Timely Diagnosis for Alzheimer’s Disease: A Literature Review on Benefits and Challenges
Abstract
Background:
Timely diagnosis of Alzheimer’s disease (AD) refers to a diagnosis at the stage when patients come to the attention of clinicians because of concerns about changes in cognition, behavior, or functioning and can be still free of dementia and functionally independent.
Objectives:
To comprehensively review existing scientific evidence on the benefits and potential challenges of making a timely diagnosis of AD.
Methods:
Relevant studies were identified by searching electronic databases (Medline, Embase) and bibliographies for studies published in English between 1 January 2000 and 2 June 2014 on the consequences of a timely diagnosis of AD.
Results:
Nine studies were identified that investigated the consequences of diagnosing AD at the initial stages; none were specifically focused on prodromal AD. A timely diagnosis potentially offers the opportunities of early intervention, implementation of coordinated care plans, better management of symptoms, patient safety, cost savings, and postponement of institutionalization. Barriers to making a timely diagnosis include stigma, suicide risk, lack of training, diagnostic uncertainty, shortage of specialized diagnostic services, and the reluctance of healthcare providers to make a diagnosis when no effective disease-modifying options are available.
Conclusions:
Despite its potential benefits, few published studies have explored the advantages or risks of a timely diagnosis of AD. In light of the cultural shift toward diagnosis at the initial stage of the disease continuum, when the patient does not yet have dementia, more investigations are needed to evaluate the benefits and address the barriers that may impede making a timely AD diagnosis.
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disease that is clinically characterized by impairment of cognitive and functional abilities together with behavioral symptoms. The diagnosis of probable AD has classically been based on clinical criteria, such as those published in 1984 by the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) [1]. According to those criteria, a definitive diagnosis of AD requires postmortem confirmation of specific neuropathological changes (accumulation of neuritic plaques and neurofibrillary tangles containing hyperphosphorylated tau proteins).
Greater understanding of the pathology and course of AD has led to it being re-conceptualized as a disease continuum (Fig. 1) [2]. It is believed that patients with AD experience a long asymptomatic (preclinical) phase in which neuropathological changes occur but cognitive ability is normal [3–5], followed by a symptomatic (prodromal or pre-dementia) phase of progressive cognitive decline before the onset of functional impairment and overt dementia [6–9]. Longitudinal follow-up studies of population-based community-dwelling individuals have shown that cognitive impairment can be detected well before the onset of dementia symptoms [10].
Moving to a timely diagnosis of AD at the prodromal stage
As more people live into older age, the number of individuals with dementia is increasing [2, 11], and the economic, health, and social care costs of dementia are escalating [11–13]. In response to this, dementia has become a priority area for coordinated action at the European Union (EU) and global level [14–16]. Many countries now have national dementia strategies and government policies that emphasize early diagnosis and intervention [2, 17–20].
In recent years, there have been calls for a cultural shift in diagnosing AD at an earlier stage, before patients have crossed the threshold into dementia. The Alzheimer Cooperative Valuation in Europe (ALCOVE) project has proposed that diagnosis should generally occur earlier than is currently common practice, at a time when patients and their family first notice changes in cognitive function and can use the information to make sense of what is happening, make lifestyle changes, and plan for the future; the term “timely diagnosis” is used to reflect this [21]. This is not the only definition proposed for timely diagnosis; it can also be described as the diagnosis made at the right time for the individual patient, irrespective of the disease stage. For the current review, we defined “timely diagnosis” of AD as the diagnosis made at a time when individuals first become worried enough to seek help and come to the attention of clinicians because of concerns about changes in cognition, behavior, or functioning not necessarily resulting in dementia, and we extended the literature search to diagnosis at the prodromal or pre-dementia stage of AD (T2 and T3 in Fig. 1) [2, 3]. “Timely” diagnosis of AD differs from “early” diagnosis (T1 in Fig. 1), which would require population or targeted screening to identify people in the asymptomatic phase of AD [22].
The International Working Group (IWG) has proposed a new concept of AD with new diagnostic criteria based on the presence of biomarkers, allowing the identification of a prodromal stage (also called the “pre-dementia stage of AD”) and of preclinical states for AD [23, 24]. Based on these criteria, AD is now considered a clinico-biological entity that can be identified in vivo and no more reference to the dementia threshold is needed for the diagnosis of AD. In line with this new approach, the revised criteria for the clinical diagnosis of AD by the National Institute on Aging and Alzheimer Association (NIA-AA) also considered a symptomatic pre-dementia phase of AD, referred to as “Mild cognitive impairment due to AD” [25, 26]. The IWG research criteria permit diagnosis of AD at the prodromal phase before patients have developed dementia [27]; these require evidence of both specific clinical features and in vivo biological evidence of an underlying abnormal pathology that is well-defined and detected using biomarkers (Table 1). Although these new researchcriteria require further validation, recent findings suggest that they have good specificity and are feasible for use in the clinical setting for the diagnosis of AD at the prodromal stage [28]. Efforts are also being made to harmonize the different clinical diagnostic criteria for AD [29].
Potential benefits or risks of timely diagnosis of AD
There are many possible benefits of a timely and accurate diagnosis of AD for patients, caregivers, and society [11, 22]. One of the main theoretical advantages of a timely diagnosis at the prodromal stage is the opportunity to achieve added value from earlier treatment or intervention with disease-modifying therapy in a clinical trial before the onset of dementia. This is entirely speculative at present as no effective disease-modifying therapies are available. Early intervention has the potential to improve the quality of life of patients and their informal family caregivers, both of whom are often relieved once the patient is diagnosed [30, 31]. A timely diagnosis at the prodromal stage may also improve patient access to support services or pathways of care and enable planning for the future.
On the other hand, there are a number of conceivable risks or challenges associated with a timely diagnosis of AD, including ethical issues, competency questions, discrimination, and stigmatization [32–35]. Another concern is misdiagnosis, which can lead to inappropriate treatment of patients who could take unnecessary medications for AD or not receive correct therapy for potentially treatable disorders [36]. The monetary costs to society of establishing systems for timely diagnosis and intervention may also be burdensome.
Objectives
Our aim was to review the research literature to identify whether there are studies that demonstrate the benefits and potential risks of a timely diagnosis of AD for individuals who exhibit changes in cognition, behavior, or function but are not yet clearly demented.
Methods
Search strategy and study selection
A comprehensive literature search was performed to identify publications that investigated the benefits and risks of a timely diagnosis of AD at the prodromal (pre-dementia) stage. First, a broad literature search on the electronic databases Medline and Embase was performed, from their start dates to November 20, 2013, using the following search terms: “early diagnosis” (subject heading) AND “Alzheimer OR dementia”. The term “early diagnosis” was used for the search to ensure capture of all relevant articles in this area as the terms “timely diagnosis”, “prodromal AD”, and “pre dementia” have only recently been introduced and defined. Items selected for further assessment were articles published in the English language since January 1, 2000 (except for the inclusion of a few relevant papers published in the late 1990s). This provided 451 records, including studies, reviews, editorials, letters, and commentaries. Meeting abstracts were not systematically searched, but the authors included any relevant meeting abstracts that they were aware of. The titles and abstracts of all 451 records identified were independently examined by at least two of the authors and rated on a 4-point scale (1 = limited, 2 = acceptable, 3 = good, 4 = excellent) for potential relevance for inclusion in this review. For any articles considered possibly eligible or where uncertainty existed, the full text articles were obtained and screened for relevance. Further studies identified from the reference lists of the full articles reviewed and from other sources were obtained and examined to see if they should also be included. One study was identified this way.
Eligible articles were those reporting the results of studies investigating the benefits or challenges of a timely diagnosis of AD. These studies could be quantitative (e.g., cost studies) or qualitative (e.g., surveys, focus groups), and there were no geographical exclusions. However, studies examining the development of or cost/benefit of the tools used to make an early/timely diagnosis (e.g., biomarkers) were not included. Of the 45 references selected by the authors, and assessed further for eligibility, nine were studies or surveys pertaining to the consequences of a timely diagnosis of AD and were included in the results.
