Neurosyphilis Initially Misdiagnosed as Behavioral Variant Frontotemporal Dementia: Life-Changing Differential Diagnosis
Abstract
Diagnosing neurosyphilis can be challenging and it may be misdiagnosed as behavior variant frontotemporal dementia, given its affinity for the frontal and temporal lobes. Here we present a model case, who, in his 40 s, was initially misdiagnosed with behavioral variant frontotemporal dementia based on extreme self-neglect and disinhibition over six months and frontal lobe atrophy. He was later diagnosed as neurosyphilis with positive syphilis tests in his cerebrospinal fluid. He underwent penicillin treatment and fully recovered. Relatively rapid cognitive decline, particularly if young, should prompt physicians to consider neurosyphilis as a treatable dementia, which could completely change a patient’s life.
INTRODUCTION
While neurosyphilis is not a common cause of dementia, it is important to highlight that it falls under the category of treatable and reversible dementias [1, 2]. However, the diagnosis of neurosyphilis is challenging because of diverse and unspecific clinical signs [3, 4] as well as its rarity [2]. Most cases with syphilis have been treated since the advent of penicillin treatment and the development of neurosyphilis has become rare [5]. It, however, has come back into the spotlight, particularly with regard to its concurrent occurrence with infection of human immunodeficiency virus (HIV) [6]. Since then, cases of neurosyphilis have been occasionally reported, whether or not they are concurrent with HIV. In recent years, the incidence of syphilis has been below 30 cases per 100,000 population in most countries [7]. For instance, as of July 2023, Japan recorded an incidence rate of 2.97 cases per 100,000 population (https://www.niid.go.jp/niid/images/epi/syphilis/2023q2/syphilis2023q2.pdf). The annual incidence of neurosyphilis is somewhat low, with a range of 0.16 to 2.1 cases per 100,000 population in the Netherlands [8].
Although damage caused by neurosyphilis can occur in any area of the brain or the spine, the frontal lobe and temporal lobes are particularly susceptible [9]. As such, there is a possibility of misdiagnosing neurosyphilis as behavioral variant frontotemporal dementia (bvFTD), the atrophy of which is found in frontal and/or anterior temporal lobes and features mainly behavior abnormalities. This fact probably aligns with the historical records that before the discovery of antibiotics, neurosyphilis accounted for 15% of psychiatric inpatients who were hospitalized mainly due to behavior abnormalities [5]. However, there have been few reports on the case of neurosyphilis that have been initially misdiagnosed as bvFTD. To the best of our knowledge, there are only two reports on such a case [10, 11]. Although these cases provide valuable information, there are certain aspects to consider. In their cerebrospinal fluid (CSF) analysis during treatment, Prynn et al. [10] and Caroppo et al. [11] found negative results for syphilis infection, while serum screening exams for syphilis infection returned positive. Additionally, while both cases showed significant cognitive improvement shortly after treatment, Prynn et al. [10] did not conduct follow-up neuropsychological testing or provide a detailed behavioral description. In the case of Caroppo et al. [11], the man’s behavioral disorders resurfaced 2½ years aftertreatment.
Distinguishing between bvFTD and other neurological or psychiatric conditions presents an intricate diagnostic challenge [12, 13]. Reciprocal errors in differential diagnosis can arise, entailing either the misclassification of bvFTD as alternative conditions or the erroneous identification of other disorders as bvFTD [12, 13]. The former pattern, wherein bvFTD is misdiagnosed as other conditions, encompasses psychiatric conditions like mood disorders, anxiety disorders, or psychotic disorders [12], as well as neurological conditions including Alzheimer’s disease [14], alcohol-related dementia [15], and Lyme disease [16]. Notably, around 50% of individuals with bvFTD initially receive a psychiatric diagnosis [12]. Conversely, the latter pattern, involving misdiagnosis of other diseases as behavioral variant frontotemporal dementia, encompasses primarily psychiatric conditions, along with neurological conditions such as Creutzfeldt-Jakob disease [17] and genetic white-matter disorders like hereditary diffuse leukoencephalopathy with spheroids or adult-onset leukoencephalopathy with axonal spheroids and pigmented glia [18]. A study indicates that nearly half of initial frontotemporal dementia diagnoses are reclassified after follow-up, with a significant proportion being reclassified as psychiatric diagnoses [13]. Among the latter pattern, of particular note are treatable dementias like neurosyphilis, which demand meticulous attention from clinicians, as appropriate treatment can dramatically alter the lives of affected patients.
