Association Between Psoriasis and Dementia: A Retrospective Cohort Study
Abstract
Background:
To date, no large study has examined the relationship between psoriasis and dementia in Germany.
Objective:
The aim of this study was to assess the association between psoriasis and the risk of all-cause dementia in patients followed in general practices in Germany.
Methods:
This retrospective cohort study is based on longitudinal data from the IQVIATM Disease Analyzer database and included patients with an initial diagnosis of psoriasis between January 1995 and December 2014 in 1,173 general practices in Germany. Patients without psoriasis were matched individually (1:1) to psoriasis patients using propensity scores. The main outcome of the study was the cumulative incidence of dementia diagnoses within up to 15 years of the index date. Univariate Cox proportional regression models were used to assess the relationship between psoriasis or psoriatic arthritis and dementia.
Results:
The present study included 10,583 patients with a diagnosis of psoriasis and 10,583 controls without psoriasis. After 15 years of follow-up, 22.0% of the psoriasis patients and 19.1% (p < 0.001) of the non-psoriasis patients developed dementia. The incidence rate of dementia in 1,000 person-years was 15.0 in psoriasis patients and 11.9 in the non-psoriasis cohort. Psoriasis was significantly associated with a dementia risk (HR: 1.24; 95% CI: (1.14–1.35); p < 0.001). The association was stronger in patients with PsA (HR: 1.35; 95% CI: (0.98–1.86)) but this was not significant (p = 0.070).
Conclusion:
The present study found a positive association between psoriasis and all-cause dementia in patients in general practices in Germany.
INTRODUCTION
Dementia is a progressive neurological disorder which eventually manifests itself in the patient’s loss of function and independence [1]. There are different types of dementia with varying underlying pathologies [2]. The most common forms are Alzheimer’s disease (AD), vascular dementia, Lewy body dementia, and frontotemporal dementia [2]. Due to demographic changes and population aging, the prevalence of dementia is growing [2]. While there were approximately 1.7 million patients with dementia living in Germany in 2019, this number is likely to double by 2060 [3]. This poses an economic burden on the German health care system: the economic costs for Germany were approximately 34 billion euro in 2016 but could increase to 90 billion euro by 2060 [3].
There is increasing evidence to support the idea that inflammatory processes underlie many pathological stages of AD [4] and that neuroinflammation is present in patients with AD and other dementias [2]. Inflammatory biomarkers have been found in the brains of patients with AD postmortem [5]. Systemic inflammation in the body can contribute to the pathogenesis of AD in the brain: pro-inflammatory cytokines are activated as an immune response and can stimulate neurons and microglia in the brain [4]. Pathways include the blood-brain barrier or the vagal nerve [4]. The blood-brain barrier becomes more permeable with increasing age [6], but also due to chronic systemic inflammation, allowing the peripheral immune cells to invade the central nervous system and induce neuroinflammatory processes [4].
Psoriasis is a chronic, immune-mediated inflammatory disease which predominantly affects the skin and the joints [7]. It is a complex genetic disease which can be triggered by different risk factors [7]. The resultant chronic inflammation leads to hyperproliferation of the keratinocytes in the skin [7]. The most common form is plaque psoriasis which affects approximately 80–90% of the patients and is characterized by plaque with silver white scales [8]. Other forms include pustular psoriasis or guttate psoriasis [8]. Around 30% of psoriasis patients are also affected by psoriatic arthritis (PsA) [9], which manifests in joint swelling and pain [8].
Moreover, the chronic inflammatory processes involved may be a mechanism linking psoriasis and AD. Indeed, a genetic association between psoriasis and AD has previously been found in a genetic epidemiological study [10]. Consequently, the relationship between chronic psoriasis and dementia has been examined and summarized in four reviews and/or meta-analyses over the past two years. These four reviews support the hypothesis that psoriasis is associated with dementia, although one of the prospective studies analyzed an inverse association [11]. Most of the included studies have been conducted in Asia, the United States, or Europe and the majority were retrospective cohort studies. Between 3 and 7 studies were included in the quantitative analyses [12–14] and 8 studies were reported in the systematic review by Zhao et al. [15]. Although some of the studies have been reported multiple times, the reviews and meta-analyses included 14 different studies in total [12–14]. Of these, the largest retrospective cohort studies in Europe were by Li et al., with over 100,000 psoriasis patients included between January 1964 and December 2010 [16], and Dregan et al., with over 90,000 psoriasis patients included between January 2002 and January 2013 and a mean follow-up time of 4 years [17]. The only prospective cohort study performed in Europe included 311 psoriasis patients in the Netherlands [11] with patient recruiting starting in 1990. Outside of Europe, one of the largest retrospective cohort studies included in the reviews was based on data from the Korean National Health Insurance System database and included over 535,000 psoriasis patients and over 2.6 million non-psoriasis patients with a median follow-up time of 3.35 years [18]. To the best of our knowledge, no study in clinical practice to date has examined the relationship between psoriasis and dementia in Germany. It was therefore the aim of this large retrospective cohort study to assess whether psoriasis is associated with an increased risk of all-cause dementia in patients followed in general practices (GP) in Germany.
METHODS
Database
This retrospective cohort study used data from the representative IQVIATM Disease Analyzer database, which contains basic demographic variables, diagnoses, and prescriptions. The data from this national panel of GPs and specialist practices are collected in anonymous format by the company and are constantly quality controlled. Diagnoses are coded using ICD-10 (International Classification of Diseases, 10th revision) codes and therapies are documented using ATC (Anatomical Therapeutic Chemical classification system) codes. The database contains back data as far back as 1992. Coverage is about 3% of all outpatient practices in Germany [19]. It has previously been shown that the database is valid and suitable for epidemiological studies, and that the panel of general and specialist practices is representative for Germany [19]. The database has also been used in previous studies focusing on dementia [20–23]. As this study only considered GP practices (1,293 in total), only the diagnoses and therapies documented by GPs were included.
