Analysis of the Inhibitory Elements in the p5 Peptide Fragment of the CDK5 Activator, p35, CDKR1 Protein
Abstract
Besides the hallmark pathology of amyloid plaques and neurofibrillary tangles, it is well documented that cyclin-dependent kinase 5 (CDK5), a critical neuronal protein kinase in nervous system development, function, and survival, when deregulated and hyperactivated induces Alzheimer’s disease (AD) and amyotrophic lateral sclerosis and Parkinson’s disease-like phenotypes in mice. In a recent study, we demonstrated that p5, a small, truncated fragment of 24 amino acid residues derived from the CDK5 activator protein 35 (NCK5A, p35), selectively inhibited deregulated CDK5 hyperactivity and ameliorated AD phenotypes in model mice. In this study, we identified the most inhibitory elements in the p5 peptide fragment. Each amino acid residue in p5 was systematically replaced with its homologous residues that may still be able to functionally substitute. The effects of these p5 peptide analogs were studied on the phosphotransferase activities of CDK5/p35, CDK5/p25, ERK1, and GSK3β. The mimetic p5 peptide (A/V substitution at the C-terminus of the peptide) in the sequence, KNAFYERALSIINLMTSKMVQINV (p5-MT) was the most effective inhibitor of CDK5 kinase activity of 79 tested mimetic peptides including the original p5 peptide, KEAFWDRCLSVINLMSSKMLQINA (p5-WT). Replacement of the residues in C-terminus end of the peptide affected CDK5 phosphotransferase activity most significantly. These peptides were strong inhibitors of CDK5, but not the related proline-directed kinases, ERK1 and GSK3β.
INTRODUCTION
Phosphorylation of neuronal cytoskeletal proteins is topographically and stably regulated during nervous system development and function. Although protein kinases substrates and regulators are synthesized in the soma, phosphorylation of cytoskeletal proteins such as neurofilaments is consigned to axons [1–4]. While studying the protein kinases involved in compartment-specific phosphorylation in neurons, we identified the cell cycle-like kinase, cyclin-dependent kinase 5 (CDK5), as a major kinase responsible for the phosphorylation of proline- directed Ser/Thr repeats in the C- terminus tail domains of human neurofilament proteins [5]. CDK5 is unique among the CDK family of protein kinases; its activity is primarily restricted to neuronal cells due to its neuron specific activators CDKR1 (also known as p35) and CDKR2 (also known as p39). CDK5 is a multifunctional kinase that targets more than a hundred proteins including other protein kinases and phosphatases essential to neuronal development, function, and survival [2, 6–8].
In recent years, we and others have shown that CDK5 is deregulated and hyperactivated in the brains of patients expressing several neurodegenerative disorders such as Alzheimer’s disease (AD) and amyotrophic lateral sclerosis (ALS) [9–13]. A hypothesis has been proposed that CDK5 deregulation arises in stressed neurons (oxidative, amyloid-β, glutamate excitotoxic, or inflammatory), accompanied by increase in Ca +2 influx, calpain activation followed by proteolytic cleavage of the p35 activator into a p10 N-terminal fragment and a p25 hyperactivator that stably binds and hyperactivates CDK5 in a CDK5/p25 complex [13–17]. Such complexes have been detected in AD brains, and they may contribute, in part, to the formation of the hyperphosphorylated neurofilament and tau tangles, and the appearance of amyloid plaques and neuronal apoptosis, all of which are hallmarks of AD pathology. Accordingly, CDK5/p25 has been identified as a potential therapeutic target for AD and other neurodegenerative disorders that share a similar pattern of CDK5 hyperactivation [17].
Currently, most therapeutic approaches that target the deregulated CDK5/p25 complex have focused primarily on drugs like Roscovitine that inhibit by interfering with the ATP binding domain of CDK5 [18–20]. These drugs, however, lack specificity, since all kinases including cell cycle CDKs, are vulnerable at the ATP binding site. During the course of our studies on the basis of CDK5/p25 crystal structure, the amino acid residues interacting between CDK5 and p25 chains within 3.5 Angstroms were identified (unpublished data). This analysis identified two peptides derived as truncations of the p35 regulator, a larger 126 amino acid fragment (CIP) and a shorter 24 amino acid peptide (p5). In vitro, these peptides inhibited CDK5/p35 and CDK5/p25, respectively, whereas in rodent cortical neurons, only the deregulated CDK5/p25 was specifically inhibited without affecting the endogenous CDK5/p35 activity [21]. We considered these peptides as potential therapeutic candidates for rescuing neurodegenerative disorders in model mice that share the hyperactivated CDK5-induced phenotypes.
In a recent study we demonstrated p5 has a higher inhibitory activity compared to CIP. In the present study, to further understand p5’s inhibitory role, we undertook the synthesis of analogues of the parent peptide p5-WT (KEAFWDRCLSVINLMSSKMLQINA) in which each amino acid was individually replaced with homologous residues that may still be able to functionally substitute. This analysis generated 78 mimetic peptides. The effects of these peptides on recombinant human CDK5/p25 phosphotransferase activity were evaluated. In addition to CDK5/p25, the actions of these p5 peptide analogs on the phosphotransferase activities of CDK5/p35, ERK1, and GSK3β were also measured. From these studies, we identified a mimetic p5 peptide, KNAFYERALSIINLMTSKMVQINV (p5-MT), that may feature sufficiently distinct epitopes such that it would not be recognized by antibodies that would react with endogenous p35 and its proteolytic fragments. In addition, p5-MT showed more potent inhibitory activity toward CDK5 compared to p5-WT.
MATERIALS AND METHODS
Quality control and reagents
The various recombinant protein kinase targets employed in the target profiling process were sourced from Signal Chem Pharmaceuticals, Inc. (Richmond, BC, Canada). Quality control testing was routinely performed on each of the protein kinase targets to ensure compliance to acceptable standards. [γ–33P]ATP was purchased from PerkinElmer.
