The Abnormal Alternations of Brain Imaging in Patients with Chronic Obstructive Pulmonary Disease: A Systematic Review
Abstract
Background:
Cognitive impairment (CI) is an important extrapulmonary complication in patients with chronic obstructive pulmonary disease (COPD). Multimodal Neuroimaging Examination can display changes in brain structure and functions in patients with COPD.
Objective:
The purpose of this systematic review is to provide an overview of the variations in brain imaging in patients with COPD and their potential relationship with CI. Furthermore, we aim to provide new ideas and directions for future research.
Methods:
Literature searches were performed using the electronic databases PubMed, Scopus, and ScienceDirect. All articles published between January 2000 and November 2021 that met the eligibility criteria were included.
Results:
Twenty of the 23 studies focused on changes in brain structure and function. Alterations in the brain’s macrostructure are manifested in the bilateral frontal lobe, hippocampus, right temporal lobe, motor cortex, and supplementary motor area. The white matter microstructural changes initially appear in the bilateral frontal subcortical region. Regarding brain function, patients with COPD exhibited reduced frontal cerebral perfusion and abnormal alterations in intrinsic brain activity in the bilateral posterior cingulate cortex, precuneus, right lingual gyrus, and left anterior central gyrus. Currently, there is limited research related to brain networks.
Conclusion:
CI in patients with COPD may present as a type of dementia different from Alzheimer’s disease, which tends to manifest as frontal cognitive decline early in the disease. Further studies are required to clarify the neurobiological pathways of CI in patients with COPD from the perspective of brain connectomics based on the whole-brain system in the future.
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is a common chronic lung disease, with shortness of breath, chronic cough, and expectoration as the main symptoms. It is characterized by incompletely reversible airflow limitation associated with an abnormal inflammatory response of the lungs to noxious particles or gases. It affects more than 300 million people worldwide [1], representing the third leading cause of death and the fifth cause of disability. In addition, COPD is a complex multisystem disease with various extrapulmonary complications, including osteoporosis, metabolic syndrome, diabetes, cognitive impairment (CI), etc. [2–5]. Studies have reported that the morbidity due to CI in patients with COPD is generally higher than in healthy individuals with similar clinical characteristics. Approximately 36% of patients with COPD would experience CI, while the prevalence of CI in the general population is 12–16.7% [6]. CI affected the functional ability and disease management of patients with COPD, which increased mortality and posed a significant burden on public health and the social economy [7].
In contrast to recognized types of dementia, such as Alzheimer’s disease (AD), the CI in patients with COPD is domain-specific and impairs attention, memory, executive function, language, and information processing, while the general cognitive ability is retained [8–9]. Previous studies have reported that COPD may promote the development of CI through the following mechanisms: tissue hypoxemia, cerebrovascular regulation disorder, hypercapnia, systemic inflammation, and oxidative stress [10]. With the development of neuroimaging, based on the quantitative and qualitative evaluation of magnetic resonance imaging (MRI), researchers discovered that patients with COPD had brain abnormalities in many aspects including brain structure, brain function, and brain network. Brain imaging changes can be utilized to investigate the mechanism of CI in patients with COPD, which has attracted a great deal of attention. However, relevant research was relatively rare, and the findings were partly conflicting. To our knowledge, no study has comprehensively and systematically analyzed and summarized the findings of various clinical imaging literature in the form of a systematicreview.
This revised review aims to summarize the abnormal alternations of brain imaging in patients with COPD from previous studies and to provide novel ideas and recommendations for further research in the future. Furthermore, by establishing the relationship between CI and the changes in brain imaging, we sought to investigate the potential neurobiological pathways underlying CI in patients with COPD.
METHODS
Search strategy
Two experienced investigators independently searched the PubMed, Scopus, and ScienceDirect databases for preliminary studies related to brain imaging of patients with COPD from January 2000 to November 2021. The retrieved document met the inclusion criteria for participants, interventions, comparators, and outcomes (PICO), which were as follows: Participants, patients diagnosed with COPD and without other lung diseases or other diseases known to affect cognition, such as liver failure, cardiovascular diseases, and nervous system diseases; Interventions, neuroimaging examination; Comparators, subjects without COPD; and Outcomes, comparison of structural brain changes and functional brain changes. Search terms include: “COPD”, “chronic obstructive pulmonary disease”, “emphysema”, “clinical imaging”, “cognitive impairment”, and “dementia”. A manual search was performed for relevant references from the selected articles and published reviews.
Selection criteria
The inclusion criteria were a clinical trial, epidemiological study, observational study, cohort study, or case-control study. The patient’s race, nationality, and course of illness were not restricted. Included studies examined the relationship between alternations of brain imaging and reduced cognitive function in patients with COPD. The clinical imaging methods used in the studies had no restrictions. Studies that only investigated clinical imaging methods in patients with COPD were excluded. Case reports, conference abstracts, reviews, dissertations, theses, tutorials, and interviews, as well as those published in languages other than English, Spanish, or French were also excluded. Two researchers independently screened the literature, extracted the data, and cross-checked them. If there were any differences, it was settled through discussion or negotiation with a third party. We read the title of the text first when screening the literature. After the irrelevant literature was excluded, the abstract and full text were further read to determine whether to include them.
Drawing method
BrainNet Viewer toolbox (https://helab.bnu.edu.cn/brainnet-viewer/) was used to display brain regions with cortical atrophy in patients with COPD using automated anatomical labeling. Using the JHU-ICBM-labels-1 mm white matter (WM) atlas as a template, we used the FSLeyes package (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSLeyes) to map WM fiber bundles with altered integrity.
RESULTS
Characteristics of the studies and population
In November 2021, when the literature search was finalized, we identified a total of 132 articles preliminary. After reading the titles, abstracts, and full texts, 109 articles were excluded. Twenty-three original studies were included in this review, all of which were case-control studies. The literature screening process and findings are presented in Fig. 1. One of the 23 studies included in this systematic review categorized participants into cognitively high- and low-performing groups based on their cognitive function. The remaining 22 studies all set normal control (NC) groups, thirteen of which selected patients with stable COPD as research objects; five studies divided subjects per the severity of COPD; subjects in four studies were divided into a hypoxemic group and a non-hypoxemic group. Fourteen studies were conducted in China, four in the United Kingdom, and one each in the Netherlands, Italy, Turkey, United States of America, and South Korea.
Fig. 1
Flowchart of literature search and selection of the articles.
CI in COPD
To date, numerous studies have indicated that patients with COPD have an increased prevalence of CI. Recently, a meta-analysis of cohort studies involving a total of 39,392 patients showed that patients with COPD have an increased risk of dementia, with a hazard ratio of 1.46 [11]. Various factors combine to promote CI in patients with COPD, including hypoxemia, lifestyle factors such as a lack of physical activity, and comorbidities such as obstructive sleep apnea and depression [12]. According to a cohort study, this association was more pronounced among smokers and patients with impaired glucose tolerance [13]. In addition, the severity of CI in patients with COPD can vary according to factors such as sex, age, region, duration of disease, and disease severity. In a meta-analysis of cohort studies, subgroup analysis on sex determined that the association between COPD and dementia was stronger in male patients than in female patients [11]. Compared with developed countries, the association between COPD and CI is weaker in low- and middle-income countries. According to a study based on the Mayo Clinic Study of Aging, there was a dose-response relationship between the duration of COPD and the odds of any mild CI (MCI). Individuals who have had COPD for more than 5 years had the greatest risk for MCI [14]. The degree of CI varied depending on the severity of COPD. After taking acute disease into account, the degree of CI in patients with deteriorating conditions was higher than that in patients with stable COPD [15]. Similarly, a study that recruited 27 patients with mild to moderate COPD, 35 patients with severe COPD, and 27 control subjects suggested that Mini-Mental State Examination (MMSE) scores were correlated with the disease severity of COPD [16].
CI in patients with COPD has a domain-specific feature, which is concentrated on attention, memory, executive function, psychomotor function, visuospatial/constructional abilities, and language [17]. To determine the subtypes of CI in patients with COPD, a study involving 45 patients with moderate-to-severe COPD, and 50 healthy controls indicated that patients with COPD with MCI were predominantly non-amnestic MCI single-domain subtypes, with attention and executive dysfunction predominating [9]. Through a cross-sectional observational study, Cleutjens et al. found that patients with COPD had general CI and CI in the domains of psychomotor speed and executive functioning including planning and cognitive flexibility by a detailed neuropsychological assessment and six core tests from the Maastricht Aging Study. However, there was no difference in working memory and verbal memory between the two groups [8]. On the contrary, another study with a large study population of 43,039 persons demonstrated that persons with obstructive lung disease had poor performance in prospective memory, visuospatial memory, numeric short-term memory, and information processing. However, the association between lung function and CI was weak [18]. A large-scale prospective study monitored 1,425 cognitively healthy individuals at 15-month intervals to assess the association between COPD and risk for MCI. The findings indicated that a diagnosis of COPD significantly increased the risk for non-amnestic MCI by 83% but not for amnestic MCI [14]. This requires further investigation in future studies.
