Baseline Telomere Length and Effects of a Multidomain Lifestyle Intervention on Cognition: The FINGER Randomized Controlled Trial
Abstract
Leukocyte telomere length (LTL) is a biomarker of aging, and it is associated with lifestyle. It is currently unknown whether LTL is associated with the response to lifestyle interventions. The goal is to assess whether baseline LTL modified the cognitive benefits of a 2-year multidomain lifestyle intervention (exploratory analyses). The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) was a 2-year randomized controlled trial including 1,260 people at risk of cognitive decline, aged 60–77 years identified from the general population. Participants were randomly assigned to the lifestyle intervention (diet, exercise, cognitive training, and vascular risk management) and control (general health advice) groups. Primary outcome was change in cognition (comprehensive neuropsychological test battery). Secondary outcomes were changes in cognitive domains: memory, executive functioning, and processing speed. 775 participants (392 control, 383 intervention) had baseline LTL (peripheral blood DNA). Mixed effects regression models with maximum likelihood estimation were used to analyze change in cognition as a function of randomization group, time, baseline LTL, and their interaction. Intervention and control groups did not significantly differ at baseline. Shorter LTL was related to less healthy baseline lifestyle. Intervention benefits on executive functioning were more pronounced among those with shorter baseline LTL (p-value for interaction was 0.010 adjusted for age and sex, and 0.007 additionally adjusted for baseline lifestyle factors). The FINGER intervention cognitive benefits were more pronounced with shorter baseline LTL, particularly for executive functioning, indicating that the multidomain lifestyle intervention was especially beneficial among higher-risk individuals.
INTRODUCTION
Leukocyte telomere length (LTL) is a biomarker of aging and aging-related diseases, representing cells’ ‘biological age’, as opposed to ‘chronological age’ [1]. Telomeres are nucleoprotein structures at the end of eukaryotic chromosomes that protect chromosomes from end-to-end fusion and damage [2]. While LTL shortens during aging, there is inter-individual variability in the rate of LTL change over time, and determinants of LTL include both genetic and non-genetic factors [1, 2]. A broad range of non-genetic factors have been linked to shorter LTL, including chronic psychological stress and related psychiatric conditions, unhealthy dietary habits and altered nutrition-related biomarkers, physical inactivity, smoking, and obesity [1, 2]. Because of the variety of such non-genetic factors, LTL shortening may represent a proxy for the overall exposure to risk factors promoting disease [2].
As these risk factors are often shared by several chronic late-life conditions, it is perhaps not surprising that LTL shortening has been related to the increased risk of, for example, cardiovascular conditions, diabetes, various cancers, poor immune function, and mortality [2]. Neurodegenerative conditions and dementia have also been associated with LTL [1]. A recent meta-analysis reported that patients with Alzheimer’s disease (AD) had shorter telomere length (measured in leukocytes or other tissue) compared to controls [3]. In addition, links between genetic determinants of shorter telomere length and AD have been reported [37, 38].
However, the significance of LTL across the cognitive continuum between normal aging and dementia is less clear. Dementia-related diseases such as AD have a long preclinical phase, and brain pathology can start decades before dementia onset. Differentiating between normal aging and high-risk states or preclinical disease stages is still challenging, and studies focusing on mild cognitive impairment (MCI) have had conflicting results. Shorter LTL was reported among patients with MCI [4], but both shorter and longer LTL have been linked to increased risk of subsequent MCI [5]. Other studies showed that the progression from MCI to dementia was not associated with LTL [4, 6]. Concerning cognition, some studies have reported that longer LTL was associated with better performance on various cognitive domains including executive functioning, attention, psychomotor speed, working memory, episodic memory, and general mental ability [7–10]. Also, LTL attrition was inversely related to global cognition and specific cognitive sub-domains [11]. However, other studies showed modest or no associations of LTL with various cognitive domains [12–16]. Such discrepancies may be due to varying age ranges and timing, and differing methods for LTL and cognitive assessments [5, 11].
Cognitive impairment and dementia have become a major public health challenge [17], and it is essential to find effective preventive interventions, as well as identify individuals who are most likely to benefit from them. Given that LTL may be regarded as a proxy for overall exposure to risk factors for cognitive impairment and dementia, determining if and how pre-intervention LTL might modify intervention effects is particularly important. Few studies have so far investigated LTL in the context of clinical trials, and none have focused on cognitive outcomes.
The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER), a 2-year randomized controlled trial, investigated the effects of a multidomain lifestyle intervention versus regular health advice among at-risk older adults from the general population [18]. Significant intervention benefits were reported on overall cognitive performance (primary outcome), executive functioning and processing speed (secondary outcomes), and an abbreviated memory score including more complex memory tasks (post-hoc analyses). The aim of the present study is to assess whether baseline LTL modifies these cognitive benefits. The initial trial protocol did not specifically include LTL, and thus analyses are exploratory. Based on previous literature, we hypothesized that individuals with the shortest LTL would benefit most from the intervention.