Study population
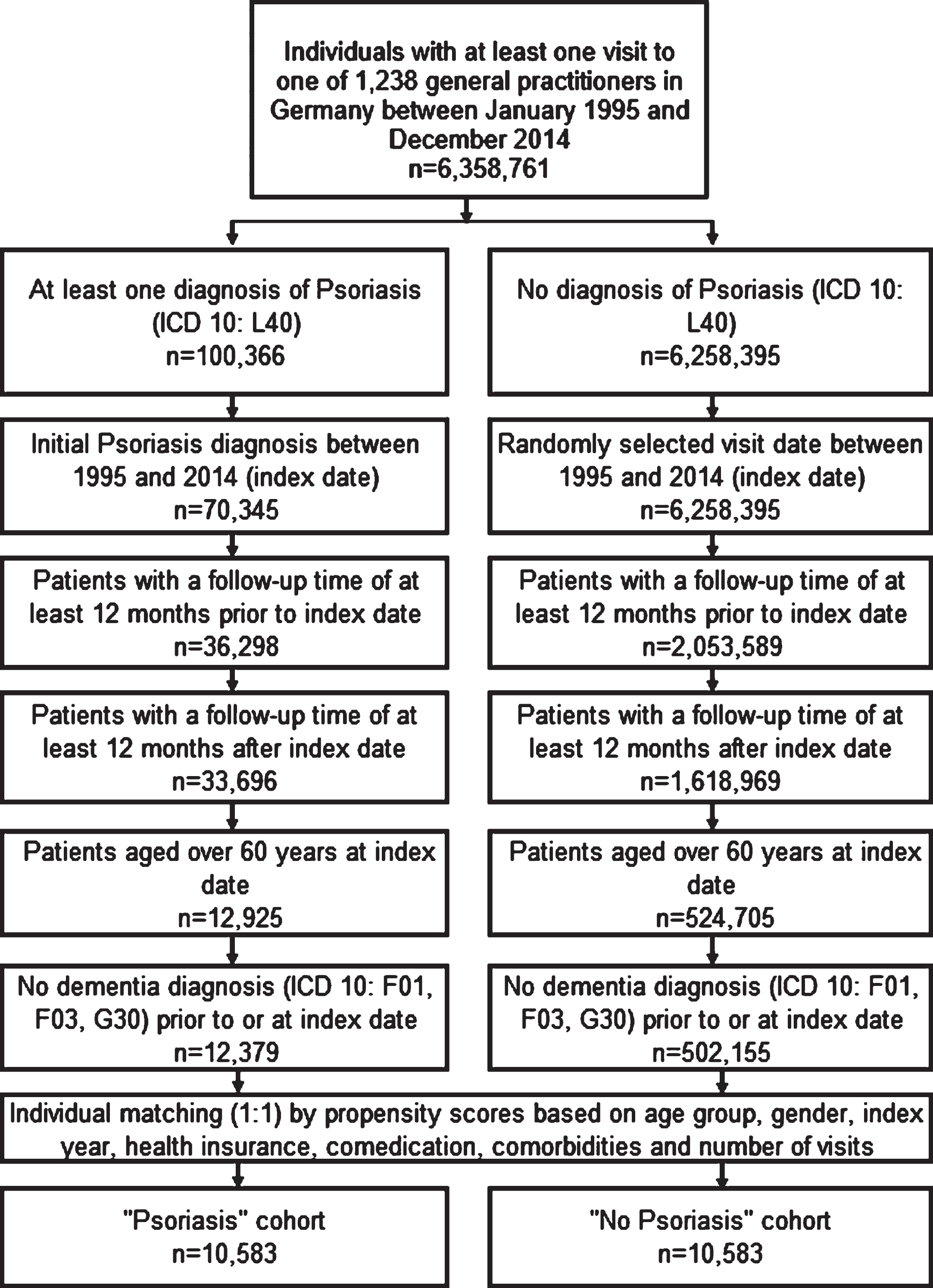
This study included patients of over 60 years with an initial diagnosis of psoriasis (ICD-10: L40) between January 1995 and December 2014 and no previous documented psoriasis diagnosis. These patients were followed in 1,173 GP practices in Germany (starting on the index date). The further inclusion criteria are shown in Fig. 1. For patients without psoriasis, a randomly selected doctor visit date between January 1995 and December 2014 was used as the index date, and follow-up started on this index date. Patients without psoriasis were matched individually (1:1) using a greedy algorithm to psoriasis patients using propensity scores based on age, sex, index year, health insurance type, and comorbidities documented any time before the index date including diabetes, hypertension, stroke including transient ischemic attack (TIA), depression, coronary heart disease, and intracranial injury known to be associated with dementia. The matching procedure also considered five different drug types prescribed any time before the index date as these are also known to be associated with dementia: antidepressants [ATC N06A0], proton pump inhibitors (PPI) [A02B2], statins [C10A1], antiepileptics [N03A0], and antiparkinsonian medication [N04A0]. Finally, the number of doctor visits per year between the index date and the diagnosis of dementia or loss to follow-up was considered as part of the matching procedure since patients with chronic diseases have a higher probability of receiving new diagnoses. A sub-group was also added, consisting of patients with a diagnosis of PsA (ICD-10: L40.5) between January 1995 and December 2014; this group was compared to the PsO patients. Patients were followed up from the index date until their first dementia diagnosis or their last visit to the respective GP practice.
Fig. 1
Selection of study patients and patient flow.
Study outcomes and variables
The study outcome was the cumulative incidence of all-cause-dementia (ICD-10: F00-F03, G30) within up to 15 years of the index date. Finally, the same analysis was conducted to examine the association between PsA (ICD-10: L40.5) and dementia.
Statistical analyses
Kaplan-Meier curves were used to show the dementia incidence. Additionally, univariate Cox proportional regression models were used to assess the relationship between psoriasis and dementia. p-values < 0.05 were considered statistically significant. Analyses were carried out using SAS version 9.4 (SAS Institute, Cary, USA).
Ethical aspect
The database used includes only anonymized data in compliance with the regulations of the applicable data protection laws. German law allows the use of anonymous electronic medical records for research purposes under certain conditions. According to this legislation, it is not necessary to obtain informed consent from patients or approval from a medical ethics committee for this type of observational study that contains no directly identifiable data. Because patients were only queried as aggregates and no protected health information was available for queries, no Institutional Review Board approval was required for the use of this database or the completion of this study.