Neuroimaging changes in COPD
Previous studies on brain imaging changes in patients with COPD mainly focus on abnormal brain structure changes. Of the 23 studies we included, thirteen examined structural changes in the brain. The basic information of the articles is presented in Table 1. Seven studies used functional MRI (fMRI) and single photon emission computed tomography (SPECT) to explore abnormal brain function changes in patients with COPD (Table 2). Five studies on the topological properties of the brain network in patients with COPD are presented in Table 3. Two articles discussed both structural and network changes in the brain.
Table 1
Description of the included studies on chronic obstructive pulmonary disease (COPD) and brain structural changes
| Author Year | Country | Sample Size | Mean Age (y) | Cognitive Assessment | Neuroimaging changes | Correlations |
| Li 2013 [22] | China | N = 116 mild-to-moderate (37) and severe (48) patients with COPD, NC (31) | 67.6 | MMSE scores: severe COPD group < mild-to-moderate COPD group < control group | 3.0T MRI: hippocampal atrophy in the two groups compared with NCs. | The HCV was positively correlated with the MMSE scores in the mild-to-moderate COPD group (r = 0.47, p < 0.01) and the total patients with COPD (r = 0.36, p < 0.01). |
| Cleutjens 2017 [23] | Netherlands | N = 55 cognitively low-performing (30), cognitively high-performing (25) | 60.6 | The MoCA and MMSE scores of patients with COPD were considerably lower than those of NCs. | 3.0T MRI: No group differences were reported in SVD features and HCV. | No relationship between HCV and cognitive function was observed. |
| Yin 2019 [25] | China | N = 115Severe (26), moderate (29), and mild (29) COPD, NC (31) | 60.9 | The four groups had significantly different MoCA item scores for visuospatial executive function, attention, abstraction, delayed recall, and orientation. | 3.0T MRI:1. “Patients with moderate COPD had atrophy of the left middle frontal gyrus and right opercular part/triangular part of the inferior frontal gyrus”.2. WM changes were mainly present in the superior and posterior corona radiata, corpus callosum, and cingulum. | 1. The grey matter densities in the left superior frontal gyrus and right orbital part of the inferior frontal gyrus was positively correlated with MoCA scores. 2. The MD and RD values of the body of the corpus callosum and the AxD value of the bilateral superior corona radiata were negatively correlated with MoCA scores. |
| Chen 2016 [26] | China | N = 50patients with stable COPD (25), NCs (25) | 69 | Two groups differed significantly in MMSE scores, figure memory and visual reproduction. | 3.0T MRI:“Patients with COPD had significantly reduced cortical thickness in motor, parietal, and prefrontal cortices”.The reduced surface area in the dorsal ventral prefrontal cortex and Broca’s area. | 1. Decreased CTh in parietofrontal networks strongly correlated with visuospatial construction impairment in patients with COPD.2. The thinner dorsolateral prefrontal cortex best predicted poorer performance and was associated with lower arterial oxygen saturation. |
| Dodd 2012 [29] | UK | N = 50 patients with stable non-hypoxemic COPD (25) and NCs (n = 25) | 68.0 | Patients with COPD had a worse performance for processing speed, working memory, and executive function.2. The MMSE score was significantly worse in COPD. | 3.0T MRI, DTI:Patients with COPD had significantly reduced WM microstructural integrity, which amounted to 46% of the total WM skeleton. | Not covered |
| Ryu 2013 [27] | South Korea | N = 30 subjects with severe (6) and moderate (12) COPD, NCs (n = 12) | 63.92 | There were significantly worse in the severe COPD group than both the control and moderate COPD groups for language-related function, visuospatial function, and frontal executive function. | 3.0T MRI, DTI:Patients with COPD had abnormal changes in the bilateral frontal, parietal, right temporal, and right occipital subcortical white matter, occipitofrontal fasciculus, and corticospinal tract. | Not covered |
| Savage 2018 [24] | UK | N = 50 patients with stable non-hypoxemic COPD (25) and NCs (25) | 67.84 | Patients with COPD had defects in executive function, working memory, verbal memory, and processing speed. | 3.0T MRI:Patients with COPD had greater atrophic frontal areas than those in the NCs. However, there was no difference between the two groups in terms of temporal or hippocampal atrophy. | There are no significant correlations between regional atrophy and cognitive performance. |
| Spilling 2019 [28] | UK | N = 50 patients with stable COPD (27) and non-COPD smokers (23) | 63 | Patients with COPD had higher HADS-total scores and lower MoCA – total scores. | 3.0T MRI:Patients with COPD had lower normalized GMV. | Mood was associated with markers of WM microstructural damage. |
| Wang 2017 [20] | China | N = 120 patients with COPD (60) and NCs (60) | 53.68 | The patients with COPD had similar MMSE scores compared with NCs.The patients with COPD had reduced MoCA scores for visuospatial, executive, naming, and memory functions. | 3.0T MRI:In PrCU, bilateral CAL, right STG/MTG, bilateral FG, and right IPL, patients with COPD had significantly decreased GMVs. | Not covered |
| Wang 2020 [19] | China | N = 85 patients with stable COPD (45) and NCs (40) | 67.93 | Patients with COPD showed CI in visuospatial/executive, naming, attention, language, abstraction, delayed recall, and orientation. | 3.0T MRI:Patients with COPD had reduced GMV in the left SMG/PreCG, bilateral pMCC, right MOG, and right SMG. | There was no correlation found between GMV and cognition function. |
| Zhang 2013 [21] | China | N = 50 patients with stable COPD (25) and NCs (25) | 68.6 | Patients with COPD had poorer performance in the MMSE, visual reproduction, and figure memory tests. | 3.0T MRIPatients with COPD showed reduced GMV in the frontal cortex, right anterior insula, CiG, right Tha/pulvinar, right caudate, right putamen, right PHG, and left amygdala. | The GMV in the inferior triangular frontal cortex in patients with COPD was significantly correlated with the picture memory score. |
| Zhang 2012 [62] | China | N = 50 patients with stable COPD (25) and NCs (25) | 68.6 | Patients with COPD had significantly lower scores in MMSE test, visual reproduction and figure memory. | 3.0T MRI DTI:Patients with COPD had significantly lower FA in the SCR, SLF, ILF, bilateral OR, bilateral LG, left PHG and fornix. | No correlation was found between impairment of WM microstructural integrity and CI. |
| Borson 2015 [63] | USA | N = 27 patients with stable COPD (18) (oxygen users [n = 9] and nonusers [n = 9]) and NCs (9) | 68.4 | Patients with COPD had significantly lower scores in DRS-2 Total, Digit symbol test, and Logical memory test. | structural MRI and MRSBrain atrophy, number and estimated volume of white matter hyperintensities did not differ between two groups. | Not covered |
AxD, axial diffusivity; CIG, cingulate gyrus; CAL, calcarine; CTh, cortical thickness; FG, fusiform gyrus; HADS, Hospital Anxiety and Depression Scale; HCV, hippocampal volume; ILF, inferior longitudinal fasciculus; IPL, inferior parietal lobule; LG, lingual gyri; L-MFG, left middle frontal gyrus; MD, mean diffusivity; MRS, magnetic resonance spectroscopy; MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment; MOG, middle occipital gyrus; NC, normal control; OR, optic radiation; PHG, parahippocampal gyrus; pMCC, posterior midcingulate cortex; PrCU, precuneus; RD, radial diffusivity; R-O, right opercular part/triangular part of the inferior frontal gyrus; SCR, superior corona radiata; SMG/PreCG, supramarginal gyrus/precentral gyrus; SLF, superior longitudinal fasciculus; STG/MTG, superior temporal gyrus/middle temporal gyrus; SVD, small vessel disease; Tha, thalamus.
