Time Investment for Program Implementation to Manage Neuropsychiatric Symptoms: An Observational Longitudinal Study in In-Home and Residential Care Settings
Abstract
Background:
There are no studies on how the same psychosocial dementia care program is adapted to both in-home and residential care settings.
Objective:
To evaluate the time investment required by professionals to implement a psychosocial dementia care program to manage neuropsychiatric symptoms.
Methods:
A prospective observational study design was used. The program consisted of 1) a one-day training course, 2) three interdisciplinary discussion meetings in five months, and 3) a web-based tool for the continued assessment of neuropsychiatric symptoms. Care professionals implemented the intervention in in-home (19 in-home care management agencies and 14 multiple in-home service providers) and residential care settings (19 group homes and eight nursing homes) in Japan from October 2019 to February 2020. The level of neuropsychiatric symptoms for the participants was evaluated using the Neuropsychiatric Inventory (NPI: 0–144). The time investment was reported by participating professionals. A total of 125 persons with dementia were included at baseline.
Results:
Neuropsychiatric symptoms were significantly decreased at the final follow-up in all types of providers (Cohen’s drm = 0.44–0.61). The mean (SD) time required for the five-month implementation was 417.9 (219.8) minutes. There was a mean (SD) decrease of 8.6 (14.0) points in the total NPI score among the 103 persons with completed interventions. The time investment was significantly lower in in-home care management agencies than in group homes, and lower in follow-ups than at baseline assessment.
Conclusion:
The program implementation may incur a substantial time investment regardless of setting. An additional benefit scheme to reward the time investment would be helpful to encourage implementation until the follow-ups.
INTRODUCTION
As the number of people with dementia increases globally [1], dementia has become a public health priority [2, 3]. Dementia has significant social and economic implications in terms of direct medical and social care costs, and the costs of informal care [4–6]. Neuropsychiatric symptoms contribute to the overall costs of dementia care, both in the community [7, 8] and in care homes [9]. Neuropsychiatric symptoms are highly prevalent in persons with dementia [10, 11], resulting in negative health consequences, including lower quality of life [12], increased psychological suffering [13], higher psychotropic drug use [14], shorter time to institutionalization [5], and increased mortality [15]. Furthermore, family caregivers suffer from a higher burden and elevated risk of being sick [13]. As neuropsychiatric symptoms are considered expressions of distress [16–18], psychosocial interventions are globally recommended as first-line treatments to target the underlying causes [2, 19, 20].
Psychosocial interventions of neuropsychiatric symptoms involve the provision of care tailored to the individual’s needs, considering their life context [21]. The underlying causes can lie in different life areas, including psychiatric aspects. Therefore, dementia case management models should enable interdisciplinary team approaches, integrate complex networks of care professionals, and respond appropriately to the unmet needs of persons with dementia [2, 20, 22]. The Swedish BPSD-registry was launched in November 2010 to improve the care of persons with dementia by focusing on psychosocial interventions for neuropsychiatric symptoms [15]. The BPSD-registry is based on an interdisciplinary team approach, and was primarily established for use in nursing homes. Adapted forms of the program have been developed in Denmark [23], the Netherlands [24], and Japan [25].
The Japanese adaptation of the BPSD-registry, called DEMBASE®, has been developed with a focus on care managers of in-home service users with dementia. The results of a cluster-randomized controlled trial indicated a reduction in neuropsychiatric symptoms upon application of the adapted program [25], with a medium observed effect size (Cohen’s dppc = 0.52). Based on the results, the program was implemented for routine dementia care in the Tokyo metropolitan area in April 2018. However, several in-home care managers raised barriers to the interdisciplinary approach, as it is not usually incorporated into their daily practice with in-home service providers [26]. The program is expected to incur substantial time investment, particularly from interdisciplinary discussion meetings to evaluate neuropsychiatric symptoms, identify unmet needs, and establish an action plan. The time investment required across various care settings warrants further investigation to design a rewarding system that can facilitate program implementation. Most psychosocial interventions require minimal to moderate financial investment by family caregivers in in-home settings as well as care professionals in residential care settings [27]. However, there is scarce evidence for care professionals’ management of the neuropsychiatric symptoms of dementia in home-based practices. Furthermore, there are no studies on how the same psychosocial dementia care program is adapted to both in-home and residential care settings. Uncovering differences could have global implications for the dissemination of psychosocial interventions for neuropsychiatric symptoms in people with dementia across various care settings.
This study evaluates the time investment required to implement a psychosocial dementia care program for neuropsychiatric symptoms in both in-home and residential care settings. Our research questions were as follows:
(1) Are neuropsychiatric symptoms reduced via the program implementation in both residential and in-home care settings?
(2) Is care professionals’ time investment higher in in-home than residential care settings?
MATERIALS AND METHODS
Study design
A prospective, observational study design was adopted.
Participants
Care professionals working as long-term service providers were invited to participate in the program by four nationwide voluntary organizations in joint commission with the Ministry of Health, Labour and Welfare (Fig. 1). Each organization selected candidate providers and asked for participation within three regions (Chiba, Kanagawa, and Saitama) around the Tokyo metropolitan area. The three regions were selected to enable employees to participate in the training course held in Tokyo and return home the same day. The directors of providers asked their employees (care professionals) to participate.
Fig.1
Participant recruitment, registration, and implementation of the psychosocial dementia care program. Care professionals working as long-term service providers were invited to participate in the program by four nationwide voluntary organizations. Each organization selected candidate providers and asked for participation. The directors of providers asked their employees (care professionals) to participate. In-home care management agencies have care managers who handle monthly care plans for in-home care users and work independently from in-home care service providers such as day care centers and home help. Multiple in-home service providers offer home-based care services to older adults in the community. Group homes offer small-scale, homelike accommodation for residents with mild to moderate dementia. Nursing homes offer permanent residence for older adults with and without dementia who are stable but require regular nursing care. Each provider organization was asked to recruit 20 professionals and 40 persons with dementia.