Both enzymes (CDK5/p25 and CDK5/p35) were co-expressed together by baculovirus in Sf9 insect cells using an N-terminal GST tag and purified. Since they were co-expressed together, they were added to the reaction mixture as CDK5/p25 and CDK5/p35 complexes at the same time. The assay conditions for protein kinases were optimized to yield acceptable enzymatic activity. In addition, the assays were optimized to give a high signal-to-noise ratio.
Protein kinase assays
A radioisotope assay format was used for profiling an evaluation of the kinase under investigation and all assays were performed in a designated radioactive working area. Protein kinase assays were carried out at 30°C for 20 min in a final volume of 25 μl according to the following assay reaction recipe:
Set 1:
Component 1. 5 μl of diluted active CDK5/p35 and CDK5/p25 (final assay concentrations of 14 and 15 nM, respectively).
Component 2. 5 μl of assay solution of calf thymus histone H1 (final assay concentration 1 mg/ml).
Component 3. 5 μl of kinase assay buffer (concentrations: 25 mM MOPS, pH 7.2, 12.5 mM β-glycerophosphate, 25 mM MgCl2, 5 mM EGTA, 2 mM EDTA and 0.25 mM dithiothreitol).
Component 4. 5 μl of [γ–33P]ATP (250 μM stock solution, 0.8 μCi).
Component 5. 5 μl of p5 (p5-WT) or p5-MT (p5 A/V) peptide.
Set 2:
Components 1–4 were same as in Set 1.
Component 5. 5 μl of peptide inhibitor (various concentrations) or 10% DMSO.
Each assay was initiated by the addition of [γ–33P] and, after 20 min incubation, was terminated by spotting 10 μl of the reaction mixture onto Multi screen phosphocellulose P81 plate. The Multi screen phosphocellulose P81 plate was washed 3 times for approximately 15 min each time in a 1% phosphoric acid solution. The radioactivity on the P81 plate was counted in the presence of scintillation fluid in a Trilux scintillation counter. Blank control was set up which included all the assay components except the addition of the appropriate substrate (replaced with equal volume of assay dilution buffer). The corrected activity for protein kinase was determined by removing the blank control value. Each experiment was repeated twice in duplicate.
All other materials were of standard laboratory grade. The 79 peptides analogs were produced by SPOT synthesis as large spots and were based on a selected substitution analysis based on the original sequence KEAFWDRCLSVINLMSSKMLQINA and shown in Supplementary Table 1.
RESULTS AND DISCUSSION
By systematic alteration of each amino acid residue in the parent peptide sequence with a homologous amino acid residue, it should be possible to identify the most crucial amino acid positions. It may be feasible to even identify amino acids that may improve the binding of p5 analogues to CDK5. These are mimetic substitutions that preserve and may improve upon the inhibitory activity of the p5 peptide toward CDK5.
Furthermore, the resulting analogues may also be immunologically distinct, which could foster their differentiation from endogenous p35 and its derivatives with antibodies. The actual sequence of p5 cannot be used to create an immunizing peptide that can distinguish between p5 and a shorter peptide with the same sequence. By identifying the most critical amino acids for inhibition, a modified inhibitory peptide may be created to elicit antibodies that could be used to track its distribution in the brain in future immunohistochemistry experiments.
SPOT synthesis of p5 peptide analogues was undertaken as the method of choice for this purpose as it would be the most cost-effective way to identify the critical amino acid residues in p5 that permit it to compete with p25 and/or p35 and inhibit CDK5 more effectively. As shown in Supplementary Tables 1 and 2, using the 78 mimetic peptides of p5, our studies revealed that the most critical amino acid residues for the inhibitory effect of p5 on CDK5/p25 were located in the last four amino acids in the p5 parent peptide (QINA). Replacement of A to V and M (A/V, M) provided for the most effective inhibitory residues substitutions. Conversely, substitution of Q to N, E, or D were the most stimulatory replacements, increasing activity by almost 80% . Equivalent stimulation is seen in the replacement of I to G, and N to G, E, or D.
The initial aim of these experiments was to determine which of the various peptides tested exhibited reduced inhibitory activity so as to identify the most critical amino acids for the interaction. It was also interesting to define replacement amino acids that could inhibit the phosphotransferase activity of CDK5/p25 better than the original peptide p5-WT (sequence KEAFWDRCLSVINLMSSKMLQINA; Peptide-1).
The phosphotransferase activity of CKD5/p25 protein kinase in the presence of each of the 79 peptides listed in Table 1 was assayed by employing the standardized radioactive assay methodology as described in Materials and Methods. The results observed as activity (cpm) are presented in Supplementary Tables 1 and 2. The intra-assay variability was determined to be less than 10%.
The profiling data for the 79 inhibitory peptides assayed against CDK5/p25 in Table 1 are arranged based on lowest to highest counts per minute (cpm). The % Activity and % Change from Control (CFC) values were determined using the original Peptide-1 (sequence KEAFWDRCLSVINLMSSKMLQINA) as the control (100% ) and measuring all other counts relative to this. With this method, only four peptides were identified that yielded lower counts against CDK5/p25 than the original peptide sequence. Peptides-77 and 78 showed the most inhibition of CDK5/p25 compared to the control p5-WT peptide, at −26 and −25% , respectively. Peptides-15 and 76 also showed moderate reductions of −8 and −7% . Most of the other peptides gave higher counts (cpm) ranging from +0 to +79% greater than observed with the parent p5 peptide with the histone H1 substrate. The four peptides that gave the highest counts were Peptides-26, 62, 64, and 70. The altered amino acids residues in these particular peptides were apparently the most critical for the CDK5 inhibitory activity of p5.