METHODS
Study design and participants
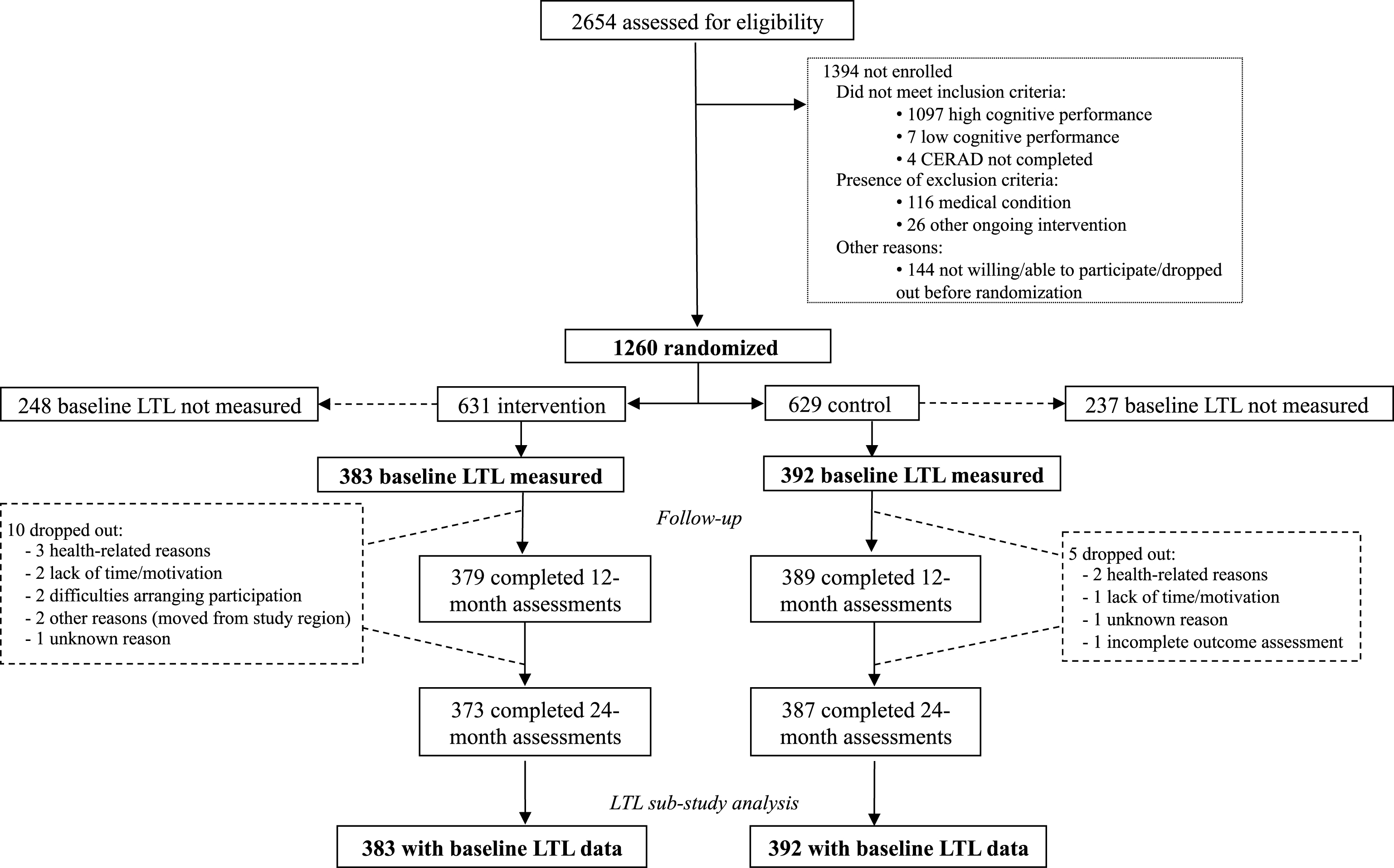
LTL measurements were planned in a sub-sample of FINGER participants selected according to the order of randomization (provided that blood samples were available and DNA could be extracted). The study population comprised 775 (383 in the intervention and 392 in the control group) of the 1,260 FINGER study participants (Fig. 1). The FINGER trial protocol, recruitment characteristics, primary results, and safety data have been reported previously [18–20]. In brief, participants were recruited between September 7, 2009, and November 24, 2011 from former non-intervention population-based health-monitoring surveys [19, 21]. Eligibility criteria included: age 60–77 years; CAIDE (Cardiovascular Risk Factors, Aging and Dementia) Dementia Risk Score of six or more points (range is 0–15 points, based on age, sex, education, systolic blood pressure, body mass index, total cholesterol, and physical activity); and cognitive screening performed using the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD-NB) neuropsychological battery [22] to select individuals with cognitive performance at the mean level or slightly lower than expected for age according to Finnish population norms [19]. Exclusion criteria were: previously diagnosed dementia; suspected dementia following clinical assessment at the screening visit; MMSE <20 points; disorders affecting safe participation/cooperation; severe loss of sensory capacities and concurrent participation in another trial. FINGER adhered to the declaration of Helsinki and was approved by the Coordinating Ethics Committee of the Hospital District of Helsinki and Uusimaa. Participants gave written informed consent at the screening and baseline visits.
Fig.1
Trial profile for the LTL sub-study. CERAD = Consortium to Establish a Registry for Alzheimer’s Disease. LTL = leucocyte telomere length.
Intervention
Participants were randomly assigned to the intensive multidomain intervention group, or regular health advice (i.e., control) group (1 : 1 ratio). Allocations were computer-generated in blocks of four (two individuals randomly allocated to each group) by the study nurse at each site. Blinding was ensured as much as possible. Outcome assessors were fully blinded to group allocation and they were not involved in any other tasks in the study.
All participants (control and intervention groups) met the study nurse at screening, baseline, and at 6, 12, and 24 months after randomization for measurements of blood pressure, weight, height, body mass index, and hip/waist circumference. All participants met the study physician at screening, and at 24 months for a detailed medical history and physical examination. At baseline, the study nurse gave oral and written information and advice on healthy diet and physical, cognitive, and social activities that are beneficial for management of vascular risk factors and disability prevention. Blood samples were collected at baseline. Laboratory test results were mailed to participants, along with written information about the clinical significance of measurements, and advice to contact primary health care if needed.
The intervention group additionally received four intervention components [18, 20]. The nutritional intervention component included individual and group sessions supervised by study nutritionists, and was based on the Finnish Nutrition Recommendations [18, 23]. The exercise training program followed international guidelines [24]. Any physical activity was promoted. Exercise was led by study physiotherapists at the gym, including individually tailored programs for progressive muscle strength training and aerobic exercise, and postural balance exercises [18, 20]. Cognitive training was led by psychologists and included group sessions and individual training. Individual training was computer-based, using a web-based in-house developed program including tasks adapted from validated protocols [25]. Additionally, social activities were stimulated through the group meetings of all intervention components. For management of metabolic and vascular risk factors, national evidence-based guidelines were used [26–28]. This included additional meetings with the study nurse (at 3, 9, and 18 months), and the study physician (at 3, 6, and 12 months) for measurements of blood pressure, weight, height, and hip/waist circumference, physical examinations, and lifestyle recommendations. Study physicians did not prescribe medications, but advised participants to contact their own physician/clinic as appropriate.
Cognitive outcomes
Cognition was assessed with an extended version of the neuropsychological test battery (NTB) [29] at baseline, 12, and 24 months by study psychologists. The primary outcome was change in cognitive performance measured by the NTB total score, a composite score based on 14 test results (calculated as z-scores standardized to the baseline mean and standard deviation (SD)), with higher scores suggesting better performance) [20]. Secondary outcomes included NTB domain z-scores for memory, processing speed, and executive functioning. The memory domain included visual paired associates test, immediate and delayed recall, logical memory immediate and delayed recall, and word list learning and delayed recall. The processing speed domain included letter digit substitution test, concept shifting test (condition A), and Stroop test (condition 2). The executive functioning domain included category fluency test, digit span, concept shifting test (condition C), trail making test (shifting score B–A), and a shortened 40-stimulus version of the original Stroop test (interference score 3–2). Post-hoc analyses were performed for an abbreviated memory domain including four memory tests with longer delayed recall (30 min instead of 5 min) and requiring more complex processing.
