Diagnosing Dementia in the Clinical Setting: Can Amyloid PET Provide Additional Value Over Cerebrospinal Fluid?
Abstract
Cerebrospinal fluid (CSF) measures of amyloid and tau are the first-line Alzheimer’s disease biomarkers in many clinical centers. We assessed if and when the addition of amyloid PET following CSF measurements provides added diagnostic value. Twenty patients from a cognitive clinic, who had undergone detailed assessment including CSF measures, went on to have amyloid PET. The treating neurologist’s working diagnosis, and degree of diagnostic certainty, was assessed both before and after the PET. Amyloid PET changed the diagnosis in 7/20 cases. Amyloid PET can provide added diagnostic value, particularly in young-onset, atypical dementias, where CSF results are borderline and diagnostic uncertainty remains.
INTRODUCTION
Differentiating the underlying pathological causes of dementia is important but often challenging. Amyloid PET can identify fibrillar amyloid-β (Aβ) deposition, a core aspect of Alzheimer’s disease (AD) pathology, in vivo [1]. However, cost, availability, and paucity of evidence that it changes management are factors that limit its clinical use. Proposed “appropriate use” criteria suggest that amyloid PET may be used in unexplained mild cognitive impairment, young-onset dementia, and atypical dementia syndromes [2, 3]. CSF measures of amyloid and tau provide an alternate means of substantiating a molecular diagnosis of AD [4], with broadly similar diagnostic performance to amyloid PET when either is used in isolation [5]. In many clinical centers, CSF measures remain the first line AD molecular biomarker.
While previous studies assessing the use of amyloid PET in the clinical setting have found it to provide added diagnostic value, patients included in these studies have often had incomplete prior investigative work-up, in some cases without MRI, neuropsychometry, and/or CSF [6–9]. Few studies have assessed the utility of amyloid PET in circumstances where it may prove most useful, i.e., in cases fulfilling good use criteria in whom a CSF has been performed and an equivocal/uncertain result is obtained and there remains clinical equipoise as to whether the patient has AD pathology or not. In this study, we aimed to determine in a real life clinical setting whether amyloid PET can provide added diagnostic value beyond CSF measurements alone; and in what specific clinical circumstances the addition of amyloid PET is likely to provide the most benefit.
MATERIALS AND METHODS
Twenty patients with a range of different dementia syndromes were recruited from a Specialist Cognitive Disorders Service (Supplementary Table 1). Ten had memory-led syndromes; the remainder had a range of other presentations including posterior cortical atrophy (n = 5), primary progressive aphasia (PPA) (n = 4), and behavioral change (with the patient becoming impulsive and socially disinhibited) (n = 1). As part of their routine clinical evaluation, each patient had been seen by an experienced cognitive neurologist, and been investigated with neuropsychological testing, MR brain imaging and lumbar puncture, with samples analyzed for Aβ42, total tau, and p-tau (INNOTEST ELISAs, Fujirebio, Ghent Belgium). At the time of testing, the normal clinical ranges for CSF measures in use were: Aβ42 >450 ng/L, tau:Aβ42 ratio <1, p-tau <68 ng/L. Tau and Aβ42 cut-points were those in use clinically at our center at the time of the study, and were based on reference ranges of healthy controls and ability to distinguish clinically diagnosed AD from FTD; the p-tau cut-point was that determined by the kit manufacturer.
As part of a research study, each patient subsequently had an F18 florbetapir PET scan on a Siemens 3T PET/MR unit, with a 50-min dynamic acquisition commencing immediately after intravenous injection of 370 MBq of florbetapir. A volumetric T1-weighted MRI scan was acquired concurrently. A single static PET image, reconstructed from the last 10 min of the PET acquisition, was used for analysis. Three nuclear medicine physicians, blinded to the diagnosis, visually rated the images as positive/negative according to standard criteria.
With the benefit of the psychology, MRI, and CSF results, but prior to the PET scan, the treating clinician was asked to give their diagnosis and degree of diagnostic certainty (on a percentage scale). The clinician was subsequently provided with the PET scan result, which with their consent was also shared with the patient, and asked again to give the diagnosis/diagnostic certainty. The study received ethical approval and all patients gave written informedconsent.
