Metformin and Dementia Risk: A Systematic Review with Respect to Time Related Biases
Abstract
Background:
When studying drug effects using observational data, time-related biases may exist and result in spurious associations. Numerous observational studies have investigated metformin and dementia risk, but have reported inconsistent findings, some of which might be caused by unaddressed time-related biases. Immortal time bias biases the results toward a “protective” effect, whereas time-lag and time-window biases can lead to either a “detrimental” or “protective” effect.
Objective:
To conduct a systematic review examining time-related biases in the literature on metformin and dementia.
Methods:
The electronic databases PubMed, Web of Science, and ProQuest were searched for the terms “Metformin” AND (“dementia” OR “Alzheimer’s Disease” OR “cognitive impairment"). These databases were searched from inception through 09/24/2021. Only English language articles and human research were eligible.
Results:
Seventeen studies were identified: thirteen cohort studies, two case-control studies, and two nested case-control studies. Eleven (64.7%) studies reported a reduced risk of dementia associated with metformin use; two (11.8%) suggested metformin increased dementia risk, while four (23.5%) concluded no significant associations. Eight (61.5%) of thirteen cohort studies had immortal time bias or did not clearly address it. Fifteen (88.2%) of seventeen reviewed studies had time-lag bias or did not clearly address it. Two (50.0%) of four case-control studies did not explicitly address time-window bias. The studies that addressed most biases concluded no associations between metformin and dementia risk.
Conclusion:
None of the reviewed studies clearly addressed relevant time-related biases, illustrating time-related biases are common in observational studies investigating the impact of anti-diabetic medications on dementia risk.
INTRODUCTION
Dementia is not a specific disease but a range of symptoms associated with cognitive impairment that affects people’s ability to think, remember, and make decisions, and that can interfere with daily activities. The most common type of dementia is Alzheimer’s disease (AD). In 2021, an estimated 6.2 million people in the U.S. aged 65 years and older had AD and related dementias [1]. In 2017, AD was listed as the sixth leading cause of death among the U.S. population and the fifth leading cause of death in adults aged 65 years and older [2]. Between 2000 and 2019, the mortality rate of AD increased 110%, from 17.6 to 37.0 per 100,000 people [3]. Additional common types of dementia include dementia with Lewy bodies (DLB), vascular dementia, Parkinson’s disease dementia (PDD), frontotemporal dementia (FTD), limbic-predominant age-related TDP-43 encephalopathy (LATE), and mixed dementia [4]. Different protein build-ups are found in different types of dementia. Previous autopsy studies supported that the presence of amyloid-β and tau-protein are associated with AD [5, 6]. The protein α-synuclein is strongly associated with DLB and PDD [7, 8], tau with TDP-43, and a host of other proteins are associated with FTD and LATE [9].
Numerous studies have investigated the association between dementia risk and metformin. Metformin, a biguanide, is the preferred first-line medication for treatment of type 2 diabetes mellitus [10]. It has been shown to decrease insulin resistance, improve glycemic control, and can be safely combined with other antidiabetic medications [11]. Observational studies investigating the association between metformin and dementia risk have found inconsistent results. A systematic review and a meta-analysis synthesizing the available evidence on the relationship between metformin use with dementia risk concluded that the majority of observational studies supported a reduced risk of dementia associated with metformin use [12]. However, in a separate meta-analysis, which included only three studies that the authors deemed to be highly qualified, no significant association between metformin use and the risk for developing dementia was found [13].
Observational studies using administrative databases have been popular in pharmacoepidemiology. Using administrative databases can provide timely answers and reduce the limitations that clinical trials may have, such as ethical considerations, costs, or feasibility. However, there are inherent methodological challenges in the utilization of observational data that, if not specifically and robustly addressed in the study design and analytic methods, can introduce different time-related biases, such as immortal time bias, time-lag bias, and time-window bias [14]. Immortal time bias often produces effect estimates biased toward an apparent “protective” effect of the medication on the disease of interest, whereas time-lag and time-window biases can lead to either a “detrimental” or “protective” effect. Unaddressed time-related biases might contribute to the inconsistent results of previous studies examining metformin and dementia risk.
To better understand the impact of metformin on the risk of dementia, we conducted a systematic review to determine if time-related biases have been addressed in previous observational studies that examined the association between metformin use and dementia risk among patients with diabetes.
MATERIAL AND METHODS
Selection criteria
Studies were included if they fulfilled the following eligibility criteria: 1) the original article was published in English; and included 2) individuals with diabetes but without any type of dementia at baseline; 3) participants using metformin at any dose for any duration compared with participants with no antidiabetic medications or other active antidiabetic agents other than metformin; 4) a major outcome of time to dementia or any type of dementia, or mild cognitive impairment; 5) quantitative measures of association between metformin use and the risk of dementia or other relevant outcomes with their 95% confidence intervals (CIs) or p-value being reported; and were 6) observational studies, including cohort studies and case-control studies. Exclusion criteria were: 1) publication was a review, case report, animal study, or letter; 2) studies that used a cross-sectional study design; 3) studies that did not clearly define exposure groups and comparison groups; 4) studies that did not clearly define major outcomes; and 5) duplicated studies. Cross-sectional studies were not eligible in the present review because cross-sectional studies analyze data from a population at a specific point in time, for which time-related biases are not applicable.
Data extraction
The electronic databases PubMed, Web of Science, and ProQuest were searched for the terms “Metformin” AND (“dementia” OR “Alzheimer’s Disease” OR “cognitive impairment”). No date restrictions were applied; databases were searched from inception through 09/24/2021. Titles and abstracts were screened first. Articles identified as relevant then received a full-text review. Those articles that met the inclusion and exclusion criteria were identify as the final eligible studies.
For each eligible study, the following data were extracted: first author, year of publication, study design, study population, exposure group, comparator, primary outcome, statistical methods, and measures of associations. If adjusted relative risks were also provided, the fully adjusted relative risks was extracted. Searches of the Cochrane database and PROSPERO did not produce any completed or pending reviews on metformin and dementia risk concerning time-related biases. PROSPERO accepted the registration of this systematic review on 04/08/2021 (ID#: CRD42021240034). Data were extracted between 04/09/2021 and 09/24/2021.
Evaluation of time-related biases
Immortal time bias is very common in observational cohort studies of drug effects and is only applicable to cohort studies. It is introduced when time zero or cohort entry is not the same as the date of the first prescription and the immortal time is included as part of the exposure time [15]. In this scenario, participants must be outcome-free until their first prescription is fulfilled. If the exposure is defined as a time-invariant binary variable, the period between time zero and the first prescription (referred to as immortal time) is included as part of the time exposed; while in fact, individuals are not exposed during this period. Thus, the period between time zero and the first prescription date is immortal, which results in immortal time bias (Fig. 1).
Fig. 1
Immortal time bias. a) The entire follow-up duration, including immortal time, is classified into the exposure group, leading to immortal time bias, which can incorrectly show metformin having a protective effect on dementia risk. b) Immortal person time in the exposure group was classified into the unexposed group, showing a proper method to classify exposed and unexposed groups at time zero.
