Language Markers of Dementia and Their Role in Early Diagnosis of Alzheimer’s Disease: Exploring Grammatical and Syntactic Competence via Sentence Repetition
Abstract
Background:
Earlier research focuses primarily on the cognitive changes due to Alzheimer’s disease (AD); however, little is known with regard to changes in language competence across the lifespan.
Objective:
The present study aims to investigate the decline of language skills at the grammatical and syntactic levels due to changes in cognitive function.
Methods:
We administered the Litmus Sentence Repetition Task (SRT) to 150 native speakers of Greek who fall into five groups: 1) young healthy speakers, 2) cognitively intact elder healthy speakers, 3) speakers with subjective cognitive impairment (SCI), 4) speakers with mild cognitive impairment (MCI); and 5) speakers with AD dementia at the mild/moderate stages. All participants underwent a physical and neurological examination and cognitive screening with a standardized neuropsychological battery to assess cognitive status comprehensively and evaluate aspects like working memory, executive function, attention and memory to appropriately classify them.
Results:
The data analysis revealed that the SRT had high discriminatory value in the development of AD; specifically, both accuracy and grammaticality indices were related to cognitive decline. Additionally, syntax significantly affected the performance of speakers with structures such as clitics being particularly challenging and in most structures the performance of speakers with MCI drops significantly compared to speakers with SCI.
Conclusions:
Linguistic indices revealed subtle early signs of cognitive decline that can be helpful in the early detection of AD, thus facilitating the clinical process offering support to language-based assessment tools such as sentence repetition, a non-invasive type of assessment to evaluate symptoms of AD.
INTRODUCTION
Alzheimer’s disease (AD)1 is an irreversible neurodegenerative disease responsible for the majority of dementia cases and is characterized by a progressive decline of cognitive functions, with linguistic ability also affected. Spoken language is a rich source of information on an individual’s linguistic status since it allows us to evaluate varied linguistic domains such as the lexicon and properties relating to lexical access and processing along with the grammar component and information on morphosyntactic ability. Unfortunately, the utility of naturalistic spoken language as a quantitative measure up until recently remained limited yet current research has turned in that direction.2–6 Often, though, language decline in AD is reported as heterogeneous due to dementia severity variability in the clinical populations tested, variants of AD such as the aphasic ones, the small number of speakers examined and the wide range of performance of patients especially in the early phases of AD.2,7,8 Aphasic variants of AD, namely, logopenic variant primary progressive aphasia, represent distinct clinical presentations characterized by specific language impairments such as anomia (difficulty to recall words), agrammatism (difficulty with grammar), or semantic paraphasias (substituting incorrect but semantically related words).9 Differentiating between aphasic variants and other types of dementia, such as frontotemporal dementia, vascular dementia, or primary progressive aphasia allows us to better understand the broad spectrum of language-related cognitive impairments seen in AD that explains the heterogeneity attested in datasets.10,11 The present study though focuses on typical AD data and not aphasic variants of AD; the term will be used hereafter to refer to typical AD cases.
Most descriptions of the clinical and cognitive features of AD highlight deficits mainly in memory ability and note the relative preservation of language skills particularly in the early stages, however, the in-depth understanding of the language deficits of people with dementia due to AD and especially of speakers with mild cognitive impairment (MCI) who run the risk of converting to AD12 requires our attention. Moreover, the exploration of those deficits is of particular interest in largely understudied languages such as Greek (for datasets available in other languages, see relevant review papers).13–16
Earlier studies on the language breakdown in AD have noted that as the disease progresses any linguistic aspect can be affected, for example phonological, lexical, syntactic, or discursive features.17 Specifically, in the first phase of AD, patients mainly lack lexical-semantic aspects of the language such as naming things or finding the right words; meanwhile in the moderate and severe phases of AD, communication starts to be non-fluent, which eventually ends up in the breakdown of comprehension.18,19 Consequently, linguistic decline in AD dementia has been associated primarily with impairments tapping on semantic and pragmatic levels exemplified via lexical access difficulties, semantic/phonemic paraphasias and repetitions.20,21 While analysis of language skills such as naming of pictures and objects in isolation provides information on lexical access, analysis of language in connected speech provides different information which is particularly relevant in dementia due to AD as a result of the broad cognitive nature of the disorder. As such, the examination of the morphosyntactic operation is necessary.
Earlier studies identified the syntactic domain as relatively unimpaired in AD dementia at least until the advanced stage of the disease.22–25 Recent work though suggests that morphosyntactic skills may not be as preserved as initially thought. Longitudinal data7 suggest that despite individual heterogeneity, the AD speakers showed a progressive decline in the proportion of verbs with inflection. Additionally, syntactic errors, specifically verb agreement errors, are prominent features of language decline in mild AD.26 In the same light, a recent study on 10 Greek speaking patients with AD employed a sentence completion task to examine tense, aspect and person/number agreement and found that aspect agreement caused the most difficulties, followed by tense, and person/number agreement,27 while another study reports lexical aspect difficulties.28 Data elicited by Greek speakers with mild to moderate AD using a single-picture description task suggest systematic differences in lexical variation and syntactic complexity between the clinical and control groups.29
To better interpret though the data provided by control and clinical groups, it is necessary to consider the type of methodology used for the elicitation of spoken language, which is critical especially when attempting to measure morphosyntactic ability.15,30 Employing narrative discourse elicitation tasks, such as picture descriptions, allow the speaker to select the grammatical components they feel competent using while more structured experimental tasks such as sentence completion and sentence repetition require the use of specific structures, which allows us to test syntactic features in a target like manner. Sentence repetition, the structured language elicitation task selected by the present study, is an experimental design used to measure verbal working memory ability along with language ability. Sentence repetition does tap on cognitive ability and as such it is valuable in the examination of populations that experience changes in their cognitive ability both in typical development and in clinical populations.31–35
Within the area of linguistic research, sentence repetition is a tool that is often used to assess different linguistic levels and language production skills at the sentential level; the Litmus SRT employed in the present study focuses on the role of syntax, while other SRTs may be assessing other linguistic conditions depending on their design. The task for the participant is to repeat sentence provided by the examiner, consequently the information content (lexical items) of the sentence is given and the participant needs to reconstruct its surface representation to be able to repeat the sentence accurately.35 An early study that examined the role of syntax in sentence repetition in AD35 explored syntactic complexity via three parameters; canonical/non-canonical thematic role assignment, right/left branching of embedded relative clauses and number of verbs testing 13 speakers with mild to moderate AD; their analysis revealed difficulties for the AD group due to syntactic complexity, specifically in canonical sentences that were left branching and noncanonical ones with right branching, while the number of verbs did not appear to affect their performance.
Beales et al.32 on the other hand, employed the repetition task from the Arizona Battery for Communication Disorder of Dementia (ABCD), which contains 10 phrases/sentences that are grammatically correct but not semantically or syntactically meaningful and developed an error evaluation schema for omissions and substitutions at the beginning, middle and end of the sentence. Their analysis highlights that speakers with AD (N: 4) opt for ending omissions and unrelated word substitutions. Furthermore, in a recent study reporting data from 13 French speakers with AD,31 researchers administered a sentence repetition span task with testing items including three to nine content words; note that this experimental design does not address syntactic complexity, rather the working-memory ability of the participant. Their analysis showed that while performance dropped as sentential length increased, the number of content words as a measure was not sufficient as a diagnostic criterion for the progress of AD. Considering this earlier work, despite the limited data due to the small number of speakers, linguistic decline may be detected via sentence repetition; yet this type of methodology has the potential to track language changes in speakers provided that it manipulates specific linguistic features keeping the working memory requirements constant.
Yet the question is open with regard to how early on can linguistic decline be identified. Research on speakers with MCI suggests that language impairment may occur; confrontation naming and semantic verbal fluency tasks might be able to differentiate individuals with MCI from healthy older adults.36 However, there are some controversial findings. Some studies suggest that the visual stimulus naming test is not suitable for detecting early AD or distinguishing between healthy elders and speakers with MCI,37 while other findings demonstrate that semantic verbal fluency tasks are useful for differentiating between healthy elderly and AD groups but were less able to accurately differentiate the MCI groups from healthy elderly adults or people with AD.38 Turning to other language components affected by MCI, research suggests mild discursive difficulties, impairments in sentence comprehension, impaired recall and reading comprehension39,40 with no detailed description though of the grammatical skills of speakers living with MCI.
Turning to online measurements and processing speed in language tasks, often speakers with MCI appear to have high accuracy levels similar to healthy controls and, thus, their performance appears unaffected, yet they differ in their speed of language processing. For Greek speakers with MCI, see 41; for other languages, see 42–44. Lastly, data from healthy aging and subjective cognitive impairment (SCI) suggests that speakers retain the microstructural aspects of language, such as morphosyntactic properties, with no significant communicative difficulties.45,46 Even though a number of studies explore the language abilities in healthy aging, SCI and MCI, to the best of our knowledge there is no study yet employing sentence repetition to assess changes in grammatical competence in these groups. Considering the above, there is an urgent need to extent language assessment in MCI and preclinical groups so as to provide a comprehensive evaluation of language function, particularly in detecting mild or emerging changes so that we can inform clinical decision-making processes in the detection of MCI and potentially other cognitive impairments; the present study will attempt to contribute to this endeavor.