Baseline LTL measurement
LTL was measured from DNA extracted from peripheral blood. Blood samples were collected at the baseline visit, before the start of the intervention. The LTL measurements were carried out and quality control was performed at the laboratory of Associate Professor Iiris Hovatta at the Department of Biosciences, Viikki Biocentre, University of Helsinki, Finland. A quantitative real-time polymerase chain reaction-based method was used [30] as described previously [30–32], with β-hemoglobin (Hgb) as a single copy reference gene. Separate reactions for telomere and Hgb reaction were carried out in paired 384-well plates in which matched sample well positions were used. Ten nanograms of DNA were used for each reaction, performed in triplicate. Every plate included a 7-point standard curve, was used to perform absolute quantification of each sample. Samples and standard dilutions were transferred into the plates using a multichannel pipet and dried overnight at room temperature. A specific reaction mix for telomere reaction included 270 nM tel1b primer (5’-CGGTTT(GTTTGG)5GTT-3’) and 900 nM tel2b primer (5’-GGCTTG(CCTTAC)5CCT-3’), 0.2X SYBR Green I (Invitrogen), 5 mM DTT (Sigma-Aldrich), 1% DMSO (Sigma-Aldrich), 0.2 mM of each dNTP (Fermentas), and 1.25 U AmpliTaq Gold DNA polymerase (Applied Biosystems) in a total volume of 15 μl AmpliTaq Gold Buffer II supplemented with 1.5 mM MgCl2. Hgb reaction mix included 300 nM Hgb1 primer (5’-GCTTCTGACACAACTGTGTTCACTAGC-3’) and Hgb2 primer (5’-CACCAACTTCATCCACGTTCACC-3’) in a total volume of 15 μl of iQ SyBrGreen supermix (BioRad). The cycling conditions for telomere amplification were: 10 min at 95°C followed by 25 cycles at 95°C for 15 s and 54°C for 2 min with signal acquisition. The cycling conditions for Hgb amplification were: 95°C for 10 min followed by 35 cycles at 95°C for 15 s, 58°C for 20 s, 72°C for 20 s with signal acquisition. Reactions were performed with CFX384 Real-Time PCR Detection System (Bio-Rad). Melt-curve analysis was carried out at the end of the run to ensure specific primer binding.
Bio-Rad CFX Manager software was used to perform quality control, and samples with SD >0.5 between triplicates were omitted from the analysis. Plate effect was taken into account by analyzing five genomic DNA control samples on every plate. The telomere and Hgb signal values were normalized separately to the mean of these control samples before taking the T/S ratio (the relative LTL). The control samples were used for calculating the coefficient of variation (CV) value that was 8.35% for the T/S.
Statistical analyses
Zero-skewness log-transformations were applied to skewed NTB components, and z-scores for cognitive tests were standardized to the baseline mean and SD. NTB total score and domain scores for memory, processing speed, executive functioning and abbreviated memory were calculated by averaging individual NTB component z-scores as previously described [18].
Comparisons between FINGER participants with and without available LTL data, and between intervention and control groups in the LTL population were performed using chi-square and t-tests as appropriate. Linear or binary logistic regression was used to investigate associations of various baseline population characteristics with LTL (continuous zero-skewness log-transformed variable), adjusted for age. These analyses were also conducted with LTL categorized into tertiles, and age-adjusted means (standard errors) or age-adjusted proportions (standard errors) for various population characteristics are shown for each LTL tertile group.
Mixed effects regression models with maximum likelihood estimation were used to analyze change in cognitive scores as a function of randomization group, time, baseline LTL, and group × time × LTL interaction. LTL was included in the analyses as a continuous variable (zero-skewness log-transformed). Model 1 was adjusted for age and sex (added as covariates). Model 2 was additionally adjusted for baseline population characteristics showing significant associations with LTL. Model 3 was similar to model 2, but using age-and sex-adjusted LTL values (i.e., recalculated as residuals from linear regression with baseline LTL as dependent variable, and age and sex as independent variables). Analyses were also conducted with the original baseline LTL variable categorized into tertiles, with the highest tertile as reference. Level of significance was <5% in all analyses, and Stata software version 13 (Stata Statistical Software: Release 13. College Station, TX: StataCorp LP) was used.
RESULTS
Population characteristics
The 775 FINGER participants included in the present study had a significantly higher level of education, lower systolic blood pressure, and higher baseline total NTB, memory, processing speed, and executive functioning scores than the remaining 485 participants without LTL data (Table 1). Differences in all cognitive test scores were significant at the 1-year visit. At the 2-year visit, participants with baseline LTL data had significantly higher executive functioning score, with no other cognitive differences compared with participants without LTL data (Table 1).
