Fifteen Years of the Australian Imaging, Biomarkers and Lifestyle (AIBL) Study: Progress and Observations from 2,359 Older Adults Spanning the Spectrum from Cognitive Normality to Alzheimer’s Disease
Abstract
Background:
The Australian Imaging, Biomarkers and Lifestyle (AIBL) Study commenced in 2006 as a prospective study of 1,112 individuals (768 cognitively normal (CN), 133 with mild cognitive impairment (MCI), and 211 with Alzheimer’s disease dementia (AD)) as an ‘Inception cohort’ who underwent detailed ssessments every 18 months. Over the past decade, an additional 1247 subjects have been added as an ‘Enrichment cohort’ (as of 10 April 2019).
Objective:
Here we provide an overview of these Inception and Enrichment cohorts of more than 8,500 person-years of investigation.
Methods:
Participants underwent reassessment every 18 months including comprehensive cognitive testing, neuroimaging (magnetic resonance imaging, MRI; positron emission tomography, PET), biofluid biomarkers and lifestyle evaluations.
Results:
AIBL has made major contributions to the understanding of the natural history of AD, with cognitive and biological definitions of its three major stages: preclinical, prodromal and clinical. Early deployment of Aβ-amyloid and tau molecular PET imaging and the development of more sensitive and specific blood tests have facilitated the assessment of genetic and environmental factors which affect age at onset and rates of progression.
Conclusion:
This fifteen-year study provides a large database of highly characterized individuals with longitudinal cognitive, imaging and lifestyle data and biofluid collections, to aid in the development of interventions to delay onset, prevent or treat AD. Harmonization with similar large longitudinal cohort studies is underway to further these aims.
INTRODUCTION
The Australian Imaging, Biomarkers and Lifestyle (AIBL) Study assembled and assessed an Inception cohort of 1,112 individuals from late 2006 to mid-2008 with the intention of conducting re-assessments every 18 months to determine the extent to which their baseline cognitive profile, demographic factors, Aβ-amyloid brain load, blood and cerebrospinal fluid (CSF) biomarkers, genetic and lifestyle factors could predict their future cognitive function and clinical status with respect to the development of Alzheimer’s disease (AD) [1, 2]. The evolution of the Inception cohort at baseline, 18, 36, 54, 72, 90, 108, and 126 months follow-up, was enriched with recruitment of 1,247 new participants to compensate for attrition (Enrichment cohort), yielding the current database of 2,359 participants with 8,592 person-contact years.
The AIBL Study grew out of the Melbourne Aging Study, initiated by Currie et al. in the 1990s [3–6]. In the mid-2000s, the Commonwealth Scientific and Industrial Research Organisation (CSIRO) successfully defended its patents concerning the use of fast wireless local area network (WLAN) technology, developed within its radio astronomy program. At the time WLAN was being used by just about every Wi-Fi-enabled laptop computer in the world. The ensuing multi-million-dollar settlements with many companies (including Apple, Microsoft, HP, Dell, Nintendo, and Netgear) were transferred into CSIRO’s Science and Industry Endowment Fund (SIEF). This gave Richard Head, then Chief of the CSIRO Division of Human Nutrition, the opportunity to initiate and fund a consortium to develop AIBL with a strong emphasis on cognitively normal (CN) individuals in their 70s and application of the then-emerging technology of Aβ-amyloid positron emission tomography (PET) imaging.
Individuals with AD, mild cognitive impairment (MCI), and those classified as CN were enrolled into the Study in order to address the following aims: 1) To improve understanding of the pathogenesis and diagnosis of AD using psychometric, neuroimaging and biomarker techniques, with a focus on early diagnosis; 2) To examine lifestyle and diet factors that may be involved in the pathogenesis of AD. Here, we review the accomplishments of this 15-year-long observational longitudinal cohort Study towards addressing these aims, and more.
MATERIALS AND METHODS
Ethics approval
The AIBL study, including the follow-up protocol and subsequent amendments and revisions to the protocol, was approved by the institutional human research ethics committees of Austin Health, St Vincent’s Health, Hollywood Private Hospital and Edith Cowan University. All volunteers gave written informed consent before participating in study assessments, and the study was conducted in accordance with the Helsinki Declaration of 1975.
Subjects
AIBL is a two-site study: Melbourne, Victoria (60%) and Perth, Western Australia (40%). Initially 1,112 individuals were recruited at baseline [1] and reassessed at 18 [2], 36, 54, 72, 90, 108, and 126 months (Inception cohort).
At baseline, subjects were classified as AD dementia (n = 211), MCI (n = 133), and CN (n = 768). The major exclusion criteria were age < 60 years, a history of non-AD dementia, schizophrenia, bipolar disorder, current depression with a short Geriatric Depression Scale score above 5/15 (for CN individuals), Parkinson’s disease, symptomatic stroke, current uncontrolled or life threatening medical illness, diagnosed obstructive sleep apnea (OSA), past head injury with over one hour of post-traumatic amnesia, or alcohol use above two standard drinks per day for women or four per day for men.
Over the duration of this study, the Inception cohort was enriched with an additional 1,247 subjects (719 CN, 282 MCI, 230 AD, and 18 with dementia other than AD or cognitive impairment not suspected to be due to AD; Enrichment cohort) both to compensate for cohort attrition and to address new research questions. In 2018, the exclusion criterion for diagnosed OSA was amended to exclude only those who were not treated with continuous positive airway pressure ventilation.
All cognitive and clinical data were considered by a review panel, prior to participants being allocated into one of five diagnostic groups: AD dementia by NINCDS-ADRDA criteria, MCI by Winblad et al. [7] and Petersen et al. [8] criteria, CN subjective memory complainers (SMC), CN non-memory complainers (NMC), and other (dementia other than AD or cognitive impairment not suspected to be due to AD), details of which are outlined below. SMC status was determined by the subject’s ‘yes’ or ‘no’ response to the single question “Do you have difficulty with your memory?”.
Assessments took place at three locations in Melbourne and at two locations in Perth, depending on whether the participants were to undergo brain imaging and where they lived. Some participants (especially some with AD dementia), were assessed by AIBL staff at home. All assessments were conducted in the morning, after an overnight fast. Weight, height, abdominal girth, sitting blood pressure and pulse were measured, followed by the drawing of 80 mL of blood. Participants were then provided with breakfast, before undergoing cognitive and mood assessments, as described below.
Cognitive and mood assessments
Cognitive and mood assessments were performed by trained staff, most of whom were qualified neuropsychologists, and all of whom had undergone extensive training in the assessment techniques. Assessors were blind to the Aβ-amyloid scan status when they undertook reviews. The length of a typical assessment was around two hours. A more comprehensive account of the mood assessments, cognitive battery and the rationale behind the selection of individual items has been detailed previously [1]. Briefly, the full assessment battery comprised the Mini-Mental State Examination (MMSE) [9], California Verbal Learning Test –Second edition (CVLT-II) [10], Logical Memory I and II (WMS; Story 1 only) [11], D-KEFS verbal fluency [12], 30-item Boston Naming Test (BNT) [13], Wechsler Test of Adult Reading (WTAR) [14] (the WTAR was not performed at the 72 month follow-up), Digit Span and Digit Symbol-Coding subtests of the Wechsler Adult Intelligence Scale –Third edition (WAIS–III) [15], the Stroop task (Victoria version) [16], and the Rey Complex Figure Test (RCFT) [17]. As detailed in Table 1, individual test scores were used to construct cognitive domain composite scores for episodic recall memory, recognition memory, executive function, language, attention and processing speed, as well as a composite referred to as the AIBL PACC (Preclinical Alzheimer Cognitive Composite). Participants also completed the computerized Cogstate battery (www.cogstate.com), and for each Cogstate test the speed (reaction time in milliseconds) and accuracy (number of correct responses given) of performance was recorded. A Cogstate Continuous Paired Associate Learning Task was also included to assess visual paired associate learning and for this the accuracy of performance was calculated by totaling the number of errors made in each round of the task.
Table 1
Cognitive domain composite score constituents
| Cognitive domain composite | Tests |
| Episodic recall memory | CVLT-II Long Delay Free Recall |
| Logical Memory 2 | |
| Rey Complex Figure Long Delay Free Recall | |
| Recognition memory | CVLT-II ‘d prime’ |
| Rey Complex Figure recognition total correct | |
| Executive function | Letter Fluency |
| Category switching total correct | |
| Language | BNT |
| Category Fluency | |
| Attention and processing speed | Digit Symbol-Coding |
| Digit Span | |
| AIBL PACC | MMSE |
| Digit Symbol-Coding | |
| CVLT-II Long Delay Free Recall | |
| Logical Memory 2 |
AIBL PACC, Australian Imaging, Biomarkers and Lifestyle Study Preclinical Alzheimer Cognitive Composite; BNT, Boston Naming Test; CVLT-II, California Verbal Learning Test –Second edition; MMSE, Mini-Mental State Examination.
Both the 15-item version of the Geriatric Depression Scale (GDS-15) [18] and the Hospital Anxiety and Depression Scale (HADS) [19, 20] were administered at all visits. For participants with a diagnosis of AD dementia, MCI, and for some CN participants where decline was suspected, an informant was asked to provide additional information about the functional performance of the research participant, and to complete the 16-item version of the Informant Questionnaire on Cognitive Decline (IQCODE) [21].
Dementia severity was rated for all participants using the Clinical Dementia Rating scale (CDR) [22], on the basis of information obtained from cognitive testing, direct questioning of the participant, and information from an informant and/or from the participant’s treating clinician (for those diagnosed with AD or MCI). The total CDR rating (range 0–3) and the CDR ‘sum of boxes’ score (range 0–18) were generated for each participant for each visit. The CDR rating for each participant was also reviewed in detail by an experienced CDR rater and clinician (DA) to ensure consistency and accuracy of classifications.
As detailed above, the AIBL neuropsychological battery is extensive, and multiple publications have focused on development and validation of composite scores to expedite assessment and clinical classification, and to provide prognostic information (e.g., [23–26]). However, given that AIBL participants range from CN to dementia, such composites have never been proposed as a substitute for comprehensive assessment, but have been posited as differentially sensitive at specific disease stages, and of selective use in development of clinical trial designs, for example, when participants from a narrower clinical spectrum are targeted (see Cognition section of the Results for more information).
Clinical review and the diagnosis of AD dementia or MCI
Monthly clinical review panel meetings were conducted to discuss the diagnostic classification for all potential AD or MCI participants. This included individuals with a probable baseline diagnosis of AD or MCI, and those initially classed as CN whose diagnostic status required further consideration. This latter group included participants who demonstrated any of the following: MMSE score < 28/30, failure on the Logical Memory test (as per Alzheimer’s Disease Neuroimaging Initiative (ADNI) criteria), other evidence of possibly significant cognitive difficulty on neuropsychological testing, a CDR score of 0.5 or greater, medical history suggestive of the presence of illnesses likely to impair cognitive function, informant or personal history suggestive of impaired cognitive function, or consumption of medications or other substances that could affect cognition. A consensus diagnosis was assigned for each such participant, using internationally agreed diagnostic criteria according to both DSM-IV (American Psychiatric Association, 1994) and ICD-10 (World Health Organization, 1992). Where appropriate, ICD-10 dementia severity rating (WHO, 1992), NINCDS-ADRDA AD diagnosis (probable or possible) and MCI classifications (amnestic/non- amnestic, single/multi-domain) were applied.
MCI diagnoses were made according to a protocol based on the criteria of Winblad et al. [7] which are informed by the criteria of Petersen et al. [8]. Consistent with Winblad criteria, all participants classified with MCI had either personally, or through an informant, reported memory difficulties. Participants with a baseline clinical diagnosis of MCI were required to demonstrate a score 1.5 SD or more below the age-adjusted mean on at least one neuropsychological test applied at the time of the AIBL reassessment in order to be retained in the MCI category. Individuals who were classed as CN at baseline had to fulfil the more stringent criterion of impairment on two or more cognitive tests at a level at least 1.5 SD below the age-adjusted mean, in addition to having reported memory difficulties, to be classified as MCI at follow-up. The greater stringency applied to assigning individuals presenting as CN to the MCI category at follow-up was decided upon after extensive discussion, is justified by the acknowledged mutability of MCI diagnoses, and replicates our practice when diagnosing MCI at baseline. Individuals with MCI were then characterized as being of the amnestic or non-amnestic type, and also whether impairment was single- or multi-domain, based on the profile of impairment in the AIBL battery of neuropsychological tests. All participants classified with MCI presented with intact activities of daily living and exhibited no clear evidence of significant impairment in their social or occupational functioning.
The clinical review panel comprised old age psychiatrists, a neurologist, a geriatrician, and neuropsychologists. A quorum was formed by three members including at least one medically qualified and at least one neuropsychologist member. The panel conferred monthly by telephone conference, and all meetings were attended by five or more panel members. Diagnoses were made blind to the results of AIBL neuroimaging data and apolipoprotein E (APOE) genotype but were sometimes informed by (non-Aβ-amyloid) imaging conducted for independent clinical reasons. Summary neuropsychological data were available to the diagnostic panel.
Medical history and medication use
At baseline, participants (or their carer if the subject was cognitively impaired) completed a detailed questionnaire regarding personal medical history, medication use and smoking, and questions about current and past alcohol and illicit drug use: this information was reviewed and updated as needed at follow-up assessments.
Brain imaging
At baseline, neuroimaging using magnetic resonance imaging (MRI) and PiB-PET was performed in 288 Inception cohort participants (178 CN, 57 MCI, and 53 mild AD); these findings have been reported in detail elsewhere [27]. At subsequent timepoints, additional Inception cohort participants underwent Aβ-amyloid imaging using PiB, NAV4694, flutemetamol, florbetapir, or florbetaben. All Enrichment cohort participants were invited to undergo brain imaging from baseline onwards. Individuals who had been imaged were invited to return for further scans at 18-month intervals, so the rate of Aβ-amyloid accumulation and brain atrophy could be determined. Tau-PET neuroimaging commenced in 2013, using the AV1451 and MK6240 tracers.
MRI scans were mainly acquired at three different scanning centers, two in Melbourne using a Siemens 3T Trio (41%of scans) and a Siemens 3T Skyra (25%) scanner, and one in Perth using a Siemens 3T Verio (16%) and a Siemens 1.5T Avanto (13%) scanner. The scans include a 3D MPRAGE (Magnetization Prepared Rapid Acquisition Gradient Echo) image (voxel size 1.2×1×1 mm3, repetition time/ echo time = 2300/ 2.98, flip angle = 9°). A 3D T2-weighted FLuid Attenuation Inversion Recovery (FLAIR) sequence was included in the image acquisition protocol, which was acquired using two different sets of parameters: 1) in-plane resolution 0.98×0.98 mm, slice thickness 0.9 mm, repetition time/ echo time/ inversion time =6000/ 420/ 2100, flip angle = 120°, field-of-view 240×256, and 176 slices; 2) in-plane resolution 0.5×0.5 mm with in-plane interpolation (factor of 2) enabled, slice thickness 1.0 mm, repetition time/ echo time/ inversion time = 5000/ 355/ 1800, flip angle = 120°, field-of-view 256×256, and 160 slices. Gradient Recalled Echo (GRE) images used for SWI (Susceptibility-Weighted Imaging) and QSM (Quantitative Susceptibility Mapping) were acquired with 0.93×0.93 mm in-plane resolution and 1.75 mm slice thickness, repetition time/ echo time = 27/ 20 ms, flip angle = 20°, and field-of-view 240×256, for 80 slices [28].
Blood samples
Fasting blood samples (80 mL) were collected and processed as described previously [1] for the purpose of routine clinical pathology testing, and biobanking. For biobanking, samples were processed to yield whole blood, serum, plasma containing ethylenediaminetetraacetic acid (EDTA), plasma containing lithium heparin, white blood cells, red blood cells, and platelets. The EDTA collection tubes and washing buffers contained pre-added prostaglandin E1 at a final concentration of 33 ng/mL to prevent platelet activation. Blood fractions were aliquoted into volumes ranging from 100μL to 1 mL in screwcap 2D barcoded polypropylene Nunc Cryotubes (NUN374088; Nalge Nunc International, Rochester, NY, USA). Samples were snap-frozen, prior to being transferred to a liquid nitrogen facility for long-term storage.
Routine clinical pathology tests (full blood examination, urea, electrolytes, creatinine, glucose, calcium, iron studies, total cholesterol and cholesterol subfractions, homocysteine, liver and thyroid function tests, vitamin B12, folate, ceruloplasmin, hormone levels) ceased after the 72-month follow-up due to financial constraints, and a diminishing utility of these results for research purposes. Basic clinical pathology test results were available to the diagnostic review panel if needed, and results outside the normal range were flagged for immediate attention and reporting to the participant’s regular doctor.
