Long-Term Effects of the Multicomponent Program BrainProtect® on Cognitive Function: One-Year Follow-Up in Healthy Adults
Abstract
Background:
Age-related neuronal changes impact cognitive integrity, which is a major contributor to health and quality of life. The best strategy to prevent cognitive decline and Alzheimer’s disease is still debated.
Objective:
To investigate the long-term effects of the eight-week multicomponent training program BrainProtect® on cognitive abilities compared to general health counseling (GHC) in cognitively healthy adults in Germany.
Methods:
Healthy adults (age ≥50 years) previously randomized to either GHC (n = 72) or BrainProtect (intervention group, IG, n = 60) for eight-weeks (once weekly, 90 minutes, group-based) underwent a comprehensive neuropsychological test battery and health-related quality of life (HRQoL) evaluation 3- and 12-months after intervention end.
Results:
Dropout rates were n = 8 after 3 months and n = 19 after 12 months. No significant long-term effect of BrainProtect was observed for the primary endpoint Consortium to Establish a Registry for Alzheimer’s Disease (CERAD-Plus) total score. Logical reasoning was significantly improved (p = 0.024) 12 months after completion of the training program in IG participants compared to the GHC group independent of sex, age, education, diet, and physical activity. In IG participants, thinking flexibility (p = 0.019) and confrontational naming (p = 0.010) were improved 3 months after completing the intervention compared to the GHC group, however, after conservative Bonferroni adjustment, significance was lost.
Conclusions:
BrainProtect® independently improved logical reasoning compared to GHC up to 12 months after cognitive training’s end in healthy adults. To uncover the long-term clinical significance of multicomponent cognitive training in healthy adults, studies with larger sample size and frequent follow up visits are necessary.
INTRODUCTION
Modern advances in disease treatment and prevention have significantly increased the average life expectancy in developed countries.1 Nonetheless, recent studies show that living in an increasingly older society comes with drawbacks, most notably increased prevalence of age-related neurodegenerative diseases, multimorbidity, and related disability.1 While mild cognitive impairment (MCI) as a prodromal stage of early Alzheimer’s disease (AD)2 and recent discoveries on subjective cognitive impairment (SCI) point out at significant conversion rates of SCI to MCI and of MCI to AD3, a large body of literature highlights the benefits of several strategies able to counteract this progression.4,5 Age-related neural changes predominantly affect cognitive abilities such as memory, executive functions as well as attention span.6 However, aging and age-related changes are multifactorial and vary inter- and intra-individually, which leads to underdiagnosis of cognitive decline and therapeutic challenges.7 Indeed, a highly effective preventive approach in cognitive decline is a personalized, value-based strategy including control of modifiable risk factors such as diabetes, hypertension, and depression as well as actions aimed at improving nutrition, physical exercise, social interaction and cognitive activity.8–14
Within this frame, the protective effect of cognitive training on neuropsychological functions during aging has recently gained much attention.15 The efficiency of cognitive training appears to be based on the changes in functional connectivity enabled by the training, especially when it targets multiple domains instead of just a single one.16 Indeed, multicomponent interventions including cognitive training, physical activity and nutritional counseling have shown beneficial effects for cognitive performance in older individuals.17 Despite the ongoing debate on the effectiveness of cognitive training against dementia development, the latter condition is not curable. In addition, the large majority of patients with dementia are older women18 with multimorbidity,19 in which differential diagnosis of dementia is often challenging. These aspects, together with the upcoming rising dementia cases, as well as multifactoriality, multimorbidity patterns and consequences of dementia, render the primary prevention of cognitive decline a public health priority. The present investigation aimed at evaluating the long-term effects of the eight-week multicomponent cognitive training program BrainProtect20 in healthy adults as well as clinical and demographic characteristics possibly associated with any observed beneficial effects of BrainProtect up to 12 months after intervention’s end.
METHODS
Study design and participants
The present analysis used data from a randomized controlled trial (RCT) of the multicomponent training program BrainProtect 2.0,21 whose participants underwent re-evaluation 3 and 12 months after study end. Healthy adults (age ≥50 years) previously randomized to either general health counseling (GHC) (n = 72) or BrainProtect (intervention group, IG, n = 60) for eight weeks (once weekly, 90 minutes, group-based) underwent a comprehensive neuropsychological test battery and health-related quality of life (HRQoL) assessment prior to the program and immediately after its end as well as 3- and 12-months later. The follow-up visits took place during the first wave of the SARS-CoV-2 pandemic, resulting in an unforeseen delay in the analyses. While the RCT results have been published elsewhere,21 the present study concerns the analysis of the 3- and 12-months follow-up data.
In order to be included in the RCT, participants had to be free from cognitive impairment (Montreal Cognitive Assessment,22 MoCA, ≥26 points in total) and depressive mood (Beck Depression Inventory,23 BDI, ≤9 points in total).21 Participants with insufficient German language skills, impaired hearing and vision, life-threatening illness or substance abuse other than nicotine were excluded as previously described.21 The RCT21 was approved by the Ethics Committee of the University Hospital of Cologne in Germany (18–289) and corresponds to the Declaration of Helsinki.
Assessments and BrainProtect training
Participants, randomized 1:1 into the IG or the GHC group, were blinded until baseline completion, like BrainProtect trainers. Afterwards, participants were instructed not to reveal their group assignment to any of the outcome evaluators, who remained blinded to intervention assignment throughout the study.21
At baseline, all participants underwent a comprehensive neuropsychological test battery, HRQoL evaluation and a lifestyle questionnaire. The MoCA22 was used as a screening tool for clinical cognitive disorders whereas the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD-Plus Battery)24 and BrainProtect battery20 were used to assess cognitive performance in all its components. The BDI23 was utilized as a screening tool to exclude depressive mood. In addition, the questionnaires Instrumental Activities of Daily Living (IADL),25 Activities of Daily Living (ADL),26 Mini Nutritional Assessment short-form (MNA-SF),27 European Quality of Life 5 Dimensions 5 Level (EQ-5D-5L),28 and a lifestyle questionnaire of the German Association for Memory Training Bundesverband Gedächtnistraining, BVGT e.V., (http://www.bvgt.de) containing questions about sociodemographic variables as well as state of health, preexisting diseases (diabetes, arterial hypertension, etc.), permanent medication, diet and physical activity and reasons for participating in the cognitive training program were collected.21
The IG underwent the eight-week BrainProtect training taking place as weekly 90-min sessions in groups of up to a maximum of 15 persons.21 GHC participants received a psychoeducational letter on lifestyle and memory skills once weekly for eight weeks21 based on current scientific recommendations in existing guidelines.7,12,29 As previously described, BrainProtect training sessions focused on skills including structuring, concentrating, perceiving, recognizing connections as well as phrasing and finding words.20,21 Furthermore, retentiveness, logical thinking, flexibility of thought, imagination and creativity, judgement, and associative thinking were evaluated by means of the BrainProtect battery.20,21 Activating motor exercises were performed during the training sessions, which included nutritional counseling units.21 At the end of the eight-week BrainProtect training, a post-testing by means of CERAD-Plus battery and BrainProtect battery took place as previously described.20,21 Box 1 provides an overview of the BrainProtect battery training tasks, including an example of each task and the maximum number of points achievable. The type of tasks trained in the intervention did not change in the post- and follow-up tests, but different words and numbers were used in each session. The BrainProtect battery is not validated to date.
Box 1: Overview of the BrainProtect battery training tasks with examples
| Task type | Task content | Maximum score |
| Thinking flexibility | Participants were asked to match a word printed in mirror writing with a picture to form a noun within one minute and 30 seconds | 8 points |
| Concentration | Participants were requested to find a maximum number of identical letters from a box to form a solution word within two minutes | 11 points |
| Learning | Participants were given a timeframe of two minutes to look at a city map and then tick all the objects they remembered on a list within one minute | 10 points |
| Working memory | Participants were dictated five arithmetical tasks involving addition and subtraction, which they had to solve in their heads by reversing the arithmetic signs (for example 1+2-3=?>1-2+3 = 2) | 10 points |
| Perception | Participants were asked to underline a particular word as many times as possible within a figure consisting of many words strung together within one minute | 10 points |
| Logical reasoning | Participants had to find the association between different concepts within one minute (example ‘short relates to long like small to ... ?’) | 10 points |
| Imagination | Participants had two minutes to form sentences from the initials of 10 car license numbers which were grammatically correct and complete | 10 points |
| Structured thinking | Participants were given an empty shelf in which they had to correctly arrange 12 items within two minutes according to the corresponding instructions | 12 points |
Long-term evaluation at 3 and 12 months after training completion
To determine BrainProtect’s long-term effects, participants were re-invited to a follow-up visit 3 and 12 months after intervention’s end. At both visits, all participants underwent CERAD-Plus battery, BrainProtect battery as well as HRQoL evaluation. At second follow-up visit, the MoCA and BDI were also assessed in all participants. Within 30 days after the second follow-up visit, personal events potentially impacting physical and mental health during the study period, such as new diagnoses and drug therapies, hospitalizations, psychological stressors, as well as significant lifestyle changes (nutrition, physical exercise, additional cognitive training, social activities, sleep habits, and general well-being) were collected by phone.
