Vascular Brain Lesions, Cognitive Reserve, and Their Association with Cognitive Profile in Persons with Early-Stage Cognitive Decline
Abstract
Background:
Cognitive reserve may protect against the effects of brain pathology, but few studies have looked at whether cognitive reserve modifies the adverse effects of vascular brain pathology.
Objective:
We determined if cognitive reserve attenuates the associations of vascular brain lesions with worse cognition in persons with subjective concerns or mild impairment.
Methods:
We analyzed 200 participants aged 50–90 years from the Comprehensive Assessment of Neurodegeneration and Dementia (COMPASS-ND) study. Cognition was measured using the Montreal Cognitive Assessment and a neuropsychological test battery. High vascular lesion burden was defined as two or more supratentorial infarcts or beginning confluent or confluent white matter hyperintensity. Cognitive reserve proxies included education, occupational attainment, marital status, social activities, physical activity, household income, and multilingualism.
Results:
Mean age was 72.8 years and 48% were female; 73.5% had mild cognitive impairment and 26.5% had subjective concerns. Professional/managerial occupations, annual household income≥$60,000 per year, not being married/common law, and high physical activity were independently associated with higher cognition. Higher vascular lesion burden was associated with lower executive function, but the association was not modified by cognitive reserve.
Conclusion:
Markers of cognitive reserve are associated with higher cognition. Vascular lesion burden is associated with lower executive function. However, cognitive reserve does not mitigate the effects of vascular lesion burden on executive function. Public health efforts should focus on preventing vascular brain injury as well as promoting lifestyle factors related to cognitive reserve, as cognitive reserve alone may not mitigate the effects of vascular brain injury.
INTRODUCTION
The cognitive reserve hypothesis postulates that markers of enhanced brain development and function, such as greater early-life education, can mitigate the cognitive effects of brain pathologies [1]. Compared to individuals with lower cognitive reserve, when individuals with higher reserve develop cognitive disorders studies show that their brain pathology is more severe [1–4]. This suggests that higher reserve conferred some resistance to the deleterious effects of the pathology [1]. This is often cited as a motivating factor for public health efforts to promote higher education, reduce social isolation, and increase engagement in cognitively demanding leisure activities to reduce risk of later-life dementia.
A limitation of literature on cognitive reserve is that it has mostly been tested in the context of Alzheimer’s disease (AD) pathology, where the hypothesis has gained some support. There are few studies on whether cognitive reserve can mitigate the adverse effects of vascular brain pathology, such as white matter hyperintensities (WMHs) [5]. This is important because vascular pathology is the second biggest contributor to dementia, present in the brain of most persons with AD, mild cognitive impairment (MCI), and dementia. Furthermore, most studies of cognitive reserve focus on global cognition or memory. There is relatively little information about the impact of cognitive reserve on executive functions—the most common cognitive domain affected by cerebrovascular disease.
We analyzed data from patients with early stage impairment in the Comprehensive Assessment of Neurodegeneration and Dementia (COMPASS-ND) study [6] to test the hypothesis that cognitive reserve attenuates the associations of vascular brain lesions, such as WMHs and brain infarcts, with the degree of cognitive impairment. First, we examined the association of proxies of cognitive reserve with cognition. We then tested if vascular brain lesions were associated with cognition, divided into domains of memory, executive function, and processing speed. Lastly, we determined whether a composite cognitive reserve score modifies the association between vascular brain lesions and the cognitive profile.
METHODS
Study population
COMPASS-ND is a multi-site longitudinal cohort study recruiting participants with MCI, AD, vascular MCI (V-MCI), mixed dementia, Lewy body dementia, Parkinson’s disease dementia, Parkinson’s disease MCI, frontotemporal dementia, primary progressive aphasia, subjective cognitive impairment (SCI), and cognitively intact elderly [6]. Participants in this study were recruited from 22 participating memory clinics across Canada. Data from the first 409 participants were analyzed.
For this analysis, we used cross-sectional data from participants with either MCI, V-MCI, or SCI. Participants were 50–90 years old, proficient in English or French (measured by the Language Experience and Proficiency Questionnaire (LEAP-Q) [7, 8]), lived within 1 h of the study site, and had a study partner who saw the participant at least weekly. Participants were recruited from 2016 to 2019. According to COMPASS-ND inclusion and exclusion criteria, participants were required to have a Montreal Cognitive Assessment (MoCA) score greater than 12, no history of ongoing drug or alcohol misuse, and no other significant chronic brain diseases. For the purpose of this study, patients with MCI were recruited into the V-MCI group if clinical CT or MRI neuroimaging showed two or more supratentorial infarcts [9]. Of 409 participants, we excluded 223 participants that had diagnoses other than SCI, MCI, or V-MCI, 1 participant with missing data on ethnicity, 1 participant with missing data on the vascular lesion burden score, 1 participant with missing data on infarcts, and 20 participants with missing data on cognitive reserve, leaving 200 total participants for our analysis. All 200 participants had MRI data and at least one cognitive assessment (all 200 had MoCA, memory, and executive function measures, and 199 had processing speed measures).
Participants provided written consent. The study received local ethical approval from the participating centers’ Institutional Review Boards.
Assessment
Demographic information, medical history, lifestyle, and cognitive reserve factors were collected through extensive self-reported questionnaires. Clinical assessments and physical exams were completed by a clinician [6].
We defined these variables as potential proxies of cognitive reserve: education, occupational attainment, physical activity, social activity, marital status, multilingualism, and annual household income. They were selected a priori based on literature searches [1, 10–16].
