Cognitive Trajectories in Preclinical and Prodromal Alzheimer’s Disease Related to Amyloid Status and Brain Atrophy: A Bayesian Approach
Abstract
Background:
Cognitive decline is a key outcome of clinical studies in Alzheimer’s disease (AD).
Objective:
To determine effects of global amyloid load as well as hippocampus and basal forebrain volumes on longitudinal rates and practice effects from repeated testing of domain specific cognitive change in the AD spectrum, considering non-linear effects and heterogeneity across cohorts.
Methods:
We included 1,514 cases from three cohorts, ADNI, AIBL, and DELCODE, spanning the range from cognitively normal people to people with subjective cognitive decline and mild cognitive impairment (MCI). We used generalized Bayesian mixed effects analysis of linear and polynomial models of amyloid and volume effects in time. Robustness of effects across cohorts was determined using Bayesian random effects meta-analysis.
Results:
We found a consistent effect of amyloid and hippocampus volume, but not of basal forebrain volume, on rates of memory change across the three cohorts in the meta-analysis. Effects for amyloid and volumetric markers on executive function were more heterogeneous. We found practice effects in memory and executive performance in amyloid negative cognitively normal controls and MCI cases, but only to a smaller degree in amyloid positive controls and not at all in amyloid positive MCI cases.
Conclusions:
We found heterogeneity between cohorts, particularly in effects on executive functions. Initial increases in cognitive performance in amyloid negative, but not in amyloid positive MCI cases and controls may reflect practice effects from repeated testing that are lost with higher levels of cerebral amyloid.
INTRODUCTION
Rates of cognitive decline are a key outcome for longitudinal follow-up and monitoring the effect of interventions in Alzheimer’s disease (AD). In addition, cognitive decline serves as a reference for assessing the predictive accuracy of biomarkers such as pathological accumulation of amyloid or brain atrophy. In addition to linear trajectories, loss of initial increases in performance representing practice effects from repeated testing may be a sensitive indicator of early pathology [1–4]. Thus, cognitive decline does not necessarily follow a linear course, and the effects of external predictors or effectors of change may also be nonlinear. Therefore, allowing for non-linearity both in trajectories of cognitive decline and in effects of external variables may increase the ability to uncover biologically meaningful effects, including practice effects.
Bayesian approaches have become increasingly popular for modeling change in imaging biomarkers of AD [5], and have been applied to study trajectories of atrophy [6], or functional connectivity [7] over time. Bayesian analysis has major advantages over classical frequentist analysis [8]. It allows explicit modelling and checking a priori assumptions on the shape of cognitive trajectories and predictive parameters. This is particularly interesting if one uses several cohorts so that the shape parameters from one cohort can be used as a prior for another cohort. In addition, Bayesian analysis allows for a direct evaluation of the evidence in favor and against competing hypotheses [9, 10].
In the current study, we used Bayesian linear and nonlinear joint mixed effect models with selected distribution functions to determine the association of key pathological markers of AD with longitudinal rates of cognitive decline as well as practice effects in cognitively healthy people and people with mild cognitive impairment (MCI) or subjective cognitive decline (SCD). Specifically, we tested the association of amyloid load as potential upstream event of AD pathology [11–13], as well as atrophy of cholinergic basal forebrain [1] and hippocampus as potential downstream markers of neurodegeneration [14] with rates of memory and executive function change. To allow testing the generalizability of findings assessments included three independent samples, one from the ADNI cohort [15], one from the AIBL cohort [16] and one from the DELCODE cohort [17], with a total of 1,514 participants.
We tested the following two sets of hypotheses:
First, we expected that amyloid positivity as well as basal forebrain atrophy would be associated with less favorable cognitive performance over time in executive [18, 19] and memory function [1, 20], whereas hippocampus atrophy was expected to be predominantly associated with memory function decline [21, 22]. We used Bayesian random effects meta-analysis to determine replicability and heterogeneity of results across cohorts [23].
Secondly, based on previous assessments of practice effects [1–4], we expected that cognitively normal individuals would show benefits from repeated testing, which could be captured by polynomial rather than linear models of change. We also expected that this effect would be attenuated in the presence of amyloid positivity [24] and brain volume reductions. Considering these effects eventually will help increasing the power of studies monitoring effects of disease or interventions on cognitive decline.
MATERIAL AND METHODS
Data sources
Part of the data was obtained from the ADNI-1, ADNI-GO, and ADNI-2 cohorts from the ADNI database (http://adni.loni.usc.edu/). The ADNI was launched in 2003 by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, the Food and Drug Administration, private pharmaceutical companies and non-profit organizations, with the primary goal of testing whether neuroimaging, neuropsychological, and other biologic measurements can be used as reliable in-vivo markers of AD pathogenesis [15]. A comprehensive description of ADNI and up-to-date information is available at HTTP://www.adni-info.org. All procedures performed in the ADNI studies involving human participants were in accordance with the ethical standards of the institutional research committees and with the 1964 Helsinki declaration and its later amendments. Written informed consent was obtained from all participants and/or authorized representatives and the study partners before any protocol-specific procedures were carried out in the ADNI studies.
Another part of the data was obtained from the Australian Imaging, Biomarker & Lifestyle Flagship Study of Ageing (AIBL) [16]. This study was launched in 2006. Data were obtained from the AIBL LONI website (https://ida.loni.usc.edu/login.jsp?project = AIBL&page = HOME). Written informed consent was obtained from all participants and/or authorized representatives and the study partners before any protocol-specific procedures were carried out in the AIBL study.
Finally, another part of the data was obtained from the DZNE-Longitudinal Cognitive Impairment and Dementia Study (DELCODE) study, conducted by the Deutsches Zentrum für Neurodegenerative Erkrankungen (DZNE) [17]. DELCODE is an ongoing German multicenter observational study initiated in 2014 on predementia AD that aims to characterize early disease stages, in particular SCD, improve upon prognostics of disease progression and identify new markers for preclinical AD (https://www.dzne.de/forschung/studien/klinische-studien/delcode/). All participants or their representatives provided written informed consent. The study protocol was approved by the local institutional review boards and ethical committees of the participating centers. It was conducted in accord with the Helsinki Declaration of 1975 and its later amendments.
Participants
From the ADNI cohort we identified cognitively normal subjects and participants with MCI with amyloid PET and MRI scans available at baseline and at least one cognitive follow-up. Detailed inclusion criteria for diagnostic categories can be found at the ADNI web site (http://adni.loni.usc.edu/methods/). Cognitively normal subjects had MMSE scores between 24–30 (inclusive), a CDR = 0, were non-depressed, non-MCI, and non-demented, and reported no subjective memory concerns. MCI subjects had MMSE scores between 24–30 (inclusive), a subjective memory concern reported by subject, informant, or clinician, objective memory loss measured by education and age adjusted scores on delayed recall, a CDR = 0.5, absence of significant levels of impairment in other cognitive domains, essentially preserved activities of daily living, and an absence of dementia. Starting with ADNI-GO, MCI cases were further divided into late and early stages of MCI based on the WMS-R Logical Memory II Story A score. Here, we combined both subtypes into a single category of MCI to be consistent with ADNI-1 cases. ADNI participants are followed-up yearly using clinical, neuropsychological, and imaging assessment.
For replication purposes we used baseline and longitudinal data from AIBL and DELCODE. From AIBL we identified cognitively normal participants and MCI cases with MRI and amyloid PET scans available at baseline and at least one cognitive follow-up. Different to ADNI and DELCODE, AIBL MCI participants fulfilled criteria by Winblad et al. [25] and Petersen et al. [26], including not only amnestic, but also non-amnestic MCI cases. Major exclusion criteria for AIBL were age < 60 years, a history of non-AD dementia, schizophrenia, bipolar disorder, current depression with a short Geriatric Depression Scale score above 5/15 (for cognitively normal individuals), Parkinson’s disease, symptomatic stroke, current uncontrolled or life threatening medical illness, diagnosed obstructive sleep apnea, past head injury with over one hour of post-traumatic amnesia, or alcohol use above two standard drinks per day for women or four per day for men. AIBL participants are followed-up every 18 months using clinical, neuropsychological and imaging assessment.
From DELCODE we derived two samples. One sample with MRI scans available at baseline and at least one cognitive follow-up and one subsample with cerebrospinal fluid (CSF) available at baseline and at least one cognitive follow-up. Both samples consisted of older healthy controls, cognitively normal first degree relatives of patients with dementia, and participants with amnestic MCI or subjective cognitive decline (SCD). Participants with AD dementia from DELCODE were excluded from our analyses. DELCODE excluded people with a current major depressive episode, past or present major psychiatric disorders, neurological diseases other than AD, or unstable medical conditions [17]. SCD was defined as a persistent self-perceived cognitive decline in the absence of objective cognitive impairment as measured by the CERAD test battery, lasting at least for 6 months and being unrelated to an acute event [27]. The MCI patients met the core clinical criteria for MCI according to National Institute on Aging-Alzheimer’s Association (NIA-AA) workgroup guidelines [28]. The control participants had no objective cognitive impairment in cognitive tests, no history of neurological or psychiatric disease and did not report self-perceived cognitive decline. Relatives were defined as cognitively normal people with at least one confirmed AD case in their direct kinship. DELCODE participants are followed-up yearly using clinical, neuropsychological, and imaging assessment.
The sample size was not based on a priori power calculation but used data available from the three cohorts fulfilling the inclusion criteria.
Neuropsychological assessment
For the ADNI cohort, we used two previously defined composite measures for the investigation of memory (ADNI memory score) [29] and executive functions (ADNI executive score) [30].
For the AIBL cohort, we used the delayed recall of logical memory of the Wechsler Memory Scale-Third edition [31]. We had no access to a measure of executive function in the AIBL data repository made available to us.
For the DELCODE cohort we used the delayed recall of logical memory of the Wechsler Memory Scale-Revised as a measure of memory function, and the Wechsler Memory Scale-Revised digit span (average of digit span forward and backward) as measure of executive function. In a sensitivity analysis, we used the ratio of the trail making test B to A (TMTB/A) as measure of executive function [32].
Amyloid PET data acquisition
For the ADNI data, amyloid positivity was determined using amyloid-sensitive 18F-florbetapir PET scans. Detailed acquisition and standardized pre-processing steps of ADNI imaging data are available at the ADNI website (https://adni.loni.usc.edu/methods/). Amyloid-PET data was collected during a 50- to 70-min interval following a 370 MBq bolus injection of 18F-Florbetapir. To account for the multicentric acquisition of the data across different scanners and sites, all PET scans undergo standardized pre-processing steps within ADNI. For anatomical reference and pre-processing of the PET scans we used the corresponding structural MRI scan that was closest in time to the Florbetapir PET scan.
For the AIBL sample, each participant had an amyloid sensitive PET scan from one of the following three tracers: 11C-Pittsburgh compound-B (PIB), 18F-florbetapir, and 18F-flutemetamol. Amyloid-PET scans were collected as three to six frames of 5 min starting 40, 50, or 90 min post injection of the respective tracer. Detailed acquisition information is available at https://aibl.csiro.au/adni/imaging.html. The frames were averaged and matched to the corresponding structural MRI scan for further processing.
