The Relationship Between Hearing and Mild Behavioral Impairment and the Influence of Sex: A Study of Older Adults Without Dementia from the COMPASS-ND Study
Abstract
Background:
Hearing loss and mild behavioral impairment (MBI), both non-cognitive markers of dementia, can be early warning signs of incident cognitive decline.
Objective:
We investigated the relationship between these markers and reported the influence of sex, using non-dementia participants (n = 219; 107 females) from the Canadian Comprehensive Assessment of Neurodegeneration and Dementia (COMPASS-ND).
Methods:
Hearing was assessed with the 10-item Hearing Handicap for the Elderly–Screening (HHIE-S) questionnaire, a speech-in-noise test, screening audiometry, and hearing aid use. MBI symptoms were assessed using the Neuropsychiatric Inventory Questionnaire (NPI-Q). Multivariable linear regressions examined the association between hearing and MBI symptom severity and multiple logistic regressions examined the association between hearing and MBI domains.
Results:
HHIE-S score was significantly associated with greater global MBI symptom burden, and symptoms in the apathy and affective dysregulation domains. Objective measures of audiometric hearing loss and speech-in-noise testing as well as hearing aid use were not associated with global MBI symptom severity or the presence of MBI domain-specific symptoms. Males were older, had more audiometric and speech-in-noise hearing loss, higher rates of hearing-aid use, and showed more MBI symptoms than females, especially apathy.
Conclusion:
The HHIE-S, a subjective self-report measure that captures emotional and social aspects of hearing disability, was associated with informant-reported global MBI symptom burden, and more specifically the domains of affective dysregulation and apathy. These domains can be potential drivers of depression and social isolation. Hearing and behavior change can be assessed with non-invasive measures, adding value to a comprehensive dementia risk assessment.
INTRODUCTION
Over 50 million people worldwide live with dementia, and this number is expected to rise to 152 million by 2050 [1]. Dementia, most commonly Alzheimer’s disease (AD), results in a devastating personal cost to those affected and their carers, and a financial impact on the world economy exceeding 1 trillion USD each year [1]. Most current pharmacological approaches to target AD offer short term symptom modifying effects, but there has been growing interest in dementia prevention [2]. For preventative treatments to be more successful, they need to start earlier, with better markers to identify people at preclinical stages of disease. Hearing loss and mild behavioral impairment (MBI) have both been identified as early warning signs of risk of cognitive decline and dementia in older adults, and both have been recommended in clinical guidelines for use as non-cognitive markers of dementia [3, 4].
Hearing loss often begins in middle age with a doubling of incidence each decade and is the third most prevalent chronic condition among older adults [5]. The Lancet Commission identified hearing loss as accounting for 9% of the population attributable risk of dementia [6]. This was the first time that hearing loss, the largest of all potentially modifiable risk factors linked to dementia was featured in the Life Course model, which helped link hearing health with brain health. Despite the high prevalence of hearing loss, it is often untreated [7]. In addition to dementia risk, hearing loss is also associated with higher rates of hospitalization/rehospitalization with longer hospital stays, frailty, depression, loneliness, social isolation, and the onset of overt behavioral symptoms in long term care [8–17]. Hearing loss is more frequent in males than females, and the use of hearing aids also differs by sex [18–20]. These findings indicate a need to explore sex differences in hearing-associated risk.
MBI represents a validated neurobehavioral syndrome characterized by the de novo emergence in later life of persistent neuropsychiatric symptoms (NPS), representing a change from longstanding behaviors, as an at-risk state for all-cause dementia [21, 22]. MBI is associated with cognitive decline and incident dementia [23–26], and known dementia biomarkers including amyloid, tau, and neurodegeneration [27–31]. Notwithstanding the increased recognition of MBI, early behavioral markers are commonly dismissed as a part of normal aging, despite associations with greater caregiver burden, functional impairment, rates of institutionalization, and neuropathological burden [32]. Although not yet well studied, early data suggest that the prevalence of MBI may be higher in males versus females (14% versus 11% of a sample of cognitively normal older adults; p = 0.01) [33], necessitating sex-specific analyses of outcomes with MBI.
Few studies have examined the association between hearing and the symptoms of MBI [34], and none to our knowledge with a focus on sex differences. Our aim was to investigate the relationship between these two dementia risk markers, and to explore sex-related differences in this relationship.
MATERIALS AND METHODS
Overview
The Comprehensive Assessment of Neurodegeneration and Dementia (COMPASS-ND) [35] is a Canada-wide multi-site prospective longitudinal cohort study designed to investigate different causes of neurodegeneration and their interactions (ClinicalTrials.gov NCT03402919). In summary, the study is recruiting 2,310 adults who live with or are at risk of developing neurodegenerative diseases. Participants include persons with dementia as well as mild cognitive impairment (MCI), subjective cognitive decline (SCD), and cognitively normal (CN) controls. Participants with clinical diagnoses were primarily recruited from specialty clinics, although healthy controls and those with subjective cognitive complaints were also enrolled through non-clinical referral sources [36]. Clinical and cognitive data, sociodemographic details, biomarkers, structural brain imaging, and measures of sensory function are collected. The study is conducted with local ethical approval by institutional review boards at each of the 13 sites affiliated with the study.
Sample
This report analyzed the second data release of the COMPASS-ND study from February 2020 (n = 409). All participants without dementia and with available hearing, demographic, Neuropsychiatric Inventory Questionnaire (NPI-Q), and Montreal Cognitive Assessment (MoCA) data, were included for a total of 219 participants (49% female).
