Less Daily Computer Use is Related to Smaller Hippocampal Volumes in Cognitively Intact Elderly
Abstract
Background: Computer use is becoming a common activity in the daily life of older individuals and declines over time in those with mild cognitive impairment (MCI). The relationship between daily computer use (DCU) and imaging markers of neurodegeneration is unknown.
Objective:The objective of this study was to examine the relationship between average DCU and volumetric markers of neurodegeneration on brain MRI.
Methods: Cognitively intact volunteers enrolled in the Intelligent Systems for Assessing Aging Change study underwent MRI. Total in-home computer use per day was calculated using mouse movement detection and averaged over a one-month period surrounding the MRI. Spearman’s rank order correlation (univariate analysis) and linear regression models (multivariate analysis) examined hippocampal, gray matter (GM), white matter hyperintensity (WMH), and ventricular cerebral spinal fluid (vCSF) volumes in relation to DCU. A voxel-based morphometry analysis identified relationships between regional GM density and DCU.
Results: Twenty-seven cognitively intact participants used their computer for 51.3 minutes per day on average. Less DCU was associated with smaller hippocampal volumes (r = 0.48, p = 0.01), but not total GM, WMH, or vCSF volumes. After adjusting for age, education, and gender, less DCU remained associated with smaller hippocampal volume (p = 0.01). Voxel-wise analysis demonstrated that less daily computer use was associated with decreased GM density in the bilateral hippocampi and temporal lobes.
Conclusions: Less daily computer use is associated with smaller brain volume in regions that are integral to memory function and known to be involved early with Alzheimer’s pathology and conversion to dementia. Continuous monitoring of daily computer use may detect signs of preclinical neurodegeneration in older individuals at risk for dementia.
INTRODUCTION
Increasing engagement in life online has grown rapidly for the aging population. Approximately 60% of the population over age 65 now goes on-line (http://www.pewinternet.org/2014/04/03/older-adults-and-technology-use/). This transformation has been driven by a range of available online activities and a growing demand for conducting business through the Internet, including: email, social media, “brain fitness” training, banking, and government programs. Accordingly, the integration of computer activities into daily life is becoming increasingly common among older populations. Successful computer use likely requires the ability to effectively call upon multiple cognitive domains, including executive function, attention, and memory [1]. It is therefore conceivable that daily computer use (DCU) may be viewed as a bellwether of high level “instrumental activities of daily living” (IADL) or “everyday cognition” from which meaningful changes regarding cognitive status may be observed; changes in this IADL activity may indicate cognitive decline. This principle has been suggested in recent work relating DCU decline in persons with mild cognitive impairment (MCI) [2]. In this study, we aimed to examine in-home monitoring of DCU in relation to neurodegenerative brain changes on magnetic resonance imaging (MRI) in cognitively intact older adults. As a secondary analysis, we also examined the relationship between DCU and cognitive functions indicated by neuropsychological tests.
MATERIALS AND METHODS
Subjects
Thirty-four of 151 participants from the Intelligent Systems for Assessing Aging Change (ISAAC) longitudinal aging study [3] responded to an advertisement for an MRI research study. The research protocol was approved by the Oregon Health & Science University Institutional Review Board. Participants provided written informed consent. Subjects enrolled in ISAAC undergo assessment of daily function via remote monitoring using sensors strategically placed in the home to continuously track daily function in real time [3]. Subjects were 65 or older, living independently, and dementia-free (Mini-Mental State Examination [4] score≥24; Clinical Dementia Rating scale (CDR) [5] score < 1). Exclusion criteria included contraindications for MRI. Because the aim of this analysis was to examine DCU as a potential indicator of cognitive impairment, additional exclusion criteria included a CDR score of 0.5 [5]. Four subjects had unanalyzable MRI data due to motion. Three subjects were excluded for having a CDR = 0.5, leaving 27 subjects for analysis. As part of their participation in the ISAAC study, all participants had neuropsychological testing, including the Mini-Mental State Examination (MMSE) [4], delayed story recall [6], Trails B [7], and category fluency [8], within six months of their MRI visit.