Figure 1 summarizes the findings from analysis of the inhibitory actions of the p5-WT mimetic peptide analogs on the CDK5 phosphotransferase activity. We conclude that the most critical amino acid residues for the inhibitory effect of p25 fragment on CDK5 phosphotransferase activity in the presence of p25 activator appear to be: K1, A3, F4, R7, L9, S10, I12, N13, M15, S17, K18, M19, Q21, I22, and N23. These amino acid residues were distributed across the entire sequence, but region from residues 10 to 23 was the most essential for binding, whereas the last amino acid residue at Position 24 could be altered to actually improve the inhibitory activity of p5 peptide analogs. The most effective replacements of A at Position 24 were with the M and V amino acid residues (A24M and A24V). Most of the other amino acid residues replacements of the p5-WT sequence reduced the inhibitory effect of the peptide mimetics on p25-activated CDK5 phosphotransferase activity. The most marked losses of inhibitory activity were observed with the peptides with the S10A, N13D, L20A Q21N, I22G and N23D replacements.
The above experiments provided evidence that mimetic p5 (A/V) corresponding to Peptide-77 in Table 1 with the sequence KNAFYERALSIINLMTSKMVQINV (p5-MT) was one of the most effective inhibitors of CDK5 phosphotransferase activity of the 79 tested peptides. We next carried out a comparative study using the original wild-type p5 peptide corresponding to Peptide-1 in Table 1 with the sequence KEAFWDRCLSVINLMSSKMLQINA (p5-WT) and the p5-MT peptide to ascertain their inhibitory effects on CDK5/p35, CDK5/p25, and two more related proline-directed protein-serine/threonine kinases, GSK3β and ERK1. The results observed as % change of phosphotransferase activity at different inhibitor concentrations are presented in Table 2, while Supplementary Tables 3–6 and 7–10 contains all the raw data for these protein kinase assays with the p5-WT and p5-MT peptides, respectively. The intra-assay variability was determined to be less than 10% . Inhibition of target phosphotransferase activity by the compound gave negative values, while activation of phosphotransferase target activity gave positive values. Only values of >25% change were considered to be significant.
The profiling study for the parent peptide p5-WT against CDK5/p25 showed moderate to strong inhibition of CDK5/p25 phosphotransferase activity with increasing compound concentration (Supplementary Table 3). At 250 μM concentration of this peptide, the CDK5/p25 phosphotransferase activity was inhibited by 59% compared to control. An IC50 value of 169 μM was generated (using a graph of log inhibitor versus normalized response with variable slope with the Prism software) for this peptide against CDK5/p25 (Fig. 2).
Likewise, the profiling data for p5-WT against CDK5/p35 showed moderate to strong inhibition of CDK5/p35 activity with increasing compound concentration (Supplementary Table 4). At 250 μM concentration of this peptide, the CDK5/p35 activity was inhibited by 53% compared to control. An IC50 value of 217 μM was generated (using a graph of log inhibitor versus normalized response with variable slope with the Prism software) for this compound against CDK5/p35 (Fig. 2). These findings reveal that p5-WT inhibited both p25 and p35 activation of CDK5 fairly equally.
The profiling data for compound p5-WT against ERK1 showed much weaker inhibition of ERK1 phosphotransferase activity with increasing compound concentration (Fig. 2). At 500 μM concentration of p5-WT, the ERK1 activity was inhibited by only 25% compared to control. An IC50 value of 1322 μM was generated (using a graph of log inhibitor versus normalized response with variable slope with the Prism software) for this peptide against ERK1 (Fig. 2). The profiling data for p5-WT against GSK3β showed no inhibition of GSK3β phosphotransferase activity with increasing compound concentration (Fig. 2). At 500 μM concentration of this peptide, the GSK3β phosphotransferase activity was slightly activated by 24% compared to control.
The profiling data for the mutated peptide p5-MT against CDK5/p25 showed strong to potent inhibition of CDK5/p25 phosphotransferase activity with increasing compound concentration (Supplementary Table 7). At 100 μM concentration of this peptide, the CDK5/p25 phosphotransferase activity was inhibited by 46% compared to control. An IC50 value of 119 μM was generated (using a graph of log inhibitor versus normalized response with variable slope with the Prism software) for this compound against CDK5/p25 (Fig. 3).
The profiling data for compound p5-MT against CDK5/p35 showed strong to potent inhibition of CDK5/p35 phosphotransferase activity with increasing compound concentration (Supplementary Table 8). At 100 μM concentration of compound p5-MT, the CDK5/p35 phosphotransferase activity was inhibited by 57% compared to control. An IC50 value of 83 μM was generated (using a graph of log inhibitor versus normalized response with variable slope with the Prism software) for this compound against CDK5/p35 (Fig. 3).
The profiling data for p5-MT against ERK1 showed weak to moderate inhibition of ERK1 phosphotransferase activity with increasing compound concentrations (Supplementary Table 9). At 500 μM concentration of compound p5-MT, the ERK1 activity was inhibited by 54% compared to control. An IC50 value of 425 μM was generated (using a graph of log inhibitor versus normalized response with variable slope with the Prism software) for this compound against ERK1 (Fig. 3). The profiling data for p5-MT against GSK3β showed weak activation of this kinase with increasing compound concentration (Fig. 3). At 500 μM concentration of this peptide, the GSK3β phosphotransferase activity was activated by 40% compared to control.
CONCLUSION
After the production of 78 mimetic peptides with single amino acid substitutions of the p5-WT sequence, the p5-MT peptide with a A24V replacement was determined to be slightly more potent in its inhibition of p25 and p35 stimulation of CDK5 phosphotransferase activity (IC50= 169 to 217 μM for p25 and p35, respectively for p5-WT; IC50= 119 to 83 μM for p25 and p35, respectively for p5-MT). The p5-WT and p5-MT were more extensively tested for their actions on the related proline-directed kinases ERK1 and GSK3β, and ERK1 was modestly inhibited by the p5-MT peptide (IC50= 425 μM) and to a lesser extent by the p5-WT peptide, and the phosphotransferase activity of GSK3-β was weekly stimulated by these peptides.