MRI data acquisition
In the ADNI sample MRI data were acquired on multiple 3T MRI scanners using scanner-specific T1-weighted sagittal 3D MPRAGE sequences. Similar to the PET data, MRI scans undergo standardized preprocessing steps aimed at increasing data uniformity across the multicenter scanner platforms (see https://adni.loni.usc.edu/methods/ for detailed information on multicentric MRI acquisition and preprocessing in ADNI).
In the AIBL sample, MRI data were acquired at two sites equipped with Siemens 3T MRI scanners using ADNI-compliant T1-weighted sagittal MPRAGE sequences. See https://aibl.csiro.au/adni/imaging.html for details.
For DELCODE the MRI data were acquired from nine Siemens 3.0 Tesla MRI scanners (4 Verio, 1 Skyra, 3 TimTrio and 1 Prisma system) using identical acquisition parameters and harmonized instructions as previously described [33]. To ensure high image quality throughout the acquisition phase, all scans had to pass a semi-automated quality check during the study conduction, so that protocol deviations could be reported to the study sites, and the acquisition at the respective site could be adjusted. High-resolution T1-weighted anatomical images were obtained using a sagittal magnetization-prepared rapid gradient echo (MPRAGE) sequence (field of view 256×256 mm, matrix size 256×256, isotropic voxel size 1 mm, echo time 4.37 ms, flip angle 7°, repetition time 2500 ms, number of slices 192, parallel imaging acceleration factor 2).
Amyloid PET data pre-processing
Images were preprocessed using Statistical Parametric Mapping software version 8 (SPM8) (The Wellcome Trust Centre for Neuroimaging, Institute of Neurology, University College London) implemented in Matlab 2013. The pre-processing pipeline followed the routine previously described in [34]. First, each subject’s averaged PET frames were co-registered to their corresponding T1-weighted MRI scan. The coregistered PET images were spatially normalized to an aging/AD-specific reference template using the deformation parameters derived from the normalization of their corresponding MRI.
The regional 18F-Florbetapir-PET mean uptake values were estimated for 52 brain regions defined by the Harvard–Oxford structural atlas [35], including both cortical and subcortical regions (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Atlases). Standard uptake value ratios (SUVRCer) were computed for the 52 brain regions by dividing the mean uptake values by the mean uptake value of the whole cerebellum [34, 36–38].
For ADNI data, we used the widely used global signal cutoffs for 18F-Florbetapir-PET SUVRs of SUVRCer = 1.17 [39, 40].
For the AIBL amyloid scans, we followed the Centiloid SPM pre-processing pipeline [37], which established procedures to harmonize measures obtained from multi-tracer PET studies. First, all amyloid scans were coregistered to their corresponding T1-weighted MRI scans and spatially normalized to the MNI reference space as described above. Then, the tissue masks provided at https://www.gaain.org/centiloid-project were used to calculate the standard uptake value ratios (SUVRCL). SUVRCL measures were converted to Centiloid values as recommended for the different amyloid PET traces [37, 41, 42]. Finally, amyloid status of the participants was determined using the cutoff 24.1 CL [41].
Table 1
Demographic characteristics
| ADNI (N = 548) | N(f/m)1 | Age [y]2 mean (95% CI) | MMSE3 mean (95% CI) | Education [y]4 mean (95% CI) | Follow-up [y]5 mean (95% CI) |
| Controls | 87/205 | 73.6 (72.7–74.6) | 29.1 (28.9–29.2) | 16.6 (16.3–17.0) | 4.4 (4.2–4.7) |
| MCI | 85/171 | 71.9 (71.1–72.6) | 28.1 (27.9–28.3) | 16.1 (15.8–16.4) | 4.0 (3.8–4.1) |
| AIBL (N = 265) | N(f/m)6 | Age [y]7 mean (95% CI) | MMSE8 mean (95% CI) | Education [y]9 mean (95% CI) | Follow-up [y]10 mean (95% CI) |
| Controls | 131/100 | 72.4 (71.6–73.2) | 28.9 (28.7–29.0) | – | 2.0 (1.8–2.1) |
| MCI | 14/20 | 74.2 (72.0–76.5) | 27.0 (26.2–27.7) | – | 2.7 (2.3–3.2) |
| DELCODE (N = 416) amyloid | N(f/m)11 | Age [y]12 mean (95% CI) | MMSE13 mean (95% CI) | Education [y]14 mean (95% CI) | Follow-up [y]15 mean (95% CI) |
| Controls | 67/58 | 68.3 (67.4–69.2) | 29.4 (29.3–29.6) | 14.5 (14.0–14.9) | 2.9 (2.6–3.1) |
| SCD | 85/111 | 71.4 (70.6–72.2 | 29.2 (29.0–29.3) | 14.8 (14.4–15.2) | 2.6 (2.4–2.8) |
| MCI | 41/54 | 72.4 (71.3–73.5) | 27.0 (26.5–27.5) | 13.9 (13.3–14.5) | 2.6 (2.3–2.9) |
| DELCODE (N = 701) volumes | N(f/m)16 | Age [y]17 mean (95% CI) | MMSE18 mean (95% CI) | Education [y]19 mean (95% CI) | Follow-up [y]20 mean (95% CI) |
| Controls | 148/108 | 68.7 (68.1–69.4) | 29.5 (29.4–29.6) | 14.7 (14.3–15.0) | 3.1 (2.9–3.2) |
| SCD | 160/172 | 71.3 (70.6–71.9) | 29.2 (29.1–29.3) | 14.7 (14.4–15.1) | 2.4 (2.3–2.6) |
| MCI | 49/64 | 73.3 (72.2–74.3) | 28.0 (27.7–28.4) | 14.2 (13.7–14.8) | 2.2 (2.0–2.5) |
1Bayes factor in favor of no group effect (BF10 = 0.143); i.e., a group effect is 0.143 times less likely than the absence of such an effect. 2Bayes factor moderately in favor of a group effect (BF10 = 3.291). 3Bayes factor extremely in favor of a group effect (BF10 = 2.4 * 108). 4Bayes factor shows no conclusive evidence of a group effect (BF10 = 0.90). 5Bayes factor moderately in favor of a group effect (BF10 = 7.6). 6Bayes factor in favor of the absence of a group effect (BF10 = 0.26). 7Bayes factor in favor of a group effect (BF10 = 8.6). 8Bayes factor in favor of a group effect (BF10 = 2.6*1056). 9Data on education years were not available through the AIBL LONI website. 10Bayes factor strongly in favor of a group effect (BF10 = 21.2). 11Bayes factor in favor of the absence of a group effect (BF10 = 0.14). 12Bayes factor extremely in favor of a group effect (BF10 = 5.73*106). 13Bayes factor extremely in favor of a group effect (BF10 = 1.6*1028). 14Bayes factor in favor of the absence of a group effect (BF10 = 0.72). 15Bayes factor in favor of the absence of a group effect (BF10 = 0.37). 16Bayes factor shows no conclusive evidence of a group effect (BF10 = 0.80). 17Bayes factor extremely in favor of a group effect (BF10 = 3.9 * 109). 18Bayes factor extremely in favor of a group effect (BF10 = 1.5 * 1023). 19Bayes factor in favor of the absence of a group effect (BF10 = 0.07). 20Bayes factor extremely in favor of a group effect (BF10 = 1.14* 109).
Brain volume measurements
The T1-weighted anatomical images were preprocessed using the Computational Anatomy Toolbox (CAT12, v9.6/r7487[43] for Statistical Parametric Mapping 12 (SPM12, v12.6/r1450, Wellcome Centre for Human Neuroimaging, London, UK). The images were segmented into grey matter, white matter, and CSF, followed by spatial normalization to the default CAT12 brain template in Montreal Neurological Institute (MNI) reference space using the Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra (DARTEL) algorithm. During this step, the images were resliced to an isotropic voxel size of 1.5 mm, and modulated to adjust for expansion and shrinkage of the grey matter tissue. For basal forebrain volumetry, we applied a mask containing the cholinergic nuclei of the basal forebrain [44] to derive the raw basal forebrain volumes. For hippocampus volumetry we used the harmonized hippocampus segmentation protocol, an internationally driven effort under the auspices of the Alzheimer’s Association [45], implemented into an automated volumetry pipeline to ease processing of larger numbers of cases [46]. The raw basal forebrain and hippocampus volume estimates were proportionally scaled to total intracranial volume, i.e., the sum of the grey matter, white matter and CSF maps from the CAT12 segmentation, to adjust for head size.
We dichotomized the brain volumes according to the following procedure: We determined volume W-scores by regressing out age and sex, and used the 15% percentile of the volume W-scores in the healthy control group as threshold for the binary split; i.e., values corresponding to W-scores below the 15% percentile of the control group were classified as smaller and volumes corresponding to W-scores above the 15% percentile as larger volumes.
CSF sampling and AD biomarker assessment
For the DELCODE data, amyloid positivity was determined using CSF Aβ 42 levels. Biomaterial sampling procedures and biomarker measurements for DELCODE have been described elsewhere [17]. In brief, material was sampled by trained study personal following standard operating procedures (SOP) for collection and storage of samples. CSF samples were aliquoted after centrifugation and stored at –80°C until analysis.
AD markers had been determined using commercially available kits according to vendor specifications: V-PLEX Aβ Peptide Panel 1 (6E10) Kit (K15200E) and V-PLEX Human Total Tau Kit (K151LAE) (Mesoscale Diagnostics LLC, Rockville, USA), and Innotest Phospho-Tau(181P) (81581; Fujirebio Germany GmbH, Hannover, Germany). Amyloid positivity was determined based on CSF Aβ 42 levels in the DELCODE data. The cut-off for abnormal concentrations of Aβ 42 (<496 pg/ml) was derived from the literature, which applied the respective assays [47].
Statistical analysis
Demographic characteristics were compared between diagnostic groups using Bayesian ANOVA and contingency tables as appropriate. We used Jeffreys’ Amazing Statistics Program (JASP Version 0.11), available at jasp-stats.org, to calculate the models. We report the Bayes Factor (BF10) quantifying evidence against the null hypothesis.
Prediction of cognitive change by amyloid status and volumes was done using Bayesian generalized mixed effects models with time nested within individuals with random slope and intercept terms, and longitudinal cognitive scores as outcomes. We compared fit of non-Gaussian versus Gaussian models for the dependent variables using posterior predictive checks. We compared 3rd (and 2nd) degree polynomial interaction terms of time by amyloid (and volume) with only a linear interaction term for time using leave one out cross validation in the R library “loo”. These analyses were conducted using library “brms” in R, accessed through R Studio, version 1.1.463. We used the “brms” default uninformative flat priors and in a sensitivity analysis compared outcomes when using very weakly informed priors with a normal distribution of mean 0 and standard deviation of 10.
