Antiepileptic Drug Use Is Associated with an Increased Risk of Pneumonia Among Community-Dwelling Persons with Alzheimer’s Disease-Matched Cohort Study
Abstract
Background:
Antiepileptic drugs (AEDs) have sedative properties which may lead to an increased risk of pneumonia.
Objectives:
To investigate whether incident AED use is associated with an increased risk of pneumonia among community-dwelling persons with Alzheimer’s disease (AD). In addition, we determined the risk according to duration of AED use and specific AEDs.
Methods:
Persons with AD were identified from the MEDALZ dataset which includes all community-dwelling persons who received a clinically verified diagnosis of AD during 2005-2011 in Finland (N=70,718). New AED users were identified with one-year washout period. A matched cohort (1 : 1, N=5,769, matching criteria age, gender, and time since AD diagnoses) of nonusers was formed. Data from nationwide registers included dispensed medications which were modelled with PRE2DUP method, hospitalizations, and causes of death. The association between AED use and hospital admission or death due to pneumonia was analyzed with Cox proportional hazard models.
Results:
AED use was associated with an increased risk of pneumonia (adjusted HR 1.92, 95% CI 1.63-2.26; incidence rate per 100 person-years 12.58, 95% CI 12.49-12.66 during AED use and 6.41, 95% CI 6.37-6.45 during nonuse). The highest risk was observed during the first month of use (aHR 3.59, 95% CI 2.29-5.61) and the risk remained elevated until two years of use. Of specific drug substances, phenytoin, carbamazepine, valproic acid, and pregabalin were associated with an increased risk.
Conclusion:
Antiepileptic drug use may increase the risk of pneumonia which is concerning as persons with AD have elevated risk of pneumonia.
INTRODUCTION
Cognitive disorders causing dementia are an increasing public health concern worldwide [1]. The number of dementia cases is estimated to double every 20 years due to population aging as aging is the major risk factor for these conditions. Alzheimer’s disease (AD) is the most common form of dementia, accounting for 60-70% of all cases. Due to progressive cognitive impairment and decreasing independence in activities of daily living, dementia disorders and AD result in substantial healthcare and societal costs [2].
Behavioral and psychological symptoms of dementia (BPSD) are common among persons with AD [3, 4]. Clinical care guidelines list antiepileptic drugs (AEDs), in addition to psychotropic drugs as one possible option for BPSD pharmacotherapy. The Finnish guideline states that oxcarbazepine and valproic acid can be used to treat agitation and aggression and pregabalin as a possible treatment for anxiety. However, they are not recommended as a first-line treatment, and non-pharmacological therapies as well as possible indications for other psychotropics (such as serotonin selective reuptake inhibitors and second generation antipsychotics) should be considered before AEDs [4]. Further, more recent data shows that valproic acid is ineffective for agitation in dementia and may be associated with unacceptable rate of adverse effects [5]. Besides epilepsy and BPSDs, AEDs may be used for various other indications of which neuropathic pain is most frequent in older population [6].
Incidence of hospital-treated pneumonia increases strongly with age, and pneumonia is a common reason for hospitalization among older population [7]. Pneumonia is one of the leading causes for hospitalization among persons with AD [8]. As persons with dementia also are at increased risk of mortality from pneumonia[9], it is important to assess possible factors increasing the risk of incident pneumonia in this population. Of medications affecting the central nervous system, use of antipsychotics[10]. and more recently also benzodiazepines[11] have been associated with an increased risk of pneumonia, among persons of all ages and also among persons with AD. To our knowledge, only one previous study has assessed the risk of pneumonia associated with AED use, and found that among persons with bipolar disorder, the risk was evident for antipsychotics but not for AEDs, specifically valproic acid and carbamazepine [12]. AEDs may have sedative effects[13, 14] especially among older persons, and sedation increases the risk of aspiration[15] which in turn may lead to increased risk of pneumonia. As the use of AEDs is relatively common (5%) among persons with AD [16], it is important to investigate if AEDs are related to higher pneumonia risk among them.
As recent evidence has linked several serious adverse events to antipsychotic use among older persons with dementia, antiepileptic drugs may be considered as alternatives for treatment of BPSDs [17]. However, there is a lack of evidence on safety aspects of antiepileptic drug use among persons with dementia. The objective of this study was to investigate whether incident antiepileptic drug use is associated with an increased risk of pneumonia among community-dwelling persons with AD. In addition, we determined the risk according to duration of AED use and specific AEDs.
MATERIALS AND METHODS
Study cohort
The MEDALZ (Medication use and Alzheimer’s disease) cohort was utilized in this study to identify persons with AD [18]. The MEDALZ includes all community-dwelling persons who were diagnosed with AD between 2005 and 2011 in Finland (70,718). Persons with AD were identified from Special Reimbursement register which includes data on persons entitled for higher reimbursement of medication due to chronic diseases. The Finnish Current care guideline recommends that all persons with clinically verified AD should be prescribed anti-dementia drugs (acetylcholinesterase inhibitors or memantine) if there is no contraindication for use [4]. The diagnostic process of AD is conducted according to a predefined protocol set by the Social Insurance Institution of Finland (SII), including symptoms consistent with AD, exclusion of alternative diagnoses, and computed tomography or MRI scan according to the NINCDS-ADRDA[19] and DSM-IV criteria. Certificate describing clinical findings and fulfillment of criteria, confirmed by a geriatrician or neurologist is sent to SII which grants special reimbursement if the criteria are fulfilled. AD diagnoses recorded in the Special Reimbursement register have high positive predictive value for AD (97.1, 95% CI 84.7-99.9) [20].
