EEG Abnormalities During Delirium as a Prodromal Feature of Dementia with Lewy Bodies: A Case Report
Abstract
Background:
A 79-year-old woman was admitted to the Neurology Clinic of the University of Chieti-Pescara for a syncope. At admission, the occurrence of an acute stroke was ruled out. Her cognitive status was unimpaired. After three days from the hospitalization, the patient experienced an episode of mixed delirium.
Objective:
The present case report shows a case of delirium-onset dementia with Lewy bodies (DLB) with a specific electroencephalographic (EEG) pattern from its prodromal stage.
Methods:
Delirium was assessed by 4AT test. During the hospitalization, the patient underwent a quantitative EEG (QEEG) with spectral analysis. At six months from the episode of delirium, she was tested by neuropsychological evaluation, QEEG, and 18F-fluorodeoxyglucose PET/CT to assess the onset of a possible cognitive decline.
Results:
At baseline, the QEEG exam showed a dominant frequency (DF) in the pre-alpha band (7.5 Hz) with a dominant frequency variability (DFV) of 2 Hz. This pattern is typical of DLB at early stage. After six months, she reported attention deficits in association with cognitive fluctuation and REM sleep behavior disorder. The neurological examination revealed signs of parkinsonism. Cognitive status resulted to be impaired (MoCA = 15/30). QEEG recording confirmed the presence of a DLB-typical pattern (DF = 7.5 Hz, DFV = 2.5 Hz). The 18F-FDG-PET/CT showed a moderate bilateral posterior hypometabolism (occipital and temporal cortex), with relative sparing of the posterior cingulate cortex compared to cuneus/precuneus (Cingulate Island sign), and mild bilateral hypometabolism in frontal regions (suggestive of a DLB diagnosis).
Conclusion:
EEGs may represent supportive and validated biomarkers for delirium-onset prodromal DLB.
INTRODUCTION
Dementia with Lewy bodies (DLB) is clinically defined by the presence of dementia, visual hallucinations (VH), cognitive fluctuations (CF), REM sleep behavior disorder (RBD), and parkinsonism, which are all considered its core clinical features [1]. DLB is the second most common cause of neurodegenerative cognitive decline, and accounts for up to 20–30% of all forms of dementia [2].
Considering research efforts in identifying prodromal biomarkers for DLB, recent clinical consensus criteria have been established for its prodromal stages, classified into three different clusters based on the main early clinical manifestations: mild cognitive impairment (MCI-LB), psychiatric-onset DLB, and delirium-onset DLB [3]. A fourth cluster represented by idiopathic RBD is now defined. However, even though clinical criteria for MCI-LB have been validated, specific consensus criteria for psychiatric and delirium clusters have not been defined yet.
Case presentation
A 79-year-old woman with five years of education accessed our Emergency Unit for a syncope. Her medical records included chronic atrial fibrillation, hypertension, chronic obstructive pulmonary disease, left coxarthrosis, and depression. The patient was under treatment with oral direct anticoagulant (apixaban), angiotensin receptor blocker, bisoprolol, furosemide, salmeterol/fluticasone, and bromazepam. The neurological examination was normal. Her cognitive and functional performances were unimpaired. The dosage of apixaban activity was in range. The computed tomography (CT) of the brain was negative, whereas angiography CT showed a lack of opacification of the P1 tract of the right posterior cerebral artery. Due to apixaban therapy, the patient was not eligible for systemic fibrinolysis. According to interventional radiology, eligible criteria for mechanical thrombolysis were also not met.
The patient was admitted to our Neurology Clinic, and a brain CT was repeated after 48 h, which was negative for recent ischemic lesions. After three days from the hospitalization, the patient experienced an episode of mixed delirium, assessed by 4AT test [4] and represented by a combination of hyperactive (i.e., restlessness, psychomotor agitation, and aggressiveness) and hypoactive symptoms (i.e., reduced motor activity and drowsiness). The symptoms were treated with benzodiazepine and quetiapine. A spectral analysis electroencephalogram (QEEG) was performed (Fig. 1a), which showed a dominant frequency (DF) in the pre-alpha band (7.5 Hz) with a dominant frequency variability (DFV) of 2 Hz, typical of the early stages of DLB [5, 6]. At discharge, after seven days, her neurological examination was completely negative.
