Association Between the Cholinesterase Inhibitor Donepezil and the Cholinergic Precursor Choline Alphoscerate in the Treatment of Depression in Patients with Alzheimer’s Disease
Abstract
Background:
Depressive symptoms are common in Alzheimer’s disease (AD) patients and are associated with an increased functional decline. Selective serotonin reuptake inhibitor antidepressants showed a limited efficacy.
Objective:
The purpose of this work was to evaluate if a higher brain cholinergic stimulation induced by the association between the acetylcholinesterase inhibitor donepezil and the cholinergic precursor choline alphoscerate has any effect on depression in AD patients.
Methods:
Patients were selected among those recruited in the ASCOMALVA (association between the cholinesterase inhibitor donepezil and the cholinergic precursor choline alphoscerate in AD) trial. Depressive symptoms were investigated in 90 AD patients through the neuropsychiatric inventory at baseline and after 3, 6, 9, 12, 18, and 24 months of treatment. Patients were randomized in a group association therapy (45 subjects) receiving donepezil 10 mg plus choline alphoscerate 1,200 mg/day, and a group monotherapy (45 subjects) receiving donepezil 10 mg/day plus placebo. Based on the results of the MMSE at the recruitment patients were divided into 3 groups: severely impaired (score < 15); moderately impaired (score 19-16); mild-moderately impaired (score 24-20).
Results:
Depression symptoms were significantly lower (p < 0.05) in patients treated with donepezil plus choline alphoscerate compared to patients treated with donepezil alone. Subjects of the group having mild to moderate cognitive impairment were those more sensitive to the association treatment.
Conclusion:
Depression symptoms of AD patients in the mild to moderate stage probably could to benefit of a stronger cholinergic stimulation induced by associating donepezil with the cholinergic precursor choline alphoscerate.
INTRODUCTION
Depressive symptoms are common in Alzheimer’s disease (AD) patients, their prevalence ranging from 16% to 45% and represents one of the most common behavioral and psychological symptoms of dementia (BPSD) [1–3]. Subjects most often show some of them as the reduction in positive affect and pleasure, isolation, social withdrawal, and irritability [4], that lower their and their caregiver’s quality of life [5, 6]. Depression is associated with institutionalization [7] by increasing the risk of behavioral disturbances, functional decline [8], and mortality [9, 10].
Depression is a symptom of AD but may also represents a trait of the prodromal phase of the disease as well as a risk factor for AD [11]. Based on these observations, it has been suggested that both in depression and in AD there is the implication of common neurological pathways [12–15]. Moreover, white matter lesions of vascular type [16], hippocampal atrophy, and inflammatory phenomena which are present both in depression and AD, probably represent the common neuropathological substrate of the two disorders [17].
For patients with AD and depression, depression treatment should be initiated as soon as possible. Selective serotonin reuptake inhibitors (SSRIs) [18], particularly citalopram and sertraline, are still the first line drugs for treating depressive symptoms in AD, but they have limited efficacy. Systematic reviews and meta-analyses did not report enough evidence to support this practice [19, 20]. For example, citalopram has demonstrated effect on delusions, anxiety, and irritability, but not on depression/dysphoria [21] and the use of this drug is associated with cognitive worsening [22]. Sertraline was more effective than placebo, in the long term [23]. The Cochrane review on depression in dementia shows, globally, weak evidence of efficacy for these treatments [24], and other drugs such as antipsychotics and anticonvulsants have not shown more effect [19].
In contrast, the acetylcholinesterase inhibitor (AChE-I) donepezil leads to a long-term improvement in depression/dysphoria, anxiety, and apathy/indifference [25–27]. The administration of a stronger cholinergic stimulation by associating to the AChE-I donepezil to the cholinergic precursor choline alphoscerate, significantly reduced apathy in AD [28]. This effect is probably due to the higher brain acetylcholine bioavailability that this precursor/inhibitor association induces. These results, which are in agreement with previous data on choline alphoscerate [29], can be theoretically explained by an involvement of the cholinergic system in depressive symptoms of AD subjects.
In this work, we have compared the effect of treatment with the AChE-I donepezil alone or plus choline alphoscerate compared on depressive symptoms in AD patients. The analysis was done on AD patients recruited for the randomized trial ASCOMALVA (Association between the cholinesterase inhibitor Donepezil and the cholinergic precursor of choline alphoscerate in Alzheimer’s disease) [30]. Analysis included 24 months of observation.
MATERIALS AND METHODS
In the double-blind randomized trial ASCOMALVA [30, 31] out of 113 patients recruited, 71 subjects had depression symptoms at the baseline, and 19 patients developed depression symptoms during the period of observation. In these patients, randomly allotted to the treatment with donepezil 10 mg/day plus choline alphoscerate 1,200 mg/day (Association Therapy group, AT) or with donepezil 10 mg/day plus placebo (Monotherapy group, MT) the effect of pharmacological treatment on depression were assessed comparatively.
The mean age of patients was 77±7 years and the Mini-Mental State Examination (MMSE) score at baseline was between 14 and 24. All patients and their caregivers signed an informed consent.
Depression was investigated at the recruitment by an informal interview, and by the Frequency x Severity depression subtest of the Neuropsychiatric Inventory (NPI) at baseline and after 3, 6, 9, 12, 18 and 24 months. Caregiver’s distress was measured by the NPI-Distress of depression subtest.
Cognitive functions were investigated by the MMSE and Alzheimer’s Disease Assessment Scale Cognitive subscale (ADAS-Cog). MMSE is the best-known and the most often used short screening tool for providing an overall measure of cognitive impairment in clinic and research settings.
The ADAS-Cog is considered the gold standard for assessing the efficacy of antidementia treatments in clinical trial on patients with AD.
The main results for the primary and secondary outcome measures of the ASCOMALVA trial are reported elsewhere [30, 31].
