Mild Cognitive Disorder in Post-COVID-19 Syndrome: A Retrospective Cohort Study of 67,000 Primary Care Post-COVID Patients
Abstract
Background:
Little is known about the impact of COVID-19 on mild cognitive disorder.
Objective:
The aim of this retrospective cohort study was to investigate whether COVID-19 diagnosis is associated with subsequent mild cognitive disorder (MCD) compared to acute upper respiratory infections (AURI).
Methods:
This retrospective cohort study used data from the Disease Analyzer database (IQVIA) and included 67,046 patients with first-time symptomatic or asymptomatic COVID-19 diagnoses in 1,172 general practices in Germany between March 2020 and September 2021. Diagnoses were based on ICD-10 codes. Patients diagnosed with AURI were matched to 67,046 patients with COVID-19 using propensity scores based on sex, age, index month, and comorbidities. The index date was the diagnosis date for either COVID-19 or AURI. Associations between the COVID-19 and MCD were studied using conditional Poisson regression models.
Results:
The incidence of MCD was 7.6 cases per 1,000 person-years in the COVID-19 group and 5.1 cases per 1,000 person-years in the AURI group (IRR = 1.49, 95% CI = 1.22–1.82). The incidence rate ratio decreased strongly with increasing age from 10.08 (95% CI = 4.00–24.42) in the age group≤50 to 1.03 (95% CI = 0.81–1.31) in the age group > 70. In addition, the association between COVID-19 and MCD was significant in women (IRR: 1.70, 95% CI: 1.34–2.16) but not in men (IRR: 1.08, 95% CI: 0.75–1.56).
Conclusion
The incidence of MCD was low but significantly higher in COVID-19 than in AURI patients, especially among younger patients. If a cognitive disorder is suspected, referral to a specialist is recommended.
INTRODUCTION
Coronavirus disease 2019 (COVID-19) is a viral disorder caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which emerged in China in December 2019 and has since spread rapidly across the world [1, 2]. COVID-19 is associated with pulmonary and extra-pulmonary complications (gastrointestinal, cardiovascular, and neurologic) [1, 3, 4].
Previous research has shown overwhelmingly that the ongoing COVID-19 pandemic has had harmful effects on mental health [5–8]. In addition to depressive and anxiety disorders and fatigue, cognitive disorders are also among the consequences of COVID-19. A 2022 review by Premrai found that cognitive dysfunctions (brain fog, memory problems, attention deficit disorder) were among the main features of post-COVID-19 syndrome, in addition to a variety of psychiatric manifestations [9]. Ceban et al. included 43 studies in their 2022 meta-analysis on COVID-19 and cognitive impairment, concluding that the proportion of individuals with cognitive impairment 12 or more weeks after COVID-19 diagnosis was more than 20% [10].
However, these studies are subject to some limitations that need to be considered. First, the reviews included studies completed during the first few months of the COVID-19 pandemic. It is possible that the impact of COVID-19 diagnosis on mental health changed throughout the different waves. Secondly, the impact of COVID-19 on mild cognitive disorder (MCD) has rarely been studied, unlike that of other respiratory diseases [11, 12]. Cognitive impairment may be related to both biological processes [13–15] and social conditions, specific limiting quarantine conditions or stressful life circumstances that may occur under lockdown conditions [16]. Many studies were conducted in hospitals, specialized COVID wards and research institutions, while research rarely focused on the primary care perspective [17]. Only a few studies have reported on the situation in general practitioner (GP) practices. In a French study of 1,209 GPs, the most common symptoms reported were respiratory problems (60.6%), psychological problems (42.8%), and anosmia and dysgeusia (40.8%). Memory disorders were less common, occurring in 13% of patients [18]. By contrast, 72% reported cognitive disorders in a neurological outpatient clinic [19]. Finally, given these limitations, it makes sense also to use the scale advantages of a large health research database.
As the number of people infected with COVID-19 is still very high worldwide, and it can therefore be expected that the number of people with COVID-19 complications including cognitive disorders is also high, research on the possible association between this virus and cognitive impairment is very important.
Therefore, the aim of this retrospective cohort study was to investigate whether COVID-19 diagnosis is associated with a significant increase in the incidence of MCD in German primary care patients compared with other acute upper respiratory infection diagnoses using the large German database.
METHODS
Database
This retrospective cohort study was based on data from the Disease Analyzer database (IQVIA), which contains drug prescriptions, diagnoses, and basic medical and demographic data obtained directly and in anonymous format from computer systems used in the practices of GPs and specialists [20]. The database covers approximately 3% of all outpatient practices in Germany. Diagnoses (according to International Classification of Diseases, 10th revision [ICD-10]), prescriptions (according to Anatomical Therapeutic Chemical [ATC] classification system), and the quality of the reported data is monitored by IQVIA. In Germany, the sampling methods used to select physicians’ practices are appropriate for obtaining a representative database of general and specialized practices. It has previously been shown that the panel of practices included in the Disease Analyzer database is representative of general and specialized practices in Germany [20]. Finally, this database has already been used in previous studies focusing on COVID-19 [21, 22] as well as MCD [23, 24].