Registers
For the MEDALZ cohort [18], data from several nationwide register has been collected; Prescription register (years 1995-2015), Special Reimbursement register (1972-2015), Hospital Discharge register (1972-2015), and socioeconomic data since 1970 and causes of death 2005-2015 from Statistics Finland. All registers are linkable with personal identification number which has been assigned for each resident. The Prescription register includes information on all reimbursed drug purchases from Finnish community pharmacies to all residents. Drugs used during stays in hospitals and public nursing homes are not recorded in the Prescription register. Data in this register is categorized according to Anatomical Therapeutic Chemical–classification system (ATC) codes[21] and purchased amount is recorded also as Defined Daily Doses (DDD) which is the assumed average maintenance dose per day for a drug used for its main indication in adults. Hospital care periods with corresponding discharge diagnoses are recorded in the Hospital Discharge register. Other comorbid conditions were also identified from the Special Reimbursement register including diagnoses both from inpatient and outpatient care physicians.
Exposure
Antiepileptics were defined according to ATC class N03A. AEDs are reimbursed in Finland, for all residents and higher reimbursement for those with diagnosed epilepsy. They are available only as prescription drugs. AED use was modelled with ‘From prescription drug purchases to drug use periods’ (PRE2DUP) method from drug purchases recorded in the Prescription register data [22]. Each ATC code for each person is modelled separately. The method is based on calculation of sliding averages of daily dose in DDDs according to individual drug use patterns. It takes into account on hospitalizations (when drugs are provided by the caring unit and not recorded in the register), stockpiling of drugs and changing dose. The method is guided with ATC-code and Nordic Article Number (vnr-number, i.e., package identifier) based parameters which restrict joining of purchases according to clinically relevant minimum and maximum doses. For single purchases, the method uses typical durations of the purchased package in the study sample. To retrieve duration of “any AED use”, overlapping drug use periods of AED substances were combined and continuous duration of use was constructed.
Outcome
Pneumonia diagnoses were retrieved from the Hospital Discharge register and Causes of death register data. Pneumonia was defined as ICD-10 codes J100, J110, J12, J13, J14, J15, J16, J18, and only the first event after AD diagnoses was considered for each person. Thus, pneumonia events in this study represent only those, which led to hospitalization or death (i.e., more severe cases).
Study setting
Incident antiepileptic users were identified as those initiating AED use after diagnoses of AD and not using AEDs within one-year washout period (new user design). Persons hospitalized for >50% of the washout period and those who were hospitalized/institutionalized for >90 days at the end of the washout period were also excluded as drug exposure cannot be accurately defined for hospitalized persons. As active cancer and its treatment are major risk factors for pneumonia, we excluded persons with active cancer based on the following definition; hospitalization due to cancer (ICD-10 codes C00-C97; or surgical procedures related to cancer as NOMESCO codes AAG50, AX, HA0, PJO, QA0, QB0, QC0, QD0, QW0, QX0, WA, WB, WC, WD, WE, WF0, WFO, ZX0) or antineoplastic medication use (ATC codes L01, L02, L03AA, L03AB01, L03AB04, L03AB05, L03AC, L03AX (excluding L03AX13), L04AA10, L04AA18, L04AA34, L04AX02, L04AX03, L01BA01; except methotrexate use in persons with rheumatoid arthritis), within a year preceding AED initiation [23]. Persons hospitalized due to pneumonia within 6 months before AED initiation were excluded to ensure new pneumonia events in the analyses. In addition, persons hospitalized/institutionalized the entire follow-up period were excluded. These exclusions are described in detail in Figure 1.
Fig.1
Flow chart of defining incident antiepileptic drug (AED) initiators and matched nonusers for this study.
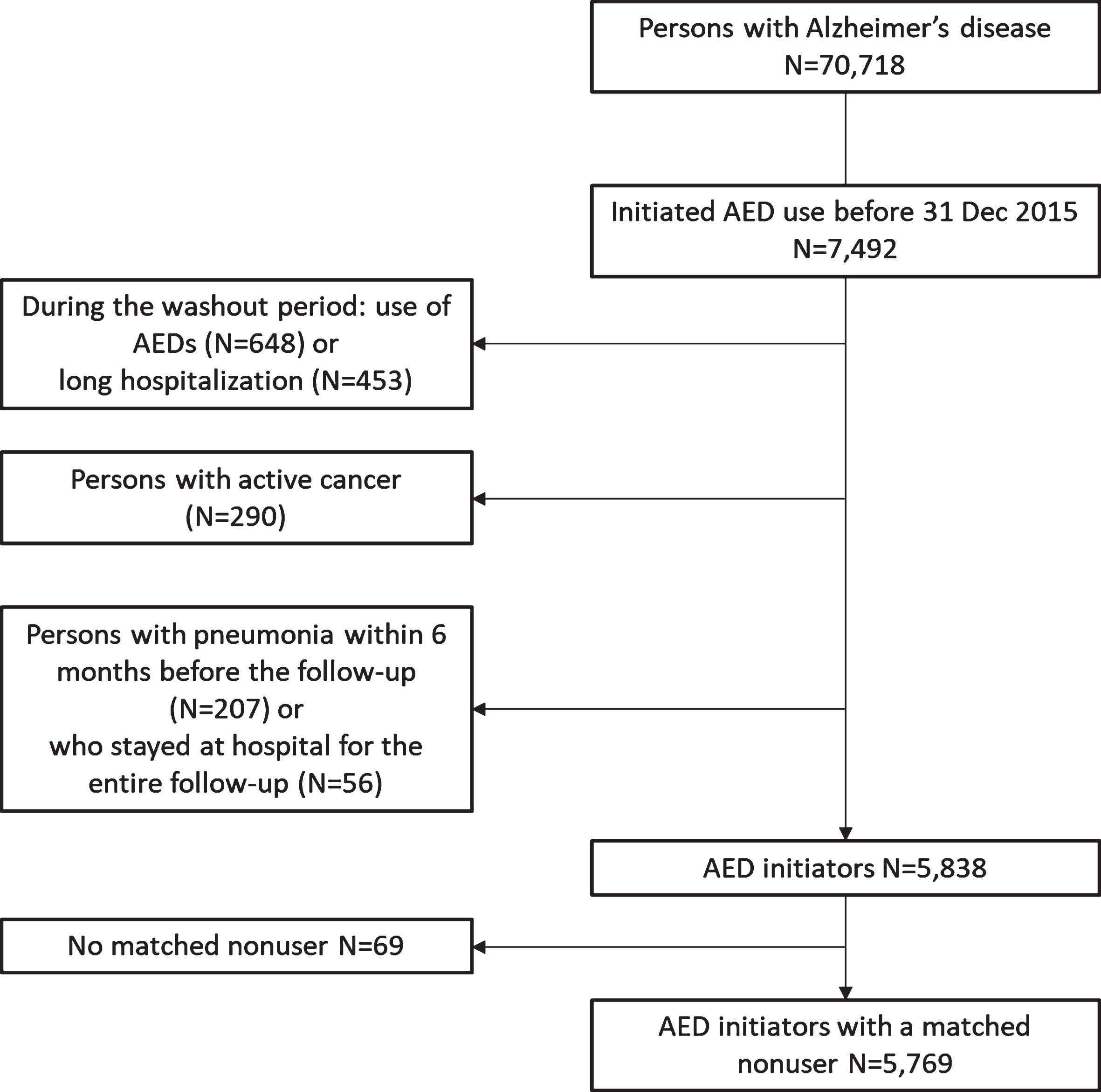
For each incident AED user, a nonuser with AD was matched (1 : 1) to form a matched cohort. Nonusers were matched at the initiation date of AED use, based on age (±2 years), gender and time since AD diagnoses (±90 days) with incidence density sampling. The same exclusion criteria were applied to nonusers as described for AED users. Age, gender, and time since AD diagnoses were utilized in the matching because they were judged to have the greatest impact on risk of pneumonia[24, 25]. We excluded 69 users without a matched nonuser. The follow-up started on the date of AED initiation for users (n=5769) and the corresponding matching date for nonusers (referred as the index date).