Table 1
Characteristics of the FINGER participants with and without baseline TL measurements available
| Characteristics at baseline | Total | LTL available | LTL not available | p |
| n | n = 775 | n = 485 | ||
| Demographic characteristics | ||||
| Age at the baseline visit (y) | 1260 | 69.2±4.7 | 69.4±4.6 | 0.459 |
| Sex (women, %) | 1260 | 363 (46.8) | 225 (46.4) | 0.877 |
| Education (y) | 1258 | 10.1±(3.4) | 9.7±(3.5) | 0.022 |
| Vascular factors | ||||
| Systolic blood pressure (mmHg) | 1249 | 139.0±16.2 | 141.8±15.9 | 0.003 |
| Diastolic blood pressure (mmHg) | 1249 | 80.3±9.5 | 80.5±9.5 | 0.704 |
| Fasting plasma glucose (mmol/l) | 1257 | 6.1±0.9 | 6.1±0.9 | 0.706 |
| Body mass index (kg/m2) | 1249 | 28.1±4.8 | 28.3±4.6 | 0.417 |
| History of hypertension | 1246 | 492 (64.2) | 329 (68.5) | 0.118 |
| History of diabetes | 1253 | 104 (13.5) | 61 (12.7) | 0.688 |
| Lifestyle factors | ||||
| Physical activity 2 or more times/week (%) | 1247 | 553 (71.9) | 330 (69.0) | 0.278 |
| Current smokers (%) | 1255 | 70 (9.1) | 44 (9.1) | 0.965 |
| Alcohol drinking at least once/week (%) | 1252 | 350 (45.5) | 206 (42.7) | 0.347 |
| Fish intake at least twice/week (%) | 1253 | 401 (52.0) | 255 (52.9) | 0.758 |
| Daily intake of vegetables (%) | 1257 | 490 (63.4) | 286 (59.1) | 0.127 |
| Baseline Cognition* | ||||
| NTB total score | 1259 | 0.04±0.6 | –0.08±0.6 | <0.001 |
| Executive functioning | 1258 | 0.05±0.7 | –0.11±0.7 | <0.001 |
| Processing speed | 1259 | 0.04±0.8 | –0.07±0.8 | 0.016 |
| Memory | 1259 | 0.03±0.6 | –0.05±0.7 | 0.024 |
| Abbreviated memory | 1237 | 0.02±0.7 | –0.04±0.8 | 0.153 |
| 1-year Follow-up Cognition* | ||||
| NTB total score | 1166 | 0.18±0.6 | 0.01±0.6 | <0.001 |
| Executive functioning | 1161 | 0.11±0.7 | –0.05±0.07 | <0.001 |
| Processing speed | 1166 | 0.13±0.9 | –0.05±0.8 | <0.001 |
| Memory | 1167 | 0.26±0.8 | 0.10±0.8 | 0.001 |
| Abbreviated memory | 1127 | 0.15±0.78 | –0.02±0.8 | <0.001 |
| 2-year Follow-up Cognition* | ||||
| NTB total score | 1120 | 0.23±0.7 | 0.16±0.7 | 0.079 |
| Executive functioning | 1115 | 0.13±0.7 | 0.03±0.7 | 0.024 |
| Processing speed | 1118 | 0.13±0.9 | 0.05±0.9 | 0.182 |
| Memory | 1121 | 0.37±0.8 | 0.32±0.8 | 0.368 |
| Abbreviated memory | 1093 | 0.25±0.8 | 0.19±0.9 | 0.253 |
Values are means±SD unless otherwise specified. Differences between groups with and without available LTL data were analyzed with chi-square and t-tests as appropriate. *Scores on the NTB total score, executive functioning, processing speed, memory, and abbreviated memory are mean values of z scores of the cognitive tests included in each cognitive outcome. Higher scores indicate better performance.
Characteristics of the intervention (n = 383) versus control (n = 392) groups among FINGER participants with available baseline LTL data are shown in Table 2. The intervention group tended to have lower baseline NTB total score (p = 0.053), memory score (p = 0.051), and abbreviated memory score (p = 0.087) compared with the control group.
Table 2
Baseline characteristics of the FINGER participants with baseline TL measurements available
| Characteristics at baseline | Total | Intervention | Control | p |
| n | n = 383 | n = 392 | ||
| Demographic characteristics | ||||
| Age at the baseline visit (y) | 775 | 69.5±4.6 | 69.0±4.8 | 0.187 |
| Sex (women, %) | 775 | 167 (43.6) | 196 (50.5) | 0.074 |
| Education (y) | 774 | 10.1±(3.4) | 10.2±(3.4) | 0.472 |
| Baseline LTL | 775 | 1.06±0.3 | 1.06±0.3 | 0.501 |
| Vascular factors | ||||
| Systolic blood pressure (mmHg) | 768 | 140.1±17.2 | 140.5±16.4 | 0.603 |
| Diastolic blood pressure (mmHg) | 768 | 80.7±10.1 | 81.2±9.7 | 0.492 |
| Fasting plasma glucose (mmol/l) | 775 | 6.1±0.9 | 6.1±0.9 | 0.445 |
| Body mass index (kg/m2) | 766 | 28.4±4.7 | 27.8±4.8 | 0.141 |
| History of hypertension | 766 | 255 (67.3) | 237 (61.2) | 0.081 |
| History of diabetes | 772 | 56 (14.7) | 48 (12.3) | |
| Lifestyle factors | ||||
| Physical activity 2 or more times/week (%) | 769 | 275 (72.2) | 278 (71.7) | 0.870 |
| Current smokers (%) | 773 | 40 (10.5) | 30 (7.7) | 0.175 |
| Alcohol drinking at least once/week (%) | 770 | 179 (46.9) | 171 (44.1) | 0.438 |
| Fish intake at least twice/week (%) | 771 | 207 (54.2) | 194 (49.9) | 0.230 |
| Daily intake of vegetables (%) | 773 | 241 (63.1) | 249 (63.7) | 0.864 |
| Baseline Cognition* | ||||
| NTB total score | 774 | 0.01±0.6 | 0.08±0.6 | 0.053 |
| Executive functioning | 774 | 0.02±0.7 | 0.08±0.7 | 0.214 |
| Processing speed | 774 | –0.01±0.8 | 0.09±0.8 | 0.126 |
| Memory | 774 | –0.01±0.7 | 0.07±0.6 | 0.051 |
| Abbreviated memory | 762 | –0.02±0.8 | 0.07±0.7 | 0.087 |
| 1-year Follow-up Cognition* | ||||
| NTB total score | 768 | 0.15±0.6 | 0.21±0.7 | 0.181 |
| Executive functioning | 764 | 0.09±0.7 | 0.14±0.7 | 0.318 |
| Processing speed | 768 | 0.12±0.8 | 0.15±0.9 | 0.470 |
| Memory | 769 | 0.21±0.8 | 0.30±0.7 | 0.113 |
| Abbreviated memory | 743 | 0.13±0.78 | 0.17±0.8 | 0.463 |
| 2-year Follow-up Cognition* | ||||
| NTB total score | 760 | 0.22±0.7 | 0.25±0.7 | 0.521 |
| Executive functioning | 757 | 0.13±0.7 | 0.14±0.7 | 0.871 |
| Processing speed | 759 | 0.13±0.9 | 0.13±0.9 | 0.969 |
| Memory | 761 | 0.34±0.8 | 0.40±0.8 | 0.280 |
| Abbreviated memory | 743 | 0.24±0.8 | 0.25±0.8 | 0.839 |
Values are means±SD unless otherwise specified. Differences between intervention and control groups were analyzed with chi-square and t-tests as appropriate. *Scores on the NTB total score, executive functioning, processing speed, memory, and abbreviated memory are mean values of z scores of the cognitive tests included in each cognitive outcome. Higher scores indicate better performance.
Age-adjusted associations between various population characteristics and LTL are shown in Table 3. As expected, shorter LTL was related to older age and less healthy lifestyle indicated by the total number of healthy lifestyle factors (physically active, non-smoker, lower alcohol consumption, higher intake of fish and vegetables). There were no significant associations with cognition or other baseline characteristics.