All cognitive reserve variables were dichotomized. High education was defined as having a university degree (undergraduate degree, some graduate school, or graduate degree) versus technical school/community college, some university, high school or less. High occupational attainment was defined as professional, managerial, or qualified non-manual occupations, marital status was married/common-law versus other, participation in social activities was defined as engagement in social activities once a month or more, high annual household income was defined based on the median income (≥$60,000 per year), and the language variable was defined as multilingual (one or more language) versus monolingual. High physical activity was defined as a z-score in the 75% percentile (top quartile) on the Physical Activity Scale for the Elderly (PASE) [17]. This z-score was obtained by standardizing the PASE score to previously published normative data (1993) [17].
Cognition was assessed using a cognitive screening tool (MoCA) [18] and neuropsychological assessments. All neuropsychological assessments were first converted to z-scores based on the means and standard deviations of the total study population. A memory composite z-score was then created by using the mean between the total recall z-score component of the Brief Visuospatial Memory Test and the trial raw z-score of the Rey Auditory Verbal Learning Test [19, 20]. An executive function composite z-score was created by using the mean between the trail making part B z-score component of the Reitan Trail Making Test (A & B) and the Letter Fluency total raw z-score of the Delis-Kaplan Executive Function System [21, 22]. A processing speed composite z-score was created by using the mean between the trail making part A z-score of the Reitan Trail Making Test (A & B) and the digit symbol raw z-score of the Wechsler Adult Intelligence Scale-III Digit Symbol-Coding [21, 23].
A 3T MRI was used in this study and sequences were standardized across MRI machines [6, 24]. The following sequences were used: 3D T1, PD/T2, FLAIR, gradient echo, resting state fMRI, and DTI [6]. MRI vascular lesions were identified visually centrally by readers at either the University of Calgary and Sunnybrook Hospital, Toronto [6, 9] according to Standards for Reporting Vascular Changes on Neuroimaging [25]. WMH was grade according to the Fazekas scale [26], summing the periventricular and subcortical scores to give a total range of 0-6. High WMH was defined as total Fazekas score 4-6.
Vascular brain injury was defined as the presence of either brain infarcts or high WMH. Additionally, we analyzed a combined variable, termed “high vascular lesion burden”, defined as the presence of two or more supratentorial brain infarcts or beginning confluent or confluent WMH on the Age-Related White Matter Changes scale [27], which was hypothesized to be a threshold for a potential vascular contribution to cognitive impairment [9].
Statistical analysis
Total MoCA score was treated as a continuous variable. Memory, executive function, and processing speed variables were converted to z-scores and treated as continuous variables.
We first tested the association between each of the seven cognitive reserve variables, MoCA, and the three cognitive domains. To test this, we built separate multiple linear regression models for each cognitive reserve variable and each individual cognitive domain, adjusting each model for age, sex, and education. We then ran the same analysis in a fully adjusted model, adjusting for age, sex, and all cognitive reserve predictor variables. Collinearity between cognitive reserve variables was assessed by calculating variation inflation factors, and variables with inflation factor > 5 were removed from the model. An analysis was also performed to determine if the association between marital status and cognition was modified by sex. After finding that multilingualism was associated with worse, not better, MoCA we performed a secondary analysis on a revised total MoCA score removing 3 items that are more heavily dependent on language (naming, letter fluency, and sentence repetition).
A single cognitive reserve score was calculated based on the cognitive reserve candidate variables that were associated with either memory, executive function, or processing speed in the previous fully-adjusted analysis. Based on the relative size of the beta coefficients, a cognitive reserve composite score was created by allocating 3 points for professional/managerial/qualified non-manual occupations, and 2 points each for annual household income≥$60,000 per year, not being married/common law, and high physical activity. These four variables were used in the composite because they were the only four variables associated with any of the cognitive domains during our initial fully-adjusted analysis.
We tested the association between markers of vascular brain injury (infarcts, WMH, and high vascular lesion burden) and cognitive outcomes by building separate linear regression models for each exposure and outcome, adjusting for age, sex, and education. We also tested whether cognitive reserve predicts infarcts, high WMH, or high vascular lesion burden using separate multiple logistic regression models, adjusted for age and sex.
We then tested for effect modification to determine if cognitive reserve modifies the association between markers of VBI and cognitive outcomes. We analyzed only the markers of VBI that were associated with cognition in previously analyses. The models for effect modification included terms for age, sex, VBI variable, cognitive reserve, and the interaction of VBI with cognitive reserve. Education was not included as a separate term because it was included in the cognitive reserve score. Separate models were created for each cognitive outcome (MoCA total score, memory, processing speed, and executive functions).
Sensitivity analyses were performed to test the robustness of our results. We tested the impact of: a) creating a cognitive reserve score without dichotomizing any of the variables, b) stratifying or controlling for SCI and MCI status, c) using education alone as a proxy of cognitive reserve, and d) removing marital status from the cognitive reserve score.
A p-value of less than 0.05 was considered significant; because these analyses were considered exploratory, no adjustment was made for multiple hypothesis testing. Statistical analyses were conducted using SAS v9.4 (SAS Institute, Cary, NC).
RESULTS
Table 1 shows the characteristics of the study population. Of the 200 participants, 48% were female, 6% had V-MCI, 67.5% had MCI, and 26.5% had SCI. The mean age was 72.8 with standard deviation 6.8, 51% had a university degree, 86% had professional/managerial occupations or qualified non-manual occupations, 60% had an annual household income≥60K, 57.5% were multilingual, and 74% were married/common law. Brain infarcts were seen in 19.5% of participants had brain infarcts, high WMH in 22%, and vascular brain lesions (either infarcts or high WMH) in 36%. The mean MoCA score was 24.5±3.3.