Hearing variables
Hearing was measured with the 10-item Hearing Handicap Inventory for the Elderly–Screening Version (HHIE-S) [37–39] self-report measure, a speech-in-noise test (Canadian Digit Triplet Test; CDTT) [40], screening audiometry and hearing aid use. The HHIE-S measures the social and emotional effects of hearing disability. Scores range from 0 (no hearing handicap) to 40 (significant hearing handicap). As the HHIE-S is a self-report measure, it could be viewed as a subjective measure of hearing. Using an adaptive procedure, the CDTT is designed to find the speech recognition threshold in noise that corresponds to the signal-to-noise ratio at which combinations of 3 digits are identified correctly 50% of the time. Lower signal-to-noise ratios indicate better speech in noise hearing ability. Screening audiometry used Pass/Fail scoring for 2000-Hz pure-tones presented at 2 discrete input levels to yield six hearing loss categories: Category 1 – Normal Hearing: 25 dB HL detected in both ears; Category 2 – Mild 1 : 25 dB HL detected in one ear, 40 dB HL detected in other ear; Category 3 – Mild 2 : 40 dB HL detected in both ears; Category 4 – Moderate 1 : 25 dB HL detected in one ear, 40 dB HL not detected in other ear; Category 5 – Moderate 2 : 40 dB HL detected in one ear, 40 dB HL not detected in other ear; Category 6 – Moderate 3 : 40 dB HL not detected in either ear. Hearing aid use was assessed with a Yes/No question: “Do you use hearing aids?”.
Behavior variables
The severity of global and domain-specific MBI was approximated using the NPI-Q with a published algorithm [41]. Informant-reported total MBI symptom severity, as well as domain scores to capture the presence or absence of NPS symptoms, were tabulated for the five MBI domains of apathy, affective dysregulation, impulse dyscontrol, social inappropriateness, and psychosis. Participants were considered positive in a domain if they scored > 0.
Analyses
To describe the participant characteristics, continuous variables were reported in mean (SD); range and categorical variables were reported in n (%). Comparisons between sexes were conducted with independent-samples t-tests for continuous variables and chi-square or Fisher’s exact tests, as appropriate, for categorical variables. Cross-tabulation analyses were used to investigate if the distribution of NPS in any and each domain of MBI differed for people with no significant versus any degree of self-reported hearing disability. Multivariable linear regressions were conducted to examine the association between the hearing measures and global MBI burden, adjusting for age, education, MoCA score, and a sex by hearing variable interaction term. The a priori statistical plan was to remove the interaction term from the model and include sex as a covariate if the interaction was not statistically significant. Conversely, if the interaction term was statistically significant, sex-stratified regression models would be fitted. Using a similar methodology, multivariable logistic regressions were used to investigate whether the hearing variables were associated with the presence/absence of MBI domains. Recognizing that the distribution of global MBI symptom severity is often positively skewed, we applied a log transformation to the data. We acknowledge that log transformations do not introduce symmetry into the distribution of global MBI symptom severity nor do they perfectly address issues with the data that might lead to violations of assumptions about the normal distribution of residuals. However, we utilized the log transformation to address the multiplicative, as opposed to additive, nature of MBI symptom severity measured using a transformed version of the NPI-Q. Because of the limited range of each individual NPI-Q item (0–3), we have reason to believe that the true difference in MBI symptom severity between MBI scores of 5–10, for instance, are greater than the difference between MBI scores of 0–5. As such, the purpose of the log transformation is to represent the nature of this association in the linear regression model from a standpoint of scientific validity. Furthermore, the log transformation provides modest but not crucial improvements to the normality of residuals.
RESULTS
Sample characteristics stratified by sex
The demographic characteristics of the participants are shown in Table 1. Males were older and had lower scores on the MoCA, greater degrees of audiometric hearing loss, poorer speech-in-noise performance, higher rates of hearing aid use, and showed more MBI symptoms than females – especially apathy. The distribution of sexes across the cognitive subtypes also differed: 91% of males were classed as MCI with the remainder as SCD; and for women, 55% had MCI, 35% SCD and the remainder were CN.