To verify that no relevant studies on the benefits or challenges of a timely diagnosis of AD were missed, a second comprehensive search of Medline (through PubMed) and Embase (both accessed via ProQuest Dialog) from January 1, 2000 to May 21, 2014 using the search terms (timely diagnosis) AND (mild cognitive impairment OR amnestic mild cognitive impairment) was subsequently performed. After removing duplicates, the titles of the 31 records identified were assessed and nine abstracts were selected for further assessment of eligibility. None of these articles yielded findings on the consequences of a timely diagnosis of prodromal AD.
A further search of Medline and Embase (both accessed via ProQuest Dialog) to identify original clinical studies on the benefits and challenges of a timely diagnosis of AD was performed on June 2, 2014 using the following search terms: “dementia OR Alzheimer OR Alzheimer’s” AND “prodromal OR pre-dementia OR early symptoms” AND “diagnosis”. When the search was limited to clinical trials in Embase and “clinical trials” was added as a search string in Medline, 169 records remained after duplicates were removed. After assessment of the titles and abstracts, no additional studies were identified for inclusion in the results. Note that the heterogeneity of the studies identified prevented us from performing a full systematic review, including meta-analysis, according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines [37].
Figure 2 illustrates the article identification and selection process for the literature searches. Because the identified studies have different aims, combination or comparison of the data in a systematic manner was not possible. Thus, a brief narrative summary of each study is provided separately. Included studies did not undergo any quality assessment (e.g., risk of bias).
Results
Summary of literature search
Nine studies related to the benefits or challenges of a timely diagnosis of AD were identified from the literature search. Some of these studies included subjects in the pre-dementia stage of AD but none were specifically focused on diagnosing AD at the prodromal stage. One study was a survey of the psychological reactions of patients and their companions to receiving a diagnosis of dementia [38], while three studies focused on physicians or caregivers, not patients [39–41]. These included a qualitative survey of caregivers or primary care physicians (PCPs) on the perceived benefits and risks of an early recognition of dementia or Alzheimer’s dementia [39]; a survey of general practitioner (GP) attitudes toward diagnosing dementia that aimed to understand the low dementia diagnosis rate in certain regions in England [40]; and a randomized controlled study that examined the effects of a tailored educational intervention on dementia diagnosis and management in primary care [41]. Two studies were of memory clinic patients, looking at the effects of diagnosis made early in the course of the illness on institutionalization and mortality [42, 43]. Finally, three studies looked at the possible cost-benefits of an early diagnosis of dementia or Alzheimer’s dementia using simulation models and estimates of outcomes [44–46].
Perceived consequences of a timely diagnosis of AD
Using a pre/post survey design, Carpenter et al. [38] studied the reactions of 90 people and their companions upon receiving a diagnosis of dementia. The participants were people with and without memory or cognitive complaints seeking evaluation, and the companions were mostly the spouse (61% ) or child (22% ) of the person seeking dementia evaluation. Symptoms of anxiety and depression were assessed using the State-Trait Anxiety Inventory and Geriatric Depression Scale, respectively, with patients and their companions being assessed separately. Of the 90 patients who participated in the study, 31% were given a diagnosis of no dementia (Clinical DementiaRating [CDR] = 0), 46% were given a diagnosis of very mild dementia (CDR = 0.5), and 23% were given a diagnosis of mild dementia (CDR = 1). The results showed a substantial reduction in anxiety and no significant changes in depression or psychological distressin either patients or companions after receiving the diagnostic feedback, regardless of diagnostic outcome or severity [38].
In the UK, Iliffe et al. [39] surveyed 990 PCPs (including GPs, community nurses, practice nurses, community mental health nurses, and others) on the perceived benefits and risks of an early recognition of dementia in general practice. This study generated data from workshops using a nominal group approach, identifying benefits for patients, families and local services. Reduced uncertainty and the opportunity to come to terms with the diagnosis, seek support, and avoid crises were the most relevant advantages identified for patients. The benefits for families included awareness of prognosis and disease course as well as the need to organize support, plan for the future, make appropriate legal arrangements, and optimize quality of life. Better workload planning and resource allocation were the perceived advantages for local services. The hazards identified for patients and families included fear, anxiety/depression, stigma, and altered relationships, while PCPs expressed concerns about diagnostic errors and resource limitations, including shortfalls in local services, as more people received diagnoses early in the disease continuum [39].
In an online survey where GPs in primary care in Norfolk and Suffolk, England, were asked to rate the extent to which they agreed or disagreed with seven statements on dementia diagnosis, 85% of the 113 respondents agreed that it is beneficial to patients and their families to have a timely diagnosis of dementia [40]. Although this survey showed positive attitudes toward diagnosing dementia, it also found that participating GPs were less confident in their knowledge about the availability of post-diagnostic support services for people with dementia and their carers, and were dissatisfied with locally available services. The authors commented that GPs’ attitudes toward diagnosing and managing dementia were more positive since the 2009 National Audit of GPs in the UK [47].
Effects of interventions or models to facilitate a timely diagnosis of AD
Some studies have indicated that educational interventions for healthcare providers may improve the early recognition of dementia in primary care [48, 49] and, therefore, may have subsequent benefits for patients and their caregivers. However, a recent study of an educational intervention tailored to the needs of an individual primary care practice that combined practice-based workshops and computer decision support systems (EVIDEM-ED study) did not show positive results. This open-label cluster randomized controlled trial, which included more than 1000 patients with dementia seen in 23 general practices in England, had a pre/post-intervention design of educational intervention versus usual care [41]. The educational program combined timely diagnosis of dementia and psychosocial support around the time of diagnosis with components appropriate to the later stages of the disease course, while the usual care control practices were provided with a summary of the UK National Institute for Health and Care Excellence Dementia Clinical Guideline 42 [50]. The results showed no significant difference in dementia management reviews before and after the educational intervention aimed to help physicians make a timely diagnosis; there was also no change in case detection rates [41].
A multidisciplinary Memory Clinic set up in Sheffield, England, was designed to facilitate diagnosis and treatment of Alzheimer’s dementia early in the course of the disease through careful management of the diagnostic process with a focus on pre- and post-diagnostic counseling followed by psychosocial interventions. The effectiveness of this Memory Clinic model was investigated in a small retrospective cohort study of 30 patients with Alzheimer’s dementia in the “Memory Clinic treatment group” versus 30 AD patients in the control group who received standard care from other facilities. The median time to institutionalization was 26.5 months for the Memory Clinic group versus 17.5 months for the control group, demonstrating that early diagnosis of Alzheimer’s dementia can delay time to institutionalization, in this case by a median of 9 months [42]. Interpretation of this study is limited by its small sample size.
One study provides indirect evidence that diagnosis of AD at the earlier stage may prolong survival. In a cohort study of 970 patients with dementia (including 663 with AD) attending a memory clinic in France, survival analysis showed that a shorter time between first symptoms and first visit was associated with longer survival regardless of diagnosis (risk ratio = 0.7 for each year earlier the first visit occurred, p < 0.0001) [43].
Cost benefits of early identification of AD
Three studies investigated the cost-benefits of early diagnosis and treatment of Alzheimer’s dementia [44–46]. These studies questioned whether the costs associated with early identification and diagnostic evaluation can be offset by cost savings achieved by the use of early interventions that hypothetically slow disease progression and/or delay the time to institutionalization.