The present description will be limited to the analysis of the effect of pharmacological treatment on depression in the AT and MT groups of patients.
Procedures
Presence and severity of depression were measured by the depression subscale of the NPI, a well-known test which allows a semi-quantitative assessment of various behavioral symptoms of AD [32]. Depression scores were obtained by multiplying frequency to severity. The score ≥1 in the depression subfield indicates its presence in the last 4 weeks; the maximum score representing the highest level of depression is 12.
Statistics
The correlation between the age and gender of the whole sample and the severity of behavioral symptoms (NPI-F) was assessed by the Pearson’s test of correlation. The variation during treatment in depression sub-item of NPI (NPI-F, NPI-D) for each treatment group were evaluated at baseline and at each follow up analysis by contrast statement in proc mixed. Analysis of variance (ANOVA) for repeated measure with Bonferroni correction were used for the comparison between treatment groups.
Ethics
The ASCOMALVA trial was approved by the Ethics Committee of the National Hospital, “A. Cardarelli”, Naples, Italy.
RESULTS
Among the patient population of the ASCOMALVA trial (113 subjects), 90 subjects (27 males) (mean age 77±7 years) presented depression symptomatology. Forty-five patients (12 males), with a mean age of 76±7 years were in the AT group and forty-five (15 males) with a mean age of 78±7 years were in MT group. The demographic and clinic characteristics of AD patients with depression are summarized in Table 1.
Table 1
Demographic and clinic characteristics of depressed AD patients examined
| 90 SUBJECT (27 M) | |||||
| Baseline | 12th month | p versus Baseline | 24th month | p versus Baseline | |
| Age | |||||
| MT | 76±7 | ||||
| AT | 78±7 | ||||
| p versus MT | 0.10165 | ||||
| Education | |||||
| MT | 7±4 | ||||
| AT | 7±4 | ||||
| p versus MT | 0.90572 | ||||
| MMSE | |||||
| MT | 20.4±2.7 | 19.1±5.4 | 0.15901 | 17.5±5.9* | 0.00359 |
| AT | 18.4±3.6# | 16.4±4.5*# | 0.02373 | 15.3±4.6* | 6.75834E-4 |
| p versus MT | 0.00418 | 0.01252 | 0.05656 | ||
| NPI (FxS) | |||||
| MT | 24.3±17.3 | 23.2±16.3 | 0.74539 | 29.7±19.5 | 0.16677 |
| AT | 21.5±12.3 | 24.5±15.2 | 0.31748 | 26.5±12.8 | 0.06565 |
| p versus MT | 0.38364 | 0.69351 | 0.35909 | ||
| NPI (distress) | |||||
| MT | 11.5±8.1 | 12.2±8.6 | 0.66936 | 17.1±14.7* | 0.02688 |
| AT | 12.0±7.9 | 13.4±9.5 | 0.43716 | 14.8±8.6 | 0.11642 |
| p versus MT | 0.77436 | 0.53863 | 0.36297 | ||
Data are expressed as means±S.D. MT, Monotherapy (donepezil 10 mg/day + placebo); AT, Association therapy (donepezil 10 mg/day + choline alphoscerate 1,200 mg/day); MMSE, Mini-Mental State Evaluation; NPI (FxS), Neuropsychiatric Inventory frequency multiplied by severity; NPI (stress), Neuropsychiatric Inventory caregiver distress. *p < 0.05 by versus baseline, #p < 0.05 versus monotherapy.
By analyzing the items of the NPI, we have found, after two years of treatment, a significant difference in depression scores between group AT and group MT patients. An increase in the severity and frequency of depression symptoms was observed in patients of the MT group compared to the baseline (Table 2). After two years of treatment, the severity of depression was not related to the assumption of antidepressants, as reported elsewhere [33]; data of NPI and antidepressant intake were converted to qualitative data, the number of patients respondent/non respondent to therapy were confronted by chi-square test that not showed significant response (p = 0.077). The correlation of the therapy was also confronted by odds ratio for the antidepressant efficacy, data shoved not significant correlation (OR: 2.65, C.I. 0.50 to 14.12; p = 0.254).
Table 2
Analysis of depression items of the NPI depression subtest, after two years of treatment
| Subtest NPI Depression | ||||
| Baseline | 24th month | p versus Baseline | Difference from baseline (SD) | |
| Depression (FxS) in MT subject | 4.2±4.2 | 6.3±4.4* | 0.00330 | 2.1±6.4 |
| Depression (FxS) in AT subject | 4.7±4.4 | 4.6±4.2# | 0.93208 | –0.1±5.0# |
| p versus MT | 0.88708 | 0.03076 | 0.04798 | |
Data are expressed as means±S.D. For the significance of abbreviations, see legend to Table 1. *p < 0.05 versus baseline, #p < 0.05 versus monotherapy.