Here we present a potential model case of neurosyphilis, a treatable dementia, that represents the initial misdiagnosis as behavioral variant frontotemporal dementia, its accurate diagnosis with the positive syphilis infection in the CSF, and complete recovery after the treatment, the clinical course of which underscores the crucial importance of accurate differential diagnosis. Based on the clinical course of this case and past reports of similar cases, we have summarized key points for not missing the diagnosis of neurosyphilis in the discussion.
CASE PRESENTATION
Initial misdiagnosis as bvFTD
A 47-year-old man was referred to our neuropsychiatric unit in Ashikaga Red Cross Hospital from a nearby hospital due to abnormal behaviors, where he was diagnosed as having bvFTD. During the initial examination at our neuropsychiatric unit, a medical doctor (MF) gathered a comprehensive medical history, which found no prior history of medical, neurological, or psychiatric diseases, nor any obvious skin signs such as rash or gummas, which are associated with syphilis. At age 47, the patient had been working as a racehorse breeder for two decades and had never married. Over a period of six months, he began to experience a series of mishaps, such as dropping packages while on duty. Four months after the initial onset of symptoms, he caused two minor car accidents and had trouble feeding the horses. He also began to experience difficulties speaking at times. Six months after the onset of symptoms, he developed apathy, and his supervisor instructed him to visit a psychiatric clinic due to his behavioral changes. After his initial diagnosis of dementia at a local clinic, the patient was prescribed donepezil, a cholinesterase inhibitor. Two weeks later, he made a phone call to his family’s home, but his speech was difficult to understand. The following day, a community leader visited his house and found him in a state of incontinence, with feces covering his hair. The patient had been prone to agitation and violent behavior, so he had to be physically restrained and transported by ambulance to a localhospital.
During his one-month hospitalization in an internal medicine unit at the local hospital, the patient exhibited disinhibition. He tried to bite the doctor and stole and ate other patients’ meals. He displayed symptoms of euphoria but was also prone to irritability and distraction by the actions of other patients around him. He exhibited abnormal behavior, such as suddenly starting to brush his teeth when it was not necessary. Additionally, he lacked empathy and could be excessively intrusive at times. Magnetic resonance imaging (MRI) of his head revealed atrophy of the frontal lobe, and single photon emission computed tomography showed a significant decrease in blood flow in the same area. Based on these brain imaging results and symptoms such as disinhibition, apathy, and lack of empathy, the patient was diagnosed with probable bvFTD [19]. To manage his remarkable behavioral abnormalities, he was given a daily dose of 375 mg of chlorpromazine antipsychotic medication, but due to its side effects, he developed aspiration pneumonia and was treated with a daily dose of 6 g of sulbactam/ampicillin antibiotics for the three days prior to transfer to our hospital.
Upon admission to our neuropsychiatric unit, which was seven months after the onset of symptoms, the patient’s vital signs were normal. The patient’s level of consciousness is alert, and their gait and speech were normal. However, syphilis was detected during routine blood screening. Other than that, no significant findings were observed in his blood test, including a negative result for HIV antibody. There were no obvious skin signs such as rash or gummas. To investigate the cause of his symptoms further, we conducted a battery of tests, including further testing for syphilis, neurological and neuropsychological examinations, and brain imaging.
Assessments
Syphilis tests
We performed syphilis testing on both serum and CSF, including a serological test for syphilis (rapid plasma regain) and tests for treponema pallidum antibodies (treponema pallidum hemagglutination assay and fluorescent treponemal antibody absorption). These tests were conducted upon admission and at 1 month, 5 months, and 9.5 years after the start of treatment to monitor the patient’s progress.
Neurological examination
We examined the presence of typical signs and symptoms associated with neurosyphilis [2, 20], such as meningeal signs, pupillary abnormalities (Argyll-Robertson pupils), and tabes dorsalis, which is characterized by the slow degeneration of the neural tracts primarily in the dorsal root ganglia of the spinal cord.