Covariates
Covariates associated with AED use[16, 26, 27] and risk of pneumonia[28, 29] were considered based on previous studies. Comorbid conditions were identified based on diagnoses recorded in the Hospital discharge register (since 1996) and Special Reimbursement register (since 1972). Both data sources were utilized when defining coronary artery disease, asthma/COPD, and rheumatoid arthritis. Diagnoses based on special reimbursements only were epilepsy and diabetes (although antidiabetic drug use was also considered for diabetes definition). Diagnoses based solely on hospital discharges were previous cancers and pneumonia. Occupational socioeconomic status in the middle age (age of 45-55) was based on data from Statistics Finland as the highest position recorded for each person. Previous antiepileptic drug use (since 1995 until one year before start of follow-up) was derived from PRE2DUP-modelled drug use. Recent use of medications was defined during a 30-day period before start of follow-up as use of psychotropics including benzodiazepines and related drugs (BZDRs), antipsychotics and antidepressants, and other drugs including oral glucocorticoids, non-steroidal anti-inflammatory drugs (NSAIDs), and proton pump inhibitors (PPIs). Specific definitions are provided in Supplementary Table 1. Occupational socioeconomic status included missing data (no records at Statistics Finland) for about 1% of the cohort (coded as “unknown” category). Other variables did not include missing data.
Statistical analyses
The follow-up for users and their matched nonusers ended to the first of the following events; hospitalization or death due to pneumonia (outcome), long-term (>90 days) hospitalization/institutionalization period, death due to other causes, or the end of study follow-up (December 31, 2015). The follow-up also ended on discontinuation of AED use for users, and on initiation of AED use for nonusers.
Incidence rate for first-time pneumonia per 100 person-years (with 95% confidence interval) was calculated and compared between AED users and non-users. Cox proportional hazard models were utilized to investigate risk of pneumonia associated with incident AED use. Proportional hazard assumption was ascertained by Kaplan-Meier graphs. The matched design was taken into account in the models (own strata for each matched pair). In all analyses, nonusers were the reference category. Any continuous AED use was analyzed in the main analyses. For duration of AED use, time varying any AED use was modeled using categorical time-dependent variable with classes for ≤30 days, 31-180 days, 181-365 days, 366-731 days, and over 731 days of exposure. AED users were stratified according to AED substance used at the initiation, and persons starting with multiple AEDs concomitantly were excluded. The analyses were restricted to the first 2000 days of use due to sparsity of data. The level of statistical significance for drug-drug comparisons was set at p <0.0045 according to Bonferroni correction (0.05/11=0.0045, with 11 being the number of studied AEDs).
To investigate the impact of informative censoring, intention-to-treat (ITT) analyses were conducted by not censoring to discontinuation of use, initiation of use for non users or long-term hospitalization. These analyses were restricted to the first 180 days of follow-up. Whether the risk of pneumonia was related to underlying epilepsy, we excluded all persons with epilepsy from both users and nonusers, and retained the matching between these groups (resulting in 5184 users and 5184 nonusers). Any AED use as an exposure was then analyzed among this restricted cohort. In addition, we conducted sensitivity analyses with case crossover design which is a within-individual design aimed to control for fixed unmeasured confounding by comparing case period (before the event) with control period of the same person. All persons with pneumonia event and using AEDs after AD diagnoses were included in this sensitivity analyses. Two case periods were constructed (1-14 days and 1-30 days before pneumonia) and three control periods (31-45 days, 61-74 days, and 61-90 days before). The analyses were adjusted for time-dependent use of antipsychotics, benzodiazepines and related drugs, immunosuppressants (ATC L04A) and oral corticosteroids (H02AB).
Statistical analyses were performed using SAS statistical software, version 9.4 (SAS Institute, Inc., Cary, NC). Register maintainers retrieved data from the registers and only de-identified data was provided to the research team. No ethics committee approval was required according to Finnish legislation as only register-based data was used and participants were not contacted.
