Benefits of a 12-Week Non-Drug “Brain Fitness Program” for Patients with Attention-Deficit/Hyperactive Disorder, Post-Concussion Syndrome, or Memory Loss
Abstract
Background:
Non-pharmacologic interventions can potentially improve cognitive function, sleep, and/or mood in patients with attention-deficit/hyperactive disorder (ADHD), post-concussion syndrome (PCS), or memory loss.
Objective:
We evaluated the benefits of a brain rehabilitation program in an outpatient neurology practice that consists of targeted cognitive training, lifestyle coaching, and electroencephalography (EEG)-based neurofeedback, twice weekly (90 minutes each), for 12 weeks.
Methods:
223 child and adult patients were included: 71 patients with ADHD, 88 with PCS, and 64 with memory loss (mild cognitive impairment or subjective cognitive decline). Patients underwent a complete neurocognitive evaluation, including tests for Verbal Memory, Complex Attention, Processing Speed, Executive Functioning, and Neurocognition Index. They completed questionnaires about sleep, mood, diet, exercise, anxiety levels, and depression—as well as underwent quantitative EEG—at the beginning and the end of the program.
Results:
Pre-post test score comparison demonstrated that all patient subgroups experienced statistically significant improvements on most measures, especially the PCS subgroup, which experienced significant score improvement on all measures tested (p≤0.0011; dz≥0.36). After completing the program, 60% to 90% of patients scored higher on cognitive tests and reported having fewer cognitive and emotional symptoms. The largest effect size for pre-post score change was improved executive functioning in all subgroups (ADHD dz= 0.86; PCS dz= 0.83; memory dz= 1.09).
Conclusion:
This study demonstrates that a multimodal brain rehabilitation program can have benefits for patients with ADHD, PCS, or memory loss and supports further clinical trials in this field.
INTRODUCTION
Non-pharmacologic brain rehabilitation interventions that are based on promoting neuroplasticity can potentially serve as powerful tools to enhance cognitive capacity in patients with attention-deficit/hyperactivity disorder (ADHD) [1], concussion [2], cognitive impairment [3], and/or stroke [4]. Such methods include cognitive training, neurofeedback, coaching for increasing physical activity, mindfulness meditation, Mediterranean diet, and multi-disciplinary programs that combine these interventions [5–10]. Potential mechanisms of action for many of these interventions include optimizing glucose metabolism in the brain, enhancing cardiorespiratory fitness, improving cerebral blood flow, reducing inflammation in the brain, optimizing the brain glymphatic system [11], and increasing levels of brain-derived neurotrophic factor (BDNF) [12]. These interventions have been shown to increase the number of synapses throughout the brain, promote neurogenesis in the hippocampus, and enhance neuronal connections [12–14]. They can, especially when combined, reduce age-related atrophy in the brain [15] and enhance growth of specific brain regions, especially the prefrontal cortex and the hippocampus [13, 16–18].
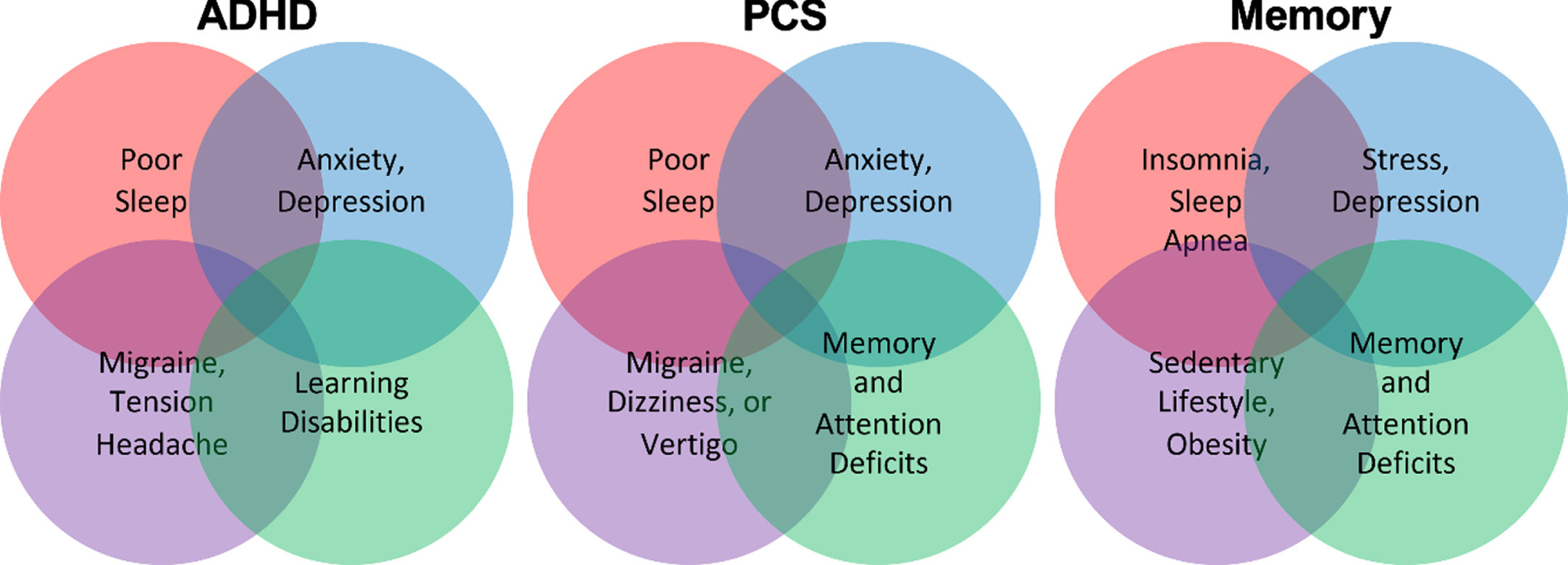
Patients with neurological conditions such as ADHD, post-concussion syndrome (PCS), and memory loss often suffer from multiple co-existing symptoms including anxiety, depression, insomnia, learning disability, and obesity due to a variable degree of brain pathology, individual genetic profile, or overall health and fitness differences (Fig. 1) [19–26]. Therefore, receiving coaching to improve their sleep, diet, and exercise may produce a subjective sense of improvement in brain functions due to improvements in physical health and/or due to psychological factors [27, 28].
Fig. 1
Patients with ADHD, PCS, and memory loss often suffer from several other symptoms and comorbidities that impact their cognitive functions.
The importance of multimodal interventions
Many of the non-drug interventions described above have been combined into multi-disciplinary programs [29–36]. A 2-year randomized controlled trial with a multidomain lifestyle intervention showed a reduction in cognitive decline based on results of comprehensive neurological testing [37, 38]. A recent 9-month trial of personalized multi-modal interventions, called “precision medicine,” to address vascular, inflammatory, infectious, and sleep issues in patients with mild cognitive impairment (MCI) or mild Alzheimer’s disease showed that more than 80% experienced improved cognitive scores [39]. A personalized set of multimodal interventions has also been used for treatment of patients with traumatic brain injury and ADHD [34, 40]. We have previously performed retrospective data analyses from patients with MCI and PCS who completed a 12-week multimodal brain rehabilitation program [41, 42]. As described below, we found that both groups of patients experienced objective and subjective improvements in cognitive capacity and symptoms.
In our first study, MCI patients received a twice-weekly brain rehabilitation program for 12 weeks. The “Brain Fitness Program” (BFP) included 45 min of brain coaching (individualized goal-based accountability coaching for behavior change to improve diet, sleep, exercise, and stress management) followed by 45 min of neurofeedback (NFB, electroencephalography (EEG)-based biofeedback to optimize brain frequencies that are associated with being calm and focused) [43, 44]; EEG-neurofeedback has been shown to be helpful for treatment of ADHD [45], PCS [46], and dementia [47]. This first study included 127 patients with MCI with an average age of 70. Results showed that 84% of patients had objective improvements in their cognitive scores, and half of those who had received brain MRIs (before and after the BFP) had experienced an increase in brain volume in the hippocampus [41].
Our second study included patients with PCS, which is defined as having persistent concussion symptoms beyond three months [2]. We analyzed data from a group of 46 PCS patients who completed our 12-week program. We found that PCS patients experienced significant improvements on objective neurocognitive tests (including those for complex attention, cognitive flexibility, and executive functioning) and a significant reduction in concussion symptoms [42]. As utilized in this second study and in the current study, the term PCS is functionally equivalent to the newer term “Persisting Symptoms after Concussion” [48].
Rationale for the current study
The current study is the natural progression of our previous findings. Both MCI and PCS are due to a combination of multiple pathological etiologies, and patients often have a spectrum of different symptoms that could include anxiety, depression, insomnia, and low executive function. Recent evidence shows that ADHD, the most common neurocognitive disorder in children (which is also prevalent in adults), is likewise caused by multiple etiologies [49]. As with MCI and PCS patients, ADHD patients also suffer from poor sleep, high levels of stress and anxiety, depression, and poor executive function [50, 51]. In line with these findings, several studies have shown that a combination of exercise, cognitive training, behavioral training, and neurofeedback can provide effective treatment for ADHD [52]. Having seen promising results in our earlier studies for patients with MCI and PCS, in this current study we wish to examine the potential benefits of our 12-week program for children and adults with ADHD. Our rationale is that lifestyle modifications can have a profound effect on brain functions in patients with a variety of neurocognitive symptoms. Further, in the current study we included patients with PCS and patients with “memory loss,” a new group that includes patients with a diagnosis of MCI or with subjective cognitive decline [53]. Our hypothesis is that this program will prove equally effective for patients with ADHD, PCS, and memory loss.
MATERIALS AND METHODS
Participants
Authorization to analyze patient data, with all personal identifiers removed, was obtained from the New England Institutional Review Board. This study is a retrospective analysis of data from de-identified patients who presented to NeuroGrow Brain Fitness center, a neurology practice located in Virginia, USA, between January 1, 2017 and December 31, 2019. Data from six patients with concussion that are included in this paper were also included in our previous publication [42]. All included patients experienced symptoms of sufficient severity to reduce their ability to function at home, school, or work environments. Patients who completed at least 10 of the total 24 treatment sessions and completed the program in up to 26 weeks were eligible. The inclusion and exclusion criteria for this data analysis were as follows:
Inclusion criteria:
- Age 7 to 80 years old.
- For the ADHD group: symptoms of ADHD, a diagnosis of ADHD based on DSM-V criteria, and abnormal scores in neurocognitive testing; and for children up to age 16, a diagnosis of ADHD supported by the Vanderbilt Assessment Scale [54].
- For the PCS group: symptoms of concussion for more than 3 months after one or more TBIs, meeting the ICD-10 criteria for a diagnosis of post-concussion syndrome [55] and abnormal scores in neurocognitive testing. Only patients who began the program at least 90 days after their TBI were included in this analysis. The ICD-10 criteria required at least three symptoms from the following list: headache, dizziness/lightheadedness, fatigue, irritability, sleep disturbances, impaired concentration, impaired memory, problems tolerating stress, emotional liability, and alcohol/substance abuse.
- For the Memory Loss group: symptoms of memory loss and abnormal neurocognitive testing scores; patients either met the criteria for a diagnosis of MCI [56] or had subjective cognitive decline [53].
Exclusion criteria:
- Diagnosis of autism.
- Neuropsychiatric conditions such as schizophrenia and dementia.
- Mild symptoms or no cognitive impairment in objective neurocognitive testing. If patients had a few mild to moderate symptoms, and/or if they had normal scores in their complete neurocognitive testing, they were not offered the opportunity to enroll in the program.
NeuroGrow Brain Fitness Program
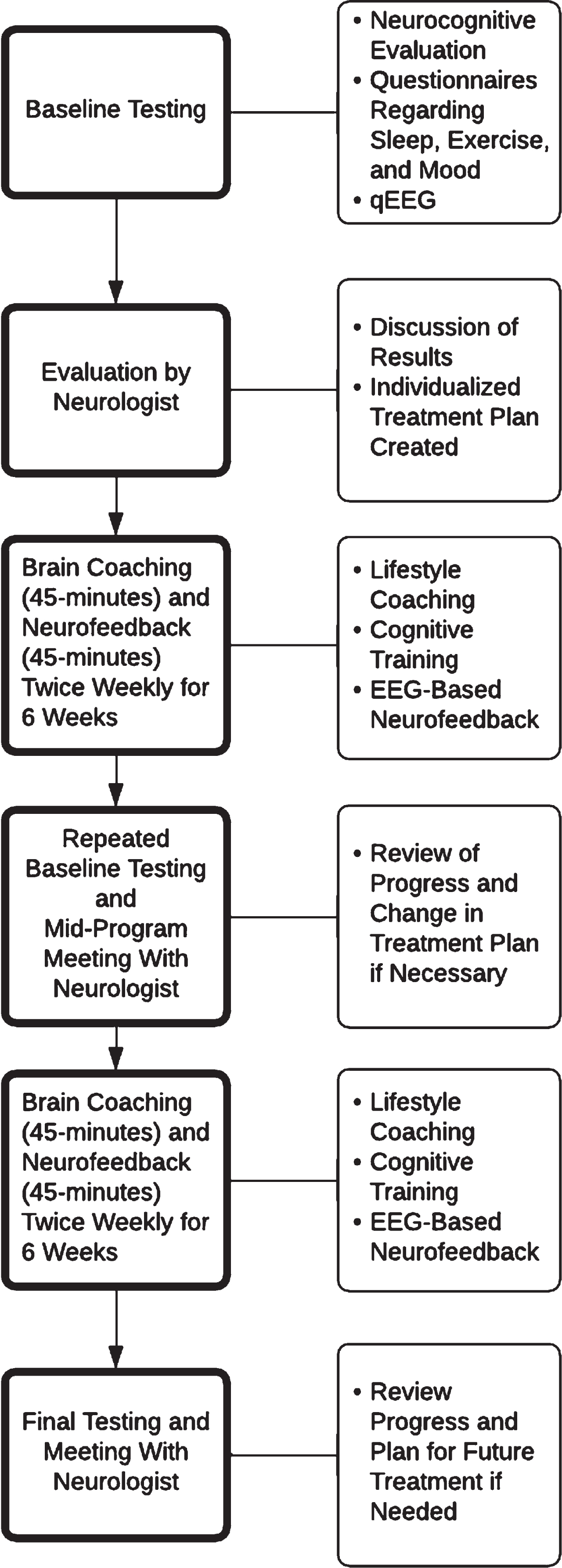
NeuroGrow Brain Fitness Program (NeuroGrow BFP) consists of a set of baseline neurological tests followed by a set of brain rehabilitation interventions over a period of 12 weeks (Fig. 2). Patients first undergo neurocognitive testing and quantitative EEG (qEEG) to establish the nature and the severity of their neurocognitive and neurobehavioral symptoms, cognitive deficits, and EEG abnormalities. They then meet with the neurologist and review their testing results and undergo a neurological evaluation. This 60-min visit includes a review of their medications and a detailed analysis of their medical history. If needed, patients may undergo further blood tests (for evaluation of possible deficiencies in vitamin B12, vitamin D, thyroid, and iron levels), sleep study (for evaluation of possible obstructive sleep apnea), or brain MRI (for evaluation of possible stroke or neurodegeneration). Patients completed a comprehensive assessment before beginning the NeuroGrow BFP.
