Low Prevalence and Clinical Effect of Vascular Risk Factors in Early-Onset Alzheimer’s Disease
Abstract
Background:
Determinants of early-onset Alzheimer’s disease (EOAD) are not well known. In late-onset AD, vascular risk factors (VRFs) are associated with earlier clinical manifestation.
Objective:
The objective of this study was to assess the putative association between VRFs and EOAD.
Methods:
We studied participants with dementia meeting criteria for EOAD (recruited into the French CoMAJ prospective cohort study from 1 June 2009 to 28 February 2014) and age-, gender-matched controls (ratio 1:3, drawn randomly from the French MONA-LISA population-based survey between 2005 and 2007). Demographic data, VRFs, comorbidities, treatments, and APOE genotypes were compared in multivariable logistic regression analyses.
Results:
We studied 102 participants with dementia (mean±standard deviation age: 59.5±3.8; women: 59.8%) and 306 controls. Compared with controls, EOAD participants had spent less time in formal education (9.9±2.9 versus 11.7±3.8 y; p < 0.0001), were less likely to be regular alcohol consumers (p < 0.0001), had a lower body mass index (–2 kg/m2; p < 0.0004), and a lower mean systolic blood pressure (–6.2 mmHg; p = 0.0036). The prevalence of APOE ɛ4 allele was higher in participants with dementia than in controls (50% versus 29.4%; p = 0.0002), as was the prevalence of depression (48% versus 32%; p < 0.001). Similar results were observed in multivariable analysis. Compared with EOAD participants lacking VRFs, EOAD participants with at least one VRF had a higher prevalence of depression (29.6% versus 53.3%, respectively; p = 0.03).
Conclusion:
The prevalence of VRFs is not elevated in EOAD patients (in contrast to older AD patients). Extensive genetic testing should be considered more frequently in the context of EOAD.
INTRODUCTION
Alzheimer’s disease (AD) remains the leading cause of dementia in all age categories - including younger patients [1]. Early-onset Alzheimer’sdisease (EOAD) is arbitrarily defined as disease onset under the age of 65. In the 45–64 age group, prevalence varies between 15 and 35 per 100,000 people [2], and annual incidence is 12 per 100,000 people [3]. According to a survey in 2010, there were 17,000 patients with EOAD in France, and 5,000 of these had developed the first symptoms before 60 years old [4].
Little is known about the risk factors of EOAD. Only 13% of cases are linked to a known autosomal-dominant mutation in the genes coding for presenilin 1 and 2 or amyloid precursor protein (PSEN1, PSEN2, or APP) [5]. The APOE ɛ4 allele is the most important genetic risk factor for sporadic AD. Nonetheless, the observation of EOAD in patients lacking the ɛ4 allele suggests that other factors may favor early onset. At present, research efforts are mainly focused on cerebrovascular aspects and cognitive reserve in older AD patients [6]. Treatment of vascular risk factors (VRFs) in older patients has been associated with slower cognitive decline and later disease onset [7]. Management of patients with EOAD might be improved by the recognition and treatment of putative disease-associated vascular component factors. However, data on environmental factors (and especially VRFs) in EOAD are scarce, despite the large number of affected patients. Some studies identified vascular and environmental risk factors to develop young onset “all-cause” dementia [8–10]. Therefore, the objective of the present study was to assess the putative relationship between VRFs and EOAD. We hypothesized that if VRFs shorten the disease onset of AD, we might observe a higher prevalence of VRFs in EOAD. The second hypothesis was that the VRFs might modify the clinical presentation in EOAD.
MATERIALS AND METHODS
Participants with dementia
We studied participants with dementia from the Cohorte Malades Alzheimer Jeunes (CoMAJ) longitudinal, multicenter cohort recruited by the multisite French National Reference Center for Early-Onset Dementia in the cities of Lille, Rouen, and Paris. The main objective of the CoMAJ study is tobetter understand the course of AD in young patients by providing multimodal data (i.e., clinical, biological, social, genetic, imaging, and neuropathological data). Inclusion criteria are onset before the age of 60 (although age at inclusion could be over 60), and a diagnosis of AD based on the International Working Group criteria [11] (with abnormalities of cerebrospinal fluid (CSF) biomarkers) and compatible with those of the National Institute on Aging -Alzheimer’s Association workgroups (NIA/AA) [12]. The age of disease onset was 60 because it is the age criteria in France to receive social allowance especially the Solidarity Allowance for the Elderly. All participants with dementia gave their written, informed consent to participation in the CoMAJ cohort. The study was approved by local investigational review boards (CPP Nord-Ouest I, CPP Paris Pitié-Salpêtrière and CPP Ile-de-France II; reference 110-05). Recruitment began in June 2009 and is ongoing.
At the first visit, we collected data on the participants’ demographics, medical history, social situation, and neuropsychological status. Unless contraindicated or refusal, participants underwent ApoE genotyping, brain MRI, fluorodeoxyglucose positron emission tomography (FDG PET), and lumbar puncture for AD biomarkers (amyloid beta, tau, and phospho-tau) in the CSF. Assessments with EOAD participants and their primary caregivers were performed (using a standardized medical work-up) at baseline and then every 6 months until death or withdrawal. To avoid bias related to the geographical origin of controls (detailed in the next section), we only included AD participants having been recruited by the Lille center between 1 June 2009, and 28 February 2014. We excluded persons with a confirmed mutation (PSEN1, PSEN2, or APP) and those who had at least one first-degree relative withEOAD.
Controls
Controls were drawn randomly from among participants in the Monitoring National du Risque Artériel (MONA-LISA) multicenter, cross-sectional, population-based study performed in three areas of France (the Lille Urban Community in northern France, the Bas-Rhin county in eastern France, and the Haute-Garonne county in southern France). In the MONA-LISA study, participants aged from 35 to 74 were randomly sampled from electoral rolls after stratification by the number of inhabitants in the locality, gender, and 10-year age group. In the period between 2005 and 2007, each area had about 500,000 inhabitants. Details of the MONA-LISA survey have been reported elsewhere [13]. To minimize the influence of geographical origin, we used data on participants from the Lille Urban Community (i.e., from much the same catchment area as the CoMAJ participants). Controls from the MONA-LISA survey were matched 3:1 for age (in 5-year age groups) and gender with participants from the CoMAJ cohort.