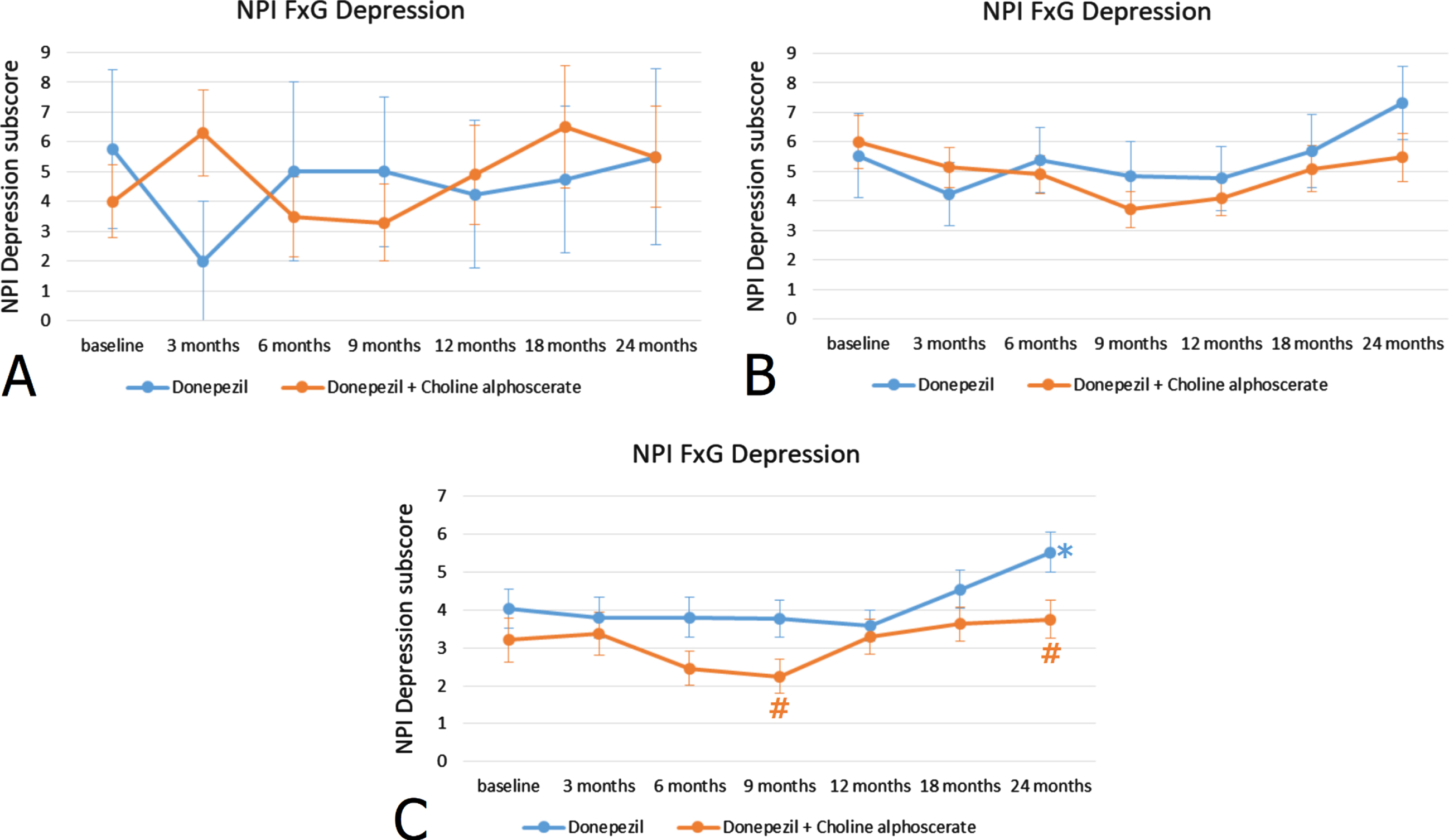
Stratification of patients according to MMSE scores at the recruitment revealed that the 23.5% of the sample included subjects with a low score (< 15) (group A), the 64.7% subjects with an intermediate score (16–19) (group B), and the remaining 11.8% of the patients had higher MMSE scores (20–24) (group C). In group C patients, those with high MMSE scores indicative of a mild-moderate cognitive impairment, the difference between the two treatments was statistically significant (Fig. 1C), whereas it was not significant in patients with low and intermediate MMSE scores (Fig. 1A, B).
Fig. 1
NPI depression subscore (frequency x severity) at the baseline and during the 24 months of observation in AD patients with MMSE score < 15 indicating severe cognitive impairment (A), with MMSE score 16–19 indicating moderate cognitive impairment (B) and MMSE score 20–24 indicating mild-moderate cognitive impairment (C). *p < 0.05 versus baseline; #p < 0.05 versus monotherapy.
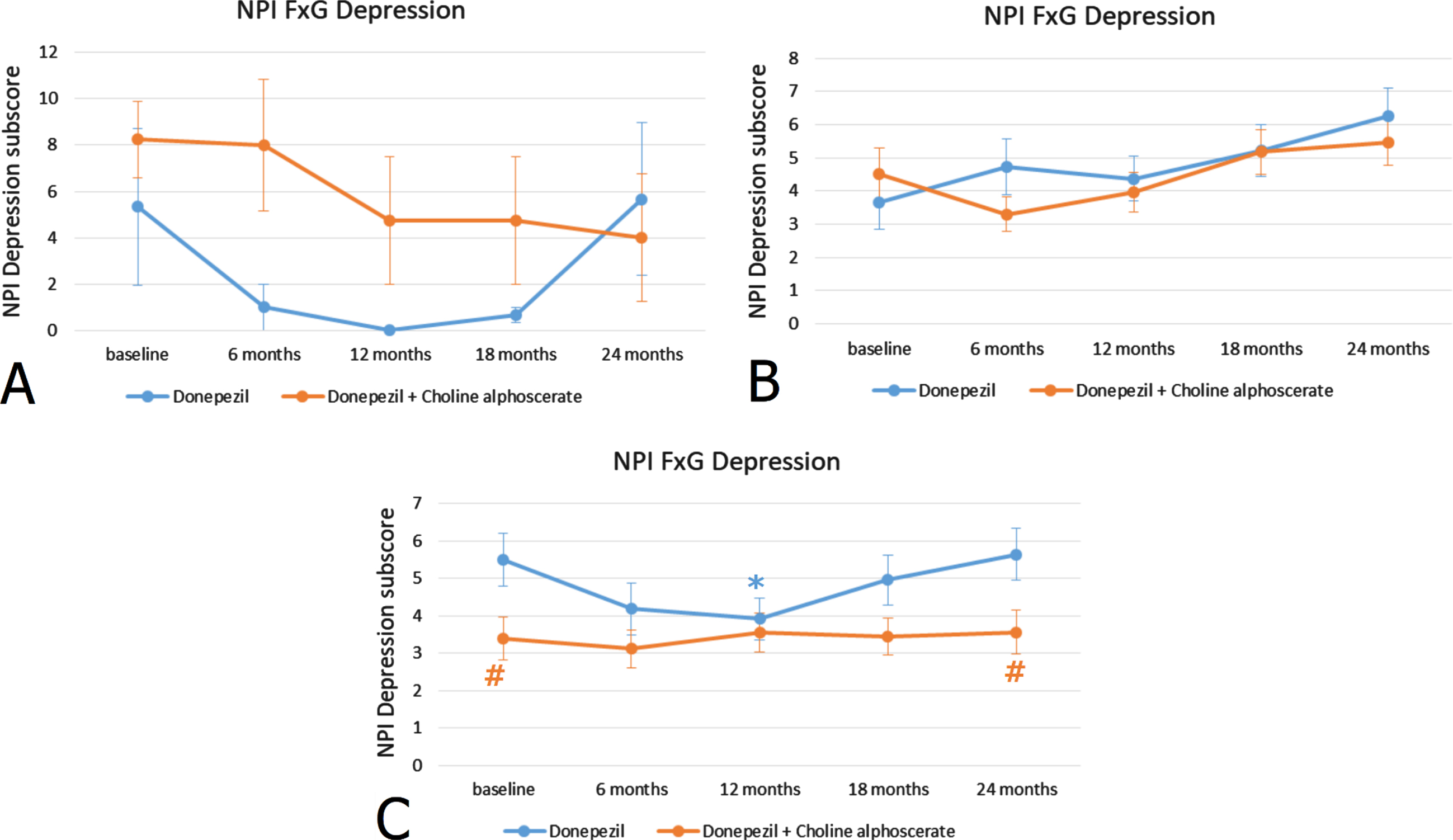
Stratification of patients according to ADAS-Cog scores revealed that the 23.5% of the sample included subjects with a high score (44–60), the 64.7% subjects with an average score (27–43) and the remaining 11.8% of the individuals had low ADAS-Cog scores (11–26). Similarly, as observed for the MMSE scores, in subjects with mild-moderate ADAS-Cog scores, the difference between monotherapy and association therapy was statistically significant (Fig. 2C). No statistically significant differences were noticeable in NPI depression scores between the two treatments in patients with moderate or severe ADAS-Cog scores (Fig. 2A, B). Depression severity was not related to the FAB score (correlation: –0,016 p = 0.699) (data not shown).
Fig. 2
NPI depression subscore (frequency x severity) at the baseline and during the 24 months of observation in AD patients with ADAS-Cog scores 44–60 indicating severe cognitive impairment (A), with ADAS-Cog scores 27–43 indicating moderate cognitive impairment (B) and ADAS-Cog scores 11–26 indicating mild-moderate cognitive impairment (C). *p < 0.05 versus baseline; #p < 0.05 versus monotherapy.
DISCUSSION
Over one-third of AD patients suffer from depressive symptoms [34–36] or from major depression [37–39]. These conditions contribute to the functional decline of these subjects [8, 40–42]. In this and other studies, the depression is more frequent in women than men, probably because women are more sensitive to depression [43–45]. Epidemiology and neuropsychology studies have shown a substantial impact of the depressive symptoms, alone or in combination, on the cognitive decline in AD [46], even if different assessments tools are employed [13]. The treatment of depressive symptoms AD is still a challenge as antidepressants, mainly belonging to the family of SSRI, have a limited effect [47], suggesting the presence in AD patients of a wider involvement of neurotransmitter systems than in patients showing depression only.