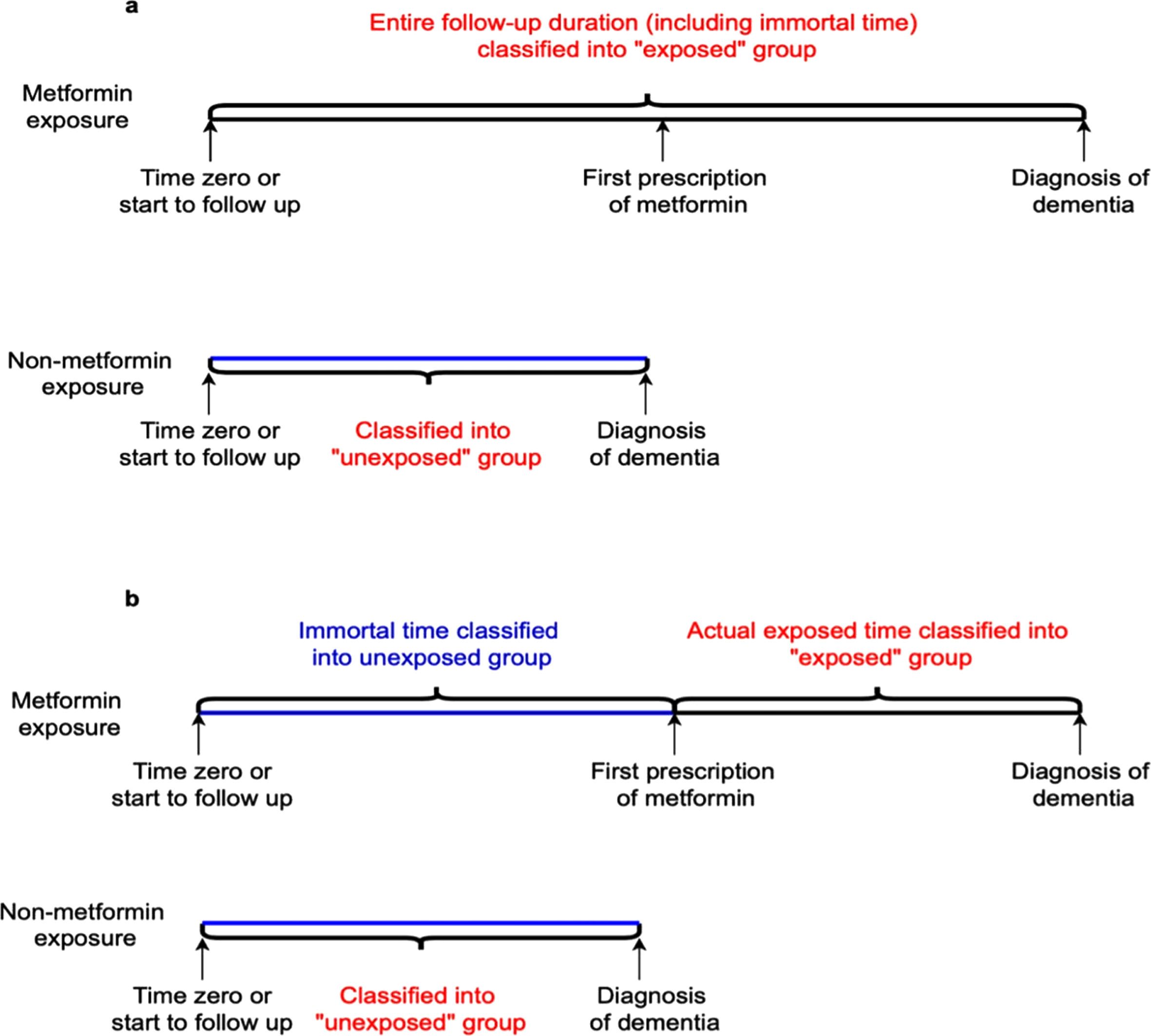
In addition to different dates of time zero and first prescription, immortal time bias happens when the investigators use cumulative duration or prescription of metformin during the entire follow-up as the exposures to estimate the associations between metformin and dementia risk [16]. When the cutoff for the cumulative duration category is large (e.g., 10 years), patients in the exposed group must be outcome-free before the cutoff time, which leads to immortal time bias. Applying time-vary covariates methods to correctly define time zero as the date of first prescription fulfilled is a feasible way to avoid immortal time bias [17]. Generally, immortal time bias results in effect estimates biased toward an apparent protective effect of the medications.
Time-lag bias is introduced when participants are at different stages of the disease [18]. For example, if diabetes duration is not adjusted for or matched, a first-line therapy may be compared with a second or third-line therapy. In that case, time-lag bias exists because patients with second or third-line therapy are more likely to have a longer duration of diabetes than those with first-line therapy, and a longer duration of diabetes is associated with a higher incidence of dementia (Fig. 2) [19, 20]. Adjusting for or matched on diabetes duration is a potential solution for addressing time-lag bias.
Fig. 2
Time-lag bias. a) First-line therapy compared with second-line therapy, which implies patients who use second-line therapy can have a longer diabetes duration than those with first-line therapy. In this case, time-lag bias is likely to occur for participants with first-line therapy because longer duration of diabetes is associated with a higher risk of dementia. b) The appropriate comparison should ensure participants in metformin and non-metformin therapies are on a similar stage of diabetes.
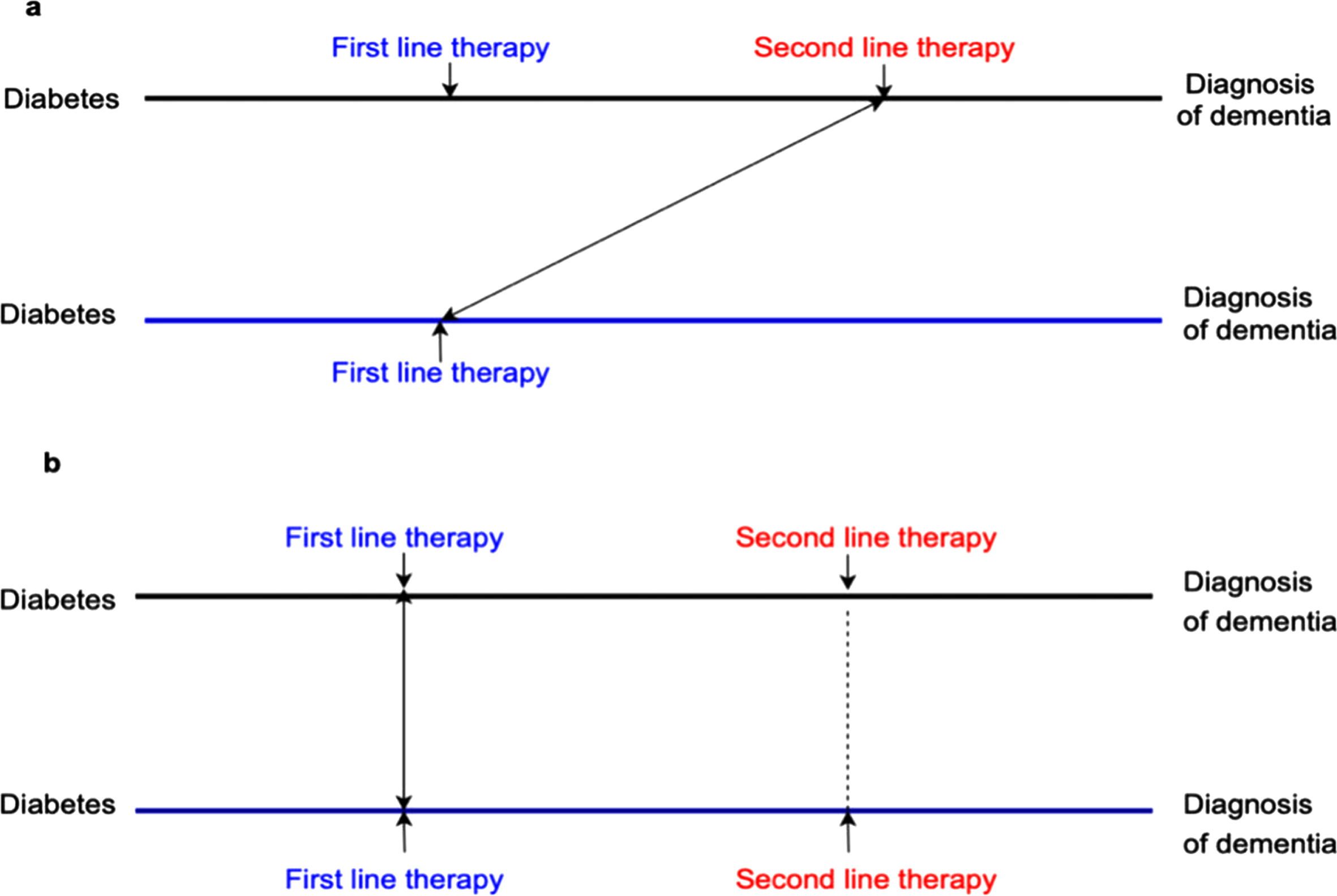
Generally, time-lag bias can bias the results toward either a protective or detrimental effect of the medications. For example, when the exposure is a first-line therapy, but the comparison group included a second or third-line therapy, time-lag bias occurs and biases the results toward a protective effect of the exposure. Conversely, when the comparison group is a first-line therapy, but the exposure is a second or third-line therapy, time-lag bias biases the results toward a detrimental effect.