Cerebrospinal fluid samples
In a subgroup of participants CSF was collected in the morning by routine lumbar puncture after overnight fasting, employing a protocol similar to that recommended by the Alzheimer’s Biomarkers Standardization Initiative [29]. CSF was directly collected into polypropylene tubes (Greneir Bio-One188271), and immediately placed on wet ice. The tubes were gently mixed by repeated inversion and CSF was centrifuged within 45 min (2000×g, 4°C, for 10 min). The supernatant was removed into a new polypropylene tube and gently inverted three times to avoid possible gradient effects. Samples were then aliquoted into screwcap 2D barcoded polypropylene Nunc Cryotubes (NUN374088) and frozen within 1 h of lumbar puncture, prior to being transferred to a liquid nitrogen facility for long-term storage. The remaining cell pellet was resuspended in 100μL of CSF, and also stored in Nunc Cryotubes in liquid nitrogen. Polypropylene tubes were used for the sampling and storage to minimize reported plastic surface effects [29].
CSF was tested routinely using the INNOTEST® kit assay (Innogenetics, now Fujirebio Europe N.V., Ghent, Belgium) for Abeta 1–42 (Aβ42), total tau (tTau) and phospho-tau 181 (pTau181), with thresholds for CSF biomarker positivity determined using a rank order approach (0.10 fractile for Aβ42 and 0.90 fractile for tTau and pTau181) against a Pittsburgh Compound B (PiB) Standardized Uptake Value Ratio (SUVR) of 1.4 (or equivalent in other tracers) [30]. Participants with three or more collections of CSF have also been analyzed using automated Elecsys® assays (Roche Diagnostics) for Aβ42, Aβ1–40, tTau, and pTau181 [31].
Lifestyle measures
All participants were asked to complete the International Physical Activity Questionnaire (IPAQ) [32], the Cancer Council of Victoria Food Frequency Questionnaire (CCVFFQ) [33], and the Pittsburgh Sleep Quality Index (PSQI) [34]. The IPAQ collects data on self-reported physical activity undertaken during the previous seven days, the CCVFFQ assesses usual dietary intake over the preceding 12 months, and the PSQI captures information in relation to the previous month’s sleep habits. IPAQ data were collected at each timepoint, CCVFFQ data were collected at alternating timepoints; i.e., baseline, 36-months, 72-months, etc. PSQI data were collected at each timepoint, from 72-months onwards for the Inception cohort, and from baseline onwards for Enrichment cohort participants. Objective measures of physical activity and body composition were also obtained from a subset of the cohort using ActiGraph Uni-axial Accelerometers and Dual Energy X-Ray Absorptiometry (DXA) scans respectively.
Genetic data
QIAamp DNA Blood Maxi Kits (Qiagen, Hilden, Germany) were used for the extraction of DNA from 5 mL of whole blood, as per the manufacturer’s instructions. Neat DNA was aliquoted and stored at –80°C for downstream analyses.
APOE and brain-derived neurotrophic factor (BDNF; Val66Met) genotypes were determined by TaqMan® genotyping assays (Life Technologies, USA). Specifically, for APOE rs7412 (Assay ID: C____904973_10) and rs429358 (Assay ID: C___3084793_20) and for BDNF, rs6265 (Assay ID: C__11592758_10). These were carried out on a QuantStudioTM 12K Flex Real-Time-PCR system (Applied Biosystems™, USA), as per the manufacturer’s instructions.
Genome-wide single nucleotide polymorphism (SNP) array data were derived on 1357 AIBL participants. Genome-wide analysis was performed on 976,713 SNPs (including 273,000 exome variants and an additional 13,000 custom content SNPs) on the OmniExpressHumanExome + BeadChip (Illumina, USA). Genetic markers were mapped to human genome reference hg19 and standard quality control (QC) was undertaken at marker and sample levels in PLINK [35]. Markers that were duplicated, with < 95%call rate, and/or unmappable were removed, and samples with call rate < 98%, mismatch gender and/or high heterozygosity rate were removed. Post-QC, 948,720 markers and 1315 samples were included in the pre-imputed AIBLgene (ver1.0) dataset. This dataset was further imputed to the 1000 Genomes Project Phase 3 [36] using the Michigan Imputation Server [37]. At the time of manuscript submission, the remaining AIBL Enrichment cohort and additional samples, for QC and cross-platform validation, were undergoing gen-omewide SNP array processing using the Axiom Precision Medicine Diversity Array (Applied Biosystems™).
Whole exome sequencing was undertaken on 1μg of DNA, extracted as described above, from 500 AIBL participants. Exonic sequence enrichment was performed by hybridisation using the SureSelect Human All Exon V5 (51 Mb) Kit (Agilent Technologies, USA). Resulting sequence libraries were sequenced on the Illumina HiSeq2500 using 100 bp paired-end read chemistry with a minimum of 36x mapped coverage. Four hundred and ninety-seven samples progressed from raw sequencing through to sequence alignment. The Burrows-Wheeler Aligner (BWA) was used for the alignment of raw sequence data to the human genome reference (build GRCh37, 1kG reference) [38], and the open-source Picard Tools (available from: http://picard.sourceforge.net.) was used for the removal of PCR/optical duplicates. Realignment of data around indel positions and recalibration of base quality scores was performed using the Genome Analysis ToolKit 3.3 (GATK3.3) [39]. The GATK HaploTypeCaller was used for per-sample genotyping according to GATK Best Practices. Finally, the annotation of per-sample variants was performed using Ensembl’s Variant Effect Predictor (VEP) [40].
Genome-wide methylation analysis was performed on 726 AIBL participants at a single timepoint (AIBL Inception participants at 18-month follow-up: 318 CN, 34 MCI, and 123 AD; AIBL Enrichment participants at baseline: 153 CN, 60 MCI, and 38 AD). Prior to bisulfite conversion, extracted DNA was processed through Amicon® Ultra-0.5 Centrifugal Filters (Merck, USA), following manufacturer’s instructions, to remove any guanidine isothiocyanate introduced during the extraction process. Cleaned DNA was first standardized to 500 ng using a method previously described [41] before undergoing bisulphite conversion using the Illumina recommended protocol for the EZ DNA Methylation Kits (ZYMO Research, USA). Bisulfite converted DNA samples were hybridized to the eight sample HumanMethylationEPIC BeadChip Array using the Infinium HD Methylation protocol (Illumina, USA). The Meffil R package [42] was used for data QC and normalization as previously described [41], with technical variation removed by functional normalization [43]. At the time of manuscript submission, a subset of 400 of the above 726 participants also have samples, from two additional timepoints, undergoing the same workflow. This will generate a 400-sample longitudinal genome-wide peripheral DNA methylation dataset.
RESULTS
Major achievements (including publications and citations)
AIBL data have confirmed the diagnostic and prognostic power of Aβ-amyloid imaging [44], permitted direct calculation of the very slow accumulation rate of AD-related pathologic change [45, 46], shown the best measures for tracking very early cognitive decline [47, 48], and revealed a wide window for early intervention [45]. These early estimates of Aβ-amyloid-related cognitive change provided a basis for estimation of power for therapeutic trials in pre-symptomatic AD [49]. Prospective AIBL data have also shown the powerful interaction of several genes, either independently or combined in genetic profiles or polygenic risk scores (PRS), on cognitive decline in preclinical and prodromal stages of AD [45, 47, 50–52] suggesting that the genetic architecture of AD progression may differ from that of risk. With additional genetic data and detailed lifestyle analysis now emerging from the cohort, AIBL is revealing further important genetic and lifestyle interactions (e.g., [53, 54]) that will guide future tailored early intervention and prevention trials and place AIBL at the forefront of the emerging field of lifestyle genomics (LGx) in AD.
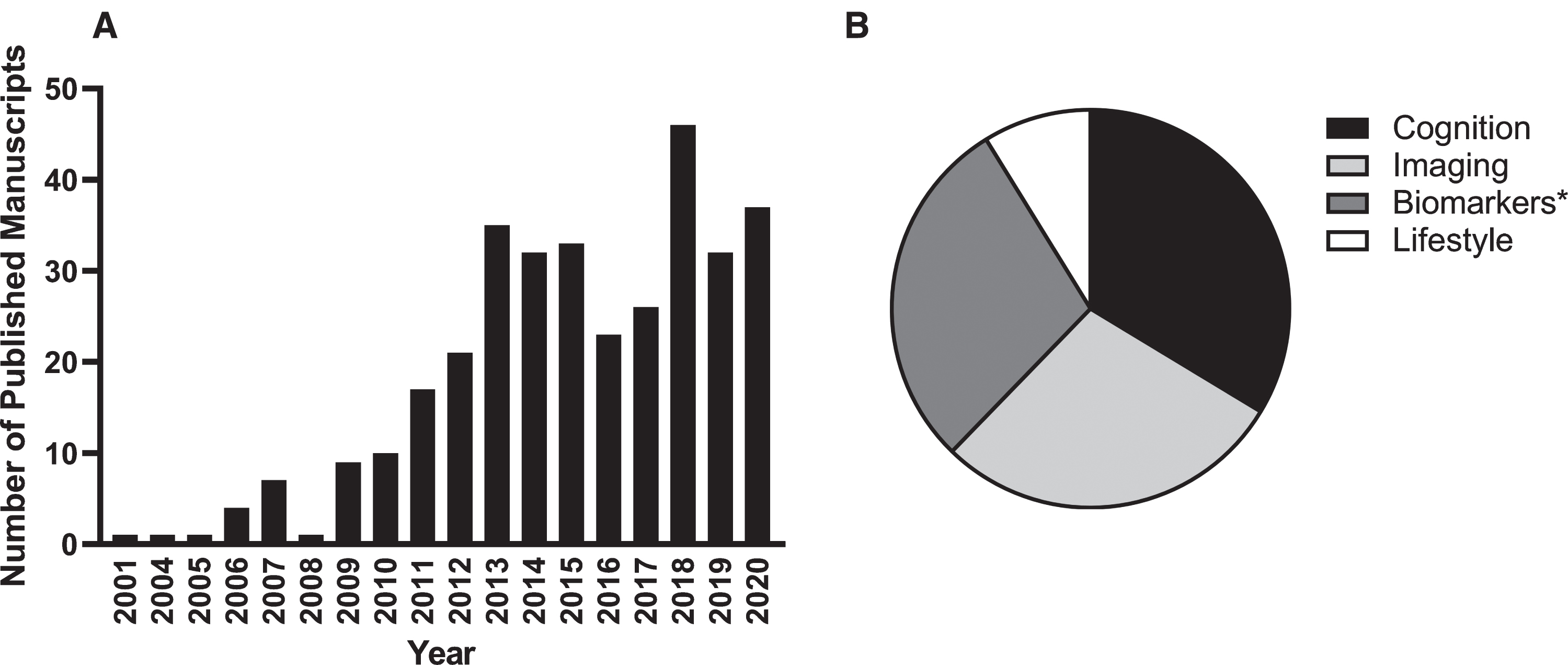
Table 2 lists the 10 highest cited papers produced using AIBL data. Most relate to the nosology of AD, particularly in its preclinical stages, and the utility and applicability of PET as a biomarker of Aβ-amyloid and tau accumulation. Supplementary Table 1 lists each of the 341 AIBL-related manuscripts either published in peer reviewed journals or accepted for publication as of 31 December 2020. The highest percentage (33%) focus on issues related to the nature and magnitude of cognitive changes in preclinical, prodromal and clinical AD, 28%relate to biomarkers (including genetics), 28%to neuroimaging, and 11%to lifestyle (Fig. 1).
Table 2
Top AIBL Study and AIBL investigator-co-authored papers, determined via combining Google Scholar Citations, ISI, Altmetrics, and FWCI scores
| Citation | Impact Measures |
| Sperling RA, Aisen, PS, Beckett LA, Bennett DA, Craft S, Fa gan AM, Iwatsubo T, Jack CR, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo M, Thies B, Morrson-Bogorad M, Wagster MV, Phelps CH (2011) Toward defining the preclinical stages of Alzheimer’s disease: recommendations of the National Institute on Aging and the Alzheimer’s Association workgroup. Alzheimers Dement 7, 280–292. | Citations: 5352 |
| ISI: 2940 | |
| Altmetrics: 136 | |
| FWCI: 120.29 | |
| Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, DeKosky ST, Gauthier S, Selkoe D, Bateman R, Cappa S, Crutch S, Engelborghs S, Frisoni GB, Fox NC, Galasko D, Habert MO, Jicha GA, Nordberg A, Pasquier F, Rabinovici G, Robert P, Rowe CC, Salloway S, Sarazin M, Epelbaum S, de Souza LC, Vellas B, Visser PJ, Schneider L, Stern Y, Scheltens P, Cummings JL (2014) Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol 13, 614–629. | Citations: 2136 |
| ISI: 1181 | |
| Altmetrics: 110 | |
| FWCI: 48.40 | |
| Rowe CC, Ng S, Ackermann U, Gong SJ, Pike K, Savage G, Cowie TF, Dickinson KL, Maruff P, Darby D, Smith C, Woodward M, Merory J, Tochon-Danguy H, O’Keefe G, Klunk WE, Mathis CA, Price JC, Masters CL, Villemagne VL (2007) Imaging β-amyloid burden in aging and dementia. Neurology 68, 1718–1725. | Citations: 1121 |
| ISI: 760 | |
| Altmetrics: 9 | |
| FWCI: 0.63 | |
| Villemagne VL, Burnham S, Bourgeat P, Brown B, Ellis KA, Salvado O, Szoeke C, Macaulay SL, Martins RN, Maruff P, Ames D, Rowe CC, Masters CL (2013) Amyloid β deposition, neurodegeneration and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol 12, 357–367. | Citations: 1312 |
| ISI: 80 | |
| Altmetrics: 88 | |
| FWCI: 41.86 | |
| Jessen F, Amariglio RE, van Boxtel M, Breteler M, Ceccaldi M, Chételat G, Dubois B, Dufouil C, Ellis KA, van der Flier WM, Glodzik L, van Harten AC, de Leon MJ, McHugh P, Mielke MM, Molinuevo JL, Mosconi L, Osorio RS, Perrotin A, Petersen RC, Rabin LA, Rami L, Reisberg B, Rentz DM, Sachdev PS, dela Sayette V, Saykin AJ, Scheltens P, Shulman MB, Slavin MJ, Sperling RA, Stewart R, Uspenskaya O, Vellas B, Visser PJ, Wagner M and the Subjective Decline Initiative (SCD-I) Working Group (2014) A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement 10, 844–852. | Citations: 1122 |
| ISI: 551 | |
| Altmetrics: 20 | |
| FWCI: 27.31 | |
| Rowe CC, Ellis KA, Rimajova M, Bourgeat P, Pike KE, Jones G, Fripp J, Tochon-Danguy H, Morandeau L, O’Keefe G, Price R, Raniga P, Robins P, Acosta O, Lenzo N, Szoeke C, Salvado O, Head R, Martins R, Masters CL, Ames D, Villemagne VL (2010) Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging 31, 1275–1283. | Citations: 894 |
| ISI: 600 | |
| Altmetrics: 36 | |
| FWCI: 18.56 | |
| Pike KE, Savage G, Villemagne VL, Ng S, Moss SA, Maruff P, Mathis CA, Klunk WE, Masters CL, Rowe CC (2007) β-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer’s disease. Brain 130, 2837–2844. | Citations: 818 |
| ISI: 583 | |
| Altmetrics: 15 | |
| FWCI: 16.68 | |
| Villemagne VL, Pike KE, Chételat G, Ellis KA, Mulligan R, Bourgeat P, Ackermann U, Jones G, Szoeke C, Salvado O, Martins R, O’Keefe G, Mathis CA, Klunk WE, Ames D, Masters CL, Rowe CC (2011) Longitudinal assessment of Aβ and cognition in aging and Alzheimer’s disease. Ann Neurol 69, 181–192. | Citations: 749 |
| ISI: 484 | |
| Altmetrics: 11 | |
| FWCI: 21.99 | |
| Ellis KA, Bush AI, Darby D, De Fazio D, Foster J, Hudson P, Lautenschlager NT, Lenzo N, Martins RN, Maruff P, Masters CL, Milner A, Pike K, Rowe CC, Savage G, Szoeke C, Taddei K, Villemagne VL, Woodward M, Ames D and the AIBL Research Group (2009) The Australian Imaging, Biomarkers and Lifestyle (AIBL) study of ageing: methodology and baseline characteristics of 1112 individuals recruited for a longitudinal study of Alzheimer’s disease. Int Psychogeriatr 21, 672–687. | Citations: 519 |
| ISI: 320 | |
| Altmetrics: 6 | |
| FWCI: 10.44 | |
| Villemagne VL, Fodero-Tavoletti MT, Masters CL, Rowe CC (2015) Tau imaging: early progress and future directions. Lancet Neurol 14, 114–124. | Citations: 412 |
| ISI: 248 | |
| Altmetrics: 2 | |
| FWCI: 14.10 |
FWCI, Field-Weighted Citation Impact; ISI, International Scientific Indexing.