A different mechanism leading to depression and involving acetylcholine (ACh) has been hypothesized in the past [48]. This hypothesis has been revisited on the basis of the results coming from studies of neuroimaging and functional evaluation in AD patients and depressed subjects [49]. These studies have demonstrated that the areas mainly involved in mood regulation are the hippocampus and the subcortical networks of the frontal lobe [50]. These brain areas are structurally and functionally damaged in AD patients showing depression, particularly in the presence of relevant vascular risk factors [35, 47]. Hence, ACh may have a significant impact not only on attention and learning but on mood regulation too [52–54] through a quite complex mechanism consisting in the coordination of the neural networks.
Neuropathological studies have also reported in AD patients with depression, the presence of white matter lesions in subcortical and frontal areas [16]. These aspects are attributed to the coexistence of neurodegeneration and vascular impairment in these subjects which represent a large majority of AD patients [55]. Choline alphoscerate has demonstrated to have a neuroprotective effect and has shown a clear benefit on the cognitive and behavioral symptoms in AD subjects with cerebrovascular injury [30, 31]. Unlike the other cholinergic precursors, choline alphoscerate is probably capable of being rapidly absorbed and crossing the blood-brain barrier acting as a donor of metabolically active choline in the brain and basal forebrain cholinergic structures involved cognitive activities which are particularly sensitive to ischemia [56]. A recent study reported that administration of it, would protect the brain by reducing transthyretin deposition (an amyloidogenic protein) and preventing neuroinflammation [57].
The coexistence of both neurodegenerative and vascular injury occurred also in our sample of subjects. In fact our patients which had AD diagnosed according to the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) criteria [58], for being included in the ASCOMALVA trial [30] were required to show brain ischemic lesions documented by neuroimaging techniques and to have at least two vascular risk factors (as hypertension, diabetes, obesity, ischemic heart disease, hypercholesterolemia, hyperhomocysteinemia, smoking, previous cerebrovascular events, family history of cardio-cerebrovascular diseases). On the other hand, as reported in systematic reviews and meta-analysis, patients treated previously with SSRI, did not have relevant improvement of depressive symptoms [19, 24, 59].