ACKNOWLEDGMENTS
The authors thank Dr. Philip Grant for helpful discussions. This work was supported by the intramural research program of the U.S. National Institutes of Health (NIH), NINDS.
Authors’ disclosures available online (http://j-alz.com/manuscript-disclosures/15-0412r2).
Appendices
The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JAD-150412.
REFERENCES
1 | Shetty KT, Link WT, Pant HC(1993) cdc2-like kinase from rat spinal cord specifically phosphorylates KSPXK motifs in neurofilament proteins: Isolation and characterizationProc Natl Acad Sci U S A90: 68446848 |
2 | Tsai LH, Delalle I, Caviness VSJr, Chae T, Harlow E(1994) p35 is a neural-specific regulatory subunit of cyclin-dependent kinase 5Nature371: 419423 |
3 | Grant P, Pant HC(2002) Topographic regulation of kinase activity in Alzheimer’s disease brainsJ Alzheimers Dis4: 269281 |
4 | Binukumar BK, Shukla V, Amin ND, Reddy P, Skuntz S, Grant P, Pant HC(2013) Topographic regulation of neuronal intermediate filaments by phosphorylation, role of peptidyl-prolyl isomerase 1: Significance in neurodegenerationHistochem Cell Biol140: 2332 |
5 | Pant HC, Veeranna (1995) Neurofilament phosphorylationBiochem Cell Biol73: 575592 |
6 | Cheng K, Ip NY(2003) Cdk5: A new player at synapsesNeurosignals12: 180190 |
7 | Ohshima T, Mikoshiba K(2002) Reelin signaling and Cdk5 in the control of neuronal positioningMol Neurobiol26: 153166 |
8 | Ohshima T, Ward JM, Huh CG, Longenecker G, Veeranna , Pant HC, Brady RO, Martin LJ, Kulkarni AB(1996) Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal deathProc Natl Acad Sci U S A93: 1117311178 |
9 | Lee MS, Tsai LH(2003) Cdk5: One of the links between senile plaques and neurofibrillary tangles?J Alzheimers Dis5: 127137 |
10 | Lim AC, Qi RZ(2003) Cyclin-dependent kinases in neural development and degenerationJ Alzheimers Dis5: 329335 |
11 | Monaco EA3rd(2004) Recent evidence regarding a role for Cdk5 dysregulation in Alzheimer’s diseaseCurr Alzheimer Res1: 3338 |
12 | Monaco EA3rd, Vallano ML(2005) Role of protein kinases in neurodegenerative disease: Cyclin-dependent kinases in Alzheimer’s diseaseFront Biosci10: 143159 |
13 | Patrick GN, Zukerberg L, Nikolic M, de la Monte S, Dikkes P, Tsai LH(1999) Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegenerationNature402: 615622 |
14 | Kesavapany S, Li BS, Pant HC(2003) Cyclin-dependent kinase 5 in neurofilament function and regulationNeurosignals12: 252264 |
15 | Shukla V, Skuntz S, Pant HC(2012) Deregulated Cdk5 activity is involved in inducing Alzheimer’s diseaseArch Med Res43: 655662 |
16 | Zheng YL, Amin ND, Hu YF, Rudrabhatla P, Shukla V, Kanungo J, Kesavapany S, Grant P, Albers W, Pant HC(2010) A 24-residue peptide (p5), derived from p35, the Cdk5 neuronal activator, specifically inhibits Cdk5-p25 hyperactivity and tau hyperphosphorylationJ Biol Chem285: 3420234212 |
17 | Tsai LH, Lee MS, Cruz J(2004) Cdk5, a therapeutic target for Alzheimer’s disease?Biochim Biophys Acta1697: 137142 |
18 | Wu PC, Tai MH, Hu DN, Lai CH, Chen YH, Wu YC, Tsai CL, Shin SJ, Kuo HK(2008) Cyclin-dependent kinase inhibitor roscovitine induces cell cycle arrest and apoptosis in rabbit retinal pigment epithelial cellsJ Ocul Pharmacol Ther24: 2533 |
19 | Yan Z, Chi P, Bibb JA, Ryan TA, Greengard P(2002) Roscovitine: A novel regulator of P/Q-type calcium channels and transmitter release in central neuronsJ Physiol540: 761770 |
20 | Zhang B, Tan VB, Lim KM, Tay TE, Zhuang S(2007) Study of the inhibition of cyclin-dependent kinases with roscovitine and indirubin-3’-oxime from molecular dynamics simulationsJ Mol Model13: 7989 |
21 | Amin ND, Albers W, Pant HC(2002) Cyclin-dependent kinase 5 (cdk5) activation requires interaction with three domains of p35J Neurosci Res67: 354362 |
Figures and Tables
Fig.1
Mutational analysis of the p5-WT peptide to define the critical amino acids for its inhibitory action on CDK5 phosphotransferase activity. Replacement amino acids are graphed according to their actions on CDK5 kinase activity as a percentage of the effect of the parent p5-WT peptide, which served as a control. Single letter designations are used for each amino acid and these are colored in part according to their charge and hydrophobicity. The corresponding amino acid residue positions are shown in the bottom X-axis.
