The Importance of Diagnosing and the Clinical Potential of Treating Obstructive Sleep Apnea to Delay Mild Cognitive Impairment and Alzheimer’s Disease: A Special Focus on Cognitive Performance
Abstract
Obstructive sleep apnea (OSA) is a highly frequent sleep disorder in the middle-aged and older population, and it has been associated with an increased risk of developing cognitive decline and dementia, including mild cognitive impairment (MCI) and Alzheimer’s disease (AD). In more recent years, a growing number of studies have focused on: 1) the presence of OSA in patients with MCI or AD, 2) the link between OSA and markers of AD pathology, and 3) the role of OSA in accelerating cognitive deterioration in patients with MCI or AD. Moreover, some studies have also assessed the effects of continuous positive airway pressure (CPAP) treatment on the cognitive trajectory in MCI and AD patients with comorbid OSA. This narrative review summarizes the findings of studies that analyzed OSA as a risk factor for developing MCI and/or AD in the middle-aged and older populations with a special focus on cognition. In addition, it describes the results regarding the effects of CPAP treatment in hampering the progressive cognitive decline in AD and delaying the conversion to AD in MCI patients. Considering the importance of identifying and treating OSA in patients with MCI or AD in order to prevent or reduce the progression of cognitive decline, further larger and adequately powered studies are needed both to support these findings and to set programs for the early recognition of OSA in patients with cognitive impairment.
INTRODUCTION
A growing number of studies have documented an association between obstructive sleep apnea (OSA) and the increased risk of cognitive decline and Alzheimer’s disease (AD) [1–3]. OSA is a highly frequent sleep disorder, and it is characterized by repeated episodes of upper airways obstruction during sleep, causing intermittent hypoxia (IH), sleep fragmentation (SF), and excessive daytime sleepiness (EDS). Polysomnography (PSG) is considered the gold standard tool for OSA diagnosis [4], which is made through the assessment of physiological signals during sleep, namely from oronasal airflow, respiratory effort, and pulse oximetry. Further, the apnea-hypopnea index (AHI) reflects OSA severity degree and is defined as the number of respiratory events (apneas and hypopneas) per hour of sleep. OSA syndrome is diagnosed when patients present an AHI≥5 events/h on PSG coupled with reporting symptoms of impaired daytime function, or AHI≥15 events/h [5]. The global recommendation is to start OSA treatment as soon as the diagnosis is made [5]. It has been recently evidenced that OSA can impair brain and body health since it has been associated with diabetes, hypertension, metabolic syndrome, and depression [6–15]. Therefore, OSA represents a growing healthcare problem affecting middle-aged and older adults with several clinical consequences [6, 9–13, 15–20]. Consistently, OSA is associated with different medical disorders, and it has also been recognized as a risk factor for AD; moreover, OSA is a common comorbidity in patients with mild cognitive impairment (MCI) as well as AD dementia (ADD), with rates of prevalence up to 40%[21, 22].
The overall prevalence of OSA in the general population is still to be re-defined worldwide, since it has been estimated to be between 2 and 4%, with an increased rate in middle-aged and older adults [23–25]. Consistently, a recent population-based study reported a prevalence of moderate-to-severe sleep-disordered breathing of 23.4%in women and 49.7%in men [6].
OSA treatment can depend on disease severity, symptoms and contributing causes. The treatment of choice for OSA is continuous positive airway pressure (CPAP), although surgical approaches, oral devices, pharmacological therapies, and oropharyngeal muscles stimulators have emerged as an efficacious treatment against OSA [26–31]. Past literature, following the evidence that OSA can impair cognition, thus causing attention deficit and memory impairment, demonstrated that CPAP can restore cognitive performances both in young and in cognitively unimpaired patients [32]. On the other hand, in spite of some recent evidence stating that OSA can increase the risk of developing cognitive impairment and dementia (AD or vascular dementia), several studies have focused on proving that CPAP treatment can improve neuropsychological performances also in patients with cognitive impairment [33–36]. Hence, any intervention that enhances cognition in patients with dementia is likely to have a wider impact, since improving daily function may lead to a patients’ greater independence, lower caregiver burden, and lesser nursing assistance and social support.
In more recent years, growing attention was given to the associations between OSA, markers of AD pathology, and the development of cognitive impairment and the progression to dementia. Regarding the prevalence of OSA in AD, a quantitative meta-analytical study [37] documented that patients with AD have a five times higher likelihood of presenting OSA when compared to cognitively unimpaired individuals of similar age. This meta-analysis, based on five cross-sectional studies, detected that around 50%of patients with AD experienced OSA at some time after their initial diagnosis. However, few studies were evaluated and all were conducted in the late 1980s [37].
Most of the narrative reviews published in the last year focused on the possible explanatory mechanisms linking OSA to dementia, as well as explored dementia biomarkers in OSA [3, 38–40].
Indeed, OSA has been currently considered a risk factor for AD [41], since it can induce or aggravate neurodegenerative mechanisms such as amyloid-β (Aβ) pathology [16, 18], oxidative stress, synaptic dysfunction, and neuroinflammation [40].
There were also other systematic and meta-reviews focused on how OSA affects specific neurocognitive domains in adults; nevertheless, the findings reported were inconsistent [42, 43] and sometimes non-conclusive [44, 45]. The only meta-review focusing on OSA and cognition in older adults reported a weak association between OSA and cognitive impairment, concluding that only specific populations of OSA patients may be at risk of cognitive decline [46]. Recently, a systematic review [47], including 68 studies, documented that OSA is frequently associated with mild impairment in attention, memory, and executive functions in middle-aged adults, whereas it is not associated with any particular pattern of cognitive impairment in older adults. This systematic review [47] also examined some randomized controlled trials (RCTs) evaluating the effects of CPAP treatment and suggesting the relation between improvement of sleep parameters (slow-wave sleep –SWS and EDS) and cognitive performances in AD patients with OSA. Other studies highlighted the effects of CPAP treatment in modifying AD biomarkers among older adults with comorbid OSA and AD. In spite of taking all this recently published literature into account [32, 38], the present review will not be focusing on AD biomarkers changes in patients with OSA, but it will focus instead exclusively on AD-related cognitive function in patients with OSA and the effects of CPAP on these cognitive domains in middle-aged and older adults.
Hence, the current narrative review of the literature aims at combining two important aspects linking OSA to MCI or AD: on the one hand, it analyses the impact of OSA on the possible development of MCI and/or AD in the middle-aged and adult populations from a cognitive point of view; on the other hand, it describes the effects of CPAP treatment in hampering the progressive cognitive decline in AD and delaying the clinical conversion to AD in MCI patients. The methodology of the study is described in Box 1.
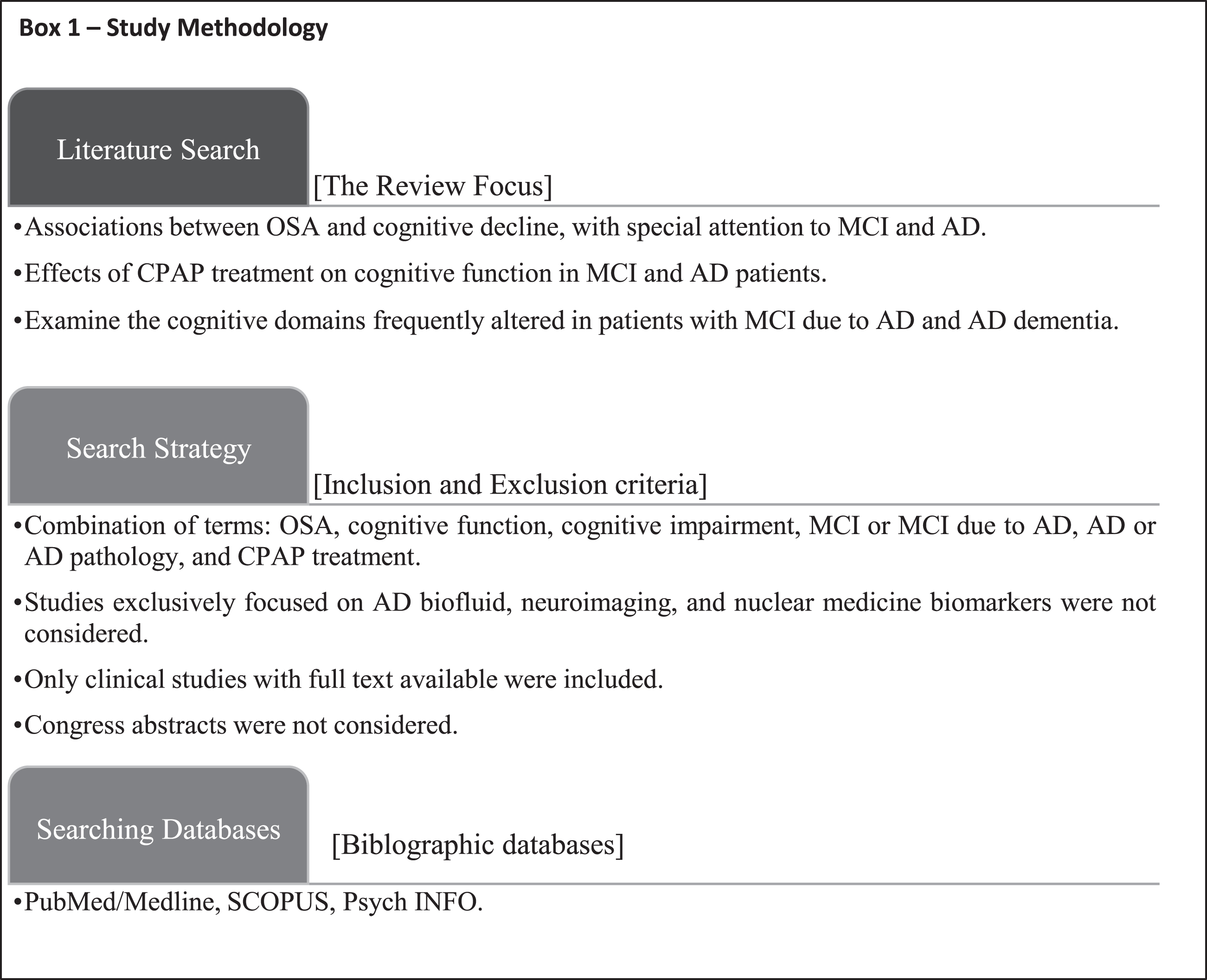
OSA AS A RISK FOR DEVELOPING MCI AND AD
Several studies have examined the relation between OSA and MCI in the last decade. However, studies focusing on the association between OSA and AD diagnosis are few and mainly conducted in community samples and nursing homes. Table 1 presents the summary findings from 17 studies that considered the relation between OSA and cognitive functioning in MCI and AD patients, which will be discussed in detail in this section.