Demographic data and VRFs
Only data collected at inclusion were considered in the present study. Participants with dementia and controls were investigated using the same procedures.
We prospectively collected data on demographics (age, gender, and level of education), any family history of vascular diseases and/or late-onset dementia, comorbidities, regular alcohol consumption (more than 3 standard drinks per day), vascular diseases (myocardial infarction, stroke, and atrial fibrillation), depression (diagnosed by a physician or use of antidepressant drugs), anxiety, epilepsy, any ongoing treatments, and the following VRFs:
• Hypertension, defined as a history of elevated blood pressure (BP) (diagnosed by a primary care physician), use of BP-lowering medication, or elevated BP measured at inclusion (systolic BP ≥140 mmHg or diastolic BP ≥90 mmHg).
• Hypercholesterolemia, defined as a fasting total cholesterol ≥6.2 mmol/L or a low-density lipoprotein cholesterol level ≥4.1 mmol/L (diagnosed by a primary care physician) or use of cholesterol-lowering medication.
• Hypertriglyceridemia, defined as a triglyceride level ≥2.3 mmol/L (diagnosed by a primary care physician) or use of triglyceride-lowering medication.
• Diabetes mellitus, defined as a history of diabetes mellitus (diagnosed by a primary care physician), with a fasting glucose >7 mmol/L or use of antidiabetic medication.
• Smoking, categorized as a current or former smoker.
• Body mass index (BMI), calculated as the body weight (kg) divided by the square of the height (m2) on inclusion (underweight: BMI<19 kg/m2; normal weight: 19 kg/m2≤BMI < 25 kg/m2; overweight: 25 kg/m2≤BMI < 30 kg/m2; obesity: BMI ≥30 kg/m2).
The following information on the participant’s medical history and clinical presentation were recorded for participants with dementia only: date of symptom onset, awareness of symptoms, date of the first consultation in our center, date of diagnosis, Mini Mental State Examination (MMSE) score [14] at first visit and at diagnosis; time interval between first symptoms and inclusion in the CoMAJ cohort; neurological signs at inclusion; results of a standard neuropsychological assessment at inclusion (the MMSE; the Clinical Dementia Rating Scale [15], the Mattis Dementia Rating Scale [16], or Severe Impairment Battery [17] if MMSE score was lower than 10; the Frontal Assessment Battery [18], the NeuroPsychiatric Inventory [19], the Disability Assessment of Dementia [20], the Instrumental Activities in Daily Living Scale [21] and the Zarit Burden Interview [22]) and clinical presentation (an amnestic typical presentation or an atypical presentation, with executive dysfunction, language disturbance or visuospatial presentation [12], based on the patient’s medical history and neuropsychological profile).
APOE genotyping
At inclusion, a blood sample was collected with a PyroMark ApoE kit (Biotage, Uppsala, Sweden), and then sent to the Department of Genetics at Rouen University Medical Centre/INSERM U1079 (Rouen, France) for determination of the participant’s APOE genotype.
Statistical analysis
Quantitative variables were expressed as the mean±standard deviation (after confirmation of a normal distribution), and categorical variables were expressed as the number (percentage). Means were compared using Student’s t-test, and frequencies were compared using a chi-squared test (with Yates’ correction) or Fisher’s exact test.
We first compared EOAD participants to controls by performing a multivariable conditional logistic regression with age, gender, educational level, smoking status, APOE ɛ4 status, regular alcohol consumption, BMI, blood pressure, blood-pressure-lowering medication, hypercholesterolemia, hypertriglyceridemia, lipid-lowering medication, and diabetes mellitus as dependent variables.
We also tested EOAD participant versus control differences in the VRF profile for the following EOAD subgroups: above-median versusbelow-median MMSE score; amnestic versus non-amnestic clinical presentation; presence versus absence of neurological signs, presence versus absence of a family history of AD history, and presence versus absence of at least one APOE ɛ4 allele. To this end, we used a multivariable multinomial logistic regression analysis to compare EOAD subgroups (outcome) with all the controls, by using all the variables selected in the multivariable conditional logistic regression.
We then investigated the link between clinical presentation of EOAD participants and VRF by analyzing data only in participants with dementia, by considering each VRF one by one. To compare participants who presented the factor and participants who did not present it according to a numerical parameter, a Student’s t-test or a Wilcoxon test was realized. Categorical variables were compared by using chi-squared test or Fisher’s exact test. Variables that emerged significant at p < 0.05 in univariable analyses were then included in a multivariable logistic regression model adjusted for age, gender, and educational level. For hypercholesterolemia, APOE ɛ4 status was added in adjustment’s variables. The same method was used to compare EOAD participants without VRF and EOAD participants with at least one VRF.
All statistical analyses were performed with SAS software (version 9.3, SAS Institute Inc., Cary, NC, USA). The threshold for statistical significance was set to p < 0.05.
RESULTS
One hundred and two participants with dementia from the CoMAJ cohort and 306 controls from the MONA-LISA cohort were included in the present study (Fig. 1 and Table 1). All but one of the EOAD participants and all but 8 of the controls had been genotyped for APOE.
Fig.1
Study flow chart.