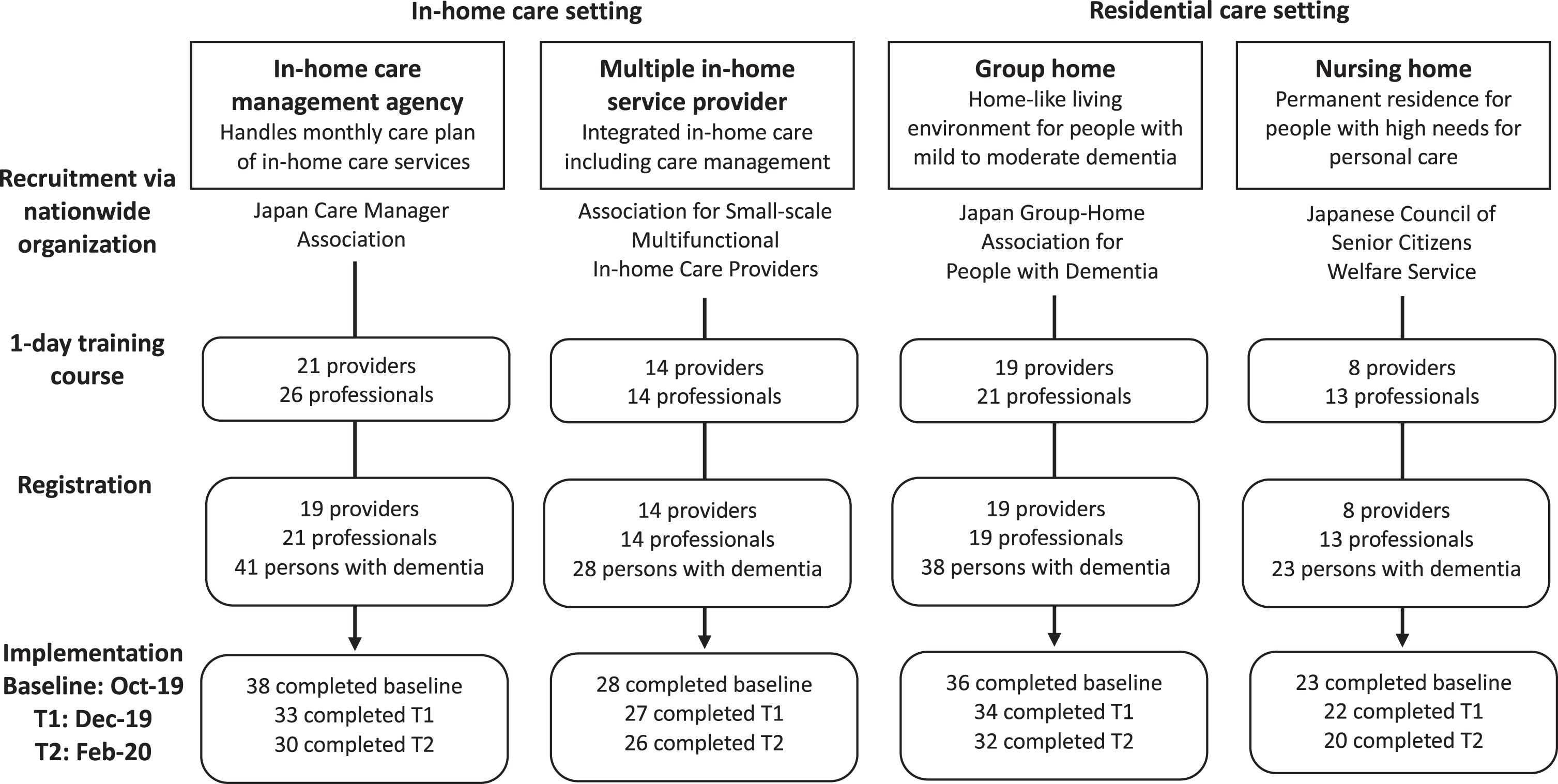
Multiple in-home service providers, group homes, and nursing homes were sampled for comparison with in-home care management agencies. In-home care management agencies have care managers who handle monthly care plans for in-home care users and work independently from in-home care service providers such as day care centers and home help. Multiple in-home service providers offer home-based care services to older adults in the community. Group homes offer small-scale, homelike accommodation for residents with mild to moderate dementia. Nursing homes offer a permanent residence for older adults with and without dementia who are stable but require regular nursing care. Nursing home residents have greater personal care needs but lower healthcare needs than group home residents [28]. In-home care service users are those in-home care management agencies or multiple in-home service providers. Residential care settings in this study comprised group homes and nursing homes. We hypothesized that in-home care management agencies would invest more time in program implementation than the other three types of providers, because they provide care management to users independently from service providers, thus increasing the difficulty of holding interdisciplinary discussion meetings [26].
The sample size was calculated for the analysis of variance in time investment for repeated measures between factors, using G*Power 3.1.9.7 [29, 30]. Assuming an alpha level of 0.05 and 95% power, 132 participants were required to observe an effect size of 0.3 (Cohen’s f, medium) in time investment. To allow for a 16% dropout rate based on a previous study in Tokyo [25, 31], the sample size was set at 157 persons with dementia. Therefore, each provider organization was asked to recruit 20 professionals and 40 persons with dementia, to collate a total of 160 persons with dementia at baseline. It was intended that each type of provider has an equal number of participants.
Intervention
The program comprised 1) a one-day training course, 2) interdisciplinary discussion meetings, 3) a web-based tool for ongoing behavioral assessments, and 4) a three-hour debriefing meeting.
Training course
The training course guided 1) the process of interdisciplinary meetings for the evaluation of neuropsychiatric symptoms, specification of unmet needs using a 23-item checklist, and establishment of an action plan using an interdisciplinary approach; 2) implementation of the action plan; and 3) use of a web-based tool. The training course was based on consideration of neuropsychiatric symptoms as communicating unmet needs [16–18]. Psychosocial interventions with goal-setting, such as providing pleasant activities, outdoor activities, and removal of environmental triggers were recommended to address these unmet needs based on global evidence [22]. The web-based tool was explained to each participating professional during the training course, held at the end of September 2019.
Interdisciplinary discussion meeting
Once the training course was completed, the professionals held an interdisciplinary discussion meeting with other care professionals to evaluate the neuropsychiatric symptoms of each participant with dementia, to specify their unmet needs, and to establish an interdisciplinary action plan to meet those needs. The medications prescribed to each participant with dementia were also assessed. These components were included to promote a plan-do-study-act (PDSA) cycle, developed according to the team-based dementia case management model. The PDSA cycle is widely used as a quality improvement method in healthcare settings [32]. If the level of neuropsychiatric symptoms assessed was not reduced during follow-up evaluations, care professionals reviewed unmet needs and revised the action plan during the discussion meeting. Unmet needs, contents of the action plan, and type of caregivers involved in the meeting were categorized and recorded in the web-based tool (Supplementary Table 1). The categories of unmet needs were developed in reference to the findings on associations between neuropsychiatric symptoms and basic physical needs [33] and environmental sources of discomfort [34, 35].
Web-based tool
The web-based tool provided a visualization of longitudinal changes in neuropsychiatric symptoms measured by the Neuropsychiatric Inventory to inform interdisciplinary decision-making. The professionals input the information collected during the discussion meeting using the web-based tool. The individual characteristics of persons with dementia were recorded at registration, including birth year and month, sex, and type of dementia.
Debriefing meeting
Participating professionals attended a three-hour debriefing meeting in mid-November 2019, six weeks after the training. They were divided into groups of four to six members to share their experiences with the program.
Data collection
A questionnaire completed on the day of training evaluated the attitudes towards persons with dementia, self-reported competence in dementia care, and care professionals’ personal characteristics. The care professionals responded to the questionnaire before the training course.
Care professionals assessed the neuropsychiatric symptoms and prescribed medication for each person with dementia. They input the information on the web-based tool at baseline (October 2019), after two months (T1 = December 2019), and after four months (T2 = February 2020). Care professionals also reported, via paper questionnaire, the time investment required to implement the program per person at each time of assessment.
Ethical considerations
The study protocol was approved by the Ethics Board of the Tokyo Metropolitan Institute of Medical Science (number 19–26). The research was conducted in accordance with the principles of the Declaration of Helsinki (version November 2013) and is in agreement with the law regarding medical-scientific research in humans. Written informed consent was obtained from each person with dementia and/or by proxy (typically, next of kin) as appropriate. This trial is registered at the UMIN Clinical Trials Registry (UMIN000037254).