Consequently, there is a gap in research with regard to the detailed linguistic profiling of speakers of Greek experiencing early cognitive changes and cognitive decline due to AD. Despite the fact that research on language ability in AD has received a lot of attention in recent years; datasets currently available contain data primarily from English speakers and there is no dataset on typical AD speakers of Greek available in the Dementia Talkbank (https://dementia.talkbank.org/), hence we consider this area of research significant. The present study aims at extracting information on the grammar features of language production both in unimpaired and clinical Greek speaking populations in attempt to map language and cognition across the dementia continuum. Our goal is to disentangle healthy aging and AD effects in language skills and possibly identify the grammatical indicators for the early diagnosis of AD. To this aim, we employ the Litmus Sentence Repetition Task (SRT) to explore language changes along the healthy aging – AD dementia continuum. SRTs are highly structured complex linguistic tasks that test language at a variety of levels, i.e., language perception, lexicon, morphosyntax, and language production;33,34 as such, an SRT provides a global measurement of verbal abilities and it enables us to track language decline.
The Litmus SRT was initially developed to assess grammatical development, yet we selected it as a tool for the adult populations for the following reasons: 1) it is designed to assess syntactic operations in Greek and as such it can provide information on grammatical and syntactic competence; 2) the sentential length is constant and as such it allows us to see if different structures have an effect in the performance of participants rather than testing incremental length that relates primarily to working-memory ability; 3) word frequency, a factor often affecting lexical access, has been controlled for in the development of test items; 4) grammatical phenomena acquired later in life appear to be more sensitive to linguistic decline, since the syntactic structures included in the task include both early and later acquired structures, it allows us to test possible linguistic decline in aging adult speakers and adult speakers with MCI and AD. For similar findings, see also first language attrition data in cognitively intact multilingual speakers.47–49 Currently, there are no normative data of the Litmus SRT available for adult speakers, yet the present data can be considered as preliminary evidence in that direction. For developmental datasets of data elicited via the Litmus SRT in various languages, see https://www.litmus-srep.info/.
MATERIALS AND METHODS
Participants
For the purposes of the present study, we informed the local community on the study with an open call for participation and we recruited 150 Greek-speaking participants in collaboration with the University General Hospital of Thessaloniki AHEPA and the Greek Association of Alzheimer’s Disease and Related Disorders (GAADRD; Alzheimer Hellas https://www.alzheimer-hellas.gr/index.php/el/). Ethical consideration was given to all aspects of the study adhering to the principles of the World Medical Association’s Declaration of Helsinki and the Ethics Committee of the host institution (AUTH Ethical Approval N 272183/2020). Informed consent and demographic information were obtained from all participants. We tested 150 speakers, 30 speakers per group: 1) young healthy adult speakers (yHC), 2) cognitively intact elder healthy speakers (eHC), 3) speakers with SCI, 4) speakers with MCI, and 5) speakers with AD dementia at the mild/moderate stages. See demographic information in Table 1. With regard to age, considering that we are looking across the lifespan, the groups differ to each other (F (4,145) = 319.830, p < 0.001) with eHC and SCI groups matched in age, the MCI group marginally matched to SCI group; pair comparisons including either yHC or AD: p < 0.001. Turning to years of education, the groups differ to each other (F (4,145) = 30.242, p < 0.001), yet eHC, SCI, MCI groups are matched; yHC > eHC, SCI, MCI > AD (pair comparisons: yHC versus MCI/AD: p < 0.001).
Table 1
Demographic information of study’s participants
| N Female | N Male | Sign. | ||
| Sex | yHC | 20 | 10 | n.s. |
| eHC | 22 | 8 | ||
| SCI | 23 | 7 | ||
| MCI | 20 | 10 | ||
| AD | 21 | 9 | ||
| M | SD | |||
| Age | yHC | 26.96 | 4.82 | ** |
| eHC | 66.76 | 6.96 | ||
| SCI | 64.00 | 6.13 | ||
| MCI | 71.46 | 7.01 | ||
| AD | 79.83 | 6.20 | ||
| M | SD | |||
| Years of education | yHC | 17.16 | 0.87 | ** |
| eHC | 15.53 | 2.59 | ||
| SCI | 15.20 | 2.91 | ||
| MCI | 14.30 | 2.69 | ||
| AD | 10.50 | 2.71 |
yHC, young healthy adult speakers; eHC, cognitively intact elder healthy speakers; SCI, Subjective Cognitive Impairment; MCI, Mild Cognitive Impairment; AD, Alzheimer’s disease; M, mean; SD, standard deviation; *p < 0.05; **p < 0.001; n.s. not significant.
Diagnosis of healthy controls, individuals with SCI, MCI, and AD was determined by two neurologists with expertise on neurodegenerative diseases based on structural magnetic resonance imaging (MRI), medical history, neuropsychological tests, and the neurological examination. All participants underwent a clinical evaluation and a detailed neuropsychological assessment.50 The neuropsychological battery aimed to assess cognitive status comprehensively and evaluate aspects like working memory, executive functioning, attention and memory so that we appropriately group the participants.1 Specifically, participants diagnosed with AD met the criteria outlined by the National Institute of Neurological and Communication Disorders and Stroke/Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) for probable AD,1,51 as well as the Diagnostic and Statistical Manual of Mental Disorders (DSM-V) criteria for dementia of Alzheimer type.83 Participants with MCI fulfilled the Petersen criteria52 and they were all individuals with single domain amnestic MCI (aMCI), while the SCI group met IWG-2 Guidelines1 as well as the latest suggestions proposed by the SCD-I Working Group.53 Moreover, the identification of SCI participants further included self-perceived memory decline compared to other cognitive functions and in reference to others of the same age occurring during the past five years as determined by the individuals’ medical history and an informant report, at an age cut-off of 60. Regarding the preclinical stages, we tried to eliminate possible confounding factors based on blood tests (hormonal disorders, vitamin deficiency, etc.) and structural MRI (vascular/demyelinating lesions, tumors, anatomical variations, etc.). We considered all the above for the recruitment process, since they are factors that could affect our sample performance and signal elicitation. Additional inclusion criteria for the SCI and HC groups were to have a normal medical, neurological, and neuropsychological examination.
For the cognitive screening of yHC and eHC groups the following tasks were employed: Raven’s Progressive Matrices (RPM), Mini-Mental State Examination (MMSE – Greek version),54 Montreal Cognitive Assessment (MoCA),55 Memory Alteration Test (M@T),56 Verbal and Semantic Fluency Test (FAS),57 Digit Backwards Recall (Wechsler Adult Intelligence Scale, WAIS-IV),58 Verbal Stroop,59 and Non-Verbal Stroop Card Sorting Test.60 The individual scores of the yHC and eHC speakers were within the cut-off scores set for typical populations and validated their inclusion in these two control groups; scores provided in Table 2.
Table 2
Cognitive screening of control speakers
| Task | yHC | eHC | Sign. | ||
| M | SD | M | SD | ||
| RPM | 50.35 | 4.98 | 42.54 | 9.13 | * |
| MMSE – Greek version | 29.83 | 0.44 | 29.16 | 0.88 | * |
| MoCA | 28.64 | 1.31 | 27.00 | 1.70 | ** |
| M@T | 48.89 | 1.14 | 46.66 | 2.25 | ** |
| Semantic fluency test (FAS) | 22.19 | 7.34 | 18.56 | 3.99 | n.s. |
| Verbal fluency test (FAS) | 14.22 | 3.36 | 13.46 | 3.13 | n.s. |
| Digit backwards recall (WAIS-IV) | 27.06 | 8.17 | 24.04 | 6.19 | n.s. |
| Verbal stroop card sorting test – words/colors | 54.11 | 12.73 | 48.06 | 12.47 | n.s. |
| Non-verbal stroop card sorting test | 27.17 | 8.16 | 18.51 | 4.10 | * |
yHC, young healthy adult speakers; eHC, cognitively intact elder healthy speakers; M, mean; SD, standard deviation; RPM, Raven’s Progressive Matrices; MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment; M@T, Memory Alternation Test; WAIS-IV, Wechsler Adult Intelligence Scale; *p < 0.05; **p < 0.001; n.s. not significant.