Table 1
Studies’ characteristics and main findings: OSA and MCI/AD
| Authors, Year Published | Study Design, Setting and Country | Participants | OSA assessment | Cognitive Assessment: Tests and Diagnostic Criteria | Adjusted Variables | Main Findings | |||||
| Patients | Controls | ||||||||||
| N | Gender | N | Age | N | Age | ||||||
| Chang et al., 2013 [56] | Longitudinal, community based, Taiwan | 8,464 | 5,034 M 3,450 F | 1,414 OSA | 40%–40–49 y 30.9%–50–59 y 15.5%–60–69 y 13.6%–≥70 y | 7,050 Non-OSA | 40%–40–49 y 30.9%–50–59 y 15.5%–60–69 y 13.6%–≥70 y | Clinical diagnosis (ICD-9) | Dementia diagnosis based on ICD-9 criteria | Age, sex, CVD comorbidities, income, urbanization | OSA was associated with increased dementia risk within 5 years, and OSA was found to be gender, age, and time-dependent. |
| Dlugaj et al., 2014 [7] | Cross-sectional, population-based, Germany | 1,793 | 919 M 874 F | M = 63.8 (SD = 7.5) | AHI | Neuropsychological test battery to assess memory and executive functions | OSA model adjusted: Age, sex, education.Poor sleep quality model adjusted also: BMI, diabetes, systolic blood pressure, coronary heart disease, APOE e4, smoking, antidepressant use, benzodiazepine use, severe depressive symptoms and pure alcohol intake. | OSA not associated with MCI or MCI subtypes. Poor sleep quality was associated with MCI. | |||
| Foley et al., 2003 [59] | Cross-sectional, The Honolulu-Asia Aging Study of Sleep Apnea, Hawaii | 718 | Only men | 27.2%with ≥85y | PSGAHIESS | Cognitive impairment assessed through Cognitive Abilities Screening Instrument | Age, education, and marital status | OSA was associated with more drowsiness, but not with poor cognitive functioning. | |||
| Hoch et al., 1986 [49] | Cross-sectional, USA | 139 | 33 M 47 F | 34 AD | M = 71.5 (SD = 8.1) | 56 HC | M = 69.3 (SD = 5.4) | PSG, AHI | Dementia diagnosis based on DSM-III criteria | None | A significant association between the AHI and severity of dementia in apnea-positive AD patients, as well as in the entire sample of AD patients. |
| Hoch et al., 1989 [52] | Cross-sectional, Geriatric units, senior clubs, USA | 27 | 7 M 20 F | 15 OSA | M = 74.5 (SD = 5.1) | 12 Non-OSA | M = 70.2 (SD = 5.6) | PSG, AHI | AD diagnosis was made through NICNDSS-ADRDA and DSM-III criteria,Global cognition (MMSE and CDR) | None | Overnight mental status deterioration was not associated with measures of OSA. |
| Jorge et al. 2020 [62] | Longitudinal, Cognitive Disorders Unit,Spain | 125 | 72 F 53 M | 81 OSA | M = 74.0 | 44 Non-OSA | M = 76.0 | PSG,AHI | Neuropsychological test battery to assess memory, language and praxis. | Age, sex, body mass index, HTA, pharmacological treatment and Alzheimer status | No significant difference in any cognitive subdomains at 12 months and no differences among OSA severity groups in patients with mild to moderate AD. |
| Kim et al., 2011 [48] | Cross-sectional, Clinic,South Korea | 60 | 42 M 18 F | 30 MCI | M = 67.4 (SD = 3.8) | 30 HC | M = 68.0 (SD = 4.1) | PSG,AHI | MCI diagnosis was made using CERAD-K criteriaNeuropsychological battery to assess executive function, language, memory and visuospatial construction | Gender | A significant association between severe OSA and impaired language function in MCI patients. |
| Lee et al., 2019 [57] | Longitudinal, community,Republic of Korea | 4,362 | 3,332 M 1,030 F | 727 OSA | 48.8% – 40–49 y 34.9% – 50–59 y 14.2% – 60–69 y 2.06% – ≥70 y | 3,635 Non-OSA | 49.7% – 40–49 y 30.4% – 50–59 y 14.0% – 60–69 y 2.94% – ≥70 y | NHIS record of clinical diagnosis | Dementia diagnosis based on ICD-10 criteria | Sex, age, CVD, hypertension, Type 2 Diabetes, depression, BMI, smoking status, physical activity, and drinking | Patients with OSA were 1.575 times more likely to develop AD than those without OSA |
| Lutsey et al., 2016 [60] | Longitudinal, community, USA | 966 | 469 M 497 F | M = 61.3 (SD = 5.0) | Home PSQ, AHI | Executive function, memory and language were assessed through DigitSymbol Substitution Test,Delayed Word Recall Test Word Fluency Test | Age, sex, field centre, education; ethanol intake, smoking status, leisure-time physical activity, APOEe4, BMI, high-sensitivity C-reactive protein, diabetes mellitus, hypertension, and prevalent coronary heart disease, heart failure, or stroke. | OSA category and additional indices of sleep were not associated with a change in any cognitive test. | |||
| Lutsey et al., 2018 [61] | Longitudinal, Community, USA | 1,667 | 790 M 877 F | 818 OSA | M = 63.6 (SD = 5.4) | 849 | M = 62.0 (SD = 5.5) | Home PSQ, AHI | Dementia and MCI were assessed through TICSm and neurocognitive exam | Age, sex, education, field centre, physical activity, drinking, smoking status, leisure time, APOE e4, BMI | Late-midlife severe OSA was associated with all-cause and ADD in later life. |
| Osorio et al., 2015 [2] | Prospective, ADNI cohort, USA | 195 | 138 M 57 F | 12 MCI 35 AD | M = 74.6 (SD = 5.8)M = 73.1 (SD = 6.6) | 50 MCI 98 AD | M = 72.1 (SD = 5.9)M = 71.9 (SD = 6.9) | Self-reported | MCI and AD diagnosis made by a clinician | APOE e4 status, sex, education, BMI, depression, cardiovascular disease, hypertension, diabetes, and age | A significant association between OSA and earlier age at cognitive decline. |
| Reynolds et al., 1985 [50] | Cross-sectional, USA | 61 | 19 M 42 F | 21AD | M = 70.3 (SD = 7.9) | 23 HC | M = 69.3 (SD = 5.6) | PSG, AHI | Dementia diagnosis based on DSM-III criteria, MMSE, CDR and a modified Hachinski Ischemia score | Sex | A significant association between OSA and dementia in women. |
| Reynolds et al., 1987 [51] | Cross-sectional, USA | 30 | 3 M 27 F | 15 | M = 73.3 (SD = 9.1) | 15 | M = 72.6 (SD = 7.8) | 24 Chanel polygraphs | Dementia diagnosis based on DSM-III criteria, MMSE, CDR and a modified Hachinski Ischemia score | None | No association between OSA and dementia. |
| Smallwood et al., 1983 [53] | Cross-sectional, Media Solicitation and AD Centers, USA | 55 | 45 M 10 F | 15 | M: M = 65.5 (SD = 2.3)F: M = 69.5 (SD = 4.4) | 40 | M: M = 60 (SD = 1.31) F: M = 65.5 (SD = 2.2) | AHIRespiratory inductive plethysmo-graphy | AD diagnosis based on DSM-III criteria, Neurological examination | Age, sex | No relation between OSA and dementia. |
| Tsai et al. 2020 [58] | Retrospective cohort study, Taiwan | 19,890 | 13,110 M 6,780 F | 3,978 OSA | 70.5%: 40–59 y29.5%:≥60 y | 15,912 Non-OSA | 70.5%: 40–59 y29.5%:≥60 y | ICD-9 CM, NHIRD CPAP, surgeries, drugs | AD diagnosis based on ICD-9 CM criteria | Sex, age, urbanization level, income, and comorbidities. | OSA was independently associated with an increased risk of AD. Treatment for OSA reduces the AD risk in OSA patients. |
| Yaffe et al., 2011 [54] | Longitudinal, Community, USA | 298 | Women Only | 105 OSA | M = 82.6 (SD = 3.1) | 193 Non-OSA | M = 82.1 (SD = 3.2) | PSG, AHI > 15 | Neuropsychological test battery to assess cognitive impairment: Global, Attention, Executive Function, Memory, MMSE | Age, race, BMI, education, smoking, diabetes, hypertension, antidepressant use, benzodiazepine use, non-diazepam anxiolytics use | Higher Hypoxemia had a higher risk of developing MCI or dementia over a 5y follow-up. Sleep fragmentation and duration not associated with cognitive impairment. |
| Yaffe et al., 2015 [55] | Retrospective cohort study, USA | 179,738 | Men Only | 4,107AD14,380 D2,715 VaD5,82 LBD | ≥55y | Clinical diagnosis | AD and Dementia diagnosis based on ICD-9 criteria | Age, CVD comorbidities, obesity, depression, income, education | Those with OSA had a 20%and 27%increased risk for AD and dementia, respectively. | ||
AD, Alzheimer’s disease; AHI, Apnea-Hypopnea Index; BMI, body mass index; CDR, Clinical Dementia Rating; CERAD-K, Korean version of Consortium to Establish a Registry for Alzheimer’s Disease; CPAP, continuous pulmonary airway pressure; CVD, cardiovascular disease; D, dementia; DSM-III, Diagnostic and Statistical Manual of Mental Disorders, third edition; APOE, apolipoprotein epsilon4; ESS, Epworth Sleepiness Scale; F, female; ICD-9/10, International Classification of Diseases ninth/tenth edition AD criteria; LBD, Lewy body dementia; M, male; MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination; N, number of participants; NINCDS-ADRDA, National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association; NHIRD, National Health Insurance Research Database; OSA, obstructive sleep apnea; PSQI, Pittsburg Sleep Quality Index; PSG, Polysomnography; RCT, randomized clinical trial; TICSm, Telephone interview for cognitive status; VaD, vascular dementia.
Considering the very limited literature investigating the prevalence of OSA in patients with MCI, Dlugaj et al. [7], using a community-based sample, found a similar OSA prevalence in patients with and without MCI (27%and 26%, respectively), and no association between MCI or MCI sub-types and OSA-severity. Similarly, Kim and colleagues [48] found no association between MCI and the AHI in a clinic-based sample of patients. However, the authors documented that higher AHI was associated with lower language test performance among individuals with MCI and it was not present in controls, suggesting that OSA can mainly impair language in patients with MCI.