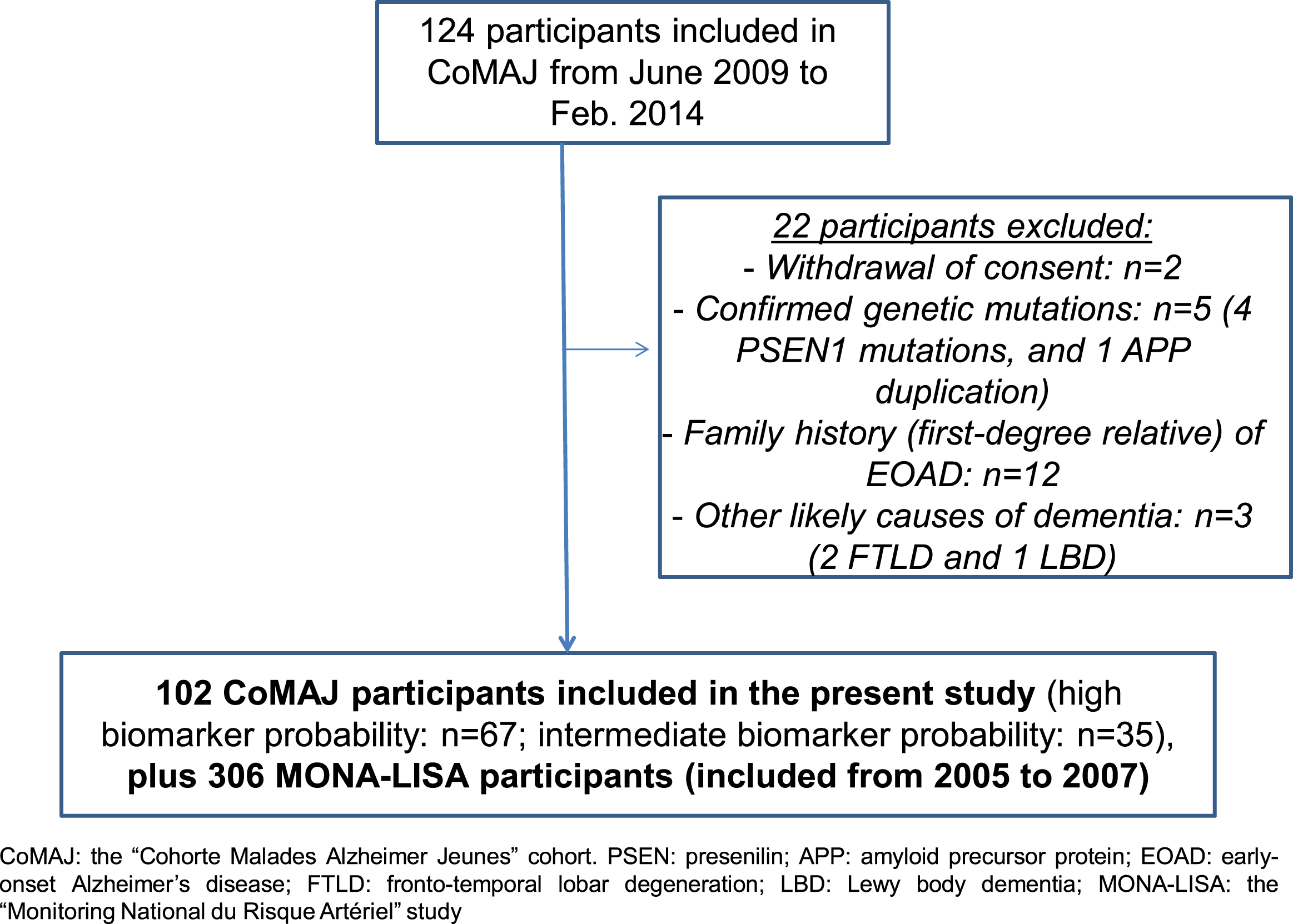
Table 1
The EOAD participant’s clinical characteristics at inclusion
| EOAD participants | |
| (n = 102) | |
| Women | 61 (59.8) |
| Family history | |
| Late onset dementia | 42 (41.2) |
| Vascular diseases | 35 (34.3) |
| Disease onset | |
| Age at onset* | 53.7±3.2 |
| Amnesia as first symptom | 65 (63.7) |
| Awareness of cognitive disorders | 64 (62.8) |
| Disease diagnosis | |
| Age at diagnosis* | 57.6±3.3 |
| MMSE score at diagnosisΔ | 19.8±5.1 |
| Time interval between first signs and | 3.9±0.1 |
| diagnosis* | |
| Age at first visit to memory clinic* | 57.8±3.7 |
| MMSE score at first visit to memory clinic | 18.6±6.5 |
| Inclusion in the CoMAJ study | |
| Time interval between first symptoms | 5.9±3.3 |
| and inclusion* | |
| MMSE score at inclusion | 14.0±7.9 |
| CDR score≤1 at inclusion | 54 (54.0) |
| Abnormal neurological signs | 42 (41.6) |
| Typical amnestic neuropsychological profile | 67 (67.0) |
EOAD, early-onset Alzheimer’s disease; *, years; categorical variables are reported as the number (percentage); quantitative variables are reported as the mean±standard deviation (SD); Δ, the only variable with a high proportion of missing data (for 26 patients); the proportion of missing data was below 5% for all other variables; MMSE, Mini-Mental State Examination; CoMAJ, the “Cohorte Malades Alzheimer Jeunes” cohort; CDR, Clinical Dementia Rating.
Comparisons of EOAD participants with controls (Table 2)
On average, EOAD participants had spent 2 years less in formal education than controls (p < 0.0001). Regular alcohol consumption was less frequent in EOAD participants (p < 0.0001). Compared with controls, EOAD participants had a lower BMI (by 2 points, on average; p = 0.0004) and lower systolic (p = 0.0036) and diastolic (p < 0.0001) BP, and were less likely to have hypertriglyceridemia (p = 0.0108). The prevalence of an APOE ɛ4 allele was higher in EOAD participants than in controls (50% versus 29.4%; p = 0.0002), and the proportion of homozygous ɛ4 carriers was also higher in EOAD participants than in controls (14.3% versus 1.3%; p < 0.0001). The distribution in all possible allele pairs was detailed in Table 3. EOAD participants were seven times more likely to suffer from depression (p < 0.0001) and were more likely to be taking psychotropic drugs (p < 0.0001). In the multivariable analysis, educational level, alcohol consumption, BMI, systolic BP, and hypertriglyceridemia were inversely related to EOAD, whereas BP-lowering medication and APOE ɛ4 carrier status remained positively associated with EOAD (Table 4).