Fig. 1
AIBL-related publications by calendar year (A) and by primary research focus (B). At the time of writing, more than 340 AIBL-related manuscripts have been published since study commencement. *Biomarker publications include those related to fluid biomarkers (blood, cerebrospinal fluid), buccal cells and genetics.
Cohort reassessment
Table 3 details characteristics of the cohort at baseline, while Fig. 2 shows the total number of participants from both the Inception and Enrichment cohorts assessed at baseline and reassessed at 18, 36, 54, 72, 90, 108, and 126 months (the baseline and 18 month data for the Inception cohort have been published previously [1, 2]) and both the initial diagnostic category to which each volunteer was assigned at baseline and the diagnostic category after each reassessment and clinical review. For ease of interpretation the NMC and SMC categories have been combined into a single CN group in this complex figure, as there was no difference in the rate of attrition, deaths or change in cognitive status between these two groups over time, and there was considerable movement of participants between these two categories across timepoints. Collectively, these assessments equate to 8,592 person-years of investigation (Table 4).
Table 3
Characteristics of the cohort at baseline
| CN | MCI | AD | Other | |
| N | 1487 | 413 | 441 | 18 |
| Age, y | 71.1 | 73.8 | 75.1 | 68.5 |
| Female, % | 57.2 | 48.7 | 55.9 | 27.8 |
| APOE ɛ4 carrier, % | 28.1 | 53.6 | 64.5 | 25.0 |
| Education, y | 13.1 | 12.2 | 12.1 | 13.2 |
| MMSE | 28.6 | 26.0 | 20.5 | 22.2 |
| CDR Global | 0.03 | 0.50 | 0.91 | 0.78 |
Data are represented as means unless otherwise stated. AD, Alzheimer’s disease; APOE, Apolipoprotein E, CDR, Clinical Dementia Rating; CN, Cognitively Normal; MCI, Mild Cognitive Impairment; MMSE, Mini-Mental State Examination; Other, Dementia other than AD or cognitive impairment not suspected to be due to AD.
Fig. 2
Consort diagram illustrating the study cohort composition from baseline to 126 months. The numbers include Inception and Enrichment cohort participants. Solid black arrows indicate study participants remaining within a clinical classification, across timepoints. Broken black arrows indicate the transition of study participants from one clinical classification to another, across timepoints. Tabulated information details loss to follow up of participants due to death or study withdrawal, or those who missed the previous assessment but returned for the current assessment timepoint. AD, Alzheimer’s disease; CN, cognitively normal; MCI, mild cognitive impairment; Other, dementia other than AD or cognitive impairment not suspected to be due to AD.
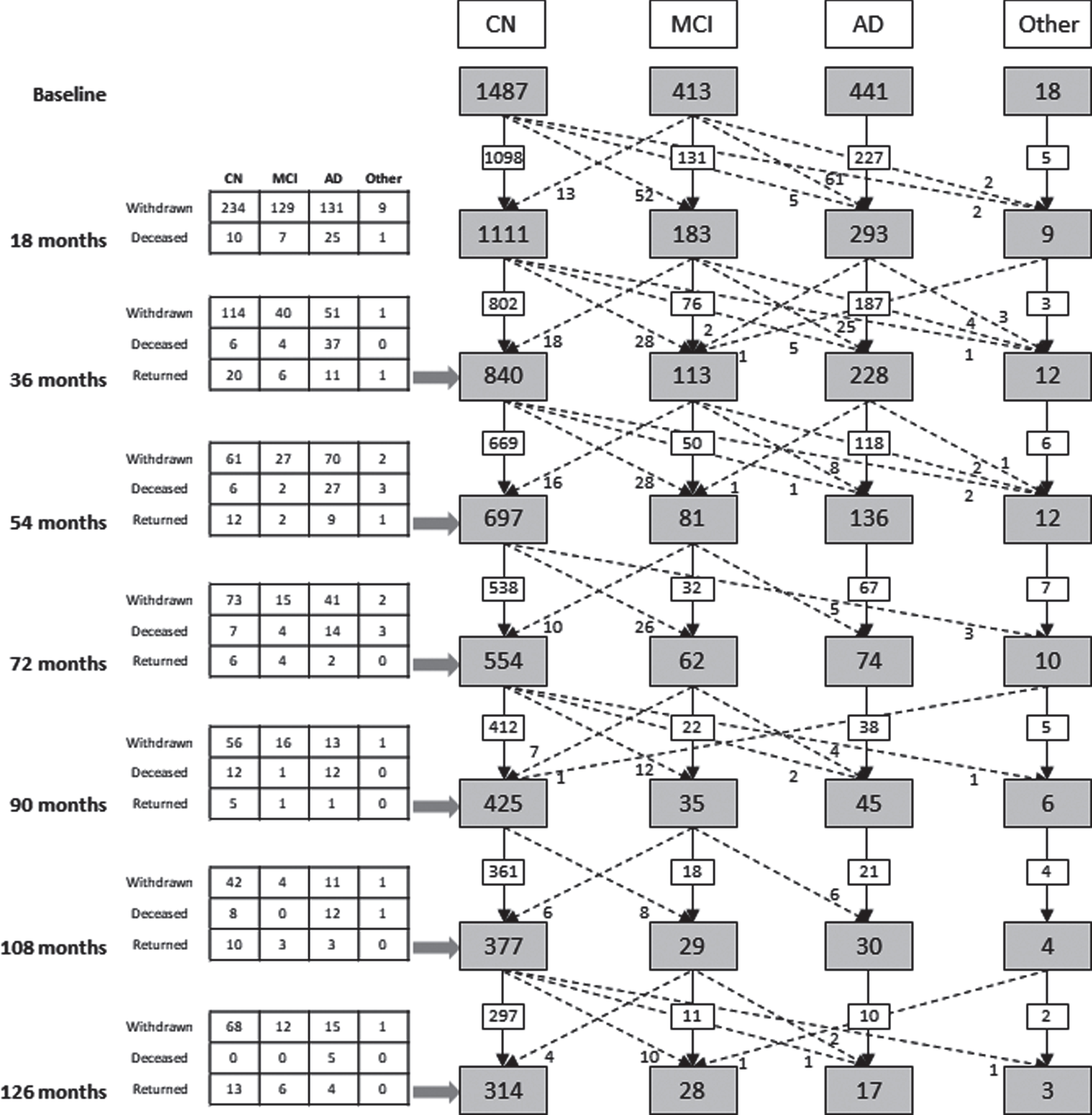
Table 4
Person-contact years
| Clinical Classification | |||||
| CN | MCI | AD | Other | Total | |
| Years | 6,477 | 796.5 | 1,234.5 | 84 | 8,592 |
AD, Alzheimer’s disease; CN, cognitively normal; MCI, mild cognitive impairment; Other, dementia other than AD or cognitive impairment not suspected to be due to AD.
Of the 1,112 participants in the Inception cohort, follow-up data were collected for 89.6%at 18 months, 80.6%at 36 months, 73.5%at 54 months, 64.5%at 72 months, 58.0%at 90 months, 52.6%at 108 months, and 32.6%at 126 months. Of note, due to financial constraints, participants willing to undergo brain scans were prioritized for assessment at 126 months; hence the significant reduction in the number of assessments at this timepoint.
Of the 133 Inception cohort baseline MCI participants, 56 (42%) developed AD by the 126-month timepoint, whilst three developed vascular dementia, one developed frontotemporal dementia (FTD) and one developed Parkinson’s disease dementia. Three of the original MCI subjects were classified as MCI throughout the study, whilst eight MCI subjects were reclassified as CN at least once in the study follow-up sequence, with three of these reverting to MCI by their last follow-up visit and one transitioning to AD.
Thirty of 768 Inception cohort participants (3.9%) who were CN at baseline developed AD by 126 months and 19 of these were classed as having MCI on at least one follow-up visit prior to the development of AD. Two CN subjects developed vascular dementia by the 126-month follow-up, one developed progressive supranuclear palsy and one FTD. Fifty-eight subjects (7.6%) who were CN at baseline developed MCI by 126 months but had not progressed to exhibit dementia, and a further twenty-one subjects (2.7%) developed MCI but reverted to CN status. Two hundred and ninety (37.8%) were classed as CN at every follow-up they attended.
Of the 441 individuals (Inception plus Enrichment combined) classified as AD at baseline, by 54 months, four had transitioned to dementia other than AD or cognitive impairment not suspected to be due to AD (Other), and three to MCI. No other transitions were observed for those with a baseline clinical classification of AD.
The Enrichment cohort has now reached 1,247 individuals, with 622 individuals having undergone at least one follow-up assessment included in this report. The Enrichment cohort initially focused on recruiting CN participants to investigate the preclinical stages of AD, however, from 2016 onwards the Enrichment cohort has increased participant recruitment via referral from memory specialists with a focus on MCI and early AD; these more recent recruits subsequently drop out at a higher rate to participate in therapy trials. Overall, the Enrichment cohort has maintained the percentage of AD participants at 18%and has seen the percentage of MCI participants increase from 12%(Inception) to 22%(Enrichment); greatly increasing the cross-sectional dataset of PET-Aβ-amyloid imaging and replenishing biobank stores.
Unsurprisingly, for both the Inception and Enrichment cohorts combined, the number of deaths at any timepoint was greatest in those classified with dementia due to AD, and those with AD were most likely to drop out of the study. By 126 months only 5 of the 211 Inception cohort AD subjects were still engaged with the study: 97 were known to have died and 109 had withdrawn and their current status was unknown.
Overall, for Inception and Enrichment cohorts combined, attrition was 1203 persons (of 2359, or 51%). Of these, 216 were known to have died and 987 failed to return. Of those who failed to return, 290 (29%) offered no specific reason, 133 (14%) were uninterested, 107 (11%) cited concomitant physical or medical illness, 88 (9%) found it too difficult logistically or had moved away, 65 (7%) were too cognitively impaired, 43 (4%) found it psychologically distressing, 32 (3%) reported being too busy, and 21 (2%) were unwilling to undergo PET neuroimaging or CSF collection. One hundred and eight subjects (11%) were withdrawn for other reasons (e.g., they entered a therapy trial, data collection was incomplete) and 100 (10%) were unable to be contacted.
Cognition
As impairment in cognition is the cornerstone manifestation of AD in both the preclinical and clinical stages, studies arising from the AIBL cohort confirmed the nature and magnitude of cognitive impairment in adults with MCI and dementia in this well characterized sample. These studies were the first to use prospective analyses to identify how abnormally high levels of Aβ-amyloid (Aβ+), detected via PET were associated with a subtle but relentless decline in memory and other aspects of higher cognition in CN older adults; even though these adults showed no change in their clinical status over the same time period [48, 55, 56]. From the opposite perspective we were also the first to demonstrate that in CN older adults, Aβ+status detected using a plasma Aβ composite biomarker successfully predicted decline in memory and executive function, and that the magnitude of this decline was equivalent to that observed when Aβ+status was classified using PET neuroimaging [57]. Over the course of 126 months, it also became clear that the CN older adults who had met the rigorous AIBL inclusion/exclusion criteria but who had normal levels of brain Aβ-amyloid (Aβ-), showed no decline in cognition over the same time-period, challenging the notion of age-related cognitive decline [58, 59].
The AIBL study was also the first to make use of computerized assessments of cognition in their experimental designs [60]. Data from the Cogstate Brief Battery (CBB), applied repeatedly in the AIBL study, showed that Aβ-amyloid-related cognitive decline could be detected on the basis of relatively brief assessments of visual learning and working memory in both preclinical and prodromal AD, and that this decline was associated strongly with loss of volume in medial temporal lobe brain regions [25, 61]. Furthermore, consistent with our observations using conventional neuropsychological tests, decline in performance on the CBB measures of learning and working memory was increased further in Aβ+ individuals who also carried at least one APOE ɛ4 allele [62]. The brevity of the CBB resulted in its use in an AIBL sub-study that sought to determine whether sensitivity to AD-related change was increased with reassessments conducted more frequently than the 18-month time period used for AIBL. Results from this ‘Rate Of Change Sub-study’ (AIBL-ROCS), confirmed that increased numbers of reassessments (i.e., > 8) improved sensitivity to Aβ-amyloid-related cognitive change in both preclinical and prodromal AD [63]. These data provided the foundation for the use of the CBB in other studies of AD genesis (e.g., the Mayo study of Health and Aging [64]) and in clinical trials designed to treat AD by clearing Aβ-amyloid prior to the development of cognitive impairment (e.g., [65, 66]).
As the AIBL sample and the number of reassessments increased, we identified that known genetic risk factors for AD, such as the APOE ɛ4 allele and variation in the BDNF Val66Met polymorphism, substantially influenced the course of cognitive decline, brain volume loss and clinical disease progression [47, 67]. For example, sophisticated modelling showed that the rate of decline in memory associated with Aβ+status increased with age, and this increase was greater in APOE ɛ4 carriers [67]. These studies also showed that decline in cognition through the preclinical and prodromal stages of AD was associated more strongly with changes in brain volume loss, than with increasing Aβ-amyloid levels [61]. As the duration of follow-up increased past 72, to 90 months, which meant that the number of re-assessments conducted also increased, the presentation of Aβ-amyloid-related cognitive dysfunction changed. Trajectories of memory performance in preclinical AD became flat (i.e., with slopes of zero), while performance in matched Aβ- adults improved [68]. This failure to benefit from experience observed in preclinical AD led to the development of experimental models which have now shown that the earliest and most severe cognitive impairment in preclinical AD manifests as a reduction in learning, as opposed to impairment in memory [69]. Moreover, with the knowledge that it is possible to identify cognitive dysfunction in Aβ- CN adults, we have sought to challenge our cognitive models further by recruiting and assessing middle-aged adults who are genetically at-risk of developing AD (i.e., APOE ɛ4 carriers). This has led to the development of the Healthy Brain Project, which is an online prospective cohort study designed to survey and test a large number of individuals with a family history of dementia [70]. Initial studies have already demonstrated that cognitive impairment in middle-aged APOE ɛ4 homozygotes can be readily detected using online tests of learning.
PET- Aβ-amyloid/tau
The number of PET-Aβ-amyloid scans conducted by calendar year, by clinical classification and PET-Aβ status (-ve/+ve), as well as by clinical classification progression group (CN to MCI to AD) is shown in Tables 5, 6, and 7, respectively. To date 1685 participants have undergone at least one Aβ-amyloid brain scan with 518 individuals undergoing three or more scans.
Table 5
Number of PET-Aβ-amyloid scans conducted each calendar year
| Collection * | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | Total |
| T0 | 4 | 109 | 175 | 0 | 17 | 32 | 135 | 315 | 105 | 207 | 257 | 201 | 90 | 38 | 1,685 |
| T1 | 0 | 0 | 20 | 171 | 33 | 2 | 2 | 88 | 194 | 119 | 59 | 79 | 135 | 39 | 941 |
| T2 | 0 | 0 | 0 | 1 | 47 | 119 | 11 | 2 | 2 | 36 | 97 | 99 | 76 | 28 | 518 |
| T3 | 0 | 0 | 0 | 0 | 0 | 12 | 90 | 32 | 7 | 1 | 2 | 10 | 50 | 9 | 213 |
| T4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 41 | 53 | 14 | 6 | 0 | 0 | 1 | 115 |
| T5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 11 | 52 | 17 | 12 | 2 | 1 | 95 |
| T6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 24 | 22 | 4 | 50 |
| T7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 0 | 6 |
| Total | 4 | 109 | 195 | 172 | 97 | 165 | 238 | 478 | 372 | 429 | 438 | 425 | 381 | 120 | 3,623 |
*Positron Emission Tomography (PET)-Aβ-amyloid collection is case-wise; i.e., T0 represents the first time a participant is imaged but may not correspond to their baseline cognitive assessment, T1 represents the second time a participant is imaged but may not correspond to their 18 month follow-up cognitive assessment, etc. The following Aβ-amyloid tracers were utilized: Pittsburgh Compound B (PiB), NAV4694, flutemetamol, florbetapir, or florbetaben.
