An Open-Label, Pilot Study of Daratumumab SC in Mild to Moderate Alzheimer’s Disease
Abstract
We conducted a small, open-label, pilot study of daratumumab to explore target engagement, safety, and potential efficacy in patients with mild to moderate Alzheimer’s disease. Daratumumab SC 1800 mg was given subcutaneously weekly for 8 weeks, then every 2 weeks for 16 weeks. Flow cytometry to measure the CD38+ proportion of CD8 + CD4- T cells and cognitive assessments were performed at baseline, day 176, and day 246. Daratumumab significantly reduced CD38 + CD8 + CD4- T cells after 24 weeks and this effect persisted 11 weeks thereafter. There was no hematological toxicity or unexpected adverse events. Responder analysis showed no improvement on cognitive outcome measures.
INTRODUCTION
Daratumumab is a human IgG1 kappa monoclonal antibody that targets CD38, a multi-functional enzyme that hydrolyzes nicotinamide adenine dinucleotide (NAD) and is involved in cell signaling.1 NAD is a co-enzyme found in all living cells. NAD levels decrease during aging and are involved in age-related metabolic decline.2,3 CD38 levels increase with age and correlate with NAD decline.4 In CD38 knockout mice, tissue levels of NAD are significantly increased.5 When CD38 knockout mice were crossed with APPswePS1ΔE9 transgenic mice, they showed significant reductions in amyloid-β (Aβ) plaque load and soluble Aβ levels, and this correlated with improved spatial learning.6 CD38 expression on CD8 + T cells, indicative of activation, is significantly increased in the blood of early Alzheimer’s disease (AD) patients as compared with age-matched controls, and activated T cells are capable of trafficking into the brain and exerting cytotoxic effects.7 CD38 has been implicated in brain neurodegeneration and neuroinflammation.8,9 CD38 affects regulation of the amount and function of activated microglia, with important consequences for injury and repair processes in the brain.10 Cerebrospinal fluid levels of microRNAs that reduce CD38 expression are significantly decreased in AD patients compared to age-matched controls.11 CD38 immunoreactivity localizes to the perikarya and dendrites of many neurons within the human brain, suggesting that CD38 is involved in signal transduction.12 CD38 immunoreactivity in intracellular neurofibrillary tangles and neuropil threads has been observed on AD brain postmortem pathology.13 CD38 inhibitor intervention has been shown to restore the level of NAD+ in the hippocampus and cortex of APP/PS1 mice, alleviate the disorder of energy metabolism, enhance the effect of Iba1+ microglia in clearing senile plaque deposits, reduce the neuroinflammatory response, and improve the learning and memory ability of APP/PS1 mice.14 Accordingly, CD38 may be a novel target for AD treatment.
Daratumumab and hyaluronidase-fihj is FDA-approved for the treatment of multiple myeloma and light chain (AL) amyloidosis. Daratumumab is able to cross the blood-brain barrier,15 and has broad-ranging immunomodulatory effects on non-plasma cells that express CD38.16,17 We conducted a small, open-label, phase 2a pilot study of daratumumab to explore target engagement, safety and tolerability, and potential efficacy signal in patients with mild to moderate AD.
MATERIALS AND METHODS
This was a single-site open-label study enrolling subjects≥55 to≤85 years of age with a diagnosis of probable AD dementia according to NIA-AA criteria, MMSE score≥15 and≤26, confirmed amyloid positive by PET scan.
Eligible subjects were treated with daratumumab SC 1800 mg (daratumumab 1800 mg with rHuPH20 30,000 units) subcutaneous infusion (15 mL over approximately 3 to 5 min) once weekly for 8 weeks followed by daratumumab SC 1800 mg every 2 weeks for 16 weeks. To minimize the risk of post-injection reactions, subjects were premedicated with montelukast 10 mg orally 1 to 3 h prior to the first dose of daratumumab, diphenhydramine 25 mg orally 1 to 3 h prior to each dose, acetaminophen 1000 mg orally 1 to 3 h prior to each dose, and dexamethasone 20 mg orally 1 to 3 h prior to the first 3 doses, 10 mg orally 1 to 3 h prior to all subsequent doses, and 4 mg daily orally the day after each dose. To prevent herpes zoster reactivation, patients were also treated with oral acyclovir 400 mg daily beginning the day after the first dose and continuing until 12 weeks after the final dose.