Table 3
Population characteristics by LTL tertile groups
| Characteristics | Short LTL | Middle LTL | Long LTL | p* |
| tertile | tertile | tertile | ||
| (n = 273) | (n = 251) | (n = 251) | ||
| Demographic characteristics | ||||
| Age at baseline (y) | 69.9 (0.3) | 69.1 (0.3) | 68.5 (0.3) | 0.002 |
| Women, N (%) | 116 (42.5) | 115 (45.8) | 132 (52.6) | 0.064 |
| Education (y) | 10.0 (0.2) | 10.3 (0.2) | 10.1 (0.2) | 0.883 |
| Baseline vascular factors | ||||
| Systolic blood pressure (mmHg) | 138.1 (1.0) | 139.5 (1.0) | 139.5 (1.0) | 0.220 |
| Diastolic blood pressure (mmHg) | 79.5 (0.6) | 80.7 (0.6) | 80.6 (0.6) | 0.116 |
| Fasting plasma glucose (mmol/l) | 6.1 (0.1) | 6.1 (0.1) | 6.1 (0.1) | 0.212 |
| BMI (kg/m2) | 27.8 (0.3) | 28.2 (0.3) | 28.3 (0.3) | 0.377 |
| History of hypertension, % (SE) | 65.8 (2.9) | 61.1 (3.1) | 65.6 (3.0) | 0.839 |
| History of diabetes, % (SE) | 14.3 (2.1) | 14.0 (2.2) | 12.1 (2.1) | 0.430 |
| History of myocardial infarction, % (SE) | 5.9 (1.4) | 5.6 (1.5) | 5.1 (1.4) | 0.326 |
| History of stroke, % (SE) | 5.7 (1.4) | 4.4 (1.3) | 5.0 (1.4) | 0.655 |
| Baseline TL | 0.8 (0.01) | 1.0 (0.01) | 1.4 (0.01) | <0.001 |
| Baseline lifestyle factors | ||||
| Physical activity at least twice/week, % (SE) | 67.0 (2.9) | 76.0 (2.7) | 73.1 (2.8) | 0.087 |
| Current smokers, % (SE) | 8.9 (1.8) | 9.8 (1.8) | 8.5 (1.7) | 0.685 |
| Alcohol drinking at least once/week, % (SE) | 47.9 (3.0) | 48.7 (3.1) | 39.7 (3.1) | 0.027 |
| Fish intake at least twice/week, % (SE) | 52.5 (3.0) | 52.0 (3.1) | 51.5 (3.2) | 0.442 |
| Daily intake of vegetables, % (SE) | 62.9 (2.9) | 58.8 (3.1) | 68.5 (2.9) | 0.082 |
| At least 4 healthy lifestyle factors, % (SE) | 41.2 (2.9) | 46.5 (3.1) | 57.2 (3.1) | <0.001 |
| Baseline cognition | ||||
| NTB total score | 0.04 (0.03) | 0.05 (0.03) | 0.03 (0.03) | 0.377 |
| Executive functioning | 0.05 (0.04) | 0.05 (0.04) | 0.05 (0.04) | 0.552 |
| Processing speed | 0.03 (0.05) | 0.07 (0.05) | 0.02 (0.05) | 0.740 |
| Memory | 0.05 (0.04) | 0.04 (0.04) | 0.02 (0.04) | 0.312 |
| Abbreviated memory | 0.04 (0.04) | 0.03 (0.05) | 0.01 (0.05) | 0.258 |
| 1-year Follow-up Cognition | ||||
| NTB total score | 0.18 (0.04) | 0.19 (0.04) | 0.17 (0.04) | 0.452 |
| Executive functioning | 0.10 (0.04) | 0.15 (0.04) | 0.09 (0.04) | 0.335 |
| Processing speed | 0.12 (0.05) | 0.12 (0.05) | 0.15 (0.05) | 0.924 |
| Memory | 0.27 (0.05) | 0.25 (0.05) | 0.26 (0.05) | 0.455 |
| Abbreviated memory | 0.17 (0.05) | 0.11 (0.05) | 0.17 (0.05) | 0.455 |
| 2-year Follow-up Cognition | ||||
| NTB total score | 0.23 (0.04) | 0.23 (0.04) | 0.24 (0.04) | 0.653 |
| Executive functioning | 0.11 (0.04) | 0.15 (0.04) | 0.15 (0.04) | 0.994 |
| Processing speed | 0.13 (0.05) | 0.10 (0.05) | 0.15 (0.05) | 0.808 |
| Memory | 0.39 (0.05) | 0.36 (0.05) | 0.36 (0.05) | 0.414 |
| Abbreviated memory | 0.26 (0.05) | 0.23 (0.05) | 0.25 (0.05) | 0.380 |
Values are age-adjusted means (standard errors, SE) from linear regressions with population characteristics as dependent variables and baseline LTL tertiles and age as independent variables. % (SE) are age-adjusted proportions and standard errors from binary logistic regressions with population characteristics as dependent variables and baseline LTL tertiles and age as independent variables. *p-values are shown for the abovementioned models with baseline LTL as continuous variable (zero-skewness log-transformed).
Baseline LTL and intervention effects on cognition
Table 4 summarizes the impact of baseline LTL on primary and secondary cognitive end points from baseline to 24 months (adjusted for age and sex). The difference between intervention and control groups per year (intervention×time interaction) was significant among individuals in the shortest LTL tertile (but not the other tertiles) for all cognitive domains except memory. Overall p-value for the intervention×time×LTL interaction (LTL as continuous variable) was significant for executive functioning (p = 0.010), indicating more improvement with shorter baseline LTL. A similar trend was found for NTB total score (p = 0.101). Findings were very similar after additionally adjusting for healthy lifestyle at baseline (Table 4, Model 2), and when using recalculated (age- and sex-adjusted) LTL values (Table 4, Model 3).