Table 1
Characteristics of the study population
| Characteristic | % Mean (SD) | n/N |
| Female | 48.0% | 96/200 |
| Age, y | 72.8 (6.8) | 200 |
| Ethnicity | — | — |
| White | 88.0% | 176/200 |
| Asian | 4.5% | 9/200 |
| South Asian | 2.5% | 5/200 |
| Black and Other | 5.0% | 10/200 |
| Diagnosis | — | —- |
| Mild Cognitive Impairment | 67.5% | 135/200 |
| Subjective Cognitive Impairment | 26.5% | 53/200 |
| Vascular Mild Cognitive Impairment | 6.0% | 12/200 |
| MoCA score | 24.5 (3.3) | 200 |
| Brain Infarcts | 19.5% | 39/200 |
| WMH Fazekas Score 4 to 6 | 22.0% | 44/200 |
| Vascular Lesion Burden | 36.0% | 72/200 |
| Diabetes | 11.0% | 22/200 |
| Hypertension | 36.5% | 73/200 |
| Stroke | 2.01% | 4/199 |
| Vascular Risk Factors | 50.5% | 101/200 |
| University Degree | 51.0% | 102/200 |
| Professional/Managerial Occupation | 86.0% | 172/200 |
| Annual Household Income≥60K | 60.0% | 120/200 |
| Married/Common-Law | 74.0% | 148/200 |
| Multilingual (2 or More) | 57.5% | 115/200 |
| Normal/High Social Activity | 65.0% | 130/200 |
| High Physical Activity | 25.5% | 51/200 |
WMH, white matter hyperintensity; MoCA, Montreal Cognitive Assessment; Vascular Risk Factors: Having one or more diagnoses of Diabetes, Hypertension, Stroke, Transient Ischemic Attack, Heart Attack or Congestive Heart Failure, Atrial Fibrillation, or Angina. High physical activity was defined as≥75Th Percentile z-score on the Physical Activity Scale for the Elderly.
Table 2
Association of cognitive reserve proxies with cognition
| MoCA | Memory | Executive function | Processing speed | |||||
| β | 95% CL | β | 95% CL | β | 95% CL | β | 95% CL | |
| University Degree | 1.17 | 0.27, 2.06 | 0.05 | –0.18, 0.28 | 0.17 | –0.06, 0.40 | 0.14 | –0.08, 0.36 |
| Occupation | 2.72 | 1.42, 4.01 | 0.23 | –0.10, 0.57 | 0.61 | 0.27, 0.94 | 0.52 | 0.19, 0.85 |
| Household Income | 0.78 | –0.14, 1.71 | 0.00 | –0.23, 0.24 | 0.41 | 0.17, 0.64 | 0.23 | 0.00, 0.45 |
| Multilingual | –0.77 | –1.69, 0.15 | –0.14 | –0.37, 0.09 | –0.00 | –0.24, 0.24 | 0.08 | –0.14, 0.31 |
| Married | –1.52 | –2.58, –0.45 | –0.32 | –0.59, –0.06 | –0.27 | –0.54, 0.01 | –0.13 | –0.40, 0.14 |
| Social Activities | –0.23 | –1.19, 0.73 | 0.05 | –0.19, 0.29 | 0.18 | –0.06, 0.43 | 0.20 | –0.03, 0.44 |
| Physical Activity | 0.73 | –0.33, 1.79 | 0.08 | –0.19, 0.35 | 0.30 | 0.03, 0.57 | 0.26 | 0.00, 0.52 |
Beta coefficient for MoCA is the estimated different in points, otherwise it represents the estimated difference in z score. Adjusted for age, and education. MoCA, Montreal Cognitive Assessment.
Table 3
Association of predictors with cognition; fully adjusted model
| MoCA | Memory | Executive function | Processing speed | |||||
| β | 95% CL | β | 95% CL | β | 95% CL | β | 95% CL | |
| University Degree | 0.90 | –0.02, 1.82 | 0.04 | –0.21, 0.29 | 0.05 | –0.19, 0.28 | 0.02 | –0.21, 0.25 |
| Occupation | 2.38 | 1.03, 3.73 | 0.24 | –0.13, 0.60 | 0.56 | 0.21, 0.90 | 0.51 | 0.16, 0.86 |
| Household Income | 0.87 | –0.03, 1.77 | 0.03 | –0.21, 0.28 | 0.37 | 0.14, 0.61 | 0.16 | –0.07, 0.39 |
| Multilingual | –1.04 | –1.92, –0.16 | –0.15 | –0.39, 0.09 | –0.04 | –0.27, 0.19 | 0.05 | –0.17, 0.28 |
| Married | –1.67 | –2.70, –0.65 | –0.32 | –0.60, –0.05 | –0.36 | –0.63, –0.10 | –0.16 | –0.43, 0.10 |
| Social Activities | –0.36 | –1.26, 0.53 | 0.05 | –0.19, 0.30 | 0.12 | –0.11, 0.35 | 0.18 | –0.05, 0.41 |
| High Physical Activity | 0.78 | –0.23, 1.78 | 0.08 | –0.19, 0.36 | 0.31 | 0.05, 0.57 | 0.31 | 0.05, 0.56 |
Adjusted for age, sex, and all predictor variables. MoCA, Montreal Cognitive Assessment.