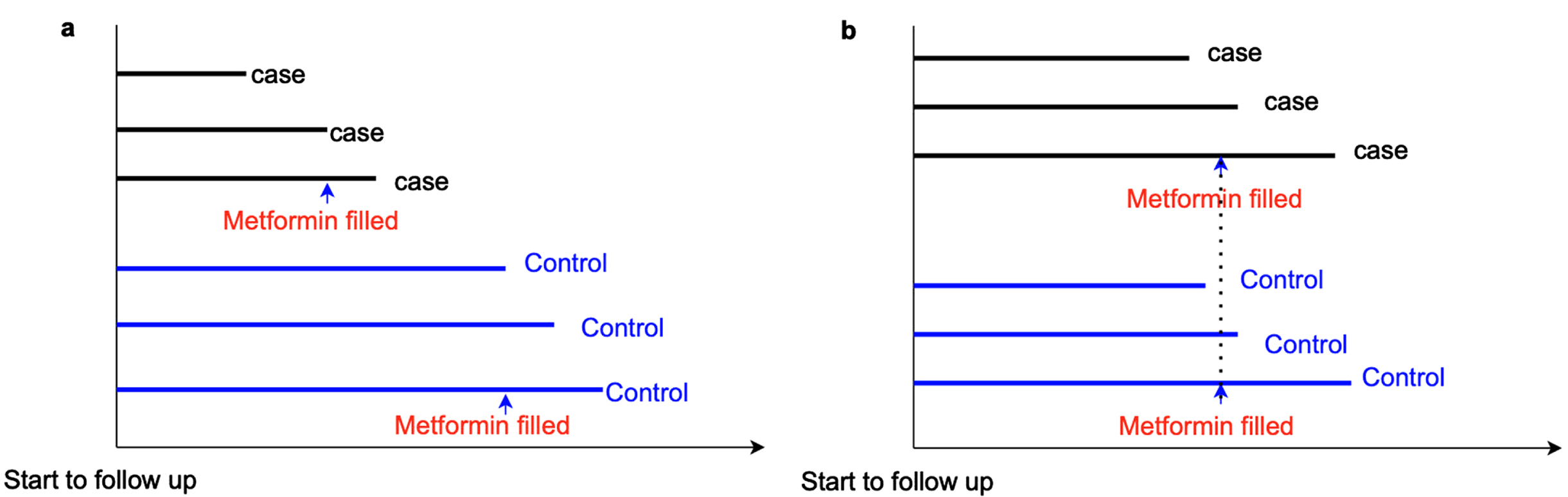
Time-window bias is specific to case-control or nested case-control studies. It happens when controls are defined as participants who do not experience the outcome during an observational period, but the observational period is not matched with that of the cases (Fig. 3). In this scenario, the exposure might be evaluated during different lengths of time intervals for cases and controls (i.e., different exposure opportunities), thus, resulting in time-window bias [21]. Similar to time-lag bias, time-window bias can also bias the results toward either a protective or detrimental effect of the medications. For instance, if controls have longer follow-up time, which means controls have greater exposure opportunities than cases, the estimates of the exposure effect will be biased toward a protective effect on the disease. Using a time-dependent sampling method to select controls in order to address time-window bias was suggested because this method ensures cases and controls have the same exposure opportunity time [21].
Fig. 3
Time window bias. a) In a case-control study, cases have a shorter time window of metformin exposure than controls, which indicates controls have a greater opportunity to receive metformin prescriptions than cases. Thus, time-window bias occurs, and it can bias the results to show metformin has a protective effect on dementia risk. b) Time-window bias can be addressed in case-control studies if cases and controls have the same exposure opportunity time.
Assessment of methodological quality
The Good Research for Comparative Effectiveness (GRACE) checklist is a tool to assess the quality of data and methods for observational research studies of comparative effectiveness [22]. We used the GRACE checklist to evaluate the methodological quality of the included observational studies in the present review. Item M4 of the GRACE checklist was used to evaluate the presence of immortal time bias. If time zero was defined correctly, which was the date of the first prescription in exposed and comparison groups, we considered immortal time bias to be addressed clearly. Alternatively, if a study applied a time-dependent exposure analysis, immortal time bias was also considered to be addressed because a time-varying analysis ensures all patients were classified as the unexposed group until the onset of anti-medication usage (i.e., time zero). We added two more questions to the GRACE checklist to evaluate the studies more comprehensively with a focus on time-related biases. M6 asked “Was time-window bias clearly addressed?” We considered time-window bias to be clearly addressed if cases and controls were matched on or adjusted for the duration of follow-up, or more specifically, duration of exposure opportunity time. Time-window bias was also considered to be addressed if studies applied a time-dependent analysis since a time-dependent method could ensure an equal time window to measure exposure for cases and controls. M7 asked “Was time-lag bias clearly addressed?” Time-lag bias was considered addressed if diabetes duration was matched or adjusted for. Additionally, we added two more questions to evaluate the precisions of reviewed studies. M8 asked “Was the follow-up period long enough for outcomes to occur?” A follow-up period of five years or longer was considered an acceptable length of time for investigating metformin exposure and dementia risk. Item M9 asked “Is follow-up of cohorts’ adequate (i.e., participants lost to follow up unlikely to introduce serious bias)?” If the percentage of loss to follow-up or non-response rate was≤20%, we considered the follow-up of the cohort was adequate [23]. The revised GRACE consists of six questions to assess the quality of the data and nine items to evaluate the methods used in study design and analysis. For each item, we assigned a score: Sufficient or Yes (+1), Insufficient or No or not enough information (0), Not applicable (NA). The potential range of the revised GRACE score is: 0 to 14.
RESULTS
Literature search outcomes
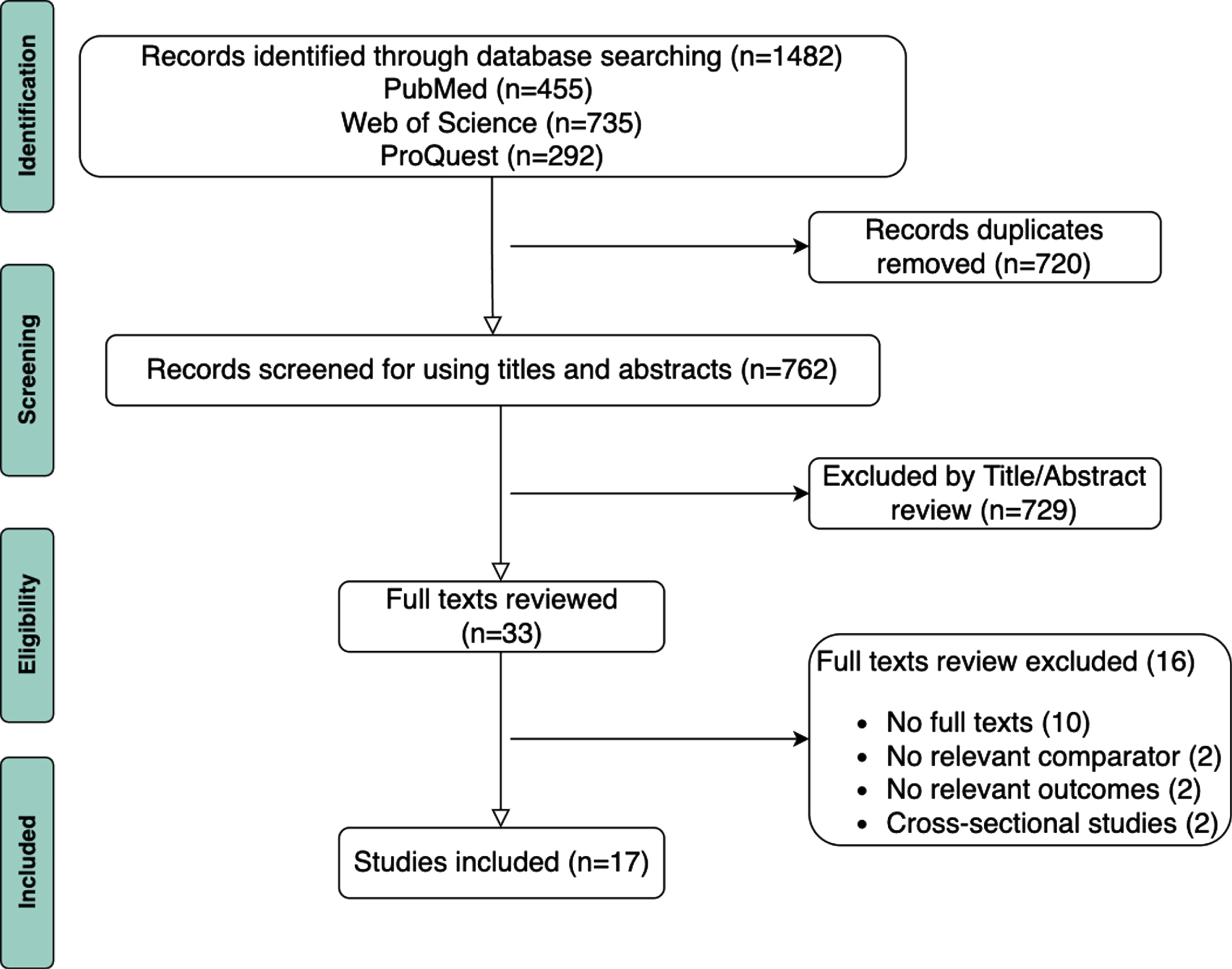
We identified 1,482 articles in PubMed, Web of Science, and ProQuest, but 720 articles were removed after de-duplication. A total of 729 articles were excluded after title and abstract screening. The remaining 33 articles were eligible for full-text reviews. Among those 33 articles, 16 were excluded during full-text review due to the absence of full texts (n = 10), the lack of a relevant comparator (n = 2), non-relevant outcomes (n = 2), and having a cross-sectional study design (n = 2). A total of 17 articles met the inclusion criteria, including 13 cohort studies [16, 24–35], two case-control studies [36, 37], and two nested case-control studies [38, 39]. Figure 4 illustrates the inclusion flowchart based on Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2009 flowchart diagram [40].