Although more studies have been conducted in patients with AD, they date back to the 1980s and reported controversial results; two studies [49, 50] demonstrated a significant association between AD and OSA diagnoses, whereas three studies did not find any relations [51–53]. As aforementioned, a recent meta-analysis including these five studies [37] concluded that the aggregate odds ratio for OSA in patients with AD was five times higher when compared to cognitively unimpaired individuals of similar age. Moreover, high AHI was related to worse cognitive and functional status, suggesting that the severity of OSA increases along AD progression. Another recent meta-analysis reported that individuals with sleep disturbances (insomnia, sleep-disordered breathing, EDS, sleep-wake rhythm disorders) have a higher risk of developing all-cause dementia, AD, and vascular dementia [41]. In particular, a sub-analysis showed that both snoring and OSA were associated with an increased risk of all-cause dementia, AD, and vascular dementia. However, many of the cited studies used self-reported measures to assess OSA and few longitudinal studies were included. Among them, Yaffe and colleagues [54] in a sample of older women found that those diagnosed with OSA by PSG had an increased risk of developing MCI or dementia over a 5-year follow-up. In another study including only older adult male veterans, the authors reported a significant association between OSA and a higher risk of AD, vascular dementia and other dementias [55]. Similarly, two large longitudinal community-based studies proposed the same results in the Asian population [56, 57]. In particular, Lee et al. [57], from a national representative cohort data, found that patients with OSA were almost 1.58 times more likely to develop AD than those without OSA. In addition, a recent retrospective study [58], including 3,978 OSA patients and 15,912 non-OSA patients, reported that OSA was independently and significantly associated with a higher incidence of AD, with an average period of AD detection from the time of OSA diagnosis estimated as 5.44 years. However, the literature also presents some studies not showing a clear and significant association between OSA and the development of cognitive impairment and AD [59–61]. In keeping with that, Lutsey and colleagues [61] analyzed a group of OSA patients that were followed for over 15 years and did not find a significant association between OSA and risk of dementia. However, when using adjudicated outcomes (i.e., syndromic dementia and MCI as adjudicated by an expert panel), severe OSA (AHI≥30) was associated with a higher risk of all-cause dementia and AD dementia, but these associations were reduced after controlling for cardiovascular risk factors. Moreover, a recent study documented a non-significant role of comorbid OSA on the longitudinal deterioration of cognitive subdomains or global cognition in a group of 144 AD patients [62]. These divergent results regarding the role of OSA on AD raise questionable issues about the diagnosis and the treatment of OSA in patients with MCI or AD.
Therefore, the aforementioned data seems to be contrasting, and cannot allow for an interpretation of the causality direction between OSA and AD due to the different methodology and study designs. Nevertheless, the here presented data highlights the hypothesis of a bi-directional relation between OSA and AD. In particular, the current evidence shows that OSA is frequently comorbid in patients with MCI or AD diagnosis [2, 63]. Moreover, OSA may represent a risk factor for developing MCI and AD in middle-aged and older adults, boosting and advancing cognitive decline and possibly inducing or accelerating AD neurodegenerative processes [54, 64].
Hence, all these findings support the evidence that OSA can increase the risk of MCI or AD, but also suggest a higher incidence of OSA in MCI and AD patients, with a progressive increase of OSA prevalence along with cognitive deterioration. However, these findings need to be supported by further studies, which should overcome some of the current limitations of these previous investigations (e.g., cross-sectional studies). Further, there are still few studies focused on the risk of MCI or AD in patients with OSA, mainly because OSA is immediately treated when diagnosed, thus making it impossible to conduct longitudinal observational studies.
THE EFFECTS OF CPAP THERAPY ON COGNITION
Several studies reported that CPAP treatment can improve different cognitive deficits in adult OSA patients, namely executive function, memory, attention, and reaction time [32, 65]. Regarding AD patients, different studies explored the effects of CPAP on cognitive functioning, suggesting the possibility of hampering or retarding the transition from MCI to AD [2, 66, 67], as well as delaying the cognitive decline in AD [33–35]. Table 2 presents the summary findings from the 9 studies that explored the effects of CPAP on cognitive functioning in MCI and AD patients.
Table 2
Studies’ characteristics and main findings: CPAP on cognitive functioning in MCI and AD patients
| Authors, Year Published | Study Design, Setting and Country | Participants | OSA assessment | Cognitive Assessment:Tests and Diagnostic Criteria | Adjusted Variables | Main Findings | |||||
| N | Gender | Treatment Group | Control Group | ||||||||
| N | Mean age (SD) | N | Mean age (SD) | ||||||||
| Ancoli-Israel et al., 2008 [33] | RCT, General clinical research center, USA | 52 | 39 M 13 F | 27 | 78.6 (6.8) | 25 Placebo | 77.7 (7.7) | Rechtschaffen and Kales criteria | Neuropsychological test battery to assess global cognition, basic attention and vigilance, psychomotor speed, verbal episodic memory and executive functioning | None | After 3-weeks of CPAP for both AD groups, there was a significant improvement in the composite neuropsychological score. |
| Cooke et al., 2009 [34] | RCT, Follow-up, USA | 10 | 7 M 3 F | 5 | 5 NCCPAP | AHI, PSQI, ESS, FOSQ | Neuropsychological test battery to assess global cognition, basic attention and vigilance, psychomotor speed, verbal episodic memory and executive functioning | None | Sustained CPAP use associated with less cognitive decline in patients with AD. | ||
| Dunietz et al., 2021 [70] | Retrospective, Medicare beneficiaries, USA | 41,466 | 25,462 M 16,004 F | 30,717 | 10,749 NCCPAP | Clinical Diagnosis and issued treatment | MCI, AD and Dementia not otherwise specified were identified with ICD-9 codes | Age, sex, race, stroke, hypertension, cardiovascular disease, and depression | PAP adherence was associated with lower odds of incident diagnoses of AD. | ||
| Ligouri et al., 2021 [69] | Retrospective, Multicenter study, Italy | 24 | 16 M 8 F | 12 | 75.2 (4.9) | 12 NCCPAP | 74.4 (6.9) | AHI, ESS | Global cognition and dementia assessed through MMSE, CDR | None | Decrease in cognitive decline (CDR) between baseline and 1-year follow-up in MCI and AD patients. |
| Osorio et al. 2015 [2] | Prospective, ADNI cohort, USA | 195 | 138 M 57 F | 12 MCI 35 AD | 74.6 (5.8) 73.1 (6.6) | 50 MCI 98 AD | 72.1 (5.9) 71.9 (6.9) | Self-reported | MCI and AD diagnosis made by a clinician | APOE e4 status, sex, education, BMI, depression, cardiovascular disease, hypertension, diabetes, and age | CPAP treatment delayed age at MCI onset. |
| Richards et al. 2019 [67] | Quasi-experimental; Sleep and geriatric clinics and community Longitudinal | 54 | 34 M 24 F | 29 | 67.4 (7.2) | 25 | 73.2 (8.6) | PSG; AHI > 10 | Memory (Hopkins Verbal Learning Test-Revised), Cognitive processing speed (Digit Symbol), Global cognition (MMSE), Attention (Stroop test and Psychomotor Vigilance Task) | Age, race, and marital status | CPAP adherence in MCI and OSA patients significantly improved cognition compared with a nonadherent control group. |
| Skiba et al., 2020 [71] | Retrospective, Urban tertiary health center, USA | 96 | 63 M 33 F | 42 | 70.0 (9.4) | 24 No CPAP 30 NCCPAP | 70.1 (11.3) 71.4 (7.7) | PSG, AHI | Modified CERAD: memory, language. attention executive function Global cognition(CDR). | Age, gender, education and race | CPAP use in MCI patients with OSA was not associated with a delay in progression to dementia or cognitive decline. |
| Troussierè et al. 2014 [35] | Longitudinal, Clinic Centre, France | 23 | 14 M 9 F | 14 | 73.4 | 9 NCCPAP | 77.6 | Video PSGAHI≥30, ESS | Global cognition assessed with MMSE | None | CPAP treatment of severe OSA in mild-to-moderate AD patients was associated with slower cognitive decline over a three-year follow-up period. |
| Wang et al., 2020 [66] | Quasi-experimental clinical, Memories 1 data 1-year follow-up | 17 | 8 M 9 F | 7 | 68.4 (6.6) | 10 NCCPAP | 74.6 (9.67) | PSG | Memory (Hopkins Verbal Learning Test-Revised); Global cognition (Montreal Cognitive Assessment) Global progression (ADCS- CGIC; CDR) | None | A year ofCPAP adherence improved psychomotor/ cognitive processing speed in older adults with MCI and mild OSA. |
AD, Alzheimer’s disease; ADCS-CGIC, Alzheimer’s Disease Cooperative Study-Clinical Global Impression of Change; AHI, Apnea-Hypopnea Index;BMI, body mass index;APOE, apolipoprotein epsilon4; CERAD, Consortium to Establish a Registry for Alzheimer’s Disease; CDR, Clinical Dementia Rating; CPAP, continuous pulmonary airway pressure; CVD, cardiovascular disease; D, dementia; DSM-III, Diagnostic and Statistical Manual of Mental Disorders, third edition; ESS, Epworth Sleepiness Scale; F, female; FOSQ, Functional Outcomes Sleep Questionnaire; ICD-9/10, International Classification of Diseases ninth/tenth edition AD criteria; M, male; MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination; N, number of participants; NCCPAP, Non-compliant CPAP; OSA, obstructive sleep apnea; PAP, positive airway pressure; PSQI, Pittsburg Sleep Quality Index; PSG, polysomnography; RCT, randomized clinical trial.
Few RCTs of CPAP on AD patients were performed; however, there are currently no RCTs of CPAP in MCI patients co-affected by OSA. Cooke and colleagues [34], by selecting patients from a 6-week randomized placebo-controlled trial of CPAP in patients with mild to moderate AD, compared a group of five patients who had sustained CPAP treatment after completion of the RCT to a group of five patients who had discontinued CPAP. Results showed that sustained CPAP use was associated with less cognitive decline, stabilization of depressive symptoms and daytime somnolence, and significant improvement in subjective sleep quality. Furthermore, Ancoli-Israel et al. [33], in a randomized double-blind placebo-controlled trial study in 52 patients with mild-moderate AD and comorbid OSA, observed no changes in neuropsychological functioning between treatment groups (3 weeks of active CPAP versus 3 weeks of placebo followed by 3 weeks of CPAP) but documented in the paired analysis that after three weeks of therapeutic CPAP both groups reported an improvement in cognitive functioning. Notably, some limitations on these RCTs on AD patients need to be acknowledged, namely the statistical power and the use of small samples, which make it harder to draw conclusive assumptions on the causality of CPAP on cognitive performance.