Table 1
Participant Demographic Characteristics Stratified by Sex
| Variable | All | Females | Males | p |
| n | 219 | 107 | 112 | |
| Age | 72.2 (6.5); 50.2–87.1 | 71.2 (6.4); 50.2–84.2 | 73.2 (6.6); 60.6–87.1 | 0.027 |
| Education | 15.8 (3.6); 6.0–31.0 | 15.8 (3.6); 11.0–28.0 | 15.7 (3.9); 6.0–31.0 | 0.810 |
| MoCA Score | 24.5 (3.6); 13.0–31.0 | 25.4 (3.1); 17.0–30.0 | 23.5 (3.8); 13.0–31.0 | <0.001 |
| Cognitive Subtypes | <0.001 | |||
| CN | 10 (4.6%) | 10 (9.3%) | 0 (0.0%) | |
| SCD | 48 (21.9%) | 38 (35.5%) | 10 (8.9%) | |
| MCI | 161 (73.5%) | 59 (55.1%) | 102 (91.1%) | |
| Hearing Variables | ||||
| HA Use | 38 (17.4%) | 10 (9.3%) | 28 (25.0%) | 0.002 |
| HHIE-S | ||||
| HHIE-S Total Score | 8.1 (8.7); 0–34.0 | 8.1 (8.7); 0–34.0 | 8.7 (8.8); 0–30.0 | 0.317 |
| HHIE-S Categories | 0.509 | |||
| No Handicap | 143 (65.3%) | 73 (68.2%) | 70 (62.5%) | |
| Mild/mod Handicap | 62 (28.3%) | 29 (27.1%) | 33 (29.5%) | |
| Significant Handicap | 14 (6.4%) | 5 (4.7%) | 9 (8.0%) | |
| Audiometric Hearing Loss Categories | ||||
| 1 | 128 (58.7%) | 70 (66.0%) | 58 (51.8%) | |
| 2 | 28 (12.8%) | 15 (14.2%) | 13 (11.6%) | |
| 3 | 15 (6.9%) | 8 (7.5%) | 7 (6.2%) | |
| 4 | 3 (1.4%) | 0 (0.0%) | 3 (2.7%) | |
| 5 | 26 (11.9%) | 7 (6.6%) | 19 (17.0%) | |
| 6 | 18 (8.3%) | 6 (5.7%) | 12 (10.7%) | |
| CDTT | ||||
| CDTT Threshold (dB SNR) | –9.2 (2.9); –12.6–6.2 | –9.6 (2.8); –12.6–6.2 | –8.7 (2.9); –12.2–4.6 | 0.015 |
| Behavior Variables | ||||
| MBI Severity | ||||
| Total MBI | 1.8 (2.7) | 1.4 (2.2) | 2.2 (3.1) | 0.022 |
| MBI Domains–Presence | ||||
| Any Domain | 113 (51.6%) | 49 (45.8%) | 64 (57.1%) | 0.093 |
| Apathy | 35 (16.0%) | 10 (9.3%) | 25 (22.3%) | 0.009 |
| Affect | 77 (35.2%) | 34 (31.8%) | 43 (38.4%) | 0.305 |
| Impulse Dyscontrol | 72 (32.9%) | 31 (29.0%) | 41 (36.6%) | 0.229 |
| Social Inappropriateness | 28 (12.8%) | 13 (12.1%) | 15 (13.4%) | 0.783 |
| Psychosis | 15 (6.8%) | 4 (3.7%) | 11 (9.8%) | 0.075 |
Continuous variables reported in mean (SD); range. Categorical variables reported in n (%). Comparison tests were conducted between sexes using independent-samples t-tests for continuous variables, and chi-square tests or fisher’s exact tests, as appropriate, for categorical variables. MoCA, Montreal Cognitive Assessment; CN, cognitively normal; SCD, subjective cognitive decline; MCI, mild cognitive impairment; HA, hearing aid; HHIE-S, Hearing Handicap Inventory for the Elderly–Screening Version; CDTT, Canadian Digit Triplet Test; dB, decibel; SNR, Signal-to-Noise Ratio; MBI, mild behavioral impairment.
With the sexes combined, 65% self-reported no degree of hearing disability. However, fewer passed audiometric screening, with 59% passing in both ears and 13% passing in only one ear. Hearing aids were used by 17% of participants. MBI symptoms were identified in any domain by 52% of all participants.
Regression results
With all regression analyses, none of the sex by hearing variable interaction terms were significant meaning that even though individually audiometric hearing loss, the CDDT, and hearing aid use differed by sex, the association of any of the hearing variables with MBI did not differ by sex. With sex as a covariate, self-reported hearing disability as measured by the HHIE-S was significantly associated with greater global MBI symptom burden, and the presence of apathy and affective dysregulation, when controlling for global cognition (Table 2). However, when controlling for sex, speech-in-noise thresholds, audiometric hearing loss, and hearing aid use were not associated with global MBI symptom severity or the presence of MBI domain-specific symptoms (see Supplementary Table 1 for added details).
Table 2
Multivariable Regression Models for the Association between HHIE-S Score and MBI Burden in Non-Dementia
| Outcome Variable | Exp(β) or OR (95% CI) | p |
| Global MBI Burdena | 1.02 (1.00–1.04) | 0.002 |
| MBI Apathyb | 1.09 (1.03–1.14) | 0.002 |
| MBI Affectb | 1.08 (1.04–1.13) | <0.001 |
Models were adjusted for age, sex, years of education, hearing aids use, and MoCA score (n = 219). HHIE-S, Hearing Handicap Inventory for the Elderly–Screening Version; MBI, mild behavioral impairment; MoCA, Montreal Cognitive Assessment. a Multivariable linear regression with standardized continuous log-transformed global MBI score as the outcome variable. b Multivariable logistic regression with dichotomized MBI domain (presence/absence of symptoms) as the outcome variable.