Study population
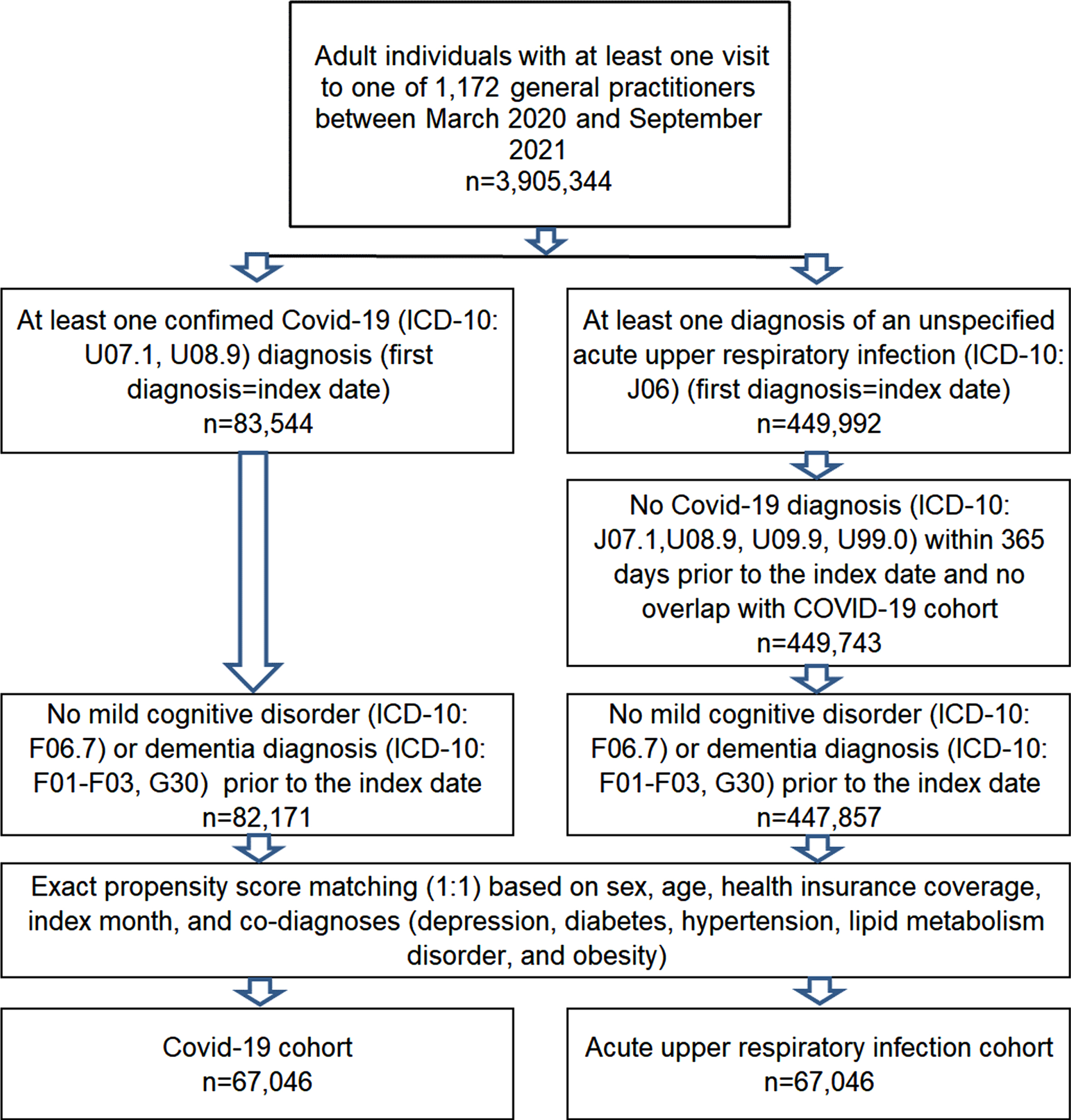
This retrospective cohort study included patients aged 18 years and over diagnosed with symptomatic or asymptomatic COVID-19 (German version of the International Classification of Diseases, 10th revision [ICD-10]: U07.1 [COVID-19 virus identified]) or U08.9 COVID-19 (personal history of COVID-19) in 1,172 German GP practices between March 2020 and September 2021 (index date). After applying the same inclusion criteria, patients diagnosed with an acute upper respiratory infection (AURI, ICD-10: J06; index date) were matched to those with COVID-19 using propensity scores based on sex, age, health insurance coverage (private, statutory), index month, and diagnoses documented within one year prior to the index date (depression, diabetes, obesity, hypertension, lipid metabolism disorder). In order to be included in the study, AURI patients had to have had no diagnosis of COVID-19 (ICD-10: U07.1, U08.9, U09.9, and U99.0) before or on the index date. Patients with a documented diagnosis of MCD (F06.7) or all-cause dementia (ICD-10: F00-F03, G30) prior to or on the index date were excluded from the analyses in order to allow the MCD incidence to be investigated. The selection of study patients is displayed in Fig. 1.
Fig. 1
Selection of study patients.
Outcomes and statistical analyses
The incidence of MCD (ICD-10: F06.7) was investigated separately in the COVID-19 and AURI groups, stratified by age group and gender.