Banerjee and Wittenberg [44] performed a cost-benefit analysis of the Croydon Memory Service Model for early diagnosis and intervention in dementia. This British service model takes a multi-disciplinary and multi-agency approach and is designed to provide an early diagnosis, information, and help for people with dementia and their families. The costs of this service, if extrapolated nationally, were estimated at £220 million per year (in 2007/2008 prices). Also, with a theoretical reduction of 6% , 10% , and 20% in residential care home admission by year 10 after introducing the service model, potential annual savings to society were estimated at approximately £150 million, £245 million, and £490 million, respectively. In addition, it was estimated that the service model need only achieve a modest increase in average quality of life (of between 0.01 and 0.02 Quality Adjusted Life Years [QALY] per person year) together with a 10% reduction in institutionalization to be considered cost-effective.
Weimer and Sager [45] performed a cost-benefit analysis (Monte Carlo model) of early identification and treatment of Alzheimer’s dementia. The model estimated the net social benefits and net fiscal savings of early intervention with drug treatment, a caregiver intervention program, and a combination of both interventions. The results showed that the net benefits could be highest when patients received a diagnosis at the initial symptomatic stage of the disease and when drug treatment was combined with caregiver intervention: the mean net social, state fiscal, and federal fiscal benefits of drug treatment plus caregiver intervention for a 70-year-old married woman with a Mini-Mental State Examination (MMSE) score of 28 were estimated as $US125,000, $16,000, and $34,000, respectively.
Getsios et al. [46] performed an economic evaluation of early assessment and treatment for AD in the UK using discrete event simulation based on data from the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD), and patient-level data from seven donepezil clinical trials to simulate AD progression and the effects of treatment intervention. The model calculates direct costs of care and the indirect costs of caregiver time over a period of 10 years. QALYs for patients and caregivers were also reported, as were incremental cost-effectiveness ratios (cost/QALY). Findings show that early assessment and treatment result in up-front costs of £4083 and £2402 per patient (2007 cost year), respectively, but this was offset by savings in patient care. The total expenditures (for drugs, early assessment, direct care, and indirect costs) were lower for the early assessment and treatment group (£204,561) versus treatment without early assessment (£209,837), or no early assessment and no treatment (£212,302). Reduced institutional care was the largest contributor to savings, and patients assessed and treated early remained in the community longer. Compared with the no assessment/treatment group, early assessment reduced the time patients spent with low cognitive ability (MMSE scores <10) by over 5 months. The analyses suggested that early assessment and treatment could have health benefits for the patient and might also be cost-effective [46].
Discussion
Members of the scientific community, stakeholders (e.g., AD associations), regulators, and policy makers are, to varying degrees, encouraging a cultural shift toward making a timely diagnosis of AD at the initial symptomatic stages of the illness. Despite this change in paradigm, our extensive and comprehensive review of the literature identified some studies highlighting the potential advantages of diagnosis and intervention early in the time course of dementia, but failed to find studies clearly focused on the benefits for patients, carers, or society of a timely diagnosis at the prodromal stage, before dementia sets in. In agreement with the 2011 World Alzheimer Report [2], we found that much of the literature outlining the benefits of timely diagnosis of AD is based on expert opinion rather than research evidence. The scarcity of published studies assessing the benefits and challenges of timely diagnosis could be due to the fact that the definition of AD as an entity that encompasses both pre-dementia and dementia phases is relatively recent and still not widely accepted.
Alternatively, some methodological issues could explain why few studies on this topic were identified. Although our search of the literature was comprehensive and the search terms used should have been broad enough to capture most publications relevant to a timely diagnosis of AD, it is possible that some studies were missed because of the terminology used. In addition, some of the identified studies assessed the potential advantages of early recognition of cognitive decline without specifying the etiology of the dementia syndromes and could have included individuals with other types of dementia or mixed pathology. Another limitation of our review is that the methodological quality of the studies was not rated, some of which may have had a high risk of bias. Also, the heterogeneity of the studies identified prevented the performance of a fully systematic review including meta-analysis, according to PRISMA guidelines [37].
Several of the studies identified assessed the possible economic benefits of early diagnosis and treatment of Alzheimer’s dementia [44–46]. These studies, however, were not evidence-based but based on models that estimated the cost-effectiveness of different theoretical interventions and/or outcomes. The significant economic impact of AD is expected to increase in the future with the trend toward diagnosis at the prodromal stage [12]. A timely diagnosis offers the opportunity of introducing psychosocial interventions that may prolong the time people spend in initial stages of the disease and delay admission to residential long-term care homes, as indicated by the effectiveness of a multidisciplinary Memory Clinic model [42]. Data from a Markov simulation model of intervention with a hypothetical disease-modifying therapy at the pre-dementia stage also support this hypothesis [51]. As the costs associated with institutionalization and long-term residential care account for a large proportion of the total care costs of AD [52, 53], savings in overall healthcare costs may be achieved by extending the time a patient can remain living in the community. However, as societal costs of caring for community-dwelling patients with AD are primarily determined by the costs of caregiver informal care [13, 54, 55], informal care costs must be taken into account together with the caregiver burden.
Although timely diagnosis and intervention are likely to incur greater up-front costs, economic modeling suggests that these may be offset by subsequent savings achieved primarily from a reduction in institutionalization; additional benefits, also demonstrated in a cohort study in France, include prolonged patient survival and improved patient/carer quality of life [43, 44, 46]. Simulation models of early intervention with hypothetical disease-modifying therapies indicate that substantial cost savings might be possible [56, 57]. For example, the introduction in the UK of a hypothetical disease modifier that would delay the onset of dementia by 5 years (from 2020 to 2025) is estimated to potentially reduce the number of people with dementia in 2030 by 36% , with a related highly significant decrease in the costs of care [58]. However, the actual cost impact of delaying disease progression from pre-dementia through mild to severe AD, and of prolonging the time spent living in the community, still remains to be determined in longitudinal studies. Thus, further investigation of the health and social care costs associated with timely diagnosis of AD at the prodromal stage is needed.
People who seek health care generally prefer active strategies and certainty about their diagnosis and prognosis, even when effective drugs are not available [59]. Many of the potential benefits of timely diagnosis and treatment of AD from the studies identified in this review are dependent on the existence of a disease-modifying medication. Unfortunately, such a medication does not yet exist and evidence-based studies will have to be performed if a proven disease-modifying treatment becomes available.
The scant evidence base regarding the benefits of timely diagnosis of AD on outcomes in patients (e.g., subsequent disease progression, time to institutionalization, death) or caregivers (e.g., burden, quality of life, depression/anxiety) from published studies allows us to only speculate on the potential clinical benefits and risks of diagnosing patients at an earlier phase in the disease continuum, when they have symptoms but have not yet developed overt dementia. Moreover, much of the following discussion is based on the assumption that a timely diagnosis of AD is feasible, even if the well-recognized problem of under-diagnosis of dementia at more severe stages in the current medical setting [2, 40] demonstrates that this is not always the case.
Among the many conceivable benefits to patients, caregivers, healthcare providers, and society of a timely diagnosis of AD (Table 2), one of the most important would appear to be that an earlier and improved diagnostic pathway may help patients avoid the “medical nomadism” that some experience during the diagnostic process, which begins with seeking help and ends with receiving a definitive diagnosis and treatment [22]. Prompt evaluation means that it might be possible to detect and treat other causes of memory problems (e.g., depression, anxiety, sleep disorders) [33]. As many older people have comorbidities, we can speculate that timely diagnosis of AD may help avoid prescription of medications that could actually worsen cognitive function.
Other important possible benefits of a timely diagnosis are that it may reduce feelings of uncertainty and anxiety in people with memory complaints and their families [38, 60], and may improve their quality of life and relationships [61]. Recent studies indicate that many people are in favor of early diagnostic testing for AD [62] and would want a potential diagnosis of AD to be disclosed to them and their relatives [61, 63]. For example, the “Value of Knowing” survey of public attitudes to early diagnosis of AD found that over 94% of the people surveyed (in each of the four European countries where the question was asked) wanted to be told if they had AD [64], and a recent qualitative study on people with cognitive decline, ranging from subjective memory problems to early dementia, showed that “the overwhelming majority of participants were keen to know their diagnosis and its long-term consequences” [60]. Moreover, as shown by Carpenter et al. [38], patients and their caregivers report a reduction in anxiety and no increase in psychological distress after receiving a diagnosis of dementia in its initial stages. In addition, a recent focus group report found that a primary driver for people with memory problems to take part in a clinical trial for prodromal AD was to obtain an unambiguous diagnosis [65]. Early detection of dementia was considered the highest priority topic for dementia research in a recent survey conducted in Scotland on AD patients, their carers, and the general public with an interest in dementia [66].