Relationship between self-reported hearing disability and MBI
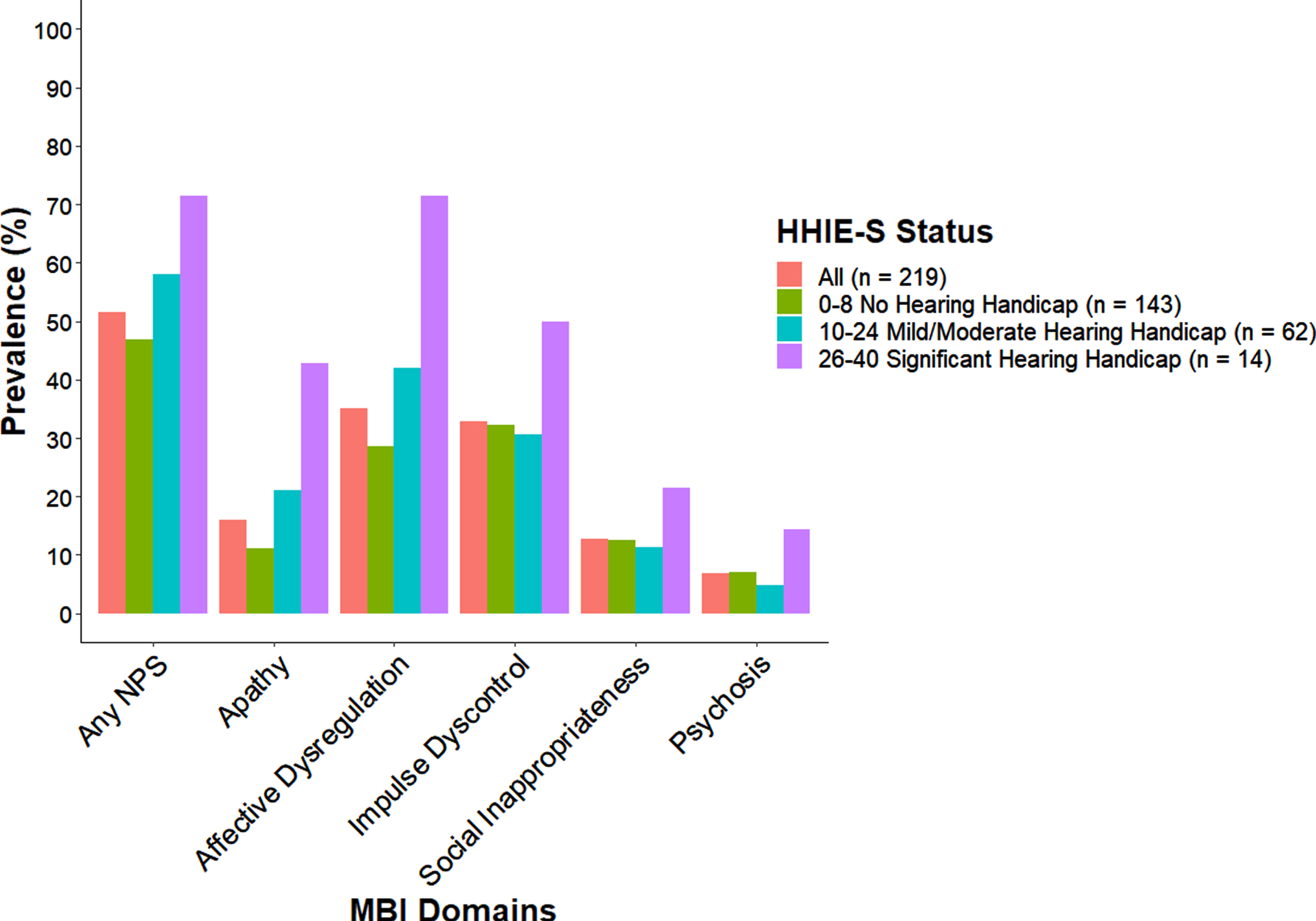
Graphing the relationship between self-reported hearing disability and MBI (see Fig. 1), people with greater self-reported hearing disability as measured by the HHIE-S were more likely to have any MBI symptoms compared to people reporting no hearing disability. While the cross-tabulation results were not significant (χ 2 = 4.53, p = 0.10), the prevalence of MBI symptoms was highest in each of the MBI domains for the group reporting significant hearing disabilities and this group consisted of more males than females) (see Supplementary Table 2 for added details about Fig. 1). Of the 14 people with significant hearing disability, 9 were male of which 7 were aided and all had audiometric hearing loss; 5 were female of which only 2 were aided and 3 had audiometric hearing loss.
Fig. 1
Distribution of MBI Prevalence by HHIE-S Status for Non-Dementia. Percentage of neuropsychiatric symptoms present on any domain and by each domain of the Mild Behavioral Impairment-Checklist for all participants (n = 219) and by HHIE-S categories: no hearing handicap, mild/moderate hearing handicap and significant hearing handicap.
DISCUSSION
Scores on the HHIE-S, a subjective self-report measure which was designed to capture the emotional and social aspects of hearing loss, were positively related to informant-reported global MBI symptom burden. This signal was driven by affective dysregulation and apathy, which might be considered as drivers of depression and social isolation seen in those with hearing loss.
In a previous study of older adults, apathy and impulse dyscontrol measured with the Mild Behavioral Impairment Checklist (MBI-C) [42], were linked to audiometric hearing loss [34]. In our study, however, sources of information for the significant relationship differed. The MBI-hearing impairment association was based on informant reported neurobehavioral symptoms, and subjective self-report of hearing disability. Our objective measure of audiometric hearing loss did not associate with MBI, and this may in part be related to the methodological differences in its measurement. The COMPASS ND study measured 2 kHz only with Pass/Fail criteria at two discrete input levels (25 & 40 dB HL) rather than the more typical measure of audiometric hearing sensitivity which calculates a pure-tone average using multiple frequencies. While anosognosia may contribute to variability in findings, this lack of awareness would only apply to the HHIE-S. In general, older adults do have a tendency to underestimate their degree of impairment as reflected by Bainbridge who showed that older adults with measurable audiometric hearing loss are less likely to self-report “at least a little trouble hearing” based on the 2007 National Health Interview Survey [43]. The self-reported HHIE-S is unique in that it captures both the impact of sensory impairment along with perceived hearing difficulty and adjustment to the social and emotional impact of hearing impairment [37, 39].
Whether the mechanism behind the association of hearing and MBI involves a common etiology versus a causal relationship is unknown. A common etiology would suggest that the same underlying neurodegenerative process could account for both hearing loss and MBI; both are prevalent in preclinical and prodromal dementia and associated with more rapid progression to AD [5, 23]. Specifically, MBI is associated with both amyloid-β and tau in cognitively normal older adults [27, 28]. Similarly, in non-dementia ADNI participants, age-related hearing loss is associated with higher levels of tau and p-tau cross-sectionally in preclinical and prodromal AD, and faster rate of increase of these two types of biomarkers in healthy controls and preclinical AD participants [44]. With the ADNI study search criteria of clinical assessments were used to identify participants with reported hearing loss. In a community sample of older adults, both pure-tone audiometry and word recognition were associated with whole brain amyloid-β [45]. Thus, both MBI and hearing impairment can occur in advance of cognitive changes and are associated with AD biomarkers. The relationship between them is uncertain, but it is also possible that both are sequelae of a shared etiology, whether it be AD proteinopathies or vascular disease [46, 47] or other unknown etiologies. Additional research is required.