Fig. 4
Flow diagram of the study selection process for systematic review.
An overview of the 17 eligible articles is provided in Table 1. Among the 17 articles, 11 articles (64.7%) reported a significantly reduced risk of dementia associated with metformin use [16, 25, 26, 28–32, 36, 38, 39]; two articles (11.8%) suggested metformin increased dementia risk [27, 37]; and four articles (23.5%) concluded no significant association between metformin use and dementia risk [24, 33–35]. Among the 13 cohort studies, immortal time bias was identified in five studies (38.5%), and another three studies (23.1%) did not address it clearly [16, 24–26, 29, 32, 33, 35]. There were 15 (88.2%) out of 17 studies identified to have time-lag bias or did not clearly address it [16, 24–35, 37, 39]. Among the four case-control and nested case-control studies, time-window bias was not explicitly addressed in two studies (50.0%) [36, 38]. A total of eight studies (47.1%) did not clearly address two relevant time-related biases, and the remaining nine studies (52.9%) did not address one time-related bias.
Table 1
Time-related biases in observational studies investigated the effects of metformin on the risk of dementia
| Studies | Study designs | Exposure (Sample size) | Comparator (Sample size) | Population (All dementia free at baselines) | Statistical methods | Outcomes | Estimated association (95% CI) | Immortal time bias addressed clearly | Time-lag bias addressed clearly | Time-window bias addressed clearly |
| Studies supporting a reduced the risk of dementia associated with metformin | ||||||||||
| R1 [25] | Cohort | T2DM patients with metformin monotherapy (1864) | Non-medication patients with T2DM (10519). Cohort without T2DM (101816) | Taiwanese, aged≥50 y. | Cox regression model | dementia | 0.76 (0.58–0.98) a | No | No | Not Applicable |
| R2 [30] | Cohort | New users of metformin monotherapy (55,859) | New users of sulfonylurea monotherapy (17,902) | African American and whites with T2DM and aged≥50 y. | Cox regression model | dementia | Whites: 0.98 (0.92–1.05) a African American: 0.77 (0.64–0.94) a | Yes | No | Not Applicable |
| R3 [31] | Cohort | New users of metformin monotherapy (64518) | New users of sulfonylurea monotherapy (21535) | VHA patients and KPW patients with T2DM, aged≥50 y. | Cox regression model | dementia | AHA: 0.93 (0.87–0.99) a KPW: 0.89 (0.74–1.07) a | Yes | No | Not Applicable |
| R4 [28] | Cohort | New users of metformin monotherapy (17200) | New users of Sulfonylureas monotherapy (11440) | US veterans aged≥65 y with T2DM. | Cox regression model | Dementia | <75 y 0.89 (0.79–0.99) a ≥75 y 0.96 (0.87–1.05) a | Yes | No | Not Applicable |
| R5 [16] | Cohort | Metformin ever users (15,676) | Metformin never users (15,676) | Taiwan’s population who aged between 25 y to 75 y. New-onset diabetes patients during 1999 and 2005. | Cox regression model | Dementia | <26.6 months: 1.279 (1.100–1.488) a 26.6–57.8 months: 0.70 (0.60–0.83) a >57.8 months: 0.39 (0.32–0.47) a | No | No | Not Applicable |
| R6 [26] | Cohort | Metformin use (combined or monotherapy)≥90 days Low users (1211) Mid users (1210) High users (1211) | Metformin use (combined or monotherapy) < 90 days (4436) | Korean National health insurance holders with DM, aged 40–79 y. | Cox regression model | Dementia | 0.97 (0.73–1.28) a 0.77 (0.58–1.01) a 0.48 (0.35–0.67) a 0.80 (0.65–0.98) a 0.61 (0.50–0.76) a 0.46 (0.36–0.58) a | No | No | Not Applicable |
| R7 [29] | Cohort | Participants with diabetes with metformin (combined or monotherapy) (67) | Metformin non-users (non-medication users or antidiabetic medications other than metformin) (56). | Australia, Sydney. Community-dwelling participants aged 70–90 y with DM. | Linear mixed model and cox regression survival analysis | Dementia. Cognitive decline; Cognitive Performance. | Dementia: 0.19 (0.04–0.85) a | No | No | Not Applicable |
| R8 [32] | Cohort | Insulin and metformin users (3053) | Insulin users but without metformin (2993) | US Veterans with T2DM, aged≥50 y, insulin users. | Cox regression model | Dementia, ND, including AD, PD, and MCI. | 2–4 y: Dementia: 0.55 (0.38–0.79) a >4 y: Dementia: 0.22 (0.13–0.37) a | No | No | Not Applicable |
| R9 [36] | Case-control | Metformin monotherapy (5826), or metformin as dual therapy with sulfonylureas (1481) | Sulfonylurea monotherapy (1415) | Germany. Cohort aged≥60 y with T2DM. | Multivariate regression models | Dementia | Metformin monotherapy: 0.71 (0.66–0.76) b Metformin+ sulfonylureas (dual therapy): 0.90 (0.89–0.92) b | Not Applicable | Yes | No |
| R10 [38] | Nested case-control | Metformin users > 3 years before AD or individuals who were only exposed to metformin during the 3-year lag period. (23,948) | Metformin non-users (non-medications or antidiabetic medications other than metformin) (5,464) | Finland. All community-dwelling people with DM in Finland. | Conditional logistic regression models | AD | Metformin ever use: 0.99 (0.94–1.05) b Metformin use > 10 y: 0.85 (0.76–0.95) b DDD > 1825 and metformin intake > 1.0 DDD/day: 0.89 (0.82–0.96) b | Not Applicable | Yes | No |
| R11 [39] | Nested case-control | Metformin ever users: 0–0.5 DDD, 0.5–0.75 DDD, 0.75–1 DDD, >1 DDD. (37,173) | Metformin never users (non-medication or antidiabetic medication other than metformin) (20,922) | Denmark. Patients in Denmark registered with T2DM in the National Diabetes Register (NDR). | Conditional logistic regression models | Dementia | 0.94 (0.89–0.99) b | Not Applicable | No | Yes |
| Studies supporting no associations between metformin and dementia risk | ||||||||||
| R12 [33] | Cohort | Diabetes patients with metformin use (2120) | Diabetes patients without metformin use (3774) | White (UK); data from the following cohorts: FHS, RS, ARIC, AGES, SALSA. | Multivariable Cox proportional hazard model | Dementia; AD | Dementia: 1.36 (0.98, 1.89) a AD: 1.61 (0.89, 2.9) a | No | No | Not Applicable |
| R13 [34] | Cohort | Diabetes patients with metformin use (1478) | Diabetes patients without metformin use (3854) | German. Sample of the largest German mandatory public health insurance company, AOK. | Cox proportional hazard models | Dementia | 0.97 (0.91–1.03) a | Yes | No | Not Applicable |
| R14 [24] | Cohort | Metformin only users (1033) | Sulfonylurea only users (796) or thiazolidinediones only users (28) | Taiwanese population, birth-year period before 1940 (≥65 y) and new-onset diabetes between January 2004 to June 2009. | Cox regression model | Dementia | 0.82 (0.52–1.28) a | No | No | Not Applicable |
| R15 [35] | Cohort | Metformin use (4978) | Diabetes patients without any anti-diabetic medication (or diabetes other therapy) (unclear about the sample size of comparators) | Taiwanese population, newly diagnosed diabetes between January 1997 and December 2007. | Cox regression model | AD | Metformin monotherapy: 0.69 (0.28–1.71) a Metformin combination therapy: 0.57 (0.26–1.26) a | No | No | Not Applicable |
| Studies supporting an increased the risk of dementia associated with metformin | ||||||||||
| R16 [27] | Cohort | Metformin use (alone or combined) (4651) | Metformin non-users, but with other anti-diabetic medications (4651) | Taiwanese population, aged > 50 y. New diagnosis of T2DM between January 1, 2000, and December 31, 2010. | Cox regression model | Dementia, PD | Dementia: 1.66 (1.35–2.04) a PD: 2.27 (1.68, 3.07) a | Yes | No | Not Applicable |
| R17 [37] | Case-control | Metformin use: 1–9, 10–29, 30–59,≥60 prescriptions or Metformin monotherapy: 1–9, 10–29,≥30 prescriptions (634) | Metformin non-users (13,538) | UK. Cohort aged≥65 y with DM | Conditional logistic regression | AD | metformin≥60: 1.71 (1.12–2.60) b 30–59:0.99 (0.68–1.44) b 10–29:1.47 (1.03–2.09) b 1–9:1.08 (0.75–1.56) b | Not Applicable | No | Yes |
aHazard ratio; bOdds ratio. HR, hazard ratio; OR, odds ratio; T2DM, Type 2 diabetes mellitus; VHA, Veterans’ Health Affairs; KPW, Kaiser Permanente Washington; ND, neurodegenerative disease; AD, Alzheimer’s disease; PD, Parkinson’s disease; MCI, mild cognitive impairment; DDD, daily defined doses; FHS, The Offspring cohort of the Framing- ham Heart Study; RS, the Rotterdam Study; ARIC, the Atherosclerosis Risk in Communities Study; AGES, the Aging Gene-Environment Susceptibility-Reykjavik Study; SALSA, Sacramento Area Latino Study on Aging; AOK, Allgemeine Ortskrankenkassen. All HRs or ORs were obtained after adjustment of potential confounders or inverse probability of treatment weighting for propensity score.