Fig. 1
Representations of both CSA and 18F-FDG-PET/CT patterns. a) Patient CSA patterns from occipital derivation at both admission and 6-month follow-up visit (left), compared to a CSA 1 pattern of a normal control (right). b) Axial 18F-FDG-PET/CT images and semiquantitative data (Cortex ID software) at 6 months from the hospitalization. Brain 18F-FDG PET study showed a moderate reduction in radiopharmaceutical uptake in the parietal, occipital, and lateral temporal bilaterally, more evident in the left hemisphere. A mild hypometabolism was also evident in the prefrontal regions bilaterally, although not reaching the statistically significant threshold at semiquantitative analyses. The uptake was also reduced at the level of the precuneus, especially on the right side, and a normal uptake at the level of the cingulate was present, describing the Cingulate Island Sign. This hypometabolic pattern was suggestive of DLB diagnosis. CSA, Compressed Spectral Array; 18F-FDG-PET/CT, 18F-fluorodeoxyglucose positron emission tomography; R, right; L, left.
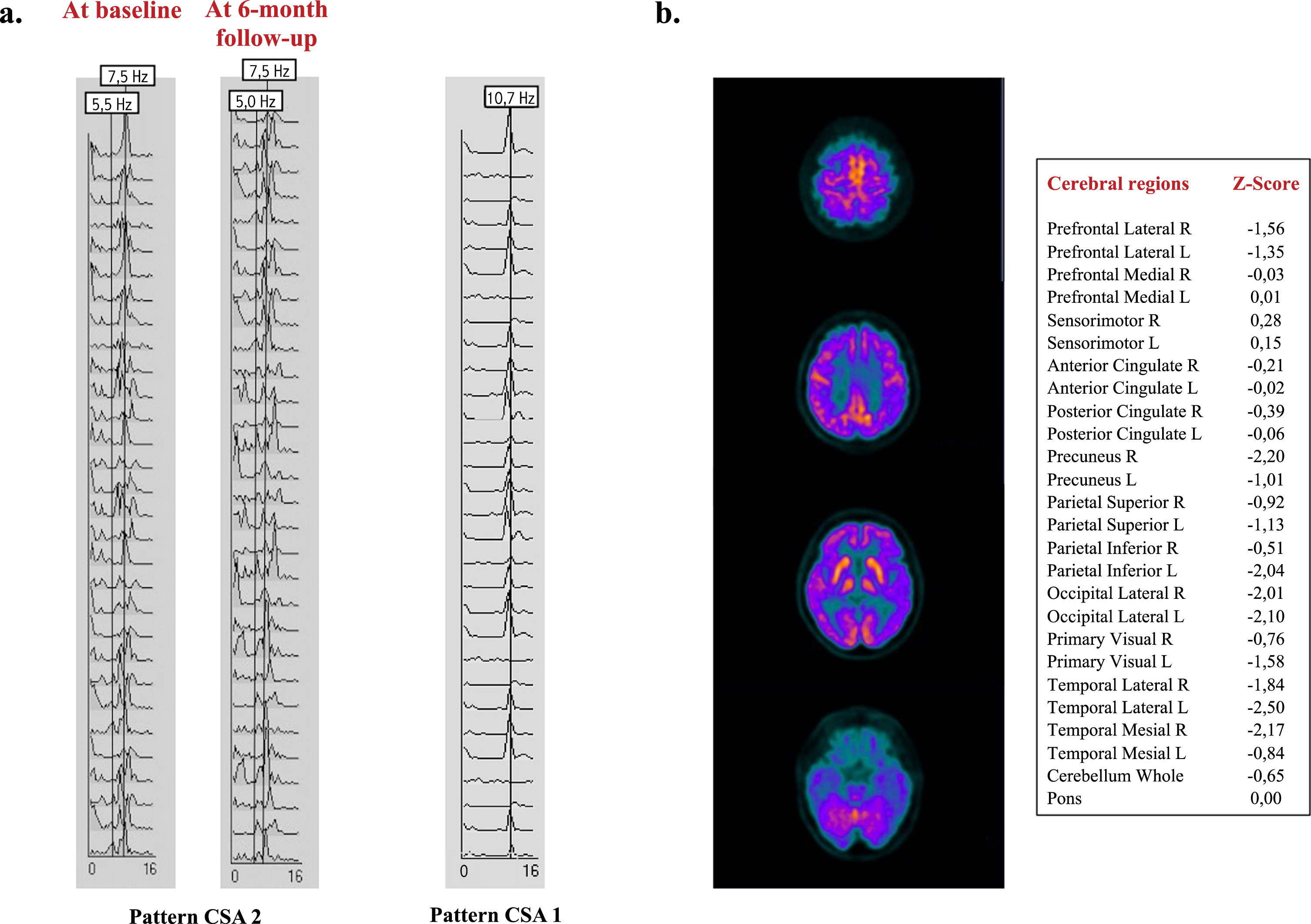
At six-month follow-up, the patient (confirmed by her relatives) reported attentive deficits with CF and the presence of RBD occurring over the past year. The neurological examination revealed moderate signs of parkinsonism (Unified Parkinson’s Disease Rating Scale, UPDRS-III = 15). Cognitive performances were tested with a complete neuropsychological assessment (Table 1), including the Montreal Cognitive Assessment (MoCA), which showed a score of 15/30 (normal value > 26/30). QEEG recording confirmed the presence of a DLB-typical pattern (DF = 7.5 Hz, DFV = 2.5 Hz) (Fig. 1a). The patient underwent a 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET)/CT, which showed a moderate bilateral posterior hypometabolism (occipital and temporal cortex), with relative sparing of the posterior cingulate cortex compared to cuneus/precuneus (Cingulate Island sign), and mild bilateral hypometabolism in frontal regions. This pattern was suggestive of DLB diagnosis (Fig. 1b).
According with a final diagnosis of probable DLB, treatment with rivastigmine patch (4.6 mg/24 h), levodopa/benserazide (100/25 mg three times a day), and clonazepam (1 mg before bedtime) was started.
MATERIALS AND METHODS
The study was performed in accordance with the declaration of Helsinki and its later amendments and our local ethical committee.
Quantitative EEG recordings