Neuropsychological examination
To assess dementia severity, we used the Japanese version of the Clinical Dementia Rating scale [21], while the Japanese version of the Mini-Mental State Examination (MMSE) [22] was used to evaluate general cognitive function. For detailed evaluation of intelligence, we used the Japanese version of the Wechsler Adult Intelligence Scale—Revised Edition [23]. To evaluate executive function, we employed a modified Wisconsin card sorting test [24] and used the number of categories achieved as an index. The first two tests were administered upon admission, while the remaining two were conducted within three days of admission. After treatment, all four tests were repeated five months later, except for the Clinical Dementia Rating scale, which was also assessed one month and 9.5 years after treatment.
Brain imaging
He underwent MRI to evaluate the structural imaging of his brain. Additionally, magnetic resonance angiography (MRA) was performed to evaluate any abnormalities in the blood vessels of the cerebral and cervical regions. To check for the presence of an aneurysm that is related to syphilis infection, echocardiography was also used. For functional imaging, we evaluated the patient using 99mTcethylcysteinate dimer single photon emission computed tomography. The results were analyzed using an easy Z score imaging system [25].
RESULTS AND SYPHILIS TREATMENT
Initial assessment
The information presented in Table 1 indicates that all syphilis tests conducted on both the patient’s serum and CSF were positive. The positive fluorescent treponemal antibody absorption test performed on his CSF was particularly suggestive of symptomatic neurosyphilis [2]. Further examination of his CSF revealed a white blood cell count of 3.3 cells/mm3, with 80% of cells being mononuclear, a protein level of 47 mg/dL, and an IgG level of 164.0 mg/L. These values were either beyond the normal range or at the upper limit of normal, indicating the presence of neurosyphilis [3]. The patient’s relatively low white blood cell count in the CSF may be attributed to the use of antibiotics for aspiration pneumonia prior to being referred to our hospital. As a result of these findings, the patient was diagnosed with an active infection of neurosyphilis.
Table 1
Patient’s progression on syphilis tests, CSF parameters, and neuropsychological tests
| Assessment | Evaluation subcategory | Upon admission (7 mo post onset) | 1 mo after treatment | 5 mo after treatment (12 mov post onset) | 9.5 y after treatment (10 y post onset) |
| Syphilis tests (Antibody titer) | Serum STS (rapid plasma regain, normal < 1) | 4 | 8 | 4 | <1 |
| Serum TPHA (normal < 80) | 20,480 | 40,980 | 20,480 | 5,120 | |
| Serum FTA-Abs (normal < 20) | 1,280 | 1,280 | 320 | 320 | |
| CSF STS (rapid plasma regain, normal < 1) | 2 | 2 | N.A. | N.A. | |
| CSF TPHA (normal < 80) | 5120 | 5120 | N.A. | N.A. | |
| CSF FTA-Abs (normal < 20) | 80 | 80 | N.A. | N.A. | |
| CSF parameters | White-cell count (per mm3) (normal < 3.3) | 3.3 | 0.3 | N.A. | N.A. |
| Total protein (mg/dl) (normal range: 15–45) | 47 | 48 | N.A. | N.A. | |
| IgG (mg/L) (normal < 60) | 164.0 | 143.0 | N.A. | N.A. | |
| Neuropsychological examination | Clinical Dementia Rating Scale score (0, absent; 3, severe) | 2 | 1 | 0 | 0 |
| MMSE (0, worst; 30, best; cut-off point, 23/24) | 19 | N.A. | 28 | N.A. | |
| WAIS-R (normal range: 70–130) | FIQ784, VIQ884, PIQ987 | N.A. | FIQ98, VIQ106, PIQ88 | N.A. | |
| WCST (number of categories achieved; 0, worst; 7, best; 4.7±1.7 for normal 40 s) | 1 | N.A. | 5 | N.A. |
mo, months; y, years; STS, serological test for syphilis; TPHA, treponema pallidum hemagglutination assay; FTA-Abs, fluorescent treponemal antibody absorption; CSF, cerebrospinal fluid; MMSE, Mini-Mental State Examination; WAIS-R: Wechsler Adult Intelligence Scale-Revised Edition; FIQ, full intelligence quotient; VIQ, verbal intelligence quotient; PIQ, performance intelligence quotient; WCST, Wisconsin Card Sorting Test; N.A., not assessed.