Table 4 shows the association between vascular brain lesions and cognitive profile. High vascular lesion burden, but not infarcts or WMH alone, was associated with lower executive function (beta coefficient -0.36, 95% CI -0.63 to -0.09, p = 0.01). Higher composite cognitive reserve score was associated with lower odds of high vascular lesion burden (OR 0.82 per additional point, 95% CI 0.68 to 0.996), but was not associated with WMH alone (OR 0.84, per additional point, 95% CI 0.68 to 1.03) or brain infarcts alone (OR 0.93, per additional point, 95% CI 0.77 to 1.14) in analyses adjusted for age and sex. Considering marital status alone, being married or in a common law partnership was associated with higher odds of high vascular lesion burden (adjusted OR 2.50, 95% CI 1.08 to 5.80) controlling for age and sex.
Table 4
Association of vascular brain lesions (Multiple Infarcts or High WMH) with cognition
| MRI brain measures | MoCA | Memory | Executive function | Processing speed | ||||
| β | 95% CL | β | 95% CL | β | 95% CL | β | 95% CL | |
| Infarcts | –0.75 | –1.92, 0.41 | 0.04 | –0.26, 0.34 | –0.08 | –0.38, 0.23 | –0.06 | –0.35, 0.23 |
| WMH | –0.989 | –2.16, 0.18 | –0.08 | –0.38, 0.22 | –0.30 | –0.60, 0.007 | –0.13 | –0.42, 0.16 |
| Vascular Lesion Burden | –0.99 | –2.04, 0.06 | –0.03 | –0.30, 0.24 | –0.36 | –0.63, –0.09 | –0.23 | –0.49, 0.03 |
Adjusted for age, sex, and education. MoCA, Montreal Cognitive Assessment; WMH, white matter hyperintensity.
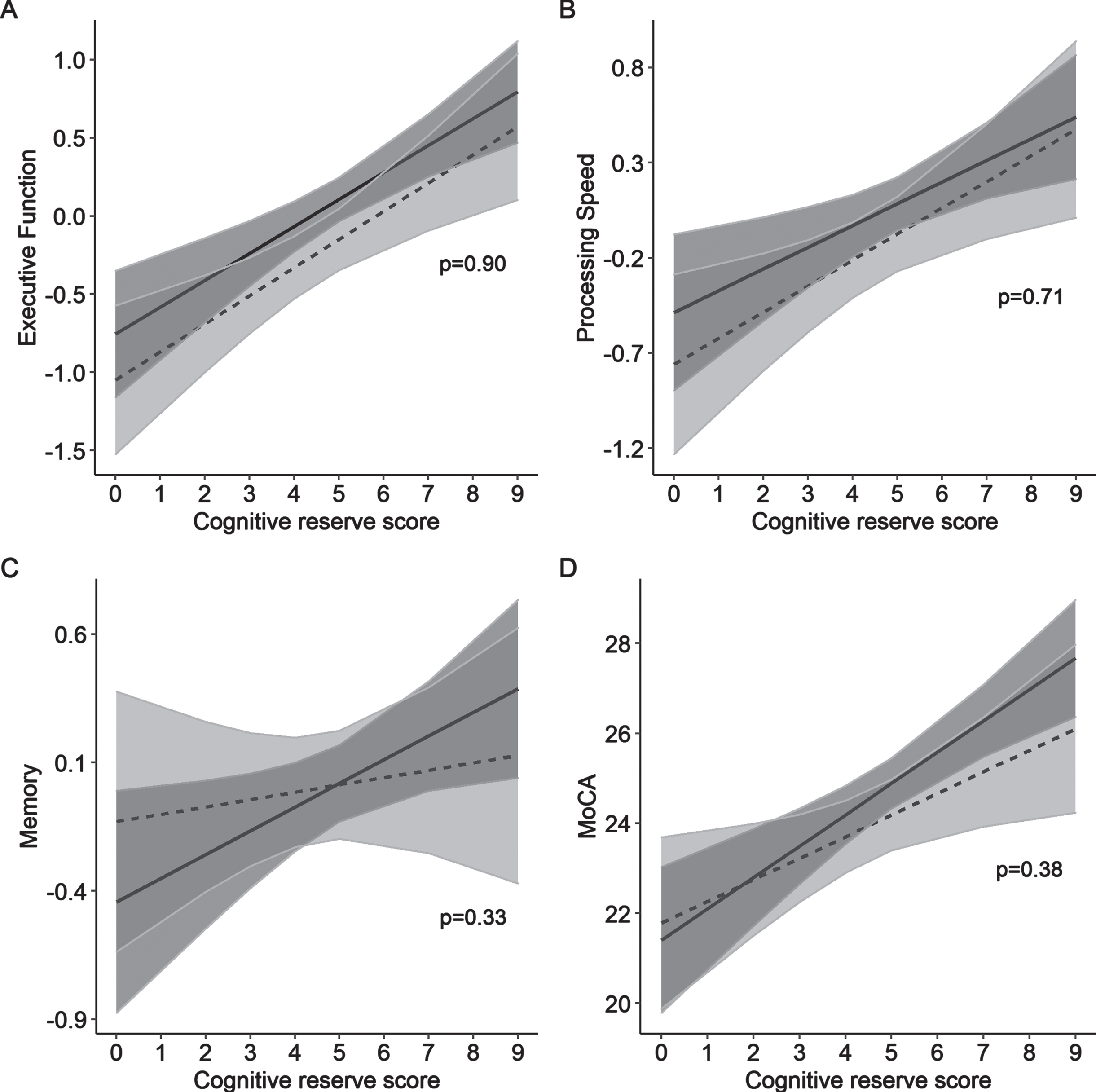
Table 5 and Fig. 1 show the modifying effects of the composite cognitive reserve score on the association between vascular lesion burden and cognitive profile. When adjusted for each other in the same model, higher cognitive reserve composite score was associated with higher executive function (p < 0.001) and high vascular lesion burden was associated with lower executive function (p = 0.046). Higher cognitive reserve composite score was associated higher processing speed (p < 0.001), memory (p = 0.03), and MoCA (p < 0.001), but high vascular lesion burden was not associated with these cognitive domains. There was no evidence of an interaction between the cognitive reserve composite score and vascular lesion burden on any of the cognitive domains or MoCA (memory: beta coefficient -0.06, 95% CI -0.19 to 0.07, interaction 0.33; executive function: beta coefficient 0.008, 95% CI -0.11 to 0.13, interaction 0.90; processing speed: beta coefficient 0.02, 95% CI –0.10 to 0.14, interaction p = 0.71; MoCA: beta coefficient -0.22, 95% CI -0.70 to 0.27, interaction p = 0.38).