The ICD-10 classification defines MCD (ICD-10: F06.7) as follows: “A disorder characterized by impairment of memory, learning difficulties, and reduced ability to concentrate on a task for more than brief periods. There is often a marked feeling of mental fatigue when mental tasks are attempted, and new learning is found to be subjectively difficult even when objectively successful. None of these symptoms is so severe that a diagnosis of either dementia or delirium can be made. This diagnosis should be made only in association with a specified physical disorder and should not be made in the presence of any of the mental or behavioral disorders classified to F10-F99. The disorder may precede, accompany, or follow a wide variety of infections and physical disorders, both cerebral and systemic, but direct evidence of cerebral involvement is not necessarily present. It can be differentiated from postencephalitic syndrome (F07.1) and postconcussional syndrome (F07.2) by its different etiology, more restricted range of generally milder symptoms, and usually shorter duration.
Baseline characteristics were compared for patients with COVID-19 and those with AURI using McNemar tests for categorical variables and Wilcoxon signed-rank tests for continuous variables. The incidence of MCD in the COVID-19 and AURI groups was estimated as the number of cases per 1,000 person-years. Kaplan-Meier curves were used to display the incidence. Differences between the COVID-19 and AURI groups within the AURI group were also studied using conditional Poisson regression models. These models were applied to the overall population and further stratified by sex and age. The results of the Poisson regression analyses are displayed as incidence rate ratios (IRRs) with 95% confidence intervals (CIs) and p-values. Due to the large patient numbers and multiple comparisons involved in this study, p-values < 0.01 were considered statistically significant. All analyses were performed using SAS 9.4 (SAS institute, Cary, USA)
RESULTS
Patient characteristics
This study included n = 67,046 patients diagnosed with COVID-19 and n = 67,046 patients diagnosed with AURI. The average follow-up time was 158 days among patients diagnosed with COVID-19 and 165 days among those diagnosed with AURI.
In total, 53.3% of patients were women, and the mean (standard deviation, SD) age was 44.6 (16.6) years. Patient sociodemographic and clinical characteristics are shown in Table 1.
Table 1
Basic characteristics of the study sample (after 1:1 matching by sex, age, private health insurance coverage, index month, and defined comorbidities)
| Variable | Proportion among individuals with COVID-19 (%) N = 67,046 | Proportion among individuals with AURI* (%) N = 67,046 | p |
| Age (Mean, SD) | 44.6 (16.6) | 44.6 (16.6) | 1.000 |
| Age 18–30 | 16,153 (24,1) | 16,153 (24,1) | 1.000 |
| Age 31–40 | 13,377 (19.9) | 13,377 (19.9) | |
| Age 41–50 | 12,609 (18.8) | 12,609 (18.8) | |
| Age 51–60 | 13,899 (20.7) | 13,899 (20.7) | |
| Age 61–70 | 6,426 (9.6) | 6,426 (9.6) | |
| Age > 70 | 4,582 (6.8) | 4,582 (6.8) | |
| Female | 35,763 (53.3) | 35,763 (53.3) | 1.000 |
| Male | 31,283 (46.7) | 31,283 (46.7) | |
| Private health insurance coverage | 2,093 (3.1) | 2,093 (3.1) | 1.000 |
| Statutory health insurance coverage | 64,953 (96.9) | 64,953 (96.9) | |
| Depression | 7,544 (11.3) | 7,544 (11.3) | |
| Diabetes | 2,937 (4.4) | 2,937 (4.4) | 1.000 |
| Obesity | 2,911 (4.3) | 2,911 (4.3) | 1.000 |
| Hypertension | 10,716 (16.0) | 10,716 (16.0) | 1.000 |
| Lipid metabolism disorder | 6,003 (9.0) | 6,003 (9.0) | 1.000 |
Proportions of patients are given in % unless otherwise indicated. AURI, acute respiratory tract infection; SD, standard deviation.