His neurological examination revealed no abnormalities, including absence of Argyll-Robertson pupil and negative Romberg sign, no decrease in tendon reflexes, no sensory or urinary dysfunction, and no signs of spinal cord damage.
His neuropsychological evaluation revealed overall cognitive impairment with a moderate dementia level. The low score on his MMSE was primarily attributed to errors in orientation and calculation, the latter of which is considered closely linked to executive function [26]. In contrast, he obtained a perfect score of 3 out of 3 points on the delayed recall subtest of the MMSE, indicating potential intact episodic memory function. His executive dysfunction was particularly strong since his intellectual function as measured by Wechsler Adult Intelligence Scale was within the normal range, yet his performance on executive function tasks measured by Wisconsin Card Sorting Test was impaired. These neuropsychological findings align with those seen in bvFTD [19] that are characterized by executive dysfunction with relatively preserved episodic memory, although our neuropsychological evaluation did not thoroughly assess episodic memory function in detail.
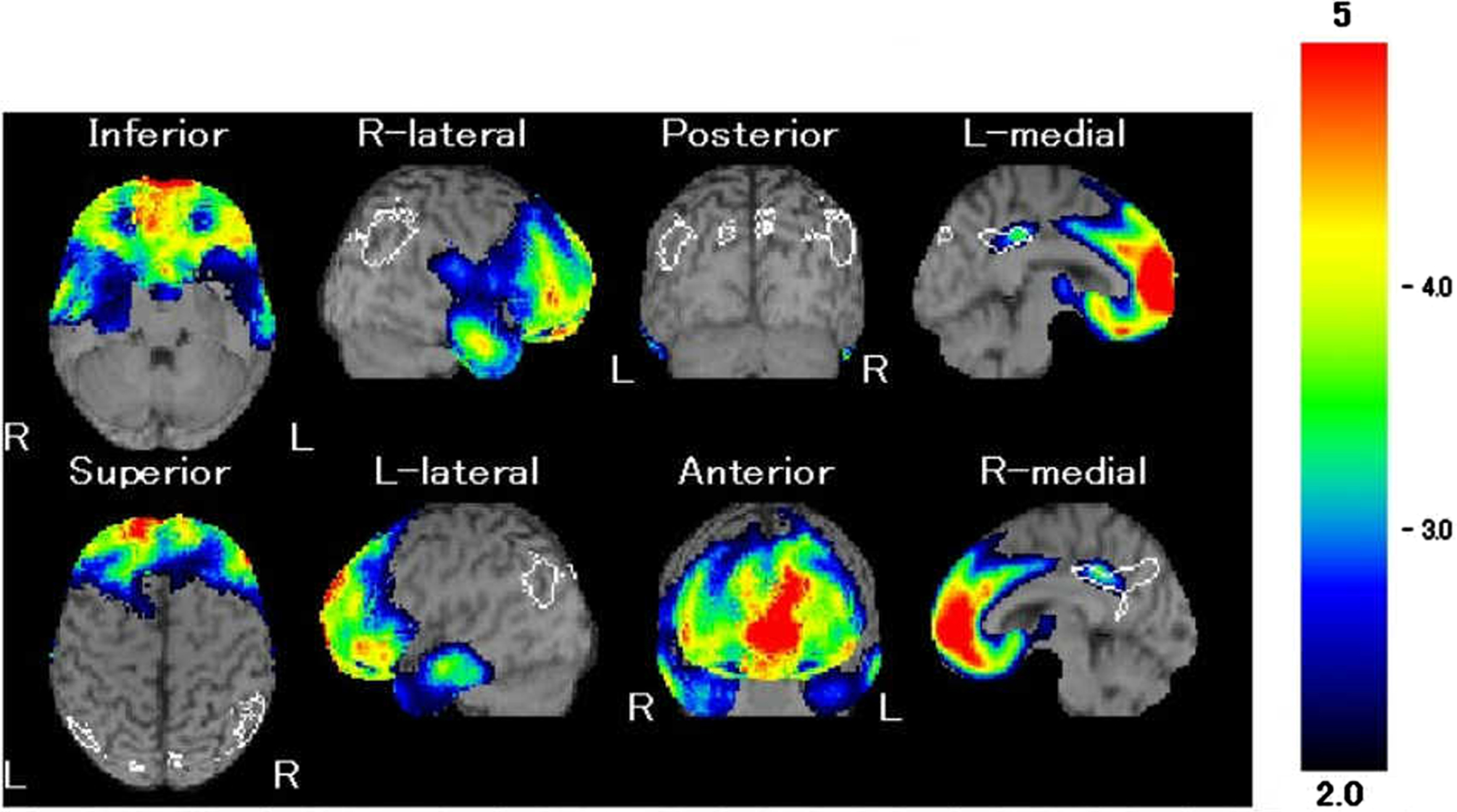
His MRI showed atrophy of the frontal lobe with no other significant findings (Fig. 1), and single photon emission computed tomography revealed a relative decrease in blood flow mainly in the same area (Fig. 2). Both MRA of his head and his echocardiography showed no evidence of vascular abnormalities.