Table 2
Description of the included studies on chronic obstructive pulmonary disease (COPD) and brain functional changes
| Author Year | Country | Sample Size | Mean Age (y) | Cognitive Assessment | Neuroimaging changes | Correlations |
| Wang 2018 [32] | China | N = 39 patients with stable COPD (19) and NCs (20) | 62.7 | The MoCA and MMSE scores of patients with COPD were significantly lower. | Rs-fMRI:Patients with COPD had significantly higher ALFF areas in the brainstem, and lower ALFF areas in the PCC and precuneus. | No correlations were observed between abnormal ALFF values and lower scores on the neuropsychological tests. |
| Antonelli Incalzi 2003 [30] | Italy | N = 43 NHC (18), HC (15), NCs (10) | 68.4 | Patients with COPD had lower verbal attainment, attention, and deductive thinking scores. | SPECT:The average perfusion decreased from NHC to HC and, then, to patients with AD. | The frequency of correctly completed neuropsychological tests was directly associated with the perfusion of anterior cortical and subcortical regions of the dominant hemisphere. |
| Zhang 2016 [33] | China | N = 50 patients with stable COPD (25) and NCs (25) | 68.0 | COPD: lower scores in visual reproduction and figure memory | rs-fMRI:In patients with COPD, the whole-brain analysis indicated significant reductions in ALFF in the bilateral PCC and right lingual gyrus, as well as an increase in ALFF in the left postcentral gyrus. | ALFF in the bilateral PCC and right lingual gyrus had positive correlations with visual reproduction. |
| Lu 2019 [34] | China | N = 54 patients with COPD (28) and NCs (26) | 63.12 | Patients with COPD performed worse in executive function than NCs. | rs-fMRI:Patients with COPD exhibited decreased ALFF value in bilateral basal ganglia areas and the right thalamus. | No meaningful correlations were found. |
| Ortapamuk 2006 [31] | Turkey | N = 28HC (8), NHC (10), NC (10) | 52.6 | Scores of verbal memory delayed recall, and attention was significantly lower in patients with COPD. | SPECT:Patients with NHC had significantly decreased perfusion indexes on the frontal ROIs.2. Perfusion indexes on frontal and parietal ROIs decreased in patients with HC | 1. Verbal memoryThe function was inversely correlated with left anterior frontal perfusion in both NHC and HC.Attention function was negatively correlated with left middle frontal lobe perfusion.3. In patients with HC, there was a significant correlation between delay recall and left parietal perfusion. |
| Xin 2019 [35] | China | N = 39 patients with stable COPD (19) and NCs (20) | 62.7 | COPD: lower scores in MoCA, MMSE, naming, visuospatial, executive, and memory testing | rs-fMRI:Patients with COPD showed significantly lower ReHo values in the left occipital lobe, right lingual, bilateral precuneus, and right precentral gyrus. | Not covered |
| Lv 2020 [64] | China | N = 62 patients with COPD (32) and NCs (30) | 71.5 | Participants with COPD had lower scores in the CVFT. | rs-fMRI:COPD patients exhibited decreased sALFF in the right basal ganglia and increased dALFF in the bilateral PHG/HG. | The increased dALFF in the left HG/PHG was associated with poor semantic-memory performance and the increased dALFF in the left HG/PHG was associated the forced vital capacity. |
ALFF, amplitude of low-frequency fluctuation; CVFT, category verbal fluency test; dALFF, dynamic amplitude of low-frequency fluctuations; sALFF, static amplitude of low-frequency fluctuations; HC, health control; HG, hippocampal gyrus; MMSE, Mini-Mental State Examination; MoCA, Montreal cognitive assessment; NC, normal control; NHC, patients with COPD without hypoxemia; PCC, posterior cingulate cortex; PHG, parahippocampal; ReHo, regional homogeneity; ROI, region of interest; SPECT, single photon emission computed tomography.
Table 3
Description of the included studies on chronic obstructive pulmonary disease (COPD) and brain networks
| Author Year | Country | Sample Size | Mean Age | Cognitive Assessment | Neuroimaging changes | Correlations |
| Hu 2018 [36] | China | N = 113 mild (29), moderate (30), patients with severe COPD (24), and NCs (30) | 60.9 | The total MoCA scores, executive function, attention, delayed recall, abstraction, and orientation were significantly different among the four groups. | Rs-fMRI:Activated brain regions in the DMN (L-PCC, L-ACC, L-SMG, L-HP, R-PHP, R-MOFC) had changes of FC. | The left PCC and left hippocampus FC levels were associated with cognitive functioning. |
| Li 2020 [65] | China | N = 39 patients with stable COPD (19) and NCs (20) | 62.7 | Patients with COPD had lower MMSE, MoCA, naming, visuospatial and executive function, and memory scores. | Rs-fMRI:The patients with COPD exhibited significantly decreased DC in the right LG, bilateral SMA, and right PCL, and decreased FC between these abnormal hubs in the L-CAL, L-LG, L-FG, right insula, R-IFG, limbic lobe, cingulate gyrus, left putamen, lentiform nucleus, right precuneus, and R-PL. | The decreased DC in the SMA was positively correlated with naming in patients with COPD. |
| Spilling 2019 [37] | UK | N = 53 patients with COPD (30) and NCs (23) | 67.2 | Patients with COPD had lower MMSE, executive function, episodic memory, processing speed, and working memory scores. | 3.0T MRI, DTI:COPD: lower global connection strength and connection density and decreased connection density in the right superior temporal gyrus | No correlation was observed |
| Wang 2020 [19] | China | N = 85 patients with stable COPD (45) and NCs (40) | 67.93 | Patients with COPD showed CI in visuospatial/executive, naming, attention, language, abstraction, delayed recall and orientation. | r-s fMRI:COPD: decreased FC within the visual network and frontoparietal network | Decreased FC values within the visual network and the frontoparietal network was positively correlated with the MoCA, language domain, and attention-domain score in patients with COPD. |
| Dodd 2012 [29] | UK | N = 50 Patients with stable non-hypoxemic COPD (25) and NCs (25) | 68.0 | Processing speed, working memory, executive function, and MMSE scores were all lower in patients with COPD. | r-s fMRI:All RSNs showed increased functional gray matter activation in COPD. | Not covered |
DC, degree centrality; DMN, default mode network; FC, functional connectivity; L-ACC, left anterior cingulated cortex; L-CAL, left cerebellum anterior lobe; L-FG, left fusiform gyrus; LG, lingual gyrus; L-HP, left hippocampus; L-LG, left lingual gyrus; L-PCC, left posterior cingulate cortex; L-SMG, left supramarginal gyrus; PCL, paracentral lobule; R-IFG, right inferior frontal gyrus; R-MOFC, right medial orbitofrontal cortex; R-PHP, right parahippocampus; R-PL, right paracentral lobule; RSN, resting-state networks; SMA, supplementary motor area.
Brain structure
Patients with COPD have structural changes in the brain, including macrostructural changes (Fig. 2) and microstructural changes (Fig. 3). At a macro scale, the structural alterations in patients with COPD are reflected in the reduction of gray matter volume (GMV) in specific brain regions, including the hippocampus, bilateral frontal lobes, and the right temporal lobe. In addition, the morphological change of gray matter is also a kind of macroscopic structural change, which is reflected in the thinning of the motor, parietal, and prefrontal cortex, and the reduction of the surface area of the dorsolateral prefrontal cortex and Broca’s area. Brain atrophy due to COPD is not global, but the atrophy of specific subregions. Wang et al. using voxel-based morphometric analysis observed that there were no significant differences in total GMV between COPD and healthy individuals by structural MRI [19]. To determine the specific areas brain regions that experience cortical atrophy, a study revealed that the patients with COPD had significantly lower GMV in the left precuneus, bilateral calcarine, right superior temporal gyrus/middle temporal gyrus, bilateral fusiform gyrus, and right inferior parietal lobule. The GMV in the calcarine was associated with the forced vital capacity (FVC) [20]. Zhang et al. found that patients with COPD showed reduced GMV in the frontal cortex, right anterior insula, cingulate gyrus, right thalamus /pulvinar, right caudate, right putamen, right parahippocampal gyrus, and left amygdala [21]. The hippocampus as part of the limbic system is responsible for the storage and conversion of long-term memory and orientation. Li et al. observed that compared with the control group, the hippocampal volume (HCV) of patients with COPD was significantly smaller. Further, it was positively correlated with the MMSE score, oxygen saturation (SaO2), and partial pressure of oxygen (PaO2) in patients with COPD [22]. There were also conflicting results on the relationship between brain structural changes and CI. Cleutjens et al. through a cross-sectional study, divided patients with COPD into two groups: cognitively low-performing and high-performing group and observed that macro brain structure changes such as cerebrovascular disease and HCV had no relationship with CI [23]. Researchers observed that the degree of regional atrophy in the frontal lobe from cerebral MR images in patients with COPD was significantly higher than that in healthy controls using a validated visual analog grading technique. However, there was no significant correlation between local atrophy and disease severity and cognitive function [24]. In a cross-sectional study, which included a total of 115 participants (26 severe, 29 moderate, and 29 patients with mild COPD and 31 individuals without COPD), it was observed that with the increase of COPD severity, the atrophy of brain areas became extensive. Patients with mild COPD had no significant structural alteration in the brain. Patients with moderate COPD had atrophy of the left middle frontal gyrus and right opercular part/triangular part of the inferior frontal gyrus. Then, patients with severe COPD exhibited the most extensive gray matter density changes, increasing parahippocampal/fusiform gyrus, supplementary motor cortex, and right thalamus [25]. This suggests that with the increase in COPD severity, the degree of local atrophy did not increase, however, the atrophy of brain areas became extensive. Cortical thinning may be a key morphological feature associated with COPD. Chen et al. studied cortical morphometry and neuropsychology in 25 patients with stable COPD and 25 age-matched controls by T1-weighted MRI scans. The findings indicated that compared to controls, patients with COPD had significantly reduced cortical thickness broadly distributed in the motor, parietal, and prefrontal cortices, and a more circumscribed surface area reduction in the dorsomedial prefrontal cortex and Broca’s area. On the contrary, these morphological changes were associated with decreased SaO2, visual memory, and drawing deficits [26].