RESULTS
The present study included 10,583 patients with psoriasis diagnosis and 10,583 controls without psoriasis (Table 1). The mean age was 69.7 years (SD = 6.9 years) in psoriasis patients and 69.8 years (SD = 6.9 years) in the cohort without psoriasis. Of the 10,583 psoriasis patients, 859 (8%) had a diagnosis of PsA between January 1995 and December 2014. The balance of the propensity score matching was assessed using the standardized mean differences (SMD), which were less than 0.00% for all covariates listed in Table 1. The median follow-up time was 8.35 years in the psoriasis cohort and 8.02 years in the non-psoriasis cohort. For PsA patients, it was 8.74 and 8.24 in the non-PsA cohort. On average, patients without psoriasis had 7.5 GP visits per year before the index date compared to 8.1 visits per year in psoriasis patients. The distribution of visits during follow-up is shown in Table 1.
Table 1
Baseline characteristics of psoriasis and non-psoriasis patients after matching (1:1)
| Variable | Psoriasis cohort (n = 10,583) | Non-psoriasis cohort (n = 10,583) | PsA cohort (n = 859) | Non-psoriasis cohort (n = 859) | Psoriasis without PsA only cohort (n = 9,724) | Non-psoriasis cohort (n = 9,724) |
| Age at baseline (Mean, SD) | 69.7 (6.9) | 69.8 (6.9) | 67.9 (6.0) | 68.4 (6.1) | 69.8 (6.9) | 70.0 (7.0) |
| Age 60–70 | 58.1% | 58.1% | 69.3% | 69.3% | 57.2% | 57.2% |
| Age 71–80 | 34.1% | 34.1% | 27.2% | 27.2% | 34.7% | 34.7% |
| Age 80+ | 7.8% | 7.8% | 3.5% | 3.5% | 8.1% | 8.1% |
| Female | 51.5% | 51.5% | 59.1% | 59.1% | 50.9% | 50.9% |
| Male | 48.5% | 48.5% | 40.9% | 40.9% | 49.1% | 49.1% |
| Private health insurance | 6.1% | 6.1% | 6.5% | 6.5% | 6.1% | 6.1% |
| Diagnoses prior to index date | ||||||
| Hypertension (I10) | 66.9% | 66.9% | 59.1% | 59.1% | 67.6% | 67.6% |
| Diabetes mellitus (E10, E11, E14) | 27.2% | 27.2% | 21.8% | 21.8% | 27.7% | 27.7% |
| Stroke incl. TIA (I63, I64, G45) | 3.6% | 3.6% | 3.0% | 3.0% | 3.6% | 3.6% |
| Depression (F32, F33) | 16.1% | 16.1% | 15.3% | 15.3% | 16.1% | 16.1% |
| Coronary heart Disease (I24, I25) | 21.3% | 21.3% | 16.0% | 16.0% | 21.8% | 21.8% |
| Intracranial injury (S06) | 0.2% | 0.2% | 0.1% | 0.1% | 0.3% | 0.3% |
| Therapies prior to index date | ||||||
| Antidepressants (N06A) | 11.6% | 11.6% | 11.6% | 11.6% | 11.6% | 11.6% |
| PPI (A02B2) | 27.8% | 27.8% | 28.6% | 28.6% | 27.8% | 27.8% |
| Statins (C10A1) | 21.8% | 21.8% | 19.0% | 19.0% | 22.0% | 22.0% |
| Antiepileptics (N03A0) | 2.4% | 2.4% | 2.0% | 2.0% | 2.4% | 2.4% |
| Antiparkinsonian (N04A0) | 0.7% | 0.7% | 0.7% | 0.7% | 0.7% | 0.7% |
| Index year | ||||||
| 1995–1999 | 3.7% | 3.7% | 2.0% | 2.0% | 3.9% | 3.9% |
| 2000–2004 | 9.5% | 9.5% | 9.8% | 9.8% | 9.4% | 9.4% |
| 2005–2009 | 28.4% | 28.4% | 27.3% | 27.3% | 28.5% | 28.5% |
| 2010–2014 | 58.4 | 58.4 | 60.9% | 60.9% | 58.2% | 58.2% |
After 15 years of follow-up, 22.0% of the psoriasis patients and 19.1% of the non-psoriasis patients developed dementia (log-rank p < 0.001) (Fig. 2). In the sub-group of patients with PsA, 18.6% of the patients and 14.3% of the non-PsA controls received a dementia diagnosis (log-rank p = 0.067) (see Fig. 3) within the same period. The sub-group without PsA showed a similar trend to the overall analysis, with 22.3% of the psoriasis patients and 19.5% of the non-psoriasis patients developing dementia within 15 years after the index date (log-rank p < 0.001) (Fig. 4).
Fig. 2
Cumulative incidence of dementia in patients with or without psoriasis within 15 years of the index date.