Recently, in a 1-year quasi-experimental trial, Richards et al. [67] found that CPAP adherence in MCI and OSA patients, when compared with a nonadherent control group, significantly improved cognition, which suggests that CPAP adherence may slow the trajectory of cognitive decline. Similarly, Wang and colleagues [66] recently reported a significant positive effect of adherent CPAP treatment on cognitive functioning in older adults with MCI and OSA (AHI≥10); however, the authors did not examine cognitive outcomes related to the different degree of OSA severity.
Several studies have documented that CPAP treatment may slow cognitive decline in mild to moderate AD patients showing severe OSA [34, 35, 68]. In particular, a small longitudinal study, including 23 patients with AD comorbid with severe OSA, reported that the cognitive decline was significantly slower in the CPAP group than in the non-CPAP group through a 3-year follow-up [35].
Some retrospective studies have also found an association between CPAP use and cognitive functioning. For example, Osorio et al. [2], using Alzheimer’s disease Neuroimaging Initiative data, documented that OSA patients had an earlier onset age of MCI or AD and that CPAP use delayed the age of MCI onset. However, in this study, OSA diagnosis and CPAP use were self-reported. Another recent, retrospective study [69], found a significant difference in cognitive and functional performances assessed with clinical dementia rating scale, between the CPAP non-compliant and the CPAP compliant group, with patients CPAP non-compliant reporting a significant longitudinal worsening in cognitive and functional performances compared to CPAP compliant patients. In line with these findings, another recent retrospective study [70], considering a large sample of patients diagnosed with OSA, documented that PAP adherence was associated with lower odds of incident diagnoses of AD. Conversely, Skiba et al. [71] reported no significant differences between CPAP compliant, CPAP non-compliant and no CPAP use on a sample of 96 MCI patients. However, when looking at the timing before conversion, it appeared evident that patients CPAP compliant (77.3 months) presented a longer time before conversion than non-compliant (52.1 months) or non-use (47.3 months) CPAP groups. Moreover, the authors found that patients with amnestic MCI had better CPAP use and shorter progression time to dementia; however, this difference became non-significant after adjusting for age, education, and race. The authors, thus, concluded that the CPAP use in MCI patients with OSA was not associated with delay in progression to dementia or cognitive decline. This negative result contrasts with most of the previous literature and must be read carefully since the criteria for OSA diagnosis were not well-defined and different treatments of OSA were considered [72].
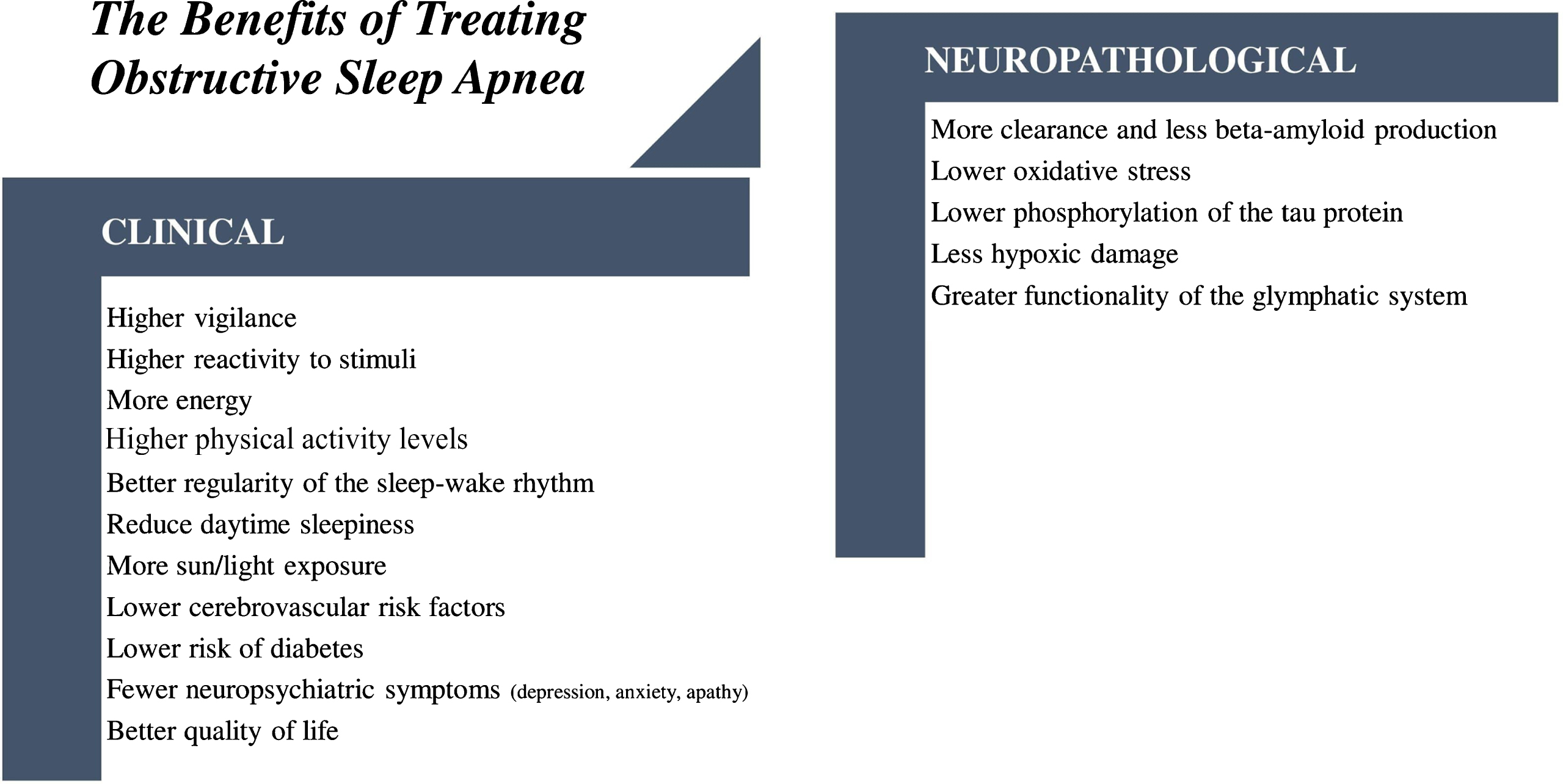
The findings of these several studies demonstrated that, despite some statistical limitations, there is sufficient evidence to suggest that CPAP treatment may be effective in improving cognition in OSA patients with MCI and AD. Moreover, treating sleep apnea holds great promise not only to enhance cognition but also to improve quality of life and delay MCI and AD progression [73, 74]. For instance, several studies demonstrated that OSA increases AD biomarker burden and as such, it is plausible to hypothesize that CPAP treatment would also result in changes of amyloid and tau AD biomarkers. Although there are still few studies, one recent report showed that untreated OSA patients present lower cerebrospinal fluid Aβ42 concentrations, and a higher T-tau/Aβ42 ratio when compared to both CPAP-treated individuals and controls [16]. Furthermore, CPAP treatment in MCI and AD patients showed also to be an effective intervention with significant positive effects in sleep architecture and key sleep parameters [34, 68], which may, in turn, reduce the risk of metabolic dysfunction, cardiovascular morbidity and mortality, as well as decrease the risk of developing depression [74]. Figure 1 summarizes the benefits of treating OSA in clinical and neuropathological aspects in patients with MCI and AD.
Fig. 1
Benefits of treating OSA in clinical and neuropathological aspects in patients with MCI and AD.
THE ROLE OF SLEEP PHYSIOLOGICAL MECHANISMS ON COGNITIVE FUNCTIONING
It is well known that sleep plays an important role in the development and maintenance of cognitive performance [75]. In particular, sleep quality and continuity has been demonstrated to ensure brain health. Healthy sleep habits promote better cognitive performance given the positive effects of both non-rapid eye movement (NREM) and REM sleep stages [75, 76]. NREM sleep, especially SWS, reactivates the hippocampal-neocortical circuits activated during a waking learning period. REM sleep is responsible for the consolidation of the new learning materials and skills [75, 76]. This coupled action of NREM and REM sleep during the night permits memory consolidation and storage. Further, a link has also been found between brain cholinergic activity, timing, and density of REM sleep and cognitive functioning [77]. Thus, considering that the AD process impairs the cholinergic network [78, 79], it has been hypothesized that deep sleep (SWS and REM) alteration can be associated with cognitive deficits in middle-aged and older adults from the early stages of AD neurodegeneration.
Sleep deprivation and disruption markedly affect the individuals’ cognitive and emotional abilities [80]. In fact, studies consistently show that not only sleep architecture and continuity, but also sleep stages and components, such as SWS, REM sleep, K-complexes, and sleep spindles, have specific and crucial roles in neurogenesis [81], synaptic plasticity [82], and next-day vigilance [83], as well as in memory formation and consolidation [84, 85]. Moreover, abnormal sleep duration (both short and long) is associated with lower brain grey matter volume in patients with OSA, suggesting the possibility of future cognitive decline [86]. OSA is characterized by IH and major changes in sleep characteristics, namely sleep fragmentation (SF), sleep deprivation, SWS disruption, and a decrease in REM sleep [87–89]. Sleep impairment due to apnea events can accelerate cognitive decline and trigger brain pathological events leading to AD neurodegeneration [90]. Figure 2 shows the possible intermediate detrimental mechanisms in the relation between OSA and cognitive deterioration.
Fig. 2
Possible intermediate detrimental mechanisms in the relation between OSA and cognitive deterioration.