Table 5
Modifying effect of cognitive reserve composite score on the association between vascular lesion burden and cognitive scores
| Interaction between cognitive reserve and vascular lesion burden | ||||
| Predictors of executive function | Without interaction | With interaction | ||
| Beta (95% CI) | p | Beta (95% CI) | p | |
| Cognitive reserve | 0.18 (0.12, 0.23) | <0.0001 | 0.17 (0.10, 0.25) | <0.0001 |
| Vascular lesion burden | –0.26 (–0.51, –0.004) | 0.04 | –0.29 (–0.93, 0.34) | 0.36 |
| Interaction | — | — | 0.008 (–0.11, 0.13) | 0.90 |
| Predictors of processing speed | Without interaction | With interaction | ||
| Beta (95% CI) | p | Beta (95% CI) | p | |
| Cognitive reserve | 0.12 (0.06, 0.18) | <0.0001 | 0.11 (0.04, 0.19) | 0.003 |
| Vascular lesion burden | –0.16 (–0.41, 0.09) | 0.21 | –0.27 (–0.91, 0.36) | 0.40 |
| Interaction | — | — | 0.02 (–0.10, 0.14) | 0.71 |
| Predictors of memory | Without interaction | With interaction | ||
| Beta (95% CI) | p | Beta (95% CI) | p | |
| Cognitive reserve | 0.07 (0.005, 0.13) | 0.03 | 0.09 (0.01, 0.17) | 0.02 |
| Vascular lesion burden | 0.006 (–0.26, 0.28) | 0.97 | 0.31 (–0.37, 0.99) | 0.36 |
| Interaction | — | — | –0.06 (–0.19, 0.07) | 0.33 |
| Predictors of MoCA | Without interaction | With interaction | ||
| Beta (95% CI) | p | Beta (95% CI) | p | |
| Cognitive reserve | 0.61 (0.38, 0.85) | <0.0001 | 0.70 (0.40, 0.996) | <0.0001 |
| Vascular lesion burden | –0.67 (–1.68, 0.35) | 0.20 | 0.38 (–2.17, 2.92) | 0.77 |
| Interaction: | — | — | –0.22 (–0.70, 0.27) | 0.38 |
Adjusted for age and sex. MoCA, Montreal Cognitive Assessment. The Cognitive Reserve Composite Score consists of 3 points for professional/managerial/qualified non-manual occupations, and 2 points each for annual household income≥60K, not being married/common law, and high physical activity. Association between cognitive reserve and cognition (executive functioning (A), processing speed (B), memory (C), and MoCA (D)), in the presence (dotted line) or absence (solid line) of vascular lesion burden. Shaded regions display 95% confidence intervals, p-values represent the interaction between vascular lesion burden and cognitive reserve score. Participants with high vascular lesion burden had lower mean executive function, processing speed, and MoCA scores. However, there was no interaction between vascular lesion burden and cognitive reserve on cognitive function, as shown by the overlapping confidence intervals and non-significant interaction p-values.
Fig. 1
Association of vascular lesion burden with cognition, across cognitive reserve composite scores.
The results were similar in sensitivity analyses using variables without dichotomizing them (Supplementary Table 1), controlling for SCI and MCI status (Supplementary Table 2), using education alone was used as the cognitive reserve proxy (Supplementary Table 3), and when marital status was removed from the cognitive reserve score (Supplementary Table 4).
DISCUSSION
In this population with early, mild symptoms or impaired cognition, we found that vascular lesion burden was associated with a cognitive profile featuring poor executive function. We found that occupational attainment, income, marital status, and physical activity were independently associated with a milder cognitive profile. However, we found that both vascular lesion burden and cognitive reserve score had independent, non-interactive effects on cognition. The results were similar when education alone was used as the cognitive reserve proxy. Thus, we found that in this population the effects of vascular lesion burden on the cognitive profile were not mitigated by higher cognitive reserve.
Vascular lesion burden was associated with a cognitive profile marked by lower executive function. This is consistent with other studies showing that in vascular cognitive impairment, executive function is often affected out of proportion to memory [28]. However, we found only trends, without significant associations, when analyzing brain infarcts alone or WMH alone. This probably reflects limitations of the sample size and suggests that infarcts and WMH have additive effects when present in combination. Our findings are only relevant to the influence of vascular lesions on cognitive profile in persons with early-stage cognitive symptoms and impairment, whereas in the general population it is known that brain infarcts and WMH are also associated with impaired processing speed and global cognition, too [29].