RESULTS
The patients had a mean±SD age of 65.5±7.6, and age of symptom onset of 59.2±6.2. CSF examination preceded scanning, with a median delay of 145 days (range 32–427). Across all subjects, CSF Aβ42 ranged between 343–1127 ng/L, tau/Aβ42 ratio 0.24–2.54, and p-tau 24–227 ng/L.
Prior to the amyloid PET, 13 patients had a diagnosis of AD, and seven of non-AD dementia. Pre-PET diagnostic certainty was 68±16%. Based on the visual PET results, 18 patients were amyloid positive and 2 patients amyloid negative, with no disagreement between the three readers for any scan. The amyloid PET result led to a change in diagnosis in 7/20 patients (5 non-amnestic, 2 amnestic) (Table 1). Six patients’ diagnoses changed from non-AD to AD; and 1 from AD to non-AD. The PET scan result led to an increase in diagnostic certainty in 18/20 patients (to a mean 84±14%). Management was changed in 8/20 patients: an acetylcholinesterase inhibitor was started/stopped (n = 4); memantine was started (n = 1); immune suppression was stopped (n = 1); and AD genetic testing was requested (n = 2).
Of the seven individuals in whom the PET led to a change in diagnosis, all had young-onset dementia (age-at-onset <65 y), and five had non-amnestic syndromes, particularly primary progressive aphasia (n = 4) (Table 1, Supplementary Table 1). Clinically, the PPA cases had each been felt to be most consistent with progressive non-fluent aphasia (PNFA), although three of the four cases did not fulfill one of the canonical PPA syndromes, having some features additionally consistent with a logopenic aphasia (e.g., anomia and word finding pauses). The seven cases in whom the PET scan altered the diagnosis had a rather lower pre-PET diagnostic certainty than the rest of the group (55% versus 76%). Also, the CSF results for these cases were generally close to the pre-determined CSF diagnostic cut-points, particularly for the tau:Aβ42 ratio (Table 1, Fig. 1).
DISCUSSION
Providing molecular evidence to support/refute a diagnosis of AD will become ever more important as we move toward an era of disease-modifying therapies. In many centers, CSF measurements remain the first-line molecular biomarker of choice. Here, we show that in certain situations, amyloid PET provides added diagnostic value above and beyond CSF measurements, leading not only to increased diagnostic certainty but also to changes in management.
We found that amyloid PET had most benefit in two particular scenarios, (1) in patients with young onset, atypical clinical syndromes; and (2) in those where there was diagnostic uncertainty despite otherwise comprehensive investigation. These findings are consistent with “appropriate use” criteria [2], but also extend on the previous guidelines by assessing the use of amyloid PET in patients who have already had CSF amyloid and tau measurements; which in most clinical centers is likely to be the situation where amyloid PET may most commonly be used.
Atypical (i.e., non-amnestic) syndromes may be underpinned by a variety of different underlying pathologies and this is particularly the case for PPA, where a tauopathy, TDP-43 proteinopathy, and AD are all possibilities. Although consensus clinical diagnostic criteria describe three major forms (PNFA, logopenic aphasia, and semantic dementia), in many cases patients do not easily fulfill any one criteria [10, 11]. Additionally, while there is good clinico-pathological correspondence in some of the PPA syndromes, notably semantic dementia and logopenic aphasia (TDP43 and AD, respectively), in nosyndrome is this perfect; and in non-fluent aphasia and undetermined aphasias ascribing a particular pathology is notoriously difficult [12, 13].