Incidence of mild cognitive disorder
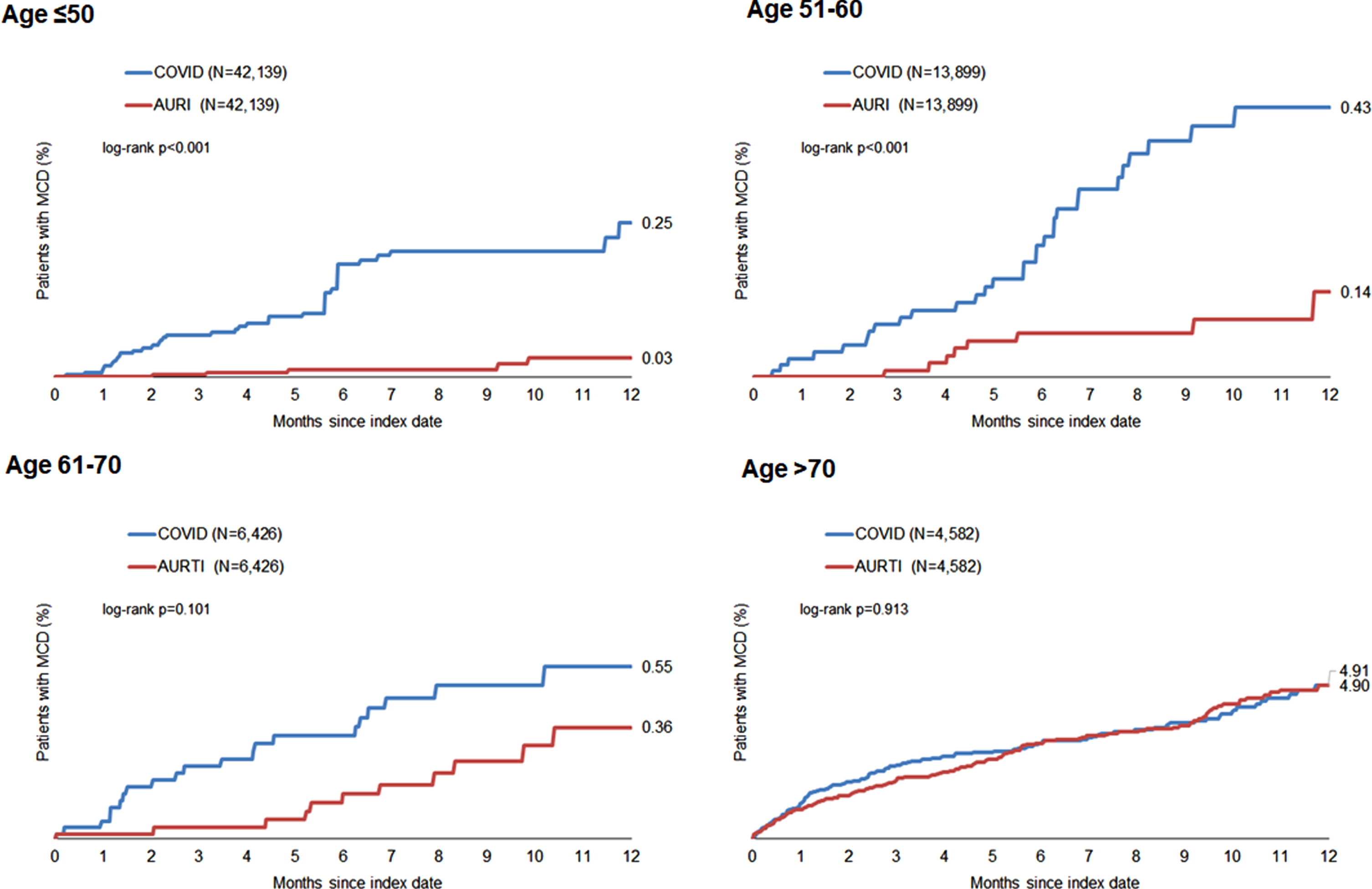
The cumulative incidence of MCD is represented in Fig. 2 and was much higher in COVID-19 than in AURI patients except in the age group > 70.
Fig. 2
Kaplan-Meier curves for incidence of mild cognitive disorder in patients with COVID-19 versus patients with upper respiratory tract infection.
The association between COVID-19 and the subsequent incident MCD diagnosis in patients is shown in Table 2. The incidence of MCD was 7.6 cases per 1,000 person-years in the COVID-19 group and 5.1 cases per 1,000 person-years in the AURI group, resulting in an increased relative risk of MCD in the COVID-19 cohort (IRR = 1.49, 95% CI = 1.22–1.82, p < 0.001).
Table 2
Association between COVID-19 and subsequent incident MCD diagnosis in patients followed in general practices in Germany (Poisson regression models)
| Cohorts | Incidence among individuals with COVID (cases per 1,000 patient years) | Incidence among individuals without AURI (cases per 1,000 patient years) | Incidence rate ratios (95% CI) | p |
| Total | 7.6 | 5.1 | 1.49 (1.22–1.82) | <0.001 |
| Age≤50 | 2.6 | 0.3 | 10.08 (4.00–24.42) | <0.001 |
| Age 51–60 | 4.4 | 1.3 | 3.56 (1.69–7.50) | <0.001 |
| Age 61–70 | 6.3 | 3.5 | 1.80 (0.89–3.68) | 0.102 |
| Age > 70 | 57.6 | 56.1 | 1.03 (0.81–1.31) | 0.829 |
| Women | 10.4 | 6.1 | 1.70 (1.34–2.16) | <0.001 |
| Men | 4.2 | 3.9 | 1.08 (0.75–1.56) | 0.682 |
The incidence rate ratio decreased strongly with increasing age from 10.08 (95% CI = 4.00–24.42) in patients under the age of 50 to 1.03 (95% CI = 0.81–1.31) in patients 70 years old or more. In terms of age, the association between COVID-19 and MCD was only significant among patients aged under 60 years. Furthermore, COVID-19 was significantly associated with MCD in women (IRR: 1.70, 95% CI: 1.34–2.16, p < 0.001) but not in men (IRR: 1.08, 95% CI: 0.75–1.56, p = 0.682) (Table 2).
DISCUSSION
This retrospective study, including more than 134,000 adults followed in general practices in Germany between March 2020 and September 2021, showed that incident mild cognitive disorder as defined in ICD-10: F06.7 was significantly more common in those diagnosed with COVID-19 than those diagnosed with an acute upper respiratory infection. However, at 7.6 cases per 1,000 person-years, the incidence rate was low and only slightly higher than that of MCD in patients with other respiratory infectious diseases. The incidence rate ratio decreased strongly with increasing age. The association between COVID-19 and MCD was only significant among patients≤50 and aged 51–60 years. Finally, COVID-19 was significantly associated with MCD in women but not men.