Fig. 1
Our patient’s T1-weighted magnetic resonance imaging. Atrophy in the bilateral frontal lobe was demonstrated.
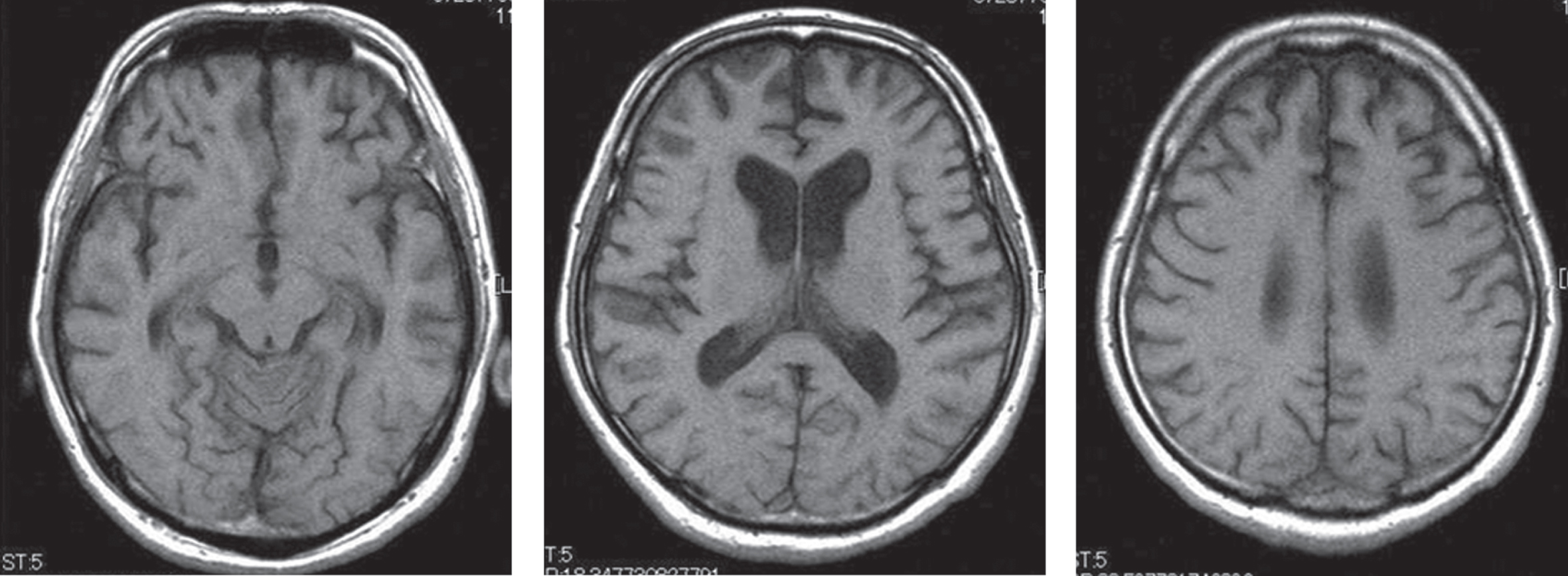
Fig. 2
Our patient’s 99mTc-ethylcysteinate dimer single photon emission computed tomography. Red indicates areas of the strongest hypoperfusion (z score ≤ –5); blue is where hypoperfusion is significant (z score of of –2 to –3) when compared with neurotypical individuals. Hypoperfusion in the bilateral frontal lobe was demonstrated.