While previous studies have found relatively high concordance between CSF measures and amyloid PET [14, 15], atypical AD syndromes may have less clear-cut CSF profiles than would normally be seen in typical AD [16]. This subtle variability between the CSF profiles of different AD syndromes, combined with the high proportion of atypical cases in our cohort, may have contributed in some cases to diagnostic uncertainty and the discrepancy between the CSF and PET results. Cases where there was most diagnostic uncertainty were often those where the CSF levels were equivocal, lying close to or even outside diagnostic cut-points. No CSF cut-point is perfect, and it is possible that the CSF cut-points in clinical use at the time of this study were overly conservative. CSF values can vary substantially if not taken, handled, and stored appropriately; and establishing CSF cut-points is difficult, as evidenced by the wide differences in normal ranges used at different clinical centers [17], leading us and others to propose the adoption of diagnostic grey-zones, and to suggest that comparing CSF and amyloid PET results may be useful in refining CSF cut-point use [18]. This study reinforces that it is just such equivocal cases— with atypical phenotypes and borderline CSF results— that addition of an amyloid brain scan may be most useful, and that so doing can alter clinical management.
Given the known incidence of cerebral amyloid deposition within the asymptomatic aging population [19], it is always necessary to consider whether a positive amyloid PET scan reflects the primary cause of a patient’s symptoms, or if it is merely reflective of a second coincident pathology. This possibility was considered by the treating clinician in each case. However, particularly considering the patients’ relatively young ages of symptom onset, the possibility of dual pathology was felt to be unlikely, again in keeping with the inclusion of young-onset dementia as an “appropriate use” criterion for amyloid PET.
Our study has a number of limitations. There were in some cases significant delays between the CSF and PET scan. However, as Aβ load is thought to plateau prior to the onset of AD symptoms, this delay is unlikely to have had a significant effect on the relative diagnostic utility of the two tests [20], and also reflects what is likely to happen in real life clinical practice. And, without pathological confirmation, we cannot know whether any diagnostic or management changes following the amyloid PET were correct or not.
This study demonstrates that amyloid PET scanning has clinical utility is some cases in addition to CSF examination, and provides evidence to support its use particularly in young-onset, atypical dementias, where CSF results are borderline and diagnostic uncertainty remains.
ACKNOWLEDGMENTS