To the best of our knowledge, this is one of the first studies demonstrating the effect of post-COVID-19 on MCD by comparing patients with COVID-19 and patients with other respiratory infections.
Interpretation of findings
Recently, a substantial amount of research has focused on the impact of COVID-19 on mental diagnoses, but this research initially did not focus on mild cognitive disorder. International and national reviews have lately pointed to the possible occurrence of mild cognitive impairment as a result of COVID-19 infection [9, 10, 25]. Interestingly and in line with our findings, an American survey study reported that COVID-19 status was predictive of worse cognitive dysfunction scores [26]. In another South American study, post-COVID-19 patients were extensively neuropsychologically tested [27]. The results confirmed that the cognitive impairment persists for months and cannot be detected with the screening instruments used in family practices alone [26, 27]. This may mean that the detection rate in German GP practices in this study was relatively low because screening tests such as the Mini-Mental State Examination Test (MMSE) or Montreal-Cognitive-Assessment-Test (MoCA) are generally used. However, it has been proven for other samples that cognitive abnormalities can also be detected using these simple test methods [28–30]. Nevertheless, it should be noted that cognitive dysfunctions can go undetected in GP practices in particular [23, 30].
Post-COVID cognitive deficits cannot be detected easily using the instruments oriented toward dementia detection and without clinical experience. The importance of the severity of the cognitive disorders concerned was pointed out in a comparison of an outpatient sample with an inpatient sample. It was shown that cognitive disorders were significantly less pronounced in those COVID patients treated as outpatients than in the patients treated as inpatients. In a recent population study in Hamburg, subclinical multi-organ affection related to pulmonary, cardiac, thrombotic, and renal function was shown in a controlled sample of 443 mildly ill patients who tested positive for SARS-CoV-2. However, no signs of structural brain damage or neurocognitive impairment were found [14]. By contrast, in a neurological outpatient clinic for patients with post-COVID-19 syndrome in predominantly mildly ill patients, mild cognitive impairment was a common symptom found in 72% of patients, with 30% of patients reporting cognitive deficits and scoring below 26 points on the MoCA [19].
One key finding of this study is that the incidence rate ratio decreased sharply with increasing age from the ≤50 to the > 70 age group. In terms of age, the association between COVID-19 and MCD was only significant in patients under the age of 60. Furthermore, COVID-19 was significantly associated with MCD in women but not in men. In German GP practices, MCD diagnoses are typically made in connection with the onset of dementia, which is contrary to the results of this study [23]. This age shift is possibly an indicator of the connection between this diagnosis and the coronavirus pandemic because in our study, it occurred primarily in the middle-aged cohorts, who are still professionally active [19]. Similar age structures have also been found internationally [28–30]. However, the details of post-COVID mild cognitive impairment in older age [31] and comorbid MCD and dementia [32] require further investigation. The predominance of the female sex described here is in line with both German and international research [9, 10, 33, 34].
The reason for the discrepancy between the very low incidence of MCD diagnoses reflected in the reality of care and documented in this study and the wider research, which reports a relatively high number of post-COVID-19 patients with cognitive impairment, is not yet clear. Firstly, it is unclear which pathomechanism leads to cognitive impairment in COVID-19. A number of very different models are being discussed [35]. Multiple direct and indirect mechanisms contribute to the clinical picture of post-COVID cognitive disorder. The role of SARS-CoV-2 neurotropism, the general pro-inflammatory state, and the psychosocial stressors associated with the pandemic all play important roles. Neurotropism is associated with several mechanisms, including retrograde neuronal transmission via the olfactory pathway, but also general hematogenous spread and the use of immune cells as vectors by the virus. The severe inflammation caused by COVID-19 is equally detrimental to cognition. Finally, the unique psychosocial impact of the pandemic has also raised concerns because of its potential cognitive consequences [35]. It is possible that a persistent pronounced cognitive performance disorder is a rare occurrence in the everyday practice of GPs in private practice because it is a rather rare secondary disease. Although testing for such disorders may be routine in special consultations or in the follow-up care of patients with severe disease progression with extracorporeal membrane oxygenation, this does not apply to routine care in general practice. Irrespective of this, however, there are also indications that quarantine measures, which are accompanied by considerable social isolation, not only have consequences concerning various psychological disorders but also affect cognitive performance [36]. Secondly, complication rates and severe courses with a high need for intensive care were more common at the beginning of the pandemic than in the further course of 2021 [37]. During this early phase of the pandemic, GPs were under immense pressure to provide care [38, 39] and had little time for detailed exploration of patients’ complaints [40]. It is also possible that COVID practice management and the physical consequences of a COVID-19 infection were more in the foreground during this early phase than the exploration or even neuropsychological testing of cognitive disorders [41]. In addition, knowledge about the long-term consequences of the virus simply was not available in primary care [39].
Public health implications and directions for future research
This study conducted in Germany found that primary care patients diagnosed with COVID-19 were at a significantly increased risk of MCD compared to their counterparts who were diagnosed with other acute upper respiratory infections. Although these preliminary data are reassuring, GPs and other health professionals should regularly assess the potential presence of MCD in patients newly diagnosed with COVID-19 even though these symptoms appear to be reported comparatively rarely in GP practices [18].