Table 6
Number of PET-Aβ-amyloid scans conducted by clinical classification and PET-Aβ status
| PET-Aβ-amyloid collection | ||||||||||
| Clinical classification and PET-Aβ status | T0 | T1 | T2 | T3 | T4 | T5 | T6 | T7 | Total | |
| CN -ve | 773 | 533 | 282 | 119 | 62 | 51 | 30 | 3 | 1,853 | |
| CN +ve | 305 | 168 | 121 | 52 | 24 | 24 | 14 | 3 | 711 | |
| MCI -ve | 103 | 53 | 25 | 12 | 6 | 4 | 1 | 0 | 204 | |
| MCI +ve | 225 | 66 | 31 | 9 | 8 | 4 | 2 | 0 | 345 | |
| AD -ve | 26 | 8 | 5 | 0 | 0 | 1 | 0 | 0 | 40 | |
| AD +ve | 231 | 95 | 44 | 15 | 9 | 5 | 3 | 0 | 402 | |
| Other -ve | 13 | 5 | 1 | 3 | 3 | 3 | 0 | 0 | 28 | |
| Other +ve | 4 | 5 | 4 | 1 | 1 | 1 | 0 | 0 | 16 | |
| Not classified* | 5 | 8 | 5 | 2 | 2 | 2 | 0 | 0 | 24 | |
| Total | 1685 | 941 | 518 | 213 | 115 | 95 | 50 | 6 | 3,623 | |
*Imaged but withdrawn from cognitive stream. ‘PET-Aβ-amyloid collection’ is case-wise; i.e., T0 represents the first time a participant is imaged but may not correspond to their baseline cognitive assessment, T1 represents the second time a participant is imaged but may not correspond to their 18 month follow-up cognitive assessment, etc. The following Aβ-amyloid tracers were utilized: Pittsburgh Compound B (PiB), NAV4694, flutemetamol, florbetapir, or florbetaben. -ve, Aβ-amyloid negative (≤20 Centiloids);+ve, Aβ-amyloid positive (> 20 Centiloids); AD, Alzheimer’s disease; CN, cognitively normal; MCI, mild cognitive impairment; Other, dementia other than AD or cognitive impairment not suspected to be due to AD; PET, positron emission tomography.
Table 7
Number of PET-Aβ scans conducted by clinical classification progression group, and PET-Aβ status
| PET-Aβ status | ||||
| Clinical classification | -ve | +ve | Total scanned | Not scanned |
| CN | 685 | 286 | 971 | 340 |
| CN/MCI-CN | 31 | 16 | 47 | 5 |
| CN-MCI | 24 | 45 | 69 | 31 |
| MCI | 70 | 148 | 218 | 61 |
| CN-AD | 3 | 22 | 25 | 12 |
| MCI-AD | 11 | 64 | 75 | 25 |
| AD | 25 | 219 | 244 | 195 |
| Other | 20 | 14 | 34 | 7 |
| Total | 869 | 814 | 1,683 | 676 |
Individuals scanned (-ve,+ve, and Total scanned columns) have undergone at least one PET scan. Clinical classification groups are defined as follows: CN, MCI, AD, Other = individuals remain in these groups throughout study participation. CN/MCI-CN = individuals move from the CN classification to MCI, then back to CN during study participation. CN-MCI = progression from CN to MCI clinical classification during study participation. CN-AD = progression from CN to AD clinical classification during study participation. MCI-AD = progression from MCI to AD clinical classification during study participation. The clinical classification changes refer to the clinical visit history of a participant, while the T0 PET scan can occur at any stage during the AIBL visit history. For example, a participant could be listed as CN-MCI-AD, but the T0 PET scan occurred at the MCI or the AD stage. The following Aβ-amyloid tracers were utilized: Pittsburgh Compound B (PiB), NAV4694, flutemetamol, florbetapir, or florbetaben. -ve, Aβ-amyloid negative (≤20 Centiloids); +ve, Aβ-amyloid positive (> 20 Centiloids); AD, Alzheimer’s disease; CN, cognitively normal; MCI, mild cognitive impairment; Other, ementia other than AD or cognitive impairment not suspected to be due to AD; PET, positron emission tomography.
The application of Aβ-amyloid PET imaging from study commencement has enabled AIBL researchers to make seminal contributions to understanding AD progression. We demonstrated that Aβ-amyloid likely accumulates for 30 years to reach the level typically present in mild AD dementia, and that abnormal levels can conceivably be detected with PET 15–20 years before the onset of dementia [46, 71], identifying a wide window for prevention trials in the asymptomatic and prodromal disease phases.
AIBL has also confirmed the role of Aβ-amyloid in conversion from MCI to AD. Over 3 years, conversion risk in the cohort was associated most strongly with a positive Aβ-amyloid scan (odds ratio (OR) 15) with a positive predictive value of 84%[45]. The Aβ-amyloid-PET scan likely becomes positive 15–20 years prior to mild dementia, a decade before other imaging and cognitive tests become abnormal [45].
The recent development of tau-PET scans and their subsequent implementation in AIBL will likely yield further important insights into the development of AD. The number of tau-PET scans conducted by calendar year and by clinical classification in AIBL is depicted in Tables 8 and 9, respectively. To date 470 participants have undergone tau imaging, with 88 of these individuals undergoing at least one follow-up tau scan.
Table 8
Number of PET-tau scans conducted each calendar year
| Collection * | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | Total |
| T0 | 15 | 50 | 32 | 9 | 134 | 230 | 470 |
| T1 | 0 | 7 | 37 | 9 | 4 | 31 | 88 |
| T2 | 0 | 10 | 8 | 18 | |||
| T3 | 0 | 0 | 4 | 4 | |||
| Total | 15 | 57 | 69 | 18 | 148 | 273 | 580 |
*Positron Emission Tomography (PET)-tau collection is case-wise; i.e., T0 represents the first time a participant is PET-tau imaged but may not correspond to their baseline cognitive assessment, T1 represents the second time a participant is PET-tau imaged but may not correspond to their 18-month follow-up cognitive assessment, etc. The following tau tracers were utilized: AV1451 and MK6240.
Table 9
Number of PET-tau scans conducted by clinical classification
| PET-tau collection | |||||
| Clinical classification | T0 | T1 | T2 | T3 | Total |
| CN | 292 | 62 | 17 | 4 | 375 |
| MCI | 97 | 16 | 0 | 0 | 113 |
| AD | 78 | 10 | 1 | 0 | 89 |
| Other | 3 | 0 | 0 | 0 | 3 |
| Total | 470 | 88 | 18 | 4 | 580 |
‘PET-tau collection’ is case-wise; i.e., T0 represents the first time a participant is PET-tau imaged but may not correspond to their baseline cognitive assessment, T1 represents the second time a participant is PET-tau imaged but may not correspond to their 18 month follow-up cognitive assessment, etc. The following tau tracers were utilized: AV1451 and MK6240. AD, Alzheimer’s disease; CN, cognitively normal; MCI, mild cognitive impairment; Other, dementia other than AD or cognitive impairment not suspected to be due to AD; PET, positron emission tomography.
Magnetic resonance imaging
Nine hundred and fifty-four subjects have undergone structural MR imaging at least once (Table 10). Structural MRI using T1W Magnetization Prepared RApid Gradient Echo (MPRage) was used to elucidate the relationship between atrophy and Aβ-amyloid both cross-sectionally [72, 73] and longitudinally [45, 74]. An association between Aβ-amyloid deposition and atrophy in asymptomatic individuals was observed, as was little-to-no overlap between the patterns of atrophy and that of Aβ-amyloid deposition. In other studies, atrophy was also related to cognition (hippocampal volume; [25]) and physical activity (hippocampal and temporal lobe volumes; [75]), as well as to BDNF and KIBRA (KIdney and BRAin expressed protein) polymorphisms [75–77]. In addition to structural sequences, the AIBL MR protocol also includes FLuid-Attenuated Inversion Recovery (FLAIR) imaging, used to assess white matter lesions. These lesions were shown to be related to cognitive decline, neurodegeneration and age, but were not associated with rate of Aβ-amyloid accumulation [78, 79].
Table 10
Number of MRI scans conducted by clinical classification and PET-Aβ status
| MRI collection | ||||||||
| Clinical classification and PET-Aβ status | T0 | T1 | T2 | T3 | T4 | T5 | T6 | T7 |
| CN -ve | 442 | 370 | 193 | 104 | 102 | 47 | 12 | 17 |
| CN +ve | 228 | 94 | 54 | 31 | 38 | 16 | 2 | 9 |
| MCI -ve | 58 | 31 | 20 | 8 | 9 | 1 | 1 | 0 |
| MCI +ve | 105 | 36 | 16 | 7 | 12 | 1 | 1 | 0 |
| AD -ve | 12 | 4 | 4 | 0 | 0 | 1 | 0 | 0 |
| AD +ve | 103 | 62 | 24 | 19 | 9 | 5 | 1 | 1 |
| Other -ve | 3 | 3 | 2 | 4 | 3 | 0 | 1 | 0 |
| Other +ve | 3 | 3 | 2 | 0 | 1 | 1 | 0 | 0 |
| Total | 954 | 603 | 315 | 173 | 174 | 72 | 18 | 27 |
‘MRI collection’ is case-wise; i.e., T0 represents the first time a participant undergoes MRI but may not correspond to their baseline cognitive assessment, T1 represents the second time a participant undergoes MRI but may not correspond to their 18-month follow-up cognitive assessment, etc. The following Aβ-amyloid tracers were utilized: Pittsburgh Compound B (PiB), NAV4694, flutemetamol, florbetapir, or florbetaben. -ve, Aβ-amyloid negative (≤20 Centiloids);+ve, Aβ-amyloid positive (> 20 Centiloids); AD, Alzheimer’s disease; CN, cognitively normal; MCI, mild cognitive impairment; MRI, magnetic resonance imaging; Other, dementia other than AD or cognitive impairment not suspected to be due to AD; PET, positron emission tomography.
A subset of AIBL participants was imaged using more advanced MRI sequences including Diffusion Weighted Imaging (DWI), Arterial Spin Labelling (ASL), and Susceptibility Weighted Imaging (SWI). DWI was used to show a reduction of specific white matter fibers in AD [80]. An increase in cerebral blood flow, as measured using ASL, was observed in preclinical AD subjects, and linked with an increase in Aβ-amyloid [81]. Higher risk of cerebral microbleeds, as measured with SWI, was evident for subjects with high Aβ-amyloid burden [82]. More recently, the SWI data were reconstructed to provide Quantitative Susceptibility Mapping (QSM) for iron deposition measurement. These data showed that QSM could predict Aβ-related cognitive decline in a mixed diagnostic group [28]. More work is required to determine whether iron plays a role in enhancing susceptibility to Aβ-mediated neurodegeneration, or if iron deposition is instead a reflection of underlying damage to the brain.
Blood biomarkers: plasma Aβ/tau, lipidomics and metabolomics
Fasted blood samples have been collected at each 18-monthly participant visit, and 587,665 blood fraction aliquots have been stored in liquid nitrogen. This number includes over 100,000 serum aliquots, 200,000 EDTA plasma aliquots, 100,000 lithium heparin plasma aliquots, 35,000 platelet aliquots, 40,000 aliquots of Ficoll-purified white blood cells (stored in either dH2O or ‘RNA later’), and 90,000 aliquots of red blood cells.
Since AIBL commenced in 2006, high on the list of priorities has been the objective of developing a robust blood test for AD. We first confirmed the presence of Aβ dimers in plasma [83] and then explored the utility of early Aβ immunoassays [53, 75, 84–89]. These results confirmed that an Aβ signal was present in plasma, and that Aβ42 decreased as the Aβ-PET signal increased. We also explored levels of ApoE [90], ApoJ [91], inflammatory markers [92], and micro-RNA [93]. New Aβ immunoassays showed improved performances [94–96] but the combination of immunoprecipitation and mass spectrometry proved to have exceptional performance [97], equivalent to CSF assays. Notably, measurement of the natural history of plasma Aβ change, via one of the new immunoassays, suggests that the trajectory of plasma Aβ change mirrors brain Aβ-amyloid accumulation as measured by PET, with a rapid phase of plasma change observable up to 6 years prior to the rapid phase of brain Aβ-amyloid accumulation [96]. CSF and plasma Aβ42 are in equilibrium, and are expected to show changes earlier than Aβ-PET since the dynamic range of CSF change is greatest at the lower ranges of Aβ-PET.
Proteomic [98], lipidomic [99] and metabolomic approaches are now being explored. Moreover, functional assays of mononuclear cell phagocytosis have indicated that defects in the innate immune response could play a role in AD [100], confirming independent genome-wide association study (GWAS) results obtained by others.
3.7Cerebrospinal fluid biomarkers: Aβ, tau, neurofilament light chain
CSF for analysis has to date been donated by 262 subjects, with 242 of these individuals having also undergone Aβ-amyloid imaging (Table 11). At the time of writing, we have collected CSF at two timepoints from 109 subjects, and at three timepoints from 33 subjects.
Table 11
Number of CSF collections by clinical classification progression group, and PET-Aβ status
| Latest PET-AβResult | ||||||
| Clinical classification | -ve | +ve | Not scanned | Total | N with ≥2 collections | N with ≥3 collections |
| CN | 104 | 54 | 11 | 169 | 78 | 27 |
| CN/MCI-CN | 4 | 5 | 0 | 9 | 7 | 3 |
| CN-MCI | 2 | 4 | 1 | 7 | 5 | 1 |
| MCI | 9 | 14 | 3 | 26 | 5 | 0 |
| CN-AD | 0 | 2 | 0 | 2 | 2 | 0 |
| MCI-AD | 1 | 6 | 1 | 8 | 4 | 2 |
| AD | 1 | 30 | 3 | 34 | 6 | 0 |
| Other | 4 | 2 | 1 | 7 | 2 | 0 |
| Total | 125 | 117 | 20 | 262 | 109 | 33 |
Clinical classification groups are defined as follows: CN, MCI, AD, Other = individuals remain in these groups throughout study participation. CN/MCI-CN = individuals move from the CN classification to MCI, then back to CN during study participation. CN-MCI = progression from CN to MCI clinical classification during study participation. CN-AD = progression from CN to AD clinical classification during study participation. MCI-AD = progression from MCI to AD clinical classification during study participation. The clinical classification changes refer to the clinical visit history of a participant, while the T0 CSF collection can occur at any stage during the AIBL visit history. For example, a participant could be listed as CN-MCI-AD, but the T0 CSF collection occurred at the MCI or the AD stage. The following Aβ-amyloid tracers were utilized to determine -ve/+ve status: Pittsburgh Compound B (PiB), NAV4694, flutemetamol, florbetapir, or florbetaben. -ve, Aβ-amyloid negative (≤20 Centiloids); +ve, Aβ-amyloid positive (> 20 Centiloids); AD, Alzheimer’s disease; CN, cognitively normal; CSF, cerebrospinal fluid; MCI, mild cognitive impairment; Other, dementia other than AD or cognitive impairment not suspected to be due to AD; PET, positron emission tomography.
Strong concordance has been observed between CSF markers (Aβ42, tau, and pTau181) and PET-Aβ-amyloid status, as measured by the Roche Elecsys and INNOtest assays [30, 31]. A high correlation of CSF biomarkers on the AlzBio3 and EUROIMMUNE platforms has also been reported [101]. Methodologically, no difference in these biomarkers was observed from CSF collected using gravity or aspiration techniques [102]. Moreover, CSF neurofilament light chain (NfL), a non-specific marker of neuronal degradation, has been shown to correlate with both clinical performance and neuropathologic changes, including reduced cortical grey matter volume, but did not correlate with Aβ-amyloid burden [103].
Thresholds for abnormal CSF levels of Aβ42, tau and pTau, compared to a PiB SUVR of 1.5, have been calculated using the INNOtest [30]. Updated thresholds are presented here using a lower PiB SUVR threshold of 1.4, and for an updated version of the INNOtest, which controls the temperature of the experiment at 25°C. Using a rank based approach (0.1 fractile for Aβ42 and 0.9 fractile for tau and pTau181) in a larger cohort of N = 115 CN PET-amyloid negative participants, with tau data age bracketed, the normal reference ranges are tau < 303.5 pg/mL (60–70 years); 378.7 pg/mL (> 70 years), pTau181 < 59.2 pg/mL (60–70 years); 73.8 pg/mL (>70 years), and Aβ42 > 656.0 pg/mL (> 60 years). A different set of cut points applies to the Elecsys platform: using optimisation of Youden’s index, based on a cohort of 202 participants, the normal thresholds are for Aβ42 > 1054 pg/mL, tau < 213 pg/mL, and pTau181 < 21.3 pg/mL [31].