Computer use assessment
Computer installation, setup, and training have been previously described [2]. Participants received a computer and Internet broadband services to facilitate data acquisition and participation. At entry, all subjects were observed and scored for proficiency on 20 computer-based tasks [3], and indicated skills sufficient enough to perform basic computer tasks, such as opening and using an internet browser, and sending and receiving emails. An online database and project tracking software examined the status of daily data transfer and quality and alerted staff to equipment malfunction [9]. Computer sessions were calculated using mouse movement data defined as any movement of more than five pixels generating a Windows event that was saved and time-stamped [2]. Total computer time per day (in minutes) was averaged over a one-month period surrounding the participant’s MRI.
MRI acquisition and analysis
MRI data were obtained using a 3.0T Siemens MRI scanner and included: T1-weighted magnetization prepared rapid gradient echo: repetition time = 2300 ms, echo time = 3.4 ms, inversion time = 1200 ms, spatial resolution = 1 mm isotropic and fluid attenuated inversion recovery: TR = 9000 ms, TE = 87 ms, TI = 2500 ms, Axial, FOV = 248 mm, slice thickness = 2 mm, number of slices = 95.
T1-weighted images were segmented into white matter, gray matter (GM), hippocampal, and ventricular cerebrospinal fluid (vCSF) masks using FreeSurfer (v.5.1), and manually edited for tissue misclassification. White matter hyperintensity (WMH) segmentation was performed using a semi-automated algorithm [10].
An exploratory VBM analysis with voxelwise GLM and cluster thresholding to correct for multiple comparisons was performed (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSLVBM). T1 images were brain extracted, non-linearly aligned to the MNI-152 template and GM extracted. All GM images were non-linearly aligned to a study specific template, modulated by the Jacobian and smoothed with an isotropic Gaussian kernel of sigma 2 mm. Cluster thresholded voxelwise GLM was used to explore the relationship between GM density and DCU while accounting for age, gender, and education.
Statistical analysis
We examined demographic differences between ISAAC participants with (n = 27) and without brain MRI (n = 117) using t-test (continuous variables) and Fisher’s exact test (categorical variables). We examined the association between mean DCU and age, geriatric depression score (GDS) [11], education and brain volumetry, including: WMH, GM, vCSF, and hippocampal volumes using Spearman’s rank order correlations. Right and left hippocampal volumes were not significantly different (paired t-test > 0.05), and were therefore combined as a total hippocampal volume. Brain volumes were divided by total intracranial volume to give % volume. A final multivariable linear regression was performed using % hippocampal volume (normally distributed) as the dependent variable and mean DCU in minutes (non-normal distribution) as the independent variable, adjusted for age, gender, and education. In a secondary analysis, we examined the relationship between mean DCU and cognitive functions using MMSE [4] for global cognition, delayed paragraph recall testing for memory, and Trails B [7] and verbal fluency [8] category (vegetables) for executive function.
RESULTS
ISAAC participants who had an MRI were not different in age, education, or gender distribution than participants without an MRI (p > 0.05) (not shown). Subject characteristics are described in Table 1. Less DCU was associated with older age (r = –0.49, p = 0.009). There was no correlation between DCU and geriatric depression score or education. The mean time between MRI and neuropsychological testing was three months (SD 1.6 months).
Relationship between DCU and % brain volumes
Less DCU was associated with smaller % hippocampal volume (Spearman’s correlation = 0.48, p = 0.01). There was no association between DCU and % WMH, % GM, or % vCSF volumes. In a final model adjusted for age, gender, and education, smaller % hippocampal volume remained associated with less DCU (p = 0.01). A second model was also adjusted for MMSE. In this model, MMSE was not associated with % hippocampal volume, while DCU remained significantly associated (p = 0.03) (Table 2).
Relationship between DCU and neuropsychological tests of global cognition, memory and executive function
After adjusting for age, gender, and education, greater DCU was associated with superior delayed recall performance (coefficient estimate = 0.03, p = 0.03). There was no relationship between DCU and either MMSE or Trails B test scores (p > 0.05). The association between DCU and category fluency was marginally significant (estimate = 0.02, p = 0.049).
Exploratory whole-brain VBM analysis
Voxel-wise analysis demonstrated that less DCU was associated with decreased GM density in the bilateral hippocampi and other temporal lobe regions (Table 3, Fig. 1). There were no significant voxel clusters demonstrating less GM density to be related to greater computer use.