Table 2
Comparisons of EOAD participants and controls
| EOAD participants (n = 102) | Controls (n = 306) | p | |
| Demographic and lifestyle characteristics | |||
| Age (y) | 59.5±3.8 | 59.5±4.2 | 0.89 |
| Women | 61 (59.8) | 183 (59.8) | 1 |
| Education level (y) | 9.9±2.9 | 11.7±3.8 | <0.0001 |
| Alcohol | 12 (11.8) | 87 (28.4) | <0.0001 |
| Smoking | |||
| Former | 30 (29.4) | 82 (26.8) | |
| Current | 8 (7.8) | 47 (15.4) | 0.16 |
| Never | 64 (62.8) | 177 (57.8) | |
| Vascular risk factors | |||
| Height (M) | 1.7±0.1 | 1.7±0.1 | 0.84 |
| Weight (kg) | 69.4±15.4 | 75.8±15.1 | 0.0003 |
| BMI (kg/m2) | 25.4±5.5 | 27.5±5.0 | 0.0004 |
| BMI <20 | 11 (11.1) | 7 (2.3) | |
| BMI <25 and ≥20 | 40 (40.4) | 96 (31.7) | 0.0003 |
| BMI <30 and ≥25 | 33 (33.3) | 126 (41.6) | |
| BMI ≥30 | 15 (15.2) | 74 (24.4) | |
| Systolic BP (mmHg) | 133.3±19.1 | 139.5±18.3 | 0.0036 |
| Diastolic BP (mmHg) | 78.0±13.3 | 83.5±10.7 | <0.0001 |
| History of hypertension | 42 (41.2) | 113 (36.9) | 0.44 |
| BP-lowering medication | 42 (41.2) | 93 (30.4) | 0.045 |
| Overall hypertension | 58 (56.9) | 179 (58.5) | 0.77 |
| Hypercholesterolemia | 44 (43.1) | 115 (37.6) | 0.32 |
| Hypertriglyceridemia | 5 (4.9) | 44 (14.4) | 0.0108 |
| Lipid-lowering medication | 26 (25.5) | 74 (24.2) | 0.79 |
| Diabetes mellitus | 5 (4.9) | 13 (4.3) | 0.78 |
| Antidiabetic medication | 5 (4.9) | 13 (4.3) | 0.78 |
| APOE genotype | |||
| APOE ɛ4 | 51 (50.0) | 90 (29.4) | 0.0002 |
| Comorbidities | |||
| Stroke | 2 (2.0) | 3 (1.0) | 0.60 |
| Heart disease | 13 (12.7) | 24 (7.8) | 0.11 |
| Depression | 48 (47.1) | 32 (7.5) | <0.0001 |
| Epilepsy | 3 (2.9) | 2 (0.7) | 0.10 |
| Ongoing medication | |||
| APA or VKA | 17 (16.7) | 31 (10.1) | 0.16 |
| Antidepressant | 47 (46.0) | 18 (5.1) | <0.0001 |
| Anxiolytic | 22 (21.6) | 23 (7.5) | <0.0001 |
| Nootropic | 5 (4.9) | 0 (0) | <0.0001 |
| Neuroleptic | 5 (4.9) | 3 (1.0) | 0.026 |
| Hypnotic | 6 (5.9) | 17 (5.6) | 0.90 |
Categorical variables are reported as the number (percentage); quantitative variables are reported as the mean±SD; EOAD, early-onset Alzheimer’s disease; BMI, body mass index; BP, blood pressure; overall hypertension, high blood pressure measured at inclusion, hypertension reported by a primary care physician, or use of a blood-pressure-lowering medication; APOE ɛ4, at least one APOE ɛ4 allele; APA, antiplatelet agent; VKA, vitamin K antagonist. Participants with dementia and controls were compared using Student’s t-test and a chi-squared or Fisher’s test.
Table 3
Distribution of allele pairs of APOE genotyping of EOAD participants and controls
| EOAD participants | Controls | p | |
| (n = 102) | (n = 306) | ||
| At least one allele E4 | 51 (50) | 90 (29.4) | 0.0002 |
| E2/E2 | 0 (0) | 2 (0.7) | |
| E2/E3 | 3 (3.1) | 30 (10.1) | |
| E2/E4 | 3 (3.1) | 9 (3.0) | <0.0001 |
| E3/E3 | 47 (48.0) | 176 (59.1) | |
| E3/E4 | 34 (34.7) | 77 (25.8) | |
| E4/E4 | 14 (14.3) | 4 (1.3) |
Variables are reported as the number (percentage). EOAD, early-onset Alzheimer’s disease EOAD participants and controls were compared using a chi-squared or Fisher’s test.
Table 4
Multivariable analysis of the association between EOAD and VRF
| Odds ratio of | 95% confidence | p | ||
| EOAD | interval | |||
| Lifestyle factors | ||||
| Educational level (high vs low) | 0.82 | 0.75 | 0.89 | <0.0001 |
| Alcohol (yes versus no) | 0.08 | 0.02 | 0.28 | <0.0001 |
| Smoking (never) | ||||
| Former | 1.25 | 0.62 | 2.52 | 0.53 |
| Current | 0.42 | 0.15 | 1.17 | 0.10 |
| Vascular risk factors | ||||
| Body mass index (kg/m2) | 0.90 | 0.85 | 0.95 | 0.0004 |
| Systolic BP (mmHg) | 0.98 | 0.96 | 0.99 | 0.004 |
| BP-lowering medication (yes versus no) | 1.93 | 1.06 | 3.51 | 0.0324 |
| Hypercholesterolemia (yes versus no) | 2.03 | 0.96 | 4.32 | 0.0652 |
| Hypertriglyceridemia (yes versus no) | 0.33 | 0.11 | 0.98 | 0.0456 |
| Lipid-lowering medication (yes versus no) | 0.72 | 0.31 | 1.68 | 0.45 |
| Diabetes mellitus (yes versus no) | 2.35 | 0.57 | 9.66 | 0.24 |
| APOE genotype | ||||
| APOE ɛ4 (yes versus no) | 2.13 | 1.23 | 3.68 | 0.0066 |
EOAD, early-onset Alzheimer’s disease; VRF, vascular risk factors; APOE ɛ4, at least one APOE ɛ4 allele; BP, blood pressure.