Outcomes
As in Falkenreck et al.,21 the primary endpoint of this investigation was the total score of CERAD-Plus test battery (CERAD-Plus total score, CERAD-TS2) at 3 and 12 months after intervention. For comparability of the participants’ results, absolute subtest-scores were converted to z-scores using the Memory Clinic program (CERAD-Plus online), considering age, gender, and education.30 This calculation of the z-scores is based on a norm population resulting from a multi-center study with n = 604 healthy control subjects (age: 55–88 years, education: 7–20 years) assessed with the German CERAD-Plus test battery.31 For accurate interpretation, the CERAD-TS2 was calculated according to Lillig et al. which is based on equally weighted z-scores for the different subtests.32
Secondary endpoints were the changes in the outcome measures CERAD-Plus and BrainProtect subcategories as well as those in MoCA, BDI and HRQoL evaluation. The change was analyzed as Δ values 12-month follow-up - baseline and 3-month follow-up – baseline, respectively. A positive delta indicates deterioration, while a negative delta indicates improvement, except for the BDI, in which a negative delta indicates deterioration and a positive delta indicates improvement. Particular attention was given to biological sex, age, years of education (school years and higher education), employment, sedentary work, family status, diet (omnivore, vegetarian, vegan), physical activity, previously diagnosed memory disorder within the family as well as past participation in cognitive training programs as well as personal events (of social, psychological, physical or lifestyle-based nature) as possible influencing factors on cognitive performance at follow-up.
Statistical analysis
The statistical analyses were carried out using IBM SPSS 28 software. Descriptive statistics are presented as absolute numbers and relative frequencies for description of categorical variables and mean (+ standard deviation, SD) or median (+ interquartile range, IQR) for continuous variables as a function of distribution.
The Shapiro-Wilk test was used to test for normal distribution. For baseline comparisons, two-sample t-tests and Mann-Whitney-U tests were applied as appropriate. Categorical data and frequencies were compared using Chi-square (χ2) test.
Data analysis was carried out according to a per-protocol (PP) approach. Only data from subjects who completed all training sessions of intervention and participated in testing at baseline, after intervention and follow-up visits 1 and 2 were considered (n = 88) and the sample size at each test time was reported. The threshold of significance was defined as 0.05 for all analyses.
Repeated measures analysis of variance (ANOVA) was utilized to compare training effects between the groups at the four test times at baseline, immediately after intervention as well as 3 and 12 months after intervention’s end. The effect size is determined by the partial eta square (
Multiple linear regression analyses were used to determine whether certain predictors had an impact on subjects’ performance after intervention. The change value Δ(12-month follow-up - baseline) of the outcome measures of IG were selected as dependent variables. Independent variables were the characteristics gender, age, years of education (school years and higher education), employment, sedentary work, family status, diet (omnivore, vegetarian, vegan), physical activity, previously diagnosed memory disorder within the family as well as past participation in cognitive training and baseline level.
Influence of the four domains of social, psychological, physical and nutritional changes during the study period on intervention’s success at the end of the trial were investigated using multiple linear regression. The change value Δ(12-month follow-up - baseline) of the outcome measures were selected as dependent variables. Independent variables were the group allocation, the sum scores of the four domains and the interaction term Group x Sum score as well as baseline level. A detailed description of the questionnaire and calculation of the sum score can be found in the Supplementary Material.
RESULTS
Of the 132 participants completing the baseline assessment, 115 attended the posttest, corresponding to a dropout rate of 12.9%.21 At 3-month follow-up, the dropout rate was 6.1% and of 107 participants a total of 12 (11%) were tested late due to the SARS-CoV-2 pandemic. At 12-month follow-up, the dropout rate was 14.4% and of 88 participants a total of 4 (4%) were tested late due to the SARS-CoV-2 pandemic. The PP analysis included therefore 88 participants. A total of 52 participants still took part in answering the final questionnaire, which corresponds to a dropout rate of 27.3%. Figure 1 shows the overall timeline of the study as well as the participants’ reasons for dropping out.
Fig. 1
Flow of Participation BrainProtect 2.0.
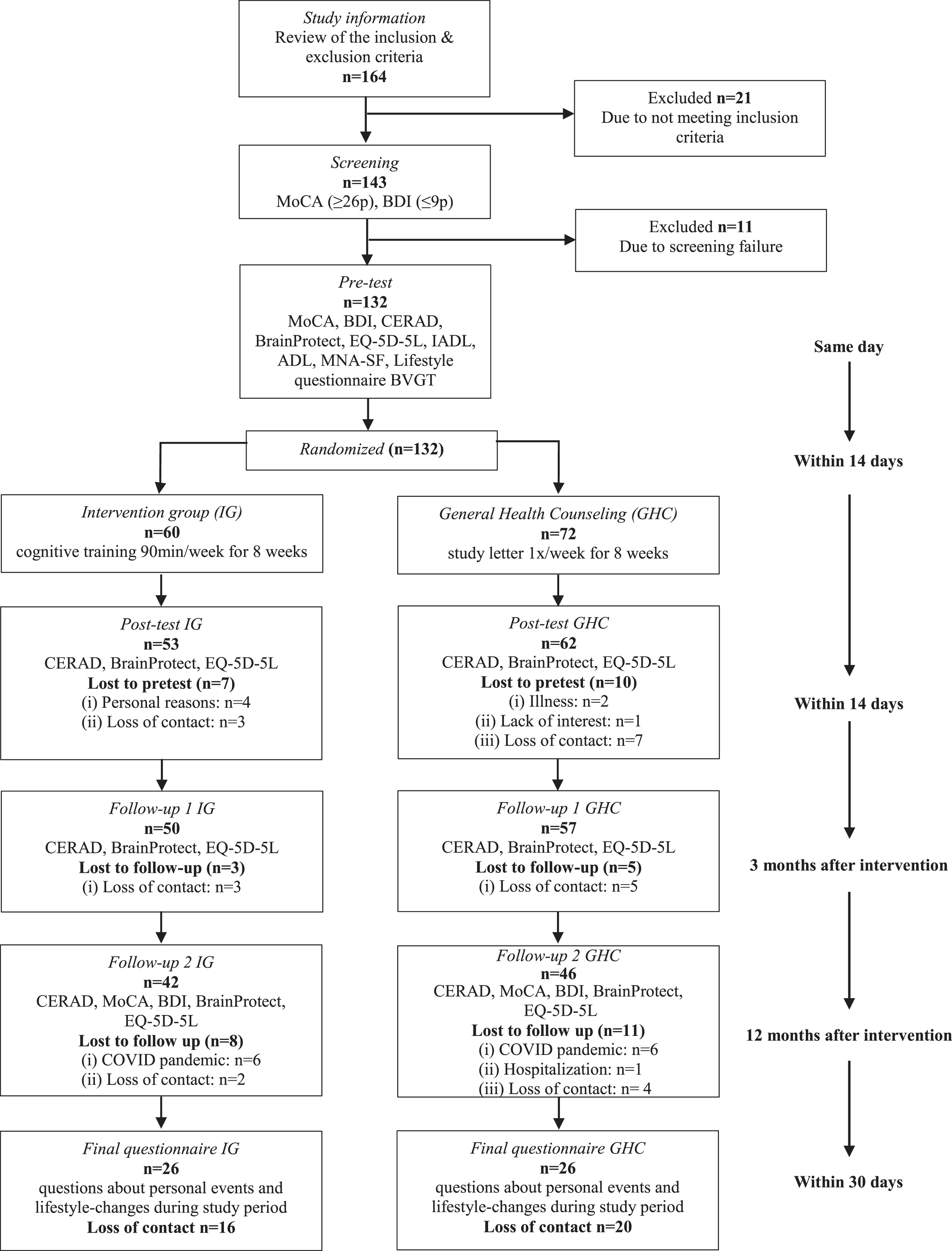
The baseline demographic characteristics of the study participants as well as demographic characteristics of the participants at posttest and 3- and 12-month follow-up are presented in Table 1.