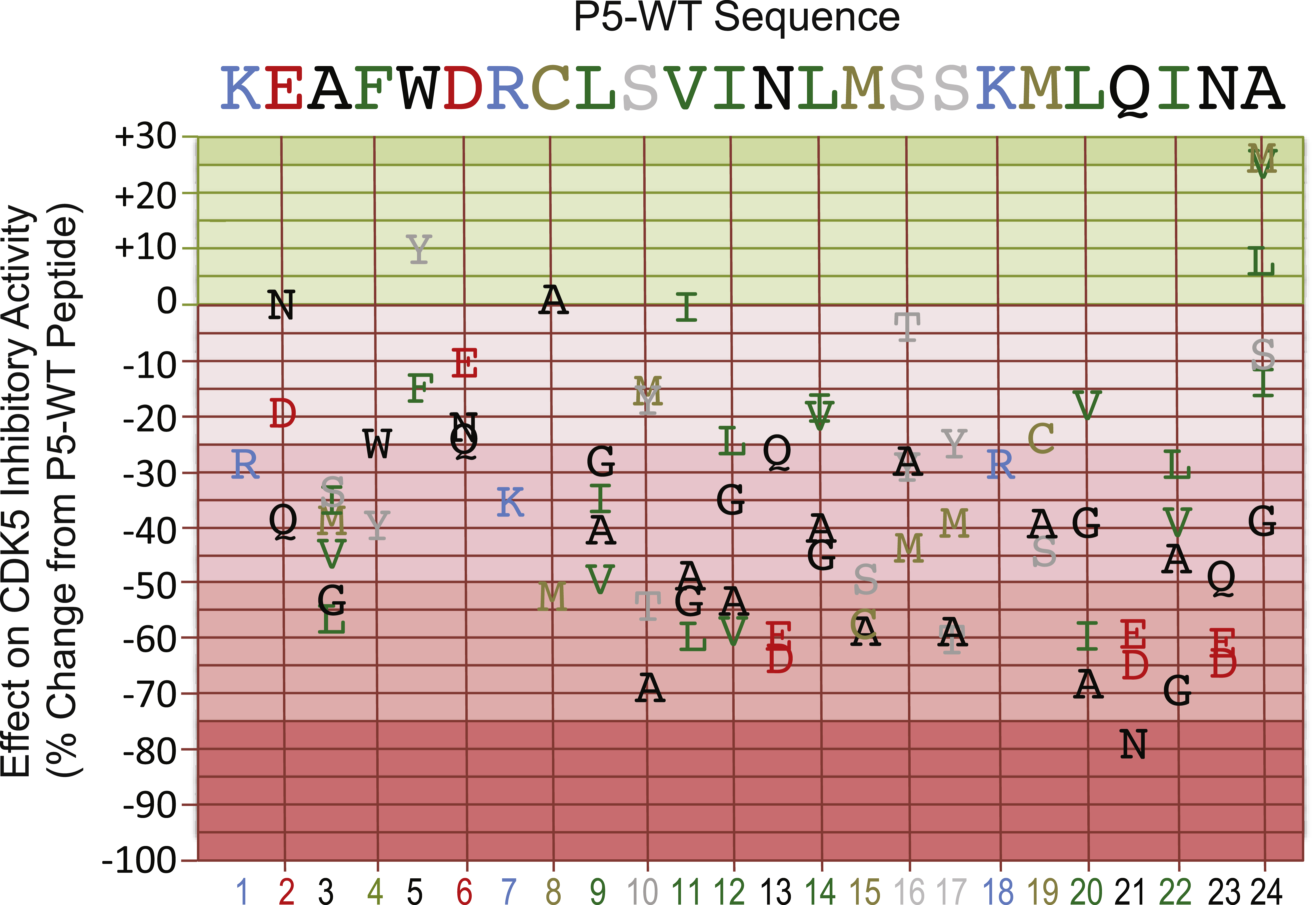
Fig.2
p5-WT peptide concentration-dependent effects on the phosphotransferase activities of CDK5, ERK1 and GSK3β. IC50 determination for compound p5-WT against protein kinases. A graph of log inhibitor versus normalized response with variable slope was generated using the Prism software. The graph showed increased inhibition of the phosphotransferase activities of CDK5 and ERK1, and increased stimulation of GSK3β with increasing compound concentration. The IC50 value for compound p5-WT against CDK5/p25 was determined to be 169 μM, the IC50 value for compound p5-WT against CDK5/p35 was determined to be 217 μM, and the IC50 value for compound p5-WT against ERK1 was determined to be 1322 μM.