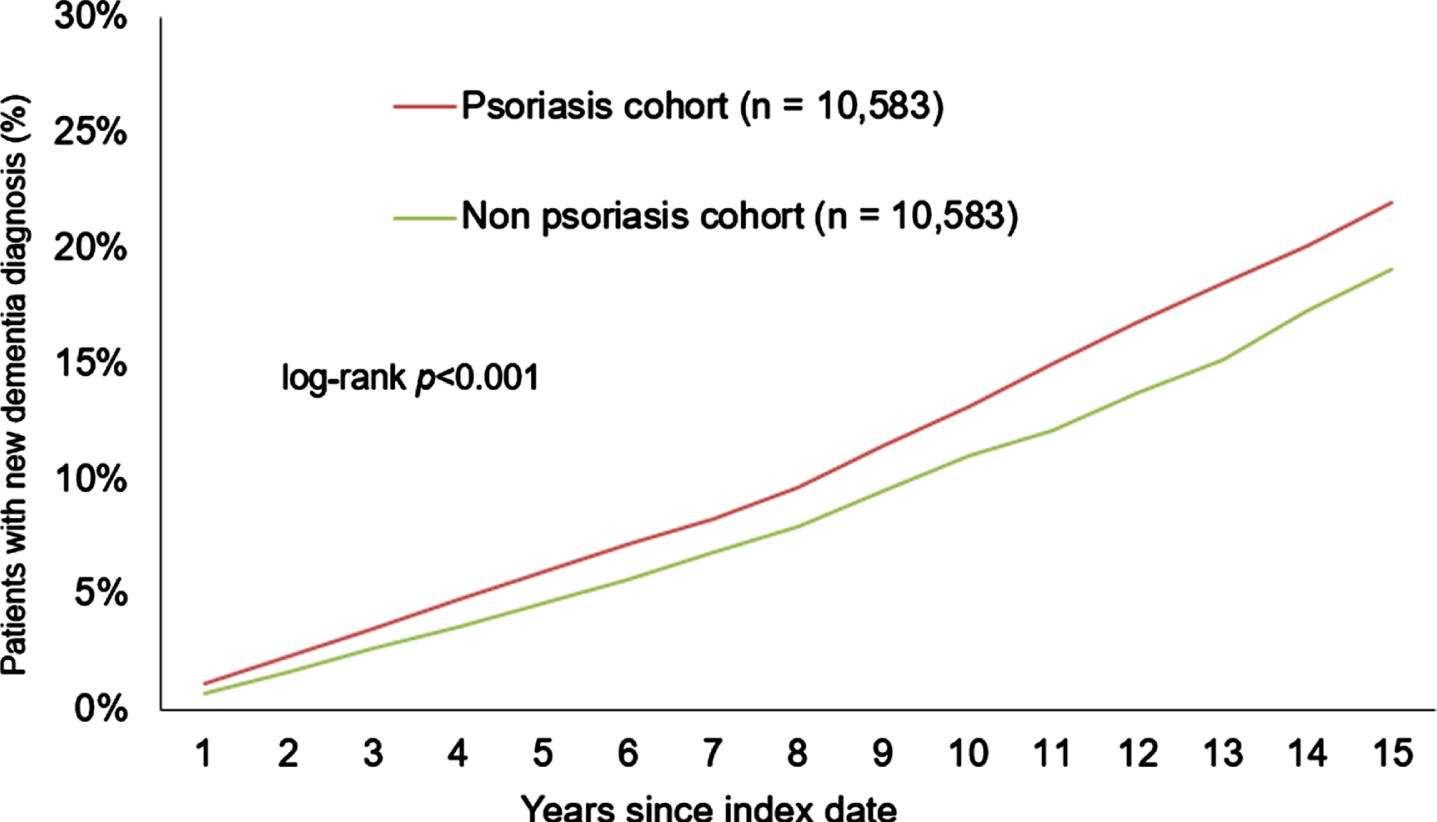
Fig. 3
Cumulative incidence of dementia in patients with or without presence of PsA within 15 years of the index date.
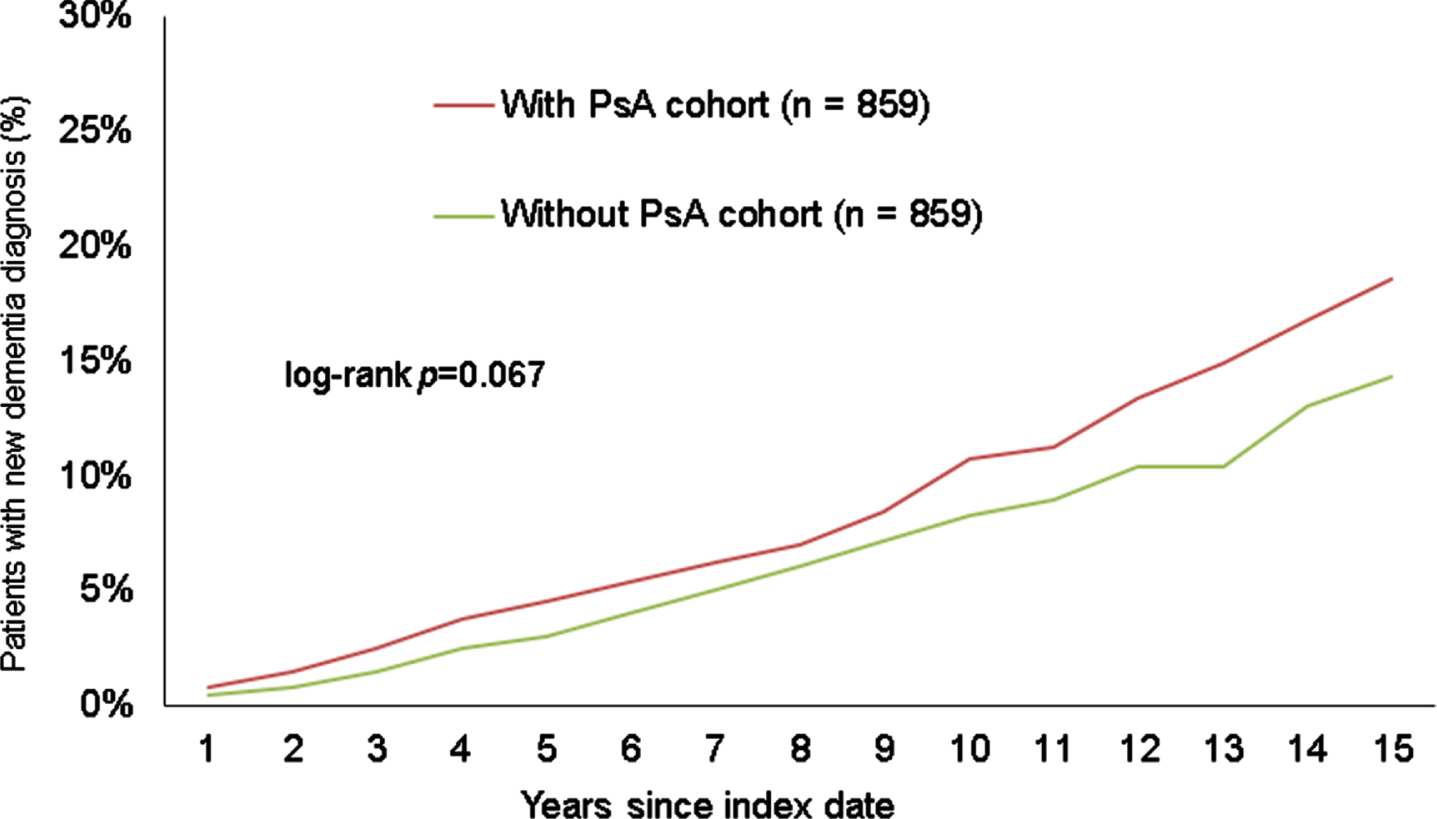
Fig. 4
Cumulative incidence of dementia in patients with or without presence of psoriasis only (PsO) within 15 years of the index date.