Associations between cognitive reserve proxies and cognition were consistent with prior literature with the exception that multilingualism was associated with worse MoCA and marital status was associated with worse cognition rather than better cognition. Lower MoCA scores in multilingual persons may have reflected difficulty with language related MoCA items, as neuropsychological scores on tests of executive function and processing speed, which are less dependent on language, did not differ from unilingual persons. Indeed, after removing three of the more language dependent MoCA items the association was no longer significant. The association of marital status with lower cognition was unexpected and differs from what has been observed in studies in the general population, where being in a martial partnership is associated with higher cognition [30]. We explored whether there were sex-specific effects of marital status on cognition and did not find any. However, marital status was associated with higher odds of having high vascular lesion burden, suggesting that married persons may be presenting at a later stage of impairment, with higher levels of brain pathology, because their spouse helps them compensate for earlier impairments.
Some prior studies suggest that cognitive reserve modifies the association between vascular pathology and cognition [2, 4, 31–34] while others do not [30, 35]. Variable findings across studies may reflect differences in study populations. Our study was composed of participants that have high occupational attainment (85.3%), the majority were married or in a common-law partnership (73.1%), almost half had a university degree (48.7%), many participants had high annual household income (60.0%) and a high proportion were multilingual (57.5%). Thus, our study participants may have had unusually high cognitive reserve, with less heterogeneity in reserve than other studies. One recently published study found that cognitive reserve did not modify the association between WMH and cognition, which is consistent with our findings [35]. The participants in that study also had a relatively high mean years of education (13.0±4.5) [35], which exceeded that reported in older studies of WMH and cognitive reserve (e.g., mean 9.6±3.8 years in the Leukoaraiosis and Disability (LADIS) study) [2]. Other studies included participants with dementia, whereas our study only included persons with SCI or MCI. Because eligibility for our study required cognitive symptoms, which are related to the study outcome (neuropsychological function), the findings may be influenced by reasons why patients presented to the clinic or the presence of other cognitive brain pathologies, and associations with risk factors may differ from what would be observed in the general population. However, the study findings are applicable to the setting in which they were observed, i.e., patients presenting to a memory clinic with cognitive symptoms without dementia.
When individuals with higher cognitive reserve develop a cognitive disorder their brain pathology may be more severe, because the additional reserve capacity has allowed the brain to compensate for some of the effects of the pathology [1]. In other words, it takes more pathology to cause cognitive impairment in persons with higher reserve. If that were the case, then in persons with a cognitive disorder and high cognitive reserve one might expect to find higher levels of brain pathology. However, in our study we found the opposite–that in mildly impaired participants, higher cognitive reserve composite score was associated with lower vascular lesion burden. This finding may be because higher socioeconomic status and higher physical activity, which are proxy variables for cognitive reserve, are also associated with lower risk of cardiovascular disease and vascular brain injury in the general population [36, 37]. However, we did find that one component of the composite score, being married or in a common law partnership, was associated with higher burden of vascular brain lesions at presentation with SCI or MCI.
Limitations of our study include the cross-sectional nature of our cohort, which does not allow us to test temporality of relationships. However, future COMPASS-ND analyses plan to include longitudinal data to better determine the temporal association of vascular brain injury with cognitive profile. The COMPASS-ND study cohort is predominantly white and well educated with high occupational attainment; it is increasingly recognized that the dementia research field requires more studies targeting patient populations that previously have been under-represented [38]. Another potential limitation of our study is that we dichotomized our cognitive reserve variables for simplicity of interpretation, which may have some impact on statistical power. However, a sensitivity analysis using the original variable categories without dichotomization showed similar associations. Our analysis of social participation was based on a simple frequency of social interactions in three categories, and it is possible that the effect of social participation would have been greater if it had been captured in greater detail. Because our study was not done in the general population, it does not provide definitive evidence on whether cognitive reserve modifies the effect of vascular brain lesions in that setting.
Findings from our study suggest that high occupational attainment, high physical activity, high annual household income, and being unmarried or not being in a common-law partnership are lifestyle factors associated with a milder cognitive profile in persons with subjective cognitive concerns or mild cognitive impairment. However, these lifestyle factors did not mitigate the effects of vascular brain injury on executive function in this study population with early-stage cognitive concerns and impairments. Targeting vascular risk factors to prevent vascular brain injury, as well as promoting cognitive reserve, will be necessary to reduce the risk for vascular cognitive impairment.
ACKNOWLEDGMENTS
The authors have no acknowledgments to report.
FUNDING
The Canadian Consortium on Neurodegeneration in Aging is supported by a grant from the Canadian Institutes of Health Research with funding from several partners. Amanpreet Badhwar is supported by Fonds de recherche du Québec Santé - Chercheur boursiers Junior 1 and Fondation Courtouis.
CONFLICT OF INTEREST
Romella Durrani, Aravind Ganesh, Jaspreet Bhangu, Sandra Black, and Vladimir Hachinski report no disclosures. Philip Barber reports research grant funding from the Heart and Stroke Foundation of Canada. Thalia Field reports a research grant from Bayer (in-kind study medication) and consulting fees from HLS Therapeutics. Rick Swartz received salary support for research from Heart and Stroke Foundation Clinician-Scientist Phase II Award, the Sunnybrook Department of Medicine and the SE Black Centre for Brain Resilience and Recovery, and research grant funding from CIHR, Heart and Stroke Foundation, National Institute of Health, and the Ontario Brain Institute. Mukul Sharma reports research support from Bayer, Bristol Myers Squibb and Janssen, and consulting fees from Bayer, Bristol Myers Squibb, Alexion, and HLS Therapeutics. Eric Smith reports consulting fees from Bayer, Biogen, and Cyclerion, and royalties from UpToDate.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/ADR-220054.