In terms of future research, further studies comparing the effects of COVID-19 on MCD with those of other acute upper respiratory infections are needed to corroborate or invalidate the present findings. It is currently not known which specific mechanism is of particular relevance for cognitive disorders associated with COVID-19. For studies in the primary care setting, it is therefore particularly important to investigate whether these disorders are persistent or transient.
Limitations
Two major strengths of this study are the large sample size and the inclusion of patients diagnosed with COVID-19 in 2020 and 2021. However, our results should be interpreted in the light of several limitations. First, no data were available on COVID-19 symptoms. Second, MCD was diagnosed using the ICD-10 classification alone, and no information is available on how this diagnosis was made. Third, since this study did not include individuals diagnosed with dementia or MCD in the year prior to the index date, we were unable to analyze the potential effects of COVID-19 diagnosis on pre-existing MCD. Fourth, given that this study only included patients followed in general practices, the study findings may not be generalizable to those diagnosed with COVID-19 in specialized practices or hospitals. Fifth, no data were available on vaccinations for COVID-19 in the study period. Sixth, the AURI cohort likely includes COVID-19 cases diagnosed outside general practice, meaning that these patients may be wrongly classified as AURI patients rather than COVID-19 cases. Seventh, COVID-19 was a very rare outcome in those aged≤50 causing very broad confidence intervals for incidence rate ratios, which may hinder the clinical interpretation of this result. Finally, it is not possible to assess the extent to which GPs were unable to examine patients more closely through test screenings such as MMSE or MoCA, due to the stresses of everyday care in the context of the pandemic [28, 42].
ACKNOWLEDGMENTS
The authors have no acknowledgments to report.
FUNDING
The authors have no funding to report.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
REFERENCES
[1] | Hu B , Guo H , Zhou P , Shi ZL ((2021) ) Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol 19: , 141–154. |
[2] | Sanyaolu A , Okorie C , Hosein Z , Patidar R , Desai P , Prakash S , Jaferi U , Mangat J , Marinkovic A ((2020) ) Global pandemicity of COVID-19: Situation report as of June 9, 2020. Infect Dis (Auckl) 14: , 1178633721991260. |
[3] | Gupta A , Madhavan MV , Sehgal K , Nair N , Mahajan S , Sehrawat TS , Bikdeli B , Ahluwalia N , Ausiello JC , Wan EY , Freedberg DE , Kirtane AJ , Parikh SA , Maurer MS , Nordvig AS , Accili D , Bathon JM , Mohan S , Bauer KA , Leon MB , Krumholz HM , Uriel N , Mehra MR , Elkind MSV , Stone GW , Schwartz A , Ho DD , Bilezikian JP , Landry DW ((2020) ) Extrapulmonary manifestations of COVID-19. Nat Med 26: , 1017–1032. |
[4] | Konturek PC , Harsch IA , Neurath MF , Zopf Y ((2020) ) COVID-19 - more than respiratory disease: A gastroenterologist’s perspective. J Physiol Pharmacol 71: , 179–189. |
[5] | Rogers JP , Chesney E , Oliver D , Pollak TA , McGuire P , Fusar-Poli P , Zandi MS , Lewis G , David AS ((2020) ) Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: A systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry 7: , 611–627. |
[6] | Huang C , Huang L , Wang Y , Li X , Ren L , Gu X , Kang L , Guo L , Liu M , Zhou X , Luo J , Huang Z , Tu S , Zhao Y , Chen L , Xu D , Li Y , Li C , Peng L , Li Y , Xie W , Cui D , Shang L , Fan G , Xu J , Wang G , Wang Y , Zhong J , Wang C , Wang J , Zhang D , Cao B ((2021) ) 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 397: , 220–232. |
[7] | Evans RA , McAuley H , Harrison EM , Shikotra A , Singapuri A , Sereno M , Elneima O , Docherty AB , Lone NI , Leavy OC , Daines L , Baillie JK , Brown JS , Chalder T , De Soyza A , Diar Bakerly N , Easom N , Geddes JR , Greening NJ , Hart N , Heaney LG , Heller S , Howard L , Hurst JR , Jacob J , Jenkins RG , Jolley C , Kerr S , Kon OM , Lewis K , Lord JM , McCann GP , Neubauer S , Openshaw PJM , Parekh D , Pfeffer P , Rahman NM , Raman B , Richardson M , Rowland M , Semple MG , Shah AM , Singh SJ , Sheikh A , Thomas D , Toshner M , Chalmers JD , Ho LP , Horsley A , Marks M , Poinasamy K , Wain LV , Brightling CE ; PHOSP-COVID Collaborative Group ((2022) ) Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): A UK multicentre, prospective cohort study. Lancet Respir Med 9: , 1275–1287. |