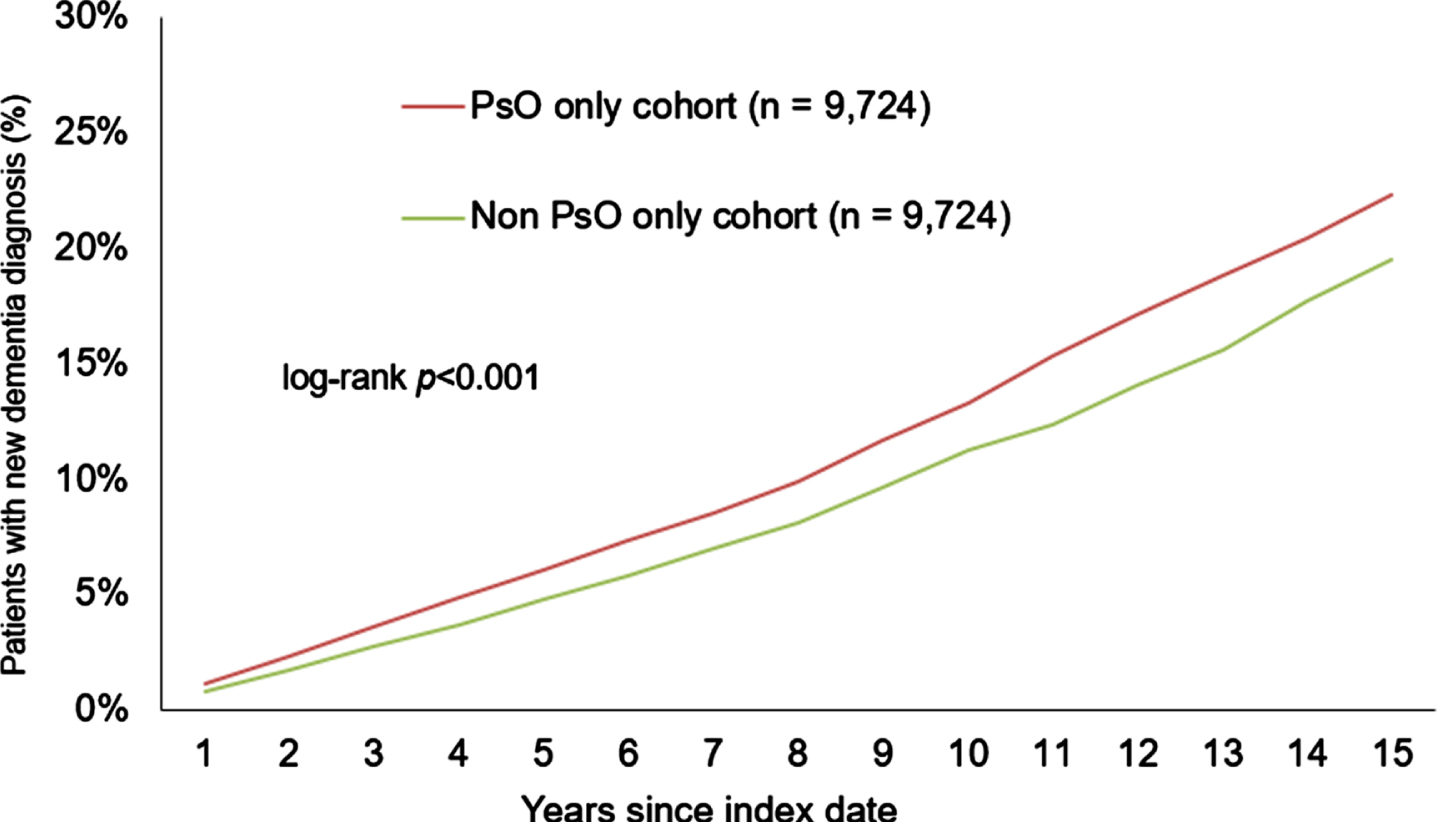
Table 2 shows the results of the Cox-regression. Psoriasis was significantly associated with a dementia risk (HR: 1.24; 95% CI: (1.14–1.35); p < 0.001). The association was stronger for PsA (HR: 1.35; 95% CI: (0.98–1.86)) but this was not significant (p = 0.070).
Table 2
Association between psoriasis and dementia stratified by sex, age, and presence of PsA and incidence rate of dementia in 1,000 person years
| HR (95% CI) | p | Incidence rate in 1,000 person years in psoriasis cohort | Number of events in psoriasis cohort | Number of person-years of follow-up in psoriasis cohort | Incidence rate in 1,000 person years in non-psoriasis cohort | Number of events in non-psoriasis cohort | Number of person-years of follow-up in non-psoriasis cohort | |
| Psoriasis | 1.24 (1.14–1.35) | <0.001 | 15.0 | 1,368 | 91,031 | 11.9 | 1,017 | 85,705 |
| PsA | 1.35 (0.98–1.86) | 0.070 | 11.9 | 92 | 7,752 | 8.6 | 62 | 7,215 |
| Psoriasis w/o PsA | 1.23 (1.13–1.34) | <0.001 | 15.3 | 1,276 | 83,279 | 12.2 | 955 | 78,490 |
| Female | 1.24 (1.11–1.38) | <0.001 | 16.1 | 763 | 47,439 | 12.8 | 572 | 44,741 |
| Male | 1.25 (1.10–1.41) | <0.001 | 13.9 | 605 | 43,592 | 10.9 | 445 | 40,964 |
| Age 60–70 | 1.23 (1.07–1.41) | 0.004 | 8.3 | 489 | 58,913 | 6.4 | 354 | 55,081 |
| Age 71–80 | 1.29 (1.15–1.45) | <0.001 | 24.6 | 682 | 27,770 | 18.6 | 492 | 26,432 |
| Age 81–90 | 1.10 (0.90–1.35) | 0.361 | 45.3 | 197 | 4,348 | 40.8 | 171 | 4,192 |
DISCUSSION
Main findings
This retrospective study including over 20,000 patients followed in German GP offices found a higher incidence rate of all-cause dementia in 1,000 person years in patients with psoriasis than in those without psoriasis. The Cox proportional hazard regression analysis revealed that psoriasis was associated with a 1.2-fold higher risk of developing dementia. The same results were found in patients with PsO while the risk was 1.4-fold in patients with PsA, although this was not significant due to the lower patient number.