Fig. 2
Distribution of cortical changes in COPD patients. Compared to the normal controls, cortical atrophy in COPD patients is concentrated in bilateral hippocampus (red), right opercular part (pink)/triangular part of the inferior frontal gyrus (green), left precuneus (purple), bilateral middle frontal gyrus (brown), right thalamus (blue), right superior (gray)/middle temporal gyrus (orange), right superior frontal gyrus (blackish green), bilateral calcarine (cyan), bilateral orbital inferior frontal gyrus (yellow), left orbital superior frontal gyrus (magenta), and right fusiform gyrus (indigo). Compared with the dominant hemispheres, the structural changes in the non-dominant hemispheres were more obvious. The lesions in the frontal lobe are prominent.
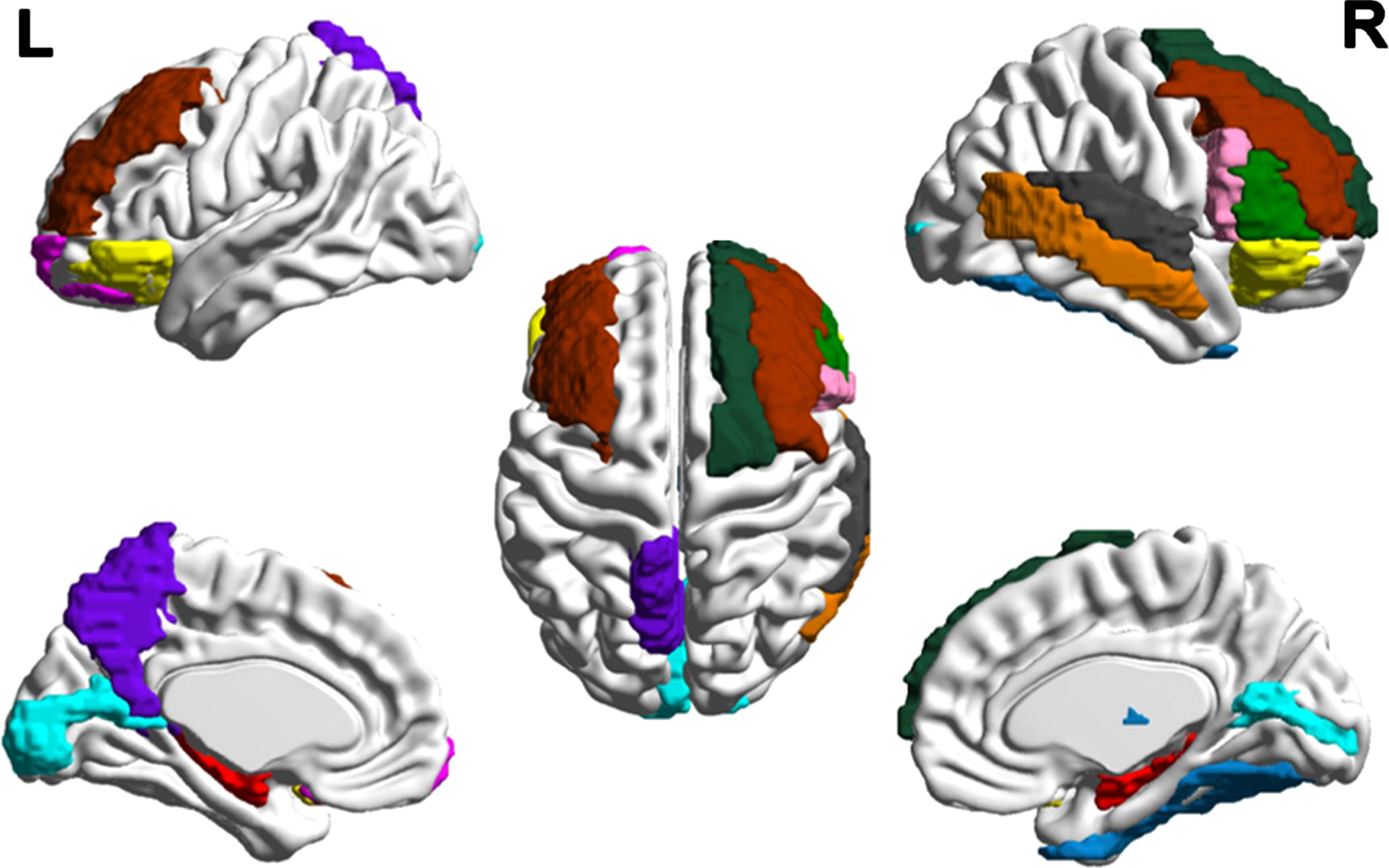
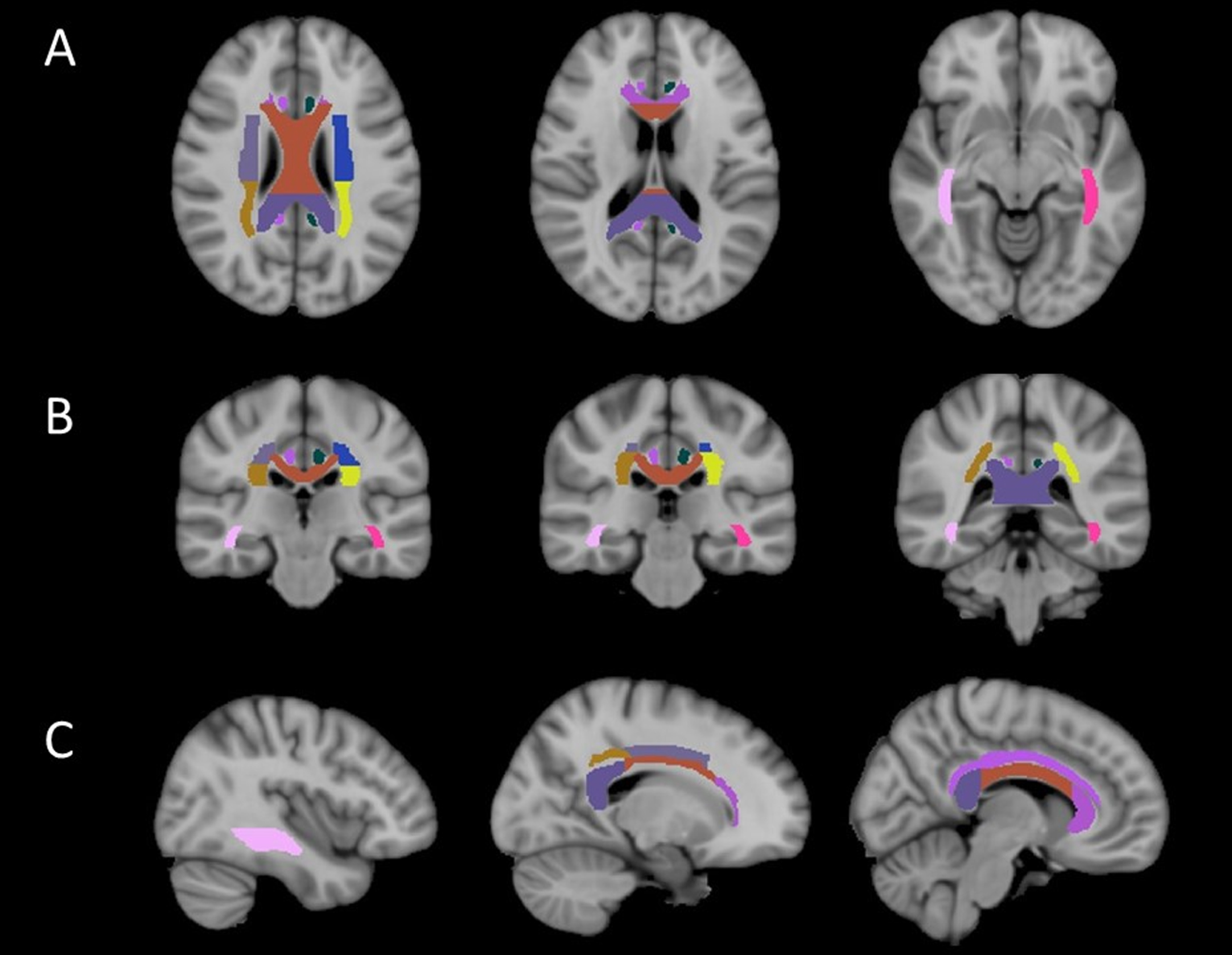
Fig. 3
Altered white matter fiber tracts in one COPD patient. The integrity of nine fiber bundles was altered: bilateral superior/posterior corona radiata, splenium/genu/body of corpus callosum, bilateral cingulum, bilateral occipitofrontal fasciculus.