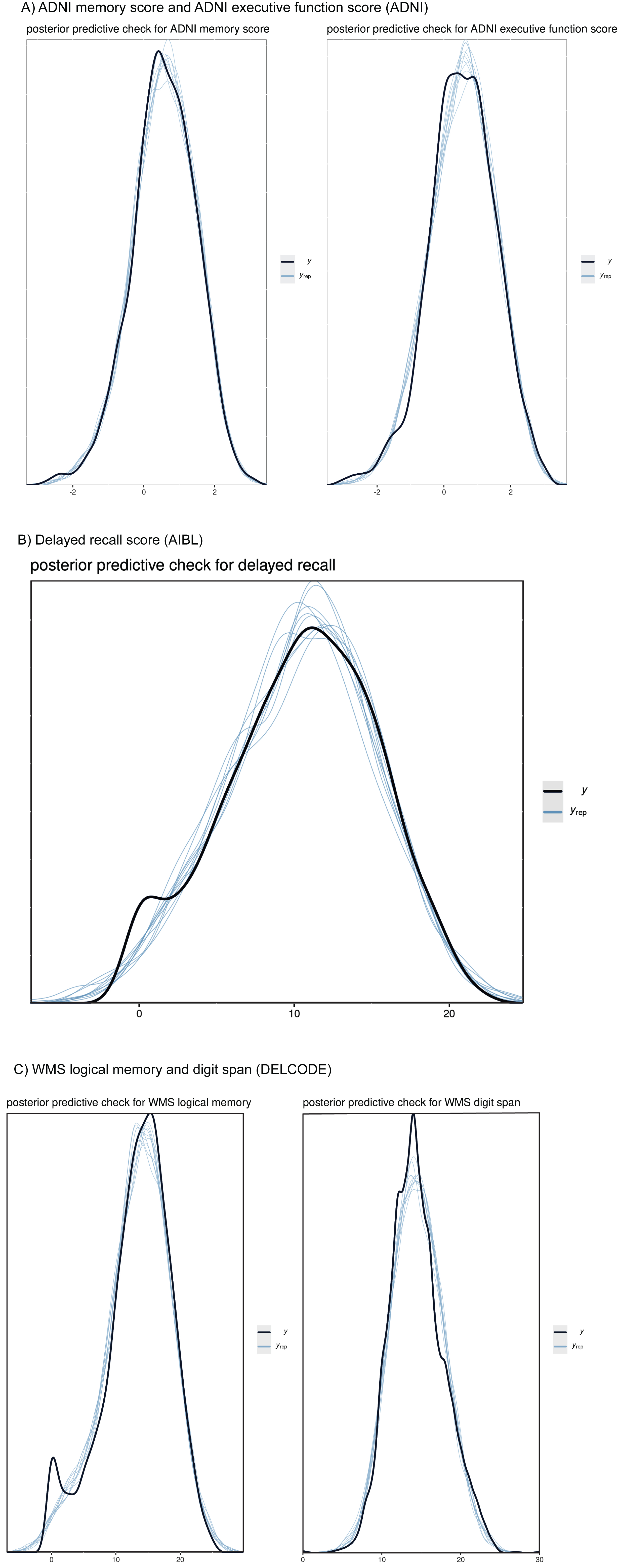
Fig. 1
Posterior predictive checks for cognitive outcomes. Plots comparing the observed outcome variables y (black line) to simulated datasets yrep (blue lines) from the posterior predictive distribution. The plots show the dependent variables ADNI memory score and ADNI executive function score (ADNI, A), delayed recall score (AIBL, B), and WMS logical memory and digit span (DELCODE, C), and 10 samples each from the posterior distribution. The posterior distribution was assessed from mixed effect regression models, predicting cognitive scores by diagnosis and its interaction term with time and age and sex with random intercept and slope, nested within individuals. We used a Gaussian distribution for all dependent variables.
We derived the individual degree of a practice effect as the slope of the individual cognitive trajectory at baseline. More formally, the slope of the trajectory at t = 0 equals the value of the first derivative of the cubic function at t = 0. If the basis function is a + bt + ct 2 + dt 3, then its 1st derivative is b + 2ct + 3dt2. Consequently, the derivative at t = 0 (i.e. the slope of the trajectory at baseline) is equal to the linear component coefficient b. This analysis was only meaningful for the ADNI data where we could fit a cubic function for the cognitive trajectories. Associations of the individual slopes of the trajectory at t = 0 with age (controlling for amyloid status) and with amyloid and volumes (controlling for age and sex) were conducted using Bayesian ANCOVA models in JASP.
We used a Bayesian random-effects meta-analysis [23] to determine domain specific effects of amyloid (and volumes) on rates of cognitive change across the three cohorts. Rates of cognitive change within each cohort were estimated from generalized mixed effects models with random slope and intercept terms, and age, sex, and diagnosis main effects as confounders. Rates of cognitive change were z-standardized within each cohort. Bayesian random effects meta-analysis was conducted using library “bayesmeta” in R [23]. For the effect size estimates we chose a weakly informed normally distributed prior with mean 0 and standard deviation of 4. The prior for the heterogeneity parameter τ was chosen as a weakly informed half-normal distribution with a standard deviation of 0.5, consistent with a previous systematic review of heterogeneity estimates across a large range of meta-analyses from the Cochrane library [48]. In a sensitivity analysis, we compared effects when using an uninformative Jeffreys prior for heterogeneity.
Details of the Bayesian analysis are summarized according to the Checklist of Bayesian Analysis Reporting Guidelines [49] in Supplementary Table 1.
RESULTS
Demographic data
We had overall 1,514 cases divided into 548 cases from ADNI, 265 cases from AIBL, and 701 cases from DELCODE (with volume measures available). From the DELCODE sample, we separately analyzed a subset of 416 cases with additionally CSF amyloid measures available. All cases had at least one follow-up cognitive testing. Detailed demographic characteristics are summarized in Table 1. Overall, age was well matched between diagnostic groups within cohorts, except for a maximum mean difference of 4.6 years in the DELCODE sample. Education and sex were very well matched between diagnostic groups across all cohorts. Follow-up times were unbalanced between diagnostic groups within cohorts with a maximum mean difference of 0.9 years in the DELCODE volume sample. AIBL data were unbalanced in respect to diagnostic groups with 231 healthy controls, but only 34 MCI cases.
Gaussian versus non-Gaussian distribution of the dependent variables
We used posterior predictive checks of the draws from the posterior distributions to check if a Gaussian function sufficiently fitted the cognitive outcomes. The posterior predictive checks showed very good fit by a Gaussian distribution for all cognitive outcomes (ADNI memory score, ADNI executive function score, WMS logical memory, WMS logical memory, WMS digit span), see Fig. 1.
Linear versus polynomial function of the predictors
Leave one out cross-validation suggested that a cubic model provided a better fit than a linear model for the ADNI amyloid and volume data (Table 2). In contrast, a cubic model did not provide a better fit than a linear model for either the AIBL or the DELCODE cohort data (Table 2). This was also true, when we fitted quadratic instead of cubic models to the AIBL and DELCODE data (data not shown).
Table 2
Leave-one-out model fit
| ADNI | amyloid | basal forebrain | hippocampus | |||
| ELPD | SE | ELPD | SE | ELPD | SE | |
| Linear model | –2727.0 | 77.2 | –2744.2 | 77.9 | –2749.0 | 78.3 |
| Cubic model | –2627.4 | 77.1 | –2634.6 | 75.1 | –2643.8 | 76.2 |
| Diff (cubic versus linear) | 99.6 | 14.3 | 109.5 | 15.8 | 105.1 | 14.4 |
| AIBL | amyloid | basal forebrain | hippocampus | |||
| ELPD | SE | ELPD | SE | ELPD | SE | |
| Linear model | –2610.8 | 23.8 | –2627.2 | 23.5 | –2623.9 | 23.9 |
| Cubic model | –2613.8 | 23.5 | –2632.2 | 22.9 | –2626.5 | 23.0 |
| Diff (cubic versus linear) | –3.0 | 4.2 | –5.0 | 3.5 | –2.7 | 4.1 |
| DELCODE | amyloid | basal forebrain | hippocampus | |||
| ELPD | SE | ELPD | SE | ELPD | SE | |
| Linear model | –3503.4 | 26.4 | –13978.0 | 70.5 | –13972.4 | 70.0 |
| Cubic model | –3526.6 | 26.3 | –13991.1 | 70.0 | –13.976.6 | 69.6 |
| Diff (cubic versus linear) | –18.6 | 6.0 | –13.1 | 6.3 | –4.3 | 7.2 |
ELPD, expected log posterior density; SE, standard error; Diff, difference in ELPD between cubic and linear model. Positive values for the ELPD difference between cubic and linear model indicate superior fit of the cubic compared with the linear model, negative values indicate superior fit of the linear model. Only ELPD differences substantially exceeding the SE of the difference should be considered to be relevant [65]. No threshold for this has been established, some authors suggest as a rule of thumb |ELPDdiff/SEdiff| > 4, highlighted in the table in bold font.
Cognitive change associated with baseline amyloid and volumes
The trajectories of cognitive change, controlled for age and sex, according to diagnosis by amyloid status and volumes, respectively, are shown in Figs. 2–4. We used the best fitting models, i.e., cubic models in time for ADNI, and linear models in time for AIBL, and DELCODE data.
Fig. 2
Cognitive change by diagnosis and amyloid status. Marginal interaction effects of time with amyloid status and diagnosis for ADNI memory and executive function in the ADNI cohort (A), delayed recall of logical memory in the AIBL cohort (B), and delayed recall of logical memory in the DELCODE cohort (C) as dependent variables in generalized mixed effect models predicting cognitive scores by diagnosis, amyloid status, and their interaction with time with random slope and intercept terms, nested within individuals. Trajectories feature the 95% credibility intervals for estimates of change. HC Abeta-, amyloid negative healthy controls; HC Abeta+, amyloid positive healthy controls.