RESULTS
The final study sample included 5769 AED users (which represent 8.2% of the original study cohort) and their 5769 age-, gender- and time since AD diagnoses matched comparison persons not using AEDs. Mean age of the matched cohort was 80 years (80.3, SD 7.9 for users and 80.4, SD 7.9 for nonusers) and most of them were women (66.7% in both groups). Median time since AD diagnoses at the initiation of AED use (or the corresponding index date for nonusers) was 862 (IQR 354-1527) days for users and 858 (IQR 354-1519) days for nonusers. Most persons (43%, n=2497) initiated with pregabalin.
Incident AED users were more likely to have coronary artery disease, diabetes, asthma/COPD, previous pneumonia, previous fracture, and epilepsy than nonusers (Table 1). They were also more likely to use psychotropic drugs, NSAIDs, PPIs, and oral glucocorticoids at the baseline than nonusers.
Table 1
| Nonuser N | User N | Nonuser % | User % | p | |
| Female gender | 3846 | 3846 | 66.67 | 66.67 | matched |
| Age (matching criteria) | matched | ||||
| <65 | 283 | 288 | 4.91 | 4.99 | |
| 65-75 | 899 | 935 | 15.58 | 16.21 | |
| 75-85 | 2882 | 2835 | 49.96 | 49.14 | |
| ≥85 | 1705 | 1711 | 29.55 | 29.66 | |
| Time since AD diagnoses | matched | ||||
| <1 year | 1480 | 1481 | 25.65 | 25.67 | |
| 1-<2 years | 1101 | 1088 | 19.08 | 18.86 | |
| 2-<3 years | 858 | 880 | 14.87 | 15.25 | |
| ≥3 years | 2330 | 2320 | 40.39 | 40.21 | |
| Socioeconomic position | <0.0001 | ||||
| High | 1969 | 1802 | 34.13 | 31.24 | |
| Medium | 3346 | 3391 | 58 | 58.78 | |
| Low | 380 | 503 | 6.59 | 8.72 | |
| Unknown | 74 | 73 | 1.28 | 1.27 | |
| Comorbidities | |||||
| Coronary artery disease | 1571 | 1768 | 27.23 | 30.65 | <0.0001 |
| Diabetes | 1053 | 1235 | 18.25 | 21.41 | <.0001 |
| Asthma/ COPD | 546 | 633 | 9.46 | 10.97 | 0.0075 |
| Rheumatoid arthritis | 237 | 279 | 4.11 | 4.84 | 0.0585 |
| Previous pneumonia | 359 | 678 | 6.22 | 11.75 | <.0001 |
| Previous fracture | 1314 | 1574 | 22.78 | 27.28 | <.0001 |
| Epilepsy | 27 | 558 | 0.47 | 9.67 | <0.0001 |
| Any previous cancer | 574 | 623 | 9.95 | 10.8 | 0.1347 |
| Recent drug use (0-30 days) | |||||
| Antipsychotics | 1042 | 1660 | 18.06 | 28.77 | <0.0001 |
| Antidepressants | 1432 | 1934 | 24.82 | 33.52 | <0.0001 |
| BZDR | 1092 | 1885 | 18.93 | 32.67 | <0.0001 |
| NSAIDs | 294 | 605 | 5.1 | 10.49 | <0.0001 |
| PPIs | 906 | 1405 | 15.7 | 24.35 | <0.0001 |
| Oral glucocorticoids | 176 | 268 | 3.05 | 4.65 | <0.0001 |
| Previous use of drugs | |||||
| Antiepileptics | 342 | 783 | 5.93 | 13.57 | <0.0001 |
BZDR, benzodiazepines and related drugs; COPD, chronic obstructive pulmonary disease; PPI, proton pump inhibitor; NSAID, non-steroidal anti-inflammatory drugs
During the median follow-up time of 176 (IQR 51-549) days for users and 839 (IQR 350-1448) days for nonusers, 806 and 994 pneumonia events were recorded, respectively. Incident AED use was associated with an increased risk of pneumonia (adjusted HR [aHR] 1.92, 95% CI 1.63-2.26) (Table 2). Incidence rate of pneumonias per 100 person-years was higher during AED use (12.58, 95% CI 12.49-12.66) than during no use of AEDs (6.41, 95% CI 6.37-6.45). The risk of pneumonia was highest during the first month of use (aHR 3.59, 95% CI 2.29-5.61) and although decreased gradually, was still elevated after 1-2 years of use (aHR 1.42, 95% CI 1.03-1.95).
Table 2
Antiepileptic drug (AED) use and risk of pneumonia among persons with Alzheimer’s disease. The reference category in all analyses is nonuse
| Number of events | Person years | Incidence rate per 100 person-years (95% CI) | Unadjusted HR (95% CI) | Adjusted HR (95% CI)a | |
| Users compared with nonusers | |||||
| Nonusers, N=5,769 | 994 | 15,498 | 6.41 (6.37-6.45) | ref | |
| Users, N=5,769 | 806 | 6,409 | 12.58 (12.49-12.66) | 2.10 (1.83-2.41) | 1.92 (1.63-2.26) |
| Duration of use categorized | |||||
| 1-30 days | 104 | 450 | 22.97 (22.53-23.41) | 3.82 (2.50-5.83) | 3.59 (2.29-5.61) |
| 31-180 days | 218 | 1481 | 14.67 (14.47-14.86) | 2.58 (2.00-3.34) | 2.40 (1.82-3.16) |
| 181-365 days | 139 | 1206 | 11.51 (11.32-11.70) | 2.09 (1.51-2.89) | 1.78 (1.26-2.52) |
| 366-731 days | 159 | 1494 | 10.63 (10.46-10.79) | 1.57 (1.17-2.10) | 1.42 (1.03-1.95) |
| >731 days | 186 | 1778 | 10.44 (10.29-10.59) | 1.39 (1.01-1.92) | 1.25 (0.88-1.77) |
| Persons with epilepsy excluded (matching retained) | |||||
| Nonusers, N= 5184 | 896 | 13,999 | 6.40 (6.36-6.44) | ref | ref |
| Users, N= 5184 | 661 | 5627 | 11.75 (11.66-11.84) | 2.01 (1.73-2.34) | 1.92 (1.63-2.27) |
| Intention-to-treat analyses (restricted to the first 180 days) | |||||
| Nonusers, N=5769 | 170 | 2731 | 6.23 (6.13-6.32) | ref | ref |
| Users, N=5769 | 435 | 2638 | 16.49 (16.33-16.64) | 2.64 (2.20-3.17) | 2.33 (1.89-2.87) |
aAdjusted for: benzodiazepine and related drug, antipsychotic, NSAID, oral glucocorticoid, PPI and antidepressant use at baseline, previous use of antiepileptics, coronary artery disease, previous cancer, epilepsy, diabetes, rheumatoid arthritis, asthma/ COPD, previous fracture, previous pneumonia, occupational socioeconomic status.