Immortal time bias
We found that eight (61.5%) of the 13 reviewed cohort studies had immortal time bias or did not clearly address it [16, 24–26, 29, 32, 33, 35]. Among those eight studies, seven (87.5%) did not correctly define time-zero [24–26, 29, 32, 33, 35], and one study (12.5%) introduced immortal time bias by examining the cumulative duration of metformin use [16]. Five (62.5%) of these eight cohort studies reported that metformin significantly reduced the risk of dementia.
Three of these seven studies had an improper time zero definition, which defined time zero as date of initial diabetes diagnosis. In this scenario, the period between time zero and the date of the first prescription was immortal, which resulted in immoral time bias. All these three studies supported metformin was significantly associated with a reduced risk of dementia [25, 26, 32]. One study defined time zero as of January 2004, which was not the date of the first prescription. Hence, the time between time zero and the first prescription was immortal. Although this study reported no significant relationships between metformin and dementia risk (Hazard ratio [HR]: 0.82, 95% CI: 0.52–1.28), the estimated HR was < 1 with a wide 95% CI, indicating a potential protective effect of metformin on dementia risk with larger sample size [24].
The remaining three studies among these seven articles did not report any information regarding time zero or the start date of follow-up [29, 33, 35]. Immortal time bias was not addressed clearly since time zero or the start of follow-up was unlikely to be the same as the first prescription of medication intake. One of these three studies reported a significantly reduced risk of dementia [29], but two studies did not find a significant association [33, 35]. One of the studies supporting no significant associations had an estimated HR was < 1 (HR: 0.69, 95% CI: 0.28–1.71) [35], but the other’s HR was > 1 (HR:1.60, 95% CI: 0.87, 2.93) [33]. Both HRs had relatively wide 95% CI, implying the sample sizes were not large enough to make a reliable conclusion. Additionally, the study with an estimated HR > 1 investigated questions by pooling analysis from five cohorts. However, these cohorts did not have the same criteria to define diabetes, and there was no information on time zero [33].
One cohort study was found to have immortal time between time zero and the cut-off used for cumulative duration [16]. This study concluded that short-term metformin use (<27.0 months) was associated with an increased the risk of dementia, but long-term metformin use (27.0–58.1 month or > 58.1 months) reduced dementia risk significantly. Immortal time bias occurred among the longest metformin use group (>58.1 months) because individuals in this group should have been dementia-free for at least 58.1 months. However, if a person had more than 58.1-month metformin exposure but was diagnosed with dementia before 27 months after time-zero, the person was likely classified into the < 27 months group rather than > 58.1 months group. Therefore, only long-term metformin users without dementia were classified into the longest metformin use group, inducing immortal time bias. This immortal time could bias results toward a protective effect of long-term metformin use on dementia risk.
Time-lag bias
Fifteen (88.2%) of the 17 reviewed studies were found to have time-lag bias or did not clearly address it since none of these studies matched or adjusted for diabetes duration, even though some compared metformin with other first-line therapies [16, 24–35, 37, 39].
Eleven studies compared metformin users with those on an unspecified antidiabetic medication or not on any antidiabetic medications, and none of these studies considered diabetes duration [16, 25–27, 29, 32–35, 37, 39]. In this case, metformin was compared with a second or third-line antidiabetic therapy. Time-lag bias occurred because an individual on a second or third-line antidiabetic therapy likely had longer duration of diabetes and thus was more likely to have a higher risk of dementia [19, 20]. Conversely, when metformin users were compared with those who were not on any antidiabetic medications, they were unlikely at the same stage of diabetes, which resulted in time-lag bias. Six of these eleven studies supported metformin significantly reducing dementia risk [16, 25, 26, 29, 32, 39], three reported no significant associations [33–35], and two studies indicated metformin was associated with an increased dementia risk [27, 37].
The remaining four studies compared metformin with other first-line antidiabetic medications but did not adjust for diabetes duration [24, 28, 30, 31]. In these studies, although the comparison groups were on the same line of antidiabetic medications, exposed and non-exposed individuals were still not guaranteed to be at the same stage of diabetes. For example, some first-line antidiabetic medication users were new-onset diabetes patients, while some other first-line users had been diagnosed with diabetes for a few years. Significantly, three of these four studies supported metformin slightly reducing the risk of dementia, while one reported no significant associations.
Time-window bias
Among the four identified case-control and nested case-control studies, two studies (50.0%) did not explicitly address time-window bias because they did not consider the duration of follow-up in their analysis [36, 38]. In this case, cases and controls might not have the same opportunity to receive metformin. One of them found that metformin use ≥10 years was associated with a reduced risk of dementia. However, cases and controls were not matched on the duration of follow-up after ten years follow-up, suggesting time-window bias was not addressed after ten years. For example, although cases and controls both had ≥10 years of diabetes duration, controls might have had a longer follow-up period than cases. Thus, controls would have greater opportunities to receive metformin prescriptions than cases, resulting in time window bias [38]. The other study concluded that metformin use was associated with a significantly reduced risk of dementia compared with non-metformin use [36]. Similarly, this study adjusted for diabetes duration in their analyses, but the follow-up period was not considered. More metformin exposures for controls than cases resulted in time window bias.
GRACE assessment
Table 2 depicts the methodological quality assessment of all studies using the GRACE checklist. The possible range of GRACE scores is 0 to 14; the mean GRACE scores of reviewed studies was 6.35±1.61. The highest score was 11.0, and the lowest score was 4.0. The study [38] that received the highest GRACE score (R10) among the 17 reviewed studies did not receive credit for three items (D3, M1, and M6). For D3 [Was the primary clinical outcome(s) measured objectively rather than subject to clinical judgment (e.g., opinion about whether the patient’s condition has improved)?], none of the 17 studies received credit because dementia diagnosis usually involves clinical judgement from the doctors. For M1 [Was the study (or analysis) population restricted to new initiators of treatment or those starting a new course of treatment?], R10 included metformin new users, short-term users, and long-term users. For M6 [If “time-window bias” was addressed clearly?], R10 did not receive credit because time-window bias was not fully addressed, which was discussed in detail above. All the other studies had a GRACE score < 10. The two studies with the highest (R10) and second-highest GRACE score (R13) addressed most time-related biases by applying time-varying methods or adjusting for diabetes duration and duration of follow-up [34, 38], and both supported no significant effects of metformin on the risk of dementia.