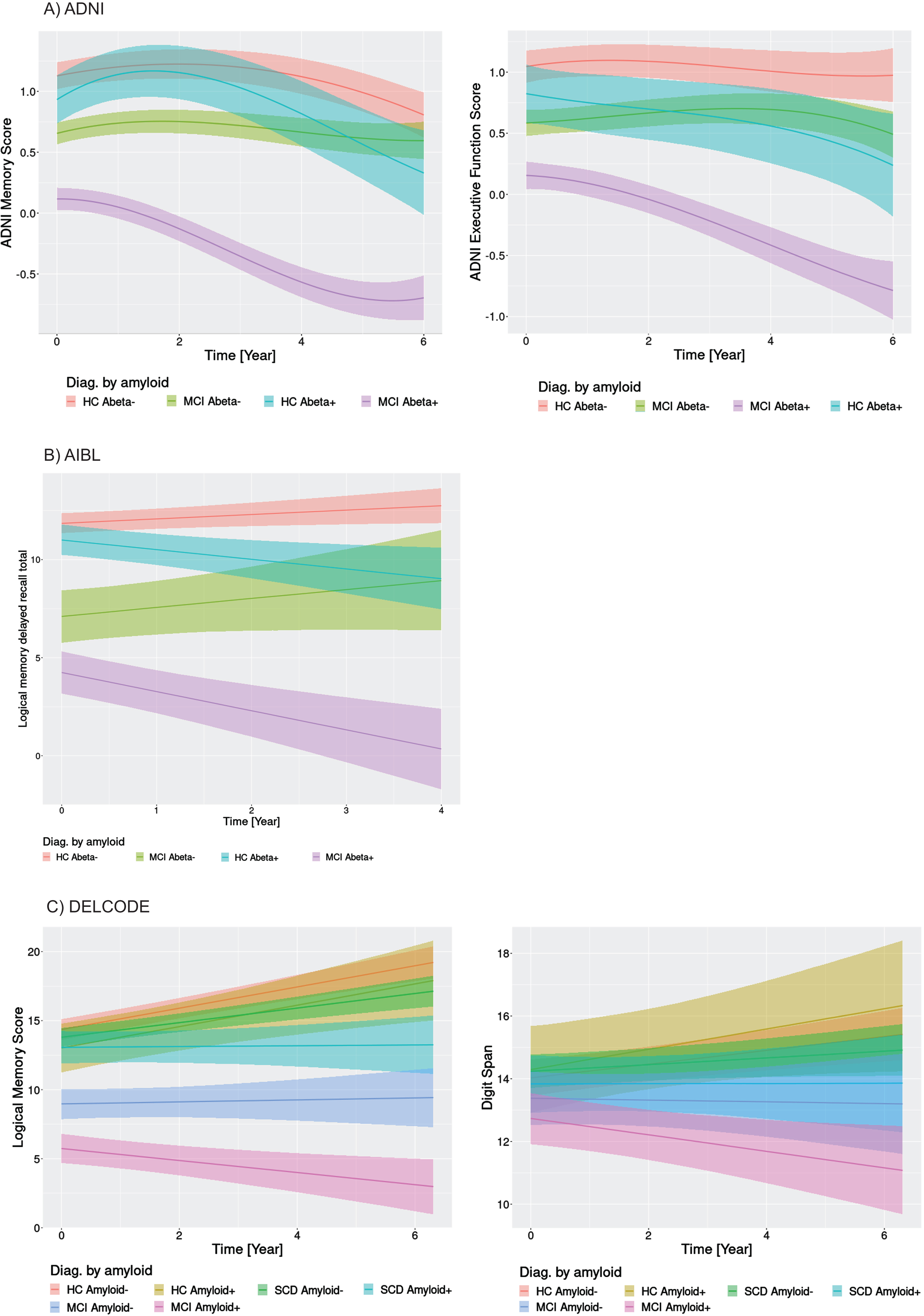
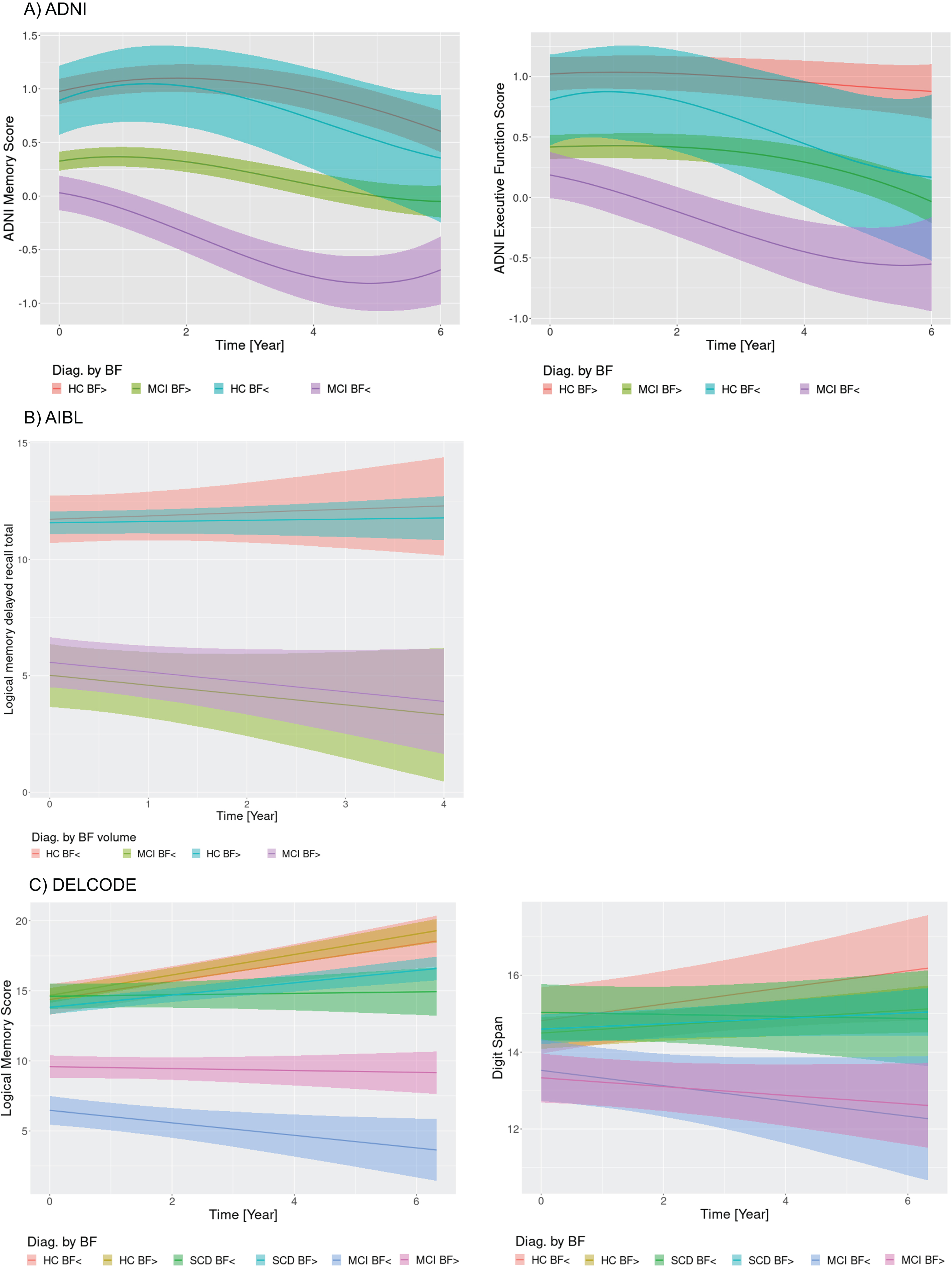
In the ADNI data (Fig. 2A), the amyloid positive MCI cases showed low baseline values of memory and executive function followed by immediate cognitive decline. The amyloid positive cognitively normal controls showed an initial increase of performance followed by pronounced cognitive decline. In contrast, MCI amyloid negative cases remained cognitive stable, whereas the amyloid negative controls showed an initial increase with reversion to baseline levels. A similar pattern arose for the AIBL delayed logical memory recall (Fig. 2B), with decline both in amyloid positive MCI and control cases, and cognitive stability or even increase in the amyloid negative MCI and control cases. Due to the linear models, we could not assess initial increases. Also, the DELCODE data for WMS logical memory recall (Fig. 2C) showed a decline both for amyloid positive MCI and SCD cases that was separated from the trajectory of the amyloid negative healthy control reference group according to the 95% credibility interval of the regression estimates. MCI amyloid negative cases also showed more pronounced decline than the amyloid negative controls. Different to the ADNI and AIBL findings, logical memory scores were widely stable or even increasing even for amyloid positive healthy controls. The digit span showed decline only for the MCI amyloid positive cases, and relatively stable to increasing performance in the other groups. Similar to the amyloid effects in the ADNI cohort, we found declining trajectories (after an intermediate increase in the controls) for the MCI and control cases with smaller basal forebrain volume, both for memory and executive function scores (Fig. 3A). Only slight decline, after intermediate improvement, occurred in the MCI and control cases with larger basal forebrain volume. In contrast, in the AIBL cohort basal forebrain volume did not differentiate trajectories of delayed recall within the MCI and the healthy controls, respectively (Fig. 3B). In the DELCODE cohort, logical memory showed more decline in amyloid MCI cases with smaller basal forebrain volumes, with stable estimates for MCI cases with larger basal forebrain volumes and increases of performance in healthy controls and SCD cases irrespective of basal forebrain volume (Fig. 3C). Digit span declined in MCI cases irrespective of basal forebrain volume, with increases in the other groups.
Fig. 3
Cognitive change by diagnosis and basal forebrain volume. Marginal interaction effects of time with basal forebrain volume (atrophy versus no atrophy) and diagnosis for ADNI memory and executive function in the ADNI cohort (A), delayed recall of logical memory in the AIBL cohort (B), and delayed recall of logical memory in the DELCODE cohort (C) as dependent variables in generalized mixed effect models predicting cognitive scores by diagnosis, basal forebrain volume, and their interaction with time with random slope and intercept terms, nested within individuals. Trajectories feature the 95% credibility intervals for estimates of change. HC BF>, healthy controls without basal forebrain atrophy; HC BF<, healthy controls with basal forebrain atrophy.