Measurements
Assessment of persons with dementia
Information was made available via the web-based tool. We collected information on neuropsychiatric symptoms and prescribed medications. The Neuropsychiatric Inventory – Nursing Home version (NPI-NH) was used to assess the incidence, frequency, and severity of neuropsychiatric symptoms. The original NPI-NH comprised 12 items to rate the frequency and severity of neuropsychiatric symptoms in persons with dementia [36–39]. Scores for each item range from 0 to 12, with higher scores indicating more severe symptoms. Frequency and severity scores are multiplied to determine a total score ranging from 0 to 144. The Japanese version of the NPI-NH has good validity and reliability [40]. The drug name and daily dosage were recorded for each prescribed medication. In this study, the presence of prescribed medication for the nervous system was used for analysis based on the Anatomical Therapeutic Chemical classification. Percentages of the prescriptions were calculated for analgesics (N02), antipsychotics (N05A), anxiolytics (N05B), hypnotics and sedatives (N05C), antidepressants (N06A), and anti-dementia drugs (N06D).
Time investment
Care professionals completed a questionnaire at baseline, T1, and T2 to report the time required to implement the program per person with dementia. The program implementation comprised 1) preparation, 2) transportation, 3) meeting, and 4) management of the web-based tool (Supplementary Table 2). The care professionals also recorded the number of other professionals who had participated in the discussion meeting, and if any professionals had been invited but declined to participate. If so, the reason for declining was also reported (Supplementary Table 3).
Assessment of care professionals
Care professionals completed a questionnaire on the day of the training course, which evaluated their self-reported competence in dementia care and their personal characteristics. Competence in dementia care was measured using the Japanese version of the Sense of Competence in Dementia Care Staff (SCIDS) scale [41], comprising 17 items on a four-point Likert scale ranging from ‘1 = not at all’ to ‘4 = very much’. The total score ranges from 17 to 68, with higher scores indicating a higher sense of confidence. The Japanese version of the SCIDS has been validated [42]. Care professionals’ personal characteristics comprised age, sex, primary work qualification, tenure in care for older adults, and educational attainment. Primary work qualification was classified into 1) care manager/social worker, or 2) nurses or other direct care workers in the multivariate analysis.
Statistical analysis
Differences in personal and professional characteristics at baseline were assessed between four provider types (in-home care management agency, multiple in-home service, group home, and nursing home). One-way analyses of variance were used for continuous variables, and chi-square tests were used for categorical variables.
Changes in neuropsychiatric symptoms per type of provider were examined using multilevel linear regression analysis, including persons with dementia and care professionals as random effects, and time of assessment as fixed effects. Panel-data format was adopted in which the same person with dementia could appear two or three times (data at baseline, T1, and T2) to utilize information on cessation cases during follow-ups. The within-subject effect size was calculated using Cohen’s drm for the NPI-NH total score. The effect size was considered low if d values varied by approximately 0.20, medium if approximately 0.50, and large if greater than 0.80 [43, 44].
Multilevel linear regression analysis was also executed to examine the associations between time investment and type of provider. The model included other independent variables from professional characteristics (primary work qualification, tenure in care for older adults, and competence in dementia care), characteristics of persons with dementia (age, sex, type of dementia, use of anti-dementia drugs and antipsychotics), and level of neuropsychiatric symptoms. The interclass correlation coefficient (ICC) for care professional was calculated to evaluate the contribution of the difference of professionals to the variance in time investment. A sensitivity analysis was employed using the multilevel model where the dependent variable comprised each category of time investment rather than total minutes. Time investment for transportation was not included as the average number of minutes was small (4.2–5.8).
Statistical significance was considered at an overall α= 0.05. Adjustments for multiple testing included the Bonferroni correction with a significance threshold at 0.0125 for neuropsychiatric symptoms per type of provider. All statistical analyses were conducted using STATA version 16.1 (StataCorp, Texas).
RESULTS
Characteristics of persons with dementia
Registration
There were 130 persons with dementia registered in the web-based tool. Of these, 125 received a complete baseline assessment; 5 did not receive baseline assessments due to consent withdrawal (n = 2), hospital admission (n = 1), acute deterioration of physical health (n = 1), and the care professional’s change in place of work (n = 1). Those who completed the baseline assessment had a mean age of 85.1 (SD = 6.5) years and included 30 men (24.0%). Eighty-six persons had a diagnosis of Alzheimer’s disease (68.8%); 65 had been prescribed anti-dementia drugs (52.0%); and 22 had been prescribed antipsychotics (17.6%). These personal characteristics at baseline did not differ by type of provider except for the level of neuropsychiatric symptoms. The average total NPI-NH score varied between 18.7 in in-home care management agencies and 32.9 in nursing home residents (Table 1).
Table 1
Baseline characteristics of persons with dementia
| Baseline, Oct 2019 N (%) or mean (SD) | In-home care | Residential care | Test statistic | p | ||
| Care management (N = 38) | Multiple in-home (N = 28) | Group home (N = 36) | Nursing home (N = 23) | |||
| Age, y | 85.1 (5.6) | 85.1 (6.0) | 84.0 (7.3) | 86.7 (7.0) | F(3) = 0.81 | 0.489 |
| Sex, male | 14 (36.8) | 5 (17.9) | 6 (16.7) | 5 (21.7) | χ2(3) = 5.14 | 0.162 |
| Alzheimer’s disease | 28 (73.7) | 16 (57.1) | 26 (72.2) | 16 (69.6) | χ2(3) = 2.40 | 0.494 |
| Prescribed medication | ||||||
| Anti-dementia drugs (N06D) | 17 (44.7) | 15 (53.6) | 22 (61.1) | 11 (47.8) | χ2(3) = 2.19 | 0.534 |
| Antipsychotics (N05A) | 5 (13.2) | 8 (28.6) | 6 (16.7) | 3 (13.0) | χ2(3) = 3.19 | 0.363 |
| Analgesics (N02) | 1 (2.6) | 5 (17.9) | 4 (11.1) | 2 (8.7) | χ2(3) = 4.44 | 0.217 |
| Hypnotics and sedatives (N05C) | 2 (5.3) | 2 (7.1) | 5 (13.9) | 1 (4.3) | χ2(3) = 2.53 | 0.470 |
| Antidepressants (N06A) | 1 (2.6) | 2 (7.1) | 5 (13.9) | 1 (4.3) | χ2(3) = 3.88 | 0.275 |
| Anxiolytics (N05B) | 3 (7.9) | 0 (0.0) | 4 (11.1) | 0 (0.0) | χ2(3) = 5.47 | 0.140 |
| Neuropsychiatric symptoms (0–144) | 18.7 (16.1)a | 25.2 (17.7) | 28.8 (18.2) | 32.9 (19.2)a | F(3) = 3.64 | 0.015 |
SD, standard deviation. Level of neuropsychiatric symptoms was assessed using the Japanese version of the Neuropsychiatric Inventory-Nursing Home version (NPI-NH). aSignificant difference between the same alphabet letter at p < 0.05, Bonferroni correction.
Follow-ups
Of the 125 persons with dementia at baseline, 116 received a complete T1 assessment, and 108 received a complete T2 assessment (Fig. 1). Seventeen persons did not receive complete follow-ups due to an unavailable schedule for the discussion meeting (n = 6), hospital admission (n = 5), change in care professional’s place of work (n = 3), acute deterioration in physical health (n = 2), or death (n = 1) during the period. The 17 cessation cases did not differ from the remaining 108 cases in baseline characteristics or type of provider.