In addition to the changes in macrostructure, there are changes in brain microstructure in patients with COPD. To better understand the changes in the macroscopic structure of the brain in patients with COPD, increasing research has focused on the alternations in the microscopic structure. Currently, most studies of WM microstructure mainly use DTI technology. Yin et al. reported that patients with COPD have decreased WM integrity in the superior and posterior corona radiata, corpus callosum, and cingulum [25]. The extent of the microstructural change in the brain may correlate with the severity of COPD. The voxel-based analysis of fractional anisotropy (FA) difference among NCs, and patients with moderate and severe COPD demonstrated that WM with reduced integrity was concentrated in bilateral frontal, right temporal, and right occipital subcortical WM. Among them, the alteration of bilateral prefrontal lobes was presented in the earlier stage of COPD. More importantly, the researchers observed a gradational change in WM integrity from NCs to patients with severe COPD [27]. Similarly, to search for possible causes of brain structural damage in patients with COPD, Spilling et al. observed that lung function was significantly correlated with both WM macrostructure and microstructure after controlling for smoking and cardiovascular risk factors [28]. Dodd et al. reported that the structural integrity of WM in patients with COPD decreased, accounting for 46% of the total WM skeleton. After controlling the cognitive test results, the difference in WM integrity between the control group and patients with COPD was no longer significant, suggesting that COPD-related CI may occur secondary to WM damage independent of smoking and other cardiovascular risk factors [29].
Brain functions
Recently, the technique of local blood flow to the brain has been used to study the brain function of patients with COPD. Neural activity can increase metabolism and local blood flow. Both fMRI and SPECT are imaging methods based on blood oxygen metabolism in the brain. They locate the cognitive functions of brain activity by measuring corresponding changes in metabolism or blood flow and oxygen levels. A cross-sectional study including 10 NCs, 15 patients with COPD with hypoxemia, and 18 of those without hypoxemia discovered that NCs and patients with non-hypoxemic COPD exhibited comparable perfusion patterns. However, the average perfusion of hypoxemic COPD declined. Interestingly, frontal lobe hypoperfusion was more prominent and decreased with the worsening of the hypoxemia, possibly indicating herald frontal-type cognitive decline [30]. To further explore the relationship between cerebral perfusion and hypoxia or CI in patients with COPD, another study demonstrated that perfusion indexes on the frontal region of interest (ROI) in patients with non-hypoxemic COPD and frontal and parietal ROIs in patients with hypoxemic COPD revealed significant decrease by an ROI-based method. In addition, these findings were correlated with the results of neuropsychological tests. Patients with hypoxemia revealed more deterioration in cerebral perfusion and cognitive performance than patients with non-hypoxemic COPD [31]. These results also implied a reduction in frontal perfusion owing to hypoxia may cause CI in patients with COPD. The decreased cerebral perfusion in patients with non-hypoxemic COPD indicated that some factors affected the brain function besides hypoxia in patients with COPD.
To evaluate the intrinsic brain activity in patients with COPD, Wang et al. used the amplitude of low-frequency fluctuation (ALFF) method to demonstrate that patients with COPD had lower ALFF areas in the cluster of the posterior cingulate cortex (PCC) and precuneus and a higher ALFF area in the brainstem than NCs, by resting-state fMRI (rsfMRI). Although no correlations were observed between abnormal intrinsic brain activity and lower scores on the neuropsychological tests, researchers observed that the higher ALFF area was positively correlated with forced expiratory volume in one second (FEV1)% predicted whereas the lower ALFF areas revealed a negative correlation with PaCO2 and positive correlation with FEV1/FVC [32]. This finding indicated that hypoxia and hypercapnia may be important causes of abnormal intrinsic brain activity. With the same imaging method, another study using whole-brain analysis and ROI analysis revealed abnormal spontaneous neuronal activities in several brain regions of patients with COPD, including bilateral PCC, right lingual gyrus, left postcentral gyrus, left precentral gyrus, and left caudate nucleus. Among these regions, the ALFF in bilateral PCC and right lingual gyrus revealed significant correlations with poor performance in visual reproduction. Interestingly, after controlling for SaO2, the group differences in ALFF in bilateral PCC and right lingual gyrus did not exist [33], suggesting that CI in patients with COPD may be attributed to hypoxia-reduced neuronal activity. Patients with COPD exhibit decreased ALFF value in bilateral basal ganglia areas and right thalamus, and aberrant ALFF value is correlated with PaO2 and pulmonary ventilation function (FEV1% pred). After supplementary oxygen inhalation, the ALFF value of basal ganglia and right thalamus significantly increased in the controls albeit not in the patients with COPD. This implies that aberrant ALFF alteration in the deep brain may be directly associated with lower PaO2 in patients with COPD [34]. Using the regional homogeneity (ReHo) method based on rsMRI, Xin et al. demonstrated that patients with COPD exhibited abnormal local synchronization of spontaneous activity, and the regions with abnormal activity were all correlated with visual processing pathways, including the left occipital lobe and the right lingual, bilateral precuneus, and right precentral gyrus. Among them, the mean lower ReHo values in the precuneus gyrus revealed a significant positive correlation with lung function and orientation function albeit a significant negative correlation with PaCO2 [35]. There is extensively organized default functional activity throughout the entire brain. However, in patients with COPD, the decreased intensity and intra-regional consistency of brain activity were concentrated in the PCC, precuneus, precentral gyrus, and right lingual gyrus.
Brain networks
In patients with stable non-hypoxic COPD, the researchers demonstrated widespread disruption of gray functional activation in the brain. Research on the resting state network based on the seed-based analysis demonstrated that, except for the visual network, six of the seven resting-state networks revealed an increased functional gray matter activation in COPD compared with controls, containing ventral-dorsal network, DMN, right frontoparietal network, left the frontoparietal network, visual network, sensorimotor network, and prefrontal network. However, after controlling for smoking, stroke risk, and cognitive function, the difference became insignificant [29]. Future research is required to determine which of the three factors specifically contributes to the rise in gray matter function activation. DMN is essential for maintaining the metabolic activity of brain tissue and for the processes of spatial orientation, internal monitoring, and memory in the resting state. A cross-sectional study involving 83 patients with COPD and 30 healthy controls revealed that fewer brain regions in DMN were activated in patients with COPD compared to the NCs. Additionally, functional connectivity (FC) of the six active brain regions was altered in patients with COPD. Among them, the FC values of the left PCC and hippocampus correlated well with cognitive functions and pulmonary function, which may be important brain regions related to CI in patients with COPD [36]. Connectivity between brain areas has increasingly been suggested to play a key role in neurophysiology. The human brain functions as a whole through the interaction of various brain regions. Therefore, it is significant to study brain connectome changes based on the whole-brain level. Researchers used 90 regions of gray matter as nodes and WM fiber tracts as edges to build a network in a cross-sectional study to examine the changes in the structural brain networks in patients with COPD. They discovered that the brain networks of these patients had significantly lower global connection strength and connection density and a trend of reduced nodal connection density and connection strength. However, there has not been any evidence linking altered brain networks to disease severity or cognitive function [37].
Comparing COPD related CI with AD
Whether COPD and AD share CI risk factors and pathogenesis is a point of interest requiring further research, as it has important implications for improving prognosis and treatment. We attempted to provide further clues by comparing CI patterns, brain imaging features, and pathological changes in patients with COPD and AD.
To verify whether COPD and AD had different CI patterns, a retrospective study indicated that patients with both AD and COPD had worse results in executive functions and a higher presence of depression than those with AD only [38]. This indicates that patients with COPD have a higher frequency of frontal deficits and behavioral disturbances. In addition, COPD was associated with an increased risk for non-amnestic MCI albeit not of amnestic MCI [14]. It is well known that the CI of AD is mainly characterized by significant memory impairment in the early stage, namely amnestic MCI, of which visual memory impairment is more significant, followed by progressive multidimensional cognitive decline [39]. Another cross-sectional study revealed that the domain-specific scores for attention, memory, and fluency were significantly lower in the AD group than in the COPD group [40]. The CI patterns of COPD and AD are different, and the affected cognitive domains are different.
The early changes in brain regions in AD occurred in the medial temporal lobe (entorhinal cortex and hippocampus), and the changes in the entorhinal cortex predicted the occurrence of AD more accurately [41, 42]. Nevertheless, the primary area of brain atrophy happening in patients with COPD was mostly reported in the frontal lobe, suggesting a frontal-type cognitive decline. Furthermore, with the increase in disease severity, the areas of brain atrophy enlarged [25]. The WM lesions of AD focused on the WM fibers closely connected with the temporal parietal lobe, such as the cingulate tract and corpus callosum [43]. The fibers with WM microstructure changes in patients with COPD also included the frontooccipital fasciculus, corpus callosum, superior and posterior corona radiata, and corticospinal system. AD was characterized by hypoperfusion of multiple brain regions and associative areas [44], which was observed to be more severe in anterior areas in patients with COPD complicated by hypoxemia. In the functional network constructed based on rsfMRI, researchers demonstrated that patients with COPD exhibited decreased FC of the visual network, frontal-parietal network, and DMN; however, it was more prone to occur in DMN in patients with AD. The small-world properties of AD patients changed [45], but not those of patients with COPD.