Table 1
Demographic characteristics of the participants at baseline, posttest, 3-month follow-up and 12-month follow-up
| Baseline demographic characteristics | Post-test | Follow-up 1 | Follow-up 2 | ||||
| Total (N = 131) | Intervention group (N = 59) | General health counseling (N = 72) | p | (N = 115) | (N = 107) | (N = 88) | |
| Age (y), median (IQR) | 68 (63–74) | 69 (64–76) | 67 (59.5–73) | 0.053 | 67 (62–73) | 67 (62–72) | 67 (63–72) |
| Female, frequency (%) | 94 (71.2) | 41 (68.3) | 53 (73.6) | 0.602 | 79 (68.7) | 72 (67.3) | 58 (65.9) |
| Education (y), median (IQR) | 17.5 (13–18) | 16 (12–18) | 18 (14–18) | 0.043 | 17.5 (13–18) | 18 (13–18) | 18 (13–18) |
| Professional degree, frequency (%) | 0.446 | ||||||
| None | 9 (6.8) | 6 (10) | 3 (4.2) | 8 (7) | 8 (7.5) | 10 (11.3) | |
| Apprenticeship | 31 (23.5) | 17 (28.3) | 14 (19.4) | 27 (23.5) | 24 (22.4) | 17 (19.3) | |
| Technical school | 19 (14.4) | 8 (13.3) | 11 (15.3) | 17 (14.8) | 17 (15.9) | 14 (15.9) | |
| University | 69 (52.3) | 27 (45) | 42 (58.3) | 60 (52.2) | 56 (52.3) | 47 (53.4) | |
| Employment, frequency (% yes) | 34 (25.8) | 12 (20) | 22 (30.6) | 0.184 | 33 (28.7) | 30 (28) | 23 (26.1) |
| Sedentary work, frequency (% yes) | 93 (70.5) | 41 (68.3) | 52 (72.2) | 0.847 | 82 (71.3) | 77 (72) | 65 (73.9) |
| Marital status, frequency (% married) | 75 (56.8) | 32 (53.3) | 43 (59.7) | 0.467 | 68 (59.1) | 62 (57.9) | 53 (60.2) |
| Living alone, frequency (% yes) | 40 (30.3) | 21 (35) | 19 (26.4) | 0.255 | 33 (28.7) | 31 (29%) | 26 (29.5) |
| Previous participation in cognitive training, frequency (% yes) | 16 (12.1) | 5 (8.3) | 11 (15.3) | 0.239 | 14 (12.2) | 12 (11.2) | 8 (9.1) |
| Self-perception of health, mean (SD) | 2.79±1.70 | 2.83±1.68 | 2.76±1.72 | 0.681 | 2.77±1.69 | 2.63±1.51 | 2.56±1.46 |
| (Indication by grades, higher values indicate worse perceived health) | |||||||
| Diagnosed memory disorder in family (% yes) | 44 (33.3) | 20 (33.3) | 24 (33.3) | 0.879 | 38 (33) | 34 (31.8) | 27 (30.7) |
| Diagnosed disease at study start (% yes) | 102 (77.3) | 46 (76.7) | 56 (77.8) | 0.979 | 89 (77.4) | 81 (75.7) | 69 (78.4) |
| Medication at study start (% yes) | 100 (75.8) | 45 (75) | 55 (76.4) | 0.534 | 85 (73.9) | 79 (73.8) | 64 (72.7) |
| Nutrition, frequency (%) | 0.694 | ||||||
| Omnivore | 112 (84.8) | 48 (80) | 64 (88.9) | 97 (84.3) | 91 (85) | 74 (84.1) | |
| Vegetarian | 8 (6.1) | 4 (6.7) | 4 (5.6) | 8 (7) | 7 (6.5) | 6 (6.8) | |
| No specification | 12 (9.1) | 8 (13.3) | 4 (5.6) | 10 (8.7) | 98 (91.6) | 8 (9.1) | |
| Regular physical activity (% yes) | 107 (81.1) | 48 (80) | 59 (81.9) | 0.959 | 93 (80.9) | 86 (80.4) | 68 (77.3) |
| Regular physical activity, frequency per week (%) | 0.885 | ||||||
| 1-2x | 46 (35.1) | 22 (37.3) | 24 (33.3) | 40 (34.8) | 38 (35.5) | 33 (37.5) | |
| 3-4x | 44 (33.6) | 18 (30.5) | 26 (36.1) | 38 (33) | 35 (32.7) | 28 (31.8) | |
| 5-7x | 18 (13.7) | 9 (15.3) | 9 (12.5) | 17 (14.8) | 15 (14) | 11 (12.5) | |
| No specification | 23 (17.6) | 10 (16.9) | 13 (18.1) | 19 (16.5) | 18 (16.8) | 15 (17.0) | |
| Regular physical activity, duration per unit (%) | 0.24 | ||||||
| 15–30 min | 9 (6.9) | 7 (11.9) | 2 (2.8) | 7 (6.1) | 7 (6.5) | 5 (5.7%) | |
| 30–60 min | 56 (42.7) | 24 (40.7) | 32 (44.4) | 51 (44.3) | 47 (43.9) | 42 (47.7%) | |
| >60 min | 36 (27.5) | 15 (25.4) | 21 (29.2) | 30 (26.1) | 29 (27.1) | 22 (25.0%) | |
| No specification | 30 (22.9) | 13 (22) | 17 (23.6) | 26 (22.6) | 23 (21.5) | 18 (20.5%) | |
| BMI, frequency (%) | 0.497 | ||||||
| <19 | 4 (3) | 2 (3.3) | 2 (2.8) | 4 (3.5) | 3 (2.8) | 2 (2.3) | |
| 19-20 | 12 (9.1) | 6 (10) | 6 (8.3) | 10 (8.7) | 10 (9.3) | 9 (10.2) | |
| 21-22 | 22 (16.7) | 13 (21.7) | 9 (12.5) | 18 (15.7) | 17 (15.9) | 14 (15.9) | |
| >23 | 94 (71.2) | 39 (65) | 55 (76.4) | 83 (72.2) | 77 (72%) | 63 (71.6) | |
| ADL-Score, mean (SD) | 5.96±0.19 | 5.93±0.25 | 5.99±0.12 | 0.115 | 5.97±0.18 | 5.97±0.17 | 5.98±0.15 |
| IADL-Score, mean (SD) | 7.99±0.09 | 7.98±0.13 | 8±0 | 0.273 | 7.99±0.09 | 7.99±0.10 | 8±0.00 |
| MNA-SF-Score, median (IQR) | 13 (12–14) | 13 (11–14) | 13.5 (12–14) | 0.274 | 13 (12–14) | 13 (12–14) | 13 (12–14) |
| EQ-5D-5L, mean (SD) | 5.71±1.47 | 5.83±1.61 | 5.61±1.35 | 0.322 | 5.70±1.48 | 5.68±1.50 | 5.66±1.49 |
| EQ-5D-5L health in %, mean (SD) | 79.77±14.53 | 77.85±16.76 | 81.38±12.9 | 0.226 | 79.19±14.64 | 80.14±14.11 | 80.81±13.48 |
| (max. 100%, higher values indicate better perceived health) | |||||||
| BDI, mean (SD) | 3.37±2.73 | 4.02±2.63 | 2.83±2.71 | 0.005 | 3.14±2.61 | 3.28±2.66 | 3.32±2.64 |
| MoCA, mean (SD) | 27.89±1.4 | 27.83±1.39 | 27.94±1.42 | 0.628 | 27.88±1.46 | 27.88±1.45 | 27.84±1.49 |
| CERAD-Plus-z total score (TS2), mean (SD) | 0.16±0.56 | 0.16±0.54 | 0.17±0.58 | 0.879 | 0.16±0.58 | 0.06±0.58 | 0.20±0.58 |
| BVGT BrainProtect total, mean (SD) | 44.97±8.85 | 42.32±7.65 | 47.18±9.23 | 0.001 | 45.12±8.73 | 45.50±8.55 | 45.53±8.50 |
Values are presented as the mean±standard deviation or median and interquartile range or frequency with percentages. For baseline comparison between groups, p-values of Mann-Whitney-U testes, independent sample t-tests or χ2-tests are reported as appropriate. Variables were previously inspected visually by qq-plots and statistically by Shapiro-Wilk tests for normal distribution. BMI, body mass index; ADL, Activities of Daily Living; IADL, Instrumental Activities of Daily Living; MNA, Mini Nutritional Assessment short-form; EQ-5D-5L, descriptive system of 5 dimensions mobility, self-care, usual activities, pain and depression; EQ-5D-5L health in %, participant’s self-rated health on a vertical visual analogue scale; BDI, Beck Depression Inventory; MoCA, Montreal Cognitive Assessment; CERAD, Consortium to Establish a Registry for Alzheimer’s Disease; BVGT, Bundesverband Gedächtnistraining e.V. BrainProtect.
Of the 88 participants included in the PP-analysis n = 58 (66%) were female and the median age was 67 years (63–72). On median, the participants had 18 education years and half of them (53%) had an academic degree, whereas 10 (11%) had no professional degree.
The IG and GHC were comparable regarding most demographic characteristics at baseline. However, there were significant differences between groups regarding years of education (p = 0.043), BDI baseline score (p = 0.005), and BrainProtect baseline score (p = 0.001). With a total of 18 years (14–18), educational years were higher in GHC than in IG with a total of 16 years (12–18). On BrainProtect battery at baseline, the IG participants had a significantly lower score than the GHC participants and reported higher BDI baseline scores than the GHC (Table 1).
The median age of the 43 dropout participants was 69 (62–78) years and the majority were women (81%). Twenty-six % were still employed and 74% entered the study with at least one non-communicable chronic condition such as arterial hypertension or diabetes. The dropout cohort’s self-perception of health via grade assignment was significantly worse (M = 3.3±2.0) than that of the PP cohort (M = 2.6±1.5). Finally, dropping-out participants were significantly older (p = 0.009), and their self-perceived health was worse (p = 0.010) compared to participants completing the study (Supplementary Table 1).