[8] | Nasserie T , Hittle M , Goodman SN ((2021) ) Assessment of the frequency and variety of persistent symptoms among patients with COVID-19: A systematic review. JAMA Netw Open 4: , e2111417. |
[9] | Premraj L , Kannapadi NV , Briggs J , Seal SM , Battaglini D , Fanning J , Suen J , Robba C , Fraser J , Cho SM ((2022) ) Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: A meta-analysis. J Neurol Sci 434: , 120162. |
[10] | Ceban F , Ling S , Lui LMW , Lee Y , Gill H , Teopiz KM , Rodrigues NB , Subramaniapillai M , Di Vincenzo JD , Cao B , Lin K , Mansur RB , Ho RC , Rosenblat JD , Miskowiak KW , Vinberg M , Maletic V , McIntyre RS ((2021) ) Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain Behav Immun 101: , 93–135. |
[11] | Jacob L , Koyanagi A , Smith L , Bohlken J , Haro JM , Kostev K ((2022) ) No significant association between COVID-19 diagnosis and the incidence of depression and anxiety disorder? A retrospective cohort study conducted in Germany. J Psychiatr Res 147: , 79–84. |
[12] | Damiano RF , Guedes BF , de Rocca CC , de Pádua Serafim A , CastroLHM , Munhoz CD , Nitrini R , Filho GB , Miguel EC , Lucchetti G , Forlenza O ((2022) ) Cognitive decline following acute viralinfections: Literature review and projections for post-COVID-19. Eur Arch Psychiatry Clin Neurosci 272: , 139–154. |
[13] | Reiken S , Sittenfeld L , Dridi H , Liu Y , Liu X , Marks AR ((2022) ) Alzheimer’s-like signaling in brains of COVID-19 patients. Alzheimers Dement 18: , 955–965. |
[14] | Petersen EL , Goßling A , Adam G , Aepfelbacher M , Behrendt CA , Cavus E , Cheng B , Fischer N , Gallinat J , Kühn S , Gerloff C , Koch-Gromus U , Härter M , Hanning U , Huber TB , Kluge S , KnoblochJK , Kuta P , Schmidt-Lauber C , Lütgehetmann M , Magnussen C , MayerC , Muellerleile K , Münch J , Nägele FL , Petersen M , RennéT , Riedl KA , Rimmele DL , Schäfer I , Schulz H , Tahir E , Waschki B , Wenzel JP , Zeller T , Ziegler A , Thomalla G , Twerenbold R , Blankenberg S ((2022) ) Multi-organ assessment in mainlynon-hospitalized individuals after SARS-CoV-2 infection: The HamburgCity Health Study COVID programme. Eur Heart J 43: , 1124–1137. |
[15] | Schweitzer F , Goereci Y , Franke C , Silling S , Bösl F , Maier F , Heger E , Deiman B , Prüss H , Onur OA , Klein F , Fink GR , DiCristanziano V , Warnke C ((2022) ) Cerebrospinalfluid analysis post-COVID-19 is not suggestive of persistent centralnervous system infection. Ann Neurol 91: , 150–157. |
[16] | Favieri F , Forte G , Agostini F , Giovannoli J , Di Pace E , Langher V , Tambelli R , Pazzaglia M , Giannini AM , Casagrande M ((2021) ) The cognitive consequences of the COVID-19 pandemic on members of the general population in Italy: A preliminary study on executive inhibition. J Clin Med 11: , 170. |
[17] | van Kessel SAM , Olde Hartman TC , Lucassen PLBJ , van Jaarsveld CHM ((2022) ) Post-acute and long-COVID-19 symptoms in patients with mild diseases: A systematic review. Fam Pract 39: , 159–167. |
[18] | Davin-Casalena B , Lutaud R , Scronias D , Guagliardo V , Verger P ((2021) ) french general practitioners frequently see patients with long-COVID. J Am Board Fam Med 34: , 1010–1013. |
[19] | Boesl F , Audebert H , Endres M , Prüss H , Franke C ((2021) ) A neurological outpatient clinic for patients with post-COVID-19 syndrome - a report on the clinical presentations of the first 100 patients. Front Neurol 12: , 738405. |
[20] | Rathmann W , Bongaerts B , Carius HJ , Kruppert Y , Kostev K ((2018) ) Basic characteristics and representativeness of the German disease analyzer database. Int J Clin Pharmacol Ther 56: , 459–466. |
[21] | Tanislav C , Kostev K ((2022) ) Investigation of the prevalence of non-COVID-19 infectious diseases during the COVID-19 pandemic. Public Health 203: , 53–57. |
[22] | Bohlken J , Kostev K , Riedel-Heller S , Hoffmann W , Michalowsky B ((2021) ) Effect of the COVID-19 pandemic on stress, anxiety, and depressive disorders in German primary care: A cross-sectional study. J Psychiatr Res 143: , 43–49. |
[23] | Bohlken J , Riedel-Heller S , Steininger G , Kostev K , Michalowsky B ((2021) ) Trends in dementia and mild cognitive impairment prevalence and incidence in German general and specialist practices between 2015 and 2019. J Alzheimers Dis 79: , 1683–1690. |
[24] | Jacob L , Bohlken J , Kostev K ((2017) ) Risk factors for mild cognitive impairment in German primary care practices. J Alzheimers Dis 56: , 379–384. |
[25] | Sanchez-Ramirez DC , Normand K , Zhaoyun Y , Torres-Castro R ((2021) ) Long-term impact of COVID-19: A systematic review of the literature and meta-analysis. Biomedicines 9: , 900. |