Some scholars believe that COPD may be one of the basic diseases of preclinical AD. The etiology of AD remains unclear, and the main pathological changes of AD are believed to include amyloid-β (Aβ) deposition, nerve fiber tangles, synaptic dysfunction, and neuronal apoptosis [46]. Based on a study published in 2015, serum Aβ40, Aβ42, and total Aβ levels were significantly increased in patients with COPD compared with NCs. In addition, Aβ levels were more elevated in patients with poor lung function [47]. Clusterin plays important biological roles in pathways relevant to neuropathology, including amyloid clearance, complement modulation, and apoptosis. A cross-sectional study observed that the pattern of the relationship between the elevated level of serum clusterin and the reduced cognitive ability in patients with COPD was similar to that observed in patients with AD [16]. S100B is a 21 kDa calcium-binding protein mainly produced and released by astrocytes. It has been studied as a biochemical marker of central nervous system injury. Previous studies have revealed that serum S100B levels are significantly elevated in patients with AD [48]. Interestingly, a similar phenomenon was observed in patients with COPD. In a cross-sectional study, the serum S100B concentration increased with the increase in disease severity, and the serum S100B level was negatively correlated with MMSE score and HCV in all patients with COPD [22]. However, in terms of the histopathological changes in the brain, a previous neuropathological study has demonstrated no specific degenerative changes, including AD in the brain of patients with COPD [49].
DISCUSSION
This review offers an overview of the clinical imaging evidence of CI in COPD patients.
We created a narrative review of the published studies on neuroimaging changes in patients with COPD, which is not only important to promote the research on relevant mechanisms but also helpful for the guidance of future research. The changes in brain macrostructure in patients with COPD were mainly manifested in the bilateral frontal lobe, hippocampus, right temporal lobe, motor cortex, and supplementary motor area. The microstructural changes in WM occur in the bilateral frontal subcortical. Further, with the progression of COPD, WM fiber damage expands to the right temporal lobe, occipital lobe subcortical, posterior, superior corona radiata, frontooccipital fasciculus, corticospinal system, cingulate tract, and corpus callosum. Existing research suggests that patients with COPD have altered intrinsic brain activity in the bilateral PCC, precuneus, right lingual gyrus, and left anterior central gyrus and reduced frontal cerebral perfusion. Brain networks are currently the subject of little research.
There may be a potential correlation between lung function impairment and brain pathology. Several hypotheses have been proposed to explain the association between COPD and higher rates of CI. First, as the main risk factor of COPD, smoking is also a risk factor for CI. Nicotine, carbon monoxide and other harmful components in tobacco can directly damage the nervous system, leading to the reduction of nerve cell function, even apoptosis [50]. In addition, smoking will accelerate the occurrence of atherosclerosis, reduce the ability of cerebral blood vessels to regulate blood flow, and lead to cerebral microcirculation disorders, thus damaging cognition [51]. COPD-related co-morbidities, such as sleep disorders, decreased physical activity, and depression can further impair cognitive function [52]. Patients with COPD are chronically deprived of quality sleep owing to poor breathing. Sleep is essential for cognitive function [53]. Sleep deprivation results in impaired molecular clearance from the human brain, dementia-related death of neurons involved in sleep function, and reduced blood flow in prefrontal cortex [17, 54]. Therefore, sleep disorders may be a cause of CI in patients with COPD. However, importantly, it seems that there is an association between CI and COPD independent of these co-morbidities. Pathophysiological changes caused by COPD itself, such as systemic inflammation, hypoxia, and hypercapnia may explain the accelerated cognitive aging [10]. Tissue hypoxemia plays a key role in the CI of patients with COPD. Pulmonary insufficiency causes decreased SaO2; further, the oxygen supply of patients with COPD is insufficient to meet the metabolic needs of the brain, causing the loss of neurons, brain atrophy, and WM degeneration, which can also affect cognition [55, 56]. Patients with COPD have high levels of pro-inflammatory cytokines, such as CRP, interleukin-6, fibrinogen, activated leukocytes, and tumor necrosis factor, which may cause cerebral vascular endothelial dysfunction and arteriosclerosis and thus CI [57]. A cross-sectional study discovered that patients with CI have lower PaO2 and higher PaCO2 than patients with normal cognition [58]. In addition, there is a correlation between PaCO2 and cognitive function in patients with COPD [59]. Based on the aforementioned evidence, PaCO2 is widely believed to play an important role in the CI of patients with COPD; however, its mechanism remains elusive.
Neuroimaging provides valuable information for the assessment of cognitive dysfunction in patients with COPD. Summarizing previous studies, we observed that brain atrophy in patients with COPD mainly occurred in the frontal lobe, which affected our logical thinking, language ability, and executive function. The middle frontal gyrus on the left is responsible for working memory and for integrating information and allocating cognitive resources. The opercular and triangular parts of the inferior frontal gyrus are collectively called the Broca area, which is the motor language center, which may explain the patient’s speech impairment. With the increase in disease severity, the areas of brain atrophy enlarged, including the parahippocampal, fusiform gyrus, supplementary motor cortex, right thalamus, and other brain areas. In terms of brain function, patients with COPD demonstrated abnormal synchrony of regional spontaneous activity in the left occipital lobe, right lingual gyrus, bilateral precuneus, and right precentral gyrus, which is involved in many high-level cognitive functions. PCC and precuneus are the core areas of the default mode network (DMN), which are associated with emotional processing, episodic memory, information processing, and other cognitive functions [60]. In addition, the precuneus is also responsible for visual processing. Lingual gyrus, fusiform gyrus and occipital cortex are all brain regions related to visual processing pathways. The precentral gyrus is involved in motor function. These brain regions are more susceptible to hypoxia [61], suggested that hypoxia is an important mechanism for COPD related CI. Pulmonary insufficiency causes decreased SaO2; further, the oxygen supply of patients with COPD is insufficient to meet the metabolic needs of the brain, resulting in the loss of neurons, brain atrophy in brain regions associated with cognitive function [56]. Zhang et al. demonstrated that the ALFF in right lingual gyrus in patients with COPD differed from which in HC group. After controlling for SaO2, the group differences did not exist [33]. Xin et al. demonstrated that the abnormal local synchronization of spontaneous activity, and the regions with abnormal activity in brain regions associated with visual processing pathways revealed a significant positive correlation with lung function [35]. Oxygen therapy improves cerebral oxygen delivery and neurovascular function to reduce the risk of MCI and dementia in patients with COPD [55].
However, the previous research findings were partly inconsistent. Patients with COPD have an increased risk of dementia. Most researches support that ‘COPD specific’ CI is dominated by non-amnestic [8, 9, 14], while some indicate a decreased memory function in patients with COPD [18]. Indeed, compared to cross-sectional studies, the longitudinal study has stronger effect and reliability, which may explain some inconsistent results. It should also be noted that a lack of standardised neuropsychological tests and variability in adjustment for confounders between studies limit the consistency of results. As an important brain area related to cognitive, hippocampus changes in the course of COPD are still controversial [22, 36]. This may be due to subjects with different disease severity and duration included in the studies. In patients with mild and moderate COPD, only a few brain areas have structural changes. With the increase of disease severity, the atrophic brain areas become widespread [25]. Therefore, the research results was inconsistent in some brain regions. There were also conflicting results on the relationship between brain imaging changes and CI. The control of comorbidity, risk factors and different imaging detection methods in different studies could affect the relationship. In addition, the inconsistency of the relationship can be explained by the fact that the cumulative impact of these small, spatially dispersed lesions, not the individual lesions, determines the impact on cognitive performance.
A beneficial cycle can be created by paying early attention to cognitive function, which can increase treatment compliance, support efficient management, and improve COPD outcomes. Therefore, finding an effective marker for early screening is extremely important. Neuroimaging provides valuable information for the diagnosis and assessment of cognitive dysfunction in patients with COPD and the mechanisms involved. Neuroimaging examination played a significant role in the early identification and screening of CI in patients with COPD. Wang et al. discovered that the alterations of the mean ALFF values in the PCC, precuneus, and brainstem in patients with COPD may distinguish COPD from NCs using the area under the curve of receiver operating characteristics [32]. Among them, the precuneus had the highest sensitivity and specificity. Another study has demonstrated that FC changes in activated brain regions in the DMN may predict CI [36].
Inevitably, this research has some limitations. First, the exciting studies of brain imaging changes in patients with COPD are mainly focused on brain structure and brain function. However, considering that human brain function is integrity that results from the interaction of various brain regions, it is necessary to study the changes in the brain network in patients with COPD from the perspective of brain connectome. Future research should focus more on how patients with COPD’s altered brain network topology attributes. Second, all the previous studies have been cross-sectional. To further clarify the relationship between COPD itself and cognitive function or brain pathological changes, long-term follow-up is necessary.