QEEG recording was performed at both baseline and 6-month follow-up visit. The exam was recorded with Ag/AgCl disk scalp electrodes from 19 scalp derivations (Fp1, Fp2, Fz, F3, F4, F7, F8, Cz, C3, C4, Pz, P3, P4, T3, T4, T5, T6, O1, and O2) placed according to the international 10–20 system. Two additional electrodes were placed on A1 and A2. EEG signal was measured at rest, and in no-task conditions. Eye movements were simultaneously monitored from two additional channels. Two pairs of bipolar recording channels for respiration and electrocardiogram were also applied. Muscular or tremor artefacts were controlled using supplementary derivations.
QEEG was acquired as a continuous signal for 30 min and visually inspected for current clinical interpretation or artefact detections. QEEG signal was stored to be epoched in off-analysis setting as series of 2-s-long epochs. Only electrodes from posterior derivations (P3, P4, Pz, O1, O2) were considered for the analysis. Fast Fourier Transform (FFT) was applied on each epoch. The obtained spectra values were processed to compute a mean power spectrum for each channel and divided automatically into four different frequency bands, as follows: delta (3–4 Hz), theta (4.5–5.5 Hz), fast theta or pre-alpha (6–7.5 Hz), and alpha (8–12 Hz).
EEG traces were quantified by the following mathematical descriptors: DF, which is the frequency where the spectral power value was greatest for each epoch, and DFV, expressing the variability of DF across the 90 analyzed epochs [5, 6].
Table 1
Neuropsychological assessment at 6-month follow-up visit
| Neuropsychological assessment | Score |
| MoCA | 15 |
| ADL | 2 |
| IADL | 2 |
| CDR | 1 |
| NPI_tot | 24 |
| NPI-VH | 0 |
| RAVLT-immediate recall | N/A |
| RAVLT-delayed recall | N/A |
| Rey-Osterrieth Figure-immediate recall | 26 |
| Rey-Osterrieth Figure -delayed recall | 5 |
| TMT-part A | 47 |
| TMT-part B | N/A |
| SCWT-time | N/A |
| SCWT-errors | N/A |
| Fonemic fluency | N/A |
| Semantic fluency | N/A |
| Noise Pareidolia Test | 0 |
For each test, the score is reported as raw scores. Scores below normal performances are reported in bold. MoCA, Montreal Cognitive Assessment; ADL, Activities of daily living; IADL, Instrumental activities of daily living; CDR, Clinical dementia rating scale; NPI, Neuropsychiatric Inventory; VH, visual hallucinations; RAVLT, Rey Auditory Verbal Learning Test; TMT, Trail Making Test; SCWT, Stroop Color and Word Test; tot, total; N/A, not applicable.