Table 2
Quality of included studies assessing the risk of dementia with metformin use
| Q. The Good Research for Comparative Effectiveness (GRACE) Checklist v5.0 (last amended in 2016) | |||||||||||||||||
| Website: https://www.graceprinciples.org/ | |||||||||||||||||
| Studies | R1 [25] | R2 [30] | R3 [31] | R4 [28] | R5 [16] | R6 [26] | R7 [29] | R8 [32] | R9 [36] | R10 [38] | R11 [39] | R12 [33] | R13 [34] | R14 [24] | R15 [35] | R16 [27] | R17 [37] |
| Major Components | Sufficient or Yes (+1), Insufficient or No or not enough information (0), Not applicable (NA) | ||||||||||||||||
| Data | |||||||||||||||||
| D1. Were treatment and/or important details of treatment exposure adequately recorded for the study purpose in the data source(s)? Note: not all details of treatment are required for all research questions. | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| D2. Were the primary outcomes adequately recorded for the study purpose (e.g., available in sufficient detail through data source(s)) | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| D3. Was the primary clinical outcome(s) measured objectively rather than subject to clinical judgment (e.g., opinion about whether the patient’s condition has improved)? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D4. Were primary outcomes validated, adjudicated, or otherwise known to be valid in a similar population? | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| D5. Was the primary outcome(s) measured or identified in an equivalent manner between the treatment/ intervention group and the comparison group(s)? | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| D6. Were important covariates that may be known confounders or effect modifiers available and recorded? | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Methods | |||||||||||||||||
| M1. Was the study (or analysis) population restricted to new initiators of treatment or those starting a new course of treatment? Efforts to include only new initiators may include restricting the cohort to those who had a washout period (specified period of medication nonuse) before the beginning of study follow-up. (New-user of first therapy or new-onset design)? | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 |
| M2. If 1 or more comparison groups were used, were they concurrent comparators? If not, did the authors justify the use of historical comparison groups? | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| M3. Were important confounding and effect-modifying variables taken into account in the design and/or analysis? (Appropriate methods to take these variables into account may include restriction, stratification, interaction terms, multivariate analysis, propensity score matching, instrumental variables, or other approaches?) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| M4. Is the classification of exposed and unexposed person-time free of “immortal time bias,” i.e., “immortal time” in epidemiology refers to a period of cohort follow-up time during which death (or an outcome that determines end of follow-up) cannot occur. (Or if immortal time bias was addressed clearly?) | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | NA | NA | NA | 0 | 1 | 0 | 0 | 1 | NA |
| M5. Were any meaningful analyses conducted to test key assumptions on which primary results are based (e.g., were some analyses reported to evaluate the potential for a biased assessment of exposure or outcome, such as analyses where the impact of varying exposure and/or outcome definitions was tested to examine the impact on results)? | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
| M6: If “time-window bias” was addressed clearly? | NA | NA | NA | NA | NA | NA | NA | NA | 0 | 0 | 1 | NA | NA | NA | NA | NA | 1 |
| M7: If “time-lag bias” was addressed clearly? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| M8: Was follow-up period long enough for outcomes to occur? | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 |
| M9: Is follow-up of cohorts’ adequate? (Participants lost to follow up unlikely to introduce serious bias) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 |
| Total scores | 4 | 7 | 7 | 7 | 6 | 4 | 7 | 6 | 6 | 11 | 6 | 6 | 8 | 4 | 6 | 7 | 6 |
Except for items regarding time-related biases, most studies did not receive credit on D2, D3, D4, D6, M3, and M9. For D2 [Were the primary outcomes adequately recorded for the study purpose (e.g., available in sufficient detail through data source(s)], outcomes were ascertained using diagnosis codes in an administrative database, but the details of the diagnosis were not captured in 14 (82.4%) of the 17 studies. For D4 [Were primary outcomes validated, adjudicated, or otherwise known to be valid in a similar population?], outcomes were not validated, adjudicated, or known to be valid in a similar population in 14 (82.4%) of the studies. For D6 [Were important covariates that may be known confounders or effect modifiers available and recorded?], 14 studies (82.4%) did not consider or adjust for diabetes durations or follow-up periods. For M3 [Were important confounding and effect-modifying variables taken into account in the design and/or analysis? (Appropriate methods to take these variables into account may include restriction, stratification, interaction terms, multivariate analysis, propensity score matching, instrumental variables, or other approaches)], 15 studies (88.2%) did not handle primary cofounders in their analysis or apply appropriate methods to take them into account. Finally, 12 studies (70.6%) did not have enough information to receive credit for M9 [Is follow-up of cohorts’ adequate?].
DISCUSSION
To the best of our knowledge, this is the first systematic review conducted to identify if time-related biases exist in previous observational studies investigating metformin use and dementia risk among patients with diabetes. Most of reviewed studies reported that metformin use was associated with a reduced risk of dementia. Most of the cohort studies reported that metformin was associated with a reduced risk of dementia; however, more than half of these studies had immortal time bias or did not clearly address it, which supports that immortal time bias usually biases the results toward a protective effect of the medication. Nearly 90% of reviewed studies had time-lag bias or did not clearly address it, and half of the reviewed case-control and nested case-control studies did not explicitly address time window bias. Notably, two studies that addressed most time-related biases concluded no significant associations between metformin use and dementia risk [34, 38]. As a result of our comprehensive systematic review, we found that the findings of these reviewed studies were inconsistent, and time-related biases were common in the studies.
Among the articles reviewed in this systematic review, one nested case-control study effectively addressed most time-related biases and received the highest GRACE score [38]. This study conducted sensitivity analyses restricting the window of medication exposure prior to the date of AD diagnosis to ten years for all participants, addressing time-window bias. Additionally, the cases were matched with controls by both age and diabetes duration (±1 year), addressing time-lag bias. The conclusion of sensitivity analyses was no significant associations between metformin use and AD risk. However, this study also found that long-term (≥10 years) and high-dose metformin use was associated with a lower risk of AD incidence among individuals with diabetes. In this analysis, time-window bias was not explicitly addressed after ten years because cases and controls were not matched on cumulative duration of metformin exposures after ten years or > 1825 daily defined doses, indicating follow-up periods after ten years were not considered. In the possible event that controls had a longer follow-up period than cases, thereby having a greater opportunity to receive a metformin prescription, the results would be biased toward a protective effect of long-term or high-dose metformin on AD risk.
The primary biological mechanism of metformin in treating diabetes is activating AMP-activated protein kinase (AMPK) to enhance the insulin sensitivity of peripheral tissues, inhibit hepatic gluconeogenesis, and reduce the intestinal absorption of glucose. AMPK also modulated tau protein, amyloid-β peptide protein precursor, and autophagy, which are all thought to be involved in the pathogenesis of AD and FTD [9, 41]. Previous investigations have found that regulating AMPK activity could mitigate α-synuclein toxicity in nigral dopamine neurons, which might contribute to treatments against PD and DLB [7, 8, 42]. Consequently, it has been hypothesized that metformin may reduce the risk of dementia and investigators have tested this hypothesis through the utilization of observational data.
A previous systematic review conducted by Campbell et al. concluded that metformin was protective against dementia among individuals who had been prescribed the medication for diabetes management [12]. However, in consideration of the potential time-related biases identified and other methodological differences, our results differed from this previous review. We found that the existing observational studies are inconclusive regarding the effects of metformin use on the risk of dementia. There are a number of significant factors that differentiate the present systematic review from the one previously conducted. First, the previous systematic review did not consider time-related biases in their analysis. Second, the reviews examined different sets of studies. Our review included five eligible studies from the former review but excluded seven studies because they were either cross-sectional studies or randomized clinical trials (RCTs). We added 12 studies to our review that were not included in the former. Two of these studies were published earlier than the cutoff date of the prior review, and the remaining ten studies were published after the cutoff date. Among these 12 studies, most concluded that metformin was associated with a reduced risk of dementia [16, 26, 28–32, 36, 38, 39], whereas several studies reported no significant associations, or an increased risk of dementia associated with metformin use [27, 33].
Our results are consistent with another previous systematic review and meta-analysis of 13 studies investigating metformin and dementia risk conducted by McMillan et al. [13]. However, only three of the 13 studies were eligible for their meta-analysis, and the investigators concluded that no significant association exists between metformin use and dementia risk. Although the findings are consistent, McMillan et al. did not consider time-related biases.
To date, only two clinical trials examining the effects of metformin on dementia or cognitive impairment have been completed. The first, a double-blind, placebo-controlled, randomized pilot study, examined the effects of 12 months of metformin intake on AD among 90 participants. Participants included in this investigation had been diagnosed with amnestic mild cognitive impairment were aged 55 to 90 years, had a body mass index of 25 kg/m2 or higher, and were not receiving diabetes-related treatments. After adjusting for the baseline difference in Alzheimer’s Disease Assessment Scale cognitive subscale, the group that was randomized to receive metformin showed significantly greater improvement in total recall only in the Selective Reminding Test (p = 0.02) [43]. No significant differences were found between metformin and the placebo in changes in other cognition-related biomarker outcomes. The second clinical trial was a small pilot study of 20 participants utilizing a randomized, double-blinded, placebo-controlled, 16-weeks crossover design to investigate the effects of metformin on AD biomarkers [44]. The results of this study were not published but were found inconclusive. To date, the completed clinical trials do not provide strong evidence to support a protective effect of metformin on dementia risk.
Although the presence of time-related biases in observational studies exploring the effects of metformin on dementia risk have not been previously investigated, these biases have been described in other fields including antidiabetic medications and cancer. In this field, a large number of observational studies had been published reporting significant reductions in the risk of different cancer types associated with metformin use; however, upon investigation, many of these studies were found to suffer from time-related biases [18, 45, 46]. Three studies that specifically applied statistical models with time-dependent covariates and new user designs to address both immortal time bias and time-window bias found no significant associations between metformin use and cancer incidence [47–49]. Regrading clinical trials, the four randomized, parallel arm, and double/quadruple masked trials found that metformin did not have a significant effect on overall survival in patients with cancer [50–53]. Contrastingly, other clinical trials found metformin to be associated with improved overall survival among patients with cancer [54–58]; however, these studies had very small sample sizes and were primarily single-arm design. The largest RCT (the MA.32) of metformin, compared to a placebo, on disease-free survival among women with early breast cancer recently published their results, reporting that metformin did not significantly improve invasive disease-free survival among the 3,649 randomized patients [59]. Therefore, there is currently not enough evidence to conclude that metformin is beneficial for patients with cancer.
Metformin is currently being studies as an anti-aging agent for various outcomes [60, 61]. The Targeting Aging with Metformin (TAME) trial is a series of nationwide, six-year clinical trials with over 3,000 older adults aged 65–79 years, which plans to test whether metformin delays the development or progression of age-related chronic diseases, such as dementia and cancer [62]. The results of the TAME trials will greatly inform us with respect to our research question: does metformin have a protective or harmful effect on the risk of dementia among adults with and without diabetes after addressing time-related biases adequately.