Sensitivity analyses (notably probing the use of psychotropic drugs and the time interval betweendisease onset and inclusion in the CoMAJ cohort) did not modify previous results (data not shown).
Likewise, subgroup analyses of EOAD participants versus controls did not reveal any significant differences, other than when comparing amnestic versus non-amnestic clinical presentations. The odds ratio [95% confidence interval] for EOAD was 3.8 [2.0–7.2] in participant carriers of an APOE ɛ4 allele with a typical amnestic presentation and only 0.8 [0.3–2.0] for participants carriers of an APOE ɛ4 allele with an atypical presentation (executive dysfunction or language or visuospatial disturbances).
Influence of VRFs in EOAD participants
EOAD participants with at least one VRF were more likely to be male than those without VRFs (46.7% versus 22.2%; p = 0.02), were more likely to have a family history of late-onset dementia (48.0% versus 22.2%; p = 0.02), and were more likely to have depression (53.3% versus 29.6%; p = 0.03). These results were confirmed by the multivariable analysis. We did not observe any other differences of clinical presentation and neuropsychological profile.
For each VRF, we then compared EOAD participants with the factor and participants without.
The disease onset in EOAD participants with hypercholesterolemia seemed to be later (54.7±2.8 versus 52.9±3.2; p = 0.0081). Yet, it did not remain significant in multivariable analysis. Smokers were more likely to be male (78.9% versus 17.2%; p < 0.0001).
Taking anxiolytic seemed to be inversely related to smoking (10.5% versus 28.1%; p = 0.0367). However, in the multivariable analysis results on anxiolytic did not persist.
There were no other significant differences for other VRFs. The low number of EOAD participants with diabetes (n = 5) prevented us from performing comparisons for this parameter.
DISCUSSION
Our results showed that VRFs were not more prevalent in EOAD participants than in age- and gender-matched controls from the same geographical area. In fact, some factors (hypertension, overweight, and obesity) were less prevalent in participants with dementia than in controls. Furthermore, VRFs did not appear to be strongly associated with the EOAD participants’ clinical presentation.
One potential study limitation related to the fact that some EOAD participants had been monitored in memory center for a long time before their inclusion in the CoMAJ cohort, whereas others were included just after their first visit in our center. Participants with dementia entered the study at different stages of dementia, which would modify the prevalence of VRFs. However, our subgroup analyses between prevalent and incident EOAD participants did not reveal any differences with regard to the duration of follow-up before inclusion.
In agreement with earlier reports [23], EOAD participants in the present study had a lower educational level than controls did. This may contribute to the low MMSE mean score at inclusion. One study has shown that cognitive decline begins 8 years earlier in less-educated patients [24]. In a Swedish cohort of 18-year-old males over a period of more than 30 years, lower cognitive performance in early adulthood was associated with an elevated risk of early-onset all-cause dementia [25]. Altogether these data suggested that educational level had a significant impact in revealing the cognitive impairment and supported the role of cognitive reserve.
In the present study, the EOAD was not associated with major VRF, consistent with a previous report which has shown that VRFs are less prevalent in EOAD than in late-onset AD [26].
The BMI and levels of obesity were clearly lower in participants with dementia than in controls. Other studies have reported low BMIs in older patients with dementia [27], or a slower midlife increase in BMI in Swedish women who developed late-onset dementia [28], and it has been suggested that weight loss might start 9 to 10 years before diagnosis [29]. Furthermore, a study of younger patients suggested that body weight was lower in EOAD patients than in patients with frontotemporal lobar degeneration (FTLD), although hyperorality and dietary changes in the latter condition contributed to weight changes [30]. The reasons for lower body weight might be related to an increase of metabolic rate, or to amyloid deposition in the leptin-receptor-containing brain structures that control appetite (such as the hypothalamus and the amygdala) in EOAD patients [31].
We did not observe significant differences in the prevalence of hypertension, regardless of whether the latter was defined as (1) a history of physician-diagnosed hypertension, (2) use of BP-lowering medication, or (3) elevated systolic and diastolic BP at inclusion. This finding might appear to run counter to the role of hypertension in cognitive decline and dementia [32] and contrasts with literature data. In an earlier study, hypertension was three times more frequent in EOAD patients than in age-matched patients with FTLD [30], suggesting that elevated systolic BP is a risk factor for EOAD. Furthermore, hypertension is a midlife risk factor for late-onset dementia and cognitive decline in the years before dementia onset [33]. In the Kungsholmen cohort of elderly patients, however, the BP started to fall 3 years before symptom onset [34]. In the present study, BP remained lower in EOAD participants than in controls after adjustment for the use of BP-lowering medications. Cognitive symptoms had been present for 3 to 4 years, and moderate dementia was already present at study inclusion; hence, the drop in BP associated with dementia might have hidden previously higher BP values. However, there was no significant difference in the proportion of history of hypertension between participants with dementia (41%) and controls (37%). Lower BP might contribute to lower brain perfusion, and thus might accelerate histopathological damage in AD. Alternatively, amyloid deposits may affect BP-regulating centers in the brain (such as the amygdala and the hypothalamus); it has been shown that atrophy of C1 neurons in AD brains is associated with lower BP [35].