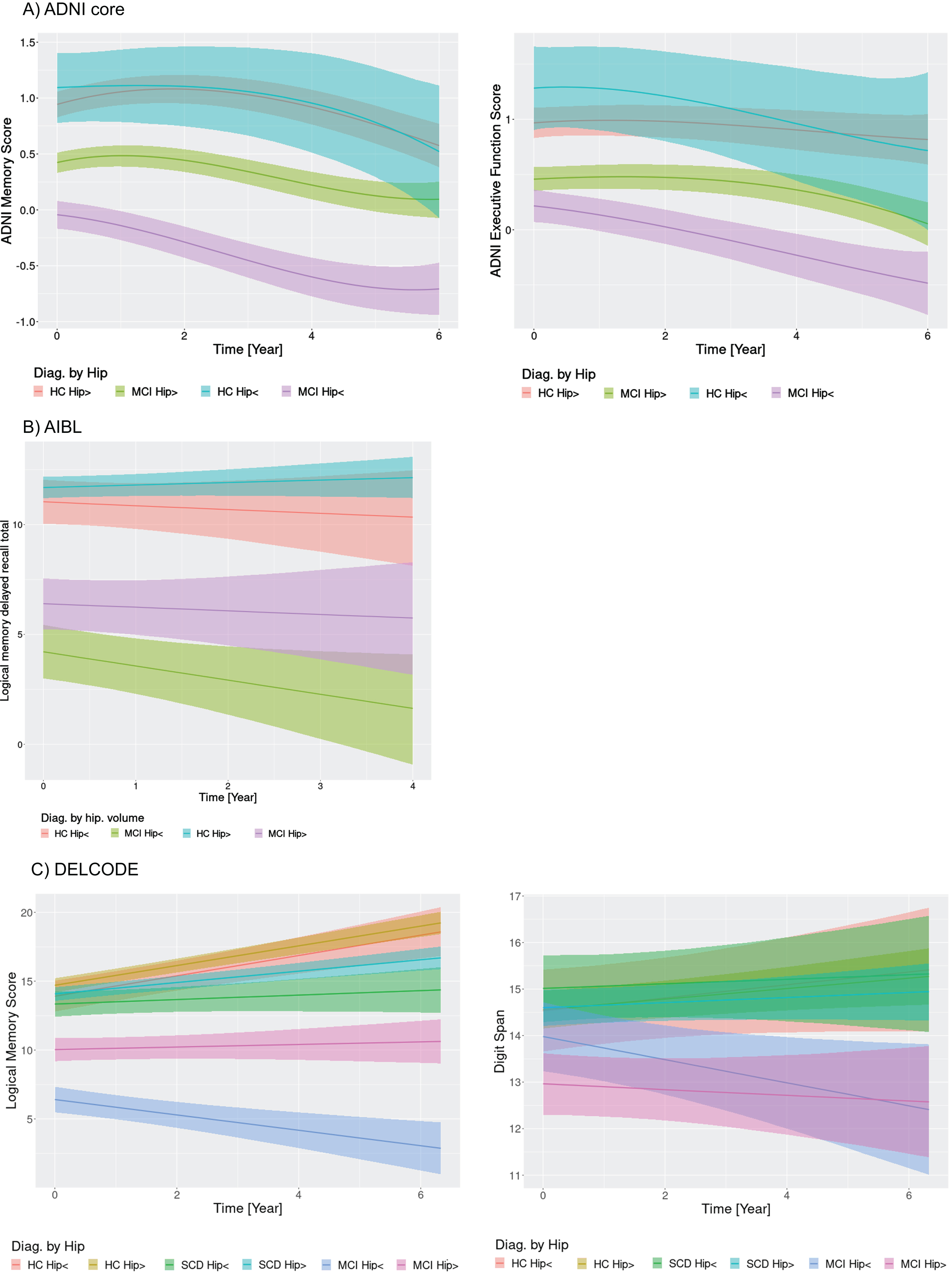
For hippocampus volume, in the ADNI cohort trajectories of memory and executive function showed more pronounced decline for MCI cases with smaller hippocampus volumes. All other groups declined as well, however, MCI cases with larger hippocampus volume and healthy controls irrespective of hippocampus volume showed less pronounced cognitive decline (Fig. 4A). Even the healthy controls with large hippocampus volume showed some degree of memory decline, but not of executive function decline. Both control groups, irrespective of hippocampus volume showed initial improvements in performance. In the AIBL cohort, smaller hippocampus volume separated between more or less pronounced decline in delayed recall both in the controls and the MCI cases (Fig. 4B). In the DELCODE cohort, similar to the basal forebrain volume, logical memory score decline was more pronounced in the MCI cases with smaller hippocampus volume (Fig. 4C). Digit span declined in MCI cases with smaller hippocampus volume, was stable in MCI cases with larger hippocampus volume and increased in the other groups.
Fig.4
Cognitive change by diagnosis and hippocampus volume. Marginal interaction effects of time with hippocampus volume (atrophy versus no atrophy) and diagnosis for ADNI memory and executive function in the ADNI cohort (A), delayed recall of logical memory in the AIBL cohort (B), and delayed recall of logical memory in the DELCODE cohort (C) as dependent variables in generalized mixed effect models predicting cognitive scores by diagnosis, hippocampus volume, and their interaction with time with random slope and intercept terms, nested within individuals. Trajectories feature the 95% credibility intervals for estimates of change. HC Hip>, healthy controls without hippocampus atrophy; HC Hip<, healthy controls with hippocampus atrophy.
When we considered the combination of diagnosis with amyloid status and volume, we found that amyloid positive MCI (control) cases with smaller basal forebrain or hippocampus volumes had most more pronounced rates of memory or executive function decline compared with amyloid negative MCI (control) cases with larger brain volumes, suggesting an additive effect of pathologies (corresponding trajectories for the ADNI cohort are shown in Supplementary Figures 1 and 2).
Practice effects
We found extreme evidence for an association between age and estimates of practice effects for both memory and executive functions, with lower estimates of the linear slope component at older ages, even after controlling for the effects of group and sex (Supplementary Tables 2A and 3B, Supplementary Figure 3). We found even stronger evidence for a group (diagnosis by amyloid or volume) effect, with lower estimates for amyloid-positive cases and for MCI cases compared with control cases, after controlling for the effects of age and sex (Supplementary Tables 2B and 3B, Supplementary Figure 4).
Meta-analysis
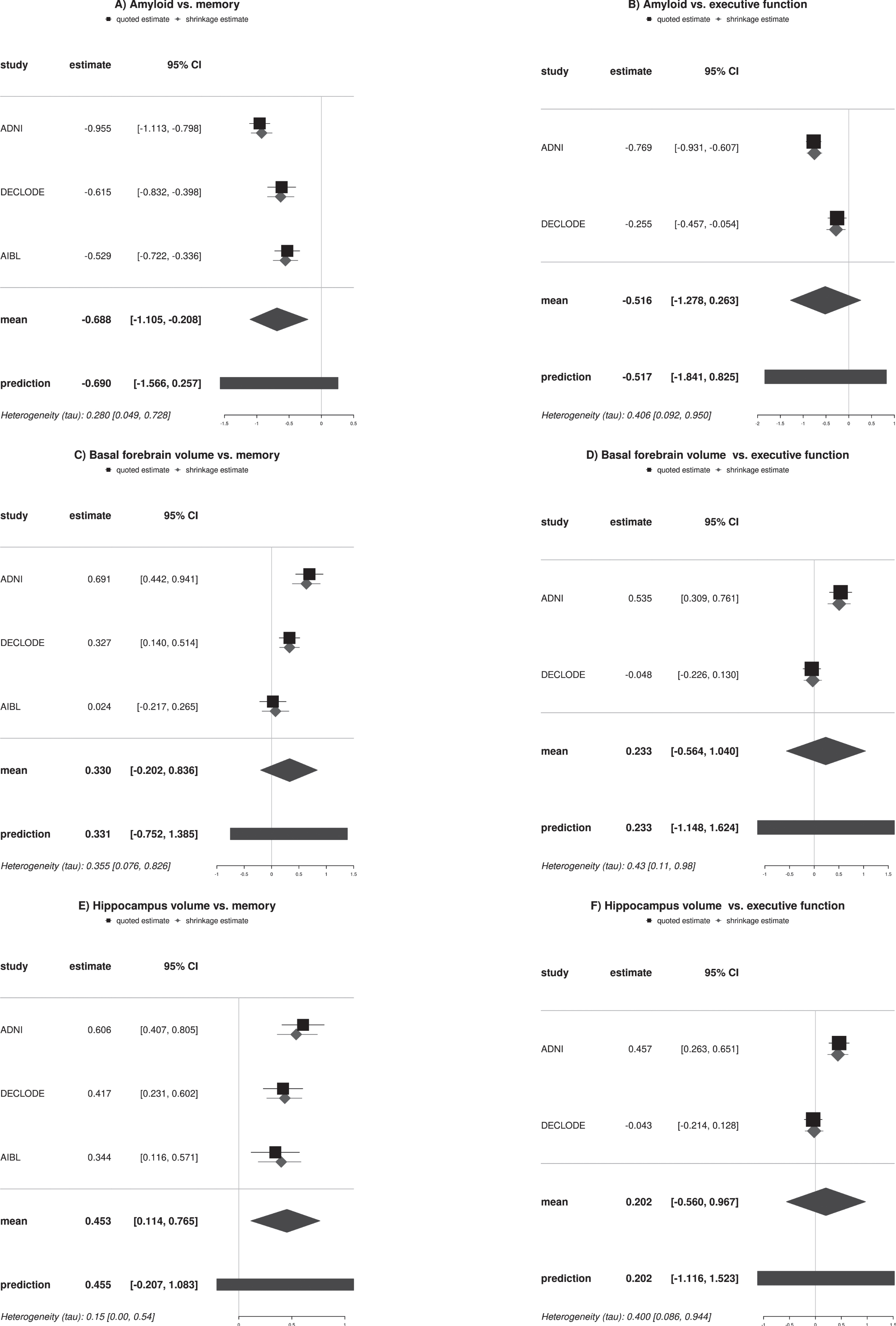
As shown in Fig. 5, mean effect size estimates across the three cohorts for the memory domain and the two cohorts for the executive function domain were in favor of an effect for amyloid and hippocampus volume on memory. The other effects size estimates included zero. In addition, the heterogeneity parameter tau was small for hippocampus and memory and medium for amyloid and memory, but was high for the other models, reflecting the small number of studies and suggesting relatively large heterogeneity across the cohorts. All results were preserved when using an uninformative Jeffreys prior for heterogeneity instead of the weakly informative prior (data not shown).
Fig. 5
Forest plots of amyloid and volume effects on cognitive outcomes. The forest plots feature the direct and indirect estimates of amyloid and volume effects separated for memory and executive outcomes across the cohorts. Estimates are based on Bayesian random effect meta-analysis models with weakly informative effect priors (normal (mean = 0, standard deviation = 4)) and heterogeneity priors (halfnormal (scale = 0.5)). 95% CI, 95% credibility interval.
DISCUSSION
We studied trajectories of cognitive decline across three independent cohorts to determine whether amyloid status and basal forebrain and hippocampus volumes were associated with domain specific cognitive decline and differences in practice effects from repeated cognitive testing [50] in healthy older people and preclinical and prodromal AD cases.
Across all cohorts, cognitive outcomes for memory and executive function were fit very well by a normal distribution. This indicates that ADNI memory and executive function score as well as WMS-R/IV delayed recall of logical memory and digit span showed little floor and ceiling effects in older cognitively normal people and prodromal AD patients. Results would likely have been different if AD dementia cases with possible floor effects or high-performing younger individuals with possible ceiling effects had been included.
Consistent with our first hypothesis and in agreement with previous studies [51, 52], we found associations of amyloid status and brain volumes with rates of cognitive decline across the three cohorts. Confirming previous studies that pooled data across cohorts [52], here we used a Bayesian random-effects meta-analysis to determine heterogeneity of results across cohorts. Amyloid status and hippocampus volume were associated with change in memory with a strong mean effect. Here, the heterogeneity parameter tau indicated small to medium heterogeneity across studies. Heterogeneity was high among executive function models. These results suggest that apparently well-established associations of amyloid and brain volumes with rates of executive function were heterogeneous across cohorts. As one potential factor, a clinical-neuropathological study suggested that episodic memory is sensitive to many neuropathologies, including AD pathology but also Lewy body disease and TDP-43 pathology, whereas working memory as measure of executive function was only late affected by the different pathologies and less consistently than episodic memory [53]. This would suggest that differences between cohorts for example in respect to stages of disease and underlying pathologies may have less effect on the heterogeneity between cohorts in estimates of rates of episodic memory than executive function change.
Meta-analysis across several cohorts allows assessing how stable effects are across cohorts which is particularly relevant for heterogeneous outcomes such as executive function. The WMS delayed logical memory scores and the ADNI composite measures of memory and executive function provided a good separation of trajectories across the three cohorts. In contrast, only the amyloid positive MCI cases had a distinct trajectory in WMS digit span, whereas the credibility intervals for the executive function trajectories of other groups in DELCODE largely overlapped. In a sensitivity analysis we used the ratio of TMT-B to A as alternative measure of executive function [32], but the overlap of trajectories was even more pronounced than for digit span. One solution to this problem could be the use of composite measures of executive function, such as the ADNI executive composite, to compensate for the high intraindividual variability of single scores. Such composite scores derived from a confirmatory factor analysis are available for the DELCODE baseline data [54, 55], but are still in progress for the longitudinal data.