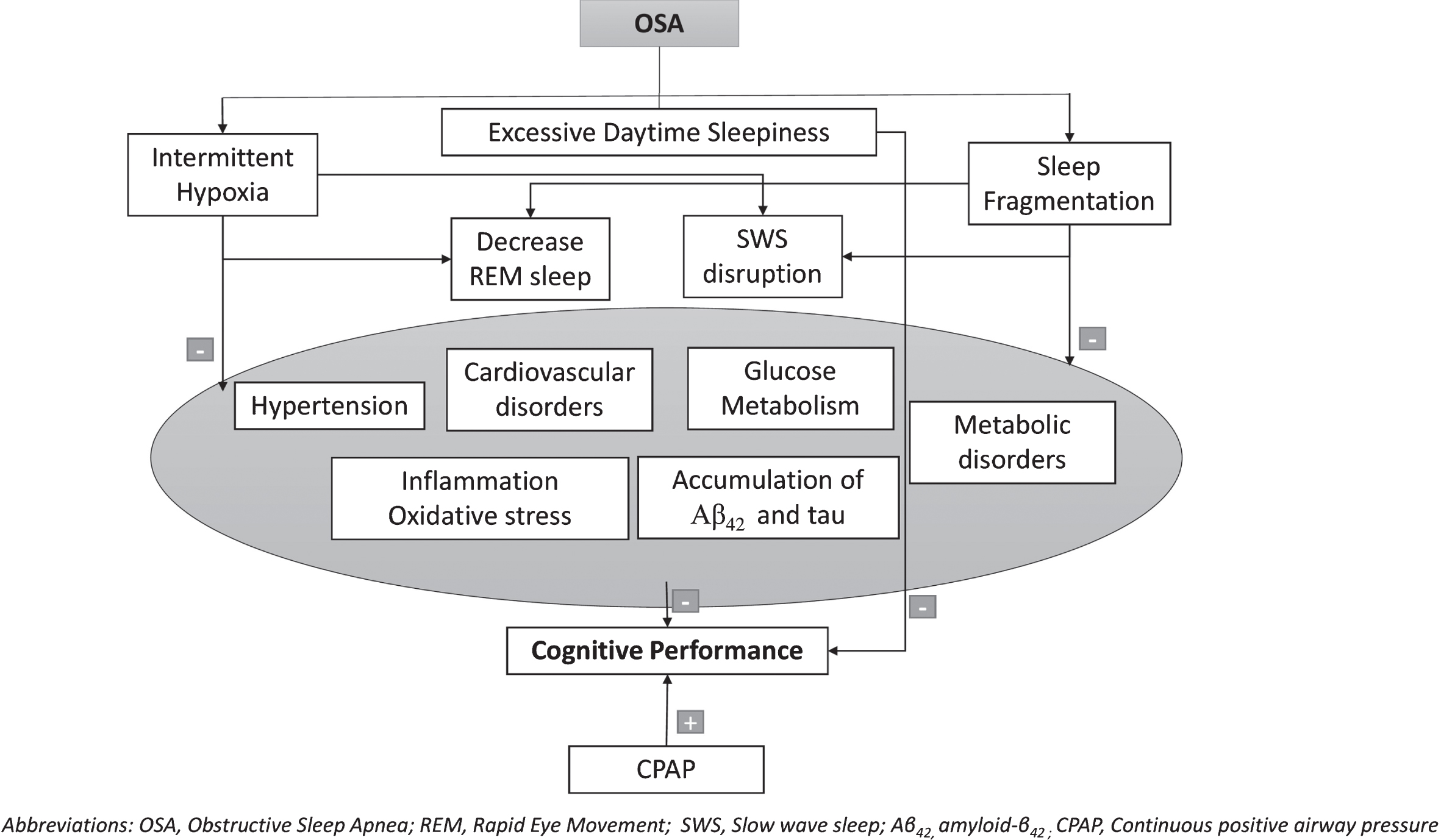
The association between hypoxemia and some cognitive deficits, including attention deficit, slow processing speed and executive dysfunctions, has been widely established in patients with OSA [91–95]. Notably, IH in OSA promotes the brain accumulation of Aβ42 and tau pathology and can induce neuronal damage [40, 96, 97]. The pathophysiological effects of IH may also trigger hypertension [98, 99], impaired glucose metabolism [100, 101], metabolic consequences [101, 102], neuroinflammation, and oxidative stress [103], which could lead to cognitive impairment and progress to AD. Consistently, a recent general population cohort study reported an association between lower mean oxygen saturation during sleep and brain atrophy in cortical and subcortical areas more sensible to hypoxemia [104]. Although the detrimental role of IH has been well established in patients with OSA, controversial results have been achieved in different studies regarding neuroinflammation; in particular, the role of interleukin-6 (IL-6) has been recently investigated in patients with OSA. Although higher levels of IL-6 can significantly improve memory and learning processes via its effects on neuronal excitability at hippocampal synapses [105, 106], as an inflammatory cytokine, its effects can also be detrimental and induce neurodegeneration [107]. Hence, it has been reported that high IL-6 blood levels in OSA patients can reflect both the negative effects of the excessive inflammatory response, triggered by intermittent hypoxia and the positive effects of neurogenesis against neurodegeneration as an attempt to counteract the pathological processes induced by OSA [69]. In line with this hypothesis, although IH parameters correlated with high IL-6 blood levels in patients with OSA, these high IL-6 blood levels give a reduced propensity to develop dementia [108].
SF is one of the well-recognized sleep parameters influencing cognition in OSA patients. For instance, different reviews have reported an association between SF and cognitive impairment in patients with OSA, specifically, the severity of SF was associated with worse performance in attention and vigilance tests [109, 110]. Executive functions were also found to be affected by SF in OSA patients. For example, Djnlagic and colleagues [111] found that OSA patients had less overnight improvement on the motor sequence learning task when compared to healthy controls. Moreover, the decline in sleep-dependent memory consolidation was associated in OSA patients with increasing age, higher arousal index and more severe sleep-disordered breathing (higher AHI). SF, actigraphy-assessed arousals and circadian rhythm disruption have also been associated with increased risk of MCI/dementia in older adults [112, 113].
Another contributing factor of OSA to cognitive impairment is EDS because of its association with slowing information speed, alteration in attention, vigilance, and memory [114]. Accordingly, EDS in patients with OSA could be related to high AHI, more SF, and low nocturnal oxygenation [114]. However, other factors may have a significant role in explaining EDS in OSA patients, such as age, sex, obesity, depression, and other medical illnesses [115].
In conclusion, OSA may promote neurodegenerative changes resulting from all three of its main consequences: IH, SF, and EDS [40, 43]. These have a deleterious impact on cortical and hippocampal networks, causing degeneration and necrosis of neurons, which leads to memory impairment, cognitive decline and progression to MCI and AD.
FUTURE PERSPECTIVE
Although some double-blinded, placebo-controlled clinical trials addressed the effects of CPAP, other RCTs are also needed in order to improve the current methodological issues, which are related to design, sample size and recruitment. Hence, larger and more powered samples, as well as different recruitment establishments (not only in OSA and CPAP therapeutic centers) will benefit empirical research the most. Moreover, future RCTs should consider other aspects besides OSA severity (mild, moderate, and severe), namely disease duration, hypoxemia and fragmented sleep severity, and presence of other comorbidities. As mentioned before, RCTs of CPAP in MCI patients with co-affected OSA should also be conducted. Lastly, future prospective studies among MCI patients should include a clear definition of the dementia disorder developed during the follow-up (AD versus other dementias), by using the current biomarkers-based criteria for diagnosis, with a regular time frame to evaluate CPAP compliance and efficacy concerning cognitive decline.
Most studies have focused on improving cognitive deficits or delaying the cognitive decline by using CPAP to treat an OSA condition; however, adherence to CPAP treatment remains the main target for studies since the poor compliance to CPAP represents an evident obstacle. Although there are other and emerging treatment options for OSA, namely specific drugs (solriamfetol and pitolisant for treating EDS associated with OSA [116, 117] or Atomoxetine and Oxybutynin for reducing OSA severity [31]), oral appliances, behavioral/lifestyle modifications, surgery and/or a combination of approaches, it is still unclear if these OSA treatment options have similar positive effects on sleep and cognitive functioning as CPAP treatment. In particular, by using an approved drug for cognitive impairment and AD, Moraes and colleagues [118] examined the effects of donepezil on OSA in a double-blind, placebo-controlled study including AD patients. The authors reported the significant improvement of AHI, oxygen saturation parameters, REM sleep duration, dementias assessments and cognitive scores in the arm of patients treated with donepezil for 3 months when compared to placebo. Hence, future studies should also consider examining the effects of these other treatments on neuropsychological functioning in MCI and AD patients, addressing the possibility of using pharmacological treatments for OSA in MCI and AD patients.
CONCLUSION
Cognitive impairment is the main clinical feature of MCI and AD patients and is commonly associated with neuropsychiatric symptoms, including sleep disorders [119, 120]. This review examined if OSA may influence cognitive functioning in patients with MCI or AD and highlighted the evidence that CPAP treatment may delay cognitive decline in these patients, although further evidence should be obtained. Taking all the present literature into account, from a clinical point of view, the need to understand the prevalence of OSA in patients with AD was clear, as well as the incidence of future AD in patients with OSA, in order to assess and manage resources to prevent ADD, delaying the transition from MCI to ADD, or slowing cognitive deterioration during the AD process by treating OSA. Accordingly, the 5th Canadian Consensus Conference highlights the importance of screening for dementia in patients with sleep apnea and recommends that OSA patients be treated with CPAP since it may improve cognition and decrease the risk of dementia [121]. However, some of these findings highlighted the importance of starting CPAP treatment early but also of improving the follow-up to monitor the compliance to this device. Therefore, CPAP may be a promising treatment for slowing cognitive decline in older adults with MCI or AD and comorbid OSA, although other pharmacological and non-pharmacological approaches should be evaluated and tested. Finally, larger and adequately powered studies are needed to corroborate the findings presented in this review.
CONFLICT OF INTEREST
The authors report no conflicts of interests or financial disclosures.