Conclusively, brain imaging alterations provide a new perspective to further understand the underlying neuropathological mechanism of CI in patients with COPD. Patients with COPD have abnormal brain structure, brain function, and brain network alternations, which may partly explain the CI in patients with COPD. Through analysis, we demonstrated that the CI in patients with COPD may be a distinct form of dementia from AD, which was more susceptible to a frontal-type cognitive decline in the early stages of the disease. Hence, more longitudinal studies are required to investigate brain connectome changes in patients with COPD in the future.
ACKNOWLEDGMENTS
The authors have no acknowledgments to report.
FUNDING
This study was supported by the National Natural Science Foundation of China (grant number: 81801680, 81801075, 81870850), and the Natural Science Foundation of Jiangsu Province (grant number: BK20180379).
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
DATA AVAILABILITY
Data sharing is not applicable to this article as no datasets were generated or analyzed during this study.
REFERENCES
[1] | Rabe KF , Watz H ((2017) ) Chronic obstructive pulmonary disease. Lancet 389: , 1931–1940. |
[2] | Agustí A ((2007) ) Systemic effects of chronic obstructive pulmonary disease: What we know and what we don’t know (but should). Proc Am Thorac Soc 4: , 522–525. |
[3] | Negewo NA , Gibson PG , McDonald VM ((2015) ) COPD and its comorbidities: Impact, measurement and mechanisms. Respirology 20: , 1160–1171. |
[4] | Meek PM , Lareau SC , Anderson D ((2001) ) Memory for symptoms in COPD patients: How accurate are their reports? Eur Respir J 18: , 474–481. |
[5] | ((1980) ) Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: A clinical trial. Nocturnal Oxygen Therapy Trial Group. Ann Intern Med 93: , 391–398. |
[6] | Torres-Sánchez I , Rodríguez-Alzueta E , Cabrera-Martos I , López-Torres I , Moreno-Ramírez MP , Valenza MC ((2015) ) Cognitive impairment in COPD: A systematic review. J Bras Pneumol 41: , 182–190. |
[7] | Lindbergh CA , Dishman RK , Miller LS ((2016) ) Functional disability in mild cognitive impairment: A systematic review and meta-analysis. Neuropsychol Rev 26: , 129–159. |
[8] | Cleutjens FA , Franssen FM , Spruit MA , Vanfleteren LE , Gijsen C , Dijkstra JB , Ponds RW , Wouters EF , Janssen DJ ((2017) ) Domain-specific cognitive impairment in patients with COPD and control subjects. Int J Chron Obstruct Pulmon Dis 12: , 1–11. |
[9] | Villeneuve S , Pepin V , Rahayel S , Bertrand JA , de Lorimier M , Rizk A , Desjardins C , Parenteau S , Beaucage F , Joncas S , Monchi O , Gagnon JF ((2012) ) Mild cognitive impairment in moderate to severe COPD: A preliminary study. Chest 142: , 1516–1523. |
[10] | Pelgrim CE , Peterson JD , Gosker HR , Schols A , van Helvoort A , Garssen J , Folkerts G , Kraneveld AD ((2019) ) Psychological co-morbidities in COPD: Targeting systemic inflammation, a benefit for both? Eur J Pharmacol 842: , 99–110. |
[11] | Wang Y , Li X , Wei B , Tung TH , Tao P , Chien CW ((2019) ) Association between chronic obstructive pulmonary disease and dementia: Systematic review and meta-analysis of cohort studies. Dement Geriatr Cogn Dis Extra 9: , 250–259. |
[12] | van Beers M , Janssen DJA , Gosker HR , Schols A ((2018) ) Cognitive impairment in chronic obstructive pulmonary disease: Disease burden, determinants and possible future interventions. Expert Rev Respir Med 12: , 1061–1074. |
[13] | Cleutjens F , Spruit MA , Ponds R , Vanfleteren L , Franssen FME , Gijsen C , Dijkstra JB , Wouters EFM , Janssen DJA ((2018) ) Cognitive impairment and clinical characteristics in patients with chronic obstructive pulmonary disease. Chron Respir Dis 15: , 91–102. |
[14] | Singh B , Mielke MM , Parsaik AK , Cha RH , Roberts RO , Scanlon PD , Geda YE , Christianson TJ , Pankratz VS , Petersen RC ((2014) ) A prospective study of chronic obstructive pulmonary disease and the risk for mild cognitive impairment. JAMA Neurol 71: , 581–588. |
[15] | Bajaj MK , Burrage DR , Tappouni A , Dodd JW , Jones PW , Baker EH ((2019) ) COPD patients hospitalized with exacerbations have greater cognitive impairment than patients hospitalized with decompensated heart failure. Clin Interv Aging 14: , 1–8. |
[16] | Li J , Huang Y , Fei GH ((2013) ) The evaluation of cognitive impairment and relevant factors in patients with chronic obstructive pulmonary disease. Respiration 85: , 98–105. |
[17] | Olaithe M , Bucks RS , Hillman DR , Eastwood PR ((2018) ) Cognitive deficits in obstructive sleep apnea: Insights from a meta-review and comparison with deficits observed in COPD, insomnia, and sleep deprivation. Sleep Med Rev 38: , 39–49. |
[18] | Cleutjens F , Spruit MA , Ponds R , Dijkstra JB , Franssen FME , Wouters EFM , Janssen DJA ((2014) ) Cognitive functioning in obstructive lung disease: Results from the United Kingdom biobank. J Am Med Dir Assoc 15: , 214–219. |
[19] | Wang W , Wang P , Li Q , Peng Z , Wang X , Wang G , Hou J , Fan L , Liu S ((2020) ) Alterations of grey matter volumes and network-level functions in patients with stable chronic obstructive pulmonary disease. Neurosci Lett 720: , 134748. |
[20] | Wang C , Ding Y , Shen B , Gao D , An J , Peng K , Hou G , Zou L , Jiang M , Qiu S ((2017) ) Altered gray matter volume in stable chronic obstructive pulmonary disease with subclinical cognitive impairment: An exploratory study. Neurotox Res 31: , 453–463. |
[21] | Zhang H , Wang X , Lin J , Sun Y , Huang Y , Yang T , Zheng S , Fan M , Zhang J ((2013) ) Reduced regional gray matter volume in patients with chronic obstructive pulmonary disease: A voxel-based morphometry study. AJNR Am J Neuroradiol 34: , 334–339. |
[22] | Li J , Fei GH ((2013) ) The unique alterations of hippocampus and cognitive impairment in chronic obstructive pulmonary disease. Respir Res 14: , 140. |
[23] | Cleutjens F , Ponds R , Spruit MA , Burgmans S , Jacobs HIL , Gronenschild E , Staals J , Franssen FME , Dijkstra JB , Vanfleteren L , Hofman PA , Wouters EFM , Janssen DJA ((2017) ) The relationship between cerebral small vessel disease, hippocampal volume and cognitive functioning in patients with COPD: An MRI study. Front Aging Neurosci 9: , 88. |
[24] | Savage CC , Dixey PHA , Pennington C , Dodd JW ((2018) ) Visual rating assessment of cerebral atrophy and its relationship with cognitive function in chronic obstructive pulmonary disease. BMJ Open Respir Res 5: , e000310. |
[25] | Yin M , Wang H , Hu X , Li X , Fei G , Yu Y ((2019) ) Patterns of brain structural alteration in COPD with different levels of pulmonary function impairment and its association with cognitive deficits. BMC Pulm Med 19: , 203. |
[26] | Chen J , Lin IT , Zhang H , Lin J , Zheng S , Fan M , Zhang J ((2016) ) Reduced cortical thickness, surface area in patients with chronic obstructive pulmonary disease: A surface-based morphometry and neuropsychological study. Brain Imaging Behav 10: , 464–476. |
[27] | Ryu CW , Jahng GH , Choi CW , Rhee HY , Kim MJ , Kim SM , Kim EJ , Choi WS ((2013) ) Microstructural change of the brain in chronic obstructive pulmonary disease: A voxel-based investigation by MRI. COPD 10: , 357–366. |
[28] | Spilling CA , Bajaj MK , Burrage DR , Ruickbie S , Thai NJ , Baker EH , Jones PW , Barrick TR , Dodd JW ((2019) ) Contributions of cardiovascular risk and smoking to chronic obstructive pulmonary disease (COPD)-related changes in brain structure and function. Int J Chron Obstruct Pulmon Dis 14: , 1855–1866. |
[29] | Dodd JW , Chung AW , van den Broek MD , Barrick TR , Charlton RA , Jones PW ((2012) ) Brain structure and function in chronic obstructive pulmonary disease: A multimodal cranial magnetic resonance imaging study. Am J Respir Crit Care Med 186: , 240–245. |