Primary outcome
PP data are presented in Table 2. No statistically significant Time (baseline versus 12-month follow-up) x Group (IG versus GHC) interaction effects were found for the primary endpoint CERAD-TS2 at both 3 and 12 months after intervention (F(3,252) = 1.15, p = 0.342,
Table 2
ANOVA with measurement repetition between IG and GHC
| Outcome | Intervention group (IG) | General health counseling (GHC) | ||||||||||||
| Baseline | Posttest | Follow-up 1 | Follow-up 2 | Baseline | Posttest | Follow-up 1 | Follow-up 2 | Time | Group | Time x Group | ||||
|
| p |
| p |
| p(p)a | |||||||||
| M | M | M | M | M | M | M | M | |||||||
| (SD) | (SD) | (SD) | (SD) | (SD) | (SD) | (SD) | (SD) | |||||||
| CERAD-Plus-z total score (TS2)b (N = 88; IG = 42, CG = 46) | 0.16 (0.54) | 0.64 (0.56) | 0.61 (0.62) | 0.42 (0.55) | 0.22 (0.62) | 0.63 (0.37) | 0.48 (0.61) | 0.52 (0.49) | 0.154 | ≤0.001 | 0.000 | 0.979 | 0.013 | 0.342 |
| Verbal fluency | –0.38 (0.84) | 0.37 (1.08) | 0.28 (1.01) | 0.21 (1.12) | –0.02 (0.87) | 0.29 (0.90) | 0.44 (1.06) | 0.36 (0.96) | 0.133 | ≤0.001 | 0.009 | 0.377 | 0.017 | 0.221 |
| Boston Naming Test (BNT) | 0.02 (1.04) | 0.48 (0.68) | 0.40 (0.83) | –0.29 (0.91) | 0.38 (0.83) | 0.55 (0.60) | 0.14 (0.90) | 0.09 (0.78) | 0.114 | ≤0.001 | 0.016 | 0.249 | 0.043 | 0.010 (0.15) |
| Mini Mental Status Examination (MMSE) | –0.62 (1.09) | 0.05 (1.03) | 0.07 (0.98) | –0.32 (0.91) | –0.43 (1.53) | –0.00 (0.94) | –0.45 (1.38) | –0.16 (0.98) | 0.040 | 0.015 | 0.002 | 0.670 | 0.022 | 0.127 |
| Wordlist (WL) total | 0.33 (1.07) | 0.78 (1.04) | 0.83 (1.13) | 0.88 (0.91) | 0.18 (0.91) | 0.73 (0.87) | 0.83 (1.10) | 0.97 (0.82) | 0.130 | ≤0.001 | 0.000 | 0.859 | 0.004 | 0.781 |
| Wordlist (WL) recall | 0.28 (1.20) | 0.74 (0.93) | 0.84 (0.86) | 0.66 (0.77) | 0.05 (1.29) | 0.63 (0.81) | 0.70 (0.92) | 0.72 (0.82) | 0.079 | ≤0.001 | 0.010 | 0.355 | 0.004 | 0.780 |
| Wordlist (WL) savings | 0.24 (1.60) | 0.25 (1.05) | 0.59 (0.88) | 0.30 (0.82) | 0.05 (2.08) | 0.27 (0.74) | 0.46 (0.97) | 0.34 (0.64) | 0.017 | 0.230 | 0.003 | 0.602 | 0.002 | 0.912 |
| Wordlist (WL) recognition | 0.04 (0.99) | 0.32 (0.53) | 0.43 (0.43) | 0.13 (0.73) | –0.28 (1.10) | 1.23 (6.16) | 0.28 (0.57) | 0.19 (0.65) | 0.025 | 0.093 | 0.003 | 0.591 | 0.013 | 0.338 |
| Constructional praxis | –0.33 (1.08) | 0.40 (0.70) | 0.12 (1.08) | –0.19 (1.23) | –0.24 (1.16) | 0.20 (0.96) | –0.36 (1.43) | –0.41 (1.29) | 0.072 | ≤0.001 | 0.019 | 0.202 | 0.013 | 0.325 |
| Constructional praxis recall | –0.30 (1.22) | 0.83 (0.76) | 0.20 (1.35) | 0.38 (1.02) | –0.12 (1.39) | 0.44 (1.01) | –0.33 (1.30) | –0.02 (1.30) | 0.110 | ≤0.001 | 0.036 | 0.079 | 0.022 | 0.131 |
| Constructional praxis saving | –0.10 (0.93) | 0.49 (0.70) | 0.12 (1.02) | 0.49 (0.94) | 0.06 (1.04) | 0.27 (0.71) | 0.01 (0.95) | 0.26 (1.00) | 0.055 | 0.002 | 0.009 | 0.381 | 0.011 | 0.425 |
| Trail Making Test (TMT) A | 0.70 (1.30) | 0.62 (1.57) | 1.02 (1.30) | 0.70 (1.16) | 0.56 (1.21) | 0.94 (1.16) | 0.91 (0.94) | 0.92 (1.00) | 0.019 | 0.173 | 0.002 | 0.696 | 0.013 | 0.325 |
| Trail Making Test (TMT) B | 0.91 (1.48) | 0.98 (1.33) | 1.04 (1.31) | 1.06 (1.72) | 0.80 (1.37) | 1.17 (1.11) | 1.27 (1.33) | 1.40 (1.37) | 0.020 | 0.162 | 0.007 | 0.435 | 0.007 | 0.611 |
| Delta TMT B_A | 0.19 (1.02) | 0.40 (1.18) | 0.04 (1.03) | 0.30 (1.36) | 0.17 (0.92) | 0.21 (1.09) | 0.26 (1.03) | 0.36 (0.93) | 0.008 | 0.540 | 0.000 | 0.903 | 0.008 | 0.570 |
| s-Words | 0.35 (1.35) | 1.04 (1.10) | 1.05 (1.17) | 0.86 (1.06) | 0.21 (1.04) | 1.01 (0.96) | 0.87 (0.95) | 1.00 (0.97) | 0.131 | ≤0.001 | 0.001 | 0.739 | 0.006 | 0.658 |
| BVGT BrainProtect total scoreb (N = 88; IG = 42, CG = 46) | 43.1 (7.31) | 51.2 (10.53) | 52.7 (11.23) | 45.2 (10.82) | 47.6 (9.19) | 52.1 (8.58) | 54.3 (10.20) | 48.9 (8.87) | 0.328 | ≤0.001 | 0.025 | 0.137 | 0.024 | 0.105 |
| Thinking flexibility | 3.7 (1.60) | 5.2 (1.91) | 4.6 (2.17) | 3.7 (1.63) | 4.5 (1.86) | 5.4 (1.73) | 4.2 (1.74) | 4.4 (1.64) | 0.136 | ≤0.001 | 0.016 | 0.240 | 0.038 | 0.019 (0.171) |
| Concentration | 7.0 (1.54) | 7.3 (1.90) | 7.3 (1.66) | 7.1 (1.99) | 7.4 (2.02) | 8.0 (1.67) | 8.1 (2.06) | 7.8 (1.66) | 0.021 | 0.133 | 0.056 | 0.027 | 0.004 | 0.803 |
| Learning | 6.7 (1.06) | 8.5 (1.30) | 8.1 (1.32) | 7.6 (1.59) | 7.1 (1.34) | 8.5 (1.06) | 8.2 (1.09) | 7.8 (0.92) | 0.310 | ≤0.001 | 0.005 | 0.494 | 0.008 | 0.563 |
| Working memory | 5.3 (2.57) | 5.7 (2.53) | 6.9 (2.62) | 5.9 (3.04) | 7.1 (2.79) | 6.5 (2.86) | 7.2 (2.65) | 7.1 (2.79) | 0.042 | 0.012 | 0.059 | 0.023 | 0.023 | 0.107 |
| Perception | 4.7 (1.82) | 6.0 (2.16) | 6.9 (2.09) | 3.8 (2.07) | 5.3 (1.92) | 6.0 (2.00) | 7.4 (2.11) | 3.8 (1.79) | 0.432 | ≤0.001 | 0.012 | 0.308 | 0.009 | 0.493 |
| Logical reasoning | 5.2 (1.75) | 6.2 (2.06) | 8.3 (2.09) | 6.3 (2.89) | 5.4 (1.56) | 5.6 (2.13) | 7.4 (2.74) | 6.5 (2.44) | 0.357 | ≤0.001 | 0.007 | 0.439 | 0.036 | 0.024 (0.216) |
| Imagination | 2.8 (1.66) | 4.1 (2.06) | 3.2 (2.01) | 3.1 (2.05) | 3.3 (1.72) | 3.9 (1.83) | 3.6 (2.00) | 3.4 (1.81) | 0.087 | ≤0.001 | 0.007 | 0.437 | 0.018 | 0.192 |
| Structured thinking | 7.7 (2.76) | 8.1 (3.10) | 7.3 (3.47) | 7.5 (3.62) | 7.4 (3.32) | 8.3 (3.12) | 8.2 (3.44) | 8.1 (3.20) | 0.010 | 0.466 | 0.007 | 0.444 | 0.008 | 0.533 |
| MoCAb (N = 88; IG = 42, CG = 46) | 27.8 (1.57) | – | – | 26.6 (2.46) | 27.9 (1.43) | – | – | 27.1 (1.912) | 0.170 | ≤0.001 | 0.010 | 0.344 | 0.005 | 0.501 |
| BDIc (N = 88; IG = 42, CG = 46) | 3.5 (2.37) | – | – | 2.1 (2.55) | 3.0 (2.78) | – | – | 2.3 (2.88) | 0.102 | 0.003 | 0.001 | 0.791 | 0.013 | 0.291 |
| EQ-5D-5Lb (N = 88; IG = 42, CG = 46) | 5.6 (1.53) | 6.0 (1.90) | 5.8 (1.46) | 6.0 (1.56) | 5.7 (1.48) | 6.1 (1.71) | 6.1 (1.581) | 5.8 (1.29) | 0.031 | 0.042 | 0.000 | 0.879 | 0.010 | 0.451 |
| Health in % b | 79.7 (14.66) | 81.3 (13.62) | 80.9 (13.82) | 80.7 (10.97) | 81.4 (12.15) | 78.8 (11.97) | 78.7 (14.63) | 77.2 (12.51) | 0.007 | 0.593 | 0.005 | 0.497 | 0.022 | 0.122 |
Data are indicated as mean standardized z-scores (CERAD) or raw scores and standard deviations (SD). CERAD, Consortium to Establish a Registry for Alzheimer’s Disease; BVGT, Bundesverband Gedächtnistraining e.V. BrainProtect; MoCA, Montreal Cognitive Assessment; BDI, Beck Depression Inventory; EQ-5D-5L, descriptive system of 5 dimensions mobility, self-care, usual activities, pain and depression; EQ-5D-5L health in %, participant’s self-rated health on a vertical visual analogue scale. (p)a, p after adjusting the significant results for multiple testing using the Bonferroni correction. bHigher values indicate better performance; clower scores indicate better performance.
Secondary outcomes
Training effects
Significant Time x Group interaction effects were seen in the CERAD-Plus subtest BNT (F(3,255) = 3.82, p = 0.010,
Pairwise comparison of logical reasoning within the IG revealed significant (p < 0.001) higher performance scores in follow-up 2 than at baseline (MDiff = 1.07, 95% -CI[0.47, 1.67]). Looking at pairwise comparison of thinking flexibility within the IG, it indicated no statistically significant higher performance scores in follow-up 1 than at baseline (MDiff = 0.32, 95% -CI[–0.30, 0.95]) and lower performance scores in follow-up 2 than at baseline (MDiff = –0.05, 95% -CI[–0.62, 0.52]). In pairwise comparison of BNT were also no statistically significant higher performance scores in follow-up 1 than at baseline (MDiff = 0.0.07, 95% -CI[–0.24, 0.39]) and lower performance scores in follow-up 2 than at baseline (MDiff = –0.30, 95% -CI[–0.62, 0.02]) seen.