Lifestyle and environmental risk factors
The number of lifestyle assessments completed at each timepoint is shown in Table 12. The vast majority of CN and MCI participants completed self-report measures of physical activity (PA), diet and sleep at each timepoint (of note, diet was assessed at alternating timepoints, and sleep measures were first administered to Inception cohort participants at the 72-month follow-up but to Enrichment cohort participants from baseline). Objective measures of PA were collected via actigraphy from a subset of Perth-based CN participants as the foundation of two consecutive PhD student projects.
Table 12
Number of lifestyle assessments
| Lifestyle Assessment Collection, months* | ||||||||
| Lifestyle Assessment | 0 | 18 | 36 | 54 | 72 | 90 | 108 | 126 |
| Self-report PA | 1,747 | 1,167 | 851 | 701 | 481 | 387 | 326 | 222 |
| Objective PA | 248 | 33 | 195 | 120 | 86 | 35 | 82 | - |
| Diet (CCV FFQ)# | 1,905 | - | 1,066 | - | 575 | - | 363 | - |
| Self-report Sleep∧ | 616 | 498 | 360 | 198 | 581 | 441 | 377 | 307 |
*Corresponds to cognitive assessment timepoint (month 0 = baseline). # Data collected at alternating timepoints. ∧ Sleep measures were administered to Inception cohort participants from 72 months onwards, and to Enrichment participants from baseline onwards. CCV FFQ, Cancer Council of Victoria Food Frequency Questionnaire; PA, physical activity.
With detailed lifestyle data emerging from the cohort, AIBL is providing an exceptional opportunity to examine the relationship of PA, diet and sleep to cognitive, neuroimaging and fluid biomarker outcomes, as well as to investigate targeted genetic factors that may influence these relationships. Cross-sectional relationships between habitual PA levels and various cognitive and brain health markers have been reported. Objectively measured PA intensity was linked to better performance on cognitive tasks assessing executive function [104]. Moreover, self-reported PA was found to be associated with lower levels of brain Aβ-amyloid in carriers of the APOE ɛ4 allele [53] and larger hippocampal volume in BDNF Val/Val homozygotes [75].
The AIBL study was first to show that adherence to a healthy Mediterranean-style diet is more strongly associated with less decline in executive function among APOE ɛ4 carriers [105], and is associated with a slower rate of Aβ-amyloid accumulation in cognitively unimpaired older adults irrespective of APOE ɛ4 status [106]. We have also reported cross-sectional associations, in CN older adults, between carbohydrate intake and cognitive function, with specific cognitive domains affected in an APOE genotype-dependent manner [107], as well as between dietary protein intake and brain Aβ-amyloid burden, with higher protein intake associated with less likelihood of Aβ+ status [108].
Insights into the relationship between sleep and brain Aβ have also been attained, with increased time to fall asleep (sleep onset latency) associated with higher brain Aβ-amyloid [109], revealing a potential target for AD prevention strategies. Furthermore, the multidisciplinary nature of AIBL has enabled the first report of genetic variations in Aquaporin-4 (AQP4) impacting the relationship between sleep and brain Aβ-amyloid [54]. Aquaporin-4 proteins are water channel proteins, located primarily in the subpial and perivascular end-feet of astrocytic processes, which play a role in the brain’s night-time ‘housekeeping’ system. This system clears waste including Aβ-amyloid and is postulated to function almost entirely during sleep [110]. Our study showed that individuals with particular genetic variations in AQP4 are susceptible to high brain Aβ-amyloid levels if they experience poor sleep [54]. These findings suggest such individuals might benefit most, in terms of reduced AD risk, from an intervention to improve their sleep, bringing personalized strategies for AD prevention a step closer.
Genetics
Table 13 details the genetic analyses undertaken on AIBL samples to date. Samples from all 2359 participants have undergone APOE genotyping, whilst subsets have either undergone or are, at the time of submission, undergoing genome-wide SNP, whole exome sequencing and cross-sectional and longitudinal genome-wide peripheral DNA methylation analyses. These data have contributed to research across all areas of AIBL, providing important genetic covariates to individual studies or furthering the understanding of key genetic variables that impact disease presentation and progression.
Table 13
Number of genetic analyses conducted by technique
| Genetic Analysis | ||||
| APOE | Genome-Wide SNP* | Whole Exome Sequencing | Genome-Wide Methylation* | |
| N | 2,359 | 1,357 | 500 | 726 |
*Samples for both genome-wide SNP and genome-wide methylation analysis are currently being expanded. APOE, Apolipoprotein E; SNP, single nucleotide polymorphism (976,713 SNPs analyzed).
AIBL has contributed to the understanding of how individual genes [47, 67, 76, 77, 111–115] make variable contributions to cognitive decline in the preclinical and prodromal stages of AD. This has been extended to enhanced understanding of accumulative genetic contributions and whether genetic profiles for risk and progression differ. In AIBL, polygenic risk scores (PRS) weighted by AD-risk effect sizes were dependent on the presence of the APOE ɛ4 allele [51]. Moreover, the association, with cognitive decline, of genetic factors that are not major AD-risk genes, such as BDNF and KIBRA, led to the development of a cognitive-gene risk profile [52]. This profile was able to identify twice as many individuals at risk of preclinical AD-related cognitive decline than APOE ɛ4-BDNF Met66 interaction alone. Further, this profile identified that genetic variation in genes (e.g., KL; Klotho) that were not independently associated with preclinical AD cognitive decline, in AIBL, contributed to delineating these risk groups. These studies were further expanded to the development of a novel PRS that incorporated weighting by effect sizes associated with decline in episodic memory [50]. As opposed to the AD-risk weighted PRS, this novel PRS was associated with cognitive decline both in the presence and absence of APOE ɛ4.
AIBL genomics has also contributed further knowledge to the association of genetic markers with both PET-Aβ-amyloid status and MRI volumetrics [51, 76, 77, 116, 117] and, in collaboration with AIBL lifestyle researchers, has helped uncover genetic underpinnings of lifestyle-phenotype relationships, with a particular focus on APOE and BDNF and their interaction with PA [53, 75], diet [105, 106], and sleep [109]. This has been expanded to investigate a priori candidates, such as variation in AQP4. This study, as stated above, suggests that genetic variation in AQP4 moderates the relationship between sleep and brain Aβ-amyloid levels. Collectively, these studies emphasize the importance of continued study of gene-lifestyle interactions, and in turn a focus on lifestyle genomics, in AD, which could lead to the development and implementation of personalized strategies for AD prevention.
Strong collaborations, external to AIBL, have also seen AIBL contribute significantly to large multi-center genetic studies that have shed further light on genetic and epigenetic factors. These have suggested a more oligogenic rather than polygenic structure to AD-risk [118] and have provided insights into the contribution of peripheral genome-wide methylation across neurodegenerative disease.
Data harmonization and sharing
AIBL data have been provided to the Global Alzheimer’s Association Interactive Network (GAAIN), and software installed, thereby enabling GAAIN users to interrogate metadata and receive cohort summaries, whereupon users can request further information (and biofluid samples; blood and CSF) if needed by submitting an Expression of Interest (EoI). This mechanism ensures worldwide sharing and utilization of AIBL data (and biofluid samples) and complements the existing framework whereby AIBL imaging scans and demographic data are available on the ADNI LONI (Laboratory of Neuro Imaging, University of Southern California) website for free download and use by researchers worldwide. Up to 3 August 2019, LONI/ GAAIN data applications had been received from over 120 companies, more than 2,000 organizations/institutes/departments, and over 3,500 individuals, spanning 81 countries. Moreover, hundreds of EoIs from academic and industry-based individuals have been approved resulting in further data and biofluid sample sharing. Data will also be provided to the National Institute on Aging-funded ADOPIC (Alzheimer’s Dementia Onset and Progression in International Cohorts) project which will harmonise data from the AIBL, ADNI, Washington University, St. Louis, and University of Washington, Seattle, cohorts to determine factors which influence cognitive decline in AD.
Capacity building
The AIBL study framework has been responsible for training researchers and clinicians of the future, by supporting multiple Honours and MSc projects, more than 25 PhD projects, as well as over 15 clinical placement students. Moreover, the study framework and/or data have been used to successfully obtain funding from the National Institutes of Health (NIH), and the National Health and Medical Research Council (Australia’s peak funding body for medical research) as well as various philanthropic and non-government entities including The Yulgilbar Foundation and the Australian Alzheimer’s Research Foundation.
DISCUSSION
The AIBL Study has collected up to 8 timepoints of data at 18-month intervals in over 2350 participants, yielding a current database of 8592 person-contact years (as of 10 April 2019). The multicenter, multidisciplinary approach and engagement of Aβ-amyloid-PET imaging from the outset has enabled AIBL researchers to make world-class contributions to understanding the natural history of AD progression.
Using data from the AIBL cohort, the very slow accumulation rate of AD-related pathologic change has been calculated [45, 46], the diagnostic and prognostic utility of Aβ-amyloid imaging [44] have been confirmed, and a wide window of opportunity for prevention trials has been identified. This vital, new knowledge has been translated to the design and implementation of trials to prevent progression to dementia from AD.
Indeed, the cohorts and technologies provided by AIBL have facilitated early intervention trials in Australia and worldwide. AIBL has enabled Australia to form a large single site for participation in the Anti-Amyloid therapy in Asymptomatic AD (A4) trial, and to be a major contributor to the Dominantly Inherited Alzheimer Network (DIAN) and DIAN Therapy studies outside of the USA. Moreover, through tracer validation, and first in human studies with colleagues in Japan and the USA, AIBL researchers have played a key role in the recent development of PET scans for tau aggregates. Serial tau imaging has been added to the investigations in a subset of AIBL participants, and this new technology promises further important insights into the development of AD, potentially opening the door to new therapeutic approaches. New AIBL-developed PET imaging analysis methods and promising blood biomarkers have also generated international patents.
To date, AIBL has enabled several promising blood biomarker candidates and panels to be identified that include Aβ-amyloid species and ApoE levels, unique inflammatory and lipid-related compounds, genomics, and micro RNA markers from exosomes [87, 90, 93, 97, 119, 120]. Another blood biomarker developed from the AIBL cohort that correlates closely with Aβ-amyloid PET classification is under licensing negotiation with a pharmaceutical company.
AIBL has provided important insights into the cognitive, genetic, and Aβ-related changes that lead to AD. Assessments of visual learning and working memory in both preclinical and prodromal AD revealed Aβ-amyloid-related cognitive decline [25, 61], which was increased further in the presence of the APOE ɛ4 allele [62]. Variation in the BDNF Val66Met polymorphism, was also shown to substantially influence the course of cognitive decline, and clinical disease progression [47]. Moreover, seminal new work from the AIBL cohort has shown that the earliest and most severe cognitive impairment in preclinical AD manifests as a reduction in learning, as opposed to impairment in memory [69]. The learning deficit was much more evident than the memory deficit in the preclinical AD subjects. This may reflect damage to those areas of the Default Mode Network where Aβ-amyloid is seen to first begin accumulating, i.e., in the precuneus, posterior cingulate and frontal cortices.
Lifestyle correlation with cognitive and neuroimaging biomarker change over time has provided support for PA and dietary modification to slow AD development. This work has highlighted the importance of PA intensity as well as volume for cognitive performance and brain health [53, 104], and has demonstrated the ability of PA and a healthy Mediterranean-style diet to mitigate the negative effects of APOE ɛ4 carriage on cognitive decline and Aβ-amyloid accumulation [53, 105, 106]. Intervention studies are required to confirm the impact of PA and a Mediterranean-style diet on the accumulation of AD pathology in the brain. AIBL has also provided novel insights into the relationship between sleep and Aβ-amyloid; revealing associations between longer sleep onset latency and higher brain Aβ-amyloid [109], as well as a moderating effect of genetic variation in Aquaporin-4 on the relationship between sleep and brain Aβ-amyloid burden [54]. Collectively, these works have revealed potential targets for AD prevention studies, as well as bringing personalized strategies for AD prevention, based on genotype, a step closer.
New genetic factors that modulate rate of cognitive decline prior to dementia have been identified and significant contributions have been made to achieving a greater understanding of the genetic architecture of both AD risk and progression, particularly how these may differ both in terms of structure and contributing factors. In addition to the APOE ɛ4 allele [45] and the BDNF Met66 allele [47] mentioned above, AIBL has also demonstrated that specific genetic profiles [52], and a novel PRS [50], are all associated with faster cognitive decline in individuals with preclinical or prodromal AD. Many of these findings have been used in the design of early intervention trials such as the NIH- and industry-funded A4 Study [49]; AIBL investigators are contributing up to 150 participants to this trial. Further exploration of the genetic architecture of AD risk and progression will also be possible as AIBL extends both its genetic and epigenetic analyses. Such exploration has already seen AIBL contribute to large multi-center studies reporting that AD risk is likely more oligogenic than polygenic in structure [118].
AIBL continues to make significant contributions to international efforts focused on improving the understanding of AD pathogenesis and diagnosis, as well as contributing to the design of both pharmacologic and non-pharmacologic intervention studies aimed at preventing dementia due to AD. AIBL data sharing has occurred via the ADNI LONI website, GAAIN, the US-based Pre-clinical AD Consortium and an EU consortium (CONNECT-ND). AIBL data are also pooled with ADNI, Japan-ADNI, DIAN, the Adult Children Study, the Wisconsin Registry for Alzheimer’s Prevention, and other initiatives to increase statistical power, as well as a variety of European consortia through the European Union Joint Programme –Neurodegenerative Disease Research (JPND). Data will also be provided to the National Institute on Aging-funded ADOPIC project. Collectively, these approaches have ensured that AIBL data have been shared, across 81 countries, with researchers based in academia and industry.
Whilst it is evident that AIBL has made significant contributions to understanding the natural history of AD, and characterizing factors which impact the rate at which AD phenotypes develop, there are some limitations that should be noted. For example, the relatively high age of CN participants at baseline precludes direct quantification of the earliest AD-related changes. Only a subset of participants provided CSF samples, with few follow-up samples collected to date, which limits longitudinal biomarker analysis in this biofluid. Moreover, the cohort is predominantly Caucasian with a relatively high level of education, which limits the generalizability of our findings.
Despite these limitations, AIBL will continue to contribute to addressing the major challenges ahead for our field, which include, defining the earliest cut-points for onset of preclinical AD, developing practical algorithms for predicting rates of progression, and determining the generalizability of current findings into ethnically/ socially diverse populations. Moreover, the attribution of degrees of cognitive impairment, and the differential diagnosis of AD from the two major co-morbidities of FTD/ hippocampal sclerosis (TAR DNA-binding protein of about 43 kDa (TDP-43) pathology) and small vessel involvement in cerebrovascular disease in the elderly population, remain unresolved. Once these issues have been addressed, the optimal approach to designing primary and secondary prevention trials will become apparent.
In summary, AIBL is at the forefront of AD research. AIBL data have contributed to the development of new diagnostic criteria for AD that permit earlier and more accurate diagnosis [121–123], and to the design of early intervention trials aimed at preventing the development of dementia from AD. AIBL has united AD researchers across Australia and as part of worldwide ADNI, AIBL has greatly increased Australian collaboration with international AD research groups. AIBL infrastructure is now also supporting recruitment for academic and industry-funded therapy trials. Further refinement and validation of blood biomarkers for AD will be undertaken, genetic and epigenetic contributions and profiles for onset and progression will be developed, lifestyle influences on cognition and AD will be prospectively tracked, and more early intervention studies will be launched. Moreover, with over 340 publications to date and 2500 citations per year, the increasing value, impact and productivity of AIBL with time is apparent, as is the increasing value of accumulating longitudinal data.
ACKNOWLEDGMENTS
The AIBL study (www.AIBL.csiro.au) is a consortium between Austin Health, CSIRO, Edith Cowan University, the Florey Institute (The University of Melbourne), and the National Ageing Research Institute. The study has received partial financial support from the Alzheimer’s Association (US), the Alzheimer’s Drug Discovery Foundation, an Anonymous foundation, the Science and Industry Endowment Fund, the Dementia Collaborative Research Centres, the Victorian Government’s Operational Infrastructure Support program, the Australian Alzheimer’s Research Foundation, the National Health and Medical Research Council (NHMRC), and The Yulgilbar Foundation. Numerous commercial interactions have supported data collection and analyses. In-kind support has also been provided by Sir Charles Gairdner Hospital, Cogstate Ltd, Hollywood Private Hospital, The University of Melbourne, and St Vincent’s Hospital. SRRS is supported by an NHMRC Investigator Grant (GNT1197315). YYL is supported by an NHMRC Career Development Fellowship (GNT1162645).