GPs and primary care health workers consider that patients and their families are the main beneficiaries of an earlier diagnosis [39, 40]. Theoretically, a timely diagnosis of AD could allow families to plan and prepare for the future. Before the onset of dementia and while insight is preserved [67], patients may have the capacity to understand what is happening and make decisions about future living/care options, treatment, and financial and legal arrangements [33, 68]. Caregivers would be able to plan and organize future support, explore outside resources, and address safety concerns (e.g., driving), financial planning, legal issues (e.g., advance directives), and caretaking arrangements [31, 39, 69]. Putative advantages of diagnosis at the earlier stages of the disease for local healthcare and social services are that they can better anticipate future demands [39]. We can postulate that recognition of AD during the prodromal stage would allow physicians to offer therapies that address specific symptoms, such as anxiety or impaired sleep, and manage medications prescribed for comorbidities that may be inadvertently exacerbating dementia or other emerging symptoms.It can be speculated that a timely diagnosis of AD would also open up the opportunity of early intervention with disease-modifying therapies if they become available. A variety of possibly disease-modifying compounds in clinical development may theoretically increase the time spent in the pre-dementia to mild AD stages. Once healthcare systems and processes that enable timely diagnosis of AD are established, data on the actual benefits to patients and their families can be collected.
Diagnosis of AD at the prodromal stage is very challenging in the current medical setting and made more difficult by numerous barriers. Some of these barriers are relevant to only patients, caregivers, healthcare providers, or society, while others could be shared by all (Table 2). Some of the existing barriers to diagnosis of dementia may also apply to the timely diagnosis of AD at the prodromal stage. In particular, stigma associated with dementia is a major issue [35], whether it be public stigma, self-stigma (which may deter individuals from seeking professional help), or family/caregiver stigma, all of which can negatively affect the quality of life of people with dementia and their caregivers [31, 70–72].
Although there are reports of patients and caregivers expressing a positive attitude toward receiving a timely diagnosis of dementia, some physicians, especially in the primary care setting, cite various reasons explaining their reluctance to give a diagnosis of AD at the initial symptomatic stages of illness. For example, they say they do not consider it is beneficial for patients’ overall health and well-being, perceive there are no effective treatments, or consider that a diagnosis early in the disease continuum may actually be harmful to patients [39, 73–75]. While some patients and families may experience negative reactions to disclosure of a diagnosis of AD, including feelings of loss, anger, uncertainty, frustration, anxiety/depression, and catastrophic thinking (although this has not been specifically investigated for patients given a diagnosis of prodromal AD) [61, 77], other reports find no such long-term after-effects from a dementia diagnosis, and conclude that individual preferences for diagnosis disclosure should be taken into account [38, 60, 78]. Concerns have been expressed that an early diagnosis of dementia may conceivably result in an increased suicide risk and request for physician-assisted suicide [79], although there is limited evidence to support this hypothesis. In a retrospective cohort study of patients aged 60 years and over with a diagnosis of dementia and who died by suicide during the study period (2001 to 2005), subgroup analysis of 136 patients found that 75% of the suicides occurred in those with a new dementia diagnosis [80]. A history of psychiatric hospitalization, diagnosis of depression, and prescription fills for antidepressant or anxiolytic medication were also associated with an increased risk of suicide [80]. Although more research is clearly needed on the risk for suicidal behavior in AD, a recent review by Draper recommended that clinicians should be sensitive to the potential for suicide in vulnerable individuals, especially in the first few years after diagnosis [81].
For healthcare providers, other possible drawbacks of diagnosis at the pre-dementia stage are an increased risk of misdiagnosis [36] and uncertainty about the rate of progression to dementia, which can vary considerably among individuals [82].
Finally, memory problems and alterations of cognitive function can cause changes in personal identity, capacity, and autonomy; these issues would presumably add to the ethical challenges associated with a timely diagnosis of AD at the prodromal stage (Table 3) [33, 35].
To achieve a timely diagnosis at the initial stage of the disease before patients exhibit dementia symptoms, existing barriers to diagnosis will have to be removed. This would require PCPs to be attuned to the early symptoms of AD, and healthcare professionals, stakeholders, and policy makers would need to take action to fill gaps in knowledge, skills, attitudes, and resources. Clinical practice guidelines will need to be modified to align with the cultural shift to a timelydiagnosis of AD at the pre-dementia stage. For example, the most recent European Federation of the Neurological Societies (EFNS) guidelines for the diagnosis and management of AD [83] do not include diagnosis at the prodromal stage, and many of the screening tests recommended for assessing global cognition and activities of daily living may not be specific or sensitive enough for identifying prodromal AD. Thus, the methods used for assessing people with memory problems to detect prodromal AD must be appropriate and accurate. Establishing a clinical diagnosis of AD is a stepwise process that is often a shared responsibility between PCPs and specialists [84]. A timely diagnosis at the pre-dementia stage requires a coordinated diagnostic process involving a multidisciplinary team approach; all of those involved will need to be aware of the subtle cognitive and non-cognitive changes that can precede the onset of dementia.
Therefore, the cultural shift toward timely diagnosis depends upon increasing public and professional awareness of the initial symptoms of AD, improving knowledge among healthcare professionals about the benefits of a timely diagnosis and early intervention, reducing the fear and stigma about dementia, normalizing the experience of dementia, and developing and implementing dementia strategies and integrated diagnostic services that enable timely diagnosis and provide post-diagnostic support, care, and interventions for patients and their families. Some of this can be achieved through public awareness campaigns as well as targeted communication and education of healthcare professionals, while others will require system-wide and structural changes at the national level.
Conclusions
We believe that timely diagnosis of AD at a time when people first seek for help being worried about changes in cognition, behavior, or functioning not necessarily resulting in dementia, has the potential to reduce the impact of no or delayed diagnosis or misdiagnosis. Timely diagnosis at the prodromal stage of the disease could offer many potential benefits to patients and caregivers, especially the opportunity to obtain treatment to control symptoms, avoid medications that may worsen symptoms, and, possibly in the future, access to interventions that slow or lessen the disease process. Patients could put into place advance care planning and make end-of-life decisions, consider changing unhealthy lifestyles, and seek better medical care. The findings of this literature review show that, at the current time, these ideas are mainly based on expert opinion and perhaps belief; evidence is lacking, and further studies are needed to demonstrate not only that a timely diagnosis is feasible, but also that it has benefits. Such evidence would support the cultural shift towards diagnosis at the pre-dementia stage of AD.
ACKNOWLEDGMENTS
The authors would like to acknowledge Dr. Deirdre Elmhirst and Dr. Amy Rothman Schonfeld (Rx Communications, Mold, UK) for medical writing assistance in the preparation of this article, funded by Eli Lilly and Company.
Authors’ disclosures available online (http://j-alz.com/manuscript-disclosures/15-0692).