Consistent with our expectation, a nonlinear model of cognitive change was superior to a linear model in the ADNI data. We found extreme evidence for an association of memory and executive function practice effects with age and amyloid and brain volume status. The amyloid negative and positive healthy controls and the amyloid negative MCI cases showed an initial increase of memory performance, indicating practice effects from repeated testing that was lost in amyloid positive MCI cases. For executive function, only amyloid negative controls and MCI cases showed evidence for a practice effect that was lost in amyloid positive controls and MCI cases. Results were similar for basal forebrain and hippocampus volumes with absence of practice effects in MCI cases with smaller volumes. These findings are consistent with the observation of a previous study that only cognitively normal people who remained cognitively stable over several years of follow up showed practice effects compared with cognitively normal people who later developed AD type cognitive impairment [3]. This previous study [3] had used linear least square estimates of slope, whereas in the current study we used the first derivative from the cubic trajectory of the individual-level change at baseline to identify practice effects. The former approach is more accessible, whereas the latter approach accounts for the likely nonlinear nature of cognitive decline and assesses the slope at baseline rather than over all or most of the observation period. Another study used continuous time structural equation modeling to determine practice effect components in longitudinal change of memory performance in aging and AD spectrum cases [56]. This approach describes cognitive change through latent factors, where the practice effect factor is partly estimated from repeated tests at each time point. This approach is more explicit in the operationalization of the practice effect than our or previous approaches; however, it requires repeated measures at each time point which are not available from all cohort repositories and not for composite measures. At the same time, it will be interesting to compare different approaches for assessing practice effect in future studies.
Another study found that absence of practice effects was predictive of more pronounced cognitive decline in MCI cases [3, 57]. Again, our finding of smaller practice effects in amyloid positive compared with amyloid negative cases agrees with this previous report, suggesting that prodromal AD cases, characterized by the presence of amyloid or progressive cognitive decline, lose the practice effect for episodic memory. In addition, we found lower practice effects with higher age, even when controlling for amyloid and diagnosis, consistent with some [56, 58], but not all [59] previous reports.
When stratifying according to basal forebrain volume, healthy controls with a larger basal forebrain volume had a higher probability for a practice effect for memory and executive performance compared with MCI cases, but not compared with controls with smaller basal forebrain volume. This indicates that not basal forebrain volume but rather control of MCI status was driving differences in practice effect. In contrast, when using hippocampus volume, only healthy controls with larger hippocampus volume showed practice effects for memory performance which was lost in MCI patients irrespective of hippocampus volume and in healthy controls with smaller hippocampus volume. This effect of hippocampus volume was much less pronounced for executive function. These findings are consistent with the association of hippocampus volume with episodic memory function [60]. They may indicate that in cognitively normal older people absence of a practice effect for episodic memory may point to hippocampal volume loss, although other factors can of course account for it as well. In a cohort of 190 cognitively normal people absence of practice effect was associated with a metabolic signature suggestive of AD type neurodegeneration in FDG-PET, irrespective of amyloid status [61]. Here, we found that practice effects for memory in healthy controls depended on hippocampus atrophy. Both FDG-PET and hippocampal volume may serve as proxy markers of neurodegeneration [62] and were associated with loss of practice effect for memory performance in cognitively healthy controls.
Interestingly, in the AIBL and DELCODE cohorts a cubic and a quadratic model were not superior or even inferior to a linear model. We can speculate that the overall increase of performance in the healthy controls and the amyloid negative MCI and (in the DECLODE sample) amyloid negative SCD cases may reflect an underlying practice effect. However, we would be reluctant to evaluate a positive slope of a linear trajectory over the entire observation period as a practice effect. This is different from an initial increase in performance after baseline, as identified by the first derivative of the polynomial trajectory of change. Factors other than a practice effects could cause positive linear slopes in performance, such as a survival effect that occurs later in the observation period.
The discrepancy findings between the ADNI cohort and the other two cohorts in the fit of linear and non-linear models may point to a requirement of non-linear models: the need of a sufficient number of time points to allow a reliable estimate of a non-linear effect. Even with a Bayesian approach we could not obtain a stable polynomial estimate for the AIBL and DELCODE samples which had at least one observation time point less compared with the ADNI data.
Our study has several limitations. First, there was no consistent outcome measure for the memory and executive domain across the three cohorts. This limited the comparability of findings between the cohorts and is mirrored in the heterogeneity estimates of the meta-analysis. At the same time, this degree of heterogeneity is prevalent across clinical cohort studies and needs to considered by the analysis strategy. Here, we chose explicit modeling of this heterogeneity in the Bayesian random effect meta-analysis, using a sensitivity analysis for the heterogeneity prior. Improving the replicability of clinical research [63] will require a major effort in harmonizing study and analysis designs and in using analyses capable of modeling heterogeneity itself. Therefore, the inclusion of multiple cohorts and the use of meta-analyses and Bayesian approaches to test the impact of a priori assumptions may help improving replicability in the future. Secondly, access to data from the AIBL cohort was limited so that we could not model executive domain changes. In future, full access to all publicly funded cohorts will be required in order to achieve maximum output for clinical research. However, it should be noted that access to German DELCODE data is also currently restricted, so we still have a long way to go to implement FAIR data sharing principles [64]. Thirdly, the method to determine amyloid status varied between cohorts. In ADNI and AIBL, we used amyloid PET data, in DELCODE CSF Aβ 42 levels. This will likely have contributed to heterogeneity, at the same time it encourages us to believe that the consistent findings for memory across cohorts reflect robust effects.
In summary, we found robust effects of amyloid status and hippocampus volume on change in memory performance across three independent cohorts, but more heterogeneous effects of basal forebrain and of all markers on executive function. Modeling non-linear trajectories of cognitive change in the ADNI data suggested initial increases of cognitive performance, possibly reflecting practice effects, in healthy controls and partly in MCI cases who were amyloid negative or had intact brain volumes. Similar effects could not be tested in the AIBL and DELCODE cohorts because the available number of time points did not allow for stable estimation of polynomial effects even in a Bayesian framework. We hope that our analyses have illustrated the power of Bayesian analysis not only to test likelihood for the absence of an effect but to directly quantify the evidence in favor or against the presence of an effect, to assess the degree of heterogeneity, and to conduct sensitivity analyses to estimate to which degree prior assumptions may influence the outcome.
ACKNOWLEDGMENTS
The DELCODE study (Study-ID: BN012) was supported and conducted by the Deutsches Zentrum für Neurodegenerative Erkrankungen (DZNE). The data samples were provided by the DELCODE study group. Details and participating sites can be found at www.dzne.de/en/research/studies/clinical-studies/delcode. The DELCODE study was supported in respect to the MR imaging by Max Delbrück Center for Molecular Medicine in the Helmholtz Association (MDC), Berlin; Center for Cognitive Neuroscience Berlin (CCNB) at Freie Universität Berlin; Bernstein Center for Computational Neuroscience (BCCN), Berlin; Core Facility MR-Research in Neurosciences, University Medical Center Goettingen; Institute for Clinical Radiology, Ludwig Maximilian University, Munich; Institute of Diagnostic and Interventional Radiology, Pediatric Radiology and Neuroradiology, Rostock University Medical Center; and Magnetic Resonance research center, University Hospital Tuebingen.
Part of the data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen Idec Inc.; Bristol-Myers Squibb Company; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Medpace, Inc.; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Synarc Inc.; and Takeda Pharmaceutical Company. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (http://www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
FUNDING
The work was supported by a grant to SJT within the CureDem funding of the Federal Ministry of Research (BMBF), grant number 01KX2130.
CONFLICTS OF INTEREST
SJT participated in scientific advisory boards of Roche Pharma AG, Biogen, Grifols, and Eisai and is member of the independent data safety and monitoring board of the Study ENVISION (Biogen).
DATA AVAILABILITY
Anonymized data from DELCODE used in this article will be made available by request from any qualified investigator through the DELCODE Steering Board for purposes of replicating procedures and results. The data from ADNI and AIBL are available through the central data repository hosted on LONI (https://ida.loni.usc.edu/login.jsp). Metrics derived from these public data for the current study, such as basal forebrain and hippocampus volumes, will be made available by request from any qualified investigator by the corresponding author.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/ADR-230027.