Neuropsychological assessment
A neuropsychological evaluation was assessed at hospital admission and at six-month follow-up visit to evaluate both cognitive performance and functional status. The neuropsychological battery included global cognitive status (MoCA [7] and Clinical Dementia Rating Scale – CDR [8]), global functioning (Activities of daily living – ADL [9], and Instrumental activities of daily living – IADL [10]), learning and long-term memory (Rey’s Auditory Verbal Learning test – RAVLT) [11], planning strategies and visuospatial memory (Rey’s Complex Figure, copy and delayed recall) [12], executive functions (Stroop color and word test – SCWT). [13], attention (Trail Making test – TMT, A and B) [14], language (phonological and categorical verbal fluency) [15], and the presence of pareidolias (Noise Pareidolia Test) [16]. The Neuropsychiatric Inventory (NPI) was administered to the subject’s caregiver to assess non-cognitive symptoms, especially VH [17].
18F-FDG PET-CT
About six months after hospitalization, the patient underwent a 18F-FDG brain scan using a PET-CT Discovery MI DR tomograph (GE Healthcare).
The patient, fasted for at least 6 h, was positioned in supine position in a quiet and soft light condition for about 15 min before 18F-FDG administration. The scan was obtained over 15 min, starting 30 min after intravenous injection of 185 MBq. The CT scan was used for attenuation correction (120 kV).
The images were assessed by both qualitative and semi-quantitative evaluation.
As regards as qualitative assessment, a lower uptake of 18F-FDG tracer compared to the overall brain radioactivity or the contralateral side (hypometabolism) was considered as significant.
The semi-quantitative analysis was performed by using CortexID (GE Healthcare), a software package that generated individual maps of hypometabolism using 3-dimensional stereotactic surface projections. Standardized uptake values were obtained for 13 regions of interest (ROI) and normalized to the pons. Individual patient’s ROI Z-scores were calculated from 294 healthy sex- and age-matched controls, with a Z-score < –2.0 as cut off for significant hypometabolism (Z-score = [mean database- mean subject]/SD database).
RESULTS
During the hospitalization, the QEEG was performed before initiating pharmacological treatment for delirium. The exam showed a CSA pattern of 2 (DF = 7.5 Hz, DFV = 2 Hz) (Fig. 1a). This CSA pattern, defined as unstable alpha with pre-alpha or theta/delta, consisted of dominant alpha (≥8 Hz) in less than 50% of epochs, mean DFV above 2 Hz, dominant pre-alpha or theta (< 8 Hz) in 40% of more of epochs [5].
After six months from the hospitalization, neuropsychological assessment, QEEG, and 18F-FDG PET-TC were performed to evaluate the possible onset of cognitive decline (Fig. 1).
During the interview to the patient and her daughter (patient’s caregiver), the presence of recent cognitive impairment with attention deficits were reported. The caregiver also reported the occurrence of sleep disturbances with dream enacting, fluctuations in alertness, and behavioral disorders (i.e., depression, aggressiveness, and anxiety). VHs were not reported.
Results of patient neuropsychological performances are reported in Table 1. The patient was alert and collaborative, well-oriented in space but not in time. Her global cognitive status was moderately impaired (MoCA = 15/30), as well as ADL and IADL showed a moderate-severe reduced functional performances (CDR = 1). Visuo-spatial abilities (Rey-Osterrieth figure, copy) were severely impaired. A moderate impairment was also observed for visuo-spatial memory (Rey-Osterrieth figure, recall). No errors were detected during the Noise Pareidolia test.
The QEEG exam at the follow-up visit confirmed a DF = 7.5 Hz with a DFV = 2.5 Hz.
In addition, 18F-FDG PET-CT showed a moderate bilateral posterior hypometabolism (occipital and temporal cortex), with relative sparing of the posterior cingulate cortex compared to cuneus/precuneus (Cingulate Island sign), and mild bilateral hypometabolism in frontal regions. This pattern, confirmed at semiquantitative analyses, was suggestive of DLB diagnosis.