Significant main effects of group with small effect sizes were observed for BrainProtect subcategories concentration (F(1,86) = 5.06, p = 0.027,
The significant main effects of time can be seen in Table 2. Of particular note were CERAD-TS2 (p < 0.001) and BrainProtect total score (p < 0.001), both with significant increasing of performance for both groups across time. Moreover, significant main effects of time were observed for BDI (p = 0.003) and HRQoL evaluation (p = 0.042), indicating increase of health well-being for both groups over time. Only significant main effects of time for MoCA (p < 0.001) pointed to a decrease of performance for both groups across time. No side effects or harms of the intervention and follow-up assessments were observed in either group.
Prediction of responsiveness to training (12-month follow-up – pretest)
Table 3 as well as Supplementary Table 3 show the results of linear regression analysis of predictors for intervention success of IG and GHC.
Table 3
Linear regression of potential influencing factors of the delta follow-up 2 - pretest (IG)
| Standardized β-coefficients of predictors (p-value) | ||||||||||||||
| Outcome Delta follow-up 2 - pretest | Baseline level | Sex | Age | Education in years | Employed | Sedentary work | Married | Divorced/separated | Single | Living alone | Diagnosed memory disorder in family | Vegetarian | Former CT participation | Physical activity |
| CERAD-Plus-z total score (TS2)a F(14,22) = 2.900, p = 0.012, adj. R2 = 0.425 | –0.688 (0.001) | –0.008 (0.963) | –0.242 (0.159) | 0.009 (0.962) | –0.022 (0.890) | 0.119 (0.440) | –0.126 (0.657) | –0.064 (0.742) | –0.191 (0.337) | 0.116 (0.622) | –0.102 (0.511) | –0.095 (0.558) | 0.007 (0.957) | 0.079 (0.597) |
| BVGT BrainProtect total scorea F(14,22) = 1.178, p = 0.355, adj. R2 = 0.065 | –0.129 (0.629) | 0.259 (0.245) | –0.059 (0.814) | 0.523 (0.025) | –0.099 (0.625) | 0.131 (0.497) | 0.367 (0.344) | –0.017 (0.942) | 0.119 (0.626) | 0.194 (0.529) | 0.072 (0.712) | –0.107 (0.594) | –0.213 (0.254) | 0.020 (0.917) |
| Thinking flexibility F(14,37) = 2.872, p = 0.005, adj. R2 = 0.339 | –0.624 (≤0.001) | 0.066 (0.603) | –0.098 (0.549) | 0.307 (0.037) | –0.070 (0.637) | 0.094 (0.488) | –0.228 (0.209) | –0.318 (0.067) | –0.166 (0.292) | 0.225 (0.244) | –0.043 (0.753) | 0.009 (0.948) | 0.012 (0.923) | –0.126 (0.334) |
| Concentration F(14,37) = 0.851, p = 0.614, adj. R2 = –0.043 | –0.147 (0.402) | 0.045 (0.777) | –0.050 (0.811) | 0.390 (0.029) | –0.144 (0.447) | 0.249 (0.151) | –0.186 (0.408) | –0.306 (0.164) | –0.136 (0.480) | 0.312 (0.210) | –0.084 (0.623) | –0.042 (0.805) | –0.006 (0.971) | –0.042 (0.797) |
| Learning F(14,37) = 1.034, p = 0.444, adj. R2 = 0.009 | –0.230 (0.158) | 0.034 (0.830) | –0.019 (0.920) | 0.295 (0.082) | –0.142 (0.438) | 0.046 (0.781) | –0.095 (0.670) | –0.300 (0.152) | –0.222 (0.230) | 0.134 (0.567) | –0.085 (0.619) | –0.023 (0.889) | –0.226 (0.161) | –0.127 (0.426) |
| Working memory F(14,37) = 1.990; p = 0.047, adj. R2 = 0.214 | –0.591 (0.001) | –0.128 (0.419) | –0.048 (0.772) | 0.480 (0.003) | –0.094 (0.577) | 0.078 (0.601) | 0.049 (0.802) | –0.176 (0.341) | –0.064 (0.693) | 0.243 (0.252) | –0.125 (0.432) | 0.069 (0.637) | –0.247 (0.108) | –0.187 (0.190) |
| Perception F(14,37) = 0.826; p = 0.637, adj. R2 = –0.050 | –0.274 (0.116) | 0.094 (0.560) | –0.080 (0.689) | 0.234 (0.188) | –0.148 (0.440) | –0.142 (0.411) | –0.045 (0.843) | –0.007 (0.974) | –0.163 (0.390) | 0.083 (0.734) | 0.051 (0.770) | 0.089 (0.597) | 0.056 (0.735) | –0.234 (0.162) |
| Logical reasoning F(14,37) = 1.242; p = 0.288, adj. R2 = 0.062 | –0.066 (0.736) | 0.066 (0.669) | 0.019 (0.921) | 0.375 (0.032) | –0.146 (0.414) | –0.159 (0.327) | –0.373 (0.085) | –0.453 (0.029) | –0.387 (0.035) | 0.337 (0.147) | –0.052 (0.750) | –0.064 (0.686) | 0.164 (0.300) | –0.139 (0.394) |
| Imagination F(14,37) = 1.452; p = 0.179, adj. R2 = 0.110 | –0.422 (0.016) | 0.128 (0.383) | 0.040 (0.819) | 0.386 (0.026) | –0.092 (0.594) | –0.161 (0.307) | –0.166 (0.433) | –0.436 (0.031) | –0.290 (0.101) | 0.331 (0.141) | –0.070 (0.659) | –0.063 (0.699) | –0.150 (0.324) | –0.119 (0.445) |
| Structured thinking F(14,37) = 1.631; p = 0.116, adj. R2 = 0.148 | –0.301 (0.117) | –0.020 (0.894) | 0.215 (0.262) | 0.251 (0.112) | 0.095 (0.571) | –0.006 (0.968) | –0.015 (0.946) | –0.428 (0.056) | –0.019 (0.908) | 0.190 (0.428) | –0.051 (0.742) | 0.070 (0.655) | –0.229 (0.134) | –0.118 (0.433) |
| MoCAa F(14,22) = 1.377, p = 0.243, adj. R2 = 0.128 | –0.457 (0.018) | –0.200 (0.319) | –0.108 (0.599) | 0.228 (0.255) | 0.154 (0.802) | 0.141 (0.448) | 0.709 (0.050) | 0.422 (0.081) | 0.291 (0.204) | 0.393 (0.185) | –0.139 (0.450) | 0.162 (0.396) | 0.007 (0.968) | 0.071 (0.704) |
| BDIb F(14,21) = 2.706, p = 0.019, adj. R2 = 0.406 | –0.652 (0.001) | 0.033 (0.844) | 0.046 (0.792) | 0.337 (0.056) | –0.143 (0.405) | –0.038 (0.807) | –0.095 (0.746) | –0.014 (0.947) | 0.023 (0.903) | –0.064 (0.786) | 0.035 (0.825) | –0.139 (0.396) | –0.022 (0.878) | –0.073 (0.666) |
| EQ-5D-5La F(14,22) = 1.235, p = 0.320, adj. R2 = 0.084 | –0.383 (0.065) | –0.267 (0.246) | –0.153 (0.475) | 0.097 (0.649) | –0.192 (0.348) | –0.299 (0.126) | 0.077 (0.829) | 0.150 (0.534) | 0.053 (0.819) | 0.104 (0.728) | 0.118 (0.540) | –0.113 (0.583) | 0.021 (0.903) | –0.112 (0.562) |
Dependent variables are defined as delta follow-up 2 minus pretest of the named outcomes; all regression models are presented; for each significant regression model, standardized regression coefficients are reported for predictors irrespective of reaching statistical significance; regression models that reached statistical significance at p < 0.05 are presented bold printed; CERAD, Consortium to Establish a Registry for Alzheimer’s Disease; BVGT, Bundesverband Gedächtnistraining e.V. BrainProtect; MoCA, Montreal Cognitive Assessment; BDI, Beck Depression Inventory; EQ-5D-5L, descriptive system of 5 dimensions mobility, self-care, usual activities, pain and depression. aHigher values indicate better performance; blower scores indicate better performance.
More years of education revealed a trend towards predicting positive responsiveness to the intervention as far as thinking flexibility (p = 0.037) and working memory (p = 0.003) were concerned. In GHC, participants with lower number of education years seemed to have more potential for improvement in the BrainProtect subcategory concentration (p = 0.021). No influence of sex, age, being employed, sedentary work, family status, living alone, diagnosed memory disorder in family, diet, former participation in cognitive training or physical activity was shown towards cognitive performance deltas in IG. In GHC, men showed a significantly higher delta in BrainProtect subcategory concentration (p = 0.049) and younger participants of GHC appeared to have more potential for improvement in BrainProtect subcategory perception (p = 0.031). Other results of the GHC are presented in Supplementary Table 3.
Prediction of influencing changes during study period
Supplementary Table 4 shows the results of linear regression analysis of changes during study period surveyed by the final questionnaire. None of the social, psychological, physical and nutritional changes during the study period affected the success of the intervention at the end of the study.
DISCUSSION
To our knowledge, this is the first investigation of the long-term, up to one-year effects of a multicomponent cognitive training program on cognitive performance of cognitively healthy persons. Similarly to the short-term results,21 the primary endpoint CERAD-Plus did not reach significance at neither 3 nor 12-month follow-up (Table 2). Several reasons may account for the lack of specific training effects using CERAD-Plus measurements. As the participants were all cognitively healthy adults, this study was more likely to identify preventive measures against cognitive decline. However, CERAD-Plus is a scientifically established instrument for diagnosing cognitive deficits associated with dementia-related diseases.24 Our participants may have not fit the target population of the CERAD-Plus and it may therefore not be sensitive enough for detecting smaller increments due to cognitive training. There is however no other comparable test that could have been used for our objective.