When persons with diagnosed epilepsy were excluded, the results remained similar (aHR 1.92, 95% CI 1.63-2.27) (Table 2). ITT analyses resulted in a similar increase in the risk of pneumonia as the main analyses (aHR 2.33, 95% CI 1.89-2.87).
Valproic acid, phenytoin, carbamazepine, and pregabaline were associated with an increased risk of pneumonia in substance specific adjusted analyses (Table 3). All these associations were statistically significant also after Bonferroni correction. Unadjusted results indicated higher risk also for oxcarbazepine and levetiracetam but these associations lost significance after adjusting for confounders.
Table 3
Antiepileptic drug substances and risk of pneumonia among persons with Alzheimer’s disease, compared with no use of all antiepileptics. Analyses were restricted to the first 2000 days of follow-up due to sparsity of data.
| N of users | N of events | Person-years | Pneumonia rate per 100 person-years (95% CI) | Unadjusted HR (95% CI) | Adjusted HR (95% CI)a | |
| Pregabalin | 2497 | 235 | 2479 | 9.48 (9.36-9.60) | 1.45 (1.26-1.68) | 1.40 (1.21-1.63) |
| Valproic acid | 1798 | 310 | 1719 | 18.03 (17.83-18.23) | 2.74 (2.40-3.12) | 2.35 (2.03-2.72) |
| Carbamazepine | 337 | 48 | 350 | 13.74 (13.35-14.12) | 2.09 (1.56-2.80) | 1.83 (1.36-2.47) |
| Oxcarbazepine | 264 | 34 | 322 | 10.57 (10.21-10.92) | 1.63 (1.15-2.29) | 1.33 (0.93-1.90) |
| Gabapentin | 318 | 24 | 275 | 8.74 (8.39-9.09) | 1.32 (0.88-1.98) | 1.21 (0.80-1.82) |
| Clonazepam | 278 | 14 | 213 | 6.59 (6.24-6.93) | 0.98 (0.58-1.66) | 0.89 (0.52-1.51) |
| Phenytoin | 119 | 22 | 150 | 14.70 (14.09-15.32) | 2.25 (1.48-3.44) | 2.17 (1.42- 3.23) |
| Levetiracetam | 81 | 11 | 82 | 13.39 (12.60-14.18) | 2.04 (1.13-3.71) | 1.72 (0.94-3.14) |
| Lamotrigine | 27 | 4 | 56 | 7.15 (6.45-7.85) | 1.11 (0.42-2.97) | 1.01 (0.38-2.70) |
| Topiramate | 9 | 1 | 4 | 23.84 (19.16-28.51) | 3.51 (0.49-24.99) | 3.80 (0.53-27.08) |
| Primidone | 5 | 1 | 3 | 31.33 (25.19-37.47) | 4.67 (0.66-33.07) | 4.33 (0.60-31.18) |
aAdjusted for: benzodiazepine and related drugs, antipsychotic, NSAID, oral glucocorticoid, PPI and antidepressant use at baseline, previous use of antiepileptics, coronary artery disease, previous cancer, epilepsy, diabetes, rheumatoid arthritis, asthma/ COPD, previous fracture, previous pneumonia, occupational socioeconomic status.
Among 5958 persons included in case-crossover analyses, AED use was consistently associated with higher odds of pneumonia, regardless of the control window, with unadjusted odds ratios (ORs) ranging from 1.84 to 1.91 (Table 4). Adjustment for time-dependent use of antipsychotics, benzodiazepines and related drugs, immunosuppressants, and oral corticosteroids did not have major impact on the results (e.g., OR 1.88, 95% CI 1.30-2.70 with case window 1-14 days and control window 31-45 days before the pneumonia).
Table 4
Case crossover analyses for risk pneumonia associated with antiepileptic use.
| Unadjusted OR (95% CI) | Adjusted OR (95% CI)a | |
| Case window 1-14 days and control window 31-45 days before the pneumonia | 1.84 (1.28-2.65) | 1.88 (1.30-2.70) |
| Case window 1-14 days and control window 61-74 days before the pneumonia | 1.91 (1.45-2.52) | 1.90 (1.44-2.51) |
| Case window 1-30 days and control window 61-90 days before the pneumonia | 1.85 (1.30-2.64) | 1.85 (1.30-2.64) |
aAdjusted for antipsychotic, benzodiazepine and related drug, immunosuppressant and oral corticosteroid use.
DISCUSSION
Incident AED use was associated with two-fold increase in risk of pneumonia in our study. The risk was highest during the first 30 days of use but remained elevated until two years of use. Of specific drugs, the risk was found for phenytoin, carbamazepine, valproic acid, and pregabalin. A previous study among persons with bipolar disorder (mean age 44) did not find an increased risk of pneumonia for AEDs, specifically valproic acid and carbamazepine [12]. However, older persons with AD (mean age 80 years) may be more sensitive to adverse effects and events associated with these drugs than adults with bipolar disorder.