There was no difference between participants with dementia and controls in the prevalence of hypercholesterolemia, which is considered to be a risk factor for late-onset dementia [36]. The lower prevalence of hypertriglyceridemia in our patients might be due to the lower BMI, although the difference was still significant after adjustment for weight. However, few of EOAD participants had hypertriglyceridemia, and so this finding should be confirmed in a larger sample. The lower prevalence of hypertriglyceridemia might also be due to the lower frequency of the APOE ɛ2 allele in patients (since the latter is known to be related to hypertriglyceridemia) [37] or differing dietary habits.
Likewise, only a few subjects had diabetes mellitus; the small sample size prevented us from performing a robust assessment of the risk in this context.
The distribution of allele pairs of APOE in our study (EOAD participants and controls) was similar to the distribution found in the French population of the GWAS study, in EOAD patients as in controls [38]. As expected from the literature data, the prevalence of the APOE ɛ4 allele was higher in patients and was more frequent in patients with amnestic profile [39]. This may be explained by a greater atrophy of the medial temporal lobe in APOE ɛ4 carriers [40].
Depression was more prevalent in participants with dementia than in controls. Other studies have reported same findings, although this might be due to the patients’ greater awareness of their impairments in activities of daily living [41]. In the Cache County Study of older AD patients, VRFs were associated with a greater risk of delusion, depression, and agitation [42]. In the present study of EOAD participants, the presence of at least one VRF was associated with depression but not with delusion or agitation.
The prevalence of regular alcohol consumption was lower in participants with dementia than in controls, in line with dietary habit changes in patients at moderate to severe stage of dementia. This finding is difficult to interpret because alcohol consumption was checked in a one-week dietary survey in the MONA-LISA study but not in the CoMAJ study (in which alcohol consumption was simply reported by the patient or his/her caregiver or family).
Our study had several strengths. To the best of our knowledge, this study was the first to have assessed and compared VRFs in a cohort of EOAD patients and a cohort of age- and gender-matched healthy controls from the same geographic area. This approach minimizes possible bias due to an influence of geographic location on the vascular profile [43]. Exhaustive, similar investigations of patient and controls enabled us to analyze lifestyle factors (such as educational level). There may still have been some recruitment bias because our memory clinic is a tertiary center. However, a large proportion of patients with EOAD are referred to our clinic because it is the reference center for this population, and before they have been treated. Our cohort had good internal and external validity because of all of the patients met the reviewed NIA/AA clinical criteria for probable AD dementia with an elevated level of certainty [12]. Our patients’ clinical presentations were also consistent with literature data on EOAD patients [44].
However, our results should be interpreted with a degree of caution because controls recruited through health surveys and epidemiological studies generally have fewer risk factors and lower disease levels than the general population [43]. In addition, survival bias might affect the association of certain VRFs with AD when prevalent cases were used. It would be interesting to see if low levels of certain VRFs (e.g., BP and BMI) are associated with an increased likelihood of EOAD by monitoring the weight and the blood pressure. Other “environmental” factors, such as social interaction and systemic inflammation markers, might contribute to an early onset of symptoms. They were not investigated in this cohort.
In conclusion, contrary to the high prevalence of some VRFs in late-onset AD patients, these VRFs were not more prevalent in EOAD patients than in controls, and they did not appear to be strongly associated with the EOAD patients’ clinical presentation. Other factors, and notably genetic factors [45], or environmental factors may be more prevalent in these patients. Lastly, clinicians should be more aware of the elevated risk of denutrition, hypotension, and depression in EOAD patients.
ACKNOWLEDGMENTS
The authors acknowledge the memory clinic network of Northern France and the Institut Pasteur de Lille for the acquisition of data and the Fondation pour la recherche sur Alzheimer, and ITMO Santé Publique for the general support. This study was funded as part of the French government’s LABEX DISTALZ program (“Development of InnovativeStrategies for a Transdisciplinary Approach to Alzheimer’s Disease”).
Authors’ disclosures available online (http://j-alz.com/manuscript-disclosures/17-0367r2).
REFERENCES
[1] | Garre-Olmo J , Genís Batlle D , del Mar Fernández M , Marquez Daniel F , de Eugenio Huélamo R , Casadevall T , Turbau Recio J , Turon Estrada A , López-Pousa S ; Registry of Dementia of Girona Study Group (ReDeGi Study Group) ((2010) ) Incidence and subtypes of early-onset dementia in a geographically defined general population. Neurology 75: , 1249–1255. |