REFERENCES
1 | McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984) Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of department of health and human services task force on Alzheimer’s disease Neurology 34: 939 944 |
2 | Prince M, Bryce R, Ferri C (2011) World Alzheimer Report 2011: The benefits of early diagnosis and intervention. Alzheimer Disease International, http://www.alz.co.uk/research/WorldAlzheimerReport2011.pdf, Accessed on January 20, 2014 |
3 | Dubois B, Feldman HH, Jacova C, Cummings JL, Dekosky ST, Barberger-Gateau P, Delacourte A, Frisoni G, Fox NC, Galasko D, Gauthier S, Hampel H, Jicha GA, Meguro K, O’Brien J, Pasquier F, Robert P, Rossor M, Salloway S, Sarazin M, de Souza LC, Stern Y, Visser PJ, Scheltens P (2010) Revising the definition of Alzheimer’s disease: A new lexicon Lancet Neurol 9: 1118 1127 |
4 | Vos SJB, Xiong C, Visser PJ, Jasielec MS, Hassenstab J, Grant EA, Cairns NJ, Morris JC, Holtzman DM, Fagan AM (2013) Preclinical Alzheimer’s disease and its outcome: A longitudinal cohort study Lancet Neurol 12: 957 965 |
5 | Bertens D, Knol DL, Scheltens P, Visser PJAlzheimer’s Disease Neuroimaging Initiative (2015) Temporal evolution of biomarkers and cognitive markers in the asymptomatic, MCI, and dementia stage of Alzheimer’s disease Alzheimers Dement 11: 511 522 |
6 | Amieva H, Le Goff M, Millet X, Orgogozo JM, Pérès K, Barberger-Gateau P, Jacqmin-Gadda H, Dartigues JF (2008) Prodromal Alzheimer’s disease: Successive emergence of the clinical symptoms Ann Neurol 64: 492 498 |
7 | Wilson RS, Leurgans SE, Boyle PA, Bennett DA (2011) Cognitive decline in prodromal Alzheimer’s disease and mild cognitive impairment Arch Neurol 68: 351 356 |
8 | Harrison J (2013) Cognitive approaches to early Alzheimer’s disease diagnosis Med Clin North Am 97: 425 438 |
9 | Villemagne VL, Burnham S, Bourgeat P, Brown B, Ellis KA, Salvado O, Szoeke C, Macaulay SL, Martins R, Maruff P, Ames D, Rowe CC, Masters CLAustralian Imaging Biomarkers, Lifestyle (AIBL) Research, Group (2013) Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: A prospective cohort study Lancet Neurol 12: 357 367 |
10 | Saxton J, Lopez OL, Ratcliff G, Dulberg C, Fried LP, Carlson MC, Newman AB, Kuller L (2004) Preclinical Alzheimer disease. Neuropsychological test performance 1.5 to 8 years prior to onset Neurology 63: 2341 2347 |
11 | Alzheimer’s Association (2011) Alzheimer’s Association Report. 2011 Alzheimer’s diseases facts and figures Alzheimers Dement 7: 208 244 |
12 | Wimo A, Jonsson L, Bond J, Prince M, Winblad BAlzheimer Disease International (2013) The worldwide economic impact of dementia 2010 Alzheimers Dement 9: 1 11 |
13 | Wimo A, Reed CC, Dodel R, Belger M, Jones RW, Happich M, Argimon JM, Bruno G, Novick D, Vellas B, Haro JM (2013) The GERAS study: A prospective observational study of costs and resource use in community dwellers with Alzheimer’s disease in three European countries – study design and baseline findings J Alzheimers Dis 36: 385 399 |
14 | ALCOVE Project (2013) The European Joint Action on Dementia. Synthesis Report 2013. ALzheimer COoperative Valuation in Europe (ALCOVE), 2013. http://www.alcove-project.eu/images/pdf/ALCOVE_SYNTHESIS_REPORT_VF.pdf, Accessed on February 18, 2014 |
15 | ILC-UK. The European Dementia Research Agenda, http://ilcuk.org.uk/files/pdf_pdf_165.pdf, February 2011, Accessed on February 18, 2014 |
16 | G8 Dementia Summit Declaration, https://www.gov.uk/government/publications/g8-dementia-summit-agreements, Published 11 December 2013, Accessed on February 18, 2014 |
17 | Department of Health (2009) Living Well with Dementia: A National Dementia Strategy. London: Department of Health. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/168220/dh_094051.pdf, Accessed on February 18, 2014 |
18 | Banerjee S (2010) Living well with dementia – development of the national dementia strategy for England Int J Geriatr Psychiatry 25: 917 922 |
19 | Alzheimer Europe. In 2012 Dementia in Europe Yearbook. http://www.alzheimer-europe.org/Policy-in-Practice2/Country-comons/National-Dementia-Strategies-diagnosis-treatment-and-research, 2012, Accessed on February 18, 2014 |
20 | Trova Norme and Concorsi Salute. Presidenza del consiglio dei ministry conferenza unificata. October 30 2014, http://www.trovanorme.salute.gov.it/norme/dettaglioAtto?id=50972&completo=true, Accessed on March 26, 2015 |
21 | Brooker D, Fontaine JL, Evans S, Bray J, Saad K (2014) Public health guidance to facilitate timely diagnosis of dementia: ALzheimer’s cooperative valuation in Europe recommendations Int J Geriatr Psychiatry 29: 682 693 |
22 | De Lepeleire J, Wind AW, Iliffe S, Moniz-Cook ED, Wilcock J, Gonzalez VM, Derksen E, Gianelli MV, Vernooij-Dassen MInterdem Group (2008) The primary care diagnosis of dementia in Europe: An analysis using multidisciplinary, multinational expert groups Aging Ment Health 12: 568 576 |
23 | Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, Delacourte A, Galasko D, Gauthier S, Jicha G, Meguro K, O’brien J, Pasquier F, Robert P, Rossor M, Salloway S, Stern Y, Visser PJ, Scheltens P (2007) Research criteria for the diagnosis of Alzheimer’s disease: Revising the NINCDS-ADRDA criteria Lancet Neurol 6: 734 746 |
24 | Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, DeKosky ST, Gauthier S, Selkoe D, Bateman R, Cappa S, Crutch S, Engelborghs S, Frisoni GB, Fox NC, Galasko D, Habert MO, Jicha GA, Nordberg A, Pasquier F, Rabinovici G, Robert P, Rowe C, Salloway S, Sarazin M, Epelbaum S, de Souza LC, Vellas B, Visser PJ, Schneider L, Stern Y, Scheltens P, Cummings JL (2014) Advancing research diagnostic criteria for Alzheimer’s disease: The IWG-2 criteria Lancet Neurol 13: 614 629 |
25 | Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH (2011) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations for the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease Alzheimers Dement 7: 270 279 |
26 | McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CRJr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH (2011) The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute of Aging-Alzheimer Association workgroups on diagnostic guidelines for Alzheimer’s disease Alzheimers Dement 7: 263 269 |
27 | Cummings JL, Dubois B, Molineuvo JL, Scheltens P (2013) International work group criteria for the diagnosis of Alzheimer disease Med Clin North Am 97: 363 368 |