AEDs have been reported to affect the immune system with several different mechanisms affecting cytokines as well as humoral and cellular immunity, e.g., valproic acid, carbamazepine, and phenytoin have been reported to have immunomodulatory effects [30]. However, the clinical relevance of these effects is uncertain, as only topiramate was significantly associated with an increased risk of infection in a recent meta-analysis [31]. Due to the small number of topiramate users, our results on topiramate are not reliable.
Sedation may lead to pneumonia by increasing aspiration risk.[15] The increased pneumonia risk observed for phenytoin, carbamazepine and valproic acid may therefore be related to sedative properties of these drugs.[13, 14, 32, 33] In addition, although pregabalin has been suggested not to have serious central nervous system (CNS) adverse effects in healthy adults,[34] it has also been shown to have sedative properties in older surgical patients[35] and adult persons with epilepsy.[36] Increased somnolence risk was reported also in systematic review by Zaccara et al. [37] As adverse effects of AEDs are most prominent during the initiation and early treatment,[38] this may explain the fact that pneumonia risk was highest during the first month of the drug use.
We lacked information on indication of AED use. In a previous Norwegian study, neuropathic pain was the most common indication for AED use among persons aged ≥60 years, with pregabaline and gabapentin being the most common AEDs for this indication [6]. It is likely that this is also the case in our study population although pregabaline may also be used for BPSDs [4]. In the Norwegian study, levetiracetam, lamotrigine, carbamazepine, and valproic acid were most common drugs for epilepsy but lamotrigine and valproic acid also for psychiatric reasons, mainly bipolar disorder [6]. In our study, lamotrigine was rarely used. Use of valproic acid, the second most commonly used AED in our study was likely for epilepsy as in the Norwegian study [6] but also for BPSDs as it and carbamazepine are mentioned as second-line options in clinical care guideline for AD [4].
Our finding on the risk of pneumonia associated with incident AED use was not explained by epilepsy. Less than 10% of users had epilepsy and sensitivity analysis excluding persons with epilepsy resulted in similar risk estimates as the main analyses. The number of persons using AEDs without epilepsy diagnosis is relatively high in our study. It is likely that AEDs are frequently prescribed to treat neuropathic pain,[6] prevalence of which is estimated to be between 7 and 10% in general population [39]. Neuropathic pain is more common in older people,[40] and AEDs can be used to treat neuropathic pain; however, their use is limited in older population due to tolerability and adverse effects [41, 42]. At least half of patients attending outpatient dementia clinics have been reported to suffer from some psychological or behavioral disturbance [43]. Therefore, it is likely that BPSDs are common also in our community-dwelling study population. AEDs may be attempted to treat BPSDs [44]. However, controlled trial data on the use of AEDs for BPSD symptoms is currently available for carbamazepine and valproate only. Carbamazepine was effective in treatment of BPSDs, especially agitation, aggression, and hostility [5]. However, low doses of valproate did not differ from placebo in the treatment of BPSD, but higher valproate doses led to frequent adverse effects such as sedation, falls, urinary tract infections, and gastrointestinal problems [5]. Although low-dose gabapentin has been found effective in BPSD treatment in a small sample,[45] AEDs cannot, in general, be recommended for treating BPSDs except for patients for whom other treatments have failed [5, 46].
Strengths and limitations
A major strength of our study was a large, nationwide cohort of community-dwelling persons with AD, leading to high generalizability of the results to community-dwelling persons with AD. The study was restricted to hospitalizations and deaths due to pneumonia. As it lacks community-treated cases, the results are representative of the more severe pneumonia cases. AED use was modelled from Prescription register data time-dependently with a validated modelling method [22]. All AEDs are reimbursed in Finland and available only with prescription and thus, they are recorded in the register. The analyses were restricted to incident AED users to avoid survival and selection biases related to prevalent users which tolerate the drug effects. The matching of a nonuser for each user aimed to control for most important predictors of pneumonia risk, i.e., age, gender, and time since AD diagnoses, as a proxy for severity of AD.
The limitations include that we lacked data on status of BPSD and cognition or progression of AD. In addition, indications for AED use such as neuropathic pain were lacking in the register-based data which may result into confounding by indication. Also, some important lifestyle factors, such as smoking, were not available in the utilized registers. Thus, residual confounding cannot be ruled out. However, we performed sensitivity analysis using self-controlled design and the results were comparable to main results, suggesting that confounding by indication and residual confounding did not fully account for the observed association. The Prescription register data does not include information on drugs used during hospital stays and thus, we restricted our analyses to the first pneumonia events and censored at long hospital care periods.
Conclusions
AED use may increase the risk of pneumonia in persons with AD, who already have an elevated risk for pneumonia. Our results further demonstrate the risk of adverse events associated with incident AED use. It should be acknowledged, that AED treatment is necessary for persons with epilepsy, but the indications have spread out to other conditions where the evidence on effectiveness and efficacy is often less convincing. Therefore, the use of AEDs in these situations should be carefully considered, and less sedative alternatives should be preferred, especially in this fragile group of aged persons.
ACKNOWLEDGMENTS
AMT was funded by Academy of Finland (grants 307232 and 295334).
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/18-0912r1).
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JAD-180912.