DISCUSSION
Findings from this cross-sectional study demonstrate a significant correlation between less DCU and smaller brain volumes in regions associated with MCI and dementia among 27 cognitively intact older adults. In this study, an additional hour of computer use time was associated with a 0.025% larger hippocampal volume. The bilateral hippocampi were strongly associated with DCU activity, areas where premorbid MRI volumes have been shown to be associated with subsequent tau pathology on autopsy [12]. These findings are consistent with previous studies showing relationships between computer use and cognitive health [1]. Using self-report measures, others have reported decreased MCI and dementia risk in those who were computer users versus non-users [13, 14]. Utilizing unobtrusive measurements of DCU, it was previously shown that, compared with cognitively intact elderly, there was a significant decline over time in the number of days and mean daily duration of computer activity in MCI subjects [2]. The new data reported here links this potential real-world activity to a plausible brain-based correlate in the medial temporal lobes. This suggests that DCU might be employed as an early indicator of everyday cognition or a behavioral indicator of neurodegeneration that can be readily quantified over time in a person’s home.
In this study, more DCU was associated with better performance on delayed recall memory testing and, to a lesser degree, executive function. Previous studies have also reported a link between computer use frequency and cognitive function, particularly in executive control [15]. Findings from this study show that memory may be more strongly associated with DCU than executive function, and are consistent with the observed MRI relationships implicating the involvement of hippocampal and mesial temporal lobe gray matter areas with computer use frequency.
This study has several limitations. The sample size is small, and findings should be considered with caution until they can be replicated in a larger cohort. These results were based on an average of over 1400 minutes of computer use per subject. Accordingly, it is possible that this type of methodology acquires sufficient data points so as to not require large numbers of participants to provide adequate power for data analysis [16]. Given its cross-sectional nature, we are not able to determine from this study a causal relationship such as: whether greater computer use is protective of future cognitive decline or accumulation of subclinical neurodegenerative pathology or reflects greater baseline brain reserve, or whether those with lower hippocampal volumes are simply less frequent computer users. A previous study has reported no difference in DCU between cognitively intact elderly and those with MCI. Those with MCI did, however, have decreased DCU when measured over time, suggesting that DCU may be a reflection of cognitive abilities and a potential indicator of meaningful cognitive change [2]. Longitudinal studies investigating this important distinction are ongoing, and future analyses will allow us to examine the trajectory of computer use activity in relation to baseline brain volumes. Lastly, our MRI cohort was composed largely of women and Caucasians, thus generalizability to a more diverse population is uncertain.
In summary, unobtrusive, continuous in-home monitoring has shown that less DCU is associated with smaller brain areas consistently associated with Alzheimer’s disease pathology among a small sample of cognitively intact older adults and may provide a means of detecting early functional changes associated with conversion to MCI and dementia.
ACKNOWLEDGMENTS
This study was sponsored by the NIH (1RO1AG036772, P30 AG008017, P30 AG024978; RO1 AG024059; R01 AG042191).
Authors’ disclosures available online (http://www.j-alz.com/manuscript-disclosures/16-0079r1).