Intuitively, causal relationships between hearing and behavior can also be envisioned. Individuals with neurodegenerative disease may have less cognitive and/or brain reserve to cope when listening is challenging, requiring increased attentional effort because of hearing loss and/or environmental noise. These hearing difficulties may result in less motivation and interest in participating in activities, withdrawal and social isolation, or apathy, with potential effects on mood and affect that may include depression. While we did not find a relationship between hearing aid use and MBI symptoms, hearing aids have shown promise as an intervention option to improve communication and reduce behavioral symptoms like depression [48]. While more research is needed, promising early findings have shown that hearing rehabilitation might reduce the risk of progression from MCI to dementia [49].
Our findings demonstrate a link between the HHIE and symptoms of MBI as well as individual MBI domains. We also found a link to sex such that more males than females were apathetic, in agreement with the literature [50]. Early studies exploring the neuropathological basis of apathy found that a combination of greater apathy, communication failure (i.e., with hearing loss), and physical disability correlated with greater neurofibrillary tangle burden in the parahippocampal, frontal, and parietal regions. This is noteworthy as recent mechanisms linking hearing loss with dementia posit an interaction between the distributed function of auditory pattern analysis in the medial temporal lobe during difficult listening situations with AD pathology [51, 52].
No association was found between MBI symptoms and performance on the speech-in-noise test, hearing aid use, or audiometric hearing loss. Even though each of these variables individually showed sex differences as shown in Table 1, regression analyses found no significant interaction between any of the hearing variables with sex. As a result, regression models were adjusted for sex. Studies have found that speech-in-noise tests, which rely on attention and memory to repeat back what is heard, have been linked with working memory and cognitive function [34]. Low uptake of hearing aids for those with hearing loss, combined with a screening audiometry approach that identified 71.5% with normal hearing in at least the better ear, may explain the lack of associations between the hearing measures and MBI symptoms. Within the group reporting significant hearing disability, 78% of males and 40% of females had hearing aids. Hearing aid use is generally higher for females [53] even though age-related hearing loss is more common, more severe, and occurs at younger ages in males [19, 54]. Had our sample included varying severities of hearing loss and more hearing aid users, we would expect greater MBI symptom burden with increasing severity of hearing loss, and lower MBI symptom burden for those who were aided, consistent with recent findings [55–57].
This study represents preliminary findings from the COMPASS-ND study. The results are limited by small sample sizes of each cognitive subtype for which the sexes are not equally distributed, and the sensitivity of the audiometry and neuropsychiatric symptom measures. With screening audiometry limited to only 2000 Hz, additional test frequencies would be needed to identify hearing loss and possible sex-related differences in the audiogram; high-frequency hearing sensitivity is better in older females and low-frequency hearing sensitivity is better in older males [19, 54]. With behavior, MBI was approximated from the NPI-Q which uses a 1-month reference period compared to the 6-month reference period of the MBI-C. This shorter reference range may result in lower specificity and signal-to-noise ratio, with transient symptomatology included in the measure of MBI. Future studies may benefit from more specific MBI case ascertainment using the MBI-C, which was developed explicitly as the case ascertainment instrument for MBI [42, 58].
Conclusions and implications
A subjective measure of self-reported hearing disability, the HHIE-S is associated with MBI symptomatology, and MBI symptoms are associated with sex. Both hearing problems and the neuropsychiatric symptoms associated with MBI are common, highlighting an opportunity for risk mitigation. In this study sample of non-demented community dwelling older adults, 46% of females and 57% of males reported at least one MBI symptom. The prevalence of hearing loss and MBI is higher in other settings, e.g., 80% in a geriatric rehabilitation acute care setting, and even higher in long term care [34, 59]. Hearing loss is often described as an invisible disability; without measurement, it can be difficult to tease apart hearing problems compared to behavior problems. In addition, hearing loss can masquerade as cognitive impairment, and cognitive test scores improve when hearing assistance is provided [59]. These issues underscore the need for the assessment of both hearing and behavior as part of dementia risk assessment, consistent with clinical guidelines [4]. The HHIE-S is freely available and easily administered, as is the MBI-C. With assessment comes the possibility of identification and intervention. While hearing rehabilitation has been shown to improve communication and reduce behavioral symptoms like depression and other neuropsychiatric symptoms, more research is required to determine if hearing rehabilitation can reduce MBI severity. Likewise, it is also possible that treating MBI could have benefits for people with hearing loss.
ACKNOWLEDGMENTS
The authors have no acknowledgments to report.
FUNDING
The Canadian Consortium on Neurodegeneration in Aging is supported by a grant from the Canadian Institutes of Health Research with funding from several partners.
CONFLICT OF INTEREST
Zahinoor Ismail has received consultation/advisory board funding from Otsuka and Lundbeck. His institution has received funds from Acadia, Biogen, and Roche. All remaining authors have no conflicts to report.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/ADR-210045.