Syphilis treatment
From the 3rd hospital day, he underwent a total of 24 million units of penicillin per day for 10 days, which did not involve Jarisch-Herxheimer reaction, a reaction with high fever that can occur in some people after the start of antibiotic treatment for syphilis infection [20]. Following this, the patient continued to take amoxicillin orally at a dosage of 1000 mg/day for six months. Within a few days of initiating treatment, the patient’s disinhibition improved, and his chlorpromazine antipsychotic medication was gradually tapered off during his hospital stay. The patient’s condition improved significantly with the treatment, and he was discharged from the hospital one month after treatment initiation (8 months post-onset). He was able to resume an independent life and returned to work 6 months after treatment (13 months post-onset) with similar competence as before.
Post-treatment assessment
The antibody titer of serum syphilis tests gradually decreased, particularly serum serological test for syphilis, which became normal 10 years post onset (Table 1). His neuropsychological function greatly improved and became within normal range 5 months after the treatment. His atrophy of the frontal lobe remained with his MRI and a relative decrease in blood flow in the frontal lobe showed a slight recovery 5 months after the treatment.
DISCUSSION
This case report presents a model case of neurosyphilis that was initially misdiagnosed as behavioral variant frontotemporal dementia, but later correctly diagnosed based on positive syphilis test results in the patient’s CSF. The patient achieved full recovery with antibiotic treatment and was able to return to work without any issues, despite remaining frontal lobe atrophy. However, it should be noted that higher cognitive demands in a job might have posed a challenge for the patient. Additionally, if the misdiagnosis had continued, there was a high probability of a poor prognosis for the patient.
What are the key indicators for suspecting neurosyphilis? Recent case reports from the last decade have shown that neurosyphilis tends to progress rapidly, typically within several months to a year [10, 27–35], as opposed to degenerative diseases, which develop slowly over several years. This was also observed in the present case, where the patient was in his 40 s, much younger than the age at which degenerative diseases are typically diagnosed. Therefore, it is essential for physicians to differentiate treatable dementia, such as neurosyphilis, from degenerative diseases, especially in cases that progress rapidly and occur at a young age. This case underscores the importance of accurate differential diagnosis, which can completely change a patient’s life.
ACKNOWLEDGMENTS
We would like to express our gratitude to the patient for consenting to the publication of this report.
FUNDING
This report did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
DATA AVAILABILITY
The datasets generated and/or analyzed during the current study are available from the corresponding author (MF) upon request.
REFERENCES
[1] | Bello VME , Schultz RR ((2011) ) Prevalence of treatable and reversible dementias: A study in a dementia outpatient clinic. Dement Neuropsychol 5: , 44–47. |
[2] | Ropper AH ((2019) ) Neurosyphilis. N Engl J Med 381: , 1358–1363. |
[3] | Ge Y , Gou X , Dong X , Peng Y , Yang F ((2022) ) Cerebrospinal fluid changes and clinical features of neurosyphilis compared with latent syphilis infection in the central nervous system: A cross-sectional study. Infect Drug Resist 15: , 5377–5385. |
[4] | Alberto C , Deffert C , Lambeng N , Breville G , Gayet-Ageron A , Lalive P , Toutous Trellu L , Fontao L ((2022) ) Intrathecal synthesis index of specific anti-treponema IgG: A new tool for the diagnosis of neurosyphilis. Microbiol Spectr 10: , e0147721. |