REFERENCES
[1] | Seelye A , Hagler S , Mattek N , Howieson DB , Wild K , Dodge HH , Kaye JA ((2015) ) Computer mouse movement patters: A potential marker of mild cognitive impairment. Alzheimers Dement 1: , 472–480. |
[2] | Kaye J , Mattek N , Dodge HH , Campbell I , Hayes T , Austin D , Hatt W , Wild K , Jimison H , Pavel M ((2014) ) Unobtrusive measurement of daily computer use to detect mild cognitive impairment. . Alzheimers Dement 10: , 10–17. |
[3] | Kaye JA , Maxwell SA , Mattek N , Hayes TL , Dodge H , Pavel M , Jimison HB , Wild K , Boise L , Zitzelberger TA ((2011) ) Intelligent systems for assessing aging changes: Home-based, unobtrusive, and continuous assessment of aging. (Suppl 1). J Gerontol B Psychol Sci Soc Sci 66: , i180–190. |
[4] | Folstein MF , Folstein SE , McHugh PR ((1975) ) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. . J Psychiatr Res 12: , 189–198. |
[5] | Morris JC ((1993) ) The Clinical Dementia Rating (CDR): Current version and scoring rules. [see comment]. . Neurology 43: , 2412–2414. |
[6] | Wechsler D ((1987) ) WMS-R Wechsler memory scale-revised manual, The Psychological Corporation, Harcourt Brace Jovanovich Inc, New York. |
[7] | Reitan RM , Wolfson D ((1985) ) The Halstead-Reitan neuropsychological test battery: Therapy and clinical interpretation, Neuropsychological Press, Tucson, AZ . |
[8] | Morris JC , Heyman A , Mohs RC , Hughes JP , van Belle G , Fillenbaum G , Mellits ED , Clark C ((1989) ) The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. . Neurology 39: , 1159–1165. |
[9] | Hayes TL , Cobbinah K , Dishongh T , Kaye JA , Kimel J , Labhard M , Leen T , Lundell J , Ozertem U , Pavel M , Philipose M , Rhodes K , Vurgun S ((2009) ) A study of medication-taking and unobtrusive, intelligent reminding. . Telemed J E Health 15: , 770–776. |
[10] | Promjunyakul N , Lahna D , Kaye JA , Dodge HH , Erten-Lyons D , Rooney WD , Silbert LC ((2015) ) Characterizing the white matter hyperintensity penumbra with cerebral blood flow measures. . Neuroimage Clin 8: , 224–229. |
[11] | Yesavage JA , Brink TL , Rose TL , Lum O , Huang V , Adey M , Leirer VO ((1982) ) Development and validation of a geriatric depression screening scale: A preliminary report. . J Psychiatr Res 17: , 37–49. |
[12] | Silbert LC , Quinn JF , Moore MM , Corbridge E , Ball MJ , Murdoch G , Sexton G , Kaye JA ((2003) ) Changes in premorbid brain volume predict Alzheimer’s disease pathology. . Neurology 61: , 487–492. |
[13] | Geda YE , Silber TC , Roberts RO , Knopman DS , Christianson TJ , Pankratz VS , Boeve BF , Tangalos EG , Petersen RC ((2012) ) Computer activities, physical exercise, aging, and mild cognitive impairment: A population-based study. . Mayo Clin Proc 87: , 437–442. |
[14] | Almeida OP , Yeap BB , Alfonso H , Hankey GJ , Flicker L , Norman PE ((2012) ) Older men who use computers have lower risk of dementia. PLoS One 7: , e44239. |
[15] | Tun PA , Lachman ME ((2010) ) The association between computer use and cognition across adulthood: Use it so you won’t lose it? . Psychol Aging 25: , 560–568. |
[16] | Dodge HH , Zhu J , Mattek NC , Austin D , Kornfeld J , Kaye JA ((2015) ) Use of high-frequency in-home monitoring data may reduce sample sizes needed in clinical trials. PLoS One 10: , e0138095. |
Figures and Tables
Fig.1
VBM analysis with threshold-free cluster enhancement and permutation testing to correct for multiple comparisons. Overlaid voxels indicate regions of decreased GM density significantly associated with less daily computer use (p < 0.05).