Moreover, we administered an extensive battery of neuropsychological assessment tools to participants of the three clinical groups.50 Specifically, we administered the following: MMSE – Greek version,54 Rey Auditory Verbal Learning Test (RAVLT),61 Rivermead Behavioral Memory Test (RBMT-story Direct & delayed recall),62 Rey Osterrieth Complex Figure Test copy and delay recall (ROCFT-copy and delayed recall),63 Trail Making Test part-B,64 Functional Cognitive Assessment Scale (FUCAS),65 Functional Rating Scale for Symptoms of Dementia (FRSSD),66 Geriatric Depression Scale (GDS),67 Neuropsychiatric Inventory (NPI),68 The Alzheimer’s Disease Assessment Scale–Cognitive Subscale – ADL Scale (ADAS-Cog),69,70 Verbal & Semantic Fluency Test (FAS),57 MoCA,55 Digit Backwards Recall (WAIS-IV),58 Verbal Stroop,59 and Non-Verbal Stroop Card Sorting Test,60 Clinical Dementia Rating (CDR),71 Royal Prince Alfred Prospective Memory Test (RPA-ProMem);72 scores provided in Table 3.
Table 3
Neuropsychological assessment of speakers with SCI, MCI, and AD
| Task | SCI | MCI | AD | Sign. | |||
| M | SD | M | SD | M | SD | ||
| MMSE – Greek version | 28.94 | 0.87 | 27.57 | 1.53 | 19.40 | 3.44 | ** |
| MoCA | 26.56 | 1.56 | 23.6 | 2.60 | not admin. | ** | |
| M@T | 45.45 | 1.75 | not admin. | not admin. | n/a | ||
| RAVLT - recall | 49.90 | 7.31 | 39.71 | 12.56 | 22.43 | 6.73 | ** |
| RBMT-story direct | 15.12 | 2.42 | 11.16 | 3.22 | 8.00 | 3.53 | ** |
| RBMT-delayed recall | 12.65 | 1.87 | 9.33 | 3.38 | 3.41 | 3.36 | ** |
| ROCFT-copy | 34.68 | 1.46 | 33.50 | 2.52 | 24.35 | 9.01 | ** |
| ROCFT-delayed recall | 19.25 | 5.05 | 13.72 | 7.61 | 3.38 | 3.62 | ** |
| Trail making test part-B | 125.75 | 44.71 | 218.25 | 112.63 | 368.62 | 112.38 | ** |
| FUCAS | 42.75 | 1.29 | 43.26 | 1.65 | 57.14 | 11.39 | ** |
| FRSSD | 2.84 | 2.18 | 2.85 | 1.81 | 8.71 | 4.97 | ** |
| GDS | n/a | n/a | 1.62 | 2.03 | 3.10 | 3.15 | n.s. |
| NPI | 1.06 | 2.32 | 2.47 | 4.45 | 8.33 | 8.38 | * |
| ADAS-Cog | 9.93 | 3.55 | 13.88 | 4.02 | 28.83 | 6.39 | ** |
| Semantic fluency test (FAS) | 18.70 | 2.74 | 17.01 | 3.26 | 8.33 | 3.06 | ** |
| Verbal fluency test (FAS) | 12.23 | 2.03 | 11.10 | 3.61 | 5.61 | 2.47 | ** |
| Digit backwards recall (WAIS-IV) | 20.13 | 4.50 | 14.94 | 3.70 | 7.50 | 3.78 | ** |
| Verbal stroop card sorting test – words/colors | 35.93 | 10.98 | 25.39 | 9.83 | 18.29 | 14.37 | ** |
| Non-verbal stroop card sorting test | 47.62 | 7.16 | 51.54 | 13.55 | 78.38 | 18.25 | ** |
| CDR | n/a | n/a | 1.10 | 0.57 | 4.68 | 2.56 | ** |
| RPA-ProMem | 2.00 | 1.26 | 2.29 | 1.04 | not admin. | n/a | |
| ADCS-ADL | not admin. | not admin. | 60.00 | 9.12 | n/a | ||
SCI, Subjective Cognitive Impairment; MCI, Mild Cognitive Impairment; AD, Alzheimer’s disease; M, mean; SD, standard deviation; MMSE – Greek version, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment; M@T, Memory Alternation Test; RAVLT, Rey Auditory Verbal Learning Test; RBMT, Rivermead Behavioral Memory Test; ROCFT, Rey Osterrieth Complex Figure Test; FUCAS, Functional Cognitive Assessment Scale; FRSSD, Functional Rating Scale for Symptoms of Dementia; GDS, Geriatric Depression Scale; NPI, Neuropsychiatric Inventory; ADAS-Cog, Alzheimer’s Disease Assessment Scale– Cognitive Subscale; WAIS-IV, Wechsler Adult Intelligence Scale; CDR, Clinical Dementia Rating; RPA-ProMem, Royal Prince Alfred Prospective Memory Test; ADCS-ADL, Alzheimer’s Disease Cooperative Study ADL Scale; *p < 0.05; **p < 0.001; n.s. not significant.
Note that following exclusion criteria apply in the present study: 1) any severe physical illness; 2) current psychiatric or neurological disorder; 3) history of drug or alcohol abuse and use of neuro-modifying drugs other than cholinesterase inhibitors or memantine in AD group; 4) having any somatic disorder that may have caused subjective or objective cognitive impairment such as a cerebrovascular accident, other neurodegenerative diseases, traumatic brain injury, brain tumor, alcohol abuse and depression or other psychiatric disorders; 5) being under treatment at least for 90 days before the assessment/experimental sessions; and 6) hearing impairment or age-related hearing loss.
Litmus SRT tool
The Litmus SRT serves as a language latent ability assessment task and has been developed within the COST Action following the guidelines outlined in 73. The tool consists of 32 test items assessing eight structures—four test items per structure. The structures are the following: subject–verb–object (SVO) sentences, sentences containing negation, structures with clitics, complement clauses, coordinated sentences, adverbial clauses, wh-questions, and relative clauses. Note that all sentences were matched for length and word frequency; examples appear in Table 4.
Table 4
Litmus SRT conditions & examples per syntactic structure
| Structure | Example |
| SVO | 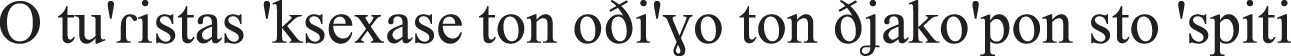 the tourist-NOM forget-PAST-PERF the guide-ACC the vacation-GEN at-the home-ACC “The tourist forgot the travel guide at home.” |
| Negation | 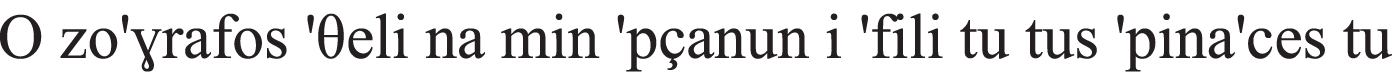 the painter-NOM want-PRES his friends-ACC to not touch his paintings-ACC “The painter does not want his friends to touch his paintings.” |
| Clitic | 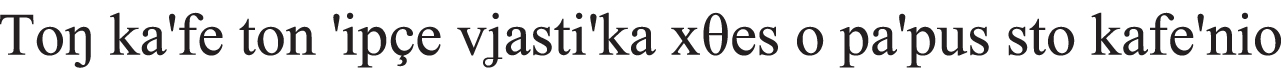 the coffee-ACC it-ACC drink-PAST-PERF in a hurry the grandfather-NOM to-the coffee shop “Yesterday, the grandfather had his coffee in the coffee shop in a hurry.” |
| Coordinated sentences | 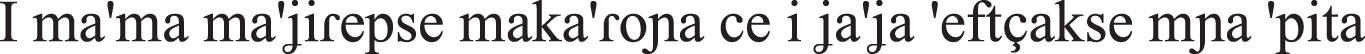 the mother-NOM cook-PAST PERF pasta-ACC and grandmother make-PAST PERF a pie-ACC “The mother- cooked pasta and the grandmother made a pie.” |
| Complement clauses |  the nurses-NOM say-PAST-PERF that the flight-ACC the doctor-GEN have-PRES delay-ACC “The nurses said that doctor’s flight had a delay.” |
| Adverbial clauses |  when the school-NOM close-PAST-PERF the summer-ACC the children-NOM run-PAST-IMPERF to-the streets-ACC “When the school closed for summer the children were running in the streets.” |
| Wh-questions | 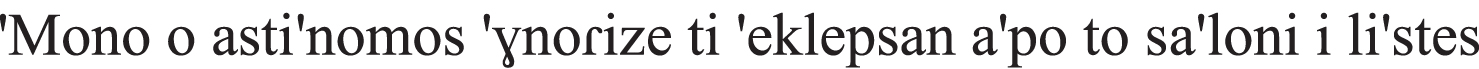 only the policeman-NOM know-PAST-IMPERF what steal-PAST from the living-room-ACC the robbers-NOM “Only the policeman knew what the robbers stole from the living-room.” |
| Relative clauses |  the newspapers-NOM write-PRES a lot-ACC about the robber-ACC that cautch-PAST the police-NOM “The newspapers write a lot about the robber that the police arrested.” |
Participants were tested in a quiet room and listened to the test items via headphones; the sentences were pre-recorded by a female native speaker of Greek and had normal intonation. The participant was asked to repeat each sentence as accurately as possible, and the examiner recorded their response. Subsequently, the recordings were transcribed and scored by two linguists for cross-checking purposes. To evaluate performance in the task we assess accuracy, grammaticality, and completeness.