[5] | Macia F , Ardagna Y ((2018) ) Historical reflections on neurosyphilis based on the 1826 treatise on general paralysis in demented patients by Louis Florentin Calmeil (1798-1895). Rev Neurol (Paris) 174: , 247–254. |
[6] | Johns DR , Tierney M , Felsenstein D ((1987) ) Alteration in the natural history of neurosyphilis by concurrent infection with the human immunodeficiency virus. N Engl J Med 316: , 1569–1572. |
[7] | Kenyon CR , Osbak K , Tsoumanis A. ((2016) ) The global epidemiology of syphilis in the past century - a systematic review based on antenatal syphilis prevalence. PLoS Negl Trop Dis 10: , e0004711. |
[8] | Daey Ouwens IM , Koedijk FD , Fiolet AT , van Veen MG , van den Wijngaard KC , Verhoeven WM , Egger JI , van der Sande MA. ((2014) ) Neurosyphilis in the mixed urban-rural community of the Netherlands. Acta Neuropsychiatr 26: , 186–92. |
[9] | Corrêa DG , de Souza SR , Freddi TAL , Fonseca APA , Dos Santos RQ , Hygino da Cruz LC Jr ((2023) ) Imaging features of neurosyphilis. J Neuroradiol 50: , 241–252. |
[10] | Prynn J , Hussain A , Winnett A. ((2016) ) Diagnosing neurosyphilis: A case of confusion. BMJ Case Rep 2016: , bcr2016216582. |
[11] | Caroppo P , Villa C , Del Sole A , Bernardi G , Carradori S , Tiraboschi P , Giaccone G , Prioni S ((2022) ) Neurosyphilis mimicking behavioral variant of frontotemporal dementia in a 59-year-old man. Cogn Behav Neurol 35: , 140–146. |
[12] | Ducharme S , Dols A , Laforce R , Devenney E , Kumfor F , van den Stock J , Dallaire-Théroux C , Seelaar H , Gossink F , Vijverberg E , Huey E , Vandenbulcke M , Masellis M , Trieu C , Onyike C , Caramelli P , de Souza LC , Santillo A , Waldö ML , Landin-Romero R , Piguet O , Kelso W , Eratne D , Velakoulis D , Ikeda M , Perry D , Pressman P , Boeve B , Vandenberghe R , Mendez M , Azuar C , Levy R , Le Ber I , Baez S , Lerner A , Ellajosyula R , Pasquier F , Galimberti D , Scarpini E , van Swieten J , Hornberger M , Rosen H , Hodges J , Diehl-Schmid J , Pijnenburg Y ((2020) ) Recommendations to distinguish behavioural variant frontotemporal dementia from psychiatric disorders. Brain 143: , 1632–1650. |
[13] | Krudop WA , Dols A , Kerssens CJ , Eikelenboom P , Prins ND , Möller C , Schouws S , Rhebergen D , van Exel E , van der Flier WM , Sikkes S , Scheltens P , Stek ML , Pijnenburg YAL ((2017) ) The pitfall of behavioral variant frontotemporal dementia mimics despite multidisciplinary application of the FTDC criteria. J Alzheimers Dis 60: , 959–975. |
[14] | Beber BC , Chaves MLF ((2013) ) Evaluation of patients with behavioral and cognitive complaints: Misdiagnosis in frontotemporal dementia and Alzheimer’s disease. Dement Neuropsychol 7: , 60–65. |
[15] | Funayama M , Nakajima A , Kurose S , Takata T ((2021) ) Putative alcohol-related dementia as an early manifestation of right temporal variant of frontotemporal dementia. J Alzheimers Dis 83: , 531–537. |
[16] | Di Battista ME , Dell’Acqua C , Baroni L , Fenoglio C , Galimberti D , Gallucci M ((2018) ) Frontotemporal dementia misdiagnosed for post-treatment Lyme disease syndrome or vice versa? A Treviso Dementia (TREDEM) registry case report. J Alzheimers Dis 66: , 445–451. |
[17] | Lin X , Xu Y , Zhen Z , Xiao K , Chen X , Yang J , Guan H , Shi Q , Dong X , Wang J , Guo Y ((2022) ) Case report: Genetic Creutzfeldt-Jakob disease with a G114V mutation and one octapeptide repeat deletion as a mimic of frontotemporal dementia. Front Neurol 13: , 888309. |
[18] | Funayama M , Sugihara M , Takata T , Mimura M , Ikeuchi T ((2019) ) Remarkable behavioural signs and progressive non-fluent aphasia in a patient with adult-onset leucoencephalopathy with axonal spheroids and pigmented glia. Psychogeriatrics 19: , 282–285. |