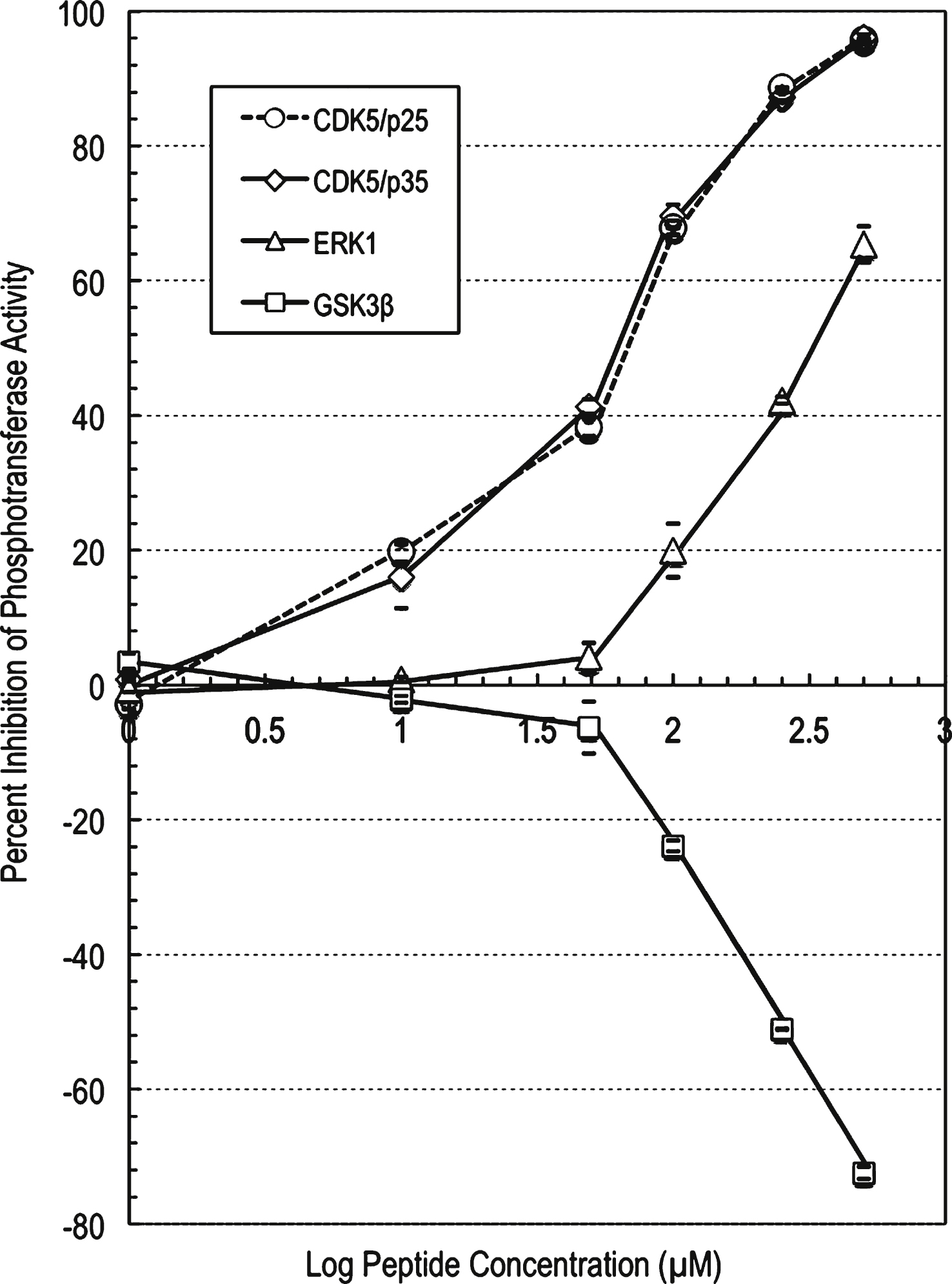
Fig.3
p5-MT peptide concentration-dependent effects on the phosphotransferase activities of CDK5, ERK1, and GSK3β. IC50 determination for compound p5-MT against protein kinases. A graph of log inhibitor versus normalized response with variable slope was generated using the Prism software. The graph showed increased inhibition of the phosphotransferase activities of CDK5 and ERK1, and increased stimulation of GSK3β with increasing compound concentration. The IC50 value for compound p5-MT against CDK5/p25 was determined to be 119 μM, the IC50 value for compound p5-MT against CDK5/p35 was determined to be 83 μM, and the IC50 value for compound p5-MT against ERK1 was determined to be 425 μM.
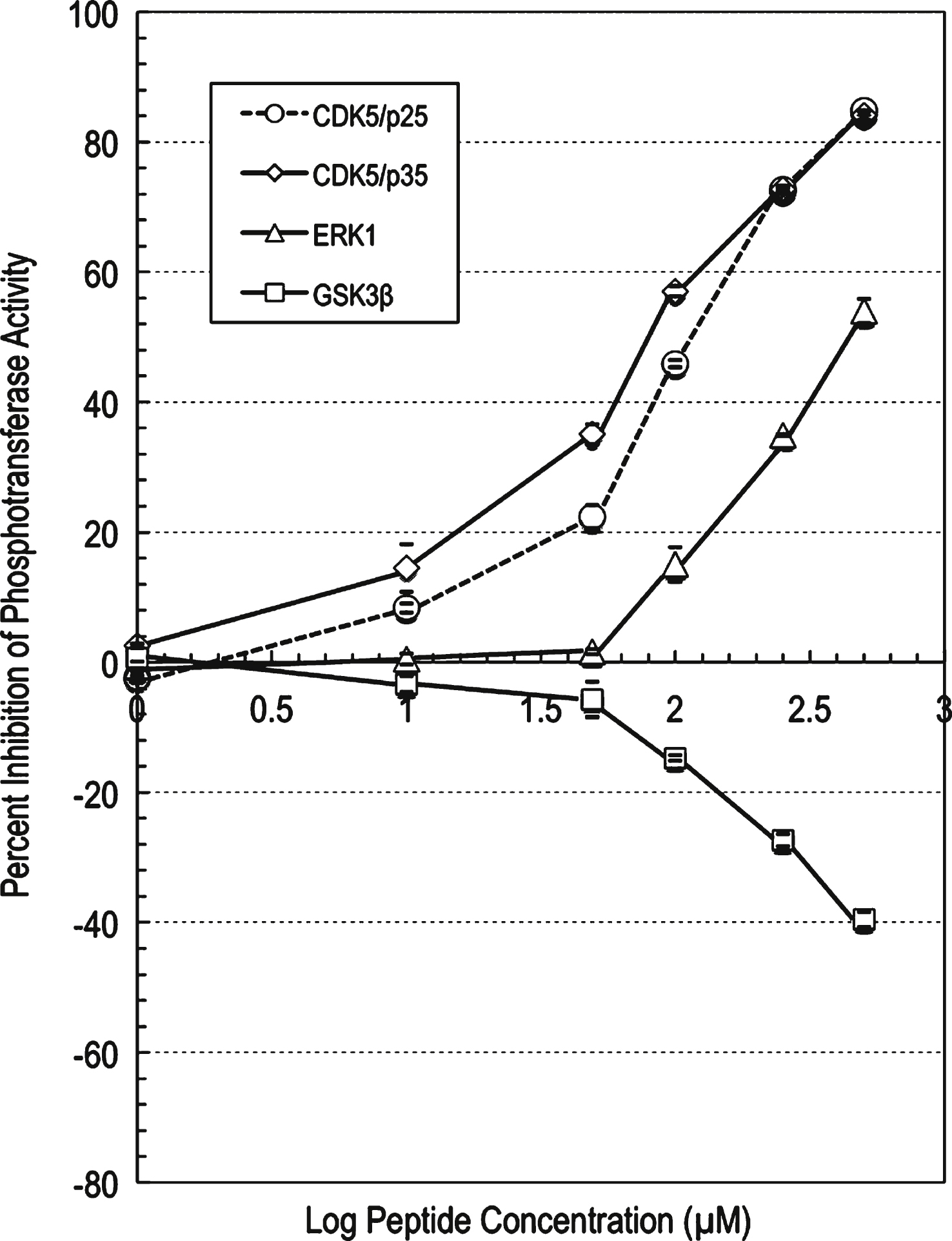
Table 1
% Activity of CDK5/p25 in the presence of inhibitory peptides using radiometric assay method arranged from lowest to highest counts (cpm), with two independent experiments in duplicates. Each assay solution contained 10% DMSO; The blank control without histone was 1,217 cpm, and with histone was 202,940 cpm. The cpm values given in the table was subtracted for the blank with histone in the presence of different peptides. The cpm value in the presence of the p5-WT peptide (#1) was taken as 100% phosphotransferase activity. The negative numbers in the percent change from control (% CFC) values correspond to even greater inhibition and positive numbers are less inhibition in the CDK5 phosphotransferase activity relative to the p5- WT peptide
| Peptide ID | Peptide Sequence | CPM | % Activity | % CFC |
| 78 | KEAFWDRCLSVINLMSSKMLQINM | 89762 | 74 | –26 |
| 77 | KEAFWDRCLSVINLMSSKMLQINV | 90305 | 75 | –25 |
| 15 | KEAFYDRCLSVINLMSSKMLQINA | 111665 | 92 | –8 |
| 76 | KEAFWDRCLSVINLMSSKMLQINL | 113022 | 93 | –7 |
| 1 | KEAFWDRCLSVINLMSSKMLQINA | 121167 | 100 | 0 |
| 20 | KEAFWDRALSVINLMSSKMLQINA | 121639 | 100 | 0 |
| 4 | KNAFWDRCLSVINLMSSKMLQINA | 122973 | 101 | 1 |
| 33 | KEAFWDRCLSIINLMSSKMLQINA | 123757 | 102 | 2 |
| 48 | KEAFWDRCLSVINLMTSKMLQINA | 126451 | 104 | 4 |
| 79 | KEAFWDRCLSVINLMSSKMLQINS | 131811 | 109 | 9 |
| 16 | KEAFWERCLSVINLMSSKMLQINA | 135248 | 112 | 12 |
| 75 | KEAFWDRCLSVINLMSSKMLQINI | 138095 | 114 | 14 |
| 14 | KEAFFDRCLSVINLMSSKMLQINA | 141068 | 116 | 16 |
| 29 | KEAFWDRCLMVINLMSSKMLQINA | 141207 | 117 | 17 |