Table 4
Impact of baseline LTL on primary and secondary cognitive end points from baseline to 24 months
| Cognitive | Baseline LTL | Difference between intervention | ||||
| end point | (tertiles) | and control groups per year | p for interaction* | |||
| Estimate (95% CI) | p | Model 1 | Model 2 | Model 3 | ||
| Primary | 0.101 | 0.080 | 0.077 | |||
| Long | –0.00 (–0.04–0.04) | 0.994 | ||||
| NTB total score | Middle | –0.00 (–0.05–0.04) | 0.817 | |||
| Short | 0.06 (0.02–0.10) | 0.006 | ||||
| Secondary | 0.470 | 0.455 | 0.411 | |||
| Long | –0.00 (–0.07–0.07) | 0.901 | ||||
| Memory | Middle | –0.03 (–0.10–0.04) | 0.383 | |||
| Short | 0.05 (–0.01–0.12) | 0.112 | ||||
| Long | 0.04 (–0.02–0.10) | 0.154 | 0.669 | 0.572 | 0.391 | |
| Processing speed | Middle | 0.01 (–0.05–0.07) | 0.754 | |||
| Short | 0.06 (0.00–0.11) | 0.042 | ||||
| Long | –0.02 (–0.07–0.04) | 0.541 | 0.010 | 0.007 | 0.014 | |
| Executive functioning | Middle | 0.02 (–0.03–0.07) | 0.492 | |||
| Short | 0.07 (0.02–0.12) | 0.006 | ||||
| Long | –0.01 (–0.08–0.06) | 0.774 | 0.268 | 0.274 | 0.212 | |
| Abbreviated Memory | Middle | 0.01 (–0.06–0.07) | 0.712 | |||
| Short | 0.08 (0.01–0.15) | 0.025 | ||||
Mixed-model repeated-measures analyses of change in cognitive scores from baseline to 24 months as a function of randomization group, time, and group×time interaction. Difference between intervention and control groups per year is adjusted for age and sex. A positive value of the estimate indicates the effect is in favor of the intervention group. *The overall p-value is shown for the group×time×LTL interaction, where baseline LTL was used as continuous variable. Model 1 is adjusted for age and sex, with zero-skewness log-transformed LTL. Model 2 is additionally adjusted for number of healthy lifestyle factors at baseline (physically active, non-smoker, lower alcohol consumption, higher intake of fish and vegetables, categorized as <4 versus ≥4 healthy lifestyle factors). Model 3 is adjusted for number of healthy lifestyle factors at baseline, and LTL is continuous with recalculated values (age- and sex-adjusted).
DISCUSSION
This study is the first to assess whether baseline LTL modified the effects of a multidomain lifestyle intervention on cognition among older adults who are at risk for cognitive decline. Results showed that the beneficial intervention effects on cognition [18] were more pronounced with shorter baseline LTL, particularly for executive functioning. The impact of shorter baseline LTL on intervention effects on other cognitive domains was less pronounced in the present study, with some trends observed for NTB total score.
Lifestyle factors such as unhealthy dietary habits, physical inactivity, or smoking have been related to shorter LTL [1], and they have also been related to increased risk of cognitive decline and dementia [33]. In the present study, shorter LTL was indeed associated with less healthy lifestyle at baseline, suggesting that FINGER participants with shorter LTL may have had more ‘room for improvement’ at the start of the lifestyle intervention. However, this did not seem to fully explain the findings, which were still present after adjustment for baseline lifestyle factors. While shorter LTL has been suggested to represent a proxy for elevated risk due to, for example, exposure to unhealthy lifestyle [2], it may also be a direct risk predictor via genetic pathways. Previous studies have suggested direct links between genetic determinants of telomere length and AD [37, 38] or cognition [34]. Such genetic pathways and their associated vulnerabilities may be independent of, or interactive with, lifestyle factors. The multidomain FINGER intervention targeted simultaneously multiple lifestyle-related, vascular, and metabolic risk factors, thus potentially mitigating several of these pathways. However, the present study cannot pinpoint the exact mechanisms behind the increased cognitive benefits among participants with shorter baseline LTL.
Interestingly, LTL was not associated with cognition, vascular factors, or history of cardio/cerebrovascular conditions at baseline in this study. A key reason may be the FINGER target population, and the trial context. The intervention was targeted towards at-risk individuals who were most likely to need it, and thus FINGER participants do not reflect the entire risk continuum (from low to high) observed in an unselected general population. In addition, individuals with dementia, substantial cognitive impairment, or serious health conditions affecting safe engagement in the intervention were excluded. This may have limited the ability to identify associations of LTL with cognition and other baseline population characteristics. However, this lack of associations also suggests that the significance of LTL is more complex than a mere proxy for exposure to risk factors promoting disease. Relations between LTL and various diseases seem to be bidirectional [2]. Telomere dysfunctions may be promoters of disease (in a highly interactive manner with other health-related factors), but they may also result from ongoing disease processes [2]. Potential activation of LTL-lengthening mechanisms has been hypothesized to be triggered by decline of LTL below a critical threshold [16].
While the present study found that individuals with shorter LTL had more intervention benefits on executive functioning, we cannot fully exclude that such benefits may have been present in other cognitive domains as well. The study was not powered to detect intervention effects by baseline LTL, and some of the 3-way interactions may have failed to reach significance due to limited statistical power. It is not yet clear if LTL-cognition associations are domain-specific. Previous observational studies have reported different findings, and conclusions are difficult to reach due to variability in cognitive tests, populations, and study designs (cognition often assessed only once, without assessment of change over time) [5, 7–16].
The strengths of the present study include the large sample of older adults at risk for cognitive impairment, the multidomain intervention with a long duration, and comprehensive cognitive assessment including multiple cognitive domains (NTB, previously used in AD clinical trials), and carefully controlled LTL measurement. However, the FINGER LTL subpopulation had better baseline cognition compared to the rest of the FINGER participants, thus limiting the generalizability of the results (i.e., whether the potential for improvement with shorter baseline LTL would still be present at somewhat lower cognition levels). Also, the FINGER trial included a 2-year intervention in an at-risk general population aged 60–77 years, and without substantial impairment at baseline [18]. We do not know if there is a time-, age-, and/or stage-limited window of opportunity for more intervention benefits with shorter baseline LTL, i.e., if this effect persists beyond two years, and if it is also present in individuals aged above 80 years and/or with clinically manifest cognitive impairment at baseline.
In addition, it is unclear which FINGER participants will develop dementia in the future, and whether baseline LTL impacts potential intervention effects on the incidence of dementia and AD in this cohort. While executive dysfunction is usually considered more typical for vascular types of cognitive impairment, a substantial decline in executive functioning has also been reported in preclinical AD [35]. The extended 7-year FINGER follow-up will facilitate assessments of dementia incidence.
The present study did not include longitudinal LTL assessments or measures of telomerase enzyme activity. Telomerase activity plays an important role in LTL maintenance, and may increase as a compensatory mechanism in response to shortened LTL [36, 37]. Future longitudinal LTL assessments will allow investigations of intervention effects on LTL, and how they relate to changes in cognition.