[19] | Rascovsky K , Hodges JR , Knopman D , Mendez MF , Kramer JH , Neuhaus J , van Swieten JC , Seelaar H , Dopper EG , Onyike CU , Hillis AE , Josephs KA , Boeve BF , Kertesz A , Seeley WW , Rankin KP , Johnson JK , Gorno-Tempini ML , Rosen H , Prioleau-Latham CE , Lee A , Kipps CM , Lillo P , Piguet O , Rohrer JD , Rossor MN , Warren JD , Fox NC , Galasko D , Salmon DP , Black SE , Mesulam M , Weintraub S , Dickerson BC , Diehl-Schmid J , Pasquier F , Deramecourt V , Lebert F , Pijnenburg Y , Chow TW , Manes F , Grafman J , Cappa SF , Freedman M , Grossman M , Miller BL ((2011) ) Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134: , 2456–2477. |
[20] | Ha T , Tadi P , Dubensky L ((2023) ) Neurosyphilis. In StatPearls [Internet]. StatPearls Publishing, Treasure Island (FL). |
[21] | Meguro K ((2004) ) Clinical Practice in Dementia. Igakushoin, Tokyo, Japan. |
[22] | Sugishita M , Hemmi I , JADNI ((2010) ) Validity and reliability of the Mini Mental State Examination—Japanese (MMSE–J): A preliminary report. Jap J Cog Neurosci 12: , 186–190. |
[23] | Shinagawa F , Kobayashi S , Fujita K , Maekawa H ((1990) ) Wechsler Adult Intelligence Scale-Revised Japanese version. Nihonbunkakagakusha, Tokyo. |
[24] | Kashima H , Kato M ((1995) ) Wisconsin Card Sorting test (Keio version). Brain Sci Mental Disord 6: , 209–216. |
[25] | Matsuda H , Mizumura S , Nagao T , Ota T , Iizuka T , Nemoto K , Kimura M , Tateno A , Ishiwata A , Kuji I , Arai H , Homma A ((2007) ) An easy Z-score imaging system for discrimination between very early Alzheimer’s disease and controls using brain perfusion SPECT in a multicentre study. Nucl Med Commun 28, 199–205. |
[26] | Lemaire P ((2010) ) Executive functions and strategic aspects of arithmetic performance: The case of adults’ and children’s arithmetic. Psychol Belg 50: , 335–352. |
[27] | Mehrabian S , Raycheva M , Traykova M , Stankova T , Penev L , Grigorova O , Traykov L ((2012) ) Neurosyphilis with dementia and bilateral hippocampal atrophy on brain magnetic resonance imaging. BMC Neurol 12: , 96. |
[28] | Landeiro L , Oliveira R , Graça J , Gouveia R ((2021) ) Traditional neurosyphilis in 21st century - tabes dorsalis, dementia paralytica, aseptic meningitis and unilateral oculomotor nerve palsy in an HIV-negative man. Cureus 13: , e18869. |
[29] | Park J , Kwon KY ((2017) ) Reversible dementia with middle cerebellar peduncle hyperintensity: 1-year follow-up of HIV-negative neurosyphilis. J Clin Neurol 13: , 437–438. |
[30] | Abdool K , Seegobin K , Ramcharan K , Alexander A , Julien-Legen L , Giddings SL , Aboh S , Rampersad F ((2016) ) Neurosyphilis with normal pressure hydrocephalus and dementia paralytica: Serial clinical, laboratory and radiological correlations in the 21st century. Neurol Int 8: , 6812. |
[31] | Rao A , Khan A , Singh K , Anderson DL , Malone ML ((2015) ) Neurosyphilis: An uncommon cause of dementia. J Am Geriatr Soc 63: , 1710–1712. |
[32] | Hagiya H , Deguchi K , Kawada K , Otsuka F ((2015) ) Neurosyphilis is a long-forgotten disease but still a possible etiology for dementia. Intern Med 54: , 2769–2773. |
[33] | Tatar ZB , Cansiz A , Köksal A , Kurt E ((2014) ) A case of neurosyphilis presenting with dementia and psychiatric symptoms. J Neuropsychiatry Clin Neurosci 26: , E39–E40. |
[34] | Stefani A , Riello M , Rossini F , Mariotto S , Fenzi F , Gambina G , Zanusso G , Monaco S ((2013) ) Neurosyphilis manifesting with rapidly progressive dementia: Report of three cases. Neurol Sci 34: , 2027–2030. |
[35] | Thiebaud PC , Banini O , Lambolez T ((2012) ) Neurosyphilis is a treatable cause of dementia not systematically searched for, but still worth to be considered. Rev Neurol (Paris) 168: , 195–196. |