We measure accuracy following the scoring procedure of the SR subtest of the Clinical Evaluation of Language Fundamentals (fourth edition) (CELF-4).74 We awarded 3 points if the participant made no errors while repeating the sentence, thus, producing a verbatim repletion of the target sentence; 2 points if their utterance included one error; 1 point, if their utterance included two errors; and 0 points for three errors or more. With regard to the accuracy index, the scoring scale ranges from 0 to 96 yet for ease of presentation data appear in % in the following section. Turning to grammaticality, the participant’s responses are evaluated with respect to whether they produced grammatically acceptable utterances or not regardless of whether their utterance was identical to the one provided. We awarded 1 point for every grammatical utterance and 0 points for every ungrammatical one. With reference to completeness, the participant’s responses are identified as complete and incomplete. We awarded 1 point for every complete utterance they produced and 0 points for every incomplete one. The grammaticality and completeness indices have a scoring scale of 0 to 32 for each participant and similarly to the accuracy index, we report data in % in the following section. Indicative examples of all types of scoring appear in Table 5.
Table 5
Examples of Accuracy, Grammaticality & Completeness Scoring
| Target sentence | |
 The mother-NOM put-PAST-PERF the shirt-ACC the girl-GEN at-the balcony-ACC “The mother put the girls’ shirts at the balcony.” | |
| Participant’s utterance | Index |
| Accuracy | |
 The mother-NOM put-PAST-PERF the shirt-ACC the girl-GEN at-the balcony-ACC | 3 points |
 The mother-NOM put-PAST-PERF the shirt-ACC the child-GEN at-the balcony-ACC | 2 points |
 The mother-NOM put-PRES the shirt-ACC the child-GEN at-the balcony-ACC | 1 point |
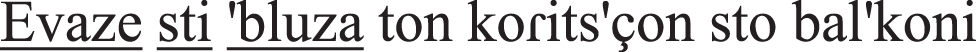 put-PAST-IMPERF on-the-shirt-ACC the girl-GEN at-the balcony-ACC | 0 points |
| Grammaticality | |
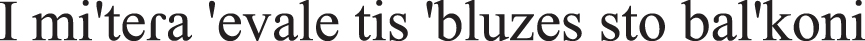 The mother-NOM put-PAST-PERF the shirt-ACC at-the balcony-ACC | 1 point |
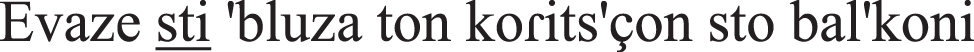 put-PAST-IMPERF on-the-shirt-ACC the girl-GEN at-the balcony-ACC | 0 points |
| Completeness | |
 The mother-NOM put-PAST-PERF the shirt-ACC the girl-GEN at-the balcony-ACC | 1 point |
 The mother-NOM put-PAST-PERF | 0 points |
Earlier studies employing the Litmus SRT have used the accuracy and grammaticality indices.33,75,76 The only measurement we added is that of Completeness so that we have a better overview of how the participants responded to the task in terms of being able to provide a complete sentence.
Data analysis
For the statistical analysis of SRT data, we used the IBM SPSS Statistics Software v. 27 (IBM Corp. Released 2020. IBM SPSS Statistics for Windows, Version 27.0. Armonk, NY: IBM Corp); statistical significance was set to two-tailed p-value <0.05. Firstly, we performed analysis of variance (one-way ANOVA) to assess the effect of Group (yHC versus eHC versus SCI versus MCI versus AD) on Accuracy, Grammaticality, and Completeness indices for overall task scores followed by Bonferroni pair group comparisons to identify significant differences. Secondly, we performed a similar analysis of variance to examine the effect of Group on the Accuracy index for the per structure scores, which was also followed by Bonferroni pair group comparisons. Next, considering that the AD group had less years of education compared to the rest of the groups of the study, we ran a Pearson’s r correlation analysis examining the relationship between Years of Education and SRT performance. Lastly, we proceeded with a receiver operating characteristic (ROC) analysis to evaluate the discriminatory ability of the task and the sensitivity of the Accuracy index in particular.
RESULTS
Language assessment data via litmus SRT
With regard to the data analysis of SRT, we examine the overall performance based on the three measures we developed, namely, the accuracy, grammaticality, and completeness indices. Table 6 summarizes the average scores per group and per index. To analyze the data, we performed an analysis of variance (ANOVA) with SRT scores as the within-subjects variable and Group as the between-subjects variable followed by Bonferroni pair group comparisons.
Table 6
Litmus SRT Scores per group
| Grammaticality | Accuracy | Completeness | ||||
| M | SD | M | SD | M | SD | |
| yHC | 99.06 | 1.86 | 95.38 | 3.82 | 100.00 | 0.00 |
| eHC | 98.13 | 2.91 | 90.76 | 6.94 | 99.38 | 2.38 |
| SCI | 96.46 | 4.24 | 88.75 | 8.63 | 99.27 | 1.58 |
| MCI | 93.13 | 5.94 | 76.87 | 16.58 | 97.60 | 3.73 |
| AD | 60.52 | 24.14 | 27.08 | 22.19 | 70.42 | 24.51 |
Values in %; M, mean; SD, standard deviation; yHC, young healthy adult speakers; eHC, cognitively intact elder healthy speakers; SCI, Subjective Cognitive Impairment; MCI, Mild Cognitive Impairment; AD, Alzheimer’s disease.
Starting with the Grammaticality index dataset, the analysis showed a main effect of Group (F (4,4644) = 203.227, p < 0.001, = 0.149). The pair group comparisons revealed that yHC, eHC, and SCI groups did not differ with regard to how grammatical their responses were, while grammaticality dropped significantly for the MCI and AD groups; Grammaticality Index: yHC, eHC, SCI > MCI > AD, p < 0.001.
Turning to the Accuracy index dataset, the analysis showed a main effect of Group (F (4,4644) = 691.604, p < 0.001, = 0.373). Further pair group comparisons revealed that all groups differed significantly to each other with the exception of eHC and SCI groups that perform similarly (yHC versus eHC: p = 0.018; all other pair comparisons p < 0.001). The accuracy index shows that there is a gradual and steady drop relating both to healthy aging effects and cognitive decline effects with speakers with AD producing accurately less than a third of their responses; Accuracy Index: yHC > eHC, SCI > MCI > AD.
Next, we analyzed the Completeness index dataset. The analysis showed a main effect of Group (F (4,4644) = 167.551, p < 0.001, = 0.126). The pair group comparisons revealed that yHC, eHC, SCI, and MCI groups perform similarly in reference to how complete their responses were while there was a significant drop in performance for speakers with AD; Completeness Index: yHC, eHC, SCI, MCI > AD, p < 0.001. Looking at the overall performance of the participants we identify the Accuracy index as the most sensitive one to track cognitive decline, followed by the Grammaticality and Completeness indices, which appear to be affected primarilyby AD.
The impact of syntax in SRT performance
Since the Accuracy index appeared to be the most sensitive to cognitive changes, as a follow-up step, we turn to the analysis of Accuracy data per structure. In this way we are able to assess the impact of syntax in how speakers perform; Table 7 summarizes the average scores per group. To analyze each dataset, we performed an analysis of variance (ANOVA) with SRT scores per Structure as the within-subjects variable and Group as the between-subjects variable followed by Bonferroni pair group comparisons.
Table 7
Accuracy per structure
| yHC | eHC | SCI | MCI | AD | ||||||
| M | SD | M | SD | M | SD | M | SD | M | SD | |
| Adverbials | 68.75 | 8.04 | 67.92 | 10.22 | 67.08 | 11.83 | 54.58 | 15.91 | 18.75 | 21.02 |
| Clitics | 67.92 | 8.80 | 61.04 | 13.90 | 56.67 | 13.53 | 43.13 | 20.78 | 12.92 | 15.30 |
| Complement clauses | 72.29 | 4.55 | 70.63 | 7.18 | 66.88 | 9.59 | 58.54 | 21.18 | 15.21 | 18.91 |
| Coordination | 73.13 | 4.69 | 70.63 | 9.16 | 70.00 | 6.44 | 68.75 | 7.70 | 27.71 | 21.88 |
| Negation | 73.96 | 2.88 | 68.54 | 9.06 | 66.88 | 12.73 | 54.79 | 15.63 | 19.38 | 19.65 |
| Relative clauses | 73.54 | 6.29 | 67.71 | 8.22 | 68.13 | 7.40 | 61.25 | 15.17 | 20.83 | 17.55 |
| SVO | 70.21 | 6.07 | 67.50 | 8.90 | 66.25 | 10.96 | 53.96 | 20.00 | 15.83 | 18.33 |
| Wh-Questions | 72.50 | 4.22 | 70.63 | 5.72 | 70.63 | 6.39 | 66.25 | 9.93 | 31.88 | 24.80 |
Values in %, M, mean; SD, standard deviation; yHC, young healthy adult speakers; eHC, cognitively intact elder healthy speakers; SCI, Subjective Cognitive Impairment; MCI, Mild Cognitive Impairment; AD, Alzheimer’s disease; SVO, subject–verb–object.