Cognitive outcome measures [11-item Alzheimer’s Disease Assessment Scale-cognitive subscale (ADAS-Cog 11), 12-item version (ADAS-Cog 12), Mini-Mental State Examination (MMSE), Clinical Dementia Rating Scale Sum of Boxes (CDR-SB), and AD Composite Score (ADCOMS)] were assessed at screening, day 85 (midpoint), day 176 (end of treatment), and day 246 (11 weeks post-treatment). Responder analysis was used to determine the number of subjects who were improved or unchanged from baseline on any of these cognitive measures following treatment. Flow cytometry to measure the CD38+ proportion of CD8 + CD4- T cells was performed at baseline, day 176, and day 246. Fresh peripheral whole blood samples in EDTA tubes were surface stained using the following combinations of fluorochrome-conjugated antibodies: anti-CD45 PerCP, anti-CD3 PE, anti-CD8 APC, and anti-CD4 V450 (BD Biosciences), and anti-CD38 FITC (provided by Janssen Scientific Affairs). Red blood cells were then lysed with BD FACS Lysing Solution and washed with stain buffer. Cells were resuspended in 1% formaldehyde. Single fluorochrome stained antibody capture beads were used to correct for spectral overlap. All samples were acquired on a FACSCanto II flow cytometer (BD Biosciences) and analyzed with FCS Express V3 (De Novo Software). Repeated measures ANOVA was utilized to evaluate the pattern of change over time.
This clinical trial was conducted in accordance with International Conference on Harmonisation Good Clinical Practice guidelines and the Declaration of Helsinki. Written informed consent was provided by all subjects or by their legally authorized representatives with subject assent. Subjects and their study partners were not compensated.
RESULTS
A total of 16 subjects were screened. There were 6 screen failures and 1 subject who withdrew consent prior to treatment, leaving 9 subjects who received at least one dose of study medication. Those subjects included 4 men and 5 women; 6 non-Hispanic White, 2 Asian, 1 Hispanic; mean age 67.3 years at study entry; and mean baseline MMSE score 22.2. One subject had a serious adverse effect while on treatment (hospitalization for COVID-19 pneumonia). Two subjects experienced mild post-injection reactions consisting of urticaria that responded to diphenhydramine and hydrocortisone, and did not recur with subsequent injections. One subject had onset of a rash 4 days after a daratumumab injection, and a few hours after having received IV iodine contrast. There was no incidence of anemia, leukocytopenia, or thrombocytopenia.
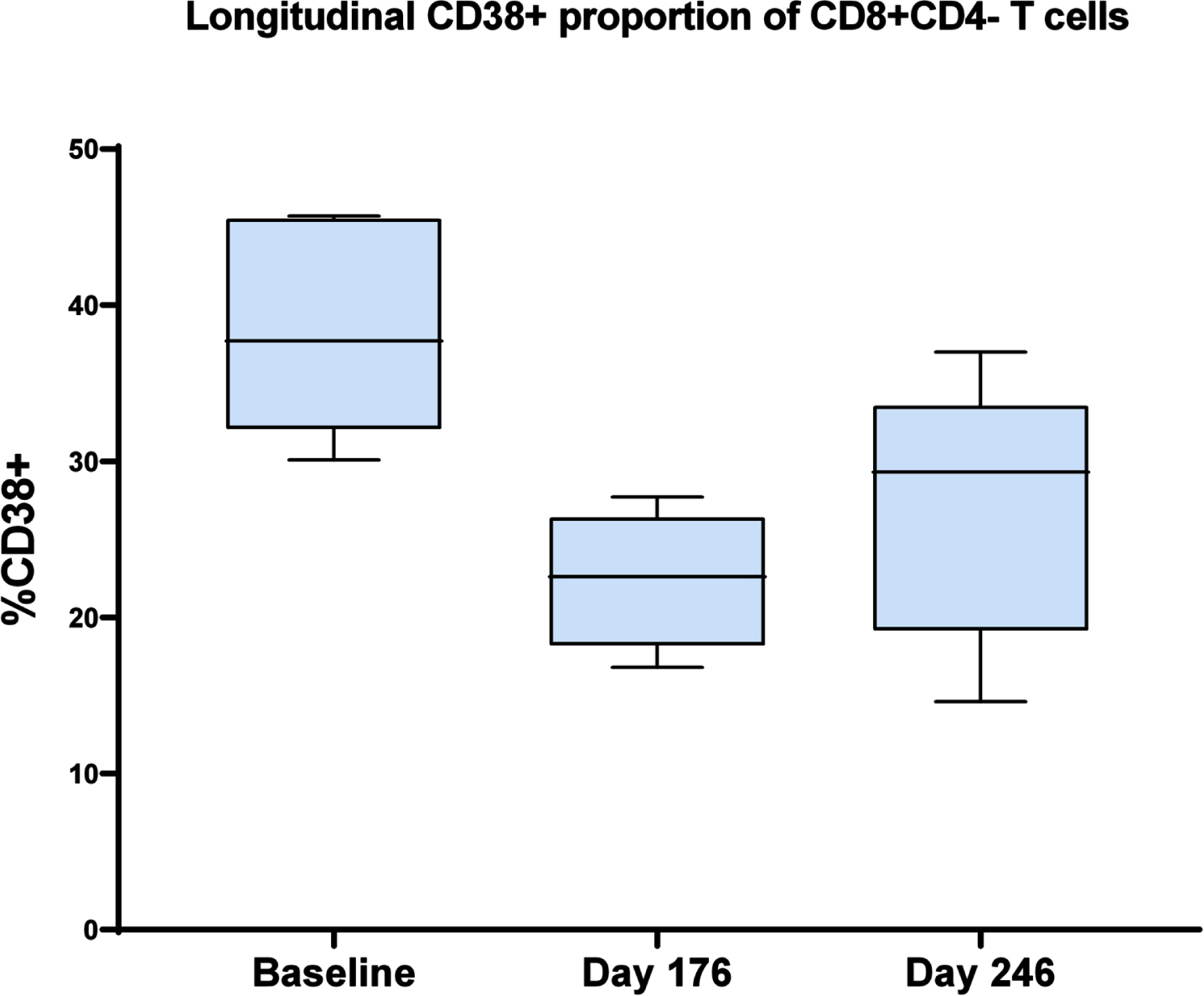