The authors acknowledge the support of the Wolfson Foundation, the NIHR Queen Square Dementia BRU, UCL/H Biomedical Research Centre, UCL ECMC, and Alzheimer’s Research UK. Florbertapir tracer was kindly provided by AVID Radiopharmaceuticals, a wholly owned subsidiary of Eli Lilly. PSJW is funded by an MRC Clinical Research Training Fellowship (MR/M003108/1). MNR and NCF are NIHR Senior Investigators.
Authors’ disclosures available online (http://j-alz.com/manuscript-disclosures/16-0302r2).
Appendices
The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JAD160302.
REFERENCES
[1] | Clark CM , Schneider JA , Bedell BJ , Beach TG , Bilker WB , Mintun MA , Pontecorvo MJ , Hefti F , Carpenter AP , Flitter ML , Krautkramer MJ , Kung HF , Coleman RE , Doraiswamy PM , Fleisher AS , Sabbagh MN , Sadowsky CH , Reiman EP , Zehntner SP , Skovronsky DM , AV45-A07 Study Group ((2011) ) Use of florbetapir-PET for imaging beta-amyloid pathology. JAMA 305: , 275–283. |
[2] | Johnson KA , Minoshima S , Bohnen NI , Donohoe KJ , Foster NL , Herscovitch P , Karlawish JH , Rowe CC , Carrillo MC , Hartley DM , Hedrick S , Pappas V , Thies WH , Alzheimer’s Association; Society of Nuclear Medicine and Molecular Imaging; Amyloid Imaging Taskforce ((2013) ) Appropriate use criteria for amyloid PET: A report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer’s Association. Alzheimers Dement 9: , e–1–16. |
[3] | Laforce R , Rosa-Neto P , Soucy JP , Rabinovici GD , Dubois B , Gauthier S ((2016) ) Canadian consensus guidelines on use of amyloid imaging in Canada: Update and future directions from the Specialized Task Force on amyloid imaging in Canada. Can J Neurol Sci 43: , 503–512. |
[4] | Blennow K , Hampel H ((2003) ) CSF markers for incipient Alzheimer’s disease. Lancet Neurol 2: , 605–613. |
[5] | Blennow K , Mattsson N , Scholl M , Hansson O , Zetterberg H ((2015) ) Amyloid biomarkers in Alzheimer’s disease. Trends Pharmacol Sci 36: , 297–309. |
[6] | Grundman M , Pontecorvo MJ , Salloway SP , Doraiswamy PM , Fleisher AS , Sadowsky CH , Nair AK , Siderowf A , Lu M , Arora AK , Agbulos A , Flitter ML , Krautkramer MJ , Sarsour K , Skovronsky DM , Mintun MA , Group AS ((2013) ) Potential impact of amyloid imaging on diagnosis and intended management in patients with progressive cognitive decline. Alzheimer Dis Assoc Disord 27: , 4–15. |
[7] | Sanchez-Juan P , Ghosh PM , Hagen J , Gesierich B , Henry M , Grinberg LT , O’Neil JP , Janabi M , Huang EJ , Trojanowski JQ , Vinters HV , Gorno-Tempini M , Seeley WW , Boxer AL , Rosen HJ , Kramer JH , Miller BL , Jagust WJ , Rabinovici GD ((2014) ) Practical utility of amyloid and FDG-PET in an academic dementia center. Neurology 82: , 230–238. |
[8] | Frederiksen KS , Hasselbalch SG , Hejl AM , Law I , Hojgaard L , Waldemar G ((2012) ) Added diagnostic value of (11)C-PiB-PET in memory clinic patients with uncertain diagnosis. Dement Geriatr Cogn Dis Extra 2: , 610–621. |