Characteristics of care professionals
Registration
A total of 74 care professionals from 62 providers participated in a 1-day training course for the program in Tokyo. Although the sample size was lower than expected, we concluded the recruitment by mid-September due to Typhoon Faxai. Of the 74 participating professionals, 7 declined to implement the program as they had been affected by Typhoon Hagibis and its aftermath in early October 2019. The remaining 67 professionals from 60 providers registered 130 persons with dementia in the program at baseline (Fig. 1). These 67 professionals completed the three-hour debriefing meeting. On average, 1.1 professionals per provider and 1.9 persons with dementia per professional were registered.
Baseline characteristics
The 67 care professionals had a mean age of 45.9 (SD = 10.3) years and included 29 men (43.3%). There were 34 direct care workers (other than nurses) (50.7%) and 30 care managers (44.8%). The mean number of months of tenure in care for older adults was 165.1 (SD = 89.1). Twenty-five professionals had graduated from university (37.3%) and 24 from vocational school or college (35.8%). The mean sense of competence in dementia care measured by SCIDS was 40.9 (SD = 6.8). Care professionals from in-home care management agencies were older, included fewer men, had a longer tenure in care for older adults, and included no professionals other than care managers. Most care professionals from group homes and nursing homes were direct care workers. There were no significant differences in measured competence in dementia care between types of providers or professionals (Table 2).
Table 2
Characteristics of care professionals
| Baseline, Sep 2019 N (%) or mean (SD) | In-home care | Residential care | Test statistic | p | ||
| Care management (N = 21) | Multiple in-home (N = 14) | Group home (N = 19) | Nursing home (N = 13) | |||
| Age, y | 51.2 (9.0)a | 44.1 (6.3) | 46.9 (9.1)b | 37.5 (12.3)a,b | F(3) = 6.08 | 0.001 |
| Sex, male | 5 (23.8) | 5 (35.7) | 13 (68.4) | 6 (46.2) | χ2(3) = 8.51 | 0.037 |
| Primary qualification for work | χ2(12) = 49.23 | <0.001 | ||||
| Professional who handles care plan/care coordination | ||||||
| Care manager | 21 (100.0) | 5 (35.7) | 2 (10.5) | 2 (15.4) | ||
| Social worker | 0 (0.0) | 0 (0.0) | 1 (5.3) | 0 (0.0) | ||
| Professional who provides care | ||||||
| Nurse | 0 (0.0) | 1 (7.1) | 0 (0.0) | 0 (0.0) | ||
| Direct care worker other than nurse | 0 (0.0) | 8 (57.1) | 16 (84.2) | 10 (76.9) | ||
| OT / PT | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Other, unspecified | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (7.7) | ||
| Educational attainment | χ2(9) = 8.76 | 0.460 | ||||
| Junior high school | 1 (4.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| High school | 4 (19.0) | 3 (21.4) | 6 (31.6) | 4 (30.8) | ||
| College, vocational school | 9 (42.9) | 4 (28.6) | 4 (21.1) | 7 (53.8) | ||
| University | 7 (33.3) | 7 (50.0) | 9 (47.4) | 2 (15.4) | ||
| Tenure in care for older adults, month | 231.1 (74.1)a,b,c | 151.4 (81.6)a | 134.7 (81.5)b | 117.5 (74.5)c | F(3) = 7.75 | <0.001 |
| Competence in dementia care (17–68) | 40.0 (7.6) | 40.4 (6.1) | 40.8 (6.0) | 43.2 (7.6) | F(3) = 0.63 | 0.598 |
SD, standard deviation. Competence in dementia care was measured by the Japanese version of the Sense of Competence in Dementia Care Staff scale. a,b,cSignificant difference between the same alphabet letter at p < 0.05, Bonferroni correction.
Intervention
At baseline, the most frequently specified unmet needs were another person bothering the person (n =67, 53.6%), sleepiness or tiredness (n = 58, 46.4%), and pain (n = 55, 44.0%). The action plan was mostly linked to pleasant activity/recreation (n = 38, 30.4%). The most frequent caregivers involved in the interdisciplinary meeting were direct care workers who were not nurses (n = 123, 98.4%) and care managers (n = 80, 64.0%) (Supplementary Table 1). On average, the baseline assessment was performed 19.8 (SD = 10.6) days after the training course. The mean number of days from baseline to T2 was 97.1 (SD = 21.9).
The average total NPI-NH score was 19.4 (SD = 16.4) at T1, and 16.0 (SD = 15.2) at T2. The total score decreased significantly from baseline to T1 and T2 in each type of provider, except for nursing homes between baseline and T1. The observed effect size at T2 was medium (0.44–0.61) at each type of provider (Table 3).
Table 3
Average total Neuropsychiatric Inventory scores by type of provider at baseline, T1, and T2
| In-home care | Residential care | |||||||
| Care management | Multiple in-home | Group home | Nursing home | |||||
| Time of assessment | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) |
| Baseline | 38 | 18.7 (16.1) | 28 | 25.2 (17.7) | 36 | 28.8 (18.2) | 23 | 32.9 (19.2) |
| T1 | 33 | 13.3 (11.2) | 27 | 17.8 (14.4) | 34 | 19.3 (13.4) | 22 | 30.9 (23.3) |
| Coefficient (95% CI) | –5.41* | –6.13* | –9.94* | –1.98 | ||||
| (–9.09, –1.73) | (–10.82, –1.44) | (–13.78, –6.09) | (–10.67, 6.70) | |||||
| T2 | 30 | 11.5 (12.1) | 26 | 16.7 (17.7) | 32 | 17.7 (14.8) | 20 | 19.3 (16.3) |
| Coefficient (95% CI) | –7.40* | –7.64* | –10.45* | –12.08* | ||||
| (–11.20, –3.59) | (–12.40, –2.89) | (–14.38, –6.52) | (–21.04, –3.12) | |||||
| Effect size, Cohen’s drm | 0.55 | 0.44 | 0.61 | 0.61 | ||||
| ICC in the null model | ||||||||
| Person with dementia | 0.304 | 0.350 | 0.308 | 0.197 | ||||
| Care professional | 0.304 | 0.350 | 0.308 | 0.197 | ||||
| Random effect in the full model | ||||||||
| Residual | 59.320 | 77.709 | 65.855 | 218.613 | ||||
| Person with dementia | 60.914 | 108.487 | 87.739 | 81.133 | ||||
| Care professional | 60.914 | 108.487 | 87.739 | 81.133 | ||||
Coefficients and 95% confidence intervals, and interclass correlation coefficient were estimated using multilevel linear regression analysis including person with dementia and care professional as random effects. Level of neuropsychiatric symptoms was assessed using the Japanese version of the Neuropsychiatric Inventory-Nursing Home version (NPI-NH). *p < 0.0125, Bonferroni significance threshold.
Time investment of care professionals
At baseline, the 119 persons with dementia had complete information on the time investment of care professionals for the program implementation. There were 111 persons with complete T1 information, and 108 with complete T2 information. The average total minutes for the five-month interventions with completed follow-ups was 417.9 (SD = 219.8) with a decrease of 8.6 points (SD = 14.0) in the NPI total score (Table 4). Two-fifths of the total time investment was spent on the discussion meeting, one-third on preparation for the meeting, and one-fifth on using the web-based tool to input evaluation of the NPI, unmet needs, and action plan determined in the meeting (Supplementary Table 2).