[26] | Becker JH , Lin JJ , Doernberg M , Stone K , Navis A , Festa JR , Wisnivesky JP ((2021) ) Assessment of cognitive function in patients after COVID-19 infection. JAMA Netw Open 4: , e2130645. |
[27] | Crivelli L , Calandri I , Corvalán N , Carello MA , Keller G , Martínez C , Arruabarrena M , Allegri R ((2021) ) Cognitiveconsequences of COVID-19: Results of a cohort study from SouthAmerica. Arq Neuropsiquiatr 80: , 240–247. |
[28] | Aiello EN , Fiabane E , Manera MR , Radici A , Grossi F , Ottonello M , Pain D , Pistarini C ((2022) ) Screening for cognitive sequelae of SARS-CoV-2 infection: A comparison between the Mini-Mental State Examination (MMSE) and the Montreal Cognitive Assessment (MoCA). Neurol Sci 43: , 81–84. |
[29] | Ferrando SJ , Dornbush R , Lynch S , Shahar S , Klepacz L , Karmen CL , Chen D , Lobo SA , Lerman D (2022) Neuropsychological, medical and psychiatric findings after recovery from acute COVID-19: A cross-sectional study. J Acad Consult Liaison Psychiatry doi: 10.1016/j.jaclp.2022.01.003 |
[30] | Valdes E , Fuchs B , Morrison C , Charvet L , Lewis A , Thawani S , Balcer L , Galetta SL , Wisniewski T , Frontera JA (2022) Demographic and social determinants of cognitive dysfunction following hospitalization for COVID-19. J Neurol Sci doi: 10.1016/j.jns.2022.120146 |
[31] | Liu YH , Wang YR , Wang QH , Chen Y , Chen X , Li Y , Cen Y , Xu C , Hu T , Liu XD , Yang LL , Li SJ , Liu XF , Liu CM , Zhu J , Li W , Zhang LL , Liu J , Wang YJ ((2021) ) Post-infection cognitive impairments in a cohort of elderly patients with COVID-19. Mol Neurodegener 16: , 48. |
[32] | Soysal P , Smith L , Trott M , Alexopoulos P , Barbagallo M , Tan SG , Koyanagi A , Shenkin S , Veronese N ; European Society of Geriatric Medicine Special Interest Group in Dementia and Systematic Reviews And Meta-Analyses ((2022) ) The effects of COVID-19 lockdown on neuropsychiatric symptoms in patients with dementia or mild cognitive impairment: A systematic review and meta-analysis. Psychogeriatrics 22: , 402–412. |
[33] | Fernández-de-Las-Peñas C , Martín-Guerrero JD , Pellicer-Valero ÓJ , Navarro-Pardo E , Gómez-Mayordomo V , Cuadrado ML , Arias-Navalón JA , Cigarán-Méndez M , Hernández-Barrera V , Arendt-Nielsen L ((2022) ) Female sex is arisk factor associated with long-term post-COVID related-symptomsbut not with COVID-19 symptoms: The LONG-COVID-EXP-CM Multicenter Study. J Clin Med 11: , 413. |
[34] | Maggi G , Baldassarre I , Barbaro A , Cavallo ND , Cropano M , Nappo R , Santangelo G ((2021) ) Age- and gender-related differences in theevolution of psychological and cognitive status after the lockdownfor the COVID-19 outbreak: A follow-up study. Neurol Sci 43: , 1521–1532. |
[35] | Ali Awan H , Najmuddin Diwan M , Aamir A , Ali M , Di Giannantonio M , Ullah I , Shoib S , De Berardis D ((2021) ) SARS-CoV-2 and the brain: What do we know about the causality of‘cognitive COVID?. J Clin Med 10: , 3441. |
[36] | Manca R , De Marco M , Venneri A The impact of COVID-19 infection and enforced prolonged social isolation on neuropsychiatric symptoms in older adults with and without dementia: A review. Front Psychiatry ((2020) ) 11: , 585540. |
[37] | Fan G , Yang Z , Lin Q , Zhao S , Yang L , He D ((2021) ) Decreased case fatality rate of COVID-19 in the second wave: A study in 53 countries or regions. Transbound Emerg Dis 68: , 213–215. |
[38] | Dutour M , Kirchhoff A , Janssen C , Meleze S , Chevalier H , Levy-Amon S , Detrez MA , Piet E , Delory T ((2021) ) Family medicine practitioners’ stress during the COVID-19 pandemic: A cross-sectional survey. BMC Fam Pract 22: , 36. |
[39] | Matenge S , Sturgiss E , Desborough J , Hall Dykgraaf S , Dut G , Kidd M (2021) Ensuring the continuation of routine primary care during the COVID-19 pandemic: A review of the international literature. Fam Pract doi: 10.1093/fampra/cmab115 |
[40] | Lokugamage AU , Bowen MA , Blair J ((2020) ) Long covid: Doctors must assess and investigate patients properly. BMJ 371: , m4583. |
[41] | Stephenson E , Butt DA , Gronsbell J , Ji C , O’Neill B , Crampton N , Tu K ((2021) ) Changes in the top 25 reasons for primary care visits during the COVID-19 pandemic in a high-COVID region of Canada. PLoS One 16: , e0255992. |
[42] | Zhao S , Shibata K , Hellyer PJ , Trender W , Manohar S , Hampshire A , Husain M ((2022) ) Rapid vigilance and episodic memory decrements in COVID-19 survivors. Brain Commun 19: , fcab295. |