The AIBL team wishes to thank all clinicians who referred patients with AD and/or MCI to the study. We also thank all those who took part as subjects in the study for their commitment and dedication to helping advance research into the early detection and causation of AD.
CONFLICT OF INTEREST
The following authors have no CoI to declare: CF, SRRS, SB, JB, PB, BMB, SCB, CC, JD, VD, KAE, LE, AF, JF, SLG, SG, RG, EH, RH, LJ, AK, FL, NTL, QXL, LL, YYL, AL, SLM, LMa, SM, LMi, MP, KP, TP, MRa, AR, JR, MRo, RR, OS, GS, BS, MS, HRS, KT, TT, CT, BT, RT, MV, MWe, MWo, YX. AIB is a shareholder in Alterity Therapeutics Ltd, Cogstate Ltd, Mesoblast Ltd, and is a paid consultant for, and has a profit share interest in, Collaborative Medicinal Development Pty Ltd. SC has received payments from Biogen for advice in relation to CSF biomarkers of Alzheimer’s disease. SML has previously been a paid consultant to Alzhyme. RNM is founder of, and owns stock in, Alzhyme, and is a co-founder of the KaRa Institute of Neurological Diseases. PM is a full-time employee of Cogstate Ltd. CLM is an advisor to Prana Biotechnology Ltd and a consultant to Eli Lilly. CCR has served on scientific advisory boards for Bayer Pharma, Elan Corporation, GE Healthcare and AstraZeneca, has received speaker honoraria from Bayer Pharma and GE Healthcare, and has received research support from Bayer Pharma, GE Healthcare, Piramal Lifesciences and Avid Radiopharmaceuticals. VLV has served as a consultant for Bayer Pharma and received research support from a NEDO grant from Japan. DA has served on scientific advisory boards for Novartis, Eli Lilly, Janssen, and Pfizer Inc.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-210005.
REFERENCES
[1] | Ellis KA , Bush AI , Darby D , De Fazio D , Foster J , Hudson P , Lautenschlager NT , Lenzo N , Martins RN , Maruff P , Masters C , Milner A , Pike K , Rowe C , Savage G , Szoeke C , Taddei K , Villemagne V , Woodward M , Ames D , AIBL ((2009) ) The Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging: Methodology and baseline characteristics of 1112 individuals recruited for a longitudinal study of Alzheimer’s disease. Int Psychogeriatr 21: , 672–687. |
[2] | Ellis KA , Szoeke C , Bush AI , Darby D , Graham PL , Lautenschlager NT , Macaulay SL , Martins RN , Maruff P , Masters CL , McBride SJ , Pike KE , Rainey-Smith SR , Rembach A , Robertson J , Rowe CC , Savage G , Villemagne VL , Woodward M , Wilson W , Zhang P , Ames D , AIBL ((2014) ) Rates of diagnostic transition and cognitive change at 18-month follow-up among 1,112 participants in the Australian Imaging, Biomarkers and Lifestyle Flagship Study of Ageing (AIBL). Int Psychogeriatr 26: , 543–554. |
[3] | Collie A , Maruff P , Shafiq-Antonacci R , Smith M , Hallup M , Schofield PR , Masters CL , Currie J ((2001) ) Memory decline in healthy older people: Implications for identifying mild cognitive impairment. Neurology 56: , 1533–1538. |
[4] | Maruff P , Collie A , Darby D , Weaver-Cargin J , Masters C , Currie J ((2004) ) Subtle memory decline over 12 months in mild cognitive impairment. Dement Geriatr Cogn Disord 18: , 342–348. |
[5] | Weaver Cargin J , Maruff P , Collie A , Masters C ((2006) ) Mild memory impairment in healthy older adults is distinct from normal aging. Brain Cogn 60: , 146–155. |
[6] | Cargin JW , Maruff P , Collie A , Shafiq-Antonacci R , Masters C ((2007) ) Decline in verbal memory in non-demented older adults. J Clin Exp Neuropsychol 29: , 706–718. |
[7] | Winblad B , Palmer K , Kivipelto M , Jelic V , Fratiglioni L , Wahlund LO , Nordberg A , Backman L , Albert M , Almkvist O , Arai H , Basun H , Blennow K , de Leon M , DeCarli C , Erkinjuntti T , Giacobini E , Graff C , Hardy J , Jack C , Jorm A , Ritchie K , van Duijn C , Visser P , Petersen RC ((2004) ) Mild cognitive impairment - beyond controversies, towards a consensus: Report of the International Working Group on Mild Cognitive Impairment. J Intern Med 256: , 240–246. |
[8] | Petersen RC , Smith GE , Waring SC , Ivnik RJ , Tangalos EG , Kokmen E ((1999) ) Mild cognitive impairment: Clinical characterization and outcome. Arch Neurol 56: , 303–308. |
[9] | Folstein MF , Folstein SE , McHugh PR ((1975) ) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: , 189–198. |
[10] | Delis D , Kramer J , Kaplan E , Ober B ((2000) ) California Verbal Learning Test-Second Edition., The Psychological Corporation, San Antonio, TX. |
[11] | Wechsler D ((1945) ) A standardised memory scale for clinical use. J Psychol 19: , 87–95. |
[12] | Delis DC , Kaplan E , Kramer JH ((2001) ) The Delis-Kaplan Executive Function System (D-KEFS), Psychological Corporation., San Antonio TX. |
[13] | Saxton J , Ratcliff G , Munro CA , Coffey EC , Becker JT , Fried L , Kuller L ((2000) ) Normative data on the Boston Naming Test and two equivalent 30-item short forms. Clin Neuropsychol 14: , 526–534. |
[14] | Wechsler D ((2001) ) Wechsler Test of Adult Reading: Examiner’s Manual. The Psychological Corporation, San Antonio, TX. |
[15] | Wechsler D ((1997) ) Wechsler Adult Intelligence Scale—Third edition (WAIS-III). Psychological Corporation, San Antonio, TX. |
[16] | Strauss E , Sherman , Spreen O ((2006) ) A compendium of neuropsychological tests: Administration, norms, and commentary (3rd Ed.). Oxford University Press, New York. |
[17] | Meyers JE , Meyers KR ((1995) ) Rey Complex Figure Test and Recognition Trial. Professional Manual, Psychological Assessment Resource, Inc. |
[18] | Sheikh JI , Yesavage JA ((1986) ) Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. Clin Gerontol 5: , 165–173. |
[19] | Snaith RP , Zigmond AS ((1986) ) The hospital anxiety and depression scale. Br Med J (Clin Res Ed) 292: , 344. |
[20] | Zigmond AS , Snaith RP ((1983) ) The hospital anxiety and depression scale. Acta Psychiatr Scand 67: , 361–370. |
[21] | Jorm AF , Jacomb PA ((1989) ) The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): Socio-demographic correlates, reliability, validity and some norms. Psychol Med 19: , 1015–1022. |
[22] | Morris JC ((1993) ) The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology 43: , 2412–2414. |
[23] | Bradfield NI , Ellis KA , Savage G , Maruff P , Burnham S , Darby D , Lautenschlager NT , Martins RN , Masters CL , Rainey-Smith SR , Robertson J , Rowe C , Woodward M , Ames D ((2021) ) Aggregation of abnormal memory scores and risk of incident Alzheimer’s disease dementia: A measure of objective memory impairment in amnestic mild cognitive impairment. J Int Neuropsychol Soc 27: , 146–157. |
[24] | Bransby L , Lim YY , Ames D , Fowler C , Roberston J , Harrington K , Snyder PJ , Villemagne VL , Salvado O , Masters CL , Maruff P for the AIBL Research Group ((2019) ) Sensitivity of a Preclinical Alzheimer’s Cognitive Composite (PACC) to amyloid beta load in preclinical Alzheimer’s disease. J Clin Exp Neuropsychol 41: , 591–600. |
[25] | Lim YY , Villemagne VL , Laws SM , Pietrzak RH , Ames D , Fowler C , Rainey-Smith S , Snyder PJ , Bourgeat P , Martins RN , Salvado O , Rowe CC , Masters CL , Maruff P , AIBL Research Group ((2016) ) Performance on the Cogstate Brief Battery is related to amyloid levels and hippocampal volume in very mild dementia. J Mol Neurosci 60: , 362–370. |
[26] | Harrington KD , Lim YY , Ellis KA , Copolov C , Darby D , Weinborn M , Ames D , Martins RN , Savage G , Szoeke C , Rowe C , Villemagne VL , Masters CL , Maruff P ((2013) ) The association of Abeta amyloid and composite cognitive measures in healthy older adults and MCI. Int Psychogeriatr 25: , 1667–1677. |
[27] | Rowe CC , Ellis KA , Rimajova M , Bourgeat P , Pike KE , Jones G , Fripp J , Tochon-Danguy H , Morandeau L , O’Keefe G , Price R , Raniga P , Robins P , Acosta O , Lenzo N , Szoeke C , Salvado O , Head R , Martins R , Masters CL , Ames D , Villemagne VL ((2010) ) Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging 31: , 1275–1283. |
[28] | Ayton S , Fazlollahi A , Bourgeat P , Raniga P , Ng A , Lim YY , Diouf I , Farquharson S , Fripp J , Ames D , Doecke J , Desmond P , Ordidge R , Masters CL , Rowe CC , Maruff P , Villemagne VL , AIBL, Salvado O , Bush AI ((2017) ) Cerebral quantitative susceptibility mapping predicts amyloid-beta-related cognitive decline. Brain 140: , 2112–2119. |
[29] | Vanderstichele H , Bibl M , Engelborghs S , Le Bastard N , Lewczuk P , Molinuevo JL , Parnetti L , Perret-Liaudet A , Shaw LM , Teunissen C , Wouters D , Blennow K ((2012) ) Standardization of preanalytical aspects of cerebrospinal fluid biomarker testing for Alzheimer’s disease diagnosis: A consensus paper from the Alzheimer’s Biomarkers Standardization Initiative. Alzheimers Dement 8: , 65–73. |
[30] | Li QX , Villemagne VL , Doecke JD , Rembach A , Sarros S , Varghese S , McGlade A , Laughton KM , Pertile KK , Fowler CJ , Rumble RL , Trounson BO , Taddei K , Rainey-Smith SR , Laws SM , Robertson JS , Evered LA , Silbert B , Ellis KA , Rowe CC , Macaulay SL , Darby D , Martins RN , Ames D , Masters CL , Collins S , AIBL Research Group ((2015) ) Alzheimer’s disease normative cerebrospinal fluid biomarkers validated in PET amyloid-beta characterized subjects from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study. J Alzheimers Dis 48: , 175–187. |
[31] | Doecke JD , Ward L , Burnham SC , Villemagne VL , Li QX , Collins S , Fowler CJ , Manuilova E , Widmann M , Rainey-Smith SR , Martins RN , Masters CL , AIBL Research Group ((2020) ) Elecsys CSF biomarker immunoassays demonstrate concordance with amyloid-PET imaging. Alzheimers Res Ther 12: , 36. |
[32] | Craig CL , Marshall AL , Sjostrom M , Bauman AE , Booth ML , Ainsworth BE , Pratt M , Ekelund U , Yngve A , Sallis JF , Oja P ((2003) ) International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 35: , 1381–1395. |
[33] | Hodge A , Patterson AJ , Brown WJ , Ireland P , Giles G ((2000) ) The Anti Cancer Council of Victoria FFQ: Relative validity of nutrient intakes compared with weighed food records in young to middle-aged women in a study of iron supplementation. Aust N Z J Public Health 24: , 576–583. |
[34] | Buysse DJ , Reynolds CF , 3rd Monk TH , Berman SR , Kupfer DJ ((1989) ) The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res 28: , 193–213. |
[35] | Purcell S , Neale B , Todd-Brown K , Thomas L , Ferreira MA , Bender D , Maller J , Sklar P , de Bakker PI , Daly MJ , Sham PC ((2007) ) PLINK: A tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81: , 559–575. |
[36] | Genomes Project C , Abecasis GR , Auton A , Brooks LD , DePristo MA , Durbin RM , Handsaker RE , Kang HM , Marth GT , McVean GA ((2012) ) An integrated map of genetic variation from 1,092 human genomes. Nature 491: , 56–65. |
[37] | Das S , Forer L , Schonherr S , Sidore C , Locke AE , Kwong A , Vrieze SI , Chew EY , Levy S , McGue M , Schlessinger D , Stambolian D , Loh PR , Iacono WG , Swaroop A , Scott LJ , Cucca F , Kronenberg F , Boehnke M , Abecasis GR , Fuchsberger C ((2016) ) Next-generation genotype imputation service and methods. Nat Genet 48: , 1284–1287. |
[38] | Li H , Durbin R ((2009) ) Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25: , 1754–1760. |
[39] | DePristo MA , Banks E , Poplin R , Garimella KV , Maguire JR , Hartl C , Philippakis AA , del Angel G , Rivas MA , Hanna M , McKenna A , Fennell TJ , Kernytsky AM , Sivachenko AY , Cibulskis K , Gabriel SB , Altshuler D , Daly MJ ((2011) ) A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet 43: , 491–498. |
[40] | McLaren W , Gil L , Hunt SE , Riat HS , Ritchie GR , Thormann A , Flicek P , Cunningham F ((2016) ) The Ensembl variant effect predictor. Genome Biol 17: , 122. |
[41] | Nabais MF , Lin T , Benyamin B , Williams KL , Garton FC , Vinkhuyzen AAE , Zhang F , Vallerga CL , Restuadi R , Freydenzon A , Zwamborn RAJ , Hop PJ , Robinson MR , Gratten J , Visscher PM , Hannon E , Mill J , Brown MA , Laing NG , Mather KA , Sachdev PS , Ngo ST , Steyn FJ , Wallace L , Henders AK , Needham M , Veldink JH , Mathers S , Nicholson G , Rowe DB , Henderson RD , McCombe PA , Pamphlett R , Yang J , Blair IP , McRae AF , Wray NR ((2020) ) Significant out-of-sample classification from methylation profile scoring for amyotrophic lateral sclerosis. NPJ Genom Med 5: , 10. |
[42] | Min JL , Hemani G , Davey Smith G , Relton C , Suderman M ((2018) ) Meffil: Efficient normalization and analysis of very large DNA methylation datasets. Bioinformatics 34: , 3983–3989. |
[43] | Fortin JP , Labbe A , Lemire M , Zanke BW , Hudson TJ , Fertig EJ , Greenwood CM , Hansen KD ((2014) ) Functional normalization of 450k methylation array data improves replication in large cancer studies. Genome Biol 15: , 503. |
[44] | Rowe CC , Bourgeat P , Ellis KA , Brown B , Lim YY , Mulligan R , Jones G , Maruff P , Woodward M , Price R , Robins P , Tochon-Danguy H , O’Keefe G , Pike KE , Yates P , Szoeke C , Salvado O , Macaulay SL , O’Meara T , Head R , Cobiac L , Savage G , Martins R , Masters CL , Ames D , Villemagne VL ((2013) ) Predicting Alzheimer disease with beta-amyloid imaging: Results from the Australian imaging, biomarkers, and lifestyle study of ageing. Ann Neurol 74: , 905–913. |
[45] | Lim YY , Ellis KA , Ames D , Darby D , Harrington K , Martins RN , Masters CL , Rowe C , Savage G , Szoeke C , Villemagne VL , Maruff P , AIBL Research Group ((2013) ) Abeta amyloid, cognition, and APOE genotype in healthy older adults. Alzheimers Dement 9: , 538–545. |