[9] | Ossenkoppele R , Prins ND , Pijnenburg YA , Lemstra AW , van der Flier WM , Adriaanse SF , Windhorst AD , Handels RL , Wolfs CA , Aalten P , Verhey FR , Verbeek MM , van Buchem MA , Hoekstra OS , Lammertsma AA , Scheltens P , van Berckel BN ((2013) ) Impact of molecular imaging on the diagnostic process in a memory clinic. Alzheimers Dement 9: , 414–421. |
[10] | Gorno-Tempini ML , Hillis AE , Weintraub S , Kertesz A , Mendez M , Cappa SF , Ogar JM , Rohrer JD , Black S , Boeve BF , Manes F , Dronkers NF , Vandenberghe R , Rascovsky K , Patterson K , Miller BL , Knopman DS , Hodges JR , Mesulam MM , Grossman M ((2011) ) Classification of primary progressive aphasia and its variants. Neurology 76: , 1006–1014. |
[11] | Grossman M ((2010) ) Primary progressive aphasia: Clinicopathological correlations. Nat Rev Neurol 6: , 88–97. |
[12] | Harris JM , Gall C , Thompson JC , Richardson AM , Neary D , du Plessis D , Pal P , Mann DM , Snowden JS , Jones M ((2013) ) Classification and pathology of primary progressive aphasia. Neurology 81: , 1832–1839. |
[13] | Mesulam MM , Weintraub S , Rogalski EJ , Wieneke C , Geula C , Bigio EH ((2014) ) Asymmetry and heterogeneity of Alzheimer’s and frontotemporal pathology in primary progressive aphasia. Brain 137: , 1176–1192. |
[14] | Landau SM , Lu M , Joshi AD , Pontecorvo M , Mintun MA , Trojanowski JQ , Shaw LM , Jagust WJ , Alzheimer’s Disease Neuroimaging Initiative ((2013) ) Comparing positron emission tomography imaging and cerebrospinal fluid measurements of beta-amyloid. Ann Neurol 74: , 826–836. |
[15] | Hake A , Trzepacz PT , Wang S , Yu P , Case M , Hochstetler H , Witte MM , Degenhardt EK , Dean RA , Alzheimer’s Disease Neuroimaging Initiative ((2015) ) Florbetapir positron emission tomography and cerebrospinal fluid biomarkers. Alzheimers Dement 11: , 986–993. |
[16] | Paterson RW , Toombs J , Slattery CF , Nicholas JM , Andreasson U , Magdalinou NK , Blennow K , Warren JD , Mummery CJ , Rossor MN , Lunn MP , Crutch SJ , Fox NC , Zetterberg H , Schott JM ((2015) ) Dissecting IWG-2 typical and atypical Alzheimer’s disease: Insights from cerebrospinal fluid analysis. J Neurol 262: , 2722–2730. |
[17] | Vos SJ , Verhey F , Frolich L , Kornhuber J , Wiltfang J , Maier W , Peters O , Ruther E , Nobili F , Morbelli S , Frisoni GB , Drzezga A , Didic M , van Berckel BN , Simmons A , Soininen H , Kloszewska I , Mecocci P , Tsolaki M , Vellas B , Lovestone S , Muscio C , Herukka SK , Salmon E , Bastin C , Wallin A , Nordlund A , de Mendonca A , Silva D , Santana I , Lemos R , Engelborghs S , Van der Mussele S , Alzheimer’s Disease Neuroimaging Initiative, Freund-Levi Y , Wallin AK , Hampel H , van der Flier W , Scheltens P , Visser PJ ((2015) ) Prevalence and prognosis of Alzheimer’s disease at the mild cognitive impairment stage. Brain 138: , 1327–1338. |