[30] | Antonelli Incalzi R , Marra C , Giordano A , Calcagni ML , Cappa A , Basso S , Pagliari G , Fuso L ((2003) ) Cognitive impairment in chronic obstructive pulmonary disease–a neuropsychological and SPECT study. J Neurol 250: , 325–332. |
[31] | Ortapamuk H , Naldoken S ((2006) ) Brain perfusion abnormalities in chronic obstructive pulmonary disease: Comparison with cognitive impairment. Ann Nucl Med 20: , 99–106. |
[32] | Wang W , Li H , Peng D , Luo J , Xin H , Yu H , Yu J ((2018) ) Abnormal intrinsic brain activities in stable patients with COPD: A resting-state functional MRI study. Neuropsychiatr Dis Treat 14: , 2763–2772. |
[33] | Zhang J , Chen J , Yu Q , Fan C , Zhang R , Lin J , Yang T , Fan M ((2016) ) Alteration of spontaneous brain activity in COPD patients. Int J Chron Obstruct Pulmon Dis 11: , 1713–1719. |
[34] | Lu CQ , Xu W , Zeng CH , Ge LY , Wang YC , Meng XP , Yu Q , Wu D , Ju S ((2019) ) Altered amplitude of low-frequency fluctuation in basal ganglia correlates to pulmonary ventilation function in COPD patients: A resting-state fMRI study. Brain Behav 9: , e01336. |
[35] | Xin H , Li H , Yu H , Yu J , Zhang J , Wang W , Peng D ((2019) ) Disrupted resting-state spontaneous neural activity in stable COPD. Int J Chron Obstruct Pulmon Dis 14: , 499–508. |
[36] | Hu X , Wang H , Tu Y , Fei M , Yin M , Fei G , Yu Y ((2018) ) Alterations of the default mode network and cognitive impairments in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 13: , 519–528. |
[37] | Spilling CA , Jones PW , Dodd JW , Barrick TR ((2019) ) Disruption of white matter connectivity in chronic obstructive pulmonary disease. PLoS One 14: , e0223297. |
[38] | Tondo G , De Marchi F , Terazzi E , Prandi P , Sacchetti M , Comi C , Cantello R ((2018) ) Chronic obstructive pulmonary disease may complicate Alzheimer’s disease: A comorbidity problem. Neurol Sci 39: , 1585–1589. |
[39] | Galton CJ , Patterson K , Xuereb JH , Hodges JR ((2000) ) Atypical and typical presentations of Alzheimer’s disease: A clinical, neuropsychological, neuroimaging and pathological study of 13 cases. Brain 123 Pt 3: , 484–498. |
[40] | Morris C , Mitchell JW , Moorey H , Younan HC , Tadros G , Turner AM ((2019) ) Memory, attention and fluency deficits in COPD may be a specific form of cognitive impairment. ERJ Open Res 5: , 00229–2018. |
[41] | Baron JC , Chételat G , Desgranges B , Perchey G , Landeau B , de la Sayette V , Eustache F ((2001) ) In vivo mapping of gray matter loss with voxel-based morphometry in mild Alzheimer’s disease. Neuroimage 14: , 298–309. |
[42] | Frisoni GB , Testa C , Zorzan A , Sabattoli F , Beltramello A , Soininen H , Laakso MP ((2002) ) Detection of grey matter loss in mild Alzheimer’s disease with voxel based morphometry. J Neurol Neurosurg Psychiatry 73: , 657–664. |
[43] | Kantarci K , Murray ME , Schwarz CG , Reid RI , Przybelski SA , Lesnick T , Zuk SM , Raman MR , Senjem ML , Gunter JL , Boeve BF , Knopman DS , Parisi JE , Petersen RC , Jack CR Jr. , Dickson DW ((2017) ) White-matter integrity on DTI and the pathologic staging of Alzheimer’s disease. Neurobiol Aging 56: , 172–179. |
[44] | Love S , Miners JS ((2016) ) Cerebrovascular disease in ageing and Alzheimer’s disease. Acta Neuropathol 131: , 645–658. |
[45] | Liu H , Hu H , Wang H , Han J , Li Y , Qi H , Wang M , Zhang S , He H , Zhao X ((2020) ) A brain network constructed on an L1-norm regression model is more sensitive in detecting small world network changes in early AD. Neural Plast 2020: , 9436406. |
[46] | Erkkinen MG , Kim MO , Geschwind MD ((2018) ) Clinical neurology and epidemiology of the major neurodegenerative diseases. Cold Spring Harb Perspect Biol 10: , a033118. |
[47] | Bu XL , Cao GQ , Shen LL , Xiang Y , Jiao SS , Liu YH , Zhu C , Zeng F , Wang QH , Wang YR , He Y , Zhou HD , Wang YJ ((2015) ) Serum amyloid-beta levels are increased in patients with chronic obstructive pulmonary disease. Neurotox Res 28: , 346–351. |
[48] | Sheng JG , Mrak RE , Griffin WS ((1994) ) S100 beta protein expression in Alzheimer disease: Potential role in the pathogenesis of neuritic plaques. J Neurosci Res 39: , 398–404. |
[49] | Cleutjens FA , Spruit MA , Beckervordersandforth J , Franssen FM , Dijkstra JB , Ponds RW , Wouters EF , Janssen DJ ((2015) ) Presence of brain pathology in deceased subjects with and without chronic obstructive pulmonary disease. Chron Respir Dis 12: , 284–290. |
[50] | Pomerleau OF ((1992) ) Nicotine and the central nervous system: Biobehavioral effects of cigarette smoking. Am J Med 93: , 2S–7S. |
[51] | Kubota K , Yamaguchi T , Abe Y , Fujiwara T , Hatazawa J , Matsuzawa T ((1983) ) Effects of smoking on regional cerebral blood flow in neurologically normal subjects. Stroke 14: , 720–724. |
[52] | Levin OS , Vasenina EE ((2019) ) [Depression and cognitive decline in elderly: Causes and consequences]. Zh Nevrol Psikhiatr Im S S Korsakova 119: , 87–94. |
[53] | Rasch B , Born J ((2013) ) About sleep’s role in memory. Physiol Rev 93: , 681–766. |
[54] | Eide PK , Vinje V , Pripp AH , Mardal KA , Ringstad G ((2021) ) Sleep deprivation impairs molecular clearance from the human brain. Brain 144: , 863–874. |
[55] | Hoiland RL , Mladinov S , Barak OF , Willie CK , Mijacika T , Stembridge M , Dujic Z , Ainslie PN ((2018) ) Oxygen therapy improves cerebral oxygen delivery and neurovascular function in hypoxaemic chronic obstructive pulmonary disease patients. Exp Physiol 103: , 1170–1177. |
[56] | Thakur N , Blanc PD , Julian LJ , Yelin EH , Katz PP , Sidney S , Iribarren C , Eisner MD ((2010) ) COPD and cognitive impairment: The role of hypoxemia and oxygen therapy. Int J Chron Obstruct Pulmon Dis 5: , 263–269. |
[57] | Názara Otero CA , Baloira Villar A ((2015) ) [The continuum of COPD and cardiovascular risk: A global scenario of disease]. Clin Investig Arterioscler 27: , 144–147. |
[58] | Yazar EE , Aydin S , Gunluoglu G , Kamat S , Gungen AC , Yildiz P ((2018) ) Clinical effects of cognitive impairment in patients with chronic obstructive pulmonary disease. Chron Respir Dis 15: , 306–314. |
[59] | Crişan AF , Oancea C , Timar B , Fira-Mladinescu O , Crişan A , Tudorache V ((2014) ) Cognitive impairment in chronic obstructive pulmonary disease. PLoS One 9: , e102468. |
[60] | Mason MF , Norton MI , Van Horn JD , Wegner DM , Grafton ST , Macrae CN ((2007) ) Wandering minds: The default network and stimulus-independent thought. Science 315: , 393–395. |
[61] | Baril AA , Gagnon K , Brayet P , Montplaisir J , De Beaumont L , Carrier J , Lafond C , L’Heureux F , Gagnon JF , Gosselin N ((2017) ) Gray matter hypertrophy and thickening with obstructive sleep apnea in middle-aged and older adults. Am J Respir Crit Care Med 195: , 1509–1518. |
[62] | Zhang H , Wang X , Lin J , Sun Y , Huang Y , Yang T , Zheng S , Fan M , Zhang J ((2012) ) Grey and white matter abnormalities in chronic obstructive pulmonary disease: A case-control study. BMJ Open 2: , e000844. |
[63] | Borson S , Scanlan J , Friedman S , Zuhr E , Fields J , Aylward E , Mahurin R , Richards T , Anzai Y , Yukawa M , Yeh S ((2008) ) Modeling the impact of COPD on the brain. Int J Chron Obstruct Pulmon Dis 3: , 429–434. |
[64] | Lv Z , Chen Q , Jiang Y , Hu P , Zhang L , Bai T , Wang K , Wang Y , Fan X ((2020) ) Abnormal static and dynamic local-neural activity in COPD and its relationship with pulmonary function and cognitive impairments. Front Hum Neurosci 14: , 580238. |
[65] | Li H , Xin H , Yu J , Yu H , Zhang J , Wang W , Peng D ((2020) ) Abnormal intrinsic functional hubs and connectivity in stable patients with COPD: A resting-state MRI study. Brain Imaging Behav 14: , 573–585. |