[2] | Harvey RJ , Skelton-Robinson M , Rossor MN ((2003) ) The prevalence and causes of dementia in people under the age of 65 years. J Neurol Neurosurg Psychiatry 74: , 1206–1209. |
[3] | Mercy L , Hodges JR , Dawson K , Barker RA , Brayne C ((2008) ) Incidence of early-onset dementias in Cambridgeshire, United Kingdom. Neurology 71: , 1496–1499. |
[4] | Pasquier F , Rollin-Sillaire A , Lebouvier T , Lebert F ((2015) ) Maladie d’Alzheimer du sujet jeune. In Démences. DOIN, ed., pp. 259–72. |
[5] | Campion D , Dumanchin C , Hannequin D , Dubois B , Belliard S , Puel M , Thomas-Anterion C , Michon A , Martin C , Charbonnier F , Raux G , Camuzat A , Penet C , Mesnage V , Martinez M , Clerget-Darpoux F , Brice A , Frebourg T ((1999) ) Early-onset autosomal dominant Alzheimer disease: Prevalence, genetic heterogeneity, and mutation spectrum. Am J Hum Genet 65: , 664–670. |
[6] | Knopman DS , Mosley TH , Catellier DJ , Coker LH ((2009) ) Atherosclerosis Risk in Communities Study Brain MRI Study, Fourteen-year longitudinal study of vascular risk factors, APOE genotype, and cognition: The ARIC MRI Study. Alzheimers Dement 5: , 207–214. |
[7] | Deschaintre Y , Richard F , Leys D , Pasquier F ((2009) ) Treatment of vascular risk factors is associated with slower decline in Alzheimer disease. Neurology 73: , 674–680. |
[8] | Nordström P , Nordström A , Eriksson M , Wahlund LO , Gustafson Y ((2013) ) Risk factors in late adolescence for young-onset dementia in men: A nationwide cohort study. JAMA Intern Med 173: , 1612–1618. |
[9] | Heath CA , Mercer SW , Guthrie B ((2015) ) Vascular comorbidities in younger people with dementia: A cross-sectional population-based study of 616|245 middle-aged people in Scotland. J Neurol Neurosurg Psychiatry 86: , 959–964. |
[10] | Cations M , Withall A , Low LF , Draper B ((2016) ) What is the role of modifiable environmental and lifestyle risk factors in young onset dementia? Eur J Epidemiol 31: , 107–124. |
[11] | Dubois B , Feldman HH , Jacova C , Hampel H , Molinuevo JL , Blennow K , DeKosky ST , Gauthier S , Selkoe D , Bateman R , Cappa S , Crutch S , Engelborghs S , Frisoni GB , Fox NC , Galasko D , Habert MO , Jicha GA , Nordberg A , Pasquier F , Rabinovici G , Robert P , Rowe C , Salloway S , Sarazin M , Epelbaum S , de Souza LC , Vellas B , Visser PJ , Schneider L , Stern Y , Scheltens P , Cummings JL ((2014) ) Advancing research diagnostic criteria for Alzheimer’s disease: The IWG-2 criteria. Lancet Neurol 13: , 614–629. |
[12] | McKhann GM , Knopman DS , Chertkow H , Hyman BT , Jack CR Jr , Kawas CH , Klunk WE , Koroshetz WJ , Manly JJ , Mayeux R , Mohs RC , Morris JC , Rossor MN , Scheltens P , Carrillo MC , Thies B , Weintraub S , Phelps CH ((2011) ) The diagnosis of dementia due to Alzheimer’s disease:Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: , 263–269. |
[13] | Wagner A , Sadoun A , Dallongeville J , Ferrières J , Amouyel P , Ruidavets JB , Arveiler D ((2011) ) High blood pressure prevalence and control in a middle-aged French population and their associated factors: The MONA LISA study. J Hypertens 29: , 43–50. |
[14] | Folstein M , Anthony JC , Parhad I , Duffy B , Gruenberg EM ((1985) ) The meaning of cognitive impairment in the elderly. J Am Geriatr Soc 33: , 228–235. |
[15] | Hughes CP , Berg L , Danziger WL , Coben LA , Martin RL ((1982) ) A new clinical scale for the staging of dementia. Br J Psychiatry 140: , 566–572. |
[16] | Gardner R Jr , Oliver-Muñoz S , Fisher L , Empting L ((1981) ) Mattis Dementia Rating Scale: Internal reliability study using a diffusely impaired population. J Clin Neuropsychol 3: , 271–275. |
[17] | Hugonot-Diener L , Verny M , Devouche E , Saxton J , Mecocci P , Boller F ((2003) ) [Abridged version of the severe impairment battery (SIB)]. Psychol Neuropsychiatr Vieil 1: , 273–283. |
[18] | Lebert F , Pasquier F , Souliez L , Petit H ((1998) ) Frontotemporal behavioral scale. Alzheimer Dis Assoc Disord 12: , 335–339. |
[19] | Cummings JL , Mega M , Gray K , Rosenberg-Thompson S , Carusi DA , Gornbein J ((1994) ) The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology 44: , 2308–2314. |
[20] | Gélinas I , Gauthier L , McIntyre M , Gauthier S ((1999) ) Development of a functional measure for persons with Alzheimer’s disease: The disability assessment for dementia. Am J Occup Ther 53: , 471–481. |
[21] | Lawton MP , Brody EM ((1969) ) Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 9: , 179–186. |
[22] | Bocquet H , Pous J , Charlet JP , Grand A ((1996) ) [Measuring the burden for carers of dependent elderly with the Zarit inventory]. Rev Epidemiol Sante Publique 44: , 57–65. |
[23] | Picard C , Pasquier F , Martinaud O , Hannequin D , Godefroy O ((2011) ) Early onset dementia: Characteristics in a large cohort from academic memory clinics. Alzheimer Dis Assoc Disord 25: , 203–205. |
[24] | Amieva H , Mokri H , Le Goff M , Meillon C , Jacqmin-Gadda H , Foubert-Samier A , Orgogozo JM , Stern Y , Dartigues JF ((2014) ) Compensatory mechanisms in higher-educated subjects with Alzheimer’s disease: A study of 20 years of cognitive decline. Brain 137: , 1167–1175. |
[25] | Nyberg J , Åberg MA , Schiöler L , Nilsson M , Wallin A , Torén K , Kuhn HG ((2014) ) Cardiovascular and cognitive fitness at age 18 and risk of early-onset dementia. Brain 137: , 1514–1523. |