Previous preclinical studies have shown that choline alphoscerate alone or in association with an AChE-I enhances cholinergic transmission [30, 31, 60, 61]. Based on these observations, we have evaluated if a higher amount of acetylcholine obtained by associating donepezil and choline alphoscerate might have a favorable effect on depression in subjects with AD. In general, our study has shown a stability of the depressive symptoms in subjects receiving donepezil plus choline alphoscerate for 24 months, whereas patients receiving donepezil only had a worsening of these symptoms. The more pronounced effect was found in subjects with mild-moderate cognitive impairment and not in those with moderate and severe impairment. It is probable that the more pronounced effect of the association therapy occurs in the presence of a relatively limited damage of the cholinergic system. A more relevant impairment of the brain cholinergic system probably exceeds the possibility of compensating this imbalance by increasing cholinergic tone and making more ACh available. Furthermore, it cannot be excluded that connections among cholinergic, glutamatergic [62, 63], and monoaminergic pathways [64] in the subcortical networks of the frontal lobe, might be involved in n the pathophysiology of affective disorders.
Our data could seem conflicting with the large use, particularly in the past years, of anticholinergic drugs for depression. Recent studies have suggested that depression may be relieved by the cholinergic stimulation of a subtype of nicotinic receptors, the alpha7 receptor, through the improvement of hippocampal function [65]. It is also well known that the hippocampus is involved in depression as well as in cognitive dysfunctions. In AD it can represent a crossroad between behavioral and cognitive symptoms [66]. Furthermore, SSRI indirectly may act on the cholinergic system by increasing brain ACh levels by inhibiting the cholinesterase [67]. In this perspective, it cannot be excluded that a pronounced cholinergic stimulation induced by the association cholinergic precursor and inhibitor might help to preserve the networks involved in mood regulation.
This study has strengths and weaknesses: the longtime of observation and treatment (24 months) represent strength points, while the size of the sample and the post hoc nature of the data are weak points. We are also aware that even the adequacy of the term Depression—as it is defined by the Diagnostic and Statistical Manual of Mental Disorders (DSM)—may be inadequate to the symptoms of AD. In fact, it has been suggested that the depressive symptoms might be better grouped in the category “affective syndrome” which includes apathy, anxiety, and irritability.
Depressive symptoms were measured by one scale only, the NPI subtest depression frequency x severity, which is due to the fact that the study of depressive symptoms was a secondary outcome of ASCOMALVA trial. On the other hand, the NPI cannot be considered an exhaustive test for measuring depression in AD, although no other tools are available for assessing depressive symptoms in AD.
Increasing evidence suggests a crucial role of brain membrane lipids (n-3 polyunsaturated fatty acids, glycerolipids, glycerophospholipids, and sphingolipids) in depression- and anxiety-related behaviors [68]. It cannot be excluded that the beneficial effect elicited by choline alphoscerate in depression symptoms in AD may be due besides to the induction of an increase of ACh bioavailability, to an activity on neuronal reparatory mechanisms promoted by brain membrane lipids.
ACKNOWLEDGMENTS
The authors thank their own institutions for allowing them to perform this non-profit investigation. The complimentary supply of choline alphoscerate and placebo solution and the support of PhD bursaries by MDM S.p.A. (Monza, Italy) is gratefully acknowledged.
FUNDING
The authors have no funding to report.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
REFERENCES
[1] | Chi S , Wang C , Jiang T , Zhu XC , Yu JT , Tan L ((2015) ) The prevalence of depression in Alzheimer’s disease: A systematic review and meta-analysis. Curr Alzheimer Res 12: , 189–198. |
[2] | Panza F , Frisardi V , Capurso C , D’Introno A , Colacicco AM , Imbimbo BP , Santamato A , Vendemiale G , Seripa D , Pilotto A , Capurso C , Solfrizzi V ((2010) ) Late-life depression, mild cognitive impairment, and dementia: Possible continuum? Am J Geriatr Psychiatry 18: , 98–116. |
[3] | Verkaik R , Nuyen J , Schellevis F , Francke A ((2007) ) The relationship between severity of Alzheimer’s disease and prevalence of comorbid depressive symptoms and depression: A systematic review. Int J Geriatr Psychiatry 22: , 1063–1086. |