REFERENCES
[1] | Teipel SJ , Cavedo E , Lista S , Habert MO , Potier MC , Grothe MJ , Epelbaum S , Sambati L , Gagliardi G , Toschi N , Greicius MD , Dubois B , Hampel H ; INSIGHT-preAD study group; Alzheimer Precision Medicine Initiative (APMI) ((2018) ) Effect of Alzheimer’s disease risk and protective factors on cognitive trajectories in subjective memory complainers: An INSIGHT-preAD study. Alzheimers Dement 14: , 1126–1136. |
[2] | Machulda MM , Pankratz VS , Christianson TJ , Ivnik RJ , Mielke MM , Roberts RO , Knopman DS , Boeve BF , Petersen RC ((2013) ) Practice effects and longitudinal cognitive change in normal aging vs. incident mild cognitive impairment and dementia in the Mayo Clinic Study of Aging. Clin Neuropsychol 27: , 1247–1264. |
[3] | Hassenstab J , Ruvolo D , Jasielec M , Xiong C , Grant E , Morris JC ((2015) ) Absence of practice effects in preclinical Alzheimer’s disease. Neuropsychology 29: , 940–948. |
[4] | Baker JE , Lim YY , Pietrzak RH , Hassenstab J , Snyder PJ , Masters CL , Maruff P ((2017) ) Cognitive impairment and decline in cognitively normal older adults with high amyloid-beta: A meta-analysis. Alzheimers Dement (Amst) 6: , 108–121. |
[5] | Aksman LM , Scelsi MA , Marquand AF , Alexander DC , Ourselin S , Altmann A , for A ((2019) ) Modeling longitudinal imaging biomarkers with parametric Bayesian multi-task learning. Hum Brain Mapp 40: , 3982–4000. |
[6] | Cespedes MI , Fripp J , McGree JM , Drovandi CC , Mengersen K , Doecke JD ((2017) ) Comparisons of neurodegeneration over time between healthy ageing and Alzheimer’s disease cohorts via Bayesian inference. BMJ Open 7: , e012174. |
[7] | Dai T , Guo Y , Alzheimer’s Disease Neuroimaging Initiative ((2017) ) Predicting individual brain functional connectivity using a Bayesian hierarchical model. Neuroimage 147: , 772–787. |
[8] | Temp AGM , Lutz MW , Trepel D , Tang Y , Wagenmakers EJ , Khachaturian AS , Teipel S ((2021) ) How Bayesian statistics may help answer some of the controversial questions in clinical research on Alzheimer’s disease. Alzheimers Dement 17: , 917–919. |
[9] | Wagenmakers EJ , Marsman M , Jamil T , Ly A , Verhagen J , Love J , Selker R , Gronau QF , Smira M , Epskamp S , Matzke D , Rouder JN , Morey RD ((2018) ) Bayesian inference for psychology. Part I: Theoretical advantages and practical ramifications. Psychon Bull Rev 25: , 35–57. |
[10] | Gigerenzer G , Krauss S , Vitouch O ((2004) ) The null ritual: What you always wanted to know about significance testing but were afraid to ask. In The Sage Handbook of Quantitative Methodology for the Social Sciences, Kaplan D, ed. Sage, Thousand Oaks, CA, pp. 391–408. |
[11] | Doraiswamy PM , Sperling RA , Johnson K , Reiman EM , Wong TZ , Sabbagh MN , Sadowsky CH , Fleisher AS , Carpenter A , Joshi AD , Lu M , Grundman M , Mintun MA , Skovronsky DM , Pontecorvo MJ ; AV45-A11 Study Group; AV45-A11 Study Group ((2014) ) Florbetapir F 18 amyloid PET and 36-month cognitive decline: A prospective multicenter study. Mol Psychiatry 19: , 1044–1051. |
[12] | Landau SM , Mintun MA , Joshi AD , Koeppe RA , Petersen RC , Aisen PS , Weiner MW , Jagust WJ , Alzheimer’s Disease Neuroimaging Initiative ((2012) ) Amyloid deposition, hypometabolism, and longitudinal cognitive decline. Ann Neurol 72: , 578–586. |
[13] | Teipel S , Gustafson D , Ossenkoppele R , Hansson O , Babiloni C , Wagner M , Riedel-Heller S , Kilimann I , Tang Y ((2022) ) Alzheimer’s disease - standard of diagnosis, treatment, care, and prevention. J Nucl Med 63: , 981–985. |
[14] | Aschenbrenner AJ , Gordon BA , Benzinger TLS , Morris JC , Hassenstab JJ ((2018) ) Influence of tau PET, amyloid PET, and hippocampal volume on cognition in Alzheimer disease. Neurology 91: , e859–e866. |
[15] | Weiner MW , Aisen PS , Jack CR , Jr. , Jagust WJ , Trojanowski JQ , Shaw L , Saykin AJ , Morris JC , Cairns N , Beckett LA , Toga A , Green R , Walter S , Soares H , Snyder P , Siemers E , Potter W , Cole PE , Schmidt M , Alzheimer’s Disease Neuroimaging Initiative ((2010) ) The Alzheimer’s disease neuroimaging initiative: Progress report and future plans. Alzheimers Dement 6: , 202–211 e207. |
[16] | Ellis KA , Bush AI , Darby D , De Fazio D , Foster J , Hudson P , Lautenschlager NT , Lenzo N , Martins RN , Maruff P , Masters C , Milner A , Pike K , Rowe C , Savage G , Szoeke C , Taddei K , Villemagne V , Woodward M , Ames D ((2009) ) The Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging: Methodology and baseline characteristics of 1112 individuals recruited for a longitudinal study of Alzheimer’s disease. Int Psychogeriatr 21: , 672–687. |
[17] | Jessen F , Spottke A , Boecker H , Brosseron F , Buerger K , Catak C , Fliessbach K , Franke C , Fuentes M , Heneka MT , Janowitz D , Kilimann I , Laske C , Menne F , Nestor P , Peters O , Priller J , Pross V , Ramirez A , Schneider A , Speck O , Spruth EJ , Teipel S , Vukovich R , Westerteicher C , Wiltfang J , Wolfsgruber S , Wagner M , Duzel E ((2018) ) Design and first baseline data of the DZNE multicenter observational study on predementia Alzheimer’s disease (DELCODE). Alzheimers Res Ther 10: , 15. |
[18] | Yoo HS , Jeon S , Cavedo E , Ko M , Yun M , Lee PH , Sohn YH , Grothe MJ , Teipel S , Hampel H , Evans AC , Ye BS ((2022) ) Association of beta-amyloid and basal forebrain with cortical thickness and cognition in Alzheimer and Lewy body disease spectra. Neurology 98: , e947–e957. |
[19] | Teipel SJ , Cavedo E , Hampel H , Grothe MJ , Alzheimer’s Disease Neuroimaging Initiative, Alzheimer Precision Medicine Initiative ((2018) ) Basal forebrain volume, but not hippocampal volume, is a predictor of global cognitive decline in patients with Alzheimer’s disease treated with cholinesterase inhibitors. Front Neurol 9: , 642. |
[20] | Schmitz TW , Nathan Spreng R , Alzheimer’s Disease Neuroimaging Initiative ((2016) ) Basal forebrain degeneration precedes and predicts the cortical spread of Alzheimer’s pathology. Nat Commun 7: , 13249. |
[21] | Dore V , Villemagne VL , Bourgeat P , Fripp J , Acosta O , Chetelat G , Zhou L , Martins R , Ellis KA , Masters CL , Ames D , Salvado O , Rowe CC ((2013) ) Cross-sectional and longitudinal analysis of the relationship between Abeta deposition, cortical thickness, and memory in cognitively unimpaired individuals and in Alzheimer disease. JAMA Neurol 70: , 903–911. |
[22] | Stoub TR , Rogalski EJ , Leurgans S , Bennett DA , deToledo-Morrell L ((2010) ) Rate of entorhinal and hippocampal atrophy in incipient and mild AD: Relation to memory function. Neurobiol Aging 31: , 1089–1098. |
[23] | Rover C ((2020) ) Bayesian random-effects meta-analysis using the bayesmeta R package. J Stat Softw 93: , 1–51. |
[24] | Schindler SE , Jasielec MS , Weng H , Hassenstab JJ , Grober E , McCue LM , Morris JC , Holtzman DM , Xiong C , Fagan AM ((2017) ) Neuropsychological measures that detect early impairment and decline in preclinical Alzheimer disease. Neurobiol Aging 56: , 25–32. |
[25] | Winblad B , Palmer K , Kivipelto M , Jelic V , Fratiglioni L , Wahlund LO , Nordberg A , Backman L , Albert M , Almkvist O , Arai H , Basun H , Blennow K , de Leon M , DeCarli C , Erkinjuntti T , Giacobini E , Graff C , Hardy J , Jack C , Jorm A , Ritchie K , van Duijn C , Visser P , Petersen RC ((2004) ) Mild cognitive impairment–beyond controversies, towards a consensus: Report of the International Working Group on Mild Cognitive Impairment. J Intern Med 256: , 240–246. |
[26] | Petersen RC , Smith GE , Waring SC , Ivnik RJ , Tangalos EG , Kokmen E ((1999) ) Mild cognitive impairment: Clinical characterization and outcome. Arch Neurol 56: , 303–308. |
[27] | Jessen F , Amariglio RE , van Boxtel M , Breteler M , Ceccaldi M , Chetelat G , Dubois B , Dufouil C , Ellis KA , van der Flier WM , Glodzik L , van Harten AC , de Leon MJ , McHugh P , Mielke MM , Molinuevo JL , Mosconi L , Osorio RS , Perrotin A , Petersen RC , Rabin LA , Rami L , Reisberg B , Rentz DM , Sachdev PS , de la Sayette V , Saykin AJ , Scheltens P , Shulman MB , Slavin MJ , Sperling RA , Stewart R , Uspenskaya O , Vellas B , Visser PJ , Wagner M , Subjective Cognitive Decline Initiative Working Group ((2014) ) A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement 10: , 844–852. |
[28] | Albert MS , DeKosky ST , Dickson D , Dubois B , Feldman HH , Fox NC , Gamst A , Holtzman DM , Jagust WJ , Petersen RC , Snyder PJ , Carrillo MC , Thies B , Phelps CH ((2011) ) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: , 270–279. |
[29] | Crane PK , Carle A , Gibbons LE , Insel P , Mackin RS , Gross A , Jones RN , Mukherjee S , Curtis SM , Harvey D , Weiner M , Mungas D , Alzheimer’s Disease Neuroimaging Initiative ((2012) ) Development and assessment of a composite score for memory in the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Brain Imaging Behav 6: , 502–516. |
[30] | Gibbons LE , Carle AC , Mackin RS , Harvey D , Mukherjee S , Insel P , Curtis SM , Mungas D , Crane PK ((2012) ) A composite score for executive functioning, validated in Alzheimer’s Disease Neuroimaging Initiative (ADNI) participants with baseline mild cognitive impairment. Brain Imaging Behav 6: , 517–527. |
[31] | Albrecht MA , Szoeke C , Maruff P , Savage G , Lautenschlager NT , Ellis KA , Taddei K , Martins R , Masters CL , Ames D , Foster JK , AIBL Research Group ((2015) ) Longitudinal cognitive decline in the AIBL cohort: The role of APOE epsilon4 status. Neuropsychologia 75: , 411–419. |
[32] | Arbuthnott K , Frank J ((2000) ) Trail making test, part B as a measure of executive control: Validation using a set-switching paradigm. J Clin Exp Neuropsychol 22: , 518–528. |
[33] | Teipel SJ , Dyrba M , Ballarini T , Brosseron F , Bruno D , Buerger K , Cosma NC , Dechent P , Dobisch L , Duzel E , Ewers M , Fliessbach K , Haynes JD , Janowitz D , Kilimann I , Laske C , Maier F , Metzger CD , Munk MH , Peters O , Pomara N , Preis L , Priller J , Ramirez A , Roy N , Scheffler K , Schneider A , Schott BH , Spottke A , Spruth EJ , Wagner M , Wiltfang J , Jessen F , Heneka MT ((2022) ) Association of cholinergic basal forebrain volume and functional connectivity with markers of inflammatory response in the Alzheimer’s disease spectrum. J Alzheimers Dis 85: , 1267–1282. |