[18] | Weston PS , Paterson RW , Modat M , Burgos N , Cardoso MJ , Magdalinou N , Lehmann M , Dickson JC , Barnes A , Bomanji JB , Kayani I , Cash DM , Ourselin S , Toombs J , Lunn MP , Mummery CJ , Warren JD , Rossor MN , Fox NC , Zetterberg H , Schott JM ((2015) ) Using florbetapir positron emission tomography to explore cerebrospinal fluid cut points and gray zones in small sample sizes. Alzheimers Dement (Amst) 1: , 440–446. |
[19] | Johnson KA , Sperling RA , Gidicsin CM , Carmasin JS , Maye JE , Coleman RE , Reiman EM , Sabbagh MN , Sadowsky CH , Fleisher AS , Murali Doraiswamy P , Carpenter AP , Clark CM , Joshi AD , Lu M , Grundman M , Mintun MA , Pontecorvo MJ , Skovronsky DM , AV45-A11 study group ((2013) ) Florbetapir (F18-AV-45) PET to assess amyloid burden in Alzheimer’s disease dementia, mild cognitive impairment, and normal aging. Alzheimers Dement 9: , S72–S83. |
[20] | Villemagne VL , Burnham S , Bourgeat P , Brown B , Ellis KA , Salvado O , Szoeke C , Macaulay SL , Martins R , Maruff P , Ames D , Rowe CC , Masters CL ((2013) ) Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: A prospective cohort study. Lancet Neurol 12: , 357–367. |
Figures and Tables
Fig.1
A comparison of CSF values for those with positive and negative amyloid PET scans: Scatter plots show CSF results for (A) Aβ42, (B) total tau, (C) tau:Aβ42, and (D) p-tau for PET positive and negative cases. For two individuals, p-tau measurement had not been possible. Those individuals in whom the amyloid PET scan led to a change in diagnosis are indicated by an X next to the corresponding CSF value.
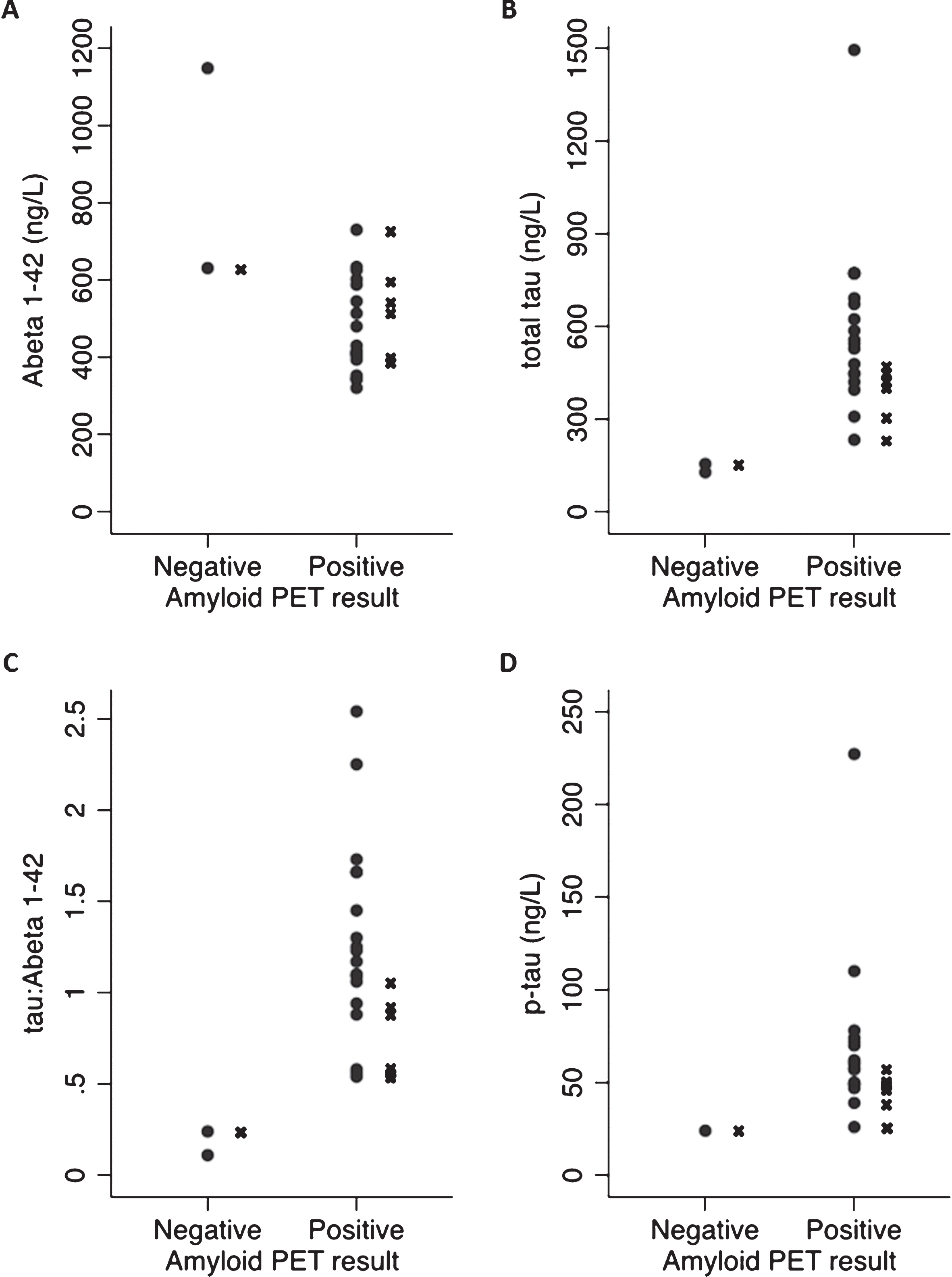
Table 1
Details of the patients in whom there was a change in diagnosis following the PET scan: Clinical information, including presenting symptom, CSF results, PET result, and details of change in diagnosis and diagnostic certainty (comparing before PET and after PET). The normal ranges for CSF measures used at our center were: Aβ1-42 >450 ng/L, tau:Aβ1-42 ratio <1, p-tau <68 ng/L
| Patient ID | Presenting symptom | CSF measures | Florbetapir PET result | Change in diagnosis | Diagnostic certainty | ||
| Aβ1-42 | tau:Aβ | p-tau | |||||
| 1 | Speech difficulty | 513 | 1.06 | 61 | Positive | FTD to AD | 40% → 60% |
| 4 | Speech difficulty | 544 | 0.56 | 39 | Positive | FTD to AD | 60% → 80% |
| 5 | Episodic memory | 630 | 0.24 | 24 | Negative | AD to depression | 50% → 90% |
| 6 | Speech difficulty | 403 | 0.58 | 26 | Positive | FTD to AD | 80% → 60% |
| 10 | Behavioral change | 729 | 0.54 | 49 | Positive | FTD to AD | 40% → 60% |
| 16 | Episodic memory | 393 | 0.94 | 50 | Positive | Autoimmune to AD | 50% → 60% |
| 20 | Speech difficulty | 601 | 0.88 | 57 | Positive | FTD to AD | 65% → 95% |