[4] | Charney DS , Reynolds CF , Lewis L , Lebowitz BD , Sunderland T , Alexopoulos GS , Blazer DG , Katz IR , Meyers BS , Arean PA , Borson S , Brown C , Bruce ML , Callahan CM , Charlson ME , Conwell Y , Cuthbert BN , Devanand DP , Gibson MJ , Gottlieb GL , Krishnan KR , Laden SK , Lyketsos CG , Mulsant BH , Niederehe G , Olin JT , Oslin DW , Pearson J , Persky T , Pollock BG , Raetzman S , Reynolds M , Salzman C , Schulz R , Schwenk TL , Scolnick E , Unutzer J , Weissman MM , Young RC , Depression and Bipolar Support Alliance ((2003) ) Depression and bipolar support alliance consensus statement on the unmet needs in diagnosis and treatment of mood disorders in late life. Arch Gen Psychiatry 60: , 664–672. |
[5] | Allan LM , Ballard CG , Rowan EN , Kenny RA ((2009) ) Incidence and prediction of fall in dementia: A prospective study in older people. PLoS One 4: , e5521. |
[6] | Liu S , Li C , Shi Z , Wang X , Zhou Y , Liu S , Liu J , Yu T , Ji Y ((2017) ) Caregiver burden and prevalence of depression, anxiety and sleep disturbances in Alzheimer’s disease caregivers in China. Clin Nurs 26: , 1291–1300. |
[7] | Gaugler JE , Yu F , Krichbaum K , Wyman JF ((2009) ) Predictors of nursing home admission for persons with dementia. Med Care 47: , 191–198. |
[8] | Lyketsos CG , Steele C , Baker L , Galik E , Kopunek S , Steinberg M , Warren W ((1997) ) Major and minor depression in Alzheimer’s disease: Prevalence and impact. J Neuropsychiatry Clin Neurosci 9: , 556–61. |
[9] | Burns A , Lewis G , Jacoby R , Levy R ((1991) ) Factors affecting survival in Alzheimer’s disease. Psychol Med 21: , 363–370. |
[10] | Suh GH , Kil Yeon B , Shah A , Lee JY ((2005) ) Mortality in Alzheimer’s disease: A comparative prospective Korean study in the community and nursing homes. Int J Geriatr Psychiatry 20: , 26–34. |
[11] | Ownby RL , Crocco E , Acevedo A , Vineeth J , Loewenstein DA ((2006) ) Depression and risk for Alzheimer’s disease: Systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry 63: , 530–538. |
[12] | Byers AL , Yaffe K ((2011) ) Depression and risk of developing dementia. Nat Rev Neurol 7: , 323–331. |
[13] | Quattropani MC , Lenzo V , Armieri V , Filastro A ((2018) ) The origin of depression in Alzheimer disease: A systematic review. Riv Psichiatr 53: , 18–30. |
[14] | Sacuiu S , Insel PS , Mueller S , Tosun D , Mattsson N , Jack CR Jr , DeCarli C , Petersen R , Aisen PS , Weiner MW , Mackin RS , Alzheimer’s Disease Neuroimaging Initiative ((2016) ) Chronic depressive symptomatology in mild cognitive impairment is associated with frontal atrophy rate which hastens conversion to Alzheimer dementia. Am J Geriatr Psychiatry 24: , 126–135. |
[15] | Vilalta-Franch J , López-Pousa S , Llinàs-Reglà J , Calvó-Perxas L , Merino-Aguado J , Garreolmo J ((2012) ) Depression subtypes and 5-year risk of dementia and Alzheimer disease in patients aged 70 years. Int J Geriatr Psychiatry 28: , 341–350. |
[16] | Yatawara C , Lee D , Ng KP , Chander R , Ng D , Ji F , Shim HY , Hilal S , Venketasubramanian N , Chen C , Zhou J , Kandiah N ((2019) ) Mechanisms linking white matter lesions, tract integrity, and depression in Alzheimer disease. Am J Geriatr Psychiatry 27: , 948–959. |
[17] | De Winter FL , Emsell L , Bouckaert F , Claes L , Jain S , Farrar G , Billiet T , Evers S , Van den Stock J , Sienaert P , Obbels J , Sunaert S , Adamczuk K , Vandenberghe R , Laere KV , Vandenbulcke M ((2017) ) No association of lower hippocampal volume with Alzheimer’s disease pathology in late-life depression. Am J Psychiatry 174: , 237–245. |
[18] | Patel K , Abdool PS , Rajji TK , Mulsant BH ((2017) ) Pharmacotherapy of major depression in late life: What is the role of new agents? Expert Opin Pharmacother 18: , 599–609. |
[19] | Lozupone M , La Montagna M , D’Urso F , Piccininni C , Sardone R , Dibello V , Giannelli G , Solfrizzi V , Greco A , Daniele A , Quaranta N , Seripa D , Bellomo A , Logroscino G , Panza F ((2018) ) Pharmacotherapy for the treatment of depression in patients with alzheimer’s disease: A treatment-resistant depressive disorder. Expert Opin Pharmacother 19: , 823–842. |
[20] | Zhuo C , Xue R , Luo L , Ji F , Tian H , Qu H , Lin X , Jiang R , Tao R ((2017) ) Efficacy of antidepressive medication for depression in Parkinson disease: A network meta-analysis. Medicine (Baltimore) 96: , e6698. |
[21] | Leonpacher AK , Peters ME , Drye LT , Makino KM , Newell JA , Devanand DP , Frangakis C , Munro CA , Mintzer JE , Pollock BG , Rosenberg PB , Schneider LS , Shade DM , Weintraub D , Yesavage J , Lyketsos CG , Porsteinsson AP , CitAD Research Group ((2016) ) Effects of citalopram on neuropsychiatric symptoms in Alzheimer’s dementia: Evidence from the citAD study. Am J Psychiatry 173: , 473–480. |
[22] | Drye LT , Spragg D , Devanand DP , Frangakis C , Marano C , Meinert CL , Mintzer JE , Munro CA , Pelton G , Pollock BG , Porsteinsson AP , Rabins PV , Rosenberg PB , Schneider LS , Shade DM , Weintraub D , Yesavage Y , Lyketsos CG , CitAD Research Group ((2014) ) . Changes in QTc interval in the citalopram for agitation in Alzheimer’s disease (CitAD) randomized trial. PLoS One 9: , e98426. |