[26] | Carotenuto A , Rea R , Colucci L , Ziello AR , Molino I , Carpi S , Traini E , Amenta F , Fasanaro AM ((2012) ) Late and early onset dementia: What is the role of vascular factors? A retrospective study. J Neurol Sci 322: , 170–175. |
[27] | Gustafson DR , Bäckman K , Waern M , Ostling S , Guo X , Zandi P , Mielke MM , Bengtsson C , Skoog I ((2009) ) Adiposity indicators and dementia over 32 years in Sweden. Neurology 73: , 1559–1566. |
[28] | Gustafson DR , Bäckman K , Joas E , Waern M , Östling S , Guo X , Skoog I ((2012) ) 37 years of body mass index and dementia: Observations from the prospective population study of women in Gothenburg, Sweden. J Alzheimers Dis 28: , 163–171. |
[29] | Knopman DS , Edland SD , Cha RH , Petersen RC , Rocca WA ((2007) ) Incident dementia in women is preceded by weight loss by at least a decade. Neurology 69: , 739–746. |
[30] | Atkins ER , Bulsara MK , Panegyres PK ((2012) ) Cerebrovascular risk factors in early-onset dementia. J Neurol Neurosurg Psychiatry 83: , 666–667. |
[31] | Harvey J , Shanley LJ , O’Malley D , Irving AJ ((2005) ) Leptin: Aotential cognitive enhancer? Biochem Soc Trans 33: , 1029–1032. |
[32] | Hughes TM , Sink KM ((2016) ) Hypertension and its role in cognitive function: Current evidence and challenges for the future. Am J Hypertens 29: , 149–157. |
[33] | Power MC , Weuve J , Gagne JJ , McQueen MB , Viswanathan A , Blacker D ((2011) ) The association between blood pressure and incident Alzheimer disease: A systematic review and meta-analysis. Epidemiology 22: , 646–659. |
[34] | Qiu C , von Strauss E , Winblad B , Fratiglioni L ((2004) ) Decline in blood pressure over time and risk of dementia: A longitudinal study from the Kungsholmen project. Stroke 35: , 1810–1815. |
[35] | Burke WJ , Coronado PG , Schmitt CA , Gillespie KM , Chung HD ((1994) ) Blood pressure regulation in Alzheimer’s disease. J Auton Nerv Syst 48: , 65–71. |
[36] | Solomon A , Kivipelto M , Wolozin B , Zhou J , Whitmer RA ((2009) ) Midlife serum cholesterol and increased risk of Alzheimer’s and vascular dementia three decades later. Dement Geriatr Cogn Disord 28: , 75–80. |
[37] | Perron P , Brisson D , Santuré M , Blackburn P , Bergeron J , Vohl MC , Després JP , Gaudet D ((2007) ) Apolipoprotein E and lipoprotein lipase gene polymorphisms interaction on the atherogenic combined expression of hypertriglyceridemia and hyperapobetalipoproteinemia phenotypes. J Endocrinol Invest 30: , 551–557. |
[38] | Genin E , Hannequin D , Wallon D , Sleegers K , Hiltunen M , Combarros O , Bullido MJ , Engelborghs S , De Deyn P , Berr C , Pasquier F , Dubois B , Tognoni G , Fiévet N , Brouwers N , Bettens K , Arosio B , Coto E , Del Zompo M , Mateo I , Epelbaum J , Frank-Garcia A , Helisalmi S , Porcellini E , Pilotto A , Forti P , Ferri R , Scarpini E , Siciliano G , Solfrizzi V , Sorbi S , Spalletta G , Valdivieso F , Vepsäläinen S , Alvarez V , Bosco P , Mancuso M , Panza F , Nacmias B , Bossù P , Hanon O , Piccardi P , Annoni G , Seripa D , Galimberti D , Licastro F , Soininen H , Dartigues JF , Kamboh MI , Van Broeckhoven C , Lambert JC , Amouyel P , Campion D ((2011) ) APOE and Alzheimer disease: A major gene with semi-dominant inheritance. Mol Psychiatry 16: , 903–907. |
[39] | van der Flier W , Schoonenboom S , Pijnenburg Y , Fox N , Scheltens P ((2006) ) The effect of APOE genotype on clinical phenotype in Alzheimer disease. Neurology 67: , 526–527. |
[40] | Hashimoto M , Yasuda M , Tanimukai S , Matsui M , Hirono N , Kazui H , Mori E ((2001) ) Apolipoprotein E epsilon 4 and the pattern of regional brain atrophy in Alzheimer’s disease. Neurology 57: , 1461–1466. |
[41] | van Vliet D , de Vugt ME , Köhler S , Aalten P , Bakker C , Pijnenburg YA , Vernooij-Dassen MJ , Koopmans RT , Verhey FR ((2013) ) Awareness and its association with affective symptoms in young-onset and late-onset Alzheimer disease: A prospective study. Alzheimer Dis Assoc Disord 27: , 265–271. |
[42] | Steinberg M , Hess K , Corcoran C , Mielke MM , Norton M , Breitner J , Green R , Leoutsakos J , Welsh-Bohmer K , Lyketsos C , Tschanz J ((2014) ) Vascular risk factors and neuropsychiatric symptoms in Alzheimer’s disease: The Cache County Study. Int J Geriatr Psychiatry 29: , 153–159. |
[43] | 3C Study Group ((2003) ) Vascular factors and risk of dementia: Design of the Three-City Study and baseline characteristics of the study population. Neuroepidemiology 22: , 316–325. |
[44] | Joshi A , Ringman JM , Lee AS , Juarez KO , Mendez MF ((2012) ) Comparison of clinical characteristics between familial and non-familial early onset Alzheimer’s disease. J Neurol 259: , 2182–2188. |
[45] | Nicolas G , Wallon D , Charbonnier C , Quenez O , Rousseau S , Richard A-C , Rovelet-Lecrux A , Coutant S , Le Guennec K , Bacq D , Garnier J-G , Olaso R , Boland A , Meyer V , Deleuze J-F , Munter HM , Bourque G , Auld D , Montpetit A , Lathrop M , Guyant-Maréchal L , Martinaud O , Pariente J , Rollin-Sillaire A , Pasquier F , Le Ber I , Sarazin M , Croisile B , Boutoleau-Bretonnière C , Thomas-Antérion C , Paquet C , Sauvée M , Moreaud O , Gabelle A , Sellal F , Ceccaldi M , Chamard L , Blanc F , Frebourg T , Campion D , Hannequin D ((2016) ) Screening of dementia genes by whole-exome sequencing in early-onset Alzheimer disease: Input and lessons. Eur J Hum Genet 24: , 710–716. |