REFERENCES
[1] | Terpening Z , Lewis SJG , Yee BJ , Grunstein RR , Hickie IB , Naismith SL ((2015) ) Association between sleep-disordered breathing and neuropsychological performance in older adults with mild cognitive impairment. J Alzheimers Dis 46: , 157–165. |
[2] | Osorio RS , Gumb T , Pirraglia E , Varga AW , Lu SE , Lim J , Wohlleber ME , Ducca EL , Koushyk V , Glodzik L , Mosconi L , Ayappa I , Rapoport DM , De Leon MJ ((2015) ) Sleep-disordered breathing advances cognitive decline in the elderly. Neurology 84: , 1964–1971. |
[3] | Liguori C , Maestri M , Spanetta M , Placidi F , Bonanni E , Mercuri NB , Guarnieri B ((2021) ) Sleep-disordered breathing and the risk of Alzheimer’s disease. Sleep Med Rev 55: , 1–16. |
[4] | Woods CE , Usher KJ , Jersmann H , Maguire GP ((2014) ) Sleep disordered breathing and polysomnography in Australia: Trends in provision from 2005 to 2012 and the impact of home-based diagnosis. J Clin Sleep Med 10: , 767–772. |
[5] | Thorpy M International classification of sleep disorders. In Sleep disorders medicine. Springer, pp. 475–484. |
[6] | Heinzer R , Vat S , Marques-Vidal P , Marti-Soler H , Andries D , Tobback N , Mooser V , Preisig M , Malhotra A , Waeber G , Vollenweider P , Tafti M , Haba-Rubio J ((2015) ) Prevalence of sleep-disordered breathing in the general population: The HypnoLaus study. Lancet Respir Med 3: , 310–318. |
[7] | Dlugaj M , Weinreich G , Weimar C , Stang A , Dragano N , Wessendorf TE , Teschler H , Winkler A , Wege N , Moebus S , Möhlenkamp S , Erbel R , Jöckel K-H ((2014) ) Sleep-disordered breathing, sleep quality, and mild cognitive impairment in the general population. J Alzheimers Dis 41: , 479–497. |
[8] | Kasai T ((2012) ) Sleep apnea and heart failure. J Cardiol 60: , 78–85. |
[9] | Khot SP , Morgenstern LB ((2019) ) Sleep and stroke. Stroke 50: , 1612–1617. |
[10] | Liguori C , Mercuri NB , Izzi F , Romigi A , Cordella A , Piccirilli E , Viola S , Costa S , Sbraccia P , Marciani MG , Tarantino U , Placidi F ((2016) ) Obstructive sleep apnoea as a risk factor for osteopenia and osteoporosis in the male population. Eur Respir J 47: , 987–990. |
[11] | Neighbors CLP , Noller MW , Song SA , Zaghi S , Neighbors J , Feldman D , Kushida CA , Camacho M ((2018) ) Vitamin D and obstructive sleep apnea: A systematic review and meta-analysis. Sleep Med 43: , 100–108. |
[12] | Mentek M , Aptel F , Godin-Ribuot D , Tamisier R , Pepin JL , Chiquet C ((2018) ) Diseases of the retina and the optic nerve associated with obstructive sleep apnea. Sleep Med Rev 38: , 113–130. |
[13] | Liguori C , Izzi F , Mercuri NB , Romigi A , Cordella A , Tarantino U , Placidi F ((2017) ) Vitamin D status of male OSAS patients improved after long-term CPAP treatment mainly in obese subjects. Sleep Med 29: , 81–85. |
[14] | Fraser L.C ((2014) ) Obstructive sleep apnea and optic neuropathy: Is there a link? Curr Neurol Neurosci Rep 14: , 1–9. |
[15] | Upala S , Sanguankeo A , Congrete S ((2016) ) Association between obstructive sleep apnea and osteoporosis: A systematic review and meta-analysis. Int J Endocrinol Metab 14: , 129–140. |
[16] | Liguori C , Mercuri NB , Izzi F , Romigi A , Cordella A , Sancesario G , Placidi F ((2017) ) Obstructive sleep apnea is associated with early but possibly modifiable Alzheimer’s disease biomarkers changes. Sleep 40: , zsx011. |
[17] | Liguori C , Palmieri MG , Pierantozzi M , Cesareo M , Romigi A , Izzi F , Marciani MG , Oliva C , Mercuri NB , Placidi F ((2016) ) Optic Nerve dysfunction in obstructive sleep apnea: An electrophysiological study. Sleep 39: , 19–23. |
[18] | Liguori C , Mercuri NB , Nuccetelli M , Izzi F , Cordella A , Bernardini S , Placidi F ((2019) ) Obstructive sleep apnea may induce orexinergic system and cerebral β-amyloid metabolism dysregulation: Is it a further proof for Alzheimer’s disease risk? Sleep Med 56: , 171–176. |
[19] | Shi Y , Chen L , Chen T , Li L , Dai J , Lui S , Huang X , Sweeney JA , Gong Q ((2017) ) A meta-analysis of voxel-based brain morphometry studies in obstructive sleep apnea. Sci Rep 7: , 10095. |
[20] | Huang X , Tang S , Lyu X , Yang C , Chen X ((2019) ) Structural and functional brain alterations in obstructive sleep apnea: A multimodal meta-analysis. Sleep Med 54: , 195–204. |
[21] | Gehrman PR , Martin JL , Shochat T , Nolan S , Corey-Bloom J , Ancoli-Israel S ((2003) ) Sleep-disordered breathing and agitation in institutionalized adults with Alzheimer disease. Am J Geriatr Psychiatry 11: , 426–433. |
[22] | Díaz-Román M , Pulopulos MM , Baquero M , Salvador A , Cuevas A , Ferrer I , Ciopat O , Gómez E ((2021) ) Obstructive sleep apnea and Alzheimer’s disease-related cerebrospinal fluid biomarkers in mild cognitive impairment. Sleep 44: , zsaa133. |
[23] | Bixler EO , Vgontzas AN , Lin HM , Ten Have T , Rein J , Vela-Bueno A , Kales A ((2001) ) Prevalence of sleep-disordered breathing in women: Effects of gender. Am J Respir Crit Care Med 163: , 608–613. |
[24] | Young T , Palta M , Dempsey J , Peppard PE , Nieto FJ , Hla KM ((2009) ) Burden of sleep apnea: Rationale, design, and major findings of the Wisconsin sleep cohort study. Wis Med J 108: , 246–249. |
[25] | Zamarrón Sanz C , Gude F , Otero Y , Alvarez JM , Golpe A , Rodriguez JR ((1999) ) Prevalence of sleep disordered breathing and sleep apnea in 50- to 70-year-old individuals. Respiration 66: , 317–322. |
[26] | Hoffstein V ((2007) ) Review of oral appliances for treatment of sleep-disordered breathing. Sleep Breath 11: , 1–22. |
[27] | Ohtsuka K , Baba R , Yamasawa W , Shirahama R , Hattori Y , Senoura H , Betsuyaku T , Fukunaga K ((2021) ) The effectiveness of nasal airway stent therapy for the treatment of mild-to-moderate obstructive sleep apnea syndrome. Respiration 100: , 193–200. |
[28] | Certal V , Nishino N , Camacho M , Capasso R ((2013) ) Reviewing the systematic reviews in OSA surgery. Otolaryngol Head Neck Surg 149: , 817–829. |
[29] | Jain N , Rodin J , Boon MS , Huntley CT ((2020) ) A systematic approach to the evaluation and Management of Obstructive Sleep Apnea: The Jefferson protocol. Am J Otolaryngol 95: , 102866. |
[30] | Ferguson KA , Cartwright R , Rogers R , Schmidt-Nowara W ((2006) ) Oral appliances for snoring and obstructive sleep apnea: A review. Sleep 29: , 244–262. |
[31] | Taranto-Montemurro L , Messineo L , Sands SA , Azarbarzin A , Marques M , Edwards BA , Eckert DJ , White DP , Wellman A ((2019) ) The combination of atomoxetine and oxybutynin greatly reduces obstructive sleep apnea severity a randomized, placebo-controlled, double-blind crossover trial. Am J Respir Crit Care Med 199: , 1267–1276. |
[32] | Wang G , Goebel JR , Li C , Hallman HG , Gilford TM , Li W ((2020) ) Therapeutic effects of CPAP on cognitive impairments associated with OSA. J Neurol 267: , 2823–2828. |
[33] | Ancoli-Israel S , Palmer BW , Cooke JR , Corey-Bloom J , Fiorentino L , Natarajan L , Liu L , Ayalon L , He F , Loredo JS ((2008) ) Cognitive effects of treating obstructive sleep apnea in Alzheimer’s disease: A randomized controlled study. J Am Geriatr Soc 56: , 2076–2081. |
[34] | Cooke JR , Ayalon L , Palmer BW , Loredo JS , Corey-Bloom J , Natarajan L , Liu L , Ancoli-Israel S ((2009) ) Sustained use of CPAP slows deterioration of cognition, sleep, and mood in patients with Alzheimer’s disease and obstructive sleep apnea: A preliminary study. J Clin Sleep Med 5: , 305–309. |
[35] | Troussière AC , Charley CM , Salleron J , Richard F , Delbeuck X , Derambure P , Pasquier F , Bombois S ((2014) ) Treatment of sleep apnoea syndrome decreases cognitive decline in patients with Alzheimer’s disease. J Neurol Neurosurg Psychiatry 85: , 1405–1408. |
[36] | Liguori C , Placidi F ((2018) ) Is it time to consider obstructive sleep apnea syndrome a risk factor for Alzheimer’s disease? Am J Respir Crit Care Med 197: , 855–856. |
[37] | Emamian F , Khazaie H , Tahmasian M , Leschziner GD , Morrell MJ , Hsiung GYR , Rosenzweig I , Sepehry AA ((2016) ) The association between obstructive sleep apnea and Alzheimer’s disease: A meta-analysis perspective. Front Aging Neurosci 8: , 78. |
[38] | Mullins AE , Kam K , Parekh A , Bubu OM , Osorio RS , Varga AW ((2020) ) Obstructive sleep apnea and its treatment in aging: Effects on Alzheimer’s disease biomarkers, cognition, brain structure and neurophysiology. Neurobiol Dis 145: , 105054. |
[39] | Baril AA , Carrier J , Lafrenière A , Warby S , Poirier J , Osorio RS , Ayas N , Dubé MP , Petit D , Gosselin N ((2018) ) Biomarkers of dementia in obstructive sleep apnea. Sleep Med Rev 42: , 139–148. |
[40] | Daulatzai MA ((2015) ) Evidence of neurodegeneration in obstructive sleep apnea: Relationship between obstructive sleep apnea and cognitive dysfunction in the elderly. J Neurosci Res 93: , 1778–1794. |
[41] | Shi L , Chen SJ , Ma MY , Bao YP , Han Y , Wang YM , Shi J , Vitiello MV , Lu L ((2018) ) Sleep disturbances increase the risk of dementia: A systematic review and meta-analysis. Sleep Med Rev 40: , 4–16. |