Interpretation of findings
Although there have been no previous German studies examining the association between psoriasis and dementia based on a large patient cohort and using real-world data to date, this subject has already been discussed and summarized in a number of systematic reviews and meta-analyses [12–15]. All four of these reviews support the hypothesis that psoriasis is associated with an increased risk of dementia, which is in line with the findings from the present study. The meta-analysis performed by Charoenngam et al. (2021) included 6 studies from Europe and Asia and found a pooled risk ratio of 1.16 (95% CI 1.04–1.30) but with a high degree of statistical heterogeneity [12]. Similar results can be observed in the meta-analysis by Liu et al. (2020), which found a pooled risk ratio of 1.14 (95% CI 1.06–1.24) in 7 studies from East Asia, Europe, and the US included in the work [13]. In addition, one of the case-control studies included, which was based on data from the Taiwan National Health Insurance database, also evaluated the risk of dementia in patients with PsA [24], and the meta-analysis summarized that the risk ratio was 2.20 (95% CI 1.29–3.78) for PsA [13]. This is in line with the finding of our study indicating that the hazard ratio was higher for patients with PsA (HR 1.35 (95% CI 0.98–1.86). This result was not significant in the present study, possibly due to the low patient number in the relevant cohorts. Nonetheless, the two meta-analyses both included the studies by Huang et al. [25] conducted in Taiwan in 2019 and Pezzolo et al. [11] based on data from the Rotterdam study (2018). The third meta-analysis by Lam et al. [14] included 3 studies which were also reported in the first meta-analysis mentioned above [11, 25, 26] as part the quantitative analysis to assess the risk of any dementia type in psoriasis patients and found a pooled hazard ratio of 1.13 (0.91–1.41) [14]. The authors excluded the prospective study by Pezzolo et al. from a sensitivity analysis [11] which led to a pooled HR of 1.29 (1.24–1.34) for all cause-dementia and a better degree of heterogeneity in the meta-analysis [14]. This is similar to the results of our study (HR 1.24 (95% CI 1.14–1.35)). Finally, the review by Zhao et al. (2021) found an increased risk of dementia in psoriasis patients in 6 of the 8 studies included [15].
The European studies reported in the meta-analyses and review were conducted in the UK (Dregan et al. [17], Wotton et al. [26], Abuabara et al. [27]), Sweden (Li et al. [16]), the Netherlands (Pezzolo et al. [11]), and Denmark (Leisner et al. [28]). The prospective study by Pezzolo et al. was the only study that reported a negative association between psoriasis and dementia (adjusted HR: 0.5, (95% CI 0.28–0.91)) [11]. Nevertheless, the number of patients with dementia in the psoriasis cohort was small, i.e., 15 patients out of 318 participants with psoriasis. In the study by Dregan et al. the results were not significant [17] and in the study by Leisner et al. the result was only significant for vascular dementia [28]. Please see Table 3 for a comparison of the different European studies included in the reviews.
Table 3
Main findings of European studies examining the association between psoriasis and dementia
| Study | Present study | Dregan et al. [17] | Wotton et al. [26] | Li et al. [16] | Pezzolo et al. [11] | Abuabara et al. [27] | Leisner et al. [28] |
| Country | Germany | United Kingdom | United Kingdom | Sweden | Netherlands | United Kingdom | Denmark |
| Study design | Retrospective cohort study | Retrospective cohort study | Retrospective cohort study | Retrospective cohort study | Prospective cohort study | Retrospective cohort study | Retrospective cohort study |
| Patient N | 10,538 in PsO cohort and in non-PsO cohort | 91,789 in PsO cohort (mild and severe), 308,843 in comparator cohort | Approximately 7,000,000 controls | 108,607 psoriasis patients | 311 in PsO cohort, 9,305 in non-PsO cohort | 3,603 in severe PsO cohort, 14,330 in non-PsO cohort | 13,675 in PsO cohort, 141,040 in non-PsO-cohort |
| Main findings | HR: 1.24; 95% CI: (1.14–1.35) | Mild PsO: HR 1.06; 95% CI: (0.95–1.19); severe PsO: HR 1.64; 95% CI: (0.99–2.74) | RR (rate ratio): 1.29; 95% CI: (1.25–1.34) | SIR (standardized incidence ratio): 1.18; 95% CI: (1.10–1.27) | HR 0.54; 95% CI: (0.32–0.90) | Increased risk of death from dementia: HR 3.64 (1.36–9.72) | AD: HR 1.14; 95% CI: (0.87–1.50); vascular dementia: HR: 1.73; 95% CI: (1.21–2.47) |
A cross-sectional study from Israel based on a health service database that included over 200,000 patients with and without psoriasis found a negative association between psoriasis and dementia (OR: 0.80 (95% CI 0.80–0.91)) [29]. However, in contrast to the present study, the patients were younger (mean age 48.9 years among both the general population and the psoriasis patients) and were not followed up until development of dementia but instead, the prevalence of dementia was assessed [29]. This may explain the difference in results.
In general, there are several plausible pathways via which psoriasis may influence the development of dementia. First, neuroinflammation can be found in patients with AD and other dementia [2]. Psoriasis is an immune-mediated disease with overactivity in the cells of the immune system where psoriatic cytokines are produced [8]. Preclinical experiments have shown that these cytokines could trigger neuroinflammation and thus contribute to the pathogenesis of AD [4]. As the blood-brain barrier becomes more permeable with increasing age [6] but also due to constant peripheral inflammation in the body [4], the cytokines can activate the immune cells of the brain, namely the microglia, and start a cascade of immune responses in the brain that can ultimately lead to AD [4]. On the other hand, genetics appear to play an important role in the relationship between psoriasis and AD which is the most common form of dementia [10]: The genetic epidemiology study conducted by Yokoyama et al. in 2015 found a single-nucleotide polymorphism (SNP) that was associated with both psoriasis and AD [10]. Furthermore, carriers of the Apolipoprotein E ɛ4 allele have an increased risk of developing AD compared to carriers of the common allele variant ɛ3 [30]. This ɛ4 allele was also more common in patients with chronic plaque psoriasis and guttate psoriasis in the epidemiological study performed by Campalani et al. in 2006 [31]. A few years later in 2010, a study reported that the Apolipoprotein E ɛ4 allele is also associated with the disease severity of psoriasis [32].