DISCUSSION
The present case shows the occurrence of an episode of delirium prior to a clinical diagnosis of probable DLB [1]. Of note, before her diagnosis, the patient showed an episode of mixed delirium, which is a well-known prodromal manifestation of DLB [3]. Even though definite diagnostic criteria for delirium-onset DLB have not been validated, recent findings reported as the prevalence of delirium prior to dementia is significantly higher in patients with DLB (25%) than other neurodegenerative disease, as Alzheimer’s disease (AD) (7%) [18]. Indeed, delirium symptoms are typically characterized by the presence of an altered conscious status, CF, dissociative, and psychotic manifestations. All these conditions tend to resemble the clinical features observed in DLB patients, especially CF, which are considered as a core clinical criterium for DLB diagnosis [3].
Together with clinical symptoms, revealing cognitive decline, CF, RBD, and parkinsonian signs, the patient diagnosis was also supported by a suggestive 18F-FDG hypometabolic pattern, which is currently considered as a supportive feature for DLB diagnosis [1].
As a novelty, the present case increases the insight into another supportive biomarker for DLB, such as EEG. In DLB patients, from their prodromal disease stages, EEG studies showed a progressive appearance of a pre-alpha or theta rhythm, a main hallmark of the thalamic derangement involved in DLB pathogenesis [5, 6]. A thalamic imbalance drives the so-called thalamocortical dysrhythmia [19–21], which is supposed to be responsible for both specific EEG patterns and symptoms (i.e., CF, VH, and RBD) observed in these patients.
EEG might be considered in the future as a supportive and validated biomarker for prodromal DLB, also for delirium-onset cluster. However, further EEG investigations, evaluating a large sample of patients who experienced delirium, are needed to support this evidence.
In prodromal delirium-onset DLB, the future introduction of specific diagnostic criteria, as well as validated biomarkers, might represent a relevant source for evaluating the disease at early stages to improve both diagnostic and prognostic management and to avoid treating delirium with typical neuroleptic potentially dangerous in the presence of a synucleinopathy. Additionally, the unveiling of novel and effective biomarkers could provide possible targeted therapies for prodromal DLB.
ACKNOWLEDGMENTS
The authors have no acknowledgments to report.
FUNDING
The authors have no funding to report.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
REFERENCES
[1] | McKeith IG , Boeve BF , Dickson DW , Halliday G , Taylor J-P , Weintraub D , Aarsland D , Galvin J , Attems J , Ballard CG , Bayston A , Beach TG , Blanc F , Bohnen N , Bonanni L , Bras J , Brundin P , Burn D , Chen-Plotkin A , Duda JE , El-Agnaf O , Feldman H , Ferman TJ , Ffytche D , Fujishiro H , Galasko D , Goldman JG , Gomperts SN , Graff-Radford NR , Honig LS , Iranzo A , Kantarci K , Kaufer D , Kukull W , Lee VMY , Leverenz JB , Lewis S , Lippa C , Lunde A , Masellis M , Masliah E , McLean P , Mollenhauer B , Montine TJ , Moreno E , Mori E , Murray M , O’Brien JT , Orimo S , Postuma RB , Ramaswamy S , Ross OA , Salmon DP , Singleton A , Taylor A , Thomas A , Tiraboschi P , Toledo JB , Trojanowski JQ , Tsuang D , Walker Z , Yamada M , Kosaka K ((2017) ) Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology 89: , 88–100. |
[2] | Hansen L , Salmon D , Galasko D , Masliah E , Katzman R , DeTeresa R , Thal L , Pay MM , Hofstetter R , Klauber M ((1990) ) The Lewy body variant of Alzheimer’s disease: a clinical and pathologic entity. Neurology 40: , 1–8. |
[3] | McKeith IG , Ferman TJ , Thomas AJ , Blanc F , Boeve BF , Fujishiro H , Kantarci K , Muscio C , O’Brien JT , Postuma RB , Aarsland D , Ballard C , Bonanni L , Donaghy P , Emre M , Galvin JE , Galasko D , Goldman JG , Gomperts SN , Honig LS , Ikeda M , Leverenz JB , Lewis SJG , Marder KS , Masellis M , Salmon DP , Taylor JP , Tsuang DW , Walker Z , Tiraboschi P ((2020) ) Research criteria for the diagnosis of prodromal dementia with Lewy bodies. Neurology 94: , 743–755. |
