Exploring the Potential Association Between Self-Reported Psychological Stress and Cerebrospinal Fluid Biomarkers of Alzheimer’s Disease in Midlife: A Cross-Sectional Study
Abstract
Psychological stress is associated with dementia risk. However, the underlying mechanisms are unclear. This cross-sectional study examined the association between self-reported psychological stress and cerebrospinal fluid (CSF) biomarkers of Alzheimer’s disease and neurodegeneration in 73 cognitively unimpaired middle-aged adults from the Healthy Brain Project (mean age = 58±7 years). Linear regression analyses did not reveal any significant associations of psychological stress with CSF amyloid-β42, phosphorylated tau-181, total tau, or neurofilament light chain. Cohen’s f2 effect sizes were small in magnitude (f2≤0.08). Further research is needed to replicate our findings, particularly given that the sample reported on average low levels of stress.
INTRODUCTION
Epidemiological studies suggest that psychological stress, defined as an individual’s subjective experience of stress, is associated with an increased risk of cognitive decline and dementia [1–3]. However, the mechanisms that link stress to dementia are unclear. Clarifying the pathophysiological processes that may underlie this relationship will improve our understanding of disease biology that leads to dementia, such as Alzheimer’s disease (AD), and potentially inform new avenues for dementia prevention.
Mouse models of AD indicate that chronic behavioural stress (e.g., restraint or isolation) is associated with greater amyloid-β (Aβ) and tau in cortical and subcortical regions, suggesting a direct link between stress and AD pathophysiology [4–6]. However, studies seeking to clarify the relationship between stress and AD biomarkers in humans remain equivocal.
Two longitudinal autopsy studies found no association between the personality trait of neuroticism (i.e., vulnerability to stress) and the number of Aβ plaques and tau neurofibrillary tangles at postmortem examination [7, 8]. In contrast, others have shown that higher neuroticism levels were associated with more advanced spread of neurofibrillary tangles at postmortem [9]. However, the use of neuroticism as a proxy of stress does not directly capture one’s appraisal or symptoms of stress.
In a sample of middle-aged women, greater levels of self-reported psychological stress were associated with higher cerebrospinal fluid (CSF) levels of total tau (t-tau) and Aβ40 in late-life, but not with more specific AD biomarkers (i.e., Aβ42 or phosphorylated tau [p-tau]) [10]. Also in women, greater midlife stress was associated with general biomarkers of neurodegeneration, indicated by significantly higher CSF levels of visinin-like protein-1 and myelin basic protein, with a trend towards higher levels of neurofilament light chain (NfL) [11]. Together, these findings suggest that stress may be associated with more general markers of neuronal injury as compared to AD-specific biomarkers [11].
Given limited studies have investigated the association between self-reported psychological stress and in vivo AD biomarkers in women, it is important to examine this relationship further, including in a more diverse sample (e.g., both sexes). Further, to inform future interventions, it is particularly important to examine these relationships in midlife, where risk factors can be modified before the clinical onset of symptoms. Consequently, we aimed to examine the cross-sectional relationship between self-reported psychological stress and CSF levels of Aβ42, t-tau, p-tau181, and NfL in cognitively unimpaired middle-aged adults. We hypothesised that greater stress levels would be associated with lower CSF Aβ42 and higher CSF p-tau181, t-tau, and NfL.
METHODS
Participants
The Healthy Brain Project (HBP; healthybrainproject.org.au) is a prospective web-based study of adults aged 40–70 years at study entry [12]. The current study draws from a subsample of HBP participants who attended an in-person assessment at the Royal Melbourne Hospital (Victoria, Australia). Apolipoprotein E (APOE) ɛ4 carriers in the larger web-based sample were preferentially invited to the in-person assessment, to enrich the sample with individuals at genetic risk for AD. As a result of this targeted recruitment, the in-person subsample had a high proportion of APOE ɛ4 carriers (41%) and a family history of dementia (80%). Inclusion criteria included being willing and able to travel to the hospital for the assessment and having no known contraindications for neuroimaging and lumbar puncture procedures. Participants with a self-reported history of cognitive impairment were excluded. In total, 82 participants completed the in-person assessment, from which CSF was obtained from 77 participants. Of those with available CSF, four were missing APOE genotype data. Seventy-two completed the Perceived Stress Scale (PSS) as part of the larger web-based HBP study, and 65 completed the Depression, Anxiety, and Stress Scale (DASS) as part of their in-person visit.