Table 4
Time investment of professionals for program implementation by type of provider
| Total N | Mean (SD) | In-home care | Residential care | |||||||
| Care management | Multiple in-home | Group home | Nursing home | |||||||
| N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | |||
| Minutes | ||||||||||
| Baseline | 119 | 193.7 (103.3) | 38 | 184.5 (71.1) | 25 | 172.4 (118.0) | 33 | 219.6 (126.6) | 23 | 194.8 (92.4) |
| T1 | 111 | 126.5 (77.6) | 33 | 133.2 (85.5) | 27 | 101.9 (60.8) | 30 | 151.8 (87.6) | 21 | 111.3 (58.2) |
| T2 | 108 | 101.2 (58.7) | 30 | 102.9 (53.4) | 26 | 64.7 (21.4) | 32 | 127.7 (77.0) | 20 | 123.5 (41.9) |
| Total | 103 | 417.9 (219.8) | 30 | 420.4 (190.4) | 24 | 311.0 (153.6) | 29 | 508.2 (281.6) | 20 | 411.4 (172.4) |
| Total cost, yen | 103 | 28836.6 | 30 | 26421.0 | 24 | 25122.3 | 29 | 31824.9 | 20 | 32584.0 |
| (14912.1) | (15231.3) | (13269.6) | (15006.9) | (15479.1) | ||||||
| Change in NPI | 103 | 8.6 (14.0) | 30 | 8.2 (11.1) | 24 | 6.6 (15.5) | 29 | 9.3 (13.3) | 20 | 10.3 (17.6) |
Estimated total cost accounted for time investment, national average wage, and number of participants in the discussion meeting. Level of neuropsychiatric symptoms was assessed using the Japanese version of the Neuropsychiatric Inventory-Nursing Home version (NPI-NH).
The average number of professionals who participated in the baseline meeting was 3.1 (SD = 1.9). Forty-eight persons (40.3%) experienced other professionals declining to participate in the meeting at baseline. The most frequent reason for declining to participate was time constraints. The percentage of professionals declining to participate did not differ by type of provider (χ2(6) = 12.08, p = 0.060) (Supplementary Table 3).
The multilevel linear regression analysis showed a significantly lower time investment at T1 and T2 than at baseline. The time investment among in-home care management agencies was significantly lower than that in group homes. There were significant associations of lower time investment with both a shorter tenure in care for older adults and greater competence in dementia care. The ICC for professionals was 0.280 (Table 5). Sensitivity analyses using each category instead of total time investment displayed a significantly higher time investment for preparation and use of the web-based tool among group homes than in-home care management agencies. Time investment for preparation was also significantly higher among nursing homes than care management agencies. There were significant associations between a lower time investment for preparation or use of the web-based tool and shorter tenure in care for older adults. Time investment for preparation was significantly lower among care professionals whose primary qualifications were nurses or other direct care workers, and professionals with greater competence in dementia care (Supplementary Table 4).
Table 5
Factors relating to time investment for the program implementation
| Coefficient (95% confidence interval) | Total minutes |
| Type of provider, reference = in-home care management | |
| Multiple in-home service provider | 7.97 (–32.39, 48.33) |
| Group home | 59.83* (16.80, 102.85) |
| Nursing home | 36.20 (–9.68, 82.08) |
| Time of evaluation, reference = baseline | |
| T1 | –66.92* (–80.43, –53.41) |
| T2 | –91.72* (–105.34, –77.61) |
| Characteristics of care professionals | |
| Primary qualification, care manager/social worker | 14.61 (–21.32, 50.54) |
| Tenure in care for older adults, month | 0.23* (0.06, 0.39) |
| Competence in dementia care (17–68) | –2.29* (–4.09., –0.49) |
| Characteristics of persons with dementia | |
| Age at baseline, year | 0.70 (–1.17, 2.57) |
| Sex, male | 21.59 (–7.52, 50.70) |
| Type of dementia, Alzheimer’s disease | 15.79 (–10.75, 42.33) |
| Prescribed medication | |
| N05A: antipsychotics | –6.33 (–35.74, 23.09) |
| N06D: anti-dementia drugs | 1.17 (–21.86, 24.21) |
| Level of neuropsychiatric symptoms (0–144) | 0.18 (–0.35, 0.72) |
| Random effect | |
| Residual | 2512.072 |
| Person with dementia | 1599.603 |
| Care professional | 1599.603 |
Multilevel linear regression analysis including person with dementia and care professionals as random effects. Interclass correlation coefficient inn the full model was 0.280 for care professional. Competence in dementia care was measured by the Japanese version of the Sense of Competence in Dementia Care Staff scale. Level of neuropsychiatric symptoms was assessed using the Japanese version of the Neuropsychiatric Inventory-Nursing Home version (NPI-NH). *p < 0.05.
DISCUSSION
The level of neuropsychiatric symptoms declined significantly in group home and nursing home residents as well as in in-home service users. The effect size of the reduction in neuropsychiatric symptoms was medium (0.44–0.61) for each type of provider. The time investment for program implementation among in-home care management agencies was significantly lower than for group homes and smaller at follow-ups than at baseline. To the best of our knowledge, this is the first study to have uncovered the time investment of a psychosocial dementia care program for neuropsychiatric symptoms both in in-home and residential care settings. Our findings have implications for exploring dissemination strategies for psychosocial dementia care interventions. Further, professionals with shorter tenure in care for older adults and those with greater competence in dementia care took significantly less time for implementation than professionals with longer tenure and lower competence.
Contrary to our expectations, the time investment was not higher among in-home care management agencies than in other types of providers. Furthermore, the time investment among in-home care management agencies was significantly lower than that in group homes. Although group home residents did not show a significant difference in their level of neuropsychiatric symptoms from in-home service users, persons with dementia living in group homes tended to have more severe neuropsychiatric symptoms than in-home service users [45]. Thus, care professionals may have needed more time for discussions to identify unmet needs and devise an action plan. It is globally recommended to start advance care planning to share the person’s values, goals, and preferences regarding the rest of life at the time of dementia diagnosis [19]; to date, however, there is no legislation or regulations regarding advance care planning in Japan [46]. The small-scale homelike environment in group homes could have added another disadvantage to program implementation. Although this environment has positive aspects such as increased decision authority among nursing staff [47], it has barriers in terms of integrated care for nursing staff working alone for a large part of the day [48]. Whereas the lack of regular multidisciplinary meetings is perceived as a facilitator to psychosocial management of neuropsychiatric symptoms [49], it could also escalate the time required for care professionals to become acquainted with the DEMBASE® program. It would be beneficial to explore the incorporation of advance care planning in the program implementation to make information more available to specify unmet needs and form an action plan.