In conclusion, baseline LTL modified the effects of a 2-year multidomain lifestyle intervention on changes in cognitive performance. The cognitive benefits of the FINGER intervention were more pronounced in people with shorter baseline LTL, particularly for executive functioning. Considering that short LTL has been associated with poor cognitive performance and dementia, it is very promising that the multidomain lifestyle intervention was especially beneficial among individuals with higher risk.
ACKNOWLEDGMENTS
The authors would like to thank the entire FINGER study group for all their valuable contributions to the design and implementation of the FINGER trial. The authors would also like to thank the FINGER participants for their time and efforts. Jenni Lahtinen is thanked for her help in telomere length measurement.
Shireen Sindi receives postdoctoral funding from the Fonds de la recherche en santé du Québec (FRSQ) (27139), including its renewal (31819). A. Solomon receives research funding from the Academy of Finland (287490, 294061) and ALF grants 20130507, 20150589. Miia Kivipelto receives research support from the Alzheimer’s Research & Prevention Foundation, Academy of Finland (SALVE and 278457), the Swedish Research Council for Joint Program of Neurodegenerative Disorders– prevention (MIND-AD), Alzheimerfonden (Sweden), Center for Innovative Medicine (CIMED) at Karolinska Institutet South Campus, AXA Research Fund, Knut and Alice Wallenberg Foundation (Sweden), Stiftelsen Stockholms sjukhem (Sweden), Konung Gustaf V:s och Drottning Victorias Frimurarstiftelse (Sweden). H. Soininen receives funding from EU 7th framework collaborative project grant (HATICE), Academy of Finland for Joint Program of Neurodegenerative Disorders– prevention (MIND-AD), UEF Strategic funding for UEFBRAIN (Finland), and EVO/VTR funding from Kuopio University Hospital (Finland). Riitta Antikainen EVO grants of Oulu University Hospital and Oulu City Hospital (Finland).
The funding sources had no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Authors’ disclosures available online (http://j-alz.com/manuscript-disclosures/17-0123r1).
REFERENCES
[1] | Eitan E , Hutchison ER , Mattson MP ((2014) ) Telomere shortening in neurological disorders: An abundance of unanswered questions. Trends Neurosci 37: , 256–263. |
[2] | Blackburn EH , Epel ES , Lin J ((2015) ) Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science 350: , 1193–1198. |
[3] | Forero DA , Gonzalez-Giraldo Y , Lopez-Quintero C , Castro-Vega LJ , Barreto GE , Perry G ((2016) ) Meta-analysis of telomere length in Alzheimer’s disease. J Gerontol A Biol Sci Med Sci 71: , 1069–1073. |
[4] | Moverare-Skrtic S , Johansson P , Mattsson N , Hansson O , Wallin A , Johansson JO , Zetterberg H , Blennow K , Svensson J ((2012) ) Leukocyte telomere length (LTL) is reduced in stable mild cognitive impairment but low LTL is not associated with conversion to Alzheimer’s disease: A pilot study. Exp Gerontol 47: , 179–182. |
[5] | Roberts RO , Boardman LA , Cha RH , Pankratz VS , Johnson RA , Druliner BR , Christianson TJ , Roberts LR , Petersen RC ((2014) ) Short and long telomeres increase risk of amnestic mild cognitive impairment. Mech Ageing Dev 141-142: , 64–69. |
[6] | Zekry D , Herrmann FR , Irminger-Finger I , Ortolan L , Genet C , Vitale AM , Michel JP , Gold G , Krause KH ((2010) ) Telomere length is not predictive of dementia or MCI conversion in the oldest old. Neurobiol Aging 31: , 719–720. |
[7] | Der G , Batty GD , Benzeval M , Deary IJ , Green MJ , McGlynn L , McIntyre A , Robertson T , Shiels PG ((2012) ) Is telomere length a biomarker for aging: Cross-sectional evidence from the west of Scotland? PLoS One 7: , e45166. |
[8] | Ma SL , Lau ES , Suen EW , Lam LC , Leung PC , Woo J , Tang NL ((2013) ) Telomere length and cognitive function in southern Chinese community-dwelling male elders. Age Ageing 42: , 450–455. |
[9] | Valdes AM , Deary IJ , Gardner J , Kimura M , Lu X , Spector TD , Aviv A , Cherkas LF ((2010) ) Leukocyte telomere length is associated with cognitive performance in healthy women. Neurobiol Aging 31: , 986–992. |
[10] | Yaffe K , Lindquist K , Kluse M , Cawthon R , Harris T , Hsueh WC , Simonsick EM , Kuller L , Li R , Ayonayon HN , Rubin SM , Cummings SR , Health ABCS ((2011) ) Telomere length and cognitive function in community-dwelling elders: Findings from the Health ABC Study. Neurobiol Aging 32: , 2055–2060. |
[11] | Cohen-Manheim I , Doniger GM , Sinnreich R , Simon ES , Pinchas R , Aviv A , Kark JD ((2015) ) Increased attrition of leukocyte telomere length in young adults is associated with poorer cognitive function in midlife. Eur J Epidemiol 31: , 147–157. |
[12] | Devore EE , Prescott J , De Vivo I , Grodstein F ((2011) ) Relative telomere length and cognitive decline in the Nurses’ Health Study. Neurosci Lett 492: , 15–18. |
[13] | Harris SE , Deary IJ , MacIntyre A , Lamb KJ , Radhakrishnan K , Starr JM , Whalley LJ , Shiels PG ((2006) ) The association between telomere length, physical health, cognitive ageing, and mortality in non-demented older people. Neurosci Lett 406: , 260–264. |
[14] | Martin-Ruiz C , Dickinson HO , Keys B , Rowan E , Kenny RA , Von Zglinicki T ((2006) ) Telomere length predicts poststroke mortality, dementia, and cognitive decline. Ann Neurol 60: , 174–180. |
[15] | Mather KA , Jorm AF , Anstey KJ , Milburn PJ , Easteal S , Christensen H ((2010) ) Cognitive performance and leukocyte telomere length in two narrow age-range cohorts: A population study. BMC Geriatr 10: , 62. |
[16] | Hochstrasser T , Marksteiner J , Humpel C ((2012) ) Telomere length is age-dependent and reduced in monocytes of Alzheimer patients. Exp Gerontol 47: , 160–163. |