Fig. 2
Flowchart showing the sequence of steps for patients who complete the NeuroGrow Brain Fitness Program.
The Neurocognitive Evaluation of this assessment consists of a battery of objective cognitive tests obtained from CNS Vital Signs (CNSVS.com). This computer-based battery of cognitive tests compares a patient’s scores to those of age-matched controls. The specific cognitive domain tests used for this study include: Neurocognition Index, Verbal Memory, Complex Attention, Processing Speed, and Executive Functioning [57]. CNS VS scores are ranked into five possible categories: “Very Low” (Standard score <70), “Low” (Standard score 70–79), “Low Average” (Standard score 80–89), “Average” (Standard score 90–109), and “Above Average” (Standard score >109) [57].
The Neurocognitive Evaluation also includes a set of eight questionnaires to explore all aspects of a patient’s brain related symptoms and their ability to feel well, sleep well, and function well on a daily basis:
1. NeuroGrow’s “Brain Fitness Calculator” is a questionnaire that checks for factors associated with optimal brain health. Patients rank their level of exercise, diet, brain vitamin consumption, sleep quality, social engagement, extracurricular activities/hobbies, attitude, and mood on a scale of 1 to 5, with one being very low and 5 being very high. The text has been published previously [42]. The final score can range from 15 to 75, with a score of >50 considered an optimal brain fitness.
2. NeuroGrow’s Neurocognitive Symptom checklist is a questionnaire with a list of 15 questions regarding cognitive symptoms that can be seen in patients with PCS, ADHD, and/or memory loss. Patients report if they are having difficulty with paying attention, calculating, concentrating, making decisions, multitasking, navigation, processing information quickly, understanding instructions, finding words during conversations, verbal expression, short-term memory, remembering names, reading comprehension, planning ahead, and organization, and the text has been published previously [42]. Patients rate their symptom severity from 1–10 with 1 being very mild and 10 being very severe. The total score can range from 15 to 150, with scores below 60 considered optimal.
3. NeuroGrow’s NeuroBehavioral Symptom checklist is a questionnaire with a list of 20 questions regarding neurobehavioral symptoms that can be seen in patients with PCS, ADHD, and/or memory loss. Patients report if they are having difficulty falling asleep, difficulty staying asleep through the night, pain issues, hypersensitivity, headaches, tremors, fatigue, mood swings, obsessive thoughts, compulsive behavior and/or thoughts, depression (feeling sad), difficulty with socializing, general anxiety, hyperactivity, agitation symptoms, impulsive behavior, low motivation and apathy issues, anger issues, frustration issues, and irritability issues, and the text has been published previously [42]. Patients rate their symptom severity from 1–10, with 1 being very mild and 10 being very severe. Total score can range from 20 to 200, with scores below 80 considered optimal.
4. Epworth Sleepiness Scale (ESS) is a commonly used questionnaire with eight questions about a patient’s experience of daytime sleepiness, with answers ranging from 0–3 based on the severity of each symptom. Total scores of 0–5 indicate low daytime sleepiness, while 16–24 indicate excessive daytime sleepiness [58, 59].
5. Pittsburgh Insomnia Rating Scale (PIRS20) is a 20 item self-report questionnaire that measures the quality of sleep over the preceding seven days. The total scores can range from 0–60, with scores above 20 suggesting progressively higher severity of insomnia [60].
6. The Vanderbilt is a questionnaire for parents of children ages 6–12 that helps to determine the severity of their ADHD symptoms. It is a commonly used questionnaire which was developed by Mark Wolraich at the Oklahoma Health Sciences Center [54].
7. The Beck Anxiety Inventory (BAI) is a commonly used questionnaire with 21 self-reported items regarding anxiety symptoms. Total scores can range from 0–63 [61, 62].
8. The Beck Depression Inventory-II (BDI-II) is a self-report scale for depression with 21 items, with a total possible score range of 0–63 [63].
In addition to a comprehensive NeuroCognitive Evaluation, patients undergo an evaluation to check the pattern of their brain oscillations with a testing procedure called qEEG, also known as brain mapping. qEEG measures brain frequencies from 1 Hz to 32 Hz and compares it with a normative database [64]. Conditions such as ADHD, depression, insomnia, and anxiety are often associated with specific abnormal patterns on qEEG [64]. The two software packages used for data collection in this study were Discovery from BrainMaster Technologies (https://www.BrainMaster.com) and TruScan from Deymed Diagnostic (Deymed.com). The collected EEG data were then analyzed by using normative databases and software provided by NeuroGuide (https://www.appliedneuroscience.com). The equipment and software from BrainMaster Technologies, Deymed Diagnostic, and Neuroguide have all been FDA cleared. The EEG protocol included recordings with eyes closed for 5 min. We obtained high quality qEEGs with removal of artifacts per standard protocols and impedance of the electrodes set <5 ohm.
After completing the Neurocognitive Evaluation and qEEG, patients meet with the neurologist (author M.F., with 20 years of experience in academic and clinical neurology) to discuss results of these tests, review the nature and severity of their presenting symptoms, and undergo a neurological examination. The neurologist made the diagnosis of ADHD, PCS, and memory loss per guidelines of American Academy of Neurology and DSM-V criteria [53, 65]. For inclusion in the memory loss group, patients either met the criteria for MCI or for subjective cognitive decline, and experienced objective deficits on neurocognitive testing with regards to verbal memory and/or working memory. Patients who met the required criteria for the NeuroGrow BFP received all necessary information about the program and were offered the option to enroll.
Brain coaching and EEG-neurofeedback
Patients who participate in the NeuroGrow BFP receive a combination of EEG-Neurofeedback and brain coaching for 45 min each (totaling 90 min), twice weekly for 12 weeks. Comprehensive health programs that include an accountability coach can improve patients’ motivation and program adherence [66]. NeuroGrow brain coaches provide patients with targeted cognitive training through the use of computer-based brain games and/or hands-on games that are designed to challenge specific cognitive domains such as attention, memory, processing speed, or executive function. These include chess, sudoku, checkers, scrabble, Bananagram, Rush Hour, Spot-it, and Blink. Such brain games have been showed to improve cognitive function in patients with cognitive deficits [67–69]. Patients with concerns about their memory receive focused brain training on how to memorize a list of 100 words. For patients who prefer computer-based brain games, we use a brain game App called HappyNeuronPro (HappyNeuronPro.com). A full list of the brain games utilized in the Brain Fitness Program is included in Supplementary Figure 1.
Brain coaches also encourage patients to improve their lifestyle so that they sleep better, have a healthier diet, reduce their stress, and exercise regularly. During their first sessions, brain coaches provide patients with a hand-out that includes an overview of the scientific evidence about how certain lifestyle modifications can improve brain health and cognitive capacity as well as general recommendations and resources on how they can incorporate these changes in their lives [70]. They are also informed about a book on this topic [14] if they are interested in obtaining further details about the scientific basis of the recommendations they receive. Their progress throughout the program is monitored and documented on a weekly basis.
Brain coaches at NeuroGrow have a bachelor’s or master’s degree in neuroscience or psychology. Additionally, they undergo extensive training with the neurologist and pass two in-house exams before they are allowed to see patients. Brain coaches help each patient incorporate the unique and individual treatment protocols for brain coaching interventions that are ordered by the neurologist, with the focus on improving the patient’s specific set of symptoms. Brain coaches also encourage patients enthusiastically to incorporate habits and lifestyle factors that are associated with better cognitive function and brain health (described below).
Patients with high levels of anxiety receive training in stress management, Heart Rate Variability (HRV) biofeedback, and meditation. They are encouraged to have a positive attitude, stress less, and use phone apps such as Headspace (http://www.headspace.com) and Calm (http://www.calm.com), as well as the Muse biofeedback device (http://www.choosemuse.com), to practice different forms of mediation. After gaining a level of comfort with their introductory meditation exercises, they learn more advanced methods of meditation in the form of transcendental meditation. Patients who have difficulty learning to meditate receive HRV biofeedback during their brain coaching sessions. Those with poor sleep receive general counseling for better sleep hygiene. If patients have symptoms suggestive of obstructive sleep apnea, they are referred to a sleep lab for further testing and treatment.
Patients with sedentary lifestyles are encouraged to gradually increase the frequency and intensity of their physical activities. Vigorous exercise for a minimum of 45 min, four times a week (preferably through an activity they enjoy) is recommended if patients are physically healthy, as tolerated. This recommendation is directly from the United States Department of Health and Human Services 2008 Physical Activity Guidelines [71]. Brain coaches discuss patients’ current level of exercise at each session and encourage them to increase their level of exercise during their weekly meetings, if needed.
Neurocognitive test results guide the cognitive rehabilitation for each patient. If a patient has low scores for executive function, they are assigned to perform brain games that stimulate frontal lobe functions. If a patient has low scores on memory, they are assigned to complete brain-training assignments, which may include memorizing a list of 100 words. Patients are also encouraged to challenge their cognitive functions at home with specific brain games from HappyNeuronPro; these brain games have been used in more than 60 research and clinical studies, according to the publisher’s website, which maintains an updated list (https://www.happyneuronpro.com/en/research/clinical-studies/).
Regarding diet, patients are introduced to a Mediterranean diet, which consists of fruits, vegetables, whole grains, olive oil, nuts, and fish two to three times a week. Taking omega-3 fatty acid supplements (containing 1000–1500 mg/day of DHA+EPA) is strongly encouraged. We provide patients with education regarding the benefits of the Mediterranean diet for improving their cognitive function [72]. Many patients also receive general advice regarding portion size, weight management, and fitness, if needed.
Brain coaches closely monitor the progress of each patient toward better fitness, better sleep, lower stress, healthier diet, meditation, brain exercises, and having a positive attitude through weekly questionnaires, and mark them in a booklet (called the “The Passport To A Sharper Brain” [73]. The subjective responses of patients to a series of questions, their “Brain Health Score” (Fig. 3), in this booklet help brain coaches track patients’ progress toward individualized set goals each week. Their progress with regards to the number of words they memorize every week, how well they sleep, how closely they follow a heart-healthy Mediterranean diet, how positive they are in their daily activities, how often they practice meditation, and how calm and relaxed they are on a daily basis is also recorded in this booklet. Brain coaches act as enthusiastic cheerleaders when patients succeed in meeting goals and as encouraging accountability partners when patients fall short.
Fig. 3
Brain coaching and the Brain Health Score. Brain coaches closely monitor the progress of each patient toward their individual goals for better fitness, better sleep, lower stress, healthier diet, meditation, brain exercises, and having a positive attitude. During each brain coaching session, brain coaches discuss the patient’s self-reported Brain Health Score for the previous week, which marks the degree of progress made toward these goals. Brain coaches act as enthusiastic cheerleaders when patients succeed in meeting goals and as encouraging accountability partners when patients fall short.

Brain coaches meet with the lead neurologist in the program every week to discuss each patient’s progress, whether the patient has failed to improve steadily, or if there are challenges with any aspects of their progress. Such issues could include a patient’s persistent insomnia challenges despite receiving general recommendations for sleep hygiene and taking over-the-counter herbal sleep supplements, or persistent anxiety or stress management issues despite general strategies such as regular physical exercise, meditation, and receiving cognitive behavioral therapy. The neurologist will then contact the patient to discuss possible solutions to the problem, which may include prescribing medications for a short period of time.
Objective assessments to monitor patient progress
At the mid-way point of their program, patients repeat their neurocognitive evaluation and qEEG brain mapping. They then meet with the neurologist to discuss their test results and progress towards symptom improvement. Based on this conversation, the second part of their program is modified, if needed. For example, some patients may receive more intensive training for verbal memory or executive functioning (based on the results of the neurocognitive testing), or they may receive more encouragement to improve their diet or sleep (based on their responses to questionnaires). Since mid-way evaluations do not occur at the same time across all patients, these data were not included in this study. At the end of the program, patients complete their final round of testing and meet with the neurologist to review their progress. If needed, the neurologist may suggest further at home brain training or other interventions to help patients fully recover from their baseline symptoms.
Statistical analysis
If a patient’s pre- or post-program score on a given measure was listed as invalid, both their pre- and post-program scores for that measure were discarded and not used for analysis. All t-tests completed were calculated using Microsoft Excel for Mac version 16.54 and were paired and 2-tailed. For this study, three separate diagnosis groups of patients (ADHD, PCS, and memory) were analyzed on 12 separate measures (five CNS VS domains: Neurocognition Index, Verbal Memory, Complex Attention, Processing Speed, and Executive Functioning, ESS, PIRS20, BAI, BDI-II, Brain Fitness Calculator Score, Neurocognitive Symptoms, and Neurobehavioral Symptoms), for a total number of hypotheses tested, m = 36. To control for multiple testing, we utilized Bonferroni correction to set the level of significance, αbonf= 0.0014. Effect sizes for t-tests reported in this analysis are Cohen’s dz (effect size for paired differences) and calculated as they were in [74]. The standard benchmarks used were as follows, small (0.2), medium (0.5), and large (0.8) effect size.
Reliable Change (RC) was calculated for CNS VS tests using the Jacobson-Truax method [75]. For these calculations, Intraclass Correlation Coefficient (ICC) values from the first to the third instance of taking the test from the publication [76] were utilized as the measure of test-retest reliability. RC was calculated for the CNS VS domains Verbal Memory, Complex Attention, Processing Speed, and Executive Functioning just as in our previous study [42]. As in that publication, the normative mean and standard deviation for CNS VS domains (M = 100, SD = 15) was taken from CNS VS materials [57], the normal range was defined as within two standard deviations of the normative mean (score ≥70), and the abnormal range was defined as greater than two standard deviations of the normative mean (score <70) [77].
The following formulas were used to calculate RC
An |RC| >1.96 is considered reliable change. Using this metric, a patient would need a pre- to post-program increase (or decrease) in score of at least the following number of points to be considered reliable (1.96×S(diff)): Verbal Memory = 32 points; Complex Attention = 26 points; Processing Speed = 24 points; Executive Function = 26 points. To determine whether individual patients experienced reliable changes in score that were of clinical significance, we restricted the RC calculations to patients who were in the “abnormal” range for each domain before the program (a score of <70) and also determined whether they improved sufficiently to be within the “normal” range for that domain after the program (a score of ≥70) [77]. These analyses were performed with Microsoft Excel.