One subject withdrew consent after 3 treatment visits, 1 subject was withdrawn by the investigator after having missed 7 consecutive visits due to the COVID pandemic, and 1 subject was withdrawn due to a possibly related adverse event (rash), leaving 6 evaluable subjects who completed treatment. Daratumumab significantly reduced the CD38+ proportion of CD8 + CD4- T cells after 24 weeks of treatment and this effect persisted 11 weeks thereafter (repeated measures ANOVA F2,13 = 8.61, p < 0.005) (Fig. 1). On responder analysis, no subjects showed improvement with treatment on any of the cognitive outcome measures. Mean progression from screening to day 176 was 3.7 on ADAS-Cog 11, 4.3 on ADAS-Cog 12, 3.8 on MMSE, 1.2 on CDR-SB, and 0.13 on ADCOMS.
Fig. 1
The CD38+ proportion of CD8 + CD4- T cells at baseline, day 176 (end of treatment), and day 246 (11 weeks post-treatment). Repeated measures ANOVA F2,13 = 8.61, p < 0.005.
DISCUSSION
Daratumumab showed evidence of target engagement as manifested by robust and persistent reduction in the CD38+ proportion of CD8 + CD4- T cells. It was generally safe and well-tolerated in this study population, with no evidence of hematological toxicity and no unexpected adverse events. In this small pilot study, there was no signal of clinical improvement based on responder analysis. Limitations of this study include the small sample size and open-label design. The duration of treatment was only 24 weeks, and the dose of daratumumab was not increased above the standard dose used in the treatment of patients with multiple myeloma, for whom considerations of hematological toxicity and tumor lysis syndrome could be dose-limiting. Furthermore, our study population consisted of patients with mild to moderate AD dementia, rather than earlier stages of disease.
Conclusions
In this limited pilot study, daratumumab significantly and persistently reduced the CD38+ proportion of CD8 + CD4- T cells in patients with mild to moderate AD and appeared to be safe and well-tolerated in this study population. Future studies would be needed to ascertain whether CD38 may prove to be a viable target for AD treatment.
AUTHOR CONTRIBUTIONS
Marc Lawrence Gordon (Conceptualization; Investigation; Methodology; Supervision; Writing – original draft); Erica Christen (Investigation; Project administration); Lynda Keehlisen (Investigation); Michelle Gong (Investigation); Fung Lam (Investigation; Methodology); Luca Giliberto (Investigation); Jesus Gomar (Formal analysis; Writing – review & editing); Jeremy Koppel (Investigation; Writing – review & editing).
ACKNOWLEDGMENTS
The investigators are profoundly grateful to the subjects and their study partners for participating in this study. We would also like to recognize the late Dr. Peter Davies for his contribution to the initial conceptualization of this study, and for his sustaining mentorship, kindness, and support.