28 | Molinuevo JL, Rami L (2013) Applying the IWG research criteria in clinical practice: Feasibility and ethical issues Med Clin North Am 97: 477 484 |
29 | Morris JC, Blennow K, Froelich L, Nordberg A, Soininen H, Waldemar G, Wahlund LO, Dubois B (2014) Harmonized diagnostic criteria for Alzheimer’s disease: Recommendations J Intern Med 275: 204 213 |
30 | Boise L, Morgan DL, Kaye J, Camicioli R (1999) Delays in the diagnosis of dementia: Perspectives of family caregivers Am J Alzheimers Dis Other Demen 14: 20 26 |
31 | De Vugt ME, Verhey FR (2013) The impact of early dementia diagnosis and intervention on informal caregivers Prog Neurobiol 110: 54 62 |
32 | Iliffe S, Manthorpe J (2004) The hazards of early recognition of dementia: A risk assessment Aging Ment Health 8: 99 105 |
33 | Mattsson N, Brax D, Zetterberg H (2010) To know or not to know: Ethical issues related to early diagnosis of Alzhiemer’s disease Int J Alzheimers Dis 2010: 841941 |
34 | Milne A (2010) Dementia screening and early diagnosis: The case for and against Health Risk Soc 12: 65 76 |
35 | Gauthier S, Leuzy A, Racine E, Rosa-Neto P (2013) Diagnosis and management of Alzheimer’s disease: Past, present and future ethical issues Prog Neurobiol 110: 102 113 |
36 | Gaugler JE, Ascher-Svanum H, Roth DL, Fafowora T, Siderowf A, Beach TG (2013) Characteristics of patients misdiagnosed with Alzheimer’s disease and their medication use: An analysis of the NACC-UDS database BMC Geriat 13: 137 |
37 | Moher D, Liberati A, Tetzlaff J, Altman DGGroup PRISMA (2009) Preferred reporting items for systematic reviews and meta-anlayses: The PRISMA statement Ann Intern Med 151: 264 269 |
38 | Carpenter BD, Xiong C, Porensky EK, Lee MM, Brown PJ, Coats M, Johnson D, Morris JC (2008) Reaction to a dementia diagnosis in individuals with Alzheimer’s disease and mild cognitive impairment J Am Geriatr Soc 56: 405 412 |
39 | Iliffe S, Manthorpe J, Eden A (2003) Sooner or later? Issues in the early diagnosis of dementia in general practice: A qualitative study Family Pract 20: 376 381 |
40 | Fox M, Fox C, Cruickshank W, Penhale B, Poland F, Steel N (2014) Understanding the dementia diagnosis gap in Norfolk and Suffolk: A survey of general practitioners Qual Prim Care 22: 101 107 |
41 | Wilcock J, Iliffe S, Griffin M, Jain P, Thuné-Boyle I, Lefford F, Rapp D (2013) Tailored education intervention for primary care to improve the management of dementia: The EVIDEM-ED cluster randomized trial Trial 14: 397 |
42 | Littlewood C, Seymour J, Owen V (2010) Does treating Alzheimer’s disease early, delay institutionalisation? Int J Geriatr Psychiatry 25: 1307 1309 |
43 | Bruandet A, Richard F, Bombois S, Maurage CA, Deramecourt V, Lebert F, Amouyel P, Pasquier F (2009) Alzheimer disease with cerebrovascular disease and vascular dementia: Clinical features and course compared with Alzheimer disease J Neurol Neurosurg Psychiatry 80: 133 139 |
44 | Banerjee S, Wittenberg R (2009) Clinical and cost effectiveness of services for early diagnosis and intervention in dementia Int J Geriatr Psychiatry 24: 748 754 |
45 | Weimer DL, Sager MA (2009) Early identification and treatment of Alzheimer’s disease: Social and fiscal outcomes Alzheimers Dement 5: 215 226 |
46 | Getsios D, Blume S, Ishak KJ, Maclaine G, Hernandez L (2012) An economic evaluation of early assessment for Alzheimer’s disease in the United Kingdom Alzheimers Dement 8: 22 30 |
47 | National Audit Office. Improving dementia services in England – an interim report. GP survey results. 2010. http://www.nao.org.uk/wp-content/uploads/2010/01/091082.pdf, Accessed on July 17, 2014 |
48 | Downs M, Turner S, Bryans M, Wilcock J, Keady J, Levin E, O’Carroll R, Howie K, Iliffe S (2006) Effectiveness ofeducational interventions in improving detection and management of dementia in primary care: Cluster randomised controlled study BMJ 332: 692 696 |
49 | Koch T, Iliffe S (2011) Dementia diagnosis and management: A narrative review of changing practice Br J Gen Pract 61: e513 e525 |
50 | National Institute for Health and Care Excellence (NICE) (2006) Dementia. Supporting people with dementia and their carers in health and social care. NICE clinical guideline 42. http://www.nice.org.uk/guidance/cg42 |
51 | Budd D, Burns LC, L’Italien G, Lapuerta P (2011) Impact of early intervention and disease modification in patients with predementia Alzheimer’s disease: A Markov model simulation ClinicoEcon Outcomes Res 3: 189 195 |
52 | Quentin W, Riedel-Heller SG, Luppa M, Rudolph A, König HH (2010) Cost-of-illness studies of dementia: A systematic review focusing on stage dependency of costs Acta Psychiatr Scand 121: 243 259 |
53 | Leicht H, Heinrich S, Heider D, Bachmann C, Bickel H, van den Bussche H, Fuchs A, Luppa M, Maier W, Mösch E, Pentzek M, Rieder-Heller SG, Tebarth F, Werle J, Weyerer S, Wiese B, Zimmermann T, König HHAgeCoDe study group (2011) Net costs of dementia by disease stage Acta Psychiatr Scand 124: 384 395 |
54 | Costa N, Ferlicoq L, Derimeaux-Burel H, Rapp T, Garnault V, Gillette-Guyonnet S, Andrieu S, Vellas B, Lamure M, Grand A, Molinier L (2013) Comparison of informal care time and costs in different age-related dementias: A review Biomed Res Int 2013: 852368 |
55 | Rapp T, Andrieu S, Molinier L, Grand A, Cantet C, Mullins CD, Vellas B (2012) Exploring the relationship between Alzheimer’s disease severity and longitudinal costs Value Health 15: 412 419 |
56 | Barnett JH, Lewis L, Blackwell AD, Taylor M (2014) Early intervention in Alzheimer’s disease: A health economic study of the effects of diagnostic timing BMC Neurology 14: 101 |
57 | Knapp M, Comas-Herrera A, Wittenberg R, Hu B, King D, Rehill A, Adelaja B (2014) Scenarios of dementia care: What are the impacts on cost and quality of life? June 2014. http://www.pssru.ac.uk/pdf/dp2878.pdf, Accessed on July 17, 2014 |
58 | Lewis F, Schaffer SK, Sussex J, O’Neill P, Cockcroft L (2014) The trajectory of dementia in the UK - making a difference London Consulting Report, Office of Health Economics |
59 | Detsky AS (2011) What patients really want from health care JAMA 306: 2500 2501 |
60 | Samsi K, Abley C, Campbell S, Keady J, Manthorpe J, Robinson L, Watts S, Bond J (2014) Negotiating a labyrinth: Experiences of assessment and diagnostic journey in cognitive impairment and dementia Int J Geriatr Psychiatry 29: 58 67 |
61 | Werner P, Karnieli-Miller O, Eidelman S (2013) Current knowledge and future directions about the disclosure of dementia: A systematic review of the first decade of the 21st century.e74-e Alzheimers Dement 9: 88 |