Demographic information was compared between patients in the three possible diagnosis subcategories using ordinary one-way ANOVAs (for continuous variables) and a two-way 2×3 chi-square test (for sex). Repeated measures, 2 way grouped ANOVAs were used to determine the effect of diagnosis subgroup on test score change from pre- to post- program; 12 separate tests/measures were considered in this analysis, and αbonf= 0.004. All ANOVAs and chi-square tests were performed using Graphpad Prism 9 for Macintosh, version 9.1.2.
RESULTS
Demographics and details of brain fitness program
The 223 patients who completed the program included 71 patients with ADHD (this group is abbreviated as “ADHD”), 88 patients with PCS (this group is abbreviated as “PCS”), and 64 patients with memory loss (this group is abbreviated as “memory”) (Table 1). This 12-week program included EEG-Neurofeedback and brain coaching, twice weekly, with the goal of completing 24 sessions of each. An overview of the complete BFP is shown in Fig. 2. The mean time spent to complete the BFP by patients in this study was 16.2 weeks (SD = 3.2 weeks). Time spent in the program, broken down by diagnosis subgroup, is shown in Table 1; we performed a one-way ANOVA and found no evidence for a difference in mean weeks spent in the program by patients in different subgroups (F = 1.503; p = 0.2248). The average number of neurofeedback sessions completed by patients during the BFP was 23.8 (SD = 3.7); there was no evidence for a difference in mean neurofeedback sessions between the three subgroups (F = 0.2969; p = 0.7434). The average number of brain coaching sessions completed was 23.4 (SD = 3.5); there was no evidence for a difference in mean sessions completed between the three subgroups (F = 0.4657; p = 0.6283).
Table 1
Demographics of patients with ADHD, PCS, or memory loss who completed the brain fitness program along with the number of treatment sessions they received
| All Patients | Patients Divided into Diagnosis Subgroups | |||
| ADHD | PCS | Memory | ||
| Total Patients | 223 | 71 | 88 | 64 |
| Male (%) | 46.6% | 63.4% | 38.6% | 39.1% |
| Female (%) | 53.4% | 36.6% | 61.4% | 60.9% |
| sAge in Years (M (SD)) | 36.9 (19.3) | 24.6 (15.3) | 37.1 (16.6) | 50.5 (17.7) |
| Age Ranges (%) | 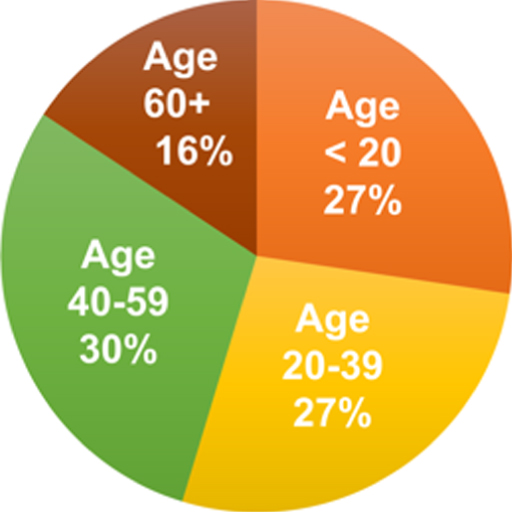 | 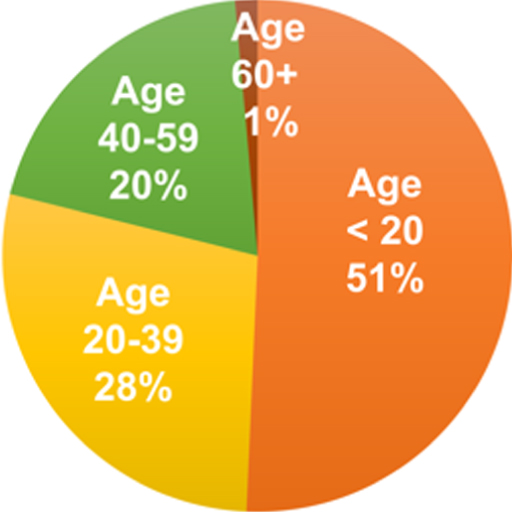 | 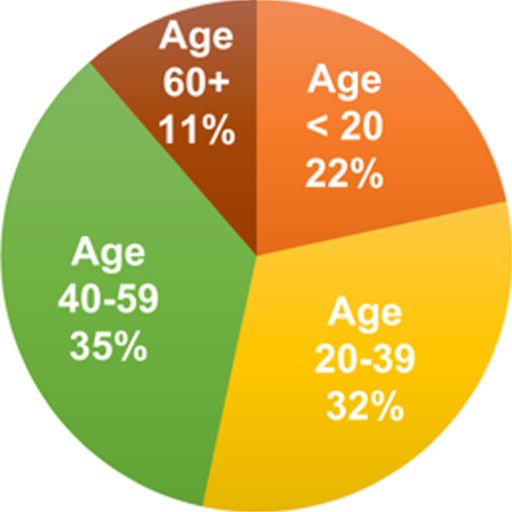 | 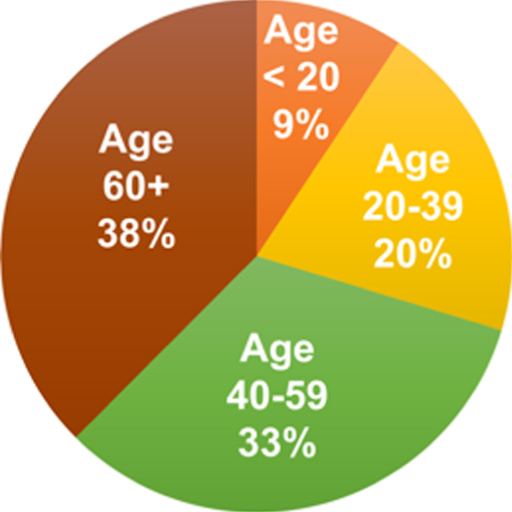 |
| # of Brain Coaching Sessions (M (SD)) | 23.4 (3.5) | 23.1 (2.8) | 23.5 (4.0) | 23.6 (3.6) |
| # of Neurofeedback Sessions (M (SD)) | 23.8 (3.7) | 23.6 (2.8) | 23.8 (3.7) | 24.1 (4.6) |
| # of Weeks in Program (M (SD)) | 16.2 (3.2) | 16.7 (3.3) | 16.2 (3.1) | 15.8 (3.2) |
Mean age of patients at start of program and the percentage of patients in each diagnosis subgroup by sex and age category are listed in Table 1. There was a significant difference in mean age at start of program between the three groups (F = 41.04; p < 0.0001). The mean age for patients in the ADHD diagnosis subgroup was 24.6 years (SD = 15.3), the mean age for the PCS subgroup was 37.1 years (SD = 16.6), and the mean age for the memory subgroup was 50.5 years (SD = 17.7). The male/female frequency distribution was significantly different between the three diagnosis subgroups (χ2 = 11.74; df = 2; p = 0.0028).
Improvements in cognitive scores
For patients in the ADHD, PCS, and memory subgroups, scores for neurocognitive tests taken before the program were compared to those taken after the program using paired t-tests (Table 2). For all three diagnosis groups, the mean pre-program score for the CNS VS domain Neurocognition Index, which is a summary of multiple cognitive domains, was within the “Low Average” range (Table 2, Fig. 4). Following completion of the program, the mean scores for all three groups were now within the “Average” range. For each of the three subgroups, the average change from pre- to post-treatment was a significant increase (improvement) in score, with p < 0.0001 and dz≥0.75. For the ADHD group, Mdiff= 9.7 points; for the PCS group, Mdiff= 9.2 points; and for the memory group, Mdiff= 9.5 points. Similar findings were noted for the four other CNS VS domains included in this study (Verbal Memory, Complex Attention, Processing Speed, and Executive Functioning). With the exception of the Verbal Memory domain for the ADHD group, for which the mean change in score (Mdiff= 6.7 points; p = 0.0027) was not significant after Bonferroni correction, all three groups experienced an increase in score for each domain, p≤0.0011.
Fig. 4
Mean CNS VS domain scores before and after treatment. For patients in the ADHD, PCS, and memory diagnosis groups, mean test scores are shown before and after the brain fitness program (error bars are standard error of the mean). For all these domains, an increase in score indicates improvement. In all cases except for the ADHD group Verbal Memory domain, the mean change in score from pre- to post-program (Table 2) was a significant increase (improvement).
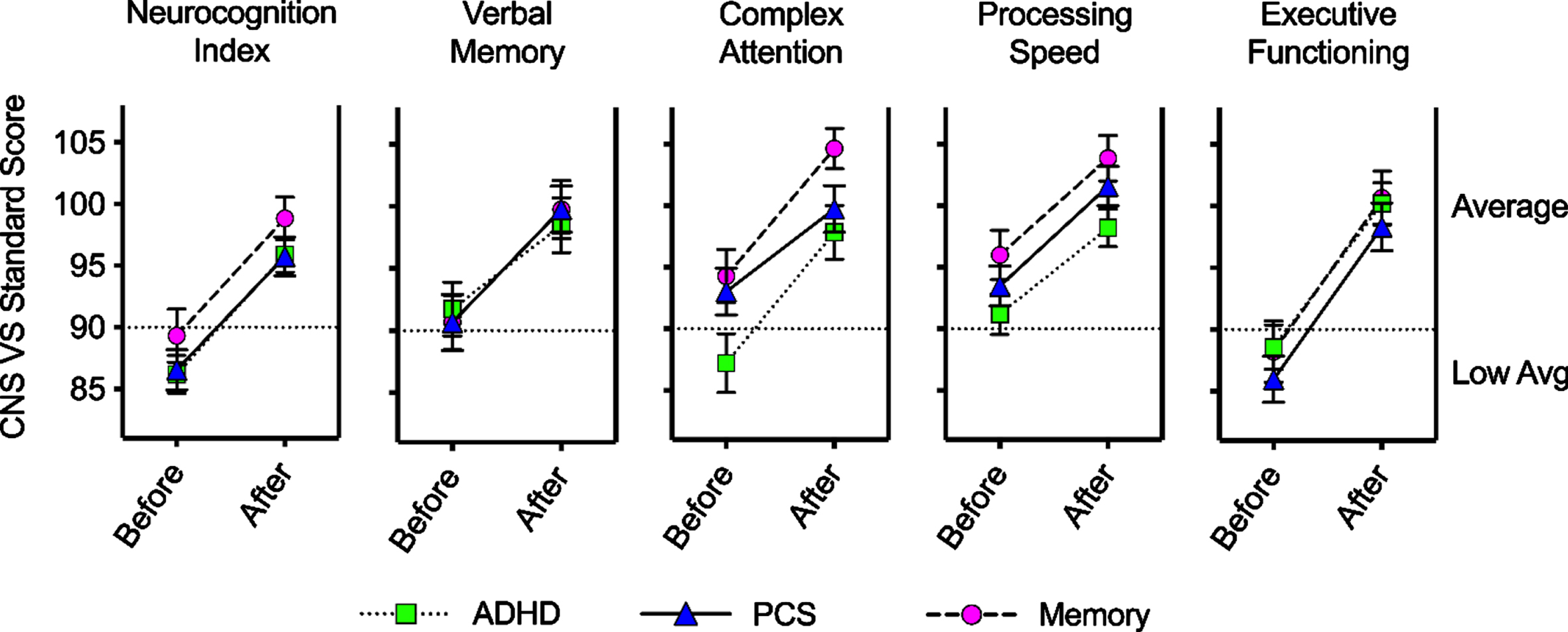
Table 2
Paired T-test results and effect sizes for patients in major diagnosis subgroups
| ADHD | PCS | Memory | ||||||||||||||||
| Pre | Post | Change | Paired t-test | Pre | Post | Change | Paired t-test | Pre | Post | Change | Paired t-test | |||||||
| Measure | n | M (SD) | M (SD) | Mdiff (SDdiff) | p | dz | n | M(SD) | M (SD) | Mdiff (SDdiff) | p | dz | n | M (SD) | M (SD) | Mdiff (SDdiff) | p | dz |
| Neurocog. Index | 70 | 86.2(12.8) | 95.9 (11.9) | 9.7 (12.0) | <0.0001* | 0.81 | 87 | 86.6 (15.2) | 95.7 (14.7) | 9.2 (12.2) | <0.0001* | 0.75 | 64 | 89.3 (17.4) | 98.8 (13.8) | 9.5 (10.2) | <0.0001* | 0.93 |
| Verbal Memory | 71 | 91.7 (18.2) | 98.5 (18.6) | 6.7 (18.3) | 0.0027 | 0.37 | 88 | 90.7 (21.1) | 99.8 (17.6) | 9.1 (19.2) | <0.0001* | 0.47 | 64 | 90.7 (17.6) | 99.8 (18.8) | 9.1 (20.5) | 0.0007* | 0.44 |
| Complex Attention | 69 | 87.2 (19.7) | 97.8 (18.1) | 10.6 (19.3) | <0.0001* | 0.55 | 86 | 93.0 (17.5) | 99.7 (17.6) | 6.7 (18.4) | 0.0011* | 0.36 | 63 | 94.3 (17.2) | 104.6 (13.0) | 10.3 (13.7) | <0.0001* | 0.76 |
| Processing Speed | 71 | 91.2 (14.1) | 98.2 (12.8) | 7.0 (10.5) | <0.0001* | 0.66 | 88 | 93.5 (15.3) | 101.6 (15.1) | 8.1 (13.5) | <0.0001* | 0.60 | 64 | 96.0 (16.1) | 103.8 (14.8) | 7.8 (12.2) | <0.0001* | 0.64 |
| Executive Function | 71 | 88.6 (15.3) | 100.2 (14.1) | 11.6 (13.5) | <0.0001* | 0.86 | 88 | 86.0 (17.6) | 98.3 (18.2) | 12.3 (14.9) | <0.0001* | 0.83 | 64 | 88.2 (19.8) | 100.7 (17.5) | 12.4 (11.4) | <0.0001* | 1.09 |
| Brain Fitness | 70 | 47.8 (10.8) | 55.2 (9.0) | 7.4 (9.3) | <0.0001* | 0.80 | 86 | 43.8 (9.7) | 53.6 (8.9) | 9.8 (10.9) | <0.0001* | 0.90 | 64 | 46.1 (7.6) | 54.0 (10.8) | 7.8 (10.4) | <0.0001* | 0.76 |
| ESSa | 36 | 6.9 (4.5) | 5.0 (3.5) | –1.9 (3.0) | 0.0006* | –0.63 | 73 | 7.9 (4.9) | 6.2 (4.5) | –1.8 (3.9) | 0.0002* | –0.46 | 60 | 7.3 (4.8) | 5.8 (4.8) | –1.5 (5.1) | 0.0258 | –0.30 |
| PIRS20a | 36 | 30.9 (12.1) | 20.4 (11.7) | –10.5 (13.0) | <0.0001* | –0.81 | 73 | 29.1 (12.2) | 21.3 (12.2) | –7.9 (12.3) | <0.0001* | –0.64 | 60 | 26.0 (16.0) | 17.8 (11.5) | –8.2 (12.5) | <0.0001* | –0.65 |
| BAIa | 35 | 17.2 (12.9) | 8.1 (7.4) | –9.2 (9.6) | <0.0001* | –0.95 | 73 | 17.8 (10.5) | 11.8 (8.3) | –5.9 (7.5) | <0.0001* | –0.79 | 60 | 14.0 (11.5) | 10.0 (13.9) | –4.1 (14.6) | 0.0347 | –0.28 |
| BDI-IIa | 36 | 15.9 (10.3) | 8.3 (8.5) | –7.6 (8.1) | <0.0001* | –0.93 | 72 | 16.2 (9.3) | 11.7 (7.8) | –4.5 (6.1) | <0.0001* | –0.74 | 60 | 14.2 (9.2) | 8.7 (10.4) | –5.5 (11.3) | 0.0004* | –0.49 |
| Neurocog. Symptoms | 70 | 76.0 (25.7) | 58.5 (23.9) | –17.5 (24.7) | <0.0001* | –0.71 | 87 | 88.4 (29.3) | 62.7 (29.2) | –25.7 (26.8) | <0.0001* | –0.96 | 62 | 79.4 (26.8) | 58.8 (27.3) | –20.6 (23.3) | <0.0001* | –0.88 |
| Neurobeh. Symptoms | 69 | 79.7 (34.8) | 56.8 (27.0) | –23.0 (29.5) | <0.0001* | –0.78 | 86 | 89.4 (33.7) | 64.2 (29.5) | –25.2 (29.1) | <0.0001* | –0.87 | 61 | 77.3 (33.7) | 52.2 (26.2) | –25.2 (27.1) | <0.0001* | –0.93 |
aOnly adult patients are included for this measure; *Significant change at αbonf= 0.0014. Large effect sizes (Cohen’s dz) are bolded. For measures: Neurocognition Index through Brain Fitness, an increase in score indicates improvement. For measures: ESS through Neurobehavioral Symptoms, a decrease in score indicates improvement.