[42] | Aloia MS , Arnedt JT , Davis JD , Riggs RL , Byrd D ((2004) ) Neuropsychological sequelae of obstructive sleep apnea-hypopnea syndrome: A critical review. J Int Neuropsychol Soc 10: , 772–785. |
[43] | Beebe DW , Groesz L , Wells C , Nichols A , Mcgee K ((2003) ) The neuropsychological effects of obstructive sleep apnea: A meta-analysis of norm-referenced and case-controlled data. Sleep 26: , 298–307. |
[44] | Kilpinen R , Saunamäki T , Jehkonen M ((2014) ) Information processing speed in obstructive sleep apnea syndrome: A review. Acta Neurol Scand 129: , 209–218. |
[45] | Vaessen TJA , Overeem S , Sitskoorn MM ((2015) ) Cognitive complaints in obstructive sleep apnea. Sleep Med Rev 19: , 51–58. |
[46] | Cross N , Lampit A , Pye J , Grunstein RR , Marshall N , Naismith SL ((2017) ) Is obstructive sleep apnoea related to neuropsychological function in healthy older adults? A systematic review and meta-analysis. Neuropsychol Rev 27: , 389–402. |
[47] | Bubu OM , Andrade AG , Umasabor-Bubu OQ , Hogan MM , Turner AD , de Leon MJ , Ogedegbe G , Ayappa I , Jean-Louis G G , Jackson ML , Varga AW , Osorio RS ((2020) ) Obstructive sleep apnea, cognition and Alzheimer’s disease: A systematic review integrating three decades of multidisciplinary research. Sleep Med Rev 50: , 101250. |
[48] | Kim SJ , Lee JH , Lee DY , Jhoo JH , Woo JI ((2011) ) Neurocognitive dysfunction associated with sleep quality and sleep apnea in patients with mild cognitive impairment. Am J Geriatr Psychiatry 19: , 374–381. |
[49] | Hoch CC , Reynolds CF 3rd, Kupfer DJ , Houck PR , Berman SR , Stack JA ((1986) ) Sleep-disordered breathing in normal and pathologic aging. J Clin Psychiatry 47: , 499–503. |
[50] | Reynolds CF , Kupfer DJ , Taska LS , Hoch CC , Sewitch DE , Restifo K , Spiker DG , Zimmer B , Marin RS , Nelson J ((1985) ) Sleep apnea in Alzheimer’s dementia: Correlation with mental deterioration. J Clin Psychiatry 46: , 257–261. |
[51] | Reynolds CF , Kupfer DJ , Hoch CC , Houck PR , Stack JA , Berman SR , Campbell PI , Zimmer B ((1987) ) Sleep deprivation as a probe in the elderly. Arch Gen Psychiatry 44: , 982–990. |
[52] | Hoch CC , Reynolds CF , Nebes RD , Kupfer DJ , Berman SR , Campbell D Clinical significance of sleep-disordered breathing in Alzheimer’s disease: Preliminary data. J Am Geriatr Soc 37: , 138–144. |
[53] | Smallwood RG , Vitiello MV , Giblin EC , Prinz PN ((1983) ) Sleep apnea: Relationship to age, sex, and Alzheimer’s dementia. Sleep 6: , 16–22. |
[54] | Yaffe K , Laffan AM , Harrison SL , Redline S , Spira AP , Ensrud KE , Ancoli-Israel S , Stone KL ((2011) ) Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA 306: , 613–619. |
[55] | Yaffe K , Nettiksimmons J , Yesavage J , Byers A ((2015) ) Sleep quality and risk of dementia among older male Veterans. Am J Geriatr Psychiatry 23: , 651–654. |
[56] | Chang WP , Liu ME , Chang WC , Yang AC , Ku YC , Pai JT , Huang HL , Tsai SJ ((2015) ) Sleep apnea and the risk of dementia: A population-based 5-year follow-up study in Taiwan. PLoS One x8: , 6–13. |
[57] | Lee JE , Yang SW , Ju YJ , Ki SK , Chun KH ((2019) ) Sleep-disordered breathing and Alzheimer’s disease: A nationwide cohort study. Psychiatry Res 273: , 624–630. |
[58] | Tsai MS , Li HY , Huang CG , Wang RYL , Chuang LP , Chen NH , Liu CH , Yang YH , Liu CY , Hsu CM , Cheng WN , Lee LA ((2020) ) Risk of Alzheimer’s disease in obstructive sleep apnea patients with or without treatment: Real-world evidence. Laryngoscope 130: , 2292–2298. |
[59] | Foley DJ , Masaki K , White L , Larkin EK , Monjan A , Redline S ((2003) ) Sleep-disordered breathing and cognitive impairment in elderly Japanese-American men. Sleep 26: , 596–599. |
[60] | Lutsey PL , Bengtson LGS , Punjabi NM , Shahar E , Mosley TH , Gottesman RF , Wruck LM , MacLehose RF , Alonso A ((2016) ) Obstructive sleep apnea and 15-year cognitive decline: The atherosclerosis risk in communities (ARIC) study. Sleep 39: , 309–316. |
[61] | Lutsey PL , Misialek JR , Mosley TH , Gottesman RF , Punjabi NM , Shahar E , MacLehose R , Ogilvie RP , Knopman D , Alonso A ((2018) ) Sleep characteristics and risk of dementia and Alzheimer’s disease: The Atherosclerosis Risk in Communities Study. Alzheimers Dement 14: , 157–166. |
[62] | Jorge C , Targa A , Benítez ID , Dakterzada F , Torres G , Minguez O , Carnes A , Pujol M , Gibert A , López R , Gaeta AM , Dalmases M , Farré R , Sánchez-de-la-Torre M , Barbé F , Piñol-Ripoll G ((2020) ) Obstructive sleep apnoea and cognitive decline in mild-to-moderate Alzheimer’s disease. Eur Respir J 56: , 520–523. |
[63] | Guarnieri B , Adorni F , Musicco M , Appollonio I , Bonanni E , Caffarra P , Caltagirone C , Cerroni G , Concari L , Cosentino FII , Ferrara S , Fermi S , Ferri R , Gelosa G , Lombardi G , Mazzei D , Mearelli S , Morrone E , Murri L , Nobili FM , Passero S , Perri R , Rocchi R , Sucapane P , Tognoni G , Zabberoni S , Sorbi S ((2012) ) Prevalence of sleep disturbances in mild cognitive impairment and dementing disorders: A multicenter Italian clinical cross-sectional study on 431 patients. Dement Geriatr Cogn Disord 33: , 50–58. |
[64] | Liguori C , Placidi F , Izzi F , Spanetta M , Mercuri NB , Di Pucchio A ((2020) ) Sleep dysregulation, memory impairment, and CSF biomarkers during different levels of neurocognitive functioning in Alzheimer’s disease course. Alzheimers Res Ther 12: , 1–13. |
[65] | Rosenzweig I , Glasser M , Crum WR , Kempton MJ , Milosevic M , McMillan A , Leschziner GD , Kumari V , Goadsby P , Simonds AK , Williams SCR , Morrell MJ ((2016) ) Changes in neurocognitive architecture in patients with obstructive sleep apnea treated with continuous positive airway pressure. EBioMedicine 7: , 221–229. |
[66] | Wang Y , Cheng C , Moelter S , Fuentecilla JL , Kincheloe K , Lozano AJ , Carter P , Gooneratne N , Richards KC ((2020) ) One year of continuous positive airway pressure adherence improves cognition in older adults with mild apnea and mild cognitive impairment. Nurs Res 69: , 157–164. |
[67] | Richards KC , Gooneratne N , Dicicco B , Hanlon A , Moelter S , Onen F , Wang Y , Sawyer A , Weaver T , Lozano A , Carter P , Johnson J ((2019) ) CPAP adherence may slow 1-year cognitive decline in older adults with mild cognitive impairment and apnea. J Am Geriatr Soc 67: , 558–564. |
[68] | Cooke JR , Ancoli-Israel S , Liu L , Loredo JS , Natarajan L , Palmer BS , He F , Corey-Bloom J ((2009) ) Continuous positive airway pressure deepens sleep in patients with Alzheimer’s disease and obstructive sleep apnea. Sleep Med 10: , 1101–1106. |
[69] | Liguori C , Cremascoli R , Maestri M , Fernandes M , Izzi F , Tognoni G , Scarpina F , Siciliano G , Mercuri NB , Priano L , Bonanni E , Placidi F (2021) Obstructive sleep apnea syndrome and Alzheimer’s disease pathology: May continuous positive airway pressure treatment delay cognitive deterioration? Sleep Breath, doi: 10.1007/s11325-021-02320-4 |
[70] | Dunietz GL , Chervin RD , Burke JF , Conceicao AS , Braley TJ (2021) Obstructive sleep apnea treatment and dementia risk in older adults. Sleep, doi: 10.1093/sleep/zsab076 |
[71] | Skiba V , Novikova M , Suneja A , McLellan B , Schultz L ((2020) ) Use of positive airway pressure in mild cognitive impairment to delay progression to dementia. J Clin Sleep Med 16: , 863–870. |
[72] | Maestri M , Liguori C , Bonanni E , Guarnieri B ((2020) ) PAP use in mild cognitive impairment to delay progression to dementia. J Clin Sleep Med 16: , 1397. |
[73] | Blackman J , Swirski M , Clynes J , Harding S , Leng Y , Coulthard E (2020) Pharmacological and non-pharmacological interventions to enhance sleep in mild cognitive impairment and mild Alzheimer’s disease: A systematic review. J Sleep Res, doi; 10.1111/jsr.13229 |
[74] | Cao MT , Sternbach JM , Guilleminault C ((2017) ) Continuous positive airway pressure therapy in obstuctive sleep apnea: Benefits and alternatives. Expert Rev Respir Med 11: , 259–272. |
[75] | Rasch B , Born J ((2013) ) About sleep’s role in memory. Physiol Rev 93: , 681–766. |
[76] | Cartwright RD ((2004) ) The role of sleep in changing our minds: A psychologist’s discussion of papers on memory reactivation and consolidation in sleep. Learn Mem 11: , 660–663. |
[77] | Spruyt K , Gozal D ((2010) ) Sleep in children: The evolving challenge of catching enough and quality Zzz’s. In Sleep, Health and Society: From Aetiology to Public Health, Cappuccio FP, Miller MA, Lockley SW, eds. Oxford, pp. 215–238. |
[78] | Mesulam M , Shaw P , Mash D , Weintraub S ((2004) ) Cholinergic nucleus basalis tauopathy emerges early in the aging-MCI-AD continuum. Ann Neurol 55: , 815–828. |
[79] | Bohnen NI , Grothe MJ , Ray NJ , Müller MLTM , Teipel SJ ((2018) ) Recent advances in cholinergic imaging and cognitive decline—revisiting the cholinergic hypothesis of dementia. Curr Geriatr Rep 7: , 1–11. |
[80] | Krause AJ , Simon Ben E , Mander BA , Greer SM , Saletin JM , Goldstein-Piekarski AN , Walker MP ((2017) ) The sleep-deprived human brain. Nat Rev Neurosci 18: , 404–418. |