Moreover, the present analysis showed that, compared to GHC, the BrainProtect training is associated to significant improvements in logical reasoning up to 12 months (Fig. 2C) as well as to significant improvements in thinking flexibility and confrontational naming up to 3 months (Fig. 2A-B) after completion of training.
Fig. 2
Development of mean over time. Subtests of CERAD: A) z-Scores: BNT; Subtests of BrainProtect: B) BVGT BrainProtect (thinking flexibility); and C) BVGT BrainProtect (logical reasoning) at the pre- and posttest and follow-up 1 after 3 months and follow-up 2 after 12 months for IG and GHC; presented with standard error; mean and standard deviation are presented in Table 2. CERAD, Consortium to Establish a Registry for Alzheimer’s Disease; BNT, Boston Naming Test; BVGT, Bundesverband Gedächtnistraining e.V. BrainProtect.
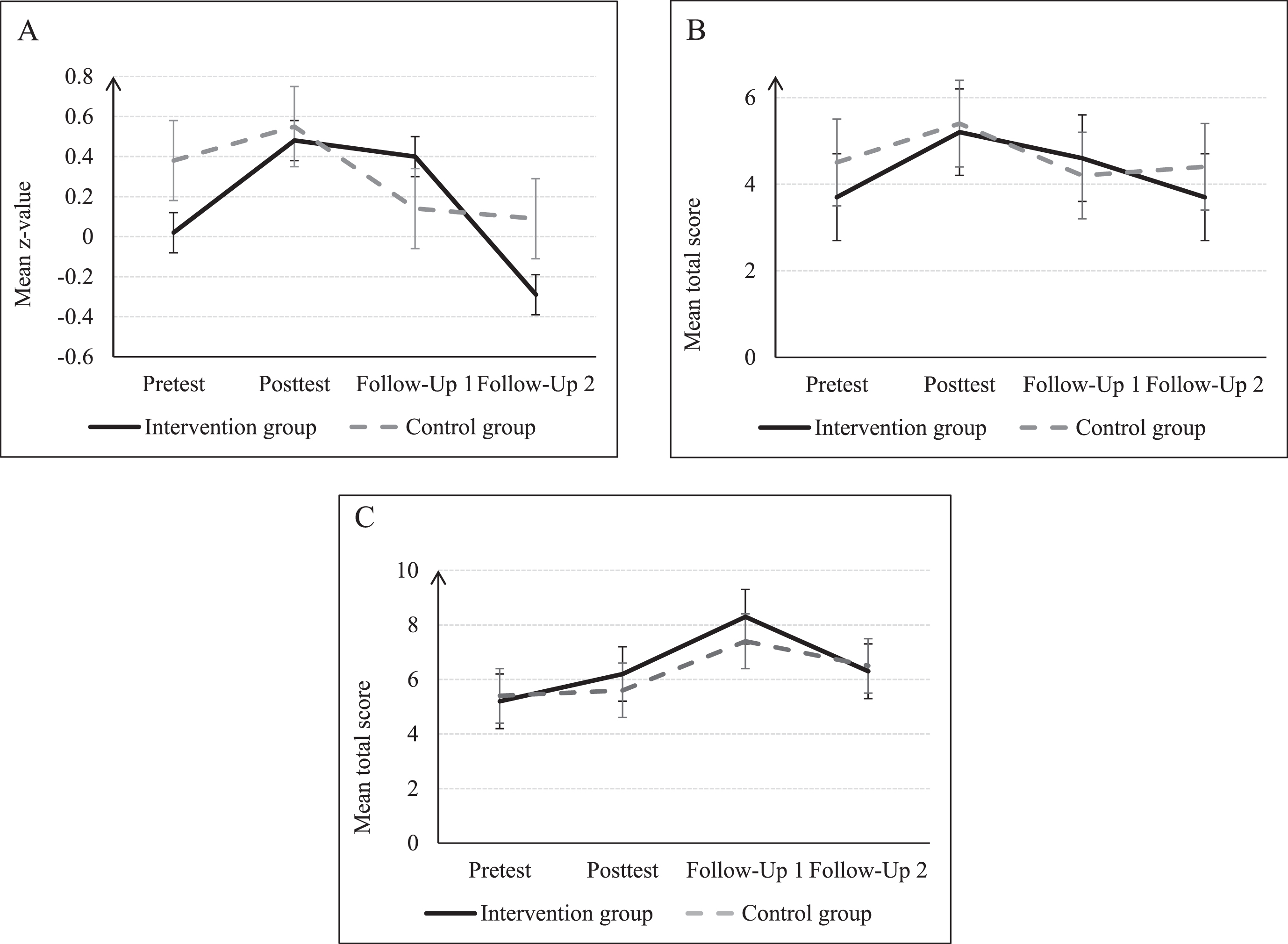
Although the significant training effects remained significant after adjustment by depressive symptoms and years of education (Supplementary Table 2), significance was lost after adjustment by Bonferroni correction. Another important result of the present study is that response to intervention at 12-month follow-up was influenced, as far thinking flexibility and working memory are concerned, by higher education, while none of the other hypothesized predictors (gender, age, employment, sedentary work, family status, diet, physical activity, previously diagnosed memory disorder within the family, past participation in cognitive training and baseline level) affected such response (Table 3).
The definition of cognitive reserve as “adaptability that helps to explain differential susceptibility of cognitive abilities or day-to-day function to brain aging, pathology, or insult.”34 implies that each person seems to cope differently with age- and disease-related changes in cognitive function based on individual burdens and behaviors throughout one’s life span.35 This evidence is strongly indicative for the need of multicomponent interventions for the prevention of age-related cognitive decline36,37 and the long-term results of this study in the context of secondary endpoint partially demonstrate the success of this type of intervention.
After adjusting by years of education and depressive symptoms, BrainProtect significantly improved logical reasoning in IG participants up to 12 months after intervention’s end (Fig. 2C) (Table 2). Although significance was lost after Bonferroni correction, this result is deemed important as it sheds light on possible training developments focusing on long-term delay of cognitive impairment. The Bonferroni correction was applied to prevent Type I errors in the context of multiple comparisons, but it represents a very conservative method of adjustment.38 Even if the result before adjustment should be interpreted cautiously, it demonstrates a tendency of training enhanced cognitive functions that persist and will have positive effects not only on cognition but also on quality of life in the context of logical reasoning. Cristofori et al. describe reasoning as “the core of the generalization and abstraction processes that enable concept formation and creativity”.39 Reasoning belongs to the executive functions of individuals and thus represents a fundamental cognitive property for day-to-day life and social functioning.39 The ACTIVE trial also showed that logical reasoning can be enhanced by cognitive training, even after 10 years40 and thus underlines the effectiveness and importance of cognitive interventions in adults.
Executive functions in general can be significantly influenced by cognitive training in healthy older people.41 BrainProtect subcategory thinking flexibility, which is another executive function and fundamental to basic human thinking39 and CERAD-Plus subcategory BNT measuring confrontational naming showed a significant difference between IG and GHC 3 months after intervention’s end (Fig. 2A-B) (Table 2). A previous study concluded that confrontational naming ability differs significantly between younger and older people and that poorer ability of confrontational naming is an impact of normal ageing.42 Hedden maintained that executive functions are particularly affected by volumetric reductions in the prefrontal cortex, which are part of the neurocognitive processes of normal cognitive aging.43 Although age-related deterioration in confrontational naming seems to be considered normal, our result suggests that this can be partly counteracted preventively by multicomponent intervention.
Participants’ years of education seemed to predict better outcome in BrainProtect subcategories thinking flexibility and working memory. Lövdén et al.44 also reported a positive correlation between earlier years of education and cognitive function in later life. Otherwise, no significant predictors of training success were found, suggesting that none of the other variables included in the analysis may be regarded as systematically predicting training outcome. Such homogenous effects regardless of participant’s characteristics were also observed in the FINGER-trial, in which sociodemographic data, vascular risk and cognition did not influence response to the intervention.45
So far, there is no consensus on how long cognitive training should last to be effective. Cognitive training with lower weekly frequencies46 and sessions shorter than 0.5 h in a larger total number seem to be most effective, although positive effects of cognitive interventions are still small.47 This is consistent with the results of another meta-analysis in which weekly training sessions for≥two months were found to be more effective.41 These findings indicate that the future training duration of BrainProtect must be adjusted.
Considering that depressive symptoms within the IG were more distinctive at baseline than after 12 months and HRQoL also showed an increase within the IG, it can be assumed that the intervention had positive effects on participant’s mental health, though without significance (Table 2). The retrospective analyses from BrainProtect 1.0 already indicated that more than half of the participants reported a significantly improved well-being after the intervention20 and also eight-week intervention’s short-term outcomes showed a significantly better HRQoL for the IG whereas the GHC reported a worsening.21 This may be consistent with the findings of Pitkala et al.48 that well-being, cognition, and health are related to social interaction, which the IG was able to perceive. Moreover, patient-related outcome measures (PROMs) such as emotions, self-efficacy and motivation are highly relevant for both training and intervention outcomes49 and provide determining factors.50
Older age and worse self-perception of health in dropout cohort were significant and seem to be a reason for early completion of the trial (Supplementary Table 1). However, further differences especially regarding clinical and neuropsychological parameters were not present between dropouts and the PP-cohort, indicating that personal reasons rather than clinically relevant variables influenced protocol adherence.
The multidomain intervention BrainProtect includes short physical exercises at the beginning and during the intervention. Kalbe et al.51 determined that cognitive training plus physical exercises are not superior to pure cognitive training in healthy adults but that both lead to cognitive gains. Therefore, the significance of physical activity needs to be considered when thinking about healthy ageing in general and especially in cognitive decline.11 Nevertheless, BrainProtect did not include aerobic exercises, which seem to positively counteract the age-related reduction of brain structure and accumulation of neurotoxic factors52 and might be supplemented in the future.