For the effect sizes of these pre-post changes in score, the CNS VS domain with the largest effect sizes for all three groups was Executive Functioning, with large effect sizes for all (for ADHD, dz= 0.86; for PCS, dz= 0.83; and for memory, dz= 1.09). The Neurocognition Index domain had a medium effect size for patients with PCS (dz= 0.75) and a large effect size for patients with ADHD (dz= 0.81) and memory deficits (dz= 0.93). Verbal Memory domain scores significantly improved after the program in the PCS (dz= 0.47) and memory (dz= 0.44) diagnosis groups, with small effect sizes for both.
Improvement in symptoms
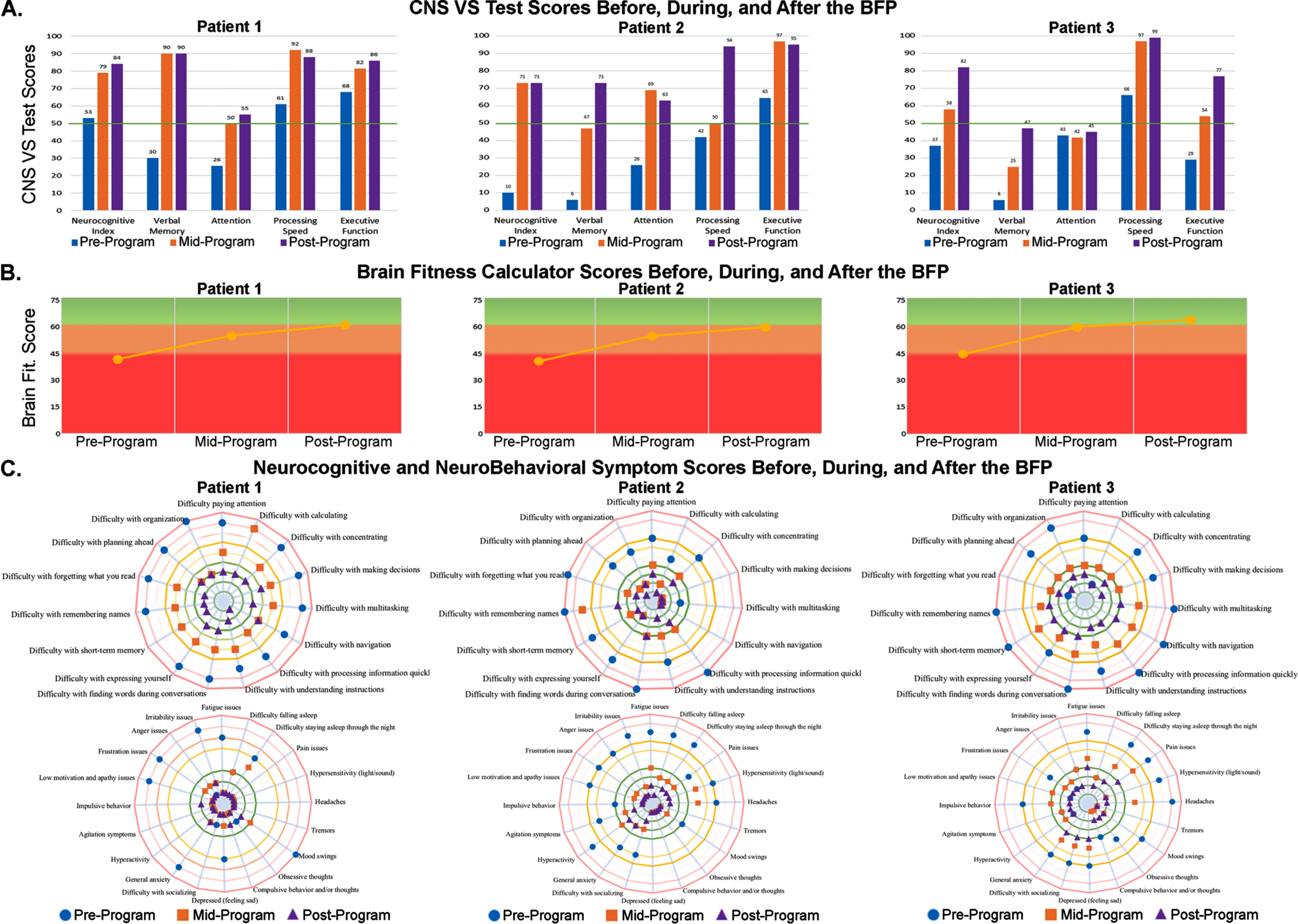
Patients completed several questionnaires to determine the nature and severity of their brain-related symptoms. These included four validated and standard questionnaires regarding sleep and mood (the ESS, PIRS20, BAI, and BDI-II) and three questionnaires which were designed at NeuroGrow Brain Fitness Center (The Brain Fitness Calculator, Neurocognitive Symptoms, and Neurobehavioral Symptoms; the full text for these questionnaires was published in [42]). An example of three representative patients with memory loss who experienced improvement in cognitive scores as well as a wide range of “Neurocognitive and Neurobehavioral Symptoms” is shown in Fig. 5.
Fig. 5
Raw data from three patients who completed the NeuroGrow BFP. Pre-program, post-program, and mid-program test scores and questionnaire responses are shown for three patients with memory loss: Patient 1, a 44-year-old woman (left panels of A, B, and C); Patient 2, a 38-year-old man (center panels of A, B, and C); and Patient 3, a 55-year-old woman (right panels of A, B, and C). A) CNS VS Neurocognitive testing at pre-program shows that these patients had particularly low scores for Verbal Memory at baseline. Scores were higher (better) at mid-point in the program and after completing the BFP. B) The Brain Fitness Calculator score was higher (better) at the mid-point and after completing the program when compared to their baseline (pre-program) score for all three patients. C) A pair of spider diagrams display numeric test scores for the Neurocognitive Symptoms Checklist (top circle) and NeuroBehavioral Symptoms Checklists (bottom circle). For both checklists, lower scores represent fewer/less severe symptoms and are graphed closer to the center of the diagram. This diagram demonstrates the specific questions that each patient responds to, and how these values decreased (improved) at mid-point and after completing the BFP. These spider diagrams highlight the important fact that each patient who reports having memory loss suffers from a unique constellation of symptoms and comorbidities, at different levels of severity. The goal of the program is for the reported symptoms of patients at their post-program visit (purple triangles) fall in the green target zone of these circles.
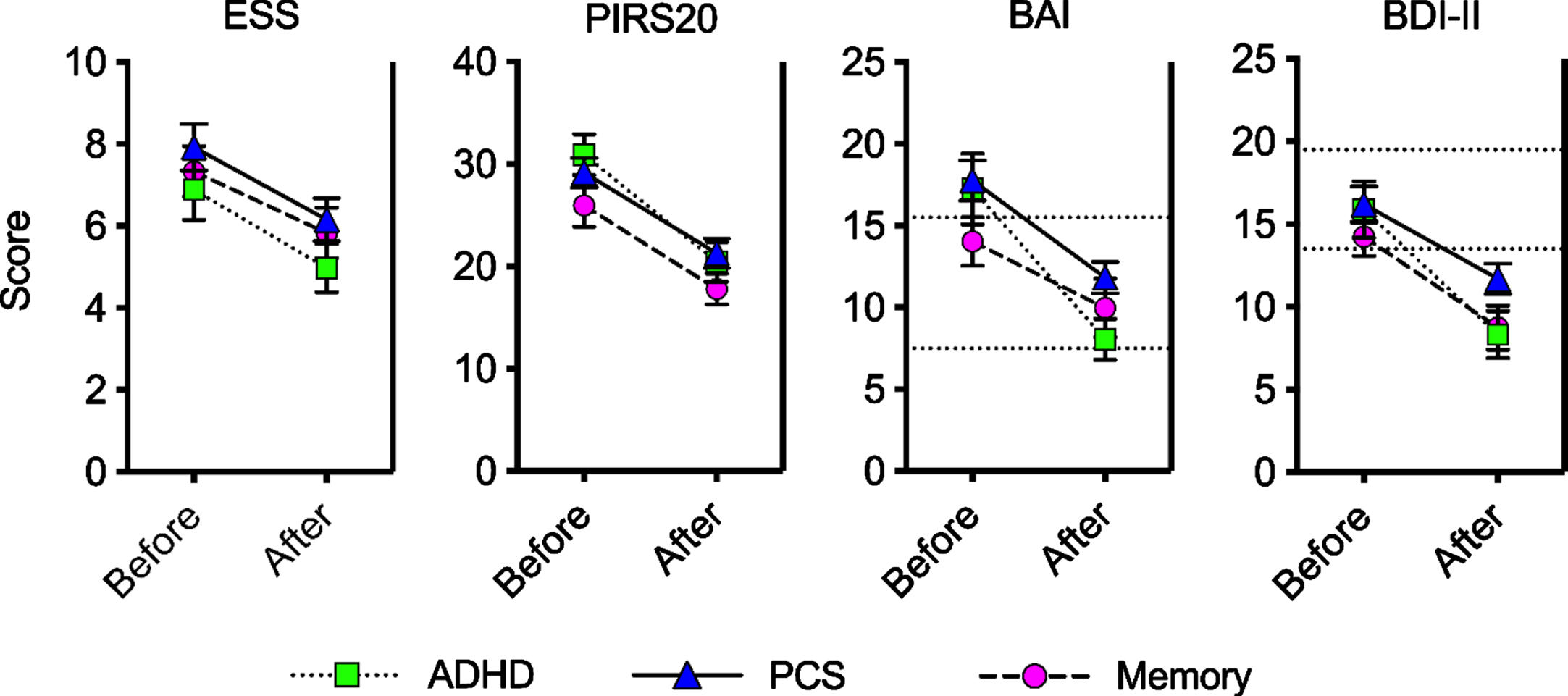
For the validated questionnaires on sleep and mood, mean pre- and post-program scores for all three diagnosis groups are shown graphically in Fig. 6. For the ADHD group and PCS group, the mean change in score from pre- to post-program was a significant increase (improvement) on all four questionnaires (p≤0.0006; Table 2). For the memory group, there was a significant increase in score for the PIRS20 and the BDI-II (p≤0.0004). For the ESS and the BAI, there was no significant mean change in score after Bonferroni correction for multiple testing.
Fig. 6
Mean scores before and after treatment on measures taken only by adult patients. For adult patients in the ADHD, PCS, and memory diagnosis groups, mean test scores are shown before and after the brain fitness program (error bars are standard error of the mean). For all these measures, a decrease in score indicates improvement. Scores on the ESS range from 0–24, and scores from 1–10 are within the normal daytime sleepiness range. Scores on the PIRS20 range from 0 to 60. Scores on the BAI range from 0–63, and the horizontal dotted lines represent the divisions between minimal (0–7), mild (8–15) and moderate (16–25) anxiety. Scores on the BDI-II range from 0 to 63, and the horizontal dotted lines represent the divisions between minimal (0–13), mild (14–19), and moderate (20–28) depression. In all cases except for the memory group ESS and BAI, the mean change in score from pre- to post-program (Table 2) was a significant decrease (improvement).