Conclusions
In our review, the findings from previous observational studies are inconsistent regarding the association between metformin and dementia risk. We found all reviewed studies had some type of time-related biases or did not address them explicitly, illustrating time-related biases are common in the observational studies investigating the impacts of antidiabetic medications on dementia risk. The studies that did address most time-related biases found no significant associations between metformin use and dementia risk. Time-related biases can be eliminated or alleviated using appropriate study designs and analysis methods. Future observational studies should use more rigorous study designs and appropriate statistical analyses to avoid or reduce time-related biases.
ACKNOWLEDGMENTS
We thank Sara Mumby, a Publication Manager and Media Coordinator at Colorado School of Public Health, for revising the manuscript to improve the accuracy, clarity, and consistency in English writing.
FUNDING
This work was supported by National Institute on Aging (R01AG061189 to L. J. and J. O.). The content of this report is solely the responsibility of the authors and does not necessarily represent the official views of the funding organization.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
REFERENCES
[1] | Rajan KB , Weuve J , Barnes LL , McAninch EA , Wilson RS , Evans DA ((2021) ) Population estimate of people with clinical Alzheimer’s disease and mild cognitive impairment in the United States (2020–2060). Alzheimers Dement 17: , 1966–1975. |
[2] | Murphy SL , Xu J , Kochanek KD , Arias E (2018) Mortality in the United States, 2017. NCHS Data Brief, pp. 1-8. |
[3] | U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics (2020) About Underlying Cause of Death, 1999-2019. https://wonder.cdc.gov/ucd-icd10.html. |
[4] | Alzheimer’s Association ((2021) ) Alzheimer’s disease facts and figures. Alzheimers Dement 17: , 327–406. |
[5] | Joachim C , Morris J , Selkoe D ((1988) ) Clinically diagnosed Alzheimer’s disease: Autopsy results in 150 cases. Ann Neurol 24: , 50–56. |
[6] | Clark CM , Xie S , Chittams J , Ewbank D , Peskind E , Galasko D , Morris JC , McKeel DW , Farlow M , Weitlauf SL ((2003) ) Cerebrospinal fluid tau and β-amyloid: How well do these biomarkers reflect autopsy-confirmed dementia diagnoses?. Arch Neurol 60: , 1696–1702. |
[7] | Donaghy PC , McKeith IG ((2014) ) The clinical characteristics of dementia with Lewy bodies and a consideration of prodromal diagnosis. Alzheimers Res Ther 6: , 1–12. |
[8] | Outeiro TF , Koss DJ , Erskine D , Walker L , Kurzawa-Akanbi M , Burn D , Donaghy P , Morris C , Taylor J-P , Thomas A ((2019) ) Dementia with Lewy bodies: An update and outlook. Mol Neurodegener 14: , 1–18. |
[9] | Nelson PT , Dickson DW , Trojanowski JQ , Jack CR , Boyle PA , Arfanakis K , Rademakers R , Alafuzoff I , Attems J , Brayne C ((2019) ) Limbic-predominant age-related TDP-43 encephalopathy (LATE): Consensus working group report. Brain 142: , 1503–1527. |
[10] | Inzucchi SE , Bergenstal RM , Buse JB , Diamant M , Ferrannini E , Nauck M , Peters AL , Tsapas A , Wender R , Matthews DR ; American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD) ((2012) ) Management of hyperglycemia in type 2 diabetes: A patient-centered approach: Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 35: , 1364–1379. |
[11] | Cusi K , DeFronzo R ((1998) )Metform, A review of its metabolic effects. Diabetes Rev 6: , 89–131 . |
[12] | Campbell JM , Stephenson MD , De Courten B , Chapman I , Bellman SM , Aromataris E ((2018) ) Metformin use associated with reduced risk of dementia in patients with diabetes: A systematic review and meta-analysis. J Alzheimers Dis 65: , 1225–1236. |
[13] | McMillan JM , Mele BS , Hogan DB , Leung AA ((2018) ) Impact of pharmacological treatment of diabetes mellitus on dementia risk: Systematic review and meta-analysis. BMJ Open Diabetes Res Care 6: , e000563. |
[14] | Suissa S , Dell’Aniello S ((2020) ) Time-related biases in pharmacoepidemiology. Pharmacoepidemiol Drug Safety 29: , 1101–1110. |
[15] | Suissa S ((2008) ) Immortal time bias in pharmacoepidemiology. Am J Epidemiol 167: , 492–499. |
[16] | Chin-Hsiao T ((2019) ) Metformin and the risk of dementia in type 2 diabetes patients. Aging Dis 10: , 37. |
[17] | Shintani AK , Girard TD , Arbogast PG , Moons KG , Ely EW ((2009) ) Immortal time bias in critical care research: Application of time-varying Cox regression for observational cohort studies. Crit Care Med 37: , 2939. |
[18] | Suissa S , Azoulay L ((2012) ) Metformin and the risk of cancer: Time-related biases in observational studies. Diabetes Care 35: , 2665–2673. |
[19] | Amidei CB , Fayosse A , Dumurgier J , Machado-Fragua MD , Tabak AG , van Sloten T , Kivimäki M , Dugravot A , Sabia S , Singh-Manoux A ((2021) ) Association between age at diabetes onset and subsequent risk of dementia. JAMA 325: , 1640–1649. |
[20] | Rawlings AM , Sharrett AR , Albert MS , Coresh J , Windham BG , Power MC , Knopman DS , Walker K , Burgard S , Mosley TH ((2019) ) The association of late-life diabetes status and hyperglycemia with incident mild cognitive impairment and dementia: The ARIC study. Diabetes Care 42: , 1248–1254. |
[21] | Suissa S , Dell’Aniello S , Vahey S , Renoux C ((2011) ) Time-window bias in case-control studies: Statins and lung cancer. Epidemiology 22: , 228–231. |
[22] | Dreyer NA , Bryant A , Velentgas P ((2016) ) The GRACE checklist: A validated assessment tool for high quality observational studies of comparative effectiveness. J Manag Care Spec Pharm 22: , 1107–1113. |
[23] | Haynes RB , Sackett DL , Richardson WS , Rosenberg W , Langley GR ((1997) ) Evidence-based medicine: How to practice & teach EBM. CMAJ 157: , 788. |
[24] | Cheng C , Lin C-H , Tsai Y-W , Tsai C-J , Chou P-H , Lan T-H ((2014) ) Type 2 diabetes and antidiabetic medications in relation to dementia diagnosis. J Gerontol A Biol Sci Med Sci 69: , 1299–1305. |
[25] | Hsu C-C , Wahlqvist ML , Lee M-S , Tsai H-N ((2011) ) Incidence ofdementia is increased in type 2 diabetes and reduced by the use ofsulfonylureas and metformin. J Alzheimers Dis 24: , 485–493. |
[26] | Kim Y , Kim H-S , Lee J-w , Kim Y-S , You H-S , Bae Y-J , Lee H-c , Han Y-E , Choi E-A , Kim J ((2020) ) Metformin use in elderly population with diabetes reduced the risk of dementia in a dose-dependent manner, based on the Korean NHIS-HEALS cohort. Diabetes Res Clin Pract 170: , 108496. |
[27] | Kuan Y-C , Huang K-W , Lin C-L , Hu C-J , Kao C-H ((2017) ) Effects of metformin exposure on neurodegenerative diseases in elderly patients with type 2 diabetes mellitus. Prog Neuropsychopharmacol Biol Psychiatry 79: , 77–83. |
[28] | Orkaby AR , Cho K , Cormack J , Gagnon DR , Driver JA ((2017) ) Metformin vs sulfonylurea use and risk of dementia in US veterans aged≥65 years with diabetes. Neurology 89: , 1877–1885. |
[29] | Samaras K , Makkar S , Crawford JD , Kochan NA , Wen W , Draper B , Trollor JN , Brodaty H , Sachdev PS ((2020) ) Metformin use is associated with slowed cognitive decline and reduced incident dementia in older adults with type 2 diabetes: The Sydney Memory and Ageing Study. Diabetes care 43: , 2691–2701. |
[30] | Scherrer JF , Morley JE , Salas J , Floyd JS , Farr SA , Dublin S ((2019) ) Association between metformin initiation and incident dementia among African American and white veterans health administration patients. Ann Fam Med 17: , 352–362. |