REFERENCES
[1] | World Health Organization ((2012) ) Dementia: a public health priority, World Health Organization. |
[2] | Association A ((2011) ) Alzheimer’s disease facts and figures. Alzheimers Dement 7: , 208–244. |
[3] | ((2010) ) Practice guideline for the treatment of patients with major depressive disorder, American Psychiatric Association. |
[4] | Finnish Medical Society Duodecim ((2010) ) Memory Disorders. Current Care Guideline [in Finnish with English summary], The Finnish Medical Society Duodecim, Helsinki. |
[5] | Masopust. J , Protopopová D , Vališ M , Pavelek Z , Klímová B ((2018) ) Treatment of behavioral and psychological symptoms of dementias with psychopharmaceuticals:a review. Neuropsychiatr Dis Treat 14: , 1211–1220. |
[6] | Baftiu A , Feet SA , Larsson PG , Burns ML , Henning O , Saetre E , Molden E , Granas AG , Johannessen SI , Landmark CJ ((2018) ) Utilisation and polypharmacy aspects of antiepileptic drugs in elderly versus younger patients with epilepsy:A pharmacoepidemiological study of CNS-active drugs in Norway, 2004-2015. Epilepsy Res 139: , 35–42. |
[7] | Simonetti AF , Garcia-Vidal C , Carratalà J ((2014) ) Management of community-acquired pneumonia in older adults. Ther Adv Infect Dis 2: , 3–16. |
[8] | Rudolph JL , Zanin NM , Jones RN , Marcantonio ER , Fong TG , Yang FM , Yap L , Inouye SK ((2010) ) Hospitalization in community-dwelling persons with Alzheimer’s disease: frequency and causes. J Am Geriatr Soc 58: , 1542–1548. |
[9] | Foley NC , Affoo RH , Martin RE ((2015) ) A systematic review and meta-analysis examining pneumonia-associated mortality in dementia. Dement Geriatr Cogn Disord 39: , 52–67. |
[10] | Tolppanen AM , Koponen M , Tanskanen A , Lavikainen P , Sund R , Tiihonen. J , Hartikainen S , Taipale H ((2016) ) Antipsychotic use and risk of hospitalization or death due to pneumonia in persons with and those without Alzheimer disease. Chest 150: , 1233–1241. |
[11] | Taipale H , Tolppanen A-M , Koponen M , Tanskanen A , Lavikainen P , Sund R , Tiihonen. J , Hartikainen S ((2017) ) Risk of pneumonia associated with incident benzodiazepine use among community-dwelling adults with Alzheimer disease. Can Med Assoc J 189: , E519–E529. |
[12] | Yang S-Y , Liao Y-T , Liu H-C , Chen WJ , Chen C-C , Kuo C-J ((2013) ) Antipsychotic drugs, mood stabilizers, and risk of pneumonia in bipolar disorder: a nationwide case-control study. J Clin Psychiatry 74: , e79–e86. |
[13] | Park S-P , Kwon S-H ((2008) ) Cognitive effects of antiepileptic drugs. J Clin Neurol 4: , 99–106. |
[14] | Eddy CM , Rickards HE , Cavanna AE ((2011) ) The cognitive impact of antiepileptic drugs. Ther Adv Neurol Disord 4: , 385–407. |
[15] | Loeb M , McGeer A , McArthur M , Walter S , Simor AE ((1999) ) Risk factors for pneumonia and other lower respiratory tract infections in elderly residents of long-term care facilities. Arch Intern Med 159: , 2058–2064. |
[16] | Bell JS , Lönnroos E , Koivisto AM , Lavikainen P , Laitinen M-L , Soininen H , Hartikainen S ((2011) ) Use of antiepileptic drugs among community-dwelling persons with Alzheimer’s disease in Finland. J Alzheimers Dis 26: , 231–237. |
[17] | Ballard CG , Gauthier S , Cummings JL , Brodaty H , Grossberg GT , Robert P , Lyketsos CG ((2009) ) Management of agitation and aggression associated with Alzheimer disease. Nat Rev Neurol 5: , 245–255. |
[18] | Tolppanen A-M , Taipale H , Koponen M , Lavikainen P , Tanskanen A , Tiihonen. J , Hartikainen S ((2016) ) Cohort profile: the Finnish Medication and Alzheimer’s disease (MEDALZ) study. BMJ Open 6: , e012100. |
[19] | McKhann G , Drachman D , Folstein M , Katzman R , Price D , Stadlan EM ((1984) ) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34: , 939–944. |
[20] | Solomon A , Ngandu T , Soininen H , Hallikainen MM , Kivipelto M , Laatikainen T ((2014) ) Validity of dementia and Alzheimer’s disease diagnoses in Finnish national registers. Alzheimers Dement 10: , 303–309. |
[21] | WHO Collaborating Centre for Drug Statistics Methodology ((2018) ) The Anatomical Therapeutic Chemical (ATC) classification system. Oslo, Norway. https://www.whocc.no/atc/structure_and_principles/ Accessed on September 22, 2018. |
[22] | Tanskanen A , Taipale H , Koponen M , Tolppanen A-M , Hartikainen S , Ahonen R , Tiihonen J ((2015) ) From prescription drug purchases to drug use periods – a second generation method (PRE2DUP). BMC Med Inform Decis Mak 15: , 21. |
[23] | Hamina A , Taipale H , Tanskanen A , Tolppanen A-M , Karttunen N , Pylkkänen L , Tiihonen. J , Hartikainen S ((2017) ) Long-term use of opioids for non-malignant pain among community-dwelling persons with and without Alzheimer’s disease in Finland. Pain 158: , 252–260. |
[24] | Wada H , Nakajoh K , Satoh-Nakagawa T , Suzuki T , Ohrui T , Arai H , Sasaki H ((2001) ) Risk factors of aspiration pneumonia in Alzheimer’s disease patients. Gerontology 47: , 271–276. |