All participants provided written informed consent and study approval was obtained from the Melbourne Health Human Research Ethics Committee. Data collection took place from November 2018 to February 2020.
Measures
Psychological stress
The DASS and PSS self-report questionnaires were used to capture different stress characteristics. These questionnaires were selected as they measure both subjective symptoms of stress [13] and levels of perceived stress [14].
Participants completed the 42-item DASS as part of their in-person visit (i.e., the same day as CSF collection). Scores for each subscale (i.e., Depression, Anxiety, and Stress) were totalled separately. Stress was measured using the DASS-Stress subscale, with higher scores indicating higher levels of stress (range 0–42). Of those who completed the DASS, one participant was excluded from the analysis because of missing responses.
Participants completed the 10-item PSS to measure self-reported perceived stress as part of the web-based HBP study [14]. The outcome was the total score, with higher scores indicating greater levels of perceived stress (range 0–40). The mean time interval between completion of the PSS and CSF sampling was 2.07±0.36 years.
CSF biomarkers
CSF samples were obtained by lumbar puncture in the L3/L4 or L4/L5 intervertebral space and transferred on wet ice for processing. CSF samples were centrifuged at 2000xg for 10 min at 4°C. The supernatant was then transferred to a new polypropylene tube and gently inverted several times to avoid possible gradient effects. Samples were stored in 0.5 mL aliquots at –80°C pending analysis. CSF levels of Aβ42, t-tau, and p-tau181 were measured by immunoassay (Roche Elecsys®) and CSF levels of NfL were measured by ELISA (UmanDiagnostics, Umeå, Sweden). All analyses were conducted at the National Dementia Diagnostics Laboratory (The Florey Institute of Neuroscience and Mental Health, University of Melbourne, Australia). Twenty-five participants (34%) had Aβ42 levels above the maximum detection limit and were reported as > 1,700 pg/mL.
Participant characteristics
Demographic information (including age, sex, education, and race) was measured through self-report questionnaires. Race was dichotomised into White and non-White (African, Asian, Latin American, Indigenous Australian, Pacific Islander, or not specified) groups, given that some categories were not represented, and others comprised less than 1.5% of the sample. Participants provided a saliva sample to enable APOE genotyping via TaqMan assays (Life Technologies).
Statistical analysis
The associations between self-reported stress and AD biomarkers were estimated using separate multiple linear regression analyses for each biomarker (i.e., Aβ42, p-tau181, t-tau, and NfL levels). All models were adjusted for age, sex, education, race, and APOE ɛ4 carriage. Models using the PSS total score as the predictor were also adjusted for the time interval between the completion of the web-based PSS and the in-person assessment. Reported coefficients are unstandardised. Statistical significance was set at p < 0.05. Cohen’s f2 was calculated as an effect size for individual predictors, with values of 0.02, 0.15, and 0.35 considered as small, medium, and large effects, respectively [15]. All analyses were performed using R version 4.1.0 [16].
Sensitivity analysis
As one-third of the sample had Aβ42 levels above the maximum limit of detection, Tobit regression models were used to examine the associations between self-reported stress and CSF Aβ42, as these models are more suitable for outcome variables subject to censoring. Regression coefficients for these models are interpreted on the latent (uncensored) Aβ42 scale, rather than the observed (censored) Aβ42 scale.
RESULTS
Table 1 summarises the demographic and clinical characteristics of the sample. On average, participants reported low levels of stress. Using published cut-off scores [13], 92% of the sample had stress levels that fell in the ‘normal’ range. Scores on the PSS were moderately correlated with scores on the DASS-Stress subscale (r = 0.50, p < 0.001).