The data analysis showed a main effect of Group (F (4,4609) = 724.443, p < 0.001, = 0.386) replicating the group differences found at the previous analysis of overall scores with all groups differing to each other with the exception of eHC and SCI groups; yHC > eHC, SCI > MCI > AD; yHC versus eHC: p = 0.001; all other pair comparisons p < 0.001). The analysis also revealed a main effect of Structure (F (7,4609) = 28.084, p < 0.001, = 0.041) with best performance for Wh-questions (84.6%) and Coordination (84.3%), followed by Relative clauses (79%). Performance drops in Negation (77.3%), Complement clauses (76.7%), Adverbials (74.9%), and SVO (73.6%)—yet it is similar in these structures—while the lowest performance is found at Clitics (65.1%); Wh-questions, Coordination > Relative CL > Negation, Complement clauses, Adverbials, SVO > Clitics; p < 0.05 for all pair comparisons. Finally, the analysis showed a significant interaction between Group and Structure; F (28,4609) = 2.918, p < 0.001, = 0.017. This interaction allowed us to proceed with between group comparisons per structure with findings summarized in Table 8.
Table 8
Between group comparisons per structure - > for every significant drop in performance in btw group comparisons; all pairs p< 0.001 unless otherwise stated
| Group effect | Btw group differences | |
| Adverbials | F (4,577) = 76.979, p < 0.001, = 0.348 | yHC, eHC, SCI > MCI > AD |
| Clitics | F (4,578) = 75.413, p < 0.001, = 0.343 | yHC > eHC, SCI > MCI > AD yHC versus eHC, SCI: p = 0.006 SCI versus MCI: p = 0.001 |
| Complement clauses | F (4,575) = 146.686, p < 0.001, = 0.505 | yHC, eHC, SCI > MCI > AD SCI versus MCI: p = 0.018 |
| Coordination | F (4,575) = 93.465, p < 0.001, = 0.394 | yHC, eHC, SCI, MCI > AD |
| Negation | F (4,570) = 87.499, p < 0.001, = 0.380 | yHC, eHC, SCI > MCI > AD SCI versus MCI: p = 0.005 |
| Relative clauses | F (4,574) = 100.171, p < 0.001, = 0.411 | yHC, eHC, SCI > MCI > AD SCI versus MCI: p = 0.041 |
| SVO | F (4,582) = 114.706, p < 0.001, = 0.441 | yHC, eHC, SCI > MCI > AD |
| Wh-Questions | F (4,578) = 66.654, p < 0.001, = 0.316 | yHC, eHC, SCI, MCI > AD |
yHC, young healthy adult speakers; eHC, cognitively intact elder healthy speakers; SCI, Subjective Cognitive Impairment; MCI, Mild Cognitive Impairment; AD, Alzheimer’s disease; SVO, subject–verb–object.
The analysis per structure showed that the Clitics structure is the most challenging one as it reveals effects relating to both healthy aging and cognitive decline related to AD dementia. Moreover, the three clinical groups of the study, SCI, MCI, and AD groups differed in their performance with regard to Adverbials, Complement clauses, Negation, Relative clauses, and SVO, while only the AD group’s Accuracy index dropped significantly in Coordination and Wh-question structures. These findings suggest that 1) the grammatical component in language is indeed affected by cognitive decline and AD dementia and 2) particular structural elements, e.g., Clitics, appear to be more vulnerable to those effects as opposed to others, e.g., Coordination.
The impact of years of education in SRT performance of speakers with AD
Since the AD group had less years of education compared to the rest of the groups of the study, as a follow-up step we explored whether speakers with AD that had more years of education would perform better compared to the ones with less years of education. To this aim, we ran three Pearson’s r correlation analyses, one for the Grammaticality data, one for the Accuracy data and one for Completeness data testing the possible relationship between Years of Education and overall performance and performance by structure type.
The analysis revealed that there was no correlation between Years of Education and overall performance in Grammaticality, Accuracy, and Completeness Indices. When looking by structure type, the analyses showed a positive moderate correlation between Years of Education and performance in Complement and Relative Clauses in the Accuracy data (Complement CL: r (30) =0.438, p = 0.016; Relative CL: r (30) =0.431, p = 0.017) and a similar one between Years of Education and performance in Coordinated Sentences in the Grammaticality data (r (30) =0.387, p = 0.035). Given that the Years of Education did not appear to affect the overall performance in SRT of speakers with AD, and only moderately in a few cases when looking per structure, the correlation data suggest that the deterioration in cognitive ability and linguistic competency is such in this group that it overrides individual differences relating to educational background.
The discriminatory ability of the Litmus SRT
Considering that the Litmus SRT appears to be sensitive to the (non)clinical status of speakers, we further explored the discriminatory ability of the task testing the sensitivity of the Accuracy index to cognitive changes in our dataset. Specifically, we examined the Area Under the Curve (AUC), the sensitivity and specificity via ROC analysis and we identified the best threshold value to discriminate the groups. The results of the ROC analysis are summarized in Table 9 and Fig. 1.
Table 9
Sensitivity and specificity of accuracy index of the litmus SRT for each group compared to the other groups
| Groups | AUC (%) | Sign. | Threshold Value | Sensitivity (%) | Specificity (%) |
| yHC versus eHC | 72.7% | * | 87.5 | 83.3% | 46.7% |
| yHC versus SCI | 76.3% | ** | 86.5 | 86.7% | 43.3% |
| yHC versus MCI | 92.6% | ** | 85.5 | 90.0% | 73.3% |
| yHC versus AD | 100% | ** | 84.5 | 96.7% | 100% |
| eHC versus SCI | 55.9% | n.s. | 83.5 | 80.0% | 33.3% |
| eHC versus MCI | 79.8% | ** | 81.5 | 86.7% | 53.3% |
| eHC versus AD | 99.9% | ** | 80.5 | 90.0% | 100% |
| SCI versus MCI | 74.7% | * | 76.5 | 86.7% | 46.7% |
| SCI versus AD | 99.8% | * | 70.5 | 93.3% | 100% |
| MCI versus AD | 95.1% | ** | 49.5 | 90.0% | 83.3% |
Fig. 1
ROC Curves per group pair.
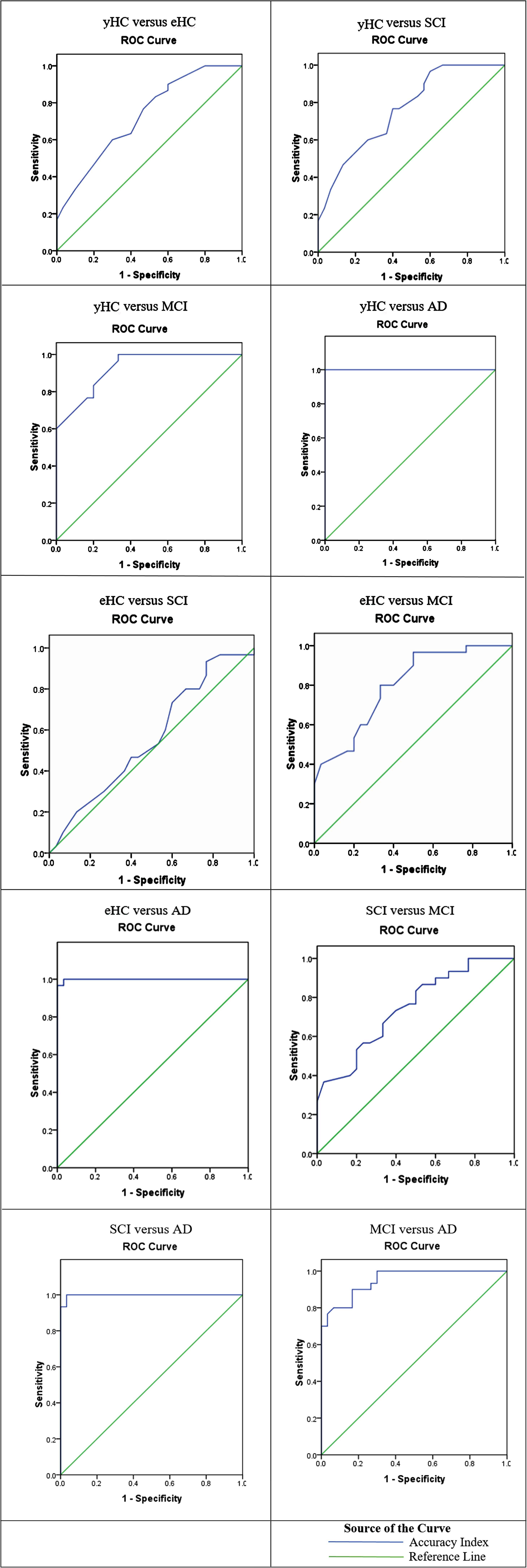
The ROC analysis showed that the SRT is a quite sensitive task that can differentiate the groups of the study. Specifically, there is 72.7% probability that it can identify healthy aging effects (yHC versus eHC) and 55.9% probability that it can discriminate between eHC and SCI; note that in the case of the latter pair there is limited clinical utility, which also reflects the ANOVA findings in the previous section. Turning to the clinical group comparisons, the probability raises significantly since there is 79.8% probability that it can discriminate between eHC and MCI, 74.7% probability that it can discriminate between SCI and MCI and 95.1% probability that it can discriminate between MCI and AD. See Table 9 for complete set of values. These results suggest that there is clinical utility for the SRT as an add-on diagnostic tool since it can track the cognitive decline due to AD.