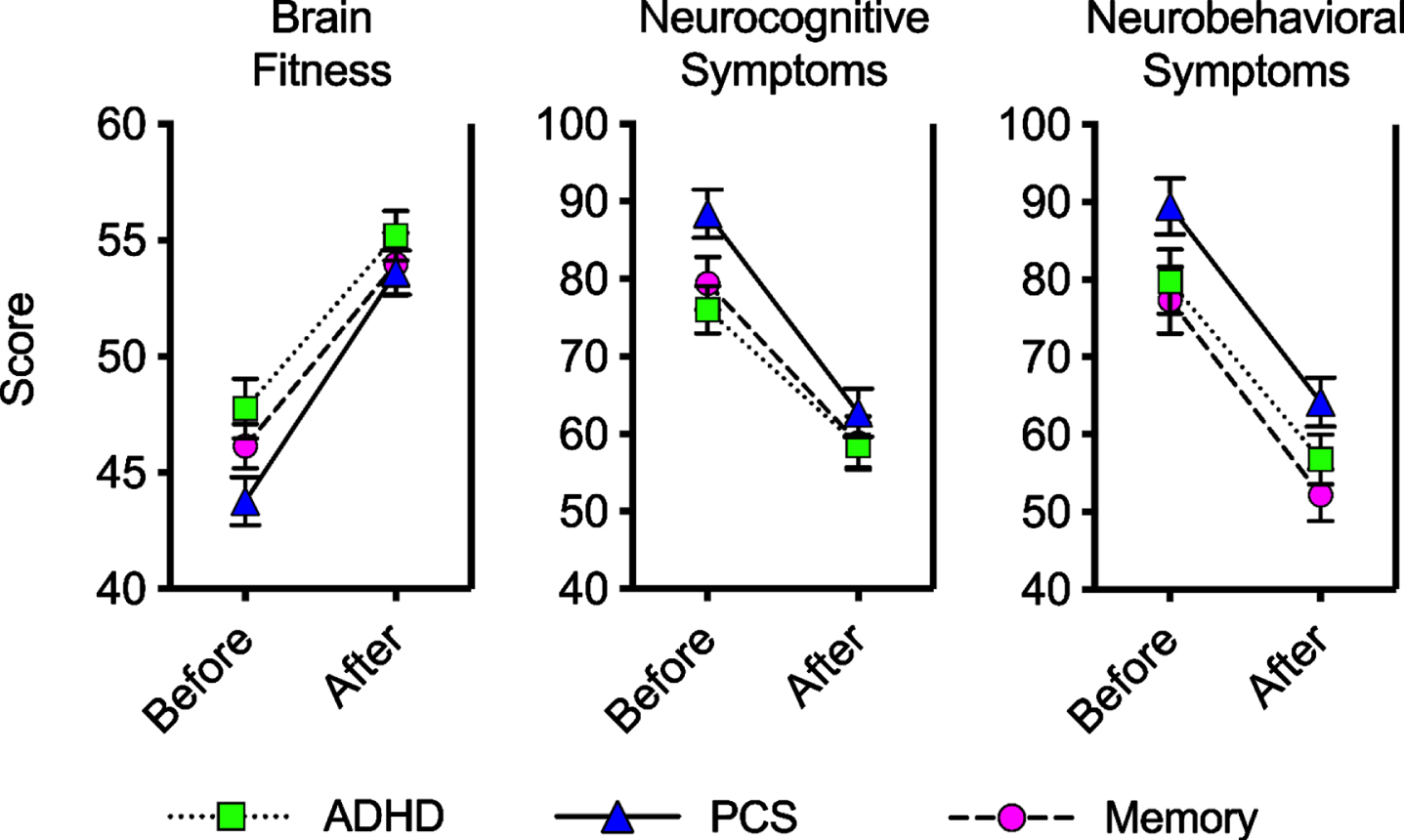
For the Brain Fitness Calculator Score (Table 2, Fig. 7), the mean change in score from pre- to post-program was a significant increase (improvement) for all three diagnosis groups (p ? 0.0001). For the ADHD diagnosis group, the effect size for this score change was large (dz = 0.80), for the PCS diagnosis group, the effect size was large (dz = 0.90), and for the memory group, the effect size was medium (dz = 0.76). The Neurocognitive Symptoms and Neurobehavioral Symptoms questionnaires allow for the improvement of symptoms to be tracked in patients during the BFP. For both Neurocognitive Symptoms and Neurobehavioral Symptoms, the mean change in score from pre- to post-program was a significant decrease (improvement) for all three diagnosis groups (p ≤.0001) with medium (ADHD) or large (PCS and memory) effect sizes.
Fig. 7
Mean scores before and after treatment on in-house tests. For patients in the ADHD, PCS, and memory diagnosis groups, mean test scores are shown before and after the Brain Fitness Program (error bars are standard error of the mean). Brain Fitness scores can range from 15 to 75, and an increase in score indicates improvement. Scores on the Neurocognitive Symptoms scale range from 15 to 150, and a decrease in score indicates improvement. Scores on the Neurobehavioral Symptoms scale range from 20 to 200, and a decrease in score indicates improvement. In all cases, the mean change in score from pre- to post-program (Table 2) was a significant score improvement.
Meaningful change in scores for individual patients
The percent of patients in the ADHD, PCS, or memory loss groups whose post-program scores was higher in the direction of improvement by at least one point when compared to their pre-program test scores are shown in Fig. 8. To determine whether individual patients experienced reliable score changes of clinical significance on the CNS VS domains Verbal Memory, Complex Attention, Processing Speed, and Executive Functioning, we utilized the Jacobson-Truax Reliable Change (RC) measure (see Methods). Using this metric, an individual patient would need a pre- to post-program increase (or decrease) in score of at least the following number of points to be considered a “reliable change”: Verbal Memory = 32 points; Complex Attention = 26 points; Processing Speed = 24 points; Executive Function = 26 points. Using this method, it is only possible to determine whether or not any “reliable change” in score is of “clinical significance” for patients who began the program with scores in the abnormal range for that test (see Methods). The percentage of patients in each diagnosis group who experienced a “reliable score improvement of clinical significance” is graphed in Fig. 8B and summarized below.
Fig. 8
Score changes by diagnosis category. A) Percent of patients in the ADHD, PCS, or memory loss groups whose post-program scores were “better” (in the direction of improvement according to each specific test) than their pre-program test scores. B) Percent of patients who scored less than 70 on their baseline test who improved by at least the RCI. These patients can be considered to have experienced reliable change of clinical importance. Below each bar, the number of patients in that diagnosis category who scored below 70 on the pre-program test is written.
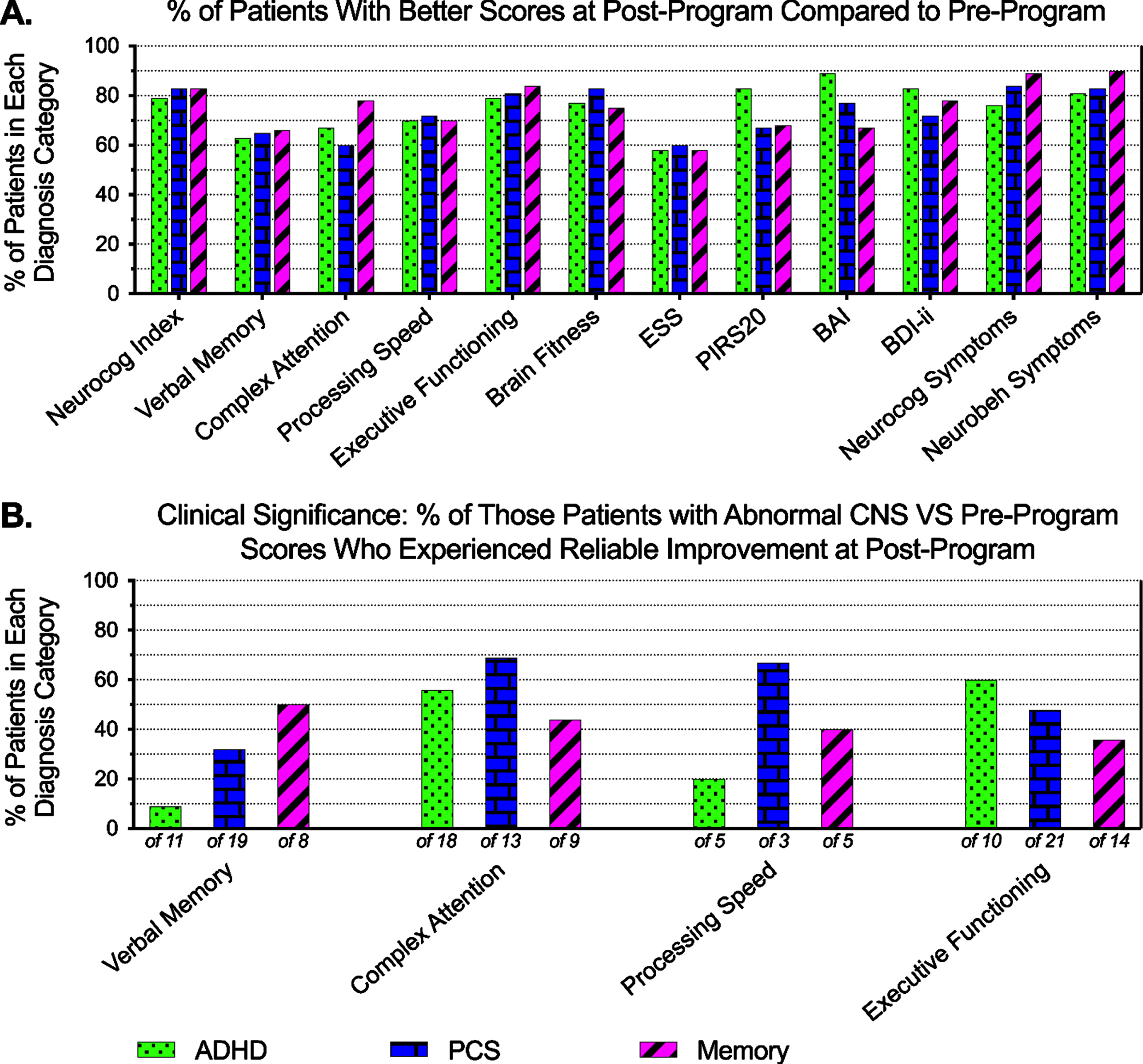
For the ADHD group, 11 patients (15%) were in the abnormal range for Verbal Memory before treatment; of these, 9% (one patient) experienced reliable improvement in score of clinical significance. For Complex Attention, 18 patients (26%) were in the abnormal range before treatment; of these, 56% experienced reliable improvement in score of clinical significance. For Processing Speed, 5 patients (7%) scored in the abnormal range before the program; of these, 20% (one patient) experienced reliable improvement of clinical significance. For Executive Functioning, 10 patients (14%) scored in the abnormal range before the program; of these, 60% experienced reliable improvement of clinical significance.
For the PCS group, 19 patients (22%) scored in the abnormal range for the Verbal Memory domain before the program; of these, 32% experienced a reliable improvement in score of clinical significance. For the Complex Attention domain, 13 patients (15%) scored in the abnormal range before the program; of these, 69% experienced a reliable improvement of clinical significance. For Processing Speed, 3 patients (3%) scored in the abnormal range before the program; of these, 66% experienced a reliable improvement of clinical significance. For Executive Functioning, 21 patients (24%) scored in the abnormal range before the program; of these, 48% experienced an improvement in score of clinical significance.
For the memory group, 8 patients (13%) scored in the abnormal range in Verbal Memory domain before the program; of these, 50% experienced an improvement in score of clinical significance. For Complex Attention, 9 patients (14%) were in the abnormal range before treatment; of these, 44% experienced a reliable improvement in score of clinical significance. For Processing Speed, 5 patients (8%) were in the abnormal range before the program; of these, 40% (two patients) experienced a reliable improvement in score of clinical significance. For Executive Functioning, 14 patients (22%) scored in the abnormal range before the program; of these, 40% experienced a reliable score improvement of clinical significance.
No evidence for differential effects of the program on different diagnosis subgroups
For each test/measure, a 2-way repeated measures ANOVA was performed to determine the effect of diagnosis subgroup (ADHD, PCS, or memory) on test score change from pre- to post- program. After Bonferroni correction for m = 12 tests, there was no evidence that diagnosis subgroup had an effect on score change over the course of the NeuroGrow BFP for any measure tested (p≥0.0242; further data not shown).
DISCUSSION
Patients with ADHD, PCS, or memory loss who completed the NeuroGrow Brain Fitness Program experienced significant improvement in scores for CNS VS Neurocognition Index, Complex Attention, Processing Speed, and Executive Functioning. Patients in the ADHD group did not experience improvements in the Verbal Memory domain. In this study, we included patients who completed at least 10 of the 24 total treatment sessions (twice weekly, for 12 weeks) in less than 26 weeks. After receiving one-on-one coaching to improve their lifestyle choices, brain training, and neurofeedback, 60% to 90% of patients reported having fewer sleep, mood, anxiety, and a list of other neurocognitive and neurobehavioral symptoms. Patients also experienced higher post-program “brain fitness” scores, which is an in-house measure of optimal brain function and quality of life [42].
The degree of improvement in cognitive scores and symptoms, which had medium to large effect sizes, were similar in all three patient groups; there was no evidence from repeated measures ANOVAs that the NeuroGrow BFP had different effects on patients in different diagnosis subgroups. Using the Jacobson-Truax RC method to determine whether individual patients experienced improvements, a subset of patients in each diagnosis category experienced reliable improvement of clinical significance in each of the CNS VS domains examined (ranging from 9% to 69% of those who began the study in the abnormal range for each test).
The focus of our initial research was to determine if a multimodal set of interventions can help patients with MCI [41]. In 2016, we published our findings, which showed that 84% of these patients had improvements in their cognitive capacity and that half of patients who had before-and-after quantitative MRIs had a significant increase in the size of their hippocampus [41]. We then tested the hypothesis that the same interventions can help patients who have had persistent concussion symptoms for months to years. In 2020, we published the results of our study in patients with PCS, which showed 89% had better cognitive scores at the end of this 12-week brain rehabilitation program [42]. In this study, we report that 80% of children and adults with ADHD gained improvement in their cognitive scores. We also found that 80% of patients with memory loss and 80% of patients with PCS in our current patient population had higher scores in their post-program testing. These findings are consistent with results from our earlier studies and those of a recent clinical trial, which showed more than 80% of patients with MCI or mild Alzheimer’s disease who received a set of personalized multi-modal interventions had better cognitive scores after 9 months of treatment [39].
Similar level of improvements in ADHD, PCS, and memory loss groups
The finding that there was no evidence for a difference in the level of improvement experienced by these three diverse patient populations in their cognitive performance from our multimodal set of interventions justifies starting larger clinical trials, similar to the FINGER study [78], to determine the biological mechanisms underlying these improvements. It is possible that the superior cognitive capacity in one group could be due to anatomical changes, higher levels of BDNF, or lower levels of cortisol, whereas in another group it could be due to better physical health and/or psychological well-being. As such, in our future studies we plan to obtain more quantitative data from our patients, including brain MRIs and blood biomarkers such as BDNF, cortisol, and markers of inflammation.
Benefits of a multimodal program for patients with ADHD
Our findings, along with results from other studies for patients with ADHD, PCS, or memory loss contribute to the growing evidence that a multi-disciplinary set of interventions is more likely to provide effective results than treatment protocols that focus only on one intervention. In particular, this is the case for clinical trials for ADHD, for which specific outcome measures and treatment protocols have been well established [79, 80]. A NIMH study found that children with ADHD who received a combination of treatments with medication and multi-component behavioral therapy gained more improvements in their symptoms than those who received either of these treatments alone [81]. A study that compared benefits of neurofeedback and taking ADHD medication found that children who received a combination of these two interventions had better outcomes [82]. Finally, a recent systematic review of nine randomized clinical trials concluded that a combination of neurofeedback, ADHD medication, physical activity, behavioral therapy training, or attention training with brain-computer interactions is more effective than mono-therapy [80].
In our future studies, we plan to compare the exact amount of brain training each patient receives (both during their brain coaching sessions in our office and during their brain training with computer games at their own home) to their degree of success in improving cognitive scores and symptoms. We will also include a waitlist control group with no interventions and a placebo group (which will receive sham neurofeedback treatment) as well as individual groups which will receive only neurofeedback treatment, only coaching for lifestyle enhancement, and only targeted cognitive training. Comparing the results from this group with those from the group that receives two or three interventions will help determine the if a multimodal treatment protocol is more effective than a mono-therapy protocol.