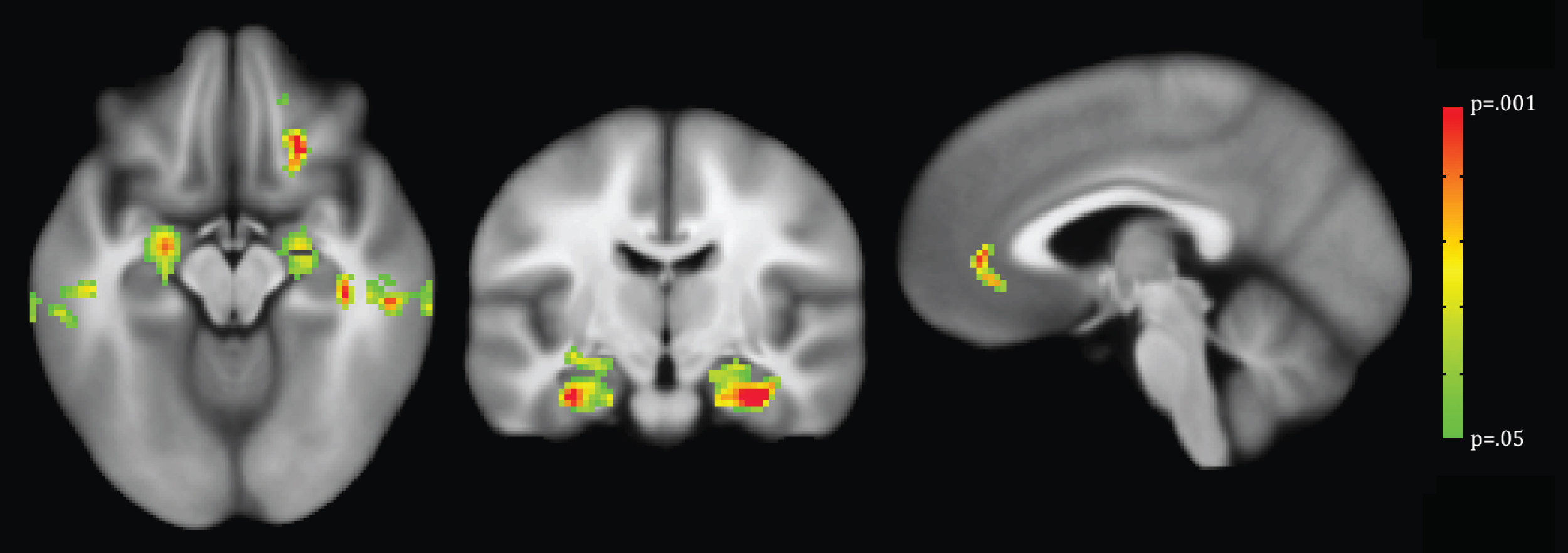
Table 1
Participant characteristics (n = 27)
| Mean (SD) | Range | |
| Age (y) | 86.7 (5.9) | 69.8 –97.9 |
| Mini-Mental State Exam | 28.3 (1.5) | 24 –30 |
| Delayed Paragraph Recall | 11.7 (4.5) | 0 –19 |
| Verbal Fluency (# vegetables named in 1 min) | 12 (3.4) | 7 –19 |
| Trails B (s to complete test) | 137.7 (59.6) | 55 –300 |
| Education (y) | 14.7 (2.0) | 12 –18 |
| Gender (% women) | 93 | NA |
| Race | NA | |
| % Caucasian | 85 | |
| % African American | 4 | |
| % Asian | 11 | |
| Mean Daily Computer Use during one month (min/day) | 51.3 (60.0) [median = 17.0] | 0 –182.9 |
Table 2
Results of multivariate linear regression models with outcome being the percent of hippocampal volume out of intracranial volume
| Model 1 | Model 2 | |||
| Variable | Coefficient | p-value | Coefficient | p-value |
| Age | –0.002 | 0.29 | –0.002 | 0.39 |
| Gender | 0.02 | 0.17 | 0.02 | 0.25 |
| Education (y) | 0.002 | 0.68 | 0.002 | 0.75 |
| DCU (min/day) | 0.0004 | 0.01 | 0.0004 | 0.03 |
| MMSE | 0.003 | 0.66 | ||
Model 1: controlling for age, gender, and education. Model 2: controlling for age, gender, education, and MMSE (Mini-Mental State Exam).
Table 3
Regions in which VBM identified significantly reduced GM density in comparison with less daily computer use (p < 0.05)
| Talairach Region | Coordinates (center of mass) | Cluster size | ||||
| Side | X | Y | Z | Voxels | Volume (cm3) | |
| Hippocampus* | L | 27.0 | 9.9 | –20.5 | 778 | 6.22 |
| Hippocampus* | R | –25.3 | 7.7 | –17.8 | 472 | 3.78 |
| Middle Temporal Gyrus* | L | 55.6 | 27 | –9.0 | 372 | 2.98 |
| Middle Temporal Gyrus | R | –58.7 | 28.3 | –13.2 | 325 | 2.60 |
| Anterior Cingulate | L+R | 6.1 | –38.7 | 3.3 | 290 | 2.32 |
| Middle Frontal Gyrus | L | 20.1 | –25.3 | –8.1 | 256 | 2.05 |
*remained significant after cluster thresholding