| 28 | KEAFWDRCLYVINLMSSKMLQINA | 141312 | 117 | 17 |
| 61 | KEAFWDRCLSVINLMSSKMVQINA | 141773 | 117 | 17 |
| 41 | KEAFWDRCLSVINIMSSKMLQINA | 142416 | 118 | 18 |
| 42 | KEAFWDRCLSVINVMSSKMLQINA | 145310 | 120 | 20 |
| 3 | KDAFWDRCLSVINLMSSKMLQINA | 145721 | 120 | 20 |
| 18 | KEAFWNRCLSVINLMSSKMLQINA | 149428 | 123 | 23 |
| 59 | KEAFWDRCLSVINLMSSKCLQINA | 150317 | 124 | 24 |
| 17 | KEAFWQRCLSVINLMSSKMLQINA | 151097 | 125 | 25 |
| 34 | KEAFWDRCLSVLNLMSSKMLQINA | 151937 | 125 | 25 |
| 13 | KEAWWDRCLSVINLMSSKMLQINA | 152154 | 126 | 26 |
| 53 | KEAFWDRCLSVINLMSYKMLQINA | 152906 | 126 | 26 |
| 38 | KEAFWDRCLSVIQLMSSKMLQINA | 154117 | 127 | 27 |
| 50 | KEAFWDRCLSVINLMASKMLQINA | 154975 | 128 | 28 |
| 2 | REAFWDRCLSVINLMSSKMLQINA | 155431 | 128 | 28 |
| 25 | KEAFWDRCGSVINLMSSKMLQINA | 155679 | 128 | 28 |
| 56 | KEAFWDRCLSVINLMSSRMLQINA | 155892 | 129 | 29 |
| 49 | KEAFWDRCLSVINLMYSKMLQINA | 156497 | 129 | 29 |
| 67 | KEAFWDRCLSVINLMSSKMLQLNA | 156827 | 129 | 29 |
| 11 | KESFWDRCLSVINLMSSKMLQINA | 163262 | 135 | 35 |
| 37 | KEAFWDRCLSVGNLMSSKMLQINA | 164154 | 135 | 35 |
| 22 | KEAFWDRCISVINLMSSKMLQINA | 165242 | 136 | 36 |
| 19 | KEAFWDKCLSVINLMSSKMLQINA | 166245 | 137 | 37 |
| 8 | KEIFWDRCLSVINLMSSKMLQINA | 166249 | 137 | 37 |
| 63 | KEAFWDRCLSVINLMSSKMGQINA | 166937 | 138 | 38 |
| 74 | KEAFWDRCLSVINLMSSKMLQING | 167174 | 138 | 38 |
| 5 | KQAFWDRCLSVINLMSSKMLQINA | 167318 | 138 | 38 |
| 68 | KEAFWDRCLSVINLMSSKMLQVNA | 167911 | 139 | 39 |
| 10 | KEMFWDRCLSVINLMSSKMLQINA | 168848 | 139 | 39 |
| 58 | KEAFWDRCLSVINLMSSKALQINA | 170159 | 140 | 40 |
| 55 | KEAFWDRCLSVINLMSMKMLQINA | 170786 | 141 | 41 |
| 12 | KEAYWDRCLSVINLMSSKMLQINA | 171316 | 141 | 41 |
| 24 | KEAFWDRCASVINLMSSKMLQINA | 171581 | 142 | 42 |
| 43 | KEAFWDRCLSVINAMSSKMLQINA | 172298 | 142 | 42 |
| 51 | KEAFWDRCLSVINLMMSKMLQINA | 174387 | 144 | 44 |
| 57 | KEAFWDRCLSVINLMSSKSLQINA | 175515 | 145 | 45 |
| 9 | KEVFWDRCLSVINLMSSKMLQINA | 175785 | 145 | 45 |
| 44 | KEAFWDRCLSVINGMSSKMLQINA | 176026 | 145 | 45 |
| 69 | KEAFWDRCLSVINLMSSKMLQANA | 178232 | 147 | 47 |
| 72 | KEAFWDRCLSVINLMSSKMLQIQA | 181214 | 150 | 50 |
| 23 | KEAFWDRCVSVINLMSSKMLQINA | 181448 | 150 | 50 |
| 45 | KEAFWDRCLSVINLSSSKMLQINA | 182017 | 150 | 50 |
| 30 | KEAFWDRCLSAINLMSSKMLQINA | 184482 | 152 | 52 |
| 21 | KEAFWDRMLSVINLMSSKMLQINA | 185771 | 153 | 53 |