[4] | Bellelli G , Morandi A , Davis DHJ , Mazzola P , Turco R , Gentile S , Ryan T , Cash H , Guerini F , Torpilliesi T , Santo F Del , Trabucchi M , Annoni G , MacLullich AMJ ((2014) ) Validation of the 4AT, a new instrument for rapid delirium screening: A study in 234 hospitalised older people. Age Ageing 43: , 496–502. |
[5] | Bonanni L , Thomas A , Tiraboschi P , Perfetti B , Varanese S , Onofrj M ((2008) ) EEG comparisons in early Alzheimer’s disease, dementia with Lewy bodies and Parkinson’s disease with dementia patients with a 2-year follow-up. Brain 131: , 690–705. |
[6] | Bonanni L , Perfetti B , Bifolchetti S , Taylor J-P , Franciotti R , Parnetti L , Thomas A , Onofrj M ((2015) ) Quantitative electroencephalogram utility in predicting conversion of mild cognitive impairment to dementia with Lewy bodies. Neurobiol Aging 36: , 434–445. |
[7] | Nasreddine ZS , Phillips NA , Bédirian V , Charbonneau S , Whitehead V , Collin I , Cummings JL , Chertkow H ((2005) ) The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53: , 695–699. |
[8] | Hughes AJ , Daniel SE , Kilford L , Lees AJ ((1992) ) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55: , 181–184. |
[9] | Katz S ((1963) ) Studies of illness in the aged. JAMA 185: , 914. |
[10] | Lawton M , Brody E ((1969) ) Assessment of older people: selfmaintaining and instrumental activities of daily living. Gerontologist 9: , 179–186. |
[11] | Carlesimo GA , Caltagirone C , Gainotti G , Nocentini U ((1995) ) Batteria per la valutazione del deterioramento mentale (parte II): standardizzazione e affidabilità diagnostica nell’identificazione dei pazienti affetti da sindrome demenziale. |
[12] | Caffarra P , Vezzadini G , Dieci F , Zonato F , Venneri A ((2002) ) Rey-Osterrieth complex figure: normative values in an Italian population sample. Neurol Sci 22: , 443–447. |
[13] | Caffarra P , Vezzadini G , Dieci F , Zonato F , Venneri A ((2002) ) Una versione abbreviata del test di Stroop: Dati normativi nella popolazione Italiana. Nuova Riv Neurol 12: , 111–115. |
[14] | Giovagnoli AR , Del Pesce M , Mascheroni S , Simoncelli M , Laiacona M , Capitani E ((1996) ) Trail Making Test: Normative values from 287 normal adult controls. Ital J Neurol Sci 17: , 305–309. |
[15] | Novelli G , Papagno C , Capitani E , Laiacona M , Vallar G , Cappa SF ((1986) ) Tre test clinici di ricerca e produzione lessicale: taratura su soggetti normali. Arch Neurol Psicol Psichiatria 47: , 477–506. |
[16] | Mamiya Y , Nishio Y , Watanabe H , Yokoi K , Uchiyama M , Baba T , Iizuka O , Kanno S , Kamimura N , Kazui H , Hashimoto M , Ikeda M , Takeshita C , Shimomura T , Mori E ((2016) ) The Pareidolia Test: a simple neuropsychological test measuring visual hallucination-like illusions. PLoS One 11: , e0154713. |
[17] | Cummings JL ((1997) ) The Neuropsychiatric Inventory: Assessing psychopathology in dementia patients. Neurology 48: (5 Suppl 6), S10–16. |
[18] | Vardy E , Holt R , Gerhard A , Richardson A , Snowden J , Neary D ((2014) ) History of a suspected delirium is more common in dementia with Lewy bodies than Alzheimer’s disease: A retrospective study. Int J Geriatr Psychiatry 29: , 178–181. |
[19] | Onofrj M , Espay AJ , Bonanni L , Delli Pizzi S , Sensi SL ((2019) ) Hallucinations, somatic-functional disorders of PD-DLB as expressions of thalamic dysfunction. Mov Disord 34: , 1100–1111. |
[20] | Delli Pizzi S , Franciotti R , Taylor JP , Thomas A , Tartaro A , Onofrj M , Bonanni L ((2015) ) Thalamic involvement in fluctuating cognition in dementia with Lewy bodies: Magnetic resonance evidences. Cereb Cortex 25: , 3682–3689. |
[21] | Mahowald MW , Schenck CH ((1991) ) Status dissociatus - A perspective on states of being. Sleep 14: , 69–79. |