62 | Blendon RJ, Benson JM, Wikler EM, Weldon KJ, Georges J, Baumgart M, Kallmyer BA (2012) The impact of experience with a family member with Alzheimer’s disease on views about the disease across five countries Int J Alzheimer Dis 2012: 903645 |
63 | Riva M, Caratozzolo S, Cerea E, Gottardi F, Zanetti M, Chilovi BV, Cristini C, Padovani A, Rozzini L (2014) Diagnosis disclosure and advance care planning in Alzheimer disease opinions of a sample of Italian citizens Aging Clin Exp Res 26: 427 434 |
64 | Alzheimer Europe Public attitudes about diagnosis. http://www.alzheimer-europe.org/Research/Value-of-Knowing/Public-attitudes-about-diagnosis, 2011, Accessed on November 24, 2014 |
65 | Lawrence V, Pickett J, Ballard C, Murray J (2014) Patient and carer views on participating in clinical trials for prodromal Alzheimer’s disease and mild cognitive impairment Int J Geriatr Psychiatry 29: 22 31 |
66 | Law E, Starr JM, Connelly PJ (2013) Dementia research – what do different public groups want? A survey by the Scottish Dementia Clinical Research Network Dementia 12: 23 28 |
67 | Antoine P, Pasquier F (2013) Emotional and psychological implications of early AD diagnosis Med Clin North Am 97: 459 475 |
68 | Holt GR (2011) Timely diagnosis and disclosure of Alzheimer disease gives patients opportunities to make choices South Med J 104: 779 780 |
69 | Tarawneh R, Holtzman DM (2012) The clinical problem of symptomatic Alzheimer disease and mild cognitive impairment Cold Spring Harb Perspect Med 2: a006148 |
70 | Garand L, Lingler JH, O’Conner KO, Dew MA (2009) Diagnostic labels, stigma and participation in research related to dementia and mild cognitive impairment Res Gerontol Nurs 2: 112 121 |
71 | Werner P, Goldstein D, Buchbinder E (2010) Subjective experience of family stigma as reported by children of Alzheimer’s disease patients Qual Health Res 20: 159 169 |
72 | Werner P, Mittleman MS, Goldstein D, Heinik J (2012) Family stigma and caregiver burden in Alzheimer’s disease Gerontologist 52: 89 97 |
73 | Vernooij-Dassen MJ, Moniz-Cook ED, Woods RT, De Lepeleire J, Leuschner A, Zanetti O, de Rotrou J, Kenny G, Franco M, Peters V, Iliffe S (2005) Factors affecting timely recognition and diagnosis of dementia across Europe: From awareness to stigma Int J Geriatr Psychiatry 20: 377 386 |
74 | Hansen EC, Hughes C, Routley G, Robinson AL (2008) General practitioners’ experiences and understanding of diagnosing dementia: Factors impacting on early diagnosis Soc Sci Med 67: 1776 1783 |
75 | Koch T, Iliffe Sfor the EVIDEM-ED project (2010) Rapid appraisal of barriers to the diagnosis and management of patients with dementia in primary care: A systematic review BMC Fam Prac 11: 52 |
76 | Sharvill NJ (2012) What are the benefits of an early diagnosis? [Letter] BMJ 344: e2747 |
77 | Bunn F, Goodman C, Sworn K, Rait G, Brayne C, Robinson L, McNeilly E, Iliffe S (2012) Psychosocial factors that shape patient and carer experiences of dementia diagnosis and treatment: A systematic review of qualitative studies PLoS Med 9: e1001331 |
78 | Robinson L, Gemski A, Abley C, Bond J, Keady J, Campbell S, Samsi K, Manthorpe J (2011) The transition to dementia – individual and family experiences of receiving a diagnosis: A review Int Psychogeriatr 23: 1026 1043 |
79 | Draper B, Peisah C, Snowdon J, Brodaty H (2010) Early dementia diagnosis and the risk of suicide and euthanasia Alzheimers Dement 6: 75 82 |
80 | Seyfried LS, Kales HC, Ignacio RV, Conwell Y, Valenstein M (2011) Predictors of suicide in patients with dementia Alzheimers Dement 7: 567 573 |
81 | Draper BM (2015) Suicidal behavior and assisted suicide in dementia Int Psychogeriatr 27: 1601 1611 |
82 | Ward A, Tadiff S, Dye C, Arrighi HM (2013) Rate of conversion from prodromal Alzheimer’s disease to Alzheimer’s dementia: A systematic review of the literature Dement Geriatr Cogn Disord Extra 3: 320 332 |
83 | Hort J, O’Brien JT, Gainotti G, Pirttila T, Popescu BO, Rektorova I, Sorbi S, Scheltens PEFNS Scientist Panel on Dementia (2010) EFNS guidelines for the diagnosis and management of Alzheimer’s disease Eur J Neurol 17: 1236 1248 |
84 | Iliffe S, Robinson L, Brayne C, Goodman C, Rait G, Manthorpe J, Ashley PDeNDRoN Primary Care Clinical Studies Group (2009) Primary care and dementia: 1. Diagnosis, screening and disclosure Int J Geriatr Psychiatry 24: 895 901 |
85 | Turner S, Iliffe S, Downs M, Wilcock J, Bryans M, Levin E, Keady J, O’Carroll R (2004) General practitioner’s knowledge, confidence and attitudes in the diagnosis and management of dementia Age Ageing 33: 461 467 |
86 | Waldemar G, Phung KT, Burns A, Georges J, Hansen FR, Iliffe S, Marking C, Rikkert MO, Selmes J, Stoppe G, Sartorius N (2007) Access to diagnostic evaluation and treatment for dementia in Europe Int J Geriatr Psychiatry 22: 47 54 |
87 | Cahill S, Clark M, O’Connell H, Lawlor B, Coen RF, Walsh C (2008) The attitudes and practices of general practitioners regarding dementia diagnosis in Ireland Int J GeriatrPsychiatry 23: 663 669 |
88 | Bradford A, Kunik ME, Schulz P, Williams SP, Singh H (2009) Missed and delayed diagnosis of dementia in primary care: Prevalence and contributing factors Alzheimer Dis Assoc Disord 23: 306 314 |
89 | Ahmad S, Orrell M, Iliffe S, Gracie A (2010) GPs’ attitudes, awareness, and practice regarding early diagnosis of dementia Br J Gen Pract 60: e360 e365 |
90 | Jones RW, Mackell J, Berthet K, Knox S (2010) Assessing attitudes and behaviours surrounding Alzheimer’s disease in Europe: Key findings of the Important Perspectives on Alzheimer’s Care and Treatment (IMPACT) survey J Nutr Health Aging 14: 525 530 |
91 | Martinez-Lage P, Frolich L, Knox S, Berthet K (2010) Assessing physician attitudes and perceptions of Alzheimer’s disease across Europe J Nutr Health Aging 14: 537 544 |
92 | Manthorpe J, Samsi K, Campbell S, Abley C, Keady J, Bond J, Watts S, Robinson L, Warner J, Iliffe S (2013) From forgetfulness to dementia: Clinical and commissioning implications of diagnostic experiences Br J Gen Pract 63: e69 e75 |
Figures and Tables
Fig.1
Timeline of AD progression and diagnosis points on the disease continuum (adapted from Prince et al. [2]).
![Timeline of AD progression and diagnosis points on the disease continuum (adapted from Prince et al. [2]).](https://content.iospress.com:443/media/jad/2016/49-3/jad-49-3-jad150692/jad-49-3-jad150692-g001.jpg)
Fig.2
Flow chart of publication identification and selection.