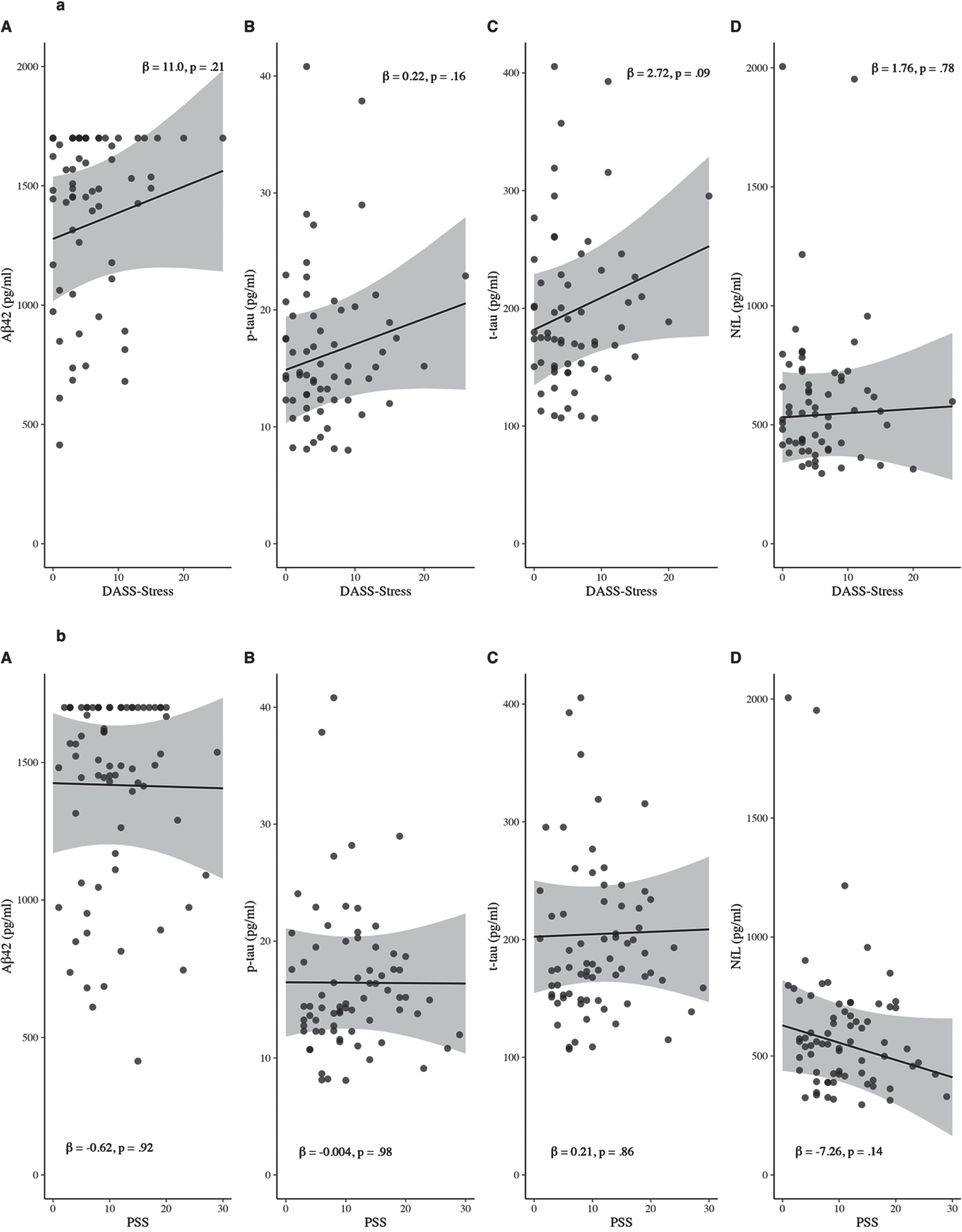
In separate adjusted linear regression models, there was no evidence for an association of self-reported psychological stress as measured by the DASS-Stress subscale with Aβ42 (β= 10.96, 95% confidence interval [95% CI] = –6.44–28.35, p = 0.21), p-tau (β= 0.22, 95% CI = –0.09–0.52, p = 0.16), t-tau (β= 2.72, 95% CI = –0.42–5.87, p = 0.09), or NfL (β= 1.76, 95% CI = –10.98–14.50, p = 0.78) (Fig. 1a). All associations were weak in magnitude (Cohen’s f2≤0.04).
There was also no significant association between self-reported stress as measured by the PSS and Aβ42 (β= –0.62, 95% CI = –13.40–12.16, p = 0.92), p-tau (β= –0.004, 95% CI = –0.24–0.23, p = 0.98), t-tau (β= 0.21, 95% CI = –2.19–2.61, p = 0.86), or NfL (β= –7.26, 95% CI = –16.84–2.33, p = 0.14) (Fig. 1b). All associations were small in magnitude (Cohen’s f2≤0.08).