[81] | Meerlo P , Mistlberger RE , Jacobs BL , Craig Heller H , McGinty D ((2009) ) New neurons in the adult brain: The role of sleep and consequences of sleep loss. Sleep Med Rev 13: , 187–194. |
[82] | Cirelli C ((2013) ) Sleep and synaptic changes. Curr Opin Neurobiol 23: , 841–846. |
[83] | Van Dongen HPA , Dinges DF ((2005) ) Sleep, circadian rhythms, and psychomotor vigilance. Clin Sports Med 24: , 237–249. |
[84] | Born J , Wilhelm I ((2012) ) System consolidation of memory during sleep. Psychol Res 76: , 192–203. |
[85] | De Gennaro L , Gorgoni M , Reda F , Lauri G , Truglia I , Cordone S , Scarpelli S , Mangiaruga A , D’Atri A , Lacidogna G , Ferrara M , Marra C , Rossini PM ((2017) ) The fall of sleep K-complex in Alzheimer disease. Sci Rep 7: , 39688. |
[86] | Kim REY , Abbott RD , Kim S , Thomas RJ , Yun C-H , Kim H , Johnson H , Shin C (2021) Sleep duration, sleep apnea, and gray matter volume. J Geriatr Psychiatry Neurol, doi: 10.1177/0891988720988918. |
[87] | Heinzer R , Gaudreau H , Décary A , Sforza E , Petit D , Morisson F , Montplaisir J ((2001) ) Slow-wave activity in sleep apnea patients before and after continuous positive airway pressure treatment: Contribution to daytime sleepiness. Chest 119: , 1807–1813. |
[88] | McArdle N , Douglas NJ ((2001) ) Effect of continuous positive airway pressure on sleep architecture in the sleep apnea-hypopnea syndrome: A randomized controlled trial. Am J Respir Crit Care Med 164: , 1459–1463. |
[89] | Ratnavadivel R , Chau N , Stadler D , Yeo A , McEvoy RD , Catcheside PG ((2009) ) Marked reduction in obstructive sleep apnea severity in slow wave sleep. J Clin Sleep Med 5: , 519–524. |
[90] | Stern Y ((2002) ) What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc 8: , 448–460. |
[91] | Quan SF , Chan CS , Dement WC , Gevins A , Goodwin JL , Gottlieb DJ , Green S , Guilleminault C , Hirshkowitz M , Hyde PR , Kay GG , Leary EB , Nichols DA , Schweitzer PK , Simon RD , Walsh JK , Kushida CA ((2011) ) The association between obstructive sleep apnea and neurocognitive performance—The Apnea Positive Pressure Long-term Efficacy Study (APPLES). Sleep 34: , 303–314. |
[92] | Beebe DW , Gozal D ((2002) ) Obstructive sleep apnea and the prefrontal cortex: Towards a comprehensive model linking nocturnal upper airway obstruction to daytime cognitive and behavioral deficits. J Sleep Res 11: , 1–16. |
[93] | Findley LJ , Barth JT , Powers DC , Wilhoit SC , Boyd DG , Suratt PM ((1986) ) Cognitive impairment in patients with obstructive sleep apnea and associated hypoxemia. Chest 90: , 686–690. |
[94] | Engleman H , Joffe D ((1999) ) Neuropsychological function in obstructive sleep apnoea. Sleep Med Rev 3: , 59–78. |
[95] | Bedard MC , Montplaisir J , Richer F , Rouleau I , Malo J ((1991) ) Obstructive sleep apnea syndrome: Pathogenesis of neuropsychological deficits. J Clin Exp Neuropsychol 13: , 950–964. |
[96] | Sun X , He G , Qing H , Zhou W , Dobie F , Cai F , Staufenbiel M , Huang LE , Song W ((2006) ) Hypoxia facilitates Alzheimer’s disease pathogenesis by up-regulating BACE1 gene expression. Proc Natl Acad Sci U S A 103: , 18727–18732. |
[97] | Zhang X , Zhou K , Wang R , Cui J , Lipton SA , Liao FF , Xu H , Zhang YW ((2007) ) Hypoxia-inducible factor 1α (HIF-1α)-mediated hypoxia increases BACE1 expression and β-amyloid generation. J Biol Chem 282: , 10873–10880. |
[98] | Peppard PE , Young T , Palta M , Skatrud J ((2000) ) Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 342: , 1378–1384. |
[99] | Morrell MJ , Finn L , Hyon K , Peppard PE , Badr MS , Young T ((2000) ) Sleep fragmentation, awake blood pressure, and sleep-disordered breathing in a population-based study. Am J Respir Crit Care Med 162: , 2091–2096. |
[100] | Pallayova M , Steele KE , Magnuson TH , Schweitzer MA , Hill NR , Bevans-Fonti S , Schwartz AR ((2010) ) Sleep apnea predicts distinct alterations in glucose homeostasis and biomarkers in obese adults with normal and impaired glucose metabolism. Cardiovasc Diabetol 9: , 83. |
[101] | Seetho IW , Wilding JPH ((2014) ) Sleep-disordered breathing, type 2 diabetes and the metabolic syndrome. Chron Respir Dis 11: , 257–275. |
[102] | ((2011) ) Metabolic disorders associated with obstructive sleep apnea in adults. Adv Cardiol 46: , 67–138. |
[103] | Lavie L ((2003) ) Obstructive sleep apnoea syndrome - An oxidative stress disorder. Sleep Med Rev 7: , 35–51. |
[104] | Marchi NA , Ramponi C , Hirotsu C , Haba-Rubio J , Lutti A , Preisig M , Marques-Vidal P , Vollenweider P , Kherif F , Heinzer R , Draganski B ((2020) ) Mean oxygen saturation during sleep is related to specific brain atrophy pattern. Ann Neurol 87: , 921–930. |
[105] | Gruol DL ((2015) ) IL-6 regulation of synaptic function in the CNS. Neuropharmacology 96: , 42–54. |
[106] | Arisi GM ((2014) ) Nervous and immune systems signals and connections: Cytokines in hippocampus physiology and pathology. Epilepsy Behav 38: , 43–47. |
[107] | Heese K ((2017) ) Functional repertoire of interleukin-6 in the central nervous system - A review. Restor Neurol Neurosci 35: , 693–701. |
[108] | Baril A-A , Beiser AS , Redline S , McGrath ER , Gottlieb DJ , Aparicio H , Seshadri S , Himali JJ , Pase MP ((2021) ) Interleukin-6 interacts with sleep apnea severity when predicting incident Alzheimer’s disease dementia. J Alzheimers Dis 79: , 1451–1457. |
[109] | Bucks RS , Olaithe M , Eastwood P ((2013) ) Neurocognitive function in obstructive sleep apnoea: A meta-review. Respirology 18: , 61–70. |
[110] | Morisson F , Décary A , Petit D , Lavigne G , Malo J , Montplaisir J ((2001) ) Daytime sleepiness and EEG spectral analysis in apneic patients before and after treatment with continuous positive airway pressure. Chest 119: , 45–52. |
[111] | Djonlagic I , Guo M , Matteis P , Carusona A , Stickgold R , Malhotra A ((2014) ) Untreated sleep-disordered breathing: Links to aging-related decline in sleep-dependent memory consolidation. PLoS One 9: , e85918. |
[112] | Lim ASP , Kowgier M , Yu L , Buchman AS , Bennett DA ((2013) ) Sleep fragmentation and the risk of incident Alzheimer’s disease and cognitive decline in older persons. Sleep 36: , 1027–1032. |
[113] | Schlosser Covell GE , Dhawan PS , Lee Iannotti JK , Hoffman-Snyder CR , Wellik KE , Caselli RJ , Woodruff BK , Wingerchuk DM , Demaerschalk BM ((2012) ) Disrupted daytime activity and altered sleep-wake patterns may predict transition to mild cognitive impairment or dementia: A critically appraised topic. Neurologist 18: , 426–429. |
[114] | Zhou J , Camacho M , Tang X , Kushida CA ((2016) ) A review of neurocognitive function and obstructive sleep apnea with or without daytime sleepiness. Sleep Med 23: , 99–108. |
[115] | Bixler EO , Vgontzas AN , Lin HM , Calhoun SL , Vela-Bueno A , Kales A ((2005) ) Excessive daytime sleepiness in a general population sample: The role of sleep apnea, age, obesity, diabetes, and depression. J Clin Endocrinol Metab 90: , 4510–4515. |
[116] | Subedi R , Singh R , Thakur RK , K C B , Jha D , Ray BK ((2020) ) Efficacy and safety of solriamfetol for excessive daytime sleepiness in narcolepsy and obstructive sleep apnea: A systematic review and meta-analysis of clinical trials. Sleep Med 75: , 510–521. |
[117] | Dauvilliers Y , Verbraecken J , Partinen M , Hedner J , Saaresranta T , Georgiev O , Tiholov R , Lecomte I , Tamisier R , Lévy P , Scart-Gres C , Lecomte JM , Schwartz JC , Pépin JL , Attali V , Bourgin P , Gagnadoux F , Baharloo F , Berg S , Polo O , Hedner JA , Peker Y , Blau A , Borreguero DG , Cuesta FJP , Belev G , Ivanov Y , Metev H , Petkova D , Dokic D , Marku MI , Kaeva BJ , Jankovic S , Kopitovic I , Vukcevic M ((2020) ) Pitolisant for daytime sleepiness in patients with obstructive sleep apnea who refuse continuous positive airway pressure treatment a randomized trial. Am J Respir Crit Care Med 201: , 1135–1145. |
[118] | Moraes W , Poyares D , Sukys-Claudino L , Guilleminault C , Tufik S ((2008) ) Donepezil improves obstructive sleep apnea in Alzheimer disease: A double-blind, placebo-controlled study. Chest 133: , 677–683. |
[119] | Liguori C , Mercuri NB , Nuccetelli M , Izzi F , Bernardini S , Placidi F ((2018) ) Cerebrospinal fluid orexin levels and nocturnal sleep disruption in Alzheimer’s disease patients showing neuropsychiatric symptoms. J Alzheimers Dis 66: , 993–999. |
[120] | Scaricamazza E , Colonna I , Sancesario GM , Assogna F , Orfei MD , Franchini F , Sancesario G , Mercuri NB , Liguori C ((2019) ) Neuropsychiatric symptoms differently affect mild cognitive impairment and Alzheimer’s disease patients: A retrospective observational study. Neurol Sci 40: , 1377–1382. |
[121] | Ismail Z , Black SE , Camicioli R , Chertkow H , Herrmann N , Laforce R , Montero-Odasso M , Rockwood K , Rosa-Neto P , Seitz D , Sivananthan S , Smith EE , Soucy JP , Vedel I , Gauthier S ((2020) ) Recommendations of the 5th Canadian Consensus Conference on the diagnosis and treatment of dementia. Alzheimers Dement 16: , 1182–1195. |