[46] | Villemagne VL , Pike KE , Chetelat G , Ellis KA , Mulligan RS , Bourgeat P , Ackermann U , Jones G , Szoeke C , Salvado O , Martins R , O’Keefe G , Mathis CA , Klunk WE , Ames D , Masters CL , Rowe CC ((2011) ) Longitudinal assessment of Abeta and cognition in aging and Alzheimer disease. Ann Neurol 69: , 181–192. |
[47] | Lim YY , Villemagne VL , Laws SM , Pietrzak RH , Snyder PJ , Ames D , Ellis KA , Harrington K , Rembach A , Martins RN , Rowe CC , Masters CL , Maruff P ((2015) ) APOE and BDNF polymorphisms moderate amyloid beta-related cognitive decline in preclinical Alzheimer’s disease. Mol Psychiatry 20: , 1322–1328. |
[48] | Lim YY , Maruff P , Pietrzak RH , Ames D , Ellis KA , Harrington K , Lautenschlager NT , Szoeke C , Martins RN , Masters CL , Villemagne VL , Rowe CC , AIBL Research Group ((2014) ) Effect of amyloid on memory and non-memory decline from preclinical to clinical Alzheimer’s disease. Brain 137: , 221–231. |
[49] | Sperling RA , Rentz DM , Johnson KA , Karlawish J , Donohue M , Salmon DP , Aisen P ((2014) ) The A4 study: Stopping AD before symptoms begin? Sci Transl Med 6: , 228fs213. |
[50] | Porter T , Burnham SC , Savage G , Lim YY , Maruff P , Milicic L , Peretti M , Ames D , Masters CL , Martins RN , Rainey-Smith S , Rowe CC , Salvado O , Taddei K , Groth D , Verdile G , Villemagne VL , Laws SM ((2018) ) A polygenic risk score derived from episodic memory weighted genetic variants is associated with cognitive decline in preclinical Alzheimer’s disease. Front Aging Neurosci 10: , 423. |
[51] | Porter T , Burnham SC , Milicic L , Savage G , Maruff P , Lim YY , Li QX , Ames D , Masters CL , Rainey-Smith S , Rowe CC , Salvado O , Groth D , Verdile G , Villemagne VL , Laws SM , AIBL Research Group ((2018) ) Utility of an Alzheimer’s disease risk-weighted polygenic risk score for predicting rates of cognitive decline in preclinical Alzheimer’s disease: A prospective longitudinal study. J Alzheimers Dis 66: , 1193–1211. |
[52] | Porter T , Villemagne VL , Savage G , Milicic L , Ying Lim Y , Maruff P , Masters CL , Ames D , Bush AI , Martins RN , Rainey-Smith S , Rowe CC , Taddei K , Groth D , Verdile G , Burnham SC , Laws SM ((2018) ) Cognitive gene risk profile for the prediction of cognitive decline in presymptomatic Alzheimer’s disease. Pers Med Psychiatry 7: , 14–20. |
[53] | Brown BM , Peiffer JJ , Taddei K , Lui JK , Laws SM , Gupta VB , Taddei T , Ward VK , Rodrigues MA , Burnham S , Rainey-Smith SR , Villemagne VL , Bush A , Ellis KA , Masters CL , Ames D , Macaulay SL , Szoeke C , Rowe CC , Martins RN ((2013) ) Physical activity and amyloid-beta plasma and brain levels: Results from the Australian Imaging, Biomarkers and Lifestyle Study of Ageing. Mol Psychiatry 18: , 875–881. |
[54] | Rainey-Smith SR , Mazzucchelli GN , Villemagne VL , Brown BM , Porter T , Weinborn M , Bucks RS , Milicic L , Sohrabi HR , Taddei K , Ames D , Maruff P , Masters CL , Rowe CC , Salvado O , Martins RN , Laws SM , AIBL Research Group ((2018) ) Genetic variation in Aquaporin-4 moderates the relationship between sleep and brain Abeta-amyloid burden. Transl Psychiatry 8: , 47. |
[55] | Lim YY , Maruff P , Pietrzak RH , Ellis KA , Darby D , Ames D , Harrington K , Martins RN , Masters CL , Szoeke C , Savage G , Villemagne VL , Rowe CC , AIBL Research Group ((2014) ) Abeta and cognitive change: Examining the preclinical and prodromal stages of Alzheimer’s disease. Alzheimers Dement 10: , 743–751 e741. |
[56] | Lim YY , Ellis KA , Harrington K , Pietrzak RH , Gale J , Ames D , Bush AI , Darby D , Martins RN , Masters CL , Rowe CC , Savage G , Szoeke C , Villemagne VL , Maruff P ((2013) ) Cognitive decline in adults with amnestic mild cognitive impairment and high amyloid-beta: Prodromal Alzheimer’s disease? J Alzheimers Dis 33: , 1167–1176. |
[57] | Lim YY , Maruff P , Kaneko N , Doecke J , Fowler C , Villemagne VL , Kato T , Rowe CC , Arahata Y , Iwamoto S , Ito K , Tanaka K , Yanagisawa K , Masters CL , Nakamura A ((2020) ) Plasma amyloid-beta biomarker associated with cognitive decline in preclinical Alzheimer’s disease. J Alzheimers Dis 77: , 1057–1065. |
[58] | Harrington KD , Schembri A , Lim YY , Dang C , Ames D , Hassenstab J , Laws SM , Rainey-Smith S , Robertson J , Rowe CC , Sohrabi HR , Salvado O , Weinborn M , Villemagne VL , Masters CL , Maruff P , AIBL Research Group ((2018) ) Estimates of age-related memory decline are inflated by unrecognized Alzheimer’s disease. Neurobiol Aging 70: , 170–179. |
[59] | Dang C , Harrington KD , Lim YY , Ames D , Hassenstab J , Laws SM , Yassi N , Hickey M , Rainey-Smith SR , Robertson J , Rowe CC , Sohrabi HR , Salvado O , Weinborn M , Villemagne VL , Masters CL , Maruff P , AIBL ((2019) ) Superior memory reduces 8-year risk of mild cognitive impairment and dementia but not amyloid beta-associated cognitive decline in older adults. Arch Clin Neuropsychol 34: , 585–598. |
[60] | Maruff P , Lim YY , Darby D , Ellis KA , Pietrzak RH , Snyder PJ , Bush AI , Szoeke C , Schembri A , Ames D , Masters CL , AIBL ((2013) ) Clinical utility of the Cogstate Brief Battery in identifying cognitive impairment in mild cognitive impairment and Alzheimer’s disease. BMC Psychol 1: , 30. |
[61] | Lim YY , Pietrzak RH , Bourgeat P , Ames D , Ellis KA , Rembach A , Harrington K , Salvado O , Martins RN , Snyder PJ , Masters CL , Rowe CC , Villemagne VL , Maruff P ((2015) ) Relationships between performance on the Cogstate Brief Battery, neurodegeneration, and Abeta accumulation in cognitively normal older adults and adults with MCI. Arch Clin Neuropsychol 30: , 49–58. |
[62] | Lim YY , Villemagne VL , Pietrzak RH , Ames D , Ellis KA , Harrington K , Snyder PJ , Martins RN , Masters CL , Rowe CC , Maruff P , Australian Imaging Biomarkers and Lifestyle (AIBL) Research Group ((2015) ) APOE epsilon4 moderates amyloid-related memory decline in preclinical Alzheimer’s disease. Neurobiol Aging 36: , 1239–1244. |
[63] | Lim YY , Pietrzak RH , Ellis KA , Jaeger J , Harrington K , Ashwood T , Szoeke C , Martins RN , Bush AI , Masters CL , Rowe CC , Villemagne VL , Ames D , Darby D , Maruff P ((2013) ) Rapid decline in episodic memory in healthy older adults with high amyloid-beta. J Alzheimers Dis 33: , 675–679. |
[64] | Mielke MM , Machulda MM , Hagen CE , Edwards KK , Roberts RO , Pankratz VS , Knopman DS , Jack CR Jr. , Petersen RC ((2015) ) Performance of the CogState computerized battery in the Mayo Clinic Study on Aging. Alzheimers Dement 11: , 1367–1376. |
[65] | Bateman RJ , Benzinger TL , Berry S , Clifford DB , Duggan C , Fagan AM , Fanning K , Farlow MR , Hassenstab J , McDade EM , Mills S , Paumier K , Quintana M , Salloway SP , Santacruz A , Schneider LS , Wang G , Xiong C , DIAN-TU Pharma Consortium for the Dominantly Inherited Alzheimer Network ((2017) ) The DIAN-TU Next Generation Alzheimer’s prevention trial: Adaptive design and disease progression model. Alzheimers Dement 13: , 8–19. |
[66] | Papp KV , Rentz DM , Maruff P , Sun CK , Raman R , Donohue MC , Schembri A , Stark C , Yassa MA , Wessels AM , Yaari R , Holdridge KC , Aisen PS , Sperling RA ((2021) ) The Computerized Cognitive Composite (C3) in an Alzheimer’s Disease Secondary Prevention Trial. J Prev Alzheimers Dis 8: , 59–67. |
[67] | Lim YY , Kalinowski P , Pietrzak RH , Laws SM , Burnham SC , Ames D , Villemagne VL , Fowler CJ , Rainey-Smith SR , Martins RN , Rowe CC , Masters CL , Maruff PT ((2018) ) Association of beta-amyloid and apolipoprotein E epsilon4 with memory decline in preclinical Alzheimer disease. JAMA Neurol 75: , 488–494. |
[68] | Baker JE , Pietrzak RH , Laws SM , Ames D , Villemagne VL , Rowe CC , Masters CL , Maruff P , Lim YY ((2019) ) Visual paired associate learning deficits associated with elevated beta-amyloid in cognitively normal older adults. Neuropsychology 33: , 964–974. |
[69] | Lim YY , Baker JE , Bruns L Jr., Mills A , Fowler C , Fripp J , Rainey-Smith SR , Ames D , Masters CL , Maruff P ((2020) ) Association of deficits in short-term learning and Abeta and hippocampal volume in cognitively normal adults. Neurology 95: , e2577–e2585. |
[70] | Lim YY , Yassi N , Bransby L , Properzi M , Buckley R ((2019) ) The healthy brain project: An online platform for the recruitment, assessment, and monitoring of middle-aged adults at risk of developing Alzheimer’s disease. J Alzheimers Dis 68: , 1211–1228. |
[71] | Villemagne VL , Burnham S , Bourgeat P , Brown B , Ellis KA , Salvado O , Szoeke C , Macaulay SL , Martins R , Maruff P , Ames D , Rowe CC , Masters CL , Australian Imaging Biomarkers and Lifestyle (AIBL) Research Group ((2013) ) Amyloid beta deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: A prospective cohort study. Lancet Neurol 12: , 357–367. |
[72] | Bourgeat P , Chetelat G , Villemagne VL , Fripp J , Raniga P , Pike K , Acosta O , Szoeke C , Ourselin S , Ames D , Ellis KA , Martins RN , Masters CL , Rowe CC , Salvado O , AIBL Research Group ((2010) ) Beta-amyloid burden in the temporal neocortex is related to hippocampal atrophy in elderly subjects without dementia. Neurology 74: , 121–127. |
[73] | Chetelat G , Villemagne VL , Bourgeat P , Pike KE , Jones G , Ames D , Ellis KA , Szoeke C , Martins RN , O’Keefe GJ , Salvado O , Masters CL , Rowe CC , Australian Imaging Biomarkers and Lifestyle Research Group ((2010) ) Relationship between atrophy and beta-amyloid deposition in Alzheimer disease. Ann Neurol 67: , 317–324. |
[74] | Dore V , Villemagne VL , Bourgeat P , Fripp J , Acosta O , Chetelat G , Zhou L , Martins R , Ellis KA , Masters CL , Ames D , Salvado O , Rowe CC ((2013) ) Cross-sectional and longitudinal analysis of the relationship between Abeta deposition, cortical thickness, and memory in cognitively unimpaired individuals and in Alzheimer disease. JAMA Neurol 70: , 903–911. |
[75] | Brown BM , Bourgeat P , Peiffer JJ , Burnham S , Laws SM , Rainey-Smith SR , Bartres-Faz D , Villemagne VL , Taddei K , Rembach A , Bush A , Ellis KA , Macaulay SL , Rowe CC , Ames D , Masters CL , Maruff P , Martins RN , AIBL Research Group ((2014) ) Influence of BDNF Val66Met on the relationship between physical activity and brain volume. Neurology 83: , 1345–1352. |
[76] | Lim YY , Villemagne VL , Laws SM , Ames D , Pietrzak RH , Ellis KA , Harrington K , Bourgeat P , Bush AI , Martins RN , Masters CL , Rowe CC , Maruff P , AIBL Research Group ((2014) ) Effect of BDNF Val66Met on memory decline and hippocampal atrophy in prodromal Alzheimer’s disease: A preliminary study. PLoS One 9: , e86498. |
[77] | Porter T , Burnham SC , Dore V , Savage G , Bourgeat P , Begemann K , Milicic L , Ames D , Bush AI , Maruff P , Masters CL , Rowe CC , Rainey-Smith S , Martins RN , Groth D , Verdile G , Villemagne VL , Laws SM ((2018) ) KIBRA is associated with accelerated cognitive decline and hippocampal atrophy in APOE epsilon4-positive cognitively normal adults with high Aβ-amyloid burden. Sci Rep 8: , 2034. |
[78] | Yassi N , Hilal S , Xia Y , Lim YY , Watson R , Kuijf H , Fowler C , Yates P , Maruff P , Martins R , Ames D , Chen C , Rowe CC , Villemagne VL , Salvado O , Desmond PM , Masters CL ((2020) ) Influence of comorbidity of cerebrovascular disease and amyloid-β on Alzheimer’s disease. J Alzheimers Dis 73: , 897–907. |
[79] | Xia Y , Yassi N , Raniga P , Bourgeat P , Desmond P , Doecke J , Ames D , Laws SM , Fowler C , Rainey-Smith SR , Martins R , Maruff P , Villemagne VL , Masters CL , Rowe CC , Fripp J , Salvado O , AIBL ((2020) ) Comorbidity of cerebrovascular and Alzheimer’s disease in aging. J Alzheimers Dis 78: , 321–334. |
[80] | Mito R , Raffelt D , Dhollander T , Vaughan DN , Tournier JD , Salvado O , Brodtmann A , Rowe CC , Villemagne VL , Connelly A ((2018) ) Fibre-specific white matter reductions in Alzheimer’s disease and mild cognitive impairment. Brain 141: , 888–902. |
[81] | Fazlollahi A , Calamante F , Liang X , Bourgeat P , Raniga P , Dore V , Fripp J , Ames D , Masters CL , Rowe CC , Connelly A , Villemagne VL , Salvado O , Australian Imaging Biomarkers and Lifestyle (AIBL) Research Group ((2020) ) Increased cerebral blood flow with increased amyloid burden in the preclinical phase of Alzheimer’s disease. J Magn Reson Imaging 51: , 505–513. |
[82] | Yates PA , Desmond PM , Phal PM , Steward C , Szoeke C , Salvado O , Ellis KA , Martins RN , Masters CL , Ames D , Villemagne VL , Rowe CC , AIBL Research Group ((2014) ) Incidence of cerebral microbleeds in preclinical Alzheimer disease. Neurology 82: , 1266–1273. |
[83] | Villemagne VL , Perez KA , Pike KE , Kok WM , Rowe CC , White AR , Bourgeat P , Salvado O , Bedo J , Hutton CA , Faux NG , Masters CL , Barnham KJ ((2010) ) Blood-borne amyloid-beta dimer correlates with clinical markers of Alzheimer’s disease. J Neurosci 30: , 6315–6322. |
[84] | Lui JK , Laws SM , Li QX , Villemagne VL , Ames D , Brown B , Bush AI , De Ruyck K , Dromey J , Ellis KA , Faux NG , Foster J , Fowler C , Gupta V , Hudson P , Laughton K , Masters CL , Pertile K , Rembach A , Rimajova M , Rodrigues M , Rowe CC , Rumble R , Szoeke C , Taddei K , Taddei T , Trounson B , Ward V , Martins RN , AIBL Research Group ((2010) ) Plasma amyloid-beta as a biomarker in Alzheimer’s disease: The AIBL study of aging. J Alzheimers Dis 20: , 1233–1242. |
[85] | Lambert JC , Dallongeville J , Ellis KA , Schraen-Maschke S , Lui J , Laws S , Dumont J , Richard F , Cottel D , Berr C , Ames D , Masters CL , Rowe CC , Szoeke C , Tzourio C , Dartigues JF , Buée L , Martins R , Amouyel P ((2011) ) Association of plasma Aß peptides with blood pressure in the elderly. PLoS One 6: , e18536. |