REFERENCES
[1] | Stern Y ((2012) ) Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol 11: , 1006–1012. |
[2] | Jokinen H , Melkas S , Madureira S , Verdelho A , Ferro JM , Fazekas F , Schmidt R , Scheltens P , Barkhof F , Wardlaw JM , Inzitari D , Pantoni L , Erkinjuntti T ((2016) ) Cognitive reserve moderates long-term cognitive and functional outcome in cerebral small vessel disease. J Neurol Neurosurg Psychiatry 87: , 1296–1302. |
[3] | Stern Y , Alexander GE , Prohovnik I , Stricks L , Link B , Lennon MC , Mayeux R ((1995) ) Relationship between lifetime occupation and parietal flow: Implications for a reserve against Alzheimer’s disease pathology. Neurology 45: , 55–60. |
[4] | Pinter D , Enzinger C , Fazekas F ((2015) ) Cerebral small vessel disease, cognitive reserve and cognitive dysfunction. J Neurol 262: , 2411–2419. |
[5] | Durrani R , Hill MD , Smith EE ((2020) ) Preventing covert brain infarct-related cognitive impairment and dementia. Can J Neurol Sci 47: , 456–463. |
[6] | Chertkow H , Borrie M , Whitehead V , Black SE , Feldman HH , Gauthier S , Hogan DB , Masellis M , McGilton K , Rockwood K , Tierney MC , Andrew M , Hsiung GR , Camicioli R , Smith EE , Fogarty J , Lindsay J , Best S , Evans A , Das S , Mohaddes Z , Pilon R , Poirier J , Phillips NA , MacNamara E , Dixon RA , Duchesne S , MacKenzie I , Rylett RJ ((2019) ) The comprehensive assessment of neurodegeneration and dementia: Canadian Cohort Study. Can J Neurol Sci 46: , 499–511. |
[7] | Kaushanskaya M , Blumenfeld HK , Marian V ((2020) ) The Language Experience and Proficiency Questionnaire (LEAP-Q): Ten years later. Biling (Camb Engl) 23: , 945–950. |
[8] | Marian V , Blumenfeld HK , Kaushanskaya M ((2007) ) The Language Experience and Proficiency Questionnaire (LEAP-Q): assessing language profiles in bilinguals and multilinguals. J Speech Lang Hear Res 50: , 940–967. |
[9] | Smith EE , Duchesne S , Gao F , Saad F , Whitehead V , McCreary CR , Frayne R , Gauthier S , Camicioli R , Borrie M , Black SE ((2021) ) Vascular contributions to neurodegeneration: Protocol of the COMPASS-ND study. Can J Neurol Sci 48: , 799–806. |
[10] | Scarmeas N , Levy G , Tang MX , Manly J , Stern Y ((2001) ) Influence of leisure activity on the incidence of Alzheimer’s disease. Neurology 57: , 2236–2242. |
[11] | Stern Y , Gurland B , Tatemichi TK , Tang MX , Wilder D , Mayeux R ((1994) ) Influence of education and occupation on the incidence of Alzheimer’s disease. JAMA 271: , 1004–1010. |
[12] | Petot GJ , Vega U , Traore F , Fritsch T , Debanne SM , Friedland RP , Lerner AJ ((2007) ) Height and Alzheimer’s disease: findings from a case-control study. J Alzheimers Dis 11: , 337–341. |
[13] | Hakansson K , Rovio S , Helkala EL , Vilska AR , Winblad B , Soininen H , Nissinen A , Mohammed AH , Kivipelto M ((2009) ) Association between mid-life marital status and cognitive function in later life: population based cohort study. BMJ 339: , b2462. |
[14] | Craik FI , Bialystok E , Freedman M ((2010) ) Delaying the onset of Alzheimer disease: Bilingualism as a form of cognitive reserve. Neurology 75: , 1726–1729. |
[15] | Bourassa KJ , Memel M , Woolverton C , Sbarra DA ((2017) ) Social participation predicts cognitive functioning in aging adults over time: comparisons with physical health, depression, and physical activity. Aging Mental Health 21: , 133–146. |
[16] | Reed BR , Dowling M , Farias ST , Sonnen J , Strauss M , Schneider JA , Bennett DA , Mungas D ((2011) ) Cognitive activities during adulthoodare more important than education in building reserve. J IntNeuropsychol Soc 17: , 615–624. |
[17] | Washburn RA , Smith KW , Jette AM , Janney CA ((1993) ) The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol 46: , 153–162. |
[18] | Nasreddine ZS , Phillips NA , Bedirian V , Charbonneau S , Whitehead V , Collin I , Cummings JL , Chertkow H ((2005) ) The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53: , 695–699. |
[19] | Tam JW , Schmitter-Edgecombe M ((2013) ) The role of processing speed in the Brief Visuospatial Memory Test - revised. Clin Neuropsychol 27: , 962–972. |
[20] | Schoenberg MR , Dawson KA , Duff K , Patton D , Scott JG , Adams RL ((2006) ) Test performance and classification statistics for the Rey Auditory Verbal Learning Test in selected clinical samples. Arch Clin Neuropsychol 21: , 693–703. |
[21] | Reitan RM ((1958) ) Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills 8: , 271–276. |
[22] | Homack S , Lee D , Riccio CA ((2005) ) Test review: Delis-Kaplan executive function system. J Clin Exp Neuropsychol 27: , 599–609. |