REFERENCES
[1] | Prince MJ ((2015) ) World Alzheimer Report 2015: the global impact of dementia: an analysis of prevalence, incidence, cost and trends, Alzheimer’s Disease International, London. |
[2] | Montero-Odasso M , Ismail Z , Livingston G ((2020) ) One third of dementia cases can be prevented within the next 25 years by tackling risk factors. The case “for” and “against”. Alzheimers Res Ther 12: , 1–5. |
[3] | Montero-Odasso M , Pieruccini-Faria F , Ismail Z , Li K , Lim A , Phillips N , Kamkar N , Sarquis-Adamson Y , Speechley M , Theou O , Verghese J , Wallace L , Camicioli R ((2020) ) CCCDTD5 recommendations on early non cognitive markers of dementia: A Canadian consensus. Alzheimers Dement (N Y) 6: , e12068. |
[4] | Ismail Z , Black SE , Camicioli R , Chertkow H , Herrmann N , Laforce R Jr. , Montero-Odasso M , Rockwood K , Rosa-Neto P , Seitz D , Sivananthan S , Smith EE , Soucy JP , Vedel I , Gauthier S , CCCDTD5 participants ((2020) ) Recommendations of the 5th Canadian Consensus Conference on the diagnosis and treatment of dementia. Alzheimers Dement 16: , 1182–1195. |
[5] | Lin FR , Metter EJ , O’Brien RJ , Resnick SM , Zonderman AB , Ferrucci L ((2011) ) Hearing loss and incident dementia. Arch Neurol 68: , 214–220. |
[6] | Livingston G , Sommerlad A , Orgeta V , Costafreda SG , Huntley J , Ames D , Ballard C , Banerjee S , Burns A , Cohen-Mansfield J ((2017) ) Dementia prevention, intervention, and care. Lancet 390: , 2673–2734. |
[7] | Chadha S , Kamenov K , Cieza A ((2021) ) The world report on hearing, 2021. Bull World Health Organ 99: , 242. |
[8] | Reed NS , Altan A , Deal JA , Yeh C , Kravetz AD , Wallhagen M , Lin FR ((2019) ) Trends in health care costs and utilization associated with untreated hearing loss over 10 years. JAMA Otolaryngol Head Neck Surg 145: , 27–34. |
[9] | Sharma RK , Chern A , Golub JS ((2021) ) Age-related hearing loss and the development of cognitive impairment and late-life depression: a scoping overview. Seminars in Hearing, Thieme Medical Publishers, Inc., pp. 010–025. |
[10] | Yamada Y , Vlachova M , Richter T , Finne-Soveri H , Gindin J , van der Roest H , Denkinger MD , Bernabei R , Onder G , Topinkova E ((2014) ) Prevalence and correlates of hearing and visual impairments in European nursing homes: results from the SHELTER study. J Am Med Dir Assoc 15: , 738–743. |
[11] | Buttery AK , Busch MA , Gaertner B , Scheidt-Nave C , Fuchs J ((2015) ) Prevalence and correlates of frailty among older adults: findings from the German health interview and examination survey. BMC Geriatr 15: , 1–9. |
[12] | Doba N , Tokuda Y , Goldstein NE , Kushiro T , Hinohara S ((2012) ) A pilot trial to predict frailty syndrome: the Japanese Health Research Volunteer Study. Exp Gerontol 47: , 638–643. |
[13] | Kamil RJ , Betz J , Powers BB , Pratt S , Kritchevsky S , Ayonayon HN , Harris TB , Helzner E , Deal JA , Martin K ((2016) ) Association of hearing impairment with incident frailty and falls in older adults. J Aging Health 28: , 644–660. |
[14] | Kamil RJ , Li L , Lin FR ((2014) ) Association between hearing impairment and frailty in older adults. J Am Geriatr Soc 62: , 1186–1188. |
[15] | Lorenzo-López L , López-López R , Maseda A , Buján A , Rodríguez-Villamil JL , Millán-Calenti JC ((2019) ) Changes in frailty status in a community-dwelling cohort of older adults: The VERISAÚDE study. Maturitas 119: , 54–60. |
[16] | Naharci MI , Engstrom G , Tappen R , Ouslander JG ((2016) ) Frailty in four ethnic groups in South Florida. J Am Geriatr Soc 64: , 656–657. |
[17] | Ng TP , Feng L , Nyunt MSZ , Larbi A , Yap KB ((2014) ) Frailty in older persons: multisystem risk factors and the Frailty Risk Index (FRI). J Am Med Dir Assoc 15: , 635–642. |
[18] | Mick PT , Hämäläinen A , Kolisang L , Pichora-Fuller MK , Phillips N , Guthrie D , Wittich W ((2021) ) The prevalence of hearing, vision, and dual sensory loss in older Canadians: an analysis of data from the Canadian longitudinal study on aging. Can J Aging 40: , 1–22. |
[19] | Nolan LS ((2020) ) Age-related hearing loss: Why we need to think about sex as a biological variable. J Neurosci Res 98: , 1705–1720. |
[20] | Staehelin K , Bertoli S , Probst R , Schindler C , Dratva J , Stutz EZ ((2011) ) Gender and hearing aids: Patterns of use and determinants of nonregular use. Ear Hear 32: , e26–e37. |
[21] | Ismail Z , Smith EE , Geda Y , Sultzer D , Brodaty H , Smith G , Agüera-Ortiz L , Sweet R , Miller D , Lyketsos CG ((2016) ) Neuropsychiatric symptoms as early manifestations of emergent dementia: provisional diagnostic criteria for mild behavioral impairment. Alzheimers Dement 12: , 195–202. |