Table 1
Participant characteristics
| Participant characteristics | N | M±SD |
| Age, y | 73 | 58.2±6.9 [43–68] |
| Female, n (%) | 73 | 49 (67.1%) |
| Education, y | 73 | 16.3±3.6 [9–24] |
| White, n (%) | 73 | 60 (82.2%) |
| Family history of dementia | 73 | 58 (79.5%) |
| APOE ɛ4 carrier, n (%) | 73 | 30 (41.1%) |
| MMSE total score | 73 | 28.8±1.2 [26–30] |
| PSS score | 72 | 10.9±6.4 [1–29] |
| DASS score | ||
| Depression | 62 | 2.6±3.5 [0–14] |
| Anxiety | 65 | 1.7±1.9 [0–8] |
| Stress | 64 | 6.0±5.3 [0–26] |
| Aβ42, pg/ml | 73 | 1405.2±344.4 [413.9–1700.0] |
| p-tau181, pg/ml | 73 | 16.2±6.1 [8.0–40.8] |
| t-tau, pg/ml | 73 | 194.2±63.7 [106.6 –405.4] |
| NfL, pg/mL | 73 | 596.4±292.3 [295.3 –2005.5] |
Aβ, amyloid-beta; APOE, apolipoprotein E; DASS, Depression, Anxiety, and Stress Scale; MMSE, Mini-Mental State Examination; NfL, neurofilament light chain; PSS, Perceived Stress Scale; p-tau, phosphorylated tau; t-tau, total tau.
Sensitivity analysis
Tobit regression models did not substantially change the pattern of results. No significant associations were found between stress and levels of latent (uncensored) CSF Aβ42 using either the DASS-Stress (β= 21.99, 95% CI = –2.38–46.35, p = 0.08) or the PSS (β= –0.73, 95% CI = –17.84–16.37, p = 0.93).
DISCUSSION
We examined the cross-sectional relationship between self-reported psychological stress and CSF AD biomarkers in cognitively unimpaired middle-aged adults, which few studies have previously investigated. The hypothesis that greater stress levels will be associated with lower CSF Aβ42 and higher CSF p-tau181, t-tau, and NfL was not supported. Associations between psychological stress and biomarkers of AD and neurodegeneration were non-significant and small in magnitude. Therefore, despite previous observations that psychological stress is associated with an increased risk of dementia [1, 3], there was no evidence of an association between psychological stress and CSF AD biomarkers in our midlife sample.
The absence of a relationship between psychological stress and AD biomarkers has been observed previously [10]. In 79 middle-aged women (mean age = 49), midlife stress was not associated with CSF Aβ42 or p-tau in late-life. However, midlife stress was significantly associated with higher CSF t-tau levels 25 years later [10]. In the current sample of men and women, the lack of association between stress and t-tau may be because we measured AD biomarkers cross-sectionally in midlife. Levels of t-tau are considered biomarkers of general neurodegeneration, with changes shown to occur closer to dementia onset than changes in Aβ [17]. As such, psychological stress may have weaker associations with biomarkers of neurodegeneration when measured in midlife. Similarly, we did not observe a significant association between stress and NfL levels. Future studies should measure CSF biomarkers longitudinally from midlife to determine if stress relates to the progression of AD and neurodegeneration over time.
The differing findings may also relate to differences in stress measurement. Compared to our study, Johansson et al. (2018) measured stress over a longer period, which may have yielded a more stable estimate of stress. The idea that chronic stress, relative to acute stress, might be more strongly related to AD biomarkers is supported by recent research reporting that biological markers reflective of chronic stress (i.e., allostatic load) are associated with lower levels of CSF Aβ42 in dementia-free adults [18]. However, despite neuroticism providing a relatively stable and chronic estimate of stress reactivity [7], the relationship between neuroticism and AD pathology remains equivocal [7–9]. This may be owing to the complex nature of psychological stress, which likely involves an interaction between genetic, environmental, and psychological factors [19].
Fig. 1
Scatterplots of the associations of psychological stress as measured by DASS-Stress (a) and PSS (b) with CSF (A) Aβ42, (B) p-tau181, (C) t-tau, and (D) NfL. Predicted values were computed at the mean for continuous covariates and at the mode for categorical covariates. Beta-coefficients are unstandardised. Aβ, amyloid-beta; DASS, Depression, Anxiety, and Stress Scale; NfL, neurofilament light chain; p-tau, phosphorylated tau; PSS, Perceived Stress Scale; t-tau, total tau.