FUNDING
Funding for this study, study medication, and anti-CD38 FITC for flow cytometry were provided by Janssen Scientific Affairs, LLC. Janssen had no role in the design and conduct of the study; in the collection, analysis, and interpretation of data; or in the preparation of the manuscript, although they had the opportunity to review the manuscript prior tosubmission.
CONFLICT OF INTEREST
M.L. Gordon has received compensation for participating in Advisory Board meetings for Corium and Labcorp. He has been a clinical trial site Principal Investigator for AbbVie, Alector, Bristol Myers Squibb, Eisai, and Novo Nordisk (payments made to institution). J. Koppel has received grant support from NIA, NIMH, and Alzheimer’s Foundation of America, and has been a clinical trial site Principal Investigator for Karuna and Otsuka (payments made to institution). J. Gomar has received grant support from NIA and Alzheimer’s Association (payments made to institution). E. Christen, L. Keehlisen, M. Gong, F. Lam, and L. Giliberto have no disclosures.
DATA AVAILABILITY
The data supporting the findings of this study are openly available in NCT04070378 at https://clinicaltrials.gov.
REFERENCES
1. | Morandi F , Airoldi I , Marimpietri D , et al. CD38, a receptor with multifunctional activities: from modulatory functions on regulatory cell subsets and extracellular vesicles, to a target for therapeutic strategies. Cells (2019) ; 8: : 1527. |
2. | Imai S and Guarante L. NAD+ and sirtuins in aging and disease. Trends Cell Biol (2014) ; 24: : 464–471. |
3. | Verdin E. NAD+ in aging, metabolism, and neurodegeneration. Science (2015) ; 350: : 1208–1213. |
4. | Camacho-Pereira J , Tarragó SG , Chini CCS , et al. CD38 dictates age-related NAD decline and mitochondrial dysfunction through an SIRT3-dependent mechanism. Cell Metab (2016) ; 23: : 1127–1139. |
5. | Askoy P , Escande C , White TA , et al. Regulation of SIRT 1 mediated NAD dependent deacetylation: a novel role for the multifunctional enzyme CD38. Biochem Biophys Res Commun (2006) ; 349: : 353–359. |
6. | Blacher E , Dadali T , Bespalko A , et al. Alzheimer’s disease pathology is attenuated in a CD38-deficient mouse model. Ann Neurol (2015) ; 78: : 88–103. |
7. | Zhang R , Miller RG , Madison C , et al. Systemic immune system alterations in early stages of Alzheimer’s disease. J Neuroimmunol (2013) ; 256: : 38–42. |
8. | Guerreiro S , Privat AL , Bressac L , et al. CD38 in neurodegeneration and neuroinflammation. Cells (2020) ; 9: : 471. |
9. | Mohamed W , Kumar J , Alghamdi BS , et al. Neurodegeneration and inflammation crosstalk: Therapeutic targets and perspectives. IBRO Neurosci Rep (2020) ; 14: : 95–110. |
10. | Mayo L , Jacob-Hirsch J , Amariglio N , et al. Dual role of CD38 in microglial activation and activation-induced cell death. J Immunol (2008) ; 181: : 92–103. |
11. | Denk J , Boelmans K , Siegismund C , et al. MicroRNA profiling of CSF reveals potential biomarkers to detect Alzheimer’s disease. PLoS One (2015) ; 10: : e0126423. |
12. | Mizuguchi M , Otsuka N , Sato M , et al. Neuronal localization of CD38 antigen in the human brain. Brain Res (1995) ; 697: : 235–240. |
13. | Otsuka K , Mizuguchi M , Aizawa T , et al. Immunoreactivity in Alzheimer’s neurofibrillary tangles. Brain Pathol (1994) ; 4: : 558. |
14. | Hu Y , Huang Y , Xing S , et al. Aβ promotes CD38 expression in senescent microglia in Alzheimer’s disease. Biol Res (2022) ; 55: : 10. |
15. | Vercruyssen M , El Hachem G and Maerevoet M. The daratumumab crosses the blood brain barrier. Clin Lymphoma Myeloma Leuk (2018) ; 18: : S289. |
16. | Krejcik J , Casneuf T , Nijhof IS , et al. Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood (2016) ; 128: : 384–394. |
17. | Hill E , Morrison C , and Kazandjian D . Daratumumab: A review of current indications and future directions. Semin Oncol (2022) ; 49: : 48–59. |