Similarly, with regards to stress reduction, some patients were trained to meditate, while others were offered to practice HRV biofeedback– depending on their interest and preference. This was necessary, as all the interventions in the program were tailored to the preferences and needs of individual patients. Some patients found it difficult to meditate, though they enjoyed participating in HRV biofeedback, and vice versa. In future studies, it would be optimal to keep track of the exact amount of stress reduction treatment each patient received. Patients should also be monitored 6 months to 12 months after they complete the program to determine whether the benefits of this program are long-lasting. Finally, the interventions in NeuroGrow BFP are intended to increase levels of BDNF, improve cerebral blood flow, promote neurogenesis, lower levels of cortisol, and promote physical growth in the hippocampus and prefrontal cortex. In order to determine whether or not this was the case, future studies will need to measure baseline and final levels of biomarkers for neuroplasticity, including levels of BDNF and cortisol and perform pre-post quantitative MRIs. These findings will help determine how closely biological markers correspond to improvements in cognitive scores and/or brain-related symptoms.
Combination of cognitive and behavioral symptoms in all patient subgroups
Patients who presented with symptoms of ADHD, PCS, or memory loss often suffered some degree of anxiety, depression, and insomnia or daytime hypersomnolence, as measured by BAI, BDI-ii, PIRS, and ESS. Though the focus of the NeuroGrow BFP was not solely to improve anxiety, mood, or sleep issues for patients, the results indicate that patients in all three diagnosis categories experienced improvements in their emotions, sleep pattern, and daytime alertness. These improvements, which may or may not include mechanisms related to neuroplasticity, may have contributed to their overall sense of well-being and having better cognitive capacity. Given that they had parallel improvements in their objective cognitive test scores, it is also possible that their improved cognitive capacity may have reduced their anxiety, enhanced their productivity, and in turn these led to better sleep at night and more energy during the day. As evidenced in higher brain fitness scores, most patients who completed this program reported having better self-esteem.
Brain fitness calculator
In an attempt to assess a combination of cognitive and non-cognitive issues as well as measures related to quality of life for each patient, we used an in-house questionnaire called the “Brain Fitness Calculator” [42]. This questionnaire includes 15 questions about sleep, mood, positive attitude, social engagement, cognitive stimulation, extracurricular activities and hobbies, as well as exercise, diet, and compliance with taking omega-3 supplements. Our results show that approximately 80% of patients in this study had higher brain fitness scores after they completed our program, in parallel to their improvements for specific cognitive domains and responses to other more commonly used questionnaires with a high degree of reliability and validity, such as BAI, BDI-ii, PIRS, and ESS [83–86]. As such, this instrument for evaluating global change in a patient with ADHD, PCS, and memory loss merits further evaluation in future studies.
A personalized set of interventions
Importantly, patients with ADHD, PCS, and memory loss experience a broad range of brain-related symptoms and comorbidities (Fig. 1). For example, patients with memory loss may also have varying degrees of depression, executive function deficits, slow thinking, insomnia, anxiety, or headaches [87]. Many of our patients with memory loss who also had significant difficulty sleeping, or had high levels of anxiety, sad mood, or attention issues did not meet the criteria for a formal diagnosis of major depressive disorder, general anxiety disorder, ADHD, and/or insomnia. Our encouraging results suggest that there is a need to recognize the broad spectrum of cognitive and non-cognitive symptoms that affect patients who present to a neurology practice with a single complaint such as poor memory and the importance of providing a multimodal and individualized set of interventions that treats all of their symptoms.
Limited pharmaceutical interventions are available for treating the multitude of symptoms in patients diagnosed with ADHD, PCS, or memory loss, and these available medications often produce only suboptimal and temporary benefits [88, 89]. These patients would likely have better outcomes if all of their individual cognitive deficits and particular behavioral symptoms (such as anxiety or insomnia) were identified, and then treated in a personalized set of interventions that aim to boost brain repair and growth. One reason for the apparent success of the NeuroGrow program is an emphasis on a holistic and personalized approach to address all of each patient’s cognitive and non-cognitive concerns. We identified the specific issues for each patient and provided them with targeted brain training and rehabilitation treatment such as lifestyle modifications, sleep counseling, and exercise coaching. Our results indicate that such individualized interventions can provide measurable objective improvements in both cognitive scores and symptoms.
Examples of patients with memory loss who completed the NeuroGrow BFP
Figure 5 illustrates how three different patients with memory loss can have a completely different set of co-morbidities and parallel cognitive deficits on formal neurocognitive evaluations. Patient 1 (left panels of Fig. 5A–C) is a 44-year-old woman whose CNS VS neurocognitive testing shows her Verbal Memory score is at 30th percentile for her age. Her brain fitness score is low (red zone) at baseline. She reports a level of 9 out of 10 (blue dots on the outer edge of the “neurocognitive” spider diagram) for difficulty with forgetting what she reads, expressing herself, concentrating, difficulty planning ahead, calculating, and several other cognitive functions. She also reports a level of 9 out of 10 (blue dots on the outer edge of “neurobehavioral” spider diagram) for difficulty with irritability, low motivation, general anxiety, and mood swings.
After six weeks of the BFP, her cognitive scores, brain fitness score, and many of her neurobehavioral symptoms improve, but she reports she still has a level of 4 out of 10 (orange squares in the yellow range of the neurocognitive spider diagram) difficulty with remembering names, expressing herself, paying attention, understanding instructions, and many of her other baseline neurocognitive symptoms. By the time she completes this 12-week program, all her cognitive scores are above the 50th percentile for her age, her brain fitness score is in the normal range (green), and all her reported symptoms are minimal (purple triangles in the green zone).
Patient 2 (center panels of Fig. 5A–C) is a 38-year-old man who also reports having poor memory. His baseline neurocognitive testing shows his CNS VS Verbal Memory score at baseline is at 6th percentile for his age. His brain fitness score is low (red zone). Although he reports suffering from memory-related symptoms, he does not appear to have marked complaints about his attention, executive function, navigation, or verbal fluency symptoms. His overall load of neurobehavioral symptoms is high, and he reports a level of 8 out of 10 difficulty falling asleep and staying asleep. As with the first patient, his cognitive scores, brain fitness score, and all of his brain-related symptoms improve by the mid-point in the program and reach normal levels by the end of the program.
Patient 3 (right panels of Fig. 5A–C) is a 55-year-old woman whose baseline CNS VS Verbal Memory score is at 6th percentile for her age, her brain fitness score is in the intermediate range (orange), and her brain-related symptoms include memory, executive function, navigation, and word-finding issues. Her neurobehavioral symptoms include fatigue, difficulty staying asleep, headaches, and hypersensitivity to light and sound. She too shows improvement in her mid-program assessment and marked resolution of her symptoms by the time she completes the program.
These three examples illustrate how patients who present to a neurology practice with concerns about their memory (and have memory deficits on objective neurocognitive tests) often have a constellation of different symptoms, most of which are interconnected (e.g., having anxiety leads to difficulty sleeping, which in turn leads to difficulty remembering things during the day). The challenges for neurologists or primary care physicians who treat patients with ADHD, PCS, and memory loss are to index all of their patients’ brain-related issues and address them in a holistic program.
Targeted cognitive training in a multimodal set of interventions
We provided intensive cognitive training for our patients through a series of brain games that were both challenging and enjoyable for our individual patients. The emphasis was on trying to push patients beyond their comfort zone while keeping the sessions enjoyable. A combination of hands on and computer-based games were used to challenge patients gradually at every visit (Supplementary Figure 1). For each of the hands-on games for each patient, their brain coach documented the level of the game he/she had reached each week. Their advances in doing more difficult brain games on the computer-based brain games were also documented and monitored. Depending on the level of interest from the patient, brain coaches also assigned “homework,” which consisted of doing more of the assigned hands-on or computer-based brain games at home.
There are mixed results regarding the meaningful benefits of cognitive training for patients with brain related symptoms [90]. Some studies have shown great benefits [91] while others demonstrate lack of any benefits [92, 93]. Given the concept of neuroplasticity and the fact that brain training has been shown to improve cortical thickness [94, 95] and cognitive capacity [96], the negative reports may be in part due to methodological issues. Reports that indicate a lack of benefit from brain training often do not provide targeted cognitive stimulation for the specific cognitive domain that the patient suffers from.
For example, if a patient has poor verbal memory, a generalized brain training program may not produce any benefits for him/her, whereas specific cognitive stimulation that targets improving verbal memory would. As such, it is important to focus on a patient’s specific cognitive impairment and construct an individualized rehabilitation program to address that specific domain. Moreover, even targeted cognitive training in isolation may provide only modest improvement, especially if a patient also suffers from sleep, anxiety, or depressed mood [97]. Targeted brain training provides more robust results when combined with interventions such as improving sleep, improving diet and exercise, and reducing anxiety. In our study, we found that anxiety, mood, and sleep issues were quite common in our three patient groups. We also noted parallel improvements in cognitive scores and improvements in sleep and neurobehavioral symptoms. Our findings support the notion that cognitive training is more effective when patients receive holistic treatment that addresses both cognitive and emotional/behavioral issues [97].
Benefits of non-drug interventions for patients with ADHD
The most effective treatment for ADHD is the use of medications such as methylphenidate or amphetamine salts [79]. These medications often produce remarkable improvement in cognitive performance and ADHD symptoms within hours. However, the benefits from taking a single dose do not last beyond one day, and not all children or adults can tolerate their side effects and complications. Their long-term daily use is associated with anorexia, weight loss, insomnia, and addiction [79]. Non-pharmacological treatments for ADHD include cognitive behavioral therapy, cognitive training, increasing physical exercise, and neurofeedback [79, 80].
Several randomized clinical trials have shown that neurofeedback is equally effective as treatment with stimulant ADHD medications, and that it provides long-lasting results with no significant side effects. A randomized clinical trial in 104 children comparing neurofeedback with cognitive therapy found both interventions improve ADHD symptoms, though the neurofeedback treatment appeared to be more effective than cognitive therapy [98]. Unlike the medication group, the neurofeedback group still had better performance in objective cognitive tests six months after the completion of the treatment protocol. These findings suggest that the most effective intervention, with the least side effects, would be a program that combines neurofeedback with cognitive training and lifestyle modifications to increase physical fitness. The positive findings for our patients with ADHD who received EEG-based neurofeedback, targeted cognitive training, and lifestyle coaching in our program are in line with these expected findings. We now need larger clinical trials with appropriate control groups to confirm our preliminary findings.
Emphasis on interventions for improving neuroplasticity in the brain
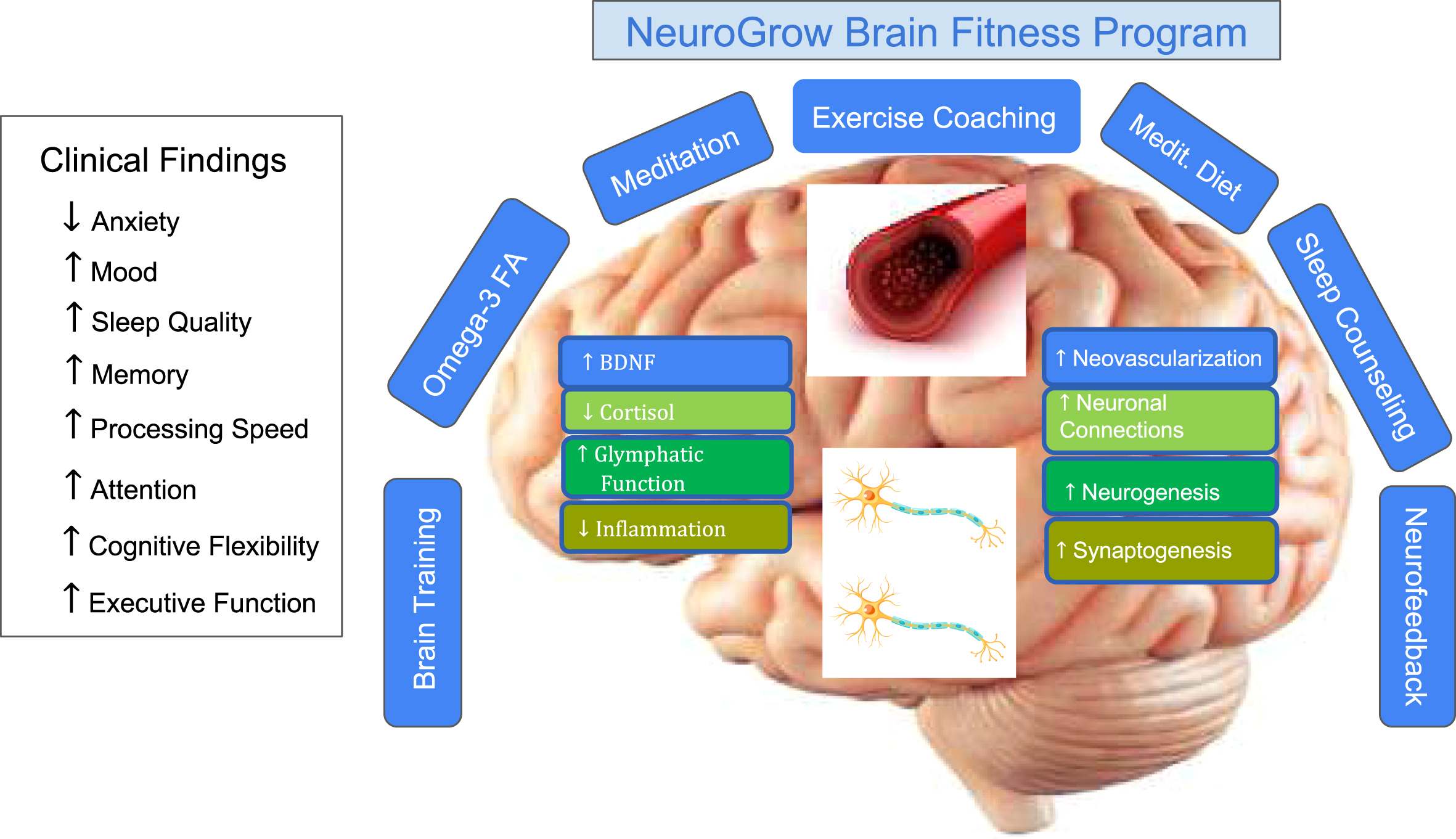
An overview of the Brain Fitness Program is shown in Fig. 2. Figure 9 shows the main components of the NeuroGrow BFP and its theoretical basis for improving clinical symptoms in patients through improving neuroplasticity in their brain. The neuroplasticity literature has provided compelling evidence that lifestyle modifications such as exercise, sleeping well, minimizing stress, and eating a Mediterranean diet can enhance the number of blood vessels in the brain, promote synaptogenesis, and trigger neurogenesis [13, 14, 38]. These changes in the brain appear to be mediated through increasing levels of BDNF and a reduction in cortisol levels as well as improvements in glymphatic function and a reduction in inflammation [13, 18, 99, 100]. Targeted brain training, HRV biofeedback, and neurofeedback have also been shown to improve attention, memory, and sleep patterns [44].