[31] | Scherrer JF , Salas J , Floyd JS , Farr SA , Morley JE , Dublin S ((2019) ) Metformin and sulfonylurea use and risk of incident dementia. Mayo Clin Proc 94: , 1444–1456. |
[32] | Shi Q , Liu S , Fonseca VA , Thethi TK , Shi L ((2019) ) Effect of metformin on neurodegenerative disease among elderly adult US veterans with type 2 diabetes mellitus. BMJ Open 9: , e024954. |
[33] | Weinstein G , Davis-Plourde KL , Conner S , Himali JJ , Beiser AS , Lee A , Rawlings AM , Sedaghat S , Ding J , Moshier E ((2019) ) Association of metformin, sulfonylurea and insulin use with brain structure and function and risk of dementia and Alzheimer’s disease: Pooled analysis from 5 cohorts. PloS One 14: , e0212293. |
[34] | Heneka MT , Fink A , Doblhammer G ((2015) ) Effect of pioglitazone medication on the incidence of dementia. Ann Neurol 78: , 284–294. |
[35] | Huang C-C , Chung C-M , Leu H-B , Lin L-Y , Chiu C-C , Hsu C-Y , Chiang C-H , Huang P-H , Chen T-J , Lin S-J ((2014) ) Diabetes mellitus and the risk of Alzheimer’s disease: A nationwide population-based study. PloS One 9: , e87095. |
[36] | Bohlken J , Jacob L , Kostev K ((2018) ) Association between the use of antihyperglycemic drugs and dementia risk: A case-control study. J Alzheimers Dis 66: , 725–732. |
[37] | Imfeld P , Bodmer M , Jick SS , Meier CR ((2012) ) Metformin, other antidiabetic drugs, and risk of Alzheimer’s disease: A population-based case–control study. J Am Geriatr Soc 60: , 916–921. |
[38] | Sluggett JK , Koponen M , Bell JS , Taipale H , Tanskanen A , Tiihonen J , Uusitupa M , Tolppanen A-M , Hartikainen S ((2020) ) Metformin and risk of Alzheimer’s disease among community-dwelling people with diabetes: A national case-control study. J Clin Endocrinol Metab 105: , e963–e972. |
[39] | Wium-Andersen IK , Osler M , Jørgensen MB , Rungby J , Wium-Andersen MK ((2019) ) Antidiabetic medication and risk of dementia in patients with type 2 diabetes: A nested case–control study. Eur J Endocrinol 181: , 499–507. |
[40] | Moher D , Liberati A , Tetzlaff J , Altman DG , PRISMA Group ((2009) ) Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med 6: , e1000097. |
[41] | Salminen A , Kaarniranta K , Haapasalo A , Soininen H , Hiltunen M ((2011) ) AMP-activated protein kinase: A potential player in Alzheimer’s disease. J Neurochem 118: , 460–474. |
[42] | Bobela W , Nazeeruddin S , Knott G , Aebischer P , Schneider BL ((2017) ) Modulating the catalytic activity of AMPK has neuroprotective effects against α-synuclein toxicity. Mol Neurodegener 12: , 1–19. |
[43] | Luchsinger JA , Perez T , Chang H , Mehta P , Steffener J , Pradabhan G , Ichise M , Manly J , Devanand DP , Bagiella E ((2016) ) Metformin in amnestic mild cognitive impairment: Results of a pilot randomized placebo controlled clinical trial. J Alzheimers Dis 51: , 501–514. |
[44] | US National Library of Medicine, ClinicalTrial: NCT01965756, https://ClinicalTrials.gov/show/NCT01965756, September 21, 2017. |
[45] | Suissa S , Azoulay L ((2014) ) Metformin and cancer: Mounting evidence against an association. Diabetes Care 37: , 1786–1788. |
[46] | Suissa S ((2017) ) Metformin to treat cancer: Misstep in translational research from observational studies. Epidemiology 28: , 455–458. |
[47] | Azoulay L , Dell’Aniello S , Gagnon B , Pollak M , Suissa S ((2011) ) Metformin and the incidence of prostate cancer in patients with type 2 diabetes. Cancer Epidemiol Biomarkers Prev 20: , 337–344. |
[48] | Smiechowski BB , Azoulay L , Yin H , Pollak MN , Suissa S ((2013) ) The use of metformin and the incidence of lung cancer in patients with type 2 diabetes. Diabetes Care 36: , 124–129. |
[49] | Mamtani R , Pfanzelter N , Haynes K , Finkelman BS , Wang X , Keefe SM , Haas NB , Vaughn DJ , Lewis JD ((2014) ) Incidence of bladder cancer in patients with type 2 diabetes treated with metformin or sulfonylureas. Diabetes Care 37: , 1910–1917. |
[50] | Kordes S , Pollak MN , Zwinderman AH , Mathôt RA , Weterman MJ , Beeker A , Punt CJ , Richel DJ , Wilmink JW ((2015) ) Metformin in patients with advanced pancreatic cancer: A double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol 16: , 839–847. |
[51] | Pimentel I , Lohmann AE , Ennis M , Dowling RJ , Cescon D , Elser C , Potvin K , Haq R , Hamm C , Chang MC ((2019) ) A phase II randomized clinical trial of the effect of metformin versus placebo on progression-free survival in women with metastatic breast cancer receiving standard chemotherapy. Breast 48: , 17–23. |
[52] | Nguyen MM , Martinez JA , Hsu C-H , Sokoloff M , Krouse RS , Gibson BA , Nagle RB , Parnes HL , Cordova C , Chow HS ((2018) ) Bioactivity and prostate tissue distribution of metformin in a pre-prostatectomy prostate cancer cohort. Eur J Cancer Prev 27: , 557. |
[53] | Chak A , Buttar NS , Foster NR , Seisler DK , Marcon NE , Schoen R , Cruz-Correa MR , Falk GW , Sharma P , Hur C ((2015) ) Metformin does not reduce markers of cell proliferation in esophageal tissues of patients with Barrett’s esophagus.-. Clin Gastroenterol Hepatol 13: , 665–672. e664. |
[54] | Brown JR , Chan DK , Shank JJ , Griffith KA , Fan H , Szulawski R , Yang K , Reynolds RK , Johnston C , McLean K , Uppal S , Liu JR , Cabrera L , Taylor SE , Orr BC , Modugno F , Mehta P , Bregenzer M , Mehta G , Shen H , Coffman LG , Buckanovich RJ ((2020) ) Phase II clinical trial of metformin as a cancer stem cell-targeting agent in ovarian cancer. JCI Insight 5: , e133247. |
[55] | Dowling RJ , Niraula S , Chang MC , Done SJ , Ennis M , McCready DR , Leong WL , Escallon JM , Reedijk M , Goodwin PJ ((2015) ) Changes in insulin receptor signaling underlie neoadjuvant metformin administration in breast cancer: A prospective window of opportunity neoadjuvant study. Breast Cancer Res 17: , 1–12. |
[56] | Galsky MD , Shahin M , Jia R , Shaffer DR , Gimpel-Tetra K , Tsao C-K , Baker C , Leiter A , Holland J , Sablinski T ((2017) ) Telemedicine-enabled clinical trial of metformin in patients with prostate cancer. JCO Clin Cancer Inform 1: , 1–10. |
[57] | Lord SR , Cheng W-C , Liu D , Gaude E , Haider S , Metcalf T , Patel N , Teoh EJ , Gleeson F , Bradley K ((2018) ) Integrated pharmacodynamic analysis identifies two metabolic adaption pathways to metformin in breast cancer. Cell Metab 28: , 679–688. e674. |
[58] | Ramos-Peñafiel C , Olarte-Carrillo I , Cerón-Maldonado R , Rozen-Fuller E , Kassack-Ipiña JJ , Meléndez-Mier G , Collazo-Jaloma J , Martínez-Tovar A ((2018) ) Effect of metformin on the survival of patients with ALL who express high levels of the ABCB1 drug resistance gene. J Transl Med 16: , 1–9. |
[59] | Goodwin PJ , Chen BE , Gelmon KA , Whelan TJ , Ennis M , Lemieux J , Ligibel JA , Hershman DL , Mayer IA , Hobday TJ , Bliss JM , Rastogi P , Rabaglio-Poretti M , Mukherjee SD , Mackey JR , Abramson VG , Oja C , Wesolowski R , Thompson AM , Rea DW , Stos PM , Shepherd LE , Stambolic V , Parulekar WR ((2022) ) Effect of metformin vs placebo on invasive disease–free survival in patients with breast cancer: The MA.32 randomized clinical trial. JAMA 327: , 1963–1973. |
[60] | Martin-Montalvo A , Mercken EM , Mitchell SJ , Palacios HH , Mote PL , Scheibye-Knudsen M , Gomes AP , Ward TM , Minor RK , Blouin M-J ((2013) ) Metformin improves healthspan and lifespan in mice. Nat Commun 4: , 1–9. |
[61] | Fahy GM , Brooke RT , Watson JP , Good Z , Vasanawala SS , Maecker H , Leipold MD , Lin DT , Kobor MS , Horvath S ((2019) ) Reversal of epigenetic aging and immunosenescent trends in humans. Aging Cell 18: , e13028. |
[62] | The Targeting Aging with Metformin (TAME) Trial, https://www.afar.org/tame-trial. |