| 36 | KEAFWDRCLSVANLMSSKMLQINA | 186090 | 154 | 54 |
| 31 | KEAFWDRCLSGINLMSSKMLQINA | 186098 | 154 | 54 |
| 6 | KEGFWDRCLSVINLMSSKMLQINA | 186614 | 154 | 54 |
| 27 | KEAFWDRCLTVINLMSSKMLQINA | 189548 | 156 | 56 |
| 7 | KELFWDRCLSVINLMSSKMLQINA | 190811 | 157 | 57 |
| 47 | KEAFWDRCLSVINLCSSKMLQINA | 191850 | 158 | 58 |
| 35 | KEAFWDRCLSVVNLMSSKMLQINA | 192606 | 159 | 59 |
| 46 | KEAFWDRCLSVINLASSKMLQINA | 193507 | 160 | 60 |
| 65 | KEAFWDRCLSVINLMSSKMLEINA | 194286 | 160 | 60 |
| 54 | KEAFWDRCLSVINLMSAKMLQINA | 194650 | 161 | 61 |
| 52 | KEAFWDRCLSVINLMSTKMLQINA | 195577 | 161 | 61 |
| 60 | KEAFWDRCLSVINLMSSKMIQINA | 196418 | 162 | 62 |
| 39 | KEAFWDRCLSVIELMSSKMLQINA | 196444 | 162 | 62 |
| 73 | KEAFWDRCLSVINLMSSKMLQIEA | 197176 | 163 | 63 |
| 32 | KEAFWDRCLSLINLMSSKMLQINA | 197903 | 163 | 63 |
| 40 | KEAFWDRCLSVIDLMSSKMLQINA | 198851 | 164 | 64 |
| 71 | KEAFWDRCLSVINLMSSKMLQIDA | 199652 | 165 | 65 |
| 66 | KEAFWDRCLSVINLMSSKMLDINA | 199850 | 165 | 65 |
| 62 | KEAFWDRCLSVINLMSSKMAQINA | 203269 | 168 | 68 |
| 26 | KEAFWDRCLAVINLMSSKMLQINA | 204497 | 169 | 69 |
| 70 | KEAFWDRCLSVINLMSSKMLQGNA | 207194 | 171 | 71 |
| 64 | KEAFWDRCLSVINLMSSKMLNINA | 217093 | 179 | 79 |
Table 2
Comparison of the p5-WT and p5-MT peptides for their inhibitory activities toward diverse proline-directed protein kinases. CDK5/p35, CDK5/p25, and two more related proline-directed protein-serine/threonine kinases, GSK3β and ERK1, were tested in duplicate at six different concentrations of these peptides (1 to 500 μM) using a radiometric assay method to determine their IC50 values
| A. Peptide p5-WT | ||||||
| Target ID | % Activity Change 1 μM | % Activity Change 10 μM | % Activity Change 50 μM | % Activity Change 100 μM | % Activity Change 250 μM | % Activity Change 500 μM |
| CDK5/p25 | 0 | –13 | –20 | –41 | –59 | –72 |
| CDK5/p35 | 2 | –2 | –10 | –29 | –53 | –76 |
| ERK1 | 0 | 0 | –3 | –6 | –11 | –25 |
| GSK3β | –3 | –1 | 0 | 8 | 19 | 24 |
| B. Peptide p5-MT | ||||||
| Target ID | % Activity Change 1 μM | % Activity Change 10 μM | % Activity Change 50 μM | % Activity Change 100 μM | % Activity Change 250 μM | % Activity Change 500 μM |
| CDK5/p25 | 2 | –8 | –22 | –46 | –73 | –85 |
| CDK5/p35 | –3 | –14 | –35 | –57 | –73 | –84 |
| ERK1 | 1 | 0 | –2 | –15 | –35 | –54 |
| GSK3β | –1 | 3 | 6 | 15 | 27 | 40 |