Considering the increased life expectancy worldwide and older age as one of the main risk factors for cognitive decline,7 the preservation of cognitive integrity is becoming increasingly important. The finding that BrainProtect has already shown positive effects in cognitively healthy adults thus supports the assertion of cognitive training as a preventive measure.29 Non-pharmacological interventions, such as multicomponent training containing nutrition, physical activity and cognitive training,53 can slow the progression of cognitive decline54 or help maintain cognitive integrity leading to the preservation of quality of life, especially before the onset of symptoms.55 As already mentioned at the beginning, SCI, which affects 50–80% of people aged ≥70 years,56 converts to MCI, which is a prodromal stage of AD.3 Since there is no cure for dementia, prevention is important not only for the health and quality of life of those affected and their relatives, but also because of the enormous socio-economic costs associated with it. Therefore, it can be said that BrainProtect shows potential as a preventive tool against cognitive decline with the long-term results of this study, but certainly still has potential for improvement regarding aerobic exercises and dietary tips.
Limitations and strengths of the study
Inherent limitations of the trial must be considered when interpreting the previously presented results. The average participant is a married, educated, non-working, active and healthy woman in her sixties who comes from the Cologne area and therefore the results are only partly transferable to a general population older than 50 years. Therefore, an increased sample size as well as recruitment from additional communities is required in the future, to enhance generalizability to other populations.
As CERAD-Plus is a neurocognitive test-battery designed for the assessment of dementia-related illness,24 the results of our healthy participants might have to be interpreted differently than in cognitively affected persons. However, it must be noted that CERAD-Plus was able to determine significant changes in cognitive performance in healthy subjects after the intervention21 as well as after 3 months (Table 2), indicating a steady effect of BrainProtect on mental functioning. Moreover, ceiling effects57 also should be considered at this point, since especially in the subcategories BNT, wordlist total and recall as well as constructive recall the maximum score was already reached by the majority at baseline, thus changes in the course could not be reflected. The statistical phenomenon of “regression to the mean” (RTM) has also to be mentioned, which may be observed especially in repeated measurements within the same observation unit.58 However, to counteract the statistical artifact of RTM,58,59 participants were randomly allocated to IG or GHC, and ANCOVA analysis was performed with significant baseline differences as covariates.
Retraining-effects of CERAD-Plus must be taken into account since the tasks did not change at the different test points and the participants thus knew to a certain extent which tasks they had to work on or even still knew terms by memorization. However, although the task types of BrainProtect remained unchanged, different words, phrases or pictures were used, so that the subjects could not fall back on rote learning.
The final questionnaire was limited by the poor accessibility despite contacting the participants for three times. However, no significant changes during the study period affecting intervention’s success at the end of the study were found.
The cognitive training took place exclusively as group training with specially qualified memory trainers, intended for motivation and training success, but restrictive in implementing individually from home. However, the opportunity of computerized cognitive training46,60 could supplement group training from home to reduce the number of group sessions but to ensure regular cognitive training.
Due to the SARS-CoV-2 pandemic, many of the participants expressed their great fear of infection which was, in many cases, the reason for non-participating or a delayed testing due to the lockdown. Moreover, the test had to take place in a different location than before which led to great confusion or lack of accessibility for some subjects. Crivelli et al.61 found a lower general cognition in COVID-19 recovered patients compared to healthy controls. This finding and the fear of participants as well as distance and wearing a medical mask may have had impact on the results of the last follow-up.
Conclusions
The eight-week BrainProtect training program did not improve the primary endpoint of global cognitive function (CERAD-Plus). Nevertheless, it may have the potential of improving participant’s cognitive functions, as especially logical reasoning seems to be improved after 12 months regardless of individual characteristics. Also thinking flexibility and confrontational naming were improved for at least 3 months after intervention, disregarding the fact that none of the significances were present after Bonferroni adjustment.
Since BrainProtect appears to be a potential prevention tool against cognitive decline in healthy adults, additional studies are needed in the future to analyze the optimal duration of the BrainProtect training program and its effects after a period of more than one year with a larger sample size. Moreover, BrainProtect needs to be further scientifically validated.
AUTHOR CONTRIBUTIONS
Michelle Celine Kunkler (Data curation; Formal analysis; Investigation; Methodology; Writing – original draft); Julia Maria Falkenreck (Data curation; Formal analysis; Methodology); Anja Ophey (Data curation; Formal analysis; Methodology; Writing – review & editing); Katharina Dencker (Methodology); Andrea Friese (Data curation); Petra Jahr (Data curation); Elke Kalbe (Formal analysis; Methodology; Writing – review & editing); Gereon Nelles (Formal analysis; Methodology; Supervision; Writing – review & editing); M. Cristina Polidori (Formal analysis; Methodology; Supervision; Writing – original draft; Writing – review & editing).
ACKNOWLEDGMENTS
The authors are grateful to the study participants and to the trainers of the BVGT e.V.
FUNDING
The Bundesverband Gedächtnistraining e.V. covered the costs associated to trainers’ BrainProtect sessions with the study participants.
CONFLICT OF INTEREST
M. Cristina Polidori is an Editorial Board Member of the Journal of Alzheimer’s Disease. M. Cristina Polidori is a member of the advisory board of memodio (multidomain app for the treatment of MCI) and an Editorial Board Member of Aging Research Reviews and of the ‘Deutsche Medizinische Wochenschrift’. M. Cristina Polidori receives the royalties for the publication of two books: the latest edition of ‘Paziente Anziano - Paziente Geriatrico - Medicina della Complessit‘a’, EdiSES 2020 and ‘Ratgeber Altern - Es ist nie zu spät’, Elsevier 2021. M. Cristina Polidori is a member of the supervisory board and curatorship of Diakonie Michaelshoven e.V.
M. Cristina Polidori and Elke Kalbe are members of the scientific advisory board of the Bundesverband Gedächtnistraining e.V.
Elke Kalbe has received grants from the German Ministry of Education and Research, Brandau-Laibach Stiftung, Germany, RheinEnergie Stiftung, Germany, consulting fees from Memodio GmbH, Germany, Kyowa Kirin Services Ltd, UK, lecture fees from Biogen GmbH, Germany, EISAI GmbH, Germany, Abbvie GmbH, Germany, KoJ Gehörtraining, Switzerland, licence fees from Prolog GmbH, Germany; all outside the submitted work.
Gereon Nelles is a board member of the Berufsverband Deutscher Nervenärzte (BVDN) e.V. Gereon Nelles is a member of the advisory board of memodio (multidomain app for the treatment of MCI).
Petra Jahr is the regional manager ‘Nordrhein-Westfalen West‘ of the Bundesverband Gedächtnistraining e.V.
Andrea Friese is the former educational manager of the Bundesverband Gedächtnistraining e.V.
Anja Ophey received grants of the Koeln Fortune Program (grant-no. 329/2021), Faculty of Medicine, University of Cologne, and the “Novartis-Stiftung für therapeutische Forschung” and speaking honoraria of ProLog Wissen GmbH, all outside the submitted work.
Julia Maria Falkenreck is a member of the Bundesverband Gedächtnistraining e.V.
The other authors have no conflict of interest to report.
DATA AVAILABILITY
The data supporting the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/ADR-230199.