DISCUSSION
The present study aimed to examine the role of language markers in the early assessment of cognitive changes due to AD. Specifically, we focused on the investigation of the decline of linguistic ability due to changes in cognition of speakers along the healthy aging–AD dementia continuum employing the Litmus SRT tool, which is a highly structured complex language task that assesses language production, serves as a global measurement of verbal abilities33,34,75 and provides information on the impact of syntactic properties in the performance of speakers. We tested young adults and cognitively intact healthy elder speakers along with speakers with SCI, MCI and mild/moderate AD dementia. In normal aging, individuals experience slight cognitive changes that may (or not) impact linguistic ability;34 consequently, we included both a younger and an older healthy control group so as to address any possible healthy aging effects in the SRT performance and disentangle them from effects relating to cognitive changes due to AD dementia. To evaluate the impact of the cognitive decline in clinical populations and track changes in language competence, our study recruited speakers at the preclinical silent phase of SCI during which individuals may notice changes that are not detectable via cognitive screening tools, speakers with MCI, amnestic type, who experience cognitive changes which are clinically observable in one cognitive domain and also speakers with mild/moderate AD who live with severe cognitive impairment.1,6 We have attempted to explore language performance along a continuum of speakers of Greek; this is one of the novelties and main contributions of the study; meanwhile, it raises a number of potential difficulties in how this data may be interpreted. To tackle this issue, we included a strict set of inclusion/exclusion criteria per group.
Earlier research has analyzed thoroughly the cognitive skills of individuals with MCI and AD; however, research on the linguistic profiling of speakers with MCI and AD dementia is limited in number and often focuses on English speakers.2,7,8,18,21,36,77 Recently, a growing body of research, though, has highlighted the value of tracking language ability in populations with AD,3–6 aphasic variants of AD,9–11 and also MCI.36,39–41 The findings on language changes so far show high variability reflecting the variation in the language testing methods employed, languages tested and number of speakers included in the analyses.2,7,8,31,32,35 Considering this gap in the literature, the present study compiled an extended pool of 150 speakers of Greek and analyzed language data collected via the Litmus SRT, a language tool assessing the role of syntax. For the analysis, we employed language indices that assess 1) overall performance (Accuracy index) along with degree of grammaticality and completeness in the language production data of the speakers tested; and 2) performance per syntactic condition.
Starting with overall performance, the data analysis showed that the Litmus SRT is a tool with high discriminatory value in the development of AD revealing significant aging and dementia effects. Starting with the Grammaticality index, the analysis showed that yHC, eHC, and SCI groups did not differ with regard to how grammatical their responses were, while grammaticality dropped significantly for the MCI and AD groups. The Completeness index analysis revealed that yHC, eHC, SCI, and MCI groups perform similarly in reference to how complete their responses of speakers were, while there was a significant drop in performance for speakers with AD. Unlike the Grammaticality and Completeness indices, the Accuracy index was shown to be the most sensitive one to track cognitive decline since as all groups differed significantly to each other with the exception of eHC and SCI groups that perform similarly. Based on this evidence, the SRT scores appear to have high discriminatory ability since we could identify both healthy aging and cognitive function effects due to AD. Our results provide support to earlier studies that identify SRTs as sensitive enough tools for age-related decline in healthy populations.78 Additionally, the difficulty to discriminate between eHC and SCI suggests that our study verifies earlier work that reports that grammatical skills of speakers with SCI as retained.46
Turning to the language skills of individuals with MCI and AD, earlier studies suggest that individuals with MCI may experience mild discursive difficulties, impairments in sentence comprehension, impaired recall and reading comprehension,39,40 while linguistic decline in AD dementia affects primarily the semantic and pragmatic levels.20,21 Thus, the syntactic grammatical domain initially was considered unimpaired in early stage AD dementia.22–25 Nonetheless, recent work identifies some syntactic disturbances7,26,27,29 and our findings provide support in that direction as well. Specifically, our data analysis showed that syntax has an impact on how accurately participants responded overall, and specific structures appear to be significantly affected by cognitive decline and AD. Note also that the significant drop in performance by speakers with AD irrespective of index type provides further support to earlier studies that identified language changes via SRT methodology.31,32,35 Lastly, the ROC analysis of the Accuracy index verified the discriminatory value of the SRT, particularly for the clinical groups of the study, and suggests that clinical decision-making can be improved by including language assessment.
Turning to the role of syntax and the effect of different structures in the performance of speakers of Greek, participants with AD appear to retain high accuracy in Wh-questions and Coordination structures, while their accuracy performance dropped significantly in structures such as Clitics. This finding reveals that when a speaker with AD is asked to produce a complex structure involving clitic doubling or clitic left-dislocation, they opt for the omission of the clitic element which does not lead to ungrammaticality in Greek; the clitic in such structures copies the DP argument of the sentence and as such the information content of the utterance remains the same at the expense though of discourse considerations – on the syntax of clitics see studies.79-81 Clitic structures have been shown to be particularly challenging both in typical and atypical development33,82 and the present dataset indicates that it is an equally challenging syntactic feature for speakers with cognitive decline. This evidence suggests that certain grammar areas are more vulnerable to change compared to others and future research needs to focus on the specific grammatical features that appear to be more or less vulnerable across different languages. It is also of interest to point out that we identify changes in language performance relating to syntax in speakers with MCI with their performance dropping significantly in relation to speakers with SCI in most structures, e.g., complement clauses, clitics, relative clauses, and SVO. This finding suggests that MCI screening needs to include language assessment as an integral component of the diagnostic process for MCI since it can implement the clinical evaluation early on.
Nonetheless, the present study has some limitations. Recruiting speakers across the spectrum of cognitive decline, ranging from healthy aging, SCI and MCI to AD, can be challenging. We attempted to address variability in the dataset, such as the possible impact of years of education in AD, the correlation analysis showed that the deterioration in cognitive ability and linguistic competency in this group is so robust that it overrides any individual differences relating to educational background. However, this point needs to be further verified in much more extensive research with the use of other language elicitation methods, more or less structured ones and a possibly larger data pool; we treat this finding as a preliminary one that needs be further assessed. Furthermore, considering that anatomically, the diffuse pathology of the temporal lobe in AD could explain partially the language deficits with the repetition retention in early stages of AD,84 the assessment of other structures, either in a SRT format or other type of structured experiment would further our understanding of grammatical decline in speakers with MCI and AD. Lastly, there is no follow-up yet that would allow us to examine the performance of our participants longitudinally; a cohort study would be particularly valuable in enhancing our research findings and assist in more accurate differential diagnoses since monitoring changes in language abilities over time can inform adjustments to treatment strategies and help track disease progression.
To conclude, considering the pervasive role of language in daily living, along with the fact that language-based AD dementia assessment is one of the least intrusive ways to assess changes in symptoms of cognitive decline and AD dementia,2 the findings of the present study offer further support in this direction. Future work is required to provide the detailed mapping of the relationship between cognitive and linguistic skills along the dementia continuum. The present study provides a dataset in that direction; yet it tests one language, using one language tool. As a follow-up step, we will be exploring other areas of language production by speakers across the healthy aging–AD dementia continuum in an attempt to enrich language databases and potentially identify linguistic indicators related to the decline of cognitive skills due to aging and AD which can facilitate significantly the clinical process and possibly lead to the development of language intervention protocols.
AUTHOR CONTRIBUTIONS
Maria Kaltsa (Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Supervision; Validation; Writing – original draft; Writing – review & editing); Anthoula Tsolaki (Validation; Writing – original draft; Writing – review & editing); Ioulietta Lazarou (Data curation; Formal analysis; Methodology; Writing – original draft); Ilias Mittas (Investigation; Methodology; Software; Validation); Mairi Papageorgiou (Supervision; Validation; Writing – review & editing); Despina Papadopoulou (Supervision; Visualization; Writing – review & editing); Ianthi Maria Tsimpli (Visualization; Writing – original draft; Writing – review & editing); Magda Tsolaki (Conceptualization; Methodology; Writing – review & editing).
ACKNOWLEDGMENTS
The authors have no acknowledgments to report.