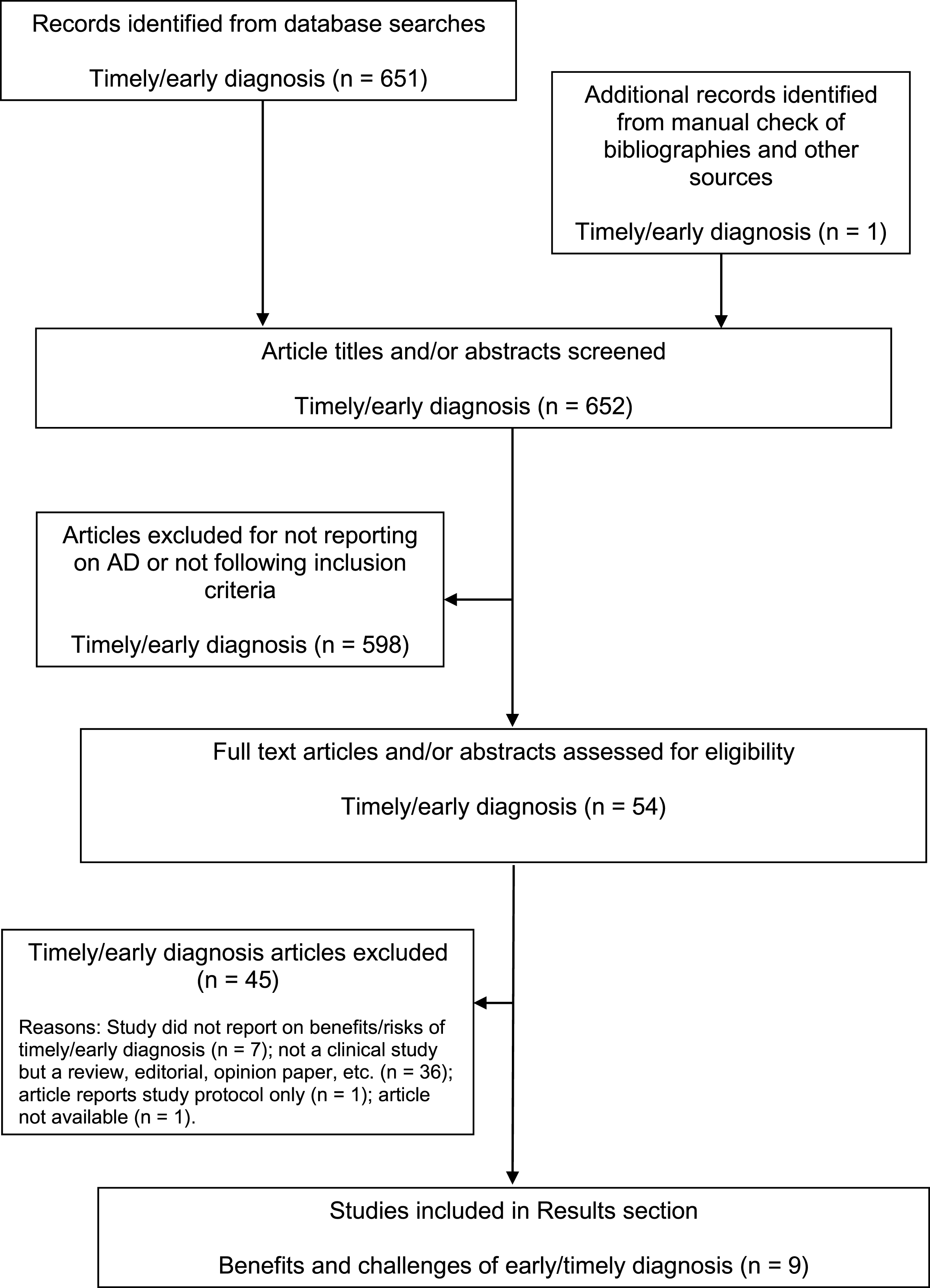
Table 1
IWG-2 criteria for the diagnosis of typical AD
| A plus B at any stage | |
| A. Specific clinical phenotype | • Presence of an early and significant episodic memory impairment (isolated or associated with other cognitive or behavioral changes that are suggestive of a mild cognitive impairment or of a dementia syndrome) that includes the following features: |
| • Gradual and progressive change in memory function reported by patient or informant over more than 6 months | |
| • Objective evidence of an amnestic syndrome of the hippocampal type, *based on significantly impaired performance on an episodic memory test with established specificity for AD, such as cued recall with control of encoding test | |
| B. In vivo evidence of AD pathology (one of the following) | • Decreased Aβ1-42 together with increased T-tau or P-tau in cerebrospinal fluid |
| • Increased tracer retention on amyloid PET | |
| • AD autosomal dominant mutation present (in presenilin 1 (PSEN1), presenilin 2 (PSEN2), or amyloid precursor protein (APP) |
Table source: [24]; *Hippocampal amnestic syndrome might be difficult to identify in the moderately severe to severe dementia stages of the disease, in which in vivo evidence of AD pathology might be sufficient in the presence of a well-characterized dementia syndrome.
Table 2
Benefits of and challenges to timely diagnosis of AD for patients, families, healthcare providers, and society
| Benefits | Challenges |
| Shared | |
| Can promote shared management | Stigma |
| Encourages planning of future care, including design of a specific, individualized treatment and management plan | Denial Belief that memory problems are a normal part of aging causes delays in seeking help |
| Allows prompt evaluation and treatment of reversible causes of impairment | Lack of awareness about signs and symptoms of dementia |
| Allows potential management of symptoms with medications or other interventions | Financial constraints or negative effects of reimbursement systems |
| Helps facilitate treatment or management of coexisting medical conditions that may worsen cognitive function and prevents prescription of medications for co-existing conditions that may impair cognitive function | Cultural beliefs impact degree of perceived stigma and support alternative explanations of symptoms Limited preventative or treatment options Shortage of specialist diagnostic services Lack of clinically proven biomarkers or diagnostic test |
| Enables the inclusion of patients in clinical trials for researching new treatments | |
| Aids management of possible behavioral symptoms | |
| Encourages the development of coping strategies to handle future changes in patient’s function | |
| Could reduce the overall costs of dementia | |
| Could postpone institutionalization into residences and nursing homes | |
| Could reduce dangerous and challenging behavior (e.g., traffic accidents, etc.) |
| Patients | |
| Can reduce anxiety by addressing concerns about early symptoms | Normalization of symptoms (belief that memory problems are a normal part of aging causes delays in seeking help) |
| Can improve quality of life, social skills, and future security | Prioritization of physical health problems over mental health problems |
| Enables the organization of timely counseling and social support | Financial limitations |
| Risk of suicide | |
| Fear and anxiety | |
| Social isolation | |
| Worry about loss of competency (e.g., driver’s license, work, control of finances) | |
| Impaired decision making | |
| Limited access to healthcare services, long wait times | |
| Desire for autonomy, fear of loss of independence | |
| Desire to maintain pre-dementia identity | |
| Personality characteristics, including coping strategies | |
| Age |
| Families/Caregivers | |
| Provides answers to concerns about cognitive and functional impairment of the relative, which may help to reduce anxiety | Normalization of symptoms (belief that memory problems are a normal part of aging causes delays in seeking help) |
| Enables the search for solutions in care and emotional burden of disease. Care plans can be initiated. | Fear and anxiety Apprehension about changes to family dynamics Desire to sustain autonomy of affected person |
| Opens up access to social support, resources and psycho-educational groups earlier, reducing caregiver strain | Time constraints Financial concerns Reluctance to take on caregiving burden |
| Allows anticipation and prevention of future problems (e.g., risk of falling, financial mismanagement, vulnerability to scams) | Limited access to treatment centers |
| Physical distance between patient and family members may impair recognition of early symptoms and/or accessing care | |
| Patient resistance | |
| Discordance between decision makers |
| Healthcare Providers | |
| Lays groundwork for a relationship with caregivers and family of patient, and fosters the provision of appropriate advice and information to the patient and family | Lack of knowledge in identifying early symptoms of AD PCPs lack confidence in ability to diagnose AD Misdiagnosis or diagnostic uncertainty |
| Stimulates liaison with colleagues in secondary and tertiary care regarding patient care and management | Prioritization of physical health problems over mental health problems |
| Poor communication skills | |
| Reluctance to disclose diagnosis | |
| Time constraints | |
| Limited reimbursement | |
| Poor care coordination | |
| Variability in patient’s response to diagnosis |
| Society | |
| Reduction in overall healthcare costs | Lack of healthcare personnel capable of making timely diagnosis |
| Greater public safety with reduction of dangerous and challenging behaviors (e.g., traffic accidents) | Shortage of adequate support services |
Table sources: [2, 11, 22, 30, 39, 68, 73–77, 84–92].
Table 3
Ethical challenges for healthcare providers associated with a timely diagnosis of prodromal AD
| • Consequences of disclosure of a diagnosis of AD in persons with minimal symptoms who have full insight |
| • Diagnostic uncertainty based on biomarkers that have not been fully validated; no “gold standard” biomarker or specific diagnostic threshold values currently available |
| • Social stigma of very mild AD |
| • Repeated assessment of competence |
| • Cost of diagnostic tests |
| • Access to and cost of treatment |
| • Exclusion criteria for treatment |
| • Cost/benefit of drugs; reimbursement issues and rules about discontinuation |
| • Competency of patient to consent to participate in research studies |
| • Use of placebo in randomized clinical trials for new drugs |
| • Impact on patient’s autonomy and capacity (e.g., insurance premiums, driving license) |
| • Decision-making competence and judging future best interests; conflicts may arise as personality and sense of identity can change as disease progresses |
| • Determination of whether a patient is safe and has adequate family or other social support and long-term care plan; for those who do not, providing help to secure medical and social support services |