Strengths and limitations
The present study is the first real-world study including over 20,000 patients followed in GP practices in Germany that has examined the association between psoriasis and all-cause dementia to date. The study also has an adequately long follow-up time to capture a dementia diagnosis in patients. In addition, the use of real-world data helps to prevent a recall bias in the results since the diagnostic behavior of the physicians is captured in the database. Finally, the validity and representativeness of the Disease Analyzer database and its suitability for epidemiological studies has been demonstrated previously [19].
However, the findings of the present study should be interpreted in light of several limitations. First, patients cannot be followed between different practices, so patients who change physicians can no longer be tracked. Second, the study only included patients attending GP practices to follow up for dementia diagnoses and did not include patients treated by dermatologists, and there is no information about further treatments for psoriasis by skin specialists. It is important to highlight this fact, since the biological adalimumab, which is approved as a treatment option for psoriasis and PsA [33], has been shown to have neuroprotective effects in a study utilizing a mouse model [34]. Nevertheless, the study by Kostev et al. (2019) showed that the share of biological treatments administered for psoriasis in specialist (i.e., dermatologist) practices was only 7% between April 2015 and December 2018 [35]. Furthermore, other systemic treatments for psoriasis such as methotrexate [36] have not been considered in the study although a large case-control study from 2020 found a negative association between prior methotrexate use and dementia [37]. Third, the disease severity of psoriasis is not included in the database and was therefore not considered. Fourth, the database does not include socio-economic and behavioral factors such as lifestyle, income, and education, and these factors therefore were not considered in the study. In addition, genetic characteristics are not captured in the database and were therefore not included, although genetic predisposition may influence the likelihood of developing dementia (i.e., there is a known association between the apolipoprotein E ɛ4 allele and AD [30]).
Conclusion
The present study found a positive association between psoriasis and all-cause dementia in patients in general practices in Germany. This positive association was found in patients with PsO and those with PsA. While the risk of developing dementia was higher in those patients with PsA, it was not significant. These results support the hypothesis that the chronic inflammatory disease of psoriasis may influence the pathogenesis of dementia and may therefore help provide future insights into the potential underlying factors for dementia. Patients with psoriasis should be investigated thoroughly by physicians and should be advised about the relationship between the disease and the development of dementia.
ACKNOWLEDGMENTS
The authors have no acknowledgments to report.
FUNDING
The authors have no funding to report.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
REFERENCES
[1] | Ljubenkov PA , Geschwind MD ((2016) ) Dementia. Semin Neurol 36: , 397–404. |
[2] | Raz L , Knoefel J , Bhaskar K ((2016) ) The neuropathology and cerebrovascular mechanisms of dementia. J Cereb Blood Flow Metab 36: , 172–186. |
[3] | Michalowsky B , Kaczynski A , Hoffmann W ((2019) ) [The economic and social burden of dementia diseases in Germany-A meta-analysis]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 62: , 981–992. |
[4] | Xie J , Van Hoecke L , Vandenbroucke RE ((2022) ) The impact of systemic inflammation on Alzheimer’s disease pathology. Front Immunol 12: , 796867. |
[5] | Grande G , Qiu C , Fratiglioni L ((2020) ) Prevention of dementia in an ageing world: Evidence and biological rationale. Ageing Res Rev 64: , 101045. |
[6] | Newcombe EA , Camats-Perna J , Silva ML , Valmas N , Huat TJ , Medeiros R ((2018) ) Inflammation: The link between comorbidities, genetics, and Alzheimer’s disease. J Neuroinflammation 15: , 276. |
[7] | Grän F , Kerstan A , Serfling E , Goebeler M , Muhammad K ((2020) ) Current developments in the immunology of psoriasis. Yale J Biol Med 93: , 97–110. |
[8] | Menter A ((2016) ) Psoriasis and psoriatic arthritis overview. Am J Manag Care 22: , s216–224. |
[9] | Kim WB , Jerome D , Yeung J ((2017) ) Diagnosis and management of psoriasis. Can Fam Physician 63: , 278–285. |
[10] | Yokoyama JS , Wang Y , Schork AJ , Thompson WK , Karch CM , Cruchaga C , McEvoy LK , Witoelar A , Chen C-H , Holland D , Brewer JB , Franke A , Dillon WP , Wilson DM , Mukherjee P , Hess CP , Miller Z , Bonham LW , Shen J , Rabinovici GD , Rosen HJ , Miller BL , Hyman BT , Schellenberg GD , Karlsen TH , Andreassen OA , Dale AM , Desikan RS , Alzheimer’s Disease Neuroimaging Initiative ((2016) ) Association between genetic traits for immune-mediated diseases and Alzheimer disease. JAMA Neurol 73: , 691–697. |
[11] | Pezzolo E , Mutlu U , Vernooij MW , Dowlatshahi EA , Gisondi P , Girolomoni G , Nijsten T , Ikram MA , Wakkee M ((2018) ) Psoriasis is not associated with cognition, brain imaging markers, and risk for dementia: The Rotterdam Study. J Am Acad Dermatol 85: , 671–680. |
[12] | Charoenngam N , Rittiphairoj T , Ponvilawan B , Ungprasert P ((2021) ) Patients with psoriasis have a higher risk of dementia: A systematic review and meta-analysis. Indian J Dermatol Venereol Leprol 87: , 364–370. |