[23] | Banerjee S , Hellier J , Dewey M , Romeo R , Ballard C , Baldwin R , Bentham P , Fox C , Holmes C , Katona C , Knapp M , Lawton C , Lindesay J , Livingston G , McCrae N , Moniz-Cook E , Murray J , Nurock S , Orrell M , O’Brien J , Poppe M , Thomas A , Walwyn R , Wilson K , Burns A ((2011) ) Sertraline or mirtazapine for depression in dementia (HTA-SADD): A randomised, multicentre, double-blind, placebo-controlled trial. Lancet 378: , 403–411. |
[24] | Bains J , Birks JS , Dening TR ((2002) ) Antidepressants for treating depression in dementia. Cochrane Database Syst Rev 21: , CD003944. |
[25] | Carrasco MM , Agüera L , Gil P , Moríñigo A , Leon T ((2011) ) Safety and effectiveness of donepezil on behavioral symptoms in patients with Alzheimer’s disease. Alzheimer Dis Assoc Disord 25: , 333–340. |
[26] | Cummings JL , McRae T , Zhang R ((2006) ) Donepezil-Sertraline Study Group. Effects of donepezil on neuropsychiatric symptoms in patients with dementia and severe behavioral disorders. Am J Geriatr Psychiatry 14: , 605–612. |
[27] | Tanaka M , Namiki C , Thuy DH , Yoshida H , Kawasaki K , Hashikawa K , Fukuyama H , Kita T ((2004) ) Prediction of psychiatric response to donepezil in patients with mild to moderate Alzheimer’s disease. J Neurol Sci 225: , 135–141. |
[28] | Rea R , Carotenuto A , Fasanaro AM , Traini E , Amenta F ((2014) ) Apathy in Alzheimer’s disease: Any effective treatment? ScientificWorldJournal 2014: , 421385. |
[29] | De Jesus Moreno Moreno M ((2003) ) Cognitive improvement in mild to moderate Alzheimer’s dementia after treatment with the acetylcholine precursor choline alfoscerate: A multicenter, double-blind, randomized, placebo-controlled trial. Clin Ther 25: , 178–193. |
[30] | Amenta F , Carotenuto A , Fasanaro AM , Rea R , Traini E ((2014) ) The ASCOMALVA (Association between the Cholinesterase Inhibitor Donepezil and the Cholinergic Precursor Choline Alphoscerate in Alzheimer’s Disease) Trial: Interim results after two years of treatment. J Alzheimers Dis 42: , S281–S288. |
[31] | Amenta F , Carotenuto A , Fasanaro AM , Rea R , Traini E ((2012) ) The ASCOMALVA trial: Association between the cholinesterase inhibitor donepezil and the cholinergic precursor choline alphoscerate in Alzheimer’s disease with cerebrovascular injury: Interim results. J Neurol Sci 322: , 96–101. |
[32] | Cummings JL , Mega M , Gray K , Rosenberg-Thompson S , Carusi DA , Gornbein J ((1994) ) The Neuropsychiatric Inventory. Comprehensive assessment of psychopathology in dementia. Neurology 44: , 2308–2314. |
[33] | Carotenuto A , Rea R , Traini E , Fasanaro AM , Ricci G , Manzo V , Amenta F ((2017) ) The effect of the association between donepezil and choline alphoscerate on behavioral disturbances in Alzheimer’s disease: Interim results of the ASCOMALVA Trial. J Alzheimers Dis 56: , 805–815. |
[34] | Ballard C , Bannister C , Solis M , Oyebode F , Wilcock G ((1996) ) The prevalence, associations and symptoms of depression amongst dementia sufferers. J Affect Disord 22: , 135–144. |
[35] | Enache D , Winblad B , Aarsland D ((2011) ) Depression in dementia: Epidemiology, mechanisms, and treatment. Curr Opin Psychiatry 24: , 461–472. |
[36] | Starkstein SE , Jorge R , Mizrahi R , Robinson RG ((2005) ) The construct of minor and major depression in Alzheimer’s disease. Am J Psychiatry 162: , 2086–2093. |
[37] | Galts CPC , Bettio LEB , Jewett DC , Yang CC , Brocardo PS , Rodrigues ALS , Thacker JS , Gil-Mohapel J ((2019) ) Depression in neurodegenerative diseases: Common mechanisms and current treatment options. Neurosci Biobehav 102: , 56–84. |
[38] | Migliorelli R , Teson A , Sabe L , Petracchi M , Leiguarda R , Starkstein SE ((1995) ) Prevalence and correlates of dysthymia and major depression among patients with Alzheimer’s disease. Am J Psychiatry 152: , 37–44. |
[39] | Olin JT , Schneider LS , Katz IR , Meyers BS , Alexopoulos GS , Breitner JC , Bruce ML , Caine ED , Cummings JL , Devanand DP , Krishnan KRR , Lyketsos CG , Lyness JM , Rabins PV , Reynolds CF 3rd , Rovner BW , Steffens DC , Tariot PN , Lebowitz BD ((2002) ) Provisional diagnostic criteria for depression of Alzheimer disease. Am J Geriatr Psychiatry 10: , 125–128. |
[40] | Gómez-Gallego M , Gómez-García J , Ato-Lozano E ((2017) ) The mediating role of depression in the association between disability and quality of life in Alzheimer’s disease. Aging Ment Health 21: , 163–172. |
[41] | Köhler S , van Boxtel S , Jolles J , Verhey F ((2011) ) Depressive symptoms and risk for dementia: A 9-year follow-up of the Maastricht Aging Study. Am J Geriatr Psychiatry 19: , 902–905. |
[42] | Maji T , Pluta JP , Mell T , Treusch Y , Gutzmann H , Rapp MA ((2012) ) Correlates of agitation and depression in nursing home residents with dementia. Int Psychogeriatr 24: , 1779–1789. |
[43] | LeeJ, LeeKJ, KimH ((2017) ) Gender differences in behavioral and psychological symptoms of patients with Alzheimer’s disease. Asian J Psychiatr 26: , 124–128. |
[44] | Colombo D , Caltagirone C , Padovani A , Sorbi S , Spalletta G , Simoni L , Ori A , and Zagni E ((2018) ) Gender differences in neuropsychiatric symptoms in mild to moderate Alzheimer’s disease patients undergoing switch of cholinesterase inhibitors: Aanalysis of the EVOLUTION Study. J Womens Health (Larchmt) 27: , 1368–1377. |
