ((2018) ) Is dementia incidence declining in high-income countries? A systematic review and meta-analysis. Clin Epidemiol 10: , 1233–1247. |
[10] | van Bussel EF , Richard E , Arts DL , Nooyens AC , Coloma PM , de Waal MW , van den Akker M , Biermans MC , Nielen MM , van Boven K , Smeets H , Matthews FE , Brayne C , Busschers WB , van Gool WA , Moll van Charante EP ((2017) ) Dementia incidence trend over 1992-2014 in the Netherlands: Analysis of primary care data. PLoS Med 14: , e1002235. |
[11] | Wermuth L , Joensen P , Bunger N , Jeune B ((1997) ) High prevalence of Parkinson’s disease in the Faroe Islands. Neurology 49: , 426–432. |
[12] | Wermuth L , Bech S , Petersen MS , Joensen P , Weihe P , Grandjean P ((2008) ) Prevalence and incidence of Parkinson’s disease in The Faroe Islands. Acta Neurol Scand 118: , 126–131. |
[13] | Joensen P ((2016) ) High prevalence of primary focal dystonia in the Faroe Islands. Acta Neurol Scand 133: , 55–60. |
[14] | Joensen P ((2010) ) Multiple sclerosis incidence in the Faroe Islands 1986-2007. Acta Neurol Scand 121: , 348–353. |
[15] | Binzer S , Imrell K , Binzer M , Kyvik KO , Hillert J , Stenager E ((2015) ) High inbreeding in the Faroe Islands does not appear to constitute a risk factor for multiple sclerosis. Mult Scler 21: , 996–1002. |
[16] | Eliasen EH , Ferrer M , Gaini S , Louis ED , Petersen MS ((2019) ) Prevalence of essential tremor in the Faroe Islands: A population-based study. Neuroepidemiology 52: , 227–236. |
[17] | Joensen P ((2012) ) Incidence of amyotrophic lateral sclerosis in the Faroe Islands. Acta Neurol Scand 126: , 62–66. |
[18] | Johansen T , Olaffson A ((1999) ) The Faroe Islands: A Brief Introduction, Faroese Government Office, Copenhagen. |
[19] | Als TD , Jorgensen TH , Borglum AD , Petersen PA , Mors O , Wang AG ((2006) ) Highly discrepant proportions of female and male Scandinavian and British Isles ancestry within the isolated population of the Faroe Islands. Eur J Hum Genet 14: , 497–504. |
[20] | Føroya H, https://statbank.hagstova.fo/pxweb/fo/H2/H2__IB__IB01/fo_aldbygd.px/?rxid=a23570e6-6ae5-4408-989c-a2f3586c31b8, Accessed 18 September 2018. |
[21] | Folstein MF , Folstein SE , McHugh PR ((1975) ) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: , 189–198. |
[22] | Shulman KI ((2000) ) Clock-drawing: Is it the ideal cognitive screening test? Int J Geriatr Psychiatry 15: , 548–561. |
[23] | Marc LG , Raue PJ , Bruce ML ((2008) ) Screening performance of the 15-item geriatric depression scale in a diverse elderly home care population. Am J Geriatr Psychiatry 16: , 914–921. |
[24] | Association AP ((2013) ), Diagnostic and statistical manual of mental disorders. Washington, DC. |
[25] | World Population Prospects 2017, United Nations Department of Economic and Social Affairs, https://esa.un.org/unpd/wpp/dataquery/. |
[26] | Phung TK , Waltoft BL , Kessing LV , Mortensen PB , Waldemar G ((2010) ) Time trend in diagnosing dementia in secondary care. Dement Geriatr Cogn Disord 29: , 146–153. |
[27] | Doblhammer G , Fink A , Zylla S , Willekens F ((2015) ) Compression or expansion of dementia in Germany? An observational study of short-term trends in incidence and death rates of dementia between 2006/07 and 2009/10 based on German health insurance data. Alzheimers Res Ther 7: , 66. |
[28] | Kosteniuk JG , Morgan DG , O’Connell ME , Kirk A , Crossley M , Teare GF , Stewart NJ , Bello-Haas VD , McBain L , Mou H , Forbes DA , Innes A , Quail JM ((2016) ) Simultaneous temporal trends in dementia incidence and prevalence, 2005-2013: A population-based retrospective cohort study in Saskatchewan, Canada. Int Psychogeriatr 28: , 1643–1658. |
[29] | Kivipelto M , Mangialasche F , Ngandu T ((2018) ) Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat Rev Neurol 14: , 653–666. |
[30] | Lobo A , Saz P , Marcos G , Dia JL , De-la-Camara C , Ventura T , Montanes JA , Lobo-Escolar A , Aznar S ((2007) ) Prevalence of dementia in a southern European population in two different time periods: The ZARADEMP Project. Acta Psychiatr Scand 116: , 299–307. |
[31] | Fereshtehnejad SM , Johannsen P , Waldemar G , Eriksdotter M ((2015) ) Dementia diagnosis, treatment, and care in specialist clinics in two Scandinavian countries: A data comparison between the Swedish Dementia Registry (SveDem) and the Danish Dementia Registry. J Alzheimers Dis 48: , 229–239. |
[32] | Religa D , Fereshtehnejad SM , Cermakova P , Edlund AK , Garcia-Ptacek S , Granqvist N , Hallback A , Kawe K , Farahmand B , Kilander L , Mattsson UB , Nagga K , Nordstrom P , Wijk H , Wimo A , Winblad B , Eriksdotter M ((2015) ) SveDem, the Swedish Dementia Registry - a tool for improving the quality of diagnostics, treatment and care of dementia patients in clinical practice. PLoS One 10: , e0116538. |
[33] | Waldemar G , Phung KT , Burns A , Georges J , Hansen FR , Iliffe S , Marking C , Rikkert MO , Selmes J , Stoppe G , Sartorius N ((2007) ) Access to diagnostic evaluation and treatment for dementia in Europe. Int J Geriatr Psychiatry 22: , 47–54. |
[34] | Norton S , Matthews FE , Barnes DE , Yaffe K , Brayne C ((2014) ) Potential for primary prevention of Alzheimer’s disease: An analysis of population-based data. Lancet Neurol 13: , 788–794. |