In previous research in Tokyo, where the majority of participating professionals were in-home care managers, the professionals presumed that their challenges in holding interdisciplinary discussion meetings were specific to in-home care settings [26]. In this study, however, more than half of group home and nursing home residents received declines from professionals other than participating employees to attend the discussion meeting. Time constraint was the most frequently cited reason for the decline across all types of providers, which is consistent with international findings regarding the implementation of psychosocial interventions for persons with dementia [50, 51]. Therefore, the largest time investment at the first implementation is a unique barrier to program implementation regardless of setting. Since the time investment gradually decreased from baseline to follow-ups, care professionals who managed the implementation and other professionals who participated in the discussion meeting acquired knowledge regarding the individual needs of the person with dementia, resulting in a faster implementation process. The facilitators to program implementation included visualized feedback regarding mitigated neuropsychiatric symptoms via a web-based tool that motivated care professionals only at follow-up assessments [26]. This delayed-return feature of the program may increase the risk of cessation before follow-ups when the time investment for baseline assessment is felt not to be worth the expense. In this study, the most frequent reason for cessation was time constraints for the discussion meeting. An additional benefit scheme under the public long-term care insurance program would be helpful to reward the time investment and encourage program implementation until follow-ups.
The time investment was significantly lower among care professionals with greater professional competence in dementia care. Their enhanced competence may enable care professionals to more effectively explain the program and encourage other professionals to participate in discussion meetings. Our results imply that effective program implementation may require a certain level of competence among care professionals who manage the implementation for each provider. A shorter professional tenure in the care for older adults was also significantly related to lower time investment in all categories except for the discussion meeting. More experienced professionals may have required more time to adapt to new dementia care practice. Alternatively, less experienced professionals were aware of fewer signs of unmet needs and acted sooner to determine the action plan. However, even after adjustment for competence in dementia care and tenure in care for older adults, 28% of the variance (ICC = 0.280) in time investment was accounted for by individual care professionals, implying that time investment may depend on which professional manages the implementation. Further refinement of the program components should be explored to help care professionals proceed with implementation with minimum time investment. It should be cautioned that discussion in the care team is an essential aspect and that working too fast may increase the risk of overlooking the essence of a successful action plan. Instruction on how to structure the discussion meeting would be helpful to shorten the time required for professionals.
The total time investment for program implementation was 418 minutes on average, per person, besides an 8.6-point decrease in NPI total score during the five-month period, with three interdisciplinary discussion meetings. Using the national mean wage for long-term care providers in 2018 [52], the total cost for the five-month implementation was estimated at 3353 yen for a one-point decrease in NPI score. Since the incremental cost of a one-point increase in NPI score was estimated as 30 USD per month [7] or 247–409 USD per year [53], which would be driven by caregiving time [8, 54], the program may have yielded a fair return on the time investment. It should be noted that reduction in neuropsychiatric symptoms could impact the costs of psychotropic drug use, physical/psychological examination, consultation, and treatment for the management of neuropsychiatric symptoms [55, 56]. Future research should evaluate the change in these costs by a reduction in neuropsychiatric symptoms, as well as the time investment for program implementation.
Strengths and limitations
The main strength of our study lies in including both in-home and residential care settings in the implementation of a common psychosocial dementia care program. A significant reduction in neuropsychiatric symptoms was observed in both in-home and residential care settings, which incurred a substantial time investment. However, our study has some limitations. The number of participants per type of provider did not fulfil the expected sample size due to the effect of natural disasters during the recruitment process. Care professionals were also unable to access training courses following the government’s advice to avoid large gatherings during the COVID-19 pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). As the pandemic and consequent distancing might increase neuropsychiatric symptoms of people with dementia [57, 58], the psychosocial dementia care program delivered remotely through technology should be further explored. Currently, an e-learning training course of the program has been developed in the Netherlands and Japan to enable care professionals encouraged to avoid travel due to COVID-19 to participate in the program remotely.
The time investment was based on professionals’ reports, which may have resulted in response bias toward overestimation. Because participant recruitment was conducted by nationwide provider organizations, participating professionals probably had higher competence (mean 40.9) than care professionals in Tokyo (mean 37.0) [31]. This sampling bias may have resulted in lower time investment for the program implementation and underestimation of the association between time investment and professional competence. The present study did not assess the quality of life of persons with dementia, which should be evaluated to measure the cost-effectiveness of the program in the future.
Conclusions
The program implementation incurred a substantial time investment in both in-home and residential care settings. The total time investment may have a fair return when the level of reduced neuropsychiatric symptoms is considered. However, there is a risk of cessation before follow-ups when the time investment for the baseline assessment is felt not to be worth the expense. An additional benefit scheme to reward the time investment would be helpful to encourage the program implementation until follow-ups.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interests.
ACKNOWLEDGMENTS
This work was supported by the Ministry and Welfare Bureau for the Elderly, Ministry of Health, Labour and Welfare under Project Number Tsuika-24. This work was partly supported by the Tokyo Metropolitan Institute of Medical Science. None of these funding sources were involved in the design or conduct of this study. They had no input into data collection, management, analysis, or interpretation and were not able to monitor the manuscript for presentation, review, or approval. The authors would like to thank the persons with dementia and care professionals who participated in the survey.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/ADR-200235.