[34] | Grothe MJ , Barthel H , Sepulcre J , Dyrba M , Sabri O , Teipel SJ , Alzheimer’s Disease Neuroimaging Initiative ((2017) ) In vivo staging of regional amyloid deposition. Neurology 89: , 2031–2038. |
[35] | Desikan RS , Segonne F , Fischl B , Quinn BT , Dickerson BC , Blacker D , Buckner RL , Dale AM , Maguire RP , Hyman BT , Albert MS , Killiany RJ ((2006) ) An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31: , 968–980. |
[36] | Gonzalez-Escamilla G , Lange C , Teipel S , Buchert R , Grothe MJ , Alzheimer’s Disease Neuroimaging I ((2017) ) PETPVE12: An SPM toolbox for partial volume effects correction in brain PET - Application to amyloid imaging with AV45-PET. Neuroimage 147: , 669–677. |
[37] | Klunk WE , Koeppe RA , Price JC , Benzinger TL , Devous MD Sr , Jagust WJ , Johnson KA , Mathis CA , Minhas D , Pontecorvo MJ , Rowe CC , Skovronsky DM , Mintun MA ((2015) ) The Centiloid Project: Standardizing quantitative amyloid plaque estimation by PET. Alzheimers Dement 11: , 1-15.e11–14. |
[38] | Catafau AM , Bullich S , Seibyl JP , Barthel H , Ghetti B , Leverenz J , Ironside JW , Schulz-Schaeffer WJ , Hoffmann A , Sabri O ((2016) ) Cerebellar amyloid-beta plaques: How frequent are they, and do they influence 18F-Florbetaben SUV ratios? J Nucl Med 57: , 1740–1745. |
[39] | Clark CM , Schneider JA , Bedell BJ , Beach TG , Bilker WB , Mintun MA , Pontecorvo MJ , Hefti F , Carpenter AP , Flitter ML , Krautkramer MJ , Kung HF , Coleman RE , Doraiswamy PM , Fleisher AS , Sabbagh MN , Sadowsky CH , Reiman EP , Zehntner SP , Skovronsky DM , AV45-A07 Study Group ((2011) ) Use of florbetapir-PET for imaging beta-amyloid pathology. JAMA 305: , 275–283. |
[40] | Fleisher AS , Chen K , Liu X , Roontiva A , Thiyyagura P , Ayutyanont N , Joshi AD , Clark CM , Mintun MA , Pontecorvo MJ , Doraiswamy PM , Johnson KA , Skovronsky DM , Reiman EM ((2011) ) Using positron emission tomography and florbetapir F18 to image cortical amyloid in patients with mild cognitive impairment or dementia due to Alzheimer disease. Arch Neurol 68: , 1404–1411. |
[41] | Navitsky M , Joshi AD , Kennedy I , Klunk WE , Rowe CC , Wong DF , Pontecorvo MJ , Mintun MA , Devous MD , Sr. ((2018) ) Standardization of amyloid quantitation with florbetapir standardized uptake value ratios to the Centiloid scale. Alzheimers Dement 14: , 1565–1571. |
[42] | Battle MR , Pillay LC , Lowe VJ , Knopman D , Kemp B , Rowe CC , Dore V , Villemagne VL , Buckley CJ ((2018) ) Centiloid scaling for quantification of brain amyloid with [(18)F]flutemetamol using multiple processing methods. EJNMMI Res 8: , 107. |
[43] | Kurth F , Gaser C , Luders E ((2015) ) A 12-step user guide for analyzing voxel-wise gray matter asymmetries in statistical parametric mapping (SPM). Nat Protoc 10: , 293–304. |
[44] | Kilimann I , Grothe M , Heinsen H , Alho EJ , Grinberg L , Amaro E , Jr. , Dos Santos GA , da Silva RE , Mitchell AJ , Frisoni GB , Bokde AL , Fellgiebel A , Filippi M , Hampel H , Kloppel S , Teipel SJ ((2014) ) Subregional basal forebrain atrophy in Alzheimer’s disease: A multicenter study. J Alzheimers Dis 40: , 687–700. |
[45] | Frisoni GB , Jack CR Jr , Bocchetta M , Bauer C , Frederiksen KS , Liu Y , Preboske G , Swihart T , Blair M , Cavedo E , Grothe MJ , Lanfredi M , Martinez O , Nishikawa M , Portegies M , Stoub T , Ward C , Apostolova LG , Ganzola R , Wolf D , Barkhof F , Bartzokis G , DeCarli C , Csernansky JG , deToledo-Morrell L , Geerlings MI , Kaye J , Killiany RJ , Lehericy S , Matsuda H , O’Brien J , Silbert LC , Scheltens P , Soininen H , Teipel S , Waldemar G , Fellgiebel A , Barnes J , Firbank M , Gerritsen L , Henneman W , Malykhin N , Pruessner JC , Wang L , Watson C , Wolf H , deLeon M , Pantel J , Ferrari C , Bosco P , Pasqualetti P , Duchesne S , Duvernoy H , Boccardi M , EADC-ADNI Working Group on The Harmonized Protocol for Manual Hippocampal Volumetry and for the Alzheimer’s Disease Neuroimaging Initiative ((2015) ) The EADC-ADNI Harmonized Protocol for manual hippocampal segmentation on magnetic resonance: Evidence of validity. Alzheimers Dement 11: , 111–125. |
[46] | Wolf D , Bocchetta M , Preboske GM , Boccardi M , Grothe MJ , Alzheimer’s Disease Neuroimaging Initiative ((2017) ) Reference standard space hippocampus labels according to the EADC-ADNI harmonized protocol: Utility in automated volumetry. Alzheimers Dement 13: , 893–902. |
[47] | Janelidze S , Zetterberg H , Mattsson N , Palmqvist S , Vanderstichele H , Lindberg O , van Westen D , Stomrud E , Minthon L , Blennow K , Swedish BioFINDER study group, Hansson O ((2016) ) CSF Abeta42/Abeta40 and Abeta42/Abeta38 ratios: Better diagnostic markers of Alzheimer disease. Ann Clin Transl Neurol 3: , 154–165. |
[48] | Rhodes KM , Turner RM , Higgins JPT ((2015) ) Predictive distributions were developed for the extent of heterogeneity in meta-analyses of continuous outcome data. J Clin Epidemiol 68: , 52–60. |
[49] | Kruschke JK ((2021) ) Bayesian analysis reporting guidelines. Nat Hum Behav 5: , 1282–1291. |
[50] | Goldberg TE , Harvey PD , Wesnes KA , Snyder PJ , Schneider LS ((2015) ) Practice effects due to serial cognitive assessment: Implications for preclinical Alzheimer’s disease randomized controlled trials. Alzheimers Dement (Amst) 1: , 103–111. |
[51] | Wirth M , Oh H , Mormino EC , Markley C , Landau SM , Jagust WJ ((2013) ) The effect of amyloid beta on cognitive decline is modulated by neural integrity in cognitively normal elderly. Alzheimers Dement 9: , 687–698 e681. |
[52] | Ossenkoppele R , Pichet Binette A , Groot C , Smith R , Strandberg O , Palmqvist S , Stomrud E , Tideman P , Ohlsson T , Jogi J , Johnson K , Sperling R , Dore V , Masters CL , Rowe C , Visser D , van Berckel BNM , van der Flier WM , Baker S , Jagust WJ , Wiste HJ , Petersen RC , Jack CR Jr , Hansson O ((2022) ) Amyloid and tau PET-positive cognitively unimpaired individuals are at high risk for future cognitive decline. Nat Med 28: , 2381–2387. |
[53] | Wilson RS , Yang J , Yu L , Leurgans SE , Capuano AW , Schneider JA , Bennett DA , Boyle PA ((2019) ) Postmortem neurodegenerative markers and trajectories of decline in cognitive systems. Neurology 92: , e831–e840. |
[54] | Amaefule CO , Dyrba M , Wolfsgruber S , Polcher A , Schneider A , Fliessbach K , Spottke A , Meiberth D , Preis L , Peters O , Incesoy EI , Spruth EJ , Priller J , Altenstein S , Bartels C , Wiltfang J , Janowitz D , Burger K , Laske C , Munk M , Rudolph J , Glanz W , Dobisch L , Haynes JD , Dechent P , Ertl-Wagner B , Scheffler K , Kilimann I , Duzel E , Metzger CD , Wagner M , Jessen F , Teipel SJ ((2021) ) Association between composite scores of domain-specific cognitive functions and regional patterns of atrophy and functional connectivity in the Alzheimer’s disease spectrum. Neuroimage Clin 29: , 102533. |
[55] | Wolfsgruber S , Kleineidam L , Guski J , Polcher A , Frommann I , Roeske S , Spruth EJ , Franke C , Priller J , Kilimann I , Teipel S , Buerger K , Janowitz D , Laske C , Buchmann M , Peters O , Menne F , Fuentes Casan M , Wiltfang J , Bartels C , Duzel E , Metzger C , Glanz W , Thelen M , Spottke A , Ramirez A , Kofler B , Fliessbach K , Schneider A , Heneka MT , Brosseron F , Meiberth D , Jessen F , Wagner M , DELCODE Study Group ((2020) ) Minor neuropsychological deficits in patients with subjective cognitive decline. Neurology 95: , e1134–e1143. |
[56] | Bender AR , Ganguli A , Meiring M , Hampstead BM , Driver CC ((2022) ) Dynamic modeling of practice effects across the healthy aging-Alzheimer’s disease continuum. Front Aging Neurosci 14: , 911559. |
[57] | Howieson DB , Carlson NE , Moore MM , Wasserman D , Abendroth CD , Payne-Murphy J , Kaye JA ((2008) ) Trajectory of mild cognitive impairment onset. J Int Neuropsychol Soc 14: , 192–198. |
[58] | Calamia M , Markon K , Tranel D ((2012) ) Scoring higher the second time around: Meta-analyses of practice effects in neuropsychological assessment. Clin Neuropsychol 26: , 543–570. |
[59] | Zheng B , Udeh-Momoh C , Watermeyer T , de Jager Loots CA , Ford JK , Robb CE , Giannakopoulou P , Ahmadi-Abhari S , Baker S , Novak GP , Price G , Middleton LT ((2022) ) Practice effect of repeated cognitive tests among older adults: Associations with brain amyloid pathology and other influencing factors. Front Aging Neurosci 14: , 909614. |
[60] | Van Petten C ((2004) ) Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: Review and meta-analysis. Neuropsychologia 42: , 1394–1413. |
[61] | Machulda MM , Hagen CE , Wiste HJ , Mielke MM , Knopman DS , Roberts RO , Vemuri P , Lowe VJ , Jack CR Jr , Petersen RC ((2017) ) Practice effects and longitudinal cognitive change in clinically normal older adults differ by Alzheimer imaging biomarker status. Clin Neuropsychol 31: , 99–117. |
[62] | Besson FL , La Joie R , Doeuvre L , Gaubert M , Mezenge F , Egret S , Landeau B , Barre L , Abbas A , Ibazizene M , de La Sayette V , Desgranges B , Eustache F , Chetelat G ((2015) ) Cognitive and brain profiles associated with current neuroimaging biomarkers of preclinical Alzheimer’s disease. J Neurosci 35: , 10402–10411. |
[63] | Hillary FG , Medaglia JD ((2020) ) What the replication crisis means for intervention science. Int J Psychophysiol 154: , 3–5. |
[64] | Wilkinson MD , Dumontier M , Aalbersberg IJ , Appleton G , Axton M , Baak A , Blomberg N , Boiten JW , da Silva Santos LB , Bourne PE , Bouwman J , Brookes AJ , Clark T , Crosas M , Dillo I , Dumon O , Edmunds S , Evelo CT , Finkers R , Gonzalez-Beltran A , Gray AJ , Groth P , Goble C , Grethe JS , Heringa J , t Hoen PA , Hooft R , Kuhn T , Kok R , Kok J , Lusher SJ , Martone ME , Mons A , Packer AL , Persson B , Rocca-Serra P , Roos M , van Schaik R , Sansone SA , Schultes E , Sengstag T , Slater T , Strawn G , Swertz MA , Thompson M , van der Lei J , van Mulligen E , Velterop J , Waagmeester A , Wittenburg P , Wolstencroft K , Zhao J , Mons B ((2016) ) The FAIR Guiding Principles for scientific data management and stewardship. Sci Data 3: , 160018. |
[65] | Sivula T , Magnusson M , Matamoros AA , Vehtari A ((2020) ) Uncertainty in Bayesian Leave-One-Out Cross-Validation Based Model Comparison. arXiv: Methodology arXiv:2008.10296v3 [stat.ME] 17 Mar 2022. |