REFERENCES
[1] | Tipton PW and Graff-Radford NR. Prevention of late-life dementia: What works and what does not. Pol Arch Intern Med (2018) ; 128: : 310–316. |
[2] | Anderson ND . State of the science on mild cognitive impairment (MCI). CNS Spectr (2019) ; 24: : 78–87. |
[3] | Rostamzadeh A , Bohr L , Wagner M , et al. Progression of subjective cognitive decline to MCI or dementia in relation to biomarkers for Alzheimer disease: A meta-analysis. Neurology (2022) ; 99: : e1866–e1874. |
[4] | Veronese N , Soysal P , Demurtas J , et al. Physical activity and exercise for the prevention and management of mild cognitive impairment and dementia: A collaborative international guideline. Eur Geriatr Med (2023) ; 14: : 925–952. |
[5] | Ellouze I , Sheffler J , Nagpal R , et al. Dietary patterns and Alzheimer’s disease: An updated review linking nutrition to neuroscience. Nutrients (2023) ; 15: : 3204. |
[6] | Hughes ML , Agrigoroaei S , Jeon M , et al. Change in cognitive performance from midlife into old age: Findings from the Midlife in the United States (MIDUS) study. J Int Neuropsychol Soc (2018) ; 24: : 805–820. |
[7] | Polidori MC , Nelles G , Senin U , et al. Cognitive decline. In: Roller-Wirnsberger R, Singler K and Polidori MC (eds) Learning Geriatric Medicine Practical Issues in Geriatrics. Springer, Cham, (2018) . |
[8] | Campbell NL , Unverzagt F , LaMantia MA , et al. Risk factors for the progression of mild cognitive impairment to dementia. Clin Geriatr Med (2013) ; 29: : 873–893. |
[9] | Norton S , Matthews FE , Barnes DE , et al. Potential for primary prevention of Alzheimer’s disease: An analysis of population-based data. Lancet Neurol (2014) ; 13: : 788–794. |
[10] | Polidori MC , Stahl W , Griffiths HR. Nutritional cognitive neuroscience of aging: Focus on carotenoids and cognitive frailty. Redox Biol (2021) ; 44: : 101996. |
[11] | Eckstrom E , Neukam S , Kalin L , et al. Physical activity and healthy aging. Clin Geriatr Med (2020) ; 36: : 671–683. |
[12] | Meyer AM , Podolski N , Pickert L , et al. Strategies to prevent age-related cognitive decline. Dtsch Med Wochenschr (2020) ; 145: :(3) 146–150. |
[13] | Ngandu T , Lehtisalo J , Solomon A , et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. Lancet (2015) ; 385: : 2255–2263. |
[14] | Kivipelto M , Mangialasche F , Ngandu T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat Rev Neurol (2018) ; 14: : 653–666. |
[15] | Gavelin HM , Lampit A , Hallock H , et al. Cognition-oriented treatments for older adults: A systematic overview of systematic reviews. Neuropsychol Rev (2020) ; 30: : 167–193. |
[16] | Li T , Yao Y , Cheng Y , et al. Cognitive training can reduce the rate of cognitive aging: A neuroimaging cohort study. BMC Geriatr (2016) ; 16: : 12. |
[17] | Ahn S , Chung JW , Crane MK , et al. The effects of multi-domain interventions on cognition: A systematic review. West J Nurs Res (2021) ; 1939459211032272. |
[18] | Global status report on the public health response to dementia. Geneva: World Health Organization, (2021) . |
[19] | Ben Hassen C , Fayosse A , Landré B , et al. Association between age at onset of multimorbidity and incidence of dementia: 30 year follow-up in Whitehall II prospective cohort study. BMJ (2022) ; 376: : e068005. |
[20] | Falkenreck JM , Roheger M , Weigert H , et al. BrainProtect® - A cognitive training program with nutritional and physical counseling components: A retrospective analysis of its effects in healthy individuals. Geriatric Care (2020) ; 6: :(4): https://doi.org/10.4081/gc.2020.9328. |
[21] | Falkenreck JM , Kunkler MC , Ophey A , et al. Effects of the multicomponent cognitive training program BrainProtect in cognitively healthy adults: A randomized controlled trial. J Alzheimers Dis (2023) ; 94: : 1013–1034. |
[22] | Nasreddine ZS , Phillips NA , Bédirian V , et al. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc (2005) ; 53: : 695–699. |
[23] | Beck AT , Ward CH , Mendelson M , et al. An inventory for measuring depression. Arch Gen Psychiatry (1961) ; 4: : 561–571. |
[24] | Schmid NS , Ehrensperger MM , Berres M , et al. The extension of the German CERAD neuropsychological assessment battery with tests assessing subcortical, executive and frontal functions improves accuracy in dementia diagnosis. Dement Geriatr Cogn Dis Extra (2014) ; 4: : 322–334. |
[25] | Lawton MP and Brody EM. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist (1969) ; 9: : 179–186. |
[26] | Katz S , Ford AB , Moskowitz RW , et al. Studies of illness in the aged. The index of ADL: A standardized measure of biological and psychosocial function. JAMA (1963) ; 185: : 914–919. |
[27] | Rubenstein LZ , Harker JO , Salvà A , et al. Screening for undernutrition in geriatric practice: Developing the short-form mini-nutritional assessment (MNA-SF). J Gerontol A Biol Sci Med Sci (2001) ; 56: : M366–372. |
[28] | Herdman M , Gudex C , Lloyd A , et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res (2011) ; 20: : 1727–1736. |
[29] | Risk Reduction of Cognitive Decline and Dementia: WHO Guidelines. Geneva: World Health Organization, (2019) . |
[30] | Berres M , Zehnder A , Bläsi S , et al. Evaluation of diagnostic scores with adjustment for covariates. Stat Med (2008) ; 27: : 1777–1790. |
[31] | Aebi C . Validierung der Neuropsychologischen Testbatterie CERAD-NP: Eine Multi-Center Studie. Universität Basel, Schweiz: Doktorarbeit, (2002) . |
[32] | Lillig R , Ophey A , Schulz JB , et al. A new CERAD total score with equally weighted z-scores and additional executive and non-amnestic “CERAD-Plus” tests enhances cognitive diagnosis in patients with Parkinson’s disease: Evidence from the LANDSCAPE study. Parkinsonism Relat Disord (2021) ; 90: : 90–97. |
[33] | Cohen J . Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ, USA: Lawrence Erlbaum Associates, (1988) . |
[34] | Stern Y , Arenaza-Urquijo EM , Bartrés-Faz D , et al. Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimers Dement (2020) ; 16: : 1305–1311. |
[35] | Stern Y . Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol (2012) ; 11: : 1006–1012. |
[36] | Rosenberg A , Mangialasche F , Ngandu T , et al. Multidomain interventions to prevent cognitive impairment, alzheimer’s disease, and dementia: From FINGER to World-Wide FINGERS. J Prev Alzheimers Dis (2020) ; 7: : 29–36. |
[37] | Kivipelto M , Mangialasche F , Snyder HM , et al. World-Wide FINGERS Network: A global approach to risk reduction and prevention of dementia. Alzheimers Dement (2020) ; 16: : 1078–1094. |
[38] | Chen SY , Feng Z , Yi X. A general introduction to adjustment for multiple comparisons. J Thorac Dis (2017) ; 9: : 1725–1729. |
[39] | Cristofori I , Cohen-Zimerman S and Grafman J. Executive functions. Handb Clin Neurol (2019) ; 163: : 197–219. |
[40] | Rebok GW , Ball K , Guey LT , et al. Ten-year effects of the advanced cognitive training for independent and vital elderly cognitive training trial on cognition and everyday functioning in older adults. J Am Geriatr Soc (2014) ; 62: : 16–24. |
[41] | Chiu HL , Chu H , Tsai JC , et al. The effect of cognitive-based training for the healthy older people: A meta-analysis of randomized controlled trials. PLoS One (2017) ; 12: : e0176742. |
[42] | Tsang HL, Lee TM. The effect of ageing on confrontational naming ability. Arch Clin Neuropsychol (2003) ; 18: : 81–89. |
[43] | Hedden T and Gabrieli JD. Insights into the ageing mind: A view from cognitive neuroscience. Nat Rev Neurosci (2004) ; 5: : 87–96. |
[44] | Lövdén M , Fratiglioni L , Glymour MM , et al. Education and cognitive functioning across the life span. Psychol Sci Public Interest (2020) ; 21: : 6–41. |
[45] | Rosenberg A , Ngandu T , Rusanen M , et al. Multidomain lifestyle intervention benefits a large elderly population at risk for cognitive decline and dementia regardless of baseline characteristics: The FINGER trial. Alzheimers Dement (2018) ; 14: : 263–270. |
[46] | Lampit A , Hallock H and Valenzuela M. Computerized cognitive training in cognitively healthy older adults: A systematic review and meta-analysis of effect modifiers. PLoS Med (2014) ; 11: : e1001756. |
[47] | Mewborn CM , Lindbergh CA and Stephen Miller L. Cognitive interventions for cognitively healthy, mildly impaired, and mixed samples of older adults: A systematic review and meta-analysis of randomized-controlled trials. Neuropsychol Rev (2017) ; 27: : 403–439. |
[48] | Pitkala KH , Routasalo P , Kautiainen H , et al. Effects of socially stimulating group intervention on lonely, older people’s cognition: A randomized, controlled trial. Am J Geriatr Psychiatry (2011) ; 19: : 654–663. |
[49] | Ryan RM , Lynch MF , Vansteenkiste M , et al. Motivation and autonomy in counseling, psychotherapy, and behavior change: A look at theory and practice. Couns Psychol (2011) ; 39: : 193–260. |
[50] | Meyer AM , Bartram MP , Antczak P , et al. A tailored discharge program improves frailty and mood in patients undergoing usual rehabilitative care: A randomized controlled trial. J Am Med Dir Assoc (2022) ; 23: : 1962.e1961–1962.e1913. |
[51] | Kalbe E , Rohere M , Paluszak K , et al. Effects of a cognitive training with and without additional physical activity in healthy older adults: A follow-up 1 year after a randomized controlled trial. Front Aging Neurosci (2018) ; 10: : 407. |
[52] | Cheng ST . Cognitive reserve and the prevention of dementia: The role of physical and cognitive activities. Curr Psychiatry Rep (2016) ; 18: : 85. |
[53] | Dominguez LJ , Veronese N , Vernuccio L , et al. Nutrition, physical activity, and other lifestyle factors in the prevention of cognitive decline and dementia. Nutrients (2021) ; 13: : 4080. |
[54] | Rabin LA , Smart CM and Amariglio RE. Subjective cognitive decline in preclinical Alzheimer’s disease. Annu Rev Clin Psychol (2017) ; 13: : 369–396. |
[55] | Krivanek TJ , Gale SA , McFeeley BM , et al. Promoting successful cognitive aging: A ten-year update. J Alzheimers Dis (2021) ; 81: : 871–920. |
[56] | Jessen F , Amariglio RE , Buckley RF , et al. The characterisation of subjective cognitive decline. Lancet Neurol (2020) ; 19: : 271–278. |
[57] | Wang L , Zhang Z , McArdle JJ , et al. Investigating ceiling effects in longitudinal data analysis. Multivariate Behav Res (2009) ; 43: : 476–496. |
[58] | Barnett AG , van der Pols JC , Dobson AJ. Regression to the mean: What it is and how to deal with it. Int J Epidemiol (2005) ; 34: : 215–220. |
[59] | Smoleń T , Jastrzebski J , Estrada E , et al. Most evidence for the compensation account of cognitive training is unreliable. Memory Cogn (2018) ; 46: : 1315–1330. |
[60] | Gates NJ , Rutjes AW , Di Nisio M , et al. Computerised cognitive training for 12 or more weeks for maintaining cognitive function in cognitively healthy people in late life. Cochrane Database Syst Rev (2020) ; 2: : CD012277. |
[61] | Crivelli L , Palmer K , Calandri I , et al. Changes in cognitive functioning after COVID-19: A systematic review and meta-analysis. Alzheimers Dement (2022) ; 18: : 1047–1066. |