FUNDING
The data used for this paper is part of a larger project, Project DemLENS ‘Linguistic Perspectives on Dementia: an investigation of lexical, syntactic and content complexity in the narratives of Greek speaking patients with Mild Cognitive Impairment and Alzheimer’s Disease’ https://demlens.enl.auth.gr/. The research project is supported by the Hellenic Foundation for Research and Innovation (H.F.R.I.) under the “2nd Call for H.F.R.I. Research Projects to support Post-Doctoral Researchers” (Award No 163 | Project No 98109 | PI: Maria Kaltsa).
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
DATA AVAILABILITY
The data supporting the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
1. | Eyigoz E , Mathur S , Santamaria M , et al. Linguistic markers predict onset of Alzheimer’s disease. EClinicalMedicine (2020) ; 28: : 100583. |
2. | Dubois B , Feldman HH , Jacova C , et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol (2024) ; 13: : 614–629. |
3. | Fraser KC , Meltzer JA and Rudzicz F. Linguistic features identify Alzheimer’s disease in narrative speech. J Alzheimers Dis (2016) ; 49: : 407–422. |
4. | Petti U , Baker S and Korhonen A. A systematic literature review of automatic Alzheimer’s disease detection from speech and language. J Am Med Inform Assoc (2020) ; 27: : 1784–1797. |
5. | Benııtez-Burraco A and Ivanova O. Language in healthy and pathological ageing: Methodological milestones and challenges. Int J Lang Commun Disord (2024) ; 59: : 4–12. |
6. | Lindsay H , Tröger J and König A. Language impairment in Alzheimer’s disease—robust and explainable evidence for ad-related deterioration of spontaneous speech through multilingual machine learning. Front Aging Neurosci (2021) ; 13: : 642033. |
7. | Ahmed S , Haigh AMF , de Jager CA , et al. Connected speech as a marker of disease progression in autopsy-proven Alzheimer’s disease. Brain (2013) ; 136: : 3727–3737. |
8. | Duong A , Giroux F , Tardif A , et al. The heterogeneity of picture-supported narratives in Alzheimer’s disease. Brain Lang (2005) ; 93: : 173–184. |
9. | Beber BC , Kochhann R , da Silva BM , et al. Logopenic aphasia or Alzheimer’s disease: Different phases of the same disease? Dement Neuropsychol (2014) ; 8: : 302–307. |
10. | Rogalski E , Sridhar J , Rader B , et al. Aphasic variant of Alzheimer disease: clinical, anatomic, and genetic features. Neurology (2016) ; 87: : 1337–1343. |
11. | Weekes BSH . Aphasia in Alzheimer’s disease and other dementias (ADOD): Evidence from Chinese. Am J Alzheimers Dis Other Demen (2020) ; 35: : 1533–3175. |
12. | Geslani DM , Tierney MC , Herrmann N , et al. Mild cognitive impairment: an operational definition and its conversion rate to Alzheimer’s disease. Dement Geriatr Cogn Disord (2005) ; 19: : 383–389. |
13. | Mueller KD , Hermann B , Mecollari J , et al. Connected speech and language in mild cognitive impairment and Alzheimer’s disease: A review of picture description tasks. J Clin Exp Neuropsychol (2018) ; 40: : 917–939. |
14. | Slegers A , Filiou RP , Montembeault M , et al. Connected speech features from picture description in Alzheimer’s disease: A systematic review. J Alzheimers Dis (2018) ; 65: : 519–542. |
15. | Varlokosta S , Fragkopoulou K , Arfani D , et al. Methodologies for assessing morphosyntactic ability in people with Alzheimer’s disease. Int J Lang Commun Disord (2024) ; 59: : 38–57. |
16. | Williams E , McAuliffe M and Theys C (2021) Language changes in Alzheimer’s disease: a systematic review of verb processing. Brain Lang (2021) ; 223: 105041. |
17. | Groves-Wright K , Neils-Strunjas J , Burnett R , et al. A comparison of verbal and written language in Alzheimer’s disease. J Commun Disord (2004) ; 37: : 109–130. |
18. | Tang-Wai DF and Graham NL. Assessment of language function in dementia. Geriatr Aging (2008) ; 11: : 103–110. |
19. | Klimova B and Kuca K. Speech and language impairments in dementia. J Appl Biomed (2016) ; 14: : 97–103. |
20. | Croisile B , Ska B , Brabant MJ , et al. Comparative study of oral and written picture description in patients with Alzheimer’s disease. Brain Lang (1996) ; 53: : 1–19. |
21. | Kavé G and Dassa A. Severity of Alzheimer’s disease and language features in picture descriptions. Aphasiology (2018) ; 32: : 27–40. |
22. | Kaprinis S and Stavrakaki S. Morphological and syntactic abilities in patients with Alzheimer’s disease. Brain Lang (2007) ; 1: : 59–60. |
23. | Kavé G and Levy Y. Morphology in picture descriptions provided by persons with Alzheimer’s disease. J Speech Lang Hear Res (2003) ; 46: : 341–352. |
24. | Kempler D , Curtiss S and Jackson C. Syntactic preservation in Alzheimer’s disease. J Speech Hear Res (1987) ; 30: : 343–350. |
25. | Kemper S , LaBarge E , Ferraro FR , et al. On the preservation of syntax in Alzheimer’s disease. Evidence from written sentences. Arch Neurol (1993) ; 50: : 81–86. |
26. | Sajjadi SA , Patterson K , Tomek M , et al. Abnormalities of connected speech in semantic dementia vs Alzheimer’s disease. Aphasiology (2012) ; 26: : 847–866. |
27. | Fyndanis V , Manouilidou C , Koufou E , et al. Agrammatic patterns in Alzheimer’s disease: Evidence from tense, agreement, and aspect. Aphasiology (2013) ; 27: : 178–200. |
28. | Manouilidou C , Roumpea G , Nousia A , et al. Revisiting aspect in mild cognitive impairment and Alzheimer’s disease: evidence from Greek. Front Commun (2020) ; 5: : 434106. |
29. | Rentoumi V , Paliouras G , Danasi E , et al. Automatic detection of linguistic indicators as a means of early detection of Alzheimer’s disease and of related dementias: A computational linguistics analysis. In 8th IEEE International Conference on Cognitive Infocommunications (CogInfoCom 2017), Debrecen, Hungary, 11–14 September 2017, pp. 000033–000038. IEEE. |
30. | Faitaki F and Murphy VA. Oral language elicitation tasks in applied linguistics research. In: McKinley J and Rose H (eds) The Routledge handbook of research methods in applied linguistics. Routledge, (2019) , pp. 360–369. |
31. | Arslan S , Plonka A , Mouton A , et al. Sentence repetition span in primary progressive aphasia and Alzheimer’s disease: insights from preliminary results. Front Commun (2022) ; 7: : 934487. |
32. | Beales A , Whitworth A , Cartwright J , et al. Profiling sentence repetition deficits in primary progressive aphasia and Alzheimer’s disease: Error patterns and association with digit span. Brain Lang (2019) ; 194: : 1–11. |
33. | Kaltsa M , Prentza A and Tsimpli IM. Input and literacy effects in simultaneous and sequential bilinguals: The performance of Albanian–Greek-speaking children in sentence repetition. Int J Biling (2020) ; 24: : 159–183. |
34. | Polišenská K , Chiat S and Roy P. Sentence repetition: What does the task measure? Int J Lang Commun Disord (2015) ; 50: : 106–118. |
35. | Small JA , Kemper S and Lyons K. Sentence repetition and processing resources in Alzheimer’s disease. Brain Lang (2000) ; 75: : 232–258. |
36. | Taler V and Phillips NA. Language performance in Alzheimer’s disease and mild cognitive impairment: a comparative review. J Clin Exp Neuropsychol (2008) ; 30: : 501–556. |
37. | Testa JA , Ivnik RJ , Boeve B , et al. Confrontation naming does not add incremental diagnostic utility in MCI and Alzheimer’s disease. J Int Neuropsychol Soc (2004) ; 10: : 504–512. |
38. | Lopez-Higes R , Prados JM , Galindo-Fuentes M , et al. Semantic verbal fluency of animals in amnesia-type mild cognitive impairment. Rev Neurol (2014) ; 58: : 493–499. |
39. | Drummond C , Coutinho G , Fonseca RP , et al. Deficits in narrative discourse elicited by visual stimuli are already present in patients with mild cognitive impairment. Front Aging Neurosci (2015) ; 7: : 96. |
40. | Johnson M and Lin F. Communication difficulty and relevant interventions in mild cognitive impairment: implications for neuroplasticity. Top Geriatr Rehabil (2014) ; 30: : 18–34. |
41. | Kaltsa M , Papadopoulou D , Gialaouzidis M , et al. The processing of lexical ambiguity: the effect of mild cognitive impairment and Alzheimer’s disease in the monolingual lexicon. In Proceedings of 14th International Conference of Greek Linguistics (ICGL 14) (eds Markopoulous T, Vlachos C, Archakis A, etc.), (2021) , pp. 467–476. Patra: Patra University Press. |