REFERENCES
[1] | PrinceM, WimoA, GuerchetM, AliGC, WuYT, PrinaM ((2015) ) World Alzheimer Report 2015. The Global Impact of Dementia. An Analysis of Prevalence, Alzheimer’s Incidence, Cost and Trends. Alzheimer’s Disease International, London. |
[2] | Livingston G , Sommerlad A , Orgeta V , Costafreda SG , Huntley J , Ames D , Ballard C , Banerjee S , Burns A , Cohen-Mansfield J , Cooper C , Fox N , Gitlin LN , Howard R , Kales HC , Larson EB , Ritchie K , Rockwood K , Sampson EL , Samus Q , Schneider LS , Selbæk G , Teri L , Mukadam N ((2017) ) Dementia prevention, intervention, and care. Lancet 390: , 2673–2734. |
[3] | World Health Organization ((2017) ) Global action plan on the public health response to dementia 2017-2025. WHO Document Production Services, Genova. |
[4] | Dodel R , Belger M , Reed C , Wimo A , Jones RW , Happich M , Argimon JM , Bruno G , Vellas B , Haro JM ((2015) ) Determinants of societal costs in Alzheimer’s disease: GERAS study baseline results. Alzheimers Dement 11: , 933–945. |
[5] | Belger M , Haro JM , Reed C , Happich M , Argimon JM , Brunom G , Dodel R , Jones RW , Vellas B , Wimo A ((2019) ) Determinants of time to institutionalisation and related healthcare and societal costs in a community-based cohort of patients with Alzheimer’s disease dementia. Eur J Health Econ 20: , 343–355. |
[6] | Nakanishi M , Igarashi A , Ueda K , Brnabic AJM , Treuer T , Sato M , Kahle-Wrobleski K , Meguro K , Yamada M , Mimura M , Arai H ((2020) ) Costs and resource use associated with community-dwelling patients with Alzheimer’s disease in Japan: Baseline results from the prospective observational GERAS-J study. J Alzheimers Dis 74: , 127–138. |
[7] | Herrmann N , Lanctôt KL , Sambrook R , Lesnikova N , Hébert R , McCracken P , Robillard A , Nguyen E ((2006) ) The contribution of neuropsychiatric symptoms to the cost of dementia care. Int J Geriatr Psychiatry 21: , 972–976. |
[8] | Costa N , Wübker A , De Mauléon A , Zwakhalen SMG , Challis D , Leino-Kilpi H , Hallberg IR , Stephan A , Zabalegui A , Saks K , Molinier L , Wimo A , Vellas B , Sauerland D , Binot I , Soto ME ; RightTimePlaceCare consortium ((2018) ) Costs of care of agitation associated with dementia in 8 European countries: Results from the RightTimePlaceCare study. J Am Med Dir Assoc 19: , 95.e1–95.e10. |
[9] | Gola AB , Morris S , Candy B , Davis S , King M , Kupeli N , Leavey G , Moore K , Nazareth I , Omar R , Vickerstaff V , Jones L , Sampson EL ((2020) ) Healthcare utilization and monetary costs associated with agitation in UK care home residents with advanced dementia: A prospective cohort study. Int Psychogeriatr 32: , 359–370. |
[10] | Lyketsos CG , Lopez O , Jones B , Fitzpatrick AL , Breitner J , DeKosky S ((2002) ) Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: Results from the cardiovascular health study. JAMA 288: , 1475–1483. |
[11] | Steinberg M , Shao H , Zandi P , Lyketsos CG , Welsh-Bohmer KA , Norton MC , Breitner JC , Steffens DC , Tschanz JT ; Cache County Investigators ((2008) ) Point and 5-year period prevalence of neuropsychiatric symptoms in dementia: The Cache County Study. Int J Geriatr Psychiatry 23: , 170–177. |
[12] | Samus QM , Rosenblatt A , Steele C , Baker A , Harper M , Brandt J , Mayer L , Rabins PV , Lyketsos CG ((2005) ) The association of neuropsychiatric symptoms and environment with quality of life in assisted living residents with dementia. Gerontologist 45: , S19–26. |
[13] | Bakker AJEM ((2010) ) Integrative Reactivation and Rehabilitation to Reduce Multiple Psychiatric Symptoms of Psychogeriatric Patients and Caregiver Burden. Thesis VUmc, Amsterdam. |
[14] | Maust DT , Langa KM , Blow FC , Kales HC ((2017) ) Psychotropic use and associated neuropsychiatric symptoms among patients with dementia in the USA. Int J Geriatr Psychiatry 32: , 164–174. |
[15] | Bränsvik V , Granvik E , Minthon L , Nordström P , Nägga K ((2020) ) Mortality in patients with behavioural and psychological symptoms of dementia: A registry-based study. Aging Ment Health, doi: 10.1080/13607863.2020.1727848. |
[16] | Algase DL , Beck C , Kolanowski A , Whall A , Berent S ((1996) ) Need-driven dementia compromised behavior: An alternative view of disruptive behavior. Am J Alzheimers Dis Other Demen 11: , 10–19. |
[17] | Cohen-Mansfield J , Werner P ((1995) ) Environmental influences on agitation: An integrative summary of an observational study. Am J Alzheimers Dis Other Demen 10: , 32–39. |
[18] | Kovach CR , Noonan PE , Schlidt AM , Wells T ((2005) ) A model of consequences of need-driven, dementia-compromised behavior. J Nurs Scholarsh 37: , 134–140. |
[19] | van der Steen JT , Radbruch L , Hertogh CM , de Boer ME , Hughes JC , Larkin P , Francke AL , Jünger S , Gove D , Firth P , Koopmans RT , Volicer L ; European Association for Palliative Care (EAPC) ((2014) ) White paper defining optimal palliative care in older people with dementia: A Delphi study and recommendations from the European Association for Palliative Care. Palliat Med 28: , 197–209. |
[20] | Zuidema SU , Smalbrugge M , Bil WME , Geelen R , Kok RM , Luijendijk HJ , van der Stelt I , van Strien AM , Vink MT , Vreeken HL ((2018) ) Multidisciplinaire Richtlijn probleemgedrag bij mensen met dementie. [Multidisciplinary Guideline problem behaviour in dementia.] Verenso, NIP, Utrecht. |
[21] | Vernooij-Dassen M , Vasse E , Zuidema S , Cohen-Mansfield J , Moyle W ((2010) ) Psychosocial interventions for dementia patients in long-term care. Int Psychogeriatr 22: , 1121–1128. |
[22] | Lord K , Berefotd-Dent J , Rapaport P , Burton A , Leverton M , Walters K , Lang I , Downs M , Manthorpe J , Boex S , Jackson J , Ogden M , Cooper C ((2020) ) Developing the New Interventions for independence in Dementia Study (NIDUS) theoretical model for supporting people to live well with dementia at home for longer: A systematic review of theoretical models and Randomised Controlled Trial evidence. Soc Psychiatry Psychiatr Epidemiol 55: , 1–14. |
[23] | Sundhedsstyrelsen ((2019) ) BPSD-modellen. Metode til målrettet pleje af beboere med demens og adfærdsmæssige og psykiske symptomer. [BPSD-model. Method for targeted care of residents with dementia and behavioural and psychological symptoms]. Sundhedsstyrelsen, Copenhagen. |
[24] | Bakker AJEM , Ziylan C ((2019) ) De-STIP-methodiek ‘De gepersonaliseerde stapsgewijze integrale aanpak van probleemgedrag bij mensen met dementie.’ [The STIP-methodology ‘The personalised step-by-step integrated approach to problem behavior among people with dementia.’]. https://www.hogeschoolrotterdam.nl/onderzoek/projecten-en-publicaties/zorginnovatie/samenhang-in-zorg/De-STIP-methodiek/project. |
[25] | Nakanishi M , Endo K , Hirooka K , Granvik E , Minthon L , Nägga K , Nishida A ((2018) ) Psychosocial behaviour management programme for home-dwelling people with dementia: A cluster-randomized controlled trial. Int J Geriatr Psychiatry 33: , 495–503. |
[26] | Nakanishi M , Ziylan C , Bakker TJ , Granvik E , Nägga K , Nishida A ((2020) ) Facilitators and barriers associated with the implementation of a Swedish psychosocial dementia care programme in Japan: A secondary analysis of qualitative and quantitative data. Scand J Caring Sci, doi: 10.1111/scs.12854. |