[25] | Rivero-Calle I , Pardo-Seco. J , Aldaz P , Vargas DA , Mascarós E , Redondo E , Díaz-Maroto JL , Linares-Rufo M , Fierro-Alacio MJ , Gil A , Molina J , Ocaña D , Martinón-Torres F , Vargas D , Mascarós E , Redondo E , Díaz-Maroto JL , Linares-Rufo M , Gil A , Molina J , Ocaña D , Rivero-Calle I ((2016) ) Incidence and risk factor prevalence of community-acquired pneumonia in adults in primary care in Spain (NEUMO-ES-RISK project). BMC Infect Dis 16: , 1–8. |
[26] | Garrard J , Cloyd J , Gross C , Hardie N , Thomas L , Lackner T , Graves N , Leppik I ((2000) ) Factors associated with antiepileptic drug use among elderly nursing home residents. J Gerontol Med Sci 55A: , M384–M392. |
[27] | Johnell K , Fastbom J ((2011) ) Antiepileptic drug use in community-dwelling and institutionalized elderly: a nationwide study of over 1,300,000 older people. Eur J Clin Pharmacol 67: , 1069–1075. |
[28] | Juthani-Mehta M , De Rekeneire N , Allore H , Chen S , O’Leary JR , Bauer DC , Harris TB , Newman AB , Yende S , Weyant RJ , Kritchevsky S , Quagliarello V ((2013) ) Modifiable risk factors for pneumonia requiring hospitalization of community-dwelling older adults: The health, aging, and body composition study. J Am Geriatr Soc 61: , 1111–1118. |
[29] | Dang TT , Majumdar SR , Marrie TJ , Eurich DT ((2014) ) Recurrent pneumonia: a review with focus on clinical epidemiology and modifiable risk factors in elderly patients. Drugs Aging 32: , 13–19. |
[30] | Godhwani N , Bahna SL ((2016) ) Antiepilepsy drugs and the immune system. Ann Allergy Asthma Immunol 117: , 634–640. |
[31] | Zaccara G , Giovannelli F , Giorgi FS , Franco V , Gasparini S , Tacconi FM ((2017) ) Do antiepileptic drugs increase the risk of infectious diseases? A meta-analysis of placebo-controlled studies. Br J Clin Pharmacol 83: , 1873–1879. |
[32] | Nadkarni S , Devinsky O ((2005) ) Psychotropic effects of antiepileptic drugs. Epilepsy Curr 5: , 176–181. |
[33] | Ijff DM , Aldenkamp AP ((2013) ) Cognitive side-effects of antiepileptic drugs in children, Elsevier B.V. |
[34] | Hindmarch I , Trick L , Ridout F ((2005) ) A double-blind, placebo- and positive-internal-controlled (alprazolam) investigation of the cognitive and psychomotor profile of pregabalin in healthy volunteers. Psychopharmacology (Berl) 183: , 133–143. |
[35] | Mathiesen O , Jacobsen LS , Holm HE , Randall S , Adamiec-Malmstroem L , Graungaard BK , Holst PE , Hilsted KL , Dahl JB ((2008) ) Pregabalin and dexamethasone for postoperative pain control: A randomized controlled study in hip arthroplasty. Br J Anaesth 101: , 535–541. |
[36] | Ciesielski AS , Samson S , Steinhoff BJ ((2006) ) Neuropsychological and psychiatric impact of add-on titration of pregabalin versus levetiracetam: A comparative short-term study. Epilepsy Behav 9: , 424–431. |
[37] | Zaccara G , Gangemi P , Perucca P , Specchio L ((2011) )The adverse event profile of pregabalin: A systematic review and meta-analysis of randomized controlled trials. Epilepsia 52: , 826–836. |
[38] | Cramer JA , Mintzer S , Wheless. J , Mattson RH ((2010) ) Adverse effects of antiepileptic drugs: A brief overview of important issues. Expert Rev Neurother 10: , 885–891. |
[39] | Van Hecke O , Austin SK , Khan RA , Smith BH , Torrance N ((2014) ) Neuropathic pain in the general population: A systematic review of epidemiological studies. Pain 155: , 654–662. |
[40] | Rapo-Pylkkö S , Haanpää M , Liira H ((2015) ) Neuropathic pain among community-dwelling older people: A clinical study in Finland. Drugs Aging 32: , 737–742. |
[41] | Fine PG ((2012) ) Treatment guidelines for the pharmacological management of pain in older persons. Pain Med 13: (Suppl 2), S57–S66. |
[42] | Abdulla A , Adams N , Bone M , Elliott AM , Gaffin. J , Jones D , Knaggs R , Martin D , Sampson L , Schofield P , British Geriatric Society ((2013) ) Guidance on the management of pain in older people. Age Ageing 42: (Suppl 1), i1–57. |
[43] | Zaudig M ((2000) ) A risk-benefit assessment of risperidone for the treatment of behavioural and psychological symptoms in dementia. Drug Saf 23: , 183–195. |
[44] | APA. Work Group on Alzheimer’s Disease and other Dementias, Rabins PV , Blacker D , Rovner BW , Rummans T , Schneider LS , Tariot PN , Blass DM ; Steering Committee on Practice Guidelines, McIntyre JS , Charles SC , Anzia DJ , Cook IA , Finnerty MT , Johnson BR , Nininger JE , Schneidman B , Summergrad P , Woods SM , Berger J , Cross CD , Brandt HA , Margolis PM , Shemo JP , Blinder BJ , Duncan DL , Barnovitz MA , Carino AJ , Freyberg ZZ , Gray SH , Tonnu T , Kunkle R , Albert AB , Craig TJ , Regier DA , Fochtmann LJ ((2007) ) American Psychiatric Association practice guideline for the treatment of patients with Alzheimer’s disease and other dementias. Second edition. Am J Psychiatry 164: (12 Suppl), 5–56. |
[45] | Cooney C , Murphy S , Tessema H , Freyne A ((2013) ) Use of low-dose gabapentin for aggressive behavior in vascular and mixed vascular/Alzheimer dementia. J Neuropsychiatry Clin Neurosci 25: , 120–125. |
[46] | Kales HC, Gitlin LN, Lyketsos CG, Detroit. Expert Panel on Assessment and Management of Neuropsychiatric Symptoms of Dementia (2014) Management of neuropsychiatric symptoms of dementia in clinical settings: recommendations from a multidisciplinary expert panel. J Am Geriatr Soc 62: , 762–769. |