[22] | Creese B , Ismail Z ((2022) ) Mild behavioral impairment: measurement and clinical correlates of a novel marker of preclinical Alzheimer’s disease. Alzheimers Res Ther 14: , 1–5. |
[23] | Ismail Z , McGirr A , Gill S , Hu S , Forkert ND , Smith EE ((2021) ) Mild behavioral impairment and subjective cognitive decline predict cognitive and functional decline. J Alzheimers Dis 80: , 459–469. |
[24] | Gill S , Mouches P , Hu S , Rajashekar D , MacMaster FP , Smith EE , Forkert ND , Ismail Z , Initiative AsDN ((2020) ) Using machine learning to predict dementia from neuropsychiatric symptom and neuroimaging data. J Alzheimers Dis 75: , 277–288. |
[25] | Matsuoka T , Ismail Z , Narumoto J ((2019) ) Prevalence of mild behavioral impairment and risk of dementia in a psychiatric outpatient clinic. J Alzheimers Dis 70: , 505–513. |
[26] | Creese B , Brooker H , Ismail Z , Wesnes KA , Hampshire A , Khan Z , Megalogeni M , Corbett A , Aarsland D , Ballard C ((2019) ) Mild behavioral impairment as a marker of cognitive decline in cognitively normal older adults. Am J Geriatr Psychiatry 27: , 823–834. |
[27] | Johansson M , Stomrud E , Insel PS , Leuzy A , Johansson PM , Smith R , Ismail Z , Janelidze S , Palmqvist S , van Westen D , Mattsson-Carlgren N , Hansson O ((2021) ) Mild behavioral impairment and its relation to tau pathology in preclinical Alzheimer’s disease. Transl Psychiatry 11: , 76. |
[28] | Lussier FZ , Pascoal TA , Chamoun M , Therriault J , Tissot C , Savard M , Kang MS , Mathotaarachchi S , Benedet AL , Parsons M , Qureshi MNI , Thomas EM , Shin M , Dion LA , Massarweh G , Soucy JP , Tsai IH , Vitali P , Ismail Z , Rosa-Neto P , Gauthier S ((2020) ) Mild behavioral impairment is associated with beta-amyloid but not tau or neurodegeneration in cognitively intact elderly individuals. Alzheimers Dement 16: , 192–199. |
[29] | Miao R , Chen HY , Gill S , Naude J , Smith EE , Ismail Z ; Alzheimer’s Disease Neuroimaging Initiative ((2021) ) Plasma β-amyloid in mild behavioural impairment-neuropsychiatric symptoms on the Alzheimer’s continuum. J Geriatr Psychiatry Neurol, doi: 10.1177/08919887211016068. |
[30] | Naude J , Gill S , Hu S , McGirr A , Forkert N , Monchi O , Stys P , Smith EE , Ismail Z ((2020) ) Plasma neurofilament light: a marker of cognitive decline in mild behavioural impairment. J Alzheimers Dis 76: , 1017–1027. |
[31] | Gill S , Wang M , Mouches P , Rajashekar D , Sajobi T , MacMaster FP , Smith EE , Forkert ND , Ismail Z , Alzheimer’s Disease Neuroimaging Initiative ((2021) ) Neural correlates of the impulse dyscontrol domain of mild behavioral impairment. Int J Geriatr Psychiatry 36: , 1398–1406. |
[32] | Lanctôt KL , Amatniek J , Ancoli-Israel S , Arnold SE , Ballard C , Cohen-Mansfield J , Ismail Z , Lyketsos C , Miller DS , Musiek E , Osorio RS , Rosenberg PB , Satlin A , Steffens D , Tariot P , Bain LJ , Carrillo MC , Hendrix JA , Jurgens H , Boot B ((2017) ) Neuropsychiatric signs and symptoms of Alzheimer’s disease: New treatment paradigms. Alzheimers Dement (N Y) 3: , 440–449. |
[33] | Wolfova K , Creese B , Aarsland D , Ismail Z , Corbett A , Ballard C , Hampshire A , Cermakova P ((2021) ) Sex differences in the association of mild behavioral impairment with cognitive aging. medRxiv, doi: 10.1101/2021.05.20.21257514. |
[34] | Gosselin PA , Ismail Z , Faris PD , Benkoczi CL , Fraser TL , Cherry SW , Faulkner TI , Islam MS ((2019) ) Effect of hearing ability and mild behavioural impairment on MoCA and memory index scores. Can Geriatr J 22: , 165–170. |
[35] | Chertkow H , Borrie M , Whitehead V , Black SE , Feldman HH , Gauthier S , Hogan DB , Masellis M , McGilton K , Rockwood K ((2019) ) The comprehensive assessment of neurodegeneration and dementia: Canadian cohort study. Can J Neurol Sci 46: , 499–511. |
[36] | Smith EE , Duchesne S , Gao F , Saad F , Whitehead V , McCreary CR , Frayne R , Gauthier S , Camicioli R , Borrie M ((2021) ) Vascular contributions to neurodegeneration: protocol of the COMPASS-ND Study. Can J Neurol Sci, doi: 10.1017/cjn.2021.19. |
[37] | Ventry IM , Weinstein BE ((1982) ) The hearing handicap inventory for the elderly: a new tool. Ear Hear 3: , 128–134. |
[38] | Demers K ((2013) ) Hearing Screening in Older Adults. The Hartford Institute for Geriatric Nursing. College of Nursing, New York University. |
[39] | Humes LE ((2021) ) An approach to self-assessed auditory wellness in older adults. Ear Hear 42: , 745. |
[40] | Giguère C , Lagacé J , Ellaham NN , Pichora-Fuller MK , Goy H , Bégin C , Alary É , Bowman R ((2020) ) Development of the Canadian Digit Triplet Test in English and French. J Acoust Soc Am 147: , EL252–EL258. |