Fig. 9
Summary of the NeuroGrow Brain Fitness Program interventions and the mechanisms by which this multi-disciplinary set of interventions may improve clinical symptoms. In NeuroGrow’s 12-week Brain Fitness Program, patients receive EEG-based neurofeedback, which is a form of biofeedback to help normalize brain oscillations. They also receive life coaching (to improve their sleep, meditate, exercise more, eat a Mediterranean diet, and take omega-3 fatty acid supplements) as well as targeted brain training (to improve their memory, attention, executive function, processing speed, and other cognitive domains). The combination of these interventions can potentially increase levels of brain-derived neurotrophic factor (BDNF), reduce levels of cortisol, enhance glymphatic function, and reduce inflammation in the brain. They may also increase the number of blood vessels in the brain (neo-vascularization), promote neurogenesis, ameliorate connectivity in the brain, and result in a higher number of synapses. However, it is also possible that these interventions improve cognitive capacity and symptoms of patients with memory loss, ADHD, and PCS through other mechanisms such as better physical health and enhanced psychological factors. More studies with data from brain MRIs and blood biomarkers are needed to establish the exact mechanisms for improvements noted in these patient populations.
Strength and limitations
Strengths of this study included having a large group of patients from different age groups from a community-based population. Moreover, only patients who had disruptive symptoms and abnormalities in objective cognitive tests were included in this study. Their progress was monitored both by improvements in their computer-based cognitive evaluations (objective findings) and responses to a series of comprehensive questionnaires (subjective findings). The questionnaires included widely used assessment scales for anxiety and mood (BAI and BDI-ii) and sleep (ESS and PIRS) as well as 35 questions in the neurocognitive and neurobehavioral checklists and 15 questions in the Brain Fitness assessment. Finally, using identical tests and questionnaires for patients with ADHD, PCS, and memory loss allowed the parallel evaluation of the NeuroGrow BFP in these three groups of patients.
The main limitation of this study is the lack of a control or placebo group. Though it is difficult to find a comparable group of patients with the exact same profile of cognitive deficits and brain-related symptoms, studying a larger group of patients– and comparing them with a wait-list group– may make it possible to do a more definitive assessment of the NeuroGrow BFP. There was also a lack of consistency in the brain games that were used for different patients in each group. Some patients were trained to memorize a list of 100 words, while others were trained with hands-on brain games that improves executive function or processing speed. Because the program was personalized and each patient received targeted brain training and given that each patient had a variable degree of preferences for computer-based versus hands-on brain games and variable degree of enthusiasm to improve their cognitive capacity, very few patients received an identical dose of brain training. An additional limitation is the fact that patients of different ages were included in the study. To control for this, we have focused on the CNS VS battery of cognitive tests (which compares each patient’s performance to average values for individuals of the same age) and considered only adult patients in all statistical tests for the measures ESS, PIRS20, BAI, and BDI-ii.
In summary, we found that patients who completed the NeuroGrow Brain Fitness Program (twice-weekly brain training sessions for 12 weeks) experienced significant improvement in their symptoms and objective neurocognitive test scores from a personalized set of interventions through brain coaching and neurofeedback. These preliminary findings appear to show that multimodal interventions which are known to increase neuroplasticity in the brain, when personalized, can have benefits for patients with cognitive symptoms from a variety of neurological conditions. They support the need for more research into such transdiagnostic interventions for the treatment of patients with ADHD, PCS, or memory loss.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Rachel Tittle for her contributions in statistical analysis and edits of this manuscript. We also thank Ms. Kayleah Groeneveld for her help with our data analysis, and Ms. Pritty Dwivedy for her assistance in making the summary figure.
FUNDING
There was no grant from public or commercial funding agencies provided for this research. The funding for this study was provided by NeuroGrow Brain Fitness Center. Dr. Fotuhi, the owner of NeuroGrow, was involved in the process of data analysis, writing, editing, approval, and the decision to publish.
CONFLICT OF INTEREST/DISCLOSURE STATEMENT
The authors have no conflict of interest to report. The first two authors (M.F. and N.K.) are employees of NeuroGrow Brian Fitness Center, a private neurology practice located in Northern Virginia.
DATA AVAILABILITY
The data in this manuscript are available to be shared. Please send your request to Dr. Majid Fotuhi ([email protected]).
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/ADR-220091.
REFERENCES
[1] | Nimmo-Smith V , Merwood A , Hank D , Brandling J , Greenwood R , Skinner L , Law S , Patel V , Rai D ((2020) ) Non-pharmacological interventions for adult ADHD: A systematic review. Psychol Med 50: , 529–541. |
[2] | Ryan LM , Warden DL ((2003) ) Post concussion syndrome. Int Rev Psychiatry 15: , 310–316. |
[3] | Yamaguchi H , Maki Y , Yamagami T ((2010) ) Overview of non-pharmacological intervention for dementia and principles of brain-activating rehabilitation. Psychogeriatrics 10: , 206–213. |
[4] | Su Y , Yuki M , Otsuki M ((2020) ) Non-pharmacological interventions for post-stroke fatigue: Systematic review and network meta-analysis. J Clin Med 9: , 621. |
[5] | Cernich AN , Kurtz SM , Mordecai KL , Ryan PB ((2010) ) Cognitive rehabilitation in traumatic brain injury. Curr Treat Options Neurol 12: , 412–423. |
[6] | Shaffer J ((2016) ) Neuroplasticity and clinical practice: Building brain power for health. Front Psychol 7: , 1118. |
[7] | Ballarini T , Melo van Lent D , Brunner J , Schroder A , Wolfsgruber S , Altenstein S , Brosseron F , Buerger K , Dechent P , Dobisch L , Duzel E , Ertl-Wagner B , Fliessbach K , Freiesleben SD , Frommann I , Glanz W , Hauser D , Haynes JD , Heneka MT , Janowitz D , Kilimann I , Laske C , Maier F , Metzger CD , Munk M , Perneczky R , Peters O , Priller J , Ramirez A , Rauchmann B , Roy N , Scheffler K , Schneider A , Spottke A , Spruth EJ , Teipel SJ , Vukovich R , Wiltfang J , Jessen F , Wagner M , DELCODE study group ((2021) ) Mediterranean diet, Alzheimer disease biomarkers and brain atrophy in old age Neurology 96: , e2920–2932. |
[8] | Feart C , Samieri C , Barberger-Gateau P ((2010) ) Mediterranean diet and cognitive function in older adults. Curr Opin Clin Nutr Metab Care 13: , 14–18. |
[9] | Fu J , Tan LJ , Lee JE , Shin S ((2022) ) Association between the mediterranean diet and cognitive health among healthy adults: A systematic review and meta-analysis. Front Nutr 9: , 946361. |
[10] | Scarmeas N , Stern Y , Mayeux R , Manly JJ , Schupf N , Luchsinger JA ((2009) ) Mediterranean diet and mild cognitive impairment. Arch Neurol 66: , 216–225. |
[11] | Mestre H , Mori Y , Nedergaard M ((2020) ) The brain’s glymphatic system: Current controversies. Trends Neurosci 43: , 458–466. |
[12] | Gaitan JM , Boots EA , Dougherty RJ , Oh JM , Ma Y , Edwards DF , Christian BT , Cook DB , Okonkwo OC ((2019) ) Brain glucose metabolism, cognition, and cardiorespiratory fitness following exercise training in adults at risk for Alzheimer’s disease. Brain Plast 5: , 83–95. |
[13] | Fotuhi M , Do D , Jack C ((2012) ) Modifiable factors that alter the size of the hippocampus with ageing. Nat Rev Neurol 8: , 189–202. |
[14] | Fotuhi M , Antoniades CB ((2013) ) Boost your brain: The new art and science behind enhanced brain performance, HarperOne, San Francisco. |
[15] | Yu F , Mathiason MA , Han S , Gunter JL , Jones D , Botha H , Jack C Jr ((2021) ) Mechanistic effects of aerobic exercise in Alzheimer’s disease: Imaging findings from the pilot FIT-AD Trial. Front Aging Neurosci 13: , 703691. |
[16] | Erickson KI , Voss MW , Prakash RS , Basak C , Szabo A , Chaddock L , Kim JS , Heo S , Alves H , White SM , Wojcicki TR , Mailey E , Vieira VJ , Martin SA , Pence BD , Woods JA , McAuley E , Kramer AF ((2011) ) Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A 108: , 3017–3022. |
[17] | Ghaziri J , Tucholka A , Larue V , Blanchette-Sylvestre M , Reyburn G , Gilbert G , Levesque J , Beauregard M ((2013) ) Neurofeedback training induces changes in white and gray matter. Clin EEG Neurosci 44: , 265–272. |
[18] | Fotuhi M , Hachinski V , Whitehouse PJ ((2009) ) Changing perspectives regarding late-life dementia. Nat Rev Neurol 5: , 649–658. |
[19] | Lam B , Masellis M , Freedman M , Stuss DT , Black SE ((2013) ) Clinical, imaging, and pathological heterogeneity of the Alzheimer’s disease syndrome. Alzheimers Res Ther 5: , 1. |
[20] | Delano-Wood L , Bondi MW , Sacco J , Abeles N , Jak AJ , Libon DJ , Bozoki A ((2009) ) Heterogeneity in mild cognitive impairment: Differences in neuropsychological profile and associated white matter lesion pathology. J Int Neuropsychol Soc 15: , 906–914. |
[21] | Giraldo DL , Sijbers J , Romero E , Alzheimer’s Disease Neuroimaging Initiative ((2021) ) Quantification of cognitive impairment to characterize heterogeneity of patients at risk of developing Alzheimer’s disease dementia. Alzheimers Dement (Amst) 13: , e12237. |
[22] | Langdon S , Goedhart E , Inklaar M , Oosterlaan J , Konigs M ((2023) ) Heterogeneity of persisting symptoms after sport-related concussion (SRC): Exploring symptom subtypes and patient subgroups. J Neurol 270: , 1512–1523. |
[23] | Kenzie ES , Parks EL , Bigler ED , Lim MM , Chesnutt JC , Wakeland W ((2017) ) Concussion as a multi-scale complex system: An interdisciplinary synthesis of current knowledge. Front Neurol 8: , 513. |
[24] | Luo Y , Weibman D , Halperin JM , Li X ((2019) ) A review of heterogeneity in attention deficit/hyperactivity disorder (ADHD). Front Hum Neurosci 13: , 42. |
[25] | Mostert JC , Onnink AMH , Klein M , Dammers J , Harneit A , Schulten T , van Hulzen KJE , Kan CC , Slaats-Willemse D , Buitelaar JK , Franke B , Hoogman M ((2015) ) Cognitive heterogeneity in adult attention deficit/hyperactivity disorder: A systematic analysis of neuropsychological measurements. Eur Neuropsychopharmacol 25: , 2062–2074. |
[26] | Vos M , Rommelse NNJ , Franke B , Oosterlaan J , Heslenfeld DJ , Hoekstra PJ , Klein M , Faraone SV , Buitelaar JK , Hartman CA ((2022) ) Characterizing the heterogeneous course of inattention and hyperactivity-impulsivity from childhood to young adulthood. Eur Child Adolesc Psychiatry 31: , 1–11. |
[27] | Mintzer J , Donovan KA , Kindy AZ , Lock SL , Chura LR , Barracca N ((2019) ) Lifestyle choices and brain health. Front Med (Lausanne) 6: , 204. |
[28] | Mace RA , Greenberg J , Stauder M , Reynolds G , Vranceanu AM ((2022) ) My Healthy Brain: A multimodal lifestylerogram to promote brain health. Aging Ment Health 26: , 980–991. |
[29] | Salzman T , Sarquis-Adamson Y , Son S , Montero-Odasso M , Fraser S ((2022) ) Associations of multidomain interventions with improvements in cognition in mild cognitive impairment: A systematic review and meta-analysis. JAMA Netw Open 5: , e226744. |
[30] | Brasser M , Fruhholz S , Schneeberger AR , Ruschetti GG , Schaerli R , Haner M , Studer-Luethi B ((2022) ) A randomized controlled trial study of a multimodal intervention vs. cognitive training to foster cognitive and affective health in older adults. Front Psychol 13: , 866613. |
[31] | Solomon A , Stephen R , Altomare D , Carrera E , Frisoni GB , Kulmala J , Molinuevo JL , Nilsson P , Ngandu T , Ribaldi F , Vellas B , Scheltens P , Kivipelto M , European Task Force for Brain Health Services ((2021) ) Multidomain interventions: State-of-the-art and future directions for protocols to implement precision dementia risk reduction. A user manual for Brain Health Services-part 4 of 6. Alzheimers Res Ther 13: , 171. |
[32] | Chalfont G , Milligan C , Simpson J ((2020) ) A mixed methods systematic review of multimodal non-pharmacological interventions to improve cognition for people with dementia. Dementia (London) 19: , 1086–1130. |
[33] | Jaganathan KS , Sullivan KA ((2020) ) Moving towards individualised and interdisciplinary approaches to treat persistent post-concussion symptoms. EClinicalMedicine 18: , 100230. |
[34] | Anderson V , Rausa VC , Anderson N , Parkin G , Clarke C , Davies K , McKinlay A , Crichton A , Davis GA , Dalziel K , Dunne K , Barnett P , Hearps SJ , Takagi M , Babl FE ((2021) ) Protocol for a randomised clinical trial of multimodal postconcussion symptom treatment and recovery: The Concussion Essentials study. BMJ Open 11: , e041458. |
[35] | Nguyen JVK , McKay A , Ponsford J , Davies K , Makdissi M , Drummond SPA , Reyes J , Willmott C ((2022) ) Interdisciplinary Rehabilitation for Concussion Recovery (i-RECOveR): Protocol of an investigator-blinded, randomised, case series with multiple baseline design to evaluate the feasibility and preliminary efficacy of a 12-week treatment for persistent post-concussion symptoms. Pilot Feasibility Stud 8: , 198. |