[17] | Winblad B , Amouyel P , Andrieu S , Ballard C , Brayne C , Brodaty H , Cedazo-Minguez A , Dubois B , Edvardsson D , Feldman H , Fratiglioni L , Frisoni GB , Gauthier S , Georges J , Graff C , Iqbal K , Jessen F , Johansson G , Jonsson L , Kivipelto M , Knapp M , Mangialasche F , Melis R , Nordberg A , Rikkert MO , Qiu C , Sakmar TP , Scheltens P , Schneider LS , Sperling R , Tjernberg LO , Waldemar G , Wimo A , Zetterberg H ((2016) ) Defeating Alzheimer’s disease and other dementias: A priority for European science and society. Lancet Neurol 15: , 455–532. |
[18] | Ngandu T , Lehtisalo J , Solomon A , Levalahti E , Ahtiluoto S , Antikainen R , Backman L , Hanninen T , Jula A , Laatikainen T , Lindstrom J , Mangialasche F , Paajanen T , Pajala S , Peltonen M , Rauramaa R , Stigsdotter-Neely A , Strandberg T , Tuomilehto J , Soininen H , Kivipelto M ((2015) ) A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. Lancet 385: , 2255–2263. |
[19] | Ngandu T , Lehtisalo J , Levalahti E , Laatikainen T , Lindstrom J , Peltonen M , Solomon A , Ahtiluoto S , Antikainen R , Hanninen T , Jula A , Mangialasche F , Paajanen T , Pajala S , Rauramaa R , Strandberg T , Tuomilehto J , Soininen H , Kivipelto M ((2014) ) Recruitment and baseline characteristics of participants in the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER)-a randomized controlled lifestyle trial. Int J Environ Res Public Health 11: , 9345–9360. |
[20] | Kivipelto M , Solomon A , Ahtiluoto S , Ngandu T , Lehtisalo J , Antikainen R , Backman L , Hanninen T , Jula A , Laatikainen T , Lindstrom J , Mangialasche F , Nissinen A , Paajanen T , Pajala S , Peltonen M , Rauramaa R , Stigsdotter-Neely A , Strandberg T , Tuomilehto J , Soininen H ((2013) ) The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER): Study design and progress. Alzheimers Dement 9: , 657–665. |
[21] | Saaristo T , Peltonen M , Keinanen-Kiukaanniemi S , Vanhala M , Saltevo J , Niskanen L , Oksa H , Korpi-Hyovalti E , Tuomilehto J , Group F-DDS ((2007) ) National type 2 diabetes prevention programme in Finland: FIN-D2D. Int J Circumpolar Health 66: , 101–112. |
[22] | Morris JC , Heyman A , Mohs RC , Hughes JP , van Belle G , Fillenbaum G , Mellits ED , Clark C ((1989) ) The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology 39: , 1159–1165. |
[23] | National Nutrition Council ((2005) ) Finnish nutrition recommendations: Diet and physical activity in balance. |
[24] | Nelson ME , Rejeski WJ , Blair SN , Duncan PW , Judge JO , King AC , Macera CA , Castaneda-Sceppa C , American College of Sports Medicine, American Heart Association ((2007) ) Physical activity and public health in older adults: Recommendation from the American College of Sports Medicine and the American Heart Association. Circulation 116: , 1094–1105. |
[25] | Dahlin E , Neely AS , Larsson A , Backman L , Nyberg L ((2008) ) Transfer of learning after updating training mediated by the striatum. Science 320: , 1510–1512. |
[26] | Working group appointed by the Finnish Medical Society Duodecim and the Finnish Hypertension Society ((2009) ) Hypertension: Current care summary. |
[27] | Working group appointed by the Finnish Medical Society Duodecim and the Medical Advisory Board of the Finnish Diabetes Society ((2007) ) Diabetes: Current care summary. |
[28] | Working group appointed by the Finnish Medical Society Duodecim and the Finnish Society of Internal Medicine ((2009) ) Dyslipidaemias: Current care summary. |
[29] | Harrison J , Minassian SL , Jenkins L , Black RS , Koller M , Grundman M ((2007) ) A neuropsychological test battery for use in Alzheimer disease clinical trials. Arch Neurol 64: , 1323–1329. |
[30] | Cawthon RM ((2002) ) Telomere measurement by quantitative PCR. Nucleic Acids Res 30: , e47. |
[31] | Kananen L , Surakka I , Pirkola S , Suvisaari J , Lonnqvist J , Peltonen L , Ripatti S , Hovatta I ((2010) ) Childhood adversities are associated with shorter telomere length at adult age both in individuals with an anxiety disorder and controls. PLoS One 5: , e10826. |
[32] | Kao HT , Cawthon RM , Delisi LE , Bertisch HC , Ji F , Gordon D , Li P , Benedict MM , Greenberg WM , Porton B ((2008) ) Rapid telomere erosion in schizophrenia. Mol Psychiatry 13: , 118–119. |
[33] | Sindi S , Mangialasche F , Kivipelto M ((2015) ) Advances in the prevention of Alzheimer’s Disease. F1000Prime Rep 7: , 50. |
[34] | Hagg S , Zhan Y , Karlsson R , Gerritsen L , Ploner A , van der Lee SJ , Broer L , Deelen J , Marioni RE , Wong A , Lundquist A , Zhu G , Hansell NK , Sillanpaa E , Fedko IO , Amin NA , Beekman M , de Craen AJM , Degerman S , Harris SE , Kan KJ , Martin-Ruiz CM , Montgomery GW , Neuro CCWG , Adolfsson AN , Reynolds CA , Samani NJ , Suchiman HED , Viljanen A , von Zglinicki T , Wright MJ , Hottenga JJ , Boomsma DI , Rantanen T , Kaprio JA , Nyholt DR , Martin NG , Nyberg L , Adolfsson R , Kuh D , Starr JM , Deary IJ , Slagboom PE , van Duijn CM , Codd V , Pedersen NL ((2017) ) Short telomere length is associated with impaired cognitive performance in European ancestry cohorts. Transl Psychiatry 7: , e1100. |
[35] | Lim YY , Snyder PJ , Pietrzak RH , Ukiqi A , Villemagne VL , Ames D , Salvado O , Bourgeat P , Martins RN , Masters CL , Rowe CC , Maruff P ((2016) ) Sensitivity of composite scores to amyloid burden in preclinical Alzheimer’s disease: Introducing the Z-scores of Attention, Verbal fluency, and Episodic memory for Nondemented older adults composite score. Alzheimers Dement (Amst) 2: , 19–26. |
[36] | Chan SR , Blackburn EH ((2004) ) Telomeres and telomerase. Philos Trans R Soc Lond B Biol Sci 359: , 109–121. |
[37] | Lin J , Epel E , Blackburn E ((2012) ) Telomeres and lifestyle factors: Roles in cellular aging. Mutat Res 730: , 85–89. |