[86] | Doecke JD , Laws SM , Faux NG , Wilson W , Burnham SC , Lam CP , Mondal A , Bedo J , Bush AI , Brown B , De Ruyck K , Ellis KA , Fowler C , Gupta VB , Head R , Macaulay SL , Pertile K , Rowe CC , Rembach A , Rodrigues M , Rumble R , Szoeke C , Taddei K , Taddei T , Trounson B , Ames D , Masters CL , Martins RN ((2012) ) Blood-based protein biomarkers for diagnosis of Alzheimer disease. Arch Neurol 69: , 1318–1325. |
[87] | Burnham SC , Faux NG , Wilson W , Laws SM , Ames D , Bedo J , Bush AI , Doecke JD , Ellis KA , Head R , Jones G , Kiiveri H , Martins RN , Rembach A , Rowe CC , Salvado O , Macaulay SL , Masters CL , Villemagne VL ((2014) ) A blood-based predictor for neocortical Aβ burden in Alzheimer’s disease: Results from the AIBL study. Mol Psychiatry 19: , 519–526. |
[88] | Rembach A , Stingo FC , Peterson C , Vannucci M , Do KA , Wilson WJ , Macaulay SL , Ryan TM , Martins RN , Ames D , Masters CL , Doecke JD ((2015) ) Bayesian graphical network analyses reveal complex biological interactions specific to Alzheimer’s disease. J Alzheimers Dis 44: , 917–925. |
[89] | Burnham SC , Rowe CC , Baker D , Bush AI , Doecke JD , Faux NG , Laws SM , Martins RN , Maruff P , Macaulay SL , Rainey-Smith S , Savage G , Ames D , Masters CL , Wilson W , Villemagne VL ((2016) ) Predicting Alzheimer disease from a blood-based biomarker profile: A 54-month follow-up. Neurology 87: , 1093–1101. |
[90] | Gupta VB , Laws SM , Villemagne VL , Ames D , Bush AI , Ellis KA , Lui JK , Masters C , Rowe CC , Szoeke C , Taddei K , Martins RN , AIBL Research Group ((2011) ) Plasma apolipoprotein E and Alzheimer disease risk: The AIBL study of aging. Neurology 76: , 1091–1098. |
[91] | Gupta VB , Doecke JD , Hone E , Pedrini S , Laws SM , Thambisetty M , Bush AI , Rowe CC , Villemagne VL , Ames D , Masters CL , Macaulay SL , Rembach A , Rainey-Smith SR , Martins RN ((2016) ) Plasma apolipoprotein J as a potential biomarker for Alzheimer’s disease: Australian Imaging, Biomarkers and Lifestyle study of aging. Alzheimers Dement (Amst) 3: , 18–26. |
[92] | Pedrini S , Gupta VB , Hone E , Doecke J , O’Bryant S , James I , Bush AI , Rowe CC , Villemagne VL , Ames D , Masters CL , Martins RN ((2017) ) A blood-based biomarker panel indicates IL-10 and IL-12/23p40 are jointly associated as predictors of β-amyloid load in an AD cohort. Sci Rep 7: , 14057. |
[93] | Cheng L , Doecke JD , Sharples RA , Villemagne VL , Fowler CJ , Rembach A , Martins RN , Rowe CC , Macaulay SL , Masters CL , Hill AF , Australian Imaging Biomarkers and Lifestyle (AIBL) Research Group ((2015) ) Prognostic serum miRNA biomarkers associated with Alzheimer’s disease shows concordance with neuropsychological and neuroimaging assessment. Mol Psychiatry 20: , 1188–1196. |
[94] | Fandos N , Pérez-Grijalba V , Pesini P , Olmos S , Bossa M , Villemagne VL , Doecke J , Fowler C , Masters CL , Sarasa M ((2017) ) Plasma amyloid β 42/40 ratios as biomarkers for amyloid β cerebral deposition in cognitively normal individuals. Alzheimers Dement (Amst) 8: , 179–187. |
[95] | Doecke JD , Pérez-Grijalba V , Fandos N , Fowler C , Villemagne VL , Masters CL , Pesini P , Sarasa M ((2020) ) Total Aβ(42)/Aβ(40) ratio in plasma predicts amyloid-PET status, independent of clinical AD diagnosis. Neurology 94: , e1580–e1591. |
[96] | Burnham SC , Fandos N , Fowler C , Pérez-Grijalba V , Dore V , Doecke JD , Shishegar R , Cox T , Fripp J , Rowe C , Sarasa M , Masters CL , Pesini P , Villemagne VL ((2020) ) Longitudinal evaluation of the natural history of amyloid-β in plasma and brain. Brain Commun 2: , fcaa041. |
[97] | Nakamura A , Kaneko N , Villemagne VL , Kato T , Doecke J , Doré V , Fowler C , Li QX , Martins R , Rowe C , Tomita T , Matsuzaki K , Ishii K , Ishii K , Arahata Y , Iwamoto S , Ito K , Tanaka K , Masters CL , Yanagisawa K ((2018) ) High performance plasma amyloid-β biomarkers for Alzheimer’s disease. Nature 554: , 249–254. |
[98] | Ashton NJ , Nevado-Holgado AJ , Barber IS , Lynham S , Gupta V , Chatterjee P , Goozee K , Hone E , Pedrini S , Blennow K , Schöll M , Zetterberg H , Ellis KA , Bush AI , Rowe CC , Villemagne VL , Ames D , Masters CL , Aarsland D , Powell J , Lovestone S , Martins R , Hye A ((2019) ) A plasma protein classifier for predicting amyloid burden for preclinical Alzheimer’s disease. Sci Adv 5: , eaau7220. |
[99] | Lim WLF , Huynh K , Chatterjee P , Martins I , Jayawardana KS , Giles C , Mellett NA , Laws SM , Bush AI , Rowe CC , Villemagne VL , Ames D , Drew BG , Masters CL , Meikle PJ , Martins RN ((2020) ) Relationships between plasma lipids species, gender, risk factors, and Alzheimer’s disease. J Alzheimers Dis 76: , 303–315. |
[100] | Gu BJ , Huang X , Ou A , Rembach A , Fowler C , Avula PK , Horton A , Doecke JD , Villemagne VL , Macaulay SL , Maruff P , Fletcher EL , Guymer R , Wiley JS , Masters CL ((2016) ) Innate phagocytosis by peripheral blood monocytes is altered in Alzheimer’s disease. Acta Neuropathol 132: , 377–389. |
[101] | Doecke JD , Rembach A , Villemagne VL , Varghese S , Rainey-Smith S , Sarros S , Evered LA , Fowler CJ , Pertile KK , Rumble RL , Trounson B , Taddei K , Laws SM , Macaulay SL , Bush AI , Ellis KA , Martins R , Ames D , Silbert B , Vanderstichele H , Masters CL , Darby DG , Li QX , Collins S , AIBL Research Group ((2018) ) Concordance between cerebrospinal fluid biomarkers with Alzheimer’s disease pathology between three independent assay platforms. J Alzheimers Dis 61: , 169–183. |
[102] | Rembach A , Evered LA , Li QX , Nash T , Vidaurre L , Fowler CJ , Pertile KK , Rumble RL , Trounson BO , Maher S , Mooney F , Farrow M , Taddei K , Rainey-Smith S , Laws SM , Macaulay SL , Wilson W , Darby DG , Martins RN , Ames D , Collins S , Silbert B , Masters CL , Doecke JD , the AIBL Research Group ((2015) ) Alzheimer’s disease cerebrospinal fluid biomarkers are not influenced by gravity drip or aspiration extraction methodology. Alzheimers Res Ther 7: , 71. |
[103] | Dhiman K , Gupta VB , Villemagne VL , Eratne D , Graham PL , Fowler C , Bourgeat P , Li QX , Collins S , Bush AI , Rowe CC , Masters CL , Ames D , Hone E , Blennow K , Zetterberg H , Martins RN ((2020) ) Cerebrospinal fluid neurofilament light concentration predicts brain atrophy and cognition in Alzheimer’s disease. Alzheimers Dement (Amst) 12: , e12005. |
[104] | Brown BM , Peiffer JJ , Sohrabi HR , Mondal A , Gupta VB , Rainey-Smith SR , Taddei K , Burnham S , Ellis KA , Szoeke C , Masters CL , Ames D , Rowe CC , Martins RN , AIBL research group ((2012) ) Intense physical activity is associated with cognitive performance in the elderly. Transl Psychiatry 2: , e191. |
[105] | Gardener SL , Rainey-Smith SR , Barnes MB , Sohrabi HR , Weinborn M , Lim YY , Harrington K , Taddei K , Gu Y , Rembach A , Szoeke C , Ellis KA , Masters CL , Macaulay SL , Rowe CC , Ames D , Keogh JB , Scarmeas N , Martins RN ((2015) ) Dietary patterns and cognitive decline in an Australian study of ageing. Mol Psychiatry 20: , 860–866. |
[106] | Rainey-Smith SR , Gu Y , Gardener SL , Doecke JD , Villemagne VL , Brown BM , Taddei K , Laws SM , Sohrabi HR , Weinborn M , Ames D , Fowler C , Macaulay SL , Maruff P , Masters CL , Salvado O , Rowe CC , Scarmeas N , Martins RN ((2018) ) Mediterranean diet adherence and rate of cerebral Abeta-amyloid accumulation: Data from the Australian Imaging, Biomarkers and Lifestyle Study of Ageing. Transl Psychiatry 8: , 238. |
[107] | Gardener SL , Rainey-Smith SR , Sohrabi HR , Weinborn M , Verdile G , Fernando W , Lim YY , Harrington K , Burnham S , Taddei K , Masters CL , Macaulay SL , Rowe CC , Ames D , Maruff P , Martins RN , AIBL Research Group ((2017) ) Increased carbohydrate intake is associated with poorer performance in verbal memory and attention in an APOE genotype-dependent manner. J Alzheimers Dis 58: , 193–201. |
[108] | Fernando WMADB , Rainey-Smith SR , Gardener SL , Villemagne VL , Burnham SC , Macaulay SL , Brown BM , Gupta VB , Sohrabi HR , Weinborn M , Taddei K , Laws SM , Goozee K , Ames D , Fowler C , Maruff P , Masters CL , Salvado O , Rowe CC , Martins RN , AIBL Research Group ((2018) ) Associations of dietary protein and fiber intake with brain and blood amyloid-beta. J Alzheimers Dis 61: , 1589–1598. |
[109] | Brown BM , Rainey-Smith SR , Villemagne VL , Weinborn M , Bucks RS , Sohrabi HR , Laws SM , Taddei K , Macaulay SL , Ames D , Fowler C , Maruff P , Masters CL , Rowe CC , Martins RN , AIBL Research Group ((2016) ) The relationship between sleep quality and brain amyloid burden. Sleep 39: , 1063–1068. |
[110] | Xie L , Kang H , Xu Q , Chen MJ , Liao Y , Thiyagarajan M , O’Donnell J , Christensen DJ , Nicholson C , Iliff JJ , Takano T , Deane R , Nedergaard M ((2013) ) Sleep drives metabolite clearance from the adult brain. Science 342: , 373–377. |
[111] | Lim YY , Laws SM , Villemagne VL , Pietrzak RH , Porter T , Ames D , Fowler C , Rainey-Smith S , Snyder PJ , Martins RN , Salvado O , Bourgeat P , Rowe CC , Masters CL , Maruff P ((2016) ) Abeta-related memory decline in APOE epsilon4 noncarriers: Implications for Alzheimer disease. Neurology 86: , 1635–1642. |
[112] | Lim YY , Rainey-Smith S , Lim Y , Laws SM , Gupta V , Porter T , Bourgeat P , Ames D , Fowler C , Salvado O , Villemagne VL , Rowe CC , Masters CL , Zhou XF , Martins RN , Maruff P ((2017) ) BDNF Val66Met in preclinical Alzheimer’s disease is associated with short-term changes in episodic memory and hippocampal volume but not serum mBDNF. Int Psychogeriatr 29: , 1825–1834. |
[113] | Lim YY , Villemagne VL , Laws SM , Ames D , Pietrzak RH , Ellis KA , Harrington KD , Bourgeat P , Salvado O , Darby D , Snyder PJ , Bush AI , Martins RN , Masters CL , Rowe CC , Nathan PJ , Maruff P , Australian Imaging , Biomarkers and Lifestyle (AIBL) Research Group ((2013) ) BDNF Val66Met, Abeta amyloid, and cognitive decline in preclinical Alzheimer’s disease. Neurobiol Aging 34: , 2457–2464. |
[114] | Porter T , Burnham SC , Milicic L , Savage G , Maruff P , Lim YY , Ames D , Masters CL , Martins RN , Rainey-Smith S , Rowe CC , Salvado O , Groth D , Verdile G , Villemagne VL , Laws SM ((2019) ) Klotho allele status is not associated with Abeta and APOE epsilon4-related cognitive decline in preclinical Alzheimer’s disease. Neurobiol Aging 76: , 162–165. |
[115] | Porter T , Burnham SC , Milicic L , Savage G , Maruff P , Sohrabi HR , Peretti M , Lim YY , Weinborn M , Ames D , Masters CL , Martins RN , Rainey-Smith S , Rowe CC , Salvado O , Groth D , Verdile G , Villemagne VL , Laws SM ((2019) ) COMT val158met is not associated with Abeta-amyloid and APOE epsilon4 related cognitive decline in cognitively normal older adults. IBRO Rep 6: , 147–152. |
[116] | Lim YY , Williamson R , Laws SM , Villemagne VL , Bourgeat P , Fowler C , Rainey-Smith S , Salvado O , Martins RN , Rowe CC , Masters CL , Maruff P , AIBL ((2017) ) Effect of APOE genotype on amyloid deposition, brain volume, and memory in cognitively normal older individuals. J Alzheimers Dis 58: , 1293–1302. |
[117] | Vacher M , Porter T , Villemagne VL , Milicic L , Peretti M , Fowler C , Martins R , Rainey-Smith S , Ames D , Masters CL , Rowe CC , Doecke JD , Laws SM ((2019) ) Validation of a priori candidate Alzheimer’s disease SNPs with brain amyloid-beta deposition. Sci Rep 9: , 17069. |
[118] | Zhang Q , Sidorenko J , Couvy-Duchesne B , Marioni RE , Wright MJ , Goate AM , Marcora E , Huang KL , Porter T , Laws SM , Australian Imaging Biomarkers and Lifestyle (AIBL) Study; Sachdev PS , Mather KA , Armstrong NJ , Thalamuthu A , Brodaty H , Yengo L , Yang J , Wray NR , McRae AF , Visscher PM ((2020) ) Risk prediction of late-onset Alzheimer’s disease implies an oligogenic architecture. Nat Commun 11: , 4799. |
[119] | Rembach A , Faux NG , Watt AD , Pertile KK , Rumble RL , Trounson BO , Fowler CJ , Roberts BR , Perez KA , Li QX , Laws SM , Taddei K , Rainey-Smith S , Robertson JS , Vandijck M , Vanderstichele H , Barnham KJ , Ellis KA , Szoeke C , Macaulay L , Rowe CC , Villemagne VL , Ames D , Martins RN , Bush AI , Masters CL , AIBL research group ((2014) ) Changes in plasma amyloid beta in a longitudinal study of aging and Alzheimer’s disease. Alzheimers Dement 10: , 53–61. |
[120] | Faux NG , Rembach A , Wiley J , Ellis KA , Ames D , Fowler CJ , Martins RN , Pertile KK , Rumble RL , Trounson B , Masters CL , AIBL Research Group; Bush AI ((2014) ) An anemia of Alzheimer’s disease. Mol Psychiatry 19: , 1227–1234. |
[121] | Dubois B , Feldman HH , Jacova C , Hampel H , Molinuevo JL , Blennow K , DeKosky ST , Gauthier S , Selkoe D , Bateman R , Cappa S , Crutch S , Engelborghs S , Frisoni GB , Fox NC , Galasko D , Habert MO , Jicha GA , Nordberg A , Pasquier F , Rabinovici G , Robert P , Rowe C , Salloway S , Sarazin M , Epelbaum S , de Souza LC , Vellas B , Visser PJ , Schneider L , Stern Y , Scheltens P , Cummings JL ((2014) ) Advancing research diagnostic criteria for Alzheimer’s disease: The IWG-2 criteria. Lancet Neurol 13: , 614–629. |
[122] | Albert MS , DeKosky ST , Dickson D , Dubois B , Feldman HH , Fox NC , Gamst A , Holtzman DM , Jagust WJ , Petersen RC , Snyder PJ , Carrillo MC , Thies B , Phelps CH ((2011) ) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: , 270–279. |
[123] | Sperling RA , Aisen PS , Beckett LA , Bennett DA , Craft S , Fagan AM , Iwatsubo T , Jack CR Jr., Kaye J , Montine TJ , Park DC , Reiman EM , Rowe CC , Siemers E , Stern Y , Yaffe K , Carrillo MC , Thies B , Morrison-Bogorad M , Wagster MV , Phelps CH ((2011) ) Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: , 280–292. |