[36] | Rejani TG , Oommen A , Srinath S , Kapur M ((2012) ) Efficacy of multimodal intervention for children with attention deficit hyperactivity disorder (ADHD)—an Indian study. J Behav Brain Sci 02: , 117–127. |
[37] | Kivipelto M , Mangialasche F , Ngandu T ((2018) ) Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat Rev Neurol 14: , 653–666. |
[38] | Livingston G , Huntley J , Sommerlad A , Ames D , Ballard C , Banerjee S , Brayne C , Burns A , Cohen-Mansfield J , Cooper C , Costafreda SG , Dias A , Fox N , Gitlin LN , Howard R , Kales HC , Kivimaki M , Larson EB , Ogunniyi A , Orgeta V , Ritchie K , Rockwood K , Sampson EL , Samus Q , Schneider LS , Selbaek G , Teri L , Mukadam N ((2020) ) Dementiaprevention, intervention, and care: 2020 report of the LancetCommission. Lancet 396: , 413–446. |
[39] | Toups K , Hathaway A , Gordon D , Chung H , Raji C , Boyd A , Hill BD , Hausman-Cohen S , Attarha M , Chwa WJ , Jarrett M , Bredesen DE ((2022) ) Precision medicine approach to Alzheimer’s disease: Successful pilot project. J Alzheimers Dis 88: , 1411–1421. |
[40] | Conners CK , Epstein JN , March JS , Angold A , Wells KC , Klaric J , Swanson JM , Arnold LE , Abikoff HB , Elliott GR , Greenhill LL , Hechtman L , Hinshaw SP , Hoza B , Jensen PS , Kraemer HC , Newcorn JH , Pelham WE , Severe JB , Vitiello B , Wigal T ((2001) ) Multimodal treatment of ADHD in the MTA: An alternative outcome analysis. J Am Acad Child Adolesc Psychiatry 40: , 159–167. |
[41] | Fotuhi M , Lubinski B , Trullinger M , Hausterman N , Riloff T , Hadadi M , Raji CA ((2016) ) A personalized 12-week “brain fitness program” for improving cognitive function and increasing the volume of hippocampus in elderly with mild cognitive impairment. J Prev Alzheimers Dis 3: , 133–137. |
[42] | Fotuhi M , Dwivedy P , Yeom LH , Nadeem I , Ebadi AY , Miles M , Tittle RK ((2020) ) Retrospective analysis of a comprehensive concussion recovery program. J Rehabil 86: , 20–31. |
[43] | Thatcher RW , Lubar JF , Koberda JL ((2019) ) Z-score EEG biofeedback: Past, present, and future. Biofeedback 47: , 89–103. |
[44] | Meeuwsen KD , Groeneveld KM , Walker LA , Mennenga AM , Tittle RK , White EK ((2021) ) Z-score neurofeedback, heart rate variability biofeedback, and brain coaching for older adults with memory concerns. Restor Neurol Neurosci 39: , 9–37. |
[45] | Riesco-Matias P , Yela-Bernabe JR , Crego A , Sanchez-Zaballos E ((2021) ) What do meta-analyses have to say about the efficacy of neurofeedback applied to children with ADHD? Review of previous meta-analyses and a new meta-analysis. J Atten Disord 25: , 473–485. |
[46] | Surmeli T , Eralp E , Mustafazade I , Kos IH , Ozer GE , Surmeli OH ((2017) ) Quantitative EEG neurometric analysis-guided neurofeedback treatment in postconcussion syndrome (PCS): Forty cases. How is neurometric analysis important for the treatment of PCS and as a biomarker? Clin EEG Neurosci 48: , 217–230. |
[47] | Surmeli T , Eralp E , Mustafazade I , Kos H , Ozer GE , Surmeli OH ((2016) ) Quantitative EEG neurometric analysis-guided neurofeedback treatment in dementia: 20 cases. How neurometric analysis is important for the treatment of dementia and as a biomarker? Clin EEG Neurosci 47: , 118–133. |
[48] | Broshek DK , Pardini JE , Herring SA ((2022) ) Persisting symptoms after concussion: Time for a paradigm shift. PM R 14: , 1509–1513. |
[49] | Nunez-Jaramillo L , Herrera-Solis A , Herrera-Morales WV ((2021) ) ADHD: Reviewing the causes and evaluating solutions. J Pers Med 11: , 166. |
[50] | Mayes SD , Calhoun SL , Bixler EO , Vgontzas AN , Mahr F , Hillwig-Garcia J , Elamir B , Edhere-Ekezie L , Parvin M ((2009) ) ADHD subtypes and comorbid anxiety, depression, and oppositional-defiant disorder: Differences in sleep problems. J Pediatr Psychol 34: , 328–337. |
[51] | Zhang SY , Qiu SW , Pan MR , Zhao MJ , Zhao RJ , Liu L , Li HM , Wang YF , Qian QJ ((2021) ) Adult ADHD, executive function, depressive/anxiety symptoms, and quality of life: A serial two-mediator model. J Affect Disord 293: , 97–108. |
[52] | Lambez B , Harwood-Gross A , Golumbic EZ , Rassovsky Y ((2020) ) Non-pharmacological interventions for cognitive difficulties in ADHD: A systematic review and meta-analysis. J Psychiatr Res 120: , 40–55. |
[53] | Garcia-Ptacek S , Cavallin L , Kareholt I , Kramberger MG , Winblad B , Jelic V , Eriksdotter M ((2014) ) Subjective cognitive impairment subjects in our clinical practice. Dement Geriatr Cogn Dis Extra 4: , 419–430. |
[54] | Wolraich ML , Lambert W , Doffing MA , Bickman L , Simmons T , Worley K ((2003) ) Psychometric properties of the Vanderbilt ADHD diagnostic parent rating scale in a referred population. J Pediatr Psychol 28: , 559–567. |
[55] | Polinder S , Cnossen MC , Real RGL , Covic A , Gorbunova A , Voormolen DC , Master CL , Haagsma JA , Diaz-Arrastia R , von Steinbuechel N ((2018) ) A multidimensional approach to post-concussion symptoms in mild traumatic brain injury. Front Neurol 9: , 1113. |
[56] | Petersen RC , Negash S ((2008) ) Mild cognitive impairment: An overview. CNS Spectr 13: , 45–53. |
[57] | CNS Vital Signs brief interpretation guide, https://www.cnsvs.com/WhitePapers/CNSVS-BriefInterpretationGuide.pdf, Accessed May 29. |
[58] | Johns MW ((1991) ) A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep 14: , 540–545. |
[59] | Johns MW , Hocking B ((1997) ) Daytime sleepiness and sleep habits of Australian workers. Sleep 20: , 844–849. |
[60] | Moul DE , Pilkonis PA , Miewald JM , Carey TJ , Buysse DJ ((2002) ) Preliminary study of the test-retest reliability and concurrent validities of the Pittsburgh Insomnia Rating Scale (PIRS). Sleep 25: , A246–247. |
[61] | Beck AT , Epstein N , Brown G , Steer RA ((1988) ) An inventory for measuring clinical anxiety: Psychometric properties. J Consult Clin Psychol 56: , 893–897. |
[62] | Beck AT , Steer RA ((1993) ) Beck Anxiety Inventory Manual, Psychological Corporation, San Antonio, TX. |
[63] | Beck AT , Steer RA , Brown GK ((1996) ) Manual for the Beck Depression Inventory-II, Psychological Corporation, San Antonio, TX. |
[64] | Budzynski TH , Budzynski HK , Evans JR , Abarbanel A ((2009) ) Introduction to quantitative EEG and neurofeedback: Advanced theory and applications Academic Press/Elsevier, Amsterdam. |
[65] | Permenter CM , Fernandez-de Thomas RJ , Sherman AL ((2023) ) Postconcussive syndrome. In StatPearls, Treasure Island (FL). |
[66] | Kivela K , Elo S , Kyngas H , Kaariainen M ((2014) ) The effects of health coaching on adult patients with chronic diseases: A systematic review. Patient Educ Couns 97: , 147–157. |
[67] | Combourieu Donnezan L , Perrot A , Belleville S , Bloch F , Kemoun G ((2018) ) Effects of simultaneous aerobic and cognitive training on executive functions, cardiovascular fitness and functional abilities in older adults with mild cognitive impairment. Ment Health Phys Act 15: , 78–87. |
[68] | Kletzel SL , Sood P , Negm A , Heyn PC , Krishnan S , Machtinger J , Hu X , Devos H ((2021) ) Effectiveness of brain gaming in older adults with cognitive impairments: A systematic review and meta-analysis. J Am Med Dir Assoc 22: , 2281–2288 e2285. |
[69] | Lim CG , Lee TS , Guan C , Sheng Fung DS , Cheung YB , Teng SS , Zhang H , Krishnan KR ((2010) ) Effectiveness of a brain-computer interface based programme for the treatment of ADHD: A pilot study. Psychopharmacol Bull 43: , 73–82. |
[70] | Fotuhi M , Intervention and Information for Optimizing Your Brain Function, https://neurogrow.com/wp-content/uploads/2021/04/Interventions-and-Information-for-our-website.pdf, Accessed May 29, 2023. |
[71] | Office of Disease Prevention and Health Promotion, 2008 Physical activity guidelines for Americans summary, https://health.gov/paguidelines/guidelines/summary.aspx, Accessed December 23, 2017. |
[72] | Chatterjee P , Kumar DA , Naqushbandi S , Chaudhary P , Khenduja P , Madan S , Fatma S , Khan MA , Singh V ((2022) ) Effect of Multimodal Intervention (computer based cognitive training, diet and exercise) in comparison to health awareness among older adults with Subjective Cognitive Impairment (MISCI-Trial)-a pilot randomized control trial PLoS One 17: , e0276986. |
[73] | Fotuhi M , the Passport to a Sharper Brain, https://neurogrow.com/wp-content/uploads/2022/12/The-Passport-Booklet-.pdf, Accessed May 29 2023. |
[74] | Lakens D ((2013) ) Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front Psychol 4: , 863. |
[75] | Jacobson NS , Truax P ((1991) ) Clinical significance: A statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol 59: , 12–19. |
[76] | Littleton AC , Register-Mihalik JK , Guskiewicz KM ((2015) ) Test-retest reliability of a computerized concussion test: CNS Vital Signs. Sports Health 7: , 443–447. |
[77] | Chomycz S , Schmidt F ((2016) ) Practice guidelines for the assessment of clinically significant treatment outcomes in the children’s mental health system. J Evid Inf Soc Work 13: , 236–248. |
[78] | Rosenberg A , Mangialasche F , Ngandu T , Solomon A , Kivipelto M ((2020) ) Multidomain interventions to prevent cognitive impairment, Alzheimer’s disease, and dementia: From FINGER to World-Wide FINGERS. J Prev Alzheimers Dis 7: , 29–36. |
[79] | Catala-Lopez F , Hutton B , Nunez-Beltran A , Page MJ , Ridao M , Macias Saint-Gerons D , Catala MA , Tabares-Seisdedos R , Moher D ((2017) ) The pharmacological and non-pharmacological treatment of attention deficit hyperactivity disorder in children and adolescents: A systematic review with network meta-analyses of randomised trials. PLoS One 12: , e0180355. |
[80] | Sampedro Baena L , Fuente GAC , Martos-Cabrera MB , Gomez-Urquiza JL , Albendin-Garcia L , Romero-Bejar JL , Suleiman-Martos N ((2021) ) Effects of neurofeedback in children with attention-deficit/hyperactivity disorder: A systematic review. J Clin Med 10: , 3797. |
[81] | The MTA Cooperative Group ((1999) ) A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. The MTA Cooperative Group. Multimodal treatment study of children with ADHD. Arch Gen Psychiatry 56: , 1073–1086. |
[82] | Duric NS , Assmus J , Gundersen D , Duric Golos A , Elgen IB ((2017) ) Multimodal treatment in children and adolescents with attention-deficit/hyperactivity disorder: A 6-month follow-up. Nord J Psychiatry 71: , 386–394. |
[83] | Fydrich T , Dowdall D , Chambless DL ((1992) ) Reliability and validity of the beck anxiety inventory. J Anxiety Disord 6: , 55–61. |
[84] | Levin BE , Llabre MM , Weiner WJ ((1988) ) Parkinson’s disease and depression: Psychometric properties of the Beck Depression Inventory. J Neurol Neurosurg Psychiatry 51: , 1401–1404. |
[85] | Backhaus J , Junghanns K , Broocks A , Riemann D , Hohagen F ((2002) ) Test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom Res 53: , 737–740. |
[86] | Johns MW ((1992) ) Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep 15: , 376–381. |
[87] | Leddy JJ , Sandhu H , Sodhi V , Baker JG , Willer B ((2012) ) Rehabilitation of concussion and post-concussion syndrome. Sports Health 4: , 147–154. |
[88] | Johansson B , Wentzel AP , Andrell P , Ronnback L , Mannheimer C ((2017) ) Long-term treatment with methylphenidate for fatigue after traumatic brain injury. Acta Neurol Scand 135: , 100–107. |
[89] | Hurwitz M , Lucas S , Bell KR , Temkin N , Dikmen S , Hoffman J ((2020) ) Use of amitriptyline in the treatment of headache after traumatic brain injury: Lessons learned from a clinical trial. Headache 60: , 713–723. |
[90] | Harvey PD , McGurk SR , Mahncke H , Wykes T ((2018) ) Controversies in computerized cognitive training. Biol Psychiatry Cogn Neurosci Neuroimaging 3: , 907–915. |
[91] | Jaeggi SM , Buschkuehl M , Jonides J , Shah P ((2011) ) Short- and long-term benefits of cognitive training. Proc Natl Acad Sci U S A 108: , 10081–10086. |
[92] | Sala G , Gobet F ((2019) ) Cognitive training does not enhance general cognition. Trends Cogn Sci 23: , 9–20. |
[93] | Stojanoski B , Lyons KM , Pearce AAA , Owen AM ((2018) ) Targeted training: Converging evidence against the transferable benefits of online brain training on cognitive function. Neuropsychologia 117: , 541–550. |
[94] | Engvig A , Fjell AM , Westlye LT , Moberget T , Sundseth O , Larsen VA , Walhovd KB ((2010) ) Effects of memory training on cortical thickness in the elderly. Neuroimage 52: , 1667–1676. |
[95] | Duerden EG , Laverdure-Dupont D ((2008) ) Practice makes cortex. J Neurosci 28: , 8655–8657. |
[96] | Schweizer S , Hampshire A , Dalgleish T ((2011) ) Extending brain-training to the affective domain: Increasing cognitive and affective executive control through emotional working memory training. PLoS One 6: , e24372. |
[97] | Barman A , Chatterjee A , Bhide R ((2016) ) Cognitive impairment and rehabilitation strategies after traumatic brain injury. Indian J Psychol Med 38: , 172–181. |
[98] | Steiner NJ , Frenette EC , Rene KM , Brennan RT , Perrin EC ((2014) ) In-school neurofeedback training for ADHD: Sustained improvements from a randomized control trial. Pediatrics 133: , 483–492. |
[99] | Brigadski T , Lessmann V ((2020) ) The physiology of regulated BDNF release. Cell Tissue Res 382: , 15–45. |
[100] | von Holstein-Rathlou S , Petersen NC , Nedergaard M ((2018) ) Voluntary running enhances glymphatic influx in awake behaving, young mice. Neurosci Lett 662: , 253–258. |