The observation in the current study that stress was not associated with CSF Aβ42 is consistent with previous research [10]. While stress may not directly influence Aβ42 levels, stress may increase dementia risk through other non-AD neurodegenerative pathways. For example, stress or poor coping strategies in response to stress (i.e., smoking, overeating, or physical inactivity), might increase the risk of vascular cognitive impairment and dementia, independent of AD. Indeed, midlife chronic stress has been associated with a greater burden of white matter lesions in late-life [20]. Vascular mechanisms may also partially explain the aforementioned association between allostatic load and Aβ42, given the measurement of allostatic load incorporates multiple indicators of vascular health (e.g., blood pressure, cholesterol levels, and triglycerides)[18].
There are several limitations to our study. Given the relatively small sample size and the health of the sample, stress scores had a restricted range, with most participants endorsing low levels of stress. Consequently, the analysis may have lacked sufficient power to detect any relationship between more severe levels of stress and AD pathology. It will be important to replicate this study in a sample with greater variation in self-reported stress levels. Further, the cross-sectional nature of this study prevented the investigation of whether psychological stress increases the likelihood of developing AD pathology in late-life.
Stress is a common experience in midlife, with middle-aged adults facing numerous competing demands (e.g., financial, health, relationships, workplace, and caregiving). This study found no relationships between stress and CSF biomarkers of AD and neurodegeneration. However, further research is needed to determine if these associations change when individuals are followed over longer periods. It will also be important to explore whether psychological stress is linked to incident dementia through non-AD pathways (e.g., cerebral small vessel disease).
ACKNOWLEDGMENTS
We thank the Healthy Brain Project study group (healthybrainproject.org.au) and all our participants for their commitment to combating dementia and Alzheimer’s disease.
FUNDING
The Healthy Brain Project (healthybrainproject.org.au) is funded by the National Health and Medical Research Council (GNT1158384, GNT1147465, GNT1111603, GNT1105576, GNT1104273, GNT1158384, and GNT1171816), the National Institute of Health (NIH-PA-13-304), the Alzheimer’s Association (AARG-17-591424, AARG-18-591358, AARG-19-643133), the Dementia Australia Research Foundation, the Bethlehem Griffiths Research Foundation, the Yulgilbar Alzheimer’s Research Program, the National Heart Foundation of Australia (102052), and the Charleston Conference for Alzheimer’s Disease. We thank our study partners (PearlArc, SRC Innovations, Cogstate Ltd and Cambridge Cognition) for their ongoing support.
LB is supported by a Dementia Australia Research Foundation PhD scholarship. RB is supported by a National Institutes of Health K99-R00 award (K99AG061238) and an Alzheimer’s Association Research Fellowship (AARF-20-675646). TC is supported by the Australian Research Council (FT220100294). YYL is supported by a National Health and Medical Research Council of Australia Career Development Fellowship (GNT1162645) and a National Health and Medical Research Council of Australia Emerging Leadership 2 Investigator Grant (GNT2009550). MP is supported by a National Health and Medical Research Council of Australia Emerging Leadership 2 Investigator Grant (GTN2009264).
CONFLICT OF INTEREST
TC reports personal fees/honoraria for lectures from Roche. KF, LC, LB, RB, NY, YYL, and MP have no known conflicts of interest to disclose.