[13] | Liu L , Chen S , Li H , Qiang Y , Sun X , Zhou Y , Xing M , Luo Y , Ru Y , Ding X , Kuai L , Li B , Li X ((2020) ) Association between psoriasis and dementia: Current evidence. Front Aging Neurosci 12: , 570992. |
[14] | Lam M , Khorvash R , Drucker AM ((2021) ) Association between psoriasis and risk of dementia: A systematic review and meta-analysis. J Am Acad Dermatol 84: , 790–792. |
[15] | Zhao J , Li T , Wang J (2021) Association between psoriasis and dementia: A systematic review. Neurologia (Engl Ed), doi: 10.1016/j.nrl.2020.12.007 |
[16] | Li X , Sundquist J , Zöller B , Sundquist K ((2018) ) Dementia and Alzheimer’s disease risks in patients with autoimmune disorders. Geriatr Gerontol Int 18: , 1350–1355. |
[17] | Dregan A , Chowienczyk P , Gulliford MC ((2015) ) Are inflammation and related therapy associated with all-cause dementia in a primary care population? J Alzheimers Dis 46: , 1039–1047. |
[18] | Kim M , Park HE , Lee S-H , Han K , Lee JH ((2020) ) Increased risk of Alzheimer’s disease in patients with psoriasis: A nationwide population-based cohort study. Sci Rep 10: , 6454. |
[19] | Rathmann W , Bongaerts B , Carius H-J , Kruppert S , Kostev K ((2018) ) Basic characteristics and representativeness of the German Disease Analyzer database. Int J Clin Pharmacol Ther 56: , 459–466. |
[20] | Kostev K , Bohlken J , Jacob L ((2019) ) Association between migraine headaches and dementia in more than 7,400 patients followed in general practices in the United Kingdom. J Alzheimers Dis 71: , 353–360. |
[21] | Bohlken J , Jacob L , Kostev K ((2017) ) Association between anti-dementia treatment persistence and daily dosage of the first prescription: A retrospective analysis in neuropsychiatric practices in Germany. J Alzheimers Dis 58: , 37–44. |
[22] | Zingel R , Bohlken J , Riedel-Heller S , Barth S , Kostev K ((2021) ) Association between low-density lipoprotein cholesterol levels, statin use, and dementia in patients followed in German general practices. J Alzheimers Dis 79: , 37–46. |
[23] | Zingel R , Bohlken J , Kostev K ((2021) ) Association between inflammatory bowel disease and dementia: A retrospective cohort study. J Alzheimers Dis 80: , 1471–1478. |
[24] | Chen K-T , Chen Y-C , Fan Y-H , Lin W-X , Lin W-C , Wang Y-H , Lin L , Chiou J-Y , Wei JC-C ((2018) ) Rheumatic diseases are associated with a higher risk of dementia: A nation-wide, population-based, case-control study. Int J Rheum Dis 21: , 373–380. |
[25] | Huang K-L , Yeh C-C , Wu S-I , Huang K-Y , Yang Y-H , Kuo T-Y , Liang H-Y , Lee Y-C , McIntyre RS , Lee Y , Weng J-C , Chen VC-H ((2019) ) Risk of dementia among individuals with psoriasis: A nationwide population-based cohort study in Taiwan. J Clin Psychiatry 80: , 18m12462. |
[26] | Wotton CJ , Goldacre MJ ((2017) ) Associations between specific autoimmune diseases and subsequent dementia: Retrospective record-linkage cohort study, UK. J Epidemiol Community Health 71: , 576–583. |
[27] | Abuabara K , Azfar RS , Shin DB , Neimann AL , Troxel AB , Gelfand JM ((2010) ) Cause-specific mortality in patients with severe psoriasis: A population-based cohort study in the U.K. Br J Dermatol 163: , 586–592. |
[28] | Leisner MZ , Riis JL , Schwartz S , Iversen L , Østergaard SD , Olsen MS ((2019) ) Psoriasis and risk of mental disorders in Denmark. JAMA Dermatol 155: , 745–747. |
[29] | Kridin K , Lindner D , Shalom G , Piaserico S , Babaev M , Freud T , Comaneshter D , Cohen AD ((2020) ) Psoriasis and dementia: A cross-sectional study of 121,801 patients. Acta Derm Venereol 100: , adv00250. |
[30] | Liu C-C , Liu C-C , Kanekiyo T , Xu H , Bu G ((2013) ) Apolipoprotein E and Alzheimer disease: Risk, mechanisms and therapy. Nat Rev Neurol 9: , 106–118. |
[31] | Campalani E , Allen MH , Fairhurst D , Young HS , Mendonca CO , Burden AD , Griffiths CEM , Crook MA , Barker JNWN , Smith CH ((2006) ) Apolipoprotein E gene polymorphisms are associated with psoriasis but do not determine disease response to acitretin. Br J Dermatol 154: , 345–352. |
[32] | Coto-Segura P , Coto E , Alvarez V , Morales B , Soto-Sánchez J , Corao AI , Santos-Juanes J ((2010) ) Apolipoprotein epsilon4 allele isassociated with psoriasis severity. Arch Dermatol Res 302: , 145–149. |
[33] | Iaconi A , Feldman SR , Balkrishnan R ((2010) ) Psoriasis and its treatment with adalimumab. Expert Opin Biol Ther 10: , 133–152. |
[34] | Park J , Lee S-Y , Shon J , Kim K , Lee HJ , Kim KA , Lee B-Y , Oh S-H , Kim NK , Kim OJ ((2019) ) Adalimumab improves cognitive impairment, exerts neuroprotective effects and attenuates neuroinflammation in an Aβ1-40-injected mouse model of Alzheimer’s disease. Cytotherapy 21: , 671–682. |
[35] | Kostev K , Madelung M ((2019) ) Prescription-based prevalence of biological therapy in patients with psoriasis, rheumatoid arthritis, and inflammatory bowel diseases. Biologicals 61: , 52–54. |
[36] | Sbidian E , Chaimani A , Afach S , Doney L , Dressler C , Hua C , Mazaud C , Phan C , Hughes C , Riddle D , Naldi L , Garcia-Doval I , Le Cleach L ((2020) ) Systemic pharmacological treatments for chronic plaque psoriasis: A network meta-analysis. Cochrane Database Syst Rev 1: , CD011535. |
[37] | Newby D , Prieto-Alhambra D , Duarte-Salles T , Ansell D , Pedersen L , van der Lei J , Mosseveld M , Rijnbeek P , James G , Alexander M , Egger P , Podhorna J , Stewart R , Perera G , Avillach P , Grosdidier S , Lovestone S , Nevado-Holgado AJ ((2020) ) Methotrexate and relative risk of dementia amongst patients with rheumatoid arthritis: A multi-national multi-database case-control study. Alzheimers Res Ther 12: , 38. |