[27] | Scales K , Zimmerman S , Miller SJ ((2018) ) Evidence-based nonpharmacological practices to address behavioral and psychological symptoms of dementia. Gerontologist 58: , S88–102. |
[28] | Nakanishi M , Hattori K , Nakashima T , Sawamura K ((2014) ) Health care and personal care needs among residents in nursing homes, group homes, and congregate housing in Japan: Why does transition occur, and where can the frail elderly establish a permanent residence? J Am Med Dir Assoc 15: , 76.e1–6. |
[29] | Faul F , Erdfelder E , Lang AG , Buchner A ((2007) ) G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39: , 175–191. |
[30] | Faul F , Erdfelder E , Buchner A , Lang AG ((2009) ) Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav Res Methods 41: , 1149–1160. |
[31] | Nakanishi M , Hirooka K , Imai Y , Inoue S , Yukari Y , Katayama C , Miyamoto Y , Shindo Y , Ueno H , Toya J , Takano Y , Nishida A ((2018) ) Dementia care competence among care professionals and reduced challenging behavior of home-dwelling persons with dementia: A pre- and post-intervention data analysis. J Alzheimers Dis 64: , 515–523. |
[32] | Taylor MJ , McNicholas C , Nicolay C , Darzi A , Bell D , Reed JE ((2013) ) Systematic review of the application of the plan-do-study-act method to improve quality in healthcare. BMJ Qual Saf 23: , 290–298. |
[33] | Bédard A , Landreville P , Voyer P , Verreault R , Vézina J ((2011) ) Reducing verbal agitation in people with dementia: Evaluation of an intervention based on the satisfaction of basic needs. Aging Ment Health 15: , 855–865. |
[34] | Cohen-Mansfield J , Thein K , Marx MS , Dakheel-Ali M , Jensen B ((2013) ) Sources of discomfort in persons with dementia. JAMA Intern Med 173: , 1378–1379. |
[35] | Cohen-Mansfield J , Thein K , Marx MS , Dakheel-Ali M , Jensen B ((2015) ) Sources of discomfort in persons with dementia: Scale and initial results. Behav Neurol 2015: , 732832. |
[36] | Cummings JL , Mega M , Gray K , Rosenberg-Thompson S , Carusi DA , Gornbein J ((1994) ) The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology 44: , 2308–2314. |
[37] | Cummings JL ((1997) ) The Neuropsychiatric Inventory: Assessing psychopathology in dementia patients. Neurology 48: , S10–16. |
[38] | Kaufer DI , Cummings JL , Christine D , Bray T , Castellon S , Masterman D , MacMillan A , Ketchel P , DeKosky ST ((1998) ) Assessing the impact of neuropsychiatric symptoms in Alzheimer’s disease: The Neuropsychiatric Inventory Caregiver Distress Scale. J Am Geriatr Soc 46: , 210–215. |
[39] | Wood S , Cummings JL , Hsu MA , Barclay T , Wheatley MV , Yarema KT , Schnelle JF ((2008) ) The use of the Neuropsychiatric Inventory in nursing home residents. Characterization and measurement. Am J Geriatr Psychiatry 8: , 75–83. |
[40] | Shigenobu K , Hirono N , Tabushi K , Ikeda M ((2008) ) Validity and reliability of the Japanese Version of the Neuropsychiatric Inventory-Nursing Home Version (NPI-NH). Brain Nerve 60: , 1463–1469. |
[41] | Schepers AK , Orrell M , Shanahan N , Spector A ((2012) ) Sense of Competence in Dementia Care Staff (SCIDS) scale: Development, reliability, and validity. Int Psychogeriatr 24: , 1153–1162. |
[42] | Nakanishi M , Endo K , Hirooka K , Nakashima T , Morimoto Y , Granvik E , Minthon L , Nägga K , Nishida A ((2018) ) Dementia behaviour management programme at home: Impact of a palliative care approach on care managers and professional caregivers of home care services. Aging Ment Health 22: , 1057–1062. |
[43] | Cohen J ((1988) ) Statistical Power Analysis for the Behavioral Sciences. Routledge Academic, New York. |
[44] | Lakens D ((2013) ) Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front Psychol 4: , 863. |
[45] | Nakanishi M , Hirooka K , Morimoto Y , Nishida A ((2017) ) Quality of care for people with dementia and professional caregivers’ perspectives regarding palliative care in Japanese community care settings. Int J Geriatr Psychiatry 32: , 1342–1351. |
[46] | Nakanishi M , Nakashima T ((2014) ) Features of the Japanese national dementia strategy in comparison with international dementia policies: How should a national dementia policy interact with the public health- and social-care systems? Alzheimers Dement 10: , 468–476.e3. |
[47] | Willemse BM , Depla MFIA , Smit D , Pot AM ((2014) ) The relationship between small-scale nursing home care for people with dementia and staff’s perceived job characteristics. Int Psychogeriatr 26: , 805–816. |
[48] | Verbeek H , Zwakhalen SM , van Rossum E , Kempen GI , Hamers JP ((2012) ) Small-scale, homelike facilities in dementia care: A process evaluation into the experiences of family caregivers and nursing staff. Int J Nurs Stud 49: , 21–29. |
[49] | Appelhof B , Bakker C , van Duinen-van den Ijssel JCL , Zwijsen SA , Smalbrugge M , Verhey FRJ , de Vugt ME , Zuidema SU , Koopmans RTCM ((2018) ) Process evaluation of an intervention for the management of neuropsychiatric symptoms in young-onset dementia. J Am Med Dir Assoc 19: , 663–671. |
[50] | Dugmore O , Orrell M , Spector A ((2015) ) Qualitative studies of psychosocial interventions for dementia: A systematic review. Aging Ment Health 19: , 955–967. |
[51] | Mangiaracina F , Chattat R , Farina E , Saibene FL , Gamberini G , Brooker D , Evans SC , Evans SB , Szcześniak D , Urbanska K , Rymaszewska J , Hendriks I , Dröes RM , Meiland FJ ((2017) ) Not re-inventing the wheel: The adaptive implementation of the meeting centres support programme in four European countries. Aging Ment Health 21: , 40–48. |
[52] | Wage and Labour Welfare Statistics Office ((2019) ) Basic Survey on Wage Structure. Ministry of Health, Labour, and Welfare, Tokyo. |
[53] | Murman DL , Colenda CC ((2005) ) The economic impact of neuropsychiatric symptoms in Alzheimer’s disease: Can drugs ease the burden? Pharmacoeconomics 23: , 227–242. |
[54] | Rattinger B , Sanders CL , Vernon E , Schwartz S , Behrens S , Lyketsos CG , Tschanz JT ((2019) ) Neuropsychiatric symptoms in patients with dementia and the longitudinal costs of informal care in the Cache County population. Alzheimers Dement (N Y) 5: , 81–88. |
[55] | Bray J , Brooker D , Latham I , Wray F , Baines D ((2019) ) Costing resource use of the Namaste Care Intervention UK: A novel framework for costing dementia care interventions in care homes. Int Psychogeriatr, doi: 10.1017/S1041610218002314. |
[56] | van Duinen-van den Ijssel JCL , Bakker C , Smalbrugge M , Zwijsen SA , Adang E , Appelhof B , Zuidema SU , de Vugt ME , Verhey FRJ , Koopmans RTCM ((2020) ) Cost-consequence analysis of an intervention for the management of neuropsychiatric symptoms in young-onset dementia: Results from the BEYOND-II study. Int J Geriatr Psychiatry 35: , 131–137. |
[57] | Livingston G , Huntley J , Sommerlad A , Ames D , Ballard C , Banerjee S , Brayne C , Burns A , Cohen-Mansfield J , Cooper C , Costafreda SG , Dias A , Fox N , Gitlin LN , Howard R , Kales HC , Kivimäki M , Larson EB , Ogunniyi A , Orgeta V , Ritchie K , Rockwood K , Sampson EL , Samus Q , Schneider LS , Selbæk G , Teri L , Mukadam N ((2020) ) Dementia prevention, intervention, and care: 2020 report of the lancet Commission. Lancet 396: , 413–446. |
[58] | Boutoleau-Bretonnière C , Pouclet-Courtemanche H , Gillet A , Bernard A , Deruet AL , Gouraud I , Mazoue A , Lamy E , Rocher L , Kapogiannis D , Haj ME ((2020) ) . The effects of confinement on neuropsychiatric symptoms in Alzheimer’s disease during the COVID-19 crisis. J Alzheimers Dis 76: , 41–47. |