DATA AVAILABILITY
The data supporting the findings of this study are available on reasonable request. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
[1] | Franks KH , Bransby L , Saling MM , Pase MP ((2021) ) Association of stress with risk of dementia and mild cognitive impairment: A systematic review and meta-analysis. J Alzheimers Dis 82: , 1573–1590. |
[2] | Franks KH , Rowsthorn E , Bransby L , Lim YY , Chong TTJ , Pase MP ((2022) ) Association of self-reported psychological stress with cognitive decline: A systematic review. Neuropsychol Revdoi: 10.1007/s11065-022-09567-y. |
[3] | Stuart KE , Padgett C ((2020) ) A systematic review of the association between psychological stress and dementia risk in humans. J Alzheimers Dis 78: , 335–352. |
[4] | Dong H , Goico B , Martin M , Csernansky CA , Bertchume A , Csernansky JG ((2004) ) Modulation of hippocampal cell proliferation, memory, and amyloid plaque deposition in APPsw (Tg2576) mutant mice by isolation stress. Neuroscience 127: , 601–609. |
[5] | Jeong YH , Park CH , Yoo J , Shin KY , Ahn SM , Kim HS , Lee SH , Emson PC , Suh YH ((2006) ) Chronic stress accelerates learning and memory impairments and increases amyloid deposition in APPV717I-CT100 transgenic mice, an Alzheimer’s disease model. FASEB J 20: , 729–731. |
[6] | Lee KW , Kim JB , Seo JS , Kim TK , Im JY , Baek IS , Kim KS , Lee JK , Han PL ((2009) ) Behavioral stress accelerates plaque pathogenesis in the brain of Tg2576 mice via generation of metabolic oxidative stress. J Neurochem 108: , 165–175. |
[7] | Wilson RS , Arnold SE , Schneider JA , Kelly JF , Tang Y , Bennett DA ((2006) ) Chronic psychological distress and risk of Alzheimer’s disease in old age. Neuroepidemiology 27: , 143–153. |
[8] | Wilson RS , Evans DA , Bienias JL , de Leon CFM , Schneider JA , Bennett DA ((2003) ) Proneness to psychological distress is associated with risk of Alzheimer’s disease. Neurology 61: , 1479–1485. |
[9] | Terracciano A , Iacono D , O’Brien RJ , Troncoso JC , An Y , Sutin AR , Ferrucci L , Zonderman AB , Resnick SM ((2013) ) Personality and resilience to Alzheimer’s disease neuropathology: A prospective autopsy study. Neurobiol Aging 34: , 1045–1050. |
[10] | Johansson L , Kern S , Zetterberg H , Blennow K , Börjesson-Hansson A , Rosengren L , Guo X , Skoog I ((2018) ) Midlife stress in relation to late-life cerebrospinal fluid biomarkers of Alzheimer’s disease: A 25-year follow-up study. Dement Geriatr Cogn Disord 46: , 90–99. |
[11] | Johansson L , Sacuiu S , Kern S , Guo X , Zetterberg H , Blennow K , Zettergren A , Skoog I ((2019) ) Longstanding psychological stress in relation to biomarkers of neuronal dysfunction in cerebrospinal fluid: A 25-year follow-up study in women. Neurobiol Aging 80: , 111–115. |
[12] | Lim YY , Yassi N , Bransby L , Properzi M , Buckley R ((2019) ) The Healthy Brain Project: An online platform for the recruitment, assessment, and monitoring of middle-aged adults at risk of developing Alzheimer’s disease. J Alzheimers Dis 68: , 1211–1228. |
[13] | Lovibond SH , Lovibond PF ((1995) ) Manual for the Depression Anxiety Stress Scales, Psychology Foundation of Australia, Sydney, Australia. |
[14] | Cohen S , Kamarck T , Mermelstein R ((1983) ) A global measure of perceived stress. J Health Soc Behav 24: , 385–396. |
[15] | Cohen J ((1977) ) NY. Statistical power analysis for the behavioral sciences, Academic Press, New York. |
[16] | Core Team R ((2019) ) R Foundation for Statistical Computing, Vienna, Austria. |
[17] | Jack CR Jr , Bennett DA , Blennow K , Carrillo MC , Dunn B , Haeberlein SB , Holtzman DM , Jagust W , Jessen F , Karlawish J , Liu E , Molinuevo JL , Montine T , Phelps C , Rankin KP , Rowe CC , Scheltens P , Siemers E , Snyder HM , Sperling R ((2018) ) NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14: , 535–562. |
[18] | Adedeji DO , Holleman J , Juster R-P , Udeh-Momoh CT , Kåreholt I , Hagman G , Aspö M , Adagunodo S , Håkansson K , Kivipelto M , Solomon A , Sindi S ((2023) ) Longitudinal study of Alzheimer’s disease biomarkers, allostatic load, and cognition among memory clinic patients. Brain Behav Immun Health 28: , 100592. |
[19] | Epel ES , Crosswell AD , Mayer SE , Prather AA , Slavich GM , Puterman E , Mendes WB ((2018) ) More than a feeling: A unified view of stress measurement for population science. Front Neuroendocrinol 49: , 146–169. |
[20] | Johansson L , Skoog I , Gustafson DR , Olesen PJ , Waern M , Bengtsson C , Björkelund C , Pantoni L , Simoni M , Lissner L , Guo X ((2012) ) Midlife psychological distress associated with late-life brain atrophy and white matter lesions: A 32-year population study of women. Psychosom Med 74: , 120–125. |