42. | Beltrami D , Gagliardi G , Rossini Favretti R , et al. Speech analysis by natural language processing techniques: a possible tool for very early detection of cognitive decline? Front Aging Neurosci (2018) ; 10: : 369. |
43. | Fraser KC , Lundholm Fors K , Eckerström M , et al. Predicting MCI status from multimodal language data using cascaded classifiers. Front Aging Neurosci (2019) ; 11: : 205. |
44. | Mazaheri A , Segaert K , Olichney J , et al. EEG oscillations during word processing predict MCI conversion to Alzheimer’s disease. Neuroimage Clin (2018) ; 17: : 188–197. |
45. | Madhavan KM , McQueeny T , Howe SR , et al. Superior longitudinal fasciculus and language functioning in healthy aging. Brain Res (2014) ; 1562: : 11–22. |
46. | Lundholm-Fors K , Fraser K and Kokkinakis D. Automated syntactic analysis of language abilities in persons with mild and subjective cognitive impairment. In: Ugon A, Karlsson D, Klein GO, et al. (eds) Building Continents of Knowledge in Oceans of Data: The Future of Co-Created eHealth. Amsterdam: IOS Press, (2018) , pp. 705–709. |
47. | Gallo F , Bermudez-Margaretto B , Shtyrov Y , et al. First language attrition: What it is, what it isn’t, and what it can be. Front Hum Neurosci (2021) ; 15: : 686388. |
48. | Kaltsa M , Tsimpli IM and Rothman J. Exploring the source of differences and similarities in L1 attrition and heritage speaker competence: Evidence from pronominal resolution. Lingua (2015) ; 164: : 266–288. |
49. | Yılmaz G and Schmid MS. First language attrition and bilingualism. In: Miller D, Bayram F, Rothman J, et al. (eds) Bilingual cognition and language: The state of the science across its subfields. Amsterdam/Philadelphia: John Benjamins Publishing Company, (2018) , pp. 225–249. |
50. | Tsolaki M , Tsatali M , Gkioka M , et al. Memory clinics and day care centers in Thessaloniki, Northern Greece: 30 years of clinical practice and experience. Front Neurol (2021) ; 12: : 683131. |
51. | McKhann G , Drachman D , Folstein M , et al. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology (1984) ; 34: : 939–944. |
52. | Petersen RC , Knopman DS , Boeve BF , et al. Mild cognitive impairment: Ten years later. Arch Neurol (2009) ; 66: : 1447–1455. |
53. | Molinuevo JL , Rabin LA , Amariglio R , et al. Implementation of subjective cognitive decline criteria in research studies. Alzheimers Dement (2017) ; 13: : 296–311. |
54. | Fountoulakis KN , Tsolaki M , Chantzi H , et al. Mini mental state examination (MMSE): A validation study in Greece. Am J Alzheimers Dis (2000) ; 15: : 342–345. |
55. | Konstantopoulos K , Vogazianos P and Doskas T. Normative data of the Montreal Cognitive Assessment in the Greek population and parkinsonian dementia. Arch Clin Neuropsychol (2016) ; 31: : 246–253. |
56. | Lazarou I , Moraitou D , Papatheodorou M , et al. Adaptation and validation of the Memory Alteration Test (M@T) in Greek middle-aged, older, and older-old population with subjective cognitive decline and mild cognitive impairment. J Alzheimers Dis (2021) ; 84: : 1219–1232. |
57. | Kosmidis MH , Vlahou CH , Panagiotaki P , et al. The verbal fluency task in the Greek population: Normative data, and clustering and switching strategies. J Int Neuropsychol Soc (2004) ; 10: : 164–172. |
58. | Wechsler D . Wechsler Adult Intelligence Scale–Fourth Edition (WAIS–IV). San Antonio, TX: The Psychological Corporation, (2008) . |
59. | Zalonis I , Christidi F , Bonakis A , et al. The Stroop effect in Greek healthy population: Normative data for the Stroop Neuropsychological Screening Test. Arch Clin Neuropsychol (2009) ; 24: : 81–88. |
60. | Koch C and Roid G. Manual for the nonverbal Stroop card sorting task. Wood Dale, IL: Stoelting Company, (2012) . |
61. | Messinis L , Tsakona I , Malefaki S , et al. Normative data and discriminant validity of Rey’s Verbal Learning Test for the Greek adult population. Arch Clin Neuropsychol (2007) ; 22: : 739–752. |
62. | Wilson B , Cockburn J , Baddeley A , et al. The development and validation of a test battery for detecting and monitoring everyday memory problems. J Clin Exp Neuropsychol (1989) ; 11: : 855–870. |
63. | Osterrieth PA . Le test de copied’une figure complexe; contribution à l’étude de la perception et de la mémoire. Arch Psychol (1944) ; 30: : 206–356. |
64. | Tombaugh TN . Trail Making Test A and B: Normative data stratified by age and education. Arch Clin Neuropsychol (2004) ; 19: : 203–214. |
65. | Kounti F , Tsolaki M and Kiosseoglou G. Functional cognitive assessment scale (FUCAS): A new scale to assess executive cognitive function in daily life activities in patients with dementia and mild cognitive impairment. Hum Psychopharmacol (2006) ; 21: : 305–311. |
66. | Tsolaki M , Fountoulakis K , Nakopoulou E , et al. Alzheimer’s Disease Assessment Scale: The validation of the scale in Greece in elderly demented patients and normal subjects. Dement Geriatr Cogn Disord (1997) ; 8: : 273–280. |
67. | Yesavage JA and Sheikh JI. Geriatric depression scale (GDS): Recent evidence and development of a shorter version. Clin Gerontol (1986) ; 5: : 165–173. |
68. | Cummings JL , Mega M , Gray K , et al. The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology (1994) ; 44: : 2308. |
69. | Mohs RC , Knopman D , Petersen RC , et al. Development of cognitive instruments for use in clinical trials of antidementia drugs: Additions to the Alzheimer’s Disease Assessment Scale that broaden its scope. Alzheimer Dis Assoc Disord (1997) ; 2: 11 Suppl 2: S13–S21. |
70. | Rosen WG , Mohs RC and Davis KL. A new rating scale for Alzheimer’s disease. Am J Psychiatry (1984) ; 141: : 1356–1364. |
71. | Morris JC . The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology (1993) ; 43: : 2412–2414. |
72. | Radford KA , Lah S , Say MJ , et al. Validation of a new measure of prospective memory: The Royal Prince Alfred Prospective Memory Test. Clin Neuropsychol (2011) ; 25: : 127–140. |
73. | Marinis T and Armon-Lotem S. Sentence repetition. In: Armon-LotemS, deJong J, MeirN (eds) Methods for assessing multilingual children: Disentangling bilingualism from Language Impairment. Bristol, UK: Multilingual Matters, (2015) , pp. 95–124. |
74. | Semel E , Wiig EH and Secord WA. Clinical evaluation of language fundamentals – Fourth Edition (CEFL-4). Toronto, Canada: The Psychological Corporation, Harcourt Assessment Company, (2003) . |
75. | Andreou M , Tsimpli IM , Masoura E , et al. Cognitive mechanisms of monolingual and bilingual children in monoliterate educational settings: Evidence from sentence repetition. Front Psychol (2021) ; 11: : 613992. |
76. | Prentza A , Tafiadis D , Chondrogianni V , et al. Validation of a Greek Sentence Repetition task with typically developing monolingual and bilingual children. J Psycholinguist Res (2022) ; 51: : 373–395. |
77. | Ferris SH and Farlow M. Language impairment in Alzheimer’s disease and benefits of acetylcholinesterase inhibitors. Clin Interv Aging (2013) ; 8: : 1007–1014. |
78. | Schum RL and Sivan AB. Verbal abilities in healthy elderly adults. Appl Neuropsychol (1997) ; 4: : 130–134. |
79. | Philippaki-Warburton I , Varlokosta S , Georgiafentis M , et al. Moving from theta-positions: Pronominal clitic doubling in Greek. Lingua (2004) ; 114: : 963–989. |
80. | Roussou A and Tsimpli IM. On Greek VSO again! J Linguist (2006) ; 42: : 317–354. |
81. | Marchis M and Alexiadou A. The syntax of clitics revisited: Two types of clitics. Lingua (2013) ; 127: : 1–13. |
82. | Chondrogianni V , Marinis T , Edwards S , et al. Production and on-line comprehension of definite articles and clitic pronouns by Greek sequential bilingual children and monolingual children with specific language impairment. Appl Psycholinguist (2015) ; 36: : 1155–1191. |
83. | American Psychiatric Association, DSM-5 Task Force. Diagnostic and statistical manual of mental disorders: DSM-5™ (5th ed.). Washington, DC: American Psychiatric Publishing, Inc., (2013) . |
84. | López-Barroso D , Paredes-Pacheco J , Torres-Prioris MJ , et al. Brain structural and functional correlates of the heterogeneous progression of mixed transcortical aphasia. Brain Struct Funct (2023) ; 228: : 1347–1364. |