[23] | Joy S , Kaplan E , Fein D ((2004) ) Speed and memory in the WAIS-III Digit Symbol–Coding subtest across the adult lifespan. Arch Clin Neuropsychol 19: , 759–767. |
[24] | Duchesne S , Chouinard I , Potvin O , Fonov VS , Khademi A , Bartha R , Bellec P , Collins DL , Descoteaux M , Hoge R , McCreary CR , Ramirez J , Scott CJM , Smith EE , Strother SC , Black SE , CIMA-Q group and the CCNA group ((2019) ) The Canadian Dementia Imaging Protocol: harmonizing national cohorts. J Magn Reson Imaging 49: , 456–465. |
[25] | Wardlaw JM , Smith EE , Biessels GJ , Cordonnier C , Fazekas F , Frayne R , Lindley RI , O’Brien JT , Barkhof F , Benavente OR , Black SE , Brayne C , Breteler M , Chabriat H , Decarli C , de Leeuw FE , Doubal F , Duering M , Fox NC , Greenberg S , Hachinski V , Kilimann I , Mok V , Oostenbrugge R , Pantoni L , Speck O , Stephan BC , Teipel S , Viswanathan A , Werring D , Chen C , Smith C , van Buchem M , Norrving B , Gorelick PB , Dichgans M ((2013) ) Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 12: , 822–838. |
[26] | Fazekas F , Chawluk JB , Alavi A , Hurtig HI , Zimmerman RA ((1987) ) MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol 149: , 351–356. |
[27] | Wahlund LO , Barkhof F , Fazekas F , Bronge L , Augustin M , Sjogren M , Wallin A , Ader H , Leys D , Pantoni L , Pasquier F , Erkinjuntti T , Scheltens P ((2001) ) A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke 32: , 1318–1322. |
[28] | Sachdev P , Kalaria R , O’Brien J , Skoog I , Alladi S , Black SE , Blacker D , Blazer DG , Chen C , Chui H , Ganguli M , Jellinger K , Jeste DV , Pasquier F , Paulsen J , Prins N , Rockwood K , Roman G , Scheltens P , Internationlal Society for Vascular Behavioral and Cognitive Disorders ((2014) ) Diagnostic criteria for vascular cognitive disorders: a VASCOG statement. Alzheimer Dis Assoc Disord 28: , 206–218. |
[29] | Debette S , Markus HS ((2010) ) The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ 341: , c3666. |
[30] | Durrani R , Friedrich MG , Schulze KM , Awadalla P , Balasubramanian K , Black S , Broet P , Busseuil D , Desai D , Dummer T , Dick A , Hicks J , Iype T , Kelton D , Kirpalani A , Lear SA , Leipsic J , Li W , McCreary CR , Moody AR , Noseworthy MD , Parraga G , Poirier P , Rangarajan S , Szczesniak D , Szuba A , Tardif JC , Teo Mbbch K , Vena JE , Zatonska K , Zimny A , Lee DS , Yusuf S , Anand SS , Smith EE ((2021) ) Effect of cognitive reserve on the association of vascular brain injury with cognition: analysis of the PURE and CAHHM studies. Neurology 97: , e1707–e1716. |
[31] | Dufouil C , Alperovitch A , Tzourio C ((2003) ) Influence of education on the relationship between white matter lesions and cognition. Neurology 60: , 831–836. |
[32] | Nebes RD , Meltzer CC , Whyte EM , Scanlon JM , Halligan EM , Saxton JA , Houck PR , Boada FE , Dekosky ST ((2006) ) The relation of white matter hyperintensities to cognitive performance in the normal old: education matters. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 13: , 326–340. |
[33] | Brickman AM , Siedlecki KL , Muraskin J , Manly JJ , Luchsinger JA , Yeung L , Brown TR , DeCarli C , Stern Y ((2011) ) White matter hyperintensities and cognition: Testing the reserve hypothesis. Neurobiol Aging 32: , 1588–1598. |
[34] | Vemuri P , Lesnick TG , Przybelski SA , Knopman DS , Preboske GM , Kantarci K , Raman MR , Machulda MM , Mielke MM , Lowe VJ , Senjem ML , Gunter JL , Rocca WA , Roberts RO , Petersen RC , Jack CR Jr. , ((2015) ) Vascular and amyloid pathologies are independent predictors of cognitive decline in normal elderly. Brain 138: , 761–771. |
[35] | Arola A , Laakso HM , Pitkanen J , Koikkalainen J , Lotjonen J , Korvenoja A , Erkinjuntti T , Melkas S , Jokinen H ((2021) ) Associations of cognitive reserve and psychological resilience with cognitive functioning in subjects with cerebral white matter hyperintensities. Eur J Neurol 28: , 2622–2630. |
[36] | Willey JZ , Moon YP , Paik MC , Yoshita M , Decarli C , Sacco RL , Elkind MS , Wright CB ((2011) ) Lower prevalence of silent brain infarcts in the physically active: the Northern Manhattan Study. Neurology 76: , 2112–2118. |
[37] | Addo J , Ayerbe L , Mohan KM , Crichton S , Sheldenkar A , Chen R , Wolfe CD , McKevitt C ((2012) ) Socioeconomic status and stroke: an updated review. Stroke 43: , 1186–1191. |
[38] | Gilmore-Bykovskyi AL , Jin Y , Gleason C , Flowers-Benton S , Block LM , Dilworth-Anderson P , Barnes LL , Shah MN , Zuelsdorff M ((2019) ) Recruitment and retention of underrepresented populations in Alzheimer’s disease research: A systematic review. Alzheimers Dement (N Y) 5: , 751–770. |