[41] | Sheikh F , Ismail Z , Mortby ME , Barber P , Cieslak A , Fischer K , Granger R , Hogan DB , Mackie A , Maxwell CJ , Menon B , Mueller P , Patry D , Pearson D , Quickfall J , Sajobi T , Tse E , Wang M , Smith EE , investigators Pr ((2018) ) Prevalence of mild behavioral impairment in mild cognitive impairment and subjective cognitive decline, and its association with caregiver burden. Int Psychogeriatr 30: , 233–244. |
[42] | Ismail Z , Aguera-Ortiz L , Brodaty H , Cieslak A , Cummings J , Fischer CE , Gauthier S , Geda YE , Herrmann N , Kanji J , Lanctot KL , Miller DS , Mortby ME , Onyike CU , Rosenberg PB , Smith EE , Smith GS , Sultzer DL , Lyketsos C , NPS Professional Interest Area of the International Society of to Advance Alzheimer’s Research and Treatment (NPS-PIA of ISTAART) ((2017) ) The Mild Behavioral Impairment Checklist (MBI-C): A rating scale for neuropsychiatric symptoms in pre-dementia populations. J Alzheimers Dis 56: , 929–938. |
[43] | Bainbridge KE , Wallhagen MI ((2014) ) Hearing loss in an aging American population: extent, impact, and management. Annu Rev Public Health 35: , 139–152. |
[44] | Xu W , Zhang C , Li J-Q , Tan C-C , Cao X-P , Tan L , Yu J-T , Alzheimer’s Disease Neuroimaging Initiative ((2019) ) Age-related hearing loss accelerates cerebrospinal fluid tau levels and brain atrophy: a longitudinal study. Aging (Albany NY) 11: , 3156. |
[45] | Golub JS , Sharma RK , Rippon BQ , Brickman AM , Luchsinger JA ((2021) ) The association between early age-related hearing loss and brain β-amyloid. Laryngoscope 131: , 633–638. |
[46] | Miao R , Chen H-Y , Robert P , Smith EE , Ismail Z , Group MS ((2021) ) White matter hyperintensities and mild behavioral impairment: Findings from the MEMENTO cohort study. Cereb Circ Cogn Behav 2: , 100028. |
[47] | Eckert MA , Kuchinsky SE , Vaden KI , Cute SL , Spampinato MV , Dubno JR ((2013) ) White matter hyperintensities predict low frequency hearing in older adults. J Assoc Res Otolaryngol 14: , 425–433. |
[48] | Cosh S , Helmer C , Delcourt C , Robins TG , Tully PJ ((2019) ) Depression in elderly patients with hearing loss: current perspectives. Clin Interv Aging 14: , 1471. |
[49] | Bucholc M , McClean PL , Bauermeister S , Todd S , Ding X , Ye Q , Wang D , Huang W , Maguire LP ((2021) ) Association of the use of hearing aids with the conversion from mild cognitive impairment to dementia and progression of dementia: A longitudinal retrospective study. Alzheimers Dement (N Y) 7: , e12122. |
[50] | Lanctôt KL , Agüera-Ortiz L , Brodaty H , Francis PT , Geda YE , Ismail Z , Marshall GA , Mortby ME , Onyike CU , Padala PR ((2017) ) Apathy associated with neurocognitive disorders: recent progress and future directions. Alzheimers Dement 13: , 84–100. |
[51] | Griffiths TD , Lad M , Kumar S , Holmes E , McMurray B , Maguire EA , Billig AJ , Sedley W ((2020) ) How can hearing loss cause dementia?. Neuron 108: , 401–412. |
[52] | Eckert MA , Vaden KI Jr , Dubno JR ((2019) ) Age-related hearing loss associations with changes in brain morphology. Trends Hear 23: , 2331216519857267. |
[53] | Sarant J , Harris D , Busby P , Maruff P , Schembri A , Lemke U , Launer S ((2020) ) The effect of hearing aid use on cognition in older adults: can we delay decline or even improve cognitive function?. J Clin Med 9: , 254. |
[54] | Dubno JR , Eckert MA , Lee F-S , Matthews LJ , Schmiedt RA ((2013) ) Classifying human audiometric phenotypes of age-related hearing loss from animal models. J Assoc Res Otolaryngol 14: , 687–701. |
[55] | Kim AS , Morales EEG , Amjad H , Cotter VT , Lin FR , Lyketsos CG , Nowrangi MA , Mamo SK , Reed NS , Yasar S ((2021) ) Association of hearing loss with neuropsychiatric symptoms in older adults with cognitive impairment. Am J Geriatr Psychiatry 29: , 544–553. |
[56] | Brewster KK , Hu M-C , Wall MM , Brown PJ , Zilcha-Mano S , Roose SP , Stein A , Golub JS , Rutherford BR ((2021) ) Age-related hearing loss, neuropsychological performance, and incident dementia in older adults. J Alzheimers Dis 80: , 855–864. |
[57] | Brewster KK , Hu M-C , Zilcha-Mano S , Stein A , Brown PJ , Wall MM , Roose SP , Golub JS , Rutherford BR ((2021) ) Age-related hearing loss, late-life depression, and risk for incident dementia in older adults. J Gerontol A Biol Sci Med Sci 76: , 827–834. |
[58] | Creese B , Griffiths A , Brooker H , Corbett A , Aarsland D , Ballard C , Ismail Z ((2020) ) Profile of mild behavioral impairment and factor structure of the Mild Behavioral Impairment Checklist in cognitively normal older adults. Int Psychogeriatr 32: , 705–717. |
[59] | McCreedy EM , Weinstein BE , Chodosh J , Blustein J ((2018) ) Hearing loss: Why does it matter for nursing homes?. J Am Med Dir Assoc 19: , 323–327. |