[45] | Cohen D , Eisdorfer C , Gorelick P , Luchins D , Freels S , Semla T , Paveza G , Shaw H , Ashford JW ((1993) ) Sex differences in the psychiatric manifestations of Alzheimer’s disease. J Am Geriatr Soc 41: , 229–232. |
[46] | Gasser AI , Salamin V , Zumbach S ((2018) ) Late life depression or prodromal Alzheimer’s disease: Which tools for the differential diagnosis? Encephale 44: , 52–58. |
[47] | Orgeta V , Tabet N , Nilforooshan R , Howard R ((2017) ) Efficacy of antidepressants for depression in Alzheimer’s disease: Systematic review and meta-analysis. J Alzheimers Dis 58: , 725–733. |
[48] | Janowski DS , ElYousef MK , Davis JM , Sekerke HJ ((1972) ) A cholinergic adrenergic hypothesis of mania and depression. Lancet 2: , 632–635. |
[49] | Mineur YS , Cahuzac EL , Mose TN , Bentham MP , Plantenga ME , Thompson DC , Picciotto MR ((2018) ) Interaction between noradrenergic and cholinergic signaling in amygdala regulates anxiety- and depression-related behaviors in mice. Neuropsychopharmacology 43: , 2118–2125. |
[50] | Aznar S , Knudsen GM ((2011) ) Depression and Alzheimer’s disease: Is stress the initiating factor in a common neuropathological cascade? J Alzheimers Dis 23: , 177–193. |
[51] | Chi S , Yu JT , Tan MS , Tan L ((2014) ) Depression in Alzheimer’s disease: Epidemiology, mechanisms, and management. J Alzheimers Dis 42: , 739–755. |
[52] | Higley MJ , Picciotto MR ((2014) ) Neuromodulation by acetylcholine: Examples from schizophrenia and depression. Curr Opin Neurobiol 29: , 88–95. |
[53] | Mineur YS , Obayemi A , Wigestrand MB , Fote GM , Calarco CA , Li AM , Picciotto MR ((2013) ) Cholinergic signaling in the hippocampus regulates social stress resilience and anxiety- and depression-like behavior. Proc Natl Acad Sci U S A 110: , 3573–3578. |
[54] | Picciotto MR , Higley MJ , Mineur YS ((2012) ) Acetylcholine as a neuromodulator: Cholinergic signaling shapes nervous system function and behavior. Neuron 76: , 116–129. |
[55] | Bidzan M , Bidzan L , Pachalska M ((2014) ) Neuropsychiatric symptoms in patients with Alzheimer’s disease with a vascular component. Ann Agric Environ Med 21: , 412–415. |
[56] | Parnetti L , Mignini F , Tomassoni D , Traini E , Amenta F ((2007) ) Cholinergic precursors in the treatment of cognitive impairment of vascular origin: Ineffective approaches or need for re-evaluation? J Neurol Sci 257: , 264–269. |
[57] | Matsubara K , Okuda M , Shibata S , Miyaki S , Ohkubo T , Izu H , Fujii T ((2018) ) The delaying effect of alpha-glycerophosphocholine on senescence, transthyretin deposition, and osteoarthritis in senescence-accelerated mouse prone 8 mice. Biosci Biotechnol Biochem 82: , 647–653. |
[58] | McKhann G , Drachman D , Folstein M , Katzman R , Price D , Stadlan EM ((1984) ) Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34: , 939–944. |
[59] | Dudas R , Malouf R , McCleery J , Dening T ((2018) ) Antidepressants for treating depression in dementia. Cochrane Database Syst Rev 8: , CD003944. |
[60] | Amenta F , Tayebati SK , Vitali D , Di Tullio MA ((2006) ) Association with the cholinergic precursor choline alphoscerate and the cholinesterase inhibitor rivastigmine: An approach for enhancing cholinergic neurotransmission. Mech Ageing Dev 127: , 173–179. |
[61] | Tayebati SK , Di Tullio MA , Tomassoni D , Amenta F ((2009) ) Neuroprotective effect of treatment with galantamine and choline alphoscerate on brain microanatomy in spontaneously hypertensive rats. J Neurol Sci 283: , 187–194. |
[62] | Gipson KE , Yeckel MF ((2007) ) Coincident glutamatergic and cholinergic inputs transiently depress glutamate release at rat schaffer collateral synapses. J Neurophysiol 97: , 4108–4119. |
[63] | Henter ID , Sousa RT , Oro PW , Brunoni AR , Zarate CA , Machado-Vieira R ((2017) ) Mood therapeutics: Novel pharmacological approaches for treating depression. Expert Rev Clin Pharmacol 10: , 153–166. |
[64] | Dilsaver SC ((1986) ) Cholinergic mechanisms in depression. Brain Res 396: , 285–316. |
[65] | Zhao D , Xu X , Pan L , Zhu W , Fu X , Guo L , Lu Q , Wang J ((2017) ) Pharmacologic activation of cholinergic alpha7 nicotinic receptors mitigates depressive-like behavior in a mouse model of chronic stress. J Neuroinflammation 14: , 234. |
[66] | Demir EA , Tutuk O , Dogan H , Tumer C ((2019) ) Depression in Alzheimer’s disease: The roles of cholinergic and serotonergic systems. In Alzheimer’s disease, WisniewskiT, ed. Codon Publications, Brisbane (AU), pp. 223–235. |
[67] | Nisa MU , Munawar MA , Iqbal A , Ahmed A , Ashraf M , Gardener QA , Khan MA ((2017) ) Synthesis of novel 5-(aroylhydrazinocarbonyl) escitalopram as cholinesterase inhibitors. Eur J Med Chem 138: , 396–406. |
[68] | Müller CP , Reichel M , Mühle C , Rhein C , Gulbins E , Kornhuber J ((2015) ) Brain membrane lipids in major depression and anxiety disorders. Biochim Biophys Acta 1851: , 1052–1065. |




